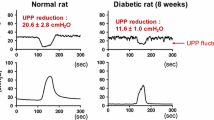
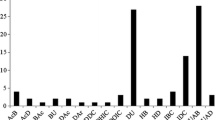
Abstract
Overactive bladder syndrome (OAB) is a symptom-based diagnosis characterised by the presence of urinary urgency. It is highly prevalent and overlaps with the presence of bladder contractions during urine storage, which characterises the urodynamic diagnosis of detrusor overactivity. Animal models are needed to understand the pathophysiology of OAB, but the subjective nature of the symptomcomplex means that interpretation of the findings in animals requires caution. Because urinary urgency cannot be ascertained in animals, surrogate markers such as frequency, altered toileting areas, and non-micturition contractions have to be used instead. No model can recapitulate the subjective, objective, and related factors seen in the clinical setting. Models used include partial bladder outlet obstruction, the spontaneous hypertensive rat, the hyperlipidaemic rat, various neurological insults and some gene knock-outs. Strengths and weaknesses of these models are discussed in the context of the inherent difficulties of extrapolating subjective symptoms in animals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adenosine triphosphate
- Animal models
- Detrusor overactivity
- Knock-out studies
- Nitric oxide
- Oestrogen
- Overactive bladder syndrome
- Prostaglandin
- Transgenic
- Urgency
- Uroplakin
1 Introduction
Overactive bladder syndrome (OAB) is a clinical diagnosis based on the presence of the symptom of urinary urgency. The subjective nature of the defining symptom is a crucial consideration since it is therefore not possible to ascertain the syndrome’s presence or otherwise in an animal. The discussion below must be read with the consideration of the inherent limitations of relying on surrogate markers to infer a situation akin to the clinical scenario. Seemingly more objective is the concept of detrusor overactivity (DO), the urodynamic observation of phasic bladder contractions during the filling phase. On the face of it, this represents a more sound foundation for developing animal models. However, such contractions appear to be normal in many animal species. Indeed in humans, contractions that would be labelled as DO during conventional urodynamics are often observed when people with normal lower urinary tracts are studied with ambulatory urodynamics. Accordingly, the use of terms such as OAB and DO in animal contexts risks misinterpretation of the underlying processes. Nonetheless, animal models are an essential adjunct in endeavouring to address the considerable clinical challenges of OAB.
2 The Problem
OAB is a symptom-based diagnosis defined by the International Continence Society (ICS) as urgency with or without urgency incontinence usually with daytime frequency and nocturia (Abrams et al. 2002a). This definition presumes that infection and other causes for the storage symptoms have been excluded. The key symptom of OAB is urgency, defined as a sudden compelling desire to void, which is difficult to defer (Abrams et al. 2002b). Urgency with at least one of the other storage symptoms listed above is essential for the diagnosis of OAB to be made. Synonyms for the condition include urgency syndrome and urgency–frequency syndrome. OAB is a diagnosis of exclusion as there is no pathognomonic criterion available to confirm the diagnosis.
It is a common condition with an estimated overall prevalence of 11.8%, comprising 10.8% of men and 12.8% of women (Irwin et al. 2006a). Although the condition affects all ages, the prevalence of OAB increases with ageing (Irwin et al. 2006a). OAB symptoms have been shown in numerous studies to have a significant negative impact on the health-related quality of life, emotional well-being and work productivity of affected individuals (Abrams et al. 2000; Irwin et al. 2006b). OAB can be socially disabling as it foments low self-esteem and embarrassment, which can in turn lead to depression and withdrawal from social activities. Increased night-time voiding may cause significant sleep pattern disturbances, resulting in fatigue. Urgency incontinence is associated with an increase in the number of falls and fractures in the elderly population (Brown et al. 2000).
DO is a urodynamic observation characterised by involuntary contractions during the filling phase of cystometry and these can be spontaneous or provoked (Abrams et al. 2002b). Although the symptom of urgency described by OAB patients often correlates with DO seen on cystometry, it must be emphasised that the two terms are not synonymous with each other. A retrospective study published in 2006 found that only 64% of patients diagnosed with OAB using the new ICS definition had DO demonstrable on urodynamics (Hashim and Abrams 2006). The study also showed that more than 30% of patients with DO did not have OAB (Hashim and Abrams 2006), and it is therefore understandable why the bladder has previously been described as an “unreliable witness” (Blaivas 1996).
3 Pathophysiology of OAB
The current understanding of the pathophysiology of OAB is limited, and much of what is known about the clinical aetiology of the condition has been derived from epidemiological data. The mechanisms involved are complex and comprise both peripheral and central nervous system factors. Research into the pathogenesis of OAB is hampered by the fact that it is a symptom-based diagnosis, and consequently, studies in this field have focussed on abnormalities of afferent signalling and the mechanisms underlying DO. Changes in afferent return and signal processing are presumed to be the basis of urgency, whereas cystometrically determined DO is likely to be a contributing factor for incontinence in many OAB sufferers.
Regulation of normal micturition is complex and involves both spinal and supra-spinal control mechanisms. The pontine micturition centre is the prime determinant of lower urinary tract function as it sets the volume at which the lower urinary tract switches from storage to voiding mode, thereby effectively determining maximum bladder capacity. The main factor causing the switch between these two phases is the level of afferent activity arising from the bladder. The afferent nerves conveying sensory information from the bladder to the central nervous system comprise at least two major populations. Myelinated Aδ-fibres are mechanosensitive and are probably responsible for the sensation of bladder fullness. They are activated by both low (non-nociceptive) and high (nociceptive) intravesical pressures. Unmyelinated C-fibres are the second type of afferent and are activated by cold, heat or irritation of the bladder mucosa. C-fibres have primarily nociceptive functions and do not usually respond to bladder distention, but may do so under pathological conditions. It has been proposed that DO and OAB may arise when the levels of afferent activity are inappropriately high for any given degree of bladder distention (Abrams and Drake 2007).
Three main hypotheses have been proposed to explain the pathophysiological basis of DO: neurogenic (de Groat 1997), myogenic (Brading 1997; Brading and Turner 1994) and peripheral autonomy (integrative) (Drake et al. 2001). The neurogenic theory places the fundamental problem in the central nervous system, whereas the latter two hypotheses focus on the periphery (Drake et al. 2006). The neurogenic hypothesis proposes that DO is caused by neural plasticity within the central nervous system, which gives rise to generalised, nerve-mediated excitation of detrusor muscle (de Groat 1997). This may result from damage to descending inhibitory pathways arising from higher brain centres, inappropriate expression of primitive spinal bladder reflexes, or as a result of sensitisation of afferent terminals (de Groat 1997).
The myogenic hypothesis suggests that DO results from changes within the bladder smooth muscle that lead to increased excitability and likelihood of spontaneous contraction with enhanced electrical coupling between muscle cells (Brading 1997). In keeping with this, studies have revealed characteristic ultrastructural changes associated with DO, which could potentially facilitate the propagation of electrical activity and contraction over a wider than normal proportion of the detrusor muscle (Haferkamp et al. 2003a, b).
The integrative hypothesis arises from the observation that the bladder shows complex autonomous activity when descending suppressive influences are removed (Coolsaet et al. 1993; Drake et al. 2003a, b). Resulting localised contractions and stretches (micromotions) have been demonstrated in several animal models (Drake et al. 2003a). These findings have led to the suggestion that the detrusor muscle is functionally modular in arrangement, conceptually determined by a myovesical plexus, analogous to the myenteric plexuses, which control gut motility. While localised modular activity during bladder filling may contribute to the normal generation of sensory information, exaggerated responses may give rise to urinary urgency. In keeping with this, an increased frequency in micromotion activity has been correlated with reported sensations of urgency in symptomatic women in the absence of intravesical pressure changes (the so-called “sensory urgency”) (Drake et al. 2005; Van Os-Bossagh et al. 2001). The hypothesis goes on to suggest that while activity in individual modules would have little effect on intravesical pressure, the enhanced coordination of modular activity through the myovesical plexus would lead to contraction of a more substantial proportion of the bladder wall resulting in the emergence of DO (Drake et al. 2001). The integrative hypothesis proposes that other cell types in addition to muscle (interstitial cells, urothelium and peripheral nerves) contribute to normal generation of localised spontaneous activity, and thus low pressure sensing of filling state; in symptomatic people, the localised micromotions are exaggerated, giving rise to urgency, and the same cell types also lead to the wider propagation of spontaneous activity, seen as DO.
