Abstract
This chapter summarizes major aspects of N-nutrition in plants. N distribution within a plant varies widely according to the organ, the development stage, and mostly to the environmental conditions. Within the cell, the different N forms are stored in different compartments and the pool sizes are controlled in contrasting manner. Plants can take up nitrate, ammonium, urea, and other organic N forms. Various transporters for these compounds have been characterized, and the localization and properties of these proteins give rise to a complex pattern of N fluxes within the plant. The further assimilation of nitrate is well described, but the in planta role of all proteins, as for example GS1 and GDH, is far from being evident. Some are involved in N remobilization which is an important N source for example during seed filling.
Regulation of N assimilation occurs at the transcriptional and post-transcriptional levels, and regulation of the different steps is highly coordinated. However, only very few molecular players are known. As a special case in N-signaling, NO, a side product of N assimilation, is considered in some detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nitrate Reductase
- Nitrate Reductase Activity
- Nitrate Uptake
- Nitrate Transporter
- Supra Molecular Structure
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Nitrogen is the mineral nutrient required in highest amounts by plants and is most frequently limiting growth and yield. Inorganic or organic N forms participate to plant nutrition in a variable extent depending on plant parameters as well as soil characteristics. In temperate climatic conditions, inorganic N forms are predominant, and fertilizers are often supplied as nitrate, ammonium, or urea (http://www.fertilizer.org/ifa/). However, the soil solution may contain different organic N forms such as soluble proteins or amino acids derived from proteolytic processes. A variety of plant species are able to use organic N forms in artic, boreal, temperate, Mediterranean shrub-land, or alpine natural ecosystems (reviewed in Näsholm et al. 2009). In legume plants, atmospheric N2 is fixed in the nodule, a specialized organ resulting from the interaction between bacteria and roots (Gordon et al. 2001). In the same manner, nutrient use efficiency is increased by symbioses between fungi and plants, the mycorrhizal system being involved in nutrient uptake and the plant partner providing reduced carbon to the fungus (Martin et al. 2001).
Although such symbioses are important in natural ecosystems, this chapter describes only direct N uptake by root cells. We give an overview of (1) N distribution within the plant and more precisely within a plant cell, (2) the molecular elements involved in different fluxes or in assimilatory steps, and (3) the regulatory mechanisms that control these processes. N metabolites, such as nitrate, ammonium, and glutamate act as signal molecules as well. However, this is out of the scope of this chapter and has been reviewed recently (Walch-Liu et al. 2005); instead, we extend this chapter by (4) a detailed description of the synthesis and mode of action of NO.
2 Distribution of N Forms in Plant Cells
2.1 N in Different Tissues
The N forms and N quantities within a plant vary widely according to the organ, the development stage, and the environmental conditions. The root is obviously the predominant organ where large exchanges of a variety of N forms occur between root cells and the soil solution. The differential expression and localization of channel- or transporter proteins (see below) led to a complex picture of the root cellular organization, with specialized uptake functions for lateral root caps or epidermis/cortex, and horizontal transport toward the vasculature for endodermis/pericycle and stele lines (see below 1.2.2 ammonium transport). Inorganic N forms can then enter the xylem to be transported to the shoots. N assimilation and remobilization take place in roots and shoots, and organic N forms are then distributed to sinks organs (Brouquisse et al. 2001).
2.2 N Cellular Distribution
Within the cell, N forms are stored in different compartments (Fig. 1).
N Storage in different compartments. Nitrate and ammonium enter the cell and can be either stored in the vacuole, transported to other tissues, or assimilated in the cytosol and the chloroplast. Small italic letters: nitrogen assimilation steps. Capital letters: enzymes. Capital italic letters: genes. NR: Nitrate reductase. NiR: nitrite reductase/ GS: Glutamine synthethase. GOGAT: glutamate synthase, AMT: ammonium transporter. NRT: nitrate transporter; CLC: chloride channel
Cytoplasmic ammonium pools originate not only from ammonium uptake across the plasma membranes but also from amino acid catabolism occurring during photorespiration in illuminated leaves (Leegood et al. 1996) or in senescent tissues (Matsson and Schjoerring 2003). Ammonium concentrations have been measured using analysis of 1H-coupled 14N-NMR signals (review in Mesnard and Ratcliffe 2005) or with ammonium-selective microelectrodes (Wells and Miller 2000). In both cases, the cytoplasmic ammonium concentrations were no more than a few millimolar (8–15 mM), but this concentration could be increased in maize roots when ammonium assimilation was blocked (Lee and Ratcliffe 1991). In vacuoles, ammonium concentrations vary between 1 and 45 mM in nonstressed plants (Miller et al. 2001), indicating a possible role of this compartment for the storage of ammonium.
The global nitrate concentrations in leaves or roots are highly dependent on external N supply, and nitrate, among all N-compounds, disappears most quickly in response to N starvation (Richard-Molard et al. 2008). The pool of nitrate associated with purified chloroplasts remains remarkably constant under various conditions (Schröppel-Maier and Kaiser 1988). In contrast, the vacuolar nitrate pools show a positive correlation with the external nitrate supply (Miller and Smith 2007; van der Leij et al. 1998). The pool size varies also with the cell type and is higher in epidermal than in mesophyll cells in barley leaves (Karley et al. 2000) and higher in cortical compared to epidermal cells in barley roots. In roots, remobilization of vacuolar nitrate occurs more slowly from cortical cells than from epidermal cells (van der Leij et al. 1998). This tissue heterogeneity revealed by single-cell techniques implies that knowledge obtained for vacuoles from one type of tissue cannot be necessarily transferred to vacuoles from other tissues, as also shown for gene expression (Gifford et al 2008). A striking characteristic of the cytosolic NO −3 pool is its low size (in the order of 3–4 mM). In contrast to vacuoles, cytosolic nitrate is maintained at a remarkably stable value that is independent of changes in the external nitrate concentration (Miller and Smith 2007; van der Leij et al. 1998 ).
The global amino acid contents in leaves depend on external N supplies and can vary from 150 to 45 nmol/mgDM when Arabidopsis plants are fed with 10 or 3 mM nitrate, respectively (Loudet et al. 2003). Subcellular volumes and amino acid concentrations have been analyzed using non-aqueous fractionation in spinach (Winter et al. 1994), barley (Winter et al. 1993), or potato (Leidreiter et al. 1995). In all cases, the concentration of amino acids is much lower in the vacuoles than in the cytosol (1.7/40 mM for glutamate in barley, for example). These concentrations are quite similar between cytosol and stroma.
3 N Fluxes Within a Plant Cell
3.1 Nitrate and Nitrite Fluxes
Two nitrate transport systems have been shown to co-exist in plants and act co-ordinately to take up nitrate from the soil solution and distribute nitrate within the whole plant (Fig. 2) (review in Daniel-Vedele et al. 1998 ; Tsay et al. 2007).
It is generally assumed that the NRT1 gene family mediates the root low-affinity transport system (LATS), with the exception of the AtNRT1.1, which is a dual affinity transporter (Wang et al. 1998; Liu et al. 1999). In Arabidopsis, 53 genes belong to the NRT1 family. Among them 51 genes are expressed and exhibit different tissue expression patterns in the whole plant (Tsay et al. 2007), suggesting a specialized and unique function for at least some of them. The most extensively studied gene is the first one isolated, AtNRT1.1 (formerly Chl1; Tsay et al. 1993). The gene is expressed in epidermis of the root tips and in the cortex and endodermis in the more mature part of the root (Huang et al. 1996) but also accumulates in nascent organs (Guo et al. 2001). AtNRT1.1 is also considered as a nitrate sensor that could regulate other processes like regulation of other components of nitrate uptake (Krouk et al. 2006), stomatal opening (Guo et al. 2001), relieving of seed dormancy (Alboresi et al. 2005), or stimulation of root proliferation by nitrate (Remans et al. 2006a). Beside this gene, the AtNRT1.2 gene is constitutively expressed only in the root epidermis and belongs to the constitutive low-affinity system (Huang et al. 1999). AtNRT1.5 is located on the plasma membrane of root pericycle cells close to the xylem. The protein is a low-affinity, pH-dependent bidirectional nitrate transporter and is involved in long distance transport of nitrate from the root to the shoot (Lin et al. 2008). The AtNRT1.4 gene is only expressed in leaf petioles, and the nitrate content is twice lower in the petiole of the mutant compared to that of the wild type (Chiu et al. 2004). Recently, AtNRT1.6 was shown to be involved in embryo development. The gene is expressed in the vascular tissue of the silique. Expression in oocytes and mutant phenotypes suggest that the protein could deliver nitrate from maternal tissue to the developing embryo (Almagro et al. 2008). A striking particularity of the NRT1 family is that certain members belonging to the group II (reviewed in Tsay et al. 2007) are able to transport not only nitrate but also di or tripeptides in heterologous systems, while OPT proteins transport tetra/pentapeptides.
The high-affinity transport system (HATS), acting when the external nitrate concentration is low, relies on the activity of the so called NRT2 family (reviewed in Williams and Miller 2001). AtNRT2.1 is a major component of the iHATS in Arabidopsis, as shown by the fact that a mutant disrupted for the AtNRT2.1 gene has lost up to 75% of the inducible high-affinity NO −3 uptake activity and showed a lower leaf nitrate content (Cerezo et al. 2001; Filleur et al. 2001). As a consequence, growth of these mutants is severely impaired at low NO −3 concentration (Orsel et al. 2004; Orsel et al. 2006). Li and coworkers showed that the AtNRT2.2 makes only a small contribution to iHATS under normal growth conditions (Li et al. 2007).
