Abstract
In this chapter, we discuss the metabolic changes relevant for the production of reactive oxygen and nitrogen species (ROS and RNS) in plant tissues during oxygen deprivation. It is notable too, that at times the oxidative damage does not take place during the oxygen deficiency period but only after the restoration of normal oxygen supply to the tissues. This is mainly due to the fact that ROS may be formed immediately after oxygen re-enters the tissues. The level of oxygen in the tissues naturally depends on the outside concentration and diffusion rate but is also under metabolic control. Two hypotheses on the regulation of internal O2 concentration in the cells through the control of respiration rely on a regulation of glycolytic pathway and pyruvate availability and mitochondrial electron transport chain by NO and ROS balance. Both adaptive strategies aim to decrease the respiratory capacity and to postpone complete anoxia. This chapter also describes the interaction of the many antioxidants found in plant tissues and oxidative stress and oxidative damage caused by waterlogging and oxygen deprivation. If the antioxidative protection is still capable of detoxifying the ROS formed, damage is minimal, but if not, then considerable damage can take place very suddenly. Many of the ROS and RNS species act as signalling agents acting in the regulation of metabolic events leading to tolerance or, e.g. in the case of aerenchyma development, into programmed cell death. The balance between O2 transport to hypoxic tissues, regulatory adaptations, anoxic metabolites, ROS–RNS chemistry and signalling, determines the survival of plants under waterlogging and oxygen deprivation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Almost two decades of oxidative stress studies in plants under diverse biotic and abiotic stress factors, has yielded a concept of ROS being ubiquitous stress markers and signalling species (Bailey-Serres and Chang 2005; Miller et al. 2008; Van Breusegem et al. 2008). Oxygen deprivation stress stands somewhat apart in the universal and quite concise picture of stress responses. Intrinsic contradiction between low oxygen concentration on the one hand, and the well documented ROS production under these conditions on the other hand, has promoted studies on the biochemistry underlying ROS formation under oxygen deprivation and during reoxygenation (Yan et al. 1996; Blokhina et al. 1999; Biemelt et al. 1998; Biemelt et al. 2000; Blokhina et al. 2001, 2003; Fukao and Bailey-Serres 2004). Such biochemical adjustments are closely related to plant survival under hypoxia and inherently connected with the activation of remaining oxygen. Among the main processes relevant for occurrence of oxidative stress under hypoxia are preservation of energy (Greenway and Gibbs 2003) and regulation of the internal O2 concentration (Borisjuk et al. 2007; Zabalza et al. 2009). Moreover, during the last years a vast amount of evidence has accumulated on the regulatory role of reactive nitrogen species (RNS) in hypoxic metabolism (Qiao and Fan 2008). These metabolic adjustments have a common crosspoint – mitochondrial metabolism affected by the lack of oxygen and by hypoxic metabolites (Bailey-Serres and Voesenek 2008; Benamar et al. 2008; Igamberdiev and Hill 2009).
Multiple routes for hypoxic ATP production and consumption have been elucidated recently: pyrophosphate-dependent glycolysis (Huang et al. 2008), anaerobic nitrite-dependent ATP synthesis (Stoimenova et al. 2007), and a reverse reaction for ATP synthesis – anaerobic ATP hydrolysis (St-Pierre et al. 2000). The above-mentioned NO can exert a dual effect on cell energetics, depending on the target and localization: inhibition of key mitochondrial enzymes cytochrome oxidase (COX), aconitase and upregulation of alternative oxidase, sustaining of NADH turnover via hemoglobin-dependent reaction cascade, and termination of the lipid peroxidation (LP) cascade (discussed in more detail in the text).
Two hypotheses on the regulation of internal O2 concentration in the cells through the control of respiration rely on a regulation of glycolytic pathway and pyruvate availability (Geigenberger 2003; Zabalza et al. 2009) and mitochondrial ETC by NO and ROS balance (Borisjuk et al. 2007). Both adaptive strategies aim to decrease the respiratory capacity and to postpone complete anoxia. They also emphasize the key role of adenylate energy charge of the tissue (Bailey-Serres and Voesenek 2008). These routes are inherently connected with the mitochondria and hypoxic oxidative stress. However, several non-mitochondrial enzyme reactions are capable of producing ROS (and RNS) in a stress-dependent manner (Blokhina et al. 2003). The phenomenon of oxidative stress under hypoxia has found its affirmation in microarray studies on the whole genome response to hypoxia. Indeed, expression of a wide range of ROS- and redox-related transcripts is induced by the lack of oxygen (Klok et al. 2002; Branco-Price et al. 2005; Loreti et al. 2005; Branco-Price et al. 2008; van Dongen et al. 2009).
Elegant new methods for the determination of oxygen levels inside plant organs and tissues have given new possibilities for accurate description of the regulation of respiration very precisely. This is discussed further in Sect. 7.4.
2 Anoxia: Metabolic Events Relevant for ROS Formation
2.1 “Classic” Metabolic Changes Under Oxygen Deprivation Related to ROS Formation
Studies on morphological and metabolic adaptations to oxygen deprivation stress in plants have a long history, and are very well documented, with a number of excellent reviews available on the subject (Gibbs and Greenway 2003; Greenway and Gibbs 2003; Visser et al. 2003; Fukao and Bailey-Serres 2004; Bailey-Serres and Voesenek 2008; Jackson 2008; Sairam et al. 2008). Here we will only discuss changes in metabolic events brought about by oxygen deprivation which can potentially support the progression of oxidative stress in oxygen-limited environments and on reoxygenation.
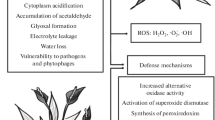
An induction of alcoholic fermentation can be observed under normoxic conditions as part of regulatory mechanism controlling O2 consumption and, hence, O2 concentration in the tissue (Zabalza et al. 2009). Controlling pyruvate availability for respiration underlies the organism’s ability to avoid complete depletion of O2 i.e. anoxia. Energy status of the tissue has been shown to be the key regulatory switch in the regulation of the pyruvate level and, hence, the inhibition of respiration rate (Zabalza et al. 2009), Fig. 7.1. Threshold ATP concentration has been also discussed as a regulatory trigger for membrane integrity, i.e. when ATP level falls below 10 μM membrane lipids are hydrolysed to FFA (Rawyler et al. 2002), the process which can have detrimental consequences for mitochondrial functioning (see Sect. 7.2.2).
Anoxia-induced metabolic changes regulating ATP level, internal O2 concentration and favourable for ROS and RNS formation. Upregulated metabolic pathways and accumulating metabolites are highlighted in red. AcAld, acetaldehyde; ETC, electron transport chain; LP, lipid peroxidation; Hb, hemoglobin; PPi, pyrophosphate. Enzymes are omitted for clarity of the picture. Decreasing availability of O2 leads to acidification of cytoplasm (pH down to 6.8) and repression of TCA cycle. Non-functional TCA cycle is unable to sustain respiration when O2 concentration is not yet limiting for COX. However, accumulating succinate can support ROS-producing reverse e− transport from mitochondrial complex II to complex I. Acidification optimizes enzymatic production of nitrite and NO and promotes the release of ferrous ions from proteins. NO facilitates this process by acting on FeS clusters of enzymes. Nitrite can serve as an e− acceptor in mitochondrial ETC, sustaining anoxic ATP synthesis. Accumulating acetaldehyde, one of the products of induced fermentation pathway, serves as a substrate for xanthine oxidoreductase, an enzyme capable for both NO and O −•2 generation. NO formed affects both negatively and positively a number of reactions: inhibitis aconitase (a TCA cycle enzyme), catalase and COX – the latter two enzymes are important for the control of ROS level. Positive effects of NO under anoxia are represented by its interaction with hypoxically-induced hemoglobin in a NADH regenerative cycle and by transcriptional upregulation of AOX. Pyrophosphate-dependent glycolysis is efficient (in terms of theoretical ATP production) in supporting energy status and relies on upregulation of cytosolic reversible PPi-dependent phosphofructokinase and pyruvate phosphate dikinase. Induced conventional (as opposite to PPi-induced) glycolytic pathway is responsible not only for ATP synthesis but also for the regulation of pyruvate available for respiration. High pyruvate stimulates respiration and facilitates the onset of anoxia. Control of pyruvate level can be achieved through pyruvate kinase and phosphoenolpuryvate carboxylase, entry of pyruvate into TCA cycle is regulated by pyruvatedehydrogenase complex. The respiration rate is adjusted according to ATP level and internal O2 concentration. ETC is the source of ROS when not fully operational. ROS and NO produced in different hypoxia-induced reactions can exert feedback regulation of respiration rate and other signalling functions, directly oxidize biological molecules. The oxidative damage by ROS is exemplified on the graph by lipid peroxidation which is induced by Fe2+ ions and also embraces NO-induced lipid modification. ATP is essential for the integrity of lipids and for prevention of FFA release
It seems that multiple mechanisms for glycolytic flux regulation exist in plants and control adenylate energy charge, O2 availability, the rate of mitochondrial respiration, and ROS formation in ETC. A switch to pyrophosphate-dependent glycolysis due to upregulation of cytosolic reversible PPi-dependent phosphofructokinase (PFK-PPi) and pyruvate phosphate dikinase (PPDK) in anoxia tolerant plants has been suggested recently as an alternative to conventional glycolysis. Theoretically five ATP vs two ATP molecules are produced in PPi-dependent and conventional glycolysis, respectively (Huang et al. 2008). The dynamic association of glycolytic enzymes with mitochondria, glycolytic support of mitochondrial respiration and regulatory substrate channelling has been shown recently (Graham et al. 2007). Moreover, since both PPi-dependent enzymes are reversible, the increased level of PPi can affect the mitochondrial energy production directly through an engagement of proton pumping inner mitochondrial membrane pyrophosphatase (Vianello and Macrì 1999). Diminished PPi level in transgenic potato expressing E. coli pyrophosphatase has resulted in decreased glycolytic activity, lower ATP levels and negatively affected overall survival of plants (Mustroph et al. 2005). In addition to its role in NO formation, nitrite accumulated under oxygen deprivation can act as an electron acceptor in ETC, providing the sink mechanism for reduced NAD(P)H and sustaining ATP synthesis comparable with that of glycolysis (Stoimenova et al. 2007). The additional ATP formed would slow down the demand for mitochondrial ETC, and the concurrent production of mitochondrial ROS.
