Abstract
Cell contact-dependent inhibition and regulation of immune responses play an essential role in balancing the need for rapid and efficient responses to a wide variety of pathological challenges, while at the same time maintaining self-tolerance. Much attention has been given to immune synapses that lead to the activation of, for example, cell-mediated cytotoxicity, and here we compare the supramolecular dynamics of synapses that lead to inhibition or regulatory functions. We focus on natural killer cells where such different synapses have been best studied. An emergent principle is that inhibition or regulatory responses are commonly achieved by selective recruitment of signalling proteins to the synapse and exclusion of membrane-proximal intracellular proteins needed for activation. We also discuss evidence that an inhibitory synapse triggers or maintains effector cells in a migratory configuration, which serves to break the synapse before the steps needed for effector cell activation can be completed. This model implies that the concept of kinetic-proofreading, previously used to describe activation of individual T-cell receptors, can also apply in determining the outcome of intercellular conjugation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Natural Killer Cell
- Inhibitory Receptor
- Inhibitory Synapse
- Human Natural Killer Cell
- Intercellular Contact
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Many of the key cell surface molecules involved in immune cell surveillance have been identified and an important new scientific frontier is to understand where and when each protein–protein interaction occurs to regulate cell functions. Thus, imaging has a major role to play in contemporary cell biology and one interesting theme to emerge is that immune cell communication is often accompanied by the segregation of proteins into micrometre-scale domains at an intercellular contact or immune synapse (IS) (Davis 2006). More recently, it has been shown that kinases, adaptors and antigen receptors accumulate at synapses within micron- or sub-micron-scaled structures termed microclusters. T- and B-cell receptor signalling, for example, is initiated in such microclusters (Bunnell et al. 2002; Campi et al. 2005; Yokosuka et al. 2005) and these signals are terminated as microclusters move from the periphery to the centre of the IS (Harwood and Batista 2008; Seminario and Bunnell 2008; Varma et al. 2006; Yokosuka et al. 2005). In natural killer (NK) cells, phosphorylation of inhibitory killer cell immunoglobulin (Ig)-like receptors (KIR) is restricted to microclusters (Treanor et al. 2006) implicating that inhibitory signalling is also restricted to microclusters. The emerging new paradigm is that interactions between immune cell kinases, adaptors and other proteins are at least in part controlled by the dynamics of supramolecular assemblies rather than isolated protein–protein interactions that are commonly depicted in textbook diagrams of immune receptor signalling pathways. While this has been widely discussed for immune cell activation, here we review what happens at IS where inhibitory or regulatory signals dominate.
2 Definition of Inhibitory Immune Synapses
It is well established that inhibitory receptor functions are crucial to maintaining self-tolerance and control responses spatially and temporally while allowing rapid and efficient responses when appropriate (Long 1999). This is particularly evident from the data associating inhibitory receptor dysfunction, or genetic variations in inhibitory receptor expression, with susceptibility to a variety of diseases, including autoimmunity, viral infection and cancer (Chouaib et al. 2002; Pritchard and Smith 2003; Rajagopalan and Long 2005). Broadly, inhibitory receptors can be divided into two classes based on the presence or absence of cytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs). Inhibition by ITIM-containing receptors is initiated by tyrosine phosphorylation and recruitment of Src homology 2 (SH2) domain-containing phosphatases SHP-1 and/or SHP-2 or the SH2 domain-containing inositol phosphatase (SHIP) (Daeron et al. 2008; Long 2008). Engagement of inhibitory receptors changes the micrometre-scale organization of proteins at the IS, compared to activating interactions to form a so-called “inhibitory immune synapse” (Davis and Dustin 2004). An inhibitory synapse is not merely a transient intercellular contact at which little happens. Such transient interactions may occur when T cells briefly interact with target cells or APCs that lack a relevant peptide/MHC and they do not involve assembly of an IS. Rather, an inhibitory synapse can be defined as an intercellular contact at which the encounter causes proteins to segregate into micrometre-scale domains and where directed signalling serves to terminate or prevent immune cell activation.
Such inhibitory immune synapses were first proposed for NK cells conjugated to EBV-transformed B cells that were protected from lysis by expression of class I MHC protein recognized by inhibitory NK-cell receptors (Davis et al. 1999). It is broadly accepted that expression of class I MHC protein facilitates self-tolerance by NK cells and that conversely, viral-infected or tumour cells can become susceptible to lysis by NK cells via decreased expression of self class I MHC protein (Karre et al. 1986). Commonly referred to as the “missing self-hypothesis” (Ljunggren and Karre 1990), this provides a conceptual framework in which the importance of an inhibitory IS is well-documented.
Recently, ligation of inhibitory receptors has been shown to assemble distinct synaptic structures in many other immune cell interactions, including those involving T cells, B cells and macrophages (Dietrich et al. 2001; Fourmentraux-Neves et al. 2008; Guerra et al. 2002; Henel et al. 2006; Schneider et al. 2008; Sohn et al. 2008; Tsai and Discher 2008). For example, subpopulations of T cells express inhibitory receptors of the KIR family, the C-type lectin-like heterodimer CD94/NKG2A, the Ig-like transcript (ILT) 2 or members of the CD28:B7 Ig superfamily, such as CTLA-4 (CD154) (Peggs et al. 2008; Ugolini and Vivier 2000). Engagement of these receptors negatively regulates signalling through the T-cell antigen receptor (Chouaib et al. 2002; Peggs et al. 2008; McMahon and Raulet 2001; Snyder et al. 2002; Ugolini and Vivier 2000; van Bergen et al. 2004) and influences the supramolecular organization of the IS (Dietrich et al. 2001; Fourmentraux-Neves et al. 2008; Guerra et al. 2002; Henel et al. 2006; Schneider et al. 2008). The B cell inhibitory receptor FcγRIIB similarly disrupts formation of an activating synapse in response to membrane bound antigen (Sohn et al. 2008). The formation of a phagocytic synapse between macrophages and red blood cells is inhibited by binding of the inhibitory receptor SIRPα (CD172a) to its ligand CD47 (Tsai and Discher 2008). These data extend the concept of the inhibitory synapse to other cellular interactions and demonstrate its broad relevance.
