Abstract
Bladder problems are frequently disorders of control, which is exercised from the brain. In such disorders, brain responses to bladder events are abnormal; therapy is accompanied by regional changes that may be measured by functional imaging and used to monitor the effect of treatment. The regional responses may be understood in terms of a tentative model of the bladder control system. The model helps also to interpret alterations in brain behavior (as imaged by functional scanning) that occur when afferent signals from bladder or urethra are changed experimentally or by an underlying disorder or treatment, for example, overactive bladder (urge/urgency incontinence). Successful treatment may either increase the ability to cope with the problem or may be curative. The direction of treatment-induced change of abnormal brain responses can distinguish these two possibilities and shed light on the therapeutic mechanism. In addition, brain activity in regions such as insula or dorsal anterior cingulate cortex may be regarded as a proxy for sensations such as desire to void or urgency, which are otherwise difficult to define or measure. Monitoring of brain responses in these regions offers an obvious way to test the effect of drugs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain
- Bladder control
- Bladder filling sensation
- Functional brain imaging
- Urgency
- Urge urinary incontinence
1 Introduction
Disorders of voluntary bladder control are prevalent and are of two main types: inability to empty the bladder (retention, urinary) and inability to store urine, manifested as the triad of symptoms making up the overactive bladder syndrome overactive bladder (OAB) (frequency, urgency, and urge or incontinence, urge(ncy)). In some cases, the symptoms are associated with neurological diseases such as multiple sclerosis or spinal cord injury, or with neuropathy (e.g., in longstanding diabetes), or with other peripheral changes (e.g., prostatic obstruction). Nevertheless in many cases the underlying pathology is unknown. Until recently the cause of such disorders has been sought in the peripheral organs or the afferent signals that they generate. For example, Fowler’s syndrome (unexplained urinary retention in younger women) has been ascribed to an overactive urethral sphincter, while overactive bladder, as the name suggests, is usually supposed to be a manifestation of an underlying bladder abnormality, at least if no obvious pathological cause can be identified.
Correspondingly, and especially in OAB, most animal models and pharmacological approaches to therapy have focused on the bladder or on bladder afferents. Yet these symptoms are manifestations of loss of voluntary control of the bladder, and control is exerted by an extensive spinal and supraspinal neuronal network, of which the peripheral organs and their innervation form only a small part. On the face of it, pathology of the control system seems equally or more likely to be responsible for bladder control problems than an intrinsic bladder defect. This possibility was for a long time ignored, however, because of the lack of technical means to study it. Undoubtedly, this neglect has limited the range of potential therapeutic targets.
Recent advances in functional brain imaging, and especially the widespread accessibility of these techniques during the past decade, have provided a basic understanding of the supraspinal bladder control system, which makes it possible to recognize normal and abnormal states of the brain and monitor response to therapy (Kavia et al. 2005; Griffiths and Tadic 2008). Functional imaging has great advantages: the ability to monitor the control system itself rather than just the behavior of the peripheral organs and their afferent signals; and the ability to do this in human beings, with or without disease, instead of experimental animals. Indeed, good animal models of OAB symptoms such as urge incontinence are difficult to devise because lack of communication makes it difficult to draw the critical distinction between involuntary loss of bladder control and voluntary voiding, or even to know whether such a distinction is meaningful in an animal. Furthermore, functional imaging may enable us to recognize neural correlates of clinically important but difficult-to-define concepts, thus offering easily measured proxy markers that might be used to monitor therapeutic effect. As we shall see moreover the direction of the changes in brain activity brought about by therapy provides helpful information about the therapeutic mechanism.
Functional brain imaging may be carried out by a number of techniques, including SPECT scanning (single photon emission tomography), PET scanning (positron emission tomography), and fMRI (functional magnetic resonance imaging). All provide an indirect measure of regional cerebral blood flow, believed to represent local neuronal activity. One potential problem that should be borne in mind when interpreting functional imaging studies is that neuronal activity may be either excitatory or inhibitory: without further information it is not possible to distinguish these two possibilities. Both SPECT and PET require injection of a radioactive tracer. SPECT has relatively poor temporal and spatial resolution. PET is good for examining long-lived states and slow changes in brain activity and has been used to investigate both storage and voiding. fMRI has better temporal and spatial resolution than PET. It is relatively inexpensive and noninvasive, but produces a weak and noisy signal. It is good for examining relatively short events, but frequent repetitions are required in order to average out random errors due to noise. This requirement has so far precluded fMRI measurements during actual voiding [imagined voiding has been examined (Kuhtz-Buschbeck et al. 2005)], but repetitive infusion and withdrawal of a small amount of water in and out of the bladder has proved a fruitful way of mimicking bladder filling so as to investigate the storage phase (Griffiths et al. 2005). (Technically, the fMRI BOLD [blood oxygen level dependent] signal during withdrawal of fluid is subtracted from the signal during infusion, and the result is averaged over numerous repetitions.) Limitation to the storage phase has not proved a problem because disorders of bladder control such as OAB are disorders of storage.
