Abstract
The assessment of circulatory failure and shock constitutes the most frequent indication for performing an echocardiographic study in the intensive care unit. In addition to its diagnostic capabilities, echocardiography allows a close monitoring of the effects of the applied therapies.
When a patient presents hypotension or signs of hypoperfusion, the role of echocardiography is to determine the main cause and suggest therapies that could improve the hemodynamic situation. Diagnostic algorithms are proposed. As cardiovascular alterations are rarely pure (e.g., septic shock in a patient with cardiac disease), we have to create a hierarchy among the potential causes, differentiating the main factor from other contributing factors. The clinical relevance of fortuitous echocardiographic diagnoses (e.g., underlying chronic cardiomyopathy or valvulopathy) needs to be addressed.Whether this abnormality is severe enough to explain the hemodynamic pattern of the patient and is compatible with the clinical presentation may be challenging to determine. Regarding therapies, the order of the interventions has to be prioritized, when considering we have to ask ourselves the following questions: is this abnormality severe enough to explain the hemodynamic pattern of the patient, and is it compatible with the clinical scenario?
Regarding therapies, we also have to prioritize the order of the interventions, always considering the clinical scenario (e.g., in a septic patient with impaired cardiac function and preload responsiveness, fluids should be prioritized unless the patient also suffers from severe respiratory distress). More importantly, echocardiographic evaluation should never be performed as a single shot: it is best used when it also evaluates the effects of the selected interventions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Right Ventricular
- Left Ventricular Systolic Dysfunction
- Circulatory Failure
- Massive Pulmonary Embolism
- Predict Fluid Responsiveness
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Circulatory failure encompasses a broad range of clinical scenarios, primarily involving the occurrence of shock in critically ill patients with frequently associated comorbidities. The primary goal of acute therapeutic management is to restore rapidly an adequate tissue perfusion and oxygen delivery in order to meet the patient’s metabolic demand. If shock persists after initial resuscitation, a comprehensive evaluation of hemodynamic status is crucial in identifying the prime mechanism of circulatory failure and guiding acute care in patients requiring more advanced and prolonged support.
Echocardiography Doppler is an unparalleled technique that provides functional and morphological information about the heart and great vessels [1]. By providing real-time images, it directly depicts the response and tolerance to therapy, which are not accessible using other “blind” technologies [2]. The assessment of circulatory failure and shock constitutes the most frequent indication for performing an echocardiographic study in intensive care unit (ICU) settings. In addition to its diagnostic capability, echocardiography allows for a close monitoring of ultrasound-guided therapeutic changes.
The present chapter describes the use of Doppler echocardiography for the diagnosis and management of circulatory failure and presents an overview of decision algorithms. It is important to note that the algorithms proposed here are unable cover the great complexity and number of different clinical situations. Circulatory failure after cardiac surgery, severe trauma, or associated with the acute aortic syndrome is detailed in a specific section (see Part. 5).
1 Definitions
Clinical findings suggestive of shock are typically associated with the presence of hypotension together with abnormal heart rate and signs of tissue hypoperfusion, such as altered mental status, decreased urine output, skin mottling, and cold extremities [3]. Shock results from inadequate tissue perfusion and oxygen delivery with respect to organ needs, which ultimately lead to cellular dysoxia. Accordingly, shock has recently been defined by an international consensus conference as a life-threatening, generalized maldistribution of blood flow, resulting in failure to deliver and/or utilize adequate amounts of oxygen, leading to tissue dysoxia [4]. Importantly, the conference recommended that the presence of hypotension (i.e., systolic blood pressure <90 mmHg or decrease of at least 40 mmHg from baseline or mean arterial pressure <65 mmHg) was not required to define shock [4]. Although hemodynamic instability is most frequently present in the development of shock, clinical signs of tissue hypoperfusion and/or biological markers of inadequate tissue perfusion – e.g., decreased central venous oxygen saturation (ScvO2) or mixed venous oxygen saturation (SvO2), increased blood lactate, increased base deficit, and low pH – can be observed in the absence of hypotension [4].
