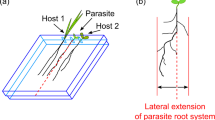
Abstract
Parasitic and carnivorous plants that adopt a heterotrophic lifestyle encounter novel environmental challenges that are shared with other heterotrophs, such as the need to locate hosts or lure prey and the need to overcome the defenses of their intended victims. These challenges are particularly acute for holoparasitic plants that depend entirely on their hosts for nutrients and other resources. In response to these challenges, holoparasitic plants employ a variety of strategies to locate and identify appropriate hosts. Root parasites such as Striga and Orobanche produce large numbers of tiny seeds that germinate only in response to host-derived chemical cues localized to the immediate vicinity of host roots. Other parasites, such as dodders (Cuscuta), produce relatively few large seeds that store sufficient resources for the parasitic seedling to "forage" for nearby hosts. Here we describe recent research on the mechanisms underlying these host-location strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.I 1 Introduction
6.I.1 1.1 Plant Behavior
If the concept of plant "behavior" is in some sense provocative, or even controversial, it is likely because behavior can easily seem, on first reflection, to be exactly the quality that animals possess and plants do not. A reasonable definition of the common-sense notion of behavior might be, "things that organisms do." And, to the casual observer, plants often don't seem to be doing much. Even Aristotle—who was manifestly not a casual observer—attributed to plants only the qualities of growth, reproduction, and decay, while reserving the powers of perception and locomotion for animals. More recent observers, aided by the tools of modern science, have shown that plants are not nearly so passive as they appear at first glance. Plants perceive the environments around them in myriad ways, as the examples described throughout this volume amply document. Plants also locomote, though over distances and timescales that are not always readily apparent to human observers.
Whether these activities of plants—or some subset of them—should be called behavior is a matter of intellectual perspective, the key question being whether such usage tends to illuminate the real and important commonalities between plants and animals or to obfuscate significant differences. The answer depends largely on which aspects of the phenomena we wish to emphasize. A mechanistic definition of behavior, drawing on work in animal systems, that makes explicit reference to muscles and nerves will necessarily exclude the actions of plants no matter how rapid or complex they might be. However, while such a definition might be criticized on grounds of utility or historical precedence, it cannot be argued that such a restrictive definition is incoherent, for there are obviously profound differences in the ways that plants and animals respond to and interact with their environment, and these distinctions are worth noting.
However, we prefer to emphasize the evolutionary function of behavior as an adaptive mechanism by which organisms achieve a better fit to dynamic and unpredictable environments by acquiring and responding to external information in ecological time. Thus, we are amenable to the recently proposed definition of plant behaviors as morphological or physiological responses to events or environmental changes that are rapid relative to the lifetime of an individual (Silvertown and Gordon 1989; Silvertown 1998; Karban 2008). As Karban (2008) points out, this definition is similar to commonly used descriptions of phenotypic plasticity in plants (Bradshaw 1965)—behavior under this definition being a form of phenotypic plasticity, occurring in response to a stimulus, that is relatively rapid and potentially reversible (Silvertown and Gordon 1989). It is likely, in fact, that plant responses occupy a continuum of rapidity and reversibility along which it may prove difficult to draw clear-cut distinctions. At one end of this continuum, the active foraging of the seedling of a parasitic dodder vine, for example, would likely satisfy even the most common-sense notion of behavior—if Aristotle had seen a time lapse video of a dodder seedling searching for a host he would likely have reconsidered the classification cited above. In contrast, the dependence of seed germination in other parasitic plants on exposure to chemical cues derived from the roots of host plants fits somewhat less easily with either an intuitive notion of behavior or with the technical definition described above. Nevertheless, it obviously makes sense to address these plant strategies together since, as we will discuss below, they serve fundamentally similar ecological functions as mechanisms of host location.
We should note, however, that such ambiguity is not unique to plant systems. The quiescence that eggs of some aquatic invertebrates exhibit—which we will discuss below as being analogous to the contingent germination strategies of plants—and from which they emerge only in response to environmental cues signaling the presence of favorable ecological conditions, also may not obviously fit with our commonsense notion of behavior. However, it is certainly the sort of thing that behavioral ecologists might study, which suggests another less technical but potentially useful definition of plant behavior: plant behaviors are those things that plants do which people who are interested in behavior might be keen to know about.
6.I.2 1.2 The Behavior of Parasitic Plants
Whatever definition we employ, we are likely to find that the behavior and ecology of plants most closely approaches those of animals in plant groups that adopt a parasitic or carnivorous habit. In their migration up the food chain, these plants encounter novel environmental challenges that are shared with other heterotrophs, such as the need to identify and locate organisms on which to feed and the need to overcome the defenses of their hosts or prey. This is especially the case for holoparasitic plants, which have forsaken the autotrophic habit entirely and derive their sustenance exclusively from their hosts. This similarity in the lifestyles of heterotrophic plants and animals was noted as early as the tenth century by an Arabian scholar, who described the actions of a parasitic plant, most likely a member of the genus Cuscuta, as corresponding "to those of the animal soul while its body remains that of a plant … for it attaches itself to trees, seeds, and thorns, and feeds itself as the worm from the juices of its host plant, thus with its soul carrying out the actions of animals" (Dieterici 1861 in Kuijt 1969).
In this chapter we will focus on the most distinctive "behavioral" characteristics of parasitic plants: their responses to environmental cues associated with the location and exploitation of host plants. Parasitic plants perceive and respond to cues from their host at many stages of development. In some cases, cues indicating the proximity of the host are required for the germination of seeds (Boumeester et al. 2003). Following germination, the radical of the parasitic seedling must grow toward and contact the body of the host plant, and this process also may be guided by the reception of chemical or other cues from the host (e.g., Runyon et al. 2006). Upon contacting the surface of the host, the attachment of the parasite and the penetration of host tissues (haustorium formation) are initiated and guided by the perception of host secondary metabolites (Yoder 2001; this volume). This chapter will focus primarily on the means by which parasites are able to find their hosts, as efficient host location is a particularly pressing problem for holoparasitic species, which depend entirely on the host for resources and thus must rapidly attach to a host following seed germination or else perish when the stored nutrients from the endosperm are exhausted (Butler 1995). The mechanisms underlying haustoria formation and the creation of a connection to the xylem of the host plant are addressed in more detail in the chapter on hemiparasitic plants.
