Abstract
At over 1000 km in length, the Mesoamerican Reef (MAR) is a near-continuous reef system within four different countries: Mexico, Guatemala, Belize, and Honduras. MAR is the largest reef system in the Northern Hemisphere and is comprised of fringing and barrier reefs and offshore atolls, which extend into deeper water where mesophotic coral ecosystems (MCEs; 30–150 m depth) are found. Scientific studies of MCEs in the MAR began in the 1970s, and despite a rapid increase in marine research throughout the region in recent years, MCE ecological research has been restricted to a few locations and has been focused on hard (scleractinian) corals and fish communities. Hard corals have been documented at a maximum depth of 102 m in the MAR. However, hard corals do not represent the dominant benthic community at mesophotic depths in most cases. The benthic organisms providing structural habitat on many of the known MAR MCEs are octocorals, sponges, black corals, and calcareous macroalgae. Studies on MAR fish communities showed that a large proportion of fish species are found on both shallow reefs and MCEs. The limited data available suggests that MCEs are likely widespread along the MAR. There is evidence of the negative effects of fisheries, sedimentation, harvesting of black corals, and invasive lionfish on MAR MCEs. Improved identification and increase of biological and ecological studies of MCEs, coupled with an extension of scope to include mesophotic habitats in managed areas, should be undertaken to enhance their protection in the region.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
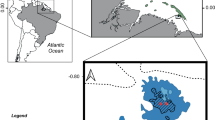
The Mesoamerican Reef (MAR) is a coral reef ecosystem approximately 1000 km long located on the western margin of the Caribbean Sea. It extends south from Cabo Catoche, Mexico, at the northern tip of the Yucatan Peninsula and runs along the coasts of Belize and Guatemala, before ending at the Bay Islands and fringing coastal reefs of Honduras (Fig. 5.1). The MAR is the largest reef system in the Northern Hemisphere and the second largest in the world, surpassed only by the Great Barrier Reef in Australia. The northern MAR consists of 300 km of fringing reefs along the east coast of the Yucatan Peninsula and extensive reefs around Cozumel Island and the Banco Chinchorro Atoll (Ardisson et al. 2011). The Belizean section at the center of the MAR spans approximately 250 km from Ambergris Caye to the Gulf of Honduras and encompasses a large barrier reef with hundreds of cays, along with three atolls seaward of the barrier reef (Turneffe Atoll, Glover’s Reef, and Lighthouse Reef) (Rützler and Macintyre 1982). Guatemala has the shortest MAR coastline, only 150 km long, with large river outflows limiting reef development (Wilkinson 2008; Kramer et al. 2015). In Honduras, the MAR runs from east to west, with fringing reefs along the Honduran Caribbean north coast, and extensive reef dominated offshore banks. Reefs are also associated with continental shelf islands (e.g., Cayos Cochinos) and islands on the continental shelf edge (e.g., the Bay Islands of Roatan, Guanaja, and Utila).
The MAR region comprises a vast linked system of coastal habitats and is considered a biodiversity hotspot. The MAR has high ecological, aesthetic, and cultural and economic value while sustaining more than two million people from four countries (Chollett et al. 2017), making it an international conservation priority. In this chapter, we review the current state of knowledge of mesophotic coral ecosystems (MCEs; 30–150 m depth) in the MAR region.
1.1 Research History
The first MAR scientific cruises recording marine diversity began in the late nineteenth century. Hundreds of species, including corals and fishes from depths down to 3000 m, were described based on the collections by the US Coast Survey vessel Blake (1877–1880) expeditions. However, it wasn’t until the 1970s that extensive taxonomic and ecological studies of MAR reefs began (Miloslavich et al. 2010). Observational studies using submersibles constitute the earliest modern qualitative analyses conducted on MAR MCEs (James and Ginsburg 1979). These early surveys often reported the zonation of different taxonomic groups across a depth gradient and included interesting species-specific observations alongside more general reef geomorphology. The first dedicated MAR MCE biodiversity survey conducted was fish observations reported by Colin (1974), who recorded many unidentifiable fish species off Belize. In the 1990s, shallow-reef surveys began to extend into the upper-mesophotic zone (30–60 m), for example, in Roatan (Fenner 1993) and in Cayos Cochinos (Guzman 1998). Also at this time, in Cozumel, species-specific studies on black corals (Antipatharia) were conducted driven by harvesting pressures for the jewelry industry (Padilla and Lara 2003; Padilla Souza 2004). More recent work has used combinations of technical diving, remote camera drops, and submarines to survey detailed fish and benthic community structure on MCEs in Mexico (Gress et al. 2018) and Honduras (Andradi-Brown et al. 2016c, d; Gress et al. 2017; Laverick et al. 2017). Other recent work has considered biases in fish detection caused by survey technique choice on MCEs in Honduras (Andradi-Brown et al. 2016d, 2017a). Despite this growing body of MAR MCE research, investigations have been confined to a few locations, and no studies have addressed whether recorded biodiversity patterns are representative of the whole region.
2 Environmental Setting
Sedimentation is a major problem for the MAR as a whole, which is exposed to terrestrial runoff annually (Chérubin et al. 2008). MAR health is affected by the proximity to shore, and the levels of riverine and sediment input, caused by land clearance activities (Harborne et al. 2001). At the southern end of the MAR (Fig. 5.1), reefs are subject to twice-yearly pulses of terrestrial runoff, caused by both the initial runoff impact itself, but then also by this runoff being cycled in a gyre that returns the pollutants to the reefs several months later (Paris and Chérubin 2008). Off Guatemala, the most established and best-studied reefs are the carbonated banks of Punta Manabique, which are dominated by sediment-tolerant coral species, because of the high sediment input (Wilkinson 2008; Kramer et al. 2015). The coastline of the Yucatan Peninsula lacks terrestrial freshwater input from surface rivers, as its limestone structure results in an outflow of freshwater through underground river systems accessible through sinkholes (Spalding et al. 2001). Although it is not clear how this affects sedimentation rates on the northern MAR.
Other environmental parameters are poorly surveyed in the MAR. Currents in the region can be strong, especially in the north, with average speeds of 0.61 and 0.54 m s−1 at 35 m depth and 0.54 and 0.47 m s−1 at 105 m depth when measured at specific points in 2006 and 2007, respectively (Muhling et al. 2013). In Belize, on the reef off Carrie Cay in June, photosynthetically active radiation readings of 28.1 W m−2 and 17.1 W m−2 were recorded in 30 m and 39 m depths, respectively, compared to 86.6 W m−2 in 12 m (Shick et al. 1996).