3 Formation of Inhibitory Synapses
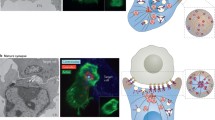
The inhibitory NK-cell IS is the best studied inhibitory IS and may be considered “prototypic” (see Fig. 1 for a summary of molecular processes at the inhibitory NK-cell IS). Inhibition of NK-cell activity through engagement of inhibitory receptors is essential to provide protection of “self” (Ljunggren and Karre 1990) because, in contrast to T- and B-cells, NK-cell activation does not depend on antigen receptors and is independent of prior sensitization or priming. Inhibitory receptors expressed on human NK cells include the ITIM-containing class I MHC protein binding KIRs and CD94/NKG2A (Lanier 2005). These inhibitory receptors and their ligands rapidly cluster at an inhibitory IS (Davis et al. 1999; Dietrich et al. 2001; Egen and Allison 2002; Eriksson et al. 1999b; Fourmentraux-Neves et al. 2008; Henel et al. 2006; Standeven et al. 2004). Interestingly, clustering of KIR and its class I MHC protein ligands is largely a spontaneous process triggered by binding alone. Accumulation of these proteins does not require receptor signalling or ligation of adhesion molecules (Fassett et al. 2001; Faure et al. 2003) and is largely independent of actin reorganization or ATP-driven cellular processes (Almeida and Davis 2006; Carlin et al. 2001; Davis et al. 1999; Standeven et al. 2004). Indeed, insect cell transfectants expressing class I MHC protein, considered to not express any other ligands for NK cells, can trigger efficient clustering and tyrosine phosphorylation of KIR at the NK-cell IS (Faure et al. 2003). Intriguingly, clustering and phosphorylation of KIR does require the presence of divalent cations such as Zn2+ (Davis et al. 1999; Fan et al. 2000; Fassett et al. 2001; Rajagopalan and Long 1998; Rajagopalan et al. 1995; Vales-Gomez et al. 2001), although the molecular basis for this remains unclear. It still remains to be established if efficient spontaneous clustering of inhibitory NK-cell receptors and ligands is essential for their function but it is tempting to speculate that the rapid spontaneous clustering of inhibitory receptors and ligands is important in keeping NK-cell responses tightly controlled. In contrast, however, recruitment of the inhibitory receptor CTLA-4 to the T-cell IS is proportional to the strength of the TCR stimulus (Egen and Allison 2002). This difference must reflect an interesting distinction in the functions and/or mechanisms by which these different inhibitory receptors operate but this requires further investigation.
Comparison of the inhibitory and cytolytic NK cell IS. Contacts between NK cells and target cells that are protected from NK cell attack result in the formation of an inhibitory synapse with recruitment of inhibitory receptors bound to their respective MHC ligands (Davis et al. 1999; Eriksson et al. 1999b). Initially, inhibitory receptors appear in small clusters that move from the periphery to the centre of the IS to form larger aggregates during IS maturation (Oddos et al. 2008). Concomitantly, the phosphatases SHP-1 and SHP-2 and the kinase Lck accumulate. In the early inhibitory IS, cytoskeletal proteins talin and F-actin and GM1-rich microdomains are detectable (Masilamani et al. 2006; Treanor et al. 2006; Vyas et al. 2002, 2004). Lck, F-actin, talin and GM1-rich microdomains are excluded from the inhibitory IS at later time points, while SHP-1 and SHP-2 remain (Vyas et al. 2002, 2004). Inhibitory receptors segregate from integrins and arrange in different patterns across the synapse being either homogeneously distributed, ring shaped, or containing multiple exclusions (Almeida and Davis 2006; Carlin et al. 2001; Davis et al. 1999; Vyas et al. 2004). Activating receptors, e.g., CD2 and 2B4, are present within the inhibitory IS (Schleinitz et al. 2008). Finally, the NK cell detaches from the target and moves away. Target cells that display reduced expression of class I MHC protein or increased expression of activating NK cell ligands will activate NK cell cytotoxicity. Early stages of the cytolytic IS are characterized by actin reorganization, the accumulation of GM1-rich microdomains and the recruitment of kinases and adapter molecules including Lck, Syk, ZAP-70 and SLP-76 (Orange et al. 2003; Vyas et al. 2001, 2002, 2004). During maturation, f-actin reorganizes to form a ring in the periphery of the IS, while the MTOC and lytic granules polarize towards the centre (Culley et al. 2009; McCann et al. 2003; Orange et al. 2003; Orange et al. 2002; Vyas et al. 2001). Inhibitory receptors can be still present in cytolytic IS but cluster in multifocal patterns (Almeida and Davis 2006; Schleinitz et al. 2008)
The supramolecular organization of class I MHC protein across an inhibitory NK-cell IS can form a single cluster, a ring, or a cluster containing multiple regions where class I MHC protein is excluded (Almeida and Davis 2006; Carlin et al. 2001; Oddos et al. 2008). These configurations are dynamic and interchangeable (Almeida and Davis 2006). However, these patterns are less clear for peripheral blood NK-cell clones compared to larger immortal NK-cell lines and thus, it seems unlikely that class I MHC protein being organized in a single or multiple foci, for example, has any direct influence on the outcome of the cell–cell interaction (Almeida and Davis 2006). Instead, recent evidence points to the extent of co-localisation or segregation between different receptor/ligand pairs within the organized IS being the important issue. For example, the level of expression of HLA-C on target cells determined its supramolecular organization and the extent of segregation from ICAM-1 (CD54) at the NK-cell IS (Almeida and Davis 2006). Strikingly, for individual peripheral blood NK clones, specific thresholds in the level of target cell HLA-C needed to cause segregation of HLA-C from ICAM-1 at the IS, directly correlated with the threshold needed to functionally inhibit cytotoxicity (Almeida and Davis 2006). Thus, organization of HLA-C at the IS, determined by its level of expression, may directly influence NK-cell inhibition by regulating the proximity of activating and inhibitory receptors. This would be consistent with earlier studies, using mAb cross-linking, demonstrating that co-clustering of activating and inhibitory receptors was required for inhibition (Blery et al. 1997).
