Abstract
Nicotinamide phosphoribosyltransferase (Nampt ) is a key nicotinamide adenine dinucleotide (NAD) biosynthetic enzyme in mammals, converting nicotinamide into nicotinamide mononucleotide (NMN ), an NAD intermediate. First identified in humans as a cytokine pre-B-cell colony enhancing factor (PBEF ) and subsequently described as an insulin-mimetic hormone visfatin , Nampt has recently excited the scientific interest of researchers from diverse fields, including NAD biology, metabolic regulation, and inflammation. As an NAD biosynthetic enzyme, Nampt regulates the activity of NAD-consuming enzymes such as sirtuins and influences a variety of metabolic and stress responses. Nampt plays an important role in the regulation of insulin secretion in pancreatic β-cells. Nampt also functions as an immunomodulatory cytokine and is involved in the regulation of inflammatory responses. This chapter summarizes the various functional aspects of Nampt and discusses its potential roles in diseases, with special focus on type 2 diabetes mellitus (T2DM).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the pathogenesis of type 2 diabetes mellitus (T2DM), the tight regulation of insulin sensitivity and secretion is dysbalanced. This is caused by both environmental and genetic factors.
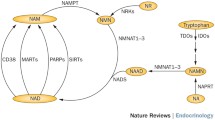
While the physiological significance of nicotinamide phosphoribosyltransferase (Nampt) in obesity, T2DM, and other metabolic disorders is still unclear, the intracellular nicotinamide adenine dinucleotide (NAD) biosynthetic function of Nampt is well characterized. In the NAD biosynthetic pathway from nicotinamide , Nampt (EC 2.4.2.12) catalyzes the rate-limiting step, namely the transfer of a phosphoribosyl group from 5-phosphoribosyl-1-pyrophosphate (PRPP) to nicotinamide, forming nicotinamide mononucleotide (NMN ) and pyrophosphate (PPi) (Rongvaux et al. 2002) (Fig. 1). NMN is then converted into NAD by the isoenzymes nicotinamide mononucleotide adenylyltransferase (Nmnat, EC 2.7.7.1) 1 – 3 (Fig. 1). While tryptophan and nicotinic acid are also precursors for NAD biosynthesis in mammals, nicotinamide is predominantly used to synthesize NAD (Magni et al. 1999; Rongvaux et al. 2003). NAD is a coenzyme with a well-established role in cellular redox reactions. Recently, several lines of evidence have implicated NAD biochemistry in a broad range of biological functions. For example, NAD is used as a substrate in a number of important signaling pathways in mammalian cells, including poly(ADP-ribosyl)ation in DNA repair (Ménissier de Murcia et al. 2003), mono-ADP-ribosylation in both the immune response and G-protein-coupled signaling (Corda and Di Girolamo 2003), and synthesis of cyclic ADP-ribose and nicotinate adenine dinucleotide phosphate (NAADP) in intracellular calcium signaling (Lee 2001). Furthermore, NAD and its derivatives also play important roles in transcriptional regulation (Lin and Guarente 2003). In particular, the discovery that yeast and mammalian Sir2 (silent information regulator 2 ) proteins require NAD for their deacetylase activity (Imai et al. 2000) has drawn much attention to this novel regulatory role for NAD.
NAD biosynthetis from nicotinamide. The rate-limiting step in mammalian NAD biosynthesis from nicotinamide is the transfer of a phosphoribosyl residue from 5-phosphoribosyl-1-pyrophosphate (PRPP) to nicotinamide catalyzed by nicotinamide phosphoribosyltransferase (Nampt ) to produce nicotinamide mononucleotide (NMN ), which is then converted into NAD by nicotinamide mononucleotide adenylyltransferase (Nmnat)
Although Nampt enzymatic activity was reported and characterized as early as 1957 (Preiss and Handler 1957), the gene encoding Nampt was first identified in Haemophilus ducreyi in 2001 (Martin et al. 2001). Since then, several groups have characterized the enzymological features of mammalian Nampt (Revollo et al. 2004; van der Veer et al. 2005). The K m value of Nampt for nicotinamide is ~1 μM, and it does not use nicotinic acid as a substrate (Revollo et al. 2004). The crystal structure of Nampt has been determined, which clearly demonstrates that this protein belongs to the dimeric class of type II phosphoribosyltransferases (Khan et al. 2006; Kim et al. 2006; Wang et al. 2006).
Whereas the biochemical and structural basis of Nampt as an intracellular NAD biosynthetic enzyme has been well established, this protein seems to have several other physiological functions. Human Nampt was originally characterized as a cytokine named pre-B-cell colony enhancing factor (PBEF ) (Samal et al. 1994). Nampt was also claimed to function as an insulin-mimetic adipocytokine, predominantly secreted from visceral fat and therefore named visfatin (Fukuhara et al. 2005). We will discuss these different functional aspects of Nampt and its potential roles in a variety of pathophysiological conditions including type 2 diabetes.
2 Nampt and Metabolic Disorders
The function of intracellular Nampt (iNampt) as NAD biosynthetic enzyme has been well characterized. In contrast, the physiological role of extracellular Nampt (eNampt) has been a matter of much debate. At least three different functions have been assigned to eNampt – an insulin-mimetic, a cytokine -like, and a function as an NMN producing enzyme (Pilz et al. 2007; Revollo et al. 2007a; Sethi 2007; Yang et al. 2006). It is not clear which of these functions is important in what physiological context. There is also no study that has demonstrated in what way eNampt enters extracellular space and what distinguishes iNampt and eNampt on the molecular level. This makes it difficult to investigate the role that iNampt and eNampt play in various pathophysiological metabolic states as is discussed in more detail below.
