Abstract
Atmospheric air at sea level contains 30 times more O2, when compared to fully O2-saturated water and, in addition, the O2 content of water is ever-changing. The gill systems of fish are highly efficient for O2 extraction, but this cannot save the animal if its O2 supply is insufficient. This explains why air-breathing in fish has evolved in at least 60 independent lines. Lungfish and bichirs (Polypteriidae) possess true lungs, whereas other air-breathing organs (ABOs) can be derived from the swimbladder as in the gar pike (Lepidosteus) and the bowfin (Amia). In Hypostomus sp. (Loricariidae) the ABO is a modified part of the digestive system. The functions of gills, ABOs and lungs all depend on surfactants. Aerial breathing increases with activity and/or reduced O2 availability in the water. In addition, increases of temperature result in larger air-breathing efforts. These responses are adjusted by O2 receptors, located in the gills, whereas the role CO2/H+-receptors is minor in actinopterygian fish.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Air and Water as Respiratory Media
Compared to air, water is an oxygen poor environment, because the O2 solubility in water is low. At 20°C, the O2 concentration in the fully saturated water is 0.007 (l−1 l−1 against 0.2095 (l−1 l−1) in air, which implies that air-breathers have 30 times more O2 available when compared to aquatic breathers in fully saturated water. The relative density and the viscosity of water and air should also be taken into account. The water density is no less then 800 times higher compared to the density of air (water: 1.00 kg l−1; air: 0.0013 kg l−1) and the water viscosity is 50-fold higher than atmospheric air at sea level. The joint analysis of these variables gives an impression of the difficulties faced by water-breathers to maintain an aerobic metabolism. The low O2 availability in the aquatic environment forces water-breathers to ventilate the respiratory surface area a much higher volume of inert mass with high density and viscosity, which leads to a high metabolic cost of gill ventilation. Gills are absent in most aquatic invertebrates, but they may ventilate some specific part of the body. Fish possess a highly sophisticated countercurrent system, in which the blood passes the gills in the opposite direction to the inspired and expired water flow. It should also be pointed out that the O2 content in water depends on photosynthesis, which changes on a daily and seasonal basis. Further determinants of O2 levels are water movement, currents, changes in temperature, and salinity (Dejours 1981).
The metabolic cost of gill ventilation in water-breathing fish is species-specific. It ranges between 3.7 and 5.7% of the total oxygen uptake (\(\dot {{\rm V}}{\rm O}_2\)) in the sharksucker, Echeneis naucrates, which is an unusually low value (Steffensen and Lomholt 1983), while the highest value (43%) was measured in the tench, Tinca tinca (Schumann and Piiper 1966). Gill ventilation consumed about 13–22% of the total \(\dot {{\rm V}}{\rm O}_2\) in carp, Cyprinus carpio, 10–25% in trout, Onchorhynchus mykiss (Hughes and Shelton 1962; Hughes and Saunders 1970), and 22–28% in plaice, Pleuronectes platessa (Edwards 1971). The cost of ventilation may increase in response to hypoxia, since hyperventilation is an attempt to compensate for the reduced O2 level. The metabolic cost of gill ventilation in normoxic Nile tilapia, Oreochromis niloticus, is about 3% in normoxia, increasing to 20% when the inspired PO2 falls to 35 mmHg (Fernandes and Rantin 1994). Likewise, the hypoxia-tolerant and sedentary erythrinid fish traíra, Hoplias malabaricus, spends 3% of its total \(\dot {{\rm V}}{\rm O}_2\) to ventilate its gills when in normoxia, but hypoxia (PwO2 = 25 mmHg) increases the cost of ventilation to no less than 13% (Rantin et al. 1992).
Air-breathing organs (ABOs) have evolved in at least 60 independent lines of teleost and holeost fish (Graham 1997). Fish of bimodal respiration have large advantages when exposed to hypoxic water. Transition to air-breathing will alleviate the hypoxic conditions, which obviously increases the chances for survival. In addition, air-breathing assures access to a steady O2 supply at a much higher concentration.
1.2 Evolution of the Atmosphere
The early atmosphere contained hydrogen and helium, which the solar wind gradually removed, after which CO2 became the prevalent gas. This prevalence of CO2 is still predominant in the atmospheres of Venus and Mars (Stearns and Hoekstra 2005). Geochemical research indicates that evolution of photosynthesis occurred in precursors to the cyanobacteria, which suggests that the catalyzing reaction of the photosystem II oxidizing complex emerged some 3 billion years ago (Dismukes et al. 2001), and this event would gradually lead to an ozone protection resulting from the photosynthesis. In addition, Dismukes et al. (2001) point out that oxidation of water is one of the most challenging multielectron reactions in biology.
Estimates for the atmospheric O2 levels are available from the beginning of the Silurian period to the end of the Permian period, which ranges from 444 to 250 million years ago. There is evidence that atmospheric O2 levels in the early Devonian could have reached up to 25%, exceeding our actual level of 20.95%. The middle and late Devonian periods were characterized by reduced O2 levels (13% O2), but then between 340 and 250 million years ago, the O2 levels rose and might have reach up to 35% (Berner and Canfield 1989; Scott and Glasspool 2006). As pointed out by Dudley (1998), it is unlikely that 35% O2 was exceeded, because this value is an approximate threshold for spontaneous combustion of the atmosphere. Recent studies agree with an O2 peak of up to 25% in the early Devonian, followed by low O2 levels of about 15% during the beginning of the Late Devonian (Frasnian) period (Clack 2007). Based on Dehadrai and Tripathi (1976), Daniels et al. (2004) proposed that Devonian bony fish may have developed aerial respiration due to low oxygen levels. The message of this short section is that ambient O2 availability is a principal determinant of the levels of metabolism and of new strategies for respiratory function; one of these is bimodal respiration.
1.3 Hypoxia and Hypercarbia
Based on Junk (1984), Nelson et al. (2007) listed some adverse conditions for O2 uptake, which are particularly common in tropical and subtropical regions, and these are: (1) respiratory rates may become larger than the production of O2 by photosynthesis, (2) a stagnant air/water interface impedes an adequate distribution of O2, and (3) a poor light penetration decreases photosynthesis.
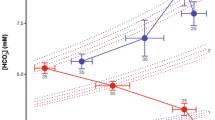
By comparison, the atmosphere above us seems a stable source of O2. At sea level and 20°C, the PO2 would be about 155 mmHg with 20.95% O2. The atmospheric source of O2 will obviously be advantageous for fish exposed to frequent reductions of ambient O2 levels. Hypercarbia is the condition of elevated CO2 levels in the environment, whereas hypercapnia is elevated CO2 levels within the body. Temperature is also an important factor, since higher temperatures increase the metabolic demands, and, the higher the temperature, the larger are the increases of gill ventilation in response to hypoxia (Glass et al. 1990). An ever-changing O2 availability is a challenge to the O2-oriented respiratory control of teleost and holeost fish (cf. Dejours 1981), and each species has a critical PO2 threshold (PcO2) below which the normal \(\dot {{\rm V}}{\rm O}_2\) can no longer be maintained. Holeosts and teleosts compensate for an excess of CO2 (hypercarbia) by active increases of plasma [\({\rm HCO}_3^-\)] levels, which returns pHa towards the normal (normocarbic) value. About 90% of acid–base relevant ions are mediated by specialized cells within the gill epithelia, and the kidney contributes the rest (Heisler and Claiborne 1986).