2 Indications of echocardiography
Recent American guidelines recommend performing a transthoracic (TTE) or transesophageal echocardiography (TEE) to evaluate hypotension or hemodynamic instability of uncertain or suspected cardiac etiology with an appropriateness score [5]. The recent international consensus conference postulates that TTE can be safely considered in patients sustaining persistent shock despite aggressive initial therapy [4]. Using modern upper-end platforms, TTE has been reliably shown to exclude a cardiac source of shock in ventilated critically ill patients [6]. Nevertheless, accumulated experience in ICUs routinely using echocardiography as a first-line imaging technique for the assessment of circulatory failure suggests that TTE frequently fails to provide adequate imaging quality and unambiguous findings in mechanically ventilated patients [7, 8]. In these cases, TEE is the preferred approach [5]. When contraindications are carefully excluded, esophageal intubation is safe in critically ill patients who are mechanically ventilated [9]. TEE provides further insight into central hemodynamics, such as in examining the superior vena cava, which indirectly allows assessment of volume status (see Chap. 4).
3 Advantages and limitations of echocardiography
Several studies have shown the additional value of echocardiography in patients presenting with circulatory failure who were assessed using right-heart catheterization [10–14] or the single-indicator transpulmonary thermodilution technique (PiCCO system) [15]. This is presumably related to: (1) the limitations of the thermodilution technique for accurate measurement of cardiac output [16, 17] and identification of the failing ventricle in low-flow states [8, 15]; (2) the inability of cardiac filling pressures to predict fluid responsiveness reliably [18]. Whether echocardiography and continuous monitoring technologies should be jointly used in a multimodal approach ultimately to treat these complex patients more effectively remains to be determined (see Chap. 22).
Doppler echocardiography suffers from substantial limitations. As with every imaging modality, cardiac ultrasonography is an intermittent, yet easily repeatable, operator-dependent technique. Adequate image acquisition and interpretation of echocardiographic findings are of utmost importance to ensure diagnostic accuracy and provide a satisfactory guide to therapy in unstable ICU patients. These require a tailored training, especially when performed in the ICU environment by noncardiologists [19]. Focused, goal-oriented TTE [20] and TEE [14, 21] have proved efficient after limited training to provide adequate answers to simple, limited clinical questions. Nevertheless, the assessment of patients sustaining complex shock requires a more comprehensive hemodynamic evaluation (see Chap. 21).
4 Diagnostic algorithms
To accommodate all nuances of interpretation, the proposed algorithms should be considered within the context of the following important assumptions. First, the algorithms should be used in light of the clinical situation and are unable to cover the great complexity and number of clinical situations encountered in the ICU environment. Second, the indications of TTE and TEE are assumed to be adequate with regard to their respective diagnostic accuracy (see Chap. 1). Third, the echocardiographic study is performed and interpreted by a trained intensivist, who is aware of the adequate conditions for using this diagnostic modality, as described below.
4.1 Conditions of use
Echocardiographic results have to be incorporated within a sound medical framework, which takes into account medical history, current clinical situation, and ongoing therapies (e.g., fluid loading, vasopressor or inotropic support). In the absence of overt echocardiographic abnormalities, concordant abnormal findings or out-of-range indices should be gathered to identify more confidently the leading mechanism of shock. The clinical relevance of fortuitous echocardiographic diagnoses (e.g., underlying chronic cardiomyopathy, or valvulopathy) should carefully be examined in patients assessed for circulatory failure. Since the hemodynamic profile is influenced by both the pathological process and therapeutic interventions, the profile observed in an unstable patient may rapidly change over a short period. For example, transient left ventricular (LV) systolic dysfunction is commonly observed at the acute phase of septic shock and in many other clinical circumstances encountered in ICU patients. The diagnostic algorithms proposed below should be used in the specific clinical context of each patient to prompt diagnosis of the origin of shock and determine the appropriate therapy (Fig. 10.1).