As a symptom-based diagnosis, more recent thinking has shifted emphasis towards sensory mechanisms. The concept of “afferent noise” signifies the existence of normal and pathological mechanisms capable of modulating the amount of neuronal traffic in bladder-related sensory nerves for any given filling volume (Andersson 2002; Gillespie 2005), such as urothelial interactions with subjacent interstitial cells and nerve endings (Birder 2005; Birder and de Groat 2007), or alterations in transmitter profiles in peripheral nerves. In addition, central nervous system circuitry, including the limbic system and other neural processes underpinning conscious attention, has a major impact on the effects perceived by the individual. Functional brain imaging techniques are beginning to yield intriguing insights into the relevant brain areas, the potential matrix of brain centres regulating the processing of lower urinary tract sensory perception and motor responses and the recognised interactions with other organ systems.
It is clear from the above that the mechanisms that underlie OAB and DO are still incompletely understood. The pathogenesis of OAB is multifactorial, differing for different individuals, and altering over time in any one person. Normal bladder function is dependent on the intricate interplay of neural, myogenic and other cell types, and disruption of any of these may lead to lower urinary tract dysfunction, if not compensated for elsewhere in the control hierarchy. It is therefore not surprising that increasingly complex hypothetical insights into bladder function and control are emerging; consequently, animal models are a vital tool to study their validity.
4 Current Management of OAB
For the majority of OAB sufferers, treatment is aimed at symptom relief rather than cure; consequently, OAB is now recognised as a chronic medical condition. The principles of treatment are to reduce urinary urgency so as to decrease the number of episodes of incontinence and improve urinary frequency, nocturia and voided volumes (Hashim and Abrams 2007). Once alternative pathologies have been excluded, the treatment of individuals diagnosed with OAB involves lifestyle advice, behavioural training and pharmacological and surgical interventions. Lifestyle interventions include fluid restriction, avoidance of foods with a high water content, reduction of evening fluid and caffeine intake, smoking cessation and weight loss (Bulmer et al. 2001; Hannestad et al. 2003). Bladder training, supplemented by pelvic floor muscle exercises, aims to suppress involuntary detrusor contractions through feedback inhibition as intentional contraction of the pelvic floor muscles can inhibit detrusor contraction (Yamaguchi et al. 2009). Antimuscarinic medication is the mainstay of treatment for OAB symptoms, but a significant proportion of patients do not respond to therapy, or will discontinue drug treatment because of its side effects.
Patients whose OAB symptoms fail to respond to optimised first line measures should be managed in a specialist centre within the settings of a multidisciplinary team. Previously, standard alternatives for sufferers with refractory symptoms involved major reconstructive surgery, of which the main options are augmentation cystoplasty (Bramble 1982, 1990) or urinary diversion into a urostomy (Singh et al. 1997). These procedures have the potential for significant morbidity and risk, and long-term satisfactory outcome is uncertain. Surgery is viewed as the last resort, but it has a role to play in the management of sufferers with severe symptoms and who have failed to respond to all other treatments.
Several treatments have since been developed and adopted for use to bridge the gap between conservative and surgical interventions. These include intravesical injections of Botulinum toxin, tibial nerve stimulation and sacral neuromodulation. They are warranted in patients for whom quality of life is significantly impaired by severe symptoms, but who are unwilling to accept the risks associated with surgery or are medically insufficiently healthy to contemplate general anaesthesia. Despite the seemingly wide range of treatments available, a significant proportion of patients with OAB continue to be troubled by their symptoms, and therefore a wider range of more effective therapies are required.
5 Importance of Animal Models
Our understanding of lower urinary tract function remains incomplete as human studies and research using human material are inherently limited because of the legal, ethical and moral implications associated with such investigations. Much of our knowledge of bladder function has come from in vitro research such as that using muscle strips (Buckner et al. 2002; Oh et al. 1999; Yamanishi et al. 2000; Hashitani et al. 2001), but findings from these types of experiments are difficult to extrapolate to whole bladder function. Over the past century, experimental work looking at bladder function in humans has involved in vivo cystometric investigations on willing patients and volunteers as well as molecular and cellular research using biopsy material (Gillespie 2005). Advances in diagnostic and functional imaging have allowed brain mapping (Griffiths et al. 2007, 2009) in human subjects, and although this has been informative, the results generated by these studies are by their nature correlational.
Attempts to discover the origins of urgency, urgency incontinence and DO have been the major forces driving research into lower urinary tract function (Gillespie 2005). Improved understanding of these phenomena may lead to the discovery of more effective treatments for storage symptoms and OAB. Increasing use of animal models to study the integrative physiology of the lower urinary tract and its control may go some way to achieving this, but there are important limitations (see below). Hypotheses typically need animal models to test derived predictions. Animal modelling thus provides a crucial link to the clinical context, and its use in basic and clinical research is a necessary precursor to safe and ethically sound research in humans. It can be used to generate novel directions of research and corroborate findings obtained by other means/studies. Animal models are the way in which the results from simpler in vitro research can be tested in “intact” biological systems without direct human experimentation. This is important because findings obtained under artificial in vitro conditions may not apply in vivo, and the results generated using one cell type may not be applicable to other cell lines. It allows assessment of the relevance of any in vitro study findings to the living whole organism.
Animal models can be classified as exploratory, explanatory or predictive, depending on the type of information that they are designed to yield (Hau 2008). Exploratory models are used to study physiological processes and pathological mechanisms of action in order to generate novel ideas and theories about biological function. These hypotheses then require validation by replicating the findings in other models before returning to human studies to ensure that they are relevant to the disease or physiological process being studied. Explanatory models are developed and applied in an attempt to improve our understanding of the importance and relevance of findings generated by other research and the aim of studies using these models is to provide an explanation for a biological process. Predictive models are those used to discover and quantify the impact of novel treatments and therapeutic agents and to assess their toxicity to the living organism (Table 1).
The majority of animal models used in research have been developed to study the aetiology, pathogenesis, natural history and potential treatments for human disorders. The findings they generate may drive medical advances and understanding, but the information gained must be interpreted within the limitations of the model. The validation required will depend on how closely the model reproduces a disease or condition (Table 2). A homologous model is one in which the animal replicates the symptoms, aetiology and natural history of the human condition (Hau 2008). Isomorphic models resemble the human phenotype, but the underlying mechanism in the animal is different from the clinical setting (Hau 2008). Most models in current use are neither homologous nor isomorphic, but are partial as they fail to mimic the human condition they are modelling but bear enough similarities to allow their use for studying aspects of a disease or its treatments (Mogensen and Holm 1989). Irrespective of the type of model used, careful validation of both the model itself and the results it generates ensure that any findings are correctly extrapolated from animal to human; too much weight should not be given to findings from any one animal model.
6 Difficulties Interpreting Results from Studies Using Animal Models
The fact that animal models often fail to predict the efficacy of novel therapeutic agents in patients highlights the difficulties in extrapolating data from animals to humans. Ideally, animal models should reproduce all the facets of the human condition, but it is inconceivable that any single animal model will replicate all the signs, symptoms, mechanisms, and consequences of a disease/condition including the physiological and pathological changes that occur. In the context of OAB, the range of facets of the clinical condition clearly exceeds the realistic characteristics of an animal model (Table 3). A model is usually only relevant for a limited number of aspects of the human condition. Rather than viewing modelling as an attempt to replicate the human condition exactly, a more productive approach involves developing specific models each tailored to answering a particular experimental hypothesis. When viewed as tools rather than replicas, the value of animal models that do not recapitulate the entire human disease becomes more obvious.
