Abstract
Although arbuscular mycorrhizal (AM) symbioses are considered to be mutualistic, plant benefit is not always immediately obvious. Non-responsiveness in terms of growth and phosphorus (P) nutrition is observed in a wide variety of plant species, including natives and some widely cultivated crops (e.g. cereals). Non-responsiveness is primarily attributed to variations in the exchange of carbon (C) and P between the symbionts. Here, we explore recent insights into P uptake in non-responsive plants. The AM pathway of P uptake can be functional in non-responsive plants, as shown by fungal 32/33P uptake, which has raised questions regarding functionality of the direct uptake pathway. As the mechanisms for P uptake via AM and direct uptake pathways are revealed, we can begin to explore functional differences at the molecular level. Identifying factors which influence AM responsiveness will provide critical insights for future crop breeding efforts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 7.1 Introduction
Symbiotic interactions of AM fungi with roots of land plants are widespread. They occur in both natural and agricultural ecosystems and probably involve ~80% of land plants (Smith and Read 1997). Conventionally, the symbiosis is considered to be a mutualism, based upon the reciprocal exchange of nutrients. The fungi are obligate symbionts, relying on plants as sole sources of carbon (C). In return, plants receive nutrients such as phosphorus (P) and nitrogen, which are taken up from the soil by external hyphae of the fungi (see Chapter 6 by Ferrol and Pérez-Tienda). The evolutionary conservation and widespread occurrence of AM symbioses are testaments to the importance of AM in plant function. Indeed, AM symbioses have been demonstrated to improve disease tolerance (see Chapter 9 by Pozo et al.), increase drought resistance and decrease the accumulation of heavy metals (see Chapter 8 by González-Guerrero et al.), although it has been suggested that these benefits have evolved relatively recently (Fitter 2006). Improved P nutrition is still considered the primary benefit of AM symbioses, and total plant P uptake and growth response are the most commonly reported measures of AM function. Plant responses to AM colonisation are diverse, ranging from large positive increases in growth and P uptake to growth depressions (Tawaraya 2003). This diversity in responsiveness is considered to reflect the diversity in function of different plant–AM fungal combinations. This review brings together recent advances in measurement of the contributions of the AM fungus to plant P nutrition and current knowledge of molecular processes of P uptake in AM plants, with particular emphasis on situations where plants show low or no positive responses to the symbiosis.
7.2 7.2 The Challenge of P Uptake from Soil and Symbiotic Interfaces
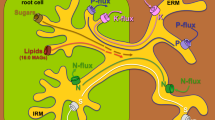
AM plants have two possible pathways for the acquisition of P from the soil solution, the direct uptake pathway via the root epidermis and root hairs, and the AM pathway via external fungal hyphae and colonised cortical cells (Fig. 7.1a). The total P content of an AM plant results from the combined operation of these pathways. Although the direct pathway has been studied extensively in model systems such as Arabidopsis thaliana [which is constitutively non-mycorrhizal (NM)], the prevalence of AM in most natural and agricultural environments suggests that an understanding of the integration of the two pathways is essential to provide a realistic picture of plant nutrient uptake. The following section briefly outlines the processes involved in P uptake in an AM plant and the interfaces at which P transfer occurs.
Schematic representation of P uptake in an AM root. (a) P uptake from soil solution is mediated by plant and fungal Pi:H+ symporters expressed in external hyphae or root epidermis and root hairs. Rapid uptake of P by roots leads to formation of a depletion zone, fungal hyphae extend beyond depletion zones taking up P and transporting it to intracellular arbuscules (Arb) in colonised cortical cells (CC). (b) P taken up by the fungus is released into the interfacial apoplast via an unknown mechanism. Plant P uptake across the plant plasma membrane (PPM) at symbiotic interfaces is also mediated by Pi:H+ symporters in competition with AM fungal retrieval
Both plants and AM fungi acquire P from the soil solution as inorganic orthophosphate (Pi). Although the total quantity of P in the soil can be high, the concentration of Pi in the soil solution rarely exceeds 10 μM (Bieleski 1973). In contrast, cytosolic P concentrations are within the millimolar range (Mimura 1999). Uptake of Pi from soil therefore requires an active, high-affinity transportation system. Over the last decade, plant and fungal Pi transporters involved in P uptake from soil have been identified (see Smith et al. 2003a and references therein). These transporters share high sequence similarity and together are members of the Pi:H+ symporter family. Functional characterisation predicts high-affinity kinetics, consistent with uptake of Pi from the low concentrations in the soil solution. Pi transporters have been localised to the root epidermis and root hairs and to the extraradical hyphae (Fig. 7.1a). Both plant and fungal Pi transporters respond to Pi concentrations in the environment and to the P status of the organism (Mimura 1999; Maldonado-Mendoza et al. 2001).