Large-scale reef geomorphology changes across the MAR affect the habitat availability for MCEs in different parts of the region. On the northern MAR, the insular shelf area is narrow off the western coast of Cozumel, at around 500 m width, but wide, at 2000 m width, off the northern and eastern coast (Günther 1990; Muckelbauer 1990) (Fig. 5.2). For the central MAR, the continental side of the barrier reef is separated from the mainland by a lagoon which ranges from 15 to 50 km in width and extends to 65 m in depth (Purdy et al. 1975; Miller and Macintyre 1977; James and Ginsburg 1979). The central MAR also encompasses four atolls, which have steep exposed drop-offs that extend to submarine canyons and the Cayman Trench (Etnoyer et al. 2015). The southern MAR is composed of coastal fringing reefs, reef banks on the approximately 75-km-wide continental shelf, and a series of reefs associated with islands on the shelf edge (Harborne et al. 2001).
Map and depth profiles across a shallow reef to MCE depth gradient for the MAR. The colors of the lines in the depth profiles match the colored dots in the map to indicate survey locations. Locations were southwest (red) and northwest (yellow) Roatan, northern (dark blue) and southern (light blue) Cozumel, and west (dark green) and southwest (light green) Glover’s Reef. Cozumel profiles are derived from multibeam data from the Texas A&M University (http://gcoos.tamu.edu/products/topography/SRTM30PLUS.html) and from bathymetric mapping conducted in September 2016 by Jacobo Caamal from Comunidad y Biodiversidad A.C, Mexico. Glover’s Reef profiles are redrawn from Figs. 3, 4, and 5 in James and Ginsburg (1979). Roatan profiles are derived from multibeam bathymetric mapping of the Roatan Peninsula conducted 9–10 July 2013 using Falkor’s Kongsberg EM302 and EM 710 accessed via NOAA’s National Geophysical Data Center (https://schmidtocean.org/serendipitous-side-trip). Roatan depths shallower than 40 m are taken from profiles redrawn from Mehrtens et al. (2001) and linear interpolation to the multibeam dataset
In Cozumel, three terraces can be found on the western shelf, at 5, 10, and 20 m depth, where the insular shelf edge begins (Muckelbauer 1990). On the eastern side, terraces have been observed at 3, 10, 20, 30, and 50 m depth. Below the western side terraces, the insular slope drops at an angle of 75–80° to a depth of 400 m, while on the eastern side, it drops near vertical to a depth of >1000 m (Muckelbauer 1990). In Belize, James and Ginsburg (1979) performed multiple submersible dives near Glover’s Reef and Tobacco, South Water, and Queen Cays down to depths of 300 m. Through exploration of these reefs, James and Ginsburg (1979) described the morphology of two distinct styles of platform margins found in the central MAR: (i) reef to shallow basin and (ii) reef to oceanic trough. They found that reef margins near a shallow basin typically exhibited a steep transition below spur-and-groove structure down to a step at approximately 30–37 m depth, except near South Water Cay, where the step is absent. Continuing seaward from the base of the step, a sandy slope progressively transitions to the top of a wall. In some locations, they observed a break in the slope near 45 m (Fig. 5.2). Belizean mesophotic depths near oceanic troughs either have a similar profile to shallow basin reef sites or, for the east side of Glover’s Reef, lack a distinct step and sandy slope. Instead, the east side of Glover’s Reef has a convex brow at 25–60 m depth, where a vertical wall begins and extends >100 m depth. On the southeastern margin of Glover’s Reef, the seaward portion of the reef exhibits a 45° slope, which terminates in a vertical wall at 30–40 m depth (Fig. 5.2).
In Honduras, on the south shore of Utila, the third largest Bay Island, shallow reefs form a spur-and-groove system, which transitions from a gentle reef slope to a flat seabed at approximately 30–40 m depth (Andradi-Brown et al. 2016c). Off the north shore of Utila, there is an extensive platform at 6 m depth, with the reef dropping near-vertically from approximately 8 m to >100 m depth. Several terraces, approximately 20 m wide, have been observed to break this steep wall at depths of 30, 55, and 70 m (Andradi-Brown et al. 2016b). On Roatan, at the southwest end of the island, there is a shallow terrace from the island edge that gently slopes to approximately 10 m depth, where the edge of the continental shelf edge steeply drops (Mehrtens et al. 2001; Fig. 5.2). Off the northwest of the island, there is an extensive terrace approximately 500 m wide at a depth of 40–60 m (Fig. 5.2).
3 Habitat Description
Patterns in reef geomorphology affects the MCE communities found across the MAR at different depths. Many MCE communities are found far from land on large continental shelf areas. This includes on reef banks, such as off Honduras (Harborne et al. 2001), and also on extensive patch reef communities, which have been observed in the Belize Reef lagoon (James and Ginsburg 1979; Miller and Macintyre 1977). In some cases, coastal shallow fringing reefs, exhibiting spur-and-groove structures, transition into MCE patch reef communities. For example, off the south shore of Utila, patch reefs in the 35–60 m depth range vary in area between 1 and 500 m2, and some raise to heights up to 3 m above the surrounding seabed (Fig. 5.3). These Utilian patch reefs share many species with the adjacent reef slope at 25–30 m depth (Andradi-Brown et al. 2016c). Shallow fringing reefs extend along most of the MAR coastline; however, little information on adjacent MCE communities is available.
On the steep walls associated with shelf edges, the most extensive MCEs have developed on terraces. For example, on the eastern side of Cozumel, the terraces at 30 and 50 m depth have hard coral (scleractinian) communities (Muckelbauer 1990), while off the north shore of Utila, extensive mesophotic hard coral communities have been recorded on terraces at depths of 30, 55, and 70 m (Andradi-Brown et al. 2016b; Laverick et al. 2017). Terraces, however, do not always contain well-developed MCEs. In northwest Roatan, the extended platform at 40–60 m depth is mainly covered with sand and a few scattered small MCE patch reefs, while at the terrace edge, MCEs are more extensive (Fig. 5.4b).
(a) Multibeam three-dimensional depth profile from the southwest coast of Roatan, showing images of the reefs at 20 m intervals from 30 to 150 m depth. In this area MCEs are located on steep slopes (Photo credit: Erika Gress and Karl Stanley). (b) Multibeam three-dimensional depth profile from the northwest coast of Roatan. Images show MCEs at 30, 40, and 60 m depth. The extended platform at 40–60 m depth is mainly covered with sand and a few scattered small MCE patch reefs (Photo credit: Erika Gress). See Fig. 5.2 legend for locations and bathymetry sources
MCE communities can also form as continuous reefs across the depth gradient, for example, on the steep slopes on the western side of Cozumel (Gress et al. 2018) and in southwest Roatan (Fig. 5.4a). Steep slopes and walls rapidly reduce light levels available for benthic organisms (Brakel 1979; Baker et al. 2016). Therefore, although hard corals are present, MCEs on steeper slopes and walls tend to be dominated by heterotrophic feeding organisms such as sponges, gorgonians, and black corals, which provide most of the structural habitat (Gress et al. 2018).