The PcO2 of an exclusively water-breathing fish often reflects the \({\rm O}_2^-\) availability of the habitat. As a typical example, carp (Cyprinus carpio) is very resistant to hypoxic conditions, and its Hb–O2 dissociation curve (ODC) has a very high Hb–O2 affinity, which implies that a high O2 saturation is preserved until PaO2 falls to extremely low pressures (P50 = 9.4 mmHg at 20°C) (Albers et al. 1983), which explains its survival under severely hypoxic conditions (Soncini and Glass 2007). Conversely, dourado (Salminus maxillosos) is a river fish capable of a high swimming speed, and it is distributed in regions of Southern Brazil. At pHa 7.7, its P50 is high (21 mmHg at 25°C.) This leaves dourado vulnerable to reductions of O2, because moderately hypoxic conditions will reduce its Hb–O2 saturation and, thereby, decrease its swimming performance (Salvo-Souza et al. 2001).
1.4 Gill Function and O2 Extraction
The O2 extraction of a gas exchanger can be defined as:
An alternative expression is:
where \(\dot {{\rm V}}{\rm O}_2 = {\rm O}_2\) uptake, \(\dot {{\rm V}}_{\rm G} =\) the water flow irrigating the gill, CIO2 and CEO2 = O2 contents of the inspired and expired water (Dejours 1981). The difference \(({\rm C}_{\rm I}{\rm O}_2\hbox{--}{\rm C}_{\rm E}{\rm O}_2) \cdot {\rm C}_{\rm I}{\rm O}_2^{-1}\) is the amount of O2 extracted by the blood flow and CIO2 is the total inspired amount of O2. The degree of O2 extraction by fish gills is high and often about 85% (Lomholt and Johansen 1979). This is very high compared to the extraction of a human lung, which is only about 30%. Evidently, the gas exchange is very effective, because the blood flows in the opposite direction to the water movement, and the gas exchange takes place within the blood lacunae of the secondary lamellae (cf. Piiper 1992).
A cardio-vascular shunt is defined as a quantity of incompletely saturated blood that bypasses the gas exchanges surfaces. As the second step, the shunted blood fraction mixes into the saturated blood from the gas exchanger, and the larger this shunt, the lower Hb–O2 saturation. Such shunts are apparently small or absent in fish gills, although there is some evidence for shunted blood within the basal channels of the secondary lamellae of rainbow trout (Oncorhynchus mykiss) (Tuurala et al. 1984). On the other hand, convincing evidence against major shunts was provided by Eddy (1974), who constructed an in vivo Hb–O2 dissociation curve for tench (T. tinca). The saturation of the oxygen dissociation curve (ODC) ranged from 75 to 95%, but the in vivo arterial point was in some cases located on the upper portion of the oxygen dissociation curve (ODC), which indicates that shunts are minimal. Most teleost fish have almost hyperbolic ODCs (nHill ∼ 1.0 to 1.3), which implies that PO2, even when high, cannot saturate the blood. Therefore, the incomplete saturation can be due to the shape of the ODC and not to cardiovascular shunt (Glass and Soncini 1997). Application of hyperoxia permits calculation of the shunt fraction based on the mixed venous and arterial points. Such measurements are available for pacu (Piaractus mesopotamicus), in which the shunt fraction was less than 10%. The equation cannot distinguish between diffusion limitation and ventilation–perfusion mismatching, which leaves 10% shunt as an overestimated value (Soncini and Glass 1997). Consistent data were obtained by Gilmour and Perry (1994), when they exposed trout (O. mykiss) to an aquatic PO2 of 548 mmHg, which increased PaO2 to a range within 352–502 mmHg. The characteristics of teleost gills are impressive, but in spite of this the fish may not survive severely hypoxic conditions. This explains why air-breathing in fish has evolved within many different and, mainly, tropical taxonomic groups.
Some fish avoid hypoxia by skimming the air/water interface, which has the highest O2 level. This respiratory behavior is known as aquatic surface respiration (ASR) and is common to a number of tropical fish that inhabit lakes with frequent hypoxic conditions (Kramer and McClure 1982). An example is pacu (P. mesopotamicus). To escape from hypoxia, this species performs ASR, which is facilitated by a gradual swelling and extension of the lower lip (Saint-Paul and Bernardino 1988; Val and Almeida-Val 1995). Pacu immediately initiates ASR even in moderate hypoxia (50–70 mmHg), while the full development of swelling may take no less than 3 h or more (Rantin et al. 1998).
1.5 Air-Breathing Organs and Their Function
Respiration by a lung or an ABO requires surfactant to reduce surface tension. Daniels et al. (2004) addressed the role of surfactant in fish in two air breathers: the pirarucu (Arapaima gigas) and the tarpon (Megalops cyprionoides), along with the exclusively water-breathing snapper (Pagrus auratus). They concluded that the fish surfactants share basic features with the alveolar Type II cells of tetrapod lungs, and proposed that surfactant in lungs or swimbladders are homologous. The swimbladder and the lungs are of separate ontogenetic origin (Perry 2007), and Daniels et al. (2003) proposed that surfactant systems already existed before lungs and swimbladders evolved. It also turns out that the surfactant of Osteichthyes is uniform, and may represent the vertebrate prototype.
Adequate O2 uptake and CO2 elimination can be obtained via modifications of structure and function, which often leads to surprising solutions. In this section, we present a selection of ABOs and their function, beginning from the most ancient forms. The text takes us from real lungs to respiration using modified stomach tissue.
1.6 Polypterus senegalus (Polypteridae)
The genera Polypterus sp. and Erpetoichthys [earlier Calamoichthys] form a prominent group of ancient fish. It has been argued that they are a phylogenetically isolated group and that they are the most original extant representatives of actinoterygians (Carroll 1987), and their habitats are tropical waters in Africa. Land vertebrates, lungfish and Polypteriformes possess lungs of a ventral, posterior pharyngeal origin and these are pleisiomorphic paired structures (Perry 2007), i.e. they are ancestral trait and are not apomorphic structures.
