Abstract
Particles smaller than 100 nm in size are referred to as nanoparticles. Nanoparticles are being applied in various domains and have ventured their path in healthcare including dentistry. This chapter would describe the various types of nanoparticles, their properties, how and where they have been applied in orthodontics. The various materials whose properties can be modified by the application of nanoparticles, how new materials can be developed will be mentioned. The various tests performed while using nanoparticles, to detect the physical and biological properties of the new material, will also be summarized for easy understanding of a researcher experimenting in this area. This chapter will also talk about the advantages and disadvantages of using nanoparticles and the precautions that one needs to take while researching with nanoparticles.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Background
5.1.1 Introduction
Orthodontic treatment of malaligned teeth and jaws is being carried out since ages. Advances in material sciences have lead to development of materials that enable treatment to be carried out in all walks of life and in mutilated dentitions. Despite their broad success, the orthodontic treatment suffers from a range of limiting factors like friction, long duration of treatment, and complications like inflammation of the biological tissues, decalcification of teeth due to bacterial growth, and root resorptions. To reduce these limitations and complications researchers and manufacturers of devices are focusing on modification of surface characteristics of materials so as to conquer these problems. The research is being focused on development of coatings that are stable in the oral environment and noncytotoxic. The coatings developed are either lubricants to reduce friction or antibacterial to reduce the microbial count and problems associated with it like caries , periodontal diseases, and decalcification around the brackets. Developments are also taking place to improve the bond strength of the brackets to the enamel. Nanoparticles are also being used in photodynamic therapy to control oral infections. The purpose of this article is to examine the various applications of nanoparticles in the field of orthodontics. This chapter will guide a young researcher to invent novel coatings and materials and to experiment in the area of material sciences.
5.1.2 Definitions
British Standards Institution defines nanoparticles as those particles in which all the fields or diameters are in the nanoscale range. Whereas, nanomaterials are those material for which at least one side or internal structure is in the nanoscale [1]. An engineered nanoparticle may be defined as any intentionally produced particle that has a characteristic dimension from 1 to 100 nm and has properties that are not shared by non-nanoscale particles with the same chemical composition [2]. Nanotechnology [3] is enabling technology that deals with nanometer-sized objects. Bionanotechnology and nanobiotechnology are terms that refer to the intersection of nanotechnology and biology [4]. Given that the subject is one that has only emerged very recently, bionanotechnology and nanobiotechnology serve as blanket terms for various related technologies. These two terms are often used interchangeably. When a distinction is intended, though, it is based on whether the focus is on applying biological ideas or on studying biology with nanotechnology. Bionanotechnology generally refers to the study of how the goals of nanotechnology can be guided by studying how biological “machines” work and adapting these biological motifs into improving existing nanotechnologies or creating new ones [5, 6]. Nanobiotechnology , on the other hand, refers to the ways that nanotechnology is used to create devices to study biological systems [7].
5.1.3 Historical Background
In 1959, the late Nobel Prize-winning physicist Richard P Feynman presented a talk entitled “There’s plenty of room at the bottom” at the annual meeting of the American Physical Society at the California Institute of Technology, Pasadena, CA [3, 8]. This was the first time when the vision of nanotechnology was introduced. Prof. Kerie E. Drexeler introduced the term “nanotechnology,” which was defined by Norio Taniguchi [9] as follows: “Nanotechnology mainly consists of the processing, separation, integration, and deformation of materials by one atom or one molecule.”
The optical characteristics of nanoparticles have been used in sculptures paintings even before the fourth century AD. The most famous example is the Lycurgus cup, known as dichroic glass, that changes color when held up to the light. The opaque green cup turns to a glowing translucent red when light is shone through it internally (i.e., light is incident on the cup at 90° to the viewing direction). Analysis of the glass revealed that it contains a very small quantity of tiny (70 nm) metal crystals of Ag and Au in an approximate molar ratio of 14: 1, which give it these unusual optical properties [1, 10].
The soluble gold was mostly used for its fabulous curative powers of various diseases. The first book on colloidal gold was published in 1618 by the philosopher and medical doctor Francisci Antonii. A complete treatise on colloidal gold was published in 1718 by Helcher [11]. Nanotechnology is easily evident in various old churches where ruby red color had been used in making of stained glass window. Science had not advanced much at that time to answer this phenomenon. It was later when developments in the field of chemistry took place that an answer to this phenomenon was revealed. These vivid colors were controlled by the size and the form (or shape) of the nanoparticles of gold and silver.
Industrial production of nanomaterials saw its origins in the twentieth century. For example, nanoparticles of carbon black (tire soot) have been used in the fabrication of rubber tires of automobiles from the beginning of the twentieth century [8]. Later developments in the chemical and physical properties led to new possibilities in various fields.
Nanotechnology has come a long way to find its application in supramolecular chemistry self-assembling drug carriers and gene delivery systems [12], nanoparticles and nanocapsules, antibody technologies, polymer-drug conjugates, polymer-protein and antibody conjugates [13], nanoprecipitation, nanocrystals, emulsification technologies, liposome technology [14], in situ polymerization , tissue engineering and repair [15], dendrimer technologies [16], molecular imprinting including recent innovations in dental diagnostics, material and therapeutics [17].
The confederation of nanotechnology with the field of dentistry has given rise to new stream “nanodentistry.” The important historical events in the development of Nanotechnology are summarized in Table 5.1.
