Abstract
Intense efforts of many pharmaceutical companies and academicians in the A1 adenosine receptor (AR) field have led to the discovery of clinical candidates that are antagonists, agonists, and allosteric enhancers. The A1AR antagonists currently in clinical development are KW3902, BG9928, and SLV320. All three have high affinity for the human (h) A1AR subtype (hA1 K i < 10 nM), > 200-fold selectivity over the hA2A subtype, and demonstrate renal protective effects in multiple animal models of disease and pharmacologic effects in human subjects. In the A1AR agonist area, clinical candidates have been discovered for the following conditions: atrial arrhythmias (tecadenoson, selodenoson and PJ-875); Type II diabetes and insulin sensitizing agents (GR79236, ARA, RPR-749, and CVT-3619); and angina (BAY 68–4986). The challenges associated with the development of any A1AR agonist are to obtain tissue-specific effects but avoid off-target tissue side effects and A1AR desensitization leading to tachyphylaxis. For the IV antiarrhythmic agents that act as ventricular rate control agents, a selective response can be accomplished by careful IV dosing paradigms. The treatment of type II diabetes using A1AR agonists in the clinic has met with limited success due to cardiovascular side effects and a well-defined desensitization of full agonists in human trials (GR79236, ARA, and RPR 749). However, new partial A1AR agonists are in development, including CVT-3619 \((\mathrm{hA}_{1}\ {\rm AR}\ {K}_{\mathrm{i}} = 55\,{\mathrm{nM,\ hA}_{\rm 2A:hA}}_{{\rm 2B:hA}_{3}}\,>\,200\):1,000:20, CV Therapeutics), which have the potential to provide enhanced insulin sensitivity without cardiovascular side effects and tachyphylaxis. The nonnucleosidic A1AR agonist BAY 68–4986 (capadenoson) represents a novel approach to angina wherein both animal studies and early human studies are promising. T-62 is an A1AR allosteric enhancer that is currently being evaluated in clinical trials as a potential treatment for neuropathic pain. The challenges associated with developing A1AR antagonists, agonists, or allosteric enhancers for therapeutic intervention are now well defined in humans. Significant progress has been made in identifying A1AR antagonists for the treatment of edema associated with congestive heart failure (CHF), A1AR agonists for the treatment of atrial arrhythmias, type II diabetes and angina, and A1AR allosteric enhancers for the treatment of neuropathic pain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- A1 adenosine receptor agonists
- A1 adenosine receptor antagonists
- Acutely decompensated heart failure
- Adentri
- Cardiorenal syndrome
- Congestive heart failure
- Anti-arrhythmic agents
- Tecadenoson
- Selodenoson
- Insulin sensitizing agents
- CVT-3619
- Angina
- Capadenoson
- Allosteric enhancers
- Neuropathic pain
- Type II diabetes
- BG9928
- KW3902
- SLV320
1 Introduction
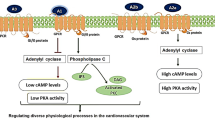
The A1 adenosine receptor (AR), a member of the P1 family of seven-transmembrane adenosine receptors, couples to Gi to decrease the secondary messenger cAMP. The P1 family of adenosine receptors consists of the members A1, A2A, A2B, and A3, which have high sequence homology with many conserved residues at the active sites; however, sufficient differences are found for each active site such that selective agonists and antagonists have been generated for each receptor subtype (Akhari et al. 2006; Dhalla et al., 2003; Fredholm et al. 2001; Jacobson and Gao 2006). The major goal of this review is twofold: to highlight the structure–affinity relationships (SAR) of A1AR antagonists, agonists, and allosteric enhancers, and to give an overview of the A1AR antagonists, agonists, and allosteric enhancers currently under development for various indications.
2 A1 Adenosine Receptor Antagonists
From the earliest reports on the physiologic effects of theophylline and adenosine to the three active clinical programs today (see Fig. 1), the study of AR ligands has a long and rich history (Baraldi et al. 2008; Jacobson and Gao 2006). There are a number of excellent reviews on A1AR antagonists (Baraldi et al. 2008; Hess 2001; Moro et al. 2006; van Galen et al. 1992; Yuzlenko and Kieć-Kononowicz 2006). Because these reviews discuss the historical development of this class of molecules, their structure–activity relationships (SARs), pharmacology, and therapeutic applications, a comprehensive review of A1AR antagonists will not be presented here. It is important to note that a number of A1AR antagonists have entered clinical trials; however, problems with high lipophilicity and corresponding low water solubility and bioavailability have limited their clinical development (Hess 2001). This section of our review will present brief overviews of the most advanced A1 adenosine receptor (A1AR) antagonists that are promising drug candidates currently in clinical trials. The discussion will address SARs around the lead molecules, highlights of pharmacology in healthy animals and disease models, and top-line human clinical trial results.
The 1,3-dialkylxanthine core has been the mainstay of A1AR antagonists since the isolation of theophylline in 1886 (Kossel 1888) (Fig. 1). One hundred years passed before replacement of the methyl substituents with n-propyl chains and the installation of cyclopentane at the C8 position led to the discovery of 1,3-dipropyl-8-cyclopentyl xanthine (DPCPX), which has been used as a radioligand and pharmacologic probe for the in vivo effects of A1AR antagonism in living systems (Shamim et al. 1988). Since then, significant effort has been directed toward garnering improvements in activity and selectivity on the well-optimized 1,3-dipropylxanthine, and has led to the discovery of two molecules that are in active clinical development programs: KW3902 (3-noradamantyl-1,3-dipropylxanthine) (Suzuki et al. 1992) and BG9928 (3-(4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-purin-8-yl)bicyclo[2.2.2]octan-1-yl)propanoic acid) (Kiesman et al. 2006a). A structurally distinct nonxanthine series based upon the adenine substructure of adenosine itself has also been developed and is represented by clinical candidate SLV320 (4-(2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)cyclohexanol) (Kalk et al. 2007). All three of these A1AR antagonists display high affinity for the A1AR and significant selectivity over the A2AAR (Table 1).
2.1 KW3902
To further characterize the hydrophobic interactions between the 8 position in the xanthine series and the A1AR binding site, Shimada et al. (1991, 1992) investigated substitutions of the 8-cyclopentyl ring in DPCPX 1 (Table 2). Clipping the cyclopentyl ring into two ethyl groups 2 led to a loss of guinea pig (gp)A1 affinity but had no effect on rat (r)A2A binding. When compared to 1, dicyclopropyl substitution 3 showed enhanced potency and selectivity versus gpA1 and rA2A receptors, respectively, while the addition of gem-dimethyl substitution 4 led to diminished gpA1 affinity but a remarkable decrease in rA2A binding ( > 170-fold decrease). Bicyclo- and tricycloalkane systems 5, 7, 8 were then examined to determine if restrictions in conformational flexibility around the cyclopentyl ring in 1 effected the antagonist activity. Interestingly, the 3-noradamantyl system 8 stood out, with increases in gpA1 affinity (K i = 1. 3 nM and high selectivity over the rA2A receptor (890-fold; Nonanka et al. 1996). Introduction of a methylene linker between the bulky polycyclic alkane in the 8 position gave compound 9 significantly reduced gpA1 affinity ( > 65-fold).
2.1.1 Animal Studies
The diuretic activity of KW3902 was examined in saline-loaded, conscious Wistar rats (Suzuki et al. 1992). The antagonist was orally administered in a saline suspension and the urine collected and analyzed for sodium content. Urine volume (UV) and urinary sodium excretion (UNaV) both increased in a dose-dependent manner, with maximal effects observed between 0.1 and 0. 4 mg kg − 1 (Fig. 2).
Dose–response for urine volume (UV) in \({\mathrm{mL} \mathrm{100g}}^{-1}6{\mathrm{h}}^{-1}\) (mean ± SEM) and urinary sodium excretion (UNaV) in \({\mu }{\mathrm{Eq} \mathrm{100g}}^{-1}\ 6{\mathrm{h}}^{-1}\) (mean ± SEM) over 6 h following oral doses of KW3902 ranging from 0.0025 to 25 mg kg − 1 in male rats
During the development of intravenously (IV) injectable formulations for KW3902, which has a solubility in water of < 1 μg mL − 1, Hosokawa et al. (2002) investigated the effects of a lipid emulsion and liposome formulation on the pharmacokinetics of KW3902 and its metabolite (M1-KW3902) in comparison to a 1 N NaOH–DMSO-containing formulation (Fig. 3). They reported no significant differences in elimination half-life (t 1 ∕ 2), area under the curve (AUC), total body clearance (CL), and mean residence time (MRT) for all of the formulations investigated. Table 3 summarizes the pharmacokinetic parameters measured for KW3902 and M1-KW3902 in the 1 N NaOH–DMSO formulation. The lipid formulation, however, did prevent the precipitation of KW3902 after IV injection, and it was suggested that this formulation may be used in further clinical studies.
