Abstract
The endocannabinoid system (ECS) plays a central role in the regulation of learning and memory processes. The fine-tuned regulation of neural transmission by the system is likely to be the mechanism underlying this important function. In this chapter, we review the data in the literature showing the direct involvement of the physiological activation of cannabinoid receptors in the modulation of different forms of learning and memory. When possible, we also address the likely mechanisms of this involvement. Finally, given the apparent special role of the ECS in the extinction of fear, we propose a reasonable model to assess how neuronal networks could be influenced by the endocannabinoids in these processes. Overall, the data reviewed indicate that, despite the enormous progress of recent years, much is still to be done to fully elucidate the mechanisms of the ECS influence on learning and memory processes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Marijuana has been used for its psychotropic effects for centuries. It was, however, only during the last decades that this topic attracted scientific interest. The discovery of the active compound of the plant (Gaoni and Mechoulam 1964), the cloning of cannabinoid receptors (Matsuda et al. 1990), the synthesis of receptor antagonists (Rinaldi-Carmona et al. 1994), the identification of endogenously produced ligands of cannabinoid receptors (Devane et al. 1992; Mechoulam et al. 1995; Sugiura et al. 1995), the identification of endocannabinoids as retrograde synaptic signalling molecules (Maejima et al. 2001; Wilson and Nicoll 2001) and the first use of cannabinoid-interfering drugs in clinical trials (Pagotto et al. 2006; Van Gaal et al. 2005) represent scientific milestones in the study of cannabinoids over the last decades. Accordingly, Fig. 1 shows the evolution of the rate of published papers during the last 50 years in relation to these key discoveries, which have boosted new waves of interest. Nowadays, the number of scientific publications on the subject has achieved an exponential rate of growth, further strengthening the enormous interest that the cannabinoid field elicits in the scientific community. The most important passages in this history were the ones related to the definition of the endocannabinoid system (ECS). The discovery of cannabinoid receptors, of their endogenous lipid ligands (endocannabinoids) and of the machinery to synthesise and degrade endocannabinoids led to the identification of an endogenous signalling system, which is emerging as a very important element in mammalian physiology and in learning and memory processes.
Number of publications on cannabinoids during the last 50 years. Data obtained from PubMed (www.ncbi.nlm.nih.gov/pubmed/), using the following search keywords: cannabinoid OR marijuana OR marihuana OR tetrahydrocannabinol OR cannabis. Key discoveries are indicated: (1) Purification of Δ9-tetrahydrocannabinol (THC) (Gaoni and Mechoulam 1964); (2) Cloning of CB1 receptors (Matsuda et al. 1990); (3) Identification of anandamide (Devane et al. 1992); (4) Synthesis of the first CB1 antagonist (Rinaldi-Carmona et al. 1994); (5) ECS and retrograde synaptic transmission (Maejima et al. 2001;Wilson and Nicoll 2001); (6) First clinical trial with cannabinoid antagonists (Van Gaal et al. 2005)
2 Expression of Cannabinoid Receptors in the Brain: Focus on CB1
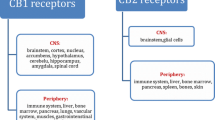
Evidence for the presence in neurons of a second cannabinoid receptor, CB2, has been shown (Van Sickle et al. 2005) and other possible targets of endocannabinoids (e.g. vanilloid receptors TRPV1, GPR55, potassium channels TASKs) are very likely involved in the functions of the ECS brain in the brain (Di Marzo et al. 2002). However, most studies dealing with the roles of the ECS in learning and memory refer to the endogenous activation of the cannabinoid receptor type 1 (CB1). Hence, this chapter will deal with the roles of the ECS in learning and memory with special emphasis on CB1 receptors. CB1 is a seven transmembrane G protein-coupled receptor (GPCR) that is expressed at very high levels in the brain. It has been calculated that the protein levels of CB1 in the brain are a good deal higher than other GPCRs and that they are comparable to those of NMDA or GABAA receptors (Howlett et al. 2002). CB1 is expressed in many different brain regions (including most of the ones classically involved in learning and memory processes), and it is present in different neuronal subpopulations (Marsicano and Lutz 1999). CB1 is generally expressed presynaptically (Freund et al. 2003), although the evidence for its presence at the somatodendritic level is steadily growing (Bacci et al. 2004). Activation of CB1 generally leads to a hyperpolarisation of neuronal membranes and to the stimulation of different intracellular signalling cascades (Straiker and Mackie 2007). Therefore, activation of CB1 generally causes an inhibition of neurotransmitter release (Freund et al. 2003). Strong evidence exists that endocannabinoids released postsynaptically signal retrogradely to presynaptic CB1 receptors, thereby providing one of the best described retrograde synaptic signalling systems in the CNS. First discovered in the hippocampus and cerebellum, this mode of endocannabinoid signalling has since been extended to the great majority of the brain regions, demonstrating the universality of the ECS as a modulatory system in the CNS. The complexity of the picture derives from the fact that the ECS can regulate, via CB1, the release of neurotransmitters that have very different and even opposite effects. The most striking example is the fact that the ECS can regulate both inhibitory GABAergic and excitatory glutamatergic transmission in the same brain regions. These diverse actions, together with the concept of an “on demand” synthesis and release of endocannabinoids (as described in other chapters), reveal a very finely regulated way of functioning of the ECS. In fact, it is believed that, during specific functions, the ECS might finely modulate several aspects of neuronal signalling at the same time.
In addition, it should be remembered that CB1 is not only expressed in the central nervous system but also at lower levels, in the retina and peripheral tissues, including the peripheral nervous system, adipocytes, hepatocytes, pancreatic cells and the gastrointestinal tract (Pacher et al. 2006). Though these organs and tissues are not classically considered in learning and memory experiments, the possible confounding effects of CB1 expression in these extra-CNS sites should be kept in mind. For instance, CB1 present in the retina should be considered when visual stimuli are used to study learning processes. Similarly, the presence of CB1 in the gastrointestinal tract might play a role, when conditioned taste aversion experiments are performed (see below). Nevertheless, CB1 is the main cannabinoid receptor involved in the functions of the ECS in the brain and this chapter will focus almost exclusively on its endogenous activation by endocannabinoids during learning and memory processes.
3 The ECS and Learning and Memory
The ability to acquire, store, retrieve and modify information concerning previous experience is a crucial function for individuals to survive and reproduce. Neuronal adaptation has been correlated with the changes in behaviour observed in response to all stages of memory. In the light of the enormous theoretical and experimental progress in the understanding of these neuronal systems (Kandel et al. 2000; Squire et al. 2008), we will modestly limit this review to the specific role of the endocannabinoid system in learning and memory. We will consider learning in terms of perception of environmental changes and the subsequent changes in intercellular communication in the brain, and memory as the relative persistence of these changes. This review is directed towards the involvement of the ECS in these processes referring to a classical and certainly oversimplified classification of the time-dependent types of memories, i.e. short-term and long-term. Concerning the latter, we will follow the classical declarative-like, procedural and emotional classification with a special focus on the different phases of these processes: encoding of information (learning), consolidation, retrieval (or recall), re-consolidation and extinction. Considering the major role of the ECS in aversive memories, we will finally propose a theoretical model, which may explain the functions of the system in the processing and extinction of aversive memories.
4 Pharmacology of Exogenous Cannabinoid Agonists and Physiology of the ECS: Important Differences in Complex Functions
There is an important body of literature on the effects of natural or synthetic cannabinoid exogenous agonists (as THC, HU210, CP55940, WIN55, 212-2) on learning and memory processes. The administration of these ligands of the CB1 receptors obviously disturbs the physiological activation of the ECS but does not always reflect a potentiation of the role of the endocannabinoids. There are indeed some physiological functions of the ECS where exposure to cannabinoid agonists simply exerts an additive effect on the physiological action of endocannabinoids, e.g. in peripheral inflammation (Massa et al. 2004). However, in complex brain processes, the effects of cannabinoid agonists rarely mimic the actions of the ECS in physiological conditions. For instance, cannabinoid agonists, including marijuana intoxication in humans and synthetic agonists in animals, are very well known to induce important alterations mainly in short-term episodic and working memory (Ranganathan and D'Souza 2006). In rats, working memory is deeply impaired by cannabinoid agonists but only sparse evidence reports that the ECS would physiologically negatively regulate working memory (Deadwyler et al. 2007; Deadwyler and Hampson 2008) by modulating the spontaneous release of endocannabinoids. However, Carter and Wang (2007b) recently suggested that the physiological activation of the ECS might even be beneficial to working memory mechanisms.