6.II 2 The Lifestyle of Parasitic Plants
Approximately 4,500 flowering plant species, representing more than 1% of all angiosperms, have evolved the ability to parasitize other plants (Nickrent 2007; Parker and Riches 1993). Parasitism appears to have evolved independently as many as 11 times in angiosperms, and parasitic forms occur in diverse plant groups in approximately 270 genera across 22 families. The common feature that all parasitic plants share is their ability to acquire some or all of their nutrients and other resources from other plants through the production of a haustorium, a structure that is able to invade host plant tissues and act as the physiological bridge through which host resources are translocated to the parasite (Kuijt 1969; Press and Graves 1995).
A distinction can be drawn between holoparasitic plants, which lack chlorophyll and obtain all of their energy, water, and nutrients from the host, and hemiparasitic plants, which obtain some of their resources from the host but also carry out photosynthesis. However, this distinction is not always clear-cut (Musselman and Press 1995). Less than 10% of all parasitic species are strict holoparasites (Heide-Jørgensen 2008), but some other parasitic groups conduct only very limited photosynthesis. The genus Cuscuta, for example, contains some species that contain very small amounts of chlorophyll along with others that contain none at all. In still other groups, individuals may possess chlorophyll only at certain stages of their life cycle. For example, the root-parasitic species in the genus Striga are achlorophyllous when below ground and only become green and photosynthetic after their emergence above the soil surface (Musselman and Press 1995).
The ecology of holoparasitic or nearly holoparasitic species can be quite distinct from that of other plants (Heide-Jørgensen 2008), including more actively photosynthetic hemiparasites. Because the absence of chlorophyll frees holoparasitic species from a dependence on light, they can inhabit low-light environments and are able to evolve life histories in which most or all of the parasite's vegetative tissue remains underground or within the host plant. The vegetative bodies of parasites in the genus Rafflesia, for example, grow entirely within the tissues of the host, with only the flowers appearing externally. Holoparasitism also renders the absorptive root system superfluous, and it is absent in most strict holoparasites and greatly reduced in the Orobanchae. A further distinction is sometimes drawn between facultative and obligate parasites, but the biological relevance of this distinction is disputable, as it is not clear that any parasitic species routinely complete development without a host under natural conditions (Heide-Jørgensen 2008). A more meaningful distinction can be drawn between stem parasites, which attach to aboveground portions of their host plants, and root parasites, which make their attachments below ground. The latter account for approximately 60% of parasitic species.
There is great disparity in the extent to which the biology and ecology of parasitic plant species have been investigated, with a large majority of the research having been done on species that pose significant problems for agriculture. These include the witchweeds (Striga spp.) and broomrapes (Orabanche spp.), which parasitize host roots, and dodders (Cuscuta spp.), which make their aboveground attachments to plant shoots. We will focus our discussion primarily on these three taxa. The Orabanche are true holoparasites, while Cuscuta, as noted above, contains both strictly holoparasitic species and species with very limited photosynthetic ability. Striga are technically hemiparasitic, as they are chlorophyllic following emergence from the soil—though the photosynthetic capacity of isolated Striga chloroplasts is quite low, indicating strong dependence on the host (Tuquet et al. 1990). However, their subterranean seedlings, which must locate hosts and initiate parasitism, lack chlorophyll and are thus functionally holoparasitic (Parker and Riches 1993)—as we will discuss below, Striga, are also dependent on host-derived cues for germination and thus cannot mature under natural conditions in the absence of the host. Moreover, the germination and host location ecologies of Striga and Orabanche are quite similar, making it convenient to discuss these taxa together.
Despite accounting for a relatively small proportion of parasitic species, holoparasitic plants—or those that are functionally holoparasitic at the stage when parasitism is initiated—have a disproportionate impact on human agriculture. The root parasites Striga, Orabanche, and Alectra can be particularly pernicious pests, as they often inflict serious damage on host plants before the latter emerge from the soil, complicating control efforts (Runyon et al. 2008). Striga spp., for example, infest an estimated two-thirds of the cereals and legumes in sub-Saharan Africa, causing annual crop losses estimated at seven billion dollars and negatively impacting the lives of more than 300 million people (Berner et al. 1995; Musselman et al. 2001; Gressel et al. 2004; Press et al. 2001). The greatest economic costs are inflicted by S. hermonthica and S. asiatica, which between them cause major damage to many of the most important cereal crops, including maize, sorghum, millet, rice and sugar cane (Parker and Riches 1993).
6.III 3 Strategies for Seed Dispersal and Host Location
6.III.1 3.1 Seed Dispersal Strategies
Given the sedentary lifestyle of plants, angiosperm dispersal is accomplished primarily by the movement of seeds (although vegetative dispersal through growth or through the movement of vegetative tissue by wind or water is frequent in some species), and plants have evolved a wide array of strategies and mechanisms for effective seed dispersal (Butler 1995). For parasitic plants, a primary objective of seed dispersal strategies is to bring the seeds into the proximity of a host. For the reasons noted above, this is an especially pressing objective for holoparasites. Heide-Jørgensen (2008) described four primary seed dispersal strategies that are employed by parasitic plants:
-
(1)
Some species produce large seeds with relatively high levels of stored resources (starch, fat, and protein) that will sustain the seedling for a limited period of time during which it will "forage" for a host. This is the strategy employed by Cuscuta spp., all the root-parasitic members of the Santalales, and several hemiparasitic Orobanchaceae (Kuijt 1969; Heide-Jørgensen 2008). This strategy entails the production of relatively large seeds, as the extent of seedling growth that can be supported by endospermic reserves present in the seed defines a critical distance beyond which a seedling has no possibility of reaching a host. For some species this distance can be quite large: seedlings of the shoot-parasitic dodder C. gronovii can search for hosts over distances of up to 35 cm (Costea and Tardif 2006). In contrast, C. pentagona seedlings rarely grow more than 10 cm before wilting (Runyon et al. 2006). For most root-parasitic holoparasites, the critical distance is probably on the order of millimeters (Salle et al.1998).