Regulation of cytoplasmic pH is another control point for anoxic metabolism and a probable upregulation of ROS production. In mammalian models cytoplasmic acidosis accelerated the peroxidation of membrane lipids in Fe2+-dependent manner. Reduced pH favours mobilisation of ferrous ions (e.g. from ferritin) and, therefore, promotes the formation O −•2 in Haber–Weiss reaction or H2O2 in Fenton reaction (Pryor et al. 2006; Hassan et al. 2009). It has been shown that more alkaline pH (in the range of pH 7.0–8.8) stimulates ROS production via mitochondrial complex 1 (Turrens and Boveris 1980) and, hence, cytoplasmic acidification (down to pH 6.8) will render ROS formation less probable. However, acidification promotes the formation of another reactive species − NO: non-enzymatically from exogenous nitrite in plants (Bethke et al. 2004) and enzymatically via cytosolic nitrate reductase and plasma membrane-bound nitrite-NO reductase (see Sect. 7.3.2).
2.2 Changes in Lipid Composition and Role of Free Fatty Acids Under Stress
Functional integrity of membrane lipids, the degree of fatty acid unsaturation, and causes of free fatty acid (FFA) liberation and regulation of LP, are issues closely connected to ROS and RNS metabolism. The maintenance of membrane lipid integrity is an important factor in plant survival under oxygen deprivation. Anoxia-tolerant plants (i.e. rice and Echinochloa phyllopogon ) are able to synthesize lipids under strict anoxia. Incorporation of radioactive label has been detected in phospholipids, glycolypids and neutral lipids but mainly in saturated fatty acids (Generosova and Vartapetian 2005). The saturation of double bonds of esterified FA has been discussed as an adaptive property (Generosova and Vartapetian 2005). There are two trends in lipid metabolism under anoxia which are important when considering ROS chemistry: increased degree of saturation and dependence of membrane integrity on ATP levels (Chirkova et al. 1989; Pavelic et al. 2000).
Polyunsaturated fatty acids (PUFA) are the targets of lipophilic RNS and ROS (discussed below) accumulating in the membranes. Increased FA unsaturation will affect the progression of both LP and nitration. These oxidative reactions can result in a number of molecular species that restructure the membrane (conjugated dienes and lipid hydroperoxides) that make the membrane less ordered and more hydrophilic). Both FA unsaturation and FA–NO interaction (discussed below) negatively affect LP (O′Donnell and Freeman 2001; Blokhina et al. 2003; Freeman et al. 2008). Falling ATP concentration under anoxia has been shown to act as a threshold regulatory switch for membrane integrity: when ATP concentration decreases below 10 µM the integrity of membrane lipids is no longer preserved and they are hydrolysed to FFA (Pavelic et al. 2000).
Liberation of FFA is indicative of severe membrane damage and manifests cell death. This process also affects mitochondria: FFAs are known to uncouple oxidative phosphorylation via a protonophore mechanism: FFA− anion forms a complex with H+ and as a neutral species moves across the membrane via the “flip-flop” mechanism (Skulachev 1998; Arcisio-Miranda et al. 2009). In case of “mild” uncoupling, when the concentration of uncoupler is extremely low, the process can exert an antioxidative function by decreasing the probability of O −•2 formation in the ETC (Skulachev 1998).
In animal systems cytochromes P450 are responsible for epoxidation of non-esterified linolenic acid, and the resulting epoxide has been shown to support mitochondrial respiration under anoxia/reperfusion (Nowak et al. 2004). In plants H2O2-dependent fatty acid epoxygenase activity has been detected in broad beans. Interestingly, the products of this reaction – epoxy fatty acids – have been shown to accumulate under the pathogen attack and to exhibit antifungal activity (Hamberg and Fahlstadius 1992).
Close association between ROS, RNS and phospholipase D (PLD) under abiotic stress conditions has been shown (Testerink and Munnik 2005). Indeed, upregulated PLD and its product phosphatidic acid induced plasma membrane NADPH oxidase under excess copper stress and, hence, were considered responsible for H2O2 accumulation and LP (Yu et al. 2008). The formation of adventitious roots in cucumber – one of the main anatomic features in the adaptation to oxygen deprivation – appeared to be mediated by auxin and NO induction of PLD and a concurrent accumulation of phosphatidic acid (Lanteri et al. 2008). Among other activation targets of phosphatidic acid in plants are protein kinases and phosphoenolpuryvate carboxylase (PEPC) (Testerink and Munnik 2005). The latter enzyme catalyzes the phosphorylation of oxaloacetate to form phosphoenolpuryvate and is upregulated during flooding. PEPC is involved in the control of pyruvate level, a metabolite shown to regulate internal O2 concentration under oxygen deprivation (see above) (Zabalza et al. 2009). Increased PEPC activity has been discussed also as an adaptive mechanism for improved CO2 assimilation under flooding (Yordanova and Popova 2007).
2.3 Modification of Lipids: LP
Oxidative modification of lipids, LP, has long been recognized as the main cause of membrane destabilisation and injury under a range of stimuli in both animals and plants. LP is a result of action of ROS on PUFA in membrane phospholipids. The hydroxyl radical, OH•, is considered as the initiation species for LP due to its high reactivity. Both OH• radical and singlet oxygen 1O2 can react with methylene groups of PUFA producing several products differing in their lifetime: conjugated dienes, lipid peroxy radicals and lipid hydroperoxides (Blokhina et al. 2003). A reaction of ROS (hydroxyl radical and singlet oxygen) with methylene groups of PUFA results in the rearrangement of the double bond and the formation of conjugated dienes (two double bonds sepatrated with a single bond), lipid peroxy (L–OO•) and alkoxy (L–O•) radicals and lipid hydroperoxides (LOOH). In turn, lipid radical species propagate or initiate (branch) a new chain of peroxidative reactions in membrane lipids (Buettner 1993).
ROS and products of LP can build up under hypoxic conditions (Biemelt et al. 2000; Blokhina et al. 2001; Santosa et al. 2007). E.g. the detection of ethane emission from submerged rice seedlings suggests oxidative damage and the formation of ROS already under hypoxia (Santosa et al. 2007) as well as apoplastic H2O2 formation (Blokhina et al. 2001). Interaction between small monomeric GTP-binding protein Rop and its negative regulator RopGAP4 (GTPase activating protein) has been shown to control H2O2 level and affect gene expression in hypoxic Arabidopsis seedlings in Ca2+-dependent manner. Rop-induced increase in H2O2 led to the induction of ADH (ethanolic fermentation) and RopGAP4 (a negative regulator of Rop) expression, thus sustaining the negative feedback regulation of oxidative stress, ethanolic fermentation and survivial under hypoxia (Baxter-Burrell et al. 2002; Van Breusegem et al. 2008). However, more prominent oxidative damage occurs upon re-admission of O2 (Yan et al. 1996; Biemelt et al. 1998; Blokhina et al. 1999; Yordanova and Popova 2007).
The main chain breaking antioxidant in biological membranes undergoing LP is tocopherol. NO can react with alkoxy and peroxy radicals thus terminating the chain reaction of LP (Wink and Mitchell 1998).
In soybean, NO donor treatment decreased the level of lipid radicals in chloroplast membranes; however, ONOO treatment resulted in an increase in LP (Jasid et al. 2006). Lipid nitration and the physiological and signalling role of nitro fatty acids have been intensively studied in animal models mostly in connection with vasorelaxation, inflammatory signalling, oxidative injury and apoptosis (Freeman et al. 2008; Rubbo and Radi 2008). The process is sustained by the lipophilicity of molecular O2 and NO: they can both accumulate three to fourfold in the lipid phase and react to produce oxidants and nitrating species (Moller et al. 2005; Freeman et al. 2008). To our knowledge there are no reports on the existence and role of nitrated lipids in plants. However, some hypoxia-related metabolic changes allow the speculation on the relevance of this process in plants. Accumulation of nitrite and its evolution to NO associated with oxygen deprivation can lead to the formation of secondary NO-derived species (•NO2, ONOO−, N2O2). The onset of cytoplasmic acidification not only favours non-enzymatic formation of NO from nitrite (Bethke et al. 2004) but also promotes protonation of NO −2 to nitrous acid HNO2 (a membrane permeable compound) with consequent nitration of proteins and unsaturated fatty acids (Freeman et al. 2008). Increased probability of Fenton reaction under hypoxia relies on the liberation of ferrous ions due to acidification (Navarre et al. 2000) and Fenton chemistry sustains nitrite oxidation to radical •NO2 (Freeman et al. 2008). Depending on O2 concentration, •NO2 can oxidize (LP) or nitrate unsaturated fatty acids in vitro. The nitration products are represented by diverse species and can include alkyl nitrites LONO and nitrolipids LNO2 (O'Donnell and Freeman 2001). Signalling properties of nitrolipids and nitro-fatty acids rely on their chemistry and encompass NO release and nitroalkylation of protein thiol and histidine residues (Freeman et al. 2008).