Given that the primary physiological functions of the mammalian bladder are urine storage and voiding at appropriate times, it is not unreasonable to presume that the general principles regulating its function should be similar among different species (Brading 2006). It is however important to be aware that there are a number of important differences in normal urinary tract structure and function between different animal species and humans. These must be considered and taken into account before extrapolating physiological and pathological findings back to humans. For example, most mammalian species, apart from humans and old world monkeys, possess a dual cholinergic and purinergic excitatory innervation to the detrusor muscle of the bladder, and this is thought to be due to the evolutionary requirement of certain species to mark their territory with urine (Craggs et al. 1986; Hashitani et al. 2000; Sibley 1984). Although nerve-mediated contractions in the human bladder are almost solely mediated by a cholinergic mechanism, normal human bladder smooth muscle cells express functional adenosine triphosphate (ATP, purinergic) receptors (Inoue and Brading 1991), and non-cholinergic non-adrenergic (NANC) excitatory transmission appears to emerge in some pathological conditions (Sibley 1984; Sjogren et al. 1982).
Another important difference is the arrangement of the parasympathetic system in certain species. Rats are commonly used for animal studies, but it must be noted that the postganglionic parasympathetic cell bodies innervating the rat bladder are found entirely in the pelvic ganglia, whereas a substantial proportion of these cell bodies are located in the bladder wall in humans and other animal species (McMurray et al. 2006). Consequently, the pattern of denervation following partial bladder outlet obstruction (BOO) differs between species (McMurray et al. 2006; Gabella and Uvelius 1990).
Acetylcholine is the primary neurotransmitter effecting bladder emptying through its action on the muscarinic receptors on detrusor muscle. Muscarinic receptors are classified based on molecular and pharmacological criteria into five subtypes (M1–M5), and detrusor muscle, like other forms of smooth muscle, exhibits a heterogeneous distribution of these receptor subtypes. Muscarinic receptors are also present in other cell types in the bladder, including interstitial cells (Gillespie et al. 2003) and the urothelium (Hanna-Mitchell et al. 2007). Studies have shown that the combined density of M2 and M3 receptors is very close to the total density of muscarinic receptors in each species studied (rat, rabbit, guinea-pig and human), with a predominance of M2 receptor expression (Wang et al. 1995). Immunoprecipitation data indicate that the M3 receptor subtype density varied from 8% in rats to 32% in humans, and that the proportion of muscarinic M2 and M3 receptor subtypes is different for the various species. The M2–M3 receptor ratio has been reported as 3:1 in the bladders from humans, rabbits and guinea-pigs and 9:1 in the rat bladder (Wang et al. 1995). Although it seems probable that there is variable expression of muscarinic receptor subtypes in the lower urinary tract of different species, recent studies have shed doubt on the validity of this immunohistological data (Pradidarcheep et al. 2009; Jositsch et al. 2009). Studies have shown that the traditional criteria used to establish the specificity of the antiserum used for immunoprecipitation studies is unable to reliably predict specificity. This is evidenced by the false positive labelling of tissues taken from muscarinic receptor gene-deficient mice (Jositsch et al. 2009). Care must therefore be taken when interpreting results of immunohistochemical studies that predate the development of the appropriate knock-out strains. There are also significant differences in the composition of the urinary bladder wall between small and large animal bladders, with variability in urodynamic features.
Almost 4,000 organisms including a variety of mammals have now had their genomes sequenced (Venter 2010), but unfortunately, large animals are often of limited value in genomic studies. Non-human primates should be ideally suited for modelling the human condition, but generating genetically modified monkeys or apes is fraught with difficulties. Even though the genomes of several non-human primates have been completely mapped out (The Chimpanzee Sequencing and Analysis Consortium 2005; Gibbs et al. 2007; Varki and Altheide 2005), manipulating specific genes for the purpose of knock-out and knock-in studies has been hampered by the low efficiency and poor reliability of the available gene transfer methods used (Chan 2004). Mice, on the other hand, though more dissimilar from humans, are ideally suited for transgenic and knock-out studies because they can be genetically manipulated with relative ease and have a shorter generation span.
The structural and functional differences discussed above are but a few examples of the interspecies variability, which are relevant to research into the lower urinary tract. It is important that these aspects are taken into account when using animal models, in order to distinguish between experimental findings that constitute general principles and are applicable to other species from those that are particular to the animal being examined. Failure to attend to species differences not only ignores the opportunity to better understand the mechanisms of diversity, but may lead researchers to make inadequately founded suppositions (Insel 2007).
Given the interspecies variability, the various animal models in current use have different strengths and weaknesses, which render them suitable for studying different aspects of disease. Animal studies can be viewed as hierarchical in nature as studies using rodents and small laboratory animals are relatively simpler to perform and maintain than studies done using larger animals and primates. Research done with small mammals is less expensive, more accessible and less time-consuming to carry out because of the shorter lifespan of these animals, but care must be taken when attempting to translate findings derived from “simple” species to more “complex” ones (Insel 2007).
Irrespective of the animal species and model used, it is vitally important to decide whether an experimental finding or change is contributory and relevant to the physiological or pathological process being studied. Whole organisms adapt to change and cope with pathological challenges through neural plasticity and cellular adaptations. Experimental findings could therefore be compensatory changes or collateral effects rather than aetiological or pathophysiological. It may also be that the changes seen are epiphenomena, being totally unrelated to the underlying pathological process and occurring by coincidence. Careful validation of findings is needed to ensure applicability of any study result to humans, and this is often an underestimated last step of published studies using animal models.
Despite the difficulties associated with animal modelling and the need for careful validation of any findings that these studies yield, there is no substitute for their use as tools to evaluate medical interventions in complex biological systems (Matute-Bello et al. 2008). To overcome the shortcomings associated with animal models, researchers must pay close attention when choosing an animal model to ensure that it is the one that is best suited for testing a hypothesis or answering a specific question. Any findings should then ideally be reproduced in more than one mammalian species before extrapolating data and contemplating research using human subjects.
6.1 The Difficulty in Developing Animal Models of OAB
Several animal models have been developed in a variety of species in an attempt to study the pathophysiology of OAB. However, OAB is a symptom-based diagnosis in which the conscious perception of urgency is key to the diagnosis. There is no way of knowing for definite whether an animal is experiencing urgency, even if pseudoaffective changes in behaviour may suggest it. Animals cannot relate their symptoms to investigators, and consequently, it is not possible technically to create an animal model of OAB. The problem is essentially the difficulty of interpolating subjectivity.
The inability to quantify urgency in animals, or assert its presence in the first place, necessitates the use of surrogate markers. That is why most experimental models have focussed on DO because this can be objectively measured at cystometry. It must be remembered however that DO is not pathognomonic of OAB, as a significant proportion of patients diagnosed with the condition have no evidence of DO on urodynamic evaluation (Hashim and Abrams 2006). Furthermore, non-micturition contractions do not necessarily equate to DO; indeed, they may be normal in some species.
Other surrogate markers that have been used in lower urinary tract research as clinically meaningful endpoints are urinary frequency, voided volumes and changes in micturition habit. An example of the latter is the fact that most rodents tend to urinate in one particular part of their confinement area, and in some models of presumed overactivity, the rodents stop doing this and begin voiding in various places (Gevaert et al. 2007). This behavioural modification is taken to be a marker of a change in their lower urinary tract whereby the rodent is unable to defer micturition and therefore has to void where it is rather than being able to make it to its usual spot in time. This is a plausible concept that may be analogous to the behavioural changes seen in OAB sufferers who identify the location of their nearest toilet when away from the comfort and safety of their home.
Research into bladder overactivity is also hampered by the lack of a biological marker (biomarker), which is specific to the lower urinary tract and reflects the activity of the disease process. Some molecules/substances have been identified and measured in the urine, but none have proven to be diagnostic. Given the likely multifactorial aetiology of OAB and the chronic nature of the condition, it will be difficult to develop such a biomarker.