What causes the segregation of different receptor/ligand pairs across the inhibitory NK-cell IS remains unproven. In the “kinetic-segregation model” for T cell receptor triggering it has been proposed that proteins can be organized according to the size of their extracellular domains (Davis and van der Merwe 2006). Accordingly at the inhibitory NK-cell IS, it has been demonstrated that larger proteins, e.g., CD43, are excluded from the IS (McCann et al. 2003). Moreover, the size of KIR-MHC protein is significantly smaller than that of LFA-1 (CD11a/C18)-ICAM-1, which is consistent with their segregation being driven by size differences (Davis 2002; Davis and van der Merwe 1996, 2006; McCann et al. 2002; Springer 1990). Such a model would also explain why the extent of segregation between these proteins was greater when their expression levels were increased (Almeida and Davis 2006). However, a prediction of this model would be that the size of the synaptic cleft would match the size of different proteins where they clustered. In contrast, at least after fixation for examination by electron microscopy, the size of the synaptic cleft varies considerably and apparently randomly, seemingly able to accommodate a range of protein sizes in close proximity (McCann et al. 2003). Thus, it is important to study in more detail whether the size of proteins influences their organization at the NK-cell IS and if so, it must be clarified whether this affects protein segregation at the level of microclusters and/or larger-scale segregation across the synapse.
While some receptor/ligand pairs can accumulate spontaneously, active receptor signalling also plays a crucial role in the organization of inhibitory synapses. Functional ITIM tyrosines and the catalytic activity of SHP-1 are required for disruption of the actin cytoskeleton and exclusion of GM1-rich microdomains from NK-cell or cytotoxic T-cell synapses (Fassett et al. 2001; Guerra et al. 2002; Lou et al. 2000; Masilamani et al. 2006). Proteins associated with GM1-rich microdomains play an essential role in the initial phosphorylation of activating NK-cell receptors (Inoue et al. 2002; Watzl and Long 2003). Thus, one way in which inhibitory NK-cell receptors can be effective, is by blocking the actin-cytoskeleton dependent recruitment of signalling proteins within GM1-rich microdomains to the IS (Endt et al. 2007; Fassett et al. 2001; Fourmentraux-Neves et al. 2008; Guerra et al. 2002; Lou et al. 2000; Masilamani et al. 2006; Sanni et al. 2004; Sohn et al. 2008; Tsai and Discher 2008; Vyas et al. 2002; Vyas et al. 2004; Watzl and Long 2003). Consistent with this model, activating receptors CD2 and 2B4 (CD244) are not inhibited from being recruited to an inhibitory NK-cell IS (Schleinitz et al. 2008), but rather are likely to be impaired in their ability to signal there. Similarly in B cells, for example, the inhibitory receptor FcγRIIB blocks association of the BCR with lipid raft-like domains and also prevents subsequent accumulation of BCR-enriched microclusters in the centre of the synapse (Sohn et al. 2008). In T cells, the inhibitory receptor CTLA-4 inhibits formation of ZAP-70 microclusters (Schneider et al. 2008). Taken together, this suggests a common principle in that inhibitory synapses still accumulate activating receptors and ligands, but specifically exclude the membrane-proximal intracellular proteins needed for activation.
4 Balancing Synapses with Kinapses and Kinetic Proofreading at the Cellular Level
The concept of a kinapse has recently been proposed to describe junctions involving moving T cells that allow signals to be integrated (Dustin 2008a, b). Kinapses lack the degree of stability characteristic for synapses and cell polarity is maintained in the direction of cell movement, rather than being orientated to face the intercellular contact. It is well established that ligation of the TCR delivers a stop signal to T cells (Dustin et al. 1997) that precedes synapse formation (Lee et al. 2002). NK cells similarly crawl over the surface of target cells, notably with higher motility for inhibitory contacts (Burshtyn et al. 2000; Davis et al. 1999; Eriksson et al. 1999a). Likewise, ligation of activating NK-cell receptors provides a stop signal that results in symmetrical spreading of NK cells over their targets, while ligation of inhibitory receptors provides a reverse-stop signal that breaks the symmetry of spreading and encourages NK-cell migration (Culley et al. 2009). Similarly, the inhibitory receptor CTLA-4 reverses the TCR-mediated stop signal (Schneider et al. 2006). Thus, an inhibitory synapse may be considered as a transient symmetrical synapse driving an effector cell to revert to its migratory kinapse configuration. PKCθ and WASp were found to favour T cells forming a kinapse or synapse, respectively (Sims et al. 2007). It would be interesting to determine if inhibitory NK-cell IS exploit these pathways in driving cells to a kinapse configuration. Indeed, inhibitory signals in NK cells are known to directly regulate cytoskeletal processes involving WASp (Krzewski et al. 2006) and Vav (Stebbins et al. 2003).
