Abstract
The gustatory system allows the brain to monitor the presence of chemicals in the oral cavity and initiate appropriate responses of acceptance or rejection. Among such chemicals are the nutrients that must be rapidly recognized and ingested for immediate oxidation or storage. In the periphery, the gustatory system consists of a highly efficient sensing mechanism, where distinct cell types express receptors that bind specifically to chemicals associated with one particular taste quality. These specialized receptors connect to the brain via dedicated pathways, the stimulation of which triggers stereotypic behavioral responses as well as neurotransmitter release in brain reward dopamine systems. However, evidence also exists in favor of the concept that the critical regulators of long-term nutrient choice are physiological processes taking place after ingestion and independently of gustation. We will appraise the hypothesis that organisms can develop preferences for nutrients independently of oral taste stimulation. Of particular interest are recent findings indicating that disrupting nutrient utilization interferes with activity in brain dopamine pathways. These findings establish the metabolic fate of nutrients as previously unanticipated reward signals that regulate the reinforcing value of foods. In particular, it suggests a role for brain dopamine reward systems as metabolic sensors, allowing for signals generated by the metabolic utilization of nutrients to regulate neurotransmitter release and food reinforcement.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The gustatory system allows the brain to monitor the presence of chemicals in the oral cavity and initiate acceptance or rejection responses accordingly. Among such chemicals are the potential fuels that must be rapidly recognized and ingested for later oxidation or storage. In fact, animals must continuously procure the substrates necessary to maintain cellular function from exogenous sources, that is, from food. A sensory system therefore evolved in which membrane receptors convey information on the presence of metabolic fuels in the oral cavity to brain circuits that control the initiation of ingestive behaviors.
However, the formation of long-term food preferences is a complex process, and different lines of evidence indicate that animals will fail to prefer foods conveying pleasant sensory cues if those are not ensued by postingestive, metabolic effects. How does the brain control food intake in such a way that previous associations between sensory properties and metabolic effects become a regulating factor during nutrient choice? One possibility is that a brain circuit exists where sensory and metabolic information will converge through independent pathways. In the following, after introducing the basic aspects related to the stimulation of brain reward systems by gustatory cues, we will review evidence in support of the idea that the midbrain dopamine system is one such candidate circuit. More specifically, we will evaluate current evidence that this brain neurotransmitter system is under the control of the nutritional state of the animal, with nutrient availability directly modulating neurotransmitter synthesis/release.
2 The Peripheral Gustatory System
The peripheral gustatory system corresponds to the anatomical substrate that links the sensory epithelium of the oral cavity to the first gustatory relay center in the brain. This includes a family of membrane proteins that function as chemical sensors, the epithelial cells hosting these sensors, and the neural afferents carrying information on sensor activation/cell depolarization to the brain. The oral chemosensory epithelia contain onion-shaped structures known as taste buds, which in turn typically host 50–100 taste receptor cells (Finger and Simon 2002). In mammals, TRCs are typically embedded in stratified epithelia and distributed throughout the oral cavity, more specifically expressed on tongue, palate, epiglottis, and esophagus (Finger and Simon 2002; Scott and Verhagen 2000; Spector and Travers 2005). The apical end of taste cells is exposed to the external environment of the oral cavity through a small opening in the epithelium called the taste pore, which is filled with microvilli. On the membranes of these microvilli are usually expressed different classes of receptors that function as oral chemosensors.
Proteins belonging to the G-protein-coupled receptor (GPCR) superfamily have been established as the receptors for sweet, l-amino acid and bitter tastants (Adler et al. 2000; Chrandrashekar et al. 2000; Liu and Liman 2003; Max et al. 2001; Montmayeur et al. 2001; Mueller et al. 2005; Perez et al. 2003; Zhang et al. 2003; Zhao et al. 2003). On the other hand, the sensations associated with the other two primary tastants, namely sour and salty, are mediated by ion channels of the transient receptor potential (TRP) (Huang et al. 2006) and epithelial sodium channel (ENaC) (Kellenberger and Schild 2002) superfamilies. Sweet taste signaling is known to be mediated by heterodimeric GPCRs and specific downstream signaling elements. More precisely, the transduction of sweet tastants is mediated by the taste genes Tas1r2 and Tas1r3, whose T1R2 and T1R3 products assemble to form the heterodimeric sweet receptor T1R2/T1R3 (Nelson et al. 2001; Zhang et al. 2003; Zhao et al. 2003). T1R2/T1R3 appears to be the one type of broadly tuned receptor that subserves detection of both natural sugars and artificial sweeteners, although it remains to be determined with exactitude whether these different classes of chemicals bind to different regions of the receptor (Nelson et al. 2001).
