Abstract
The interest in fish processing by-products and underutilized catch for the production of biofunctional food ingredients has increased in the last number of decades. These marine-derived components contain a significant quantity of protein, which is normally processed into low-value products such as animal feed, fishmeal, and fertilizer. However, due to the global demand for high-quality protein and the need for sustainable production and processing of landed material, the valorization of proteins and other nutrients from fish processing by-products has significantly increased. Fish processing by-products contain significant quantities of high-quality protein, which can be exploited as sources of essential nitrogenous nutrients and biologically active peptides. Bioactive peptides, including those from fish processing by-products, have been reported to possess the ability to beneficially modulate physiological processes associated with noncommunicable diseases. These short peptides, which are encrypted within the primary sequence of the parent protein and are released during food processing or gastrointestinal digestion, could have a role in the prevention and management of these diseases. This chapter reviews the recent literature on the processing and utilization of proteins and protein hydrolysates from fish processing by-products and underutilized fish species with a particular focus on their bioactive properties and peptide sequences.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
The ocean covers about 70% of the total earth’s surface and contains approximately 60% of all fish species. The other 40% are found in fresh water which comprises 1% of the earth’s surface. The fishing industry represents an important economic activity for many countries around the world. In 2003, marine fisheries supported 260 million jobs directly and indirectly [1]. The demand for fish and shellfish has increased throughout the world. The amount of farmed fish doubled in the last decade while that of captured fish has tended to stabilize. According to the Food and Agriculture Organization (FAO), global fish production in 2016 was estimated to be 174.1 million tonnes (mt) by both capture and aquaculture with 152.8 mt being used for human food consumption and 21.3 mt for nonfood uses [2]. It has been estimated that greater than 60% (by weight) of the fish which are processed are represented by by-products, i.e., head, skin, bones, fins, trimmings, viscera, blood, and roe [3]. The large quantity of fish by-products generated represents a potentially significant source of pollution in developing and developed countries. Since 2014, the European Commission, under Directive No 1392/2014 [4], regulates the discard of all fish caught regardless of whether they are regulated by quota, smaller than the minimum size and either dead or alive, and now imposes an obligation to land all catch.
Fish processing by-products and underutilized catch contain a significant quantity of protein which is normally processed into low-value products such as animal feed, fish meal, and fertilizer [2]. However, given the increased global demand for high-quality protein and the requirement for sustainable production and processing, there is an increasing interest in the extraction and valorization of proteins and other nutrients from fish processing by-products. The proteins within fish processing by-products represent a source of high-quality protein which can be exploited for the provision of essential nitrogenous nutrients. Therefore, various biotechnological approaches have, and are, being employed to extract valuable nutrients and bioactive compounds targeted at enhancing human health. Bioactive compounds have a role in the management and in the protection against a range of chronic noncommunicable diseases (NCDs). Furthermore, by-product proteins may be used to generate biologically active peptides (BAPs). BAPs have the ability to modulate physiological processes and thereby have a role in the prevention and management of disease.
Protein hydrolysates are obtained following the enzymatic conversion of intact proteins into peptides. These protein fragments usually contain no more than 20 amino acids. A large range of fish protein hydrolysates, generated using food-grade proteolytic preparations, such as trypsin, Alcalase® 2.4L, Flavourzyme® 500L/1000L, Corolase® PP, and Promod® 144MG, have been reported in the literature [5,6,7]. These hydrolysates, due to their physicochemical properties, are a source of amino acids [8] which have applications in human and animal nutrition, as well as in the pharmaceutical and cosmetic industries. Due to their good nutritional composition, amino acid profile, and bioactive properties, fish protein hydrolysates have many commercial applications [9].
This chapter reviews the recent literature on the processing and utilization of proteins and protein hydrolysates from fish processing by-products and underutilized fish species with a particular reference on their bioactive properties and peptide sequences.
2 Fish Protein Composition
Fish consumption is linked with many health benefits due to their high protein levels and also due to their content of unsaturated fatty acids, vitamins, and minerals [10]. Fish proteins are a particularly rich source of the essential amino acids valine and lysine [11].
The chemical composition of foods has an important role in the supply of essential nutrients for the maintenance of human health. The chemical composition of fish by-products, i.e., levels of protein, ash, and lipid, differs significantly between species.