There is little information available on the respiratory physiology of Polypteriformes. Studying a specimen of P. senegalus, Babiker (1984) reported that the fish in aerated water would surface only once or twice between 11p.m. and 3a.m., which would classify the specimen as a marginal obligatory air-breather. Juvenile specimens (10–30 g) were exclusively gill breathers, which proved fatal during aquatic hypoxia. Larger specimens (290–460 g) survived due to air-breathing, and Magid et al. (1970) measured their lung gas PO2, which increased from about 30 to 100 mmHg during inhalation, with a concomitant reduction of PaCO2 from about 14 to 9 mmHg. In this context, it should be mentioned that aerial respiration in fish increases PaCO2 and lowers pHa and, according to Dejours (1981): “the more an animal depends on pulmonary breathing, the higher its PCO2”. The reason for this is the 30-fold difference of O2 content in water and in atmospheric air. To compensate for this difference, an exclusively gill-breathing fish must ventilate a 30-fold higher volume, when compared to an air breather. A high degree of gill ventilation leaves a carp with a PaCO2 of 2–3 mmHg (Glass et al. 1990), while the PaCO2 P. senegalus would be about 3-fold higher due to additional aerial ventilation. Reductions of PwO2 markedly increased pulmonary ventilation of P. senegalus. As a second defense strategy, the O2 loss to near anoxic water was minimal, due to a shutdown of gas exchange by the gills (Lomholt and Glass 1987). This is consistent with Babiker (1984), who reported that small specimens (10–30 g) would not develop air-breathing, whereas larger individuals (> 100 g) could survive in near-anoxic water by lung ventilation.
1.7 The gars (Lepisosteus sp.) and the bowfins (Amia calva) Amiidae, Amiiformes
The gar pikes and the bowfins form a sister group which is ramified with the teleosts (Filleul and Lavoué 2001). The gar and the bowfins are restricted to North America, and their ABOs are gas bladders (Hedrick and Jones 1999). The ventilation of an ABO may seem irregular, but this is not the case for Amia. Hedrick et al. (1994) applied a spectral analysis to evaluate air-breathing behavior, and two distinct types of breaths were recorded. The first type began with an expiration followed by inhalation. A second type of breath consisted of an inhalation and the authors very reasonably suggested that this behavior represented an adjustment related to buoyancy. Upon removal of this component, a very regular pattern emerged, with an interval of 30 min between breaths at the surface (temperature 20–24°C), which indicated the presence of a central rhythm generator and/or peripheral O2-receptor input.
The effects of activity on air-breathing in Amia and L. oculatus were evaluated by Farmer and Jackson (1998), who first studied the animal at rest and then at stepwise increases of swimming speed (temp.19–23°C). It turned out that L. oculatus at rest obtained less than 2% of total O2 uptake from aerial respiration, but it turned out that swimming activity increased the contribution of O2 from air-breathing to no less than 53% of total \(\dot {{\rm V}}{\rm O}_2\). The corresponding numbers for aerial respiration in Amia were 10% at rest and no less than 66% of total \(\dot {{\rm V}}{\rm O}_2\). As the authors pointed out, the capacity to increase ventilation of the ABO is important for activity, and not only for survival in hypoxic waters.
As expected, the long-nosed gar increased ventilation of the ABO, when exposed to hypoxic water. In addition, it reduced gill ventilation, which minimized O2 losses to the water (Smatresk et al. 1986). Similar responses have been reported for Polypterus in near-anoxic water (Lomholt and Glass 1987).
There is evidence for membrane-bound carbonic anhydrase located in the ABO of Amia, and it resembles mammalian carbonic anhydrase IV as regards inhibition characteristics and membrane attachment (Gervais and Tufs 1998). Curiously, glucose is a major source of fuel in the Florida gar (L. plathyrhinchus), and the enzyme activities of its ABO resembles those of a mammalian lung, and are different from the enzymes of fish swimbladders (Frick et al. 2007). Evidently, the function of the ABO can be sophisticated, and it turned out that mechanoreceptors are present in L. oculatus (Smatresk and Azizi 1987). Two types of receptors were identified. A rapidly adapting type was identified, while a slowly adapting type turned out to be CO2-sensitive. Application of hypercarbia (6–10% CO2) decreased firing rate of the slowly adapting receptors to the slowly adapting receptors. Very similar responses have been reported for lungfish (DeLaney et al. 1983) and land vertebrates (Milsom 2002), which would suggest a very ancient origin, if the receptors turn out to be identical (Smatresk et al. 1987 ).
1.8 Channa argus (Channidae)
The snakehead fish Channa argus is carnivorous and widely distributed in temperate zone of East Asia. Ventilation of the ABO is obligatory during the summer season, whereas survival by gill ventilation alone is possible during the winter. The ABO of Channa is placed within the gill system. The first and second gill arches are perfused by the anterior ventral aorta, after which the blood traverses the bilaterally and dorsally positioned ABO. Differently, the posterior ventral aorta supplies blood to the third and fourth gill arches, bypassing the ABO (Andresen et al. 1987). Animals weighing 1–2 kg were studied at 15–25°C to evaluate the combined effects of hypoxia and temperature on aerial ventilation. Glass et al. (1986) exposed Channa to normoxia (∼155 mmHg) and hypoxia (75 and 35 mmHg). Aquatic hypoxia was tested and the responses were weak but significant at 25°C, while the response at 15°C failed to reach statistical significance. On the other hand, aerial hypoxia had a large effect on ventilation of the ABO. At 15°C, the ventilation of the ABO rose 2.8-fold with reduction of gas phase PO2 from 155 to 35 mmHg, and at 25°C the same reduction increased aerial ventilation 3.4-fold. Curiously, this response to hypoxia is also amplified by high temperature in the South American lungfish (Lepidosiren paradoxa) and in ectothermic land vertebrates, including the toad Chaunus scheideri (earlier Bufo paracnemis) (Kruhøffer et al. 1987) and the turtle Chrysemys picta bellii (Glass et al. 1983); see Fig. 1 .
This figure compares the effects of hypoxia combined with increases of temperature. a The South American lungfish Lepidosiren paradoxa (da Silva et al., in preparation). b The toad Chaunus schneideri (Kruhøffer et al. 1986). c The teleost fish Channa argus, a facultative air-breather equipped with an air-breathing organ. (Glass et al.). d The turtle Chrysemys picta bellii modified from Glass et al. (1983). These responses are similar, although they are obtained from very distant groups
In addition, Channa had a weak increase of aerial ventilation in response to aquatic hypoxia, while the South American lungfish (L. paradoxa) had no increase of pulmonary ventilation in response to aquatic O2 levels. This raises questions about the positions and roles of the involved O2 chemoreceptors.
Figure 1 clearly shows that ventilatory responses to hypoxia in air breathers become strongly reduced when temperature decreases. The dependence of exclusive water breathers may be different. Carp (Cyprinus carbio) increased gill ventilation in response to light hypoxia at 10, 20 and 25°C. These responses were significant both at 10, 20 and 25°C. Moreover, the gain of the responses could be expressed as:
Expressed in this way, it became clear that the percentage increase was independent of temperature. In other words, the control value and ventilation at a level of hypoxia were scaled up or down by the same factor, which differs from responses to gas-phase hypoxia, which fade away in response to low temperature (Glass et al. 1990; Soncini and Glass 2000).