A similar mechanism mediates the recognition of l-amino acids via the Tas1r1 and Tas1r3 genes (Nelson et al. 2002). Accordingly, the transduction of most forms of l-amino acids (i.e., with the possible exception of aromatic l-amino acids) is primarily accomplished via the G-protein-coupled heterodimeric T1R1/T1R3 receptor (Nelson et al. 2002). T1R1/T1R3 receptors are broadly tuned to signal l-amino acids (Nelson et al. 2002; Zhao et al. 2003), although it has been proposed that the human form of the receptor is more narrowly tuned to glutamate or umami taste (Maruyama et al. 2006; Rong et al. 2005). Finally, the third class of tastants mediated by GPCRs includes bitter stimuli. Bitter taste is mediated by the Tas2r genes (Bufe et al. 2005), the products of which form homodimeric (i.e., containing two identical subunits) T2R receptors. Bitter T2R receptors have been found to be both necessary and sufficient for bitter taste transduction and perception (Mueller et al. 2005).
It appears that all signaling mechanisms downstream to taste GPCRs are shared by different classes of ligands. Upon receptor binding, taste GPCR signaling is supported by gustducin, a heterotrimeric taste G-protein whose α, β, and γ constituent units are α-gustducin (McLaughlin et al. 1992), Gβ3 and Gγ13 (Huang et al. 1999), respectively. A similar pattern seems to hold for signaling events occurring downstream to G-protein signaling. This includes the taste phospholipase PLCβ2 and the nonselective ionic taste channel TRPM5, the deletion of which induces severe impairments in – if not taste blindness for – sweet, umami, and bitter transduction (Zhang et al. 2003).
Finally, salty and sour taste sensations are mediated instead by ionic receptor channels. The ENaC, particularly its subunit ENaCα, mediates behavioral attraction to sodium chloride (Chandrashekar et al. 2010; Kretz et al. 1999). On the other hand, genetic and functional studies identified one member of the TRP superfamily, the polycystic kidney disease-like ion channel PKD2L1, as necessary for sour taste transduction (Huang et al. 2006; Ishimaru et al. 2006; LopezJimenez et al. 2006).
Upon receptor activation and taste cell depolarization, neural afferents originating from branches of cranial nerves innervate the basolateral aspect of taste cells and transmit to the brain information on the identity and quantity chemicals detected by the membrane taste receptors. The chorda tympani and the greater superior petrosal branches of the VIIth (facial) cranial nerve innervate TRCs present on the anterior tongue and palate, respectively (Danilova et al. 2002; Hanamori et al. 1988), such that information on chemosensory events occurring in the oral epithelium is transduced into electrical messages on its way to the brain.
3 The Central Gustatory System
Information derived from taste-responsive cranial nerves converges onto the rostral division of the nucleus tractus solitarius (rNTS) of the medulla (Hamilton and Norgren 1984), whereas the more caudal aspect of the NTS is targeted by visceral (vagal) afferent inputs that convey information on the physiological status of the gastrointestinal system (Travagli et al. 2006). From the rNTS, taste information ascends to further brain circuitries. In rodents, axonal fibers originating in this gustatory aspect of the nucleus of the solitary tract ascend ipsilaterally to the parabrachial nucleus (PBN), establishing this pontine structure surrounding the conjunctivum brachium as the second-order gustatory relay (Norgren and Leonard 1971, 1973; Norgren and Pfaffmann 1975). From PBN, a first (“dorsal”) pathway projects to the parvicellular part of the ventroposterior medial nucleus of the thalamus (VPMpc), the taste thalamic nucleus (reviewed in Bermudez-Rattoni 2004). The second (“ventral”) pathway includes direct projections from PBN to the central nucleus of the amygdala and lateral hypothalamus. Thalamic afferents then project to the primary gustatory cortex which is defined as the VPMpc cortical target.
4 Brain Dopamine Systems and Taste Reward
The role of brain dopamine systems in mediating food reward and encoding stimulus palatability has been well established (for a schematic representation of dopamine projections in the human brain, see Fig.1). Dopamine antagonists attenuate the hedonic value of sweet-tasting nutrients, in that animals pretreated with either D1- or D2-type dopamine receptor antagonists behave toward high concentrations of sucrose solutions as if they were weaker than usual (Bailey et al. 1986; Geary and Smith 1985; Wise 2006; Xenakis and Sclafani 1981). Conversely, tasting palatable foods elevates dopamine levels in the nucleus accumbens (NAcc) of the ventral striatum (Hernandez and Hoebel 1988), a brain region largely implicated in food reinforcement (Kelley et al. 2005). In humans, striatal dopamine release directly correlates with the perceived hedonic value of food stimuli (Small et al. 2003). But is dopamine release induced by sweet palatability per se independently of carbohydrate metabolism? In fact, taste-elicited stimulation of the central dopamine systems seems to take place even in the absence of intestinal nutrient absorption: In “sham-feeding” studies, where a catheter is implanted on the stomach wall to prevent nutrients from reaching the intestinal tract, accumbens’ dopamine levels increase in proportion to the concentration of the sucrose solution used to stimulate the intraoral cavity (Hajnal et al. 2004).