4 Biodiversity
4.1 Macroalgae
With the exception of a few locations, little is known about algal coverage on MCEs in the MAR. Macroalgal cover declines with depth on reefs around Utila, Honduras, for example, from 45% of benthic habitat cover at 15 m to 8% at 40 m (Andradi-Brown et al. 2016c), and is near-absent at 70 and 85 m (Laverick et al. 2017). However, this decline may be particularly severe on Utila because of the rapid decline in hard substrata availability for the majority of surveyed MCE communities (Andradi-Brown et al. 2016c). In contrast, in Cozumel, Mexico, macroalgae broadly increases in coverage between 15 and 55 m depth (Gress et al. 2018). For example, adjacent to tourist developments, fleshy and calcareous macroalgae increased from 5% and 12% at 15 m to 21% and 24% at 55 m, respectively. Within the Cozumel protected area, calcareous macroalgae increased from 22% at 15 m to 39% at 55 m, though fleshy macroalgae declined from 10% at 15 m to 5% at 55 m. Much of the increase in calcareous macroalgae with increased depth is driven by large areas of Halimeda spp. on MCEs (Gress et al. 2018). In Belize, Halimeda spp. commonly grow on sediment slopes and on rock surfaces to a depth of approximately 75 m. Below 75 m, Halimeda spp. are rare or absent, although a solitary deep attached specimen of the genus was observed at 110 m (James and Ginsburg 1979). Crustose coralline algae (CCA) are common on MCEs formed on vertical walls in Belize from 40 to 110 m. From 75 to 90 m, CCA covers an estimated 30–50% of the exposed benthos, and the deepest known CCA in the region was reported at 250 m (James and Ginsburg 1979).
The most detailed study of mesophotic macroalgae has been conducted on upper MCEs (30–60 m) in Belize. To assemble a list of marine plants found around Glover’s Reef, Tsuda and Dawes (1974) collected specimens from six locations at 30–40 m depth. They recorded at least 15 species of green algae, 5 species of brown algae, and 8 species of red algae from mesophotic depths (Table 5.1). In addition, near Carrie Bow Cay, algae were collected from two seaward sites on the barrier reef at depths of 30–40 m (Norris and Bucher 1982). The most abundant algae found on the fore-reef slope of Carry Bow Cay to 40 m were Lobophora variegata, Stypopodium zonale, and Anadyomene stellata; Galaxaura obtusa and Kallymenia limminghei were less common but only found at deeper depths (Norris and Bucher 1982). There have also been sightings of a large bloom of Anadyomene gigantodictyon near Carrie Bow Cay, covering on average 30% of the benthos at 51–60 m deep along approximately 0.5 km section of MCE known as South Reef (Littler and Littler 2012). It is important for future work to expand algal surveys to another MAR MCE locations.
4.2 Anthozoans
Scleractinians. Zooxanthellate hard corals have been documented down to 55 m in Mexico (Gress et al. 2018), although research on deeper MCEs has not been conducted. The deepest recorded colonies to date in Belize are Montastraea cavernosa at 95 m off Tobacco Cay and Agaricia fragilis at 102 m on the eastern margin of Glover’s Reef (James and Ginsburg 1979). In Honduras, the maximum observed hard coral depths were 85 m in Utila (Laverick et al. 2017) and 91 m in Roatan (Gress, per. obs.). Similar to other geographical regions (Baker et al. 2016), hard corals take on a more plate-like morphology or are found as small individual colonies at increased depths (Graus and Macintyre 1976; Miller and Macintyre 1977; James and Ginsburg 1979).
Hard coral cover declines rapidly with depth along the slope on most studied MAR MCEs. For example, in Cozumel National Marine Park, hard coral cover was recorded as 9% at 15 m to 1% at 55 m (Gress et al. 2018). Around Utila, hard coral cover was 14% at 15 m but declined to 4% at 40 m (Andradi-Brown et al. 2016c). However, on steeper more exposed reef walls around Utila, hard coral cover has been recorded as 8%, 2%, and <1% at 55, 70, and 85 m, respectively (Laverick et al. 2017). Hard coral cover has not been quantified using transects on MCEs in Belize, though James and Ginsburg (1979) noted Orbicella annularis and Agaricia spp. between 37 and 45 m depth off Queen Cay grew in large overlapping plates, and constituted >25% of the benthic cover at all sites surveyed.
Around Cozumel, hard corals from the genera Helioseris and Agaricia were among the most common recorded at 55 m; however, colonies were small on average, with MCEs dominated by sponges, gorgonians, calcareous algae, and black coral communities (Günther 1990; Gress et al. 2018). This characteristic of few hard corals with foliose and encrusting formations at upper-mesophotic depths was reported in early qualitative observations from the 1980s on the west side of Cozumel (Jordán Dahlgren 1988; Zlatarski 2007). Other scleractinian species were documented in these early observations on Chinchorro Atoll and on the Mexican Caribbean mainland coast between 30 and 45 m depth (Table 5.1; Zlatarski 2007).
In Belize, James and Ginsburg (1979) observed hard coral species across the MCE depth gradient (Table 5.1). They reported Orbicella spp. and Agaricia spp. from 37 m down to the beginning of the wall at 65 m, followed by A. grahamae, A. fragilis, M. cavernosa, Madracis sp., Solenastrea sp., Stephanocoenia sp., and Mycetophyllia reesi at 70–80 m depth. On the upper margin of the mesophotic zone at 31 m, 42 species of hard corals have been verified from field samples (Cairns 1982). Cairns (1982) noted finding Porites astreoides to depths of >28 m, M. cavernosa to >26 m, Dichocoenia stokesii to 31 m, Scolymia cubensis from 30 to 40 m, as well as Meandrina meandrites and Scolymia lacera commonly on the fore-reef slope down to 40 m. Survey transects conducted from the seagrass flats eastward to the outer fore-reef slope just north of Carrie Bow Cay demonstrated relatively abundant plating colonies of M. cavernosa, O. annularis, Helioseris cucullata, and A. fragilis at 30 m (Rützler and Macintyre 1982). While less abundant, A. agaricites, A. lamarcki, P. porites, P. furcata, Diploria labyrinthiformis, Colpophyllia natans, and Mycetophyllia danaana were all observed near 30 m in the vicinity of Carrie Bow Cay (Rützler and Macintyre 1982).
