Abstract
The afferent innervation of the urinary bladder consists primarily of small myelinated (Aδ) and unmyelinated (C-fiber) axons that respond to chemical and mechanical stimuli. Immunochemical studies indicate that bladder afferent neurons synthesize several putative neurotransmitters, including neuropeptides, glutamic acid, aspartic acid, and nitric oxide. The afferent neurons also express various types of receptors and ion channels, including transient receptor potential channels, purinergic, muscarinic, endothelin, neurotrophic factor, and estrogen receptors. Patch-clamp recordings in dissociated bladder afferent neurons and recordings of bladder afferent nerve activity have revealed that activation of many of these receptors enhances neuronal excitability. Afferent nerves can respond to chemicals present in urine as well as chemicals released in the bladder wall from nerves, smooth muscle, inflammatory cells, and epithelial cells lining the bladder lumen. Pathological conditions alter the chemical and electrical properties of bladder afferent pathways, leading to urinary urgency, increased voiding frequency, nocturia, urinary incontinence, and pain. Neurotrophic factors have been implicated in the pathophysiological mechanisms underlying the sensitization of bladder afferent nerves. Neurotoxins such as capsaicin, resiniferatoxin, and botulinum neurotoxin that target sensory nerves are useful in treating disorders of the lower urinary tract.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Anatomy and Innervation of the Lower Urinary Tract
The storage and periodic elimination of urine are dependent upon the activity of two functional units in the lower urinary tract: (1) a reservoir (the urinary bladder) and (2) an outlet consisting of the bladder neck, urethra, and striated muscles of the external urethral sphincter (EUS) (Fig. 1) (Fowler et al. 2008; Morrison et al. 2005). These structures are in turn regulated by three sets of peripheral nerves: sacral parasympathetic (pelvic nerves), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain), and somatic nerves (pudendal nerves) distributed bilaterally (Fig. 1) (de Groat 1986; Morrison et al. 2005). The nerves consist of efferent and afferent axons originating at thoracolumbar and sacral spinal levels. Parasympathetic efferent nerves contract the bladder and relax the urethra. Sympathetic efferent nerves relax the bladder and contract the urethra. Somatic efferent nerves contract the EUS.
Sympathetic, parasympathetic, and somatic innervation of the urogenital tract of the male cat. Sympathetic preganglionic pathways emerge from the lumbar spinal cord and pass to the sympathetic chain ganglia and then via the inferior splanchnic nerves (ISN) to the inferior mesenteric ganglia (IMG). Preganglionic and postganglionic sympathetic axons then travel in the hypogastric nerve to the pelvic plexus and the urogenital organs. Parasympathetic preganglionic axons which originate in the sacral spinal cord pass in the pelvic nerve to ganglion cells in the pelvic plexus and to distal ganglia in the organs. Sacral somatic pathways are contained in the pudendal nerve, which provides an innervation to the penis, the ischiocavernosus (IC), bulbocavernosus (BC), and external urethral sphincter (EUS) muscles. The pudendal and pelvic nerves also receive postganglionic axons from the caudal sympathetic chain ganglia. These three sets of nerves contain afferent axons from the lumbosacral dorsal root ganglia. U ureter, PG prostate gland, VD vas deferens
1.1 Afferent Nerves
The afferent innervation of the human lower urinary tract arises from neurons located in the dorsal root ganglia (DRG) at S2–S4 and T11–L2 spinal segmental levels. Axons in the pelvic and pudendal nerves originate in sacral DRG, whereas those in the hypogastric nerves originate in the rostral lumbar and caudal thoracic DRG. A similar segmental organization occurs in nonhuman primates, cats (Fig. 1), and dogs. In rats, pelvic and pudendal afferent neurons are located in the sacral and most caudal lumbar DRG.
The urinary bladder, which is divided into two parts, the fundus (body) and the trigone (base or neck), consists of several layers: serosal, muscularis, lamina propria, and urothelium. Afferent axons, identified primarily by neuropeptide immunoreactivity for calcitonin-gene-related peptide (CGRP), pituitary adenylate cyclase activating polypeptide (PACAP), or substance P (SP), are distributed throughout the bladder wall (Gabella and Davis 1998; Smet et al. 1997; Uemura et al. 1973) from the serosal layer to the lamina propria, including a dense suburothelial plexus that gives rise to axons extending into the urothelium (Birder et al. 2008; Fowler et al. 2008). In the cat bladder, sacral afferents are more abundant in the muscularis than in the suburothelium and have a more uniform distribution throughout the fundus and trigone regions, whereas the lumbar afferents are localized to the trigone and are more abundant in the suburothelium than in the muscularis (Uemura et al. 1975). In human and animal bladders, peptidergic afferent axons are also located around blood vessels and in close proximity to intramural ganglion cells where they may make synaptic connections and participate in local reflex networks within the bladder wall (Gillespie et al. 2006; Smet et al. 1997).
In the urethra, the afferent nerves are also distributed between the muscle fibers, around blood vessels, in the urothelium, and in a dense suburothelial plexus (Crowe et al. 1986; Fahrenkrug and Hannibal 1998; Tainio 1993). In some species afferent nerves extend to the luminal surface of the urothelium. The striated sphincter muscle that surrounds the urethra receives a very sparse afferent innervation that is localized primarily to nerve bundles passing between the muscle bundles. Specialized tension receptors (muscle spindles) which are innervated by large-diameter myelinated group IA afferents and which are prominent in most striated muscles are absent (Gosling et al. 1981) or are present in low density (Lassmann 1984) in striated sphincter muscles.
Retrograde axonal tracing methods have identified DRG cells innervating the bladder, urethra, and EUS (Fig. 2a). Afferent nerves arising in DRG on one side of the spinal cord appear to be distributed bilaterally in the bladder wall (Chai et al. 1996). Relatively small numbers, less than 3% of the total population of neurons in an individual DRG, innervate the lower urinary tract, e.g., fewer than 3,000 sacral afferent neurons innervate the bladder of the cat (de Groat 1986; Morgan et al. 1981). The neurons are small to medium-sized (mean, 32 μm × 23 μm in the cat) and are distributed randomly throughout the DRG.
(a) Experimental methods for performing patch-clamp recordings on bladder afferent neurons obtained from rats with chronic cystitis. Chronic cystitis was induced by intraperitoneal injection of cyclophosphamide. Fluorescent dye (fast blue) injected into the bladder wall was transported via Aδ- and C-fiber bladder afferent axons to neurons in the dorsal root ganglia (DRG). L6 and S1 DRG were dissected and dissociated into single neurons by enzymatic methods. Whole-cell patch-clamp recordings were then performed on fast blue-labeled bladder afferent neurons that were identified with a fluorescence microscope. (b) Characteristics of a bladder afferent neuron (24-μm diameter, C-fiber afferent neuron, top record) exhibiting tetrodotoxin (TTX)-resistant action potentials and a bladder afferent neuron (33-μm diameter, Aδ-fiber afferent neuron, bottom record) exhibiting TTX-sensitive action potentials. The left panels are voltage responses and action potentials evoked by 30-ms depolarizing current pulses injected through the patch pipette in current-clamp conditions. Asterisks with dashed lines indicate the thresholds for spike activation. The second panels on the left side show the effects of TTX application (1 μM) on action potentials. The third panels from the left show firing patterns during membrane depolarization (700-ms duration). The panels on the right show the responses to extracellular application of capsaicin (1 μM) in voltage-clamp conditions. Note that the TTX-resistant bladder afferent neuron (a) exhibited phasic firing (i.e., one to two spikes during prolonged membrane depolarization) and an inward current in response to capsaicin, while the TTX-sensitive afferent neuron exhibited tonic firing (i.e., repetitive firing during membrane depolarization) and no response to capsaicin
When different axonal tracers are injected into multiple pelvic organs, e.g., bladder and colon, a small percentage (5–15%) of DRG neurons are doubled-labeled (Christianson et al. 2007; Keast and de Groat 1992; Malykhina et al. 2006), indicating that individual sensory neurons can innervate multiple target organs. As discussed in Sect. 12, this pattern of innervation may contribute to the phenomenon of cross-sensitization of afferent pathways and provide a mechanism by which disease in one organ can influence sensations in an adjacent organ (Christianson et al. 2007; Pezzone et al. 2005).
1.2 Central Afferent Pathways
Central projections of afferent neurons innervating the lower urinary tract and the relationship between these projections and the spinal interneurons and efferent neurons have been studied by anterograde and retrograde axonal tracing methods. Parasympathetic preganglionic neurons are located in the intermediolateral gray matter (laminae V–VII) in the sacral segments of the spinal cord (de Groat et al. 1981; Morgan et al. 1993), whereas sympathetic preganglionic neurons are located in medial (lamina X) and lateral (laminae V–VII) sites in the rostral lumbar spinal cord. EUS motoneurons are located in lamina IX in Onuf's nucleus (Thor et al. 1989b; de Groat et al. 2001; Morrison et al. 2005). Parasympathetic preganglionic neurons and EUS motoneurons send dendrites to similar regions of the spinal cord (laminae I, V–VII, and X), indicating that these sites contain important pathways for coordinating bladder and sphincter function (Morgan et al. 1993).
Afferent pathways from the lower urinary tract labeled by transganglionic transport of tracers project to discrete regions of the dorsal horn that contain the soma and/or dendrites of efferent neurons innervating the lower urinary tract (Fig. 3b). Afferent pathways from the urinary bladder of the cat (Morgan et al. 1981; de Groat 1986) and rat (Jancso and Maggi 1987; Steers et al. 1991a) project into Lissauer's tract in the lumbosacral spinal cord and then pass rostrocaudally giving off collaterals that extend through lamina I laterally and medially around the dorsal horn into deeper laminae (laminae V–VII and X) at the base of the dorsal horn. The lateral pathway, which is the most prominent projection (Fig. 3b), terminates in the region of the sacral parasympathetic nucleus (SPN) and also sends some axons medially to the dorsal commissure. Bladder afferents have not been detected in the center of the dorsal horn (laminae III–IV) or in the ventral horn. Afferent axons from the pelvic viscera of the cat passing through sympathetic nerves to the rostral lumbar segments have similar sites of termination in laminae I, V–VII, and X (Morgan et al. 1986). Although afferents are distributed primarily to the ipsilateral side of the spinal cord, an estimated 10–20% also project to the opposite side of the cord (Applebaum et al. 1980; Jänig and Morrison 1986).
(a) Summary of the events involved in chronic inflammation of the bladder and hyperexcitability of C-fiber bladder afferent neurons. The events that occur following chronic bladder inflammation (1) are indicated by sequential numbers (2–7). DRG dorsal root ganglia, 5-HT serotonin, PGE prostaglandin E, NGF nerve growth factor. (b) Primary afferent pathways to the L6 spinal cord of the rat project to the dorsal commissure (DCM), the superficial dorsal horn (DH), and the sacral parasympathetic nucleus (SPN), which contains parasympathetic preganglionic neurons. The afferent nerves consist of myelinated Aδ axons, which respond to bladder distension and contraction, and unmyelinated C-fiber axons, which respond to noxious stimuli. (c) Spinal neurons that express c-fos following the activation of bladder afferents by a noxious stimulus (acetic acid) to the bladder are located in the same regions of the L6 spinal segment that receive afferent input
Pudendal nerve afferent pathways from the EUS of the cat have central terminations that overlap in part with those of bladder afferents in lateral laminae I and V–VII and in lamina X (de Groat 1986; Thor et al. 1989b). These afferents differ markedly from other populations of pudendal nerve afferents that terminate in the deeper layers of the dorsal horn (laminae II–IV). The latter innervate sex organs as well as cutaneous and subcutaneous tissues of the perineum (Ueyama et al. 1984; Thor et al. 1989b).
The spinal neurons involved in processing afferent input from the lower urinary tract have been identified by the expression of the immediate early gene c-fos (Fig. 3c). In the rat, noxious or nonnoxious stimulation of the bladder and urethra increases the levels of Fos protein primarily in the dorsal commissure, the superficial dorsal horn, and in the area of the SPN (Birder and de Groat 1993; Birder et al. 1999; Vizzard 2000a). Noxious stimulation induces c-fos expression in a greater number of spinal neurons and in a larger number of neurons in the dorsal commissure (Fig. 3c). Some of these interneurons send long projections to the brain, whereas others make local connections in the spinal cord and participate in segmental spinal reflexes (Birder et al. 1999).
2 Histological and Chemical Properties of Afferent Nerves
Light and electron microscopy has revealed that the visceral nerves innervating the lower urinary tract are composed primarily of small myelinated (Aδ) and unmyelinated (C-fiber) axons (Hulsebosch and Coggeshall 1982; Gabella and Davis 1998; Uvelius and Gabella 1998). The cat pelvic and hypogastric nerves contain axons less than 2–3 μm in diameter, with a few larger axons 5–10 μm in diameter. The rat pelvic and hypogastric nerves contain approximately 25,000 and 21,000 axons, respectively, of which 94% are unmyelinated (Hulsebosch and Coggeshall 1982). On the other hand, the pudendal nerve in the rat contains larger-diameter myelinated as well as unmyelinated axons (Hulsebosch and Coggeshall 1982). The total number of axons in these nerves is considerably larger than the number of afferent neurons in the DRG and efferent neurons in the spinal cord sending axons into the nerves. For example, the pelvic nerve of the cat has approximately 18,000 axons (Morgan et al. 1981), compared with approximately 5,000 afferent and efferent neurons projecting into the nerve (Morgan et al. 1981). This suggests that there is considerable branching of afferent axons as they pass from the DRG into the periphery (Langford and Coggeshall 1981).
DRG neurons giving rise to myelinated Aδ-fiber and unmyelinated C-fiber axons can also be distinguished by immunohistochemical staining for neurofilament protein. Neurofilament is a cytoskeletal protein that is synthesized in cell bodies and delivered to axons by axoplasmic transport. The level of neurofilament expression is known to correlate with axonal caliber and myelination. The 200-kDa neurofilament subunit is exclusively expressed in myelinated A-fiber DRG neurons, but not in unmyelinated C-fiber neurons (Lawson et al. 1993). Approximately two thirds of bladder afferent neurons in rats are neurofilament-poor (i.e., C-fiber neurons), while the remaining one third of cells exhibit intense neurofilament immunoreactivity (Aδ-fiber neurons) (Yoshimura et al. 1998). Neurofilament immunoreactivity in bladder afferent neurons negatively correlates with the sensitivity to capsaicin (Fig. 2b). Approximately 80% of neurofilament-poor C-fiber bladder afferent neurons are sensitive to capsaicin (Yoshimura et al. 1998). The predominance of neurofilament-poor, C-fiber afferent cells in the bladder afferent population is also in line with studies using conduction velocity measurement or histological analysis of the pelvic nerve which revealed that unmyelinated C-fiber bladder afferents are more numerous than myelinated Aδ-fiber afferents in bladder afferent pathways (Hulsebosch and Coggeshall 1982; Vera and Nadelhaft 1990).
In the rhesus monkey, pelvic and pudendal nerves have axons conducting at 2–31 and 34–119 ms−1, respectively, reflecting the larger fiber diameter in the pudendal nerve (Rockswold et al. 1980a, b). Bladder afferent axons in the pelvic and hypogastric nerves in the cat have conduction velocities of 1–22 and 1–16 ms−1, respectively (Winter 1971).
