Abstract
Cabozantinib is a receptor tyrosine kinase inhibitor (TKI) with activity against a broad range of targets, including MET, RET, AXL, VEGFR2, FLT3, and c-KIT. Activity of cabozantinib towards a broad range of tumor models could be detected in several preclinical studies. Of note, cabozantinib decreases metastasis potential and tumor invasiveness when compared with placebo or agents that target VEGFR and have no activity against MET. Cabozantinib is clinically approved for the treatment of medullary thyroid cancer (MTC) and for renal cell cancer (RCC) in the second line. In MTC gain of function mutations, mutations of RET are central for tumorigenesis. Hereditary forms of MTC (MEN II) are caused by germline mutations of RET, in sporadic MTC up to 50% of cases RET mutations occur. Both MET and AXL have been described as mechanisms facilitating resistance against VEGFR-targeted tyrosine kinase therapy in clear cell RCC. Accordingly, cabozantinib has shown activity in RCC patients progressing after first-line VEGFR-TKI therapy in the pivotal METEOR trial. This phase III trial reported a benefit of 4.9 months in survival and an increase in response rate compared to standard everolimus over all patient subgroups. Of particular interest are the effects on patients with bone metastasis, which have a worse prognosis. In these patients, the beneficial effects of cabozantinib over everolimus were even more pronounced. Side effects of interest include diarrhea, hypertension, fatigue, and hand–foot syndrome.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
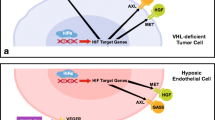
The tyrosine kinase MET is the receptor for hepatocyte growth factor (HGF), a cytokine with anti-apoptotic, pro-migratory, and mitogenic activity. Multiple signaling cascades can be recruited to the intracellular domain of MET (MEK, PI3K, and Jak/Stat) leading to their activation. Activation of MET can disrupt cell–cell contacts and facilitates cell migration (Trusolino and Comoglio 2002). These pro-migratory effects mediated by MET play an important role during embryogenesis and are also active in adults during tissue damage repair (Takayama et al. 1996). It has also been demonstrated that the HGF/MET pathway has protective activity in several degenerative diseases, including liver cirrhosis, nephropathies, and lung fibrosis (Matsumoto and Nakamura 2001; Michalopoulos and DeFrances 1997; Mizuno et al. 2001). Further, MET is expressed in endothelial cells and plays a role as in pro-angiogenic signaling (Bussolino et al. 1992) (Fig. 1).
Taken together, these properties make MET a typical candidate for oncogenic aberration and cancerous transformation by MET has been demonstrated as an early event. Since its discovery, activating changes in MET have been described in numerous cancers consisting either of activating mutations, gene duplications, or transcriptional activation that result in overexpression of wild-type MET (Table 1). The activating events in MET can either occur during primary tumorigenesis or as secondary events that drive further progression of the malignant phenotype (Trusolino and Comoglio 2002). Activating mutations have been identified in hereditary and sporadic papillary renal carcinoma (Soman et al. 1991; Schmidt et al. 1997), and overexpression of MET has been detected in many tumors, including renal cell carcinoma, pancreatic cancer, prostate cancer, NSCLC, and gastric cancer (Soman et al. 1991; Schmidt et al. 1997; Houldsworth et al. 1990; Direnzo et al. 1995). In NSCLC, MET overexpression has been shown to be a mechanism of secondary resistance during treatment with EGFR antagonists (Bean et al. 2007), and in prostate cancer, MET is upregulated during hormonal ablation and higher expression levels are also associated with progression of bone and lymph node metastasis (Sirotnak et al. 2004; Verras et al. 2007). In clear cell RCC, overexpression of MET has been implicated in resistance development against VEGFR-TKI treatment (Macher-Goeppinger et al. 2017).