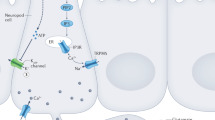
Schematic representation of dopaminergic pathways in the human brain. Analogous projections exist in both rodents and nonhuman primates. Dopaminergic neuronal cells residing in the midbrain locate within two regions of the ventral midbrain, the ventral tegmental area (“VTA”) and the pars compacta of the substantia nigra (“SNc”). The mesolimbic pathway essentially consists of targets of VTA projections; these include mainly the nucleus accumbens of the ventral striatum, the amygdalar nuclei, the lateral hypothalamus and more medial, ventral parts of the frontal lobe. The mesolimbic pathway has been traditionally associated with the control of motivated behaviors and sensory reward processing, including food intake. In contrast, the nigrostriatal pathway consists of the targets reached by dopamine projections originating from SNc. This distinct, more dorsal, dopamine system has in turn been classically associated with motor control in general and initiation of voluntary movement in particular, being noticeable that SNc dopamine neurons seem to be preferentially targeted in Parkinson’s disease. However, the functional distinctions between ventral and dorsal pathways have been blurred by recent findings showing a critical role for the dorsal striatum in feeding (see text for details)
It is therefore plausible to assume that the events leading to the stimulation of brain reward circuits via dopamine release initiate within the oral cavity, upon the activation of taste receptors. This implies that the dopamine release effect in accumbens associated with sweet taste stimulation must depend on the integrity of central taste relays conveying gustatory information to downstream brain circuits. Anatomically, whereas one group of projections from PBN reach the insular cortex via the taste thalamic relay (Norgren and Wolf 1975), a second, separate pathway reaches the amygdala, lateral hypothalamus, and the bed nucleus of the stria terminalis (Li et al. 2005; Norgren 1976). Thus, it has been shown that lesions to the PBN limbic, but not to the PBN thalamocortical, pathway blunt the dopaminergic response during intake of palatable tastants (Norgren and Hajnal 2005; Norgren et al. 2006).
More generally, the ability of pleasant sweet taste to directly stimulate brain dopamine systems appears consistent with the seemingly innate preferences to sweet taste in most species. In fact, it has been long shown that both deprived and nondeprived animals will not only avidly consume sweet solutions but also run through intricate mazes or incessantly press levers to obtain sweet rewards (Kare 1971). In humans, the innate attraction to sweetness is demonstrated by the reactions observed in children upon their first exposure to sugary solutions: newborns will immediately suck the solutions and produce characteristic facial expressions (Ganchrow et al. 1983). In rats, pups as young as 6 days old are strongly attracted to sweetness, given their robust intake responses to sweet compounds such as sucrose, lactose, and saccharin (Hall and Bryan 1981). It is also well established that other species show similar attractions to sweet compounds at early ages (Houpt et al. 1977).
5 Taste-Independent Attraction to Sugars
However, is sweetness perception required for animals to develop behavioral attractions to nutrients like sugars? This question can be more clearly addressed if the detection of certain sensory properties of distinct flavors is ablated during the experiments. One way to achieve this involves using genetically engineered animals lacking taste sensation. Following the lead open by flavor-nutrient conditioning paradigms (Sclafani and Vigorito 1987; Sclafani and Xenakis 1984), we have developed a conditioning protocol where mice are allowed to develop nutrient-specific preferences for sipper locations that had been previously associated with certain compounds (de Araujo et al. 2008). This study employed both wild-type and knockout mice lacking functional TRP channels M5 (Zhang et al. 2003). As was mentioned above, the TRPM5 ion channel is expressed in taste receptor cells (Perez et al. 2002) and is required for sweet, bitter, and amino acid taste signaling (Zhang et al. 2003). It was hypothesized in this study that sweet-blind Trpm5 knockout mice would develop a preference for spouts associated with the presentation of sucrose solutions when allowed to detect the solutions’ rewarding postingestive effects.
In fact, once the insensitivity of KO mice to the orosensory reward value of sucrose was established, we inquired whether a preference for sippers associated with caloric sucrose solutions could develop in water and food-deprived Trpm5 knockout mice when they are allowed to form an association between a particular sipper in the test chamber and the postingestive effects produced by drinking from that sipper (de Araujo et al. 2008). This was accomplished in sweet taste-naïve animals by first determining the initial side preferences using a series of preliminary two-bottle tests where both sippers contained water and by exposing animals to conditioning sessions that consisted of daily 30min free access to either water (assigned to the same side of initial bias) or sucrose (assigned to the opposite side) while access to the other sipper was blocked.
Confronting the behavioral data from both wild-type and knockout mice revealed no significant genotype×stimulus interactions, since during conditioning sessions both wild-type and knockout animals consumed significantly larger amounts of sucrose than water. In addition, during the postconditioning two-bottle tests, it was observed that both wild-type and knockout animals reversed their initial side-preference biases by drinking significantly more water from the sipper that during conditioning sessions had been associated with nutritive sucrose. Now, when the same experiments were run using the noncaloric sucrose-derived sweetener sucralose instead of sucrose, unlike the sucrose case, a significant genotype×stimulus interaction was detected since only wild-type animals consumed more sucralose than water during the conditioning sessions. Furthermore, during the two-bottle test sessions, conducted after conditioning to sucralose, knockout mice, likewise their wild-type counterparts, showed no preferences for sippers associated with the delivery of sucralose. Overall, these results provide evidence in favor of the hypothesis that postingestive effects can exert positive controls on ingestive (licking/swallowing) behaviors even in the absence of taste signaling or detection of distinct flavors. It is noticeable that even “taste-enabled” wild-type mice failed to develop preferences for sipper locations previously associated with sucralose delivery. This indicates that the mere presence of strong sweet taste input is not sufficient to induce long-term location preferences if unaccompanied by rewarding physiological effects.