2.1 eNampt and iNampt: Regulation of Pancreatic β-Cell Function
The most controversial function assigned to the Nampt protein was the insulin-mimetic activity as an adipocytokine named “visfatin ” described by Fukuhara et al. (2005). This study found that eNampt acted like insulin by binding to the insulin receptor , activating the associated downstream signaling pathway and eliciting similar biological reponses in vitro and in vivo on adipogenesis, cellular glucose uptake, and blood glucose levels. Their results immediately drew attention to a possible connection between eNampt and metabolic complications, such as obesity and T2DM. However, this paper has been retracted (Fukuhara et al. 2007). Three subsequent studies have so far provided indirect evidence for the connection between eNampt and insulin signaling (Dahl et al. 2007; Song et al. 2008; Xie et al. 2007). One group has reported an insulin-like action of eNampt on osteoblasts, which was blocked by the pretreatment with the insulin receptor kinase inhibitor HNMPA-(AM)3 (Xie et al. 2007). In another study, it has been reported that the same inhibitor blocked the effect of eNampt on matrix metalloproteinase (MMP)-9 activity in THP-1 cells and the production of TNF-α and IL-8 in PBMC (Dahl et al. 2007). A third group has found multiple actions of eNampt on cultured kidney mesangial cells. eNampt induced uptake of glucose, glucose transporter (GLUT)-1 protein expression, and synthesis of profibrotic molecules, including transforming growth factor (TGF)-β1, plasminogen activator inhibitor (PAI)-1, and type I collagen (Song et al. 2008). Whereas a potent Nampt inhibitor FK866 (Hasmann and Schemainda 2003) blocked this eNampt-mediated glucose uptake, knockdown of the insulin receptor also inhibited this effect (Song et al. 2008). Unfortunately, none of the above studies has examined whether eNampt directly binds to the insulin receptor of respective target cells and whether the NAD biosynthetic activity is required for the observed effect.
In another study, it was demonstrated that eNampt does not exert insulin-mimetic effects in vitro or in vivo, but rather exhibits robust NMN biosynthetic activity and that this eNampt-mediated NMN biosynthesis plays a critical role in the regulation of glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells in vitro and in vivo (Revollo et al. 2007b). To address the physiological significance of Nampt function in vivo, Nampt-deficient mice were generated. While Nampt homozygous (Nampt −/−) mice are embryonic lethal likely due to failure of adequate NAD biosynthesis, Nampt heterozygous (Nampt +/−) mice do not differ visibly from wild-type mice but show significant decreases in total NAD levels in tissues (Revollo et al. 2007b). Nampt +/− female mice revealed moderately impaired glucose tolerance and a significant defect in GSIS. Whereas islet morphology and size in Nampt +/− mice do not differ from control mice, further analyses of isolated primary islets revealed that Nampt +/− islets have functional defects in NAD biosynthesis and GSIS. Remarkably, insulin secretion defects in Nampt +/− mice and islets can be corrected by administration of NMN, confirming that the defects observed in Nampt +/− mice and islets are due to a lack of the NAD biosynthetic activity of Nampt. Furthermore, FK866, a potent chemical inhibitor of Nampt, significantly inhibited NAD biosynthesis and GSIS in isolated wild-type primary islets. Again, administration of NMN ameliorated defects in NAD biosynthesis and GSIS in FK866-treated wild-type islets. Thus, pancreatic β-cells require Nampt-mediated NAD biosynthesis to maintain normal NAD biosynthesis and GSIS and are capable of incorporating NMN from the extracellular space.
These findings are supported by a study on the mouse pancreatic β-cell line βTC6. Incubation with recombinant Nampt caused significant changes in the mRNA expression of several key diabetes-related genes, including upregulation of insulin, hepatocyte nuclear factor (HNF)-1β, HNF-4α, and nuclear factor-κB. Significant downregulation was seen in angiotensin-converting enzyme and UCP2. Insulin secretion was increased compared to that of control at low glucose and this increase was blocked by coincubation with the specific Nampt inhibitor FK866. Both Nampt and NMN induced activation of insulin receptor and extracellular signal-regulated kinase (ERK)1/2. Nampt-induced insulin receptor and ERK1/2 activation were inhibited by FK866 (Brown et al. 2010).
The contradictory results on the insulin-mimetic activity of Nampt could be explained by a possible crosstalk between eNampt-mediated and insulin signaling pathways in a cell type-dependent manner. eNampt could possibly bind to and activate an unidentified receptor that might indirectly affect insulin signaling. Another possibility is that Nampt-mediated NAD biosynthesis might have an impact on the insulin signaling pathway. To address these possibilities in future studies, it is critical to examine (1) whether the existence of the insulin receptor is necessary for the observed insulin-mimetic effects by using mutant cells that lack the insulin receptor, (2) whether nicotinamide , which is usually included at very high concentrations in cell culture media, is necessary to observe those effects, and (3) whether mutant Nampt proteins that lack NAD biosynthetic activity can still mediate the activity of interest in each condition. These analyses will resolve contradictory results around the claimed insulin-mimetic activity of Nampt.
In humans, it has been reported that individuals who carry specific single nucleotide polymorphism variants in the Nampt gene promoter region have lower fasting plasma insulin levels (Bailey et al. 2006; Mirzaei et al. 2009), suggesting that Nampt-mediated NAD biosynthesis might also regulate insulin secretion in humans.
Aging is one of the greatest risk factors for developing T2DM and other metabolic complications (Chang and Halter 2003; Moller et al. 2003). Sirtuins are a group of NAD -dependent enzymes that regulate metabolic responses to nutritional availability in different tissues and cellular responses to a variety of stresses and are also involved in the regulation of aging. Sirtuins deacetylate and/or ADP-ribosylate lysine residues of many target regulatory factors (Blander and Guarente 2004; Schwer and Verdin 2008). In their deacetylation reactions, sirtuins produce acetyl-ADP-ribose, nicotinamide , and deacetylated proteins. Silent information regulator 2 (Sir2), as the prototypical enzyme of this group, regulates the replicative life span of yeast mother cells (Kaeberlein et al. 1999). Strikingly, Sir2 homologues also regulate life span in worms and flies (Rogina and Helfand 2004; Tissenbaum and Guarente 2001) and, depending on the genetic background, mediate life span extension caused by caloric restriction, the only dietary regimen that can retard aging and extend life span in a wide variety of organisms (Guarente 2005). It is not yet known whether Sirt1 , the mammalian Sir2 orthologue, regulates aging and longevity in mammals. Because sirtuins absolutely require NAD for their function, NAD biosynthesis and Nampt play a critical role in the regulation of mammalian sirtuin activity.