Fish processing by-products are designated as parts of the fish which are not generally used for human consumption, e.g., head, skin, and viscera. However, the nutrients therein are recoverable and can be utilized after further processing. These components may represent between 30% and 60% by weight of the starting material. Consequently, these fish processing by-products represent a rich source of biofunctional materials such as vitamins, minerals, polysaccharides, polyunsaturated fatty acids (PUFA), enzymes, collagen, gelatin, and bioactive peptides with valuable nutraceutical, pharmaceutical, and cosmeceutical applications [12].
Proteins are characterized by their amino acid sequence and secondary structure and also by their tertiary structure, which may be highly ordered. Each type of protein has a unique structure that determines its function in the organism in addition to its technofunctional properties when utilized as a food source/ingredient [13]. The nutritional value of food proteins is determined on the basis of their essential amino acid content. Table 1 summarized the essential amino acid content of foodstuffs compared to the daily requirement. In comparison to plant proteins, proteins derived from animal sources (because of their high content of essential amino acids) are considered nutritionally superior. Of these, egg white and milk proteins (casein) are usually used as reference proteins for determination of protein quality. Proteins derived from meat and poultry muscle are also considered as a source of high-quality protein. It has been shown that the nutritional value of most fish proteins is equal to or better than casein and the quality of fish proteins may exceed that of terrestrial animal meat [16].
Myofibrillar proteins are structural proteins responsible for movement by their capacity for contraction. They represent 65–75% of the total fish muscle, while sarcoplasmic proteins (soluble proteins) represent 20–35% [17]. Myofibrillar proteins are mainly composed of actin and myosin. The motility of fish is also stabilized and regulated by other structural proteins including titin, nebulin, α-actinin, tropomyosin, and troponin (T, I, and C). The proportion and presence of these structural proteins depend on the fish species. Myosin is the major protein in fish muscle (40%); however, it is a protein easily destabilized when heated, especially the myosin from cold-water fish species. It is a large protein, with a molecular mass of 470 kDa [18], and has an unusual structure as it has both fibrous and globular properties whereas most food protein ingredients, such as proteins from egg, soy, and milk, have globular structures and have a lower molecular mass. Myosin is composed of two heavy chains of 220 kDa and two pairs of different light chains (LCs), ranging from 17 to 22 kDa [19]. The myosin molecule is approximately 160 nm in length. The heavy chains interact via two domains: a globular domain called “head” and a fibrous domain called “tail.” Actin accounts for 15–30% of the myofibrillar proteins. The monomer form of actin is a globular protein (G-actin) of 43 kDa; however, globular actin monomers polymerize to form filaments of fibrous actin (F-actin). Other small proteins associated with either actin or myosin include titin, nebulin, tropomyosin, troponin, C-proteins, and M-proteins [18]. These proteins play an important role in the stabilization of the muscle myofibril basic structure termed the sarcomere (Fig. 1).
Diagrammatic representation of the actin-myosin complex. (Modified from Gordon et al. [20])
The denaturation of myofibrillar proteins by heat, chemical, and enzymatic treatment leads to the generation of a gel [21]. The gelation properties of fish myofibrillar proteins are exploited in the food industry to produce surimi or surimi-like products. The connective tissue proteins, or stromal proteins, provide the structural elements for connective tissue. The main protein in this group is collagen which in general represents approximatively 3% of the total protein in fish muscle and is present in the skin, bone, myocommata, and swim bladder [22]. However, some species, especially Chondrichthyes species, can contain a significantly higher amount of connective tissue (10%) compared with a value of 17% found in mammalian species [23].
3 Processing of Fish Proteins
3.1 Protein Extraction
To date, several methods have been used for the extraction of proteins from various fish species. The specifics of these methods vary depending on the parameters employed, e.g., pH, temperature, agitation time, homogenization, weight-to-volume ratio, and the number of sequential extractions. The main approach used to recover proteins from fish is the so-called pH shift process. This technique employs homogenization of the fish sample in an acid (pH < 5.0) or alkaline (pH > 9.0) solution. The protein extract is then separated from the solid components by centrifugation where solids such as bones, scales, flesh, and skin are removed. Depending on the sample, a mechanical disruption process may also be employed prior to the homogenization step to disrupt the cells [24, 25]. The acid- or alkaline-solubilized protein is then precipitated by adjusting the pH to its isoelectric point. The precipitate is separated by centrifugation and a protein isolate is then obtained.