Primary mechanisms of acute circulatory failure. Doppler echocardiography allows identifying the leading mechanism of shock and helps in guiding acute therapy. It also allows prompt diagnosis of mainstream obstruction of blood flow (e.g., cardiac tamponade, massive pulmonary embolism, dynamic left outflow tract obstruction). *: Vasopressors may be indicated in the presence of isolated RV failure. Abbreviations: SVR, systemic vascular resistance; LV, left ventricle; RV, right ventricle
4.2 General approach
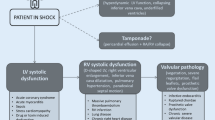
In a patient with acute circulatory failure, the first step is to determine whether cardiac output – and eventually mean blood pressure – can be significantly improved by fluid repletion, a situation commonly termed fluid or preload responsiveness (Fig. 10.2). Although overt hypovolemia can be immediately diagnosed in the presence of a severely reduced LV cavity size, the measurement of end-diastolic LV area or volume used as surrogates of ventricular preload commonly fails to predict fluid responsiveness accurately in a given patient [22]. Accordingly, “dynamic” echocardiographic parameters are better suited to evaluate preload responsiveness in ventilated patients who have frequently received variable amounts of fluid during initial resuscitation efforts (see Chaps. 6 and 7). Like other dynamic circulatory indices, respiratory variations of vena cava diameters and aortic Doppler velocities have been validated in septic patients under strict clinical conditions [18].
General algorithm illustrating the successive steps followed using echocardiography in the comprehensive assessment of an acute circulatory failure. Dashed arrows indicate second-line or rescue therapies in the absence of efficacy or poor tolerance of initial treatment. *: Cardiac tamponade is usually immediately ruled out; **: inhaled nitric oxide may be used to decrease pulmonary vascular resistance, and inotropic support may be proposed, especially if left ventricular (LV) systolic dysfunction is also present. Abbreviation: RV, right ventricle
In the absence of preload dependence, the second step is to document a potential cardiac failure (Fig. 10.2). Echocardiography allows accurate measurement of LV stroke volume using the Doppler method at the level of the aortic ring/LV outflow tract [23]. In low-flow states, cardiac ultrasonography clearly depicts which is the prominent failing ventricle (see Chap. 5).
Global LV systolic function can be readily quantified using relatively simple geometric assumptions to obtain LV ejection fraction [24] (see Chap. 8). LV systolic dysfunction may be related to a decompensated heart failure secondary to any cardiac disease, the development of an acute heart failure most frequently related to an extended/complicated myocardial infarction, or acute myocarditis; alternatively, it may be associated with septic shock regardless of its origin (Table 10.1). The presence of a significant LV remodeling (e.g., LV cavity enlargement, thin LV walls) usually reflects the presence of an underlying chronic heart disease. Regional wall-motion abnormalities are usually consistent with ischemic heart disease. Assessment of the severity of a mitral or aortic valvulopathy allows the chronic cardiac disease to be integrated into the clinical scenario. Pulmonary hypertension may be severe, especially in chronic cardiac disease. In contrast, acute heart disease is typically characterized by the presence of a normal-sized LV, with moderately elevated pulmonary artery pressures. The presence of LV regional wall-motion abnormalities is consistent with an acute myocardial infarction, an acute fulminant myocarditis, or it may reflect a severe myocardial contusion after a blunt chest trauma. LV systolic dysfunction associated with septic shock is usually characterized by an anatomically normal ventricle and low to normal filling pressures.
Right ventricular (RV) systolic function is more difficult to quantify precisely than LV systolic dysfunction (see Chap. 13). In most situations in which a failing RV is responsible for shock, its cavity is (markedly) dilated. RV sensitivity to abrupt variations in afterload may exacerbate systolic dysfunction, especially in ventilated patients with respiratory compromise and increased pulmonary vascular resistance (see Chap. 17). The presence of an associated paradoxical septal motion suggests an RV systolic overload [25]. Biventricular failure frequently requires inotropic support, whereas prominent RV systolic dysfunction may be improved by vasopressor therapy aimed at restoring an adequate level of coronary artery perfusion pressure [8]. In addition, protective ventilator settings should be considered to avoid excessive plateau pressure in ventilated patients with an overloaded RV (see Chap. 17).