The renal protective activity of KW3902 was investigated in a rat model of glycerol-induced acute renal failure (Suzuki et al. 1992). The antagonist was administered intraperitoneally (IP), and after 30 min glycerol (50% v/v in sterile saline; 0. 8 mL 100 g − 1) was injected subcutaneously. After a subsequent 24-h hold time, blood was collected and serum creatinine and urea nitrogen were determined (Table 4). Typically after glycerol injection, serum creatinine and urea nitrogen increase seven- to tenfold in rats. Pretreatment with KW3902 significantly reduced (50–60%) the negative renal effects of glycerol-induced acute renal failure (Suzuki et al. 1992).
The mechanism of the protective effects of A1AR antagonism on two additional nephrotoxic models of acute renal failure in vivo, including renal accumulation of gentamicin (Yao 2000) and radiocontrast media (RCM) (Yao et al. 2001), were examined. From these studies, it was suggested that KW3902 inhibited the action of endogenous adenosine and increased renal blood flow, which led to suppression of intrarenal accumulation of gentamicin, whereas in RCM-induced nephropathy, it prevented the drop in glomerular filtration rate (GFR); a marker for kidney function) that occurs in N-ο-nitro-l-arginine methyl ester (L-NAME) hypertensive rats. In the RCM model, it is unclear whether the action of the antagonist reduces RCM uptake and cellular toxicity by inhibiting sodium transport in the proximal tubule or by another mechanism.
Adenosine has been found, through its actions on A2ARs (presumably A2AARs), to play a protective role in the ischemic preconditioning of multiple organs, including the liver (Peralta et al. 1999), heart (Forman et al. 1993), and lung (Khimenko et al. 1995). Magata (Magata et al. 2007) recently reported that blockade of the A1AR with KW3902 attenuated hepatic ischemia-reperfusion injury in dogs. Two groups of female beagles (n = 6) underwent a 2 h total hepatic vascular exclusion; one group served as the control and the other received \(1{ \mu }{\mathrm{g} \mathrm{kg}}{}^{-1}\min^{-1}\) of KW3902 via continuous intraportal infusion for 60 min prior to ischemia. It was noted that although peripheral IV infusion of KW3902 was effective in earlier studies (Nonaka et al. 1996), no beneficial effects were seen in the hepatic ischemia-reperfusion model by this route of administration. Thus, treatment by KW3902 via the portal vein proved beneficial in a number of outcomes in the study. The two-week survival of the control group was 16.7% versus 83.3% (P < 0. 05) for the treated group. Serum alanine aminotransferase (ALT) levels were significantly inhibited by the A1AR antagonist; the control group rose rapidly to 12, 625 ± 1, 010 U L − 1 after 6 h of reperfusion, while the treated animals peaked at 24 h at 2, 352 ± 452 U L − 1. In addition, the number of infiltrating neutrophils in the hepatic tissue of the KW3902 group (41. 17 ± 11. 01 at 60 min) was significantly lower than that of the control group (63. 6 ± 23. 6 at 60 min) (Fig. 4).
The number of infiltrated neutrophils in liver tissue. Data are expressed as mean ± SEM. Group CT, negative control (n = 6); group KW, treated KW3902 (n = 6). ∗ P < 0. 05 versus group CT. Reprinted with permission from Magata et al. (2007)
Treatment with KW3902 in the liver ischemia-reperfusion model prevented the bradycardia seen in the control animals just after reperfusion, significantly increased adenine nucleotide levels in the ischemic tissues, and regulated the microcirculatory disturbances, resulting in greater hepatic tissue blood flow. It was concluded that for adenosine to protect the liver from ischemia-reperfusion injury it is necessary to block A1AR activation.
2.1.2 Clinical Studies
Building upon the earlier studies of the renal effects of another A1AR antagonist, BG9719 (8-(3-oxa-tricyclo[3.2.1.02, 4]oct-6-yl)-1,3-dipropyl-3,7-dihydropurine-2,6-dione) (Gotttlieb et al. 2002), in patients with congestive heart failure (CHF), Dittrich and colleagues (Dittrich et al. 2007) examined the renal vasodilatory effects of 30 mg doses of KW3902 in patients with ambulatory heart failure (HF). The two-way crossover study followed patients with CHF with mild renal impairment (median GFR 50 mL min − 1) and compared IV administration of placebo or KW3902 oil emulsion followed by IV furosemide (80 mg). GFR and renal plasma flow were assessed by iothalamate and para-aminohippurate clearances over 8 h. After a three- to eight-day washout period, subjects crossed over to either active treatment or placebo, again followed by furosemide. Renal plasma flow (Fig. 5) and GFR increased by 48% (P < 0. 05 vs. placebo) and 32% (P < 0. 05 vs. placebo), respectively, over baseline for 8 h postKW3902 administration, supporting the conclusion that blockade of the A1AR leads to vasodilation and increases in filtration rates in patients with HF with reduced kidney function. There also appeared to be a persistent positive effect on GFR (approx. 10 mL min − 1 increase in GFR over previous baseline) seen in the crossover patients who received KW3902 in the first dose. The pharmacokinetics of the parent compound or its metabolites could not account for the change in the baseline GFR values.
Renal plasma flow (RPF). The percent change in RPF from baseline for KW-3902 and placebo in the presence of furosemide (n = 23). Values shown are for all subjects mean ± SEM. P values reflect analysis of RPF percent change between KW-3902 and placebo using log RPF values ( ∗ P < 0. 05). Reprinted with permission from Dittrich et al. (2007)
In a related clinical investigation, Givertz et al. (2007) examined the dose-dependent effects of KW3902 on diuresis and renal function in two subsets of patients with acutely decompensated heart failure (ADHF) with either renal impairment or diuretic resistance. In the first protocol, patients with volume overload and creatinine clearance (CrCl) of \(20 - 80{\mathrm{mL} \mathrm{min}}^{-1}\) received either placebo or one of four doses (2.5, 15, 30, or 60 mg) of KW3902 as a 2 h IV infusion for up to three days of treatment. All four doses increased urine output during the 6 h period following administration (Fig. 6). There were no significant differences in systolic blood pressure or heart rate in any of the treatment groups. A transient decrease in serum creatinine was noted on day 2 of treatment for all dose levels ( − 0. 03 to \(-0.08{\mathrm{mg} \mathrm{dL}}^{-1}\) for KW3902 arms vs. \(+0.04{\mathrm{mg}\mathrm{dL}}^{-1}\) for placebo). This effect was maintained on day 4 (or the day of discharge), except for the 60 mg dose level.
Urine output in first 6 h after administration of KW3902. Cumulative urine volume (mean ± SEM) 6 h after initiation of placebo or KW-3902 in patients with acutely decompensated heart failure (ADHF) with renal impairment ( ∗ P = 0. 02 vs. placebo). Reprinted with permission from Givertz et al. (2007)
In the diuretic-resistant population, single infusions of KW3902 (10, 30, or 60 mg) were given to patients with an average baseline CrCl of 34 mL min − 1. Urine output increased for all dose levels (ranging from + 22 to \(+24{\mathrm{mL} \mathrm{h}}^{-1}\)), whereas the placebo arm saw a decrease in urine output \((-29{\mathrm{mL} \mathrm{h}}^{-1})\). The CrCl data for this subset of patients was complex. In general, the placebo arm had decreases in CrCl over 24 h with similar trending from the 60 mg dose treatment group. However, the 10 and 30 mg doses showed increases in CrCl over the 24 h period. The inverted relationship between KW3902 dose and renal function (CrCl) at high doses bears some resemblance to the dose–response relationship seen at higher doses of the A1AR antagonist BG9719 (Gottlieb et al. 2002). Whether these similarities are due to cross-activity against the other adenosine receptors in the kidney at high doses or in other tissues is unclear and may require further study. Phase III clinical trials of KW3902 in patients with ADHF are currently underway, and limited releases of the data have recently appeared (Novacardia Press Release 2007).
2.2 BG9928
At the same time that formulation development began on KW3902, another selective A1AR antagonist, BG9719, which possessed adequate pharmacologic activity, was used to demonstrate proof of concept for A1AR antagonism in animals (Pfister et al. 1997) and humans (Gottlieb et al. 2000, 2002). However, the poor pharma- ceutical properties of this molecule (low aqueous solubility and a tendency to rearrange to inactive products in both acidic and basic media) led Kiesman et al. (Kiesman et al. 2006a, b) to design more pharmaceutically acceptable antagonists by exploring the placement of polar substituents on linearly substituted 8-cycloalkyl 1,3-dipropylxanthines. For structurally related imidazolines, see Vu et al. (2006). The binding affinities of selected 8-cyclohexyl and 8-bicyclo[2.2.2]octyl xanthines are listed in Table 5.
The bicyclo[2.2.2]octyl analogs 11, 13, 15 had better A1AR binding affinities than the related cyclohexyl variants 10, 12, 14, and maintained similar A2AAR activity. A significant improvement in receptor selectivity (\(\mathrm{hA}_{2\mathrm{A}/\mathrm{hA}_{1}} = 161\) vs. 22) came with the replacement of the dimethylamino functional group in 15 with the carboxylic acid in 16. Further optimization of the bridgehead chain led to the propionic acid 18, BG9928, and single-digit nanomolar (rA1 = 1. 3 nM and hA1 = 7. 4 nM) activity and high receptor selectivities \((\mathrm{rA}_\mathrm{2A}/\mathrm{rA}_{1} = 1,880;\ \mathrm{hA}_{\mathrm{2A}/\mathrm{hA}_{1}} = 915)\).