It is very difficult to account for the reasons for these apparent discrepancies between pharmacological treatments with cannabinoid agonists and the physiological roles of the ECS. Differences between pharmacological properties of exogenous cannabinoids and endocannabinoids could account for this. Indeed, the prototypical exogenous cannabinoid agonist Δ9-tetrahydrocannabinol (THC) is only a partial agonist of CB1 receptors in vitro (Pertwee 2008) and was recently proposed to act as an antagonist in vivo (Straiker and Mackie 2007). However, this possible explanation might be valid for THC, but it is more difficult to extend to other exogenous synthetic agonists, which are very powerful full agonists of CB1 and share with THC many pharmacological effects on animals, including on learning and memory. It is, therefore, more likely that the discrepancy relies on the different spatial and temporal ways of activation of cannabinoid receptors by exogenously applied and endogenously released cannabinoids. Systemic or local intracerebral administration of agonists leads to a generalised (in the whole body or in a brain region, respectively) activation of cannabinoid receptors. The duration of this activation depends only on the pharmacokinetics of the drug and can last for hours or days. Conversely, the activation of the ECS, as described in other chapters in this volume, is tightly regulated in terms of space and time. It is believed that few cells might be under the control of the physiological activation of the ECS whereas the neighbouring ones might remain outside the influence of endocannabinoids. Moreover, the time of activation of the ECS can be very short (in the range of a few hundred milliseconds to a few minutes) thanks to very efficient systems for the degradation of endocannabinoids. It is, therefore, possible that, during specific ongoing neuronal activity such as the processing of memory traces, the ECS is activated in a limited number of brain regions (possibly only involving a few cells) within a very short time frame. This would explain why a generalised and long-lasting action of exogenously administered cannabinoid agonists does not usually reproduce the physiological functions of the ECS and can even exert opposite effects.
An example of this view can be found in a typical electrophysiological function of the ECS, the depolarization-induced suppression of inhibition (DSI) in the hippocampus, one of the most important brain regions for memory processing (Alger 2002). A short depolarisation of hippocampal pyramidal neurons induces the postsynaptic production and release of endocannabinoids, which retrogradely travel across the synaptic cleft and activate CB1 receptors present on the presynaptic terminals of GABAergic inhibitory interneurons (Alger 2002; Freund et al. 2003). This activation induces, in turn, a short-lasting (from a few seconds to a few minutes) inhibition of GABA release, thereby temporarily releasing the pyramidal cell from the GABAergic inhibitory tone. Importantly, it was shown that such a short-term form of synaptic plasticity can facilitate the induction of long-term synaptic potentiation (LTP) of the pyramidal neurons (Carlson et al. 2002). As LTP is the best acknowledged cellular model of long-term storage of information in the brain, it is very likely that DSI might somehow participate in memory processing in the hippocampus. The first evidence that the ECS is the main system responsible for the retrograde signalling component of DSI in the hippocampus derives from the seminal and simultaneous experiments of two independent groups. In both of these series of experiments, DSI was shown to be blocked by the application of a CB1 antagonist and to be “occluded” by the application of a CB1 agonist (Maejima et al. 2001; Wilson and Nicoll 2001; Fig. 2). Whereas the blockade of a phenomenon by a receptor antagonist is self-explanatory, the concept of “occlusion” by an agonist is less intuitive. By “occlusion”, it is meant that the application of an agonist compound, by occupying the available receptors, practically impedes the endogenously released endocannabinoids from binding to the same receptors. In other words, independently from the intrinsic effect of the agonist (which will induce a long-lasting decrease of GABAergic transmission), the spatially and temporally restricted action of endocannabinoids during DSI is abrogated in presence of exogenous CB1 agonists (Fig. 2). We can assume that DSI does, indeed, participate in some forms of memory processes and its temporally and spatially restricted occurrence plays a central role in its function in living animals. Thus, if we treat animals with CB1 agonists, the occurrence of DSI at the right place and at the right time will be impeded, in a very similar manner as with a CB1 antagonist. It is, therefore, possible that pharmacological applications of agonists and antagonists result in very similar impairments of complex brain functions such as learning and memory.
Depolarisation-induced depression of inhibition (DSI). Endocannabinoids released from postsynaptic CA1 pyramidal hippocampal neurons induce a transient depression of GABAergic release from inhibitory interneurons. The specificity of the involvement of the ECS in this phenomenon was shown by (a) the blockade of DSI by a CB1 antagonist and (b) its occlusion by a CB1 agonist. These data indicate that the possible physiological effects of DSI in vivo are likely abolished both by antagonists and agonists treatments. Data reproduced with permission from Wilson and Nicoll (2001)
In this chapter, we will omit the description of the effects of cannabinoid agonists on memory processes even though most of them were blocked by the administration of a CB1 receptor antagonist. We direct interested readers to extensive reviews of the subject (Castellano et al. 2003; Fride 2005; Fujiwara and Egashira 2004; Iversen 2003a; Riedel and Davies 2005). Thus, we will focus our attention on experiments in which a clear physiological involvement of the ECS has been demonstrated. The direct approaches to showing such an involvement can be summarised in three experimental paradigms: the use of cannabinoid antagonists alone, the use of mutant animals bearing alterations in the expression of genes involved in the ECS (up to now, CB1 and FAAH), and the use of drugs able to inhibit endocannabinoid degradation. In the latter case, the amount of endocannabinoids available to exert the functions of the ECS will be increased (as described in “The Life Cycle of the Endocannabinoids: Formation and Inactivation”, the chapter by Stephen P.H. Alexander and David A. Kendall, this volume). This approach presents the advantage, as compared to the application of direct cannabinoid agonists to maintain the temporal and spatial range of activation of the ECS during physiological processes: only where and when endocannabinoids are synthesised to exert a specific function, are these drugs able to slightly increase and/or prolong their activity.
The important role of the ECS in learning and memory has been already assessed in a variety of behavioural tests. We will first mention again the role of the ECS in short-term working memory, then turn to its involvement in different kinds of long-term memories: declarative-like, procedural and emotional. Given the enormous amount of data present in the literature on the subject and the space limitation of this chapter, we apologise for possible omissions.
5 Involvement of the Endocannabinoid System in Learning and Memory
5.1 The ECS and Working Memory
Working memory is a form of memory that allows access to labile information for a short period of time. In humans, a trivial example of working memory is the ability to remember a phone number from the time of reading it in the directory to actually dialling it. Natural and synthetic cannabinoid agonists are known to negatively regulate working memory in humans (for reviews, see Iversen 2003b; Ranganathan and D'Souza 2006) and in animal models (for reviews, see Castellano et al. 2003; Riedel and Davies 2005). However, there is little evidence that the physiological action of endocannabinoids could induce such impairments. While Mallet and Beninger (1998) did not observe any effect of the CB1 antagonist rimonabant on a non-match-to-position task in rats (Mallet and Beninger 1998), rats injected with rimonabant performed better in a delayed non-match-to-sample short-term memory task (Deadwyler et al. 2007) by reducing the influence of the biases encoded by the endocannabinoids in the hippocampus (Deadwyler and Hampson 2008). However, it has also been suggested that working memory would be enhanced by endocannabinoids in other tests. Indeed, Carter and Wang (2007) proposed that DSI can counteract the time-dependent decrease of accuracy of working memory in a model of spatial working memory (Carter and Wang 2007). Additional evidence comes from the use of FAAH-null mice lacking fatty acid amide hydrolase, the main enzyme involved in the degradation of the endocannabinoid anandamide (AEA). Indeed, these mice, which have tenfold increased brain levels of AEA and other fatty acid amide substrates (Cravatt et al. 2001) did not show any deficits in a working memory water maze task and even performed better on the first training session than their wild-type littermates (Cravatt et al. 2001; Varvel et al. 2006). This would argue that increased levels of endocannabinoids are not detrimental to working memory. In summary, existing studies strongly suggest that the ECS is somehow involved in working memory but it is still under discussion exactly how it affects this kind of processing.
As mentioned above, several phases of learning and memory processes can be identified. Classical theories propose that the acquisition of new information induces changes in neuronal connections that are supposed to encode a memory trace, somehow representing that information as subjectively perceived by the subject (Bailey et al. 2004; Barco et al. 2006; Dunning and During 2003; Kandel 2001). This memory trace is very labile (i.e. it can be easily disrupted through several treatments) at the beginning, and its strength increases through a process called consolidation (McGaugh 2000; Nader et al. 2000). Through consolidation, the memory is stored through long-lasting changes in neuronal connectivity and will be “retrieved” when the individual eventually needs it. More recent theories propose further complexity to the process. Every time consolidated memories are recalled, they probably switch again to a labile state. “Reactivated” traces can again be disrupted during this process. However, depending on the conditions of retrieval and on the strength of the original trace, “reactivated” memories can undergo two opposite processes. These are called “re-consolidation”, when the conditions favour the permanence of the trace (Debiec et al. 2002; Duvarci and Nader 2004; Sara 2000), and “extinction”, when the conditions indicate that the memory has no reason to persist any longer (Myers and Davis 2002; Quirk and Mueller 2008). Dissecting the neuronal mechanisms of these phases is one of the major goals of learning and memory research. In the specific case of the ECS, the tools described above (genetic and pharmacological) each present advantages and disadvantages. Genetic mutations are generally more specific than pharmacological tools, which are always liable to the risk of non-specific effects. However, any alteration in performance of mutant mice in test trials could be ascribed to a deficit during virtually any of the phases of learning and memory. On the other hand, pharmacological treatments could better differentiate these phases (acquisition, consolidation, retention, retrieval, and re-consolidation/extinction). These considerations should be kept in mind in the analysis of the different roles of the ECS in learning and memory processes.