When both significant hypovolemia and cardiac pump failure have been excluded by echocardiography, vasopressor therapy needs to be considered. In the absence of clinical improvement, LV systolic function should be reassessed echocardiographically. In septic patients, increasing systemic vascular resistance may reveal the underlying myocardial dysfunction, and the increase in LV afterload may be poorly tolerated [26, 27].
Finally, Doppler echocardiography allows the diagnosis of uncommon acute conditions that may result in circulatory failure (e.g., dynamic LV outflow tract obstruction, acute valvular regurgitation) and remain inaccessible to blind monitoring systems.
4.3 Acute circulatory failure with pulmonary venous congestion
Acute circulatory failure in conjunction with signs of pulmonary venous congestion (pulmonary edema) indicates the presence of a prominent LV failure. Cardiogenic shock is the most severe clinical presentation (see Chap. 12). The leading cause of cardiogenic shock is LV systolic dysfunction secondary to extended acute myocardial infarction. Hemodynamic confirmation relies on the presence of sustained hypotension with adequate or elevated LV filling pressures and reduced cardiac output [28]. Recent guidelines recommend that objective documentation of cardiac dysfunction related to acute heart failure has to be obtained by echocardiography [29]. First, two-dimensional echocardiography allows the identification of low-flow states related to severe LV systolic dysfunction. Second, Doppler examination confirms the presence of typically elevated LV filling pressures (see Chap. 16). Third, echocardiography provides exceptional information regarding the causal cardiomyopathy: LV remodeling and regional wall-motion abnormalities consistent with ischemic heart disease, mechanical complications of acute myocardial infarction (see Chap. 12), or noncoronary cardiac diseases, such as severe cardiomyopathies, extended myocardial contusion, fulminant myocarditis, or massive intoxication by cardiac depressant drugs [30, 31].
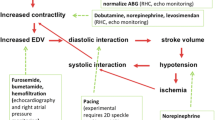
The presence of a preserved LV systolic function but elevated filling pressures suggests that an acute LV volume overload could be the origin of the circulatory failure (Fig. 10.3). The absence of dilatation of left cardiac cavities and relatively low levels of pulmonary hypertension are usually consistent with an acute condition [30]. Interestingly, echocardiography may clearly depict the cause of acute LV volume overload (e.g., acute endocarditis, ruptured papillary muscle or mitral chordae, valvular prosthesis dysfunction) and confirm its severity (see Chaps. 12 and 16). Iatrogenic volume overload is predominantly encountered in anuric patients with renal failure.
Diagnostic algorithm proposed for assessing acute circulatory failure associated with pulmonary venous congestion, i.e., elevated left ventricular (LV) filling pressures. Acute myocardial infarction is the most frequent cause of cardiogenic shock. Mechanical complications refer to LV wall or papillary muscle rupture. LV volume overload is less common and mainly related to acute valvular diseases. Abbreviation: AMI, acute myocardial infarction
4.4 Acute circulatory failure with systemic venous congestion
Acute circulatory failure in conjunction with signs of systemic venous congestion indicates the presence of a prominent RV failure, or it may reflect a mainstream obstruction of blood flow (e.g., cardiac tamponade, massive pulmonary embolism). Concomitant RV and LV systolic dysfunction associated with biventricular dilatation is usually consistent with a long-standing cardiomyopathy [32]. Patients with right-heart failure typically present with acute-onset dyspnea at rest, physical signs of peripheral congestion, and clear lung fields [29]. In this clinical setting, echocardiography helps identify the severity and origin of RV failure and promptly allows an alternative diagnosis of cardiac tamponade to be made (see Chap. 14).