The functional antagonist activity of BG9928 (Kiesman et al. 2006a) was confirmed by examining the blockade or increasing doses of the compound on the inhibitory effects of N 6-cyclopentyl adenosine (CPA) on the beat rates of isolated rat atria. Administration of the antagonist restored the atrial beat rates to their maxima and effectively blocked the negative chronotropic activity of CPA (EC50 = 16. 1 ± 7. 7 nM). In a separate set of isolated rat atria experiments, BG9928 was found to have a pA2 (antagonist potency) of 9.8.
2.2.1 Animal Studies
Single oral doses of BG9928 administered to male Sprague–Dawley rats (Fig. 7) led to increases in urine volume (UV) and sodium excretion (UNaV) with a 50% efficient dose (ED50) of approximately 15 μg kg − 1 (Kiesman et al. 2006a; Ticho et al. 2003). The increases in urinary potassium excretion were proportional to volume increases, confirming the potassium-neutral diuresis commonly observed with A1AR antagonists. The dose–response relationships are similar to those seen with KW3902 (Fig. 2); however, the magnitude of the pharmacodynamic effect is smaller in the BG9928 study than in the KW3902 study because the rats in the BG9928 model were not saline-loaded prior to treatment.
Dose–response for urine volume (UV) in mL h − 1 (mean ± SEM) and urinary sodium excretion (UNaV) in \({ \mu }{\mathrm{Eq} \mathrm{h}}^{-1}\ 100{\mathrm{g}}^{-1}\) (mean ± SEM) over 4 h following single oral doses of vehicle (0.5% carboxymethyl cellulose suspension, n = 3) or BG9928 ranging from 0.001 to 3 mg kg − 1 in rats (\(0.001{\mathrm{mg} \mathrm{kg}}^{-1},\ 0.003{\mathrm{mg} \mathrm{kg}}^{-1},\ 0.01{\mathrm{mg} \mathrm{kg}}^{-1}\), each \(n=4;\ 0.03{\mathrm{mg} \mathrm{kg}}^{-1},\ 0.1{\mathrm{mg} \mathrm{kg}}^{-1},\ 0.3{\mathrm{mg} \mathrm{kg}}^{-1}\), each \(n = 5;\ 1.0{\mathrm{mg} \mathrm{kg}}^{-1},\ 3.0{\mathrm{mg} \mathrm{kg}}^{-1}\), each n = 3). Adapted with permission from Kiesman et al. (2006a)
The single oral-dose pharmacokinetic profile of 1 mg kg − 1 BG9928 was assessed in the rat, dog, and cynomolgus monkey (Table 6). Bioavailability was nearly complete in the rat and cynomolgus monkey, slightly lower in the dog, and followed the clearance difference amongst the species. Elimination half-lives were similar in the rat and dog (3–6 h), and longer in the cynomolgus monkey (11 h).
A rat model was designed to mimic the sodium-retentive state of patients with CHF (Ticho et al. 2003). Rats were given an oral dose of 100 mg kg − 1 furosemide and placed on a low-sodium diet for up to six days. One group (n = 8) then received a single IV bolus of 30 mg kg − 1 furosemide, while the other group (n = 9) received IV furosemide and 1 mg kg − 1 of BG9928. The results are depicted in Fig. 8.
Renal protective (mean ± SEM change in glomerular filtration rate [GFR]) and natriuretic (mean ± SEM) urinary sodium excretion (UNaV) effect of 1 mg kg − 1 BG9928 administered IV in combination with furosemide (30 mg kg − 1 IV) (n = 9, circles) compared with furosemide alone \((30{\mathrm{mg} \mathrm{kg}}^{-1}\mathrm{IV})\ (n = 8,\ squares)\) in low-sodium-retention rats. Reprinted with permission from Ticho et al. (2003)
Furosemide increased natriuresis and reduced GFR by approximately 50% over baseline. The addition of BG9928 not only further increased the natriuresis \((+0.71{ \mu }{\mathrm{Eq} \min }^{-1}\ 100{\mathrm{gm}}^{-1})\) but also maintained the GFR, therefore preserving renal function. Similar data were presented for a Phase II proof-of-concept clinical trial for BG9719, a precursor compound to BG9928 (Gottlieb et al. 2002).
The interplay of A1AR antagonism and ischemic preconditioning (IPC), specifically the effects of DPCPX, BG9719, and BG9928, in an in vivo dog model of myocardial infarction was examined (Auchampach et al. 2004). The study was composed of three arms in which the dogs \((n = 6 - 12)\) were subjected to 60 min of left anterior descending coronary artery occlusion, followed by 3 h of reperfusion. Infarct size was assessed by triphenyltetrazolium chloride staining. In protocol 1, the dogs received vehicle or 1 mg kg − 1 of the antagonist as a pretreatment, followed by continuous infusion at \(10{ \mu }{\mathrm{g} \mathrm{kg}{}^{-1}\ \min }^{-1}\) over the occlusion time. In protocol 2, the dogs received the same pretreatment as before but also received four 5-min occlusion/reperfusion preconditioning cycles. In protocol 3, the antagonists were not administered until 10 min prior to release of the occlusion and continued for 1 h into the reperfusion. Figure 9 summarizes the infarct size measurements across all three protocols.
Myocardial infarct size data (infarct size expressed as a percent of the area at risk) from antagonist pretreatment (protocol 1), ischemic preconditioning (protocol 2), and antagonist at reperfusion (protocol 3). P < 0. 05 vs. control Adapted with permission from Auchampach et al. (2004)
Pretreatment with DPCPX or BG9928 reduced the myocardial infarct size by 51% and 49%, respectively, and none of the three antagonists blocked the protection of the myocardium afforded by the brief multiple-cycle IPC. In the most challenging experiment, it was found that treatment with either DPCPX or BG9928 just prior to reperfusion, a situation that more closely resembles clinical intervention in the course of treatment for myocardial infarction, reduced the infarct sizes by 43% and 45%, respectively. The study concluded that treatment with BG9928 provided cardioprotective effects that reduced infarct size and did not interfere with the protective effects of multiple-cycle IPC.
2.2.2 Clinical Studies
Greenberg et al. (2007) described the results of a placebo-controlled dose-escalation study designed to assess the pharmacokinetics and clinical effects of oral BG9928 in patients with HF. The study was conducted in 50 patients with HF, an ejection fraction of ≤ 40% documented in the past 12 months, and who were on standard therapy including angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blocker (ARB) therapy and diuretics.
The pharmacokinetics of oral BG9928 in humans compared favorably to data from the earlier animal studies (Kiesman et al. 2006a) (Table 6). BG9928 was rapidly absorbed, with a t max of 1.5–3.1 h and similar C max, t 1 ∕ 2, and clearances for days 1, 6, and 10. Steady-state AUC was reached by day 6, and the elimination half-life (14–25 h) was consistent with once-daily dosing. Patients received BG9928 (3, 15, 75, or 225 mg) or placebo orally for ten days and were evaluated for changes in sodium excretion (primary endpoint), potassium excretion, creatinine clearance, and body weight. In humans, BG9928 increased sodium excretion compared with placebo and maintained the natriuresis over the ten-day study period (Fig. 10) with little kaliuresis. These data followed the same trends seen in the previous rat and monkey studies (Kiesman et al. 2006a; Ticho et al. 2003) (Fig. 7).
Cumulative urinary sodium excretion (mEq) over the interval of 0–8 h at baseline and after placebo or BG9928 administration on days 1, 6, and 10. ∗ P < 0. 05 vs. placebo; † P < 0. 055 vs. placebo; n = 10 per group except for 15 and 75 mg dose groups on day 10 (n = 9 per group). Reproduced with permission from Greenberg et al. (2007)
Use of ACE inhibitors, ARBs, and diuretics in patients with HF can adversely affect renal function and depress GFR. Despite the significant increases in natriuresis, adjusted CrCl was unchanged over the study period for all of the treatment groups (Fig. 11), suggesting that BG9928 may have had a protective effect on renal function.
Absolute change from baseline in adjusted creatinine clearance (CrCl) \(({\mathrm{ml} \min}^{-1}\ 1.73{\mathrm{m}}^{-2})\) for the interval of 2–24 h after placebo or BG9928 administration on days 1, 6, and 10. ∗ P < 0. 05 vs. placebo; \(n = 7-10\) per group. Reproduced with permission from Greenberg et al. (2007)
Patients who received daily doses of greater than 3 mg had a reduction in body weight \((-0.6,\ -0.7,\ -0.5\mathrm{kg})\) versus a net weight gain of + 0. 3 kg for the placebo group at the end of the study (Fig. 12).
Average change in body weight (kg) from baseline to day 11 after placebo or BG9928 administration. n = 10 per group. Reproduced with permission from Greenberg et al. (2007)
Patients receiving BG9928 also showed favorable directional trends in other measures of clinical status, including New York Heart Association functional class (five BG9928-treated patients improved by one level); Cody edema score (mean change from day 1 to day 11: 0 for placebo and up to − 0. 6 for the treated groups; a negative number indicates an improvement in HF signs); and physician’s global assessment. Thus, positive effects were observed for all treated groups. However, there were no significant differences in clinical status for the short duration of the trial. This is the first clinical assessment of chronic oral dosing of an A1 AR antagonist in humans. Future studies are planned to examine clinical status and renal preservation with both oral and parenteral BG9928 in patients with HF.