3 ROS and RNS Chemistry Overview and Sources of Formation Under Lack of Oxygen
3.1 Reactive Oxygen Species
During the last two decades, chemistry of oxygen activation and its consequences for biological systems have been extensively reviewed (Mittler 2002; Sorg 2004; Blokhina and Fagerstedt 2006; Halliwell 2006; Pryor et al. 2006; Halliwell 2009,). The first step in O2 activation (one electron reduction which yields superoxide O −•2 ) requires energy; the subsequent steps can proceed spontaneously in the presence of proton and/or electron donors. The superoxide O −•2 formed is membrane impermeable and is dismutated to H2O2 by compartment specific superoxide dismutase (SOD) isoforms (Bowler et al. 1992). Due to restricted intermembrane mobility, ROS formed in the membrane-enclosed cellular compartments react “on-spot” with their oxidation targets. Therefore, distinct sites of ROS formation under different environmental stimuli might bring about the specificity of ROS signal. However, H2O2 is not a radical, is uncharged, has relatively long lifetime and is able to penetrate the membrane. This property makes H2O2 a good candidate for long-distance or intercompartment signalling species. An extremely potent oxidant hydroxyl radical OH• is considered as the most reactive ROS. It is formed in a transition metal-catalysed Fenton reaction, during decomposition of ozone in the presence of protons in the apoplast, and is considered as the species initiating the chain reaction of LP (Ohyashiki and Nunomura 2000; Pryor et al. 2006). Singlet oxygen (1O2), where one of the electrons on the outer electron sheath has changed its spin, is formed in tissues under UV-exposure and during photoinhibition in chloroplasts (Triantaphylidès and Havaux 2009). Of the ROS, hydrogen peroxide and superoxide are both produced in a number of cellular reactions including the Mehler reaction in the chloroplasts, the iron catalyzed Haber–Weiss and Fenton reaction s, photorespiration and by various enzymes such as lipoxygenases, peroxidases, NADPH oxidase, xanthine oxidase and amine oxidase (Bolwell and Wojtaszek 1997; Bolwell et al. 2002; Blokhina et al. 2003; Blokhina and Fagerstedt 2006; Pryor et al. 2006).
Several enzyme reactions have been shown to produce ROS (via the main route or as a side reaction) under the lack of oxygen (Blokhina et al. 2003). Xanthine oxidoreductase can use acetaldehyde accumulating under hypoxia (fermentation product) and hypoxanthine (ATP catabolism product) as an e− donor to perform primary activation of O2 and, hence, produce O −•2 and H2O2 (Godber et al. 2000; Harrison 2002).
The formation of ROS has been detected virtually in all cellular compartments: mitochondria, chloroplast, peroxisomes, cytoplasm (P450), plasma membrane (NADPH oxidase) and apoplast. For updates on ROS and RNS formation in peroxisomes, see the work by del Rio et al. (2006), and for mitochondria and chloroplasts, see Moller et al. (2007) and Rinalducci et al. (2008).
3.2 Reactive Nitrogen Species
The chemical properties of nitric oxide, NO, make this gas a good candidate for a signalling molecule. NO can freely penetrate the lipid bilayer and, hence, can be transported within the cell. NO is quickly produced on demand via inducible enzymatic or non-enzymatic routes. Due to its free radical nature (one unpaired electron) NO has a short half-life (in order of seconds), and can be removed easily when no longer needed (Lamattina et al. 2003; Neill et al. 2003; Besson-Bard et al. 2008; Qiao and Fan 2008). NO can also react either with O2 or O −•2 . The end products, NO2, N2O2, and peroxynitrite ONOO− all have deleterious consequences in biological systems (Wink and Mitchell 1998).
Metabolic alterations brought about by oxygen deprivation provide a possibility for increased NO levels in hypoxic tissues. At low oxygen tensions NO-generating activity of xanthine oxidoreductase is enhanced. Interestingly, under normoxic conditions xanthine oxidoreductase is capable of both NO and O −•2 formation with consequent production of ONOO− (Godber et al. 2000). It has been shown that under oxygen deprivation the accumulated nitrite can be converted to NO by several hypoxically induced enzymes, namely cytosolic nitrate reductase (can use nitrite as a substrate and convert it to NO, induced several fold under hypoxia) (Drew 1997; Yamasaki and Sakihama 2000; Rockel et al. 2002), plasma membrane-bound nitrite-NO reductase (acidic conditions under anoxia are favourable for this enzyme with pH 6.1 optimum, hemoglobin (Hb) can act as a physiological e− donor for this reaction) (Stohr and Stremlau 2006). Indeed, accumulation of NO has been shown in hypoxic alfalfa root cultures and maize cell cultures (Dordas et al. 2004). Anoxic mitochondria are capable of nitrite reduction to NO, in a process specific for root mitochondria and elevated under the lack of O2 (Gupta et al. 2005; Planchet et al. 2005).
The direct effects of NO on biological targets include reduction of free metal ions or oxidation of metals in protein complexes such as hemoglobin, and Fe-nitrosyl formation resulting in activation of guanylate cyclase and hemoxygenase, inhibition of P450, cytochrome c oxidase, aconitase and catalase, and down-regulation of ferritin (Wink and Mitchell 1998). NO exerts an inhibitory effect on aconitase, a TCA cycle enzyme which catalyses the reversible conversion of citrate to isocitrate. A cytoplasmic isoform of aconitase is also affected: NO promotes the release of iron-sulphur cluster from the active center of the enzyme (Navarre et al. 2000). Possible release of ferrous ions from the cluster, e.g. upon cytoplasmic acidification, can enhance ROS production. NO intervention with TCA cycle enzyme would also have an impact on the energy status of the cell, already affected by COX inhibition. The NO inhibitory effect on COX in potato tuber mitochondria has been shown recently (de Oliveira et al. 2008). Therefore, not only the TCA cycle enzymes but also ETC are targets for NO regulation also under oxygen deprivation stress.
On the other hand, reactions of NO with Hb allow the maintenance of NAD+ levels for the needs of glycolysis under hypoxic conditions (Igamberdiev and Hill 2004; Igamberdiev et al. 2005). Overexpression of hypoxically induced class 1 hemoglobin in maize cell cultures has resulted in the maintenance of the energy status, and these cells showed less induction of ADH and were able to maintain the redox status of the cells. Transformed alfalfa roots overexpressing Hb have lower levels of NO (Dordas et al. 2004). Hence, the regulation of NO level under oxygen deprivation can be achieved in plants via interaction with class 1 non-symbiotic hemoglobins through several routes. In a reaction with oxyhemoglobin to form nitrate and methemoglobin (Fe3+) with the latter being reduced to hemoglobin (Fe2+) in an NADPH-depending reaction (Igamberdiev and Hill 2004). Another route is interaction of NO with deoxyhemoglobin to form nitrosylhemoglobin (Dordas et al. 2003). Under low oxygen tension, nitrosylhemoglobin will represent a significant part of the Hb pool. The beneficial role of nitrate and nitrite supplementation under anoxia has been attributed to cytoplasmic alkalinisation and to NADH recycling (Stoimenova et al. 2003; Libourel et al. 2006), moreover, anoxic mitochondria have been shown to synthesize ATP in the presence of NAD(P)H and nitrite with concurrent production of NO (Stoimenova et al. 2007).
3.3 Plant Mitochondria as ROS Producers: Relevance for Oxygen Deprivation Stress
Aerobic mitochondrial metabolism relies on electron transport along the chain of inner mitochondrial membrane-associated carriers and proton extrusion to create the electrochemical gradient which is the driving force for ATP synthesis with O2 as terminal e− acceptor. This tightly coupled redox system is extremely sensitive to inhibition and/or modification of its components by pharmacological agents and different stress factors (Noctor et al. 2007). The redox misbalance leads to over reduction of e-carriers, to e-leakage and formation of ROS. Mitochondria are able to produce superoxide anion O −•2 and the succeeding H2O2 (Moller et al. 2007; Murphy 2009) due to electron leakage at the ubiquinone site − ubiquinone: cytochrome b region (Gille and Nohl 2001; Andreyev et al. 2005) − and at the matrix side of complex I (NADH dehydrogenase) (Chakraborti et al. 1999; Moller 2001). Hydrogen peroxide generation by higher plant mitochondria and its regulation by uncoupling of electron transport chain and oxidative phosphorylation have been demonstrated (Braidot et al. 1999; Grabel'nykh et al. 2006).
At the same time, mitochondria can be considered as a tool for ROS level regulation/elimination through AOX, plant uncoupling protein (UCP), and ATP-sensitive plant mitochondrial potassium channel (PmitoKATP) (Grabel'nykh et al. 2006; McDonald and Vanlerberghe 2007; Pastore et al. 2007). An antioxidant role has recently been suggested for mitochondrial uncoupling protein (UCP) which transports fatty acid anions from the inner to the outer leaflet of the membrane (Goglia and Skulachev 2003; Grabel'nykh et al. 2006). First, uncoupling itself lowers mitochondrial ROS production, and second, it is hypothesized that UCP is able to electrophoretically transport fatty acid hydroperoxides from mitochondrial matrix to the intermembrane space (Goglia and Skulachev 2003). Such extrusion preserves mtDNA and matrix proteins from contact with intermediates of LP. Indeed, overexpression of Arabidopsis uncoupling protein encoded by AtUCP1 leads to increased oxidative stress tolerance in tobacco (Brandalise et al. 2003).
Mitochondria are also responsible for retrograde ROS signalling. Importance of mitochondrial ETC complexes in the regulation of ROS-mediated abiotic stress responses have been confirmed recently by inactivation and concurrent complementation of mitochondrial pentatricopeptide repeat (PPR) domain protein, PPR40 (Zsigmond et al. 2008). The protein is associated with complex III and its malfunction resulted in ROS accumulation, SOD activation, LP and altered induction of stress-responsive genes, e.g. AOX (Zsigmond et al. 2008).
The alternative oxidase (AOX) present in plant mitochondria catalyzes four-electron reduction of O2 by ubiquinone and, hence, competes for the electrons with the main respiratory chain. Control of H2O2 formation in mitochondrial ETC is one of the functions suggested for AOX. Antisense suppression of AOX in tobacco has resulted in ROS accumulation, while overexpression lead to decreased ROS levels (Maxwell et al. 1999). Under hypoxia the expression of AOX protein has been shown to be translationally regulated, dependent on O2 concentration (Szal et al. 2003) and activated by pyruvate (Millenaar and Lambers 2003; McDonald and Vanlerberghe 2007).
Mitochondria are capable of e− transport from complex II to complex 1 (reverse electron flow) supported by succinate, a substrate for complex II (Andreyev et al. 2005). This process has been shown to increase ROS (O −•2 ) production at complex I and is regulated by ATP hydrolysis via mitochondrial ATPase confirmed by sensitivity to inhibitors of complex 1 (rotenone) and ATPase (oligomycin) (Turrens and Boveris 1980).