The following Sect. 2 continues with a description of a tentative model of the normal bladder control system. Its purpose is to set in an overall framework the many regions of the brain that respond to bladder events. There is then a summary of the potential effects of pharmacological therapy and the existing evidence concerning alterations in brain behavior (as imaged by functional scanning) when afferent signals from bladder or urethra are changed experimentally, by disease, or by treatment. There follows a more detailed description of brain responses to bladder filling in subjects with OAB and how they are altered by therapy (although unfortunately not pharmacological therapy).
2 The Bladder Control Network
The nodes of the supraspinal network that controls the bladder (the “centers” that are involved) have been established during more than a decade of functional imaging and to a large extent confirm the conclusions drawn from older animal experiments and clinical observations (Andrew and Nathan 1964; Torrens and Feneley 1982). Merely to list the centers and ascribe a function to each has rightly been labeled “phrenology.” Although the interpretation of functional scanning results has frequently not gone far beyond this point, there have been attempts to identify aspects of the connectivity of the various centers and to place them in a model network with some indication of their function and interactions (Blok 2002; Fowler et al. 2008; Tadic et al. 2008). During storage the ultimate result of the network is tonic inhibition of detrusor contraction, preventing voiding and maintaining continence. Only for voiding is this inhibition lifted.
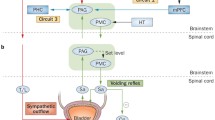
Based on these findings and some imaginative extensions, a tentative model of the bladder control network has several neural circuits working in parallel (Fig. 1). They determine whether voiding is (1) mechanically appropriate (i.e., the bladder is adequately filled), (2) emotionally safe, and (3) socially appropriate. If they concur then voiding may take place. Neural circuit 1 of the network is part of the well-known spinobulbospinal (long-loop) voiding reflex. It is driven by spinal afferent signals that are believed to originate from mechanical transducers in the bladder wall or urothelium and to increase with bladder filling (Fowler et al. 2008). Thus circuit 1 is a mechanical reflex which by itself would ensure automatic and involuntary voiding whenever the bladder volume reached a critical level. It includes regions in the brainstem (pons) and midbrain.
Diagram suggesting how cerebral control may be exercised via neural circuits fulfilling different functions, showing postulated circuits 1 (mechanical, reflex), 2 (emotional, limbic), and 3 (voluntary, social, cortical). In each circuit, specific regions known to be concerned with bladder control are shown, together with a few of the many possible interconnections. Circuit 3 is divided into 2 branches, 3P and 3S. In circuit 3P deactivation of vmPFC (see Sect. 2) inhibits the voiding reflex at the PAG, thus suppressing voiding via an ultimately parasympathetic pathway. Activation of dACC in circuit 3S suppresses voiding by a different pathway, bypassing circuit 1, that acts via the sympathetic innervation to inhibit the detrusor and contract the urethral smooth muscle. Arrow paths excitatory neural link; box paths inhibitory neural link; HC hippocampus and parahippocampal complex; mPFC medial prefrontal cortex; lPFC lateral prefrontal cortex; RI (right) insula; dACC dorsal anterior cingulate cortex; PMC pontine micturition center; PAG periaqueductal gray; Sa parasympathetic regions of sacral cord
Circuit 2 is concerned with the safety of the organism during voiding, a time of increased vulnerability. Lack of safety engenders an emotion, fear, and the derived concept anxiety. Correspondingly this circuit includes emotional (limbic) regions of the brain. It receives input from circuit 1 and spinal afferents, evaluates safety, and produces an output signal that prevents voiding unless the situation is deemed safe. It still makes no provision for voluntary control of voiding.