5.2 Shapes and Types of Nanoparticles
Different shapes of nanoparticles such as rod, rectangle, hexagon, cube, triangle, and star-shaped nanoparticles can be produced by variation of experimental parameters such as concentration of the metal precursor, reducing agents, and stabilizers and reaction conditions such as temperature, time [19]. Bulk solution synthetic methods often produce nanoparticles of multiple sizes and shapes, and low yield of the desired size and shape. Colloidal solution can generally produce particle of desired shape and size [20]. Controlling size, shape, and structural architecture of the nanocrystals requires manipulation of the kinetic and thermodynamic parameters [21]. Following are certain types of nanoparticles;
5.2.1 Carbon-Based Materials
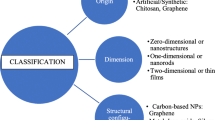
Carbon nanomaterials are one of the broadly discussed, researched, and applied of synthetic nanomaterials. Carbon nanotubes (CNTs), nanofibers (CNFs), and graphene are promising components for next-generation high-performance structural and multifunctional composite materials. The carbon nanomaterials have exceptional tensile strength, elastic modulus, electrical and thermal conductivity, and unique electronic, magnetic and optical properties. Apart from their above properties, their robust chemistry and ease of manipulation make them attractive candidates for diagnostic applications [22]. Carbon nanomaterials exist in allotropic forms such as diamond, graphene, amorphous carbon, and single-walled carbon nanotubes (SWNTs) . These materials can be classified according to the number of dimensions, which are not confined to the nanoscale range (0–100 nm), i.e., zero-dimensional (0-D) nanoparticles, one-dimensional (1-D) nanotubes, and two-dimensional (2-D) such as graphene [23] (Fig. 5.1).
5.2.2 Metal-Based Materials
Metal nanoparticles are the building blocks of the next generation of electronic, optoelectronic, biomedical, and chemical sensing devices. Among several metal nanomaterials, silver and gold nanoparticles are one of the most commercialized NPs because of their unique optical, electrical, and photothermal properties. They have wide applications in bio-sensing, diagnostic imaging, wastewater treatment, chemo-catalyst, cancer diagnosis, and therapy [24].
Metallic nanoparticles can be used as effective growth inhibitors of various microorganisms and thereby are applicable to diverse medical devices. Nanotechnology discloses the use of elemental nanoparticles as active antibacterial ingredient for dental materials. In dentistry, both restorative materials and oral bacteria are believed to be responsible for restoration failure. Metal nanoparticles can also control the formation of biofilms within the oral cavity, as a result of which it is often used as topically applied agents within dental materials [25].
5.2.3 Dendrimers
Dendrimers are perfect monodispersed macromolecules with a regular and highly branched three-dimensional architecture. Dendrimers are produced in an iterative sequence of reaction steps, in which each additional iteration leads to a higher generation material [26]. The first dendritic structures that have been thoroughly investigated and that have received widespread attention are Tomalia’s PAMAM dendrimers and Newkome’s “arborol” systems [27]. Dendrimer is grown in a stepwise manner from a central core, implying that numerous reactions have to be performed on a single molecule. Consequently, every reaction has to be very selective to ensure the integrity of the final product [28]. These nanomaterials are nanosized polymers built from branched units. The surface of a dendrimer has numerous chain ends, which can be tailored to perform specific chemical functions. This property could also be useful for catalysis. Also, because three-dimensional dendrimers contain interior cavities into which other molecules could be placed, they may be useful for drug delivery [29]. Dendritic molecules have been tested in supramolecular polymer chemistry, in medicinal chemistry, and in catalysis.
5.2.4 Nanocomposites
A nanocomposite is a multiphase solid material where one of the phases has one, two, or three dimensions in the nanometer scale, which is of less than 100 nm. Nanocomposites show unique properties, because of the nanometric size effect, compared to conventional composite even at low filler content. The organic–inorganic nanocomposites are often developed by grafting synthetic polymers on inorganic particles or by adding modified nanoparticles (NPs) into polymer matrices [30]. This leads to composite materials with improved properties. Nanoparticles can be incorporated into polymeric nanocomposites. Polymeric nanocomposites consisting of inorganic nanoparticles and organic polymers represent a new class of materials that exhibit improved performance compared to their microparticle counterparts. It is therefore expected that they will advance the field of engineering applications. The type of nanoparticles incorporated determines the properties of polymer composites. It depends on the size, shape, and concentration of the nanoparticles and their interactions with the polymer matrix [31].
5.3 Properties of Nanoparticles
The characteristic of nanoparticles like the size, shape, and surface characteristics determine the properties of the nanoparticles. Nanoparticles have properties different from microparticles due to their small size and relatively large surface area. When the size of a particle is close to or smaller than the de Broglie wavelength of the charge carrier (electrons and holes) or the wavelength of light, the periodic boundary conditions of the crystalline particle are destroyed, or the atomic density on the amorphous particle surface is changed [32]. Due to these, a lot of the physical properties of nanoparticles are quite different from bulk materials, yielding a wide variety of new applications. Optical properties of nanoparticles are due to the excitation of surface plasmons in metallic nanoparticles , this property can be used in biomedicine, energy, and environment protection technologies [33]. Magnetic properties of nanoparticles are by virtue of its external magnetic field and hence can be used for biomedical imaging and information storage technology.
The adhesion and the friction of nanoparticles play important roles in nanofabrication, lubrication, the design of micro/nano devices, colloidal stabilization, and drug delivery . Controlling the size, shape, and surrounding media of metal nanoparticles are important as many of their intrinsic properties are determined by these parameters. Particular emphasis has recently been placed on the control of shape, because, in many cases it allows properties to be fine-tuned with a greater versatility that gives the particles a unique nature. It is only within the past decade that it has become possible to control the shape of particles synthesized in solution, and numerous methods have been developed for this. Stabilizing agent also plays a role in the size of nanoparticle. The key effect of the stabilizer on the nanoparticle size lies in the initial particle nucleation stage [34]. A thermodynamically stable and mature nanoparticle can only be formed when a nucleus grows into a cluster that is larger than a certain critical size. Therefore, a fast initial nucleation is critical to the production of stable nuclei and subsequently smaller nanoparticles. The higher the temperature, larger and more polydisperse nanoparticles are obtained. Similarly, change in pH or H+ activity can impact the reduction of ions. As the reduction of H+ proceeds, the solution pH goes up, which favors the reduction of metal ions.
5.4 Synthesis of Nanoparticles
Nanoparticles may be synthesized by physical, chemical, or biological methods. Physical methods include evaporation, condensation, high gravity reactive precipitation, and laser ablation procedures. Chemical synthesis includes solvothermal methods, sol–gel conversions, chemical reduction, electrochemical techniques, photochemical reduction, and pyrolysis .
Biosynthesis of nanoparticles may be done via microorganisms, enzymes, fungi, plants, and plant extracts. Depending upon the location of nanoparticles, their synthesis via biological mode may be intracellular or extracellular. Intracellular method involves transport of ions into microbial cells to form nanoparticles in the presence of enzymes while extracellular synthesis is not within the cellular components of the organism [35] (Fig. 5.2).