The slow rate of diffusion of Pi in soil (10–8 to 10–11 cm2 s−1; Bieleski 1973) compared with rapid plant uptake results in the formation of a depletion zone adjacent to the root surface. P uptake by NM plants is therefore limited by the rate of diffusion of Pi into this zone and/or the ability of the plant to extend roots into undepleted soil. In a mycorrhizal plant, AM fungal hyphae extend beyond this depletion zone (Fig. 7.1a), their small diameter (2–15 μm) enables access to smaller soil pores than plant roots, whilst P translocation to the plant through fungal hyphae occurs at rates very much faster than the diffusion of Pi through soil. Following P uptake by extraradical hyphae, amounts in excess of metabolic requirements are transferred to the vacuoles and stored as polyphosphate (polyP). Numerous studies have investigated the dynamics of polyP sequestration, storage and transport in AM hyphae, but the mechanism of P release to the symbiotic interfaces remains obscure (see Ezawa et al. 2002).
The precise location of P transfer to the plant has received considerable attention. Intracellular arbuscules have long been considered the primary site for P transfer. These interfaces are formed when fungal hyphae penetrate the walls of root cortical cells and invaginate the plasma membranes to form the highly branched structures once considered characteristic of the symbiosis. Extension of the plasma membrane around the arbuscule provides a large increase in surface area and results in the formation of an extensive and intimate interface between the symbionts. However, not all AM fungi form arbuscules. Intracellular hyphal coils can provide an equivalent surface area to arbuscules (Dickson and Kolesik 1999) and there is now recognition that these too may play an important role in P transfer. Localisation of plant Pi transporter expression to cortical cells containing arbuscules and hyphal coils provides strong evidence for P transfer via intracellular interfaces (Harrison et al. 2002; Karandashov et al. 2004; Glassop et al. 2005). However, it is noteworthy that the fungus remains within an apoplastic compartment, with no cytoplasmic continuity between the symbionts. Transfer of P between the symbionts therefore requires both efflux from the fungus and uptake by the plant (Fig. 7.1b).
The mechanisms for efflux of P from AM fungal hyphae to the interfacial apoplast remain obscure, although specific efflux channels have been invoked to account for the considerable P release (Smith et al. 2001). P in the interfacial apoplast must be actively transported across the plasma membrane into the plant cell, presumably presenting a challenge similar to uptake from the soil, although Pi concentrations in the apoplast have not been determined. Localisation of H+-ATPases to plant membranes surrounding arbuscules (Gianinazzi-Pearson et al. 2000) and the expression of plant Pi transporters supports this model (Harrison et al. 2002; see also Chapter 6 by Ferrol and Pérez-Tienda). The plant Pi transporters expressed at symbiotic interfaces are members of the same family as the root epidermal Pi transporters; insights from their expression and functional characterisation are discussed below.
7.3 7.3 Diversity in Plant Responses to AM Colonisation
AM-induced increases in plant growth are often accompanied by significant increases in P concentration. These data provided the first evidence for the key role of P in symbiotic nutrient exchange. Typically, plants which respond positively to AM colonisation have limited capacity to grow at low P, and AM colonisation improves P uptake and results in increased growth over a broad range of external P concentrations. However, responses of plants to AM colonisation are diverse, ranging from dramatic increases in growth and P nutrition to negligible changes in growth and even growth depressions. The term mycorrhizal responsiveness (MR), calculated according to Eq. 7.1, has been developed to quantify AM-induced changes in plant growth (MGR) (Baon et al. 1993) or P uptake (MPR) (Li et al. 2008), where AM and NM refer to either dry matter or total plant P, respectively.
In the context of this review, non-responsive plants are those which demonstrate negligible or negative growth responses to AM colonisation (zero or negative MR) even at low P supply. In an extensive literature survey of growth responses of 250 AM plant species including field and forage crops, wild grasses and forbs, and trees, Tawaraya (2003) showed that non-responsive species are less common than positively responsive species, but that some were identified in all groups investigated.
Non-responsiveness of AM plants has been related to physiological traits which increase P uptake under P-limiting conditions, thereby reducing reliance on AM fungi for acquisition of P. Such traits include extensive, finely branched root systems and long root hairs (Jakobsen et al. 2005) or the ability of the plant to modify the rhizosphere thus increasing access to soil P. In Triticum aestivum and Hordeum vulgare, cultivar differences in AM responsiveness are correlated with efficiency of P acquisition or P utilisation, leading to the suggestion that breeding programs targeting improved P efficiency may have led to a decline in AM responsiveness of modern cereal cultivars (Baon et al. 1993; Zhu et al. 2001).
Whereas it is possible to make broad generalisations regarding the tendency for certain plant species to be positively responsive or non-responsive to colonization, it is important to recognise that AM responsiveness is not dependent solely upon the plant genotype. Responsiveness is also influenced by the fungal genotype (Munkvold et al. 2004) and the environmental conditions which influence the C and P status in the plant; primarily light, temperature, soil P and soil pH (Johnson et al. 1997). Diversity in plant responses is therefore considered to result from diversity in function of different AM fungus–plant interactions and is conventionally attributed to perturbations in the balance between benefit derived from increased access to growth-limiting nutrients and cost of supplying C to the fungal symbiont. C allocation to the AM fungus can account for 20% of plant photosynthates (Jakobsen and Rosendahl 1990) and, in many instances, growth depressions are attributed to this C drain (see Jakobsen 1995 and references therein).