-
(2)
A second strategy entails the production of sticky seeds that are dispersed by animals, primarily birds, and often deposited directly onto a branch of the host plant. As with the first strategy, this method of seed dispersal entails the production of relatively large seeds. This strategy is employed by the stem-parasitic loranths and mistletoes and is common among the Santales. The majority of the species that employ this strategy are hemiparasitic, and in some cases the endospermic tissues are capable of active photosynthesis, which is initiated immediately following germination. However, this strategy is also employed by the holoparasite Tristerix aphyllus, a member of the family Loranthaceae, which has a rather remarkable lifestyle (Heide-Jørgensen 2008): T. aphyllus exclusively parasitizes two columnar cacti from the southern Andes, Echinopsis chilensis and Eulychnia acida, and its seeds are dispersed by the Chilean mockingbird, Mimus thenca (Norton and Carpenter 1998; Gonzales et al. 2007). The seeds are typically deposited by the birds onto the spines of the cactus, where they adhere and then the newly germinated seedling grows up to 10 cm to bring the tip of the radicle into contact with the body wall of the cactus. After establishing itself on the host, T. aphyllus is entirely endophytic, with only its bright red inflorescences appearing on the exterior of the host, where they are pollinated by hummingbirds.
-
(3)
A third strategy is similar to the second, but involves seeds that are brought into direct contact with the host by agents other than animals, including wind and water as well as self-dispersal. Seeds of Arceuthobium, for example, are covered with sticky viscin like those of other mistletoes, but rather than being carried by birds, their dispersal is achieved by the explosiveness of the fruits (Hinds and Hawksworth 1965; Garrison et al. 2000).
-
(4)
The fourth strategy entails the production of seeds that are passively dispersed but that require exposure to stimulatory compounds from the host in order to initiate germination. This is the strategy employed by most of the holoparasitic root parasites, including Orabanche—in which the requirement for germination stimulants from the host was first observed in 1823 (Vaucher 1823)—as well as by Striga, on which a great deal of research has addressed the mechanisms underlying the stimulation of germination, as discussed in the next section. As a general rule, the host-derived exudates exploited for host recognition are active only within a few millimeters of the host roots. Consequently, this strategy entails the production of large numbers of small, long-lived seeds to enhance the probability that some seeds will come to rest in the immediate vicinity of a host.
6.IV 3.4 Seed Germination
6.IV.1 4.1 Seed Dormancy and Germination Requirements
Despite the dynamism outlined in this volume, plants cannot readily move large distances to find resources or escape harsh conditions and so must pursue other, more patient, strategies for coping with heterogeneous environments. Seed dormancy is an adaptative strategy, widely distributed among higher plants (Finch-Savage and Leubner-Metzger 2006), in which seeds enter a state of developmental quiescence, allowing time for the seeds to disperse and suspending growth until the seeds encounter a specific set of environmental conditions favorable to their development. Seed dormancy is a form of embryonic diapause, which exhibits widespread occurrence in both plants and animals. In many mammals, for example, fertilized eggs may enter a state of quiescence to await the presence of favorable conditions for development. This may occur as a matter of course, as in roe deer, where mating occurs in the fall but the development of fertilized eggs is delayed until the following spring (Sandell 1990). Or it may be contingent on specific ecological or social conditions. For example, in some mammals that produce multiple litters per year, the further development of fertilized eggs is suspended in response to the presence of physiological cues associated with lactation, indicating the presence of other dependent offspring (Lopes et al. 2004).
Diapause, embryonic or otherwise, is a common strategy employed by animals that inhabit highly variable or intermittently harsh environments. The planktonic crustacean Daphnia produces "resting" eggs that remain dormant to escape dry periods in temporary ponds or periods of intense predation in permanent ponds. The resumption of development is contingent upon exposure to environmental cues (e.g., photoperiod and temperature) associated with favorable ecological conditions (Hairston et al. 1995), and may possibly be inhibited by chemical cues indicating the presence of predatory fish (Lass et al. 2005), as has been reported for the reactivation of resting stages in dinoflagellates (Rengefors et al. 1998). Quiescent eggs of planktonic organisms may remain viable for many years, resulting in the accumulation in aquatic sediments of an "egg bank" analogous to the seed bank present in terrestrial soils (Hairston et al. 1995).
Among flowering plants, seed dormancy is the rule, and most seeds germinate only following exposure to one or more external stimuli signaling the presence of favorable growth conditions. For example, germination may depend on the presence of specific conditions relating to light, temperature, water, oxygen, and nutrients (Finch-Savage and Leubner-Metzger 2006). Parasitic plants also require permissive conditions with respect to these variables (Worsham 1987), but they face the additional challenge of needing to find a suitable host plant to parasitize—a particularly pressing issue for holoparasites and other obligately parasitic forms that must rapidly locate and attach to a host or perish. As a result, some parasitic forms are dependent on germination stimulants from the host. Even following germination, parasitic plants have been found to arrest development at a number of developmental stages, requiring signals from the host plant to continue growth. The stages at which development can be arrested include germination, haustorial initiation, host tissue penetration, physiological compatibility with the host and apical meristem development (Nickrent et al. 1979; Boone et al. 1995). However, because seed germination is the critical first committed step in the developmental process, it can be the most discriminating in terms of host selection (Boone et al. 1995).
The details of the conditions required for germination appear to be highly variable across parasitic species, with most work having been done on the economically important species of Stiga and Orabanche, and especially on the important agricultural pests S. asiatica and S. hermonthica, which attack gramineous crops, and S. gesneriodes, which parasitizes legumes (Musselman 1980; Parker 1991). The seeds of Striga are very small, measuring around 0.15 × 0.31 mm, and therefore lack the reserves for sustained growth before host attachment—it is estimated that for successful host attachment germination must take place within 3–4 mm of the host root (Ramaiah et al. 1991). To compensate for these biological restrictions, Striga spp. may produce up to 450,000 seeds per plant, with a persistence in the soil of up to ten years (Eplee 1992). Prior to germination, Striga seedlings must undergo an after-ripening period during which seeds require a certain temperature and moisture regime for a period of about two weeks before they will respond to germination stimulants. This period may involve the breakdown of phenolic compounds that act as germination inhibitors (Musselman 1980). Following the after-ripening period, the seeds require a further conditioning period during which they are exposed to adequate levels of water and oxygen in the absence of light before exposure to germination stimulants can initiate germination. White light inhibits the germination of S. asiatica both before and immediately after exposure to germination stimulants (Egley 1972). However, beyond three hours after exposure to the maize germination stimulants, the developmental process is unresponsive to light. In the absence of host-derived stimulants, the seeds maintain dormancy and can remain viable through multiple preconditioning seasons. In Orabanche, seeds may remain viable for as long as 60 years (Heide-Jørgensen 2008). As discussed below, several classes of plant-derived compounds have been suggested to have germination-stimulating activity.