6 On the Nature of the Postingestive Reward Signal
The above results raise the question of what would be the identity of the taste-independent reinforcement signal. Although there is little disagreement that postingestive factors produced by nutrients regulate food intake, much more controversial is the nature of the signal that acts on the brain as a postingestive reinforcer. Broadly speaking, the candidate signals could be classified into two major groups, related to pre- and postabsorptive postingestive events. The former group concerns those sensing mechanisms that occur before nutrient absorption but simultaneous to the arrival of nutrients to the gut. The latter group on the other hand refers to those events that occur following absorption, and nonexclusively includes a variety of signals such as fuel utilization metabolites and changes in plasma hormonal levels.
While it must be acknowledged that this is rather complex topic, currently available evidence points to a relatively weak role for preabsorptive signals in postingestive reinforcement. Regarding, for example, potential reinforcement signals generated in the stomach, early studies have shown that removing approximately 90% of the rat stomach results in gastrectomized rats being virtually as well motivated as controls during food-reinforced operant tasks (Tsang 1938). In addition, gastric vagotomy does not interfere with habitual feeding patterns in rats (Snowdon and Epstein 1970), and abdominal vagotomy did not interfere with flavor preferences conditioned by glucose-containing sugars (Sclafani and Lucas 1996).
Another possibility regards the presence of taste-like receptors in the gastrointestinal epithelium (Hofer et al. 1996). In fact, it has been shown that the taste signaling proteins α-Gustducin, T1R2, T1R3, as well as the taste ion channel TRPM5 are coexpressed in some mouse and human enteroendocrine cells (Bezençon et al. 2007; Margolskee et al. 2007). Accordingly, it is conceivable that gut cells could “taste” the contents of ingested foods and then convey (currently undetermined) signals to the brain that would function as behavioral reinforcers. Although this hypothesis deserves further attention, current evidence does not support a preponderant role for taste elements expressed in postingestive reinforcement. First we remind that Trpm5 knockout mice do assimilate the differential physiological effects between sugar and sweetener solutions and develop sipper preferences accordingly (note that these mice do not express these taste channels anywhere in the body) (de Araujo et al. 2008). In addition, α-Gustducin knockout mice increase preferences for flavors associated with fat and sugar nutrients in ways that are comparable to wild-type mice (Sclafani et al. 2007), and T1R3 knockout mice do develop robust preferences for sucrose solutions (Zukerman et al. 2009). Overall, if nutrient sensors exist in the gut to mediate postingestive reinforcement signals, these sensors are unlikely to depend on taste receptor signaling.
Finally, one possibility remains regarding the potential role of gut-derived factors, such as the peptide hormones ghrelin or GLP-1, as postingestive reinforcers. Future experiments employing flavor-nutrient conditioning paradigms (Sclafani and Xenakis 1984) on the corresponding knockout models will contribute to resolve this issue. In any case, one potential argument against the involvement of such gut factors in postingestive reinforcement relates to their nonselectivity to rewarding compounds.
The second group of candidate postingestive reinforcers would consist of physiological signals generated postabsorption. We have recently assessed the potential role of metabolic signals in taste-independent nutrient selection by comparing the behavioral responses to glucose and l-amino acids in wild-type and Trpm5 knockout mice (note that this channel mediates the tastes of sugars and l-amino acids in mice). Briefly, we have found that knockout mice, while displaying insensitivity to the tastes of glucose and l-serine during short-term tests, do develop a strong preference for glucose-associated compared to l-serine sippers over both conditioning and long-term sessions (Ren et al. 2010). In other words, animals will ingest higher quantities of, and develop preferences for, the carbohydrate glucose versus an isocaloric amino acid, even when unable to detect the distinctive flavor qualities intrinsic to each nutrient.
If the postingestive reinforcement signal is not exclusively explained by the actual number of calories ingested, which physiological cues could be playing such a role? Using indirect calorimetry measurements, we have found that these higher intake levels were closely associated with glucose oxidation levels, even more markedly than with increases in blood glucose. This finding points to a role for postabsorptive nutrient utilization in postingestive reinforcement that may be more important than any of the sensors detecting the presence of nutrients in either the gastrointestinal tract or bloodstream (Swithers and Davidson 2008). Now, these correlative measures do not necessarily provide direct evidence for the hypothesis that increased glucose intake is being primarily controlled by postabsorptive mechanisms. For example, nutrient-specific chemosensory signals and/or release of nutrient-controlling gut hormones (e.g., ghrelin, Tschop et al. 2000) might also have played a major role in shaping these behavioral responses. We have further clarified this issue by performing additional experiments where animals licked a water spout to obtain either glucose or serine infusions via a jugular catheter, thereby bypassing completely both the oral and gastrointestinal tracts. We have monitored the overall number of licks produced during the entire session and, as expected, we observed that mice licked significantly more times to water during glucose compared to during l-serine intravenous sessions. We conclude that the taste-independent differential responses to glucose and l-serine were not primarily accounted for by differential absorption rates or secretion of gut-derived factors, but rather by direct actions of nutrients on metabolism.