Channa seemed to respond to hypercarbia (range 0–8%), but this effect did not reach significant levels. Likewise, Graham and Baird (1982) reported that Hypostomus would elicit ABO breathing more easily, if hypercarbia (∼10 mmHg) was added. Moreover, juvenile specimens of the bichir (P. senegalus) increased gill ventilation in response to 0.8%CO2 in the water. Therefore, it seems that a weak CO2-related component is present, and this topic will be taken up later.
1.9 Hypostomus sp.(Loricariidae)
Facultative air-breathers ventilate exclusively use gills when in normoxic water, whereas the ABO is ventilated, when the water turns hypoxic. This applies to the armored catfish Ancistrus chagresi, Hypostomus plecostomus and Hypostomus regain, which have species-specific thresholds for the onset of aerial ventilation. Thus, A. chagresi initiated ventilation of the ABO when PwO2 fell to 33 mmHg, whereas H. plecostomus already ventilated the ABO, when PwO2 dropped to the higher threshold of 60 mmHg. Application of light hypercarbia (∼10 mmHg) changed both thresholds to the higher values 64 mmHg and 79 mmHg, which was in agreement with the relative sensibility of the two species (Graham 1982). Their ABO is modified tissue located within the stomach. The stomach tissue of the catfish H. plecostomus is quite different from that of typical fish, since the wall is transparent, and the mucosal layer is smooth, and capillaries are abundant in the arterial part of the stomach. Part of the epithelium contains respiratory epithelial cells, where the air–blood is thin (0.25–2.02 μm) (Podkowa and Goniakowska-Witalinska 2003).
Rhinelepis strigosa (cascudo preto) has been studied in some detail. It will not surface when in well-oxygenated water at 25°C, but air-breathing was initiated below a PwO2 of 30 mmHg and it peaked at 7 breaths · h−1 at 10 mmHg (Takasusuki et al. 1998), which certainly proves the efficiency of respiration by an ABO. In this context, it should be remembered that temperature and O2 levels interact, and behavioral responses may include dislocation to a lower temperature and/or a higher O2 level (Schurmann and Steffensen 1994).
Mattias et al. (1998) measured \(\dot {{\rm V}}{\rm O}_2\) and gill respiration during progressive hypoxia in the facultative air-breather H. regani. Air-breathing was absent in aerated water, but H. regani maintained aquatic \(\dot {{\rm V}}{\rm O}_2\) at 31 ml O2 kg−1 h−1 down to the critical oxygen tension (PcO2) of 34 mmHg (temperature 25°C). Gill ventilation and ventilation of the ABO reached their peak values within the PIO2 range from 56 to 25 mmHg (Fig. 2)
The threshold O2 tension for air breathing (shaded area) and air-breathing frequency (filled triangle) of H. regani with free access to the water surface and aquatic \(\dot {{\rm V}}{\rm O}_2\) (filled circle) when the access to the water surface was denied during progressive hypoxia. Arrow indicates the PcO2. Asterisks indicate significant difference compared to the normoxic values. Values are mean ± SEM. (Modified from Mattias et al. 1998)
1.10 Clarias sp.(Clariidae)
Members of the genus Clarias ventilate the ABO in a regular manner, and some species maintain a low level of air-breathing, when in normoxia. In spite of this, some studies question which species are obligatory or facultative air breathers. Most of the studies on C batractus, C. lazera, C. gariepinus and C. macrocephalus concluded that they are facultative air breathers (Hora 1935; Magid 1971; Donnelly 1973; Jordan 1976; Bevan and Kramer 1987), but some authors consider the first two species as obligatory air-breathers (Greenwood 1961; Singh and Hughes 1971).
The ABO in this genus (Fig. 3) consists of two chambers in superbranchial position and located within the posterior–dorsal part of the opercular cavity. This space is nearly filled up with arborescent gill organs, which are extensions of the second and fourth gill arches. In addition, the openings to each chamber are equipped with valves to prevent water to enter. These valves are fan shaped extensions and are located on the last gill lamellae of the interior gill arch. Aerial respiration involves a modified gill epithelium, including the inner surface of these openings, its base, the walls of the interior chamber, and the end of the arborescent surfaces (Munshi 1976; Singh et al. 1982).
Gill structures of the African catfish, Clarias gariepinus, showing: (a) general view of the four gill arches with the ABO on top (RS – right side), (b) closer look at the ABO showing the respiratory fan and the arborescent organ, which is the gas-exchange unit (RS – right side), and (c) Details of the four gill arches showing the branchial system with lamellae, ventilatory fan in the 3rd and 4th gill arches and the arborescent organ (ABO) in the 2nd and 4th gill arches (LS – left side)
The African catfish Clarias gariepinus is a facultative air-breather, which can maintain an adequate \(\dot {{\rm V}}{\rm O}_2\) down to a critical PO2 of about 55 mmHg. Below this tension, the fish increases the \(\dot {{\rm V}}_{\rm G}\) considerably, due to a larger VT. At this point the species develops hypoxic bradycardia before the air-breaths, followed by tachycardia after the surface episode (unpublished data). Figure 4 shows the frequency of air breathing (fAR) of C. gariepinus in response to graded hypoxia.
Air-breathing frequency of the African catfish, Clarias gariepinus, as a function of the inspired PO2. The arrow indicates the critical oxygen tension (PcO2 = 55 mmHg) for this species. Notice that the f AR increased significantly near the PcO2 to reach maximum values (10-fold in relation to the initial values) at a PIO2 of 20 mmHg. Asterisks represent statistical significance in relation to the normoxic condition (unpublished data)
1.11 Oxygen and CO2/H+ Receptors
The ever-changing conditions in water are matched by a highly O2-oriented respiratory control in holeost and teleost fish. Oxygen receptors are located within the gill system to monitor blood gases, or alternatively, the inspired water (Burleson and Milsom 2005a, b, 1990). These external receptors are important for homeostasis of the blood. As an example, carp (Cyprinus carbio) was exposed to light hypoxia (PwO2 = 97 mmHg), but PaO2 remained at ∼30 mmHg during both normoxia and hypoxia. Simultaneously, gill ventilation increased from 296 to 470 ml kg−1 min−1, due to stimulation by the external O2 receptors. In addition, there is evidence for [O2] receptors in carp, since gill ventilation increased 50%, after reduction of [O2]a from 6.8 to 4.4 vol% by application of CO. Carp were also exposed to hypercarbia (normoxia, 7 mmHg and 14 mmHg, which increased gill ventilation twofold. Unfortunately, the increases of gill ventilation correlated with significant reductions of [O2]a due to hypercarbia-induced Root and Bohr-shifts (Soncini and Glass 2000). Therefore, it was not possible to pin down the exact modality of the underlying receptors.