Afferent neurons innervating the lower urinary tract exhibit immunoreactivity for various neuropeptides, such as SP, CGRP, PACAP, leucine enkephalin, corticotropin releasing factor, and vasoactive intestinal polypeptide (VIP) (de Groat 1986, 1989; Maggi 1993; Keast and de Groat 1992; Vizzard 2001, 2006) as well as growth-associated protein 43 and, nitric oxide synthase (NOS) (Vizzard et al. 1996), glutamic acid, and aspartic acid (Keast and Stephensen 2000). These substances have been identified in many species and at one or more locations in the afferent pathways, including (1) afferent neurons in lumbosacral DRG, (2) afferent nerves in the peripheral organs, and (3) afferent axons and terminals in the lumbosacral spinal cord (Kawatani et al. 1985, 1986, 1996; Morrison et al. 2005). The majority (more than 70%) of bladder DRG neurons in rats appear to contain multiple neuropeptides, CGRP, SP, and PACAP being the most common. In cats, VIP is also contained in a large percentage of bladder DRG neurons (de Groat 1989).
Peptide-containing axons are distributed throughout all layers of the bladder but are particularly dense in the lamina propria just beneath the urothelium. In the spinal cord of rats and cats, peptidergic afferents are present in Lissauer's tract, in lamina I, where they are very prominent on the lateral edge of the dorsal horn and in the region of the parasympathetic nucleus (Kawatani et al. 1985, 1996; Vizzard 2001). This distribution is similar to that of the central projections of bladder afferent neurons labeled by axonal tracers (de Groat 1986; Steers et al. 1991a). Acute treatment with C-fiber afferent neurotoxins, capsaicin or resiniferatoxin, releases CGRP, SP, and PACAP in the bladder wall and can trigger inflammatory responses, including plasma extravasation or vasodilation (i.e., neurogenic inflammation) (Maggi 1993). Chronic treatment with these toxins reduces peptidergic afferent staining in the bladder wall of animals and humans, indicating that the majority of peptidergic bladder afferent nerves are capsaicin-sensitive C-fibers (Fowler et al. 2008).
Bladder afferent neurons and axons, especially C-fiber afferents, also express various receptors, including transient receptor potential vanilloid 1 (TRPV1, the capsaicin receptor), transient receptor potential ankyrin 1 (TRPA1), transient receptor potential cation channel subfamily M member 8 (TRPM8), a cold receptor, tropomyosin-related kinase A (TrkA), which responds to nerve growth factor (NGF), α and β estrogen receptors (Bennett et al. 2003), tropomyosin-related kinase B (TrkB), which responds to brain-derived neurotrophic factor (BDNF), glial cell line derived neurotrophic factor (GDNF) receptors, which respond to GDNF (GRFα1) and artemin (GRFα3) (Forrest and Keast 2008), isolectin B4 (IB4) binding sites, muscarinic receptors, endothelin receptors, and purinergic receptors (P2X2, P2X3 P2Y), receptors that can be activated by adenosine 5′-triphosphate (ATP) (Bennett et al. 1996; Everaerts et al. 2008; Streng et al. 2008;Vizzard and Boyle 1999; Zhong et al. 2003). Many of these receptors have been detected not only in axons in the bladder but also in the lumbosacral spinal cord in the same locations as the projections of bladder afferent axons.
C-fiber afferents innervating the lower urinary tract of the rat have been subdivided into two populations on the basis of lectin binding; i.e., IB4-negative, peptidergic and IB4-positive, nonpeptidergic subpopulations (Bennett et al. 1996; Yoshimura et al. 2003). The IB4-negative, peptidergic subgroup represents the largest population (70–80%) of C-fiber afferents. IB4 binding has also been used to identify different types of somatic C-fiber afferents (Averill et al.1995; Bennett et al. 1996). One type that does not exhibit IB4 binding is NGF-dependent, expresses TrkA receptors and contains neuropeptides (Averill et al. 1995), whereas a second type that binds IB4 is dependent on and expresses the GDNF family of growth factor receptors (GFRα) and is thought to be largely nonpeptidergic (Bennett et al. 1996). The IB4-binding somatic afferent neurons reportedly express a specific type of ATP receptor, P2X3 (Vulchanova et al. 1998; Guo et al. 1999), as well as TRPV1 receptors (Guo et al. 1999).
Bladder afferent neurons have a lower percentage of IB4-positive cells (30%) than somatic afferent neurons innervating the skin (50%) (Bennett et al. 1996). In addition, afferent neurons innervating the bladder or proximal urethra contain a smaller population of IB4-positive, nonpeptidergic C-fiber cells than somatic afferent neurons innervating the distal urethra (20% vs. 49% of C-fiber neurons) (Yoshimura et al. 2003). The smaller numbers of the IB4-positive bladder afferents is also reflected in the smaller numbers of GFRα receptor positive neurons. GRFα1 is present in 15.4%, GFRα3 in 8.4%, and GRFα2 in only 1% of lumbosacral bladder DRG neurons (Forrest and Keast 2008). The total percentage of GFRα-positive bladder neurons is similar to the percentage of IB4-positive bladder neurons.
The expression in bladder afferent nerves of multiple receptors indicates that sensory mechanisms in the bladder are likely to be complex and involve the summation of a variety of chemical and mechanical signaling mechanisms, many of which may interact to produce excitation, while others may produce the opposite effect and suppress afferent firing. It is clear that transient receptor potential channels such as TRPV1, TRPA1, and TRPM8, as well as TrkA receptors, P2X purinergic receptors, nicotinic and muscarinic receptors, and endothelin receptors when activated by intravesical administration of receptor agonists in in vivo experiments or by direct application to nerves in in vitro preparations can enhance afferent nerve activity (Fig. 3a), release afferent transmitters, or stimulate reflex bladder activity (Andrade et al. 2006; Avelino et al. 2002; Birder et al. 2001, 2002a; Chuang et al. 2001; Du et al. 2007; Lee et al. 2000; Nishiguchi et al. 2005; Ogawa et al. 2004; Pandita and Andersson 2002; Pandita et al. 2000; Rong et al. 2002; Streng et al. 2008; Studeny et al. 2005; Zhong et al. 2003). On the other hand, some putative transmitters/neuromodulators such as nitric oxide, nicotinic and musc-arinic agonists also appear to have inhibitory effects (Beckel et al. 2006; Kullmann et al. 2008b; Masuda et al. 2007; Ozawa et al. 1999; Pandita et al. 2000). The complex chemical modulation of bladder afferent activity may be related not only to the expression of multiple receptors on afferent nerves, but may also be due to effects on nonneural cells (urothelial cells and myofibroblasts) that can interact with afferent nerves via chemical messengers (Birder et al. 2008; Birder and de Groat 2007).
3 Anatomy and Putative Sensory Functions of the Urothelium
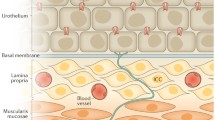
The specialized epithelial lining of the urinary tract (termed “urothelium”) which extends from the renal pelvis to the urethra is composed of at least three layers: a basal cell layer attached to a basement membrane, an intermediate layer, and a superficial apical layer with large hexagonal umbrella cells (diameters of 25–250 μm) (Birder et al. 2008). Cells in all cell layers may have direct connections to the basement membrane. Basal cells, which are thought to be precursors for other cell types, normally exhibit a low (3–6 month) turnover rate, but have an accelerated proliferation after injury (Lavelle et al. 2002).
The major function of the urothelium is to act as a barrier to block the passage of potentially noxious substances from the urine into the bladder wall. When this function is compromised during injury or inflammation, it can result in damage to the underlying tissue (neural/muscle layers), resulting in urgency, frequency, and pain during bladder distention. The superficial umbrella cells, which exhibit a number of unusual characteristics, including specialized membrane lipids, asymmetric unit membrane particles, and a plasmalemma with stiff plaques, play a prominent role in maintaining this barrier (Hu et al. 2002). The “watertight” function of the apical membrane is due in part to these specialized lipid molecules and uroplakin proteins which reduce the permeability of the urothelium to small molecules (water, urea, protons), while the tight-junction complexes reduce the movement of ions and solutes between cells.
While the urothelium has been historically viewed as primarily a “barrier,” it is becoming increasingly appreciated as a responsive structure capable of detecting physiological and chemical stimuli, and releasing a number of signaling molecules (Birder and de Groat 2007; Birder et al. 2008; de Groat 2004) (Fig. 4). Thus, urothelial cells display a number of properties similar to those of nociceptive and mechanosensitive sensory neurons and can use diverse signal-transduction mechanisms to detect physiological stimuli. The urothelium expresses “sensor molecules” (i.e., receptors/ion channels) that have been identified in afferent neurons, including receptors for bradykinin (Chopra et al. 2005), neurotrophins (TrkA and p75) (Murray et al. 2004), purines (P2X and P2Y) (Birder et al. 2004; Hu et al. 2002; Lee et al. 2000; Tempest et al. 2004), norepinephrine (α and β) (Birder et al. 1998, 2002b), acetylcholine (nicotinic and muscarinic) (Beckel et al. 2006; Chess-Williams 2002; Kullmann et al. 2008a, b), protease-activated receptors, amiloride/mechanosensitive Na+ channels (Wang et al. 2003), and a number of transient receptor potential channels (TRPV1, TRPV2, TRPV4, TRPM8) (Birder et al. 1998, 2001, 2002a,b, 2008; Stein et al. 2004; Gevaert et al. 2007). In addition, urothelial cells can release neurotransmitters and signaling molecules such as nitric oxide, ATP, acetylcholine, prostaglandins, SP, and NGF (Birder et al. 1998, 2002a, b; Ferguson et al. 1997; Yoshida et al. 2004) that influence the excitability of afferent nerves (Fig. 4). Chemicals released from urothelial cells may act directly on afferent nerves or indirectly via an action on suburothelial myofibroblasts (also referred to as “interstitial cells”) that lie in close proximity to afferent nerves. Myofibroblasts are extensively linked by gap junctions and can release chemicals that in turn act on afferent nerves (Fowler et al. 2008). Thus, it is believed that urothelial cells and myofibroblasts can participate in sensory mechanisms in the urinary tract by chemical coupling to the adjacent sensory nerves.
Receptors present in the urothelium (left side) and in sensory nerve endings in the bladder mucosa (center) and putative chemical mediators that are released by the urothelium, nerves, or smooth muscle (right side) that can modulate the excitability of sensory nerves. Urothelial cells and sensory nerves express common receptors (P2X, TRPV1, and TRPM8). Distension of the bladder activates stretch receptors and triggers the release of urothelial transmitters such as ATP, acetylcholine, and nitric oxide that may interact with adjacent nerves. Receptors in afferent nerves or the urothelium can respond to changes in pH, osmolality, high K+ concentration, chemicals in the urine, or inflammatory mediators released in the bladder wall. Neuropeptides (neurokinin A) released from sensory nerves in response to distension or chemical stimulation can act on neurokinin-2 autoreceptors to sensitize the mechanosensitive nerve endings. The smooth muscle can generate force which may influence some mucosal endings. Nerve growth factor released from muscle or urothelium can exert an acute and chronic influence on the excitability of sensory nerves via an action on TrkA receptors. ACh acetylcholine, MAChR muscarinic acetylcholine receptor, TRPV1 transient receptor potential vanilloid receptor 1 that are sensitive to capsaicin, TRPM8 menthol/cold receptor, NO nitric oxide, Trk-A tropomyosin-related kinase A receptor
4 Properties of Afferent Receptors in the Lower Urinary Tract
4.1 Sacral Afferents
The properties of small myelinated Aδ and unmyelinated C-fiber afferent axons that innervate the bladder and urethra have been studied with single-unit and multiunit recording in in vitro and in vivo preparations of various mammalian species. Aδ mechanoreceptor afferents in the pelvic nerve (Bahns et al. 1987; Downie and Armour 1992; Winter 1971; Satchell and Vaughan 1994) and sacral dorsal roots (Jänig and Morrison 1986; Häbler et al. 1993) of the cat respond to both passive distension as well as active contraction of the bladder, indicating that that they are in series tension receptors (Fig. 5). These afferents, which have conduction velocities ranging between 2.5 and 15 ms−1 (Häbler et al. 1993), are silent when the bladder is empty, but during slow filling of the bladder they display a graded increase in discharge frequency at bladder pressures above threshold, which generally is below 25 mmHg. Multiunit recordings exhibit a successive recruitment of mechanoreceptors with different thresholds during bladder filling. The maximal firing rates range from 15 to 30 Hz. All afferents behave like slowly adapting mechanoreceptors with both a dynamic and a static component of their discharge. Pressure thresholds for mechanosensitive afferents in the cat fall on the flat, compliant part of the bladder pressure-volume curve at about 25–75% of the pressure at which the curve becomes steep. These thresholds are consistent with the conditions in which humans report the first sensation of bladder filling. However, one study in cats (Downie and Armour 1992) which simultaneously measured intravesical pressure and orthogonal receptive field dimensions with a piezoelectric crystal revealed that afferent activity did not correlate with maximal bladder volume or pressure. Furthermore, activity was not linearly related to intravesical pressure, receptor field dimensions, or calculated wall tension. Thus, it was concluded that afferent receptors are also influenced by the viscoelastic properties of the bladder wall. Urethral afferents do not respond to bladder distension, but are excited by low-threshold mechanical stimulation induced by movements of a urethral catheter.
Organization of the parasympathetic excitatory reflex pathway to the detrusor muscle. The scheme is based on electrophysiological studies in cats. In animals with an intact spinal cord, micturition is initiated by a supraspinal reflex pathway passing through a center in the brainstem. The pathway is triggered by myelinated afferents (Aδ-fibers), which are connected to the tension receptors in the bladder wall. Injury to the spinal cord above the sacral segments interrupts the connections between the brain and spinal autonomic centers and initially blocks micturition. However, over a period of several weeks following cord injury, a spinal reflex mechanism emerges, which is triggered by unmyelinated vesical afferents (C-fibers); the A-fiber afferent inputs are ineffective. The C-fiber reflex pathway is usually weak or undetectable in animals with an intact nervous system. Stimulation of the C-fiber bladder afferents by instillation of ice-water into the bladder (cold stimulation) activates voiding responses in patients with spinal cord injury. Capsaicin (20–30 mg, subcutaneously) blocks the C-fiber reflex in chronic spinal cats, but does not block micturition reflexes in intact cats. Intravesical capsaicin also suppresses detrusor hyperreflexia and cold-evoked reflexes in patients with neurogenic bladder dysfunction. Glutamate is the main neurotransmitter released by Aδ and C afferent fibers at synapses in the spinal cord; C-fiber afferents additionally release neuropeptides such as substance P (SP) or vasoactive intestinal polypeptide (VIP) as neurotransmitters. In animals with an intact spinal cord, noxious stimulation can activate C-fiber afferents, which leads to a facilitation of the micturition reflex pathway
The firing of pelvic nerve Aδ afferents in the cat also reflects the size and timing of bladder contractions, and the afferent responses are larger under isovolumetric than under isotonic conditions (Jänig and Morrison 1986). When the thresholds for afferent firing were studied using small neurally evoked bladder contractions or irregular contractions that occurred spontaneously, the thresholds ranged from 5 to 15 mmHg. These pressures are within the physiological range for contractions during micturition in people with normal bladders or the pressures during involuntary detrusor contractions in people with detrusor overactivity (DO). Thus, the pelvic nerve afferents provide the central nervous system with accurate information about the size and timing of bladder contractions.