Altogether, the above findings provide support for the idea that postabsorptive mechanisms, independent of both oral and gastrointestinal sensing, ultimately mediate the higher intake and preference levels for glucose compared to amino acids. As we shall see below, a current working hypothesis relates to the possibility that nutrients providing sufficient fuel for brain metabolism may directly control neuronal activity in brain reward (dopamine) pathways, thereby reinforcing the previous behavioral sequences leading to its own intake. We will briefly describe evidence supporting a role for dopamine signaling in flavor-nutrient conditioning, and then provide evidence for a role for metabolic signals as regulators of brain dopamine pathways.
6.1 Brain Dopamine Signaling and Postingestive Conditioned Behaviors
Conditioned preferences for nutritive foods must ultimately depend on brain circuits that regulate ingestive behaviors. Among such circuits are those known to be involved in forming associations between unconditioned and conditioned reward stimuli. In fact, a role for dopamine signaling in flavor-nutrient conditioning was suggested by experiments where dopamine receptor antagonists were administered in the nucleus accumbens (the major dopamine target in ventral striatum strongly associated with feeding behaviors, see e.g., Baldo et al. (2005), Kelley et al. (2005). Rats treated with local infusions in nucleus accumbens with a D1-receptor antagonist displayed a dose-dependent reduction in intake of a flavor paired with intragastric infusions of glucose, compared to controls (Touzani et al. 2008). Interestingly, the effect of dopamine signaling antagonism on postconditioning preference tests was less compelling (Touzani et al. 2008). In any event, these results demonstrate that D1-like receptors in the nucleus accumbens are required for the acquisition, and possibly also for the expression, of glucose-conditioned flavor preferences.
On the other hand, our own findings suggest that the presence of taste or flavor stimulation is not required for the postingestive effects of foods to induce dopamine release in the nucleus accumbens. In fact, we observed by performing microdialysis in sweet-blind Trpm5 knockout mice that sugar intake per se, independently of taste signaling, was sufficient to increase extracellular dopamine levels in the nucleus accumbens (de Araujo et al. 2008). More precisely, we first found in this study that the noncaloric sweetener sucralose produced significantly higher increases in dopamine levels in wild-type compared to knockout animals. These results are consistent with a role for dopamine signaling in accumbens derived from taste stimulation alone (Hajnal et al. 2004). However, when the same comparison was performed with respect to sucrose, no differences were found between the dopamine release levels in wild-type and knockout mice. In other words, while sweet taste stimulation without caloric content only produced significant increases in accumbal dopamine levels in wild-type, caloric sucrose evoked the same levels of dopamine increase in both wild-type and knockout mice. These results therefore strongly suggest that even in the absence of taste transduction and/or palatability, nutrient intake has the ability to induce measurable tonic increases in accumbens dopamine. Not only both palatability and postingestive factors seem to independently increase dopamine levels in brain reward circuits but also the nutrient-induced increases do not require the concomitant presence of flavor inputs, as had been suggested before (Di Chiara and Bassareo 2007). Therefore, the role played by dopamine signaling in postingestive reinforcement does not seem to be restricted to the formation of learned associations between orosensory and physiological signals; rather, nutrient availability may directly influence metabolic activity in cells present in this circuit, resulting in a simple, yet efficient metabolic-sensing reward machinery.
Earlier indications that postabsorptive signals might gain direct access to dopaminergic cells have been provided by the early work by Figlewicz and colleagues, who have shown that the functional forms of both insulin and leptin receptors (Figlewicz et al. 2003), as well as of some of their substrates (Pardini et al. 2006), are richly expressed in dopaminergic neurons of the substantia nigra compacta and ventral tegmental area regions of the midbrain. In addition, leptin receptors expressed in dopaminergic neurons of the midbrain were shown to be functional and to influence dopamine release (Fulton et al. 2006; Hommel et al. 2006). However, it is currently unknown whether brain leptin receptors play a role on postingestive reinforcement. In addition, the functional implications of insulin receptor expression in dopamine neurons have been little explored. Although it has been suggested that insulin infusions in the midbrain dopamine areas “decrease” the reward value of sucrose, since mice were found to reduce overall intake of sucrose solutions upon infusion (Figlewicz 2003; Figlewicz et al. 2006), this might simply imply that insulin receptor activation in midbrain dopamine areas provide the brain with a robust signal of caloric intake. However, a preponderant role for insulin as a postingestive reward signal is challenged by the findings that diabetic (hypoinsulinemic) rats do display normal responses in flavor-nutrient conditioning paradigms (Ackroff et al. 1997). Finally, Andrews et al. (2009)demonstrated that the orexigenic gut ghrelin promotes tyrosine hydroxylase gene expression in substantia nigra concomitantly to increasing dopamine concentration in striatum, raising the possibility that changes in ghrelin levels may modulate postingestive reinforcement.