Both transient and persistent growth depressions are occasionally observed amongst positively responsive plant species. In both instances, these growth depressions can be explained with respect to C demand and P supply by the AM fungus. For example, persistent growth depressions are frequently reported at high P supply and are attributed to a shift in the balance between symbiotic cost and benefit. At high P, growth of NM plants is not limited by P and there is little benefit of increased supply via AM fungi, whilst the C cost of the fungal symbiont is maintained. This theory is supported by observations of AM citrus in which growth depression at high P was overcome by elevated CO2 supply (Jifon et al. 2002) (for further discussion see Jakobsen 1995).
As with growth depressions at high P, growth depressions at low P in non-responsive plants are often attributed to fungal C drain. Therefore, it might be expected that the magnitude of growth depression will be correlated with the extent of colonisation and, hence, C demand. However, there is an increasing body of evidence suggesting that this is not the case. As is apparent from Table 7.1, growth depressions are frequently unrelated to the degree of colonisation. The mycorrhizal growth response (MGR) of H. vulgare colonised by two AM fungi was −36% and −34% whilst colonisation was 72% and 19%, respectively (Grace et al. 2008). In T. aestivum cv. Newton, growth depressions due to Glomus mosseae or Glomus versiforme were equal (−50%, Table 7.1), but these fungi colonised 5% and 61% of the root length, respectively (Hetrick et al. 1992). Such findings have been largely ignored, but they suggest that the traditional model used to explain non-responsiveness should be re-examined.
Whereas calculation of MGR or MPR has proved useful for comparison of whole-plant responses to AM, it is important to clearly distinguish MPR from estimates of the actual contribution of the AM pathway to P uptake. Most estimates have been based on the difference in P uptake between AM and NM plants (e.g. Smith et al. 1994) and rely on the assumption that AM colonisation has no effect on the direct uptake of nutrients into plant roots. As positive values are only obtained in plants that are positively responsive to AM, such calculations have led to the notion that the AM pathway is non-functional in terms of P uptake in non-responsive plants. However, an increasing body of evidence from both radiotracer experiments and molecular studies (see below) demonstrates that the contribution of the AM pathway is not necessarily related to plant responses. The assumption that non-responsive plants receive no nutritional benefit from AM symbionts must therefore be re-examined, particularly with respect to roles and management of AM in agricultural systems and natural ecosystems.
7.4 7.4 Radiotracer Studies Enable Measurement of AM Contribution Regardless of Whole Plant Responses
Experimental designs using compartmented pots in which radiotracer is supplied in a hyphal compartment (HC) accessible only to AM fungal hyphae have been highly effective at demonstrating nutrient (P, Zn, NO3 − and NH4 +) uptake and transfer to plants via the AM pathway (Johansen et al. 1993; Pearson and Jakobsen 1993; Bürkert and Robson 1994; Tobar et al. 1994; Smith et al. 2000). As is evident from Table 7.2, 32/33P transfer via the AM pathway has been demonstrated for a range of plant and fungal combinations including both positively responsive and non-responsive interactions.
Early radiotracer experiments enabled comparison of the relative AM contributions in different plant–fungus combinations. Comparing 32P uptake in two native grasses, Hetrick et al. (1994) demonstrated that positively responsive Andropogon gerardii received less 32P than non-responsive Bromus inermis. Furthermore, in T. aestivum, the specific activity (kBq 32P mg−1 P) was lower in the positively responsive landrace Turkey, than in the non-responsive modern cultivar, Newton, indicating that the AM pathway made a greater contribution to the non-responsive cultivar (Hetrick et al. 1996). The overwhelming conclusion must be that the response, in terms of MGR or MPR, in non-responsive plants is entirely unrelated to the contribution of the AM pathway to P uptake.
Tracer studies also provide insights into the role of the fungal symbiont in determining diversity in plant responsiveness. In Cucumis sativus, Pearson and Jakobsen (1993) observed differences in plant P content (and hence MPR) depending on the identity of the AM fungal symbiont, in the order Glomus caledonium < Scutellospora calospora << Glomus invermaium. P content was unrelated to the amount of 32P transported from the HC, which increased in the order S. calospora < G. invermaium << G. caledonium. 32P transfer was not correlated with hyphal length in the HC or % colonisation, leading the authors to suggest that hyphal P uptake, P translocation and P release to the plant are likely to be important control points governing the diversity in P contribution of different AM fungal species (see Munkvold et al. 2004 for further discussion).