2.3 SLV320
Unlike the two xanthine-based A1AR antagonists described in the preceding sections, SLV320 (see Fig. 1) contains an N 6-substituted-7-deazapurine core (specifically a 2-phenyl-pyrrolopyrimidine) with an N 6-trans-cyclohexanol side chain. The published SAR data around the pyrrolopyrimidine series (Fig. 13, Table 7) are only described within a series of patent filings (Castelhano et al. 2005). Dimethyl analog 20 had equipotent A1 and A3 AR affinities, a result that contrasts strikingly with those for the earlier xanthine systems (Tables 2 and 2.5). Removal of the two methyl groups (R2 = Me) from compound 20 led to a tenfold increase in affinity for the A1AR 24 and a significant reduction in A3AR binding (22–630 nM) (Table 7). Substitution on the phenyl ring has small effects upon A1AR activity and, in general, decreased selectivity versus the A2AAR.
The functional antagonist activity of SLV320 was confirmed in experiments involving transient A1AR-mediated bradycardia in anesthetized rats. Bolus injections of adenosine (100 μg kg − 1) lowered the heart rate in rats, and subsequent pretreatment both IV and orally with the antagonist caused a dose-dependent increase back to baseline in heart rate, with ED50 values of 0.25 and 0. 49 mg kg − 1, respectively. Similar to the results for BG9928 (Greenberg et al. 2007; Ticho et al. 2003), no significant hemodynamic effects were seen in anesthetized rats (heart rate, systolic arterial pressure, or diastolic arterial pressure) with single IV bolus doses of between 0.1 and 5 mg kg − 1.
In a model of chronic renal failure and myocardial fibrosis, rats were subjected to either sham operations or removal of 5/6 of their kidneys (5/6 NX animals) (Kalk et al. 2007). The effects of SLV320 on markers for cardiomyopathy and clinical chemistry were examined. Treatment with the A1AR antagonist completely abolished higher creatinine kinase (CK) plasma levels as well as elevated ALT and aspartate aminotransferase (AST) in the nephrectomized animals (Table 8). The creatinine levels and GFR measurements of the 5/6 NX animals showed diminished renal function, as expected, when compared to the sham group. Treatment with SLV320 did not significantly lower creatinine or increase GFR. In addition, although albuminuria was higher in the 5/6 NX group, treatment with SLV320 led to a 50% reduction in albumin excretion and exerted beneficial effects on renal disease progression.
No significant differences in cardiac histology were seen between the arms of the study; however, immunohistochemistry uncovered a significant increase in collagen I and III in the untreated 5/6 NX group compared to the SLV320-treated group (Fig. 14). This study was the first to demonstrate that an A1AR antagonist inhibited markers of myocardial fibrosis without changes in blood pressure. The experiments also agree with two previous studies (Amann et al. 1998a, b) that concluded that uremia promotes cardiac fibrosis independently of hypertension.
Collagen I and III in rat hearts from nephrectomized rats versus normal controls. Values are given as mean ± SEM. Unpaired t-test was applied to detect significant differences between the study groups. ∗ ∗ P < 0. 001 vs. sham; ‡ P < 0. 001 vs. 5/6 NX. Reprinted with permission from Kalk et al. (2007)
It was recently reported that the clinical development of the oral form of SLV320 was suspended and little information is available on the results of human clinical trials with the intravenous product.
3 A1 Adenosine Receptor Agonists
Agonism at A1ARs may provide benefit for the following disease states: paroxysmal supraventricular tachycardia (PSVT)—break the atrial arrhythmia to return to sinus rhythm (Belardinelli and Lerman 1990; Belardinelli et al. 1995; DiMarco et al. 1985; Lerman and Belardinelli 1991), atrial fibrillation (AF)—provide ventricular rate control (Wang et al. 1996; Zablocki et al. 2004), type II diabetes (T2D)—lower nonesterified fatty acid (NEFA) levels and triglycerides (TG’s), as well as enhancing insulin sensitivity (Fatholahi et al. 2006; Gardner et al. 1994; Hoffman et al. 1986; Roden et al. 1996), and angina (Liu et al. 1991; Miura and Tsuchida 1999; Mizumura et al. 1996). The A1AR is found in the A–V and S–A nodes, where stimulation by an A1AR agonist results in negative dromotropic and chronotropic effects, respectively (Belardinelli et al. 1995; Wang et al. 1996). These cardiovascular (CV) effects are often side effects of A1AR agonists that are being pursued for the other indications. Multiple full A1AR agonists, tecadenoson (2R, 3S, 4R)-2-(hydroxymethyl)-5-(6-((R)-tetrahydrofuran-3-ylamino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol), selodenoson (2S, 3S, 4R)-5-(6-(cyclopentylamino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2- carboxamide) and PJ-875 are being pursued as intravenous clinical agents for the treatment of atrial arrhythmias, and the progress of these compounds will be highlighted below. In addition to the full A1AR agonists, CV Therapeutics has reported on orally bioavailable partial A1AR agonists that slow AV nodal conduction without causing third-degree AV block. For T2D, although both full and partial agonists will lower NEFA levels, a partial A1AR agonist has the potential to do so with fewer side effects (Song et al. 2002; Srinivas et al. 1997; Stephenson 1997; Wu et al. 2001). Partial A1AR agonists have the potential to provide for a selective targeted response, avoiding CV side effects (Wu et al. 2001). Plus, partial A1AR agonists may be able to avoid receptor desensitization due to overstimulation that can lead to tachyphylaxis. In T2D, overstimulation of hormone-sensitive lipase (HSL) due to enhanced beta-adrenergic agonism on adipocytes leads to elevated NEFA levels within T2D patients from the 0.4–0.5 mM range up to the 0.8–1.2 mM range. Elevated NEFA levels have been shown to decrease skeletal muscle uptake of glucose, lower insulin release from the pancreas, and increase glucose production in the liver (Boden et al. 2005; Dhalla et al. 2007a; Ferrannini et al. 1983; Green 1987; Itani et al. 2002; Sako and Grill 1990). Decreasing NEFA levels through A1AR agonism has an insulin-sensitizing effect in animal models. Several full A1AR agonists (GR79236, ARA, and RPR749) have been evaluated in animal models and in clinical trials for the treatment of T2D, and the progress and challenges of these compounds will be described. CVT-3619 (2S, 3S, 4R)-2-((2-fluorophenylthio)methyl)-5-(6-((1R, 2R)-2-hydroxycyclopentyl-amino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol), a partial agonist, is in preclinical development. The SAR leading up to the discovery of this partial agonist, as well as its efficacy in animal models of T2D, will be highlighted. The last indication for A1AR agonists that we describe in this review is the treatment of angina. One A1AR agonist (BAY 68–4986) is currently under clinical evaluation, and the progress of this compound will be described.
3.1 Intravenous Antiarrhythmic Agents: Tecadenoson, Selodenoson, Phenylsulfide, Phenylethers, PJ-875
The first A1AR agonists to enter clinical development with the exception of adenosine were the IV antiarrhythmic agents, tecadenoson (Ellenbogen et al. 2005; Peterman and Sanoski 2005; Prystowsky et al. 2003) and selodenoson (Bayes et al. 2003; ClinicalTrials.gov 2005) (Fig. 15). The N6 lipophilic substituents are the key structure features that impart high affinity and selectivity for the A1AR: N 6-(R)-3-tetrahydrofuranyl for tecadenoson (A1ARK i = 3 nM) and N 6-cyclopentyl for selodenoson (A1ARK i = 6 nM). Both compounds are similar in structure to N 6-cyclopentyl adenosine (CPA), but key structural differences are found in each molecule relative to CPA that impart beneficial pharmacological and pharmaceutical properties. For tecadenoson, the furan oxygen is favorable for imparting enhanced binding selectivity and additional solubility. For selodenoson, the 5′-N-ethyl carboxamide is favorable for both A1AR and A3 AR affinities, and it enhances oral activity. In Phase I clinical trials, both compounds were found to be safe and well tolerated at their specified IV bolus or infusion doses. With regards to efficacy, tecadenoson demonstrated favorable conversion (90%) of acute PSVT at the \(300-600{ \mu }\mathrm{g}\) bolus dose without significant adverse events. The A1AR, which is highly expressed in atrial and AV nodal tissues, exerts its effects in the heart through lowering cAMP and direct activation of the inward rectifying potassium current, IK(Ado) (Belardinelli et al. 2005). In addition, A1AR activation in the heart inhibits the catecholamine-stimulated ion currents such as pacemaker current and l-type calcium currents (Belardinelli et al. 2005). The result of A1AR activation in the heart is prolongation of the AV nodal refractory period, reducing sinoatrial pacemaker rate and shortening the action potential (Belardinelli et al. 2005). Because of the shortening of the atrial action potential duration (APD), it is not unexpected to have some atrial fibrillation (AF) after PSVT conversion, and this was found to be extremely low with tecadenoson ( < 1%), but the incidence of AF with adenosine following IV bolus is reported to be 11% and 15% in different studies (Ellenbogen et al. 2005).