Activity of unmyelinated C-fiber bladder afferent axons recorded in the sacral dorsal roots of the cat revealed only a small population (seven of 297 units) of mechanosensitive afferents that have high intravesical pressure thresholds ranging from 30 to 50 mmHg (Häbler et al. 1990). These pressures are within the range of intravesical pressures at which humans report discomfort and pain. The afferents are silent with the bladder empty, but exhibit a graded increase in firing at pressures between 30 and 100 mmHg. Among the mechanoinsensitive “silent” C-fiber units which have a mean conduction velocity of 1.4 ms−1 approximately 10% could be activated by intravesical injection of the irritant substance mustard oil (Fig. 5). After irritation of the bladder with mustard oil or turpentine oil, some mechanoinsensitive units became mechanosensitive. Some C-fiber bladder afferents also respond to cold temperatures and intravesical administration of menthol, an agent that sensitizes TRPM8 receptors (Fall et al. 1990; Jiang et al. 2002; Lindstrom et al. 2004). C-fiber bladder afferents have also been identified in the sacral ventral roots (Clifton et al. 1976) as well as in the dorsal roots.
Bladder afferent nerves have also been studied in the rat (Dmitrieva and McMahon 1996; Mitsui et al. 2001; Moss et al. 1997; Namasivayam et al.1999; Sengupta and Gebhart 1994; Shea et al. 2000; Su et al. 1997; Vera and Nadelhaft 1990; Yu and de Groat 2008), mouse (Rong et al. 2002; Daly et al. 2007; Xu and Gebhart 2008), guinea pig (Zagorodnyuk et al. 2006, 2007), and dog. In the rat, 70% of bladder afferents in the pelvic nerve have conduction velocities in the C-fiber range and 30% in the Aδ-fiber range. The mechanoreceptive afferents are subdivided into a large population (80%) of low-threshold (6 mmHg) fibers and a smaller population (20%) of high-threshold (34 mmHg) fibers. Conduction velocity does not correlate with response threshold, each population consisting of Aδ as well as C-fiber axons. The majority of axons exhibit resting activity with the bladder empty (Dmitrieva and McMahon 1996; Sengupta and Gebhart 1994; Shea et al. 2000), show a monotonic increase in firing with graded bladder distension, and slow adaptation in response to a maintained distension. Receptive fields are located in the body, base, and at the ureterovesical junction and are punctuate or oval in shape. The majority of afferents respond to distension or contraction of the bladder and therefore have been defined as tension receptors. However, some afferents with C-fiber axons respond to distension but not to contraction and have been defined as volume receptors (Morrison 1997). Some bladder afferents that do not respond to bladder distension can be excited by intravesical application of potassium or capsaicin.
In the mouse pelvic nerve, four classes of bladder afferents (serosal, muscular, muscular/urothelial, and urothelial) have been identified on the basis of responses to receptive field stimulation with different mechanical stimuli, including probing, stretch, and stroking the urothelium. Both low-threshold, representing 65–80% of the total population, and high-threshold stretch-sensitive muscular afferents are present (Daly et al. 2007; Xu and Gebhart 2008). The muscular afferents can be sensitized by application of a combination of inflammatory mediators (bradykinin, serotonin, prostaglandin, and histamine at pH 6.0) (Xu and Gebhart 2008).
In the guinea pig bladder, four classes of afferents have also been detected (Zagorodnyuk et al. 2006, 2007). These include (1) stretch-sensitive afferents in muscle which behave as in-series tension receptors, (2) tension-mucosal mechanoreceptors which can be activated by stretch, mucosal stroking with light von Frey hairs, or hypertonic solutions applied locally to the receptive fields in the mucosa, (3) stretch-insensitive afferents consisting of mucosal mechanoreceptors and chemoreceptors, and (4) muscle mechanoreceptors activated by stretch but not by mucosal stroking or by hypertonic solution or capsaicin. Removal of the urothelium does not affect the stretch-induced firing.
Muscle-mucosal mechanoreceptors are activated by both stretch and mucosal stroking, by hypertonic solution, by α,β-methylene-ATP but not by capsaicin. Stroking- and stretch-induced firing is significantly reduced by removal of the urothelium. The third class of afferents, mucosal high-responding mechanoreceptors, are stretch-insensitive but can be activated by mucosal stroking, hypertonic solution, α,β-methylene-ATP, and capsaicin. Stroking-induced activity is reduced by removal of the urothelium. The fourth class of afferents, mucosal low-responding mechanoreceptors, are stretch-insensitive but can be weakly activated by mucosal stroking but not by hypertonic solution, α,β-methylene-ATP, or capsaicin. Removal of the urothelium reduces stroking-induced firing. All four populations of afferents conducted in the C-fiber range and showed class-dependent differences in spike amplitude and duration.
4.2 Lumbar Afferents
Activity of Aδ and C-fiber bladder and urethral afferent axons with conduction velocities of 3–15 ms−1 and below 2 ms−1, respectively, has been identified in the hypogastric nerves (Winter 1971; Floyd et al. 1976), lumbar splanchnic nerves (LSN), and the lumbar white rami (Bahns et al. 1986). The receptive fields of the units are either single or multiple punctuate sites on the bladder or urethral surface or associated with blood vessels in the peritoneal attachments to the bladder base. Afferents with receptive fields on or in the bladder wall respond in a graded manner to passive distension or isovolumetric contraction at intravesical pressures ranging from 10 to 70 mmHg, with threshold pressures generally below 20 mmHg. Urethral afferents exhibit either no responses to bladder stimulation or low discharge rates at higher intravesical pressures. No functional differences between the Aδ and C-fiber afferent populations in the hypogastric nerve have been reported, except that firing rates are lower in the latter group. In contrast to pelvic nerve afferents, the hypogastric afferents are often active with the bladder empty (Winter 1971; Bahns et al. 1986).
Bladder afferents in the LSN in the mouse consist of low-threshold and high-threshold subtypes with receptive fields in the serosal and mucosal layers of the bladder (Xu and Gebhart 2008). The serosal afferents are the most abundant. Virtually all of these afferents possess small (0.5-mm), punctuate receptive fields that tend to be clustered at the base of the bladder. Some of the afferents exhibit low rates of spontaneous activity. LSN afferents do not exhibit a dynamic response to probing or adaptation during a maintained force, whereas pelvic afferents in the mouse give dynamic responses at the onset of stimulation and adaptation to a maintained stimulus.
5 Electrophysiological Properties of Afferent Neurons
Functional properties of bladder afferent neurons have been extensively investigated using patch-clamp techniques combined with retrograde axonal transport of fluorescent dyes injected into the wall of the bladder or urethra to label the neurons (Dang et al. 2005, 2008; Sculptoreanu et al. 2005a, b; Yoshimura 1999; Yoshimura and de Groat 1997, 1999; Yoshimura et al. 1996, 2001a, b, 2003 ; Zhong et al. 2003) (Fig. 2a).
5.1 Passive Membrane Properties and Action Potentials
On the basis of current clamp recordings, bladder afferent neurons are divided into two populations according to the electrical characteristics of their action potentials (Yoshimura et al. 1996) (Fig. 2b). The most common population of bladder afferent neurons (greater than 70%) exhibit high-threshold, long-duration action potentials with an inflection on the repolarization phase. These neurons are small in size and have action potentials that are resistant to application of tetrodotoxin (TTX), a Na+ channel blocker (Fig. 2b). The other population of bladder afferent neurons, which is larger in size, exhibits low-threshold, short-duration action potentials that are reversibly blocked by TTX (Fig. 2b). The population of neurons with TTX-resistant spikes usually exhibits a phasic firing pattern (i.e., one to two spike generation), while the neurons with TTX-sensitive spikes have a tonic firing pattern (i.e., multiple spikes) when stimulated with long-duration depolarizing current pulses (Fig. 2b). Since the majority of bladder afferent neurons with TTX-resistant spikes are sensitive to capsaicin (Fig. 2b), TTX-resistant neurons are likely to be the origin of C-fiber afferent axons (Yoshimura and de Groat 1999). The correlation of spike characteristics with other electrical and morphological properties of the neuron such as somal size, capsaicin sensitivity, action potential threshold, and duration has also been reported by other investigators in unspecified DRG neurons (Waddell and Lawson 1990).
Another distinctive characteristic of bladder afferent neurons with TTX-resistant action potentials is the prominent effect of 4-aminopyridine (4-AP), an A-type K+ (IA) channel blocker, on the spike threshold and firing pattern (Yoshimura et al. 1996; Yoshimura and de Groat 1999). When depolarizing currents were injected into these cells, they usually exhibited a relaxation in the membrane potential at voltages (−45 to −40 mV) below the threshold for spike activation (Fig. 2b). Since application of 4-AP suppresses this membrane potential relaxation, lowers the threshold for spike activation, and switches the phasic firing pattern to tonic firing (Yoshimura et al. 1996; Yoshimura and de Groat 1999), IA currents activated by small depolarizations from resting membrane potential are likely to contribute to high thresholds for spike activation and the phasic firing pattern in these TTX-resistant neurons. This is discussed further in Sect. 5.3.
5.2 Sodium Channels
Voltage-clamp recordings of Na+ currents in bladder afferent neurons have revealed a similar correlation between cell size and sensitivity to TTX (Yoshimura et al. 1996, 2001a). Although both TTX-resistant and TTX-sensitive Na+ currents can occur in single neurons, usually one type of current predominates. TTX-resistant currents are prominent, representing more than 85% of the total Na+ current in small bladder neurons, whereas TTX-sensitive currents represent 60–100% of the total Na+ current in large bladder afferent neurons. These two types of Na+ currents exhibit different voltage-dependence. The threshold for activation of the TTX-resistant Na+ current is shifted in the depolarizing direction by approximately 15 mV relative to the threshold of the TTX-sensitive Na+ current. Steady-state activation and inactivation of TTX-resistant Na+ currents are also displaced to more depolarized levels by 10 and 30 mV, respectively, in comparison with the value for the TTX-sensitive Na+ current. Thus, these different properties of the Na+ currents likely contribute to the higher spike thresholds in C-fiber bladder afferent neurons with TTX-resistant action potentials.
Two different Na+ channel subunits (Nav1.8 and Nav1.9) are responsible for TTX-resistant Na+ currents in DRG neurons (Novakovic et al. 1998). Nav1.8 channels, which are expressed at higher levels than Nav1.9 channels in bladder DRG neurons (Black et al. 2003), are thought to have an important role in bladder nociceptive mechanisms because intrathecal administration of an Nav1.8 antisense oligodeoxynucleotide suppresses reflex bladder hyperactivity induced by bladder irritation in addition to reducing Nav1.8 expression in lumbosacral DRG neurons and TTX-resistant Na+ currents in bladder afferent neurons (Yoshimura et al. 2001a). The relatively greater contribution of the Nav1.8 channel to bladder sensory mechanisms is in line with previous findings that the two types of TTX-resistant channels are expressed in different types of C-fiber afferent neurons: (1) Nav1.8 in peptidergic, IB4-negative neurons and (2) Nav1.9 in nonpeptidergic, IB4-positive neurons.
5.3 Potassium Channels
Several types of transient IA K+ currents are expressed in sensory neurons (Gold et al. 1996). One of these IA currents exhibits slowly inactivating decay kinetics (time constant between 150 and 300 ms) that is considerably shorter than that of other fast inactivating IA currents. This slowly inactivating IA current has a half-maximal inactivation voltage that is displaced to a more positive membrane potential when compared with that of the fast inactivating IA current. The slowly inactivating IA current is selectively expressed in small capsaicin-sensitive DRG neurons that have action potentials with inflections on the repolarization phase, whereas the fast inactivating IA current is present in large-diameter DRG neurons without action potential inflections. Bladder afferent neurons exhibit a similar distribution of two types of IA current; i.e., small neurons with TTX-resistant humped spikes exhibiting slow-inactivating IA currents and large neurons with TTX-sensitive spikes exhibiting fast inactivating IA currents (Yoshimura et al.1996; Yoshimura and de Groat 1999). In bladder afferent neurons, the steady-state inactivation of slowly inactivating IA currents is displaced by approximately 20 mV in a more depolarizing direction than fast inactivating IA currents. Thus, 20% of the slow IA current is available at the resting membrane potential between −50 and −60 mV, while the fast IA current is almost completely inactivated at this membrane potential (Yoshimura et al. 1996; Yoshimura and de Groat 1999). This is inconsistent with the current clamp recordings showing that small bladder afferent neurons exhibit a 4-AP-sensitive membrane potential relaxation during depolarization. Thus, in small C-fiber bladder afferent neurons, TTX-resistant high-threshold Na+ currents and slow IA currents contribute to the high thresholds for spike activation.
SP, which is known to act on afferent terminals in the bladder to enhance afferent firing (Morrison et al. 2005), mimics the effect of 4-AP on small-diameter DRG neurons, reducing IA currents and converting phasic firing to tonic firing (Sculptoreanu and de Groat 2007). The effects of SP are blocked by neurokinin-2 anatgonists. Similar effects are elicited by neurokinin A analogs that act selectively on neurokinin-2 receptors. However, other agonists that activate neurokinin-1 or neurokinin-3 receptors do not elicit these effects. These observations raise the possibility that neurokinins which are released from afferent nerves terminals in the bladder might participate in a positive autofeedback mechanism to increase the excitability at C-fiber afferent terminals.
5.4 Calcium Channels
Voltage-sensitive Ca2+ channels are divided into high-voltage-activated (HVA) and low-voltage-activated types according to their voltage thresholds for activation. HVA channels, which are known to be involved in neurotransmitter release from nerve terminals, are further classified into L, N, P/Q, and R subtypes on the basis of electrophysiological and pharmacological properties. N and L channels are major subtypes of HVA Ca2+ channels in both types of bladder afferent neurons. However, expression of L-type Ca2+ channels is greater in C-fiber than in Aδ bladder afferent neurons, while the proportion of N-type channels is similar in the two types of neurons (Yoshimura et al. 2001b).
HVA Ca2+ channels can be modulated by neurotransmitters. Nitric coxide donors inhibit N-type Ca2+ currents in bladder afferent neurons (Yoshimura et al. 2001b), raising the possibility that nitric oxide released from the urothelium might exert an inhibitory effect on the excitability or neurotransmitter release from afferent terminals in the bladder. Neurokinin-2 receptor activation enhances L- and N-type Ca2+ channels via protein kinase C (PKC)-induced phosphorylation in rat DRG neurons (Sculptoreanu and de Groat 2003). This effect could occur in combination with the blockade of IA channels to enhance the excitability of afferent terminals in the bladder.
Low-voltage-activated T-type Ca2+ currents, which are important in controlling cell excitability, are expressed in somatic afferent neurons innervating the urethra and pelvic floor muscles, but not in visceral afferent neurons innervating the bladder or urethra (Yoshimura et al. 2003).
5.5 Purinergic Channels
ATP or α,β-methylene-ATP activates purinergic receptors and evokes persistent inward currents in a large percentage (88%) of cultured L6–S1 (LS) bladder DRG neurons of the rat (Zhong et al. 2003; Dang et al. 2008). The remaining neurons exhibit biphasic, transient, or no responses. In contrast, the majority (66%) of cutaneous afferent neurons in L3–L4 DRG exhibit transient or biphasic responses to a purinergic agonist (Zhong et al. 2003). The subtype-selective purinergic antagonist trinitrophenyl ATP (TNP-ATP), which is a 1,000-fold more potent in inhibiting P2X2/3 receptors than P2X2 receptors, inhibits the persistent currents evoked by purinergic agonists. TNP-ATP is also effective in inhibiting ATP or α,β-methylene-ATP induced facilitation of bladder afferent nerve activity in the rat (Yu and de Groat 2008) and mouse (Rong et al. 2002). Thus, it likely that heteromeric P2X2/3 receptors are primarily involved in mediating the persistent purinergic evoked currents in bladder sensory neurons. This conclusion is supported by patch-clamp studies in unidentified DRG neurons from P2X3 and P2X2/3 knockout mice showing that homomeric P2X3 receptors mediate transient currents and heteromeric P2X2/3 receptors mediate sustained currents (Cockayne et al. 2000).