For some patients, such as those with neuropathic lower urinary tract dysfunction due to a cerebrovascular event or a spinal cord injury, the aetiology of storage symptoms is known. The inciting event can be recapitulated to induce equivalent changes in animal models. For idiopathic OAB, this is not currently possible, hindering research into mechanisms and therapy. Consequently, phenotypic (isomorphic) models are used in which an animal expressing features (signs and behavioural changes) resembling those seen in OAB sufferers are used to try and determine the underlying aetiology. Unfortunately, this approach to modelling is complicated by the fact that the bladder has a limited repertoire of responses to injury, and thus differing aetiological factors may produce a similar picture in affected individuals.
7 Animal Models of OAB
A wide range of animal species have been used for lower urinary tract research and to study urological disease. These include hamsters, mice, guinea-pigs, rats, rabbits, cats, dogs, pigs and non-human primates. The aim of this section is to provide a summary of the major types of animal models that have been used to study the mechanisms and treatments of OAB, and to highlight the areas of concern when interpreting results yielded by studies using these models.
The starting point for many of our animal models has come from what is known epidemiologically about the condition. It is well known that ageing is associated with a rise in prevalence of OAB for both genders and this has underpinned the study of ageing animals. There are recognised associations of storage symptoms and OAB with neurological conditions and BOO, and consequently, animal models have been developed with this in mind. All patients with neurological disorders have some form of consequence to their lower urinary tract function as no matter how mild their disease is, they inevitably develop a bladder-related problem. This has led to the development of large range of neurological models.
Most animal models used to study OAB are induced models, whereby a relevant pathological challenge is experimentally applied to a healthy animal. With advancing technology and the ability to genetically modify animals, there is an increasing trend towards the use of transgenic models.
7.1 Peripheral Versus Central Models
Animal models of OAB can be broadly divided into peripheral and central models based on the predominant site of the deficit. Peripheral models are those resulting from direct damage to the bladder, its peripheral innervation or blood supply, whereas central models develop following injuries to the spinal cord, brainstem or higher centres.
7.2 Induced Hypersensitivity/Inflammatory Models
These are commonly used models in which hypersensitivity and/or inflammation is induced in the bladder by either a surgical insult or chemically by instillation of a noxious substance intravesically for a short period of time. Numerous chemicals have been used for this purpose and include acetic acid, citric acid, hydrochloric acid, capsaicin, protamine sulphate, xylene and turpentine. The resulting effects are thought to be due to up-regulation and stimulation of nociceptive afferent C-fibres within the bladder wall (Fowler 2002). This leads to increased sensory activity, which is a mechanism that has been proposed as a potential cause of urgency.
Although these models have been used to try and elucidate the mechanisms leading to the development of storage symptoms, they are not a true model for OAB as symptoms arise by the entirely unrelated process of acute reactive inflammation. Indeed on histological examination, the bladder mucosa of OAB sufferers does not show evidence of the inflammatory response, which is triggered in these animals. The noxious stimuli used can affect other epithelial surfaces indicating that the effects on the urothelium are a non-specific response to injury. Thus, these models may better equate to different clinical conditions, such as infectious cystitis, radiation cystitis or ketamine cystitis. Modelling a chronic condition with an acute inflammatory challenge fails to recapitulate the mechanisms of neural plasticity and cellular adaptation, which are likely to arise in OAB clinically.
7.3 Bladder Outlet Obstruction Model
BOO is a common problem for the ageing male as a consequence of benign prostatic enlargement (BPE), in whom increasing prevalence of lower urinary tract symptoms is also apparent (Irwin et al. 2009). Storage symptoms often persist even after the obstruction is surgically corrected (Seaman et al. 1994), signifying that the association is not necessarily causative (Thomas and Abrams 2000). The mechanisms underlying the OAB seen in patients with BOO are thus not fully understood.
Effects similar to BOO in humans are relatively straightforward to replicate in animals. This has been achieved in a variety of animal species including the pig, rat, guinea-pig and rabbit by partial obstruction of the urethra using some form of ligature that either occludes/stenoses the urethra immediately or does so gradually as the animal grows (Drake et al. 2006; Jorgensen et al. 1983; Sibley 1985; Kato et al. 1988; Pampinella et al. 1997; Wolffenbuttel et al. 2001). These models show many of the structural and physiological bladder wall changes seen in human BOO, including muscle cell hypertrophy, altered responsiveness to stimuli, increased spontaneous myogenic activity with development of non-micturition contractions and enlarged sensory neurons and parasympathetic ganglia. There is also patchy denervation of detrusor muscle (Turner and Brading 1997), a key factor in the myogenic hypothesis (Brading and Turner 1994), though it is not seen in rats (Gabella and Uvelius 1990). Filling phase detrusor contractions can persist despite pharmacological blockade of peripheral neuronal activity, signifying a non-neuronal origin (Turner and Brading 1997; Igawa et al. 1992).
Many of the published studies based on research done in partial BOO animal models have used female rodents, which complicates their interpretation, given that they are derived by analogy to male BPE. Furthermore, the induced BOO is much more acute, and potentially more severe, than BPE – particularly if the urethra is crushed by a ligature tied firmly against a calibration rod. Notwithstanding, partial BOO appears to be a good model to study lower urinary tract symptoms as it can be reliably reproduced with good face and aetiological validity.
7.4 Spontaneous Hypertensive Rat
The spontaneous hypertensive rat (SHR) is a genetic model of multifactorial hypertension, which is considered to resemble human essential hypertension (McMurray et al. 2006). The SHR also exhibits abnormal bladder function and hyperactive behaviour (Jin et al. 2009). Compared to their genetic control, these animals have been shown to have a reduced bladder capacity and micturition volume, increased urinary frequency and a greater occurrence of non-voiding contractions analogous to DO (McMurray et al. 2006). This has led to the use of the SHR as a model to study DO and OAB.
The exact cause for the abnormal voiding function in the SHR is not known, but seems to involve both peripheral and spinal mechanisms. The major abnormality appears to lie in the central nervous system with changes in the noradrenergic control of the micturition reflex (Persson et al. 1998). Peripherally, there is both an increased detrusor muscle and decreased neuronal responsiveness to norepinephrine.
The majority of cystometric studies in animals measure intravesical pressure alone, so there is no way of knowing whether the bladder pressure changes recorded are actually due to transmitted rises in abdominal pressure. This uncertainty is relevant to the SHR, given the hyperactive behaviour of the rats. A study published in 2008 tackled this issue by performing simultaneous recordings of intra-abdominal and intravesical pressures in conscious male SHRs (Jin et al. 2009). The group found that the majority (76%) of the intravesical pressure rises recorded in the SHRs represented overactive detrusor waves with the remainder being caused by abdominal pressure changes (Jin et al. 2009). Although ideally simultaneous measurement of intra-abdominal and intravesical pressures should be done at cystometry, given that the vast majority of pressure changes recorded in these animals are due to non-voiding contractions of the detrusor, it seems reasonable to measure intravesical pressure alone.
7.5 The Hyperlipidaemic Rat
Epidemiological studies have shown correlation between lower urinary tract symptoms and erectile dysfunction (Ponholzer et al. 2004; Macfarlane et al. 1996; Boyle et al. 2003), suggesting that common mechanisms might be involved in the development of both these conditions. The role of hyperlipidaemia in erectile dysfunction has been extrapolated to derive a model of lower urinary tract symptoms and OAB. Rats fed with a high fat/cholesterol diet show an increase in urinary frequency and a greater number of non-voiding contractions during bladder filling on awake cystometry compared to control rats (Rahman et al. 2007; Son et al. 2007). The hyperlipidaemic rats show a significantly higher weight and cholesterol level compared to the controls, but with no statistically significant difference in glucose levels.
The underlying pathological mechanisms have not been clearly defined, but current evidence suggests that there is likely to be both a vascular and neurogenic component (Rahman et al. 2007). Chronic bladder ischaemia secondary to atherosclerosis may be one aetiological factor as prolonged moderate bladder ischaemia has been shown to be associated with detrusor contractions during bladder filling in rabbits (Azadzoi et al. 1999). There is also an up-regulation of purinergic receptors in the urothelium and bladder nerve bundles compared to control rats (Rahman et al. 2007), perhaps corresponding to the increased NANC innervation seen in ageing humans and bladder conditions (Andersson and Pehrson 2003).