Selodenoson was evaluated for the treatment of AF in a dose-ranging infusion study where it was infused for 15 min at doses of 2, 4, 6, 8, 10, and 12 μg kg − 1, where it provided for effective ventricular rate control in a dose-dependent manner with minimal side effects (Bayes et al. 2003). CV Therapeutics’ scientists have described a number of partial A1AR agonists as potential oral antiarrhythmic agents that do not cause high-degree AV block at high concentrations (Morrison et al. 2004). These partial agonists were obtained by incorporating aromatic ethers and sulfides at the 5′ position of the full agonist tecadenoson, a strategy that is known to decrease intrinsic activity with respect to GTP shift and induction of [35S]GTPγ S binding to G-protein (Yan et al. 2003). The 5′ substitution caused a significant drop in affinity for the A1AR when compared to tecadenoson, and the 5′-aromatic ethers had greater affinity and potency for the A1AR than the corresponding 5′-sulfides. Comparing the two lead molecules from both series (25 and 26) (Fig. 15), the 2-fluorophenyl ether analog 25 displayed higher affinity for the A1AR(K i = 12 nM) and sixfold greater potency (EC50 = 200 nM) in slowing AV nodal conduction than the 2-fluorophenyl sulfide 26 without causing third-degree AV block. In addition, compound 25 exhibited greater oral bioavailability (81%) relative to 26. To our knowledge, compound 25 is the most potent partial A1 AR agonist known to date; however, after oral administration, a small amount of the extremely potent full A1AR agonist tecadenoson was generated; thus, compound 25 was unacceptable for further clinical development as an oral partial A1AR agonist for chronic use. PJ-875 is a third A1AR agonist in clinical development for AF from Inotek (DailyDrugNews.com 2008). The structure of PJ-875 has not been publicly disclosed; however, Inotek’s patent application focuses on a 5′-nitrate ester of CPA with high A1AR affinity (A1ARK i = 1 nM) and a 5′-nitrate ester of tecadenoson (A1 ARK i = 10 nM) (Jagtap et al. 2005). In Phase I clinical trials, PJ-875 did not have serious side effects, and Phase II clinical trials are planned (DailyDrugNews.com 2008).
The initial clinical trials with full A1AR agonists in a controlled IV setting demonstrate that it may be possible to obtain antiarrhythmic properties with minimal CNS side effects. In addition, CV Therapeutics has discovered that the antiarrhythmic properties of a full A1AR agonist, tecadenoson, can be augmented by coadminstration of a subtherapeutic dose of a short-acting beta-blocker, esmolol, to achieve pronounced ventricular rate control effects in animal models (Belardinelli and Dhalla 2003). This combination approach of beta-blocker and A1AR agonist will be interesting to watch in the clinic. Due to the pronounced CV effects at low doses of full A1AR agonists, it is clear that a partial A1AR agonist may be required to achieve tissue selectivity for other indications such as T2D.
3.2 Insulin-Sensitizing Agents: GR79236, ARA, CVT-3619
The therapeutic use of A1 AR agonists as antilipolytic agents has been tried in the clinic; however, the CV effects mediated by the A1AR agonists are a potential obstacle to the successful use of A1AR agonists for this indication. A second challenge associated with the use of A1AR agonists as antilipolytic agents is the development of acute tolerance to the antilipolytic effects due to receptor desensitization (Dhalla et al. 2007a; IJzerman et al. 1995). One potential solution is to discover a partial A1AR agonist that is capable of eliciting a greater effect in the adipocytes than in the heart (i.e., tissue selectivity). By definition, a partial agonist is a low-efficacy ligand that, in contrast to the full agonist, elicits only a submaximal biological response, and is hence less prone to receptor desensitization.
GR79236 ((3R, 4S, 5R)-2-(6-((1S, 2S)-2-hydroxycyclopentylamino)-9H-purin -9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol) and ARA ((1S, 2R, 3R)-3-((trifl-uoromethoxy)methyl)-5-(6- (1-(5-(trifluromethyl)pyridine-2-yl)pyrrolidin-3-ylami-no)-9H-purin-9-yl)cyclopentane-1,2-diol) (Fig. 16), the two full A1AR agonists, have demonstrated that A1AR agonism can have a pronounced effect on NEFA and TG levels in both acute and chronic animal models, thus establishing the potential of this approach for the treatment of T2D (Bigot et al. 2004; Merkel et al. 1995). Plus, the use of these full A1AR agonists in clinical trials has resulted in a better understanding of the desensitization of the A1AR and some potential limitations of using a full A1AR agonist in a chronic setting. In early in vitro studies, GR79236 demonstrated that it inhibited catecholamine-induced lipolysis in adipocytes at low concentrations (Green et al. 1990; Qu et al. 1997; Webster et al. 1996). In addition, GR79236 was demonstrated to reduce NEFA levels by 50% in normal fasted rats (Qu et al. 1997). However, in a fructose-fed rat model of noninsulin-dependent diabetes, GR79236 (1 mg kg − 1 per day for eight days oral administration) did not enhance insulin sensitivity, but it did significantly lower NEFA and TGs (Webster et al. 1996). ARA, a full A1AR agonist, has both animal data and clinical trial data supporting its effects on NEFA (Bigot et al. 2004). ARA is a C-sugar wherein the ribose oxygen is replaced by a carbon, and the ribose 5′-hydroxyl group was replaced by fluoro (as in compounds 27 and 28) or a trifluoromethoxy group, as in ARA (Fig. 16). Compound 28 has lower affinity for the A1AR with its unusual disubstituted N6 substituent containing an anilino moiety and a 3-pyrrolidinyl group. This is expected, since in most models of A1AR agonist binding to the receptor, the N–H on N6 is involved in a key hydrogen-bonding interaction to the asparagine 254 side chain (IJzerman et al. 1995). ARA exhibited high affinity and selectivity for the A1AR agonist (K i = 1. 7 nM and 4.5 nM in rat brain and rat adipocytes, respectively) (Zannikos et al. 2001). ARA demonstrated some tissue selectivity, being less potent (100- to 200-fold) in inducing A1AR-mediated bradycardia than in inducing A1AR inhibition of lipolysis in rat and human adipocytes. This selective effect is most likely due to the high density and ∕ or efficiency of A1 AR coupling in adipocytes. Although ARA was effective at lowering plasma FFA when administered intravenously to fasted healthy volunteers in a Phase I clinical study, the rapid appearance of tolerance to its FFA-lowering ability was clearly evident (Zannikos et al. 2001).
These clinical findings support the need for a partial A1AR agonist.
Partial A1AR agonists were considered as an alternative to full agonists to avoid receptor desensitization. CVT-3619 ((2S, 3S, 4R)-2-((2-fluorophenylthio)methyl)-5-(6-((1R, 2R)-2-hydroxycyclopentylamino)-9H-purin-9-yl) tetrahydrofuran-3,4- diol) (Fig. 16), a selective partial A1AR agonist devoid of CV effects, is being developed by CV Therapeutics as an antilipolytic agent (Dhalla et al. 2007a; Fatholai et al. 2006). This clinical candidate was obtained by further optimization of the 5′-phenylsulfide derivatives of tecadenoson (described earlier). The binding affinity of CVT-3619 for rat epididymal adipocytes was 14 nM (K i, high affinity). CVT-3619 reduced forskolin-induced cAMP accumulation in both epididymal and inguinal adipocytes, with EC50 values of 5.9 nM and 44 nM, respectively. The maximal effect of CVT-3619 at reducing cAMP levels in adipocytes was similar to that of CPA, suggesting that CVT-3619 is a full agonist with respect to reduction of cAMP. Plus, CVT-3619 reduced the forskolin-stimulated release of NEFA from both epididymal and inguinal adipocytes, with EC50 values of 47 nM and 170 nM, respectively. However, CVT-3619 was found to be a partial agonist with respect to forskolin (1 μM)-stimulated NEFA release from epididymal and inguinal adipocytes, with only 42 and 58%, respectively, of CPA’s effect. Most likely, the presence of a large receptor reserve and/or a higher efficacy of coupling of A1AR in the adipocytes can explain the fact that CVT-3619 reduced the cAMP content of epididymal adipocytes with an EC50 value that was lower than the K i value from the binding assay, and the EC50 value to reduce the release of NEFA was also much lower than the K i. Furthermore, the high A1AR receptor reserve in the adipocyte relative to the heart can explain the 1,000-fold functional selectivity of CVT-3619 to decrease epididymal adipose tissue lipolysis in the rat (EC15 = 30 nM) relative to the atrial rate (both A1AR-mediated effects) (Fatholai et al. 2006). With respect to CV side effects, CVT -3619 \((10\mathrm{nM}-30{ \mu }\mathrm{M})\) caused only a small increase in S–H interval (6 ms) without causing second- or higher-degree AV block; however, CPA significantly prolonged the S–H interval (38 ms) and caused second- or higher-degree AV block at concentrations > 30 nM. In normal, overnight-fasted awake rats, at doses of 2.5, 5 and 10 mg kg − 1, CVT-3619 lowered FFA by 31%, 47% and 57% from baseline, respectively (Dhalla et al. 2007b). In addition, CVT-3619 significantly reduced serum TG levels and increased insulin sensitivity in rats (Dhalla et al. 2007b). The ED50 of insulin to inhibit lipolysis was potentiated fourfold by a single dose (0. 5 mg kg − 1) of CVT-3619, suggesting that CVT-3619 increases insulin sensitivity in adipose tissue. The antilipolytic effects of CVT-3619 in rats (given twice daily) were well maintained for up to six weeks of treatment, and no tachyphylaxis or receptor desensitization were observed. Based on the above data, CVT-3619 is in preclinical development by CV Therapeutics as a partial A1AR agonist for the potential treatment of T2D in order to avoid CV effects and receptor desensitization. For more information on the effects of partial A1AR agonists in diabetes and obesity, the reader is referred to Chap. 9 of this volume, “A1 Adenosine Receptor: Role in Diabetes and Obesity” (Dhalla et al.).