Circuit 3 takes input from the previous circuits and from spinal afferents to generate sensations of bladder filling (desire to void) and to evaluate the social propriety of voiding. It modifies the primary emotion, fear, derived from circuit 2, to generate secondary social emotions (Damasio 2003) such as fear of leakage (associated with urgency) and embarrassment or shame about incontinence. If voiding is judged acceptable then circuit 3 facilitates the lifting of voiding inhibition. Thus this circuit is involved in conscious sensation and voluntary control in the social setting. It includes cortical and cingulate parts of the brain. Comparison with recent publications in the cardiovascular field (Wager et al. 2009) suggests that circuit 3 should be divided into two parts, as in Fig. 1, which are the origins of pathways that become, respectively, the parasympathetic and sympathetic output controlling bladder and urethral behavior. If confirmed, this hypothesis would provide the missing link between brain activity and motor innervation at the peripheral level.
A considerable number of regions in these various circuits have been identified and some are shown in Fig. 1. Their interconnections, especially in circuit 3, are known to be numerous but are still largely unstudied in the context of bladder control. Some of the most important regions and their probable functions are as follows.
In circuit 1
-
Midbrain periaqueductal gray (PAG), receives bladder afferents (Blok and Holstege 1998)
-
Pontine micturition center (PMC; also known as Barrington’s nucleus or M-region), on excitation sends a descending signal that promotes synergic voiding by relaxing the urethral sphincter and contracting the detrusor (Blok and Holstege 1998)
In circuit 2
-
Hypothalamus (preoptic region), provides safe/unsafe signal controlling voiding (Blok and Holstege 1998)
-
Parahippocampal complex, provides emotional basis of bladder sensations; the posterior parts of the cortex seem to function similarly
In circuit 3
-
Insula, records bladder filling sensation (Craig 2002)
-
Dorsal anterior cingulate cortex, dorsal (dACC), encodes motivation, e.g., urgency to void and concurrent motor output (Critchley et al. 2003) (see also Fig. 1)
-
Medial prefrontal cortex, medial (mPFC), concerned with automatic reaction to bladder filling and decision to void in social context; produces output that inhibits voiding except when it is voluntarily desired
-
Lateral prefrontal cortex, lateral (lPFC), concerned with voluntary regulation of emotion and bladder sensation
In addition, the thalamus seems to serve as a relay/interaction station between many regions.
When the bladder is filled, these regions become excited in a predictable way (Fig. 2). The PAG is activated, suggesting reception of increased bladder afferents. The thalamus lights up, consistent with its role in relaying these signals elsewhere in the brain. The insula is activated, especially on the right, consistent with increased bladder filling sensation (desire to void). The dACC is activated, relatively weakly in normal subjects (Fig. 2) but much more strongly in those with urge incontinence (see Sect. 5.2), consistent with a role as the neural correlate of urgency (a sensation not experienced by normal subjects). The somatosensory area (Brodmann Area 40) is activated bilaterally. The mPFC and pregenual anterior cingulate cortex, pregenual ACC are deactivated, weakly in normals but more strongly in urge incontinence, suggesting a unique role in controlling the bladder (see Fig. 1) and/or in the genesis of urge incontinence (Griffiths et al. 2009).
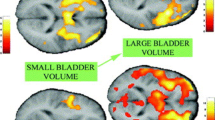
For a group of ten normal females, regions responding to bladder filling when the bladder is near full and sensation is strong. Red/yellow: activation (probability threshold P < 0.01, uncorrected for multiple comparisons); blue: deactivation (P < 0.05, uncorrected). PAG periaqueductal gray; T thalamus; RI right insula; dACC dorsal anterior cingulate cortex; mPFC medial prefrontal cortex; R right. Numbers indicate Montreal Neurological Institute (MNI) y-coordinates
3 Potential Effects of Drugs on Central Control
Up to the present there has been little actual measurement of drug effects on central control of the bladder and none using the combination of fMRI, urodynamics, and repetitive infusion/withdrawal which has proved fruitful in understanding how the control system operates (Griffiths et al. 2005; Mehnert et al. 2008; Kavia et al. 2010). Nevertheless there have been a few studies of the effects of nonpharmacological treatments on regional brain responses to bladder events (Blok et al. 2006; Kavia et al. 2010) (see also Sect. 5.5). In addition, there have been studies in situations that can be viewed as natural experiments mimicking alterations – especially alterations of bladder afferents – that might also occur with drugs used to treat overactive bladder symptoms, such as antimuscarinics or botulinum toxin. These studies offer guidance about what changes to expect in brain responses and how to interpret them. One factor that should be borne in mind when designing new studies of the effect of treatment is that successive brain scanning sessions will yield progressively different results whether or not therapy is performed, purely because of a changing memory trace in the brain related to repetition of sessions. Thus either an untreated control group should be included (note that, from a brain control point of view, placebo is not equivalent to no treatment), or else the changes in brain response that are significantly correlated with clinical success of therapy should be examined, since they show where therapy has an effect regardless of changing memory.