Under anoxic conditions mitochondrial ATPase can switch to ATP hydrolysis to maintain the decreasing proton gradient, as is the case in the skeletal muscles of anoxia tolerant frogs (St-Pierre et al. 2000). Under oxygen-limited conditions ATP use in this model can account for 9% from total ATP consumption (St-Pierre et al. 2000). To avoid the complete depletion of already limited ATP supply the enzyme is regulated by inhibitory subunit IF1 of ATPase. The inhibition occurs upon binding of IF1 to F1 subunit, is reversible and takes place under the conditions which limit oxidative phosphorylation (Rouslin 1991). In plants IF1 protein has been isolated from potato (Polgreen et al. 1995) and nucleotide sequences of cDNA for two isoforms of IF1 in rice has been described (Nakazono et al. 2000).
Besides ATP hydrolysis, plant mitochondria exhibit a number of distinct metabolic features under anoxia (reviewed by Igamberdiev and Hill 2009). Anoxic mitochondria have been shown to produce ATP utilizing NADH and nitrite (as an e− acceptor) with concurrent production of NO (Stoimenova et al. 2007). The organelles are involved in regulation of NO and NADH turnover via hypoxically induced hemoglobins to maintain redox and energy status (Igamberdiev et al. 2005; Hebelstrup et al. 2007). Apparently, the level of NO formed in anoxic mitochondria has to be tightly regulated, since NO affects the activity of ETC and matrix enzymes (as discussed above) and, besides inhibition can increase the Km for oxygen of COX (Cooper et al. 2003) and transcriptionally upregulate AOX (Huang et al. 2002). Nitrite-dependent NO production under hypoxia and the fine regulation of NO and ROS levels in mitochondria have recently been suggested as a main regulatory mechanism slowing down the respiration and preventing tissues from entering complete anoxia (Borisjuk et al. 2007; Benamar et al. 2008).
4 O2 Fluxes in Tissues and Factors Affecting O2 Concentration In Vivo
At first we have to pay attention to the definition of critical oxygen pressure for respiration (COPR). This is the lowest oxygen concentration to support maximum respiratory rate and below which the rate of O2 consumption rapidly declines. It has been estimated that the determination of COPR is very difficult through measurements of O2 consumption rates in intact or excised roots in oxygen free media, and hence a mathematical model and a polarographic method for determining COPR has been published (Armstrong et al. 2009).
In a recent study on oxygen distribution in developing seeds Borisjuk and Rolletschek (2009) argue that NO may be a key player in the oxygen balancing process in seeds and especially in the avoidance of anoxia and fermentation (Borisjuk et al. 2007; Borisjuk and Rolletschek 2009). Increasing NO concentration (synthesised from nitrite) in pea (Pisum sativum) and soybean (Glycine max) lead to reduced O2 consumption and decreased ATP availability, and hence biosynthetic activity (Borisjuk et al. 2007; Borisjuk and Rolletschek 2009). This kind of balancing of oxygen diffusion and consumption via NO can be a mechanism for avoidance of anoxia in seeds.
In a very interesting and fundamental study on oxygen consumption rates and the induction of ethanolic fermentation in pea (P. sativum) and thale grass (Arabidopsis thaliana) alcohol dehydrogenase knockout lines, it has been shown that fermentation is primarily induced by a drop in the energy status of the cells and not by the oxygen concentration per se (Zabalza et al. 2009). The fact that O2 consumption of isolated mitochondria is linear to practically zero O2 levels due to the high O2 affinity of the terminal electron acceptor COX, in comparison with the situation in intact tissues, indicates that mitochondrial respiration is regulated (possibly by substrate availability in the TCA cycle). This regulation results in slowing down of mitochondrial respiration under O2 concentration far from that limiting COX activity. Similarly, in an earlier study by van Dongen and coworkers (2003), oxygen concentrations inside Ricinus communis stems were low (7%) even while grown under normal oxygen partial pressures (21%) and resulted in adaptive changes in phloem metabolism and function (van Dongen et al. 2003).
An interesting feature in plant roots grown in stagnant solutions has been noted: A barrier for radial oxygen loss developing in the basal parts of the roots preventing O2 diffusion into the surrounding O2 deficient solution (Visser et al. 2000; Colmer 2003; Armstrong et al. 2009). The barrier seems to be constitutive and “tighter” in monocotyledonous species than in dicotyledons but this result has to be confirmed with a wider range of species (Visser et al. 2000). The barrier is formed of suberised cell walls in the exodermis of the roots in rice (Fagerstedt, unpublished data). The ability of anoxia tolerant plant species to maintain an adequate oxygen supply or to restrict oxygen loss from the tissues due to the formation of oxygen-impermeable barrier would certainly imply the formation of ROS or RNS. The reduced cellular environment, together with O2 availability (and Fe2+ liberation) create of favourable conditions for oxidative reactions producing ROS.
In a recent study on root and shoot relation, it has been noticed that root hypoxia leads to increased glycolytic flux and ethanolic fermentation in the roots but not in the leaves of grey poplar (Populus x canescens) (Kreuzwieser et al. 2009). Similarly, various biosynthetic processes were downregulated in the roots but not in the leaves and shoot growth was not hindered, which suggests that increased glycolytic flux is enough to maintain shoot growth (Kreuzwieser et al. 2009).
5 Microarray Experiments in the Study of Hypoxia-Associated Oxidative Stress
The availability of microarray technology advanced the investigation of the whole-genome response to oxygen deprivation stress (Klok et al. 2002; Branco-Price et al. 2005; Liu et al. 2005; Loreti et al. 2005; Lasanthi-Kudahettige et al. 2007; Branco-Price et al. 2008; van Dongen et al. 2009). In a number of studies both metabolic and transcriptomic changes have been assessed. Microarray data have confirmed the upregulation of transcripts coding for anaerobic proteins: about 20 proteins which function mainly in sugar metabolism, glycolysis and fermentation (sucrose synthase, fructokinase, glyceraldehyde-3-phosphate dehydrogenase, puryvate decarboxylase 1 and 2, alcohol dehydrogenase 1, lactate dehydrogenase 1, alanine aminotransferase ). Microarray studies have also revealed a number of novel transcripts implicated in signalling (ethylene receptor ETR2, calmodulin-like Ca-binding protein, heat shock proteins, MYB family transcription factors, kinases, zinc finger protein ZAT12, WRKY transcription factors )(Branco-Price et al. 2005). Earlier observations on ROS elevation resulting from oxygen deprivation and upon reoxygenation have been validated by transcriptomic analysis: indeed, expression of a wide range of ROS- and redox-related transcripts are induced by the lack of oxygen. Almost threefold transient upregulation of ascorbate peroxidase has been detected already after 0.5 h of low O2 treatment, followed by monodehydroascorbate reductase, peroxidase ATP4a and a respiratory burst oxidase protein (Klok et al. 2002; Branco-Price et al. 2005; Liu et al. 2005). Interestingly, catalase expression is downregulated or unaffected under hypoxia and low abundance of the catalase transcript in anoxic rice coleoptiles has been discussed as signalling-related and necessary for H2O2 build-up (Lasanthi-Kudahettige et al. 2007).
Regulation of ROS originating from the mitochondrial ETC is achieved by the repression of TCA cycle enzymes and by induction of alternative oxidase (see above) (Klok et al. 2002; Branco-Price et al. 2008). In corroboration with the energy saving strategy the transcripts of genes related to energy-consuming secondary processes has been shown to be downregulated, but genes responsible for ATP-producing reactions shown to be − upregulated (Klok et al. 2002; Loreti et al. 2005; van Dongen et al. 2009).
Transcripts linked to RNS metabolism constitute another group of upregulated redox-related genes: nitrate reductase, a known source of NO under oxygen deprivation (see above) and non-symbiotic hemoglobin 1 implicated in NO-dependent NADH recycling under hypoxia (Klok et al. 2002; Igamberdiev et al. 2005; van Dongen et al. 2009). Induction of class 1 hemoglobin has been detected in all experimental trials under different O2 concentrations and in a range of durations (van Dongen et al. 2009), emphasizing the importance of hypoxically induced hemoglobins in stress survival.
The response to hypoxia and reoxygenation is regulated not only by induced transcription but also by differential translation of mRNAs: the general trend being translation suppression of the majority of mRNAs, while sustaining the translation of the small group of mRNAs encoding the enzymes relevant for energy maintenance and counteraction of ROS (Ahsan et al. 2007; Bailey-Serres and Voesenek 2008; Branco-Price et al. 2008). Quick metabolic reoxygenation response has found its explanation in a reversal of the sequestered untranslated mRNA and immediate protein synthesis (Branco-Price et al. 2008).
6 Update on Antioxidant Protection
The importance of antioxidants during oxidative stress has long been recognised. The positive effect of antioxidant supplementation/upregulation has been documented in numerous studies on metabolic factors underlying anoxia tolerance (Monk et al. 1989; Noctor and Foyer 1998; Scebba et al. 1998; Blokhina et al. 1999; Boo and Jung 1999; Blokhina et al. 2000; Smirnoff 2000; Blokhina et al. 2001, 2003). The complicated antioxidant network is regulated at the site of synthesis and during transport and through interaction with reactive oxygen species. The plant antioxidant defence system is represented by a range of molecules with different chemical nature, and it is, therefore, capable of exerting the antioxidant function in both aqueous and lipid phases in live tissues. The small molecular antioxidant arsenal in plants consists of ascorbate, glutathione, thioredoxins, tocopherols and carotenoids while many of the phenolic compounds in plants also have considerable antioxidant activity (Pietta 2000; Hernandez et al. 2009). To maintain the redox status and to regenerate antioxidants in their active form an array of enzymes acts in the support of the antioxidative defences: Dehydroascorbate reductase (DHAR), thioredoxin (TRX) reductase, glutathione (GSH) reductase, lipoamide dehydrogenase, thiol transferase (glutaredoxin). The efficiency of antioxidant system is increased by direct enzymatic elimination of deleterious ROS by superoxide dismutases (SODs), catalases (CAT), and peroxidases (PRX). There are also a number of enzymes detoxifying LP products: glutathione S-transferases, phospholipid-hydroperoxide glutathione peroxidase and ascorbate peroxidase.