3.3 Angina Agents: Capadenoson (Nonnucleoside: BAY 68–4986)
Bayer chemists were first to make a key discovery that a heterocyclic class of compounds devoid of a ribose moiety can function as agonists at the adenosine receptor, although the first compounds were nonselective (Erguden et al. 2007). IJzerman and colleagues followed this with a further elaboration of the heterocyclic class of agonists in order to introduce some receptor selectivity (Chang et al. 2005). The Bayer chemists then reported the development of a compound from this very novel class of compounds. The oral A1AR agonist capadenoson (BAY 68–4986), 2-amino-6-((2-(4-chlorophenyl)thiazol-4-yl)methylthio)-4-(4-(2-hydroxyethoxy)phenyl)pyridine-3,5-dicarbonitrile (Fig. 15), was evaluated in a Phase II double-blinded, placebo-controlled multicenter study in patients with stable angina and coronary heart disease studying doses of 1, 2.5, 5, 10 and 20 mg. A 10 mg dose of capadenoson significantly reduced heart rate at peak exercise compared to placebo. Capadenoson is currently under going further studies and is anticipated to finish Phase III clinical trials by 2009 (Bays et al. 2007).
4 Allosteric Enhancers
4.1 Neuropathic Pain: T-62
A different approach to A1AR agonism is to use the endogenous adenosine levels to activate the receptor coupled with an allosteric enhancer of the A1AR. This approach has the theoretical advantage of fewer side effects, since it relies on adenosine being produced at the target tissue. In some disease states, adenosine release is a natural compensatory process to help the tissue restore balance. The A1AR allosteric enhancer will take advantage of this local adenosine release and provide activation of a local A1AR. The SAR of A1AR allosteric enhancers has evolved over many years, with major contributions from IJzerman and Baraldi (Baraldi et al. 2007; Van der Klein et al. 1999). The common structural theme that has emerged is a 2-amino-3-acyl-thiophenyl core as exemplified by the lead compound in the area, T-62 (2-amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl)(4-chlorophenyl)methanone; (Fig. 17), a compound discovered by Baraldi et al. and developed by King Pharmaceuticals for neuropathic pain (Baraldi et al. 2007; Obata et al. 2003; Pan et al. 2001). T-62 demonstrated efficacy for reducing pain hypersensitivity in a plantar surgical injury rat model (0.3–1 mcg intrathecal administration) in a dose-dependent manner. The dose of T-62 required for an antihyperalgesic effect was reduced by half when clonidine was coadministered, and this effect was 40% of the maximum possible effect. T-62 is under clinical evaluation in patients with postherpetic neuralgia experiencing pain. It will be interesting to see how the lead compound T-62 does in clinical trials of neuropathic pain, since it may drive further research in the area of A1AR allosteric enhancers.
5 Conclusion
A considerable body of research over the past 20 years in the A1AR field has resulted in the identification of clinical candidates for A1AR antagonism, agonism, and allosteric modification. From a pharmacological perspective, the developmental path for A1AR antagonists should theoretically be easier due to the challenges associated with developing A1AR agonists, such as receptor desensitization and the risk of pronounced CV and CNS side effects. With two of the three active A1AR antagonist clinical programs (KW3902 and BG9928) in Phase III human clinical trials, there is optimism in the cardiology community that an A1AR antagonist will be available for patient use in the coming years (Dohadwala and Givertz 2008). Partial A1AR agonism with CVT-3619, for example, may represent a way to avoid both CV and CNS side effects, which makes CVT-3619 an interesting compound to watch as it proceeds to the clinic. BAY 68–4986 opens up the A1AR agonist field with the advent of nonribose partial agonists that possess a longer half-life for chronic agents that are no longer limited by the high polarity of the ribose ring. The A1AR allosteric enhancer T-62 has demonstrated promising results in animal models of neuropathic pain, and is currently undergoing clinical evaluation. Based on these important scientific and clinical advances, therapeutics that target the A1AR (A1AR antagonists, A1AR agonists, and allosteric enhancers) may show long-awaited clinical success in the near future.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- ADHF:
-
Acutely decompensated heart failure
- ALT:
-
Alanine aminotransferase
- APD:
-
Action potential duration
- AR:
-
Adenosine receptor
- ARA:
-
(1S, 2R, 3R)-3-((trifluoromethoxy)methyl)-5-(6-(1-(5-(trifluro- methyl)pyridine-2-yl)pyrrolidin-3-ylamino)-9H-purin-9-yl) cyclopentane-1,2-diol
- ARB:
-
Angiotensin II receptor blocker
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the curve
- (A–V) node:
-
Atrioventricular
- BG9719:
-
(8-(3-Oxa-tricyclo[3.2.1.02, 4]oct-6-yl)-1,3-dipropyl-3, 7-dihydropurine-2,6-dione)
- BG9928:
-
(3-(4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-purin-8-yl) bicyclo[2.2.2]octan-1-yl)propanoic acid)
- cAMP:
-
Cyclic AMP
- Capadenoson:
-
(BAY 68–4986) (2-amino-6-((2-(4-chlorophenyl)thiazol-4-yl) methylthio)-4-(4-(2-hydroxyethoxy)phenyl)pyridine-3, 5-dicarbonitrile)
- CHA:
-
N 6-Cyclohexyl adenosine
- CHF:
-
Congestive heart failure
- CHO:
-
Chinese hamster ovary
- CK:
-
Creatinine kinase
- CL:
-
Total body clearance
- C max :
-
Maximal plasma concentration
- CPA:
-
N 6-Cyclopentyl adenosine
- CrCl:
-
Creatinine clearance
- CV:
-
Cardiovascular
- CVT-3619:
-
(2S, 3S, 4R)-2-((2-fluorophenylthio)methyl)-5-(6-((1R, 2R)-2-hydroxycyclopentylamino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol
- DPCPX:
-
1,3-Dipropyl-8 cyclopentylxanthine
- ED50 :
-
50% Efficient dose
- F (%):
-
% Oral bioavailability
- GFR:
-
Glomerular filtration rate
- GR79236:
-
(3R, 4S, 5R)-2-(6-((1S, 2S)-2-hydroxycyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol
- HF:
-
Heart failure
- HSL:
-
Hormone sensitive lipase
- IP:
-
Intraperitoneal
- IPC:
-
Ischemic preconditioning
- IV:
-
Intravenous
- KW3902:
-
(3-Noradamantyl-1,3-dipropylxanthine)
- L-(NAME):
-
N-ο-Nitro-l-arginine methyl ester
- MRT:
-
Mean residence time
- NEFA:
-
Nonesterified fatty acids
- PVST:
-
Paroxysmal supraventricular tachycardia
- RCM:
-
Radiocontrast media
- RPF:
-
Renal plasma flow
- SAR:
-
Structure–activity relationship
- Selodenoson:
-
(2S, 3S, 4R)-5-(6-(Cyclopentylamino)-9H-purin-9-yl)-N-ethyl-3,4-dihydroxytetrahydrofuran-2-carboxamide)
- (S–A):
-
Sinoatrial
- SLV320:
-
(4-(2-Phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-ylamino) cyclohexanol)
- t 1 ∕ 2 :
-
Half-life
- T-62:
-
(2-Amino-4,5,6,7-tetrahydrobenzo[b]thiophen-3-yl) (4-chlorophenyl)methanone
- Tecadenoson:
-
(2R, 3S, 4R)-2-(Hydroxymethyl)-5-(6-((R)-tetrahydrofuran-3-ylamino)-9H-purin-9-yl)tetrahydrofuran-3,4-diol)
- TG’s:
-
Triglycerides
- T2D:
-
Type II diabetes
- UNaV:
-
Urinary sodium excretion
- UV:
-
Urine volume
- V ds :
-
Volume of distribution
References
Akhari R, Burbiel JC, Hockemeyer J, Muller CE (2006) Recent progress in the development of adenosine receptor ligands as anti-inflammatory drugs. Curr Top Med Chem 6:1375–1399
Amann K, Breitbach M, Ritz E, Mall G (1998a) Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol 9:1018–1022
Amann K, Kronenberg G, Gehlen F, Wessels S, Orth S, Munter K (1998b) Cardiac remodeling in experimental renal failure—an immunohistochemical study. Nephrol Dial Transplant 13:1958–1966
Auchampach JA, Jin X, Moore J, Wan TC, Kreckler LM, Ge ZD, Narayanan J, Whalley E, Kiesman W, Ticho B, Smits G, Gross GJ (2004) Comparison of three different A1 adenosine receptor antagonists on infarct size and multiple cycle ischemic preconditioning in anesthetized dogs. J Pharmacol Exp Ther 308:846–856