4 Effects of Changes in Afferent Signals
4.1 Aging
In old age, detrusor overactivity and urge incontinence become very common. Among those who maintain normal bladder function, however, sensation becomes weaker with increasing age (Pfisterer et al. 2006). Cortical – especially insula – activation in response to bladder filling also becomes weaker (Griffiths et al. 2007) as one would expect if insular activation represented the neural correlate of bladder filling sensation (desire to void). Possible reasons for diminished sensation in old age include diminished bladder afferents or impaired cerebral processing. Measurements made at smaller bladder volumes show a similar reduction in insular responses, which in this case is clearly related to weaker afferent signals. Thus peripheral changes could be a contributing factor to the age-associated decrease in response. Of course, supraspinal (or spinal) changes may also contribute.
At first sight it appears that pharmacotherapy with drugs that reduce bladder afferent signals – e.g., antimuscarinics or botulinum toxin – might have a similar effect. If so, one would expect to be able to monitor this effect by measuring the decreasing insular response to bladder filling during the course of treatment. However, the above observations were made in normal subjects. The situation may be different in patients with overactive bladder problems, as discussed in Sect. 5.
4.2 Fowler’s Syndrome
Young women with Fowler’s syndrome present with unexplained urinary retention, inability to void, and reduced bladder sensation. There is an underlying overactivity of the urethral sphincter, as shown by an abnormal sphincter EMG and elevated urethral pressure (DasGupta and Fowler 2004). Based on the bladder findings, one might expect reduced bladder afferents and reduced or absent brain responses to bladder filling. In fact, however, there are widespread negative responses, especially with near-empty bladder and without treatment (Fig. 3a) (Kavia et al. 2010). The interpretation of such negative responses is still widely debated (Shih et al. 2009), but we shall refer to them as deactivations. Deactivation during bladder filling occurs even in the PAG (see Fig. 1), suggesting that the normal bladder afferents are not reaching the brain at all. It occurs also in the insula, implying that sensation is suppressed by bladder filling instead of being increased. One possible interpretation is that in Fowler’s syndrome, the overactive urethral sphincter responds to bladder filling with strong urethral afferents that inhibit bladder signals via a spinal pro-continence reflex, thus causing deactivation of many brain regions.
Results for a group of six women with Fowler’s syndrome with and without treatment by SNM. Top panels, A and B: Responses to bladder filling without treatment (with near-empty and with full bladder respectively). Bottom panels, C and D: responses after SNM. Red activation; blue deactivation; projected on surface of standard brain. Adapted from (Kavia et al. 2010) with permission
It would be interesting to compare these results with those obtained after injection of botulinum toxin in the bladder wall (a successful treatment for intractable incontinence, urge), which presumably affects bladder afferents, and therefore should reduce responses to bladder filling without evoking abnormal deactivations.
4.3 Effect of Sacral Neuromodulation in Fowler’s Syndrome
In women with Fowler’s syndrome, deactivations are less pronounced if bladder afferents are strengthened, for example, by prefilling the bladder (Fig. 3b). Therapy with sacral neuromodulation (SNM), which presumably strengthens afferent signals in a similar way, reenables voiding and improves sensation (DasGupta and Fowler 2004). Correspondingly, it reduces sacral neuromodulation and increases activation of cortical regions, as well as PAG (Kavia et al. 2010) (Fig. 3c, d). It appears therefore that treatment with SNM, although it does not cure the underlying urethral sphincter abnormality (DasGupta et al. 2005), is partially curative in the sense that it makes the brain responses more nearly normal. The effect of treatment could be monitored by determining the relative extent of deactivation vs. activation.
4.4 Ice Water (Bladder Cooling) Test
In addition to the Aδ fibers that convey afferent information about bladder filling to the CNS, there are C-fibers that transmit information about bladder pain and temperature. Instillation of cold fluid (“ice water”) into the bladder has long been used to provoke and to test for neurogenic detrusor overactivity (Geirsson et al. 1993). Brain responses to this bladder cooling (presumably mediated by C-fibers) have been measured with PET (Matsuura et al. 2002). They are different from those evoked by bladder filling using room- or body-temperature liquid, mediated by Aδ fibers. In particular, there are cortical activations in regions different from those shown in Fig. 2 and no significant changes in brainstem or PAG. These results suggest that different afferent signals indeed activate different brain regions, and possibly that thermal and pain signals may bypass the PAG and pass directly to the thalamus (Mayer et al. 2006).