7.6 Neurological Models
Injuries or diseases of the central nervous system can disrupt the voluntary control of micturition, damage descending central inhibition and cause re-emergence of primitive voiding reflexes (Andersson and Pehrson 2003). The central control of micturition is highly complex, and the voiding disturbance that develops will depend on the location and extent of the neurological injury. The coordination between the detrusor smooth muscle and the sphincter mechanism of the bladder occurs in the pontine region of the brainstem. Consequently, patients with suprapontine or cortical lesions have co-ordinated voiding mechanisms without detrusor sphincter dyssynergia (DSD) but typically have neurogenic DO (i.e., DO associated with a relevant neurological condition) on urodynamic studies (Andersson and Pehrson 2003). On the other hand, patients with spinal cord injuries will present with a variety of lower urinary tract signs and symptoms depending on the level of injury and whether it is partial or complete. Features comprise neurogenic DO, DSD and various forms of urinary incontinence.
Neurological models are not directly relevant to idiopathic OAB, but they have improved our understanding of the complex pathways controlling micturition. Given that OAB may develop as a result of increased bladder sensation and altered processing of afferent information, increased knowledge of lower urinary tract dysfunction in neurological patients is likely to be relevant to OAB and other hypersensitive bladder syndromes such as interstitial cystitis.
7.6.1 Spinal Cord Transection/Injury
This is one of the commonest neurological models used. Immediately following spinal cord injury, a spinal shock phase of varied duration is followed by disinhibition hyperreflexia below the injury, including spasticity (skeletal muscle hyperreflexia), DO (bladder hyperreflexia) and DSD (sphincter hyperreflexia). In addition to disinhibition resulting from loss of inhibitory descending pathways from higher centres, neural plasticity is relevant. Thus, nociceptive C fibres, which do not usually respond to bladder distension, emerge as functionally active in the afferent limb, underpinning part of the development of neurogenic DO (Morrison et al. 2002). This may explain the response to intravesical vanilloid therapy (Kuo et al. 2006).
7.6.2 Suprapontine Animal Models
A number of central nervous system disorders cause voiding dysfunction in humans, including cerebrovascular events, dementia, Parkinson’s disease (PD) and multiple sclerosis (MS). In stroke patients, the most common urodynamic abnormality is neurogenic DO. Animal models of cerebrovascular events have provided insights into the pathophysiology of stroke-associated OAB. Experimental cerebral infarction can be achieved by occlusion of the middle cerebral artery (MCA) in rats, producing ischaemia within the ipsilateral frontoparietal cortex and the caudate putamen (Belayev et al. 1996). This leads to the development of DO with an increase in micturition frequency and a reduction in bladder capacity (Yokoyama et al. 1997), and these changes persist for several months (Yokoyama et al. 1998a). These effects develop as soon as 30 min after MCA occlusion and it is therefore reasonable to conclude that bladder function is under tonic cortical control and that disinhibition leads to bladder overactivity (Yokoyama et al. 1998a). This is corroborated by studies of midcollicular decerebration in cats with an intact neuroaxis as this leads to a facilitatory effect on bladder function (Ruch and Tang 1956). However, decerebration of rats with MCA occlusion reduces the effects of cerebral infarction on bladder function, and this suggests that in addition to loss of tonic inhibition, the bladder overactivity that develops is mediated in part by upregulation of an excitatory pathway from the forebrain (Yokoyama et al. 2000). Intravenous administration of N-methyl-d-aspartate (NMDA) receptor antagonists reduce the effects of cerebral infarction in awake animals, indicating the involvement of glutamatergic pathways in the pathogenesis of stroke associated OAB (Yokoyama et al. 1998b). However, NMDA receptor antagonists cannot completely reverse these effects and therefore other neurotransmitter systems are likely to be involved. Pharmacological studies indicate that dopaminergic pathways are also involved in cerebral infarction-induced bladder hyperactivity, such that antagonism of D2-like receptors in MCA-occluded animals increases bladder capacity (Yokoyama et al. 1999). This is unsurprising, given that in the normal rat, activation of D1-like dopaminergic receptors inhibits micturition and activation of D2-like receptors facilitates micturition (de Groat and Yoshimura 2001).
PD is a chronic and progressive degenerative disease of the brain characterised by selective destruction of striatal dopaminergic neurons that pass from the substantia nigra pars compacta to the putamen (Gerfen 2000). The majority of patients with the condition will eventually develop voiding dysfunction, characterised by neurogenic DO and impaired relaxation of the striated urethral sphincter. Parkinsonism can be induced in monkeys by administering the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is selective for dopaminergic neurons in the substantia nigra (Burns et al. 1983). This animal model of PD has been used to study the pathogenesis of the disease and has increased our understanding of the dopaminergic pathways controlling micturition. In MPTP-treated monkeys, cystometry showed that injection of a dopamine D1 receptor agonist significantly increases the bladder volume and pressure thresholds for inducing the micturition reflex, with no corresponding effect seen in normal monkeys (Yoshimura et al. 1993). In contrast, administering a D2 receptor agonist reduced the threshold volume of the bladder, which triggers the micturition reflex in both normal and MPTP-treated monkeys (Yoshimura et al. 1993). These results suggest that in PD, the degeneration of dopaminergic neurons in the substantia nigra leads to neurogenic DO, probably as a result of a failure of activation of D1-like receptors, which then allows D2 receptors to facilitate micturition.
7.6.3 Experimental Autoimmune Encephalomyelitis Model
Voiding dysfunction is a common problem for patients with MS and develops in over 90% of patients who have had MS for more than 10 years (Andersson and Pehrson 2003). The voiding abnormalities are due mainly to spinal demyelinating lesions, but cerebral defects may contribute. Neurogenic DO is the most common abnormality seen on urodynamic testing with 70% of patients affected, and this is associated with DSD in 50% of patients (Sirls et al. 1994).
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory autoimmune condition, which targets nervous tissue and can be induced in various animal species by active immunisation with central nervous system immunogenic compounds or through passive transfer of encephalogenic T cell lines from affected animals (Petry et al. 2000). Animals with EAE can be used to study neuroinflammatory bladder dysfunction such as that which occurs with MS, though extrapolating data from these models has to take into account the uncertainties of transferring findings from an acute model to a chronic human condition. EAE is also a transient self-limiting condition that often recovers spontaneously, whereas MS is chronic and progressive. This must also be borne in mind when performing studies using this model.
7.7 Transgenic Animal Models
Advances in genetic engineering techniques have led to an increasing use of genetically modified organisms for research. Knock-out models and other transgenic animals are being used to study and understand the molecular mechanisms involved in both normal physiological processes and human disease. For example, knock-out mice have been used to determine the relative contribution of the different muscarinic receptor subtypes in normal bladder function and confirm that the M3 subtype is the dominant receptor in vivo (Matsui et al. 2002).
The progress mapping the human genome, and various non-human organisms, such as E. coli, the fruit fly and the laboratory mouse, has yielded major insights in functional genomics and proteonomics (Hau 2008). The mouse is most commonly employed, given that it is widely available, easy to maintain and has a relatively short generation span. Several transgenic mouse models that bear particular relevance to lower urinary tract physiology and dysfunction have been developed. These knock-out models will be discussed briefly below but have clearly demonstrated that molecular alterations of the urothelium, peripheral innervation and smooth muscle of the bladder leads to significant changes in its function (Hu et al. 2002).
7.7.1 Neuronal Nitric Oxide Synthase Knock-out Mouse
Compared with wild type mice, the neuronal nitric oxide synthase (nNOS) knock-out mouse demonstrates urinary frequency with no change in the total amount of urine produced (Burnett et al. 1997). This suggests changes in lower urinary tract function and is supported by the reduction in bladder capacity and induction of detrusor contractions seen in rats given intravesical nitric oxide scavengers or nitric oxide synthase inhibitors (Pandita et al. 2000; Masuda et al. 2007). Certain phosphodiesterases, through their effects on the nitric oxide-cyclic guanosine monophosphate pathway, are relevant to the lower urinary tract and may have clinical applicability (Andersson et al. 2007; Truss et al. 2000). It is not yet clear whether the changes seen in these animal models are due to bladder hypertrophy secondary to the impaired outlet relaxation or as a result of a lack of nitric oxide-induced modulation of the afferent pathways.