There are two alternative approaches for synthesis of metallic nanoparticles : the “bottom-up” approach and the “top-down” approach. In bottom-up approach, atoms or molecules are assembled to molecular structures in nanometer range. Bottom-up approach is commonly used for chemical and biological synthesis of nanoparticles. Advantage of the bottom-up approach is the enhanced possibility of obtaining metallic nanoparticles with comparatively lesser defects and more homogeneous chemical composition(s). In top-down approach, the bulk materials are gradually broken down to nanosized materials using physical (e.g., mechanical) or chemical means. A major drawback of the top-down approach is the imperfection of the surface structure. Such defects in the surface structure can have a significant impact on physical properties and surface chemistry of the metallic nanoparticles due to the high aspect ratio [36].
Synthesis by various methods by top-down and bottom-up approach has been summarized as (Table 5.2):
Nanoparticles can be made from different materials composition with different physical and chemical properties. They can be attached with a various ligands for biological targeting for different functions like contrasting agents, drug delivery vehicles, and therapeutics (Fig. 5.3).
5.5 Applications of Nanoparticles in Dentistry
Nanoparticles are used in various forms in dentistry for diagnostic and therapeutic purposes. They have been used for administering local anesthesia, for cure of dentinal hypersensitivity, to diagnosis and cure oral cancers. Nanoneedles and nanofibers have been employed for wound dressings [37]. Nanoparticles due to their property of biocidal, anti-adhesive, and delivery capabilities are being explored to prevent the formation of biofilms within the oral cavity [38]. As nanoparticles possess a greater surface-to-volume ratio, they can interact more efficiently with microbial membranes and provide considerably larger surface area for antimicrobial activity . They have been used as device coatings [39] as topically applied agents, within materials like in dental resin composites [40], cavity liners, pit and fissure sealants, cores and buildups, indirect restorations, cements, acrylic resin denture bases [41] mouth rinses, and toothpastes [39].
5.6 Applications of Nanoparticles in Orthodontics
Nanoparticles have been used for various below-mentioned purposes in orthodontics (Fig. 5.4).
5.6.1 Application as Antimicrobial Agent
Since ages efforts are being made to reduce the microbial activity in the oral cavity. Inventions are being done in the methods of sterilization or the materials are being coated or incorporated into particles thus making them self-disinfecting [42]. Plaque accumulation on fixed and removable appliances is a common problem encountered during orthodontic treatment. This bacterial growth can lead to many complications like gingivitis, periodontitis, white spot lesions, increased risk of caries , halitosis, superimposed infections, failure of TAD’s, and delayed tooth movement. To minimize these problems, nanoparticles are being incorporated in various materials.
5.6.1.1 Materials Modified by Nanoparticles
Nanoparticles as antimicrobial agents can be incorporated or applied on:
Bracket Surface
Orthodontic brackets have been coated with nitrogen-doped titanium dioxide . The activation of nitrogen-doped titanium dioxide leads to the formation of OH free radicals, superoxide ions (O2), peroxyl radicals (HO2) and hydrogen peroxide (H2O2). These chemicals, through a series of oxidation reactions, react with biological molecules such as lipids, proteins, enzymes and nucleic acids, damage biological cell structures, and also exert antimicrobial activity [43].
Resin Composite during Bonding
TiO2 nanoparticles of size 21 ± 5 nm have been blended to light cure orthodontic composite paste (Transbond XT) in 1, 2, and 3%. All the three concentrations had similar antibacterial effects [44]. ZnO-NPs when mixed with Chitosan NP in the ratio 10% (w/w) have also shown antibacterial activity when added to resin [45]. Silver and HA nanoparticles have also been added to the primer of Transbond XT in 1 and 5% concentrations and have shown good antibacterial properties [46]. 0.0100 wt% of copper NPs have also been used in resins [47].
Resin-Modified GIC
Resin-modified GIC has been improved by incorporating nanosized fluoroapatite (NFA) or fluorohydroxyapatite (NFHA) particles at 25% concentration. However, there was a significant reduction in shear bond strength . The fluoride release nearly tripled after 70 days [48]. Nanohydroxyapatite (Nano-HA) has also been added to orthodontic banding cement to prevent microleakage under orthodontic bands [49]. Zinc oxide has been added to light-cured resin-modified glass ionomer to create mixtures of 13% ZnO and 23.1% zinc oxide [50, 51].
Microimplants
Since bacterial infection has been identified as one of the major causes of titanium microimplant failures, a novel antibiotic vehicle composite, TiO2NT–PSPMA, has been synthesized via atom transfer radical polymerization ; this method improved the local antibiotic concentration and prolonged its sustainable release by loading larger amounts of antibiotic into Titanium nanotubes (TiO2 NTs) arrayed on Ti implants. Ag nanoparticles loaded into TiO2 NTs with the assistance of the ionic polymer 3-sulfopropyl methacrylate potassium salt (PSPMA) have been used. This composite perhaps could be used in future to prevent implant infection [52].
Acrylic Resins
TiO2, SiO2, and silver NPs have been added to cold-cure acrylic resins that are mainly made of polymethyl methacrylate (PMMA) . However, the cytotoxicity of this resin over long duration still needs to be verified [53,54,55].
5.6.1.2 Tests Carried Out
The following laboratory tests are carried out to check for the properties of material:
-
Antimicrobial activity: Disk agar diffusion (DAD) test
-
Cytotoxicity on cell lines: Live and dead staining, lactate production, spectroscopy
-
Distribution of nanoparticles: SEM
-
Shear bond strength: Ionstron machine and adhesive remnant index (ARI) scores
-
Microleakage: Microleakage under the bands can be assessed by the methylene blue dye
-
Insolubility of NP: Atomic absorption test
-
Bacteria test: Streptococcus mutans since it causes caries , Aggregatibacter actinomycetemcomitans since it causes periodontal diseases.