Gilmour et al. (2005) studied cardiac and respiratory responses to hypercarbia in tambaqui (Colossama macroporum). Stepwise increase of PaCO2 augmented the amplitude and frequency of ventilatory movements, which suggested the presence of CO2 receptors. The tested range was PCO2 = 7–25 mmHg, but information on conditions in the habitat would have been useful. The responses to CO2 predominated whereas the effects of pH changes were weaker. As a problem, it is difficult to distinguish between specific CO2/H+ receptors and [O2] receptors. The latter type can be stimulated by reduction of [O2] due to Bohr shifts and Root effects (cf. Soncini and Glass 2000).
Perry et al. (2004) addressed the exposure of tropical fish to large fluctuations of ambient O2 levels. One choice was the air-breathing erythrinid jeju (Hoplerythrinus unitaeniatus), an active freshwater predator, widely distributed in tropical and subtropical regions of South America (Kramer 1978; Rantin and Johansen 1984). The air-breathing organ (ABO) of jeju is the swimbladder, which is subdivided into an anterior and a posterior chamber (Fig. 5), which is subdivided into an anterior richly vascularized respiratory portion, and a nonrespiratory caudally oriented sac. As a facultative air-breather, jeju relies primarily on its gills for gas exchange as long as the water remains normoxic or moderately hypoxic, but it ventilates its ABO as a facultative option, when exposed to severe aquatic hypoxia (Kramer 1978; Stevens and Holeton 1978; Graham 1997). It turned out that this species had no release of plasma catacholamines, regardless of levels of aquatic hypoxia. With reductions of PwO2 the ventilation of the ABO increased in a hyperbolic manner down to PwO2 = 10 mmHg. On the other hand, its hypoxia-tolerant relative traira (Hoplias malabaricus) depends completely on gill ventilation, and its catacholamine levels increased no less than 18-fold, which clearly documents the advantages of an ABO. Catacholamines have been considered as a stimulus to gill ventilation (Randall and Taylor 1991), but their release failed to increase ventilation of the ABO in jeju. Oliveiro et al. (2004) evaluated the effects of aquatic hypoxia on jeju, and it turned out that the critical PO2 was PwO2 = 40 mmHg, and 50% of its time was spent at the surface, when PIO2 was reduced to 20 mmHg (Fig. 6). In this species, air-breathing was totally abolished after complete branchial denervation (cranial nerve IX to first gill arch and all branches of cranial nerve X innervating the four gill arches). This indicates that the control of air-breathing in jeju involves O2 chemoreceptors distributed on all gill arches (Lopes 2003 ).
Swimbladder of jeju, Hoplerythrinus unitaeniatus. a Intact organ showing the anterior membranous and nonvascularized portion (AC – anterior chamber) and the gas exchange unit (PC – posterior chamber with the vascularized anterior segment). b Opened bladder showing the gas exchange area (GEA). Courtesy from Dr. Marisa Narciso Fernandes (UFSCar, São Carlos, SP, Brazil)
Air breathing frequency (f AR) and time spent at the surface (T AR) of jeju, Hoplerythrinus unitaeniatus as a function of the inspired PO2. The arrow indicates the critical oxygen tension (PcO2 = 40 mmHg) for this species. Asterisks represent statistical significance in relation to the normoxic condition (modified from Oliveiro et al. 2004)
Several studies report that hypercarbia stimulates ventilation of the gills and/or an ABO, but these effects are usually weak and, therefore, hard to pin down. By contrast, the responses to hypoxia are large and predominant. The existence of central acid–base receptors in holeost and teleost fish is still a disputed topic. Hedrick et al. (1991) applied central superfusion of mock CSF to the ventricular system of Amia calva, but this failed to stimulate ventilation of the ABO.
The long-nosed gar (L. osseus) gave a different and partly controversial result. Wilson et al. (2000) reported that hypercarbia increased the frequency of the air-breathing motor output from the in vitro brain stem. Smatresk and Cameron 1982) had earlier reported a modest increase of aerial ventilation in the gar pike (Amia calva) exposed to hypercarbia. It is not a surprise that an isolated preparation can change firing characteristics, if it is isolated from a possible frequency modulator, and the discovery of central respiratory neurons is highly important.
1.12 The Air-Breathing Descendents of the Sarcopterygians
Sarcopterygians (lobe-finned fish) are in a key position in vertebrate evolution, because their descendants were the land vertebrates (Tetrapoda), the lungfish (Dipnoi) and the coelacanth (Actinistia). Currently, the most likely sister group constellation is lungfish with the tetrapods (Toyama et al. 2000; Brinkmann et al. 2004), and a fossil (Styloichthys) estimated to be 417 years old may be their last common ancestor (Zhu and Yu 2002). The South American lungfish Lepidosiren paradoxa inhabits the Amazon and Paraná–Paraguai regions, while the African lungfish Protopterus inhabits West and South Africa with four species. The Australian lungfish (Neoceratodus forsteri) inhabits rivers within the Queensland region, and it has well-developed gills combined with a simple lung (Kind et al. 2002). The gills of Protopterus and L. paradoxa are highly reduced, while the lungs are well developed, and the O2 uptake from the water is only 5% at 25°C and nil at 35°C, whereas the CO2 outputs were 50 (25°C) and 25% (35°C) (Amin-Naves et al. 2004). Lungfish have true lungs with a diffusing capacity (DLO2) is on level with that of a bullfrog (Bassi et al. 2005; Moraes et al. 2005), but the capacity for a similar-sized mammal is 16-fold higher (Takezawa et al. 1980). Just like land vertebrates, L. paradoxa and P. annectens have central chemoreceptors involved in acid–base regulation (Sanchez et al. 2001; Gilmour et al. 2007) and can be stimulated by CO2 and H+ (Amin-Naves et al. 2007a). The central chemoreceptors of L. paradoxa provide 80% of acid–base-related drive to ventilation, while 20% of the drive is provided by peripheral receptors. These relative contributions of peripheral and central CO2/H+ drives are largely identical to those of tetrapods (Amin-Naves et al. 2007b). Intrapulmonary receptors that are stimulated by stretch and inhibited by high CO2 levels are found in many land vertebrates (Milsom 2002), and are also present in L. paradoxa and Protopterus (Delaney et al. 1983). Many highly specific physiological mechanisms are evidently common to tetrapods and lungfish, which certainly back up the idea of a sister group.