It is well established that cytolytic NK cell synapses go through a series of specific stages that lead to the directed release of lytic granules (Davis 2002, 2009; Davis and Dustin 2004; Krzewski and Strominger 2008; Orange 2008; Wulfing et al 2003). Thus, the process of cellular activation can be considered as directly analogous to the model of kinetic-proofreading for triggering individual TCR signals (McKeithan 1995). There are a specific number of steps that two cells in contact must go through before lytic granules are released or other effector functions realized. These include a multitude of cellular processes, such as calcium flux, integrin-mediated tight adhesion, MTOC reorientation, translocation of granules to the synapse and many others. These steps introduce a series of time delays from initial intercellular contact until the effector function is realized, e.g. lytic granules are released. Thus, an inhibitory synapse serves to shorten the half-life of the intercellular conjugate and break the IS before these steps can be completed, preventing effector functions.
5 Unzipping the Synapse
There has been extensive research on the assembly of the IS yet relatively little attention has been given to its disassembly. Thousands of individual protein–protein interactions exist across the IS such that the disassembly of this contact cannot be trivial. Perhaps most acutely, when inhibition dominates the outcome of surveillance, e.g. at the inhibitory NK-cell IS, protein–protein interactions accumulated at the synapse must be rapidly removed or broken so that NK cells can readily move on to survey other target cells. Similarly, for cytolytic interactions involving CTL or NK cells, it is unclear how the effector cells efficiently move away from dead or dying target cells. Efficient disassembly of the synapse is important to allow cells to move between target cells and must be necessary, for example, for CTL or NK cells to sequentially kill several target cells (Bhat and Watzl 2007; Martz 1976). It has been demonstrated that some receptors are endocytosed from the IS upon ligation, e.g., the T-cell receptor (TCR) (Cemerski et al. 2008; Lee et al. 2003), but it has not been directly tested whether or not these events are important in the disassembly of the IS. Indeed, it is unclear how many protein-protein interactions would need to be removed from an IS to allow cells to move apart. Alternatively, it can be envisaged that exocytosis of the synaptic membrane from the target cells or APCs could contribute to the disassembly of the IS. This could relate to the common process of intercellular transfer of surface proteins between immune cells that can occur by several specific mechanisms (Davis 2007; LeMaoult et al. 2007). It is also unknown if specific signalling events control termination of the synapse. It is well understood that inside-out signalling leads to a high-affinity conformation of LFA-1 that in turn contributes to intercellular conjugation and assembly of the IS (Luo et al. 2007). However, it has not been tested whether or not specific signals could return LFA-1 to a lower affinity state and contribute to the disassembly of the synapse.
6 Regulatory Synapses
In addition to the autonomous interaction of NK cells with infected or transformed cells, research has expanded in recent years to study the cross-talk between NK cells and other immune cells, including monocytes, macrophages, dendritic cells and T cells. These interactions can augment or initiate NK cell responses to pathological challenges and can also shape adaptive immune responses, e.g., by triggering DC maturation (Andoniou et al. 2008; Fernandez et al. 1999; Moretta et al. 2006; Newman and Riley 2007; Raulet 2004; Strowig et al. 2008). Contact-dependent reciprocal stimulation plays an important role during these interactions and several studies have therefore investigated the organization of these intercellular contacts termed regulatory IS (Borg et al. 2004; Brilot et al. 2007; Nedvetzki et al. 2007; Pallandre et al. 2008).
7 The Regulatory NK Cell Synapse
Regulatory NK cell IS are long lasting and accumulate activating receptors, cytokine receptors and adhesion molecules (Borg et al. 2004; Brilot et al. 2007; Nedvetzki et al. 2007; Semino et al. 2005). Figure 2 summarizes current knowledge of the molecular arrangements at such regulatory NK-cell IS. In contrast to an inhibitory IS, cytoskeletal components, f-actin, fascin and talin as well as GM1-rich microdomains all accumulate at the regulatory IS (Borg et al. 2004; Brilot et al. 2007; Nedvetzki et al. 2007; Semino et al. 2005). Inhibitory receptors do still cluster at such IS and accumulate adjacent to clusters of cytokine receptors, surrounded by a ring of LFA-1 and talin (Brilot et al. 2007).Cytokines and cytokine receptors polarize towards regulatory NK-cell IS (Borg et al. 2004; Brilot et al. 2007; Semino et al. 2005). IL-18 polarizes towards the synapse of NK cells and immature DC (iDC) and stimulates secretion of HMGB1 by NK cells, which in turn is necessary to induce DC maturation (Semino et al. 2005). Mature DCs (mDC) polarize preassembled stores of IL-12 towards the NK-cell IS (Borg et al. 2004) and NK cells accumulate the high affinity subunit of the IL-15 receptor, IL-15Rα, at the IS (Brilot et al. 2007). Polarization of cytokines towards the regulatory NK-cell IS implicates an importance of synapse formation for directed cytokine secretion. This is likely to be important in a wide range of immune cell interactions. For example, co-culture experiments using a transwell membrane recently determined that IL-18 is delivered to NK cells in a contact dependent manner by Kupffer (liver macrophage-like) cells (Tu et al. 2008). As determined for T cells, a general principle is that some cytokines and chemokines are secreted multi-directionally from effector cells, to have a broad impact on inflammation, while others are secreted directionally via the IS where specific intercellular communication is required (Brilot et al. 2007).