Although these early experiments with radiotracers demonstrated transfer of 32P via the AM pathway and enabled comparison of relative transfer under particular experimental conditions, attempts to quantify the contribution of the AM pathway were confounded by large HCs distant from the plant roots, which favoured the AM plants and fungal symbionts with extensive hyphal networks (Li et al. 1991; Pearson and Jakobsen 1993). A recent advance, depending on determination of the specific activity of 32/33P in the plant and in a small radiolabeled HC, and hyphal length densities in the main pot and HC, has largely overcome these issues (Smith et al. 2003b, 2004). The experiment demonstrated significant differences in contribution of the AM pathway to Medicago truncatula, Solanum lycopersicum and Linum usitatissimum (see Table 7.2). The contribution also varied with fungal species and, as had been previously observed, was not related to percent colonisation, plant growth response or total P uptake. Of particular significance was the finding that Glomus intraradices contributed up to 100% of total plant P to both L. usitatissimum, which showed a positive growth response (MGR 1,425%), and S. lycopersicum which showed a growth depression (MGR −18%). These results clearly demonstrate that the AM pathway can be functional in non-responsive plants.
Whilst re-emphasising that responsiveness is not an appropriate measure of mycorrhizal contribution to P uptake, these results also show that the direct, epidermal P uptake pathway can be suppressed during AM symbiosis in favour of the AM pathway. One explanation for the apparent down-regulation of direct P uptake in AM plants is that the formation of the depletion zone (Fig. 7.1) occurs more rapidly in AM plants. Competition between fungal hyphae and roots for P uptake in zones close to the root could lead to rapid depletion of Pi pools accessible to root transporters and decreased overall contribution of the direct pathway. However, an alternative explanation involving changes in the expression of plant Pi transporters has also been invoked. The discovery of the transporters responsible for P uptake by each pathway has enabled further investigation of this phenomenon.
7.5 7.5 The Role of Pi Transporters in P Uptake by an AM Plant
Identification of Pi transporters involved in AM fungal and plant P uptake provides tools for further investigation of processes controlling P influx via the direct and AM pathways. Numerous reviews have focussed on the identification and functional characterisation of these transporters (Smith et al. 2003a; Karandashov and Bucher 2005; Javot et al. 2007b; see also Chapter 6 by Ferrol and Pérez-Tienda). Accordingly, we highlight significant points related to variability in P fluxes via the plant and fungal P uptake pathways. Although plant and fungal Pi transporters have been identified in both responsive and non-responsive species, there has, so far, been limited focus on differences between these species and the resultant effects on symbiotic function.
7.5.1 7.5.1 Expression of Pi Transporters in the AM Pathway
7.5.1.1 7.5.1.1 AM Fungal Pi Transporters
The AM fungal Pi:H+ symporter of G. versiforme, GvPT, is predominantly expressed in the external mycelium, with only low levels of expression in roots of the plant symbiont, M. truncatula (Harrison and van Buuren 1995). This pattern was taken as evidence for unidirectional flux of P from the fungal symbiont to the plant. In contrast, high levels of expression of the GvPT orthologue GmosPT, from G. mosseae, were observed not only in external mycelium but also in colonised roots of C. sativus (Benedetto et al. 2005). Using laser micro-dissection associated with gene expression analyses, Balestrini et al. (2007) localised intraradical expression of GmosPT in arbuscules in cortical cells of S. lycopersicum. Expression of the AM fungal H+-ATPase, GmHA5, was also identified exclusively in arbuscules in this study. The expression of both a fungal Pi transporter and a fungal H+-ATPase in arbuscules provides the first evidence for potential active P transport by fungal structures at intracellular interfaces and suggests that the fungal symbiont may compete with the plant for P in the interfacial apoplast. The differences in expression of Pi transporters from different fungi and at different locations raise important questions relating to symbiotic function. If AM fungi have different abilities to compete with plants for uptake from the interfacial apoplast this could have significant implications for overall efficiency of symbiotic P transfer in different plant–fungus combinations. Further investigation is clearly necessary. It will be interesting to determine whether the expression of fungal Pi transporters in roots is influenced by identity of host species and/or environmental conditions.
7.5.1.2 7.5.1.2 AM-Inducible Plant Pi Transporters
Plant transporters involved in Pi uptake from the interfacial apoplast have been identified in a range of plant species, including the non-responsive (or poorly responsive) H. vulgare, T. aestivum and S. lycopersicum (Glassop et al. 2005; Nagy et al. 2005). These AM-inducible Pi transporters fall into two categories, based upon their expression patterns: AM-specific Pi transporters expressed exclusively in AM roots, and AM-upregulated Pi transporters which also show low level expression in NM roots and/or shoots. RNA hybridisation and reporter-gene activity consistently demonstrate expression of AM-inducible Pi transporters in root cortical cells containing intracellular structures including arbuscules, hyphal coils and arbusculate coils (Karandashov et al. 2004; Glassop et al. 2005). In M. truncatula, immunolocalisation of the AM-specific Pi transporter MtPT4 revealed expression of protein exclusively on the plant plasma membrane surrounding arbuscules, it being strongest around mature arbuscules and coordinated with arbuscule development and decay (Harrison et al. 2002).