A recent study found that Nampt -mediated systemic NAD biosynthesis and Sirt1 activity are affected in pancreatic β-cells of aged mice. Pancreatic β-cell-specific Sirt1-overexpressing (BESTO) mice exhibited significantly enhanced GSIS and improved glucose tolerance (Moynihan et al. 2005). However, these phenotypes were completely lost in both BESTO males and females when they reached 18–24 months of age (Ramsey et al. 2008). Plasma NMN levels and Sirt1 activity in pancreatic islets were significantly reduced in those aged BESTO mice.
Consistent with this finding, NMN administration could restore enhanced GSIS and improved glucose tolerance in aged BESTO females but not in males (Ramsey et al. 2008), although the reason for the observed sex-dependent difference remained unclear. These findings suggest that an age-dependent decline in Nampt -mediated systemic NAD biosynthesis contributes to reduced Sirt1 activity in aged pancreatic islets and likely in other aged tissues. Because sirtuins have recently emerged as promising pharmaceutical targets to develop therapeutic interventions against age-associated diseases (Milne et al. 2007; Westphal et al. 2008), the systemic enhancement of NAD biosynthesis might provide another pharmacological means to activate Sirt1 and to convey benefits against metabolic diseases like T2DM (Imai and Kiess 2009).
2.2 eNampt in Human Circulation: A Biomarker for Obesity and T2DM?
Nampt possesses neither a signal sequence nor a caspase I cleavage site (Rongvaux et al. 2002). Therefore, several studies have suggested that eNampt might be released simply by cell lysis or cell death (Hug and Lodish 2005; Stephens and Vidal-Puig 2006). On the other hand, it was shown that eNampt release is governed by a highly regulated positive secretory process in a cell type-dependent manner (Revollo et al. 2007b). Fully differentiated mouse and human adipocytes are capable of secreting eNampt through a nonclassical secretory pathway , which is not blocked by inhibitors of the classical ER–Golgi secretory pathway, such as brefeldin A and monensin (Revollo et al. 2007b; Tanaka et al. 2007). There is evidence that other cell types, such as human primary hepatocytes (Garten et al. 2010) and leukocytes (D. Friebe, personal communication) are also a significant source of eNampt in human circulation.
Numerous studies have been published, addressing possible associations between plasma eNampt levels and anthropometric and metabolic parameters in obesity and T2DM. So far, results have been conflicting, showing positive, negative, or no association (Arner 2006; Revollo et al. 2007a; Sethi 2007; Stephens and Vidal-Puig 2006). For example, one study reported a positive correlation of plasma eNampt concentrations with body mass index (BMI) and percent body fat (Berndt et al. 2005), while another study found that plasma eNampt is reduced in human obesity and not related to insulin resistance (Pagano et al. 2006). Two other studies reported that higher plasma eNampt levels are independently and significantly associated with T2DM even after adjusting for known biomarkers (Chen et al. 2006; Retnakaran et al. 2008). These contradictory findings appear to be in part due to significant differences in immunoassays and the treatment and type of samples. Freeze–thaw cycles and different sample additives have considerable influence on the measurement of eNampt concentrations (Nüsken et al. 2007). Also, commercially available immunoassays differ considerably in the specificity and sensitivity of eNampt detection in human serum and plasma (Körner et al. 2007).
Looking at recent studies (as summarized in Table 1), it appears that most groups detected an elevation of plasma Nampt levels in obese subjects. However, the association between plasma eNampt levels and metabolic disorders, such as obesity and T2DM, is still unclear, and careful assessments with highly accurate assays for the measurement of eNampt will be necessary to address this critical issue.
Interestingly, in a recent study, Nampt levels in cerebrospinal fluid (CSF) were found to decrease with increasing plasma Nampt concentrations, BMI, body fat mass, and insulin resistance, indicating that the transport of Nampt across the blood–brain barrier might be impaired in obesity and that central nervous Nampt insufficiency or resistance might be linked to pathogenetic mechanisms of obesity (Hallschmid et al. 2009).
2.3 Role of Hepatic Nampt in T2DM
Hepatic insulin resistance is an underlying cause of the metabolic syndrome and the development of T2DM. In particular, the failure of insulin to suppress hepatic gluconeogenesis leads to hyperglycemia and, consequently, to the persistent stimulation of insulin production (Leclercq et al. 2007).
Sirt1 has been implicated in the regulation of hepatic glucose metabolism through the induction of gluconeogenic genes (Rodgers et al. 2005). Nampt regulates Sirt1 activity and also seems to be involved in the regulation of insulin signaling in the liver, as has been shown in animal studies. One group reported upregulation of Sirt1 and PPAR-α together with increasing NAD levels and Nampt activity in fasting mice (Hayashida et al. 2010). In rats that were injected with a Nampt overexpression plasmid insulin sensitivity was increased, while total cholesterol plasma levels decreased. The animals also displayed an enhanced insulin receptor substrate (IRS)-1 tyrosine phosphorylation in response to insulin as well as increased mRNA expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) and sterol regulatory element-binding protein 2 (SREBP-2) in the liver and adipose tissues (Sun et al. 2009). SIRT1 protein and activity was increased by treatment with metformin through an adenosine monophosphate kinase (AMPK)-mediated increase in gene expression of Nampt in db/db mice (Caton et al. 2010).