Nitrate can also be exported from the cytosolic pool by an efflux mechanism. Segonsac and co-workers have identified an Arabidopsis excretion transporter, localized at the plasma membrane of cortical root cells and encoded by the NAXT1 gene belonging to the NRT1 family (Segonzac et al. 2007).
Regarding vacuolar nitrate pools, classical experiments using indirect assay of H+ transport provided evidence for the presence of a NO −3 /H+ antiporter in the tonoplast (Schumaker and Sze 1987). Recently, De Angeli et al. (2006) demonstrated that the AtCLCa protein, localized in the vacuolar membrane, behaves as a NO −3 /H+ exchanger, allowing the accumulation of nitrate within the vacuole. Residues important for nitrate/proton coupling have been identified in plant and mammalian CLC transporters (Eun-Yeong et al. 2009; Zifarelli and Pusch 2009). Insertion mutants within the AtCLCa gene exhibit normal development but show a reduced capacity to store nitrate but not other anions (Geelen et al. 2000). This phenotype was also recently found when the expression of the vacuole-located nitrate transporter AtNRT2.7 was affected. This AtNRT2 gene is expressed in aerial organs and also highly induced in dry seeds. In two allelic atnrt2.7 mutants, less nitrate is accumulated in the seed. In contrast, seeds from plants overexpressing the AtNRT2.7 coding region accumulate more nitrate, and as a consequence they are less dormant than the corresponding wild type seeds (Chopin et al. 2007).
Finally, little is known on potential channels or transporters that could be involved in fluxes towards the chloroplast (reviewed in Weber et al. 2005). Fusion proteins with the GFP marker revealed the chloroplastic subcellular localization of the AtCLCe protein. The atclce mutants display a phenotype linked both to photosynthesis (Marmagne et al. 2007) and nitrate content (Monachello et al. 2009). The flux of nitrite, the product of nitrate reduction in the cytosol, into the chloroplast could also play a role in the flux of nitrate towards the chloroplast and thus in the homeostasis of cytosolic nitrate. A nitrite transporter belonging to the NRT1 family has been recently identified in cucumber and Arabidopsis (Sugiura et al. 2007).
3.2 Ammonium Fluxes
Since the cloning of the first gene involved in ammonium transport (Ninnemann et al. 1994), five other genes belonging to the same family were found in Arabidopsis (Gazzarrini et al. 1999; Sohlenkamp et al. 2000), ten in rice (Sonoda et al. 2003), a species adapted to ammonium nutrition, and 14 in poplar (Couturier et al. 2007). Focusing on the results obtained in Arabidopsis, kinetics properties of the AMT proteins expressed in oocytes showed Km values ranging from 34 mM for AMT1;1 (Wood et al. 2006) to 140 mM for AMT1;2 (Neuhäuser et al. 2007). Among the six genes, AMT1;1, AMT1;2, AMT1;3, and AMT2;1 are highly expressed in roots (Loqué and von Wirén 2004) and encode proteins that are located in the plasma membranes (Loqué et al. 2006; Yuan et al. 2007). In order to analyze the function of each of this genes separately in planta, physiological and ammonium influx studies were carried out on single, double, triple, and quadruple mutants (Yuan et al. 2007). Additive contribution of AMT1;1 and AMT1;3 was shown, while a second saturable transport is thought to be coded by the AMT1;5 gene. A complex picture is now emerging from these studies (Fig. 3). There is a spatial organization of AMT1 proteins, the transporters possessing the highest ammonium affinities being located in outer root cells or root hairs where they can uptake ammonium from the soil solution (AMT1;1, AMT1;3, AMT1;5). The lower affinity of AMT1;2 and its location in the endodermis along the root hair zone suggest a function in the retrieval of ammonium that is released from the cortex, or that enters the root via the apoplastic route.
Model summarizing the functions of AMT1-type transporters in high-affinity ammonium uptake in Arabidopsis roots (from Yuan et al. 2007). This schematic representation shows the contribution to ammonium uptake and spatial expression in root tissues of AMT1;1, AMT1;3, AMT1;5 (all in red), and AMT1;2 (blue) under nitrogen deficiency. AMT-dependent ammonium influx is proportionally represented by the size of their arrows. rhizo, rhizodermis; co, cortex; endo, endodermis; peric, pericycle; xyl, xylem
The electrochemical gradient between vacuole and cytosol would drive NH3 import to and NH +4 export out of the vacuole. Indeed, tonoplast intrinsic proteins of the TIP family were shown to play a role in NH3 transport into the vacuole (Loqué et al. 2005). Vacuolar loading with NH +4 should require an electrogenic ammonium transporter, which has not yet been identified.
3.3 Urea Transport
Although urea is the major nitrogen form supplied as fertilizer in agricultural plant production, its uptake by plant roots or leaves before its hydrolysis has been a matter of debate for a long time. However, studies in crop plants (Merigout et al. 2008a) and Arabidopsis (review in Kojima et al. 2006) showed the uptake of urea. The identification of the high-affinity urea transporter AtDUR3 by Liu et al. (2003a) and of the AtTIP urea permeases (Liu et al. 2003b) led to new insights regarding the molecular basis of urea uptake in plants. Growth of mutant lines carrying T-DNA insertions in AtDUR3 is impaired when urea is the sole nitrogen source. (Kojima et al. 2007). Physiological and transcriptomic analyses were performed in Arabidopsis plant to assess the interactions between urea and ammonium or nitrate uptake and assimilation (Merigout et al. 2008b).
3.4 Organic N Transport
So far, plant putative amino acid transporters have been identified as members of at least five gene families, comprising for example in Arabidopsis at least 67 genes (reviewed in Ortiz-Lopez et al. 2000; Rentsch et al. 2007). We will focus here on amino acid transporters shown to be clearly involved in uptake or distribution of amino acids within the plant.
Forward and reverse approaches were used to identify transporters involved in root amino acid uptake (Hirner et al. 2006; Svennerstam et al. 2007). Both studies led to the conclusion that LHT1 (Lysine/histidine transporter) is crucial for root uptake of acidic and neutral amino acids. The AAP1 protein was also shown to transport uncharged amino acids, but only when they are supplied at high concentrations in the external medium (Lee et al. 2007b). Uptake of cationic amino acids like l-Lys or l-Arg is mediated by AAP5 within the concentration range relevant for field conditions (Svennerstam et al. 2008). Näsholm et al. (2009) suggests a hypothetic mode of root amino acid uptake in nonmycorrhizal plants. Although expression of many seed amino acid transporters precedes storage protein synthesis during seed maturation, only a few organic N transporters, among them AtOPT3, have been shown to be essential for seed loading or development (Stacey et al. 2002).
Intracellular transport is expected to be important particularly in the case of amino acid transport. Indeed, plastids are key compartments for amino acid biosynthesis, some of them being exclusively synthesized there (phenylalanine, tyrosine, tryptophan, and lysine) whereas others (glutamine, aspartate, and serine) are produced in multiple compartments. Strikingly, only one protein, Dit2.1, is so far clearly localized at the inner envelope membrane and functions as a glutamate/malate exchanger, essential for the photorespiratory pathway (Renné et al. 2003). Similarly, only transporters for basic amino acids have been localized in the mitochondrial membrane (Catoni et al. 2003; Hoyos et al. 2003). Some transporters have been localized at the tonoplast and their function remains to be demonstrated. The concentration of amino acids in the vacuole is lower than in the cytosol, but so far a vacuolar export system has been shown only in Chara vacuoles (Martinoia et al. 2000).
4 N Assimilation Pathways
As described before, the main nitrogen sources taken up by higher plants are nitrate or ammonium as inorganic N sources, and eventually amino acids under particular conditions. Here, we will briefly describe the main steps of nitrate or ammonium assimilation in growing cells and summarize recent results obtained for source organs when N is remobilized.
4.1 N Assimilation
A global overview of N assimilation in plants is given in Fig. 1. Nitrogen assimilation requires the reduction of nitrate to ammonium, followed by ammonium assimilation into amino acids.
Nitrate reduction into nitrite is catalysed in the cytosol by the enzyme nitrate reductase (NR). This enzyme is a homodimer, each monomer being associated with three prosthetic groups: flavin adenine dinucleotide, a haem, and a molybdenum cofactor (MoCo). Characterization of mutants resistant to chlorate, which can be reduced into toxic chlorite by NR, identified two classes of genes, the NIA genes encoding the NR apoenzyme and the CNX genes encoding the MoCo cofactor (Pelsy and Caboche 1992; Crawford and Arst 1993). Since 1993, a lot of work has been done to characterize the NR in different species (reviewed in Meyer and Stitt 2001). Although the NR enzyme is thought to be localized in the cytosol (Solomonson and Barber 1990), an association with the plasma membrane (PM-NR) has been found in some species like in corn roots (Chen and Wang 1995) or barley roots (Ward et al. 1989). The structural characteristics and the potential role of this PM-NR have been intensively studied in Chlorella by Tischner and collaborators (reviewed in Tischner 2000). Nitrite is then translocated to the cytosol where it is reduced to ammonium by the second enzyme of the pathway, nitrite reductase (NiR). The NII genes encoding the NiR enzyme have been cloned from various species, the number of genes varying from one to two copies (Meyer and Stöhr 2002).