In Honduras, the most detailed mesophotic hard coral community composition analysis was conducted by Laverick et al. (2017) down to 85 m depth around Utila (Table 5.1). A total of 26 hard coral species were recorded on MCEs, with the majority of these species also found on shallow reefs. A. grahamae, A. undata, M. formosa, and M. senaria were only found below 25 m, while the only MCE coral species found exclusively deeper than 40 m was M. pharensis. In addition to those species, H. cucullata, M. cavernosa, Siderastrea siderea, and S. intersepta were also recorded reaching 70 m or deeper (Laverick et al. 2017). Also in Utila, there is evidence that historically extensive Agaricia spp. colonies were present at 70 m depth, although the majority of these colonies were dead when observations were conducted in 2015 (Laverick et al. 2017). In Roatan, benthic surveys were conducted by Mehrtens et al. (2001) down to 40 m, recording Agaricia spp., Porites spp., Diploria spp., and Montastrea sp. in the 30–40 m range.
Antipatharians. Black corals make up a major component of some MAR MCEs. In Cozumel, which has long been famed for its extensive MCE black coral populations, six black coral species have been recorded (Padilla Souza 2004). In Honduras, black corals have been observed on MCEs to >50 m depth around Barbareta Island (Fonseca et al. 2004), though their exact population density and composition has not been established. Around Cayos Cochinos, Honduras, five species of black coral have been recorded from surveys down to 35 m depth (Guzman 1998).
4.3 Sponges
Sponges have been poorly studied on most MCEs in the MAR. The few studies conducted suggest sponge cover on MCEs is highly variable. For example, in the Cozumel National Marine Park, sponge cover increased with depth from 25% at 15 m to 39% at 55 m (Gress et al. 2018). One study in Utila reported sponge cover declining with increased depth, from 4% at 15 m to 1% at 40 m (Andradi-Brown et al. 2016c), though another study recorded sponge cover of approximately 20% at several Utilian sites in the 40–70 m depth range (Laverick et al. 2017). Despite many publications on sponges from the Belize Barrier Reef, most sponge research has been limited to shallow reefs, which exhibit high sponge abundance and diversity (Rützler 2012). On the outer fore reef at Carrie Bow Cay, demosponges were recorded at about 10% cover, with 29 species found deeper than 30 m (Diaz and Rützler 2001). On the south of Carrie Bow Cay, the giant barrel sponge Xestospongia muta has been observed on the fore-reef slope to depths >60 m and was found to spawn deeper than 20 m (Ritson-Williams et al. 2005). Sponges make up a major component of benthic cover on some MAR MCEs, but further research is required to understand the complexity of observed patterns.
4.4 Fishes
Fish communities on MCEs in the MAR have been well documented, with several published studies. The earliest observations came from Belize below 50 m at Tobacco Reef, Queen Cays, and Glover’s Reef (Colin 1974). On Utila, fish species richness, abundance, and biomass declined with increased depth across a 5–40 m depth gradient, though individual fish of several species were larger on deeper reefs when compared to shallower reefs (Andradi-Brown et al. 2016c). On the deeper portions of the Belize Barrier Reef, 33 fish species were recorded comprising 15 families (Colin 1974). At Glover’s Reef transects were conducted at 90 m, where the commonest fishes observed were Gramma melacara, Gramma sp., Lipogramma klayi, Liopropoma mowbrayi, and Serranus luciopercanus (Colin 1974). More recent fish studies have focused on the reefs of Utila (Andradi-Brown et al. 2016b, c, d, 2017a, b, d). Common fishes observed on Utilian reefs in the 70–85 m depth range include the Sunshine fish (Chromis insolata) and blackfin snapper (Lutjanus buccanella) (Andradi-Brown et al. 2016b). Another deeper observation from Honduras includes the hourglass basslet (Lipogramma levinsoni) at 140 m off Roatan (Baldwin et al. 2016). The majority of fish species observed at 40 m around Utila are depth-generalist species, also found on adjacent shallow reefs (Andradi-Brown et al. 2016c). More detailed fish community surveys have been conducted to look at changes in reef fish biomass across a depth gradient. For example, several species of Scaridae were found at reduced proportions of community biomass on MCEs (Andradi-Brown et al. 2016c). Six species of fish, spanning several trophic groups, increased body size on MCEs compared to shallow reefs, Acanthurus coeruleus, Chromis cyanea, Thalassoma bifasciatum, Clepticus parrae, Ocyurus chrysurus, and Scarus iserti (Andradi-Brown et al. 2016c). Additionally, several locally threatened species, such as Caribbean reef sharks (Carcharhinus perezii), which are absent from shallow fringing reefs, were observed at depths >50 m (Andradi-Brown et al. 2016b). Other shark species, such as black nose shark (Carcharhinus acronotus), not previously reported in fisheries-independent monitoring data from Utilian fringing reefs have been observed at >40 m depth (Andradi-Brown et al. 2016d).
In Cozumel, Mexico, fish communities have been characterized by Gress et al. (2018). Of the total fish species identified, 9% were present only on MCEs, while 43% were recorded on both shallow and mesophotic reefs, including many commercially important fish species. This is similar to Honduras, where the majority of fish at 40 m were also present on shallow reefs (Andradi-Brown et al. 2016c). Of the major commercially important fishes in Cozumel, three families (Acanthuridae, Haemulidae, and Millidae) showed lower biomass at 55 m compared to 15 m, while one family showed greater biomass (Pomacanthidae) (Gress et al. 2018).
Non-native lionfish (Pterois spp.) have widely colonized shallow reefs and MCEs across the region (Andradi-Brown et al. 2017b, d). In 2010, lionfish were observed on Roatan MCEs at 122 m, making this one of the earliest MCE invasive lionfish records for Honduras (Schofield 2010). More recent observations from Roatan have extended into the upper-bathyal zone (200–1000 m depth), with lionfish observed down to 250 m (Gress et al. 2017). In Mexico, lionfish have been observed at 55 m (Gress et al. 2018) and in Belize at depths of 50 m (Voss and Eckert, pers. obs.), though it is likely that they extend deeper.
Across the MAR region, Muhling et al. (2013) studied the vertical depth distribution of fish larvae. While these samples were not directly taken on the reef, in many cases, they were taken in locations adjacent to reefs in Mexico and Belize. Muhling et al. (2013) reported larvae for common reef fish species such as Thalassoma bifasciatum were mostly restricted to shallow waters (< 50 m) during the day, with a few individuals in the 50–75 m range. At night individuals were collected down to the 75–100 m range. Many other common reef fish genera such as Sparisoma, Scarus, and Acanthurus larvae were identified as well but found to be limited to 40–60 m depth (Muhling et al. 2013). A reef-associated planktonic light-trapping study was conducted on shallow reefs (15 m) and MCEs (40 m) on Utila, finding no differences in fish larvae abundance between depths (Andradi-Brown et al. 2017c). However, these results are affected by limited sampling time, so do not account for known seasonality in fish larval recruitment (Luckhurst and Luckhurst 1977).