6.IV.2 4.2 Germination Stimulants
6.IV.2.i 4.2.1 Strigolactones
Strigol, the first germination-stimulating compound to be positively identified (Cook et al. 1966, 1972 ), was initially purified from hydroponically grown roots of cotton plants—a false host of Striga that stimulates seed germination but does not support development of the parasite—and was found to stimulate seed of S. lutea, eliciting 50% germination at concentrations as low as 10−5 ppm in water. Subsequently, a structural analog of strigol, sorgolactone, was isolated from sorghum, a true host of Striga (Hauck et al. 1992), while strigol itself was found to be present in the true hosts maize and millet (Siame et al. 1993). A chemically similar compound, alectrol, was identified from cowpea (Müller et al. 1992). Later, alectrol and another naturally occurring strigolactone, orabanchol, were found to serve as stimulants for Orabanche seed germination in response to root exudates of red clover.
Butler (1995) proposed the name "strigolactones" for this class of compounds. To date, nine naturally occurring strigolactones have been identified in plant root exudates (Akiyama and Hayashi 2008; Bouwmeester et al. 2007; Xie et al. 2007, 2008a, b; Matsuura et al. 2008). Strigol-like compounds have also been found in a number of medicinal plant species that are not known to be hosts or false hosts for parasitic weeds (Yasuda et al. 2003), suggesting that production of strigolactones may be widespread among plants. Strigolactones are typically present in root exudates in low quantities (cotton seedlings reportedly secreted ∼15 pg of strigol per day; Sato et al. 2005), and several different strigalactones are present in most plants, with the ratios of compounds present varying from one species to another and even among varieties of individual species (Awad et al. 2006).
Structurally, a strigolactone comprises a tricyclic lactone that is connected, via an enol ether bond, to a methylbutenolide ring, and they were long regarded as sesquiterpenoids. However, Matusova et al. (2005) recently demonstrated the involvement of the carotenoid pathway in strigolactone biosynthesis, through a series of experiments employing carotenoid mutants of maize, and inhibitors of isoprenoid pathways on maize, sorghum and cowpea. Specifically, the tricyclic lactone was shown to be derived from the C40 carotenoids that originate from the plastidic, nonmevalonate methylerythritol phosphate (MEP) pathway.
Following the discovery of the role of strigol in stimulating the germination of parasitic plant seeds, a number of structural bioactivity studies aimed at elucidating the mode of action of strigolactones and the developing synthetic analogs that might be used to induce "suicidal germination" of parasitic plant seeds in agricultural systems (e.g., Johnson et al 1981; Mangnus and Zwanenburg 1992; Mangus et al. 1992a, b; Bergmann et al. 1993; Kranz et al. 1996) led to the synthesis of a variety of synthetic strigolactone analogs, some of which stimulate germination in both Striga and Orabanche (Worsham 1987; Stewart and Press 1990, Bergmann et al. 1993). Among these were the so-called GR ("germination releaser") compounds that were first described by Johnson et al. (1976, 1981; see also Humphrey et al. 2006).
These structural analogs have variable rates of activity, with GR-7 and GR-24 having the strongest stimulatory effect on germination (Bergmann et al. 1993), and GR-24 came to be used as a standard positive control for studies of germination activity (Humphrey et al. 2006). Based on the results of numerous structure–activity studies, including those cited above, Mangnus and Zwanenburg (1992) proposed a tentative model for the molecular mechanism underlying the germination-stimulating activity of strigolactones. The model hypothesized a receptor-mediated process in which a nucleophilic group present at the receptor site attacks the enol bridge of the strigolactone molecule, with elimination of the D-ring serving as the mechanism for biological activation. This model is consistent with observed variation in the germination-stimulating activities of synthetic strigolactone analogs, but has not been confirmed by direct evidence as yet (Humphrey et al. 2006).
6.IV.2.ii 4.2.2 Strigolactones as Host-Location Cues for AM Fungi
An Initiation of the symbiosis relies on the establishment of a network of connections between the roots of the host plant and the fungal hyphae, and entails extensive hyphal branching, presumably in response to chemical cues released by the host roots. Akiyama et al. (2005) demonstrated that the chemical factor responsible for inducing this branching is the strigolactone 5-deoxystrigol. Moreover, several other naturally occurring strigolactones, as well as GR24, were found to induce hyphal branching at similar concentrations.
It has been proposed that the emergence of strigolactone production during the evolution of strigolactone production as a host-location signal allowing AM fungi to find host roots may have provided an opportunity for later evolving parasitic weeds to co-opt it for their own ends (Bouwmeester et al. 2007, Akiyama and Hayashi 2008). This notion is supported by the observation that plant families where germination stimulant activity is relatively unreported tend to include plants which do not associate with AM fungi (Humphrey et al. 2006). The discovery of orobanchol in the root exudates of Arabidopsis thaliana, a nonhost of AM fungi but a host of O. aegyptiaca (Goldwasser et al. 2008), suggests, however, that strigolactones may be distributed beyond the host range of AM fungi.
6.IV.2.iii 4.2.3 Sesquiterpene Lactones
These compounds, which share some structural similarities with strigolactones, are widely distributed in plants and have been shown to have a variety of biological activities, including potential allelopathy (Macías et al. 2006). Several naturally occurring sesquiterpenes were shown to stimulate germination of Striga seeds (Fischer et al 1989). More recently, Macías and colleagues (2006) found that several sesquiterpene lactones induced germination of the seeds of O. cumana but not those of O. crenata or O. ramosa (de Luque et al. 2000; Galindo et al. 2002). O. cumana is a specialist parasite of sunflowers, which are known to contain large amounts of sesquiterpene lactones (Bouwmeester et al. 2003), and the response of O. cumana to these compounds (parthenolides) may represent a specific evolutionary response by this specialist parasite in addition to any naturally occurring recognition of strigolactones (Humphrey et al. 2006).
6.IV.2.iv 4.2.4 SXSg and the Debate Over Germination Stimulation by Sorghum
Following the discovery of strigol in cotton, but prior to the isolation of strigolactones from true hosts of Striga and Orobanche, a chemically different compound, the hydroquinine derivative dihydrosorgoleone, was isolated from sorghum root exudates and reported to have germination-stimulating activity (Chang et al. 1986). This compound is also commonly referred to as SXSg (Sorghum xenognosin of Striga germination). Lynn et al. (1981) introduced the term "xenognosis" to refer to the process of host recognition though the perception of host-derived chemical signals and "xenognosin" to refer to the signals by which recognition is achieved; however, the potential of this terminology for general utility appears to have been somewhat compromised by its subsequent close association with dihydrosorgoleone and with the position that this compound, to the specific exclusion of strigolactones, is "the" sorghum xenognosin (e.g., Boone et al. 1995; Palmer et al. 2004).