5.6.2 Application as Lubricating Agent to Reduce Friction
Friction is one of the major deterrents present in alignment or retraction of teeth during orthodontic treatment. High friction leads to anchorage loss, increased duration of treatment, and increased waiting list in hospitals [56, 57]. Numerous attempts were made to overcome this problem, by using wires of a different metal, shape, and size [58, 59]. Not only the wires even the bracket surfaces are coated and the ligation methods are also varied to reduce the problem of friction [60].
5.6.2.1 Nanoparticles Used
Various nanoparticles are being used as a coating in an effort to reduce friction [61,62,63].
The nanoparticles used are as follows:
-
1.
Nickel-phosphorus and tungsten disulfide (WS2)
-
2.
Co + fullerene-like WS2
-
3.
Carbone nitride (CNx)
-
4.
ZnO
-
5.
Molybdenum disulfide
-
6.
Diamond-like carbon coating and nitrocarburizing
-
7.
Polysulfone embedded with hard alumina nanoparticles for brackets
5.6.2.2 The Method of Coating
The orthodontic wires are inserted into the electroless solution of the nanoparticles which needs to be coated usually for about 30 min. A short (1 min) sonication can be used to disperse the agglomerates and ensure the stability of the suspension. Some scientists have even etched the wire with HF (20%) acid to improve the adherence of the coatings on wires. The researchers interested in this method can do further reading on electroless plating [64].
5.6.2.3 Tests Used to Determine the Efficiency of Coatings
Whenever one is coating the wires, it needs to check the strength/quality of coating in terms of adherence and the effectiveness of coating in terms of the purpose for which the coating is applied. One also needs to check how much of ions are being released once they are coated because the release of free ions can lead to cytotoxicity [65].
-
(a)
Tests used to determine reduction in friction
Tribiological assays are done using a ball on flat tribometer and the friction coefficient is determined after various cycles. Dry and wet friction tests with paraffin oil lubricant can be carried out during 50–200 cycles. Apparatus used is universal mechanical testing apparatus.
-
(b)
Tests to determine the quality of film
-
SEM/TEM/micrographs can be taken of the coated wires.
-
X-ray photoelectron spectroscopy (XPS) analyses can be done
-
Energy dispersive x-ray spectroscopy, x-ray powder diffractometry
-
-
(c)
Tests to determine the adequate adherence of the film
Scotch-tape test or bending test of the wire with a 2 cm radius of curvature
-
(d)
Test to determine corrosion behavior
Potentiodynamic polarization test and electrochemical impedance spectroscopy
-
(e)
Test to check cytotoxicity
The cytotoxicity of the coating needs to be checked on various cell lines like the human periodontal ligament fibroblast cells (HPLF), human gingival fibroblast cells (HGF), human pulp cells (HPC).
5.6.2.4 Mechanism of Action of Coating
The mechanism by which the reduction in friction is achieved can be explained by the theories suggested by Rapoport et al. [66] and Cizaire et al. [67]. At the first stage, when there is no angle between the slot and wire, the nanoparticles act as spacers and reduce the number of asperities that come in contact, resulting in a lower coefficient of friction. The friction at the wire increases as the angle increases. At this point on the coated wire, the nanoparticles from the coating are released into the tribological interface and their exfoliation occurs, resulting in the formation of a solid lubricant film on the sliding wire. The higher load at this point brings the asperities of the mating surfaces in straight contact causing the fluid (saliva in the mouth) to be squeezed out of the gap between the wire and slot, relying on the excellent tribological behavior of the solid lubricant film to allow the sliding of the archwire. When the two materials are SS, as is the case with the uncoated wire, the friction coefficient is high. The presence of nanosheets at the interface under high loads leads to a very facile sliding between these sheets, thereby reducing the coefficient of friction.
5.6.3 Fabrication of Hollow Wires
NiTi/Ni-TiO2 composite nanoparticles are being used for the fabrication of hollow wires. These wires are hollow from inside but retain the properties of NiTi wires. They are synthesized via the synthesis method called the ultrasonic spray pyrolysis (USP) . The orthodontic wire is used to obtain the precursor solution for the synthesis of spherical NiTi particles. These spherical NiTi particles are then coated over a textile or a polymer fiber via electrospinning. Then, the fiber is removed from inside thus producing a hollow wire. Bending properties of these hollow NiTi wires were performed by the three-point bending test and compared with conventional NiTi wires [68, 69].
Advantages of hollow wires:
-
1.
This wire could potentially have the shape-memory and superelasticity properties, while possibly reducing the material needed for the wire production.
-
2.
They may deliver lighter and more continuous force.
-
3.
The bending properties can be customized by inserting another wire into the hollow core.
Limitation:
It is still difficult to obtain pure NiTi particles so research is being done on different precursor solutions, gases and collection media so as to obtain pure NiTi particles.
5.6.4 Fabrication of Orthodontic Brackets
Nanoparticles are being used for fabrication of brackets like the hard alumina nanoparticles embedded in polysulfone. These brackets have strength, reduced friction and biocompatibility while maintaining the transparency of the bracket.
Brackets are also being coated with nanoparticles to improve their properties like:
-
Titanium dioxide because of its photocatalytic properties
-
Nickel-phosphorous and tungsten disulfide (WS2) nanoparticles to reduce friction.
-
ZnO and CuO nanoparticles for antibacterial properties [70, 71].
Once they are coated, the following tests are carried out to check for their properties:
-
Surface roughness is seen on atomic force microscope.
-
Compressive strength, maximum strain, and elastic modulus are checked on Instron testing machine.
-
Photocatalytic ability is checked by decolorization method with toluidine blue followed by measurement on absorption spectrometer.
5.6.5 Use of Nanoparticles in Evolving the Cell-Matrix Interface
5.6.5.1 To Improve the Primary Stability of TADs
The surface of the microimplants is being modified with the help of nanoparticles. The following nanoparticles are being used:
-
(a)
Nanostenciled rgd-gold patterns have been used to control and influence the differentiation of mesenchymal cells with implant surface. Manipulating the maturation of cell-matrix adhesions by nanopatterned surfaces allows influencing morphology, actin dynamics, migration, and ECM assembly of adhering fibroblasts [72].
-
(b)
Primary stability and partial osseointegration of TADs could be achieved by the synergistic effects from nanoclay reinforced tricalcium phosphate (TCP) nanocoating on titanium miniscrews [73].