In H. vulgare, the AM-inducible Pi transporter HvPT8 is expressed at high levels in AM roots regardless of plant responses to AM colonisation (Grace et al. 2008 corroborating 32P data which suggested a 60% contribution of the AM pathway (Table 7.2). In S. lycopersicum, investigations with a reduced mycorrhizal colonisation mutant (rmc) and its wild type progenitor demonstrated that the AM-inducible Pi transporters, LePT3 and LePT4 are only expressed in symbiotic interactions capable of 32P transfer to the plant (Poulsen et al. 2005). These data indicate that AM-inducible Pi transporters may provide useful molecular markers signalling formation of a functional symbiosis. However, there is no evidence at present to support suggestions that expression of these transporters can be correlated with P flux via the AM pathway.
The heterologous expression of AM-inducible Pi transporters in yeast has been used to functionally characterise these transporters and investigate transport kinetics. Although this system is not ideal (Smith et al. 2003a), results suggest that there may be considerable variation among different plants. The apparent Km (64 μM) for the AM-upregulated transporter StPT3 suggests fairly high-affinity transport characteristics, whereas MtPT4 demonstrated low-affinity kinetics (Km = 493–685 μM) (Harrison et al. 2002). The possible existence of both high and low-affinity AM-inducible transporters suggests that P concentrations in the interfacial apoplast may be variable. This is conceivable if different AM fungi differ in their efficiency of P release to the interfacial apoplast and/or reabsorption (see above), and is supported by observations that P retention time in fungal hyphae differs between AM species (Jakobsen et al. 1992; Smith et al. 2000) and that some AM fungi accumulate polyP in hyphae rather than releasing it directly to the plant (R. Shibata, personal communication). Thus, the plant transport system will need to be adaptable and capable of P uptake over a range of P concentrations. Such requirements may explain the existence of multiple AM-inducible transporters in some plant species (see below). Indeed, these transporters may have evolved from others involved in scavenging P from the plant apoplast during plant maturation and senescence. It will be interesting to see whether these transporters differ in their uptake capacities and whether their expression is differentially regulated by different AM fungi or at different times during development of the symbiosis.
Multiple AM-inducible Pi transporters have been identified in the Solanaceae and in Oryza sativa (Nagy et al. 2005; Glassop et al. 2007). The targeted expression analyses of Balestrini et al. (2007) in S. lycopersicum demonstrated expression of the AM-inducible LePT3 and AM-specific LePT4 transporters exclusively in arbuscule-containing cortical cells. The AM-inducible LePT5 was also expressed in non-colonised cells of AM roots and was assumed to be associated with the presence of intercellular hyphae. Nagy et al. (2005) used a transposon insertion mutant to investigate the mycorrhizal phenotype of S. lycopersicum mutants with loss-of-function of LePT4. Mycorrhizal colonisation and 33P transfer via the mycorrhizal pathway were unaffected in lept4–1 mutants, suggesting that LePT3 and LePT5 are able to compensate for loss of function of LePT4 and indicating functional overlap amongst these transporters. This is in stark contrast to recent reports of mutants from the Fabaceae, in which only a single AM-inducible transporter has been identified to date. Partial RNAi knockdown of the LjPT3 transporter of Lotus reduced both growth response and colonisation levels of mutant plants compared with vector control plants (Maeda et al. 2006), whereas in Medicago complete silencing of MtPT4 using RNAi resulted in total inhibition of the positive growth and P response usually observed for this highly responsive species (Javot et al. 2007a). Detailed observation of colonisation patterns in the MtPT4 RNAi mutant and an MtPT4 loss-of-function mutant revealed the premature collapse and senescence of arbuscules (Javot et al. 2007a). Both LjPT3 and MtPT4 appear to be crucial for transfer of P to the plant via mycorrhizal interfaces. In addition, the data of Javot et al. (2007a) suggest that fungal P supply to the plant is essential for maintenance of a compatible AM interaction. These authors hypothesised that insufficient C transfer to the fungus may be responsible for arbuscule senescence; this was supported by the observation that fungal hyphae did not proliferate outside the root of the mtpt4–1 mutant. The suggestion that P and C transfer may be intrinsically linked has been invoked by a number of authors (Woolhouse 1975; Fitter 2006). However, such linkage does not account for variations in P transfer via the AM pathway (Table 7.1), and hence with the notion that some AM fungi ‘cheat’ their hosts by acquiring C without donating P (Johnson et al. 1997; Kiers and van der Heijden 2006).
These data suggest that symbiotic function may be more tightly controlled in some plant families than in others. It will be interesting to determine whether double or triple knockout mutants of the AM-inducible Pi transporters in members of the Solanaceae display the same reduced mycorrhization phenotype as members of the Fabaceae. It is clear that, in the Fabaceae at least, AM-inducible Pi transporters play a crucial role in the integration of plant and fungal processes which lead to a compatible AM interaction. The mechanisms of this control remain to be elucidated.