Early debate about the significance of dihydrosorgoleone relative to sorgolactone in sorghum and more generally about the nature of germination stimulants in natural soil systems (e.g., Boone et al. 1995; Wigchert and Zwanenburg 1999) focused on a number of issues, including the stability and diffusability of each compound and their distributions across host lines and species. Chang et al. (1986) followed by Lynne and colleagues (Boone et al. 1995) initially argued that the observed high activity of strigol and its relative stability were incompatible with its presumed function in limiting germination to the immediate vicinity of the host roots, in contrast to the electron-rich hydroquinone SXSg, which is readily autoxidized in soil and rapidly degrades. However, it was later reported that strigol and its analogs are much less stable in the soil, presumably because of hydrolytic degradation (Babiker et al. 1987, 1988 ). Moreover, Butler (1995) proposed a limited role for SXGs precisely because of its limited water solubility and rapid oxidation. Further arguments raised against the significance of SXSg (reviewed by Wigchert and Zwanenburg 1999) included the observation that variation in SXSg production among sorghum cultivars showed little correlation with the resistance or susceptibility of those cultivars to attack by Striga (Hess et al. 1992; Olivier and Leroux 1992), whereas the pattern of resistance is better correlated with strigolactone production (Wigchert and Zwanenburg 1999). Additionally, SXSg does not appear to be present in the root exudates of maize, which is highly susceptible to Striga (Housley et al. 1987).
Countervailing these arguments is the discovery of the compound resorcinol, a methylated analog of SXSg that reportedly acts as an autoxidation stabilizer (Fate and Lynn 1996), decreasing the effective concentrations of root exudates required for germination. Lynn and colleagues (e.g., Fate and Lynn 1996; Palmer et al. 2004) argued that the relative amounts of SXSg and resorcinol, taken together, accurately predict the germination zone of S. asiatica in several sorghum varieties. Germination in maize they attributed to the activity of a labile but as yet unidentified stimulant. A secondary debate centered on the viability of a model that attempts to explain the germination-stimulating activity of strigol based on the structural similarity of its D-ring to SXSg (e.g., Lynn and Boone 1993; Boone et al. 1995; Wigchert and Zwanenburg 1999; Palmer et al. 2004).
More recently, Matusova et al. (2005)—in the same study that demonstrated a carotenoid origin for strigolactones—reported that treating plants with the carotenoid biosynthesis inhibitor fluridone resulted in the complete inhibition of Striga germination in several plants including sorghum, suggesting that SXSg and other sorgoleone quinones are not directly involved in stimulating germination. It is unclear whether or how this result can be reconciled with previous reports that claim to demonstrate germination in response to SXSg (e.g., Chang and Lynn 1986; Fate and Lynn 1996).
Lynn and colleagues previously questioned whether strigolactones were plant-derived compounds at all, suggesting that they might rather be products of bacteria inhabiting the roots of plants grown hydroponically (Boone et al. 1995), but the subsequent identification of naturally occurring strigolactones from diverse plants (described above), and particularly the demonstration of their role in the colonization of plant roots by AM fungi, would seem to rule this out. Meanwhile, no corresponding body of evidence has emerged to support a similarly widespread role for sorgoleone quinines. Thus, more recent assertions that SXSg is "necessary and sufficient to induce seed germination in Striga" (Palmer et al. 2004) do not seem tenable, particularly in light of the recent findings regarding the effects of carotenoid inhibition on seed germination described above. Thus, the current weight of evidence seems to point toward strigolactones as the primary compounds stimulating the germination of parasitic weeds, while the significance of SXSg and related compounds is uncertain (Humphrey et al. 2006).
Nevertheless, the current literature on the relative significance of SXSg and strigolactones is somewhat muddled. For example, a recent text on the biology of parasitic plants devotes significant attention to the role of SXSg as a germination stimulant (Heide-Jørgensen 2008), and a recent review addressing the role of plant root exudates in interspecific interactions refers to SXSg as "the only plant-produced Striga germination inducer that has been identified and characterized" (Bais et al. 2006). It is likely that the apparent confusion on this point derives from an unfortunate tendency in some of the recent literature to describe either SXSg or strigolactones as "the" germination stimulants for parasitic plants, while providing little context regarding the controversy and conflicting data relating to the roles of the two compounds (e.g., Keyes et al. 2001; Palmer et al. 2004; Matusova and Bouwmeester 2006).
6.V 5 Host Location and Selection by Foraging Seedlings
In contrast to the fairly extensive work on the chemicals cues responsible for the germination of parasitic plant seeds described above, relatively little research has examined the cues responsible for guiding the growth of the seedling toward its host following germination. Though host location in the root parasites Striga and Orabanche is largely accomplished by restricting germination to the immediate vicinity of plant roots, Dube and Olivier (2001) postulated that the concentration gradients of germination stimulants may also guide radical growth toward the host's roots. However, this possibility has not yet been confirmed (Matusova et al. 2005).
Foraging and host selection by parasitic plants seedlings has been best studied in the cosmopolitan genus Cuscuta (dodders), which, like Striga and Orabanche, includes a number of important agricultural pests. The dodders are among the best known of the parasitic plants because their parasitism of host stems is readily observed and because of their "extraordinary appearance and behavior" (Kuijt 1969). Mature dodder vines, which contain little or no chlorophyll, are typically yellow or bright orange and can form an extensive interlaced mass of leafless stems; the total length of the reticulated branches of a single dodder plant may approach half a mile (Dean 1942). Unlike the seeds of Striga and Orabanche, those of Cuscuta have no specialized germination requirement and rather depend on foraging by the seedling to find a host (Parker and Riches 1993). The seeds do, however, possess a thick, impervious seed coat that must be eroded by mechanical abrasion in the soil prior to germination (Lyshede 1992) and may serve to distribute the germination of seeds over time. Cuscuta seeds can remain viable for up to 50 years under ideal conditions and for at least ten years in the soil (Menke 1954). Once the seedling has emerged, foraging occurs by circumnutation, a rotational movement pattern in which the growing seedling makes a counterclockwise rotation around its axis of growth on the order of once an hour. Upon contact with the stem of a potential host plant, the Cuscuta vine winds round tightly, making up to three complete coils prior to the initiation of haustoria formation (Parker and Riches 1993). While the swollen basal part of the seedling functions like a root in absorbing water and anchoring the plant, true roots are never produced (Kuijt 1969).