Baraldi PG, Iaconinoto MA, Moorman AR, Carrion MD, Cara CL, Preti D, Lopez OC, Fruttarolo F, Tabrizi MA, Romagnoli R (2007) Allosteric enhancers for A1 adenosine receptor. Minirev Med Chem 7:559–569
Baraldi PG, Tabrizi MA, Gessi S, Borea PA (2008) Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility. Chem Rev 108:238–263
Bayes M, Rabasseda X, Prous JR (2003) Gateways to clinical trials. Methods Find Exp Clin Pharmacol 25:831–855
Bays M, Rabasseda X, Prous JR (2007) Gateways to clinical trials. Method Find Exp Clin Pharmacol 29(5):359–373
Belardinelli L, Dhalla A (2003) Method of treating arrhythmias comprising administration of an A1 adenosine agonist with a beta blocker, calcium channel blocker, or a cardiac glycoside. US Patent WO03088978
Belardinelli L, Lerman BB (1990) Electrophysiological basis for use of adenosine in the diagnosis and treatment of cardiac arrhythmias. Br Heart J 63:3–34
Belardinelli L, Shryock JC, Song Y, Wang D, Srinivas M (1995) Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J 5:359–365
Belardinelli L, Shryock JC, Wu L, Song Y (2005) Use of preclinical assays to predict risk of drug-induced torsades de pointes. Heart Rhythm 2(2 Suppl):S16–22
Bigot A, Stengelin S, Johne G, Herling A, Muller G, Hock FJ, Myers MR (2004) Novel adenosine analogues and their use as pharmaceutical agents. US Patent WO04003002
Boden G, She P Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N (2005) Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-{Kappa}B pathway in rat liver. Diabetes 54:3458–3465
Castelhano Al, McKibben B, Witter DJ (2005) Compounds specific to adenosine A1 receptor and uses thereof. US Patent 6,878,716
Chang LC, Von Kuenzel JK, Mulder-Krieger T, Spanjersberg RF, Roerink F, Van Den Hout G, Beukers MW, Brussee J, IJzerman AP (2005) A series of ligands displaying a remarkable agonistic–antagonistic profile at the adenosine A1 receptor. J Med Chem 48(6):2045–2053
ClinicalTrials.gov (2005) Aderis Pharmaceuticals: safety and efficacy study of an A1-adenosine receptor agonist to slow heart rate atrial fibrillation. http://www.clinicaltrials.gov/ct/ show/NCT00040001?order = 1
DailyDrugNews.com (2008) Inotek begins a Phase I trial of INO-8875 for the treatment of glaucoma (Daily Essentials, 20 June 2008). http://www.dailydrugnews.com
Dhalla AK, Shryock JC, Shreeniwas R, Beraldinelli L (2003) Pharmacology and therapeutic application of A1 adenosine receptor ligands. Curr Top Med Chem 3:369–385
Dhalla AK, Wong MY, Voshol PJ, Belardinelli L, Reaven GM (2007a) A1-adenosine receptor partial agonist lowers plasma FFA and improves insulin resistance induced by high-fat diet in rodents. Am J Physiol Endocrinol Metab 292:E1358–E1363
Dhalla AK, Melissa S, Smith M, Wong M, Shryock JC, Beraldinelli L (2007b) Antilipolytic activity of a novel partial A1 adenosine receptor agonist devoid of cardiovascular effects: comparison with nicotinic acid. J Pharmacol Exp Therp 321:327–333
DiMarco JP, Sellers TD, Lerman BB Greenberg ML, Berne RM, Belardinelli L (1985) Diagnostic and therapeutic use of adenosine in patients with supraventricular tachycardias. J Am Coll Cardiol 6:417–425
Dittrich HC, Gupta D, Hack TC, Dowling T, Callahan J, Thomson S (2007) The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. J Cardiac Fail 13:609–617
Dohadwala MM, Givertz MM (2008) Role of adenosine antagonism in cardiorenal syndrome. Cardiovasc Ther 26:276–286
Ellenbogen KA, O’Neill G, Prystowsky EN, Camm JA, Meng L, Lieu HD, Jerling M, Shreeniwas R, Beraldinelli L, Wolff AA (2005) Trial to evaluate the management of paroxysmal supraventricular tachycardia during an electrophysiology study with Tecadenoson. Circulation 111:3202–3208
Erguden JK, Karig G, Rosentretter U, Albrecht B, Henninger K, Hutter J, Diedrichs N, Nell P, Arndt S, Hubsch W, Knorr A, Schlemmer KH, Brohm P (2007) Substituted phenylaminothiazoles and use thereof. US Patent WO0773855
Fatholahi M, Xiang Y, Wu Y, Li Y, Wu L, Dhalla AK, Belardinelli L, Shryock JC (2006) A novel partial agonist of the A1-adenosine receptor and evidence of receptor homogeneity in adipocytes. J Pharmacol Exp Ther 317:676–684
Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA (1983) Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747
Forman M, Velasco CE, Jackson EK (1993) Adenosine attenuates reperfusion injury following regional myocardial ischemia. Cardiovasc Res 27:9–17
Fredholm BB, IJzerman AP, Jacobson KA, Klotz, KN Linden J (2001) International Union of Pharmacology. XXXV: nomenclature and classification of adenosine receptors. Pharmacol Rev 53:527–552
Gardner CJ, Twissell DJ, Coates J, Strong P (1994) The effects of GR79236 on plasma fatty acid concentrations, heart rate and blood pressure in the conscious rats. Eur J Pharmacol 257:117–121
Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC, on behalf of the CKI-201 and CKI-202 investigators (2007) The effects of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 50:1551–1560
Gottlieb SS, Skettino SL, Wolff A, Beckman E, Fisher ML, Freudenberger R, Gladwell T, Marshall J, Cines M, Bennett, DLiitschwager EB (2000) Effects of BG9719 (CVT-124), an A1-adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J Am Coll Cardiol 35:56–59
Gottlieb SS, Brater C, Thomas I, Havranek E, Bourge R, Goldman S, Dyer F, Gomez M, Bennett D, Ticho B, Beckman E, Abraham WT (2002) BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 105:1348–1353
Green A (1987) Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem 262:15702–15707
Green A, Johnson JL, Milligan G (1990) Down-regulation of Gi subtypes by prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem 265:5206–5210
Greenberg B, Thomas I, Banish D, Goldman S, Havranek E, Massie B, Zhu Y, Ticho B, Abraham WT (2007) Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure. J Am Coll Cardiol 50:600–606
Hess S (2001) Recent advances in adenosine receptor antagonist research. Expert Opin Ther Patents 11:1533–1561
Hoffman BB, Chang H, Dall’Agilo E, Reaven GM (1986) Desensitization of adenosine receptor-mediated inhibition of lipolysis. The mechanism involves the development of enhanced cyclic adenosine monophosphate accumulation in tolerant adipocytes. J Clin Invest 78:185–190
Hosokawa T, Yamauchi M, Yamamoto Y, Iwata K, Mochizuki H, Kato Y (2002) Role of the lipid emulsion on an injectable formulation of lipophilic KW-3902, a newly synthesized adenosine A1-receptor antagonist. Biol Pharm Bull 25:492–498
IJzerman AP, van der Wenden NM, van Galen PJM, Jacobson KA (1995) Molecular modeling of adenosine A1 and A2a receptors. In: Belardinelli L, Pelleg A (eds) Adenosine and adenine nucleotides: from molecular biology to integrative physiology. Kluwer, Boston, MA, pp 27–37
Itani SI, Ruderman NB, Schmieder F, Boden G (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol protein kinase C and IKappaB-alpha. Diabetes 51:2005–2011
Jacobson KA, Gao ZG (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Disc 5:247–264
Jagtap P, Szabo C, Salzman AL (2005) Purine derivatives as adenosine A1 receptor agonists and methods of use thereof. WO Patent Appl 2005/117910
Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B (2007) The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol 151:1025–1032
Khimenko PL, Moore TM, Hill LW, Wilson PS, Coleman S, Rizzo A, Taylor AE (1995) Adenosine A2 receptors reverse ischemia-reperfusion lung injury independent of beta-receptors. J Appl Physiol 78:990–996
Kiesman WF, Zhao J, Conlon PR, Dowling JE, Petter RC, Lutterodt F, Jin X, Smits G, Fure M, Jayaraj A, Kim J, Sullivan GW, Linden J (2006a) Potent and orally bioavailable 8-bicyclo[2.2.2]octylxanthines as adenosine A1 receptor antagonists. J Med Chem 49:7119–7131
Kiesman WF, Zhao J, Conlon PR, Petter RC, Jin X, Smits G, Lutterodt F, Sullivan GW, Linden J (2006b) Norbornyllactone-substituted xanthines as adenosine A1 receptor antagonists. Bioorg Med Chem 14:3654–3661
Kossel A (1888) Über eine neue Base aus dem Pflanzenreich. Chem Ber 21:2164–2167