Bladder afferent neurons in the thoracic and rostral lumbar (TL) DRG in rats exhibit different responses to purinergic agonists (Dang et al. 2005, 2008). Only 50% of these neurons respond to ATP or α,β-methylene-ATP. In addition, the predominant responses are transient or biphasic currents, in contrast to the persistent currents in LS bladder DRG neurons, suggesting that in TL bladder neurons homomeric P2X3 receptors rather than heteromeric P2X2/3 receptors mediate the ATP-evoked responses. ATP also produces a low-magnitude depolarization and fewer action potentials in TL than in LS bladder neurons.
5.6 Transient Receptor Potential Channels
Capsaicin stimulates TRPV1 receptors and evokes an inward current (Fig. 2b) and cobalt uptake in rat LS and TL bladder afferent neurons (Yoshimura et al. 2003; Dang et al. 2005) and in sacral bladder afferent neurons in the cat (Sculptoreanu et al. 2005a). In one study (Yoshimura et al. 2003) 70% of LS neurons responded to capsaicin, but in another study (Dang et al. 2005) more than 90% of the LS and TL neurons responded to capsaicin. The capsaicin-evoked inward currents are significantly smaller in TL than in LS neurons. More than 90% of capsaicin-sensitive LS neurons have TTX-resistant Na+ currents and action potentials. Nearly all capsaicin-responsive LS and TL neurons respond to α,β-methylene-ATP or acid solution (pH 5) (Dang et al. 2005); and 75% of LS neurons respond to all three stimuli, whereas only 48% of TL neurons respond to the three stimuli. Among the α,β-methylene-ATP sensitive neurons, 60% of LS neurons and 85% of TL neurons exhibit IB4 binding, indicating that not all P2X receptor expressing bladder afferents are IB4-positive.
5.7 Acid-Sensing Ion Channels
The response of bladder afferent neurons to acid solutions was analyzed to determine if it was related to activation of TRPV1 channels or acid-sensing ion channels (ASICs) (Dang et al. 2005). The large majority of LS (78%) and TL (86%) neurons respond to application of pH 5 solutions. The neurons exhibit (1) transient currents that can be separated into rapidly and slowly desensitizing components and (2) sustained currents. Transient currents occur in 20% of LS neurons and 11% of TL neurons. Sustained currents occur in 31% of LS neurons and 44% of TL neurons. The remaining neurons exhibit mixed currents. Capsazepine, a TRPV1 antagonist, significantly reduces the sustained currents without affecting the transient currents, indicating that the sustained currents are mediated in part by activation of TRPV1 channels. On the other hand, amiloride, a nonselective ASIC blocker, almost completely blocks the transient currents while reducing the sustained currents, indicating that ASIC channels are expressed in LS and TL bladder afferent neurons.
6 Role of Afferent Neurons in the Normal Control of the Lower Urinary Tract
Afferent nerves innervating the bladder, urethra, and EUS have different roles in the regulation of urine storage and elimination. Mechanosensitive afferents in the bladder are activated during bladder filling and transmit information to the brain about the degree of bladder distension and, in turn, the amount of urine stored in the bladder. Studies in healthy volunteers have shown that the first sensation of filling occurs when about 40% of bladder capacity is reached, but this sensation is indistinct and easily disregarded. The first desire to void is reported at approximately 60% of capacity and has been defined by the International Continence Society (ICS) standardization committee as “the feeling, during filling cystometry that would lead to the patient to pass urine at the next convenient moment, but voiding can be delayed if necessary.” At more than 90% of capacity, people report a strong desire to void, which is defined by ICS as a “persistent desire to void without fear of leakage.” On the basis of studies in animals which examined the effects of C-fiber afferent neurotoxins on voiding and studies in humans after transection of sympathetic or parasympathetic nerves, it appears that the normal sensations of bladder filling are dependent on Aδ afferents carried in the pelvic nerves to the sacral spinal cord (Fowler et al. 2008). These afferents are also essential for the generation of storage and voiding reflexes.
6.1 Sympathetic Storage Reflexes
Although the integrity of the sympathetic input to the lower urinary tract is not essential for the performance of micturition, it does contribute to the storage function of the bladder. Surgical interruption or pharmacological blockade of the sympathetic innervation can reduce urethral outflow resistance, reduce bladder capacity, and increase the frequency and amplitude of bladder contractions recorded under constant-volume conditions (Morrison et al. 2005). Sympathetic reflex activity is elicited by a sacrolumbar intersegmental spinal reflex pathway that is triggered by Aδ vesical afferent activity in the pelvic nerves (Fig. 6a). The reflex pathway is inhibited when bladder pressure is raised to the threshold for producing micturition (Fig. 6b). This inhibitory response is abolished by transection of the spinal cord at the lower thoracic level, indicating that it originates at a supraspinal site, possibly the pontine micturition center. Thus, the vesicosympathetic reflex represents a negative-feedback mechanism that allows the bladder to accommodate larger volumes.
Neural circuits controlling continence and micturition. (a) Urine storage reflexes. During the storage of urine, distension of the bladder produces low-level afferent firing in the pelvic nerve, which in turn stimulates (1) the sympathetic outflow to the bladder outlet (base and urethra) and (2) pudendal outflow to the external urethral sphincter. These responses occur by spinal reflex pathways and represent guarding reflexes, which promote continence. Sympathetic firing also inhibits detrusor muscle and modulates transmission in bladder ganglia. A region in the rostral pons (the pontine storage center) increases external urethral sphincter activity. (b) Voiding reflexes. During elimination of urine, intense bladder afferent firing activates spinobulbospinal reflex pathways passing through the pontine micturition center, which stimulate the parasympathetic outflow to the bladder and internal sphincter smooth muscle and inhibit the sympathetic and pudendal outflow to the urethral outlet. Ascending afferent input from the spinal cord may pass through relay neurons in the periaqueductal gray (PAG) before reaching the pontine micturition center
6.2 Urethral Sphincter Storage Reflexes
Motoneurons innervating the striated muscles of the EUS exhibit a tonic discharge which increases during bladder filling. This activity, which is termed the “guarding reflex” because it closes the urethral outlet and prevents urine leakage, is mediated by a reflex pathway in the lumbosacral spinal cord that is activated by low-level afferent input from the bladder traveling in the pelvic nerve (Fig. 6a). Electrical stimulation of Aδ afferents in the pelvic nerve of the cat and rat evokes reflex discharges in pudendal nerve motor axons innervating the EUS and increases in EUS EMG activity, indicating that Aδ bladder afferents initiate the guarding reflex (Chang et al. 2007; Thor et al. 1989a). Cold stimulation of the rat urinary bladder also induces tonic activity of the EUS EMG. This reflex response is abolished by pretreatment with capsaicin, indicating that it is dependent on bladder C-fiber afferent nerves.
Sphincter-to-bladder reflexes may also contribute to urine storage because afferent activity arising in the striated EUS muscles during contractions can suppress reflex bladder activity and, in turn, increase bladder capacity. Studies in cats and monkeys revealed that direct electrical stimulation of the EUS muscles or electrical stimulation of motor pathways to induce a contraction of the sphincters suppresses reflex bladder activity (McGuire et al. 1983; de Groat et al. 2001). Similar inhibitory responses are elicited by electrical stimulation of afferent axons in the pudendal nerve, some of which must arise in the sphincter muscles.
6.3 Voiding Reflexes
Micturition is mediated by activation of the sacral parasympathetic efferent pathway to the bladder and the urethra as well as reciprocal inhibition of the somatic pathway to the EUS (Fig. 6b). Studies in anesthetized and decerebrate cats and rats revealed that reflex activation of the bladder is mediated by a spinobulbospinal pathway passing through the pontine micturition center (PMC; Barrington's nucleus) at the level of the inferior colliculus (de Groat et al. 1981, 1993 ). The reflex pathway is activated by Aδ bladder afferents traveling in the pelvic nerve to the sacral spinal cord (Fig. 5). Activation of the PMC also induces reflex inhibition of the spinal EUS and sympathetic storage reflexes. Micturition is accompanied by relaxation of the urethral smooth muscle mediated by activation of a sacral parasympathetic pathway and release of an inhibitory transmitter, nitric oxide, in the urethra. This reflex is activated by Aδ bladder afferents in the pelvic nerve. During micturition the flow of urine through the urethra also activates urethral afferents which travel in the pudendal nerve to the sacral spinal cord and facilitate the excitatory bladder reflex pathways.
In some species (rat, mouse, dog), micturition is accompanied by rhythmic contractions of the EUS (EUS bursting) rather than complete EUS relaxation (Chang et al. 2007). The pulsatile urethral contractile activity is thought to enhance urine flow. The EUS bursting is activated by bladder distension and by Aδ bladder afferents in the pelvic nerve. The EUS bursting is dependent on input from the PMC in rats with an intact spinal cord, but is mediated by lumbosacral spinal pathways in chronic spinal cord transected rats (Chang et al. 2007).
7 Plasticity of Afferent Neurons Induced by Spinal Cord Injury
7.1 Emergence of a C-Fiber Afferent Micturition Reflex
Spinal-cord injury (SCI) rostral to the lumbosacral level eliminates voluntary and supraspinal control of voiding, leading initially to an areflexic bladder and complete urinary retention, followed by a slow development of automatic micturition and bladder hyperactivity mediated by spinal reflex pathways (de Groat and Yoshimura 2006). However, voiding is commonly inefficient owing to simultaneous contractions of the bladder and urethral sphincter (detrusor-sphincter dyssynergia, DSD).
Electrophysiological studies in cats have shown that the recovery of bladder function after SCI is mediated by a change in the afferent limb of the micturition reflex pathway and remodeling of synaptic connections in the spinal cord. In chronic spinal cats, unmyelinated C-fiber afferents rather than Aδ afferents initiate voiding; and the spinal micturition reflex occurs with a short central delay (15 ms), in contrast to the long central delay (60 ms) of the reflex in cats with an intact spinal cord (de Groat et al. 1981) (Fig. 5). These findings are supported by pharmacological studies showing that subcutaneous administration of capsaicin, a C-fiber neurotoxin, completely blocks reflex bladder contractions induced by bladder distension in chronic spinal cats, whereas capsaicin has no inhibitory effect on reflex bladder contractions in cats with an intact spinal cord (de Groat et al. 1990; Cheng et al. 1999). Thus, it is plausible that C-fiber bladder afferents which usually do not respond to bladder distension (i.e., silent C-fibers) (Häbler et al. 1990) become mechanosensitive and initiate automatic micturition after SCI.
Studies in humans have revealed an increased density of TRPV1 and P2X3 immunoreactivity as well as immunoreactivity to a pan-neuronal marker (protein gene product 9.5) in suburothelial nerves and increased TRPV1 immunoreactivity in the basal layer of the urothelium in patients with neurogenic detrusor overactivity (NDO) resulting from multiple sclerosis or various types of SCI (Brady et al. 2004a, b; Apostolidis et al. 2005a). Treatment of NDO patients with intravesical capsaicin or another C-fiber neurotoxin, resiniferatoxin, produces symptomatic improvement in a subpopulation of these patients and reduces the density of TRPV1, P2X3, and protein gene product 9.5 immunoreactive nerve fibers and urothelial TRPV1 immunoreactivity (Brady et al. 2004a, b). Injections into the bladder wall of botulinum neurotoxin type A, an agent that blocks the release of neurotransmitters from urothelial cells and from afferent and efferent nerves, also reduces NDO (Apostolidis and Fowler 2008; Popat et al. 2005; Schurch et al., 2005; Cruz and Dinis 2007) and reduces the density of TRPV1- and P2X3-immunoreactive nerves but does not alter TRPV1 and P2X3 staining in the urothelium (Apostolidis et al. 2005a, b, 2008). These results suggest that an abnormality of the C-fiber afferent innervation contributes to NDO.
The effect in cat spinal cord of VIP, a putative bladder C-fiber afferent transmitter (Morgan et al. 1999), is also changed after SCI. Intrathecal administration of VIP, which suppresses reflex bladder activity in cats with an intact spinal cord, enhances or unmasks reflex bladder activity in chronic SCI cats. In addition, VIP-immunoreactive C-fiber afferent projections to the sacral spinal cord expand and reorganize after SCI (Thor et al. 1986). This is evident as (1) a wider distribution of VIP-immunoreactive axons in lateral lamina I of the dorsal horn forming an almost continuous band of axons in the rostrocaudal direction in comparison with a discontinuous distribution in normal cats, (2) appearance of rostrocaudal axons in this region where they are not normally present, (3) more extensive contralateral projections to lamina I, and (4) a more extensive ipsilateral projection to lateral lamina VII which contains bladder preganglionic neurons. These observations raise the possibility that C-fiber bladder afferents sprout and contribute to the synaptic remodeling in the spinal micturition reflex pathway that occurs after SCI.
Changes in morphology and neuropeptide expression in C-fiber afferents have also been detected in the rat after SCI. The changes include (1) somal hypertrophy of bladder afferent neurons (45–50% increase in cross-sectional area) in the LS DRG (Kruse et al. 1995; Yoshimura and de Groat 1997; Yoshimura et al. 1998), (2) increase in expression of PACAP immunoreactivity in bladder DRG neurons and expansion of PACAP-immunoreactive afferent axons in the lumbosacral spinal cord (Zvarova et al. 2005), (3) expansion of CGRP-containing primary afferent fibers in the spinal cord (Weaver et al. 2006), and (4) increase in Fos protein expression in the spinal cord in response to bladder distension (Vizzard 2000a).
Six weeks after SCI in the rat, PACAP immunoreactivity markedly increases in spinal segments and DRG (L1, L2, L6, S1) involved in micturition reflexes, but no changes occur in adjacent spinal segments (L4–L5) (Zvarova et al. 2005). PACAP immunoreactivity increases in the superficial laminae (I–II) of the relevant spinal segments and in a fiber bundle extending ventrally from Lissauer's tract in lamina I along the lateral edge of the dorsal horn to the SPN in the LS spinal segments. This is the same region in which VIP immunoreactivity increases in the cat after SCI (Thor et al. 1986). After SCI, PACAP immunoreactivity increases in cells in the L1, L2, L6, and S1 DRG and the percentage of bladder afferent cells expressing PACAP immunoreactiviy also significantly increases. These studies raise the possibility that upregulation of PACAP expression in C-fiber bladder afferent neurons may contribute to urinary bladder dysfunction or reemergence of primitive voiding reflexes after SCI.
The possible role of PACAP as a transmitter in bladder afferent pathways prompted an investigation of the physiological effects of PACAP on micturition reflex pathways in the spinal cord. Intrathecal administration of PACAP-27 (Ishizuka et al. 1995) or PACAP-38 (Yoshiyama and de Groat 2008a, b) in spinal cord intact unanesthetized rats decreases bladder capacity, decreases micturition volume, and increases micturition pressure, whereas PACAP-38 (Yoshiyama and de Groat 2008a, b) reduces EUS EMG activity. Thus, in spinal cord intact rats, PACAP-38 seems to facilitate micturition by enhancing the parasympathetic excitatory pathway to the bladder and inhibiting the somatic excitatory pathway to the EUS.
The effects of PACAP-38 in chronic SCI rats are somewhat different. During continuous-infusion cystometrograms in SCI rats, intrathecal injections of PACAP-38 decrease the amplitude of bladder contractions and suppress EUS EMG activity (Yoshiyama and de Groat 2008a). This unexpected result is most reasonably attributed to the combined effect of PACAP-38 on bladder and sphincter, where the excitatory effect of PACAP-38 on bladder activity is masked by a simultaneous inhibitory effect on the EUS that in turn blocks DSD and reduces urethral outlet resistance. This would indirectly lower intravesical pressure during voiding.