6.2 Glucose Metabolism and Dopamine Signaling
Alternatively, a mechanism which would allow dopamine neurons to sense changes in physiological state refers to the possibility that these cells function as glucosensors, i.e., may change intracellular activity in response to the availability of extracellular glucose. More specifically, we directly addressed the possibility that dopamine neurons of the midbrain are sensitive to glucose utilization rates based on the finding (mentioned above) that higher intake levels of glucose compared to the nongluconeogenic amino acid l-serine were strongly associated with glucose oxidation levels (Ren et al. 2010).
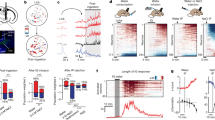
The first step consisted of showing that intragastric infusions (i.e., completely bypassing the oral cavity) of glucose produce different effects on dopamine release compared to similar infusions of l-serine (see details in Fig.2) (Ren et al. 2010). More precisely, intragastric infusions of glucose produced significantly higher levels of dopamine release in accumbens compared to isocaloric infusions of serine; we stress in particular the significant decreases in dopamine levels in accumbens following serine infusions, an effect that we relate to the lower levels of serine intake in KO animals. Furthermore, since dopamine signaling in dorsal striatum has also been implicated in feeding behavior (Sotak et al. 2005), we have in addition assessed the effects produced by intragastric infusions of glucose and serine on dorsal striatum dopamine levels. Whereas no significant decreases in dopamine levels were observed during l-serine infusions, significant increases were associated with glucose infusions. These microdialysis measures thus provided evidence that nutrient-specific dopamine release can be initiated upon direct stimulation of the gastrointestinal tract.
Intragastric infusions of glucose and serine induce differential brain dopamine responses. (a) Schematic showing the general arrangement where animals implanted with microdialysis probes are infused intragastrically with different nutrient levels, aiming at analyzing orosensory-independent monoamine release following food intake. (b) Overall percent changes in dopamine levels produced by glucose and serine intragastric infusions. In the nucleus accumbens, glucose infusions were associated with significantly higher levels of extracellular dopamine when directly compared to serine infusions (*two-sample t test p<0.03). In addition, significant decreases in dopamine levels were observed following serine infusions (**one-sample t test against 100% p<0.02). (c) Whereas glucose infusions were consistently associated with increased dopamine levels across samples, relative decreases produced by serine infusions were marked in particular at the third sample (***p<0.04). (d) In dorsal striatum, significant increases in dopamine levels were associated with glucose (*one-sample t test against 100% p=0.009), but not serine (p<0.09), infusions. (e) Across samples, significant increases in dopamine levels were observed only during glucose infusions at 30min after infusion onset (**one-sample t test against 100% p<0.04)
6.3 Disrupting Glucose Metabolism Inhibits Dopamine Release in Dorsal Striatum
To address the issue of whether the metabolism of glucose is relevant or not to nutrient-specific differences in dopamine release, we have designed another experiment where wild-type mice were fitted with a microdialysis probe in the striatum as well as with a jugular venous catheter. After measuring baseline dopamine levels, we have infused a bolus of the antimetabolic glucose analog, 2-deoxy-d-glucose (henceforth “2-DG”) via the jugular catheter for 6min and monitored dopamine levels for 1h after the injection. This was then followed by an intravenous glucose infusion lasting for 10min. We then reasoned that, if glucose metabolism is indeed relevant for the increased brain dopamine levels observed in striatum upon glucose ingestion, then an infusion of 2-DG should result in significant decreases in extracellular dopamine levels. In addition, such inhibitory effects of 2-DG on dopamine release must be reversed or at least attenuated by a subsequent intravenous glucose infusion that would contribute to restore normal rates of glucose oxidation.
In fact, intravenous infusions of 2-DG produced robust decreases in extracellular dopamine levels in striatum (34.7±9.5% dopamine concentration of initial baseline). In addition, and also consistent with our earlier predictions, the subsequent intravenous infusions of glucose resulted in a reversal of this effect, with overall dopamine concentration levels reaching 74.5±26.7% of the original baseline levels within 30min. Importantly, we observed that glucose infusions produced a striking increase in striatal dopamine levels when the comparison is made with respect to the levels observed after 2-DG infusions. Therefore, glucose provision following inhibition of glucose utilization produces a strong relative increase in dopamine levels that are higher than those observed when no glucose utilization inhibition is employed.
6.4 Glucose Solutions Acquire Higher Reward Value When Contributing to Reinstate Glucose Oxidation
So far, we have been implicitly assuming that relative changes in extracellular dopamine levels reflect the reinforcing potency of a nutrient even in the absence of taste receptor signaling. Therefore, we are now forced to conclude that ingesting glucose following an injection of 2-DG must significantly increase the reward value of glucose compared to following a vehicle injection, even in sweet-insensitive Trpm5 knockout animals. In other words, glucose solutions must be assigned a superior reward value when counteracting the effects of inhibiting glucose oxidation. We tested this hypothesis by measuring glucose intake in KO mice following an intraperitoneal injection of either 2-DG or vehicle (saline). We have found in effect that, after 2-DG injections, knockout mice produced a significantly higher number of licks to glucose compared to after saline injections. Therefore, within 30min of 2-DG administration, glucose acquired higher reward value when reinstating glucose oxidation levels, a finding that was entirely consistent with the dopamine measurements following 2-DG and glucose infusions described above.