Evidence suggests that dodder vines are able to "choose" among potential hosts and are more likely to accept hosts of high nutritional quality (Kelly 1990, 1992; Kelly and Horning 1999; Koch et al. 2004). For example, Kelly (1992) found that individual stems of C. europaea transplanted onto various host plants were more likely to "accept" hosts of host of high nutritional status and to "reject" (grow away from) lower-quality hosts, although the cues that guide these preferences have not been established. The host preferences of Cuscuta spp. can induce changes in plant community structure and diversity where they become established (e.g., Pennings and Callaway 1996, 2002).
Runyon et al. (2006) recently demonstrated that foraging seedlings of C. pentagona use host plant-derived chemicals to locate their hosts. Chemotropism had previously been suggested to play a role in host location by Cuscuta (Bϋnning and Kaut 1956) but had never been firmly established. In the more recent study, seedlings were shown to exhibit directed growth toward blends of volatile chemicals emitted by the host plants tomato and impatiens as well as the nonhost wheat (Cuscuta spp. cannot successfully parasitize grasses). However, seedlings exhibited a preference for volatiles from tomato over those from wheat, suggesting a role for chemical cues in host discrimination. Seedlings were also found to exhibit a directed growth response to a number of individual compounds present in the tomato blend, including α-pinene, β-phellandrene, and β-myrcene (which was also present in the wheat blend). One compound from the wheat blend, (Z)-3-hexenyl acetate, was found to be repellent, inducing an aversive growth response.
Light cues have also been implicated in Cuscuta foraging. Because chlorophyll primarily absorbs red light, but reflects and transmits far-red light, foraging Cuscuta seedlings may perceive chlorophyllous neighbors as far-red objects or as regions with a high ratio of far-red to red light (Smith 1994). Orr et al. (1996) reported phototropism toward far-red light in (C. planiflora) seedlings. Benvenuti et al. (2004) documented a phototropic response of C. pentagona seedlings to light transmitted by leaves of sugar beet, and reported a stronger response to leaves with higher chlorophyll contents. Light cues have also been shown to influence the coiling of the dodder vine around the host and prehaustoria formation (e.g., Haidar et al. 1997).
References
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Akiyama K, Hayashi H (2008) Plastid-derived strigolactones show the way to roots for symbionts and parasites. New Phytol 178:695–698
Awad AA, Sato D, Kusumoto D, Kamioka H, Takeuchi Y, Yoneyama K (2006) Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. J Plant Growth Regul 48:221–227
Babiker AGT, Hamdoun AM, Rudwan A, Mansi NG, Faki HH (1987) Influence of soil-moisture on activity and persistence of the strigol analog Gr-24. Weed Res 27:173–178
Babiker AGT, Ibrahim NE, Edwards WG (1988) Persistence of Gr7 and Striga germination stimulant(s) from Euphorbia aegyptiaca Boiss in soils and in solutions. Weed Res 28:1–6
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interations with plants and other organisms. Annu Rev Plant Biol 57:233–266
Benvenuti S, Dinelli G, Bonetti A (2004) Germination ecology of Leptochloa chinensis: a new weed in the Italian rice agro-environment. Weed Res 44:87–96
Bergmann C, Wegmann K, Frischmuth K, Samson E, Kranz A, Weigelt D, Koll P, Welzel P (1993) Stimulation of Orobanche crenata seed germination by (+)-strigol and structural analogs dependence on constitution and configuration of the germination stimulants. J Plant Physiol 142:338–342
Berner DK, Kling JG, Singh BB (1995) Striga research and control: a perspective from Africa. Plant Dis 79:652–660
Besserer A, Puech-Pages V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Becard G, Sejalon-Delmas N (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PloS Biol 4:1239–1247
Boone LS, Fate G, Chang M, Lynn DG (1995) Seed germination. In: Graves JD, Press MC (eds) Parasitic plants. Chapman and Hall, London, pp 14–38
Bouwmeester HJ, Matusova R, Sun ZK, Beale MH (2003) Secondary metabolite signalling in host–parasitic plant interactions. Curr Opin Plant Biol 6:358–364
Bouwmeester HJ, Roux C, Lopez-Raez JA, Becard G (2007) Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci 12:224–230
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Adv Genet 13:115–155
Bunning E, Kautt R (1956) On chemotropism of the seedlings of C. europaea. Biol Zentralbl 75:356–359
Butler LG (1995) Chemical communication between the parasitic weed Striga and its crop host: a new dimension in allelochemistry. In: Inderjit K, Dakshini MM, Einhellig FA (eds) Allelopathy: organisms, processes and application (ACS Symposium Series). ACS, Washington, DC, pp 158–168
Chang M, Lynn DG (1986) The haustorium and the chemistry of host recognition in parasitic angiosperms. J Chem Ecol 12:561–579
Cook CE, Whichard LP, Turner B, Wall ME (1966) Germination of witchweed (Striga lutea Lour): isolation and properties of a potent stimulant. Science 154:1189–1190
Cook CE, Coggon P, McPhail AT, Wall ME, Whichard LP, Egley GH, Luhan PA (1972) Germination stimulants. 2. Structure of strigol: potent seed germination stimulant for witchweed (Striga lutea Lour). J Am Chem Soc 94:6198–6199
Costea M, Tardif FJ (2006) The biology of Canadian weeds. 133. Cuscuta campestris Yuncker, C. gronovii Willd. ex Schult., C. umbrosa Beyr. ex Hook., C. epithymum (L.) L. and C. epilinum Weihe. Can J Plant Sci 86:293–316
de Luque AP, Galindo JCG, Macias FA, Jorrin J (2000) Sunflower sesquiterpene lactone models induce Orobanche cumana seed germination. Phytochemistry 53:45–50
Dean HL (1942) Total length of stem developed from a single seedling of Cuscuta. Proc Iowa Acad Sci 49:127–128
Dube MP, Olivier A (2001) Striga gesnerioides an its hosts, the cowpea: interaction and methods of control. Can J Bot 79:1225–1240