-
(c)
Ultrafine grain-sized titanium (UFG Ti) obtained by severe plastic deformation presents a bright potential for biomedical applications because it provides the strength of titanium alloys without toxic alloying elements, such as Al and V that, by dissolving away from the implant, may be harmful to human health. The osseointegration of these UFG Ti microimplants is also found to be superior [74].
5.6.5.2 To Improve the Stability of the Newly Forming Bone
Patients with clefts many a times require a bone graft to fill the defects. Bioactive and biodegradable poly (lactide-co-glycolide)/bioactive glass/hydroxyapatite (PBGHA) and poly (lactide-co-glycolide)/bioactive glass (PBG) nanocomposite coatings have been tested. They have an ability to serve as a scaffold or template to guide the newly forming bone along its surfaces thus promoting its stability. BGs also serve as synthetic biocompatible osteoconductive bone substitutes, with bone bonding capacity and documented antibacterial and angiogenesis-promoting properties. The following laboratory tests are carried out to check for the quality of coating:
-
The nanocomposite coatings are characterized by scanning electron microscopy, X-ray diffraction, and atomic force microscopy.
-
Mechanical stability of the prepared nanocomposite coatings can be studied by intramedullary implantation of coated Kirschner wires (K-wires) into rabbit tibia [75].
5.6.5.3 To Fill Defects in Damaged Bone
Nanosized hydroxyapatite particles can be converted into injectable paste with the help of neutral phosphate buffer which is self-setting at 37 °C in 20 min. Stability of the injectable hydroxyapatite has been confirmed in aqueous medium as well as in human blood [76].
5.6.5.4 To Enhance the Formation of Bone
Titanium nanotubes with crystallized Ag2O nanoparticles with diameters ranging from 5 nm to 20 nm embedded in them have been found to significantly enhance the functions of many cell types including osteoblasts thus having promising applications orthodontics. This leads to controlled release of Ag and hence long-lasting antibacterial activity without showing cytotoxicity . It has even shown some favorable effects on promoting cell spreading and can be used as a biomedical coating on devices [77].
5.6.6 Nanomaterials as Nanofillers in Orthodontics
Nanoparticles of reduced size are being used as fillers to reduce polymerization shrinkage and to improve the mechanical properties of strength. There are two types of fillers: nanoclusters and nanoparticles [78]. They are synthesized by techniques such as flame pyrolysis , flame spray pyrolysis, and sol–gel processes. Nanosized filler particles have been incorporated into the composite matrix and glass ionomer cements to form nanocomposites and nanoionomers [78,79,80,81]. The following nanoparticles are being used as fillers:
-
Silica nanosized filler particles (10 wt%, particle diameter <7 nm) are being added to orthodontic adhesives [40].
-
Titanium dioxide and zirconia due to their very high refractive indices and less weight of material are very useful [82].
-
Nanozirconia is also being used in ionomer cements to improve properties like esthetics (e.g., low visual opacity), polish retention, and radiopacity as compared to previously known glass ionomer compositions. The nanozirconia is surface modified with silanes to aid in the incorporation of the nanozirconia into ionomer compositions [83].
5.6.7 Nanoparticles as Enamel Remineralizing Agents
Demineralization and white spot lesions are common problems encountered during and post-orthodontic treatment. Nanoparticles are being used for remineralization of decalcified enamel.
Nanohydroxyapatite has been developed as a paste. Calcium nanophosphate crystals which are smaller than 100 nm, lead to improved bioactivity of the product, resulting from the increase in surface area and wettability of HA (hydroxyapatite) nanoparticles and thus form a protective layer on the enamel surface and provides protection against erosion [84].
5.6.8 Self-healing Materials
Research is being directed toward the fabrication of self-healing materials or materials that could mimic the biologic system and fill the cracks or damages on their own. When a crack appears near the network, the healing fluid or precursor can flow to the damaged region and fill the fissure. This fluid can be stored in bubbles which can be incorporated in the material.
This reservoir, upon exposure to air, polymerizes as a result of crack formation and closes the crack, thus maintaining the structural integrity of the material. This concept can be applied to polymer brackets and archwires. The autopolymerized monomer can be incorporated in nanosized bubbles and can be integrated with the material. Fracture of the bracket or wire would induce bursting of the nanobubbles and exposure of the monomer to air, thereby resulting in polymerization and filling of the crack-induced gap [85, 86].
5.6.9 Nanoparticles in Bioimaging
One of the potential applications of nanoparticles is also in the field of bioimaging as contrast agents. The advantages of nanoparticles over existing contrast agents include tunable physical (e.g., optical and magnetic) properties, high stability (e.g., against photobleaching), possibility of targeted delivery, and specific binding via chemical functionalization, multimodality (ability to combine several functions in one particle), high sensitivity, and selectivity. They can be used in bioimaging techniques such as optical and confocal microscopy, NIR imaging, magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET), single-photon emission CT (SPECT), and ultrasound imaging. The current challenges include potential nanotoxicity and biocompatibility issues [87].
5.7 Mechanism of Action of Various Nanoparticles
Mechanism of antibacterial activity of metallic nanoparticles is not exactly known. However, the presence of positive charge over the metal ion is crucial for this antimicrobial action which aids in the electrostatic attraction between the negatively charged bacterial wall and the positively charged metallic nanoparticles [88]. Nanoparticles can enter the cell after binding to a specific receptor target. There are several factors which can regulate the behavior of nanomaterials at the nano–biointerface. Such as the shape of nanoparticles directly influences uptake into cells: Rods show the highest uptake, followed by spheres, cylinders, and cubes when the synthesized nanoparticles are larger than 100 nm [89]. In studies with sub-100-nm nanoparticles, spheres show an appreciable advantage over rods. In fact, at this size range, increasing the aspect ratio of nanorods seems to decrease total cell uptake. The size-dependent uptake of nanoparticles is likely related to the membrane-wrapping process. Small nanoparticles have less ligand-to-receptor interaction than do larger nanoparticles (Table 5.3).
5.8 Toxicity
The rapid developments in the field of nanotechnology also bring with them the concerns related to toxicity through new sources of exposure like inhalation, ingestion, and injection. Not only that there are serious environmental implications but also associated with the manufactured nanomaterials.