It would be interesting to study responses to bladder filling in patients with painful bladder syndrome; presumably regions similar to those activated by ice water would respond.
5 Overactive Bladder Syndrome: Urge Incontinence, Urgency, and Detrusor Overactivity
5.1 Overactive Bladder: Symptoms and Urodynamics
In patients with OAB symptoms, urodynamic testing frequently reveals involuntary bladder contractions (detrusor overactivity (DO)) during bladder filling. The contractions may be associated with the sensation of urgency (see Sect. 5.2) or with urine leakage (detrusor overactivity incontinence). Moreover cystometric capacity may be limited by the onset of DO, because further filling is hindered by strong bladder sensation or urine leakage. Together, these observations mimic the symptoms of the overactive bladder syndrome and therefore DO is often regarded as the cause of OAB. Although DO itself is usually viewed clinically as idiopathic, the terms used to refer to it (“detrusor overactivity” and “overactive bladder”) seem to imply an underlying bladder abnormality. This view has limited the direction of research and the choice of treatment targets for the past 50 years.
In practice, detrusor overactivity is frequently accompanied by involuntary urethral sphincter relaxation, or involuntary urethral relaxation may occur with urgency but without DO (McLennan et al. 2001). Thus the behavior of the urethra is probably just as important clinically as that of the bladder, a complication that should be borne in mind but is not emphasized in this chapter.
5.2 Urgency
As the bladder of a normal subject is filled, a sensation of desire to void is generated which fluctuates but gradually becomes stronger and more difficult to ignore (Abrams et al. 2002). It is associated with increased cortical response to bladder filling in the insula, especially on the right (see Figs. 1 and 2). The insula is believed to map afferents from the bladder and other visceral systems and thus to represent the degree of bladder filling and the strength of the desire to void (Craig 2002; Griffiths et al. 2007).
Patients with OAB symptoms, however, complain of an apparently different sensation called urgency. One of the goals of treatment is to reduce this bothersome sensation. Moreover urgency is a defining characteristic of incontinence, urge(ncy), “leakage of urine accompanied or preceded by urgency” (Abrams et al. 2002). Thus this sensation is a key symptom of the overactive bladder syndrome. In practice, however, it has been difficult to define, as is reflected in a change of definition: originally “a strong desire to void with fear of leakage [or pain]” (Abrams et al. 1988), but currently “a sudden compelling desire to void that is difficult to defer and may be accompanied or preceded by detrusor overactivity” (Abrams et al. 2002). Thus urgency seems to have several different aspects – a compelling nature, a sudden onset, and an emotional component (fear of leakage). Indeed it has been suggested that there are two types of urgency, one that is an intensification of the normal strong desire to void and another, not experienced by all patients, which is of a different nature (Blaivas et al. 2009). Clearly, it is difficult to use urgency, however important it may be, as an outcome measure for treatment of OAB if it is not unambiguously defined (Lowenstein et al. 2008). The above argument suggests that reliable clinical assessment of urgency from patient reports may remain elusive until the neural basis of the sensation – a particular state or states of brain activity – has been established. It is therefore worthwhile to find objective neural correlates of the various aspects of urgency and other sensations, which may help to identify and ultimately to more precisely define them. Such neural correlates may ultimately be viewed not so much as proxies for sensations such as urgency but their actual definitions, of which patient reports are an ambiguous reflection.
The probable neural correlate of the normal desire to void has been identified above: it is the increased activation of the insula provoked by bladder filling (Figs. 1 and 2). In female patients who suffer from urge incontinence and therefore experience urgency, however, brain responses to bladder filling are abnormal. In addition to insular activation there is a greatly enhanced response in dACC, especially if the bladder is well filled and sensation is strong (Griffiths et al. 2007) (Fig. 4). This abnormally strong reaction occurs in the absence of concurrent DO: i.e., prior to any actual loss of bladder control. dACC activation is believed to indicate an emotional response (e.g., fear of leakage) and to encode the motivation driven by this emotion (Critchley et al. 2003), as well as provide concomitant motor output that inhibits contraction of the bladder via its sympathetic innervation (see Fig. 1). Therefore increased dACC response appears to be the neural correlate of at least one aspect of urgency. At the same time, there is widespread activation of other parts of the cortex that probably indicates recruitment of other motor output (e.g., to the striated urethral sphincter) in an attempt to cope with imminent loss of control (Griffiths et al. 2005). Therefore, dACC activation can be used as a proxy for urgency or indeed as a defining characteristic of a type of urgency that includes the emotional aspect (fear) and its compelling nature but not sudden onset. Thus insular activation (by itself) seems to represent normal sensation, which has a relatively mild emotional content (Fig. 1), while addition of dACC activation appears to correspond to an intensification of the normal desire to void with greater emotional and motivational content, perhaps corresponding to Blaivas’s first type of urgency (Blaivas et al. 2009). The other aspect of urgency – sudden onset – may correspond to a different situation, the onset of an involuntary detrusor contraction (DO) or involuntary urethral relaxation, which signifies that loss of bladder control is not just threatened but is actually occurring. Interestingly, the pattern of brain activation when DO develops is apparently quite different from Fig. 4 (see Sect. 5.3).