7 Conclusion
Two conclusions may be drawn from the experimental evidence described in this chapter. First, ageusic mice unable to detect the orosensory properties of certain sugars or l-amino acids are nevertheless capable of developing nutrient-specific preferences based solely on physiological cues. Second, brain dopamine systems act as metabolic sensors, with a particular sensitivity to glucose oxidation rates. More generally, our results show that sugar-specific behavioral preferences and dopamine release will develop independently of sweetness or caloric value, while being regulated by glucose oxidation levels.
The above may contribute to explain the superior reinforcing value associated with glucose-containing sugars compared to all other classes of nutrients tested in flavor-nutrient conditioning paradigms (Ackoff 2009). In particular, these results may provide clues on whether the crucial reinforcing mechanism involves pre- or postabsorptive signals. In fact, it is conceivable that the stronger postingestive effects associated with glucose-containing sugars derive from the mere fact that neurons depend almost entirely on glucose and its derivatives for metabolic activity (Pellerin et al. 2007). This privileged access of glucose through the blood–brain barrier may underlie its superior reinforcing properties compared to other isocaloric nutrients. Future research must determine the mechanisms allowing the intracellular utilization of nutrients to regulate neurotransmitter release.
References
Ackoff K (2009) Learned flavor preferences. The variable potency of post-oral nutrient reinforcers. Appetite 51:743–746
Ackroff K, Sclafani A, Axen KV (1997) Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite 28:73–83
Adler E, Hoon MA, Mueller KL, Chrandrashekar J, Ryba NJP, Zucker CS (2000) A novel family of mammalian taste receptors. Cell 100:693–702
Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, Elsworth JD, Savitt JM, DiMarchi R, Tschoep M, Roth RH, Gao XB, Horvath TL (2009) Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci 29:14057–14065
Bailey CS, Hsiao S, King JE (1986) Hedonic reactivity to sucrose in rats: modification by pimozide. Physiol Behav 38:447–452
Baldo BA, Alsene KM, Negron A, Kelley AE (2005) Hyperphagia induced by GABAA receptor-mediated inhibition of the nucleus accumbens shell: dependence on intact neural output from the central amygdaloid region. Behav Neurosci 119:1195–1206
Bermudez-Rattoni F (2004) Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci 5:209–217
Bezençon C, le Coutre J, Damak S (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32:41–49
Bufe B, Breslin PAS, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W (2005) The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol 15:322–327
Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS (2010) The cells and peripheral representation of sodium taste in mice. Nature 464:297–301
Chrandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zucker CS, Ryba NJP (2000) T2Rs function as bitter taste receptors. Cell 100:703–711
Danilova V, Danilov Y, Roberts T, Hellekant G (2002) Sense of taste of the common marmoset: recordings from the chorda tympani and glossopharyngeal nerves. J Neurophys 88:579–594
de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA (2008) Food reward in the absence of taste receptor signaling. Neuron 57:930–941
Di Chiara G, Bassareo V (2007) Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol 7:69–76
Figlewicz DP (2003) Insulin, food intake, and reward. Semin Clin Neuropsychiatry 8:82–93
Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG (2003) Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res 964:107–115
Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW (2006) Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav 89:611–616
Finger TE, Simon SA (2002) The cell biology of lingual epithelia. In: Finger TF, Silver WL, Restrepo D (eds) The Neurobiology of Taste and Smell, Wiley-Liss, New York, pp. 287–314
Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS (2006) Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822
Ganchrow JR, Steiner JE, Daher M (1983) Neonatal facial expressions in response to different qualities and intensities of gustatory stimuli. Infant Behav Dev 6:473–484
Geary N, Smith GP (1985) Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav 22:787–790
Hajnal A, Smith GP, Norgren R (2004) Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286:R31–R37
Hall WG, Bryan TE (1981) The ontogeny of feeding in rats: IV. Taste development as measured by intake and behavioral responses to oral infusions of sucrose and quinine. J Comp Physiol Psychol 95:240–251
Hamilton RB, Norgren R (1984) Central projections of gustatory nerves in the rat. J Comp Neurol 222:560–577
Hanamori T, Miller IJ Jr, Smith DV (1988) Gustatory responsiveness of fibers in the hamster glossopharyngeal nerve. J Neurophysiol 60:478–498
Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42:1705–1712
Hofer D, Puschel B, Drenckhahn D (1996) Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93:6631–6634
Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ (2006) Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810
Houpt KA, Houpt TR, Pond WG (1977) Food intake controls in the suckling pig: glucoprivation and gastrointestinal factors. Am J Physiol 232:E510–514
Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2:1055–1062
Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS (2006) The cells and logic for mammalian sour taste detection. Nature 442:934–938
Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H (2006) Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A 103:12569–12574
Kare MR (1971) Comparative study of taste. In: Beidler LM (ed) Handbook of sensory physiology. Springer, Berlin, pp 278–292
Kellenberger S, Schild L (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82:735–767
Kelley AE, Schiltz CA, Landry CF (2005) Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav 86:11–14