7.5.2 7.5.2 P Uptake via the Direct Pathway in an AM Plant
The high affinity Pi transporters involved in Pi uptake at the root epidermis are down-regulated at high external P and responsive to the P status of the plant (Liu et al. 1998a; Rausch and Bucher 2002). Down-regulation of these transporters has also been observed during colonisation by AM fungi. In non-responsive O. sativa, six of the ten Pi transporters expressed in roots were down-regulated by AM colonisation at low P supply (Paszkowski et al. 2002). In responsive M. truncatula, a steady decline in expression of the epidermal transporters MtPT1 and MtPT2 was observed with increasing AM colonisation (Liu et al. 1998b), and MtPT1 protein levels mirrored transcript levels in this response (Chiou et al. 2001). Such concomitant changes in transcript and protein abundance provide evidence for transcriptional control of Pi transporter regulation.
It has been suggested that the down-regulation of epidermal Pi transporters in roots upon AM colonisation is primarily a function of improved P status of the plant (Burleigh and Bechmann 2002). However, few gene expression studies have included the physiological measurements that are necessary to provide further insight into this phenomenon. In a more extensive study of M. truncatula colonised by seven AM fungi, down-regulation of MtPT2 varied depending on AM fungal species (Burleigh et al. 2002). In this experiment, a low level correlation was observed between shoot P concentration in AM plants and MtPT2 expression. In H. vulgare, down-regulation of the root epidermal Pi transporters HvPT1, HvPT2 and HvPT3 was observed in conjunction with increases in tissue P content resulting from P fertilisation (Glassop et al. 2005). Expression of HvPT1 and HvPT2 was also lower in roots of AM than NM plants grown at low P, despite similar shoot and root P concentrations, whereas HvPT3 transcript levels remained quite high in AM roots. In contrast to data from responsive M. truncatula, these results suggest an AM-specific signalling pathway involved in the down-regulation of epidermal Pi transporters that is independent of the P response pathways in the plant.
Down-regulation of epidermal Pi transporters in AM roots provides a significant link with 32P uptake data indicating that the direct uptake pathway is switched off (at least partially) in some AM symbioses. A number of studies have begun to investigate this further. Colonisation of H. vulgare by G. intraradices resulted in reduced growth and P uptake relative to NM controls (Grace et al. 2008). However, plants were extensively colonised and the AM pathway contributed 60% of shoot P (Table 7.2). In this experiment, the root epidermal Pi transporters were not down-regulated (as had been previously reported; Glassop et al. 2005) and the decrease in contribution of the direct pathway could not be correlated with changes in their expression. Poulsen et al. (2005) reached a similar conclusion using S. lycopersicum, although in that case AM colonisation resulted in increases in plant growth and P uptake (Table 7.2). Interestingly, in the interaction between S. lycopersicum and G. intraradices BEG 87, the AM pathway accounted for only 20% of plant P uptake, indicating that the MPR of 116% was due to an increase in P uptake via the direct pathway. However, there was no clear correlation between the contribution of the direct uptake pathway and changes in expression of the epidermal Pi transporters. Although transcriptional regulation has been identified as an important primary control point for plant Pi transport, recent advances suggest that both post-transcriptional and post-translational modification may also be involved (Bucher 2007; see Bucher 2007 and references there in). The role of these processes in determining P fluxes by both the direct and AM uptake pathways remains to be determined.
A confounding factor in gene expression studies is the use of whole root samples for analysis of changes in gene expression. AM colonisation is non-synchronous: the plant root system is patchily colonised and colonisation units vary in age and stage of development. Sampling whole roots may mask cell-specific changes in transcript accumulation or changes in the localisation of gene expression. A number of studies have reported that expression of StPT1 and StPT2 of Solanum tuberosum is not altered in AM roots (Karandashov et al. 2004; Nagy et al. 2005). However, using a split-root system, Rausch et al. (2001) demonstrated localised down-regulation of these genes in the colonised half of the root system only. These observations, together with those of Gordon-Weeks et al. (2003) demonstrating differential expression of StPT1 and StPT2 during root development, highlight the need for targeted sampling in gene expression studies. New technologies, such as laser micro-dissection, will be critical in furthering our understanding of the role that P transporter expression plays in governing P fluxes via the plant and AM pathways. If AM fungi have differential ability to directly regulate the expression of plant Pi transporters, this may be pivotal to understanding the observed diversity in plant responses to colonisation.
7.6 7.6 Conclusions
P uptake in AM plants results from the combined operation of the direct root uptake pathway and the AM pathway. In non-responsive plants, it has been assumed that the AM pathway is non-functional and that growth depressions result from C supply to an AM fungus that confers little benefit to plant P uptake. However, it is clear from experiments utilising radiotracers that the AM pathway is not only functional in many non-responsive associations, but in some instances can take over from the direct uptake pathway in P supply to the plant. In addition, the notion that growth depressions result from AM fungal C drain is not entirely upheld. Growth depressions in non-responsive species are observed even at very low levels of AM colonisation when the C demand of the AM fungal symbiont is likely to be quite low. It has been suggested (Li et al. 2008) that this is a result of the epidermal P uptake switching off in response to fungus–plant recognition, even though P flux through the AM pathway is small. However, the down-regulation of epidermal Pi transporters is not consistently observed in AM roots. Whether this relates to methodological difficulties or real differences in plant and fungal control of gene expression remains to be determined. Improving our understanding of the signal pathways will be critical.