5.2 The ECS and Long-Term Memories
5.2.1 The ECS and “Declarative”-Type Memories
Declarative memory refers to learned facts and information that are flexible and can be accessed consciously. It is also referred to as explicit memory. It is obviously quite difficult to measure “declarative” memory in animals. However, many scientists have developed different tasks in order to assess the “memory of facts” even in very simple organisms. In this chapter, we will review the role of the endocannabinoid system in two types of this form of memory: recognition memory and spatial memory.
5.2.2 The ECS and Recognition Memory
The first evidence of the involvement of the ECS in learning and memory comes from a non-procedural recognition memory task. Recognition memory is based on the innate preference of rodents for exploring novel places, objects or congeners instead of re-exploring something they have already been exposed to, referred to as familiar. The two most common protocols used in behavioural neurosciences are the object recognition and the social recognition tasks. The pharmacological blockade of CB1 receptors resulted in a facilitation of short-term olfactory memory in a social recognition memory task (Terranova et al. 1996) and the administration of rimonabant reduced the deficits observed in aged rats and mice (Terranova et al. 1996). Two CB1-null lines obtained from different genetic backgrounds were later examined at different time intervals according to the age of the animals. While CB1-null mice performed better than the wild-type controls at younger age (6–8 weeks old), they were impaired during adulthood (3–5 months old), suggesting that the genetic blockade of CB1 receptors improves social recognition memory in young animals, but it is deleterious at older ages (Bilkei-Gorzo et al. 2005). These data might set a link between age, memory performance and activity of the ECS. However, the absence of wild-type control littermates in this study did not permit the conclusion of whether the observed age-related changes were due to an intrinsic effect of CB1 deletion in the tested mice or to altered embryonic and postnatal development due to the fact that the mothers of these animals were also full CB1-null mutants. Indeed, administration of rimonabant did not induce any effects in CD1 mice nor did AM251 in rats in the object recognition paradigm (Bura et al. 2007; Clarke et al. 2008).
Reibaud et al. (1999) extended these recognition memory results by assessing CB1-null mice in an object recognition test. Mutant mice were able to retain the memory of a known object for at least 48 h whereas the wild-type controls did not remember the familiar object after 24 h. It was again suggested that this faculty could be age-dependent, because 1-month-old and 4-month-old CB1-null mice explored selectively the novel object 24 h after the first exposure, but the difference from their wild-type controls was more obvious at the younger age (Maccarrone et al. 2002).
5.2.3 The ECS and Spatial Memory
The most frequently used cognitive tests for assessing declarative-like memories in rodents are spatial memory tasks. In a delay version of the radial-maze, in which rats have to remember and go to the only baited arm which was blocked on the previous trial, Aaron Lichtman found that the administration of the CB1 antagonist rimonabant before the acquisition phase could improve the performance of the rats. Interestingly, no effect of the drug was observed when it was administered immediately after the acquisition phase nor before the retention test (Lichtman 2000), suggesting an involvement of the ECS in the learning phase of this task. The same group recently confirmed that rimonabant injection before, but not immediately after, acquisition, or before retrieval of the same task was able to decrease the number of errors of the rats even when the first exposition to the maze and the re-exposition were separated by a longer delay. This suggests a beneficial role of the ECS in choice accuracy. Moreover, the effect of the CB1 antagonist was synergistic with that of an inhibitor of acetylcholinesterase, suggesting that the ECS and the cholinergic system may interact in this form of learning (Wise et al. 2007). Another version of the 8-arm-radial maze requires the rats to encode more information: during the test session, four arms that were baited during the acquisition phase are to be avoided, whereas the four other previously blocked arms are to be visited. In these conditions, the blockade of CB1 receptors immediately after the acquisition phase could improve consolidation processes (Wolff and Leander 2003). The role of the ECS has repeatedly been assessed in the most commonly used behavioural paradigm to test spatial memory, the Morris Water Maze. In this test, rodents have to learn to find a hidden platform in a pool by using the surrounding spatial cues. While CB1-null mice learned the task as well and as fast as the wild-type controls, they were impaired in the reversal learning phase where the platform had been moved to another location in the pool. Indeed, they repeatedly went to the previous location showing increased and non-adapted perseverance and a significant deficit in learning the new location, suggesting that the ECS is involved in extinction and/or forgetting processes (Varvel and Lichtman 2002). Consequently, the behaviour of rimonabant-treated mice and of CB1-null mice was further analysed in two extinction procedures in order to differentiate these two processes. In the massed extinction procedure with many trials in a short period of time (20 in 5 days), no effect on the extinction was observed following either genetic or pharmacological blockade of the CB1 receptor. On the contrary, neither the rimonabant-treated mice nor the CB1-null mice exhibited a proper extinction in the spaced extinction procedure with five probe-tests over several months, suggesting a role for the ECS in the suppression of unadapted behaviours (Varvel et al. 2005). Robinson et al. (2008) found that intraperitoneal administration of rimonabant affected the learning abilities of rats, whereas the intrahippocampal infusion of the antagonist led to an enhanced acquisition but had no direct effect on consolidation processes, although they kept returning to the previous platform location up to seven days after reversal training. These data again confirmed an important involvement of the ECS in this form of declarative memory. Another way to better characterise the role of the ECS in spatial learning is by using FAAH-null mice that exhibit much higher levels of AEA and other fatty acid amides or by pharmacologically inhibiting this enzyme (e.g. with OL-135). However, no marked effects of these treatments were found in any versions of the task. FAAH-null mice even acquired the working memory procedure faster in the first session, whereas the administration of OL135 did not lead to a better acquisition but to a better extinction rate (Varvel et al. 2007). Additional evidence of the role of the ECS in spatial memory was observed in food-storing black-capped chickadees. When they were intrahippocampally infused with rimonabant, their long-term performance was improved but they could not recall the most recent reward location when the reward was moved (Shiflett et al. 2004).
5.3 The ECS and Procedural Memory
In contrast to declarative memory, procedural memory is an implicit memory that can be assessed by performance rather than by conscious recollection. It deals with the knowledge of “how” tasks should be performed, and it relies on processing like priming, conditioning (when subjects learn associations between two stimuli or a stimulus and an action) and skill learning (as riding a bicycle).
5.3.1 The ECS and Operant Conditioning
As the ECS is involved in functions other than learning and memory, such as emotions and reward, one should keep in mind the possible effects of the pharmacological and/or genetic treatments on locomotion and motivation of the animals to perform the task. This is particularly true in operant conditioning tests, where subjects associate a stimulus (e.g. a light) with a learned action (e.g. lever pressing or nose-poking) to obtain a reward (e.g. food). In order to improve learning rates and to overcome motivational problems, animals are generally food restricted or presented with palatable food as a reward, and this can obviously be a confounding factor, considering the important roles of the ECS in reward and energy balance (as described in other chapters of the present book). Indeed, in one of the earliest experiments aimed at studying the effects of the rimonabant on the performance of monkeys in an operant paradigm, the authors observed a decrease of overall response rate, but no changes in the percentage of errors, suggesting a predominant effect of the drug on the motivation to perform the task over the learning process itself (Winsauer et al. 1999). Mallet and Beninger had previously found no effect of this antagonist alone in a conditional discrimination task (Mallet and Beninger 1998). However, it was more recently shown that mature 3–5-month-old CB1-null mice needed more time to learn the task as compared to younger CB1-null mice (6–8 weeks), independently from the hedonic value of the reward (Bilkei-Gorzo et al. 2005) for which the motivational aspects were not altered (Soria et al. 2005). Mutant mice lacking the expression of CB1 were also evaluated in another version of operant conditioning, the five-hole-choice task. Despite evidence for a lack of motivation, the mutant mice performed as well as their wild-type littermates. Moreover, in this task, both genotypes showed a similar decline when the reward was no longer delivered, suggesting that CB1 receptors are not crucial for the extinction of appetitive tasks (Holter et al. 2005) in operant conditioning. Similarly, Niyuhire et al. (2007) recently demonstrated that there was no difference in the extinction rate of mice treated with either vehicle or rimonabant in similar tasks. However, the ones injected with the CB1 antagonist lacked the “burst extinction” that refers to a strong response the first time the reward was not presented any more. The authors interpreted this effect as a possible impairment at the beginning of the extinction phase or in frustration-like behaviour without excluding a possible lack of motivation.
5.3.2 The ECS and Habits
When an action is repeated to reach a goal, it may become more automatic and not sensitive any more to the value of the outcome. In this case, a new habit has been formed but it is not easy for the observer to differentiate between goal-directed and habitual actions. For example, you can be assessed while typing on a French keyboard. If you are used to an English one, your “Q” will be an “A” but if you are still watching the letters, you will look for the proper one. In 2007, Hilário et al. used the operant chambers in order to assess this kind of procedural memory (Hilário et al. 2007). They first showed that an interval ratio procedure (where the reinforcer is delivered upon the first press after a delay of some seconds since the last reinforcer) led to habit actions in mice. Thus, CB1-null mice and AM251-treated mice were trained in an interval ratio procedure and tested in a devaluation paradigm, i.e. after having eaten some “free” reinforcers. In this condition, the mice with pharmacological or genetic blockade of CB1 receptors were sensitive to the devaluation procedure, indicating that they were still responding in a goal-directed manner. In this way, the authors emphasised the critical role of the endocannabinoid system in habit formation.