References
Albers C, Manz R, Muster D, Hughes GM (1983) Effects of acclimation temperature on oxygen transport in the blood of the carp, Cyprinus carpio. Respiration Physiology 52:165–179
Amin-Naves J, Giusti H, Glass ML (2004) Effects of acute temperature changes on aerial and aquatic gas exchange, pulmonary ventilation and blood gas status in the South American lungfish, Lepidosiren paradoxa. Comparative Biochemistry and Physiology A 138:133–139
Amin-Naves J, Giusti H, Hoffman A, Glass ML (2007a) Components to the acid-base related ventilatory drives in the South American lungfish Lepidosiren paradoxa. Respiratory Physiology and Neurobiology 155(1):35–40
Amin-Naves J, Giusti H, Hoffman A, Glass ML (2007b) Central ventilatory control in the South American lungfish, Lepidosiren paradoxa: contributions of pH and CO2. Journal of Comparative Physiology B 177:529–534
Andresen JH, Ishimatsu A, Johanse K, Glass ML (1987) An angiographic analysis of the central circulation in the air breathing teleost Channa argus. Acta Zoologica (Stockholm) 68:165–171
Babiker MM (1984) Development of dependence on aerial respiration in Polypterus senegalis (Cuvier) Hydrobiologia 110:351–363
Bassi M, Klein W, Fernandes MN, Perry SF, Glass ML (2005) Pulmonary oxygen diffusing capacity of the South American lungfish Lepidosiren paradoxa: Physiological values by the Bohr integration method. Physiological and Biochemical Zoology 78(4):560–569
Berner RA, Canfield DE (1989) A new model of for atmospheric oxygen over phanerozoic time. American Journal of Science 289:33–361
Bevan DJ, Kramer DL (1987) The respiratory behavior of an air-breathing catfish, Clarias macrocephalus (Clariidae). Canadian Journal of Zoology 65:348–353
Branco LG, Glass ML, Wang T, Hoffman A (1993) Temperature and central chemoreceptor drive to ventilation in toad (Bufo paracnemis) Respiration Physiology 93(3):337–346
Brinkmann H, Denk A, Zitzle J, Joss JMP, Meyer A (2004) Complete mitochondrial genome sequence of the South American and the Australian lungfish: testing of the phylogenetic performance of mitochondrial data sets for phylogenetic problems in tetrapod relationships. Journal of Molecular Evolution 59:834–848
Burleson ML, Milsom WK (1990) Propanol inhibits O2-sensitive chemoreceptor activity in trout gills. American Journal of Physiology 27:R1089–R1091
Burleson ML, Milsom WK (2005a) Cardio-ventilatory control in rainbow trout: I. Pharmocology of branchial oxygen-sensitive chemoreceptors. Respiration Physiology 100:231–238
Burleson ML, Milsom WK (2005b) Cardio-ventilatory control in rainbow trout: II. Reflex effects of exogeneous neurochemicals. Respiration Physiology 101:289–299
Carroll RL (1987) Vertebrate Palaeontology and Evolution. Freeman, New York
Clack JA (2007) Devonian climate change, breathing, and the origin of the tetrapod stem group. Integrative and Comparative Biology Advanced access 47:510–523
Daniels CB, Orgeig S, Sullivan LC, Ling N, Bennett MB, Schürch AL, Braunder CJ (2004) The origin and evolution of the surfactant system in fish: insights into the evolution of lungs and swimbladders. Physiological and Biochemical Zoology 77(5):732–749
da Silva GSF, Sanchez AP, Giusti H, Amin-Naves J, Glass ML (2006) Control of pulmonary ventilation in the lungfish Lepidosiren paradoxa: The effects of temperature and hypoxia. First International Congress of Respiratory Biology. Abstracts pp 94–95
Dehadrai PV, Tripathi SD (1976) Environment and and ecology of freshwater breathing teleost, In: GM Hughes ed. Respiration of Amphibious Vertebrates. 1st Ed. Academic, London, pp 39–72
Dejours P (1981) Principles of Comparative Respiratory Physiology. 2nd revision. Elsevier, Amsterdam
Delaney RG, Laurent P, Galante R, Pack AI, Fishman AP (1983) Pulmonary mecanoreceptors in the dipnoi lungfish Protopterus and Lepidosiren. American Journal of Physiology 244:R418–R428
Dismukes GC, Klimov VV, Baranov SV, Kozlov YN, DasGupta J, Tyryshkin A (2001) The origin of atmospheric oxygen on Earth: The innovation of oxygenic photosynthesis. Proceedings of the National Academy of Sciences 98:2170–2175
Donnelly BG (1973) Aspects of behavior in the catfish Clarias gariepinus (Pices: Clariidae), during periods of habitat desiccation. Arnoldia 6(9):1–8
Dudley R (1998) Atmospheric O2, giant Paleozoic insects and the evolution of aerial locomotor performance. Journal of Experimental Biology 201:1043–1050
Eddy FB (1974) Blood gases in the tench (Tinca tinca) in well aerated and oxygen deficient water. Journal of Experimental Biology 60:71–83
Edwards RRC (1971) An assessment of the energy cost of in gill ventilation in the plaice (Pleuronectes platessa L). Comparative Biochemistry and Physiology 40:391–398
Farmer CG, Jackson DC (1998) Air-breathing during activity in the fishes Amia calva and Lepisosteus oculatus. Journal of Experimental Biology 201:943–948
Fernandes MN, Rantin FT (1994) Relationship between oxygen availability and metabolic cost of breathing in Nile tilapia (Oreochromis niloticus): aquacultural consequences. Aquaculture 127:339–346
Filleul A, Lavoué S (2001) Basal teleosts and the question of elopomorph monophyly. Morphological and molecular approaches. Comptes rendus de l'Académie des Sciences. Série III, Sciences de la vie 324(4):393–399
Florindo LH, Leite CAC, Kalinin AL, Reid AG, Milsom WK, Rantin FT (2006) The role of branchial and orobranchial O2 chemoreceptors in the control of aquatic surface respiration in the neotropical fish tambaqui (Colossoma macroporum): progressive responses to prolonged hypoxia. Journal of Experimental Biology 209:1709–1715
Frick NT, Brystriansky JS, Ballantyne JS (2007) The metabolic organization of a primitive air-breathing fish, the Florida gar (Lepisosteus platyrhincus). Journal of Experimental Zoology 307A:7–17
Gervais RG, Tufs BL (1998) Evidence for membrane bound carbonic anhydrase in the air bladder of bowfin (Amia calva), a primitive air-breathing fish. Journal of Experimental Biology 201:2205–2212
Gilmour KM, Perry SF (1994) The effects of hypoxia, hyperoxia or hypercapnia on the acid-base disequilibrium in arterial blood of rainbow trout. Journal of Experimental Biology 192:269–284
Gilmour KM, Milsom WK, Rantin FT, Reid SG, Perry SF (2005) Cardiorespiratory responses to hypercarbia in tambaqui Colossoma macropomum: chemoreceptor orientation and specificity. Journal of Experimental Biology 208(Pt 6):1095–1107