Hepatic insulin resistance and a dysregulation of hepatic glucose and lipid metabolism are also major reasons for the development of nonalcoholic fatty liver disease (NAFLD) , which has been recognized as a component of the metabolic syndrome and can lead to liver cirrhosis. There are few studies on Nampt expression in human livers. In patients with liver cirrhosis, it was found that hepatic Nampt mRNA expression and Nampt levels in circulation were decreased compared with healthy controls (de Boer et al. 2009). In severely obese patients with NAFLD, Nampt serum levels were shown to be increased. After bariatric surgery, Nampt expression in livers and Nampt serum levels decreased as the patients lost weight (Moschen et al. 2009). Another report demonstrated a correlation of serum Nampt levels with liver histology in NAFLD. Nampt levels predicted the presence of portal inflammation in NAFLD patients (Aller et al. 2009).
From these results in animal and human studies, one can speculate that Nampt is involved in regulating glucose metabolism in hepatocytes through activation of Sirt1 . The liver also seems to be a major source for circulating Nampt, and Nampt levels in circulation are consequently influenced by impaired liver function.
3 eNampt: A Link Between T2DM and Inflammation
Human Nampt was originally identified by a screen of a human peripheral blood lymphocyte cDNA library and named pre-B-cell colony enhancing factor (PBEF , see above). This 52 kDa protein was reported to act as a presumptive cytokine that increased pre-B-cell colony forming activity together with interleukin (IL)-7 and stem cell factor (SCF) (Samal et al. 1994). iNampt is also highly expressed in human fetal membranes, amnion, and placenta. Nampt mRNA expression increases in fetal membranes after labor and in severely infected amnion membranes. Interestingly, eNampt treatment upregulates the expression of inflammatory cytokines , such as IL-6 and IL-8, in amnion-like epithelial cells. Thus, it has been speculated that eNampt might have a cytokine-like function involved in the regulation of labor and in infection-induced preterm birth (Ognjanovic and Bryant-Greenwood 2002; Ognjanovic et al. 2005).
In the pathophysiology of obesity and T2DM, it has been revealed that chronic inflammation plays an important role in the development of insulin resistance and other associated complications, such as atherosclerosis (Guest et al. 2008; Poirier et al. 2006; Schenk et al. 2008; Sowers 2003). In this regard, one might speculate that eNampt could show an association with the development of vascular inflammation induced by obesity and T2DM, instead of an association with anthropometric and metabolic parameters. Indeed, it has been shown that eNampt induces the adhesion of leukocytes to endothelial cells and aortic endothelium by activating intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 (Adya et al. 2008). This phenomenon appears to be mediated through the proinflammatory transcription factor nuclear factor-κB (NF-κB) in a reactive oxygen species (ROS)-dependent manner. The same study also revealed that eNampt significantly increases the transcriptional activity of NF-κB in human vascular endothelial cells, resulting in the activation of the MMP-2/9.
A study that was aimed at investigating the expression of Nampt in circulating blood monocytes in obese and/or type 2 diabetic human subjects found that Nampt expression was significantly upregulated in obese type 2 diabetic patients, whereas obese nondiabetics exhibited similar levels compared to lean controls (Laudes et al. 2010).
In contrast, an upregulation of Nampt mRNA expression levels in peripheral blood cells (PBCs) of obese compared with lean subjects was reported, along with a correlation of Nampt plasma levels to cholesterol, triglycerides, and hepatic enzymes in circulation (Catalán et al. 2010). Another group determined Sirt1 expression in peripheral PBMC and found that insulin resistance and metabolic syndrome were associated with low PBMC Sirt1 gene and protein expression. Sirt1 gene expression was negatively correlated with carotid intima-media thickness (CIMT). In the monocytic THP-1 cell line, high glucose and palmitate reduced Sirt1 and Nampt expression and reduced the levels of intracellular NAD through oxidative stress. These effects on Nampt and Sirt1 were prevented by resveratrol in vitro (de Kreutzenberg et al. 2010). Moreover, a direct association of Nampt with advanced carotid atherosclerosis and CIMT in patients with T2DM was determined with increased Nampt serum levels, especially in patients with carotid plaques (Kadoglou et al. 2010). Therefore, eNampt might play an important role in the progression and/or the associated complications, especially inflammatory complications, of obesity and T2DM.
eNampt has also been shown to be involved in the regulation of apoptosis as a cytokine . In human neutrophils, eNampt inhibits their apoptosis in response to various inflammatory stimuli, although this particular effect of eNampt requires the presence of iNampt to some extent (Jia et al. 2004). Recently, Nampt was identified as an essential enzyme mediating granulocyte colony-stimulating factor (G-CSF)-triggered granulopoiesis in healthy individuals and in individuals with severe congenital neutropenia. Both extracellularly and intracellularly administered Nampt induced granulocytic differentiation of CD34(+) hematopoietic progenitor cells by NAD -dependent Sirt1 activation and subsequent upregulation of G-CSF synthesis and G-CSF receptor expression (Skokowa et al. 2009).
Furthermore, serum eNampt levels are found to be upregulated in sepsis patients, and rates of neutrophil apoptosis are found to be profoundly reduced in those patients (Jia et al. 2004). In amniotic epithelial cells, eNampt treatment appears to confer protection from apoptosis as a stretch-responsive cytokine (Kendal-Wright et al. 2008). In patients with inflammatory bowel disease (Crohn’s disease and ulcerative colitis), Nampt mRNA levels are increased in colon biopsy samples, and plasma eNampt levels are elevated (Moschen et al. 2007). Because eNampt stimulates the production of proinflammatory cytokines in PBMCs and upregulates IL-6 mRNA and serum levels in vivo when given intraperitoneally to mice, it has been suggested that eNampt itself also functions as a proinflammatory cytokine (Moschen et al. 2007). Stimulation of the tumor necrosis factor family member TNF- and APOL-related leukocyte-expressed ligand (TALL)-1, which is involved in lupus-like autoimmune diseases, increased Nampt mRNA (Xu et al. 2002). Additionally, Nampt has been found to be upregulated in a variety of other immunological disorders including acute lung injury, rheumatoid arthritis, and myocardial infarction and is considered a novel mediator of innate immunity (Luk et al. 2008).