Ammonium, originating from nitrate reduction, photorespiration, or amino acid catabolism, is assimilated in the chloroplast by the so-called GS/GOGAT cycle (Lea and Miflin 2004). The glutamine synthetase fixes ammonium on a glutamate molecule to form glutamine. This glutamine reacts subsequently with 2-oxoglutarate to form two molecules of glutamate, this step being catalysed by the glutamine 2-oxoglutarate amino transferase (or glutamate synthase GOGAT). Two classes of genes code for GS: the GS2 gene, present as a single nuclear gene in all species studied so far, codes for a chloroplastic GS, involved in the assimilation of ammonium stemming from nitrate reduction or photorespiration. Conversely, the GS1 nuclear gene family codes for cytosolic GS isoforms, present in different organs such as roots or stems and thought to be involved in ammonium recycling during particular developmental steps such as grain filling or leaf senescence (reviewed in Hirel and Lea 2001; Corruzzi 2003). Two different forms of glutamate synthase are present in plants: the Fd-GOGAT and NADH-GOGAT use ferredoxin and NADH as electron donors, respectively. Fd-GOGAT is predominantly localized in leaf chloroplasts, while NADH-GOGAT is primarily located in plastids of non-photosynthetic tissues, such as roots or etiolated leaf tissues. The structural, mechanistic, and regulatory properties of GOGAT enzymes and their role in amino-acid metabolism have been recently reviewed by Suzuki and Knaff (2005).
4.2 N Remobilization
Although nitrogen uptake still operates at the reproductive stage (Gallais et al. 2007), it is generally assumed that seeds receive a large part of nitrogen from remobilization of different N forms present in source organs (Feller and Keist 1986). During senescence, a re-distribution of amino acids, free or produced by proteolysis of proteins (Patrick and Offler 2001) leads to an increase of asparagine in pea (Rochat and Boutin 1991) and an increase in glutamine in other species, in the phloem sap (Herrera-Rodriguez et al. 2006; Masclaux-Daubresse et al. 2006). Some amino acid transporters of the AAP family are putatively involved in phloem loading (see above). During these particular developmental stages, specific enzymes related to N metabolism are activated (reviewed in Masclaux-Daubresse et al. 2008). Induction of cytosolic GS1 as well as induction of glutamate dehydrogenase appears in a large variety of plants. The latter, catalysing glutamate de-amination as well as glutamate synthesis, carried out the de-amination reaction in source leaves (Masclaux-Daubresse et al. 2006). This N remobilization during senescence is also triggered in response to environmental factors such as drought, nutrient limitation, or pathogen attack (Pageau et al. 2006).
5 Regulation of N Uptake and Metabolism
N uptake by the roots and N assimilation are integrated to match the nutrient demand of the whole organism. Regulatory mechanisms that modulate the expression and/or the activity of transport systems and enzymes, according to the nutritional status of the plant and to external stimuli or stresses, ensure both rapid adjustments of metabolism and long term adaptations (Fig. 4).
Pathways of nitric oxide (NO) synthesis, and basic reactions of NO with different targets. NO can be synthesized by nitrite reduction, mediated either by NR itself of by mitochondrial electron transport (the latter only in roots). Nitrite to NO reduction requires high nitrite concentrations, which can become especially high under anoxia, when NR is highly active (dephosphorylated), and nitrite reduction is impaired. Also shown are the two oxidative pathways for NO synthesis; one is the (probably non-enzymatic) oxidation of hydroxylamines by reactive oxygen, the other one is the oxidation of l-arginine by a NOS-like activity. NO may either react directly with heme groups of enzymes forming Fe:NO adducts, or it may react with thiol groups to form nitrosothiols. At least in theory, NO may also react with superoxide radicals to form the highly reactive peroxynitrite, which may nitrosate aromatic amino acids
5.1 Regulation at the mRNA Level
Patterns for changes in mRNA abundance of many components of N uptake and N assimilation have been observed, which allow coordinated regulation of N metabolism. Two main metabolic cues operate in the control of N uptake and assimilation.
The first mechanism includes the induction by substrates and repression by endogenous N assimilates, mediating a negative feedback regulation by the N status of the whole plant (Gazzarrini et al. 1999; Cerezo et al. 2001). This results in up regulation when N is low and down regulation when N is high. Accordingly, several NRT2 and AMT1 transporters as well as NIA and NII were found to be repressed at the mRNA level by N metabolites such as amino acids (Tsay et al. 2007). Further studies using the glutamate synthase inhibitor, AZA, or exposure to NH +4 or various amino acids suggested that glutamine plays an important role in the down regulation of NRT2.1 (Nazoa et al. 2003, Zhuo et al. 1999).
In response to N deprivation, AMT1.1, AMT1.3, and AMT1.5 (Gazzarrini et al. 1999; Gansel et al. 2001; Loqué et al. 2006), as well as AtNRT2.1, AtNRT2.2, and AtDUR3, are induced (Lejay et al 1999; Scheible et al. 2004). Interestingly, two genes of the NRT2 family are slowly but steadily induced by starvation (Orsel and Krapp, unpublished data). Resupply of nitrate re-induces NRT2.1, and NRT2.2 as well as NIA and NII expression after long term starvation (Scheible et al. 2004), whereas expression of NRT2.4 and NRT2.5 is repressed by the resupply of any N source (Okamoto et al. 2003).
Transcriptional regulation of genes involved in LATS for NH +4 and NO −3 is less documented. NRT1.1 shares many regulatory features with NRT2.1. NRT1.1 is rapidly induced by nitrate and by starvation but less subjected to regulation by N metabolites (Tsay et al. 1993), while AtNRT1.2 is constitutively expressed (Huang et al. 1999); AtNRT1.5 is much more slowly induced by nitrate, and is in addition regulated by potassium. AtNRT1.1 and AtNRT1.5 are both regulated by pH (Tsay et al. 1993; Lin et al. 2008).
Global transcriptome studies (Wang et al. 2003; Scheible et al. 2004) confirmed transcriptional regulation of N uptake and assimilation by nitrate and showed a broad action spectrum of nitrate as a regulator of gene expression, coordinating for example C and N metabolism. Using NR mutants (Wang et al. 2004) it was shown that nitrate itself acts as signal. Another study (Wang et al. 2007) investigating gene regulation by nitrite showed an overlap between nitrate and nitrite regulated genes. Nevertheless, specific regulation by nitrite was shown for several genes of N uptake (e.g. NRT2.5, AMT1.3). Nitrite was already discussed by Loqué et al (2006) as signaling molecule for the regulation of NRT1.1 and NIA1.
The second major regulation of N uptake and N assimilation corresponds to the stimulation by photosynthesis (Lejay et al. 2003), which ensures that N uptake is harmonized with the C status. A major common feature is the diurnal fluctuation of N uptake and N reduction. This control has often been attributed to the regulatory action of sugars produced by photosynthesis and transported downward to the roots, as shown by the positive effect of CO2 concentration on NO –3 uptake (Gastal and Saugier 1989; Delhon et al. 1996) Diurnal fluctuations in uptake and assimilation, or stimulation by sugars, are generally correlated with the expression of genes encoding transporters and enzymes. This has been shown for NH +4 transporters (Gazzarrini et al. 1999; von Wirén et al. 2000; Lejay et al. 2003), NO –3 transporters (Lejay et al. 1999; Ono et al. 2000; Matt et al. 2001), and NR and NiR (Vincentz et al. 1993). In Arabidopsis, genes tested by Lejay et al. (2003, AtNRT2.1 and AtNRT1.1), showed 5–10 times higher expression during the light period compared with the dark period. Nitrate uptake, measured using 15NO −3 also increased after the onset of light. The increase was approx. two-fold during the photoperiod. The decrease in AtNRT2.1 and AtNRT1.1 mRNA levels and nitrate uptake during the dark period was prevented by supply of 1% sucrose to the roots, which is a further indication for the role of sugars during diurnal regulation. This regulation seems to be independent of the known sugar regulation pathways, such as hexokinase signaling (Lejay et al. 2003). Recently Lejay et al. (2008) showed that up-regulation of nitrate transporters (AtNRT2.1 and AtNRT1.1) was related to the concentration of glucose 6-phosphate. Contrary to that of the transporters, the diurnal regulation of NIA transcripts is not only governed by sugars but also by light regulation via phytochrome (Rajasekhar et al. 1988). In addition, NIA expression is controlled by signals from photosynthetic electron flow, which adds to the picture of intracellular cross-talk between chloroplasts and the nucleus (Sherameti et al. 2002).
Despite the very important regulation of transcript abundance by external and internal factors, information about the molecular players such as transcription factors, miRNA, etc. is still rather rare. Lately two bZIP (basic leucine zipper) transcription factors have been discovered as being involved in the light regulation of N metabolism (Jonassen et al. 2008): HY5 and its homolog HYH are essential for phytochrome dependent light-activated expression of NR. ChIPchip analyses showed a binding site for HY5 in the NIA2 promoter (Lee et al. 2007a). Interestingly also the NRT1.1 promoter has three binding sites for HY5, but HY5 has a negative effect on transcription in this case (Lillo 2009). However, not all light regulation of N metabolism is governed by the HY5/HYH system (Lillo 2009).
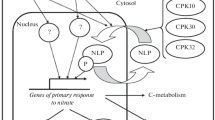
Camargo et al. (2007) identified CrNIT2 as a main regulator of NIA expression in Chlamydomonas, and Castaings et al. (2009) showed that Arabidopsis mutants in a homologous gene (NLP7) are defective in the nitrate induction of NIA genes, NRT2.1 and NRT2.2. Both proteins belong to a class of putative transcription factors homologous to a protein first identified in Medicago and essential for nodulation (NIN = nodulation inception). The CrNIT2 protein has been shown to bind to multiple sites of the NIA promoter, but no target genes are yet known for the AtNLP7 protein. Interestingly, mutants in the CIPK8 gene which encode a protein kinase (Hu et al. 2009), are also unable to fully induce expression of several genes by nitrate, such as the NIA genes, NRT2.1, NRT1.1, and several others. It is tempting to speculate that CIPK8 might be involved in the same regulation pathway than NLP7. NLP7 belongs to a gene family with nine different members, but the functions of the other NLP proteins are still unknown.