The physiological role of PACAP in the control of bladder function in chronic SCI rats was examined by administering PACAP6-38, a PAC1 receptor antagonist, during continuous-infusion cystometrograms in awake rats (Zvara et al. 2006). Intrathecal administration of the antagonist reduces premicturition contractions during bladder filling and reduces maximal voiding pressure, suggesting that activation of PAC1 receptors by endogenous PACAP contributes to the micturition reflex and bladder hyperreflexia. Capsaicin also suppresses premicturition contractions in SCI rats, indicating that they are induced by C-fiber bladder afferent pathways (Cheng et al. 1999). However, voiding contractions in SCI rats still occur, presumably triggered by capsaicin-resistant Aδ-fiber afferents.
The site and mechanism of action of PACAP on spinal micturition reflex pathways has been explored using patch-clamp recording in lumbosacral parasympathetic preganglionic neurons in spinal-slice preparations (Miura et al. 2001). The experiments revealed that PACAP-38 has direct excitatory effects on the preganglionic neurons and enhances excitatory input to the neurons, suggesting that it might act at several sites in the spinal micturition reflex pathway. In parasympathetic preganglionic neurons PACAP-38 decreases the electrical threshold for triggering action potentials, increases the number of action potentials induced by depolarizing current pulses, increases input resistance, and suppresses a 4-AP-sensitive outward current. PACAP-38 also induces spontaneous firing and increases the frequency of spontaneous excitatory postsynaptic potentials in the presence of TTX. Because excitatory synaptic inputs are mediated primarily by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and N-methyl d-aspartate glutamatergic synapses, it was proposed that PACAP-38 facilitates glutamatergic excitatory synaptic input to the parasympathetic neurons in addition to directly enhancing the excitability of the neurons by blocking K+ channels. PACAP-38 could act presynaptically to enhance the firing of excitatory interneurons and enhance glutamate release from interneuronal terminals or act postsynaptically directly on parasympathetic neurons to enhance glutamatergic currents.
Chronic spinal injury in humans also causes the emergence of an unusual bladder reflex that is elicited by infusion of cold water into the bladder (the Bors Ice Water Test) (Geirsson et al. 1993, 1994). The response to cold water does not occur in normal adults but does occur in (1) infants, (2) patients with suprasacral cord lesions, (3) patients with multiple sclerosis and Parkinson's disease, and (4) elderly patients with hyperactive bladders. Studies in animals indicate that cold temperature activates TRPM8 and possibly other temperature-sensitive receptors in bladder C-fiber afferents (Fig. 5) and/or urothelial cells (Fig. 4) (Fall et al. 1990; Stein et al. 2004). Intravesical administration of capsaicin to paraplegic patients blocks the cold-induced bladder reflexes, indicating that they are mediated by C-fiber afferents in humans as well (Geirsson et al. 1995, Jiang et al. 2002; Mazieres et al. 1998). Cold stimulation of the rat bladder also induces DSD and capsaicin pretreatment prevents this response (Cheng et al. 1997). The presence of the cold reflex in infants, its disappearance with maturation of the nervous system, and its reemergence under conditions in which higher brain functions are disrupted suggests that it may reflect a primitive spinal involuntary voiding reflex activated by C-fiber afferents.
Patients with SCI at the level of T6 or higher often exhibit autonomic dysreflexia, which is characterized by arterial pressor responses induced by visceral stimuli such as bladder distension, fecal impaction, or bladder inflammation below the level of spinal cord lesion (Fam and Yalla 1988; Weaver et al. 2006). These stimuli cause excitation of sympathetic reflex pathways and induce arteriolar vasoconstriction and high blood pressure, as well as piloerection and sweating below the level of injury. Studies in rats revealed that autonomic dysreflexia is associated with sprouting of CGRP-containing afferent nerves in the spinal cord (Weaver et al. 2006).
7.2 Role of Neurotrophic Factors
NGF has been implicated as a chemical mediator of injury or disease-induced changes in C-fiber afferent nerve excitability and reflex bladder activity (Vizzard 2000b; Yoshimura 1999). The levels of neurotrophic factors, including NGF, increase in the bladder after SCI (Vizzard 2000b, 2006) and increased levels of NGF have been detected in the lumbosacral spinal cord and DRG of rats after SCI (Seki et al. 2003). It is known that NGF upregulates PACAP expression in DRG neurons and it has been demonstrated that chronic administration of NGF into the spinal cord or chronic administration of NGF into the bladder of rats induces bladder hyperactivity and increases the firing frequency of dissociated bladder afferent neurons (Lamb et al. 2004; Seki et al. 2003; Yoshimura 1999; Yoshimura et al. 2006; Zvara and Vizzard 2007). Endogenous NGF seems to contribute to the lower urinary tract dysfunction after SCI because intrathecal application of NGF antibodies, which neutralize NGF in the spinal cord, suppresses detrusor hyperreflexia and DSD in SCI rats (Seki et al. 2002, 2004 ). This treatment with NGF antibodies produced effects similar to the effect of desensitizing C-fiber afferents with capsaicin or resiniferatoxin (Cheng et al. 1999). Intrathecal administration of NGF antibodies also blocks autonomic dysreflexia induced by bladder or distal bowel distension in SCI rats (Weaver et al. 2006). Thus, NGF and its receptors in the bladder and/or the spinal cord are potential targets for new therapies to reduce voiding dysfunction after SCI.
7.3 Changes in Firing Properties of Bladder Afferent Neurons After Spinal Cord Injury
The ionic mechanisms underlying the hyperexcitability of C-fiber bladder afferents were investigated using whole-cell patch-clamp recording in bladder DRG neurons (Yoshimura and de Groat 1997). Chronic SCI in rats produced hypertrophy of dissociated bladder DRG neurons as reflected by an increase in cell diameter and cell input capacitance. This is consistent with results from histological sections of LS DRG showing that bladder afferent neurons undergo somal hypertrophy (45–50% increase in cross-sectional area) in SCI rats (Kruse et al. 1995). In addition to neuronal hypertrophy, bladder afferent neurons from chronic SCI rats increased their excitability. In contrast to neurons from spinal cord intact rats where the majority (approximately 70%) of bladder afferent neurons exhibit high-threshold TTX-resistant action potentials (Yoshimura et al. 1996) (Fig. 2b), in chronic SCI rats, 60% of bladder afferent neurons exhibit low-threshold TTX-sensitive action potentials.
7.4 Plasticity in Sodium and Potassium Channels After Spinal Cord Injury
The alteration of electrophysiological properties in bladder afferent neurons after SCI was also reflected in changes in Na+ current distribution (Yoshimura and de Groat 1997). Consistent with the increment in the proportion of neurons with TTX-sensitive spikes, the number of bladder afferent neurons which predominantly expressed TTX-sensitive Na+ currents (60–100% of total Na+ currents) also increased. The density of TTX-sensitive Na+ currents in bladder afferent neurons significantly increased from 32.1 to 80.6 pA/pF, while TTX-resistant current density decreased from 60.5 to 17.9 pA/pF following SCI. In addition, an increase in TTX-sensitive Na+ currents was detected in some bladder afferent neurons that still retained a predominance of TTX-resistant currents (more than 50% of total Na+ currents) after SCI. These data indicate that SCI induces a switch in expression of Na+ channels from TTX-resistant type to TTX-sensitive type. Since TTX-sensitive Na+ currents have a lower threshold for activation than TTX-resistant currents, it is reasonable to assume that these changes in expression of Na+ channels in bladder afferent neurons after SCI contribute to a low threshold for spike activation in these neurons.
Bladder afferent neurons with TTX-sensitive spikes in chronic SCI rats also do not exhibit membrane potential relaxation during low-intensity depolarizing current pulses. Furthermore, the voltage responses induced by current injections are not altered by application of 4-AP as noted in cells from control animals (Yoshimura et al. 1996). Therefore, it is likely that following SCI, A-type K+ channels are suppressed in parallel with an increased expression of TTX-sensitive Na+ currents, thereby increasing the excitability of C-fiber bladder afferent neurons. If the changes occurring in afferent cell bodies also occur at peripheral receptors in the bladder or in the spinal cord, these changes could contribute to the emergence of the C-fiber-mediated spinal micturition reflex following SCI.
8 Afferent Nerves and Idiopathic Detrusor Overactivity
Urgency incontinence with underlying idiopathic detrusor overactivity (IDO) is a common condition but its fundamental cause remains to be discovered. There is accumulating evidence that aberrant afferent activity plays an important role in IDO because women with IDO, like patients with NDO (see Sect. 7.1), have increased density of suburothelial afferent nerves that are immunoreactive for SP, CGRP, TRPV1, and P2X3 (Fowler et al. 2008). Moreover, intravesical administration of resiniferatoxin delays or suppresses involuntary detrusor contractions in patients with IDO (Apostolidis et al. 2005a; Fowler et al. 2008). In addition, biopsies from patients with IDO who exhibited decreased urgency symptoms after botulinum neurotoxin type A treatment also had a normalization of the density of suburothelial TRPV1- and P2X3-immunoreactive nerves (Apostolidis et al. 2005b, 2006 ). Although IDO and NDO patients respond to neurotoxins that target afferent nerves, this does not necessarily mean that a common pathophysiological mechanism underlies both conditions.
9 Afferent Nerves and Urethral Outlet Obstruction
Plasticity of bladder afferent fibers in a setting of bladder outlet obstruction (BOO) resulting from benign prostatic hyperplasia likely plays a critical role in the subsequent manifestation of clinical symptoms. Evidence obtained from ice-water cystometry, which elicits a C-fiber-dependent spinal micturition reflex, suggests considerable C-fiber upregulation in symptomatic subjects with BOO (Chai et al. 1998; Hirayama et al. 2003). Among BOO patients with a positive ice-water test, the incidence of DO is significantly greater in those who report nocturia three times or more per night than in those who reported fewer episodes (Hirayama et al. 2003).
Histological and electrophysiological confirmation of neural plasticity in bladder afferent and bladder reflex pathways was obtained using a rat model of partial BOO. A significant increase in the size of bladder afferent and postganglionic efferent neurons innervating an enlarged bladder was documented in animals following 6 weeks of partial urethral obstruction (Steers et al. 1991a). Remodeling of the spinal cord components of the micturition reflex pathway was also evident following experimental BOO. With use of axonal labeling to identify afferent axonal projections to spinal cord, it was demonstrated that bladder afferent terminals expand to cover a larger area (60% increase) in the lateral dorsal horn and in the region of the SPN in BOO rats (Steers et al. 1991a). Electrophysiological experiments revealed that a spinal micturition reflex mechanism was unmasked in rats with BOO. An immunohistochemical analysis of the distribution and density of GAP-43 showed that this protein was increased in the spinal cord in the region of the SPN in BOO rats (Steers and Tuttle 2006). Because this protein is a marker for axonal sprouting, its upregulation provides further indirect support for morphological plasticity in afferent pathways after BOO.
Subsequent experiments using the same BOO rat model revealed that hypertrophied bladder tissue contained significantly greater amounts of NGF protein than normal bladders (Steers et al. 1991b). To determine if the changes induced by BOO were due to an action of NGF, BOO was carried out in NGF-immune animals in which endogenous NGF antibody prevents access of NGF to nerves. BOO in the NGF-immune animals does not elicit hypertrophy of bladder sensory neurons, increase in afferent projections in the spinal cord, or increase GAP-43 expression in afferent pathways (Steers et al. 1996). Removal of the urethral obstruction in BOO rats causes a partial reversal of both the elevated NGF levels in the bladder and the neuronal hypertrophy; however, bladder overactivity persists in the presence of the elevated NGF levels.
The stimulus for NGF production in the bladder is due in part to urinary retention and stretch of the bladder after BOO. Stretching bladder smooth muscle cells in vitro increases messenger RNA for NGF and stimulates the secretion of NGF (Steers and Tuttle 2006). Protein synthesis inhibitors suppress the stretch-evoked secretion. NGF levels also increase in the urothelium of BOO rats. These results indicate that mechanical stretch activates cellular machinery for the production and secretion of NGF, which in turn acts on sensory nerves in the bladder to enhance afferent input to the spinal cord and enhance reflex bladder activity.
Patch-clamp recordings from bladder sensory neurons in BOO rats have explored the mechanisms underlying the changes in afferent neuron excitability. The neurons exhibit increased amplitude and altered kinetics of TTX-sensitive Na+ currents that result in lowered firing thresholds (Steers and Tuttle 2006). An experimental drug (ICMI-136) that preferentially blocks TTX-sensitive currents reduces bladder overactivity in BOO rats.
Human bladder tissue obtained from subjects undergoing suprapubic prostatectomy for outlet obstruction had more than twice the level of NGF than tissue obtained by cystoscopy from patients who were being evaluated for conditions other than obstruction (Steers et al. 1991b). Increased levels of urinary NGF have also been detected in BOO patients exhibiting overactive bladder (OAB) symptoms. Total urinary NGF levels were low in controls (0.5 pg ml−1) and in patients with BOO without OAB symptoms (1 pg ml−1), but considerably higher in patients with BOO and OAB symptoms (41 pg ml−1) or BOO and DO (50 pg ml−1) (Liu and Kuo 2008). Following successful medical treatment with a combination of an α-adrenergic blocking agent and a 5α-reductase inhibitor that reduce symptoms, the urinary NGF levels were reduced to 3.2 pg ml−1. It was concluded that urinary NGF levels can be used as a biomarker for OAB and DO and as a method for assessing successful therapies.
10 Afferent Nerves and Cystitis
Cystitis, which is an inflammatory condition of the urinary bladder that can occur as a result of infection, radiation-induced damage, irritant chemicals in the urine, or unknown causes (painful bladder syndrome, PBS/interstitial cystitis, IC), is accompanied by pain, unusual hypersensitivity to bladder distension, edema, and accumulation of large numbers of inflammatory cells in the bladder mucosa and musculature. Activation of sensory nerves (Aδ or C-fibers) by mechanical or chemical stimuli plays a key role in this condition by transmitting signals to the central nervous system to induce painful sensations and by releasing chemicals such as tachykinins that induce or enhance inflammatory mechanisms in the periphery. Because sensitization of the relatively inexcitable bladder C-fiber afferent nerves seems to play a key role in the symptoms of cystitis, considerable attention has been focused on disease-induced plasticity in these nerves.
Histological analysis of bladders from patients with PBS/IC revealed marked edema, vasodilation, proliferation of nerve fibers, and infiltration of mast cells (Johansson and Fall 1997). Chemically induced cystitis in animals using cyclophosphamide, mustard oil, turpentine oil, low-pH solutions, or acrolein, which increase urinary frequency, is initiated by sensitizing mechanosensitive afferents and/or recruitment of afferents normally unresponsive to mechanical stimulation (i.e., silent C-fibers) (Dmitrieva and McMahon 1996; Dmitrieva et al. 1997; Häbler et al. 1990; Sengupta and Gebhart 1994) (Fig. 3a). Proinflammatory agents such as prostaglandin E2, serotonin, histamine, bradykinin, and adenosine, as well as neurotrophic factors such as NGF, which are released during chemical irritation can induce bladder hyperactivity as well as functional and chemical changes in C-fiber afferents that can lead to hyperexcitability (Dmitrieva and McMahon 1996; Gold et al. 1996) (Fig. 3a). For example, chronic chemical irritation of the bladder changes ion channel function in bladder afferent neurons and also increases the expression of various markers, including NOS (Vizzard et al. 1996), GAP-43 (Vizzard and Boyle 1999), PACAP, SP (Vizzard 2001), and protease-activated receptors (Dattilio and Vizzard 2005). The density of peptidergic afferent nerves also increases in the bladder mucosa and detrusor muscle (Dickson et al. 2006) and afferent peptidergic axons and parasympathetic efferent axons/varicosities are commonly observed in close contact, suggesting that sprouting of peripheral nerves occurs during chronic cystitis.