Nanomaterials have unique physicochemical properties, such as ultra-small size, large surface area to mass ratio, and high reactivity and these properties also influence and determine the biological responses and can often lead to toxicity . Nanoparticles have been found to cross the transplacental membrane or cross the peritoneal cavity into uterus and may affect the cranial development of embryo [93]. Endocytosis of nanoparticles can also lead to oxidative stress, which can be a concern for a number of autoimmune diseases [94]. Cytotoxicity of silver nanoparticles has also been reported a number of times, but the exact mechanism of action of silver nanoparticle is yet to be elucidated. It has been reported that silver nanoparticle is associated with the depletion of glutathione (GSH) level, increased level of ROS [95]. Ag NPs can enter via the blood-brain barrier and accumulate in different regions of the brain and this may be beneficial for drug delivery , but at the same time can increase a risk to the patient. Copper nanoparticles are often metabolized in liver and are reported to have toxic effects to hepatic and renal tissues [96]. Gold nanoparticles are also associated with male sterility, it is reported that they can affect the motility of spermatozoa by penetrating penetrate sperm cells, which could result in fragmentation [97]. Surface charge of Si NPs is also associated with the cytotoxicity toward human cells and positively charged Si NPs are 250-fold more cytotoxic compared to their negatively charged counterparts [98] (Fig. 5.5).
5.9 Conclusion
Nanoparticles have lot of potential in the field of dentistry and orthodontics per se. There is lot of research being focused on development of newer materials by the application of nanoparticles. Majority of the research is at the level of publications. The translation of this research to reality in the form of commercial products is a long journey. It is high time that researchers focus and encash on the potential of these vibrant particles as their applicability needs to be explored further in the field of dentistry.
References
Horikoshi S, Serpone N (2013) Introduction to nanoparticles. In: Microwaves in Nanoparticle Synthesis: Fundamentals and Applications, pp 1–24
Nanoscale Science Engineering and Technology Subcommittee (2004) National nanotechnology initiative: strategic plan. In: US National Science and Technology Council, 2004, pp 1–48
Feynman R (1991) There’s plenty of room at the bottom. Science 254:1300–1301
Gazit Ehud (2007) Plenty of room for biology at the bottom: an introduction to bionanotechnology. Imperial College Press, London
Imperial College Press (2007) ISBN 9781860946776 http://www.wordiq.com/definition/Bionanotechnology
Nolting B (2005) Biophysical nanotechnology. In: Methods in modern biophysics. Springer, Berlin. ISBN 3-540-27703-X
Fakruddin M, Hossain Z, Afroz H (2012) Prospects and applications of nanobiotechnology: a medical perspective. J Nanobiotechnol 10(1):31
Maheshwari S, Verma SK, Tariq M, Gaur A (2014) Nano-orthodontics revolutionizing oral health care. Indian J Oral Sci 5(3):109
Taniguchi N (1974) On the basic concept of nanotechnology. In: International Conference on Production Engineering, Tokyo, Part II, Japan Society of Precision Engineering 1974 Feb, pp 18–23
British museum collection online. Google arts and culture: The Lycurgus cup. Available from https://www.google.com/culturalinstitute/beta/asset/the-lycurgus-cup/aQG7BXUoa7xwug. Accessed 17 Jun 2016
Helcher HH (1718) Aurum Potabile oder Gold Tinstur. Johann Herbord Klossen, Breslau und Leipzig
Tian H, Chen J, Chen X (2013) Nanoparticles for gene delivery. Small 9(12):2034–2044
Jain KK (2005) Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta 358(1):37–54
Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30(11):592–599
Zhang L, Webster TJ (2009) Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 4(1):66–80
Scott RW, Wilson OM, Crooks RM (2005) Synthesis, characterization, and applications of dendrimer-encapsulated nanoparticles. J Phys Chem B 109(2):692–704
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83(5):761–769
Kreuter J (2007) Nanoparticles—a historical perspective. Int J Pharm 331(1):1
Sajanlal PR, Sreeprasad TS, Samal AK, Pradeep T (2011) Anisotropic nanomaterials: structure, growth, assembly, and functions. Nano Rev 2(1):5883
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126(28):8648–8649
De Santis CJ, Peverly AA, Peters DG, Skrabalak SE (2011) Octopods versus concave nanocrystals: control of morphology by manipulating the kinetics of seeded growth via co-reduction. Nano Lett 11(5):2164–2168
Baptista FR, Belhout SA, Giordani S, Quinn SJ (2015) Recent developments in carbon nanomaterial sensors. Chem Soc Rev 44(13):4433–4453
Ionescu MI (2011) Synthesis of One-Dimensional and Two-Dimensional Carbon Based Nanomaterials. Ir.lib.uwo.ca
Mazumder JA, Ahmad R, Sardar M (2016) Reusable magnetic nanobiocatalyst for synthesis of silver and gold nanoparticles. Int J Biol Macromol 31(93):66–74
Hamouda IM (2012) Current perspectives of nanoparticles in medical and dental biomaterials. J Biomed Res 26(3):143–151
John PR (2006) New nanotechnology research. 2006. Chapter 6: Preparation of nanomaterials in presence of branched molecules. Nova science publisher, New York, p 188
Bosman AW, Janssen HM, Meijer EW (1999) About dendrimers: structure, physical properties, and applications. Chem Rev 99(7):1665–1688
Carlmark A, Hawker C, Hult A, Malkoch M (2009) New methodologies in the construction of dendritic materials. Chem Soc Rev 38(2):352–362
Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK (2009) Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci 38(3):185–196