7.7.2 Uroplakin Knock-out Mouse
Uroplakins are integral components of the plaques that cover the apical surface of urothelial cells and are critical to the barrier function of the urothelium (Hu et al. 2002). Uroplakin IIIa and especially uroplakin II knock-out mice are associated with significant changes in bladder function including increased spontaneous activity and inter-micturition pressure and the development of non-voiding contractions on cystometry analogous to DO (Aboushwareb et al. 2009). The bladders of these knock-out mice show no significant change in their contractile response to carbachol, so an enhanced contractile response to endogenous neurotransmitters is unlikely to be a major contributing factor to the alterations in bladder function (Aboushwareb et al. 2009). On histological examination, uroplakin deficiency is associated with ultra-structural changes, which are implicated in the defective urothelial permeability seen in these models (Aboushwareb et al. 2009). These transgenic mice provide further evidence for the important role that the urothelium plays with regards to bladder physiology and dysfunction.
7.7.3 Prostaglandin Receptor Knock-out Mouse
Prostaglandins are produced by the constitutively expressed cyclooxygenase- (COX) 1 enzyme, and an inducible isozyme, COX-2. Expression of COX-2 is enhanced in models of bladder dysfunction, and prostaglandin E2 (PGE2) produced by this enzyme is increased in the urine of male and female patients with overactive bladders (Kim et al. 2005, 2006). PGE2 mediates its effects by activating the EP family (EP1–EP4 isoforms) of G-protein coupled receptors (Hao and Breyer 2008). When administered intravesically, PGE2 induces non-voiding contractions and reduces bladder capacity in humans (Schussler 1990).
PGE2 may have a physiological role to play as it is produced by detrusor muscle in response to stretch. Knock-out models of the EP3 receptor have enlarged bladder capacity, which is unrelated to urine composition or volume (McCafferty et al. 2008). These knock-out animals do not display bladder overactivity when an EP3 receptor agonist is infused intravesically. Thus, the EP3 receptor may contribute to bladder dysfunction under conditions of enhanced PGE2 release, as observed in OAB patients (McCafferty et al. 2008). The limited evidence currently available point towards an effect on peripheral afferent sensitivity.
7.7.4 Purinergic Receptor Knock-out Mouse
ATP is released from human and animal urothelium in response to mechanical stretch (Sun et al. 2001; Ferguson et al. 1997). ATP can act as a sensory neurotransmitter by binding to purinergic receptors (P2X) on suburothelial afferent nerve endings. Indeed, intravesical instillation of ATP into normal awake, freely moving rats triggers bladder overactivity, with an increased frequency and amplitude of bladder contractions (Pandita and Andersson 2002). P2X3 receptor knock-out mice appear to have reduced bladder sensation, with reduced urinary frequency and larger voided volumes (Cockayne et al. 2000). This points towards a physiological role for ATP and purinergic receptors in normal bladder function. It may be that one of the contributory mechanisms in OAB could be an augmentation of the purinergic pathway with enhanced ATP levels and up-regulation of purinergic receptor expression.
7.7.5 Oestrogen Receptor Knock-out Mouse
Epidemiological studies have implicated oestrogen deficiency as a factor contributing to the increased prevalence of lower urinary tract symptoms seen with ageing in women (Iosif and Bekassy 1984). The influence of oestrogen on the bladder has consequently been the subject of several experimental and clinical studies. Oestrogen receptors (ERs) are members of the nuclear receptor superfamily, and two different subtypes (ERα and ERβ) have been identified (Kuiper et al. 1997; Kuiper et al. 1996). They are ligand-activated transcription factors that regulate target gene expression. Both ER subtypes are expressed in the lower urinary tract, but ERβ is thought to be the predominant isoform in the bladder (Makela et al. 2000).
Studies using knock-out mice lacking either the ERα, ERβ or both receptors showed no significant differences in voiding patterns or in any parameter measured on awake control cystometry compared to wild-type animals (Schroder et al. 2003). Use of in vitro muscle strips from sacrificed animals also showed no differences in contractile response to electrical field stimulation or carbachol for any of the knock-out strains compared to control mice (Schroder et al. 2003). There is however variation in the response to capsaicin, as ERα knock-out mice did not develop the overactive cystometry patterns seen in the other ER knock-out strains and wild-type mice when capsaicin was instilled intravesically. This suggests that rather than an efferent or direct muscle effect, oestrogen may impact on lower urinary tract function through changes in afferent signalling (Schroder et al. 2003), and in keeping with this, ERs have been identified in several areas within the central nervous system known to be involved in micturition (VanderHorst et al. 1997, 1998, 2001).
Capsaicin binds the vanilloid receptor type 1 (VR-1) (Caterina et al. 1997), and this receptor is believed to play an important role in sensitisation and development of visceral pain (Wang et al. 2008). The absence of a response to capsaicin in the ERα knock-out mouse points towards an oestrogen-mediated alteration in VR-1 function and thus a possible role in pain mediation (Schroder et al. 2003). This may explain why painful bladder disorders such as interstitial cystitis are more common in women and why the symptoms of sufferers fluctuate in severity during the menstrual cycle.
The role of oestrogen in OAB is less clear. Although oestrogens have long been prescribed to treat postmenopausal lower urinary tract symptoms, the clinical efficacy of this approach has not been conclusively proven. The benefits patients experience with hormone replacement therapy may result from reversal of urogenital atrophy rather than being due to a direct action on the lower urinary tract. A double-blind placebo-controlled trial carried out in 40 postmenopausal women failed to demonstrate a statistically significant beneficial effect of an oestradiol implant over placebo in treating urinary urgency (Rufford et al. 2003). This adds further weight to the argument that oestrogen is unlikely to play a major role in OAB (Table 4).
8 Conclusions
OAB is a prevalent condition affecting the quality of life. Current management is limited to symptom relief and consequently is viewed as a chronic medical condition. If better treatments are to be developed with the eventual possibility of a cure, then an improved understanding of the integrative physiology of the lower urinary tract and its dysfunction are required.
Animal models are an important research tool for the study of whole organism physiology and pathology. Unfortunately, owing to the fact that OAB is a symptom-based diagnosis, development of an animal model of the condition is hindered by the lack of a corroborating biomarker-specific for OAB and one which predicts treatment outcome. The various models that have been used to date have different strengths and weaknesses but nevertheless have yielded findings that have advanced our understanding of lower urinary tract function.
It is unrealistic to expect that a single animal model will recapitulate all the signs, symptoms, mechanisms and consequences of a disease/condition. However, proper selection of the most appropriate model will allow most experimental questions to be addressed. Animal modelling is an iterative process in which information gained should be re-invested and used to modify and refine existing models. There is no question that animal modelling has been crucial in advancing medical knowledge. It is hoped that better treatments for OAB may be developed through ongoing development of robust animal models.