These results were recently complemented in a study in rats, suggesting a differential role of the dorsolateral and the hippocampal CB1 receptors in the extinction of habits using a T-maze task (Rueda-Orozco et al. 2008a). Indeed, the focal administration of the antagonist AM251 either in the dorsolateral striatum or in the hippocampus respectively impaired or rather facilitated the extinction of procedural memories. These data suggest that endocannabinoids in the striatum are important for extinguishing previously relevant responses and that pharmacological blockade of CB1 in the hippocampus would enhance learning in hippocampus-dependent tasks.
5.3.3 The ECS and Procedural Strategies in a Spatial Task
A similar dissociation between the hippocampal and the striatal CB1 receptors was proposed to underlie the choice of strategy to perform a Barnes maze. In this test, rodents have to visit many holes at the periphery of a circular table in order to find the drop box through which they can escape. Individuals utilised different strategies to solve the task efficiently. They can preferentially use a spatial strategy using distal cues around the maze, or a serial strategy, visiting the holes in sequence and following one direction. This latter strategy is believed to rely on procedural memory (Packard and McGaugh 1992; White and McDonald 2002). According to the dark or light phases, rodents complete the task using preferentially and respectively serial and spatial strategies (Rueda-Orozco et al. 2008b). However, during the dark phase, rats that were injected with the CB1 antagonist AM251 in the striatum at the end of the daily last trial used the serial strategy less (Rueda-Orozco et al. 2008b).
5.4 The ECS and Emotional Memory
5.4.1 The ECS and Aversive Memory
In order to survive and to adapt to their environment, animals easily learn to avoid insecure places and noxious food. Here we will review how the ECS has been involved in this kind of learning.
A possible role of the ECS was first suggested in aversive memory using the elevated T-maze. In this study, rats were placed in a starting closed arm and given the possibility to explore two open, aversive arms, which rats rapidly learn to avoid. However, rats that were injected with rimonabant acquired and consolidated this avoidance memory better (Takahashi et al. 2005).
The conditioned taste aversion (Garcia et al. 1955) was developed from the ethological observation that animals have a very good memory of noxious food, e.g. blue jays avoid monarch butterflies because of the toxins they contain (Brower and Glazier 1975). Indeed, animals learn to avoid eating or drinking a particular food or fluid because it has been previously associated with a noxious substance (e.g. lithium chloride, Welzl et al. 2001). It has been reported that the pharmacological blockade of the CB1 receptors in the insular cortex promoted memory retention and blocked its extinction without affecting re-consolidation (Kobilo et al. 2007). In a similar way, chicks that show spontaneous pecking behaviour avoid this behaviour if they have previously pecked into a bitter bead. However, when they were injected with rimonabant before the retention test, they did not avoid the bead (Adam et al. 2008).
Another way of assessing aversive memory is the step-down passive avoidance test in which a rodent is placed on the top of a small platform and as soon as it steps down it receives an electric foot shock. Subsequently, it will stay longer on the platform to avoid the “dangerous” floor. Intrahippocampal injection of AM251 after the learning phase impaired the consolidation of this experience (de Oliveira et al. 2005), confirming an important role of the ECS in this kind of memory. More classically, passive avoidance memory in rodents is assessed by exploiting their innate behaviour to go towards unlit and closed environments. In a “shuttle box”, made of one lit compartment and a dark one separated by a door, once the rodent has fled the illuminated compartment to reach the dark one, it receives an electric footshock. In the following trials, the rodents will avoid entering the dark compartment. It was recently reported that an i.p. injection of rimonabant did not induce any impairment in the acquisition of the task but the drug blocked the extinction of the avoidance response (Niyuhire et al. 2007). The lack of detectable acquisition impairment could be masked due to very strong conditioning conditions.
The “shuttle box” can be used also for active avoidance paradigms. In this case, the two compartments are identically shaped and illuminated and animals learn to flee to the other compartment when alerted (by a light or tone cue). CB1-null mice were shown to learn this task better on the fifth day of training (Martin et al. 2002). However, Bura et al. (2007) were not able to replicate these data when they wanted to evaluate the interaction of the ECS and the cholinergic system in cognitive processes. Additional studies are therefore needed to better understand the role of the ECS in this task.
In summary, the ECS seems to be deeply implicated in the significance of an aversive stimulus and can attenuate overreactions that would not be suitable to the situation.
5.4.2 The ECS and Fear Memory
Classical fear conditioning is a common paradigm to assess implicit associative memory. However, because of the very high emotional component of the procedure, we next review studies using different versions of the test.
5.4.2.1 Acquisition of Fear Memories
In fear conditioning tests, subjects form associations between a previously neutral stimulus (conditioned stimulus, CS, e.g. a tone or a context) and an aversive unconditioned stimulus (US, e.g. a footshock). After conditioning (learning), the CS induces a fear response even in the absence of the US. This behaviour can be observed and quantified, providing an indirect measure of the strength of the learning (LeDoux 2000). The quantified behaviour can vary from the natural fear responses typical of a given species (e.g. “freezing” as the absence of any movement except for the ones for respiration in rodents) to increased “startle” response. In fear conditioning protocols, most of the data published indicate that the pharmacologic blockade or the genetic deletion of CB1 receptors induces little or no effect on the acquisition of the task. In CB1-null mice, cued fear conditioning, where the fear response (freezing) is induced by a tone previously paired with a footshock, acquisition is comparable to that of wild-type controls (Cannich et al. 2004; Kamprath et al. 2006; Marsicano et al. 2002). Similarly, pharmacological blockade of CB1 was shown to have little effect on the acquisition phase of fear conditioning tasks (Kamprath et al. 2006; Marsicano et al. 2002).
However, in a context-dependent version of fear conditioning, Arenos et al. proposed that administration of a CB1 antagonist prior to conditioning is able to disrupt learning of rats (Arenos et al. 2006). Similarly, CB1-null mice did not show any fear response when re-exposed to a context in which they had previously been shocked, and mice treated with AM 251 showed a reduced peak of freezing, indicating that the ECS could play a role in the acquisition of hippocampus-dependent fear conditioning (Mikics et al. 2005). However, Suzuki et al. did not observe any disturbance of acquisition or early acquisition when they injected rimonabant before conditioning (Suzuki et al. 2004).
Moreover, Reich et al. (2008) have recently shown that administration of a CB1 antagonist prior to conditioning enhanced the freezing response of mice in both “delay” and “trace” versions of a cued fear conditioning (in the former version the CS and US are terminated at the same time, whereas in the latter version, the CS and the US are temporally separated).
5.4.2.2 Consolidation, Re-consolidation and Extinction of Fear Memories
While the ECS may or may not be involved in the acquisition of fear memories, its role in fear extinction seems to be crucial. Indeed, it was first reported in 2002 that CB1-null mice were able to learn a tone–footshock association but failed in adapting their fear response when exposed repeatedly or for a long time to the CS, whereas their wild type littermates showed a time-dependent reduction of freezing behaviour (Marsicano et al. 2002; Kamprath et al. 2006; Marsicano and Lutz 2006). The injection of rimonabant (3 mg kg−1), before the extinction training in wild-type C57/Bl6N mice confirmed that endocannabinoids, through the activation of CB1 receptors, play a major acute role in the extinction of cue-induced conditioned fear (Marsicano et al. 2002; Marsicano and Lutz 2006). Manipulating the levels of endocannabinoids by the administration of the inhibitor of endocannabinoid breakdown and uptake, AM404, Chhatwal et al. (2005) reported a dose-related enhancement of extinction. Conversely, the administration of rimonabant induced an impairment of the extinction of fear in rats (Chhatwal et al. 2005).
Besides, the infralimbic subregion of the medial prefrontal cortex seems to be a key region for the extinction of fear memories, as the focal infralimbic administration of AM 251 could block the diminution of the fear startle response in rats (Lin et al. 2009).
A precise pharmacological study was recently conducted to assess the role of the ECS at the different stages of memory (Reich et al. 2008). It was shown that the freezing response was enhanced during recall with the administration of AM251. However this was accompanied with enhanced generalised freezing. The extinction of fear memories was also further analysed and the authors suggested that the blockade of the CB1 receptors impaired the extinction expression but not extinction learning (Reich et al. 2008).