The regulatory NK-cell IS. The interaction of NK cells with DCs or macrophages can induce NK cell proliferation or cytokine secretion, without triggering cytotoxicity. The resulting IS has therefore been termed regulatory (Borg et al. 2004; Brilot et al. 2007; Nedvetzki et al. 2007; Pallandre et al. 2008). DCs polarize the MTOC and cytokines including IL-12 and IL-18 towards the IS (Borg et al. 2004). In NK cells, the IL-15Rα subunit is recruited to the contact site where it segregates from inhibitory NK-cell receptors (Brilot et al. 2007). Furthermore, adhesion molecules and their ligands, e.g. LFA-1, DC-SIGN and ICAM-3, polarize towards the cell interface (Borg et al. 2004; Brilot et al. 2007). Both, NK cells and DCs show reorganization of the actin-cytoskeleton, with cytoskeletal proteins including f-actin, talin and fascin accumulating at the synapse (Borg et al. 2004; Brilot et al. 2007). Additionally, GM1-rich microdomains enrich within the NK cell membrane in the contact area (Borg et al. 2004). Macrophages stimulate NK cell proliferation and cytokine secretion and prime NK cell cytotoxicity against susceptible target cells. This is largely dependent on the engagement of the 2B4 receptor, which is recruited to the centre of such regulatory synapses. In these contacts F-actin accumulates at the synapse from within macrophages, but not NK cells. Macrophages exposed to a high dose of LPS upregulate ligands for the NK cell receptor NKG2D and are subsequently killed by NK cells. These cytolytic NK cell–macrophage synapses accumulate NKG2D and the signalling adapters DAP10 and CD3ζ at the centre of the IS, while ICAM-1 locates to the periphery. F-actin accumulates at the contact area from within the NK cells, but not in the macrophages (Nedvetzki et al. 2007)
8 Triggering Cytokine Secretion Versus Cytolysis
There is evidence that immature DCs are susceptible to lysis by NK cells and acquire protection from lysis by maturation (Moretta et al. 2006; Strowig et al. 2008). Killing of iDCs can be triggered via the natural cytotoxicity receptor NKp30 (CD337) (Ferlazzo et al. 2002; Spaggiari et al. 2001) while increased expression of class I MHC protein during DC maturation provides protection from NK cells lysis (Carbone et al. 1999; Ferlazzo et al. 2002). Whether macrophages induce a cytolytic or regulatory NK-cell IS depends on the activation state of the macrophage in terms of the strength of TLR-4 stimulation (Nedvetzki et al. 2007). Macrophages that are stimulated with a low dose of LPS form regulatory synapses, characterized by the recruitment of the NK-cell receptor 2B4, while stimulation with a high dose of LPS induces upregulation of NKG2D (CD314) ligands on macrophages that triggers NKG2D-mediated killing (Nedvetzki et al. 2007).
It is particularly intriguing that 2B4 is recruited to the regulatory NK-cell-macrophage IS and is important for triggering cytokine secretion (Nedvetzki et al. 2007), while at other synapses, e.g., during interactions with tumour cell targets, 2B4 ligation triggers cytotoxicity (Bhat et al. 2006). Mechanisms must be in place to fine tune the function of 2B4-mediated activation. One possibility is that this depends on the synergy with other activating receptors. Indeed, only specific combinations of activating receptors can induce granule exocytosis in human NK cells (Bryceson et al. 2006). Fine tuning may also involve receptors not specifically associated with NK-cell activation. For example, the chemokine CX3CL1 influences the distribution of KIR at the NK cell–DC synapse and is able to prevent phosphorylation of its ITIMs (Pallandre et al. 2008). However, in contrast to other NK-cell activating receptors, 2B4 has been shown to directly interact with a variety of signalling molecules (Eissmann et al. 2005) and to mediate activation of granule exocytosis or cytokine secretion (Kubin et al. 1999; Nakajima et al. 1999; Tangye et al. 1999), as well as inhibition (Parolini et al. 2000; Sivori et al. 2002). Thus, the availability of downstream signalling proteins at the IS may determine the outcome of 2B4 stimulation in NK cells. In this case, regulatory synapses would function by selectively recruiting certain membrane-proximal adaptors or kinases, analogous to the restricted recruitment of signalling proteins seen at inhibitory synapses.
Overall, it is clear that there is an important role for IS in balancing activation, regulation and inhibition of immune responses. Much of what has been achieved so far is a direct result of the inter-disciplinary approach that immunologists have taken relatively recently to probe molecular recognition by individual cells. The continuing development and application of new techniques that allow intercellular communication to be probed with superior spatial and temporal resolution will enable scientists to resolve many of the outstanding issues highlighted throughout this review. Already, numerous recent super-resolution imaging techniques have the potential to directly report the spatial and temporal relationships of the key molecules (Fernandez-Suarez and Ting 2008). Perhaps most exciting is that as we continue to probe immune cell recognition with superior resolution, unexpected signalling and integration mechanisms that were not apparent at conventional diffraction-limited resolution will surely be revealed.