5.2 Regulation at the Protein Level
N metabolism has to respond fast to external stimuli. This can be achieved by rapid post-translational protein modification.
The best studied case of post-translational regulation in N metabolism is the regulation of higher plant NR. NR is inactivated via a two step process that involves phosphorylation of ser543 in spinach and the subsequent magnesium-dependent binding of an inhibitory 14-3-3 protein to NR (Bachmann et al. 1996; Moorhead et al. 1996). This activation/inactivation process is linked to the production of C assimilates that thus control NR activity (De Cires et al. 1993; Kaiser and Huber 2001). Both CDPK (calcium-dependent protein kinases) and AMPK/SNRK (SNF1-related kinase)-related protein kinases are able to phosphorylate NR at least in vitro (McMichael et al. 1995; Douglas et al. 1997; Sugden et al. 1999; Ikeda et al. 2000). The inactive phosphorylated form is re-activated by dephosphorylation probably by PP2A (MacKintosh 1992).
Protein phosphorylation may act as a trigger for protein degradation, as well as for binding of the inhibitory 14-3-3 proteins. When a modified form of NR with a truncated N-terminus that was not susceptible to post-translational dark inactivation was overexpressed, the resulting protein did not decline in the second part of the photoperiod (Nussaume et al. 1995). There is also a correlation between the phosphorylation state or the activation state of NR and the rate at which NR protein decreases (Geiger et al. 1998; Kaiser and Huber 1997; Scheible et al. 1997; Weiner and Kaiser 1999).
Post-translational regulation of nitrate transporters has recently been described. The nitrate transporter NRT1.1 is regulated by phosphorylation. When phosphorylated, AtNRT1.1 functions as a high affinity transporter, whereas it is active in the low affinity range when dephosphorylated (Liu and Tsay 2003). Recent data show that NRT1.1 acts not only as a transporter, but is also involved in N signaling (Remans et al. 2006; Walch-Liu and Forde 2008). Interestingly in one case, only the phosphorylated form is an active signaling component (Walch-Liu and Forde 2008). Nitrate transporters from the NRT2 family are also subjected to post-transcriptional regulation. First indications of putative phosphorylation of NRT2 proteins came from their amino acid sequences (Forde 2000). In addition, several of the NRT2 proteins have been identified in global phosphoprotein studies (Benschop et al. 2007). Such a post-transcriptional regulation may explain why high affinity NO −3 influx is down-regulated by NH +4 in transgenic plants expressing NpNRT2.1 cDNA under the control of a constitutive, root specific promoter (Fraisier et al. 2000). Recently, Wirth et al (2007) showed that despite strict transcriptional regulation of AtNRT2.1, NRT2.1 protein levels are rather constant in response to light, sucrose, or nitrogen treatments that strongly affect both NRT2.1 mRNA level and HATS activity. Again post-translational regulation processes are required to explain these observations. One such mechanism could correspond to the cleavage of NRT2.1 C terminus, which results in the presence of both intact and truncated proteins in the plasma membrane (Wirth et al. 2007). Several forms of the protein seem to co-exist in cell membranes (the monomer and at least one higher molecular weight complex). However, the monomer is the most abundant form of NRT2.1, and seems to be the one involved in NO3− transport (Wirth et al. 2007). Interestingly, AtNRT2.1 is only present and active at the plasma membrane in the presence of AtNAR2.1 (Orsel et al. 2006; Wirth et al. 2007). The mechanism by which NAR2.1 affects NRT2.1 is so far unknown, but might open a new level of regulation by protein stability or protein transport.
A different form of post-translational regulation has been revealed for ammonium transporters allowing rapid shut-off in order to avoid toxic accumulation of ammonium. Loqué et al (2007) showed that the soluble carboxy terminus of the oligomeric AtAMT1 serves as an allosteric regulator essential for function. It is suggested that this C terminus interacts physically with cytosolic loops in the neighboring subunit with phosphorylation as a regulating mechanism.
Less is known about nitrite transport and its regulation. In E. coli, the PII protein regulates nitrite transport. This regulation seems to be conserved in plants. The chloroplastic PII protein might be involved in the regulation of nitrite uptake by chloroplast as mutants affected in the gene exhibit a nitrite sensitive phenotype (Ferrario-Méry et al. 2005). This hypothesis was re-enforced by the increased nitrite uptake by chloroplasts isolated from PII mutants (Ferrario-Méry et al. 2008).
Several chloroplastic enzymes of nitrogen assimilation such as NIR, GS2, and Fd-GOGAT are redox regulated through the thioredoxin system (Lemaire et al. 2007; Lichter and Häberlein 1998). In addition NR is also regulated by NO, a by-product of its own activity. NO production and the broad mode of action are described in the following paragraph.
6 N- Signaling: Nitric Oxide – A Special Case
Nitrate and other low molecular weight intermediates of nitrogen metabolism are not only substrates, but also act as signals regulating the interaction between metabolic pathways of growth and differentiation, or plant interactions with the environment. Among these nitrogen signals, nitric oxide has gained specific attention during the last decade. Therefore, the role of this N-compound will be considered in more detail in context with N-metabolism.
6.1 Sources for NO in Plants
NO (+2) may be formed either by reduction of higher N-oxidation states, preferentially nitrite, or by oxidation of more reduced N-forms (for review see del Rio et al. 2004). Figure 5 summarizes pathways for NO production.
Reductive NO formation: Nitrate reduction appears always linked to the production of trace amounts of NO, originating from a one-electron reduction of nitrite. The reduction can be mediated by NR, or, at least in non-green plant tissues, by mitochondrial electron transport (Planchet et al. 2005; Gupta et al. 2005). In both cases, nitrite competes with the “normal” substrates (e.g. nitrate in the case of NR or oxygen in the case of mitochondrial ET), and therefore rather high nitrite concentrations are required for appreciable rates of NO production. Cytosolic nitrite concentrations are usually low (10–20 µM). Nevertheless, nitrate-fertilized plants emit NO into NO- free air at rates that can be detected and quantified by sensitive analytical methods such as gas-phase chemiluminescence. For example, with illuminated tobacco leaves, NO emission was 0.3 nmoles/g FW h (Rockel et al. 2002). Rates were lower in the dark, because NR activity is down regulated. As NO is rather reactive, real NO production rates inside leaf cells could be much higher, but this is not known with certainty. NR is activated by light or by anoxia in the dark, whereas nitrite reduction becomes very low under anoxia in the dark, presumably because NADPH production via oxidative pentose phosphate cycle ceases. In consequence, nitrite accumulates in anoxic cells and tissues to millimolar concentrations, and therefore anoxic NO emission can become 1,000-fold higher than in air (Rockel et al. 2002; Planchet et al. 2005). In NiR-deficient tobacco mutant leaves, which always accumulate nitrite even in air (light), NO emission was as high in air (light) as in nitrogen (dark). In NR-free nia1nia2 double mutants, NO emission in air and in nitrogen was absent (Planchet et al. 2005). The oxygen-dependent NOS reaction appeared not to contribute to this normal “bulk”-NO emission from leaves.
Plants possess yet another PM-bound NR plus a nitrite::NO reductase, which together can also produce NO (Stöhr and Stremlau 2006). No genes for these two enzymes have been identified so far, and their physiological role is still under investigation.
NO generation from nitrite may also occur non-enzymatically in acidic compartments at pH-values below 5. Such compartments might be either the mesophyll apoplast or vacuoles. While apoplastic NO formation has been localized by DAF-2 fluorescence (Bethke et al. 2004), no vacuolar NO production has been reported so far, which is actually astonishing.
Oxidative NO formation: In animals the major NO source is l-arginine, which is oxidized to NO and l-citrulline in a complex process catalyzed by the family of NOS-enzymes (nitric oxide synthases), using NADPH and O2 as further substrates. No gene homolog to the animal NOS family has been detected so far in Arabidopsis. Nevertheless, there are numerous indirect hints on NOS-like activities in plants, on the basis of effects of NOS-inhibitors, and also of enzyme activity measurements using NO measurement by EPR, of nitrite + nitrate production, or of conversion of labeled l-arginine into l-citrulline (for review see del Rio et al. 2004). Recently, an enzyme converting l-arginine to citrulline and NO has been purified from Arabidopsis shoots. The activity depends on the typical NOS-cofactors BH4 and Calmodulin (R. Tischner, pers. communication). Sequence information on that preparation may give a first insight into the nature of plant NOS.
Another substrate for oxidative NO formation are hydroxylamines, which can be oxidized by plant cells to NO, probably using superoxide and/or H2O2 as oxidants (Rümer et al. 2009). Although it is not yet clear whether the reaction is physiologically relevant, there is little doubt that plants are able to produce NO not only via nitrite reduction, but also via oxidation of amine-N.
Concentrations of NO and its “bioavailability” in cells will also depend on NO consumption (Vanin et al. 2004). NO oxidation involving reactive oxygen species (ROS), or O2-dependent oxidation catalyzed by hemoglobins (Dordas et al. 2003) should be among the most important reactions consuming NO. In addition, reversible binding of NO to thiols may be an important aspect regulating cellular NO levels (see below).