Suzuki et al. (2004) further characterised this effect, showing that the pharmacological blockade of CB1 receptors with rimonabant induces a specific impairment on extinction without affecting the consolidation or re-consolidation of fear memories. This contrasts with a later study which suggests that the endocannabinoids are involved in both consolidation and reactivation of aversive memories (Bucherelli et al. 2006). However, this discrepancy could be explained by the global (i.p. injections) vs. the local effects (local administration in the amygdala) and/or the nature of the CB1 antagonists (rimonabant vs. AM251). In a subsequent study, Suzuki et al. (2008) proposed that the endocannabinoid system was important for the de-stabilisation of reactivated contextual fear memories. Indeed, while the pharmacological blockade of protein synthesis or the genetic disruption of CREB-dependent transcription interfered with memory re-establishment following reactivation, the prior blockade of the CB1 receptors (and voltage-gated calcium channels) impeded this effect, indicating that a fear memory cannot be altered during re-stabilisation if it was not previously destabilised via the activation of the CB1 receptors (Suzuki et al. 2008).
5.4.2.3 Intracellular Cascades
During the extinction of a fear response, the levels of endocannabinoids are increased in the basolateral amygdala (Marsicano et al. 2002). In order to better understand which signalling pathways could be subsequently triggered by the activation of the ECS, Cannich et al. (2004) further analysed the molecular and cellular signature of cued fear extinction. While fear extinction induces the activation of extracellular regulated kinases (Lu et al. 2001) in wild-type animals, this effect was strongly reduced in many brain regions of the CB1-null mice, especially in the basolateral amygdala, the prefrontal cortex and the hippocampus that have been proposed to be involved in these processes (Cannich et al. 2004). Interestingly, CB1 receptors also seem to control in the same regions the expression of calcineurin, a phosphatase which was proposed to play an important role in extinction (Lin et al. 2001, 2003a, b; Mansuy et al. 1998). Therefore, both intracellular phosphorylation and dephosphorylation processes can be modulated by the activation of the CB1 receptors during fear extinction.
5.4.3 A Possible Mechanism for ECS-Dependent Extinction of Fear Memories in the Amygdala
Extinction of conditioned fear is a very complex behavioural process, and an exhaustive review of its neurobiological basis is beyond the scope of the present article. We refer the reader to excellent and extensive reviews for detailed analyses of the theoretical and experimental data present in the literature, e.g. Myers and Davis (2007). Here, we will propose a working hypothesis that might explain the neurobiological role of the endocannabinoid system in fear extinction and, at the same time, suggest a general mechanism of this important adaptive process. Extinction of fear is believed to be a behavioural process relying on multiple mechanisms (Myers and Davis 2007). For instance, both associative and non-associative learning events are probably involved in extinction (Myers and Davis 2007; Marks and Tobena 1990; Kamprath et al. 2006). Many different brain regions, including the amygdala, hippocampus, medial prefrontal cortex and ventral tegmental area have been proposed to contribute to fear extinction (Myers and Davis 2007; Pare et al. 2004; Pezze and Feldon 2004). Furthermore, several neurotransmitter systems, including GABA, glutamate, dopamine, acetycholine, ACTH, glucocorticoids, norepinephrine, brain-derived neurotrophic factor, and, as seen above, endocannabinoids, play important roles in several phases of fear extinction (reviewed in Myers and Davis 2007). Finally, fear extinction is known to involve a plethora of intracellular signalling messengers: pathways involving cAMP/PKA, ERKs, phosphatidylinositol 3-kinase (PI3K), calcium/calmodulin protein kinase II (CaMKII) and the phosphatase, calcineurin, were all proposed to be involved in the processing of fear extinction (reviewed in Myers and Davis 2007). Therefore, given the extreme complexity of the neurobiological processes involved in extinction of fear, proposing a mechanism with a general validity in different conditions might appear to be a kind of “mission impossible”. Nevertheless, considering the involvement of the ECS in fear extinction, a reductive model limited to specific neurotransmitters in a specific brain region might represent a rationale for future investigations aimed at further understanding the neuronal mechanisms of extinction. As the amygdala and its connections with other brain areas (such as the medial prefrontal cortex and the hippocampus) appear to be some of the main loci where both fear acquisition and fear extinction occur (Myers and Davis 2007; Pare et al. 2004), we will limit our attention to this particular region, keeping in mind that, since the ECS is represented in many different brain areas, similar mechanisms as described below could theoretically also occur outside of the amygdala (e.g. in the medial prefrontal cortex, which is rich in CB1 receptors and is centrally involved in extinction processes). For the sake of clarity and to limit the possible variables, our hypothesis will address specifically the modulatory role of the ECS on GABAergic and glutamatergic transmission within the amygdala. Again, the reader should keep in mind that the ECS has been shown to interact with all the neurotransmitter systems listed above that are involved in extinction and, therefore, should be aware that the reality of neuronal processes are surely much more complex than here proposed.
In many brain regions, including the amygdala, CB1 receptor activation is able to modulate both glutamatergic and GABAergic neurotransmission and synaptic plasticity (Marsicano and Lutz 2006; Chevaleyre et al. 2006; Azad et al. 2003, 2004; Domenici et al. 2006). The relative importance of GABAergic vs. glutamatergic neurotransmission in the processing of fear extinction in different brain regions is still a matter of debate in the field (Harris and Westbrook 1998; McGaugh et al. 1990; Myers and Davis 2002, 2007). Indeed, there is evidence that both these neurotransmitter systems might play an important role during several phases of extinction. On the one hand, drugs interfering with GABAA receptors can differentially alter different phases of extinction (Harris and Westbrook 1998; McGaugh et al. 1990). On the other hand, there is compelling evidence that glutamatergic transmission, and in particular activation of NMDA receptors is not only necessary for fear acquisition, but also for fear extinction (Myers and Davis 2002, 2007). The amygdala is formed by different subnuclei, among which the lateral nucleus (LA), the basal nucleus (BA) and the central nucleus (CE) are well known to contain the neuronal circuitry in great part responsible for the processing of conditioned fear responses (LeDoux 2000). For the sake of brevity and simplicity, we will refer to the LA–BA complex as the basolateral complex (BLA) and we will omit the important functional distinctions between neurons belong to each of the individual nuclei (Phelps and LeDoux 2005). The BLA (mostly the LA) is the locus where different sensory inputs (i.e. the US and the CS) converge to form the fear memory, which is likely expressed by the potentiation of particular circuits’ activity (Maren 1999). Anatomical and physiological data indicate that these circuits send information to the CE, which is the main output centre of the amygdala and is responsible, via several projections onto different areas of the brain, for the expression of the fear responses (e.g. freezing, increased startle or autonomic responses) (LeDoux 2000). Strategically located between the BLA and the CE, the intercalated cells (or intercalated cell masses, ICM) are projecting GABAergic neurons that receive excitatory inputs from glutamatergic BLA neurons and send inhibitory projections to CE neurons (Collins and Pare 1999a, b). Given their GABAergic nature, the activation of ICM neurons will lead to an inhibition of CE neurons, and this feature has been proposed to potentially participate in the extinction of fear, by the inhibition of the activity of the output nucleus of the amygdala (Pare et al. 2004). Consistently, a region that plays an important function in the storage of extinction memory, the medial prefrontal cortex (PFC) (Milad and Quirk 2002), is able to stimulate ICM neurons, e.g. as revealed by c-fos immunoreactivity (Berretta et al. 2005). In this view, fear acquisition would occur by the potentiation of the excitatory drive from BLA to CE, whereas extinction would be mediated by an inhibition of the CE, given by the GABAergic input of ICM neurons (Pare et al. 2004). Therefore, glutamatergic BLA neurons would mediate fear, whereas ICM neurons would mediate extinction by inhibiting CE outputs (Pare et al. 2004). A problematic issue with this proposal is that PFC stimulation is able not only to activate ICM neurons, but also to directly excite a certain proportion of glutamatergic BLA neurons (Likhtik et al. 2005), whereas other reports showed a PFC-mediated inhibition of BLA principal neurons, likely due to stimulation of inhibitory GABAergic interneurons (Rosenkranz and Grace 2002; Rosenkranz et al. 2003). These data suggest that, depending on the conditions, PFC can depress or activate glutamatergic neurons in the BLA. Whereas the former case fits easily with a scenario in which inhibition of glutamatergic neurons in the BLA mediates extinction, the possible excitation of the same neurons is more difficult to explain in such a scheme. Indeed, if PFC activity mediates extinction and BLA excitatory neurons mediate fear, one would expect that PFC stimulation should always inhibit, rather than excite BLA neurons. This apparent contradiction could be partially explained by the idea that a balanced activity of glutamatergic and GABAergic neurons within the amygdala could contribute to both acquisition and extinction of fear and that specific excitatory circuits exist to actively mediate fear and extinction of fear, respectively. A recent study confirmed this hypothesis. By means of single unit recordings in awake animals, Herry and colleagues (2008) showed that a certain proportion of pyramidal neurons in the basal amygdala are activated during fear acquisition, whereas another proportion is activated during fear extinction. In this balanced activity, the endocannabinoid system might play an important regulatory role. In a typical fear conditioning paradigm, an a priori neutral stimulus (often a tone, CS) is presented to the animals and immediately paired with an unpleasant stimulus (often a footshock, US). On successive presentations of the CS