Non-responsive AM plants include widely cultivated crop species. We propose that non-responsive species present the greatest potential for increasing productivity and/or yield with AM symbioses, whether by selective manipulation of growth conditions or engineered traits which increase their reliance on and responsiveness to AM. The challenge now is to discover the processes, conditions, signals or mechanisms involved in these complex interactions and to apply them to future breeding efforts. The question of why the direct and AM pathways of P uptake are not additive in non-responsive plants should be a key research focus. If the epidermal pathway is not switched off when the AM pathway is operating in a non-responsive species, we hypothesise that a positive MGR would result and agricultural benefits follow. Exploring this issue should be a focus for future research. Detailed quantitative analyses of gene expression and transporter regulation, coupled with thorough physiological measurements, will be crucial in improving our understanding of these processes and will play an important role in guiding future efforts to improve the efficiency and utilisation of P by plant systems.
References
Balestrini R, Gomez-Ariza J, Lanfranco L, Bonfante P (2007) Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol Plant Microbe Interact 20:1055–1062
Baon JB, Smith SE, Alston AM (1993) Mycorrhizal responses of barley cultivars differing in P-efficiency. Plant Soil 157:97–105
Benedetto A, Magurno F, Bonfante P, Lanfranco L (2005) Expression profiles of a phosphate transporter gene (GmosPT. ) from the endomycorrhizal fungus Glomus mosseae Mycorrhiza 15:620–627
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Phys 24:225–252
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173:11–26
Bürkert B, Robson A (1994) 65. Zn uptake in subterranean clover (Trifolium subterraneum L.) by three vesicular-arbuscular mycorrhizal fungi in a root-free sandy soil Soil Biol Biochem 26:1117–1124
Burleigh SH, Bechmann IE (2002) Plant nutrient transporter regulation in arbuscular mycorrhizas. Plant Soil 244:247–251
Burleigh SH, Cavagnaro TR, Jakobsen I (2002) Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J Exp Bot 53:1593–1601
Chiou T-J, Liu H, Harrison MJ (2001) The spatial expression patterns of a phosphate transporter (MtPT1) from Medicago truncatula. indicate a role in phosphate transport at the root/soil interface Plant J 25:281–293
Dickson S, Kolesik P (1999) Visualisation of mycorrhizal fungal structures and quantification of their surface area and volume using laser scanning confocal microscopy. Mycorrhiza 9:205–213
Ezawa T, Smith SE, Smith FA (2002) P metabolism and transport in AM fungi. Plant Soil 244:221–230
Fitter AH (2006) What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol 172:3–6
Gianinazzi-Pearson V, Arnould C, Oufattole M, Arango M, Gianinazzi S (2000) Differential activation of H+. -ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco Planta 211:609–613
Glassop D, Smith SE, Smith F (2005) Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots. Planta 222:688–698
Glassop D, Godwin RM, Smith SE, Smith FW (2007) Rice phosphate transporters associated with phosphate uptake in rice roots colonised with arbuscular mycorrhizal fungi. Can J Bot 85:644–651
Gordon-Weeks R, Tong YP, Davies TGE, Leggewie G (2003) Restricted spatial expression of a high-affinity phosphate transporter in potato roots. J Cell Sci 116:3135–3144
Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE (2008) Arbuscular Mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonisation, fungal puptake or effects on expression of plant phosphate transporter genes. New Phytologist, in press.
Harrison MJ, van Buuren ML (1995) A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378:626–632
Harrison MJ, Dewbre GR, Liu JY (2002) A phosphate transporter from Medicago truncatula. involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi Plant Cell 14:2413–2429
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hetrick BAD, Wilson GWT, Schwab AP (1994) Mycorrhizal activity in warm-season and cool-season grasses: variation in nutrient-uptake strategies. Can J Bot 72:1002–1008
Hetrick BAD, Wilson GWT, Todd TC (1996) Mycorrhizal response in wheat cultivars: relationship to phosphorus. Can J Bot 74:19–25
Jakobsen I (1995) Transport of phosphorus and carbon in VA mycorrhizas. Varma A, Hock B Mycorrhiza; structure, function, molecular biology and biotechnology. Springer, Berlin 297–324
Jakobsen I, Rosendahl L (1990) Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol 115:77–83
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum. L. 2. Hyphal transport of 32P over defined distances New Phytol 120:509–516
Jakobsen I, Chen B, Munkvold L, Lundsgaard T, Zhu YG (2005) Contrasting phosphate acquisition strategies of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant Cell Environ 28:928–938
Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007a) A Medicago truncatula. phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis Proc Natl Acad Sci USA 104:1720–1725
Javot H, Pumplin N, Harrison MJ (2007b) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant Cell Environ 30:310–322
Jifon JL, Graham JH, Drouillard DL, Syvertsen JP (2002) Growth depression of mycorrhizal citrus seedlings grown at high phosphorus supply is mitigated by elevated CO2. New Phytol 153:133–142
Johansen A, Jakobsen I, Jensen ES (1993) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum. L. 3. Hyphal transport of 32P and 15N New Phytol 124:61–68