References
Almeida CR, Davis DM (2006) Segregation of HLA-C from ICAM-1 at NK cell immune synapses is controlled by its cell surface density. J Immunol 177:6904–6910
Andoniou CE, Coudert JD, Degli-Esposti MA (2008) Killers and beyond: NK-cell-mediated control of immune responses. Eur J Immunol 38:2938–2942
Bhat R, Watzl C (2007) Serial killing of tumor cells by human natural killer cells – enhancement by therapeutic antibodies. PLoS ONE 2:e326
Bhat R, Eissmann P, Endt J, Hoffmann S, Watzl C (2006) Fine-tuning of immune responses by SLAM-related receptors. J Leukoc Biol 79:417–424
Blery M, Delon J, Trautmann A, Cambiaggi A, Olcese L, Biassoni R, Moretta L, Chavrier P, Moretta A, Daeron M et al (1997) Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J Biol Chem 272:8989–8996
Borg C, Jalil A, Laderach D, Maruyama K, Wakasugi H, Charrier S, Ryffel B, Cambi A, Figdor C, Vainchenker W et al (2004) NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood 104:3267–3275
Brilot F, Strowig T, Roberts SM, Arrey F, Munz C (2007) NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. J Clin Invest 117:3316–3329
Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 214:73–91
Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE (2002) T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol 158:1263–1275
Burshtyn DN, Shin J, Stebbins C, Long EO (2000) Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr Biol 10:777–780
Campi G, Varma R, Dustin ML (2005) Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med 202:1031–1036
Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, Karre K, Zappacosta S (1999) Recognition of autologous dendritic cells by human NK cells. Eur J Immunol 29:4022–4029
Carlin LM, Eleme K, McCann FE, Davis DM (2001) Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med 194:1507–1517
Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS (2008) The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity 29:414–422
Chouaib S, Thiery J, Gati A, Guerra N, El Behi M, Dorothee G, Mami-Chouaib F, Bellet D, Caignard A (2002) Tumor escape from killing: role of killer inhibitory receptors and acquisition of tumor resistance to cell death. Tissue Antigens 60:273–281
Culley FJ, Johnson M, Evans JH, Kumar S, Crilly R, Casasbuenas J, Schnyder T, Mehrabi M, Deonarain MP, Ushakov DS et al (2009) Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol 7:e1000159
Daeron M, Jaeger S, Du Pasquier L, Vivier E (2008) Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev 224:11–43
Davis DM (2002) Assembly of the immunological synapse for T cells and NK cells. Trends Immunol 23:356–363
Davis DM (2006) Intrigue at the immune synapse. Sci Am 294:48–55
Davis DM (2007) Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol 7:238–243
Davis DM (2009) Are we done yet? Mechanisms and functions for the duration of intercellular contacts made by lymphocytes. Nat Rev Immunol (in press)
Davis DM, Dustin ML (2004) What is the importance of the immunological synapse? Trends Immunol 25:323–327
Davis SJ, van der Merwe PA (1996) The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today 17:177–187
Davis SJ, van der Merwe PA (2006) The kinetic-segregation model: TCR triggering and beyond. Nat Immunol 7:803–809
Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL (1999) The human natural killer cell immune synapse. Proc Natl Acad Sci USA 96:15062–15067
Dietrich J, Cella M, Colonna M (2001) Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol 166:2514–2521
Dustin ML (2008a) Hunter to gatherer and back: immunological synapses and kinapses as variations on the theme of amoeboid locomotion. Annu Rev Cell Dev Biol 24:577–596
Dustin ML (2008b) T-cell activation through immunological synapses and kinapses. Immunol Rev 221:77–89
Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER (1997) Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA 94:3909–3913
Egen JG, Allison JP (2002) Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16:23–35
Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C (2005) Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244). Blood 105:4722–4729
Endt J, McCann FE, Almeida CR, Urlaub D, Leung R, Pende D, Davis DM, Watzl C (2007) Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J Immunol 178:5606–5611
Eriksson M, Leitz G, Fallman E, Axner O, Ryan JC, Nakamura MC, Sentman CL (1999a) Inhibitory receptors alter natural killer cell interactions with target cells yet allow simultaneous killing of susceptible targets. J Exp Med 190:1005–1012
Eriksson M, Ryan JC, Nakamura MC, Sentman CL (1999b) Ly49A inhibitory receptors redistribute on natural killer cells during target cell interaction. Immunology 97:341–347
Fan QR, Long EO, Wiley DC (2000) Cobalt-mediated dimerization of the human natural killer cell inhibitory receptor. J Biol Chem 275:23700–23706
Fassett MS, Davis DM, Valter MM, Cohen GB, Strominger JL (2001) Signaling at the inhibitory natural killer cell immune synapse regulates lipid raft polarization but not class I MHC clustering. Proc Natl Acad Sci USA 98:14547–14552
Faure M, Barber DF, Takahashi SM, Jin T, Long EO (2003) Spontaneous clustering and tyrosine phosphorylation of NK cell inhibitory receptor induced by ligand binding. J Immunol 170:6107–6114
Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C (2002) Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 195:343–351
Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L (1999) Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 5:405–411
Fernandez-Suarez M, Ting AY (2008) Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol 9:929–943
Fourmentraux-Neves E, Jalil A, Da Rocha S, Pichon C, Chouaib S, Bismuth G, Caignard A (2008) Two opposite signaling outputs are driven by the KIR2DL1 receptor in human CD4+ T cells. Blood 112:2381–2389