Lerman BB, Belardinelli L (1991) Cardiac electrophysiology of adenosine. Basis and clinical concepts. Circulation 83:1499–1509
Liu GS, Thornton J, Van Winkle DM, Stanely AW, Olsson RA, Downey JM (1991) Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 84:350–356
Magata S, Taniguchi M, Suzuki T, Shimamura T, Fukai M, Furukawa H, Fujita M, Todo S (2007) The effect of antagonism of adenosine A1 receptor against ischemia and reperfusion injury of the liver. J Surg Res 139:7–14
Merkel LA, Hawkins ED, Colussi DJ, Greenland BD, Smits GJ, Perrone MH, Cox BF (1995) Cardiovascular and antilipolytic effects of the adenosine agonist GR79236. Pharmacology 51:224–36
Mizumura T, Auchampach JA, Linden J, Burns RF, Gross GJ (1996) PD 81,723 an allosteric enhancer of the A1 adenosine receptor, lowers the threshold for ischemic preconditioning in dogs. Circ Res 79:415–423
Miura T, Tsuchida A (1999) Adenosine and preconditioning revisited. Clin Exp Pharm Physiol 26:92–99
Moro S, Gao Z.-G, Jacobson KA, Spalluto G (2006) Progress in the pursuit of therapeutic adenosine receptor antagonists. Med Res Rev 26:131–159
Morrison CF, Elzein E, Jiang B, Ibrahim P, Maa T, Wu L, Zeng D, Fong I, Lustig D, Leung K, Zablocki J (2004) Structure–affinity relationships of 5′-aromatic ethers and 5′-aromatic sulfides as partial A1 adenosine agonists, potential supraventricular anti-arrhythmic agents. Bioorg Med Lett 14:3793–3797
Nonaka H, Ichimura M, Takeda M, Kanda T, Shimada J, Suzuki F, Kase H (1996) KW-3902, a selective high affinity antagonist for adenosine A1 receptors. Br J Pharmacol 117:1645–1652
Novacardia, Inc. (2007) Pilot phase 3 results of Novacardia’s KW-3902 for acute congestive heart failure. In: Heart Failure Congress 2007, Hamburg, Germany, 9–12 June 2007 (see http://www.medicalnewstoday.com/articles/73876.php)
Obata H, Li X, Eisenach JC (2003) A synergistic effect of intrathecal administration of T62 and clonidine in the rat postoperative pain model. In: 33rd Annu Meet Soc Neurosci Proc, New Orleans, LA, 8–12 Nov 2003, Abstract 589.7
Pan H.-L, Xu Z, Leung E, Eisenach JC (2001) Allosteric adenosine modulation to reduce allodynia. Anesthesiology 95:416
Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpi E, Rosello-Catafau J (1999) The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology 29:126–132
Peterman C, Sanoski CA (2005) Tecadenoson: a novel, selective A1 adenosine receptor agonist. Cardiol Rev 13:315–321
Pfister JR, Bellardinelli L, Lee G, Lum RT, Milner P, Stanley WC, Linden J, Baker SP, Schreiner G (1997) Synthesis and biological evaluation of the enantiomers of the potent and selective A1-adenosine antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbonyl)]-xanthine. J Med Chem 40:1773–1778
Prystowsky EN, Niazi I, Curtis AB, Wilber DJ, Bahnson T, Ellenbogen K, Dhalla A, Bloomfield DM, Gold M, Kadish A, Fogel RI, Gonzalez MD, Belardinelli L, Shreeniwas R, Wolff AA (2003) Termination of paroxysmal supraventricular tachycardia by tecadenoson (CVT-510), a novel A1-adenosine receptor agonist. J Am Coll Cardiol 42:1098–1102
Qu X, Cooney G, Donnelly R (1997) Short-term metabolic and haemodynamic effects of GR79236 in normal and fructose-fed rats. Eur J Pharmacol 338:269–276
Roden, M, Price, TB, Perseghin, G, Petersen, KF, Rothman, DL, Cline, GW, Shulman, GI (1996) Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Investig 97: 2859–2865
Sako Y, Grill VE (1990) A 48-hours lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology 127:1580–1589
Shamim MT, Ukena D, Padgett WL, Hong O, Daly JW (1988) 8-Aryl-and 8-cycloalkyl-1,3-dipropylxanthines: further potent and selective antagonists for A1-adenosine receptors. J Med Chem 31:613–617
Shimada J, Suzuki F, Nonaka H, Karasawa A, Mizumoto H, Ohno T, Kubo K, Ishii A (1991) 8-(Dicyclopropylmethyl)-1,3 dipropylxanthine: a potent and selective adenosine A1, antagonist with renal protective and diuretic activities. J Med Chem 34:466–469
Shimada J, Suzuki F, Nonaka H, Ishii A (1992) 8-Polycycloalkyl-1,3-dipropylxanthines as potent and selective antagonists for A1-adenosine receptors. J Med Chem 35:924–930
Song Y, Wu L, Shryock JC, Beralinelli, L (2002) Selective attenuation of isoproterenol-stimulated arrhythmic activity by a partial agonist of adenosine A1 receptor. Circulation 105:118–123
Srinivas M, shryock JC, Dennis DM, Baker SP, Beraldinelli L (1997) Differential A1 adenosine receptor reserve for two actions of adenosine on guinea pig atrial myocytes. Mol Pharmacol 52:683–691
Stephenson RP (1997) A modification of receptor theory. Br J Pharmacol 120:106–120
Suzuki F, Shimada J, Mizumoto H, Karasawa A, Kubo K, Nonaka H, Ishii A, Kawakita T (1992) Adenosine A1 antagonists. 2. Structure–activity relationships on diuretic activities and protective effects against acute renal failure. J Med Chem 35:3066–3075
Ticho B, Whalley E, Gill A, Lutterodt F, Jin X, Auchampach J, Smits G (2003) Renal effects of BG9928, an A1 adenosine receptor antagonist, in rats and nonhuman primates. Drug Dev Res 58:486–492
Van der Klein PAM, Kourounakis AP, IJzerman AP (1999) Allosteric modulation of the adenosine A1 receptor. Synthesis and biological evaluation of novel 2-amino-3-benzoylthiophenes as allosteric enhancers of agonist binding. J Med Chem 42:3629–3635
van Galen PJM, Stiles GL, Michaels G, Jacobson KA (1992) Adenosine A1 and A2 receptors: structure–function relationships. Med Res Rev 12:423–471
Vu CB, Kiesman WF, Conlon PR, et al (2006) Tricyclic imidazoline derivatives as potent and selective adenosine A1 receptor antagonists. J Med Chem 49:7132–7139
Wang D, Shryock JC, Belardinelli L (1996) Cellular basis for the negative dromotropic effect of adenosine on rabbit single atrioventricular nodal cell. Circ Res 78:697–706
Webster JM, Heseltine L, Taylor R (1996) In vitro effect of adenosine agonist GR79236 on insulin sensitivity of glucose utilization in rat soleus and human rectus abdominus muscle. Biochim Biophys Acta 1316:109–113
Wu L, Belardinelli L, Zablocki JA, Palle V, Shryock JC (2001) A partial agonist of the A1-adenosine receptor selectively slows AV conduction in guinea pig hearts. Am J Physiol Heart Cir 280:H334–H343
Yan L, Burbiel JC, Maass A, Muller CE (2003) Adenosine receptor agonists from basic medicinal chemistry to clinical development. Expert Opin Emerg Drugs 8:537–576
Yao K (2000) Effect of KW-3902, a selective adenosine A1-receptor antagonist, on accumulation of gentamicin in the proximal renal tubules in rats. Yakugaku Zasshi 120:801–805
Yao K, Heyne N, Erley CM, Risler T, Osswald H (2001) The selective adenosine A1 receptor antagonist KW-3902 prevents radiocontrast media-induced nephropathy in rats with chronic nitric oxide deficiency. Eur J Pharmacol 414:99–104
Yuzlenko O, Kieć-Kononowicz K (2006) Potent adenosine A1 and A2A receptors antagonists: recent developments. Curr Med Chem 13:3609–3625
Zablocki JA, Wu L, Shryock J, Belardinelli L (2004) Partial A(1) adenosine receptor agonists from a molecular perspective and their potential use as chronic ventricular rate control agents during atrial fibrillation (AF). Curr Top Med Chem 4:839–54
Zannikos PN, Rohatagi S, Jensen BK (2001) Pharmacokinetic-pharmacodynamic modeling of the antilipolytic effects of an adenosine receptor agonist in healthy volunteers. J Clin Pharmacol 41:61–69
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kiesman, W.F., Elzein, E., Zablocki, J. (2009). A1 Adenosine Receptor Antagonists, Agonists, and Allosteric Enhancers. In: Wilson, C., Mustafa, S. (eds) Adenosine Receptors in Health and Disease. Handbook of Experimental Pharmacology, vol 193. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-89615-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-540-89615-9_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-89614-2
Online ISBN: 978-3-540-89615-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)