6.1 Low Molecular Weight Antioxidants
6.1.1 Glutathione
The tripeptide glutathione (GSH, glutamylcysteinylglycine) is an abundant compound in plant tissues being present in virtually all cell compartments: cytosol, ER, vacuole and mitochondria (Jimenez et al. 1998). GSH together with its oxidized form (GSSG) maintains the cellular redox balance, which is of great importance as it allows fine-tuning of the cellular redox environment under normal conditions and provides the basis for GSH stress signalling. Indeed, GSH has a role in redox regulation of gene expression (Wingate et al. 1988; Alscher 1989), and also in the regulation of the cell cycle (Sanchez-Fernandez et al. 1997). GSH functions under oxidative stress by scavenging cytotoxic H2O2, and reacts non-enzymatically with other ROS: singlet oxygen, superoxide radical and hydroxyl radical (Larson 1988). The central role of GSH in the antioxidative defense is due to its ability to regenerate another powerful water-soluble antioxidant, ascorbic acid, via ascorbate-glutathione cycle (Foyer and Halliwell 1976; Noctor and Foyer 1998), Fig. 7.2.
Antioxidant turnover in plant tissues. The reducing power needed comes from NAD(P)H through the several enzymes catalysing reactions in the cycles. GSH, reduced glutathione, GSSG, oxidised glutathione. Adapted and redrawn from May et al. (1998)
6.1.2 Ascorbic acid
Vitamin C is one of the most studied and powerful antioxidants (Noctor and Foyer 1998; Arrigoni and De Tullio 2000; Horemans et al. 2000). It is astonishing that the last steps in the biosynthesis of ascorbic acid (AA) have been elucidated as late as in 2000 by Smirnoff. Ascorbate has not only been detected in the majority of plant cell types and cellular organelles, but also in the apoplast. Ascorbic acid exists mostly in its reduced form (90% of the ascorbate pool) in leaves and chloroplasts (Smirnoff 2000), and its intracellular concentration can be in the high millimolar range (e.g. 20 mM in the cytosol and 20–300 mM in the chloroplast stroma (Foyer and Lelandais 1996).
AA acts in many ways in the protection of tissues against oxidative damage. It can directly scavenge superoxide, hydroxyl radicals and singlet oxygen and reduce H2O2 to water via ascorbate peroxidase reaction (Noctor and Foyer 1998). In chloroplasts AA acts in the xanthophyll cycle as a cofactor of violaxantin de-epoxidase thus sustaining dissipation of excess exitation energy (Smirnoff 2000). Albeit AA acts in the aqueous phase, it also protects membrane lipids by regenerating tocopherol from tocopheroxyl radicals (Thomas et al. 1992). In addition, AA carries out a number of non-antioxidant functions in the cell. It has been implicated in the regulation of the cell division, cell cycle progression from G1 to S phase (Liso et al. 1988; Smirnoff 1996) and cell elongation (de Tullio et al. 1999). Very recently dehydroascorbic acid has emerged as a signalling molecule regulating stomatal closure (Fotopoulos et al. 2008).
6.1.3 Tocopherol (Vitamin E)
Tocochromanols, i.e. a group of four tocopherols and four tocotrienols that collectively constitute vitamin E, is a vital nutrient in human diet only produced in photosynthetic organisms (Della Penna 2005). During the recent decade genetic engineering of the biosynthetic pathway has provided significant insights into the molecular genetics and biochemical control of tocochromanol biosynthesis in plants (Della Penna 2005). The importance of tocopherols and tocotrienols lies in the fact that they are essential components of biological membranes where they have both antioxidant and non-antioxidant functions (Kagan 1989). α-Tocopherol with its three methyl substitutes has the highest antioxidant activity of tocopherols (Kamal-Eldin and Appelqvist 1996), while β-, γ-, δ-isomers form the other three tocopherol and tocotrienols. Chloroplast membranes of higher plants contain α-tocopherol as the predominant tocopherol isomer, and are hence well protected against photooxidative damage (Fryer 1992).
The fact that makes Vitamin E especially important during the postanoxic phase in plant tissues is its chain-breaking antioxidant activity: It is able to repair oxidizing radicals directly, preventing the chain propagation step during lipid autoxidation (Serbinova and Packer 1994). The reaction between vitamin E and lipid radicals occurs in the membrane-water interphase where vitamin E donates a hydrogen ion to the lipid radical with the consequent formation of tocopheroxyl radical (TOH•) formation (Buettner 1993). Regeneration of the tocopheroxyl radical back to its reduced form can be achieved by vitamin C (ascorbate), reduced glutathione (Fryer 1992) or coenzyme Q (Kagan et al. 2000). In addition, tocopherols may act as chemical scavengers of oxygen radicals, especially singlet oxygen, and as physical deactivators of singlet oxygen by charge transfer mechanism (Fryer 1992).
It has only very recently been demonstrated for the first time that tocopherol deficiency may alter endogenous phytohormone levels, especially jasmonate, thereby reducing plant growth and triggering anthocyanin accumulation in leaves (Munné-Bosch et al. 2007). In another study on ethylene insensitive Arabidopsis mutants it has been concluded that tocopherol biosynthesis may be regulated by ethylene: The disruption of ethylene perception in ein3-1, etr1-1 and its overproduction in eto1-1 mutants of Arabidopsis thaliana correlated positively with tocopherol content (Cela et al. 2009). It was shown that a mutation in the EIN3 gene delayed the water-stress related increase in α-tocopherol and caused a reduction in the levels of this antioxidant by ca. 30% compared to the wild type. In contrast to the wild type and ein3-1 mutants, both etr1-1 and eto1-1 mutants showed a sharp (up to fivefold) increase in α-tocopherol levels during leaf aging (Cela et al. 2009).
It has also been noticed that nonenzymatic LP reprograms gene expression and activates defence markers in Arabidopsis tocopherol-deficient mutants (Sattler et al. 2006).
6.1.4 Phenolic Compounds as Antioxidants
Phenolics (flavonoids, tannins, hydroxycinnamate esters and lignin) are the largest group of secondary compounds in many plant tissues (Grace and Logan 2000). Polyphenols possess ideal structural chemistry for free radical scavenging activity, and they have been shown to be more effective antioxidants in vitro than tocopherols and ascorbate. Antioxidative properties of polyphenols arise from their high reactivity as hydrogen or electron donors, and from the ability of the polyphenol-derived radical to stabilize and delocalize the unpaired electron (chain-breaking function), as well as their ability to chelate transition metal ions (termination of the Fenton reaction) (Rice-Evans et al. 1997). Another mechanism underlying the antioxidative properties of phenolics is the ability of flavonoids to alter peroxidation kinetics by modification of the lipid packing order and to decrease fluidity of the membranes (Arora et al. 2000). These changes could sterically hinder diffusion of free radicals and restrict peroxidative reactions. Moreover, it has been shown that phenolic compounds can be involved in the hydrogen peroxide scavenging cascade in plant cells (Takahama and Oniki 1997). Induction of the biosynthesis of phenolic compounds has been shown in several articles, in connection with various stress conditions such as heavy metal stress (Sgherri et al. 2003; Mithöfer et al. 2004) and UV-B irradiation (Kondo and Kawashima 2000; Lavola et al. 2000).
6.2 Enzymes Participating in Quenching ROS
6.2.1 Superoxide Dismutase
Superoxide dismutases (SODs) are key enzymes in the antioxidative defense system of plants. SODs are present in all aerobic organisms and in all subcellular compartments susceptible of oxidative stress (Bowler et al. 1992). It is well known that enhanced formation of ROS and superoxide, O2 − •, under stress conditions may induce both protective responses and cellular damage. The scavenging of O2 − • is achieved through SODs, which catalyse the dismutation of superoxide to H2O2. This reaction has a 10–10,000-fold faster rate than spontaneous dismutation (Bowler et al. 1992). SODs are classified by their metal cofactor into FeSOD (prokaryotic organisms, chloroplast stroma) and MnSOD (prokaryotic organisms and the mitochondrion of eukaryotes) which are structurally similar, and into the structurally unrelated Cu/ZnSOD (cytosolic and chloroplast enzyme, gram-negative bacteria).
Excessive accumulation of superoxide due to the reduced activity of SOD under flooding stress has been shown by Yan and coworkers (1996). Hence, it was to be expected that SOD activities will increase and this has indeed been shown in several experiments on plants under abiotic stress conditions. An increase in total SOD activity has been detected in wheat roots under anoxia but not under hypoxia. The degree of increase positively correlated with duration of anoxia (Biemelt et al. 2000). Induction of SOD activity under hypoxia by 40–60% in roots and leaves under hypoxia of Hordeum vulgare has been shown by Kalashnikov et al. (1994). Also salt stress has an effect on SOD transcription level and it seems that in different accession of Arabidopsis the responses can be different (Attia et al. 2008).
In Arabidopsis plants under various stresses including high light, ozone fumigation, and ultraviolet-B radiation that may lead to increased oxidative stress, differential regulation of seven SOD mRNAs and four SOD proteins have been noticed (Kliebenstein et al. 1998). On the whole, antioxidant defences are induced in plants under mild oxidative stress conditions (Lee et al. 2007), while a severe stress, such as anoxia, results in antioxidant depletion or slower turnover, and hence increased oxidative damage on re-oxygenation (Blokhina et al. 2000). Very recently a view on the mechanisms for the induction of SODs in plants has emerged: It has been noticed that there is a microRNA (miRNA, a class of regulatory RNAs of c. 21 nucleotides that posttranscriptionally regulate gene expression by directing mRNA cleavage or translational inhibition) in Arabidopsis called miR398 that targets two closely related Cu/ZnSODs (Sunkar et al. 2006). The induction of these SODs is mediated by the downregulation of miR398 but the actual molecular mechanism is still unknown (Sunkar et al. 2006). Another novel mitochondrial protein, AtMTM1, has also been implicated in the activation of MnSOD in Arabidopsis (Su et al. 2007). In a recent study on the significance of MnSOD (the mitochondrial SOD) with the antisense technique, it was shown that reduced MnSOD activity results in altered mitochondrial redox balance and reduced root growth while total respiratory CO2 output did not change (Morgan et al. 2008).