alone, animals will respond with increased fear reactions (often freezing behaviour, conditioned response, CR) (LeDoux 2000). Extinction occurs upon prolonged and/or repeated presentation of the CS in the absence of the US (i.e. in “non-reinforced” conditions), and it is defined as a continuous decrease of the CR (Myers and Davis 2007). The CS is generally considered neutral, because it normally does not elicit, in absence of a previously paired US, any observable fear reaction. However, the lack of an observable response is not per se an indication that the CS is not eliciting any neural response, but only that the stimulation induced is below the threshold to induce the response chosen as the “outcome” of the experiment. Indeed, many stimuli represent rodents’ alarm signals. In other words, the auditory system is, for many species, an “alarm system”, whose main function is to alert individuals concerning possible dangers present in the environment (Marks and Tobena 1990). This intuitive observation is corroborated by the experimental observation that “neutral” tones can elicit fear reactions simply by increasing their intensity (Marks and Tobena 1990; Kamprath et al. 2006). This is probably the reason why fear conditioning is such a robust protocol, which is easily learnt by many species without the need for intensive training: animals (and rodents in particular) are “prepared” to learn fear conditioning, especially when the modality of the CS involves the auditory system (Marks and Tobena 1990). On the other hand, fear reactions are generally characterised by an inhibition of normal activity, e.g. freezing or conditioned response inhibition (LeDoux 2000). Consequently, fear responses to stimuli should also be very flexible, in order to avoid the risk of a blockade of the common activities necessary for survival (e.g. feeding, reproduction, etc.). In this sense, extinction also appears as a primary function of neuronal circuits, allowing animals to maintain the right balance between “caution” and “activity” to guarantee proper survival. In our opinion, these concepts are important to keep in mind in the attempt to explain the neuronal events occurring both during acquisition and extinction of conditioned fear, and our hypothesis is that circuits in the amygdala might exist ready-made to generate behavioural “fear” and “no fear” reactions. The endocannabinoid system might be an important regulator of the relative activity of these pre-existing circuits. A simplified vision of the anatomical organisation of the neuronal circuits within the amygdaloid complex (Fig. 3), based on physiological and anatomical data (McDonald 1998; Collins and Pare 1999a; Pare et al. 2004), suggests that two different glutamatergic circuits might exist within the BLA. One would directly send excitatory projections to the CE, whereas the second one would first activate ICM neurons, which, in turn, would inhibit CE neurons (Fig. 3a). Importantly, glutamatergic activity in the BLA is maintained under a strong control of local inhibition, which is mainly provided by GABAergic interneurons that are particularly active in this brain region (Bissiere et al. 2003) (Fig. 3a). Given this peculiar anatomical organisation, one could suggest that, in basal conditions (i.e. before conditioning), the neuronal activity conveying the information relative to the CS, deriving, in the case of a tone, from cortical and thalamic auditory areas (LeDoux 2000), might reach both “fear” and “no fear” circuits, eliciting a sub-threshold stimulation that is not sufficient to induce any relevant change in the behaviour of the animal (Fig. 3a). With the occurrence of the US, cortical sensory inputs to the BLA converge onto particular projecting neurons where the coincidence with the CS inputs elicits a long-term potentiation (LTP)-like plastic phenomenon, which is believed to mediate the acquisition of the fear conditioning (LeDoux 2000). In our model, this event would induce a plastic potentiation of the circuits mediating “fear” responses, whereas the hypothetical “no fear” circuits are left unchanged (Fig. 3b). Therefore, the successive presentation of the CS will find the “fear” pathway somehow potentiated, with the consequent stimulation of CE neurons and the behavioural and autonomic expression of the fear reaction (Fig. 3b). Sustained afferent stimulation at low intensity (900 pulses at 1 Hz) onto glutamatergic neurons in the BLA can induce a long-term depression of GABAergic inhibition onto the same neurons, which is mediated by a retrograde action of endocannabinoids likely released from the postsynaptic neurons and acting at presynaptic CB1 receptors located on GABAergic terminals (LTDi; Azad et al. 2004; Marsicano et al. 2002). During successive “non-reinforced” (i.e. in absence of the US) presentations of the CS, the continuous stimulation of “no fear” neurons might, therefore, induce a similar form of “disinhibition” at specific circuits (Azad et al. 2004). If we assume that these circuits might be the “no fear” ones, an ICM-mediated inhibition of CE neurons will occur through their potentiation (Fig. 3c). Thus, during sustained or repeated non-reinforced CS presentation, a sort of unstable balance between two different and competitive kinds of circuits in the BLA would be generated, with the start of a decrease of the conditioned fear response. In this frame, within-session extinction (Myers and Davis 2002) would result from the contrast between two opposing “potentiations”: the one of the “fear” pathways (potentiated by the previous conditioning), and the one of the “no fear” pathways (disinhibited by the CB1-dependent retrograde decrease of inhibition). As CB1 receptors can also strongly regulate glutamatergic transmission in the BLA (Azad et al. 2003), one could also hypothesize, although less electrophysiological evidence exists to support such a mechanism, that the sustained non-reinforced stimulation of glutamatergic neurons might somehow lead to a CB1-dependent inhibition (“depotentiation”) of excitatory synaptic strength in “fear” circuits, further pushing the response of the animal towards a “no fear” behavioural and autonomic reaction (Fig. 3d).
Working hypothesis of extinction processing in the amygdala. (a) Schematic representation of the amygdala, with reference to subnuclei in a cresyl-violet staining of the region (inset, with orientation). Under basal conditions, a tone presentation is not sufficient to elicit a fear response. (b) During conditioning, the simultaneous presentation of the tone (CS) and the shock (US) potentiates “fear” circuits. (c) During extinction, non-reinforced and prolonged tone presentation might cause sustained stimulation of “no-fear” pathways, which might be “disinhibited” through ECS-mediated inhibition of GABAergic transmission. (d) Simultaneously, CB1 on glutamatergic neurons might contribute to the depotentiation (habituation) of “fear” pathways. BLA, basolateral amygdala; CE, central nucleus of amygdala; ICM, intercalated cell masses. Green, glutamatergic pathways; red, GABAergic neurons; orange, potentiation; grey, depotentiation
Importantly, given the short-lasting life of endocannabinoids (see the “on demand” activation of the ECS described in other chapters of the present book) these phenomena might be in part temporary (mostly the depotentiation of “fear” pathways), thereby justifying the observation that extinction is not “erasure” of the original fear conditioning and that different conditions (e.g. spontaneous recovery, reinstatement, renewal and others) can induce a rapid re-establishment of the original response (Myers and Davis 2007).
An important issue in modern theories of extinction is the associative or non-associative nature of this behavioural phenomenon. Extinction of the fear response might rely on associative processes (“new learning”), in which animals form a new inhibitory association between the CS and the lack of the US (Myers and Davis 2002, 2007) or on non-associative mechanisms, in which the subjects simply “habituate” to the aversive stimulus and thereby decrease their response (Marks and Tobena 1990; Kamprath and Wotjak 2004; Myers and Davis 2007). This discrimination is important also for the understanding of human anxiety-related pathologies, such as phobias or post-traumatic stress disorders, in which the impairment in the ability to extinguish fear might depend on alterations in cognitive (associative learning) or non-cognitive (habituation) processes (Marks and Tobena 1990). However, the distinction between these two processes is very difficult in animal experiments, because the experimenter can observe only the decrease of the CR, which is independent of the nature of the causative neuronal processes. However, there is evidence that the ECS might participate both in the associative component of extinction (Chhatwal et al. 2005; Azad et al. 2004) and the non-associative ones (Kamprath et al. 2006). The present model might account for both these functions of the ECS in extinction of fear. On the one hand, the endocannabinoid-mediated “disinhibition” of “no fear” pathways might be somehow regarded as the “associative” component of extinction learning, in the sense that it would be mediated by an active process of “potentiation” of certain neuronal circuits. On the other hand, the proposed CB1-dependent decrease of glutamatergic transmission in the “fear” pathways would account for a form of “non-associative” habituation, in which the activation of the ECS would mediate a simple decrease in the stimulus-response reaction.
Furthermore, this model could also explain some apparently contradictory results concerning the CB1-dependent activation of intracellular pathways in the BLA during extinction (Cannich et al. 2004). In fact, as seen before, CB1 appears to be important during extinction (Cannich et al. 2004) both for the stimulation of the ERK pathway, which is believed to play a central role in synaptic potentiation (Sweatt 2001), and for the opposing action of calcineurin (phosphatase 2B, PP2B), believed to mediate certain forms of depotentiation (Mansuy 2003). The model described here would suggest that these events might be segregated in neurons specific to the “fear” and the “no fear” pathways, with ERK activation contributing to the potentiation of “no fear” neurons and calcineurin mediating the depotentiation of “fear” neurons, respectively.
As we have tried to argue, this model would present the advantage of reconciling many apparently discrepant observations present in the literature concerning the neuronal circuitry underlying extinction and, in particular, the role of the ECS in these processes. However, these ideas are still far from being proven and much experimental work is warranted to confirm or discard them and to fully understand the role of the ECS in the brain circuits mediating fear and anxiety responses.