Kango S, Kalia S, Celli A, Njuguna J, Habibi Y, Kumar R (2013) Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—a review. Prog Polym Sci 38(8):1232–1261
Camargo PH, Satyanarayana KG, Wypych F (2009) Nanocomposites: synthesis, structure, properties and new application opportunities. Mater Res 12(1):1–39
Guo D, Xie G, Luo J (2013) Mechanical properties of nanoparticles: basics and applications. J Phys D Appl Phys 47(1):013001
García MA (2011) Surface plasmons in metallic nanoparticles: fundamentals and applications. J Phys D Appl Phys 44(28):283001
He F, Zhao D (2007) Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41(17):6216–6221
Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30(11):592–599
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med 6.2:257–262
Shalumon KT, Anulekha KH, Nair SV, Nair SV, Chennazhi KP et al (2011) Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol 49(3):247–254
Allaker RP (2010) The use of nanoparticles to control oral biofilm formation. J Dent Res 89(11):1175–1186
Boldyryeva H, Umeda N, Plaksin OA, Takeda Y (2005) Kishimoto N High-fluence implantation of negative metal ions into polymers for surface modification and nanoparticle formation. Surf Coat Technol 196(1–3):373–377
Ahn SJ, Lee SJ, Kook JK (2009) Lim BS Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater 25(2):206–213
Lee CJ, Lee MS, Nam KY (2008) Inhibitory effect of PMMA denture acrylic impregnated by silver nitrate and silver nano-particles for Candida Albicans. J Korean Chem Soc 52(4):380–386
Batra P, Jyothikiran H (2013) Tips for maintaining sterilization in your orthodontic work station. Int J Orthod (Milwaukee, Wis.) 25(2):21–30
Cao B, Wang Y, Li N, Liu B, Zhang Y (2013) Preparation of an orthodontic bracket coated with an nitrogen-doped TiO(2-x)N(y) thin film and examination of its antimicrobial performance. Dent Mater J 32(2):311– 316
Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M et al (2013) Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthod 35(5):676–679
Mirhashemi A, Bahador A, Kassaee M, Daryakenari G, Ahmad Akhoundi M et al (2013) Antimicrobial effect of nano-zinc oxide and nano- chitosan particles in dental composite used in orthodontics. J Med Bacteriol 2(3–4):1–10
Akhavan A, Sodagar A, Mojtahedzadeh F, Sodagar K (2013) Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol Scand 71(5):1038–1042
Argueta Figueroa L, Scougall Vilchis RJ, Morales Luckie RA, Olea Mejia OF (2015) An evaluation of the antibacterial properties and shear bond strength of copper nanoparticles as a nanofiller in orthodontic adhesive. Aust Orthod J 31(1):42–48
Lin J, Zhu J, Gu X, Wen W, Li Q et al (2011) Effects of incorporation of nano-fluorapatite or nano- fluorohydroxyapatite on a resin-modified glass ionomer cement. Acta Biomater 7(3):1346–1353
Enan ET, Hammad SM (2013) Microleakage under orthodontic bands cemented with nano-hydroxyapatite-modified glass ionomer. Angle Orthod 83(6):981–986
Spencer CG, Campbell PM, Buschang PH, Cai J, Honeyman AL (2009) Antimicrobial effects of zinc oxide in an orthodontic bonding agent. Angle Orthod 79(2):317–322
Ramazanzadeh B, Jahanbin A, Yaghoubi M, Shahtahmassbi N, Ghazvini K, et al (2015) Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against streptococcus mutans. J Dent 16(3):200–205
Zhang M, Wei M, Wang D, Duan Y (2015) Preparation and characterization of a drug vehicle: polymer brush immobilized Ag nanoparticles onto titanium nanotubes. Mater Lett 135:51–54
Farhadian N, Mashoof RU, Khanizadeh S, Ghaderi E, Farhadian M et al (2016) Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs those wearing conventional retainers: a randomized clinical trial. Am J Orthod Dentofacial Orthop 149(2):155–160
Batra P, Miglani R (2016) Antibacterial properties of retainers with silver nanoparticles. Am J Orthod Dentofac Orthop 150(2):208–209
Sodagar A, Bahador A, Khalil S, Shahroudi AS, Kassaee MZ (2013) The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J Prosthodont Res 57(1):15–19
Wichelhaus A, Geserick M, Hibst R, Sander FG (2005) The effect of surface treatment and clinical use on friction in NiTi orthodontic wires. Dent Mater 21(10):938–945
Batra P (2017) Eisenhower box for prioritising waiting list of orthodontic patients. OHDM 16(1):1–3
Kusy RP (2000) Ongoing innovations in biomechanics and materials for the new millennium. Angle Orthod 70(5):366–376
Jyothikiran H, Shantharaj R, Batra P, Subbiah P, Lakshmi B, Kudagi V (2014) Total recall: an update on orthodontic wires. Int J Orthod (Milwaukee, Wis.) 25(3):47–56
Hain M, Dhopatkar A, Rock P (2003) The effect of ligation method on friction in sliding mechanics. Am J Orthod Dentofac Orthop 123(4):416–422
Katz A, Redlich M, Rapoport L, Wagner HD, Tenne R (2006) Self-lubricating coatings containing fullerene-like WS2 nanoparticles for orthodontic wires and other possible medical applications. Tribol Lett 21(2):135–139
Redlich M, Katz A, Rapoport L, Wagner HD, Feldman Y, Tenne R (2008) Improved orthodontic stainless steel wires coated with inorganic fullerene-like nanoparticles of WS 2 impregnated in electroless nickel–phosphorous film. Dent Mater 24(12):1640–1646
Samorodnitzky-Naveh GR, Redlich M, Rapoport L, Feldman Y, Tenne R (2009) Inorganic fullerene-like tungsten disulfide nanocoating for friction reduction of nickel–titanium alloys. Nanomedicine 4(8):943–950
Mallory GO, Hajdu JB (1990) Electroless plating: fundamentals and applications. William Andrew
Panchali B, Anam M, Jahirul m, Meryam SR, Ragini M (2016) Nanoparticles and their applications in orthodontics. Adv Dent Oral Health 2(2):1–10
Rapoport L, Leshchinsky V, Lapsker I, Volovik Y, Nepomnyashchy O, Lvovsky M, Popovitz-Biro R, Feldman Y, Tenne R (2003) Wear 255:785
Cizaire L, Vacher B, Le-Mogne T, Martin JM, Rapoport L, Margolin A, Tenne R (2002) Mechanisms of ultra-low friction by hollow inorganic fullerene-like MoS2 nanoparticles. Surf Coat Technol 160:282–287