A second approach used for protein extraction is known as the surimi process. The production of surimi consists of protein recovery from fish mince by a series of sequential steps. Washing with cold water is essential to remove water-soluble, sarcoplasmic proteins and impurities such as the skin, bones, scales, and connective tissue which can reduce the gelation ability and ultimately reduce product quality. The number of successive washes and the volume of water required vary between fish species, the freshness of the starting fish mince, and the quality of the surimi required. It is common to use 0.1–0.3% (w/v) NaCl in the washing solution to facilitate removal of water during the subsequent processing steps. Before the dewatering step, the washed minced fish still contains a large quantity of impurities, which often consist of tissues from the skin and/or internal belly flap that need to be removed. A purification step is required to obtain a product with good organoleptic quality, appearance, and product safety. The water in the surimi base obtained can be removed by a screw press, and the water content is reduced from 91% to 80–84% (w/w) [26]. This method of extraction of proteins from fish is less successful than the pH shift method as sarcoplasmic proteins are lost during the process, leading to a reduced yield. A general flow diagram for these two processes is provided in Fig. 2.
Flow diagram representing the sequential steps involved in the pH shift (a) and surimi process (b) for the extraction of proteins from fish. (Modified from Kristinsson et al. [24])
Enzyme-assisted extraction of food proteins using food-grade proteolytic preparations relies on the intrinsic properties of enzymes, i.e., high specificity and selectivity. The disadvantages of enzyme-assisted extraction are the high cost which may be an issue in the case of production at commercial scale and the inability of some enzymes to disrupt the cell membrane which may lead to low extraction yield. A new approach to overcome these issues involves the use of microwave irradiation during enzyme-assisted extraction. The procedure known as microwave-assisted enzymatic extraction (MAEE) is recognized as having the potential to increase the yield of protein extracted and, in some cases, the bioactive properties of the resulting product. Nguyen et al. (2017) recently studied the generation of fish protein hydrolysates by MAEE and the pH shift method from rainbow trout (Oncorhynchus mykiss) using the proteolytic enzyme Alcalase 2.4L® [27]. The MAEE approach was reported to improve the technofunctional and bioactive properties of the hydrolysates when compared to those generated using the pH shift method.
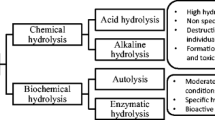
3.2 Enzymatic Hydrolysis
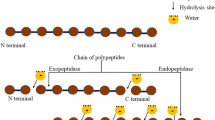
Enzymatic modification is a method which has been used to treat food proteins to improve their technofunctional, physicochemical, bioactive, and organoleptic properties without alteration of their nutritive value [28]. As the degree of hydrolysis of the protein affects the bioavailability and the bioactivity of the peptides generated, the appropriate choice of the hydrolysis conditions (e.g., temperature, pH, enzyme preparation, and enzyme to substrate ratio) is critical [29, 30]. Proteolytic enzymes can be classified according to different characteristics, e.g., proteolytic enzymes can be endo- or exo-acting proteinases/peptidases. For example, endoproteinases cleave peptide bonds within the protein and release peptides or shorter fragments, while exopeptidases remove single amino acid residues or small peptides from either the C-terminus (carboxypeptidases) or N-terminus (aminopeptidases) by cleaving the terminal peptide bonds [31].
It has been shown that enzymatically modified food proteins have improved technofunctional as well as bioactive properties compared with intact proteins [6, 32]. The generation of lower molecular mass peptides with reduced secondary structures can improve the solubility, turbidity, gelling, emulsifying, and the foaming properties as well as heat and pH stability of proteins [33]. The use of fish protein hydrolysates for their technofunctional properties has been extensively reviewed [34,35,36]. The modification observed in the technofunctional properties of food proteins following hydrolysis is highly dependent on the hydrolysis conditions, such as pH, incubation temperature, and duration of hydrolysis. Furthermore, the specificity of the enzyme used for the process and the operating parameters are responsible for the extent of hydrolysis and the peptide profile obtained. Control of the hydrolysis process is even more important when working with animal products since certain animal parts may contain endogenous proteinases [37] or even enzyme inhibitors [38]. These endogenous enzymes are mainly located in the gastrointestinal tract and are associated with the fish viscera; however, some hydrolytic enzymes, such as cathepsins, collagenases, alkaline proteases, and calcium-dependent proteinases, are located in the muscle tissue itself [39]. This endogenous proteolytic activity can increase the extent of hydrolysis independently from the exogenously added proteolytic preparations. Fortunately, the endogenous proteolytic activity in fish is generally not as high as that found in the muscle of terrestrial animals (bovine, porcine, and caprine).