6.2.2 Catalases, Peroxidases and Ascorbate Peroxidases
Catalases and peroxidases are important enzymes present in the intercellular spaces, where they can regulate the level of H2O2 (reviewed by Willekens et al. 1995). The catalase function has been explained in a review on plant antioxidants recently (Blokhina et al. 2003). In addition to catalysing the breakdown of H2O2 to water and dioxygen (catalase action), catalase can also work in a peroxidatic reaction and splits hydrogen peroxide and produces another strong oxidant, the hydroxyl radical (OH•; Elstner and Osswald 1994). OH• is a very strong oxidant and can initiate radical chain reactions with organic molecules, particularly with PUFA in membrane lipids.
In waterlogged citrus trees increases in both antioxidant enzyme activities (SOD, ascorbate peroxidase, catalase and glutathione reductase) as well as in ascorbate and glutathione have been detected (Arbona et al. 2008). Under anoxia a differential response of the peroxidase system has been observed in coleoptiles and roots of rice seedlings. A decrease in activities of cell wall-bound guaiacol and syringaldazine peroxidase activities has been reported, while soluble peroxidase activity was not affected in coleoptiles. In contrast, anoxia-grown roots have shown an increase in the cell wall-bound peroxidases (Lee and Lin 1995). Acclimation to anoxia has been shown to be dependent, at least partly, on peroxidases, which are up-regulated by anoxic stress in soybean cell cultures (Amor et al. 2000). In rice seedlings ADH and SOD activities responded non-significantly to submergence, while catalase activity increased upon re-oxygenation (Ushimaru et al. 1999).
6.2.3 Phospholipid Hydroperoxide Glutathione Peroxidase
The phospholipid hydroperoxide glutathione peroxidase (PHGPX) is an important antioxidant enzyme in the protection of biological membranes exposed to oxidative stress. It is known to be inducible under various stress conditions. This enzyme catalyses the regeneration of phospholipid hydroperoxides using the reducing power of GSH. It is localised in the cytosol and the inner membrane of mitochondria of animal cells. A cDNA clone homologous to PHGPX has been isolated from tobacco, maize, soybean, and Arabidopsis (Sugimoto et al. 1997). The PHGPX protein and its encoded gene CSA have been isolated and characterised in citrus. It has been shown that CSA is directly induced by the substrate of PHGPX under heat, cold and salt stresses, and that this induction occurs mainly via the production of ROS (Avsian-Kretchmer et al. 1999). It remains to be seen whether this gene is induced as ROS production increases after flooding or anoxia. In an earlier study, PHGPX has been suggested of being a chloroplast-localised enzyme (Mullineaux et al. 1998) preventing oxidative modification of lipids in chloroplast stroma (Baier and Dietz 1999) together with another alkyl hydroperoxide reductase, 2-Cys peroxiredoxin (Rouhier and Jacquot 2002). Recently, it has been proven to be localised in the mitochondrion (Yang et al. 2006).
7 Concluding Remarks
The paradoxic connection between oxygen deprivation and oxidative stress is substantiated by changes on transcriptional, translational and metabolic levels. Transcripts coding for both anaerobic proteins and ROS-detoxifying enzymes have been shown to be upregulated during hypoxia. ROS and RNS formation is tightly connected with hypoxia-induced pathways and metabolic adjustments during glycolysis, fermentation, cytoplasmic acidification, respiratory inhibition and the declining ATP level (Fig. 7.1). Many anoxia-induced enzymes have been shown to produce ROS or RNS under hypoxic conditions. In turn, ROS and RNS carry out a feedback regulation of e.g. respiration, ATP level and the TCA cycle. Their signalling functions rely on pH changes and Ca2+ elevation, as is the case with Rop GTPase signalling. The ability to control respiration and to preserve energy leads to manageable levels of ROS and RNS, (depending on the severity and length of oxygen deprivation), favouring cell survival. The energy preservation can be achieved via activation of glycolysis, via the PPi-dependent glycolysis (in anoxia tolerant plants) and by using of the anoxic metabolite nitrite as final electron acceptor in anoxic mitochondria. Control of the respiration rate can be realized through regulation of the glycolytic flux (to handle pyruvate levels and its entrance into the TCA cycle) and, probably, by the fine regulation of mitochondrial terminal oxidases via the dynamic equilibrium between ROS and NO. The balance between O2 transport to hypoxic tissues, regulatory adaptations, anoxic metabolites, ROS−RNS chemistry and signalling, determine the survival of plants under waterlogging and oxygen deprivation.
Abbreviations
- ADH:
-
Alcohol dehydrogenase
- AOX:
-
Alternative oxidase
- COX:
-
Cytochrome oxidase
- ETC:
-
Electron transport chain
- FFA:
-
Free fatty acids
- LP:
-
Lipid peroxidation
- NO:
-
Nitric oxide
- 1O2 :
-
Singlet oxygen
- O −•2 :
-
Superoxide anion
- ONOO− :
-
Peroxynitrite
- PFK-PPi:
-
PPi-dependent phosphofructokinase
- PLD:
-
Phospholipase D
- PEPC:
-
Phosphoenolpyruvate carboxylase
- PPDK:
-
Pyruvate phosphate dikinase
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- UCP:
-
Mitochondrial uncoupling protein
References
Ahsan N, Lee D, Lee S, Lee K, Bahk J, Lee B (2007) A proteomic screen and identification of waterlogging-regulated proteins in tomato roots. Plant Soil 295:37–51
Alscher RG (1989) Biosynthesis and antioxidant function of glutathione in plants. Physiol Plant 77:457–464
Amor Y, Chevion M, Levine A (2000) Anoxia pretreatment protects soybean cells against H2O2-induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett 477:175–180
Andreyev A, Kushnareva Y, Starkov A (2005) Mitochondrial metabolism of reactive oxygen species. Biochem (Moscow) 70:200–214
Arbona V, Hossain Z, López-Climent MF, Pérez-Clemente RM, Gómez-Cadenas A (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiol Plant 132:452–466
Arcisio-Miranda M, Abdulkader F, Brunaldi K, Curi R, Procopio J (2009) Proton flux induced by free fatty acids across phospholipid bilayers: new evidences based on short-circuit measurements in planar lipid membranes. Arch Biochem Biophys 484:63–69
Armstrong W, Webb T, Darwent M, Beckett PM (2009) Measuring and interpreting respiratory critical oxygen pressures in roots. Ann Bot 103:281–293
Arora A, Byrem TM, Nair MG, Strasburg GM (2000) Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Arch Biochem Biophys 373:102–109
Arrigoni O, de Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157:481–488
Attia H, Arnaud N, Karray N, Lachaal M (2008) Long-term effects of mild salt stress on growth, ion accumulation and superoxide dismutase expression of Arabidopsis rosette leaves. Physiol Plant 132:293–305
Avsian-Kretchmer O, Eshdat Y, Gueta-Dahan Y, Ben-Hayyim G (1999) Regulation of stress-induced phospholipid hydroperoxide glutathione peroxidase expression in citrus. Planta 209:469–477
Baier M, Dietz KJ (1999) Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci 4:166–168
Bailey-Serres J, Chang R (2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann Bot 96:507–518
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296:2026–2028
Benamar A, Rolletschek H, Borisjuk L, Avelange-Macherel M, Curien G, Mostefai HA, Andriantsitohaina R, Macherel D (2008) Nitrite–nitric oxide control of mitochondrial respiration at the frontier of anoxia. Biochim Biophys Acta Bioenergetics 1777:1268–1275
Besson-Bard A, Pugin A, Wendehenne D (2008) New insights into nitric oxide signaling in plants. Annu Rev Plant Biol 59:21–39
Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16:332–341
Biemelt S, Keetman U, Albrecht G (1998) Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol 116:651–658
Biemelt S, Keetman U, Mock H, Grimm B (2000) Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell Environ 23:135–144
Blokhina O, Chirkova TV, Fagerstedt KV (2001) Anoxic stress leads to hydrogen peroxide formation in plant cells. J Exp Bot 52:1179–1190
Blokhina O, Fagerstedt KV (2006) Oxidative stress and antioxidant defences in plants. In: Quek (ed) Oxidative stress, disease and cancer, pp 151–199
Blokhina OB, Fagerstedt KV, Chirkova TV (1999) Relationships between lipid peroxidation and anoxia tolerance in a range of species during post-anoxic reaeration. Physiol Plant 105:625–632
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Blokhina O, Virolainen E, Fagerstedt KV, Hoikkala A, Wähälä K, Chirkova TV (2000) Antioxidant status of anoxia-tolerant and -intolerant plant species under anoxia and reaeration. Physiol Plant 109:396–403
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53:1367–1376
Bolwell GP, Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant defence – a broad perspective. Physiol Mol Plant Pathol 51:347–366
Boo YC, Jung J (1999) Water deficit-induced oxidative stress and antioxidative defenses in rice plants. J Plant Physiol 155:255–261
Borisjuk L, Macherel D, Benamar A, Wobus U, Rolletschek H (2007) Low oxygen sensing and balancing in plant seeds: a role for nitric oxide. New Phytol 176:813–823
Borisjuk L, Rolletschek H (2009) The oxygen status of the developing seed. New Phytol 182:17–30
Bowler C, van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Braidot E, Petrussa E, Vianello A, Macri F (1999) Hydrogen peroxide generation by higher plant mitochondria oxidizing complex I or complex II substrates. FEBS Lett 451:347–350
Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J 56:743–755
Branco-Price C, Kawaguchi R, Ferreira RB, Bailey-Serres J (2005) Genome-wide analysis of transcript abundance and translation in Arabidopsis seedlings subjected to oxygen deprivation. Ann Bot 96:647–660
Brandalise M, Maia IG, Borecký J, AbE V, Arruda P (2003) Overexpression of plant uncoupling mitochondrial protein in transgenic tobacco increases tolerance to oxidative stress. J Bioenerg Biomembr 35:203–209
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543