Abbreviations
- ATP:
-
Adenosine triphosphate
- BOO:
-
Bladder outlet obstruction
- BPE:
-
Benign prostatic enlargement
- COX:
-
Cyclooxygenase enzyme
- DO:
-
Detrusor overactivity
- DSD:
-
Detrusor sphincter dyssynergia
- EAE:
-
Experimental autoimmune encephalomyelitis
- EP:
-
Family of G-protein coupled receptors
- ER:
-
Oestrogen receptor
- ICS:
-
International Continence Society
- MCA:
-
Middle cerebral artery
- MPTP:
-
Neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS:
-
Multiple sclerosis
- NANC:
-
Non-cholinergic non-adrenergic
- NMDA:
-
N-methyl-D-aspartate
- nNOS:
-
Neuronal nitric oxide synthase
- OAB:
-
Overactive bladder syndrome
- P2X:
-
Purinergic receptor
- PD:
-
Parkinson’s disease
- PGE2 :
-
Prostaglandin E2
- SHR:
-
Spontaneous hypertensive rat
- VR-1:
-
Vanilloid receptor type 1
References
Aboushwareb T et al (2009) Alterations in bladder function associated with urothelial defects in uroplakin II and IIIa knockout mice. Neurourol Urodyn 28(8):1028–1033
Abrams P, Drake M (2007) Overactive bladder. In: Wein AJ et al (eds) Campbell-Walsh urology. Saunders, Philadephia, pp 2079–2090
Abrams P et al (2000) Overactive bladder significantly affects quality of life. Am J Manag Care 6(11 suppl):S580–S590
Abrams P et al (2002a) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21(2):167–178
Abrams P et al (2002b) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol 187(1):116–126
Andersson KE (2002) Bladder activation: afferent mechanisms. Urology 59(5 Suppl 1):43–50
Andersson KE, Pehrson R (2003) CNS involvement in overactive bladder: pathophysiology and opportunities for pharmacological intervention. Drugs 63(23):2595–2611
Andersson KE et al (2007) Phosphodiesterases (PDEs) and PDE inhibitors for treatment of LUTS. Neurourol Urodyn 26(6 Suppl):928–933
Azadzoi KM et al (1999) Overactivity and structural changes in the chronically ischemic bladder. J Urol 162(5):1768–1778
Belayev L et al (1996) Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 27(9):1616–1622; discussion 1623
Birder LA (2005) More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol 289(3):F489–F495
Birder LA, de Groat WC (2007) Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4(1):46–54
Blaivas JG (1996) The bladder is an unreliable witness. Neurourol Urodyn 15(5):443–445
Boyle P et al (2003) The association between lower urinary tract symptoms and erectile dysfunction in four centres: the UrEpik study. BJU Int 92(7):719–725
Brading AF (1997) A myogenic basis for the overactive bladder. Urology 50(6A Suppl):57–67; discussion 68–73
Brading AF (2006) Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570(Pt 1):13–22
Brading AF, Turner WH (1994) The unstable bladder: towards a common mechanism. Br J Urol 73(1):3–8
Bramble FJ (1982) The treatment of adult enuresis and urge incontinence by enterocystoplasty. Br J Urol 54(6):693–696
Bramble FJ (1990) The clam cystoplasty. Br J Urol 66(4):337–341
Brown JS et al (2000) Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc 48(7):721–725
Buckner SA et al (2002) Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135(3):639–648
Bulmer P, Yang Q, Abrams P (2001) Does cigarette smoking cause detrusor instability in women? J Obstet Gynaecol 21(5):528–529
Burnett AL et al (1997) Urinary bladder-urethral sphincter dysfunction in mice with targeted disruption of neuronal nitric oxide synthase models idiopathic voiding disorders in humans. Nat Med 3(5):571–574
Burns RS et al (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. Proc Natl Acad Sci USA 80(14):4546–4550
Caterina MJ et al (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389(6653):816–824
Chan AW (2004) Transgenic nonhuman primates for neurodegenerative diseases. Reprod Biol Endocrinol 2:39
Cockayne DA et al (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407(6807):1011–1015
Coolsaet BL et al (1993) New concepts in relation to urge and detrusor activity. Neurourol Urodyn 12(5):463–471
Craggs MD, Rushton DN, Stephenson JD (1986) A putative non-cholinergic mechanism in urinary bladders of New but not Old World primates. J Urol 136(6):1348–1350
de Groat WC (1997) A neurologic basis for the overactive bladder. Urology 50(6A Suppl):36–52; discussion 53–56
de Groat WC, Yoshimura N (2001) Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol 41:691–721
Drake MJ, Mills IW, Gillespie JI (2001) Model of peripheral autonomous modules and a myovesical plexus in normal and overactive bladder function. Lancet 358(9279):401–403
Drake MJ, Harvey IJ, Gillespie JI (2003a) Autonomous activity in the isolated guinea pig bladder. Exp Physiol 88(1):19–30
Drake MJ et al (2003b) Partial outlet obstruction enhances modular autonomous activity in the isolated rat bladder. J Urol 170(1):276–279
Drake MJ et al (2005) Localized contractions in the normal human bladder and in urinary urgency. BJU Int 95(7):1002–1005
Drake M et al (2006) Muscarinic stimulation of the rat isolated whole bladder: pathophysiological models of detrusor overactivity. Auton Autacoid Pharmacol 26(3):261–266
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505(Pt 2):503–511
Fowler CJ (2002) Bladder afferents and their role in the overactive bladder. Urology 59(5 suppl 1):37–42
Gabella G, Uvelius B (1990) Urinary bladder of rat: fine structure of normal and hypertrophic musculature. Cell Tissue Res 262(1):67–79
Gerfen CR (2000) Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci 23(10 Suppl):S64–S70
Gevaert T et al (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117(11):3453–3462
Gibbs RA et al (2007) Evolutionary and biomedical insights from the rhesus macaque genome. Science 316(5822):222–234
Gillespie JI (2005) A developing view of the origins of urgency: the importance of animal models. BJU Int 96(suppl 1):22–28
Gillespie JI, Harvey IJ, Drake MJ (2003) Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol 88(3):343–357
Griffiths D et al (2007) Cerebral control of the bladder in normal and urge-incontinent women. Neuroimage 37(1):1–7
Griffiths DJ et al (2009) Cerebral control of the lower urinary tract: how age-related changes might predispose to urge incontinence. Neuroimage 47(3):981–986
Haferkamp A, Dorsam J, Elbadawi A (2003a) Ultrastructural diagnosis of neuropathic detrusor overactivity: validation of a common myogenic mechanism. Adv Exp Med Biol 539(Pt A):281–291
Haferkamp A et al (2003b) Structural basis of neurogenic bladder dysfunction. III. Intrinsic detrusor innervation. J Urol 169(2):555–562
Hanna-Mitchell AT et al (2007) Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci 80(24–25):2298–2302
Hannestad YS et al (2003) Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG 110(3):247–254
Hao CM, Breyer MD (2008) Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 70:357–377
Hashim H, Abrams P (2006) Is the bladder a reliable witness for predicting detrusor overactivity? J Urol 175(1):191–194; discussion 194–195
Hashim H, Abrams P (2007) Overactive bladder: an update. Curr Opin Urol 17(4):231–236
Hashitani H, Bramich NJ, Hirst GD (2000) Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol 524(Pt 2):565–79
Hashitani H et al (2001) Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol 530(Pt 2):273–286
Hau J (2008) Animal models for human disease. In: Conn PM (ed) Sourcebook of models for biomedical research. Humana, New Jersey
Hu P et al (2002) Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283(6):F1200–F1207
Igawa Y, Mattiasson A, Andersson KE (1992) Is bladder hyperactivity due to outlet obstruction in the rat related to changes in reflexes or to myogenic changes in the detrusor? Acta Physiol Scand 146(3):409–411
Imamura T et al (2008) Cold environmental stress induces detrusor overactivity via resiniferatoxin-sensitive nerves in conscious rats. Neurourol Urodyn 27(4):348–352
Inoue R, Brading AF (1991) Human, pig and guinea-pig bladder smooth muscle cells generate similar inward currents in response to purinoceptor activation. Br J Pharmacol 103(4):1840–1841
Insel TR (2007) From animal models to model animals. Biol Psychiatry 62(12):1337–1339
Iosif CS, Bekassy Z (1984) Prevalence of genito-urinary symptoms in the late menopause. Acta Obstet Gynecol Scand 63(3):257–260
Irwin DE et al (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50(6):1306–1314; discussion 1314–1315
Irwin DE et al (2006b) Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int 97(1):96–100
Irwin DE et al (2009) Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the epic study: impact of overactive bladder. Eur Urol 56:14–20
Jin LH et al (2009) Substantial detrusor overactivity in conscious spontaneously hypertensive rats with hyperactive behaviour. Scand J Urol Nephrol 43(1):3–7