Gilmour KM, Euverman, Esbaugh RM, Kenney AJ, Chew LSF, Ip YK, Perry SF. (2007) Mechanisms of acid-base regulation in the African lungfish Protopterus annectens. Journal of Experimental Biology 210:1944–1959
Glass ML, Boutilier RG, Heisler N (1983) Ventilatory control of arterial PO2 in the turtle Chrysemys picta bellii: Effects of temperature and hypoxia. Journal of Comparative Physiology 151:145–153
Glass ML, Ishimatsu A, Johansen K. (1986) Responses of aerial ventilation to hypoxia and hypercapnia. Journal of Comparative Physiology B 156:425–430
Glass ML, Andersen NA, Kruhøffer M, Williams EM, Heisler N (1990) Combined effects of environmental O2 and temperature and gases in carp (Cyprinus carbio). Journal of Experimental Biology 148:1–17
Graham JB (1997) Air-Breathing fishes: Evolution, Diversity and Adaptation. Academic, San Diego
Graham JB, Baird TA (1982) The transition to air breathing in fishes. I. Environmental effects on the facultative air breathing of Ancistrus chagresi and Hypostomus plecostomus (Loricariidae). Journal of Experimental Biology 96:53–67
Greenwood PH (1961) A revision of genus Dinotopterus BLGR. (Pisces, Clariidae) with notes on the comparative anatomy of the suprabranchial organs in the Clariidae. Bulletin of the British museum 7:215–241
Hedrick MS, Jones DR (1999) Control of gill ventilation and air-breathing in the bowfin Amia calva. Journal of Experimental Biology 202:87–94
Hedrick MS, Burleson ML, Jones DR, Milsom WK (1991) An examination of central chemosensitivity in the air-breathing fish (Amia calva). Journal of Experimental Biology 155:165–174
Hedrick MS, Katz SL, Jones DR (1994) Periodic air breathing behaviour in a primitive fish revealed by spectral analysis. Journal of Experimental Biology 197:249–436
Heisler N, Claiborne JB (1986) Acid-base regulation and ion transfers in the carp (Cyprinus carbio) PH compensation during graded long- and short-term environmental hypercapnia, and the effects of bicarbonate infusion. Journal of Experimental Biology 126:41–61
Hora SL (1935) Physiology, bionomics, and evolution of air-breathing fishes of India. Transnational Institute of Science of India 1:1–16
Hughes GM, Saunders RL (1970) Responses of respiratory pumps to hypoxia in the rainbow trout (Salmo gairdneri). Journal of Experimental Biology 53:529–545
Hughes GM, Shelton G (1962) Respiratory mechanisms and their nervous control in fish. In: Löwenstein O (ed) Advances in Comparative Physiology and Biochemistry Volume I. Academic, London, pp 275–364
Jordan (1976) The influence of body weight on gas exchange in the air-breathing fish, Clarias batrachus. Comparative Biochemistry Physiology 53(A):305–310
Junk WJ (1984) Ecology of the varzea, floodplain of Amazonian whitewater rivers. In: Sioli H (ed) The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and its Basin. W. Junk, Dordrecht, pp 215–244
Kind PK, Grigg GC, Booth DT (2002) Physiological responses to prolonged aquatic hypoxia in the Quensland lungfish. Neoceratodus forsteri. Respiratory Physiology and Neurology 132:179–190
Kramer DL (1978) Ventilation of the respiratory gas bladder in Hoplerythrinus unitaeniatus (Pisces, Characoidei, Erytrhinidae). Canadian Journal of Zoology 56:931–938
Kramer DL, Lindsey CC, Moodie GEE, Stevens ED (1978) The fishes and aquatic environment of the central Amazon basin, with particular reference to respiratory patterns. Canadian Journal of Zoology 56:717–729
Kruhøffer M, Glass ML, Abe AS, Johansen K (1987) Control of breathing in an amphibian Bufo paracnemis: effects of temperature and hypoxia. Respiration Physiology 69(2):267–275
Kramer DL, McClure M (1982) Aquatic surface respiration, a widespread adaptation to hypoxia in tropical fresh water fishes. Environmental Biology of Fish 7:47–55
Lomholt JP,Glass ML (1987) Gas exchange of air-breathing fishes in near anoxic water. Acta Physiologica Scandinavica 129:45A
Lomholt JP, Johansen K (1979) Hypoxia in carp: How it affects O2 uptake, ventilation and extraction from water. Physiological Zoology 52:38–49
Lopes JM (2003) Localização e orientação dos quimiorreceptores de O2 envolvidos no controle dos reflexos cardio-respiratórios e da respiração aérea de jeju, Hoplerythrinus unitaeniatus (Teleostei, Erytrhinidae) em resposta à hipóxia ambiental. PhD Thesis, Federal University of São Carlos, SP, Brazil
Magid AMA (1971) The ability of Clarias lazera (Pices) to survive without air-breathing. Journal of Zoology (London) 163:63–72
Magid AMA, Vokac Z, Ahmed NED (1970) Respiratory function of the swim-bladders of the primitive fish Polypterus senegalus. Journal of Experimental Biology 52:27–37
Mattias AT, Rantin FT, Fernandes MN (1998) Gill respiratory parameters during progressive hypoxia in the facultative air-breathing fish, Hypostomus regani (Loricariidae). Comparative biochemistry and physiology 120(A):311–315
Milsom WK (2002) Phylogeny of CO2/H+ chemoreception in vertebrates. Respiratory Physiology and Neurobiology 131:29–41
Moraes MFPG, Fernandes MN, Höller S, Costa OPF, Glass ML, Perry SF (2005) Morphometric comparison of the respiratory organs of the South American lungfish Lepidosiren paradoxa (Dipnoi). Physiological and Biochemical Zoology 78:546–559
Munshi JSD (1976) Gross and fine structure of respiratory organs of air-breathing fishes. In: Hughes GM (ed) Respiration of Amphibious Vertebrates. Academic, London, pp 73–104
Nelson JA, Rios FS, Sanches JR, Fernandes MN, Rantin FT (2007) Environmental influences on the respiratory physiology and gut chemistry of a facultative air-breathing, tropical herbivorous fish Hypostomus regani (Ihering, 1905). In: Fernandes MN, Rantin FT, Glass ML, Kapoor BG (eds) Fish Respiration and Environment. Science Publishers. Enfield, NH, USA, pp 191–219
Oliveiro RD, Lopes JM, Sanchez JR, Kalinin AL, Glass ML, Rantin FT (2004) Cardio-respiratory responses of the facultative air-breathing fish jeju, Hoplerythrinus unitaeniatus (Teleostei, Erythrinidae), exposed to graded ambient hypoxia. Comparative Biochemistry and Physiology A 139: 479–485
Perry SF (2007) Swimbladder-lung homology in basal osteichthyes revisited. In: Fernandes MN, Rantin FT, Glass ML, Kapoor BG (eds) Fish Respiration and the Environment. Science Publishers, Enfield, NH, USA, pp 41–55