Brain responses to bladder filling in ten urge-incontinent women (with strong sensation but in the absence of DO) (P < 0.01 uncorrected). Activation of dorsal anterior cingulate cortex (dACC) is markedly more pronounced than in normal subjects (Griffiths et al. 2007). RI right insula; PMC pontine micturition center; R right. x, y, z represent MNI coordinates of each section. Adapted from (Griffiths et al. 2007) with permission
Even at our present level of understanding, it is clear that measurement of dACC response to bladder filling offers a way to monitor therapeutically induced changes in one type or aspect of urgency. This can be done with fMRI, but in the future it may be possible to monitor dACC activation more simply with near-infrared spectroscopy, electroencephalography, or magnetoencephalography. Other parts of the brain (e.g., prefrontal cortex) may be even more accessible to such technologies and may offer opportunities for noninvasive monitoring of the effect of treatment.
Clearly, a therapy that is curative should reduce urgency and thus weaken dACC activation. On the other hand, if dACC activation is a coping reaction to threatened loss of bladder control, an alternative therapeutic mechanism would be to strengthen this reaction by reinforcing dACC activation. Thus measurement of dACC responses, and whether they are reduced or increased by successful therapy, may be used not only to monitor the progress of therapy, but also to shed light on the therapeutic mechanism.
5.3 Detrusor Overactivity
Detrusor overactivity (DO) (involuntary bladder contraction) is a urodynamic observation that by definition is identified by monitoring bladder (detrusor) pressures. Functional imaging in female subjects suggests that brain activation when DO develops is quite different from the abnormally strong dACC responses seen prior to development of DO and identified as urgency. There are several ways to perform brain-imaging of DO. One is to study brain responses to infusion and withdrawal during periods when DO (of whatever amplitude) is present; this is simple but unphysiological. Another is to study the association between bladder pressure (as it waxes and wanes with DO) and brain activity, regardless of infusion or withdrawal; this requires florid and prolonged DO which may not be easy to provoke in the scanner (see below).
Preliminary observations suggest that DO has a distinct neural signature involving reduced responses to bladder infusion in the prefrontal cortex (Griffiths et al. 2007), possibly suggesting abandonment of the attempt to control the bladder by neural circuit 3. A critical question is whether the PMC is activated during DO. If so, it would suggest that overactivity of the long-loop voiding reflex was responsible for idiopathic DO; if not, DO might be due to overactive spinal segmental reflexes of the type that is usually present in infants but suppressed in continent adults (Fowler et al. 2008). So far the observations suggest that the PMC is not activated during DO, although more measurements are required to be certain of this rather surprising result.
DO is difficult to evoke in the scanner, presumably because circuit 2 assesses the scanner environment as “unsafe” and inhibits detrusor contraction. Thus no neural correlate of DO is likely to be useful clinically for monitoring the effects of therapy. The main importance of observations made during DO would be to show that there is a difference between the neural correlate of urgency viewed as the harbinger of imminent loss of control (i.e., just prior to onset of DO) and the neural activation pattern corresponding to actual DO. The present definition of urgency confuses the two and will probably need to be revised.
5.4 Effect of Sacral Neuromodulation in Urge Incontinence
Blok has made PET observations of the acute and chronic effects of SNM on patients with intractable urge incontinence (Blok et al. 2006). The acute effects, as one might expect if SNM stimulates afferent signals, are increased brain activity in a number of locations. However, after 6 or more months of SNM, there are notable reductions in cerebral activity evoked by bladder filling, especially in dACC at a location quite near to that shown in Fig. 2. Since dACC activation is believed to represent urgency and to be part of a coping reaction to threatened loss of control, this observation is consistent with a curative effect of SNM, leading to a gradual reduction of urgency as the brain learns that loss of control is less likely to occur.