Different strategies to target MET for anticancer therapy are being followed, blockade of ligand binding by HGF antagonists or monoclonal antibodies against MET and HGF, and cell-permeable tyrosine kinase Inhibitors. The receptor tyrosine kinase AXL has recently also been implicated with tumor growth and poor prognosis. Its activation mediates proliferation, cell survival, and stem cell-like phenotype (Rankin and Giaccia 2016). Its overexpression can mediate treatment resistance against VEGFR-TKIs in renal cell carcinoma.
Cabozantinib developed by Exelixis is a broad-spectrum tyrosine kinase inhibitor with activity not only against MET but also against AXL, VEGFR2, FLT3, c-KIT, and RET. It was the first orally available MET inhibitor to enter clinical trials in 2005 (Macher-Goeppinger et al. 2017). A successful phase III trial in medullary thyroid cancer (MTC) met its primary endpoint showing a median PFS of 11.2 months for the cabozantinib arm over 4.0 months for the placebo arm (Schoffski 2012). Besides activating changes in MET, in MTC activating mutations of the proto-oncogene RET is found in hereditary and in sporadic cases. Dual kinase inhibition by cabozantinib may contribute to its clinical activity in MTC. Based on this positive trial cabozantinib was granted FDA approval for the first-line treatment of metastatic MTC in 2012. The approval for cabozantinib in clear cell RCC after failure of a previous anti-VEGF-directed therapy was obtained in 2015 after the successful METEOR trial (Choueiri et al. 2015) showed an improvement of PFS by 3.6 months and an OS advantage of almost 5 months over everolimus (Choueiri et al. 2016). At the end of 2017, cabozantinib was further approved for first-line treatment of intermediate and poor-risk clear cell RCC following the positive CABOSUN trial (Choueiri et al. 2017).
The positive phase II trials in prostate cancer, however, were not confirmed by two phase III trials, and consequently, the development for cabozantinib in prostate cancer was terminated.
Further, cabozantinib has shown clinical activity in various ongoing clinical trials in different tumor entities. The importance of MET and Axl activation for tumor progression has been confirmed in numerous cancers. Multiple tyrosine kinases might be especially beneficial in certain tumors. This review will summarize the preclinical and clinical data for cabozantinib in cancer treatment.
2 Preclinical Properties and Pharmacokinetics
Cabozantinib, initially coded XL 184, was developed by Exelixis (South San Francisco, CA). It was shown to have inhibitory activity at pharmacological doses against MET, VEGFR2, RET, KIT, FLT-3, AXL, and TIE-2 (tunica interna endothelial cell kinase-2), kinases that all play a role in the development and progression of different tumor diseases. Preclinically, MET phosphorylation was shown to be reduced in peripheral nerve sheath tumor cells by cabozantinib at low concentrations. Studies with xenografts in nude mice demonstrated reduced cell proliferation and reduced vascular density and increased apoptosis. Tumors size was decreasing in a dose-dependent manner upon treatment with cabozantinib (Yakes et al. 2011). Penetration of the blood–brain barrier was shown to be at 20% of plasma levels. In the phase I trial, the pharmacokinetics of cabozantinib were established. Oral bioavailability was demonstrated and the maximum tolerated dose was determined at 175 mg once daily (equals 140 mg free base). Peak plasma concentrations were reached after 5 h following oral administration. The half-life was shown to be 91 ± 33 h (Kurzrock et al. 2011).