Perry SF, Reid SG, Gilmour KM, Boijink CL, Lopes JM, Milsom WK, Rantin FT (2004) A comparison of adrenergic stress responses in three tropical teleosts exposed to acute hypoxia. American Journal of Physiology (Regul Integr Comp Physiol) 287(1):R188–97
Piiper J, Scheid P (1992) Modelling of gas exchange in vertebrate lungs, gills and skin. In: Wood SC, Weber RE, Hargens A, Millard, RW (eds) Physiological Adaptations in Vertebrates. Marcel Dekker, New York, pp 69–97
Podkawa D, Goniakowska-Witalinska (2003) Morphology of the air-breathing stomach of the catfish Hypostomus plecostomus. Journal of Morphology 257:147–163
Randall DJ, Taylor EW (1991) Evidence for a role of catecholamines in the control of breathing in fish. Reviews in Fish Biology and Fisheries 1:139–157
Rantin FT, Johansen K (1984) Responses of the teleost Hoplias malabaricus to hypoxia. Environmental Biology of Fishes 11:275–288
Rantin FT, Kalinin AL, Glass ML, Fernandes MN (1992) Respiratory responses to hypoxia in relation to mode of life of two erythrinid species (Hoplias malabaricus and Hoplias lacerdae). Journal of Fish Biology 41:805–812
Rantin FT, Del Rosario Guerra C, Kalinin AL, Glass ML (1998) The influence of aquatic surface respiration (ASR) on cardio-respiratory function of the serrasalmid fish Piaractus mesopotamicus. Comparative Biochemistry and Physiology A 119:991–997
Sanchez AP, Glass ML (2001) Effects of environmental hypercapnia on pulmonary ventilation of the South American lungfish. Journal of Fish Biology 58:1181–1189
Sanchez AP, Hoffman A, Rantin FT, Glass ML (2001) The relationship between pH of the cerebro-spinal fluid and pulmonary ventilation of the South American lungfish, Lepidosiren paradoxa. Journal of Experimental Zoology 290:421–425
Saint-Paul U (1988) Diurnal routine O2 consumption at different O2 concentration by Calossoma macroporum and Colossoma brachyporum. Comparative biochemistry and physiology 89A:675–682
Saint-Paul U, Bernardino G (1988) Behavioral and ecomorphological responses of neotropical pacu Piaractus mesopotamicus (Teleostei, Serrasalmidae) to oxygen-deficient waters. Experimental Biology 48:19–26
Salvo-Souza RH, Soncini R, Glass ML, Sanches JR, Rantin FT (2001) Ventilation, gill perfusion and blood gases in dourado, Salminus maxillosus Valenciennes (Teleostei, Characidae) exposed to graded hypoxia. Journal of Comparative Physiology B 171:483–862
Schumann D, Piiper J (1966) Der Sauerstoffbedarf der Atmung bei Fischen nach Messungen an der narkotisierten Schleie, Tinca tinca. Pflügers Archiv für gesamte physiologie 288:15–26
Schurmann H, Steffensen JF (1994) Spontaneous swimming activity of the Atlantic cod Gadus morhua, exposed to graded hypoxia at three temperatures. Journal of Experimental Biology 197:129–142
Scott AC, Glasspool IJ (2006) The diversification of Paleozoic fire systems and fluctuations in atmospheric oxygen concentration. Proceedings of the National Academy of Sciences 103(29):10861–10865
Singh BN, Hughes GM (1971) Respiration of an air-breathing catfish Clarias batrachus (Linn.). Journal of Experimental Biology 55:421–434
Singh BR, Mishra AP, Sheel M, Singh I (1982) Development of the air-breathing organ in the cat fish, Clarias batrachus (Linn.). Zoologischer Anzeiger, 208:100–111
Smatresk NJ, Cameron JN (1982) Respiration and acid-base physiology of the spotted gar, a bimodal breather. I. Normal values, and the response to hypoxia. Journal of Experimental Biology 96:263–280
Smatresk NJ, Burleson ML, Azizi SQ (1987) Chemoreflexive responses to hypoxia and NACN on longnose gar: evidence for two chemoreceptor loci. American Journal of Physiology 251(1pt 2):R1116–125
Stearns SC, Hoekstra RF (2005) Evolution — an introduction: 2nd edition. Oxford University Press, Oxford
Steffensen JF, Lomholt JP (1983) Energetic cost of branchial ventilation in the sharksucker, Echeneis naucrates. Journal of Experimental Biology 103:185–192
Stevens ED, Holeton GF (1978) The partitioning of oxygen uptake from air and from water by erythrinids. Canadian Journal of Zoology 56:965–969
Soncini R, Glass ML (1997) The effects of temperature and hyperoxia on arterial PO2 and acid-base status in Piaractus mesopotamicus (Holmberg). Journal of Fish Biology 51:225–233
Soncini R, Glass ML (2000) Oxygen and acid-base related drives to gill ventilation in carp. Journal of Fish Biology 56:528–541
Takasusuki J, Fernandes MN, Severi W (1998) The occurrence of aerial respiration in Rhinelepis strigosa during progressive hypoxia. Journal of Fish Biology 52:369–379
Takezawa J, Miller FJ, O'Neil JJ (1980) Single-breath diffusing capacity and lung volumes in small laboratory mammals. Journal of Applied Physiology 48(6):1052–1059
Toyama Y, Ichimiya T, Kasama-Yoshida H, Cao Y, Hasegava M, Kojima H, Tamai Y, Kurihari T (2000) Phylogenetic relation of lungfish indicated by the amino acid sequence of myelin DM20. Molecular Brain Research 8:256–259
Tuurala H, Pärt P, Nikinmaa M, Soivio A (1984) The basal channels of secundary lamellae in Salmo gairdneri gills — a non-respiratory shunt. Journal of Comparative Physiology 79A:35–39
Val AL, Almeida-Val VMF (1995) Fishes of the Amazon and their environments. Physiological and biochemical features. Springer, Heidelberg, 224 pp
Wilson RJA, Harris MB, Remmers JE, Perry SF (2000) Evolution of air-breathing and central CO2/H+-respiratory chemosensitivity: new insights from an old fish? Journal of Experimental Biology 203:3505–3512
Zhu M, Yu X (2002) A primitive fish close to the common ancestor of tetrapods and lungfish. Nature 418:767–770
Acknowledgements
Supported by Fundação de Amparo de Pesquisa do Estado de São Paulo (FAPESP) and from Conselho Nacional de Desenvolvimento Científico e Técnologico (CNPq) and Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da FMRP-USP (FAEPA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Glass, M.L., Rantin, F.T. (2009). Gas Exchange and Control of Respiration in Air-Breathing Teleost Fish. In: Glass, M., Wood, S. (eds) Cardio-Respiratory Control in Vertebrates. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-93985-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-540-93985-6_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-93984-9
Online ISBN: 978-3-540-93985-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)