In a hypotensive patient who presents with an acute coronary syndrome, a dilated RV with marked systolic dysfunction, but no relevant pulmonary hypertension, may be attributed to an RV infarction (Fig. 10.4). RV systolic pressure is not increased, as reflected by a low peak tricuspid regurgitant velocity, and associated regional wall-motion abnormality in the LV inferior wall is frequently observed [33]. In the setting of increased RV output afterload, the conjunction of significant RV dilatation and paradoxical septal motion is suggestive of acute cor pulmonale [25, 34]. RV afterloading is usually reflected by pulmonary hypertension, provided that RV systolic function can still generate a substantial systolic pressure [35]. In this clinical setting, the identification of an embolus-in-transit within right cardiac cavities or the documentation of an entrapped embolus in the proximal pulmonary artery is pathognomonic of massive pulmonary embolism (see Chap. 13). Although TEE has a greater diagnostic accuracy than TTE, it should be performed only in patients who are already ventilated since esophageal intubation may abruptly deteriorate the condition of spontaneously breathing, unstable patients. Importantly, acute cor pulmonale is consistent with massive pulmonary embolism, but it may also be observed in up to 25% of patients with acute respiratory distress syndrome [34].
Diagnostic algorithm proposed for assessing an acute circulatory failure with systemic venous congestion, i.e., elevated right ventricular (RV) filling pressures. With the exception of cardiac tamponade, the RV is typically dilated in this clinical scenario. RV failure may be precipitated by increased pulmonary vascular resistance, regardless of its origin. *: Cardiac tamponade is usually diagnosed immediately; **: provided that the RV can generate a substantial systolic pressure. Abbreviation: ARDS, acute respiratory distress syndrome
Exacerbation of chronic heart disease or chronic pulmonary hypertension can also lead to a circulatory failure with systemic venous congestion. In these patients, marked RV free-wall hypertrophy (10 mm or more) in conjunction with a dilated RV cavity is consistent with chronic cor pulmonale [25]. Severe pulmonary hypertension is usually present. Finally, RV volume overload secondary to an atrial septal defect or an organic tricuspid insufficiency (e.g., flail tricuspid leaflet, endocarditis) may contribute to RV failure.
5 Monitoring efficacy and safety of acute therapy
The therapeutic impact of echocardiography – defined as changes in therapy directly related to the procedure – is consistently high in critically ill patients assessed for acute circulatory failure [2, 7, 9]. In addition to its diagnostic capabilities, echocardiography provides real-time monitoring of both the efficacy and tolerance of therapeutic interventions derived from the noninvasive comprehensive hemodynamic assessment. Echocardiography allows precise quantification of the variations in LV stroke volume induced by a fluid challenge (Fig. 10.5) and can indirectly track variations in LV filling pressures (Fig. 10.6). When the circulatory failure persists despite the initiation of a vasopressor or inotropic support, a repeated examination may help identify the cause of treatment failure: development of LV failure secondary to increased systemic vascular resistance, dynamic LV outflow tract obstruction revealed or exacerbated by excessive inotropic stimulation, adrenergic-induced extended LV wall-motion abnormality. Echocardiography can also detect the deleterious consequences of aggressive ventilator settings in patients with severely decreased lung compliance and failing RV (see Chap. 17).