4.5 Other Biotic Components
Reef-associated MCE zooplankton studies have been conducted by Andradi-Brown et al. (2017c) in Honduras using light traps to collect plankton samples overnight. These samples suggested similar planktonic community richness and overall biomass across the depth gradient, but with high variation and differences between survey sites (Andradi-Brown et al. 2017c). Larger zooplankton organisms (>2 mm body size) were found to be more abundant on MCEs than adjacent shallow reefs. These larger organisms included groups such as decapod crab zoeae, mysid shrimps, peracarid crustaceans, and oligochaetes (Andradi-Brown et al. 2017c).
5 Ecology
The majority of detailed ecological work on MAR MCEs has been conducted on Utila and focused on hard coral or fish communities. Hard coral communities around Utila were studied by Laverick et al. (2017) across a 5–85 m depth gradient, with the aim of refining how to define a mesophotic coral community. To do this, multivariate statistical techniques were used to initially identify natural depth groupings of hard coral species based on different sites and depths and then to look at the depths of community transition between the groupings. Two groups of hard corals were found, a shallow specialist community and a depth-generalist community (Laverick et al. 2017). The shallow specialist community was dominant at 5 and 15 m, while the depth-generalist community was dominant at 40–85 m, although many coral species associated with the depth-generalist community were also present at 5 and 15 m, but at lower abundance, suggesting that other factors such as shallow-reef environmental conditions or competition with shallow specialist species could be limiting their abundance (Laverick et al. 2017).
Fish communities on MCEs contain a range of trophic groups, whose proportions depend on whether weighting by abundance or biomass. Planktivores, comprising 35% of the community, are the most numerous reef fishes at 40 m around Utila; though this is a lower proportion than the 63%, they comprise on adjacent shallow reefs (Andradi-Brown et al. 2016c). Piscivores are the largest trophic group by biomass at 40 m, making up 37% of the community, though herbivores and invertebrate feeders make up substantial community components (Andradi-Brown et al. 2016c). Herbivores are the largest group on shallow reefs; despite being present on MCEs, they decline as a proportion of the overall fish community with increased depth (Andradi-Brown et al. 2016c). On even deeper Utilian reefs in the 70–85 m range, herbivores are near-absent, and the most commonly observed fish species is the planktivorous sunshine fish (Chromis insolata) (Andradi-Brown et al. 2016b).
To better understand the relative roles of fishes in structuring benthic communities compared to other environmental variables, such as light, Andradi-Brown (2017) conducted a factorial experimental manipulation on reefs at 30–40 m depth on Utila. Results indicated that light plays a crucial role in structuring MCE benthic communities. While hard coral and sponge cover declined when fish were excluded, these results might have been caused by the reduction in water flow from the fish-exclusion cages. Therefore, despite the presence of herbivores on MCEs, they are unlikely to be the main structuring agents of Utilian MCE benthic macroalgal communities (Andradi-Brown 2017).
A wide range of fish survey techniques have been used to study MCE fish communities, both on the MAR, but also globally. Two studies have been conducted on the reefs of Utila to evaluate how the survey technique choice affects biases in recorded MCE fish communities (Andradi-Brown et al. 2016d, 2017a). Diver-operated stereo-video (DOV) transects were compared with baited remote underwater video stations (BRUVS) on both shallow reefs and MCEs to identify depth-specific biases between these techniques (Andradi-Brown et al. 2016d). BRUVS consistently recorded more species than DOV on both shallow reefs and MCEs. Some of the identified differences between both techniques were found to vary with depth, with BRUVS better able to detect smaller fish on MCEs. Broadly, BRUVS were found to be better for recording all components of fish communities but took substantially longer to analyze. As well as being much quicker to analyze, DOV was reliable for surveying key functional groups such as herbivores. A second study on Utilian reefs evaluating how method choice affects recorded fish communities compared DOV transects filmed with open-circuit SCUBA on shallow reefs and MCEs with those filmed using closed-circuit rebreather (CCR) (Andradi-Brown et al. 2017a). The minimum approach distance (MAD), the distance at which divers could approach a fish before it moved away, was measured for both dive gear types. While CCR had lower MADs, implying divers could get closer to fishes using CCR than open-circuit SCUBA, the differences were not large enough to alter the detectability of most MAR fish families surveyed. However, for many fish families, regardless of dive gear choice, divers were able to approach individuals more closely on MCEs than shallow reefs, and larger fishes were warier of divers than smaller fish.
6 Threats and Conservation Issues
Many potential MCE threats, such as fishing pressure, harvesting of key benthic species, invasive species, sedimentation, and water pollution, have been identified (Andradi-Brown et al. 2016a; Gress et al. 2017, 2018). However, there is little long-term monitoring of MCE health in the MAR, and MCEs are rarely incorporated into marine-protected areas (MPAs). In 2016, an expanded MPA encompassing the majority of the Mexican Caribbean was announced. It comprises approximately 57,000 km2 of marine habitats in the northern MAR area, with about 19,000 km2 in core zones, and the rest in buffer zones. Legislation and management plans for this vast new area are still to be announced (Diario Oficial de la Federación 2016). Since 1982, an MPA network has been established in Belize, encompassing over 3600 km2, approximately 20% of the country’s territorial sea. No-take zones comprise roughly 14% of Belize’s total established MPA area. In Guatemala, only about 62 km2 of coral reef area is known, but over 900 km2 of MPAs have been created mostly in Punta de Manabique reserve (Wilkinson 2008; Kramer et al. 2015). In Honduras, the entirety of the Bay Islands waters were designated a National Park in 1997 (Kramer et al. 2015). This protected area extends 12 nautical miles around the coasts of the Islands of Guanaja, Roatan, and Utila.