6.2 Mechanisms Through Which NO Affects Targets
In the complex cellular environment, NO may undergo various oxidation and/or dismutation reactions, yielding compounds like NO2, N2O3, the nitrosonium cation (NO+), or the nitroxyl anion (NO−). Some of these products may rapidly and reversibly nitrosylate protein- or non-protein thiols, or form nitrosyl-iron complexes with metal ions, e.g. in heme-proteins. Peroxynitrite (ONOO−) may be formed from the reaction of NO with superoxide anions. However, it is not clear to what extend the reaction occurs under natural conditions in vivo. Peroxynitrite may serve as a substrate for oxidation or nitration of aromatic amino acids. Nitration appears less easily reversible than nitrosylation. Because 3-tyrosine nitration occurs on the same position (3) that is also the site for phosphorylation, it can be assumed that tyrosine nitration has important consequence for regulation mediated via tyrosine protein kinases/phosphatases.
Cysteine-S-nitrosylation (also called nitrosation) appears as the most widespread way in which proteins are post-translationally modulated by NO (Fig. 5). More than 100 redox-sensitive proteins were identified in Arabidopsis as putative candidates for cysteine S-nitrosylation (Lindermayr et al. 2005). In animals, NO was shown to regulate by S-nitrosylation signaling-related proteins including soluble guanylate cyclase, the GTP-binding protein p21ras, Ca2+ permeable channels, and protein kinases (for review see Courtois et al. 2008, and literature cited). Already a decade before, Stamler et al. (1997) had suggested a general “nitrosylation motiv” consisting of three or four basic or acidic amino acids surrounding the regulatory cysteine, which would permit an acid-base-autocatalyzed S-nitrosylation and denitrosylation. In general, the actual nitrosylating agent appears to be the nitrosonium cation NO+, and hence S-nitrosylation would require an electron acceptor.
Glutathion in its reduced form is major cellular antioxidant. It reacts readily with NO to form the acid-stable S-nitrosoglutathione (GSNO), which may act as a NO donor to other cellular thiols. Such transnitrosation would include transfer of NO+ to another reduced thiol (Dutton et al. 2005), or RSNO may be homolytically cleaved to release free NO and disulfide (Singh et al. 1996). GSNO can be metabolized by S-nitrosoglutathione reductase (GSNOR), yielding, e.g. GSSG, hydroxylamine, and NH3 (Jensen et al. 1998). Hydroxylamine can be oxidized back to NO, probably involving ROS (Rümer et al. 2009). The relevance of GSNOR and GSNO levels for stress tolerance was recently demonstrated. Transgenic plants Arabidopsis with decreased GSNOR levels showed enhanced resistance against Peronospora parasitica correlated with higher intracellular GSNO levels (Rustérucci et al. 2007). The Arabidopsis HOT5 encoding a mutated GSNOR was unable to acquire thermotolerance and also had other important developmental defects (Lee et al. 2008).
NO also induces complex changes in the expression of many genes involved, e.g. in defense and cell death, transport, basic metabolism, and ROS production or degradation. Here again, S-nitrosylation of proteins acting as transcription factors might be the way for transcriptional control by NO. Seven families of transcription factor binding sites, among them WRKY-, GBOX-, and OCSE-elements, have been identified, which are preferentially located in the promoter regions of NO regulated genes, and co-expression of many genes can be explained by the cooperation of a set of such transcription factors (Palmieri et al. 2008).
As NO may be too short lived to diffuse via longer distances within tissues or even within single cells, it has been suggested that NO production (preferentially by NOS) and NO reception may be organized within supra molecular structures in which NO signaling occurs within highly localized environments and with minimal diffusion of free NO (Kone et al. 2003). Although this is an attractive idea, today there is no experimental evidence in context with NO that such supra molecular structures would exist and function in plants.
NO-regulated reactions in plants. The list of physiological processes in plants that are (probably) regulated by NO includes the induction of the hypersensitive response in resistance to incompatible pathogens, ABA-induced stomatal closure, seed germination and breakage of seed dormancy, iron homeostasis, flowering induction, and response to abiotic stresses such as drought, UV-B, salinity, chilling, or high temperatures (for recent reviews see Hong et al. 2008; Courtois et al. 2008; Neill et al. 2008). In spite of these many putative NO-regulated processes, today only few plant enzymes have been proven experimentally to be regulated by S-nitrosylation, hemoglobin 1, GAP-dehydrogenase, S-adenosyl synthethase, metacaspase, and potassium channels in guard cells being among them (summarized by Palmieri et al. 2008).
As mentioned, “regulatory” NO is either stemming from a NOS-like reaction or from nitrite to NO reduction. Involvement of nitrate metabolism in production of regulatory NO has been evidenced in a few cases only. For example, ABA-induced stomatal closure in Arabidopsis is impaired in the nia double mutant. Tungstate, which prevents synthesis of functional NR, also inhibited stomatal closure, whereas nitrite addition induced stomatal closure (Bright et al. 2006; Neill et al. 2008, and literature cited). Similarily, Chitosan-induced stomatal closure in Pisum sativum, which may prohibit easy entry of pathogens into the leaf, was impaired by tungstate treatment, which would again suggest some role for nitrite-dependent NO (together with NOS-derived NO) (Srivastava et al. 2009). ABA-induced stomatal closure was also reduced in a nia1::DS deletion mutant, indicating that only NIA1, but not NIA2 was required for effective ABA signal transduction (Bright et al. 2006). This is surprising, as NIA1 is thought to contribute only about 10% to total nitrite production (Wilkinson and Crawford 1991), and because a specific response to NIA1 would require a mechanism by which cells can distinguish between nitrite and NO derived from one or the other protein.
Another connection between nitrate reduction, NO production, and a physiological response seems to exist for the induction of the HR in Arabidopsis by incompatible strains of Pseudomonas syringae. Here, the HR was impaired in the nia1nia2 mutant compared to WT, and was restored by addition of nitrite (Modolo et al. 2006). However, the nia mutants had significantly lower arginine contents compared to WT, which might limit their NOS activity. Thus, it appeared possible that this was an indirect response to the low arginine and not directly related to the lack of nitrite.
Recently it was suggested that NO produced from nitrite would enhance NR activity in roots of Brassica chinensis L, thereby forming a positive feedback loop. The conclusion was based on the observation that treatment of roots with NO gas, NO donors, or NO scavengers modified extractable NR activity in the roots. In addition, treatment of purified NR or of NR in root extracts of tomato with NO in vitro also increased NR activity (Du et al. 2008; Jin et al. 2009), suggesting a direct interaction of NO with NR. It is not known yet in detail how NO modifies NR, i.e. whether NO interacts with heme-iron of the cytochrome domain or whether it forms a nitrosothiol. One consequence appears to be an increase in V max, of all partial reactions of NR.
7 Conclusion
Plants use a multitude of N forms, and their uptake, transport in the plant, and assimilation are taken care of by numerous transporters and enzymes. Their quantity, localization, and the regulation of their activity enable plants to adapt quickly and finely their N acquisition and utilization strategies to developmental and environmental changes. The availability of full genome sequences, in addition to new tools and resources for functional genomics, allows the use of systems biology in the last decade to give an entire view of this important metabolic pathway in plants. Still some effort is needed to reach a virtual plant. The in planta function of many of the proteins is still to discover and the actors implicated in the regulation on mRNA and protein levels are just about to emerge. The N metabolite NO is implicated in many regulatory processes, but its synthesis pathways and their control, as well as the exact mode of interaction of NO with multiple targets, still need to be elucidated.