Monitoring of a fluid challenge using transesophageal echocardiography. Real-time iterative measurements of left ventricular stroke volume during volume repletion allow a close tracking of cardiac response to fluid therapy. Prior to the fluid challenge, measurement of both the left ventricular outflow tract diameter (upper left, yellow double-headed arrow) and velocity-time integral of Doppler velocity profile (lower left, yellow line) allows for determination of stroke volume. After blood volume expansion, the velocity-time integral of the aortic Doppler pattern is solely measured since the surface of the aortic annulus is considered to remain constant (right panel, yellow line). In this patient, left ventricular stroke volume increased from 38 to 65 mL after the rapid infusion of 500 mL of colloids
Assessment of the efficacy (stroke volume) and tolerance (left ventricular filling pressures) of blood volume expansion using Doppler echocardiography. With serial echocar-diographic hemodynamic assessments, one can mentally construct the systolic function curve and the diastolic pressure-volume curve of the left ventricle. In this patient, the first fluid challenge resulted in a substantial increase in left ventricular stroke volume (LVSV; 38–65 mL), whereas the second blood volume expansion was unsuccessful (65–69 mL). The mitral Doppler velocity profile grossly reflects both left ventricular diastolic properties and filling pressures; the progressive alteration from “Abnormal relaxation” to “Restriction to filling” is consistent with a gradual increase in left cardiac pressures. Abbreviations: LVEDV, left ventricular end-diastolic volume; LVEDP, left ventricular end-diastolic pressure
6 Conclusion
Echocardiography has opened a window to the heart and great vessels. In patients with acute circulatory failure, echocardiography allows swift diagnosis of the leading mechanism of shock and provides a real-time visual assessment of both the efficacy and tolerance of acute therapy. In directly depicting the effects of drugs or assistance devices, including the central circulatory effects of mechanical ventilation, echocardiography is ideally suited for assessing acute circulatory failure in critically ill patients, especially in the ICU environment.
References
Vignon P (2005) Hemodynamic assessment of critically-ill patients using echocardiography. Curr Opin Crit Care 11:227–234
Price S, Nicol E, Gobson DG, Evans TW (2006) Echocardiography in the critically ill: current and potential roles. Intensive Care Med 32:48–59
Weil MH (2005) Defining hemodynamic instability. In: Pinsky MR, Payen D (eds) Functional hemodynamic monitoring. Springer-Verlag, Berlin
Antonelli M, Levy M, Andreas PJD, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, Torres A (2007) International Consensus Conference. Hemodynamic monitoring in shock and implications for management. Intensive Care Med 33:575–590
ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR (2007) Appropriateness criteria for transthoracic and transesophageal echocardiography. J Am Coll Cardiol 50:187–204
Joseph MX, Disney PJF, Da Costa R, Hutchison SJ (2004) Transthoracic echocardiography to identify or exclude cardiac cause of shock. Chest 126:1592–1597
Vignon P, Mentec H, Terré S, Gastinne H, Guéret P, Lemaire F (1994) Diagnostic accuracy and therapeutic impact of transthoracic and transesophageal echocardiography in mechanically ventilated patients in the ICU. Chest 106:1829–1834
Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F (2003) Hemodynamic instability in sepsis. Bedside assessment by Doppler echocardiography. Am J Respir Crit Care Med 168:1270–1276
Hüttemann E, Schelenz C, Kara F, Chatzinikolaou K, Reinhart K (2004) The use and safety of transesophageal echocardiography in the general ICU – A minireview. Acta Anaesthesiol Scand 48:827–836
Reichert CLA, Visser CA, Koolen JJ, Brink RBA, Van Wezel HB, Meyne NG et al (1992) Transesophageal echocardiography in hypotensive patients after cardiac operations. Comparison with hemodynamic parameters. J Thorac Cardiovas Surg 104:321–326
Jardin F, Valtier B, Beauchet A, Dubourg O, Bourdarias JP (1994) Invasive monitoring combined with two-dimensional echocardiographic study in septic shock. Intensive Care Med 20:550–554
Kaul S, Stratienko AA, Pollock SG, Marieb MA, Keller MK, Sabia PJ (1994) Value of two-dimensional echocardiography for determining the basis of hemodynamic compromise in critically ill patients: a prospective study. J Am Soc Echocardiogr 7:598–606