Management and enforcement of fisheries regulations on the southern MAR is generally weak, with overexploitation in many areas of Honduras (Gobert et al. 2005; Korda et al. 2008). Gobert et al. (2005) compared the relative contribution of the shallow-water and mesophotic fishery for snapper and grouper species throughout the Bay Islands of Honduras, finding that the mesophotic snapper fishery was significant in the region. Large (>500 mm) predatory fishes are rare on MCEs in both Cozumel and Utila, suggesting that these fisheries are having an impact on fish-length distributions (Andradi-Brown et al. 2016c; Gress et al. 2018). In Cozumel, there was little difference in MCE fish-length distributions when comparisons were made between locations inside the National Marine Park and locations without protection (Gress et al. 2018). This could suggest that depth is naturally acting as a refuge from fisheries, unfortunately, the lack of large predatory fish at both locations is likely an indicator that the existing shallow-focused marine park is not providing protection for mesophotic fish communities (Gress et al. 2018). There is some evidence of MCEs acting as fish refuges on the MAR. The north coast of Honduras historically had a large shark fishery leading to shark population declines (Box and Canty 2011). This fishery was restricted in 2010 (Box and Canty 2011), though many shark species that were historically present on Utilian shallow reefs remain absent. Recently, some of these shark species have been recorded on MCEs (Andradi-Brown et al. 2016b, d), suggesting that MCEs may offer protection for some sharks.
During 2015, Laverick et al. (2017) observed extensive dead plating Agaricia spp. at 70 m depth in Utila, suggesting there were large colonies at these depths. Andradi-Brown (2017) monitored reef areas at 30–40 m depth at one site on Utila, using permanent photo-quadrats over an 18-month period during 2015–2016. While their study was not intending to monitor background changes in reef health, they detected a slight, but significant, decline in hard coral cover over this short period (Andradi-Brown 2017). During a coral bleaching event in 2015, Laverick and Rogers (2018) conducted a transplant experiment on Utila, moving fragments of A. lamarcki between shallow and mesophotic depths along with controls. They found that fragments placed at mesophotic depths had lower bleaching rates and greater survival than fragments placed on shallow reefs. However, bleaching was observed in MCE fragments and in A. lamarcki colonies around Utila down to 35 m depth (Laverick and Rogers 2018).
Sedimentation is a major threat to reefs in the MAR region and has long been highlighted as a key issue to address (Harborne et al. 2001). In Banco Capiro, an offshore reef in Tela Bay, on the Honduran mainland, some preliminary dives looking for MCE communities were conducted to 42 m during 2015. Despite having the highest shallow-reef coral cover reported in the MAR (Bodmer et al. 2015), no MCEs were observed. Instead, at the base of the offshore bank, an extensive sediment bed was found smothering patch reefs comprised of dead coral skeletons that still had some fishes associated with them (Andradi-Brown, pers. obs.).
Cozumel has long been famed for its extensive mesophotic black coral populations and the associated black coral jewelry industry (Kenyon 1984). The majority of the jewelry is made from Antipathes caribbeana and Plumapathes pennacea, as these species form the largest colonies, with skeletons that are cut, polished, and then handcrafted into jewelry. In the Mexican Caribbean, the earliest records of black corals harvesting date from the early 1960s. While the availability of black coral jewelry on Cozumel has declined in recent years, shops selling black coral jewelry and handcrafts are still widespread (Gress, pers. obs.). Regional harvest rates reported from the 1980s to early 1990s were between 1000 and 1500 kg of gross product per year, causing overexploitation of black coral in the area (Padilla and Lara 2003). Declining black coral population densities in Cozumel have been observed since the 1970s fishers, although no population assessments were conducted until the late 1990s. In 1994, three species of black corals were included in the Mexican Official Norm that lists national threatened species under special protection: Antipathes dichotoma (wrongly cited as A. bichitoena), A. grandis, and A. ulex (wrongly cited as A. ules) (Padilla and Lara 2003). The inclusion of these three species was however a mistake, as they have not been recorded in Mexican waters on either the Atlantic or Pacific coast (Opresko and Sanchez 2005). The absence of these species was revealed by the first population assessment conducted in 1998 (Padilla Souza 2004). The only other population assessment, allowing changes in population density and colony size for the two primary harvested species to be calculated, was conducted during summer 2016 (Gress and Andradi-Brown 2018). Results indicate that the P. pennacea population density has severely declined, though the remaining mesophotic colonies are larger on average than those recorded in the 1990s, suggesting that there is low recruitment or juvenile survival rates (Gress and Andradi-Brown 2018).
Non-native lionfish are highly abundant on MCEs in some locations in the MAR, such as the Bay Islands of Honduras (Gress et al. 2017; Andradi-Brown et al. 2017b, d) but at lower densities on MCEs in other areas such as Cozumel (Gress et al. 2018). The greatest density recorded on MAR MCEs is in Utila, where lionfish reached 3.0 individuals per 250 m2 at 70 m and were found to be feeding on native fishes (Andradi-Brown et al. 2017b). While lionfish are known to exhibit ontogenetic migrations across shallow tropical marine habitats (Claydon et al. 2012), it is not clear whether these extend to MCEs. Utilian MCE lionfish behaviorally respond to divers in similar ways as lionfish populations previously exposed to culling in shallower reefs (i.e., skittish) (Andradi-Brown et al. 2017b), supporting the idea of ontogenetic migration. Yet, culling alters lionfish body-size distributions (Frazer et al. 2012), making ontogenetic migrations difficult to detect in areas with depth-restricted culling. See Andradi-Brown (2019) for a review of lionfish on MCEs. Work on Utila has identified that lionfish on MCEs are larger, and females are more mature for their body size than individuals found in adjacent shallow reefs (Andradi-Brown et al. 2017b, d). Concern has been raised whether substantial mesophotic populations could be undermining current lionfish management, which is broadly shallow-reef focused (Andradi-Brown et al. 2017d). Taken together, these observations suggest that MCE health on the southern MAR is likely declining, though more research and long-term monitoring is required to establish this across the region.
7 Conclusion
MCE research in the MAR is still in an early stage, with the majority of studies conducted restricted to a few key sites such as Cozumel, the atolls of Belize, Utila, and to a lesser extent Roatan. We were unable to identify any MCE studies from Guatemala. In addition, much of the MCE research conducted in the region has focused on upper MCEs, with few quantitative surveys conducted on lower MCEs (60–150 m). Existing studies are also site-specific, with no directly reef-associated studies incorporating multiple geographical locations along the length of the MAR to test for regional patterns. These are major knowledge gaps that future studies should address. Many threats to MCEs on the MAR are common throughout the Caribbean and wider western Atlantic, and there is an urgent need to address these. As the second largest reef system in the world, with four countries involved in management, this will inevitably be complex. However, because of their inherent biodiversity, and the potential role that MCEs may play underpinning overall reef resilience, it is crucial to better incorporate them into reef management plans.