A number of food-grade proteolytic preparations exist on the market. These arise from microbial, plant, and animal sources. Among the enzyme preparations available for the generation of protein hydrolysates from fish processing by-products, the most commonly used are Alcalase (Bacillus licheniformis), Neutrase (Bacillus amyloliquefaciens), Flavourzyme (Aspergillus oryzae), collagenase (Clostridium histolyticum), Pronase E (Streptomyces griseus), papain (Carica papaya), and bromelain (Ananas comosus) and digestive enzymes from bovine and porcine gastrointestinal tracts (e.g., pepsin, trypsin, and chymotrypsin). Moreover, fermentation and autolysis are processes which may be employed for the production of peptides by the action of the proteolytic enzymes from the product itself or from the action of the intrinsic microorganisms present. Furthermore, proteolytic enzymes have been purified from different fish species, especially from viscera for use during fish protein hydrolysate manufacture [15].
In some regions of the world, the endogenous enzymes from marine sources are used to improve the shelf life as well as the bioactive and technofunctional properties of proteins from fish and shellfish. Several examples of fermented fish, shellfish, and seafood exist for application as flavoring agents and food supplements with bioactive properties, such as Kapi, a fermented shrimp paste from Thailand, and Bakasang, a fermented fish sauce from Indonesia [40,41,42,43,44]. However, the high salt content and low pH and the presence of undesirable contaminants such as halophilic microorganisms and biogenic amines (histamine) represent some important issues for consumer safety linked to the consumption of these products. Therefore, the use of enzymatic hydrolysis with exogenously added food-grade proteolytic preparations when carried out in a controlled environment with the use of thermal treatment for enzyme inactivation and bacterial reduction represents a feasible approach for the generation of bioactive peptides from fish by-products for human consumption. Furthermore, it has been reported that the utilization of proteolytic enzymes releases a higher quantity of bioactive peptides with lower molecular masses than when fermentation processes are employed [45]. Additionally, enzymatic hydrolysis requires significantly less time to reach the desired degree of hydrolysis than fermentation processes. For example, the production of fish protein hydrolysates takes between 4 and 6 h to reach the desired degree of hydrolysis [46], while it may take weeks or months for the fermentation process to reach the equivalent extent of hydrolysis [47].
4 Characterization of Fish Peptides
Fish proteins contain peptide sequences encrypted within their primary structure. Some of these peptides have the potential to beneficially modulate some metabolic pathways and consequently may play a role in disease prevention and health enhancement. Bioactive peptides are released from proteins during normal gastrointestinal digestion which occurs in the digestive tract or during food processing with the use of proteolytic enzymes (hydrolysis) or microorganisms (fermentation) [48]. The biological activity of food-derived peptides mainly depends on their structural properties such as molecular mass and the physicochemical characteristics of the amino acids within the sequence [49]. The biological activity of peptides present in protein hydrolysates is highly dependent on the hydrolysis conditions (pH, time, temperature), the enzymes used, and the enzyme-to-substrate ratio applied [50, 51]. Therefore, careful control of these conditions during the generation of bioactive peptides is essential to optimize bioactive properties and, therefore, their ability to enhance human health.