3 Clinical Trials
3.1 Phase I
The phase I trial of cabozantinib was carried out to determine the maximum tolerated dose (MTD). Included were various solid tumor entities (Kurzrock et al. 2011). The MTD was determined at 175 mg per day. Following early reports of activity in medullary thyroid cancer (MTC), an expansion cohort for patients with MTC was added to the trial. A total of 85 patients were treated within the trial, 37 of these had MTC. Thirty-five of 37 MTC patients had measurable disease by RECIST. Of these, in 10 a partial response was confirmed and 25 had tumor shrinkage less than 30% or disease stabilization for at least 6 months. Interestingly, three patients with a confirmed response had been pretreated with vandetanib or sorafenib that also targets RET and VEGFR. This supports the hypothesis of MET being an escape mechanism to VEGFR inhibition. Tumor genotyping was performed in 31 patients with MTC an activating mutation of RET was detected in 25 of 31 patients. However, there was no correlation between mutations and clinical response. The tumor of one patient that was rapidly progressing harbored no RET mutation but an activating B-RAF mutation, which is downstream of RET and MET. In a subset of MTC patients (n = 15) analyzed for MET mutations in the tumor DNA, no mutations were detected, copy number gain was only assessed in a few samples, and three patients found to be increased. Toxicity was similar to other VEGFR tyrosine kinase inhibitors. Treatment-related adverse events (AE) were reported by 77 of 85 patients (90%). Of these, 43% reported grade 1 or 2 AEs. The most frequent AEs were diarrhea, rash, hand–foot syndrome, liver enzyme elevation, fatigue, hypertension, nausea, and mucositis. One grade 4 AE was pulmonary embolism attributed to cabozantinib. Further, dose-limiting toxicities (DLT) were hand–foot syndrome and liver enzyme elevations.
3.2 Phase III EXAM Trial
A large phase III trial in medullary thyroid cancer was directly initiated following the responses seen in phase I. The EXAM (Efficacy of XL184 in Advanced Medullary Thyroid Cancer) trial was a randomized, double-blind placebo-controlled trial (Schoffski 2012). A total of 330 patients with MTC were randomized in a 2:1 ratio to cabozantinib versus placebo. The primary endpoint was progression-free survival (PFS) and crossing over was not allowed. Secondary endpoints included overall survival (OS) and response rate. Interim results were presented at the 2012 American Society of Clinical Oncology Annual Meeting. The primary endpoint had been met with a median PFS of 7.2 months in the treatment arm versus 4.0 months in the placebo arm. The difference reached statistical significance with a hazard ratio of 0.28. One-year progression-free survival was reported as 47.3% in the treatment arm versus 7.2% in the placebo arm. All subgroups showed an increased PFS in the treatment arm, including prior treatment with TKI and RET mutational status. Overall response rate was 28% in the cabozantinib group versus 0% in the placebo group (p ≤ 0.0001), and duration of response was 14.6 months that was similar in both RET mutation-positive and RET mutation-negative patients. Overall survival data had not yet reached the required number of events for analysis, but there was no difference between the two arms at this early stage. At interim analysis, 45% of patients in the cabozantinib arm remained on treatment versus 13% in the placebo group. The primary reason for treatment discontinuation was progression of disease (20% in the treatment arm versus 60% in the placebo arm). Adverse events were the reason for discontinuation in 16 and 8% of cases, respectively. Adverse events were more common in the treatment arm with diarrhea and hand–foot syndrome of all grades in over 50% of patients. Further, fatigue, hypertension, and mucositis were also reported more often in the treatment arm. Assessment of calcitonin after 12 weeks of treatment showed a strong correlation with response. Calcitonin fell by a mean of 45% in the treatment arm and increased by a mean of 57% in the placebo arm.
3.3 Phase III Trials in Renal Cell Cancer (METEOR and CABOSUN)
The METEOR trial investigated the activity of cabozantinib in patients with clear cell renal cell carcinoma, who had received at least one line of VEGFR directed therapy. An intent to treat (ITT) population of 658 patients was randomized 1:1 between cabozantinib and everolimus. Primary endpoint was progression-free survival (PFS), and secondary endpoints were overall survival (OS) and response rate (RR). Median PFS with cabozantinib was 7.4 months and 3.8 months with everolimus leading to 42% reduction of progression in the cabozantinib arm (HR 0.58; p < 0.001). Objective response rates were 21% with cabozantinib and 5% with everolimus (p < 0.001) (Choueiri NEJM). Overall survival was also improved with 21.4 months for cabozantinib and 16.5 months for everolimus (HR 0.66 p = 0.00026) (Choueiri Lancet) (Choueiri et al. 2016). In the first line phase II trial CABOSUN, 157 patients with intermediate and poor-risk features according to IMDC criteria were randomly assigned to cabozantinib (n = 79) or sunitinib (n = 78). Cabozantinib treatment significantly increased median PFS (8.2) compared with sunitinib (5.6 months), and was associated with a 34% reduction in the rate of progression or death (hazard ratio, 0.66). The ORR was 33% for cabozantinib versus 12% for sunitinib. Grade 3 or 4 adverse events were similar for cabozantinib (67%) and for sunitinib (68%) (Choueiri et al. 2017).