Jorgensen TM et al (1983) Experimental bladder hyperreflexia in pigs. Urol Res 11(5):239–240
Jositsch G et al (2009) Suitability of muscarinic acetylcholine receptor antibodies for immunohistochemistry evaluated on tissue sections of receptor gene-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 379(4):389–395
Kato K et al (1988) The functional effect of mild outlet obstruction on the rabbit urinary bladder. J Urol 140(4):880–884
Kim JC et al (2005) Changes of urinary nerve growth factor and prostaglandins in male patients with overactive bladder symptom. Int J Urol 12(10):875–880
Kim JC et al (2006) Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175(5):1773–1776; discussion 1776
Kuiper GG et al (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93(12):5925–5930
Kuiper GG et al (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138(3):863–870
Kuo HC, Liu HT, Yang WC (2006) Therapeutic effect of multiple resiniferatoxin intravesical instillations in patients with refractory detrusor overactivity: a randomized, double-blind, placebo controlled study. J Urol 176(2):641–645
Macfarlane GJ et al (1996) The relationship between sexual life and urinary condition in the French community. J Clin Epidemiol 49(10):1171–1176
Makela S et al (2000) Differential expression of estrogen receptors alpha and beta in adult rat accessory sex glands and lower urinary tract. Mol Cell Endocrinol 170(1–2):219–229
Masuda H et al (2007) Effects of anaesthesia on the nitrergic pathway during the micturition reflex in rats. BJU Int 100(1):175–180
Matsui M et al (2002) Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 22(24):10627–10632
Matute-Bello G, Frevert CW, Martin TR (2008) Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295(3):L379–L399
McCafferty GP et al (2008) Enhanced bladder capacity and reduced prostaglandin E2-mediated bladder hyperactivity in EP3 receptor knockout mice. Am J Physiol Renal Physiol 295(2):F507–F514
McMurray G, Casey JH, Naylor AM (2006) Animal models in urological disease and sexual dysfunction. Br J Pharmacol 147(suppl 2):S62–S79
Mogensen J, Holm S (1989) Basic research and animal models in neuroscience – the necessity of co-evolution. Scand J Lab Anim Sci 16(Suppl 1):51
Morrison J, Steers WD, Brading A (2002) Neurophysiology and neuropharmacology. In: Abrams P et al (eds) Incontinence: 2nd international consultation on incontinence. Plymbridge, Plymouth, pp 85–161
Oh SJ et al (1999) Carbachol-induced sustained tonic contraction of rat detrusor muscle. BJU Int 84(3):343–349
Pampinella F et al (1997) Time-dependent remodeling of the bladder wall in growing rabbits after partial outlet obstruction. J Urol 157(2):677–682
Pandita RK, Andersson KE (2002) Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168(3):1230–1234
Pandita RK, Mizusawa H, Andersson KE (2000) Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol 164(2):545–550
Persson K et al (1998) Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. Am J Physiol 275(4 Pt 2):R1366–R1373
Petry KG et al (2000) Experimental allergic encephalomyelitis animal models for analyzing features of multiple sclerosis. Pathol Biol 48(1):47–53
Ponholzer A et al (2004) Association between lower urinary tract symptoms and erectile dysfunction. Urology 64(4):772–776
Pradidarcheep W et al (2009) Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol 379(4):397–402
Rahman NU et al (2007) An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int 100(3):658–663
Ruch TC, Tang PC (1956) Localization of brain stem and diencephalic areas controlling the micturation reflex. J Comp Neurol 106(1):213–245
Rufford J et al (2003) A double-blind placebo-controlled trial on the effects of 25 mg estradiol implants on the urge syndrome in postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct 14(2):78–83
Schroder A et al (2003) Estrogen receptor subtypes and afferent signaling in the bladder. J Urol 170(3):1013–1016
Schussler B (1990) Comparison of the mode of action of prostaglandin E2 (PGE2) and sulprostone, a PGE2-derivative, on the lower urinary tract in healthy women. A urodynamic study. Urol Res 18(5):349–352
Seaman EK et al (1994) Persistence or recurrence of symptoms after transurethral resection of the prostate: a urodynamic assessment. J Urol 152(3):935–937
Sibley GN (1984) A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol 354:431–443
Sibley GN (1985) An experimental model of detrusor instability in the obstructed pig. Br J Urol 57(3):292–298
Singh G, Wilkinson JM, Thomas DG (1997) Supravesical diversion for incontinence: a long-term follow-up. Br J Urol 79(3):348–353
Sirls LT, Zimmern PE, Leach GE (1994) Role of limited evaluation and aggressive medical management in multiple sclerosis: a review of 113 patients. J Urol 151(4):946–950
Sjogren C et al (1982) Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol 128(6):1368–1371
Son H et al (2007) New unstable bladder model in hypercholesterolemia rats. Urology 69(1):186–190
Sun Y et al (2001) Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 166(5):1951–1956
The Chimpanzee Sequencing and Analysis Consortium (2005) Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437(7055):69–87
Thomas AW, Abrams P (2000) Lower urinary tract symptoms, benign prostatic obstruction and the overactive bladder. BJU Int 85(suppl 3):57–68; discussion 70–71
Truss MC et al (2000) Initial clinical experience with the selective phosphodiesterase-I isoenzyme inhibitor vinpocetine in the treatment of urge incontinence and low compliance bladder. World J Urol 18(6):439–443
Turner WH, Brading AF (1997) Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther 75(2):77–110
Van Os-Bossagh P et al (2001) Micromotions of bladder wall in chronic pelvic pain (CPP): a pilot study. Int Urogynecol J Pelvic Floor Dysfunct 12(2):89–96
VanderHorst VG et al (1997) Estrogen receptor-immunoreactive neurons in the lumbosacral cord projecting to the periaqueductal gray in the ovariectomized female cat. Neurosci Lett 236(1):25–28
VanderHorst VG et al (1998) Estrogen receptor-alpha-immunoreactive neurons in the periaqueductal gray of the adult ovariectomized female cat. Neurosci Lett 240(1):13–16
VanderHorst VG, Meijer E, Holstege G (2001) Estrogen receptor-alpha immunoreactivity in parasympathetic preganglionic neurons innervating the bladder in the adult ovariectomized cat. Neurosci Lett 298(3):147–150
Varki A, Altheide TK (2005) Comparing the human and chimpanzee genomes: searching for needles in a haystack. Genome Res 15(12):1746–1758
Venter JC (2010) Multiple personal genomes await. Nature 464(7289):676–677
Wang P, Luthin GR, Ruggieri MR (1995) Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther 273(2):959–966
Wang ZY et al (2008) Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139(1):158–167
Wolffenbuttel KP et al (2001) Urodynamic follow-up of experimental urethral obstruction in individual guinea pigs. Neurourol Urodyn 20(6):699–713
Yamaguchi O et al (2009) Clinical guidelines for overactive bladder. Int J Urol 16(2):126–142
Yamanishi T et al (2000) The role of M(2)-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br J Pharmacol 131(7):1482–1488
Yokoyama O et al (1997) Influence of anesthesia on bladder hyperactivity induced by middle cerebral artery occlusion in the rat. Am J Physiol 273(6 Pt 2):R1900–R1907
Yokoyama O et al (1998a) Change in bladder contractility associated with bladder overactivity in rats with cerebral infarction. J Urol 159(2):577–580
Yokoyama O et al (1998b) Effects of MK-801 on bladder overactivity in rats with cerebral infarction. J Urol 159(2):571–576
Yokoyama O et al (1999) Glutamatergic and dopaminergic contributions to rat bladder hyperactivity after cerebral artery occlusion. Am J Physiol 276(4 Pt 2):R935–R942
Yokoyama O et al (2000) Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Exp Neurol 163(2):469–476
Yoshimura N et al (1993) The dopamine D1 receptor agonist SKF 38393 suppresses detrusor hyperreflexia in the monkey with parkinsonism induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP). Neuropharmacology 32(4):315–321
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Parsons, B.A., Drake, M.J. (2011). Animal Models in Overactive Bladder Research. In: Andersson, KE., Michel, M. (eds) Urinary Tract. Handbook of Experimental Pharmacology, vol 2011. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-16499-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-16499-6_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-16498-9
Online ISBN: 978-3-642-16499-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)