NGF has attracted considerable attention as a key player in the link between inflammation and altered pain signaling. NGF is expressed widely in various cells, including urothelial cells, smooth muscle cells, and mast cells, and can activate mast cells to degranulate and proliferate. In patients with PBS/IC, neurotrophins, including NGF, neurotrophin-3, and GDNF, have been detected in the urine (Okragly et al. 1999). Increased expression of NGF is also present in bladder biopsies from women with IC (Lowe et al. 1997). Thus, target organ-neural interactions mediated by an increase of neurotrophins in the bladder and increased transport of neurotrophins to the neuronal cell bodies in afferent pathways may contribute to the emergence of bladder pain in PBS/IC (Yoshimura 1999).
In the cyclophoshamide-induced chronic cystitis model in rats, increased expression of neurotrophic growth factors such as NGF, BDNF and CTNF in the bladder as well as phosphorylation of tyrosine kinase receptors (TrkA, TrkB) in bladder afferent neurons has been presented as direct evidence for increased neurotrophin-mediated signaling in chronic bladder inflammation (Qiao and Vizzard 2002; Vizzard 2000b). The enhanced neurotrophic factor mechanisms are also associated with increased phosphorylated cyclic AMP response element binding protein (CREB) in bladder afferent neurons. Phosphorylated CREB, which is a transcription factor in the neurotrophin intracellular signaling pathway, is coexpressed with phophorylated TrkA in a subpopulation of bladder afferent neurons (Qiao and Vizzard 2004). Resiniferatoxin, a C-fiber neurotoxin, reduced cyclophosphamide-induced upregulation of phosphorylated CREB in DRG cells, suggesting that cystitis is linked with an altered CREB phosphorylation in capsaicin-sensitive C-fiber bladder afferents (Qiao and Vizzard 2004). These results suggest that upregulation of phosphorylated CREB may be mediated by a neurotrophin/TrkA signaling pathway, and that CREB phosphorylation may play a role as a transcription factor in lower urinary tract plasticity induced by cystitis.
Exogenous NGF can induce bladder nociceptive responses and bladder overactivity in rats when applied acutely into the bladder lumen (Chuang et al. 2001; Dmitrieva et al. 1997) or chronically to the bladder wall or intrathecal space (Lamb et al. 2004; Seki et al. 2003; Zvara and Vizzard 2007). Conversely, application of NGF-sequestering molecules (TrkA-IgG or REN1820) can reduce referred thermal hyperalgesia elicited by bladder inflammation induced by intravesically applied turpentine oil (Jaggar et al. 1999) or bladder overactivity elicited by cyclophosphamide-induced cystitis (Hu et al. 2005), suggesting that increased NGF expression is directly involved in the emergence of bladder-related nociceptive responses in cystitis. Thus, NGF-activated mechanisms might be a potential target for the treatment of painful symptoms in PBS/IC.
Purinergic mechanisms may also contribute to the bladder dysfunction following chronic inflammation. ATP release from the urothelium is enhanced in patients and cats with PBS/IC (Birder et al. 2003, 2008; Sun et al. 2001). In conscious rats with cyclophosphamide-induced cystitis, purinergic receptor antagonists (PPADS and A-317491) reduce nonvoiding contractions and decrease voiding frequency (Ito et al. 2007). In in vitro whole bladder pelvic afferent nerve preparations from rats with cyclophosphamide-induced cystitis, afferent nerve firing induced by bladder distension or by direct electrical stimulation is markedly increased in comparison with firing in normal rats (Yu and de Groat 2008). Exogenous purinergic agonists mimic the facilitatory effects of cyclophosphamide treatment; and P2X purinergic receptor antagonists suppress the effects of purinergic agonists and cystitis. These results suggest that endogenous purinergic agonists released in the inflamed bladder can enhance the excitability of bladder afferent nerves by activating P2X receptors.
Patch-clamp studies on bladder afferent neurons from rats revealed that chronic cyclophosphamide treatment increases the currents induced by purinergic agonists in both TL and LS neurons (Dang et al. 2008). Analysis of the kinetics of the currents indicated that increased receptor expression and/or properties of homomeric P2X3 in TL neurons and P2X2/3 in LS neurons contribute to the enhanced responses during cystitis.
Cystitis also induces chemical changes in the spinal cord. Acute or chronic bladder irritation increases immediate early gene expression (c-fos) in spinal neurons (Birder and de Groat 1993) (Fig. 3) as well as an increase in GFRα1 immunoreactivity in the spinal dorsal horn and in areas associated with autonomic neurons (Forrest and Keast 2008). There was a much smaller increase in GFRα3 immunoreactivity and no change in GFRα2 immunoreactivity. Changes in spinal cord mitogen-activated-protein kinases [extracellular-signal-related kinase (ERK) 1 and ERK 2] may also play a role in the facilitation of reflex voiding after bladder inflammation. Immunohistochemical studies revealed that in noninflamed rat bladders noxious but not nonnoxious stimulation significantly increased phospho-ERK immunoreactivity (Cruz et al. 2007). However, after bladder inflammation innocuous and noxious bladder distension increased the number of spinal neurons exhibiting phospho-ERK immunoreactivity. The activation was rapid within a few minutes and transient. Desensitization of vanilloid-sensitive afferents by intravesical administration of resiniferatoxin does not decrease phospho-ERK immunoreactivity in normal or inflamed bladder preparations. ERK inhibition with intrathecal injection of PD98059 decreases reflex bladder activity and spinal c-fos expression in animals with inflamed bladders but not in normal animals (Cruz et al. 2007). The results suggest that activation of spinal cord ERK contributes to acute and chronic inflammatory pain perception and mediates reflex bladder overactivity accompanying chronic bladder inflammation.
Cystitis affects not only the bladder function but also alters somatic nociceptive mechanisms. Irritation of the bladder of mice by intravesical administration of NGF reduces the threshold for producing paw withdrawal in response to mechanical stimulation of the paw (mechanical hyperalgesia). The response occurs within 4 h after application of NGF and is blocked by administration of NGF antiserum. Systemic administration of cyclophosphamide or intravesical administration of its irritant metabolite, acrolein (Lanteri-Minet et al. 1995), produces a similar effect that occurs 24–48 h after administration of the irritants, at times when the bladder exhibits edema and areas of hemorrhage. These models of chemically induced cystitis do not influence the paw withdrawal responses induced by nociceptive thermal stimuli (thermal hyperalgesia) (Wang et al. 2008). On the other hand, bacterial cystitis or intravesical administration of turpentine oil increases thermal sensitivity. These selective sensitizing effects of different forms of cystitis on somatic sensory pathways, a phenomenon that may represent a type of visceral referred pain, raise the possibility that distinct populations of peripheral or central bladder sensory pathways can be activated by different nociceptive stimuli in the bladder. It is noteworthy that humans with bladder-associated pain also have a higher incidence of other painful disorders such as fibromyalgia. It would be interesting to determine if the location or type of somatic symptoms varies with the type of bladder disorder.
Direct evidence linking chronic bladder inflammation with functional changes in C-fiber afferents has been obtained from the rat cyclophosphamide chronic cystitis model. In this model, the electrical properties of bladder afferent neurons dissociated from L6 and S1 DRG as well as the activity of the inflamed bladder were measured. The majority of bladder afferent neurons from both control and cyclophosphamide-treated rats are capsaicin-sensitive and exhibit high-threshold TTX-resistant action potentials and Na+ currents. However neurons from rats with cystitis exhibit significantly lower thresholds for spike activation (−25.4 vs. −21.4 mV) and show tonic rather than phasic firing characteristics (12.3 vs. 1.2 action potentials per 500-ms depolarization) (Yoshimura and de Groat 1999). Other significant changes in bladder afferent neurons from cyclophosphamide-treated rats include increase in somal diameter, increase in input capacitance, and decrease in density of slowly inactivating IA currents (Yoshimura and de Groat 1999). Similar somal hyperexcitability due to reduced IA current expression after chronic tissue inflammation has also been detected in afferent neurons innervating the rat stomach or the guinea pig ileum (Stewart et al. 2003). Thus, the reduction in IA current size could be a key mechanism inducing afferent hyperexcitability and pain in pelvic visceral organs, including the bladder.
A recent study using cats with naturally occurring feline-type PBS/IC has also demonstrated that capsaicin-sensitive DRG neurons exhibit an increase in cell size and increase in firing rates to depolarizing current pulses owing to a reduction in low-threshold K+ currents (Sculptoreanu et al. 2005a). Taken together, these data indicate that chronic inflammation in PBS/IC induces both cell hypertrophy and hyperexcitability of C-fiber bladder afferent neurons. If these changes in neuronal cell bodies also occur at C-fiber afferent terminals in the bladder wall, such hyperexcitability may represent an important mechanism for inducing pain in the inflamed bladder. Therefore, suppression of C-fiber activity represents a mechanism by which to treat bladder pain. This is supported by some clinical studies showing that C-fiber desensitization induced by intravesical application of capsaicin or resiniferatoxin is effective for treating painful symptoms in patients with PBS/IC (Lazzeri et al. 1996, 2000). However, a previous prospective, randomized clinical trial using intravesical resiniferatoxin application was not effective in patients with PBS/IC (Payne et al. 2005).
Although there is little information available about the neuroplasticity of Aδ-fiber bladder afferents in PBS/IC, a previous study (Roppolo et al. 2005) using single nerve fiber recordings has documented that Aδ-fiber bladder afferents in IC cats are more sensitive to bladder pressure changes than afferents in normal cats, suggesting that, in addition to neuroplasticity of C-fiber afferents, Aδ-fiber bladder afferents might also undergo functional changes in PBS/IC.
Chronic bladder inflammation can also induce changes in functional properties of chemosensitive receptors such as TRPV1 in sensory neurons. Sculptoreanu et al. (2005b) reported that DRG neurons obtained from IC cats exhibit capsaicin-induced responses that are larger in amplitude and desensitize more slowly compared with those obtained from normal cats, and that altered TRPV1 receptor activity in IC cats is reversed by an application of an inhibitor of PKC, suggesting that PBS/IC can alter TRPV1 activity owing to enhanced endogenous PKC activity. Since TRPV1 receptors are reportedly responsible at least in part for bladder overactivity elicited by cyclophosphamide-induced cystitis (Dinis et al. 2004), enhanced activity of TRPV1 receptors could contribute to bladder pain in PBS/IC.
Studies in mice have also demonstrated a role of TRPV1 in cystitis. Systemic treatment with cyclophosphamide or intravesical administration of acrolein, the irritant metabolite of cyclophosphamide, produces not only bladder hyperactivity but also a sensitization of the paw withdrawal responses to mechanical stimulation of the paw (mechanical hyperalgesia). These responses do not occur in TRPV1 knockout mice (Charrua et al. 2007; Wang et al. 2008). However, the lack of functional TRPV1 does not inhibit the development of the histological changes associated with bladder inflammation, including submucosal edema and areas of hemorrhage, and does not alter the increased expression of various markers of cystitis (messenger RNA for NGF, endothelial NOS, cyclooxygenase-2, and bradykinin receptors) in the urothelium.
The neurotoxin saporin, which is contained in the seeds of the soapwort plant, has been used to examine the roles of two types of afferent pathways in the transmission of nociceptive sensory information from the bladder to the spinal cord of the rat. After intrathecal administration of saporin conjugated with IB4 to selectively eliminate the IB4-positive C-fiber population, the bladder overactivity induced by bladder irritation is suppressed (Nishiguchi et al. 2004). Similarly when saporin conjugated to SP is administered to eliminate neurokinin-1 receptor expressing, pain-related spinal cord neurons, the bladder overactivity induced by bladder irritation is also suppressed (Seki et al. 2005). Thus, both IB4-binding, nonpeptidergic and IB4-negative, peptidergic C-fiber afferents seem to play an important role in bladder nociceptive mechanisms even though the former represents a smaller component of the total population of bladder afferent neurons.
11 Afferent Nerves and Diabetes Mellitus
Diabetes mellitus is often accompanied by urological complications characterized by impaired sensation of bladder fullness, increased bladder capacity, reduced bladder contractility, and elevated residual urine (Kebapci et al. 2007; Yoshimura et al. 2005). These changes are attributable in part to afferent neuropathy that is evident as a reduced afferent nerve conduction velocity (Nadelhaft and Vera 1992) and reduced axonal transport of neurotrophic factors, such as NGF (Steers et al. 1994; Tong and Cheng 2007; Yoshimura et al. 2005). In streptozotocin-induced diabetic rats, an increase in bladder capacity, an increase in postvoid residual volumes, and a decrease in bladder activation by intravesical administration of an irritant chemical occurs 12 weeks after injection of streptozotocin (Sasaki et al. 2002). In the same animals, NGF levels decrease in the bladder and in the lumbosacral DRG. NGF gene therapy using a replication-defective herpes simplex virus vector injected into the bladder wall reverses these changes (Sasaki et al. 2004). These data suggest that diabetic cystopathy is related to a reduced production or axonal transport of NGF in bladder nerves leading to a defect in the functions of Aδ or C-fiber bladder afferent pathways.
12 Afferent Nerves and Interorgan Cross-Sensitization
Axonal tracing studies revealed that a subpopulation (6–21%) of afferent neurons in the lumbosacral DRG of the rat and mouse can innervate multiple pelvic organs (Christianson et al. 2007; Keast and de Groat 1992). In the rat, the larger percentage of these neurons is present in the TL DRG, whereas in the mouse the larger percentage is in the LS DRG. Subsequent studies provided evidence that chemical irritation of one organ (either the urinary bladder or the colon) can facilitate/sensitize the activity of the other organ (Pezzone et al. 2005). Electrophysiological experiments showed that acute colonic irritation can sensitize bladder C-fiber afferents to mechanical and chemical stimuli (Ustinova et al. 2006) and enhance the firing of lumbosacral spinal interneurons receiving afferent input from the bladder (Qin et al. 2005). Lumbosacral afferent neurons in the L6–S2 DRG innervating both the colon and bladder (“convergent neurons”) exhibited decreased voltage and current thresholds for action potential firing 3 days after colonic irritation with trinitrobenzene sulfonic acid (Malykhina et al. 2006). The effect persisted for 30 days in the absence of overt colonic inflammation. Colitis also enhanced the responses to capsaicin and increased the peak amplitude of TTX-resistant Na+ currents in bladder afferent neurons isolated from the L6–S2 DRG (Malykhina et al. 2004).
Changes in bladder function after colonic inflammation also appear to be mediated by a change in the cholinergic efferent pathway to the bladder (Noronha et al. 2007). During the active colonic inflammation 3 days after instillation of trinitrobenzene sulfonic acid into the colon, the bladder was not inflamed, but the contractions of bladder strips induced by electrical field stimulation or carbachol, a muscarinic receptor agonist, were reduced, while the contractions induced by KCl were not changed. During and after recovery of the colonic inflammation (15–30 days) the contractile responses of the bladder returned to normal. It was suggested that the bladder dysfunction was mediated by visceral organ cross talk induced by sensitization of a subpopulation of afferents innervating both the bladder and the colon.