Cela J, Falk J, Munné-Bosch S (2009) Ethylene signaling may be involved in the regulation of tocopherol biosynthesis in Arabidopsis thaliana. FEBS Lett 583:992–996
Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S (1999) Oxidant, mitochondria and calcium: an overview. Cell Signal 11:77–85
Chirkova TV, Sinyutina NF, Blyudzin YA, Barsky IE, Smetannikova SV (1989) Phospholipid fatty acids of root mitochondria and microsomes from rice and wheat seedlings exposed to aeration or anaerobiosis. Plant Physiol (Russian) 36:126–134
Colmer TD (2003) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann Bot 91:301–309
Cooper CE, Davies NA, Psychoulis M, Canevari L, Bates TE, Dobbie MS, Casley CS, Sharpe MA (2003) Nitric oxide and peroxynitrite cause irreversible increases in the K-m for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim Biophys Acta Bioenergetics 1607:27–34
de Oliveira HC, Wulff A, Saviani EE, Salgado I (2008) Nitric oxide degradation by potato tuber mitochondria: evidence for the involvement of external NAD(P)H dehydrogenases. Biochim Biophys Acta Bioenergetics 1777:470–476
del Rio LA, Sandalio LM, Corpas FJ, Palma JM, Barroso JB (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol 141:330–335
Della Penna D (2005) Progress in the dissection and manipulation of vitamin E synthesis. Trends Plant Sci 10:574–579
Dordas C, Hasinoff B, Rivoal J, Hill R (2004) Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta 219:66–72
Dordas C, Rivoal J, Hill RD (2003) Plant haemoglobins, nitric oxide and hypoxic stress. Ann Bot (Lond) 91:173–178
Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48:223–250
Elstner EF, Osswald W (1994) Mechanisms of oxygen activation during plant stress. Proc Royal Soc Edinb 102B:31–154
Freeman BA, Baker PRS, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M (2008) Nitro-fatty acid formation and signaling. J Biol Chem 283:15515–15519
Fotopoulos V, De Tullio MC, Barnes J, Kanellis AK (2008) Altered stomatal dynamics in ascorbate oxidase over-expressing tobacco plants suggests a role for dehydroascorbate signalling. J Exp Bot 59:729–737
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Lelandais MA (1996) A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaves mesophyll cells. J Plant Physiol 148:391–398
Fryer MJ (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15:381–392
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia – is survival a balancing act? Trends Plant Sci 9:449–456
Geigenberger P (2003) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6:247–256
Generosova IP, Vartapetian BB (2005) On the physiological role of anaerobically synthesized lipids in Oryza sativa seedlings. Russ J Plant Physiol 52:481–488
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 30:1–47
Gille L, Nohl H (2001) The ubiquinol/bc1 redox couple regulates mitochondrial oxygen radical formation. Arch Biochem Biophys 388:34–38
Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R (2000) Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275:7757–7763
Goglia F, Skulachev VP (2003) A function for novel uncoupling proteins: antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. FASEB J 17:1585–1591
Grabel'nykh OI, Kolesnichenko AV, Pobezhimova TP, Zykova VV, Voinikov VK (2006) Mechanisms and functions of nonphosphorylating electron transport in respiratory chain of plant mitochondria. Russ J Plant Physiol 53:418–429
Grace S, Logan BA (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Trans R Soc Lond B 355:1499–1510
Graham JWA, Williams TCR, Morgan M, Fernie AR, Ratcliffe RG, Sweetlove LJ (2007) Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell 19:3723–3738
Greenway H, Gibbs J (2003) Review: mechanisms of anoxia tolerance in plants. II. Energy requirements for maintenance and energy distribution to essential processes. Funct Plant Biol 30:999–1036
Gupta KJ, Stoimenova M, Kaiser WM (2005) In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. J Exp Bot 56:2601–2609
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Halliwell B (2009) The wanderings of a free radical. Free Radic Biol Med 46:531–542
Hamberg M, Fahlstadius P (1992) On the specificity of a fatty acid epoxygenase in broad bean (Vicia faba L.). Plant Physiol 99:987–995
Harrison R (2002) Structure and function of xanthine oxidoreductase: where are we now? Free Radic Biol Med 33:774–797
Hassan W, Ibrahim M, Deobald AM, Braga AL, Nogueira CW, Rocha JBT (2009) pH-Dependent Fe (II) pathophysiology and protective effect of an organoselenium compound. FEBS Lett 583:1011–1016
Hebelstrup KH, Igamberdiev AU, Hill RD (2007) Metabolic effects of hemoglobin gene expression in plants. Gene 398:86–93
Hernandez I, Alegre L, Van Breusegem F, Sergi Munne-Bosch S (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14:125–132
Horemans N, Foyer CH, Potters G, Asard H (2000) Ascorbate function and associated transport systems in plants. Plant Physiol Biochem 38:531–540
Huang S, Colmer TD, Millar AH (2008) Does anoxia tolerance involve altering the energy currency towards PPi? Trends Plant Sci 13:221–227
Huang X, von Rad U, Durner J (2002) Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta 215:914–923
Igamberdiev AU, Baron K, Manac'h-Little N, Stoimenova M, Hill RD (2005) The haemoglobin/nitric oxide cycle: Involvement in flooding stress and effects on hormone signalling. Ann Bot 96:557–564
Igamberdiev AU, Hill RD (2004) Nitrate, NO and haemoglobin in plant adaptation to hypoxia: an alternative to classic fermentation pathways. J Exp Bot 55:2473–2482
Igamberdiev AU, Hill RD (2009) Plant mitochondrial function during anaerobiosis. Ann Bot 103:259–268
Jackson MB (2008) Ethylene-promoted elongation: an adaptation to submergence stress. Ann Bot 101:229–248
Jasid S, Simontacchi M, Bartoli CG, Puntarulo S (2006) Chloroplasts as a nitric oxide cellular source. Effect of reactive nitrogen species on chloroplastic lipids and proteins. Plant Physiol 142:1246–1255
Jimenez A, Hernandez JA, Pastori G, del Río LA, Sevilla F (1998) Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol 118:1327–1335
Kagan VE (1989) Tocopherol stabilizes membrane against phospholipase A, free fatty acids, and lysophospholipids. In: Diplock AT, Machlin J, Packer L, Pryor WA (Eds) Vitamin E: biochemistry and health implications. Ann New York Acad Sci 570:121–135
Kagan VE, Fabisiak JP, Quinn PJ (2000) Coenzyme Q and vitamin E need each other as antioxidants. Lipids 214:11–18
Kalashnikov JUE, Balakhnina TI, Zakrzhevsky DA (1994) Effect of soil hypoxia on activation of oxygen and the system of protection from oxidative destruction in roots and leaves of Hordeum vulgare. Russ J Plant Physiol 41:583–588
Kamal-Eldin A, Appelqvist L-Å (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31:671–701
Kliebenstein DJ, Monde RA, Last RL (1998) Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant physiol 118:637–650
Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14:2481–2494
Kondo N, Kawashima M (2000) Enhancement of tolerance to oxidative stress in cucumber (Cucumis sativus L.) seedlings by UV-B irradiation: possible involvement of phenolic compounds and antioxidant enzymes. J Plant Res 113:311–317
Kreuzwieser J, Hauberg J, Howell KA, Carroll A, Rennenberg H, Millar AH, Whelan J (2009) Differential response of gray poplar leaves and roots underpins stress adaptation during hypoxia. Plant Physiol 149:461–473
Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Lanteri ML, Laxalt AM, Lamattina L (2008) Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-induced adventitious root formation in cucumber. Plant Physiol 147:188–198
Larson RA (1988) The antioxidants of higher plants. Phytochem 27:969–978
Lasanthi-Kudahettige R, Magneschi L, Loreti E, Gonzali S, Licausi F, Novi G, Beretta O, Vitulli F, Alpi A, Perata P (2007) Transcript profiling of the anoxic rice coleoptile. Plant Physiol 144:218–231
Lavola A, Julkunen-Tiitto R, DeE La Rosa TM, Lehto T, Aphalo PJ (2000) Allocation of carbon to growth and secondary metabolites in birch seedlings under UV-B radiation and CO2 exposure. Physiol Plant 109:260–267
Lee SH, Ahsan N, Lee KW, Lee DG, Kwark SS, Kwon SY, Kim TH, Lee BH (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164:1626–1638
Lee TM, Lin YN (1995) Changes in soluble and cell wall-bound peroxidase activities with growth in anoxia-treated rice (Oryza sativa L.) coleoptiles and roots. Plant Sci 106:1–7
Libourel IG, van Bodegom PM, Fricker MD, Ratcliffe RG (2006) Nitrite reduces cytoplasmic acidosis under anoxia. Plant Physiol 142:1710–1717
Liso R, Innocenti AM, Bitonti MB, Arrigoni O (1988) Ascorbic acid-induced progression of quiescent centre cells from G1 to S phase. New Phytol 110:469–471
Liu F, VanToai T, Moy LP, Bock G, Linford LD, Quackenbush J (2005) Global transcription profiling reveals comprehensive insights into hypoxic response in Arabidopsis. Plant Physiol 137:1115–1129
Loreti E, Poggi A, Novi G, Alpi A, Perata P (2005) A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiol 137:1130–1138
Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci U S A 96:8271–8276
May MJ, Vernoux T, Leaver C, Van Montagu M, Inze D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49:649–667
McDonald AE, Vanlerberghe GC (2007) The organization and control of plant mitochondrial metabolism. In: Plaxton WC, McManus MT (eds) Control of primary metabolism in plants. Blackwell, Oxford, pp 290–324
Millenaar FF, Lambers H (2003) The alternative oxidase: in vivo regulation and function. Plant Biol 5:2–15
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Mithöfer A, Schulze B, Boland W (2004) Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett 566:1–5
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Moller M, Botti H, Batthyany C, Rubbo H, Radi R, Denicola A (2005) Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J Biol Chem 280:8850–8854
Monk LS, Fagerstedt KV, Crawford RMM (1989) Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress. Physiol Plant 76:456–459