4 Cabozantinib Combination with Immuno-oncology
Cabozantinib is currently investigated for its activity in combination with Immune checkpoint inhibitors. The phase I study of cabozantinib and nivolumab and cabozantinib, nivolumab, and ipilimumab combinations in patients with urothelial cancer and other genitourinary tumors has already reported a good safety profile in combination with evidence for a strong synergistic activity (Nadal et al. 2017).
5 Discussion
Cabozantinib is a multi-kinase inhibitor with significant activity against MET, RET, AXL, and VEGFR2 among others. This combination of target specificity might prove especially useful in tumors where multiple target inhibitions might prevent tumor escape mechanisms to one target from getting activated. In MTC, the dual kinase inhibition of RET and MET seems to be particularly active. Both kinases have been implicated in tumorigenesis in this cancer. RET mutations are found as many as 50% of MTC tumor samples, while MET seems to be active mainly by overexpression. The influence of RET and MET was supported in the phase I trial, where the activity of cabozantinib against MTC was observed in RET-mutated and RET-unmutated tumors, alike (Kurzrock et al. 2011). The phase III exam trial confirmed a clinical benefit for the treatment of MTC patients with cabozantinib. Progression-free survival was improved to 7.2 months over 4.0 months with placebo, and cabozantinib was FDA approved in 2012 (Schoffski 2012). Cabozantinib is also active against AXL, a receptor tyrosine kinase involved in tumor promotion and therapy resistance in many cancers (Table 1) among those renal cell carcinoma a disease that also frequently displays MET over-activation as a mechanism of resistance to TKIs.
MET inhibition or multi-kinase inhibition as offered by substances like cabozantinib is a promising approach for various other tumors, where MET, AXL, and other kinases have been shown to play a role, including renal cell cancer, gastric cancer, and pancreatic cancer. Recently, MET activation has been shown to act as an escape mechanism in EGFR-mutated non-small-cell lung cancer during EGFR-targeting therapy (Bean et al. 2007). Therefore, combinations of EGFR and MET inhibitors, which have been shown to be active in vitro (Sennino et al. 2012; Nakagawa et al. 2012), are attractive approaches for clinical trials. However, excessive toxicities have shown to be a limiting factor in clinical approaches. Further, molecular mechanisms developing under the selection pressure of an existing targeted therapy have to be elucidated to further improve tailored strategies against treatment resistance (Dietz et al. 2017).
Cabozantinib is clinically effective in renal cell carcinoma and approved in the second line for clear cell renal cell carcinoma after failure of a previous anti-VEGFR therapy and for poor- and intermediate-risk patients in first line. The toxicities of cabozantinib are similar to those seen with other TKIs. Diarrhea, hand–foot syndrome, liver enzyme elevation, fatigue, and hypertension have been reported as the most common side effects. In general, side effects are mostly mild to moderate with the MTD determined by phase I and should be manageable by supportive means in clinical practice.
In conclusion, cabozantinib is a tyrosine kinase that has shown clinical activity in a variety of cancers and is approved for treatment of advanced medullary thyroid cancer and renal cell cancer. Cabozantinib was the first clinically approved MET and AXL inhibitor. Further indications remain to be elucidated, especially combinatorial approaches, e.g., with immune checkpoint inhibitors appear to be very promising. The exploratory biomarker analyses within the trials show the need for a better understanding of the pathways involved, especially of resistance and escape mechanism. And finally, for certain tumor entities, tyrosine kinase inhibitors with activity against multiple targets appear to be superior over substances that are specific only to a single target.