References
Andradi-Brown D, Laverick J, Bejarano I et al (2016a) Threats to mesophotic coral ecosystems and management options. In: Baker EK, Puglise KA, Harris PT (eds) Mesophotic coral ecosystems – a lifeboat for coral reefs? The United Nations Environment Programme and GRID-Arendal, Nairobi/Arendal, pp 67–82
Andradi-Brown DA, East A, Shepherd LM et al (2016b) Challenges and opportunities in conducting mesophotic reef research. Reef Encount 31:26–31
Andradi-Brown DA, Gress E, Wright G et al (2016c) Reef fish community biomass and trophic structure changes across shallow to upper-Mesophotic reefs in the Mesoamerican barrier reef, Caribbean. PLoS ONE 11:e0156641
Andradi-Brown DA, Macaya-Solis C, Exton DA et al (2016d) Assessing Caribbean shallow and mesophotic reef fish communities using baited-remote underwater video (BRUV) and diver-operated video (DOV) survey techniques. PLoS ONE 11:e0168235
Andradi-Brown DA (2017) Fish ecology of mesophotic coral ecosystems. Dissertation, University of Oxford
Andradi-Brown DA, Gress E, Laverick JH et al (2017a) Wariness of reef fish to passive diver presence with varying dive gear type across a coral reef depth gradient. J Mar Biol Assoc UK
Andradi-Brown DA, Grey R, Hendrix A et al (2017b) Depth-dependent effects of culling—do mesophotic lionfish populations undermine current management? R Soc Open Sci 4:170027
Andradi-Brown DA, Head CEI, Exton DA et al (2017c) Identifying zooplankton community changes between shallow and upper-mesophotic reefs on the Mesoamerican barrier reef, Caribbean. PeerJ 5:e2853
Andradi-Brown DA, Vermeij MJA, Slattery M et al (2017d) Large-scale invasion of western Atlantic mesophotic reefs by lionfish potentially undermines culling-based management. Biol Invasions 19:939–954
Andradi-Brown DA (2019) Invasive lionfish (Pterois volitans and P. miles): distribution, impact, and management. In: Loya Y, Puglise KA, Bridge T (eds) Mesophotic coral ecosystems of the world. Springer, New York, pp 931–941
Ardisson PL, May-Ku MA, Herrera-Dorantes MT, Arellano-Guillermo A (2011) El Sistema Arrecifal Mesoamericano-México: consideraciones para su designación como Zona Marítima Especialmente Sensible/The Mesoamerican Barrier Reef System-Mexico: considerations for its designation as a Particularly Sensitive Sea Area. Hidrobiológica 21:261–280
Baker E, Puglise KA, Colin PL et al (2016) What are mesophotic coral ecosystems? In: Baker EK, Puglise KA, Harris PT (eds) Mesophotic coral ecosystems – a lifeboat for coral reefs? The United Nations Environment Programme and GRID-Arendal, Nairobi/Arendal, pp 11–19
Baldwin CC, Robertson DR, Nonaka A, Tornabene L (2016) Two new deep-reef basslets (Teleostei, Grammatidae, Lipogramma), with comments on the eco-evolutionary relationships of the genus. ZooKeys 638:45–82
Bodmer MDV, Rogers AD, Speight MR et al (2015) Using an isolated population boom to explore barriers to recovery in the keystone Caribbean coral reef herbivore Diadema antillarum. Coral Reefs 34:1011–1021
Box SJ, Canty SWJ (2011) The long and short term economic drivers of overexploitation in Honduran coral reef fisheries due to their dependence on export markets. In: Proceedings of the 63rd Gulf and Caribbean Fisheries Institute, November 2010, San Juan, pp 43–51
Brakel WH (1979) Small-scale spatial variation in light available to coral reef benthos: quantum irradiance measurements from a Jamaican reef. Bull Mar Sci 29:406–413
Cairns SD (1982) Stony corals (Cnidaria: hydrozoa, Scleractinia) of Carrie Bow Cay, Belize. In: Rützler K, Macintyre IG (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, I structure and communities. Smithsonian Institution Press, Washington, DC, pp 271–302
Chérubin LM, Kuchinke CP, Paris CB (2008) Ocean circulation and terrestrial runoff dynamics in the Mesoamerican region from spectral optimization of SeaWiFS data and a high resolution simulation. Coral Reefs 27:503–519
Chollett I, Garavelli L, Holstein D, Chérubin L, Fulton S, Box SJ (2017) A case for redefining the boundaries of the Mesoamerican reef ecoregion. Coral Reefs 36:1039–1046
Claydon J, Calosso MC, Traiger SB (2012) Progression of invasive lionfish in seagrass, mangrove and reef habitats. Mar Ecol Prog Ser 448:119–129
Colin PL (1974) Observation and collection of deep-reef fishes off the coasts of Jamaica and British Honduras (Belize). Mar Biol 24:29–38
Diario Oficial de la Federación (2016) Mexican government decree. http://www.dof.gob.mx/nota_detalle.php?codigo=5464450. Accessed 1 Nov 2017
Diaz MC, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Etnoyer PJ, Brennan ML, Finamore D et al (2015) Exploration and mapping of the deep Mesoamerican reef. Oceanography 28:34–35
Fenner DP (1993) Some reefs and corals of Roatan, Cayman Brac, and Little Cayman. Atoll Res Bull 388:1–30
Fonseca A, Breedy O, Gamboa C et al (2004) Rapid ecological assessment of the reefs of Barbareta Island (Honduras) and proposed boundaries for a marine reserve. WWF Central America, Guatemala
Frazer TK, Jacoby CA, Edwards MA et al (2012) Coping with the lionfish invasion: can targeted removals yield beneficial effects? Rev Fish Sci 20:185–191
Gobert B, Berthou P, Lopez E et al (2005) Early stages of snapper–grouper exploitation in the Caribbean (Bay Islands, Honduras). Fish Res 73:159–169
Graus RR, Macintyre IG (1976) Light control of growth form in colonial reef corals: computer simulation. Science 193:4256
Gress E, Arroyo-Gerez MJ, Wright G, Andradi-Brown DA (2018) Assessing mesophotic coral ecosystems inside and outside a Caribbean marine protected area. R Soc Open Sci 5:180835
Gress E, Andradi-Brown DA, Woodall L, Schofield PJ, Stanley K, Rogers AD (2017) Lionfish (Pterois spp.) invade the upper-bathyal zone in the western Atlantic. PeerJ 5:e3683
Gress E, Andradi-Brown DA (2018) Assessing population changes of historically overexploited black corals (Order: Antipatharia) in Cozumel, Mexico. PeerJ 6:e5129
Günther A (1990) Distribution and bathymetric zonation of shell-boring endoliths in recent reef and shelf environments: Cozumel, Yucatan (Mexico). Facies 22:233–261