Type 2 diabetes mellitus (T2DM) and cardiovascular diseases are two of the main NCDs responsible for more than 0.6 and 3.9 million deaths, respectively, in Europe per annum [52]. Marine by-product-derived proteins represent a source of peptides that may have the ability to modulate specific biomarkers associated with these diseases, and therefore they have the potential for incorporation into functional food or nutraceutical products for the prevention and management of these conditions. Many studies have been conducted using several different food sources, such as bovine and camel milk, cereals, insects, and marine sources, to generate bioactive peptides with in vitro antioxidant, cardioprotective, antidiabetic, and appetite suppressant properties [53,54,55,56,57,58]. These include peptides with the ability to modulate specific pathways linked with blood pressure control and T2DM. This includes modulation of the renin-angiotensin system (inhibition of renin and angiotensin-converting enzyme (ACE)), stimulation of the incretin system (inhibition of dipeptidyl peptidase-IV (DPP-IV), and stimulation of the secretion of glucagon-like peptide (GLP-1)), as well as stimulation of the secretion of intestinal cholecystokinin, which is linked to appetite suppression in vivo [59,60,61,62]. Food protein-derived peptides have been shown to reduce oxidative stress associated with inflammation and tissue damage in vivo, which are complications generally linked to cardiovascular disease (ischemia), diabetes (diabetic food ulcer), and many other diseases such as neurodegenerative diseases and cancer [63]. The main bioactivities currently investigated for peptides from fish by-products are based on the regulation of oxidative stress and cardiovascular disease (Table 2). These include antioxidant, ACE inhibitory, renin inhibitory, and anticoagulant activities. Several reports suggest that protein hydrolysates generated from fish can modulate the immune response and inhibit cancer development, as well as having ACE inhibitory, antihypertensive, anticoagulant, ion chelating, antioxidant, antimicrobial, antiviral, and appetite suppressant activities [87, 94, 95]. These characteristics of peptides generated within marine-derived protein hydrolysates are linked to their potential as ingredients or nutraceutical products for the management of symptoms related to NCDs such as diabetes, cardiovascular diseases, cancer, and chronic allergic diseases. It has been previously reported that peptides with low molecular mass, mainly di- and tripeptides, have potent bioactive properties [96, 97], while longer peptides, with more than 20 amino acid residues, have been associated with technofunctional property improvements [98]. The most common method used to separate peptides within protein hydrolysates is reversed-phase high-performance liquid chromatography (RP-HPLC) [99]. The techniques mainly used to separate peptides on the basis of molecular weight include sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and gel permeation high-performance liquid chromatography (GP-HPLC) [100]. Furthermore, characterization of the amino acid sequence of the peptides is generally carried out using liquid chromatography systems coupled with mass spectrometry (MS) or tandem mass spectrometry (MS/MS) [54, 97, 101].
5 Bioactive Properties
Globally, the total mortality linked to NCDs was 38 million in 2012 and is estimated to reach 52 million by 2030 [102]. As mentioned in previous sections, peptides derived from proteinaceous marine processing by-products have been shown to possess a range of bioactive properties. The potential health benefits of consuming fish protein hydrolysates/peptides for the control of NCDs and oxidative stress, allergenicity and inflammation, hypertension, and cancer will therefore be outlined.
5.1 Oxidative Stress
Oxidative stress is an important factor contributing to the development and the progression of NCDs. It is characterized by the generation of reactive oxygen species (ROS), including hydroxyl radicals (•OH), superoxide radicals (O2•−), and non-radical hydrogen peroxides (H2O2). High ROS levels have been associated with the deleterious modification of nucleic acids (DNA and RNA), proteins, and lipids and have been implicated in accelerating cellular aging and several human conditions, such as atherosclerosis and neurodegenerative diseases [63]. In contrast, low ROS levels have beneficial physiological effects linked to the regulation of cell signaling, through the redox regulation of transcription factors, protein phosphorylation (kinase), and ion transfer [103]. The consumption of dietary components containing natural antioxidants (such as vitamin C, polyphenols, and carotenoids) has been shown to reduce oxidative stress by enhancing natural defenses [104]. On the other hand, oxidative stress can contribute to a reduction in the proliferation of cancer cells by inducing cell death. However, while cell death can be achieved by radical-induced oxidative stress, some cancer cells have developed resistance which indicates that cells develop sophisticated adaptation responses to oxidative stress [105]. The peptides from fish processing by-product proteins having antioxidant activity have recently been reviewed [95]. For example, Glu-Leu-Phe-Glu-Pro-Arg, a hexapeptide generated by Alcalase hydrolysis of seabass (Lates calcarifer) skin gelatin, was shown to scavenge hydrogen peroxide [106]. Table 2 provides a list of fish protein by-product hydrolysates/peptides exhibiting antioxidant activity as reported in the literature.