References
Bean J et al (2007) MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 104(52):20932–20937
Bussolino F et al (1992) Hepatocyte growth-factor is a potent angiogenic factor which stimulates endothelial-cell motility and growth. J Cell Biol 119(3):629–641
Choueiri TK et al (2015) Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1814–1823
Choueiri TK et al (2016) Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17(7):917–927
Choueiri TK et al (2017) Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol 35(6):591–597
Dietz S et al (2017) Patient-specific molecular alterations are associated with metastatic clear cell renal cell cancer progressing under tyrosine kinase inhibitor therapy. Oncotarget 8(43):74049–74057
Direnzo MF et al (1995) Overexpression and simplification of the met/HGF receptor gene during the progression of colorectal-cancer. Clin Cancer Res 1(2):147–154
Houldsworth J et al (1990) Gene amplification in gastric and esophageal adenocarcinomas. Can Res 50(19):6417–6422
Kurzrock R et al (2011) Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29(19):2660–2666
Macher-Goeppinger S et al (2017) MET expression and copy number status in clear-cell renal cell carcinoma: prognostic value and potential predictive marker. Oncotarget 8(1):1046–1057
Matsumoto K, Nakamura T (2001) Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int 59(6):2023–2038
Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276(5309):60–66
Mizuno S, Matsumoto K, Nakamura T (2001) Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int 59(4):1304–1314
Nadal R, Mortazavi A, Stein M, Pal SK, Davarpanah N, Parnes HL, Ning YM, Cordes LM, Lin J, Bagheri M, Linderberg L, Berniger M, Steinberg SM, Moore T, Lancaster T, Aviles M, Costello R, Bottaro DP, Dahut WL, Apolo AB (2017) 846O Final results of a phase I study of cabozantinib (cabo) plus nivolumab (nivo) and cabonivo plus ipilimumab (Ipi) in patients (pts) with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. Ann Oncol 28(suppl_5), 1 September 2017, mdx371.001
Nakagawa T et al (2012) Combined therapy with mutant-selective EGFR inhibitor and Met kinase inhibitor for overcoming erlotinib resistance in EGFR-mutant lung cancer. Mol Cancer Ther 11(10):2149–2157
Rankin EB, Giaccia AJ (2016) The receptor tyrosine kinase AXL in cancer progression. Cancers (Basel) 8(11)
Schmidt L et al (1997) Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 16(1):68–73
Schoffski P et al (2012) An international, double-blind, randomized, placebo-controlled phase III trial (EXAM) of cabozantinib (XL184) in medullary thyroid carcinoma (MTC) patients (pts) with documented RECIST progression at baseline. J Clin Oncol 30(15)
Sennino B et al (2012) Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2(3):270–287
Sirotnak FM et al (2004) Microarray analysis of progression to reduced prostate cancer androgen dependence: studies in unique models contrasts early and late molecular events. Mol Carcinog 41(3):150–163
Soman NR et al (1991) The TPR-MET oncogenic rearrangement is present and expressed in human gastric-carcinoma and precursor lesions. Proc Natl Acad Sci USA 88(11):4892–4896
Takayama H et al (1996) Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci USA 93(12):5866–5871
Trusolino L, Comoglio PM (2002) Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer 2(4):289–300
Verras M et al (2007) The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Can Res 67(3):967–975
Yakes FM et al (2011) Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10(12):2298–2308
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Grüllich, C. (2018). Cabozantinib: Multi-kinase Inhibitor of MET, AXL, RET, and VEGFR2. In: Martens, U. (eds) Small Molecules in Oncology. Recent Results in Cancer Research, vol 211. Springer, Cham. https://doi.org/10.1007/978-3-319-91442-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-91442-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91441-1
Online ISBN: 978-3-319-91442-8
eBook Packages: MedicineMedicine (R0)