Kretz O, Barbry P, Bock R, Lindemann B (1999) Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47:51–64
Li CS, Cho YK, Smith DV (2005) Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophys 93:1183–1196
Liu D, Liman ER (2003) Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A 100:15160–15165
LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL (2006) Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem 98:68–77
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A 104(38):15075–15080
Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD (2006) Umami responses in mouse taste cells indicate more than one receptor. J Neurosci 26:2227–2234
Max M, Shankar G, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF (2001) Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus. Sac Nat Gen 28:58–63
McLaughlin SK, McKinnon PJ, Margolskee RF (1992) Gustducin is a taste-cell specific G protein closely related to transducins. Nature 357:563–569
Montmayeur JP, Liberlis SD, Matsunami H, Buck L (2001) A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 4:492–498
Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJP (2005) The receptors and coding logic for bitter taste. Nature 434:225–229
Nelson G, Hoon MA, Chandrashekar J, Ryba NJP, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106:381–390
Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJP, Zucker CS (2002) An amino-acid taste receptor. Nature 726:1–4
Norgren R (1976) Taste pathways to hypothalamus and amygdala. J Comp Neurol 166:17–30
Norgren R, Hajnal A (2005) Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84:363–369
Norgren R, Leonard CM (1971) Taste pathways in rat brainstem. Science 173:1136–1139
Norgren R, Leonard CM (1973) Ascending central gustatory pathways. J Comp Neurol 150:217–238
Norgren R, Pfaffmann C (1975) The pontine taste area in the rat. Brain Res 91:99–117
Norgren R, Wolf G (1975) Projections of thalamic gustatory and lingual areas in the rat. Brain Res 92:123–129
Norgren R, Hajnal A, Mungarndee SS (2006) Gustatory reward and the nucleus accumbens. Physiol Behav 89:531–535
Pardini AW, Nguyen HT, Figlewicz DP, Baskin DG, Williams DL, Kim F, Schwartz MW (2006) Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res 1112:169–178
Pellerin L, Bouzier-sore AK, Aubert S, Serres S, Merle M, Costalat R, Magistretti PJ (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5:1169–1176
Perez CA, Margolskee RF, Kinnamon SC, Ogura T (2003) Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium 33:541–549
Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, De Araujo IE (2010) Nutrient selection in the absence of taste receptor signaling. J Neurosci 30:8012–8023
Rong M, He W, Yasumatsu K, Kokrashvili Z, Perez CA, Mosinger B, Ninomiya Y, Margolskee RF, Damak S (2005) Signal transduction of umami taste: insights from knockout mice. Chem Sens 30:i33–i34
Sclafani A, Lucas F (1996) Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav 60:447–453
Sclafani A, Vigorito M (1987) Effects of SOA and saccharin adulteration on polycose preference in rats. Neurosci Biobehav Rev 11:163–168
Sclafani A, Xenakis S (1984) Sucrose and polysaccharide induced obesity in the rat. Physiol Behav 32:169–174
Sclafani A, Zukerman S, Glendinning JI, Margolskee RF (2007) Fat and carbohydrate preferences in mice: the contribution of {alpha}-Gustducin and Trpm5 taste signaling proteins. Am J Physiol Regul Integr Comp Physiol 293:R1504–R1513
Scott TR, Verhagen JV (2000) Taste as a factor in the management of nutrition. Nutrition 16:874–885
Small DM, Jones-Gotman M, Dagher A (2003) Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19:1709–1715
Snowdon CT, Epstein A (1970) Oral and intragastric feeding in vagotomized rats. J Comp Physiol Psychol 71:59–67
Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD (2005) Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res 1061:88–96
Spector AC, Travers SP (2005) The representation of taste quality in the mammalian nervous system. Behav Cogn Neurosci Rev 4:143–191
Swithers SE, Davidson TL (2008) A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci 122:161–173
Touzani K, Bodnar R, Sclafani A (2008) Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci 27:1525–1533
Travagli RA, Hermann GE, Browning KN, Rogers RC (2006) Brainstem circuits regulating gastric function. Annu Rev Physiol 68:279–305
Tsang YC (1938) Hunger motivation in gastrectomized rats. J Comp Physiol Psychol 26:1–17
Tschop M, Smiley DL, Heiman ML (2000) Ghrelin induces adiposity in rodents. Nature 407:908–913
Wise RA (2006) Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci 361:1149–1158
Xenakis S, Sclafani A (1981) The effects of pimozide on the consumption of a palatable saccharin–glucose solution in the rat. Pharmacol Biochem Behav 15:435–442
Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook BWD, Zucker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112:293–301
Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erienbach I, Ryba NJP, Zuker CS (2003) The receptors for mammalian sweet and umami taste. Cell 115:255–266
Zukerman S, Touzani K, Margolskee R, Sclafani A (2009) Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Sens 35:685–694
Acknowledgments
We thank Prof Dietmar Richter for editorial assistance and Theddy Gonçalves for Fig.1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Berlin Heidelberg
About this chapter
Cite this chapter
de Araujo, I.E., Ren, X., Ferreira, J.G. (2011). Metabolic Sensing in Brain Dopamine Systems. In: Meyerhof, W., Beisiegel, U., Joost, HG. (eds) Sensory and Metabolic Control of Energy Balance. Results and Problems in Cell Differentiation, vol 52. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-14426-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-14426-4_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-14425-7
Online ISBN: 978-3-642-14426-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)