Poelaert JI, Trouerbach J, De Buyzere M, Everaert J, Colardyn FA (1995) Evaluation of transesophageal echocardiography as a diagnostic and therapeutic aid in a critical care setting. Chest 107:774–779
Benjamin E, Griffin K, Leibowitz AB, Manasia A, Oropello JM, Geffroy V et al (1998) Goal-directed transesophageal echocardiography performed by intensivists to assess left ventricular function: comparison with pulmonary artery catheterization. J Cardiothorac Vasc Anesth 12:10–15
Combes A, Berneau JB, Luyt CE, Trouillet JL (2004) Estimation of left ventricular systolic function by single transpulmonary thermodilution. Intensive Care Med 30:1377–1383
Van Grondelle A, Ditchey RV, Groves BM, Wagner WW, Reeves JT (1983) Thermodilution method overestimates low cardiac output in humans. Am J Physiol 245:H690–H692
Cigarroa RG, Lange RA, Williams RH, Bedotto JB, Hillis LD (1989) Underestimation of cardiac output by thermodilution in patients with tricuspid regurgitation. Am J Med 86:417–420
Cavallaro F, Sandroni C, Antonelli M (2008) Functional hemodynamic monitoring and dynamic indices of fluid responsiveness. Minerva Anesthesiol 74:123–135
Vieillard-Baron A, Slama M, Cholley B, Janvier G, Vignon P (2008) Echocardiography in the intensive care unit: from evolution to revolution? Intensive Care Med 34:243–249
Vignon P, Dugard A, Abraham J, Belcour D, Gondran G, Pépino F, Marin B, François B, Gastinne H (2007) Focused training for goal-oriented hand-held echocardiography performed by noncardiologist residents in the intensive care unit. Intensive Care Med 33:1684–1686
Charron C, Prat G, Caille V, Belliard G, Lefèvre M, Aegerter P, Boles JM, Jardin F, Vieillard-Baron A (2007) Validation of a skills assessment scoring system for transesophageal echocardiographic monitoring of hemodynamics. Intensive Care Med 33:1712–1718
Tousignant CP, Walsh F, Mazer CD (2000) The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg 90:351–355
Zoghbi WA, Quinones MA (1986) Determination of cardiac output by Doppler echocardiography: a critical appraisal. Herz 11:258–268
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al (2006) Recommendations for chamber quantification. Eur J Echocardiogr 7:79–108
Jardin F, Dubourg O, Bourdarias JP (1997) Echocardiographic pattern of acute cor pulmonale. Chest 111:209–217
Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F (2008) Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med 36:1701–1706
Etchecopar-Chevreuil C, François B, Clavel M, Pichon N, Gastinne H, Vignon P (2008) Cardiac morphological and functional changes during early septic shock: a transesophageal echocardiographic study. Intensive Care Med 34:243–249
Reynolds HR, Hochman JS (2008) Cardiogenic shock. Current concepts and improving outcomes. Circulation 117:686–697
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, Strömberg A, Van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J 29:2388–2442
Vignon P (2008) Assessment of critically ill patients with acute heart failure syndromes using echocardiography Doppler. In: Mebazaa A, Gheorghiade M, Zannad FM, Parrillo JE (eds) Acute heart failure. Springer-Verlag, London
Charron C, Mekontso-Dessap A, Chergui K, Rabiller A, Jardin F, Vieillard-Baron A (2005) Incidence, causes and prognosis of hypotension related to meprobamate poisoning. Intensive Care Med 31:1582–1586
Vignon P, Weinert L, Mor-Avi V et al (1999) Quantitative assessment of regional right ventricular ventricular function with Color Kinesis. Am J Respir Crit Care Med 159:1949–1959
Kinch JW, Ryan TJ (1994) Right ventricular infaction. N Engl J Med 330:1211–1217
Vieillard-Baron A, Prin S, Chergui K et al (2002) Echo-Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med 166:1310–1319
Weber KT, Janicki JS, Shroff SG et al (1983) The right ventricle: physiologic and pathophysiologic considerations. Crit Care Med 11:323–328
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Berlin Heidelberg
About this chapter
Cite this chapter
Vignon, P., Slama, M. (2011). Diagnosing the Mechanisms of Circulatory Failure. In: de Backer, D., Cholley, B., Slama, M., Vieillard-Baron, A., Vignon, P. (eds) Hemodynamic Monitoring Using Echocardiography in the Critically Ill. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-87956-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-540-87956-5_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-87954-1
Online ISBN: 978-3-540-87956-5
eBook Packages: MedicineMedicine (R0)