References
Alboresi A, Gestin C, Leydecker MT, Bedu M, Meyer C, Truong HN (2005) Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ 28:500–512
Almagro A, Lin SH, Tsay YF (2008) Chrarcterization of the Arabidopsis nitrate transporterNRT1.6 reveals a role of nitrate in early embryo development. Plant Cell 20:3289–3299
Bachmann M, Shiraishi N, Campbell WH, Yoo BC, Harmon AC, Huber SC (1996) Identification of ser-543 as the major regulatory phosphorylation site in spinach leaf nitrate reductase. Plant Cell 8:505–517
Benschop JJ, Mohammed S, O’Flaherty M, Heck AJR, Slijiper M, Menke FL (2007) Quantitative phosphoproteomics of early elicitor signalling in Arabidopsis. Mol Cell Proteomics 65:1198–1214
Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Bright J, Desikan R, Hancock JT, Weir IS, Neill JS (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Brouquisse R, Masclaux C, Feller U, Raymond P (2001) Protein hydrolysis and nitrogen remobilization in plant life. In: Lea P, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin, pp 275–294
Camargo A, Llamas A, Scnell RA, Higuera JJ, Gonzales-Ballester D, Lefebvre PA, Fernandez E, Galvan A (2007) Nitrate signalling by the regulatory gene NIT2 in Chlamydomonas. Plant Cell 19:3491–3503
Castaings L, Camargo A, Pocholle D, Gaudon V, Texier Y, Boutet-Mercey S, Taconnat L, Renou JP, Daniel-Vedele F, Fernandez E, Meyer C, Krapp A (2009) The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. Plant J 57:426–435
Catoni E, Desimone M, Hilpert M, Wipf D, Kunzae R, Schneider A, Flugge UI, Schumacher K, Frommer WB (2003) Expression pattern of a nuclear encoded mitochondrial arginine-ornithine translocator gene from Arabidopsis. BMC Plant Biol 3:1
Cerezo M, Tillard P, Filleur S, Munos S, Daniel-Vedele F, Gojon A (2001) Major alterations of the regulation of root NO -3 uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol 127:262–271
Chen J, Wang X (1995) Existence and characteristics of nitrate reductase in plamam membrane of maize roots. Sci China Ser B Chem Life Sci Earth Sci 38:564–572
Chiu C, Lin CS, Hsia AP, Su RC, Lin HL, Tsay YF (2004) Mutation of a nitrate transporter, AtNRT1:4, results in a reduced petiole nitrate content and altered leaf development. Plant Cell Physiol 45:1139–1148
Chopin F, Orsel M, Dorbe MF, Chardon F, Truong HN, Miller AJ, Krapp A, Daniel-Vedele F (2007) The Arabidopsis ATNRT2.7 nitrate transporter controls nitrate content in seeds. Plant Cell 19:1590–1602
Couturier J, Montanini B, Martin F, Brun A, Blaudez D, Chalot M (2007) The expanded family of ammonium transporters in the perennial poplar plant. New Phytol 174:137–150
Corruzzi G M (2003) Primary N-assimilation into aminoacid in Arabidopsis. I. The Arabidopsis book, Rockville, MD: American Society of Plant Biologists. doi: 10.1199/tab.0111, http://www.aspb.org/publications/arabidopsis/
Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A, Wendehenne D (2008) Nitric oxide signaling in plants: interplays with Ca2+ and proteinkinases. J Exp Bot 59:155–164
Crawford NM, Arst HN Jr (1993) The molecular genetics of nitrate assimilation in fungi and plants. Annu Rev Genet 27:115–146
Daniel-Vedele F, Filleur S, Caboche M (1998) Nitrate transport: a key step in nitrate assimilation. Curr Opin Plant Biol 1:235–239
De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442:939–943
De Cires A, De la Torre A, Delgado B, Lara C (1993) Role of light and CO2 fixation in the control of nitrate-reductase activity in barley leaves. Planta 190:277–283
Dehlon P, Gojon A, Tillard P, Passama L (1996) Diurnal regulation of NO3- uptake in soybean plants. 4. Dependence on current photosynthesis and sugar availability to the roots. J Exp Bot 47:893–900
del Rio LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65:783–792
Dordas C, Hasinoff BB, Igamberdiev AU, Manac’h N, Rivoal J, Hill RD (2003) Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa under hypoxic stress. Plant J 35:763–770
Douglas P, Pigaglio E, Ferrer A, Halford NG, MacKintosh C (1997) Three spinach leaf nitrate reductase-3-hydroxy-3-methylglutaryl-CoA reductase kinases that are regulated by reversible phosphorylation and/or Ca2+ ions. Biochem J 325:101–109
Du S, Zhang Y, Lin X, Wang Y, Tang C (2008) Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ 31:195–204
Dutton AS, Fukuto JM, Houk KN (2005) Quantum mechanical determinations of reaction mechanisms, acid base, and redox properties of nitrogen oxides and their donors. In: Packer L, Cadenas E (eds) Methods in enzymology, 396th edn. Elsevier, Amsterdam, pp 26–44
Eun-Yeong B, Zdebik AA, Jentsch TJ (2009) Residues important for nitrate/proton coupling in plant and mammalian CLC transporters. J Biol Chem. doi:10.1074/jbc.M901170200
Feller U, Keist M (1986) Senescence and nitrogen metabolism in annual plants. In: Lambers H, Neetson JJ, Stulen I (eds) Fundamental, ecological and agricultural aspects of nitrogen metabolism. Martinus Nijhoff Publishers Dordrecht, the Netherlands, pp 219–234
Ferrario-Méry S, Boutet M, Leleu O, Savino G, Hodges M, Meyer C (2005) Physiological characterization of Arabidopsis mutants affected in the expression of the putative regulatory protein PII. Planta 223:28–39
Ferrario-Méry S, Meyer C, Hodges M (2008) Chloroplast nitrite uptake is enhanced in Arabidopsis PII mutants. FEBS Lett 582:1061–1066
Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F (2001) An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett 489:220–224
Forde BG (2000) Nitrate transporters in plants: structure, function and regulation. Biochim Biophys Acta Biomembr 1465:219–235
Fraisier V, Gojon A, Tillard P, Daniel-Vedele F (2000) Constitutive expression of a putative high-affinity nitrate transporter in Nicotiana plumbaginifolia: evidence for post-transcriptional regulation by a reduced nitrogen source. Plant J 23:489–496
Gallais A, Coque M, Quilléré I, Le Gouis J, Prioul JL, Hirel B (2007) Estimating proportions of N remobilization and of post-silking N uptake allocated to maize kernels by 15N labelling. Crop Sci 47:685–691
Gastal F, Saugier B (1989) Relationship between nitrogen uptake and carbon assimilation in whole plant of tall fescue. Plant Cell Environ 12:407–416
Gansel X, Munos S, Tillard P, Gojon A (2001) Differential regulation of the NO3- and NH4+ trasnporter genes AtNRT2.1 and AtAMT1.1 in Arabidopsis: relation with long distance and local controls by N status of the plant. Plant J 26:143–155
Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB, von Wirén N (1999) Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 11:937–947
Geelen D, Lurin C, Bouchez D, Frachisse JM, Lelievre F, Courtial B, Barbier-Brygoo H, Maurel C (2000) Disruption of putative anion channel gene AtCLC-a in Arabidopsis suggests a role in the regulation of nitrate content. Plant J 21:259–269
Geiger M, Walch-Liu P, Engels C, Harnecker J, Schulze ED, Ludewig SU, Scheible WR, Stitt M (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21:253–268
Gifford LM, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD (2008) Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA 105:803–808
Gordon T, Lea PJ, Rosenberg C, Trinchnat JC (2001) Nodule formation and function. In: Lea P, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin, pp 101–146
Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56:2601–2609
Guo FQ, Wang R, Chen M, Crawford NM (2001) The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell 13:1761–17677
Herrera-Rodriguez MB, Maldonado JM, Perez-Vicente R (2006) Role of asparagine and asparagine synthetase genes in sunflower (Helianthus annuus) germination and natural senescence. J Plant Physiol 163:11061–11070
Hirel B, Lea PJ (2001) Ammonia assimilation. In: Lea P, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin, pp 79–100
Hirner A, Ladwig F, Stransky H, Okumoto S, Keinath M, Harms A, Frommer WB, Koch W (2006) Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 18:1931–1934
Hong JK, Yun BW, Kang J-G, Raja MU, Kwon E, Sorhagen K, Chu C, Wang Y, Loake GJ (2008) Nitric oxide function and signaling in plant disease resistance. J Exp Bot 59:147–154
Hoyos ME, Palmieri L, Wertin T, Arrigoni R, Polacco JC, Palmieri F (2003) Identification of a mitochondrial transporter for basic amino acids in Arabidopsis thaliana by functional reconstitution into liposomes and complementation in yeast. Plant J 33:1027–1035
Hu HC, Wang YY, Tsay YF (2009) AtCIPK8, a CBL-interating protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J 57:264–278
Huang NC, Chiang CS, Crawford NM, Tsay YF (1996) CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. Plant Cell 8:2183–2191
Huang NC, Liu KH, Lo HJ, Tsay YF (1999) Cloning and functionnal characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of a low-affiniy uptake. Plant Cell 11:1381–1392
Ikeda Y, Koizumi N, Kusano T, Sano H (2000) Specific binding of a 14–13–3 protein to autophosphorylateld WPK4, and SNF1-related wheat protein kinase, and to WPK4-phosphorylated nitrate reductase. J Biol Chem 275:31695–31700
Jensen DE, Belka GK, Du Bois GC (1998) S-nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem J 331:659–668
Jin CW, Du ST, Zhang YS, Lin XY, Tang CX (2009) Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomata (Solanum lycocarpum). Ann Bot. DOI:10.1093/mcp087
Jonassen EM, Lea US, Lillo C (2008) HY5 and HYH are positive regulators of nitrate reductase in seedlings and rosette stage plants. Planta 227:559–564
Kaiser WM, Huber SC (1997) Correlation between apparent activation state of nitrate reductase (NR), NR hysteresis and degradation of NR protein. J Exp Bot 48:1367–1374
Kaiser WM, Huber SC (2001) Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot 52:1981–1989
Karley AJ, Leigh RA, Sanders D (2000) Differential ion accumulation and ion fluxes in the mesophyll and epidermis of barley. Plant Physiol 122:835–844