Guerra N, Michel F, Gati A, Gaudin C, Mishal Z, Escudier B, Acuto O, Chouaib S, Caignard A (2002) Engagement of the inhibitory receptor CD158a interrupts TCR signaling, preventing dynamic membrane reorganization in CTL/tumor cell interaction. Blood 100:2874–2881
Harwood NE, Batista FD (2008) New insights into the early molecular events underlying B cell activation. Immunity 28:609–619
Henel G, Singh K, Cui D, Pryshchep S, Lee WW, Weyand CM, Goronzy JJ (2006) Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood 107:4449–4457
Inoue H, Miyaji M, Kosugi A, Nagafuku M, Okazaki T, Mimori T, Amakawa R, Fukuhara S, Domae N, Bloom ET et al (2002) Lipid rafts as the signaling scaffold for NK cell activation: tyrosine phosphorylation and association of LAT with phosphatidylinositol 3-kinase and phospholipase C-gamma following CD2 stimulation. Eur J Immunol 32:2188–2198
Karre K, Ljunggren HG, Piontek G, Kiessling R (1986) Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319:675–678
Krzewski K, Strominger JL (2008) The killer's kiss: the many functions of NK cell immunological synapses. Curr Opin Cell Biol 20:597–605
Krzewski K, Chen X, Orange JS, Strominger JL (2006) Formation of a WIP-, WASp-, actin-, and myosin IIA-containing multiprotein complex in activated NK cells and its alteration by KIR inhibitory signaling. J Cell Biol 173:121–132
Kubin MZ, Parshley DL, Din W, Waugh JY, Davis-Smith T, Smith CA, Macduff BM, Armitage RJ, Chin W, Cassiano L et al (1999) Molecular cloning and biological characterization of NK cell activation-inducing ligand, a counterstructure for CD48. Eur J Immunol 29:3466–3477
Lanier LL (2005) NK cell recognition. Annu Rev Immunol 23:225–274
Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS (2002) T cell receptor signaling precedes immunological synapse formation. Science 295:1539–1542
Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J et al (2003) The immunological synapse balances T cell receptor signaling and degradation. Science 302:1218–1222
LeMaoult J, Caumartin J, Carosella ED (2007) Exchanges of membrane patches (trogocytosis) split theoretical and actual functions of immune cells. Hum Immunol 68:240–243
Ljunggren HG, Karre K (1990) In search of the “missing self”: MHC molecules and NK cell recognition. Immunol Today 11:237–244
Long EO (1999) Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 17:875–904
Long EO (2008) Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev 224:70–84
Lou Z, Jevremovic D, Billadeau DD, Leibson PJ (2000) A balance between positive and negative signals in cytotoxic lymphocytes regulates the polarization of lipid rafts during the development of cell-mediated killing. J Exp Med 191:347–354
Luo BH, Carman CV, Springer TA (2007) Structural basis of integrin regulation and signaling. Annu Rev Immunol 25:619–647
Martz E (1976) Multiple target cell killing by the cytolytic T lymphocyte and the mechanism of cytotoxicity. Transplantation 21:5–11
Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE (2006) CD94/NKG2A inhibits NK cell activation by disrupting the actin network at the immunological synapse. J Immunol 177:3590–3596
McCann FE, Suhling K, Carlin LM, Eleme K, Taner SB, Yanagi K, Vanherberghen B, French PM, Davis DM (2002) Imaging immune surveillance by T cells and NK cells. Immunol Rev 189:179–192
McCann FE, Vanherberghen B, Eleme K, Carlin LM, Newsam RJ, Goulding D, Davis DM (2003) The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43, and CD45 at activating and inhibitory human NK cell immune synapses. J Immunol 170:2862–2870
McKeithan TW (1995) Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA 92:5042–5046
McMahon CW, Raulet DH (2001) Expression and function of NK cell receptors in CD8+ T cells. Curr Opin Immunol 13:465–470
Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A (2006) Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev 214:219–228
Nakajima H, Cella M, Langen H, Friedlein A, Colonna M (1999) Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol 29:1676–1683
Nedvetzki S, Sowinski S, Eagle RA, Harris J, Vely F, Pende D, Trowsdale J, Vivier E, Gordon S, Davis DM (2007) Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 109:3776–3785
Newman KC, Riley EM (2007) Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol 7:279–291
Oddos S, Dunsby C, Purbhoo MA, Chauveau A, Owen DM, Neil MAA, Davis DM, French PMW (2008) High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J 95:L66–L68
Orange JS (2008) Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 8:713–725
Orange JS, Ramesh N, Remold-O’Donnell E, Sasahara Y, Koopman L, Byrne M, Bonilla FA, Rosen FS, Geha RS, Strominger JL (2002) Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA 99:11351–11356
Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL (2003) The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA 100:14151–14156
Pallandre JR, Krzewski K, Bedel R, Ryffel B, Caignard A, Rohrlich PS, Pivot X, Tiberghien P, Zitvogel L, Strominger JL et al (2008) Dendritic cell and natural killer cell cross-talk: a pivotal role of CX3CL1 in NK cytoskeleton organization and activation. Blood 112:4420–4424
Parolini S, Bottino C, Falco M, Augugliaro R, Giliani S, Franceschini R, Ochs HD, Wolf H, Bonnefoy JY, Biassoni R et al (2000) X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med 192:337–346
Peggs KS, Quezada SA, Allison JP (2008) Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev 224:141–165
Pritchard NR, Smith KG (2003) B cell inhibitory receptors and autoimmunity. Immunology 108:263–273
Rajagopalan S, Long EO (1998) Zinc bound to the killer cell-inhibitory receptor modulates the negative signal in human NK cells. J Immunol 161:1299–1305
Rajagopalan S, Long EO (2005) Understanding how combinations of HLA and KIR genes influence disease. J Exp Med 201:1025–1029