An important observation is that acute and chronic effects of SNM are different, presumably because of the learning process mentioned above. The situation may be similar for drug therapy, which has an immediate effect on symptoms followed by a slower long-term improvement. These changes could be monitored by studying dACC response to bladder filling over the first few weeks and months of treatment.
5.5 Effect of Biofeedback Therapy on Urge Incontinence
Biofeedback therapy (BFB) is a recommended first-line conservative treatment for urge incontinence (Abrams et al. 2009). In older patients (women), it evokes relatively small changes in the properties of bladder and urethra, but seems to improve urge incontinence mainly by teaching the patients how better to control their bladder (unpublished observation). Thus it might be expected that fMRI should register the improvement in control. If BFB is curative, for example, we might expect abnormally pronounced dACC activation, representing urgency, to diminish. In fact recent preliminary results suggest that after 12 weeks of BFB, there may be significant increases in response to bladder filling among those who are successfully treated, in dACC as well as right insula and other parts of the cortex. If this result is confirmed in larger numbers of subjects, dACC activation representing urgency may prove to be a coping reaction to impending loss of control, which is reinforced by biofeedback (BFB) training. Thus therapy may not necessarily cure the condition, but may enable the individual to better cope with it, presumably reflecting the feeling of improved control evoked by successful BFB.
Pharmacotherapy for urge incontinence presumably works differently. Indeed, recent unpublished results suggest that there may be phenotypes of urge UI that respond differently to drug or behavioral treatment. In a phenotype that responds well to antimuscarinics, the drug may be curative and so may reduce abnormal dACC activation (urgency). In contrast, in a phenotype that responds well to BFB, successful treatment may improve coping by an increase in dACC activation, as described above. In either case, it is clear that changes brought about by therapy, whether drug or behavioral, can be monitored by functional brain imaging, especially of the dACC.
6 Conclusion
Symptoms such as unexplained urinary retention or the overactive bladder syndrome, including urge(ncy) incontinence, reflect defects in the voluntary control of bladder and urethra. The control system is largely cerebral, and functional brain imaging shows that the brain does indeed respond differently to bladder filling in patients with such symptoms, as opposed to normal subjects. In principle, the abnormal responses may be causal or they may represent a coping reaction to threatened loss of bladder control. Observations of the effect of therapy (but unfortunately not pharmacological therapy) indicate that some types of successful treatment may strengthen the abnormal responses, suggesting that they increase the ability to cope with the problem; while other types may normalize the responses, suggesting that they are curative, reducing a causal abnormality. Thus monitoring of regional brain activity enables one not only to follow the effect of treatment, but also sheds light on the therapeutic mechanism.
In addition, enough is now known about brain control of the bladder to identify regions that are particularly important and their functions. Moreover, activation of these same regions is significantly correlated with the severity of urge incontinence as measured clinically (by bladder diary or pad test) (Tadic et al 2010). Consequently, brain activity in certain regions (e.g., insula or dACC) can be regarded as a proxy for clinically important concepts, such as desire to void or the abnormal sensation of urgency, which are expected to change when treatment is successful but are otherwise difficult to define or measure. Monitoring of brain responses to bladder filling in these regions offers an obvious way to test the effects of drugs.
We therefore stand on the threshold of a new era in testing of pharmacological and nonpharmacological therapies which will produce an avalanche of new data. Inevitably, it will lead to new methods of treatment aimed not directly at the end organs but at their control, the seat of so many of our patients’ symptoms.