Kojima S, Bohner A, von Wirèn N (2006) Molecular mechanisms of urea transport in plants. J Membr Biol 212:83–91
Kojima S, Bohner A, Gassert B, Yuan L, von Wirén N (2007) AtDUR3 represents the major transporter for high-affinity urea transport across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J 52:30–40
Kone BC, Kuncewicz T, Zhang W, Yu ZY (2003) Protein interactions with nitric oxide synthases: controlling the right time, the right place and the right amount of nitric oxide. Am J Renal Physiol 285:178–190
Krouk G, Tillard P, Gojon A (2006) Regulation of the high-affinity NO3– uptake system by the NRT1.1 mediated NO3– demand signalling in Arabidopsis. Plant Physiol 142:1075–1086
Lea PJ, Miflin BJ (2004) Glutamate synthase and synthesis of glutamate in plants. Plant Physiol Biochem 41:555–564
Lee RB, Ratcliffe RG (1991) Observations on the subcellular distribution of the ammonium ion in maize root tissue using in-vivo 14N-nuclear magnetic resonnance spectroscopy. Planta 183:359–367
Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW (2007a) Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19:731–749
Lee YH, Foster J, Chen J, Voll LM, Weber APM, Tegeder M (2007b) AAP1transports uncharged amino acids into roots of Arabidopsis. Plant J 50:305–319
Lee U, Wie C, Fernandez BO, Feelisch M, Vierling E (2008) Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. The Plant Cell 20:786–802
Leegood RC, Lea PJ, Hauser RE (1996) Use of barley mutants to study the control of photorespiratory metabolism. Biochem Soc Trans 24:757–761
Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW (1995) Subcellular volumes and metabolites concentrations in potato (Solanum tuberosum cv Désirée) leaves. Botanica Acta 108:403–406
Lejay L, Tillard P, Lepetit M, Olive F, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two NO −3 uptake systems by N- and C-status of Arabidopsis plants. Plant J 18:509–519
Lejay L, Gansel X, Cerezo M, Tillard P, Muller C, Krapp A, von Wirén N, Daniel-Vedele F, Gojon A (2003) Regulation of root ion transporters by photosynthesis: functional importance and relation with hexokinase. Plant Cell 15:2218–2232
Lejay L, Wirth J, Pervent M, Cross JM, Tillard P, Gojon A (2008) Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol 146:2036–2053
Lemaire SD, Michelet L, Zaffagnini M, Massot V, Issakidis-Bourguet E (2007) Thioredoxins in chloroplasts. Curr Genet 51:343–365
Li W, Wang Y, Okamoto M, Crawford N, Siddiqi MY, Glass ADM (2007) Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol 143:425–433
Lichter A, Häberlein I (1998) A light-dependent redox signal participates in the regulation of ammonia fixation in chloroplasts of higher plants – ferredoxin: glutamate synthase is a thioredoxin dependent enzyme. J Plant Physiol 153:83–90
Lillo C (2009) Signalling cascades integrating light-enhanced nitrate metabolism. Biochem J 415:11–19
Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, Hsu PK, Tillard P, Lin HL, Wang YY, Tsai CB, Gojon A, Tsay YF (2008) Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20:2514–2528
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Liu KH, Huang CY, Tsay YF (1999) CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11:865–874
Liu KH, Tsay YF (2003) Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J 22:1005–1013
Liu LH, Ludewig U, Frommer WB, von Wirén N (2003a) AtDUR3 encodes a new type of high-affinity urea/H+ symporter in Arabidopsis. Plant Cell 15:790–800
Liu LH, Ludewig U, Gassert B, Frommer WB, von Wirén N (2003b) Urea transport by nitrogen-regulated tonoplast intrinsic proteins in Arabidopsis. Plant Physiol 133:1220–1228
Loqué D, von Wirén N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55:1293–1305
Loqué D, Ludewig U, Yuan L, von Wirén N (2005) Tonoplast intrinsic proteins AtTIP2;1and AtTIP2;3 facilitate NH3 transport of ammonium in plant roots. Plant Phys 137:671–680
Loqué D, Yuan L, Kojima S, Gojon A, Wirth J, Gazzarini S, Ishiyama K, Takahashi H, von Wirèn N (2006) Additive contribution of AMT1;1 and AMT1;3 to high affinity ammonium uptake across the plasma membrane of nitrogen deficient Arabidopsis roots. Plant J 48:522–534
Loqué D, Lalonde S, Looger LL, von Wiren N, Frommer WB (2007) A cytosolic trans-activation domain is essential for ammonium uptake. Nature 446:195–198
Loudet O, Chaillou S, Merigout P, Talbotec J, Daniel-Vedele F (2003) Quantitative trait loci analysis of nitrogen use efficiency in Arabidopsis. Plant Physiol 131:345–358
MacKintosh C (1992) Regulation of spinach-leaf nitrate reductase by reversible phosphorylation. Biochim Biophys Acta 1137:121–126
McMichael RW Jr, Bachmann M, Huber SC (1995) Spinach leaf sucrose-phosphate synthase and nitrate reductase are phosphorylated/inactivated by multiple protein kinases in vitro. Plant Physiol 108:1077–1082
Marmagne A, Vinauger-Douard M, Monachello D, Falcon de Longevialle A, Charon C, Allot M, Rappaport F, Wollman F, Barbier-Brygoo H, Ephritikhine G (2007) Two members of the Arabidopsis CLC (Chloride Channels) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes respectively. J Exp Bot 12:3385–3393
Martinoia E, Massonneau A, Frangne N (2000) Transport processes of solutes across the vacuolar membrane of higher plants. Plant Cell Phsyiol 41:1175–1186
Martin F, Cliquet JB, Stewart G (2001) Nitrogen acquisition and assimilation in mycorrhizal symbioses. In: Lea P, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin, pp 147–166
Masclaux-Daubresse C, Reisdorf-Cren M, Pageau K, Lelandais M, Grandjean O, Kronenberger J, Valadier MH, Feraud M, Jouglet T, Suzuki A (2006) Glutamine synthetase-glutamate synthase pathway and glutamate dehydrogenase play distinct roles in the sink-source nitrogen cycle in tobacco. Plant Physiol 140:444–456
Masclaux-Daubresse C, Reisdorf-Cren M, Orsel M (2008) Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol 10:23–36
Matsson M, Schjoerring JK (2003) Senescence-induced changes in apoplastic and bulk tissue ammonia concentrations of ryegrass leaves. New Phytol 160:489–499
Matt P, Geiger M, Walch-Liu P, Engels C, Stitt M (2001) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ 24:1119–1137
Merigout P, Gaudon V, Quilleré I, Briand X, Daniel-Vedele F (2008a) Urea use efficiency of hydroponically grown maize and wheat plants. J Plant Nutr 31:427–443
Merigout P, Lelandais M, Bitton F, Renou JP, Briand X, Meyer C, Daniel-Vedele F (2008b) Physiological and transcriptomic aspects of urea utptake and assimilation in Arabidopsis plants. Plant physiol 147:1225–1238
Mesnard F, Ratcliffe RG (2005) NMR analysis of plant nitrogen metabolism. New phytol 83:163–180
Meyer C, Stitt M (2001) Nitrate reduction and signalling. In: Lea P, Morot-Gaudry JF (eds) Plant nitrogen. Springer, Berlin, pp 37–59
Meyer C, Stöhr C (2002) Soluble and plasma membrane-bound enzymes involved in nitrate and nitrite metabolism. In: Foyer C, Noctor G (eds) Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism. Kluwer Academic, Dordrecht, pp 49–62
Miller AJ, Cookson SJ, Smith SJ, Wells DM (2001) The use of microelectrodes to investigate compartmentation and the transport of metabolized inorganic ions in plants. J Exp Bot 52:541–549
Miller AJ, Smith SJ (2008) Cytosolic nitrate ion homeostasis: could it have a role in sensing nitrogen status? Annals Bot 101:485–489
Modolo LV, Augusto O, Almeida IMG, Pinto-Maglio CAF, Oliveira HC, Seligman K, Salgado I (2006) Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant Sci 171:34–40
Monachello D, Allot M, Oliva S, Krapp A, Daniel-Vedele F, Barbier-Brygoo H, Ephritikine G (2009) The txo anions transporters AtCLCa and AtCLCe fulfil interconnecting but not redundant roles in nitrate assimilation pathways. New Phytol, in press
Moorhead G, Douglas P, Morrice N, Scarabel M, Aitken A, MacKintosh C (1996) Phosphorylated nitrate reductase from spinach leaves is inhibited by 14–3–3 proteins and activated by fusicoccin. Curr Biol 6:1104–1113
Nazoa P, Videmar J, Tranbarger TJ, Mouline K, Damiani I, Tillard P, Glass ADM, Touraine B (2003) Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: responses to nitrate, amino acids and developmental stage. Plant Mol Biol 52:689–703
Näsholm T, Kielland K, Ganeteg U (2009) Uptake of organic nitrogen by plants. New Phytol 182:31–48
Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, Morris P, Ribeiro D, Wilson I (2008) Nitric oxide, stomatal closure and abiotic stress. J Exp Bot 59:165–176
Neuhäuser B, Dynowski M, Mayer M, Ludewig U (2007) Regulation of NH4+ transport by essential cross talk between AMT monomers through carboxy tails. Plant Phys 143:1651–1659
Ninnemann O, Jauniaux J, Frommer WB (1994) Identification of a high affinity NH +4 transporter from plants. EMBO J 13:3464–3471
Nussaume L, Vincentz M, Meyer C, Boutin JP, Caboche M (1995) Posttranscriptional regulation of nitrate reductase by light is abolished by an N-terminal deletion. Plant Cell 7:611–621
Okamoto M, Vidmar JJ, Glass ADM (2003) Regulation of NRT1 and NRT2 Gene Families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol 44:304–317
Ono F, Frommer WB, von Wirén N (2000) Coordinated diurnal regulation of low-and high-affinity nitrate transporters in tomato. Plant Biol 2:17–23
Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F (2004) Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219:714–721
Orsel M, Chopin F, Leleu O, Smith SJ, Krapp A, Daniel-Vedele F, Miller AJ (2006) Characterization of a two-component high-affinity nitrate uptake system in Arabidopsis: physiology and protein-protein interaction. Plant Physiol 142:1304–1317
Ortiz-Lopez A, Chang HC, Bush DR (2000) Amino acid transporters in plants. Biochem Biophys Acta Biomembr 1465:275–280