6 General Conclusions
Many of the functions of the ECS observed in complex learning and memory processes could be explained by simple implicit forms of memory. Indeed, Kamprath et al. (2006) proposed that the deficits of the CB1-null mice in the extinction of cued fear conditioning are due to habituation-like processes. In the recognition memory task (Bilkei-Gorzo et al. 2005), old CB1-null mice or rimonabant-treated rodents could fail to habituate and would not make any distinction between familiar and novel stimuli, whereas their better performance at a younger age could be explained by a problem in habituating to the novel stimulus and they would thus remember longer. This is, however, in conflict with two studies that effectively reported that CB1-null mice habituated to an open field much faster than the wild-type controls and exhibited respectively less locomotion or less rearing (Degroot et al. 2006; Thiemann et al. 2007). However, most of the studies did not report any difference in locomotor activity or rearing behaviour (de Oliveira et al. 2005). This could be due to the time needed to observe the effects of CB1 blockade on habituation. However, it is also possible that the ECS participates in the regulation of typical associative processes of learning and memory tasks. For instance, as proposed above, the coordinate actions of the ECS on both habituation-like and associative processes could mediate the important role of endocannabinoids in extinction of aversive memories.
In attempting to understand the role of the ECS, it has been proposed that endocannabinoid signalling would be important only in situations where aversive stimuli were involved (Lutz 2002; Wotjak 2005). Indeed, the most prominent phenotype of the blockade of the ECS was obtained in the extinction of a fear response (Arenos et al. 2006; Cannich et al. 2004; Kamprath et al. 2006; Marsicano et al. 2002; Mikics et al. 2006; Suzuki et al. 2004, 2008) and in the reversal learning of a spatial task with an aversive component in the Morris Water Maze (Niyuhire et al. 2007; Robinson et al. 2008; Varvel and Lichtman 2002; Varvel et al. 2007). Moreover, it was found that the ECS was dispensable for the extinction in an appetitive task (Holter et al. 2005). In contrast with this theory, it has been very recently suggested that the ECS could also be involved in positively motivated behaviours (Hilário et al. 2007; Rueda-Orozco et al. 2008b). At this point, the ECS would rather have a major role in behavioural flexibility and/or in the ability to change attentional set, whereas it would have a limited function in initial learning. On the contrary, CB1 receptor blockade induced reduced perseveration in a strategy-shift task, indicating that the switch of strategy was even facilitated (Hill et al. 2006). The endocannabinoid system could modulate many transmitter systems that have been involved in behavioural flexibility, such as the glutamatergic (Stefani and Moghaddam 2003; Stefani et al. 2003), serotoninergic (Clarke et al. 2008) and dopaminergic (Floresco et al. 2006; Floresco and Magyar 2006) systems in the prefrontal cortex, but further analyses are still needed to characterise these interactions.
The recent idea that the ECS could be involved in the “destabilisation” of memory traces (Suzuki et al. 2008) might be very important for the interpretation of the different (and sometimes apparently discrepant) results in the literature. Indeed, if the role of the ECS is to temporarily “weaken” acquired memory, this mechanism could be also involved in the prolongation of the time for which new memories are labile during acquisition in certain conditions. This could, theoretically, explain why, in different conditions, the ECS might influence different phases of learning and memory processing.
In conclusion, the roles of the ECS in learning and memory are on the way to be dissected and clarified. However, many aspects are still obscure and further studies are mandatory both to provide knowledge of the general mechanisms of learning and also to develop novel therapeutic tools to tackle diseases characterised by improper processing and storage of information in the brain.
Abbreviations
- AEA:
-
Anandamide
- BA:
-
Basal nucleus of the amygdala
- BLA:
-
Basolateral amygdala
- CaMKII:
-
Calcium/calmodulin protein kinase II
- CE:
-
Central nucleus of the amygdale
- CR:
-
Conditioned response
- CS:
-
Conditioned stimulus
- DSI:
-
Depolarization-induced suppression of inhibition
- ECS:
-
Endocannabinoid system
- FAAH:
-
Fatty acid amide hydrolase
- GPCR:
-
G protein-coupled receptor
- ICM:
-
Intercalated cell masses
- LA:
-
Lateral nucleus of the amygdala
- LTP:
-
Long-term synaptic potentiation
- PFC:
-
Prefrontal cortex
- PI3K:
-
Phosphatidylinositol 3-kinase
- THC:
-
Δ9-Tetrahydrocannabinol
- US:
-
Unconditioned stimulus
References
Adam AS, Wenger T, Csillag A (2008) The cannabinoid CB1 receptor antagonist rimonabant dose-dependently inhibits memory recall in the passive avoidance task in domestic chicks (Gallus domesticus). Brain Res Bull 76:272–274
Alger E (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68:247–286
Arenos JD, Musty RE, Bucci DJ (2006) Blockade of cannabinoid CB1 receptors alters contextual learning and memory. Eur J Pharmacol 539:177–183
Azad SC, Eder M, Marsicano G et al. (2003) Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Memory 10:116–128
Azad SC, Monory K, Marsicano G et al. (2004) Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci 24:9953–9961
Bacci A, Huguenard JR, Prince DA (2004) Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 431:312–316
Bailey CH, Kandel ER, Si K (2004) The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron 44:49–57
Barco A, Bailey CH, Kandel ER (2006) Common molecular mechanisms in explicit and implicit memory. J Neurochem 97:1520–1533
Berretta S, Pantazopoulos H, Caldera M et al. (2005) Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132:943–953
Bilkei-Gorzo A, Racz I, Valverde O et al. (2005) Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci USA 102:15670–15675
Bissiere S, Humeau Y, Luthi A (2003) Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci 6:587–592
Brower LP, Glazier SC (1975) Localization of heart poisons in the monarch butterfly. Science 188:19–25
Bucherelli C, Baldi E, Mariottini C et al. (2006) Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learn Memory 13:426–430
Bura SA, Castane A, Ledent C et al. (2007) Genetic and pharmacological approaches to evaluate the interaction between the cannabinoid and cholinergic systems in cognitive processes. Br J Pharmacol 150:758–765
Cannich A, Wotjak CT, Kamprath K et al. (2004) CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Memory 11:625–632
Carlson G, Wang Y, Alger BE (2002) Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5:723–724
Carter E, Wang XJ (2007) Cannabinoid-mediated disinhibition and working memory: dynamical interplay of multiple feedback mechanisms in a continuous attractor model of prefrontal cortex. Cereb Cortex 17(Suppl 1):i16–i26
Castellano C, Rossi-Arnaud C, Cestari V et al. (2003) Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord 2:389–402
Chevaleyre V, Takahashi KA, Castillo PE (2006) Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29:37–75
Chhatwal JP, Davis M, Maguschak KA et al. (2005) Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30:516–524
Clarke JR, Rossato JI, Monteiro S et al. (2008) Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Memory 90(2):374–381
Collins DR, Pare D (1999a) Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J Neurosci 19:836–844
Collins DR, Pare D (1999b) Spontaneous and evoked activity of intercalated amygdala neurons. Eur J Neurosci 11:3441–3448
Cravatt BF, Demarest K, Patricelli MP et al. (2001) Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA 98:9371–9376
de Oliveira AL, de Oliveira LF, Camboim C et al. (2005) Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol Learn Memory 83:119–124
Deadwyler SA, Hampson RE (2008) Endocannabinoids modulate encoding of sequential memory in the rat hippocampus. Psychopharmacology (Berl) 198:577–586
Deadwyler SA, Goonawardena AV, Hampson RE (2007) Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharmacol 18:571–580
Debiec J, Ledoux JE, Nader K (2002) Cellular and systems reconsolidation in the hippocampus. Neuron 36:527–538
Degroot A, Kofalvi A, Wade MR et al. (2006) CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol Pharmacol 70:1236–1245
Devane WA, Hanus L, Breuer A et al. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Di Marzo V, De Petrocellis L, Fezza F et al. (2002) Anandamide receptors. Prostag Leukot Ess Fatty Acids 66:377–391
Domenici MR, Azad SC, Marsicano G et al. (2006) Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci 26:5794–5799
Dunning J, During MJ (2003) Molecular mechanisms of learning and memory. Expert Rev Mol Med 5:1–11
Duvarci S, Nader K (2004) Characterization of fear memory reconsolidation. J Neurosci 24:9269–9275
Floresco SB, Magyar O (2006) Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 188:567–585
Floresco SB, Magyar O, Ghods-Sharifi S et al. (2006) Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology 31:297–309
Freund TF, Katona I, Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066
Fride E (2005) Endocannabinoids in the central nervous system: from neuronal networks to behavior. Curr Drug Targets CNS Neurol Disord 4:633–642
Fujiwara M, Egashira N (2004) New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application. J Pharmacol Sci 96:362–366
Gaoni Y, Mechoulam R (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647
Garcia J, Kimeldorf DJ, Koelling RA (1955) Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122:157–158
Harris JA, Westbrook RF (1998) Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 140:105–115
Herry C, Ciocchi S, Senn V et al. (2008) Switching on and off fear by distinct neuronal circuits. Nature 454:600–606
Hilário MRF, Clouse E, Yin HH et al. (2007) Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci 1:6
Hill MN, Froese LM, Morrish AC et al. (2006) Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology (Berl) 187:245–259