13 Perspectives
The afferent innervation of the lower urinary tract plays an important role in both reflex and voluntary control of voiding. Myelinated Aδ afferent nerves which are mechanosensitive and respond to bladder distension are essential for the initiation of urine storage reflexes and for signaling the brain about the level of bladder filling and the need to void. On the other hand, unmyelinated C-fiber afferent nerves seem to function primarily as nociceptors that respond to noxious chemical and mechanical stimuli. Activation of these afferents triggers painful sensations as well as body defense mechanisms such as inflammation and bladder hyperactivity that eliminate infectious or irritating, potentially injurious agents from the urinary tract. C-fiber bladder afferent neurons are sensitized by a variety of endogenous substances (neurotransmitters, neurotrophic factors and inflammatory mediators) released by neural and nonneural cells within the bladder wall and exhibit a remarkable degree of morphological, chemical, and electrophysiological plasticity. Thus C-fiber bladder afferents are involved in many pathophysiological processes and are important targets for drugs and neurotoxins used to treat lower urinary tract dysfunction.
References
Andrade EL, Ferreira J, Andre E, Calixto JB (2006) Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol 72:104–114
Apostolidis A, Fowler CJ (2008) The use of botulinum neurotoxin type A (BoNTA) in urology. J Neural Transm 115:593–605
Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P (2005a) Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 65:400–405
Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P (2005b) Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol 174:977–982
Apostolidis A, Dasgupta P, Fowler CJ (2006) Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 49:644–650
Apostolidis A, Jacques TS, Freeman A, Kalsi V, Popat R, Gonzales G, Datta SN, Ghazi-Noori S, Elneil S, Dasgupta P, Fowler CJ (2008) Histological changes in the urothelium and suburothelium of human overactive bladder following intradetrusor injections of botulinum neurotoxin type A for the treatment of neurogenic or idiopathic detrusor overactivity. Eur Urol 53:1245–1253
Applebaum AE, Vance WH, Coggeshall RE (1980) Segmental localization of sensory cells that innervate the bladder. J Comp Neurol 192:203–209
Avelino A, Cruz C, Nagy I, Cruz F (2002) Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience 109:787–798
Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV (1995) Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci 7:1484–1494
Bahns E, Ernsberger U, Janig W, Nelke A (1986) Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflugers Arch 407:510–518
Bahns E, Halsband U, Janig W (1987) Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch 410:296–303
Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA (2006) Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290:F103–F110
Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB (1996) trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett 206:33–36
Bennett HL, Gustafsson JA, Keast JR (2003) Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci 105:90–100
Birder LA, de Groat WC (1993) Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. Am J Physiol 265:R326–R333
Birder LA, de Groat WC (2007) Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4:46–54
Birder LA, Apodaca G, de Groat WC, Kanai AJ (1998) Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol 275:F226–F229
Birder LA, Barrick S, Roppolo JR, Kanai A, de Groat WC, Kiss S, Buffington CA (2003) Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium Am J Physiol 285:F423–F429
Birder LA, Roppolo JR, Erickson VL, de Groat WC (1999) Increased c-fos expression in spinal lumbosacral projection neurons and preganglionic neurons after irritation of the lower urinary tract in the rat. Brain Res 834:55–65
Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ (2001) Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA 98:13396–13401
Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ (2002a) Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5:856–860
Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ (2002b) Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22:8063–8070
Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G (2004) Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 287:F1084–F1091
Birder LA, de Groat WC, Apodaca G (2008) Physiology of the urothelium. In: Schick E, Corcos J (eds) Textbook of the neurogenic bladder, 2nd edn. Taylor and Francis, London, chap 3
Black JA, Cummins TR, Yoshimura N, de Groat WC, Waxman SG (2003) Tetrodotoxin-resistant sodium channels Na(v)1.8/SNS and Na(v)1.9/NaN in afferent neurons innervating urinary bladder in control and spinal cord injured rats. Brain Res 963:132–138
Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P (2004a) P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol 46:247–253
Brady CM, Apostolidis AN, Harper M, Yiangou Y, Beckett A, Jacques TS, Freeman A, Scaravilli F, Fowler CJ, Anand P (2004b) Parallel changes in bladder suburothelial vanilloid receptor TRPV1 and pan-neuronal marker PGP9.5 immunoreactivity in patients with neurogenic detrusor overactivity after intravesical resiniferatoxin treatment. BJU Int 93:770–776
Chai TC, Steers WD, Broder SR, Rauchenwald M, Tuttle JB (1996) Characterization of laterality of innervation of the rat bladder. Scand J Urol Nephrol Suppl 179:87–92
Chai TC, Gray ML, Steers WD (1998) The incidence of a positive ice water test in bladder outlet obstructed patients: evidence for bladder neural plasticity. J Urol 160:34–38
Chang HY, Cheng CL, Chen JJ, de Groat WC (2007) Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol 292:F1044–F1053
Charrua A, Cruz CD, Cruz F, Avelino A (2007) Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol 177:1537–1541
Cheng CL, Chai CY, de Groat WC (1997) Detrusor-sphincter dyssynergia induced by cold stimulation of the urinary bladder of rats. Am J Physiol 272:R1271–R1282
Cheng CL, Liu JC, Chang SY, Ma CP, de Groat WC (1999) Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am J Physiol 277:R786–R794
Chess-Williams R (2002) Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol 22:133–145
Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA (2005) Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol 562:859–871
Christianson JA, Liang R, Ustinova EE, Davis BM, Fraser MO, Pezzone MA (2007) Convergence of bladder and colon sensory innervation occurs at the primary afferent level. Pain 128: 235–243
Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N (2001) The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165:975–979
Clifton GL, Coggeshall RE, Vance WH, Willis WD (1976) Receptive fields of unmyelinated ventral root afferent fibres in the cat. J Physiol 256:573–600
Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP (2000) Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407:1011–1015
Crowe R, Light K, Chilton CP, Burnstock G (1986) Vasoactive intestinal polypeptide-, somatostatin- and substance P-immunoreactive nerves in the smooth and striated muscle of the intrinsic external urethral sphincter of patients with spinal cord injury. J Urol 136:487–491
Cruz F, Dinis P (2007) Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn 26:920–927
Cruz CD, Ferreira D, McMahon SB, Cruz F (2007) The activation of the ERK pathway contributes to the spinal c-fos expression observed after noxious bladder stimulation. Somatosens Mot Res 24:15–20
Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D (2007) Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol 583:663–674
Dang K, Bielefeldt K, Gebhart GF (2005) Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25:3973–3984
Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF (2008) Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99:49–59
Dattilio A, Vizzard MA (2005) Up-regulation of protease activated receptors in bladder after cyclophosphamide induced cystitis and colocalization with capsaicin receptor (VR1) in bladder nerve fibers. J Urol 173:635–639
de Groat WC (1986) Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res 67:165–187
de Groat WC (1989) Neuropeptides in pelvic afferent pathways. In: Polak JM (ed) Regulatory peptides. Birkhauser, Basel, pp 334–336
de Groat WC (2004) The urothelium in overactive bladder: passive bystander or active participant? Urology 64:7–11
de Groat WC, Yoshimura N (2006) Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Eds.: Weaver LC, Polosa C. Progress in Brain Research 152:59–84
de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K (1981) Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Sys 3:135–160
de Groat WC, Kawatani M, Hisamitsu T, Cheng C-L, Ma C-P, Thor K, Steers W, Roppolo JR (1990) Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auto Nerv Sys 30(Suppl):S71–S77
de Groat WC, Booth AM and Yoshimura N (1993) Neurophysiology of micturition and its modification in animal models of human disease. In: Maggi CA (ed) The autonomic nervous system, vol 3. Nervous control of the urogenital system. Harwood, London, pp 227–289
de Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, Yoshimura N, Roppolo JR (2001) Neural control of the urethra. Scand J Urol Nephrol Suppl 207:35–43; discussion 106–125
Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A (2006) Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience 139:671–685
Dinis P, Charrua A, Avelino A, Yaqoob M, Bevan S, Nagy I, Cruz F (2004) Anandamide-evoked activation of vanilloid receptor 1 contributes to the development of bladder hyperreflexia and nociceptive transmission to spinal dorsal horn neurons in cystitis. J Neurosci 24:11253–11263
Dmitrieva N, McMahon SB (1996) Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain 66:87–97
Dmitrieva N, Shelton D, Rice AS, McMahon SB (1997) The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78:449–459
Downie JW, Armour JA (1992) Mechanoreceptor afferent activity compared with receptor field dimensions and pressure changes in feline urinary bladder. Can J Physiol Pharmacol 70:1457–1467
Du S, Araki I, Yoshiyama M, Nomura T, Takeda M (2007) Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology 70:826–831
Everaerts W, Gevaert T, Nilius B, De Ridder D (2008) On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn 27:264–273
Fahrenkrug J, Hannibal J (1998) Pituitary adenylate cyclase activating polypeptide immunor-eactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience 83:1261–1272
Fall M, Lindström S, Mazieres L (1990) A bladder-to-bladder cooling reflex in the cat. J Physiol (Lond) 427:281–300
Fam B, Yalla SV (1988) Vesicourethral dysfunction in spinal cord injury and its management. Semin Neurol 8:150–155
Ferguson DR, Kennedy I, Burton TJ (1997) ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol 505:503–511
Floyd K, Hick VE, Morrison JF (1976) Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol (Lond) 259:457–471
Forrest SL, Keast JR (2008) Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol 506:989–1002
Fowler CJ, Griffiths D, de Groat WC (2008) The neural control of micturition. Nat Rev Neurosci 9:453–466
Gabella G, Davis C (1998) Distribution of afferent axons in the bladder of rats. J Neurocytol 27:141–155
Geirsson G, Fall M, Lindstrom S (1993) The ice-water test – a simple and valuable supplement to routine cystometry. Br J Urol 71:681–685
Geirsson G, Fall M, Sullivan L (1995) Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J. Urol 154:1825–1829
Geirsson G, Lindstrom S, Fall M, Gladh G, Hermansson G, Hjalmas K (1994) Positive bladder cooling test in neurologically normal young children. J Urol 151:446–448
Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117:3453–3462
Gillespie JI, Markerink-van Ittersum M, de Vente J (2006) Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res 325:33–45
Gold MS, Shuster MJ, Levine JD (1996) Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 75:2629–2646
Gosling JA, Dixon JS, Critchley HO, Thompson SA (1981) A comparative study of the human external sphincter and periurethral levator ani muscles. Br J Urol 53:35–41
Guo A, Vulchanova L, Wang J, Li X, Elde R (1999) Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci 11:946–958
Häbler HJ, Jänig W, Koltzenburg M (1990) Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol (Lond) 425: 545–562
Häbler HJ, Jänig W, Koltzenburg M (1993) Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol 463:449–460
Hirayama A, Fujimoto K, Matsumoto Y, Ozono S, Hirao Y (2003) Positive response to ice water test associated with high-grade bladder outlet obstruction in patients with benign prostatic hyperplasia. Urology 62:909–913
Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT (2002) Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283:F1200–F1207
Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA (2005) Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173:1016–1021
Hulsebosch CE, Coggeshall RE (1982) An analysis of the axon populations in the nerves to the pelvic viscera in the rat. J Comp Neurol 211:1–10
Ishizuka O, Alm P, Larsson B, Mattiasson A, Andersson KE (1995) Facilitatory effect of pituitary adenylate cyclase activating polypeptide on micturition in normal, conscious rats. Neuroscience 66:1009–1014
Ito K, Iwami A, Katsura H, Ikeda M (2007) Therapeutic effects of the putative P2X(3)/P2X (2/3) antagonist A-317491 on cyclophosphamide-induced cystitis in rats. Naunyn Schmiedebergs Arch Pharmacol 377(4–6):483–490
Jaggar SI, Scott HC, Rice AS (1999) Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83: 442–448
Jancso G, Maggi CA (1987) Distribution of capsaicin-sensitive urinary bladder afferents in the rat spinal cord. Brain Res 418:371–376
Jänig W, Morrison JFB (1986) Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res 67:87–114
Jiang CH, Mazieres L, Lindstrom S (2002) Cold- and menthol-sensitive C afferents of cat urinary bladder. J Physiol 543:211–220
Johansson S, Fall M (1997) The pathology of interstitial cystitis. In: Sant GR (ed) Interstitial cystitis. Lippincott-Raven, Philadelphia, pp 143–151
Kawatani M, Erdman SL, de Groat WC (1985) Vasoactive intestinal polypeptide and substance P in primary afferent pathways to the sacral spinal cord of the cat. J Comp Neurol 241:327–347
Kawatani M, Nagel J, de Groat WC (1986) Identification of neuropeptides in pelvic and pudendal nerve afferent pathways to the sacral spinal cord of the cat. J Comp Neurol 249:117–132
Kawatani M, Suzuki T, de Groat WC (1996) Corticotropin releasing factor-like immunoreactivity in afferent projections to the sacral spinal cord of the cat. J Auton Nerv Syst 61:218–226
Keast JR, de Groat WC (1992) Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol 319:615–623
Keast JR, Stephensen TM (2000) Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol 424:577–587
Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C (2007) Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn 26:814–819
Kruse MN, Bray LA, de Groat WC (1995) Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auto Nerv Sys 54:215–224
Kullmann FA, Artim D, Beckel J, Barrick S, de Groat WC, Birder LA (2008a) Heterogeneity of muscarinic receptor-mediated Ca2+ responses in cultured urothelial cells from rat. Am J Physiol Renal Physiol 294:F971–F981
Kullmann FA, Artim DE, Birder LA, de Groat WC (2008b) Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci 28:1977–1987
Lamb K, Gebhart GF, Bielefeldt K (2004) Increased nerve growth factor expression triggers bladder overactivity. J Pain 5:150–156
Langford LA, Coggeshall RE (1981) Branching of sensory axons in the peripheral nerve of the rat. J Comp Neurol 203:745–750
Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D (1995) Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-fos and Krox-24 proteins. Exp Brain Res 105:220–232
Lassmann G (1984) Muscle spindles and sensory nerve endings in the urethral sphincter. Acta Neuropathol 63:344–346
Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML (2002) Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol 283:F242–F253
Lawson SN, Perry MJ, Prabhakar E, McCarthy PW (1993) Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull 30:239–243
Lazzeri M, Beneforti P, Benaim G, Maggi CA, Lecci A, Turini D (1996) Intravesical capsaicin for treatment of severe bladder pain: a randomized placebo controlled study. J Urol 156:947–952
Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D (2000) Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol 164:676–679
Lee HY, Bardini M, Burnstock G (2000) Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol 163:2002–2007
Lindstrom S, Mazieres L, Jiang CH (2004) Inhibition of the bladder cooling reflex in the awake state: an experimental study in the cat. J Urol 172:2051–2053
Liu HT, Kuo HC (2008) Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72(1):104–108
Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL (1997) Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79:572–577
Maggi CA (1993) The dual, sensory and efferent function of the capsaicin-sensitive primary sensory nerves in the bladder and urethra. In: Maggi CA (ed) Nervous control of the urogenital system, vol 1. Harwood, London, pp 383–422