Pageau K, Reisdorf-Cren M, Morot-Gaudry JF, Masclaux-Daubresse C (2006) The two senescence-related markers, GS1 (cytosolic glutamine synthetase) and GDH (glutamate dehydrogenase), involved in nitrogen mobilization, are differentially regulated during pathogen attack and by stress hormones and reactive oxygen species in Nicotiana tabacum L. leaves. J Exp Bot 57:547–557
Palmieri MC, Sell S, Hunag X, Scherf M, Werner T, Durner J, Lindermayr C (2008) Nitric-oxide responsive genes and promoters in Arabidopsis thaliana: a bioinformatics approach. J Exp Bot 59:177–186
Patrick JW, Offler CE (2001) Compartmentation of transport and transfer events in developing seeds. J Exp Bot 52:551–564
Pelsy F, Caboche M (1992) Molecular genetics of nitrate reductase in higher plants. In: Scandalios JG, Wright TRF (eds) Advances in genetics, Academic Press Inc, San Diego, CA, pp 1–40
Planchet E, Gupta KJ, Sonoda M, Kaiser WM (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41:732–743
Rajasekhar VK, Gowri G, Campbell WH (1988) Phytochrome-mediated light regulation of nitrate reductase expression in squash cotyledons. Plant Physiol 88:242–244
Remans T, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A (2006) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA 103:19206–19211
Renné P, Dressen U, Hebbeker U, Hille D, Flugge UI, Westhoff P, Weber APM (2003) The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant J 35:316–331
Rentsch D, Schmidt S, Tegeder M (2007) Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett 581:2281–2289
Richard-Molard C, Krapp A, Brun F, Ney B, Daniel-Vedele F, Chaillou S (2008) Plant response to nitrate starvation is determined by N storage capacity matched by nitrate uptake capacity in two Arabidopsis genotypes. J Exp Bot 59:779–791
Rochat C, Boutin J-P (1991) Metabolism of phloem-borne amino acids in maternal tissues of fruit of nodulated or nitrate-fed pea plants. J Exp Bot 42:207–214
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production in vivo and in vitro. J Exp Bot 53:103–110
Rümer S, Kapuganti JG, Kaiser WM (2009) Plants cells oxidize hydroxylamines to NO. J Exp Bot 60:2065–2072
Rustérucci C, Espunya MC, Díaz M, Chabannes M, Martínez CM (2007) S-nitrosoglutathione reductase affords protection against pathogens in Arabidopsis, both locally and systemically. Plant Physiol 143:1282–1292
Schröppel-Maier G, Kaiser WM (1988) Ion homeostasis in chloroplasts under salinity and mineral deficiency. Plant Physiol 87:828–832
Schumaker KS, Sze H (1987) Decrease of pH gradients in tnonplast vesicles by NO –3 and Cl-: evidence for H+-coupled anion transport. Plant Physiol 83:7490–7496
Scheible WR, Gonzalez-Fontes A, Morcuende R, Lauerer M, Geiger M, Glaab MJ, Gojon A, Schulze ED, Stitt M (1997) Tobacco mutants with a decreased number of functional NIA genes compensate by modifying the diurnal regulation of transcription, post-translational modification and turnover of nitrate reductase. Planta 203:304–319
Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Placios-Rojas N, Schindelasch D, Thimm O, Udvardi K, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism; protein synthesis, cellular growth processes and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Sherameti I, Sopory SK, Trebicka A, Pfannschmidt T, Oelmuller R (2002) Photosynthetic electron transport determines nitrate reductase gene expression and activity in higher plants. J Biol Chem 277:46594–46600
Segonzac C, Boyer JC, Ipotesi E, Szponarski W, Tillard P, Touraine B, Sommerer N, Rossignol M, Gibrat R (2007) Nitrate efflux at the root plasmam membrane: identification of an Arabidopsis excretion transporter. Plant Cell 19:3760–3777
Singh RJ, Hogg N, Joseph J, Kalyanraman B (1996) Mechanism of nitric oxide release from S-nitrosothiols. J Biol Chem 271:18596–18603
Sohlenkamp C, Shelden M, Howitt S, Udvardi M (2000) Characterization of Arabidopsis AtAMT2, a novel ammonium transporter in plants. FEBS Lett 467:273–278
Solomonson LP, Barber MJ (1990) Assimilatory nitrate reductase: functionel properties and regulation. Ann Rev Plant Physiol Plant Mol Biol 41:225–253
Sonoda Y, Ikeda A, Saiki S, von Wirén N, Yamaya T, Yamaguchi J (2003) Distinct expression and function of three ammonium transporter genes (OsAMT1;1–1;3) in rice. Plant Cell Physiol 44:726–734
Srivastava N, Gonugunta VK, Puli MR, Raghavendra AS (2009) Nitric oxide production occurs downstream of reactive oxygen species in guard cells during stomatal closure induced by chitosan in abaxial epidermis of Pisum sativum. Planta 229:757–765
Stacey MG, Koh S, Becker J, Stacey G (2002) AtOPT3, a member of the oligopeptide transporter family, is essential for embryo development in Arabidopsis. Plant Cell 14:2799–2811
Stamler JS, Toone EJ, Lipton SA, Sucher NJ (1997) (S)NO signals: translocation, regulation, and a consensus motif. Neuron 18:691–696
Stöhr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57:463–470
Sugden C, Donaghy PG, Halford NG, Hardie DG (1999) Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3- methylglutaryl Coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol 120:257–274
Sugiura M, Georgescu MN, Takahashi M (2007) A nitrite transporter associated with nitrite uptake by higher plants chloroplasts. Plant Cell Physiol 48:1022–1035
Suzuki A, Knaff DB (2005) Glutamate synthase: strucutral, mechanistic and regulatory properties, and role in the amino acid metabolism. Photosynth Res 83:191–217
Svennerstam H, Ganeteg U, Bellini C, Näsholm T (2007) Comprehensive screening of Arabidopsis mutants suggests the lysine histidine transporter 1 to be involved in plant uptake of amino acids. Plant Physiol 143:1853–1860
Svennerstam H, Ganeteg U, Näsholm T (2008) Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease. New Phytol 180:620–630
Tischner R (2000) Nitrate uptake and reduction in higher and lower plants. Plant Cell Environ 23:1005–1024
Tsay YF, Schroeder JI, Feldmann KA, Crawford NM (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72:705–713
Tsay YF, Chiu CC, Tsai CB, Ho CH, Hsu PK (2007) Nitrate transporters and peptide transporters. FEBS Lett 58:2290–2300
van der Leij M, Smith SJ, Miller AJ (1998) Remobilisation of vacuolar stored nitrate in barley root cells. Planta 205:64–72
Vanin AF, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryers MJ, Baker NR, Coopers CE (2004) Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. J Biol Chem 279:24100–24107
Vincentz M, Moureaux T, Leydecker MT, Vaucheret H, Caboche M (1993) Regulation of nitrate and nitrite reductase expression in Nicotiana plumbaginifolia leaves by nitrogen and carbon metabolites. Plant J 3:315–324
von Wirén N, Lauter FR, Ninnemann O, Gillissen B, Walch-Liu P, Engels C, Jost W, Frommer WB (2000) Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato. Plant J 21:167–175
Walch-Liu P, Filleur S, Gan YB, Forde BG (2005) Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosynth Res 83:239–250
Walch-Liu P, Forde BG (2008) Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-Glutamate-induced changes in root architecture. Plant J 54:820–828
Wang R, Liu D, Crawford NM (1998) The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA 95:15134–15139
Wang R, Okamoto M, Xing X, Crawford NM (2003) Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol 132:556–567
Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, Coruzzi G, Crawford NM (2004) Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol 136:2512–2522
Wang R, Xing X, Crawford N (2007) Nitrite acts as a transcriptome signal at micromolar concentrations in Arabidopsis roots. Plant Physiol 145:1735–1745
Ward MR, Grimes HD, Huffaker RC (1989) Latent nitrate reductase-activity associated with the plasma membrane of corn roots. Planta 177:470–475
Weber A, Schwacke R, Flügge UI (2005) Solute transporters of the plastid envelope membrane. Ann Rev Plant Biol 56:133–164
Weiner H, Kaiser WM (1999) 14–3–3 proteins control proteolysis of nitrate reductase in spinach leaves. FEBS Lett 455:75–78
Wells DM, Miller AJ (2000) Intracellular measurements of ammonium in Chara corallina using ion-selective microelectrodes. Plant Soil 221:103–106
Wilkinson J, Crawford NM (1991) Identification of the Arabidopsis CHL3 gene as the nitrate reductase structural gene NIA2. Plant Cell 3:461–471
Williams LE, Miller AJ (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Ann Rev Plant Physiol Plant Mol Biol 52:659–688
Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191:180–190
Winter H, Robinson DG, Heldt HW (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193:530–535
Wirth J, Chopin F, Santoni V, Viennois G, Tillard P, Krapp A, Lejay L, Daniel-Vedele F, Gojon A (2007) Regulation of root nitrate uptake at the NRT2.1 protein level in Arabidopsis thaliana. J Biol Chem 282:23541–23552
Wood CC, Poree F, Dreyer I, Koelher GJ, Udvardi MK (2006) Mechanisms of ammonium transport, accumulation and retention in oocytes and yeast cells expressing Arabidopsis AtAMT1;1. FEBS Lett 580:3931–3936
Yuan L, Loqué D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wirén N (2007) The organization of high-affinity ammonium uptake in Arabidopsis roots depend on the spatial arrangement and biochemical properties of AmMT1-type transporters. Plant Cell 19:2636–2652
Zhuo D, Okamoto M, Vidmar JJ, Glass ADM (1999) Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J 17:63–568
Zifarelli G, Pusch M (2009) Conversion of the 2 Cl−/1H+ antiporter CLC-5 in a NO −3 /H+ antiporter by a single point mutation. EMBO J 28:175–182
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Daniel-Vedele, F., Krapp, A., Kaiser, W.M. (2010). Cellular Biology of Nitrogen Metabolism and Signaling. In: Hell, R., Mendel, RR. (eds) Cell Biology of Metals and Nutrients. Plant Cell Monographs, vol 17. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-10613-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-10613-2_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-10612-5
Online ISBN: 978-3-642-10613-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)