Holter SM, Kallnik M, Wurst W et al. (2005) Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol 510:69–74
Howlett AC, Barth F, Bonner TI et al. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54:161–202
Iversen L (2003a) Cannabis and the brain. Brain 126:1252–1270
Iversen L (2003b) Comparing cannabis with tobacco: arithmetic does not add up. Br Med J 327:165
Kamprath K, Wotjak CT (2004) Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Memory 11:770–786
Kamprath K, Marsicano G, Tang J et al. (2006) Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci 26:6677–6686
Kandel ER (2001) The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep 21:565–611
Kandel ER, Schwartz JH, Jessel TM (2000) Principles of neural science. McGraw-Hill, New York
Kobilo T, Hazvi S, Dudai Y (2007) Role of cortical cannabinoid CB1 receptor in conditioned taste aversion memory. Eur J Neurosci 25:3417–3421
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Lichtman AH (2000) SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol 404:175–179
Likhtik E, Pelletier JG, Paz R et al. (2005) Prefrontal control of the amygdala. J Neurosci 25:7429–7437
Lin CH, Yeh SH, Lin CH et al. (2001) A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 31:841–851
Lin CH, Lee CC, Gean PW (2003a) Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol 63:44–52
Lin CH, Yeh SH, Leu TH et al. (2003b) Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci 23:1574–1579
Lin HC, Mao SC, Su CL et al. (2009) The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex 19(1):165–175
Lu KT, Walker DL, Davis M (2001) Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci 21:RC162–RC166
Lutz B (2002) Molecular biology of cannabinoid receptors. Prostag Leukot Ess Fatty Acids 66:123–142
Maccarrone M, Valverde O, Barbaccia ML et al. (2002) Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci 15:1178–1186
Maejima T, Ohno-Shosaku T, Kano M (2001) Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res 40:205–210
Mallet PE, Beninger RJ (1998) The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 140:11–19
Mansuy IM (2003) Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun 311:1195–1208
Mansuy IM, Mayford M, Jacob B et al. (1998) Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 92:39–49
Maren S (1999) Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci 22:561–567
Marks I, Tobena A (1990) Learning and unlearning fear: a clinical and evolutionary perspective. Neurosci Biobehav Rev 14:365–384
Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11:4213–4225
Marsicano G, Lutz B (2006) Neuromodulatory functions of the endocannabinoid system. J Endocrinol Invest 29:27–46
Marsicano G, Wotjak CT, Azad SC et al. (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534
Martin M, Ledent C, Parmentier M et al. (2002) Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 159:379–387
Massa F, Marsicano G, Hermann H et al. (2004) The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 113:1202–1209
Matsuda LA, Lolait SJ, Brownstein MJ et al. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564
McDonald AJ (1998) Cortical pathways to the mammalian amygdala. Prog Neurobiol 55:257–332
McGaugh JL (2000) Memory – a century of consolidation. Science 287:248–251
McGaugh JL, Castellano C, Brioni J (1990) Picrotoxin enhances latent extinction of conditioned fear. Behav Neurosci 104:264–267
Mechoulam R, Ben Shabat S, Hanus L et al. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Mikics E, Barsy B, Barsvari B et al. (2005) Behavioral specificity of non-genomic glucocorticoid effects in rats: effects on risk assessment in the elevated plus-maze and the open-field. Horm Behav 48:152–162
Mikics E, Dombi T, Barsvari B et al. (2006) The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behav Pharmacol 17:223–230
Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74
Myers KM, Davis M (2002) Behavioral and neural analysis of extinction. Neuron 36:567–584
Myers KM, Davis M (2007) Mechanisms of fear extinction. Mol Psychiatry 12:120–150
Nader K, Schafe GE, Ledoux JE (2000) The labile nature of consolidation theory. Nat Rev Neurosci 1:216–219
Niyuhire F, Varvel SA, Thorpe AJ et al. (2007) The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 191:223–231
Pacher P, Batkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462
Packard MG, McGaugh JL (1992) Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav Neurosci 106:439–446
Pagotto U, Cervino C, Vicennati V et al. (2006) How many sites of action for endocannabinoids to control energy metabolism? Int J Obes (Lond) 30(Suppl 1):S39–S43
Pare D, Quirk GJ, LeDoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9
Pertwee RG (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153:199–215
Pezze MA, Feldon J (2004) Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol 74:301–320
Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187
Quirk GJ, Mueller D (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72
Ranganathan M, D'Souza DC (2006) The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 188:425–444
Reibaud M, Obinu MC, Ledent C et al. (1999) Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharmacol 379:R1–R2
Reich CG, Mohammadi MH, Alger BE (2008) Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J Psychopharmacol. 22:761–768
Riedel G, Davies SN (2005) Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol 168:445–477
Rinaldi-Carmona M, Barth F, Heaulme M et al. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Robinson L, Killop-Smith S, Ross NL et al. (2008) Hippocampal endocannabinoids inhibit spatial learning and limit spatial memory in rats. Psychopharmacology (Berl) 198:551–563
Rosenkranz JA, Grace AA (2002) Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22:324–337
Rosenkranz JA, Moore H, Grace AA (2003) The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci 23:11054–11064
Rueda-Orozco PE, Montes-Rodriguez CJ, Soria-Gomez E et al. (2008a) Impairment of endocannabinoids activity in the dorsolateral striatum delays extinction of behavior in a procedural memory task in rats. Neuropharmacology 55(1):55–62
Rueda-Orozco PE, Soria-Gomez E, Montes-Rodriguez CJ et al. (2008b) A potential function of endocannabinoids in the selection of a navigation strategy by rats. Psychopharmacology (Berl) 198:565–576
Sara SJ (2000) Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Memory 7:73–84
Shiflett MW, Rankin AZ, Tomaszycki ML et al. (2004) Cannabinoid inhibition improves memory in food-storing birds, but with a cost. Proc Biol Sci 271:2043–2048
Soria G, Mendizabal V, Tourino C et al. (2005) Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30:1670–1680
Squire LR, Berq D, du Lac S et al. (2008) Fundamentals of Neuroscience, 3rd edn
Stefani MR, Moghaddam B (2003) Distinct contributions of glutamate receptor subtypes to cognitive set-shifting abilities in the rat. Ann N Y Acad Sci 1003:464–467
Stefani MR, Groth K, Moghaddam B (2003) Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci 117:728–737
Straiker A, Mackie K (2007) Metabotropic suppression of excitation in murine autaptic hippocampal neurons. J Physiol 578:773–785
Sugiura T, Kondo S, Sukagawa A et al. (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97
Suzuki A, Josselyn SA, Frankland PW et al. (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24:4787–4795
Suzuki A, Mukawa T, Tsukagoshi A et al. (2008) Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn Memory 15:426–433
Sweatt JD (2001) The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76:1–10
Takahashi RN, Pamplona FA, Fernandes MS (2005) The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett 380:270–275
Terranova JP, Storme JJ, Lafon N et al. (1996) Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141,716. Psychopharmacology (Berl) 126:165–172
Thiemann G, Fletcher BC, Ledent C et al. (2007) The genetic versus pharmacological invalidation of the cannabinoid CB(1) receptor results in differential effects on 'non-associative' memory and forebrain monoamine concentrations in mice. Neurobiol Learn Memory 88:416–423
Van Gaal LF, Rissanen AM, Scheen AJ et al. (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397
Van Sickle MD, Duncan M, Kingsley PJ et al. (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Varvel SA, Lichtman AH (2002) Evaluation of CB(1) Receptor Knockout Mice in the Morris Water Maze. J Pharmacol Exp Ther 301:915–924
Varvel SA, Anum EA, Lichtman AH (2005) Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 179:863–872
Varvel SA, Cravatt BF, Engram AE et al. (2006) Fatty acid amide hydrolase (-/-) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J Pharmacol Exp Ther 317:251–257
Varvel SA, Wise LE, Niyuhire F et al. (2007) Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32:1032–1041
Welzl H, D'Adamo P, Lipp HP (2001) Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res 125:205–213
White NM, McDonald RJ (2002) Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Memory 77:125–184
Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410:588–592
Winsauer PJ, Lambert P, Moerschbaecher JM (1999) Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol 10:497–511
Wise LE, Iredale PA, Stokes RJ et al. (2007) Combination of rimonabant and donepezil prolongs spatial memory duration. Neuropsychopharmacology 32:1805–1812
Wolff MC, Leander JD (2003) SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol 477:213–217
Wotjak CT (2005) Role of endogenous cannabinoids in cognition and emotionality. Mini Rev Med Chem 5:659–670
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Marsicano, G., Lafenêtre, P. (2009). Roles of the Endocannabinoid System in Learning and Memory. In: Kendall, D., Alexander, S. (eds) Behavioral Neurobiology of the Endocannabinoid System. Current Topics in Behavioral Neurosciences, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-88955-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-540-88955-7_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-88954-0
Online ISBN: 978-3-540-88955-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)