Morgan MJ, Lehmann M, Schwarzländer M, Baxter CJ, Sienkiewicz-Porzucek A, Williams TCR, Schauer N, Fernie AR, Fricker MD, Ratcliffe RG, Sweetlove LJ, Finkemeier I (2008) Decrease in manganese superoxide dismutase leads to reduced root growth and affects tricarboxylic acid cycle flux and mitochondrial redox homeostasis. Plant Physiol 147:101–114
Mullineaux PM, Karpinski S, Jiménez A, Cleary SP, Robinson C, Creissen GP (1998) Identification of cDNAS encoding plastid-targeted glutathione peroxidase. Plant J 13:375–379
Munné-Bosch S, Weiler E, Alegre L, Müller M, Düchting P, Falk J (2007) α-Tocopherol may influence cellular signaling by modulating jasmonic acid levels in plants. Planta 225:681–691
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–17
Mustroph A, Albrecht G, Hajirezaei M, Grimm B, Biemelt S (2005) Low levels of pyrophosphate in transgenic potato plants expressing E. coli pyrophosphatase lead to decreased vitality under oxygen deficiency. Ann Bot 96:717–726
Nakazono M, Imamura T, Tsutsumi N, Sasaki T, Hirai A (2000) Characterization of two cDNA clones encoding isozymes of the F1F0-ATPase inhibitor protein of rice mitochondria. Planta 210:188–194
Navarre DA, Wendehenne D, Durner J, Noad R, Klessig DF (2000) Nitric oxide modulates the activity of tobacco aconitase. Plant Physiol 122:573–582
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Mol Biol 49:249–279
Nowak G, Grant DF, Moran JH (2004) Linoleic acid epoxide promotes the maintenance of mitochondrial function and active Na+ transport following hypoxia. Toxicol Lett 147:161–175
O'Donnell VB, Freeman BA (2001) Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res 88:12–21
Ohyashiki T, Nunomura M (2000) A marked stimulation of Fe3+-dependent lipid peroxidation in phospholipid liposomes under acidic conditions. Biochim Biophys Acta Mol Cell Biol Lipids 1484:241–250
Pastore D, Trono D, Laus MN, Di Fonzo N, Flagella Z (2007) Possible plant mitochondria involvement in cell adaptation to drought stress: a case study: durum wheat mitochondria. J Exp Bot 58:195–210
Pavelic D, Arpagaus S, Rawyler A, Brandle R (2000) Impact of post-anoxia stress on membrane lipids of anoxia-pretreated potato cells. A re-appraisal. Plant Physiol 124:1285–1292
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Planchet E, Jagadis Gupta K, Sonoda M, Kaiser WM (2005) Nitric oxide emission from tobacco leaves and cell suspensions: rate limiting factors and evidence for the involvement of mitochondrial electron transport. Plant J 41:732–743
Polgreen KE, Featherstone J, Willis AC, Harris DA (1995) Primary structure and properties of the inhibitory protein of the mitochondrial ATPase (H+-ATP synthase) from potato. Biochim Biophys Acta Bioenergetics 1229:175–180
Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA (2006) Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291:R491–R511
Qiao W, Fan L (2008) Nitric oxide signaling in plant responses to abiotic stresses. J Integr Plant Biol 50:1238–1246
Rawyler A, Arpagaus S, Braendle R (2002) Impact of oxygen stress and energy availability on membrane stability of plant cells. Ann Bot 90:499–507
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Rinalducci S, Murgiano L, Zolla L (2008) Redox proteomics: basic principles and future perspectives for the detection of protein oxidation in plants. J Exp Bot 59:3781–3801
Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM (2002) Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J Exp Bot 53:103–110
Rouhier N, Jacquot J (2002) Plant peroxiredoxins: alternative hydroperoxide scavenging enzymes. Photosynth Res 74:259–268
Rouslin W (1991) Regulation of the mitochondrial ATPase in situ in cardiac muscle: role of the inhibitor subunit. J Bioenerg Biomembr 23:873–888
Rubbo H, Radi R (2008) Protein and lipid nitration: role in redox signaling and injury. Biochim Biophys Acta 1780:1318–1324
Sairam R, Kumutha D, Ezhilmathi K, Deshmukh P, Srivastava G (2008) Physiology and biochemistry of waterlogging tolerance in plants. Biol Plant 52:401–412
Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, van Montagu M, Inzé D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. PNAS 94:2745–2750
Santosa I, Ram P, Boamfa E, Laarhoven L, Reuss J, Jackson M, Harren F (2007) Patterns of peroxidative ethane emission from submerged rice seedlings indicate that damage from reactive oxygen species takes place during submergence and is not necessarily a post-anoxic phenomenon. Planta 226:193–202
Sattler SE, Mene-Saffrane L, Farmer EE, Krischke M, Mueller MJ, DellaPenna D (2006) Nonenzymatic lipid peroxidation reprograms gene expression and activates defense markers in Arabidopsis tocopherol-deficient mutants. Plant Cell 18:3706–3720
Scebba F, Sebastiani L, Vitagliano C (1998) Changes in activity of antioxidative enzymes in wheat (Triticum aestivum) seedlings under cold acclimation. Physiol Plant 104:747–752
Serbinova EA, Packer L (1994) Antioxidant properties of α-tocopherol and α-tocotrienol. Methods Enzymol 234:354–366
Sgherri C, Cosi E, Navari-Izo F (2003) Phenols and antioxidative status of Raphanus sativus grown in copper excess. Physiol Plant 118:21–28
Skulachev VP (1998) Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta Bioenergetics 1363:100–124
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol 3:229–235
Sorg O (2004) Oxidative stress: a theoretical model or a biological reality? C R Biol 327:649–662
Stohr C, Stremlau S (2006) Formation and possible roles of nitric oxide in plant roots. J Exp Bot 57:463–470
Stoimenova M, Igamberdiev AU, Gupta K, Hill RD (2007) Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 226:465–474
Stoimenova M, Libourel IG, Ratcliffe RG, Kaiser WM (2003) The role of nitrate reduction in the anoxic metabolism of roots II. Anoxic metabolism of tobacco roots with or without nitrate reductase activity. Plant Soil 253:155–167
St-Pierre J, Brand MD, Boutilier RG (2000) Mitochondria as ATP consumers: cellular treason in anoxia. PNAS 97:8670–8674
Su Z, Chai M-F, Lu P-L, An R, Chen J, Wang X-C (2007) AtMTM1, a novel mitochondrial protein, may be involved in activation of the manganese-containing superoxide dismutase in Arabidopsis. Planta 226:1031–1039
Sugimoto M, Furui S, Suzuki Y (1997) Molecular cloning and characterisation of a cDNA encoding putative phospholipid hydroperoxide glutathione peroxidase from spinach. Biosci Biotech Biochem 61:1379–1381
Sunkar R, Kapoor A, Zhu J-K (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18:2051–2065
Szal B, Jolivet Y, Hasenfratz-Sauder M, Dizengremel P, Rychter AM (2003) Oxygen concentration regulates alternative oxidase expression in barley roots during hypoxia and post-hypoxia. Physiol Plant 119:494–502
Takahama U, Oniki T (1997) A peroxide/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol Plant 101:845–852
Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10:368–375
Thomas CE, McLean LR, Parker RA, Ohlweiler DF (1992) Ascorbate and phenolic antioxidant interactions in prevention of liposomal oxidation. Lipids 27:543–550
Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14:219–228
Turrens JF, Boveris A (1980) Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 2:421–427
Van Breusegem F, Bailey-Serres J, Mittler R (2008) Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 147:978–984
van Dongen JT, Frohlich A, Ramirez-Aguilar SJ, Schauer N, Fernie AR, Erban A, Kopka J, Clark J, Langer A, Geigenberger P (2009) Transcript and metabolite profiling of the adaptive response to mild decreases in oxygen concentration in the roots of Arabidopsis plants. Ann Bot 103:269–280
van Dongen JT, Schurr U, Pfister M, Geigenberger P (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131:1529
Vianello A, Macrì F (1999) Proton pumping pyrophosphatase from higher plant mitochondria. Physiol Plant 105:763–768
Visser EJW, Colmer TD, Blom CWPM, Voesenek LACJ (2000) Changes in growth, porosity, and radial oxygen loss from adventitious roots of selected mono- and dicotyledonous wetland species with contrasting types of aerenchyma. Plant Cell Environ 23:1237–1245
Visser EJW, Voesenek LACJ, Vartapetian BB, Jackson MB (2003) Flooding and plant growth. Ann Bot 91:107–109
Ushimaru T, Kanematsu S, Shibasaka M, Tsuji H (1999) Effect of hypoxia on the antioxidative enzymes in aerobically grown rice (Oryza sativa) seedlings. Physiol Plant 107:81–187
Willekens H, Inzé D, van Montagu M, van Camp W (1995) Catalase in plants. Mol Breed 1:207–228
Wingate VPM, Lawton MA, Lamb CJ (1988) Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol 87:206–210
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med 25:434–456
Yamasaki H, Sakihama Y (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett 468:89–92
Yan B, Dai Q, Liu X, Huang S, Wang Z (1996) Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179:261–268
Yang X-D, Dong C-J, Liu J-Y (2006) A plant mitochondrial phospholipid hydroperoxide glutathione peroxidase: its precise localization and higher enzymatic activity. Plant Mol Biol 62:951–962
Yordanova R, Popova L (2007) Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiol Plant 29:535–541
Yu Z, Zhang J, Wang X, Chen J (2008) Excessive copper induces the production of reactive oxygen species, which is mediated by phospholipase D, nicotinamide adenine dinucleotide phosphate oxidase and antioxidant systems. J Integr Plant Biol 50:157–167
Zabalza A, van Dongen JT, Froehlich A, Oliver SN, Faix B, Gupta KJ, Schmalzlin E, Igal M, Orcaray L, Royuela M, Geigenberger P (2009) Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol 149:1087–1098
Zsigmond L, Rigo G, Szarka A, Szekely G, Otvos K, Darula Z, Medzihradszky KF, Koncz C, Koncz Z, Szabados L (2008) Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol 146:1721–1737
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Blokhina, O., Fagerstedt, K.V. (2010). Oxygen Deprivation, Metabolic Adaptations and Oxidative Stress. In: Mancuso, S., Shabala, S. (eds) Waterlogging Signalling and Tolerance in Plants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-10305-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-10305-6_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-10304-9
Online ISBN: 978-3-642-10305-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)