Egley GH (1972) Influence of seed envelope and growth regulators upon seed dormancy in witchweed (Striga lutea Lour). Ann Bot 36:755
Eplee RE (1992) Witchweed (Striga asiatica): an overview of management strategies in the USA. Crop Prot 11:3–7
Fate GD, Lynn DG (1996) Xenognosin methylation is critical in defining the chemical potential gradient that regulates the spatial distribution in Striga pathogenesis. J Am Chem Soc 118:11369–11376
Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Fischer NH, Weidenhamer JD, Bradow JM (1989) Dihydroparthenolide and other sesquiterpene lactones stimulate witchweed germination. Phytochemistry 28:2315–2317
Galindo JCG, de Luque AP, Jorrin J, Macias FA (2002) SAR studies of sesquiterpene lactones as Orobanche cumana seed germination stimulants. J Agric Food Chem 50:1911–1917
Garrison WJ, Miller GL, Raspet R (2000) Ballistic seed projection in two herbaceous species. Am J Bot 87:1257–1264
Goldwasser Y, Yoneyama K, Xie XA (2008) Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul 55:21–28
Gonzales WL, Suarez LH, Guinez R, Medel R (2007) Phenotypic plasticity in the holoparasitic mistletoe Tristerix aphyllus (Loranthaceae): consequences of trait variation for successful establishment. Evol Ecol 21:431–444
Gressel J, Hanafi A, Head G, Marasas W, Obilana B, Ochanda J, Souissi T, Tzotzos G (2004) Major heretofore intractable biotic constraints to African food security that may be amenable to novel biotechnological solutions. Crop Protect 23:661–689
Haidar MA, Orr GL, Westra P (1997) Effects of light and mechanical stimulation on coiling and prehaustoria formation in Cuscuta spp. Weed Res 37:219–228
Hairston NG, Van Brundt RA, Kearns CM (1995) Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76:1706–1711
Hammond WA – translator (1902) Aristotle's psychology: a treatise on the principle of life (De Anima and Parva Naturalia). Swan Sonnenschein, MacMillian, New York
Hauck C, Muller S, Schildknecht H (1992) A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J Plant Physiol 139:474–478
Heide-Jørgensen HS (2008) Parasitic flowering plants. Brill, Leiden
Hess DE, Ejeta G, Butler LG (1992) Selecting sorghum genotypes expressing a quantitative biosynthetic trait that confers resistance to Striga. Phytochemistry 31:493–497
Hinds TE, Hawksworth FG (1965) Seed dispersal velocity in 4 dwarf mistletoes. Science 148:517–519
Housley TL, Ejeta G, Cherif-Ari O, Netzly DH, Butler LG (1987) Progress towards an understanding of sorghum resistance to Striga. In: Webe H, Forstreuter W (eds) Proceedings of the 4th International Symposium on Parasitic Flowering Plants. Philipps University, Marburg, Germany, pp 411–419
Humphrey AJ, Galster AM, Beale MH (2006) Strigolactones in chemical ecology: waste products or vital allelochemicals? Nat Prod Rep 23:592–614
Johnson AW, Rosebery G, Parker C (1976) Novel approach to Striga and Orobanche control using synthetic germination stimulants. Weed Res 16:223–227
Johnson AW, Gowda G, Hassanali A, Knox J, Monaco S, Razavi Z, Rosebery G (1981) The preparation of synthetic analogs of strigol. J Am Chem Soc Perkin Trans 1:1734–1743
Karban R (2008) Plant behaviour and communication. Ecol Lett 11:727–739
Kelly CK (1990) Plant foraging: a marginal value model and coiling response in Cuscuta subinclusa. Ecology 71:1916–1925
Kelly CK (1992) Resource choice in Cuscuta europaea. Proc Natl Acad Sci USA 89:12194–12197
Kelly CK, Horning K (1999) Acquisition order and resource value in Cuscuta attenuata. Proc Natl Acad Sci USA 96:13219–13222
Keyes WJ, Taylor JV, Apkarian RP, Lynn DG (2001) Dancing together. Social controls in parasitic plant development. Plant Physiol 127:1508–1512
Koch AM, Binder C, Sanders IR (2004) Does the generalist parasitic plant, Cuscuta capestris, selectively forage in heterogeneous plant communities? New Phytol 162:147–155
Kranz A, SamsonSchulz E, Hennig L, Welzel P, Muller D, MayerFigge H, Sheldrick WS (1996) Synthesis of new strigol analogues. Tetrahedron 52:14827–14840
Kuijt J (1969) The biology of parasitic flowering plants. University of California Press, Berkeley, CA
Lass S, Vos M, Wolinska J, Spaak P (2005) Hatching with the enemy: Daphnia diapausing eggs hatch in the presence of fish kairomones. Chemoecology 15:7–12
Lopes FL, Desmarais JA, Murphy BD (2004) Embryonic diapause and its regulation. Reproduction 128:669–678
Lynn DG, Boone LS (1993) Signaling germination in Striga asiatica. In: Schultz J, Raskin I (eds) Proceedings of the 8th Annual Penn State Symposium in Plant Physiology. Waverly Inc., Baltimore, MD, 47–53
Lynn DG, Steffens JC, Kamut VS, Graden DW, Shabanowitz J, Riopel JL (1981) Isolation and characterization of the first host recognition substance for parasitic angiosperms. J Am Chem Soc 103:1868–1870
Lyshede OB (1992) Studies on mature seeds of Cuscuta pedicellata and C. campestris by electron microscopy. Ann Bot 69:365–371
Macías FA, Fernandez A, Varela RM, Molinillo JMG, Torres A, Alves P (2006) Sesquiterpene lactones as allelochemicals. J Nat Prod 69:795–800
Mangnus EM, Zwanenburg B (1992) Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogues. J Agric Food Chem 40:1066–1070
Mangnus EM, Dommerholt FJ, Dejong RLP, Zwanenburg B (1992a) Improved synthesis of strigol analog Gr24 and evaluation of the biological activity of its diastereomers. J Agric Food Chem 40:1230–1235
Mangnus EM, Vanvliet LA, Vandenput DAL, Zwanenburg B (1992b) Structural modifications of strigol analogs: influence of the B and C rings on the bioactivity of the germination stimulant Gr24. J Agric Food Chem 40:1222–1229
Matsuura H, Ohashi K, Sasako H, Tagawa N, Takano Y, Ioka Y, Nabeta K, Yoshihara T (2008) Germination stimulant from root exudates of Vigna unguiculata. Plant Growth Regul 54:31–36
Matusova R, Bouwmeester HJ (2006) The effect of host-root-derived chemical signals on the germination of parasitic plants. In: Dicke M, Takken W (eds) Chemical ecology: from gene to ecosystem. Springer, Berlin, pp 39–54
Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139:920–934
Menke HF (1954) Dodder infestation can halt certified seed production. West Feed Seed 9:24–37
Műller S, Hauck C, Schildknecht H (1992) Germination stimulants produced by Vigna unguiculata Walp Cv Saunders upright. J Plant Growth Regul 11:77–84
Musselman LJ (1980) The biology of Striga, Orobanche, and other root parasitic weeds. Annu Rev Phytopathol 18:463–489
Musselman LJ, Press MC (1995) Introduction to parasitic plants. In: Press MC, Graves JD (eds) Parasitic plants. Chapman and Hall, London, 1–13
Musselman LJ, Yoder JI, Westwood JH (2001) Parasitic plants major problem to food crops. Science 293:1434–1434
Norton DA, Carpenter MA (1998) Mistletoes as parasites: host specificity and speciation. Trends Ecol Evol 13:101–105
Nickrent DL (2007) Parasitic plant genera and species. Parasitic plant connection: http://www.parasiticplants.siu.edu/
Nickrent DL, Musselman LJ, Riopel JL, Eplee RE (1979) Haustorial initiation and non-host penetration in witchweed (Striga asiatica). Ann Bot 43:233–236
Olivier A, Leroux GD (1992) Root development and production of a witchweed (Striga Spp) germination stimulant in sorghum (Sorghum bicolor) cultivars. Weed Sci 40:542–545
Orr GL, Haidar MA, Orr DA (1996) Smallseed dodder (Cuscuta planiflora) gravitropism in red light and in red plus far-red. Weed Sci 44:795–796
Palmer AG, Gao R, Maresh J, Erbil WK, Lynn DG (2004) Chemical biology of multi-host/pathogen interactions: chemical perception and metabolic complementation. Annu Rev Phytopathol 42:439–464
Parker C (1991) Protection of crops against parasitic weeds. Crop Protect 10:6–22
Parker C, Riches CR (1993) Parasitic weeds of the world: biology and control. CAB, Wallingford
Pennings SC, Callaway RM (1996) Impact of a parasitic plant on the structure and dynamics of salt marsh vegetation. Ecology 77:1410–1419
Pennings SC, Callaway RM (2002) Parasitic plants: parallels and contrasts with herbivores. Oecologia 131:479–489
Press MC, Graves JD (1995) Parasitic plants. Chapman and Hall, London, pp 292
Press MC, Scholes JD, Riches CR (2001) Current status and future prospects for management of parasitic weeds (Striga and Orobanche). In: Riches CR, Farnham UK (eds) The world's worst weeds. British Crop Protection Council, London, pp 71–90
Ramaiah KV, Chidley VL, House LR (1991) A time-course study of early establishment stages of parasitic angiosperm Striga asiatica on susceptible sorghum roots. Ann Appl Biol 118:403–410
Rengefors K, Karlsson I, Hansson LA (1998) Algal cyst dormancy: a temporal escape from herbivory. Proc R Soc Lond B 265:1353–1358
Runyon JB, Mescher MC, De Moraes CM (2006) Volatile chemical cues guide host location and host selection by parasitic plants. Science 313:1964–1967
Runyon JB, Tooker JF, Mescher MC, De Moraes CM (2008b) Parasitic plants in agriculture: chemical ecology of germination and host-plant location as targets for sustainable control. Sustainable Agriculture Reviews - Volume 1 - Springer
Salle G, Tuquet C, Raynal-Roques A (1998) Biology of flowering parasitic plants. Compt Rend Seanc Soc Biol Filial 192:9–36
Sandell M (1990) The evolution of seasonal delayed implantation. Q Rev Biol 65:23–42
Sato D, Awad AA, Takeuchi Y, Yoneyama K (2005) Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants Striga and Orobanche, produced by cotton. Biosci Biotechnol Biochem 69:98–102
Siame BA, Weerasuriya Y, Wood K, Ejeta G, Butler LG (1993) Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J Agric Food Chem 41:1486–1491
Silvertown J (1998) Plant phenotypic plasticity and non-cognitive behaviour. Trends Ecol Evol 13:255–256
Silvertown J, Gordon DM (1989) A framework for plant behavior. Annu Rev Ecol Syst 20:349–366
Smith H (1994) Sensing the light environment the functions of the phytochrome family. In: Kendrick R, Kronenberg G (eds) Photomorphogenesis in plants, 2nd edn. Kluwer, Dordrecht,377–416
Stewart GR, Press MC (1990) The physiology and biochemistry of parasitic angiosperms. Annu Rev Plant Physiol Plant Mol Biol 41:127–151
Tuquet C, Farineau N, Salle G (1990) Biochemical composition and photosynthetic activity of chloroplasts from Striga hermonthica and Striga aspera, root parasites of field-grown cereals. Physiol Plant 78:574–582
Vaucher JP (1823) Mémoire sur la germination des orobanches. Mém MUS Hist Nat Paris 10:261–273
Worsham AD (1987) Germination of witchweed seeds. In: Musselman LJ (ed) Parasitic weeds in agriculture. CRC, Boca Raton, FL, pp 45–61
Wigchert SCM, Zwanenburg B (1999) A critical account on the inception of Striga seed germination. J Agric Food Chem 47:1320–1325
Xie X, Kusumoto D, Takeuchi Y, Yoneyama K, Yamada Y (2007) 2'-Epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J Agric Food Chem 55:8067–8072
Xie X, Yoneyama K, Kusumoto D, Yamada Y, Yokota T, Takeuchi Y (2008a) Isolation and identification of alectrol as (+)-orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry 69:427–431
Xie XN, Yoneyama K, Kusumoto D, Yamada Y, Takeuchi Y, Sugimoto Y (2008b) Sorgomol, germination stimulant for root parasitic plants, produced by Sorghum bicolor. Tetrahedron Lett 49:2066–2068
Yasuda N, Sugimoto Y, Kato M, Inanaga S, Yoneyama K (2003) (+)-Strigol, a witchweed seed germination stimulant, from Menispermum dauricum root culture. Phytochemistry 62:1115–1119
Yoder JI (2001) Host-plant recognition by parasitic Scrophulariaceae. Curr Opin Plant Biol 4:359–365
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Mescher, M.C., Smith, J., De Moraes, C.M. (2009). Host Location and Selection by Holoparasitic Plants. In: Balu¿ka, F. (eds) Plant-Environment Interactions. Signaling and Communication in Plants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-89230-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-540-89230-4_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-89229-8
Online ISBN: 978-3-540-89230-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)