References
Abrams P, Blaivas JG, Stanton S, Andersen JT (1988) The standardisation of terminology of lower urinary tract function. Neurourol Urodyn 7:403–426
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21:167–178
Abrams P, Cardozo L, Khoury S, Wein A (2009) Incontinence: 4th international consultation on incontinence, Editions 21. Health, Paris, France
Andrew J, Nathan PW (1964) Lesions of the anterior frontal lobes and disturbances of micturition and defaecation. Brain 87:233–262
Blaivas JG, Panagopoulos G, Weiss JP, Somaroo C (2009) Two types of urgency. Neurourol Urodyn 28:188–190
Blok BF (2002) Central pathways controlling micturition and urinary continence. Urology 59:13–17
Blok BF, Holstege G (1998) The central nervous system control of micturition in cats and humans. Behav Brain Res 92:119–125
Blok BFM, Groen J, Bosch JLHR, Veltman DJ, Lammersma AA (2006) Different brain effects during chronic and acute sacral modulation in urge incontinent patients with implanted neurostimulators. BJU Int 08:1238–1243
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666
Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ (2003) Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–2152
Damasio AR (2003) Looking for Spinoza: joy, sorrow, and the feeling brain. Harcourt, Orlando, FL
DasGupta R, Fowler CJ (2004) Urodynamic study of women in urinary retention treated with sacral neuromodulation. J Urol 171:1161–1164
DasGupta R, Critchley HD, Dolan RJ, Fowler CJ (2005) Changes in brain activity following sacral neuromodulation for urinary retention. J Urol 174:2268–2272
Fowler CJ, Griffiths D, De Groat WC (2008) The neural control of micturition. Nat Rev Neurosci 9:453–466
Geirsson G, Fall M, Lindstrom S (1993) The ice-water test–a simple and valuable supplement to routine cystometry. BJU 71:681–685
Griffiths D, Tadic SD (2008) Bladder control, urgency, and urge incontinence: evidence from functional brain imaging. Neurourol Urodyn 27:466–474
Griffiths D, Derbyshire S, Stenger A, Resnick N (2005) Brain control of normal and overactive bladder. J Urol 174:1862–1867
Griffiths D, Tadic SD, Schaefer W, Resnick NM (2007) Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage 37:1–7
Griffiths DJ, Tadic SD, Schaefer W, Resnick NM (2009) Cerebral control of the lower urinary tract: how age-related changes might predispose to urge incontinence. Neuroimage 47:981–986
Kavia RB, Dasgupta R, Fowler CJ (2005) Functional imaging and the central control of the bladder. J Comp Neurol 493:27–32
Kavia RB, DasGupta R, Critchley HD, Fowler CJ, Griffiths D (2010) An fMRI study of the effect of sacral neuromodulation on brain responses in women with Fowler’s Syndrome. BJU Int 105:366–372
Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP (2005) Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol 174:1477–1481
Lowenstein L, FitzGerald MP, Kenton K, Hatchett L, Durazo-Arvisu R, Mueller ER, Goldman K, Brubaker L (2008) Evaluation of urgency in women, with a validated urgency, severity and impact questionnaire (USIQ). Int Urogynecol J 20:301–307
Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T (2002) Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol 168:2035–2039
Mayer EA, Naliboff BD, Craig AD (2006) Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology 131:1925–1942
McLennan MT, Melick C, Bent AE (2001) Urethral instability: clinical and urodynamic characteristics. Neurourol Urodyn 20:653–660
Mehnert U, Boy S, Svensson J, Michels L, Reitz A, Candia V, Kleiser R, Kollias S, Schurch B (2008) Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve – an fMRI study in healthy women. Neuroimage 41:682–689
Pfisterer M, Griffiths D, Schaefer W, Resnick N (2006) The effect of age on lower urinary tract function: a study in women. J Am Geriatr Soc 54:405–412
Shih YI, Chen CV, Shyu B, Lin Z, Chiang Y, Jaw F, Chen Y, Chang C (2009) A new scenario for negative functional magnetic resonance signals: endogenous neurotransmission. J Neurosci 29:3036–3044
Tadic SD, Griffiths D, Schaefer W, Resnick NM (2008) Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage 39:1647–1653
Tadic SD, Griffiths D, Schaefer W, Cheng CI, Resnick NM (2010) Brain activity as measured by functional magnetic resonance imaging (fMRI) is related to patient reported severity of urge urinary incontinence. J Urol 183:221–228
Torrens M, Feneley RCL (1982) Rehabilitation and management of the neuropathic bladder. In: Illis LS, Sedgwick EM, Glanville HJ (eds) Rehabilitation of the neurological patient. Blackwell Scientific, Oxford
Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009) Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage 47:836–851
Acknowledgments
I am greatly indebted to Dr Stasa Tadic and Professor Clare Fowler for stimulating discussions that led to many of the ideas expressed here. Without their help and the help of many other colleagues and coworkers in Pittsburgh and London, none of these ideas would have been developed. I gratefully acknowledge the constant support and encouragement provided by Dr Neil Resnick as well as financial support from the University of Pittsburgh Competitive Medical Research Fund and from the US Public Health Service, grants R03AG25166 and R01AG020629.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Griffiths, D.J. (2011). Use of Functional Imaging to Monitor Central Control of Voiding in Humans. In: Andersson, KE., Michel, M. (eds) Urinary Tract. Handbook of Experimental Pharmacology, vol 2011. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-16499-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-16499-6_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-16498-9
Online ISBN: 978-3-642-16499-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)