Guzman HM (1998) Diversity of stony, soft and black corals (Anthozoa: Scleractinia, Gorgonacea, Antipatharia; Hydrozoa: Milleporina) at Cayos Cochinos, Bay Islands, Honduras. Rev Biol Trop 46(Supl 4):75–80
Harborne AR, Afzal DC, Andrews MJ (2001) Honduras: Caribbean coast. Mar Pollut Bull 42:1221–1235
James NP, Ginsburg RN (1979) The seaward margin of Belize barrier and atoll reefs. Blackwell, Oxford
Jordán Dahlgren E (1988) Arrecifes profundos en la Isla de Cozumel, México. An Inst Cienc del Mary Limnol. Univ Nal Auton México 15:634
Kenyon J (1984) Black coral off Cozumel. Sea Front 30:267–272
Korda RC, Hills JM, Gray TS (2008) Fishery decline in Utila: disentangling the web of governance. Mar Policy 32:968–979
Kramer P, McField M, Álvarez-Filip L, et al (2015) 2015 report card for the Mesoamerican reef. Healthy Reefs Initiative, www.healthyreefs.org
Laverick JH, Andradi-Brown DA, Rogers AD (2017) Using light-dependent scleractinia to define the upper boundary of mesophotic coral ecosystems on the reefs of Utila, Honduras. PLoS ONE 12:e0183075
Laverick JH, Rogers AD (2018) Experimental evidence for reduced mortality of Agaricia lamarcki on a mesophotic reef. Mar Environ Res
Littler MM, Littler DS (2012) Bloom of the giant anadyomene Gigantodictyon sp. nov. (Anadyomenaceae, Cladophorales) from the outer slope (25–50m) of the Belize barrier reef. J Phycol 48:60–63
Luckhurst BE, Luckhurst K (1977) Recruitment patterns of coral reef fishes on the fringing reef of Curaçao, Netherlands Antilles. Can J Zool 55:681–689
Mehrtens CJ, Rosenheim B, Modley M, Young RS (2001) Reef morphology and sediment attributes, Roatan, Bay Islands, Honduras. Carbonates Evaporites 16:131–140
Miller JA, Macintyre IG (1977) Field guidebook to the reefs of Belize. Atlantic Reef Committee, University of Miami, Miami
Miloslavich P, Díaz JM, Klein E et al (2010) Marine biodiversity in the Caribbean: regional estimates and distribution patterns. PLoS ONE 5:e11916
Muckelbauer G (1990) The shelf of Cozumel, Mexico: topography and organisms. Facies 23:185–239
Muhling BA, Smith RH, Vásquez-Yeomans L et al (2013) Larval fish assemblages and mesoscale oceanographic structure along the Mesoamerican barrier reef system. Fish Oceanogr 22:409–428
Norris JN, Bucher KE (1982) Marine algae and seagrasses from Carrie Bow Cay, Belize. In: Rützler K, Macintyre IG (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, I structure and communities. Smithsonian Institution Press, Washington, DC, pp 417–432
Opresko DM, Sanchez JA (2005) Caribbean shallow-water black corals (Cnidaria: Anthozoa: Antipatharia). Caribb J Sci 41:492–507
Padilla C, Lara M (2003) Banco Chinchorro: the last shelter for black coral in the Mexcian Caribbean. Bull Mar Sci 73:197–202
Padilla Souza C (2004) Evaluación del uso sustentable del coral negro en el Caribe Mexicano. In: Rivera Arriaga E, Villalobos Zapata GJ, Azuz Adeath I, Rosado May F (eds) El Manejo Costero en México. Universidad Autónoma de Campeche, SEMARNAT, CETYS-Universidad, Universidad de Quintana Roo, Campeche, pp 429–444
Paris CB, Chérubin LM (2008) River-reef connectivity in the Meso-American region. Coral Reefs 27:773–781
Purdy EG, Pusey WC, Wantland KF (1975) Continental shelf of Belize-regional shelf attributes. In: Wantland KF, Pusey WC (eds) Belize shelf-carbonate sediments, clastic sediments, and ecology. American Association for Petroleum Geologists, Tulsa, pp 1–40
Ritson-Williams R, Becerro MA, Paul VJ (2005) Spawning of the giant barrel sponge Xestospongia muta in Belize. Coral Reefs 24:160–160
Rützler K (2012) The role of sponges in the Mesoamerican barrier-reef ecosystem, Belize. Adv Mar Biol 61:211–271
Rützler K, Macintyre IG (1982) The habitat distribution and community structure of the barrier reef complex at Carrie Bow Cay, Belize. In: Rützler K, Macintyre IG (eds) The Atlantic barrier reef ecosystem at Carrie Bow Cay, Belize, I structure and communities. Smithsonian Institution Press, Washington, DC, pp 1–564
Schofield PJ (2010) Update on geographic spread of invasive lionfishes (Pterois volitans [Linnaeus, 1758] and P. miles [Bennett, 1828]) in the western North Atlantic Ocean, Caribbean Sea and Gulf of Mexico. Aquat Invasions 5:S117–S122
Shick JM, Lesser MP, Jokiel PL (1996) Effects of ultraviolet radiation on corals and other coral reef organisms. Glob Chang Biol 2:527–545
Spalding MD, Ravilious C, Green EP (2001) World atlas of coral reefs. University of California Press, Berkeley
Tsuda RT, Dawes CJ (1974) Preliminary checklist of the marine benthic plants from Glover’s reef, British Honduras. Atoll Res Bull 173:1–13
Wilkinson C (2008) Status of coral reefs of the world: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville
Zlatarski V (2007) Scleractinians of Yucatán peninsula, Mexico: results of 1983–1984 investigation. CICIMAR Oceánides 22:45–116
Acknowledgments
EG and DAAB were funded by the Conservation Leadership Programme to conduct MCE research in Cozumel. EG received funding from the Student Conference on Conservation Science – Miriam Rothschild Travel Bursary Programme. DAAB was funded by a Fisheries Society of the British Isles (FSBI) PhD studentship and Operation Wallacea. JV and RE were funded by NOAA through the Cooperative Institute for Ocean Exploration, Research, and Technology to conduct MCE research in Belize. We wish to thank Karl Stanley from the Roatan Institute for Deepsea Exploration for his support collecting underwater images. We also like to thank Marc Wieland from the University of Oxford for assistance with the creation of Fig. 5.1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gress, E., Voss, J.D., Eckert, R.J., Rowlands, G., Andradi-Brown, D.A. (2019). The Mesoamerican Reef. In: Loya, Y., Puglise, K., Bridge, T. (eds) Mesophotic Coral Ecosystems. Coral Reefs of the World, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-319-92735-0_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-92735-0_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92734-3
Online ISBN: 978-3-319-92735-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)