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586
Karandashov V, Bucher M (2005) Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci 10:22–29
Karandashov V, Nagy R, Wegmuller S, Amrhein N, Bucher M (2004) Evolutionary conservation of a phosphate transporter in the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 101:6285–6290
Kiers ET, van der Heijden MGA (2006) Mutualistic stability in the arbuscular mycorrhizal symbiosis: exploring hypotheses of evolutionary cooperation. Ecology 87:1627–1636
Li HY, Smith SE, Holloway RE, Zhu YG, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses. New Phytol 172:536–543
Li HY, Smith FA, Dickson S, Holloway RE, Smith SE (2008) Plant growth depressions in arbuscular mycorrhizal symbioses: not just caused by carbon drain? New Phytol 178:852–862
Li X-L, Marschner H, George E (1991) Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 136:49–57
Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG (1998a) Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol 116:91–99
Liu H, Trieu AT, Blaylock LA, Harrison MJ (1998b) Cloning and characterization of two phosphate transporters from Medicago truncatula. roots: regulation in response to phosphate and response to colonization by arbuscular mycorrhizal (AM) fungi Mol Plant Microbe Interact 11:14–22
Maeda D, Ashida K, Iguchi K, Chechetka SA, Hijikata A, Okusako Y, Deguchi Y, Izui K, Hata S (2006) Knockdown of an arbuscular mycorrhiza-inducible phosphate transporter gene of Lotus japonicus. suppresses mutualistic symbiosis Plant Cell Physiol 47:807–817
Maldonado-Mendoza IE, Dewbre GR, Harrison MJ (2001) A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices. is regulated in response to phosphate in the environment Mol Plant Microbe Interact 14:1140–1148
Mimura T (1999) Regulation of phosphate transport and homeostasis in plant cells. Int Rev Cytol 191:149–200
Munkvold L, Kjoller R, Vestberg M, Rosendahl S, Jakobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364
Nagy R, Karandashov V, Chague V, Kalinkevich K, Tamasloukht MB, Xu G, Jakobsen I, Levy AA, Amrhein N, Bucher M (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum. and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species Plant J 42:236–250
Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci USA 99:13324–13329
Pearson JN, Jakobsen I (1993) The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants measured by dual labelling with 32. P and 33P New Phytol 124:489–494
Poulsen KH, Nagy R, Gao L-L, Smith SE, Bucher M, Smith FA, Jakobsen I (2005) Physiological and molecular evidence for Pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytol 168:445–454
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37
Rausch C, Daram P, Brunner S, Jansa J, Lalol M, Leggewie G, Amrhein N, Bucher M (2001) A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 414:462–466
Ravnskov S, Jakobsen I (1995) Functional compatibility in arbuscular mycorrhizas measured as hyphal P transport to the plant. New Phytol 129:611–618
Smith FA, Jakobsen I, Smith SE (2000) Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytol 147:357–366
Smith FW, Mudge SR, Rae AL, Glassop D (2003a) Phosphate transport in plants. Plant Soil 248:71–83
Smith SE, Read DJ (1997) Mycorrhizal Symbiosis. Academic, Cambridge,
Smith SE, Dickson S, Morris C, Smith FA (1994) Transfer of phosphate from fungus to plant in VA mycorrhizas: calculation of the area of symbiotic interface and of fluxes of P from two different fungi to Allium porrum. L New Phytol 127:93–99
Smith SE, Dickson S, Smith FA (2001) Nutrient transfer in arbuscular mycorrhizas: how are fungal and plant processes integrated? Aust J Plant Physiol 28:683–694
Smith SE, Smith FA, Jakobsen I (2003b) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
Tawaraya K (2003) Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci Plant Nutr 49:655–668
Tobar R, Azcón R, Barea JM (1994) Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol 126:119–122
Woolhouse H (1975) Membrane structure and transport problems considered in relation to phosphorus and carbohydrate movement and the regulation of the endotrophic mycorrhizal associations. Sanders F, Mosse B, Tinker P Endomycorrhizas. Academic, London 209–223
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Zhu YG, Smith FA, Smith SE (2003) Phosphorus efficiencies and responses of barley (Hordeum vulgare. L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil Mycorrhiza 13:93–100
Acknowledgments
We thank Mark Tester and Olivier Cotsaftis for supervision of the project, helpful discussions and ongoing support. Emily Grace is grateful for a Commonwealth Hill postgraduate research scholarship and to the Australian Centre for Plant Functional Genomics for research support and infrastructure. Part of our research program is also supported by the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Grace, E.J., Smith, F.A., Smith, S.E. (2009). Deciphering the Arbuscular Mycorrhizal Pathway of P Uptake in Non-responsive Plant Species. In: Azcón-Aguilar, C., Barea, J., Gianinazzi, S., Gianinazzi-Pearson, V. (eds) Mycorrhizas - Functional Processes and Ecological Impact. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-87978-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-540-87978-7_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-87977-0
Online ISBN: 978-3-540-87978-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)