These studies all suggest that eNampt might function as an inflammatory cytokine . In most studies, it has not been fully addressed which activity of eNampt, NAD biosynthetic activity vs. cytokine-like activity, is responsible for the observed effects of eNampt. However, two studies have reported an enzyme-dependent proinflammatory action of Nampt : in inflammatory cells in vitro and in joints affected with rheumatoid arthritis in vivo (Busso et al. 2008) and in cultured human aortic smooth muscle cells (Romacho et al. 2009). In contrast, one group has demonstrated that eNampt protects macrophages from ER stress-induced apoptosis through its cytokine-like activity that is totally separated from its NAD biosynthetic activity (Li et al. 2008).
There is evidence that Nampt exerts its proinflammatory actions through the upregulation of monocyte chemoattractant protein (MCP)-1. MCP-1 levels were significantly increased after Nampt administration in cultured human adipocyte supernatant and serum of mice. The detectability of Nampt in serum predicted circulating MCP-1 independent of age and gender in humans (Sommer et al. 2010).
Therefore, although how eNampt exerts its cytokine -like activity in different cellular contexts still needs to be investigated, there is clear evidence that it functions as an immunomodulatory cytokine.
4 Concluding Remarks and Future Aspects
Nampt functions as an intra- and extracellular NAD biosynthetic enzyme that is important for the regulation of metabolism and stress resistance through sirtuins and other NAD-consuming regulators. On the other hand, eNampt appears to act as a cytokine , independent of its enzymatic activity, and plays a major role in the regulation of immune responses. The cytokine-like and the enzymatic functions of Nampt still need to be investigated extensively. Thorough assessments of these two roles will presumably greatly enhance our knowledge about its role in different physiological contexts. In this regard, it will also be important to analyze the mechanism and regulation of eNampt secretion and identify its putative receptor and upstream signaling.
To further clarify the effects of iNampt on cellular metabolism, it will also be important to understand the subcellular compartmentalization of Nampt and other enzymes involved in the biosynthesis and the breakdown of NAD and the regulation of their localization. Additionally, very little is known about the flux of NAD substrates, intermediates, and metabolites. Therefore, it will be critical to study not only the regulation of NAD biosynthesis and breakdown in each cellular compartment, but also the spatial and temporal dynamics of NAD metabolism at a systemic level using a metabolomics approach. For example, it will be of great interest to clarify how NMN as a product from the eNampt enzymatic reaction is distributed to target tissues, e.g., pancreatic β-cells, and how it mediates its physiological and pharmacological effects in these tissues.
To date, a substantial part of the studies on the biological functions of Nampt has been conducted in cell culture and mouse models, and the studies on possible correlations between human plasma eNampt levels and metabolic parameters have been contradictory. Therefore, more work needs to be done to elucidate the physiological relevance of the eNampt function in normal individuals and patients with metabolic and other diseases in humans.
Nampt itself or any component in Nampt-mediated systemic NAD biosynthesis could be an effective therapeutic target/reagent for the prevention and the treatment of metabolic disorders including obesity and T2DM, inflammation, and cancer. Because downstream regulators, such as sirtuins and PARPs, have pleiotropic functions, more rigorous investigations will be necessary to clarify possible benefits from the manipulation of Nampt-mediated NAD biosynthesis.
References
Adya R, Tan BK, Chen J, Randeva HS (2008) Nuclear factor B induction by visfatin in human vascular endothelial cells: role in MMP-2/9 production and activation{kappa}. Diab Care 31:758–760
Alghasham AA, Barakat YA (2008) Serum visfatin and its relation to insulin resistance and inflammation in type 2 diabetic patients with and without macroangiopathy . Saudi Med J 29:185–192
Aller R, de Luis DA, Izaola O, Sagrado MG, Conde R, Velasco MC (2009) Influence of visfatin on histopathological changes of non-alcoholic fatty liver disease . Dig Dis Sci 54:1772–1777
Araki S, Dobashi K, Kubo K, Kawagoe R, Yamamoto Y, Kawada Y, Asayama K, Shirahata A (2008) Plasma visfatin concentration as a surrogate marker for visceral fat accumulation in obese children . Obesity (Silver Spring) 16:384–388
Arner P (2006) Visfatin - a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab 91:28–30
Bailey SD, Loredo-Osti JC, Lepage P, Faith J, Fontaine J, Desbiens KM, Hudson TJ, Bouchard C, Gaudet D, Pérusse L, Vohl MC, Engert JC (2006) Common polymorphisms in the promoter of the visfatin gene (PBEF1) influence plasma insulin levels in a French-Canadian population . Diabetes 55:2896–2902
Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Stumvoll M, Blüher M (2005) Plasma visfatin concentrations and fat depot-specific mRNA expression in humans . Diabetes 54:2911–2916
Blander G, Guarente L (2004) The Sir2 family of protein deacetylases. Annu Rev Biochem 73:417–435
Brown JE, Onyango DJ, Ramanjaneya M, Conner AC, Patel ST, Dunmore SJ, Randeva HS (2010) Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells . J Mol Endocrinol 44:171–178
Busso N, Karababa M, Nobile M, Rolaz A, Van Gool F, Galli M, Leo O, So A, DeSmedt T (2008) Pharmacological inhibition of nicotinamide phosphoribosyltransferase/visfatin enzymatic activity identifies a new inflammatory pathway linked to NAD. PLoS ONE 3:e2267
Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Cienfuegos JA, Salvador J, Frühbeck G (2010) Association of increased Visfatin/PBEF/NAMPT circulating concentrations and gene expression levels in peripheral blood cells with lipid metabolism and fatty liver in human morbid obesity . Nutr Metab Cardiovasc Dis. doi:10.1016/j.numecd.2009.09.008
Caton PW, Nayuni N, Kieswich J, Khan N, Yaqoob M, Corder R (2010) Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol 205:97–106
Chang AM, Halter JB (2003) Aging and insulin secretion . Am J Physiol Endocrinol Metab 284:E7–E12
Chen CC, Li TC, Li CI, Liu CS, Lin WY, Wu MT, Lai MM, Lin CC (2007) The relationship between visfatin levels and anthropometric and metabolic parameters: association with cholesterol levels in women. Metabolism 56(9):1216–1220
Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ (2006) Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus . J Clin Endocrinol Metab 91:295–299
Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT (2008) Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 32(2):268–274
Choi KM, Kim JH, Cho GJ, Baik SH, Park HS, Kim SM (2007) Effect of exercise training on plasma visfatin and eotaxin levels . Eur J Endocrinol 157:437–442
Choi KM, Lee JS, Kim EJ, Baik SH, Seo HS, Choi DS, Oh DJ, Park CG (2008) Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease . Eur J Endocrinol 158:203–207
Corda D, Di Girolamo M (2003) Functional aspects of protein mono-ADP-ribosylation. EMBO J 22:1953–1958
Coskun A, Ozkaya M, Kiran G, Kilinc M, Arikan DC (2010) Plasma visfatin levels in pregnant women with normal glucose tolerance, gestational diabetes and pre-gestational diabetes mellitus . J Matern Fetal Neonatal Med 23:1014–1018
Dahl TB, Ynestad A, Skjelland M, Øie E, Dahl A, Michelsen A, Damås JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Frøland SS, Krohg-Sørensen K, Russell D, Aukrust P, Halvorsen B (2007) Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization . Circulation 115:972–980
de Boer JF, Bahr MJ, Böker KH, Manns MP, Tietge UJ (2009) Plasma levels of PBEF/Nampt/ visfatin are decreased in patients with liver cirrhosis . Am J Physiol Gastrointest Liver Physiol 296:G196–G210
de Kreutzenberg SV, Ceolotto G, Papparella I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP, Avogaro A (2010) Downregulation of the longevity-associated protein SIRT1 in insulin resistance and metabolic syndrome. Potential biochemical mechanisms. Diabetes 59:1006–1015
de Luis DA, Sagrado MG, Conde R, Aller R, Izaola O (2009) Relation of visfatin to cardiovascular risk factors and adipocytokines in patients with impaired fasting glucose . Nutrition. doi:10.1016/j.nut.2008.11.005
Fasshauer M, Waldeyer T, Seeger J, Schrey S, Ebert T, Kratzsch J, Lossner U, Bluher M, Stumvoll M, Faber R, Stepan H (2008) Serum levels of the adipokine visfatin are increased in preeclampsia . Clin Endocrinol (Oxf) 69:69–73
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426–430
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I (2007) Retraction. Science 318:565
García-Fuentes E, García-Almeida JM, García-Arnés J, García-Serrano S, Rivas-Marín J, Gallego-Perales JL, Rojo-Martínez G, Garrido-Sánchez L, Bermudez-Silva FJ, Rodríguez de Fonseca F, Soriguer F (2007) Plasma visfatin concentrations in severely obese subjects are increased after intestinal bypass . Obesity (Silver Spring) 15:2391–2395
Garten A, Petzold S, Barnikol-Oettler A, Körner A, Thasler W, Kratzsch J, Kiess W, Gebhardt R (2010) Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes . Biochem Biophys Res Commun 391:376–381
Gok DE, Yazici M, Uckaya G, Bolu SE, Basaran Y, Ozgurtas T, Kilic S, Kutlu M (2010) The role of visfatin in the pathogenesis of gestational diabetes mellitus. J Endocrinol Invest. doi:10.3275/6902
Guarente L (2005) Calorie restriction and Sir2 genes – towards a mechanism. Mech Ageing Dev 126:923–928
Guest CB, Park MJ, Johnson DR, Freund GG (2008) The implication of proinflammatory cytokines in type 2 diabetes. Front Biosci 13:5187–5194
Hallschmid M, Randeva H, Tan BK, Kern W, Lehnert H (2009) Relationship between cerebrospinal fluid visfatin (PBEF/Nampt) levels and adiposity in humans . Diabetes 58:637–640
Hasmann M, Schemainda I (2003) FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res 63:7436–7442
Haus JM, Solomon TP, Marchetti CM, O’Leary VB, Brooks LM, Gonzalez F, Kirwan JP (2009) Decreased visfatin after exercise training correlates with improved glucose tolerance . Med Sci Sports Exerc 41:1255–1260
Hayashida S, Arimoto A, Kuramoto Y, Kozako T, Honda SI, Shimeno H, Soeda S (2010) Fasting promotes the expression of SIRT1, an NAD(+)-dependent protein deacetylase, via activation of PPARalpha in mice . Mol Cell Biochem 339:285–292
Hofsø D, Ueland T, Hager H, Jenssen T, Bollerslev J, Godang K, Aukrust P, Røislien J, Hjelmesaeth J (2009) Inflammatory mediators in morbidly obese subjects: associations with glucose abnormalities and changes after oral glucose. Eur J Endocrinol 161:451–458
Hug C, Lodish HF (2005) Medicine. Visfatin: a new adipokine. Science 307:366–367
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase . Nature 403:795–800
Imai S, Kiess W (2009) Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes . Front Biosci 14:2983–2995
Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC (2004) Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis . J Clin Invest 113:1318–1327
Jin H, Jiang B, Tang J, Lu W, Wang W, Zhou L, Shang W, Li F, Ma Q, Yang Y, Chen M (2008) Serum visfatin concentrations in obese adolescents and its correlation with age and high-density lipoprotein cholesterol . Diabetes Res Clin Pract 79:412–418
Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Tsanikidis H, Vitta I, Karkos C, Karayannacos PE, Gerasimidis T, Liapis CD (2010) Visfatin (Nampt) and ghrelin as novel markers of carotid atherosclerosis in patients with type 2 diabetes . Exp Clin Endocrinol Diabetes 118:75–80
Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13:2570–2580
Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD (2008) Chronic stretching of amniotic epithelial cells increases pre-B cell colony-enhancing factor (PBEF/Visfatin) expression and protects them from apoptosis . Placenta 29:255–265
Khan JA, Tao X, Tong L (2006) Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol 13:582–588
Kim MK, Lee JH, Kim H, Park SJ, Kim SH, Kang GB, Lee YS, Kim JB, Kim KK, Suh SW, Eom SH (2006) Crystal structure of visfatin/pre-B cell colony-enhancing factor 1/nicotinamide phosphoribosyltransferase, free and in complex with the anti-cancer agent FK-866 . J Mol Biol 362:66–77
Körner A, Garten A, Blüher M, Tauscher R, Kratzsch J, Kiess W (2007) Molecular characteristics of serum visfatin and differential detection by immunoassays . J Clin Endocrinol Metab 92:4783–4791
Laudes M, Oberhauser F, Schulte DM, Freude S, Bilkovski R, Mauer J, Rappl G, Abken H, Hahn M, Schulz O, Krone W (2010) Visfatin/PBEF/Nampt and Resistin expressions in circulating blood monocytes are differentially related to obesity and type 2 diabetes in humans . Horm Metab Res 42:268–273
Leclercq IA, Da Silva MA, Schroyen B, Van Hul N, Geerts A (2007) Insulin resistance in hepatocyte sand sinusoidal liver cells: mechanisms and consequences. J Hepatol 47:142–156
Lee HC (2001) Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol 41:317–345
Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, Brunkan CS, Wolberger C, Imai SI, Tabas I (2008) Extracellular Nampt promotes macrophage survival via a non-enzymatic interleukin-6/STAT3 signaling mechanism . J Biol Chem 283:34833–34843
Lin SJ, Guarente L (2003) Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol 15:241–246
Luk T, Malam Z, Marshall JC (2008) Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity . J Leukoc Biol 83:804–816
Magni G, Amici A, Emanuelli M, Ruggieri S (1999) Enzymology of NAD+ synthesis . Adv Enzymol Relat Areas Mol Biol 73:135–182
Malavazos AE, Ermetici F, Cereda E, Coman C, Locati M, Morricone L, Corsi MM, Ambrosi B (2008) Epicardial fat thickness: relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity . Nutr Metab Cardiovasc Dis 18:523–530
Martin P, Shea RJ, Mulks MH (2001) Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence . J Bacteriol 183:1168–1174
Ménissier de Murcia J, Ricoul M, Tartier L, Niedergang C, Huber A, Dantzer F, Schreiber V, Amé JC, Dierich A, LeMeur M, Sabatier L, Chambon P, de Murcia G (2003) Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO J 22:2255–2263
Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bernis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliot PJ, Westphal CH (2007) Small molecule activators of Sirt1 as therapeutics for the treatment of type 2 diabetes . Nature 450:712–716
Mirzaei K, Hossein-Nezhad A, Javad Hosseinzadeh-Attar M, Jafari N, Najmafshar A, Mohammadzadeh N, Larijani B (2009) Visfatin genotype may modify the insulin resistance and lipid profile in type 2 diabetes patients. Minerva Endocrinol 34:273–279
Moller N, Gormsen L, Fuglsang J, Gjedsted J (2003) Effects of ageing on insulin secretion and action . Horm Res 60:102–104
Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H (2007) Visfatin, an adipocytokine with antiinflammatory and immunomodulating properties. J Immunol 178:1748–1758
Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, Ebenbichler CF, Stadlmann S, Moser PL, Tilg H (2009) Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol 51:765–777
Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S (2005) Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice . Cell Metab 2:80–82
Nüsken KD, Nüsken E, Petrasch M, Rauh M, Dötsch J (2007) Preanalytical influences on the measurement of visfatin by enzyme immuno assay . Clin Chim Acta 382:154–156
Nüsken KD, Petrasch M, Rauh M, Stöhr W, Nüsken E, Schneider H, Dötsch J (2009) Active visfatin is elevated in serum of maintenance haemodialysis patients and correlates inversely with circulating HDL cholesterol . Nephrol Dial Transplant 24:2832–2838
Ognjanovic S, Bryant-Greenwood GD (2002) Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes . Am J Obstet Gynecol 187:1051–1058
Ognjanovic S, Ku TL, Bryant-Greenwood GD (2005) Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium . Am J Obstet Gynecol 193:273–282
Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, Milan G, Rossato M, Federspil G, Vettor R (2006) Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans . J Clin Endocrinol Metab 91:3165–3170
Peng XD, Xie H, Zhao Q, Wu XP, Sun ZQ, Liao EY (2008) Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta 387(1–2):31–35
Pilz S, Mangge H, Obermayer-Pietsch B, März W (2007) Visfatin/pre-B-cell colony-enhancing factor: a protein with various suggested functions. J Endocrinol Invest 30:138–144
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH (2006) Obesity and cardiovascular disease: pathophysiology, evaluation and effect of weight loss. Circulation 113:898–918
Preiss J, Handler P (1957) Enzymatic synthesis of nicotinamide mononucleotide . J Biol Chem 225:759–770
Ramsey KM, Mills KF, Satoh A, Imai S (2008) Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice . Aging Cell 7:78–88
Retnakaran R, Youn BS, Liu Y, Hanley AJG, Lee NS, Park JW, Song ES, Vu V, Kim W, Tungtrongchitr R, Havel PJ, Swarbrick MM, Shaw C, Sweeney G (2008) Correlation of circulating full-lenght visfatin (PBEF/Nampt) with metabolic parameters in subjects with and without diabetes: a cross-sectional study . Clin Endocrinol (Oxf) 69:885–893
Revollo JR, Grimm AA, Imai S (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells . J Biol Chem 279:50754–50763
Revollo JR, Grimm AA, Imai S (2007a) The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals . Curr Opin Gastroenterol 23:164–170
Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S (2007b) Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme . Cell Metab 6:363–375
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and Sirt1 . Nature 434:113–118
Rogina B, Helfand SL (2004) Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA 101:15998–16003
Romacho T, Azcutia V, Vázquez-Bella M, Matesanz N, Cercas E, Nevado J, Carraro R, Rodríguez-Mañas L, Sánchez-Ferrer CF, Peiró C (2009) Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity . Diabetologia 52:2455–2463
Rongvaux A, Andris F, Van Gool F, Leo O (2003) Reconstructing eukaryotic NAD metabolism . Bioessays 25:683–690
Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F (2002) Pre-B-cell colony enhancing factor, whose expression is upregulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis . Eur J Immunol 32:3225–3234
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I (1994) Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol 14:1431–1437
Schenk S, Saberi M, Olefsky JM (2008) Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118:2992–3002
Schwer B, Verdin E (2008) Conserved metabolic regulatory functions of sirtuins . Cell Metab 7:104–112
Seo JA, Jang ES, Kim BG, Ryu OH, Kim HY, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kim NH (2008) Plasma visfatin levels are positively associated with circulating interleukin-6 in apparently healthy Korean women . Diabetes Res Clin Pract 79:108–111
Sethi JK (2007) Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? . Curr Hypertens Rep 9:33–38
Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K (2009) NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway . Nat Med 15:151–158
Sommer G, Kralisch S, Kloting N, Kamprad M, Schrock K, Kratzsch J, Tonjes A, Lossner U, Bluher M, Stumvoll M, Fasshauer M (2010) Visfatin is a positive regulator of MCP-1 in human adipocytes in vitro and in mice in vivo. Obesity (Silver Spring) 18:1486–1492
Song HK, Lee MH, Kim BK, Park YG, Ko GJ, Kang YS, Han JY, Han SY, Han KH, Kim HK, Cha DR (2008) Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol 295:F1485–F1494
Sowers JR (2003) Obesity as a cardiovascular risk factor. Am J Med 115:37S–41S
Stephens JM, Vidal-Puig A (2006) An update on visfatin/pre-B cell colony-enhancing factor, an ubiquitously expressed, illusive cytokine that is regulated in obesity . Curr Opin Lipidol 17:128–131
Sun Q, Li L, Li R, Yang M, Liu H, Nowicki MJ, Zong H, Xu J, Yang G (2009) Overexpression of visfatin/PBEF/Nampt alters whole-body insulin sensitivity and lipid profile in rats . Ann Med 41:311–320
Szamatowicz J, Kužmicki M, Telejko B, Zonenberg A, Nikolajuk A, Kretowski A, Górska M (2009) Serum visfatin concentration is elevated in pregnant women irrespectively of the presence of gestational diabetes . Ginekol Pol 80:14–18
Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M, Komuro R, Shimomura I (2007) Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem Biophys Res Commun 359:194–201
Telejko B, Kuzmicki M, Zonenberg A, Szamatowicz J, Wawrusiewicz-Kurylonek N, Nikolajuk A, Kretowski A, Gorska M (2009) Visfatin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Diabetes Res Clin Pract 84:68–75
Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227–230
Toruner F, Altinova AE, Bukan AE, Akbay E, Ersoy R, Arslan M (2009) Plasma visfatin concentrations in subjects with type 1 diabetes mellitus . Horm Res 72:33–37
Unluturk U, Harmanci A, Yildiz BO, Bayraktar M (2010) Dynamics of Nampt/visfatin and high molecular weight adiponectin in response to oral glucose load in obese and lean women . Clin Endocrinol (Oxf) 72:469–474
van der Veer E, Nong Z, O’Neil C, Urquhart B, Freeman D, Pickering JG (2005) Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation . Circ Res 97:25–34
Wang P, van Greevenbroek MM, Bouwman FG, Brouwers MC, van der Kallen CJ, Smit E, Keijer J, Mariman EC (2007) The circulating PBEF/NAMPT/visfatin level is associated with a beneficial blood lipid profile . Pflugers Arch 454:971–976
Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C (2006) Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme . Nat Struct Mol Biol 13:661–662
Westphal CH, Dipp MA, Guarente L (2008) A therapeutic role of sirtuins in diseases of aging? Trends Biochem Sci 32:555–560
Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, Zhou HD, Wu XP, Liao EY (2007) Insulin-like effects of visfatin on human osteoblasts . Calcif Tissue Int 80:201–210
Xu LG, Wu M, Hu J, Zhai Z, Shu HB (2002) Identification of downstream genes up-regulated by the tumor necrosis factor family member TALL-1. J Leukoc Biol 72:410–416
Yang H, Lavu S, Sinclair DA (2006) Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol 41:718–726
Yildiz BO, Bozdag G, Otegen U, Harmanci A, Boynukalin K, Vural Z, Kirazli S, Yarali H (2010) Visfatin and retinol-binding protein 4 concentrations in lean, glucose-tolerant women with PCOS. Reprod Biomed Online 20:150–155
Yilmaz MI, Saglam M, Qureshi AR, Carrero JJ, Caglar K, Eyileten T, Sonmez A, Cakir E, Oguz Y, Vural A, Yenicesu M, Stenvinkel P, Lindholm B, Axelsson J (2008) Endothelial dysfunction in type-2 diabetics with early diabetic nephropathy is associated with low circulating adiponectin. Nephrol Dial Transplant 23:1621–1627
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Garten, A., Petzold, S., Schuster, S., Körner, A., Kratzsch, J., Kiess, W. (2011). Nampt and Its Potential Role in Inflammation and Type 2 Diabetes. In: Schwanstecher, M. (eds) Diabetes - Perspectives in Drug Therapy. Handbook of Experimental Pharmacology, vol 203. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-17214-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-17214-4_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-17213-7
Online ISBN: 978-3-642-17214-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)