Malykhina AP, Qin C, Foreman RD, Akbarali HI (2004) Colonic inflammation increases Na+ currents in bladder sensory neurons. Neuroreport 15:2601–2605
Malykhina AP, Qin C, Greenwood-van Meerveld B, Foreman RD, Lupu F, Akbarali HI (2006) Hyperexcitability of convergent colon and bladder dorsal root ganglion neurons after colonic inflammation: mechanism for pelvic organ cross-talk. Neurogastroenterol Motil 18:936–948
Masuda H, Kim JH, Kihara K, Chancellor MB, de Groat WC, Yoshimura N (2007) Inhibitory roles of peripheral nitrergic mechanisms in capsaicin-induced detrusor overactivity in the rat. BJU Int 100:912–918
Mazieres L, Jiang C, Lindstrom S (1998) The C fibre reflex of the cat urinary bladder. J Physiol 513(2):531–541
McGuire EJ, Morrissey SG, Schichun Z, Horwinsk E (1983) Control of reflex detrusor activity in normal and spinal injured non-human primates. J Urol 129:197–199
Mitsui T, Kakizaki H, Matsuura S, Ameda K, Yoshioka M, Koyanagi T (2001) Afferent fibers of the hypogastric nerves are involved in the facilitating effects of chemical bladder irritation in rats. J Neurophysiol 86:2276–2284
Miura A, Kawatani M, de Groat WC (2001) Effects of pituitary adenylate cyclase activating polypeptide on lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res 895:223–232
Morgan C, Nadelhaft I, de Groat WC (1981) The distribution of visceral primary afferents from the pelvic nerve to Lissauer's tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. J Comp Neurol 201:415–440
Morgan C, de Groat WC, Nadelhaft I (1986) The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J Comp Neurol 243:23–40
Morgan CW, de Groat WC, Felkins LA, Zhang SJ (1993) Intracellular injection of neurobiotin or horseradish peroxidase reveals separate types of preganglionic neurons in the sacral parasympathetic nucleus of the cat. J Comp Neurol 331:161–182
Morgan CW, Ohara PT, Scott DE (1999) Vasoactive intestinal polypeptide in sacral primary sensory pathways in the cat. J Comp Neurol 407:381–394
Morrison JF (1997) The physiological mechanisms involved in bladder emptying. Scand J Urol Nephrol Suppl 184:15–18
Morrison JF, Birder L, Craggs M, de Groat WC, Downie JW, Drake M, Fowler CJ, Thor KB (2005) Neural control. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence. Health, Plymouth, pp 363–422
Moss NG, Harrington WW, Tucker MS (1997) Pressure, volume, and chemosensitivity in afferent innervation of urinary bladder in rats. Am J Physiol 272:R695–R703
Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA (2004) Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172:2434–2439
Nadelhaft I, Vera PL (1992) Reduced urinary bladder afferent conduction velocities in streptozocin diabetic rats. Neurosci Lett 135:276–8
Namasivayam S, Eardley I, Morrison JF (1999) Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int 84:854–860
Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N (2004) Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. Eur J Neurosci 20:474–482
Nishiguchi J, Hayashi Y, Chancellor MB, de Miguel F, de Groat WC, Kumon H, Yoshimura N (2005) Detrusor overactivity induced by intravesical application of adenosine 5′-triphosphate under different delivery conditions in rats. Urology 66:1332–1337
Noronha R, Akbarali H, Malykhina A, Foreman RD, Greenwood-Van Meerveld B (2007) Changes in urinary bladder smooth muscle function in response to colonic inflammation. Am J Physiol Renal Physiol 293:F1461–F1467
Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC (1998) Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci 18:2174–2187
Ogawa T, Kamo I, Pflug BR, Nelson JB, Seki S, Igawa Y, Nishizawa O, de Groat WC, Chancellor MB, Yoshimura N (2004) Differential roles of peripheral and spinal endothelin receptors in the micturition reflex in rats. J Urol 172:1533–1537
Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M (1999) Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161:438–442
Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N (1999) Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol 162:2211–2216
Pandita RK, Andersson KE (2002) Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168:1230–1234
Pandita RK, Mizusawa H, Andersson KE (2000) Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol 164:545–550
Payne CK, Mosbaugh PG, Forrest JB, Evans RJ, Whitmore KE, Antoci JP, Perez-Marrero R, Jacoby K, Diokno AC, O'Reilly KJ, Griebling TL, Vasavada SP, Yu AS, Frumkin LR (2005) Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol 173:1590–1594
Pezzone MA, Liang R, Fraser MO (2005) A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 128:1953–1964
Popat R, Apostolidis A, Kalsi V, Gonzales G, Fowler CJ, Dasgupta P (2005) A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J Urol 174:984–989
Qiao LY, Vizzard MA (2002) Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol 454: 200–211
Qiao LY, Vizzard MA (2004) Up-regulation of phosphorylated CREB but not c-Jun in bladder afferent neurons in dorsal root ganglia after cystitis. J Comp Neurol 469:262–274
Qin C, Malykhina AP, Akbarali HI, Foreman RD (2005) Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 129:1967–1978
Rockswold GL, Bradley WE, Chou SN (1980a) Innervation of the external urethral and external anal sphincters in higher primates. J Comp Neurol 193:521–528
Rockswold GL, Bradley WE, Chou SN (1980b) Innervation of the urinary bladder in higher primates. J Comp Neurol 193:509–520
Rong W, Spyer KM, Burnstock G (2002) Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol 541:591–600
Roppolo JR, Tai C, Booth AM, Buffington CA, de Groat WC, Birder LA (2005) Bladder Aδ afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol 173:1011–1015
Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, de Groat WC, Yoshimura N (2002) Diabetic cystopathy correlates with long-term decrease in nerve growth factor (NGF) levels in the bladder and lumbosacral dorsal root ganglia. J Urol 168:1259–1264
Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, Yoshimura N (2004) Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes 53:2723–2730
Satchell P, Vaughan C (1994) Bladder wall tension and mechanoreceptor discharge. Pflugers Arch 426:304–309
Schurch B, de Seze M, Denys P, Chartier-Kastler E, Haab F, Everaert K, Plante P, Perrouin-Verbe B, Kumar C, Fraczek S, Brin MF (2005) Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 174:196–200
Schurch B, Denys P, Kozma CM, Reese PR, Slaton T, Barron RL (2007) Botulinum toxin A improves the quality of life of patients with neurogenic urinary incontinence. Eur Urol 52:850–858
Sculptoreanu A, de Groat WC (2003) Protein kinase C is involved in neurokinin receptor modulation of N- and L-type Ca2+ channels in DRG neurons of the adult rat. J Neurophysiol 90:21–31
Sculptoreanu A, de Groat WC (2007) Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol 205:92–100
Sculptoreanu A, de Groat WC, Buffington CA, Birder LA (2005a) Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol 193:437–443
Sculptoreanu A, de Groat WC, Buffington CA, Birder LA (2005b) Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett 381:42–46
Seki S, Sasaki K, Fraser MO, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N (2002) Immunoneutralization of nerve growth factor in lumbosacral spinal cord reduces bladder hyperreflexia in spinal cord injured rats. J Urol 168:2269–2274
Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor M, de Groat W, Yoshimura N (2003) Detrusor overactivity induced by increased levels of nerve growth factor in bladder afferent pathways in rats. Neurourol Urodyn 22:375–377
Seki S, Sasaki K, Igawa Y, Nishizawa O, Chancellor MB, de Groat WC, Yoshimura N (2004) Suppression of detrusor-sphincter dyssynergia by immunoneutralization of nerve growth factor in lumbosacral spinal cord in spinal cord injured rats. J Urol 171:478–482
Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, de Groat WC, Chancellor MB, Yoshimura N (2005) Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol 288:F466–F473
Sengupta JN, Gebhart GF (1994) Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72:2420–2430
Shea VK, Cai R, Crepps B, Mason JL, Perl ER (2000) Sensory fibers of the pelvic nerve innervating the rat's urinary bladder. J Neurophysiol 84:1924–1933
Smet PJ, Moore KH, Jonavicius J (1997) Distribution and colocalization of calcitonin gene-related peptide, tachykinins, and vasoactive intestinal peptide in normal and idiopathic unstable human urinary bladder. Lab Invest 77:37–49
Steers WD, Tuttle JB (2006) Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 3:101–110
Steers WD, Ciambotti J, Etzel B, Erdman S, de Groat WC (1991a) Alterations in afferent pathways from the urinary bladder of the rat in response to partial urethral obstruction. J Comp Neurol 310:401–410
Steers WD, Kolbeck S, Creedon D, Tuttle JB (1991b) Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest 88:1709–1715
Steers WD, Mackway-Gerardi AM, Ciambotti J, de Groat WC (1994) Alterations in neural pathways to the urinary bladder of the rat in response to streptozotocin-induced diabetes. J Auton Nerv Syst 47:83–94
Steers WD, Creedon DJ, Tuttle JB (1996) Immunity to nerve growth factor prevents afferent plasticity following urinary bladder hypertrophy. J Urol 155:379–385
Stein RJ, Santos S, Nagatomi J, Hayashi Y, Minnery BS, Xavier M, Patel AS, Nelson JB, Futrell WJ, Yoshimura N, Chancellor MB, De Miguel F (2004) Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J Urol 172:1175–1178
Stewart T, Beyak MJ, Vanner S (2003) Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol 552:797–807
Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM (2008) Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53:391–400
Studeny S, Torabi A, Vizzard MA (2005) P2X2 and P2X3 receptor expression in postnatal and adult rat urinary bladder and lumbosacral spinal cord. Am J Physiol Regul Integr Comp Physiol 289:R1155–R1168
Su X, Sengupta JN, Gebhart GF (1997) Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 77:1566–1580
Sun Y, Keay S, De Deyne PG, Chai TC (2001) Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 166:1951–1956
Tainio H (1993) Neuropeptidergic innervation of the human male distal urethra and intrinsic external urethral sphincter. Acta Histochem 94:197–201
Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR (2004) P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93:1344–1348
Thor K, Kawatani M, de Groat WC (1986) Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Goldberger M, Gorio A, Murray M (eds) Development and plasticity of the mammalian spinal cord. Fidia research series, vol III. Fidia, Padua, pp 65–81
Thor KB, Hisamitsu T, Roppolo JR, Tuttle P, Nagel J, de Groat WC (1989a) Selective inhibitory effects of ethylketocyclazocine on reflex pathways to the external urethral sphincter of the cat. J Pharmacol Exp Ther 248:1018–1025
Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC (1989b) Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol 288:263–279
Tong YC, Cheng JT (2007) Aldose reductase inhibitor ONO-2235 restores the alterations of bladder nerve growth factor and neurotrophin receptor p75 genetic expression in streptozotocin induced diabetic rats. J Urol 178:2203–7
Uemura E, Fletcher TF, Dirks VA, Bradley WE (1973) Distribution of sacral afferent axons in cat urinary bladder. Am J Anat 136:305–313
Uemura E, Fletcher TF, Bradley WE (1975) Distribution of lumbar and sacral afferent axons in submucosa of cat urinary bladder. Anat Rec 183:579–587
Ueyama T, Mizuno N, Nomura S, Konishi A, Itoh K, Arakawa H (1984) Central distribution of afferent and efferent components of the pudendal nerve in cat. J Comp Neurol 222:38–46
Ustinova EE, Fraser MO, Pezzone MA (2006) Colonic irritation in the rat sensitizes urinary bladder afferents to mechanical and chemical stimuli: an afferent origin of pelvic organ cross-sensitization. Am J Physiol Renal Physiol 290:F1478–F1487
Uvelius B, Gabella G (1998) The distribution of intramural nerves in urinary bladder after partial denervation in the female rat. Urol Res 26:291–297
Vera PL, Nadelhaft I (1990) Conduction velocity distribution of afferent fibers innervating the rat urinary bladder. Brain Res 520:83–89
Vizzard MA (2000a) Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol 279:R295–R305
Vizzard MA (2000b) Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161:273–284
Vizzard MA (2001) Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21:125–138
Vizzard MA (2006) Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog Brain Res 152:97–115
Vizzard MA, Boyle MM (1999) Increased expression of growth-associated protein (GAP-43) in lower urinary tract pathways following cyclophosphamide (CYP)-induced cystitis. Brain Res 844:174–187
Vizzard MA, Erdman SL, de Groat WC (1996) Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol 370:191–202
Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R (1998) P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci 10:3470–3478
Waddell PJ, Lawson SN (1990) Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience 36:811–822
Wang EC, Lee JM, Johnson JP, Kleyman TR, Bridges R, Apodaca G (2003) Hydrostatic pressure-regulated ion transport in bladder uroepithelium. Am J Physiol Renal Physiol 285:F651–F663
Wang ZY, Wang P, Merriam FV, Bjorling DE (2008) Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139(1):158–167
Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA (2006) Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res 152:245–263
Winter DL (1971) Receptor characteristics and conduction velocites in bladder afferents. J Psych Res 8:225–235
Xu L, Gebhart GF (2008) Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99:244–253
Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A (2004) Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63:17–23
Yoshimura N (1999) Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol 57:583–606
Yoshimura N, de Groat WC (1997) Plasticity of Na+ channels in afferent neurones innervating rat urinary bladder following spinal cord injury. J Physiol 503:269–276
Yoshimura N, de Groat WC (1999) Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19:4644–4653
Yoshimura N, White G, Weight FF, de Groat WC (1996) Different types of Na+ and A-type K+ and A-type K+ currents in dorsal root ganglion neurons innervating the rat urinary bladder. J Physiol 494:1–16
Yoshimura N, Erdman SL, Snider MW, de Groat WC (1998) Effects of spinal cord injury on neurofilament immunoreactivity and capsaicin sensitivity in rat dorsal root ganglion neurons innervating the urinary bladder. Neuroscience 83:633–643
Yoshimura N, Seki S, de Groat WC (2001a) Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons rat urinary bladder. J Neurophysiol 86:304–311
Yoshimura N, Seki S, Novakovic SD, Tzoumaka E, Erickson VL, Erickson KA, Chancellor MB, de Groat WC (2001b) The involvement of the tetrodotoxin-resistant sodium channel Na(v)1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci 21:8690–8696
Yoshimura N, Seki S, Erickson KA, Erickson VL, Chancellor MB, de Groat WC (2003) Histological and electrical properties of rat dorsal root ganglion neurons innervating the lower urinary tract. J Neurosci 23:4355–4361
Yoshimura N, Chancellor MB, Andersson KE, Christ GJ (2005) Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int 95: 733–738
Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S (2006) Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26:10847–10855
Yoshiyama M, de Groat WC (2008a) Effects of intrathecal administration of pituitary adenylate cyclase activating polypeptide on lower urinary tract functions in rats with intact or transected spinal cords. Exp Neurol 211:449–455
Yoshiyama M, de Groat WC (2008b) The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase activating polypeptide in the neural pathways controlling the lower urinary tract. J Mol Neurosci 36(1–3):227–240
Yu Y, de Groat WC (2008) Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am J Physiol Renal Physiol 294:F1146–F1156
Zagorodnyuk VP, Costa M, Brookes SJ (2006) Major classes of sensory neurons to the urinary bladder. Auton Neurosci 126–127:390–397
Zagorodnyuk VP, Gibbins IL, Costa M, Brookes SJ, Gregory SJ (2007) Properties of the major classes of mechanoreceptors in the guinea pig bladder. J Physiol 585:147–163
Zhong Y, Banning AS, Cockayne DA, Ford AP, Burnstock G, McMahon SB (2003) Bladder and cutaneous sensory neurons of the rat express different functional P2X receptors. Neuroscience 120:667–675
Zvara P, Braas KM, May V, Vizzard MA (2006) A role for pituitary adenylate cyclase activating polypeptide (PACAP) in detrusor hyperreflexia after spinal cord injury (SCI). Ann N Y Acad Sci 1070:622–628
Zvara P, Vizzard MA (2007) Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7:1–11
Zvarova K, Dunleavy JD, Vizzard MA (2005) Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol 192:46–59
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
de Groat, W.C., Yoshimura, N. (2009). Afferent Nerve Regulation of Bladder Function in Health and Disease. In: Canning, B., Spina, D. (eds) Sensory Nerves. Handbook of Experimental Pharmacology, vol 194. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-79090-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-540-79090-7_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-79089-1
Online ISBN: 978-3-540-79090-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)