Zadno-Azizi GR, Muni KP, Bagaoisan CJ, inventors; Medtronic PercuSurge, Inc., assignee. Hollow medical wires and methods of constructing same. United States patent US 6,375,628. 2002 Apr 23
Shima Y, Otsubo K, Yoneyama T, Soma K (2002) Bending properties of hollow super-elastic Ti–Ni alloy wires and compound wires with other wires inserted. J Mater Sci - Mater Med 13(2):169–173
Ramazanzadeh B, Jahanbin A, Yaghoubi M, Shahtahmassbi N, Ghazvini K, Shakeri M, Shafaee H (2015) Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against Streptococcus mutans. J Dent 16(3):200
Sato Y, Miyazawa K, Sato N, Nakano K, Takei Y, Kawai T, Goto S (2009) Study on fabrication of orthodontic brackets with the photocatalytic function of titanium dioxide. Dent Mater J 28(4):388–395
Lutz R, Pataky K, Gadhari N, Marelli M, Brugger J et al (2011) Nano-stenciled RGD-gold patterns that inhibit focal contact maturation induce lamellipodia formation in fibroblasts. PLoS ONE 6(9):e25459
Zhou WY, Rabie AB, Wong RW, Tang B (2010) Nanocoating of montmorillonite/Mg-β-tricalcium phosphate on orthodontic titanium miniscrews. In: 2010 3rd International Nanoelectronics Conference (INEC), IEEE, pp 817–818
Elias CN, Meyers MA, Valiev RZ, Monteiro SN (2013) Ultrafine grained titanium for biomedical applications: an overview of performance. J Mater Res Tech 2(4):340–350
Mehdikhani-Nahrkhalaji M, Fathi MH, Mortazavi V, Mousavi SB, Akhavan A, Haghighat A, Hashemi-Beni B, Razavi SM, Mashhadiabbas F (2015) Biodegradable nanocomposite coatings accelerate bone healing: in vivo evaluation. Dent Res J 12(1):89
Varma NP, Garai S (2012) Sinha a synthesis of injectable and cohesive nano hydroxyapatite scaffolds. J Mater Sci Mater Med 23(4):913–919
Gao A, Hang R, Huang X, Zhao L, Zhang X et al (2014) The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 35(13):4223–4235
Uysal T, Yagci A, Uysal B, Akdogan G (2010) Are nano-composites and nano-ionomers suitable for orthodontic bracket bonding? Eur J Orthod 32(1):78–82
Hosseinzadeh Nik T, Karimzadeh A, Ayatollahi MR (2013) Bond strength of a nano-composite used for bonding ceramic orthodontic brackets. Mater Des 51:902–906
Chalipa J, Akhondi MS, Arab S, Kharrazifard MJ, Ahmadyar M (2013) Evaluation of shear bond strength of orthodontic brackets bonded with nano-filled composites. J Dent (Tehran) 10(5):461–465
Coutinho E, Cardoso MV, De Munck J, Neves AA, Van Landuyt KL et al (2009) Bonding effectiveness and interfacial characterization of a nano-filled resin-modified glass-ionomer. Dent Mater 25(11):1347–1357
Craig BD, Kolb BU, Oxman JD, Peez RF, Frank SA (2006) Inventors; 3M Innovative Properties Company, assignee. Use of nanoparticles to adjust refractive index of dental compositions. United States patent US 7090721
Kolb BU, Bui HT, Thalacker JP, Kangas LS, Oxman JD, et al (2010) Inventors; 3M Innovative Properties Company, assignee. Dental compositions containing nanozirconia fillers. United States patent US 7649029
Carvalho FG, Brasil VL, Silva Filho TJ, Carlo HL, Santos RL et al (2013) Protective effect of calcium nanophosphate and CPP-ACP agents on enamel erosion. Braz Oral Res 27(6):463–470
Cordier P, Tournilhac F, Souli_e-Ziakovic C, Leibler L (2008) Self-healing and thermoreversible rubber from supramolecular assembly. Nature 451:977–980
Eliades T (2015) Orthodontic material applications over the past century: evolution of research methods to address clinical queries. Am J Orthod Dentofac Orthop 147(5):S224–S231
Gun’ko YK (2016) Nanoparticles in bioimaging. Nanomaterials (Basel). 6(6):105
Fan TX, Chow SK, Zhang D (2009) Biomorphic mineralization: from biology to materials. Prog Mater Sci 54(5):542–659
Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ et al (2008) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618
Gu H, Fan D, Gao J, Zou W, Peng Z, Zhao Z, Ling J, LeGeros RZ (2012) Effect of ZnCl 2 on plaque growth and biofilm vitality. Arch Oral Biol 57(4):369–375
Sodagar A, Bahador A, Khalil S, Shahroudi AS, Kassaee MZ (2013) The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J Prosthodont Res 57(1):15–19
Cushing BL, Kolesnichenko VL, O’Connor CJ (2004) Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev 104(9):3893–3946
Vega-Villa KR, Takemoto JK, Y´a˜nez JA, Remsberg CM, Forrest ML, Davies NM (2008) Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev 60(8):929–938
Klimuk SK, Semple SC, Nahirney PN et al (2000) Enhancedanti-inflammatory activity of a liposomal intercellular adhesion molecule-1 antisense oligodeoxynucleotide in an acutemodel of contact hypersensitivity. J Pharmacol Exp Ther 292(2):480–488
Chung Y-C, Chen I-H, Chen C-J (2008) The surface modification of silver nanoparticles by phosphoryl disulfides for improved biocompatibility and intracellular uptake. Biomaterials 29(12):1807–1816
Meng H, Chen Z, Xing G et al (2007) Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nanocopper particles. Toxicol Lett 175(1–3):102–110
Manin OI, Nikolaev VA, Kolomi˘ıtsev AA, Lebedenko II (2007) Comparative toxicological evaluation of domestic golden alloys for soldering. Stomatologiia 86(1):64–67
Bhattacharjee S, de Haan LH, Evers NM, Jiang X, Marcelis AT, Zuilhof H et al (2010) Role of surface charge and oxidative stressin cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol 7:25–36
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Batra, P. (2018). Applications of Nanoparticles in Orthodontics. In: Chaughule, R. (eds) Dental Applications of Nanotechnology. Springer, Cham. https://doi.org/10.1007/978-3-319-97634-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-97634-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-97633-4
Online ISBN: 978-3-319-97634-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)