Rajagopalan S, Winter CC, Wagtmann N, Long EO (1995) The Ig-related killer cell inhibitory receptor binds zinc and requires zinc for recognition of HLA-C on target cells. J Immunol 155:4143–4146
Raulet DH (2004) Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol 5:996–1002
Sanni TB, Masilamani M, Kabat J, Coligan JE, Borrego F (2004) Exclusion of lipid rafts and decreased mobility of CD94/NKG2A receptors at the inhibitory NK cell synapse. Mol Biol Cell 15:3210–3223
Schleinitz N, March ME, Long EO (2008) Recruitment of activation receptors at inhibitory NK cell immune synapses. PLoS ONE 3:e3278
Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE (2006) Reversal of the TCR stop signal by CTLA-4. Science 313:1972–1975
Schneider H, Smith X, Liu H, Bismuth G, Rudd CE (2008) CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur J Immunol 38:40–47
Seminario MC, Bunnell SC (2008) Signal initiation in T-cell receptor microclusters. Immunol Rev 221:90–106
Semino C, Angelini G, Poggi A, Rubartelli A (2005) NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood 106:609–616
Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH et al (2007) Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell 129:773–785
Sivori S, Falco M, Marcenaro E, Parolini S, Biassoni R, Bottino C, Moretta L, Moretta A (2002) Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc Natl Acad Sci USA 99:4526–4531
Snyder MR, Muegge LO, Offord C, O’Fallon WM, Bajzer Z, Weyand CM, Goronzy JJ (2002) Formation of the killer Ig-like receptor repertoire on CD4+CD28null T cells. J Immunol 168:3839–3846
Sohn HW, Pierce SK, Tzeng SJ (2008) Live cell imaging reveals that the inhibitory FcgammaRIIB destabilizes B cell receptor membrane-lipid interactions and blocks immune synapse formation. J Immunol 180:793–799
Spaggiari GM, Carosio R, Pende D, Marcenaro S, Rivera P, Zocchi MR, Moretta L, Poggi A (2001) NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol 31:1656–1665
Springer TA (1990) Adhesion receptors of the immune system. Nature 346:425–434
Standeven LJ, Carlin LM, Borszcz P, Davis DM, Burshtyn DN (2004) The actin cytoskeleton controls the efficiency of killer Ig-like receptor accumulation at inhibitory NK cell immune synapses. J Immunol 173:5617–5625
Stebbins CC, Watzl C, Billadeau DD, Leibson PJ, Burshtyn DN, Long EO (2003) Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol 23:6291–6299
Strowig T, Brilot F, Munz C (2008) Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol 180:7785–7791
Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH (1999) Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J Immunol 162:6981–6985
Treanor B, Lanigan PM, Kumar S, Dunsby C, Munro I, Auksorius E, Culley FJ, Purbhoo MA, Phillips D, Neil MA et al (2006) Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol 174:153–161
Tsai RK, Discher DE (2008) Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 180:989–1003
Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS (2008) TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med 205:233–244
Ugolini S, Vivier E (2000) Regulation of T cell function by NK cell receptors for classical MHC class I molecules. Curr Opin Immunol 12:295–300
Vales-Gomez M, Erskine RA, Deacon MP, Strominger JL, Reyburn HT (2001) The role of zinc in the binding of killer cell Ig-like receptors to class I MHC proteins. Proc Natl Acad Sci USA 98:1734–1739
van Bergen J, Thompson A, van der Slik A, Ottenhoff TH, Gussekloo J, Koning F (2004) Phenotypic and functional characterization of CD4 T cells expressing killer Ig-like receptors. J Immunol 173:6719–6726
Varma R, Campi G, Yokosuka T, Saito T, Dustin ML (2006) T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25:117–127
Vyas YM, Mehta KM, Morgan M, Maniar H, Butros L, Jung S, Burkhardt JK, Dupont B (2001) Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol 167:4358–4367
Vyas YM, Maniar H, Dupont B (2002) Cutting edge: differential segregation of the SRC homology 2-containing protein tyrosine phosphatase-1 within the early NK cell immune synapse distinguishes noncytolytic from cytolytic interactions. J Immunol 168:3150–3154
Vyas YM, Maniar H, Lyddane CE, Sadelain M, Dupont B (2004) Ligand binding to inhibitory killer cell Ig-like receptors induce colocalization with Src homology domain 2-containing protein tyrosine phosphatase 1 and interruption of ongoing activation signals. J Immunol 173:1571–1578
Watzl C, Long EO (2003) Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med 197:77–85
Wulfing C, Purtic B, Klem J, Schatzle JD (2003) Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci USA 100:7767–7772
Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T (2005) Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol 6:1253–1262
Acknowledgements
We thank current members of our laboratory and F.J. Culley, M.A. Purbhoo, F.V. de Abreu, P.M.W. French, M.A.A. Neil and A.I. Magee for useful discussions. We thank N. Powell for assistance with preparing the figures. Research in our laboratory is funded by The Medical Research Council, The Biotechnology and Biological Sciences Research Council, The Wellcome Trust, a Lister Institute Research Prize and a Wolfson Royal Society Research Merit Award (to DMD).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Eissmann, P., Davis, D.M. (2010). Inhibitory and Regulatory Immune Synapses. In: Saito, T., Batista, F. (eds) Immunological Synapse. Current Topics in Microbiology and Immunology, vol 340. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-03858-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-03858-7_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-03857-0
Online ISBN: 978-3-642-03858-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)