The in vitro determination of antioxidant activity of peptides can be performed using various chemical reactions. The methods used to assess antioxidant capacity can be classified according to whether they assess the transfer of either hydrogen atoms (HAT) or electron (ET) [107]. The assays used to measure proton-donating ability are represented by the oxygen radical absorbance capacity (ORAC), hydroxyl radical, alkyl radical, and peroxide radical scavenging activity assays. The reported ORAC activity of peptides derived from fish processing by-products ranges from 5.47 to 19.74 μmol TE/μmol peptide (Table 2). Compared to milk protein-derived antioxidant peptides, the radical scavenging activity was approximately 2.1- to 7.5-fold higher than the hendecapeptide, Trp-Tyr-Ser-Leu-Ala-Met-Ala-Ala-Ser-Asp-Ile derived from a β-lactoglobulin A hydrolysate [108]. The ET-based assays used to determine the reducing capacity of an antioxidant are the Trolox equivalent antioxidant capacity (TEAC), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) radical cation (ABTS•+), FRAP (ferric reducing antioxidant power), and DPPH• (2,2-diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl radical) assays. The DPPH radical scavenging activity (EC50, concentration causing 50% of antioxidant activity) of Phe-Ile-Gly-Pro, a peptide derived from a bluefin leatherjacket skin hydrolysate, was reported to be 0.118 mg/mL. Interestingly, Song et al. (2011) reported that a potent pepsin hydrolysate from half-fin anchovy (Setipinna taty) possessed a DPPH EC50 value of 4.46 μg/mL, while the synthetic antioxidant butylated hydroxytoluene (BHT) is reported to exhibit an EC50 value of 22.78 μg/mL [78]. The antioxidant potency of bioactive peptides has been attributed to the presence of specific amino acids therein especially histidine residues. This has been attributed to the chelating properties and the radical-trapping properties of the imidazole ring. Furthermore, the presence of hydrophobic amino acid residues in peptides has been associated with an increase in their accessibility to hydrophobic targets [109, 110].
5.2 Allergenicity and Inflammation
Allergy is a hypersensitivity response associated with an immune system reaction toward an allergen. The major types of allergies include life-threatening anaphylaxis; food, drug, and insect allergies; asthma; rhinitis; angioedema; eczema; urticaria; and eosinophilic disorders [111]. Allergic reactions/diseases can be caused by environmental factors, such as pollution, as well as genetic factors. The global prevalence of allergic diseases is increasing with 200–250 million people suffering from food allergy and about 300 million people suffering from asthma causing 250,000 deaths annually [112]. The diagnosis of allergy is commonly performed using blood or cutaneous tests. The cutaneous (skin prick) test is carried out by introducing a series of punctures on a subject’s skin which are loaded with different suspected allergens. The signs of inflammation, indicative of an allergenic response, are then observed. Inflammation is one of the first responses of the immune system to a challenge/infection [113]. The increase in blood flow into the tissue causes redness, swelling, heat, and pain. Inflammation occurs when damaged tissues or infected cells release eicosanoids and cytokines. Eicosanoids, which include prostaglandins, dilate blood vessels and increase the temperature locally, while leukotrienes recruit white blood cells (leukocytes). Cytokines, including interleukins and chemokines, are responsible for the recruitment and communication between leukocytes at the site of infection, while interferons shut down protein synthesis in infected cells [114]. Cytotoxic and growth factors can also be released by the damaged tissues. These molecules have the ability to recruit immune cells to the site of challenge/infection and contribute to the healing of damaged tissue following removal of the antigen [115].
The human immune system reacts against various chemical and biological threats by two separate but interconnected systems. The first defense system, called the innate immune system, consists of cells and proteins present in the circulation system, and it is activated in the presence of an exogenous threat. This system includes mucosal epithelial barriers, dendritic cells, and leukocytes [116]. The second system, called the adaptive immune system, is a specific system which involves B- and T-cells and the production of specific antibodies against the detected threat. The adaptive immune system is activated when the innate immune system is insufficiently effective or when it has been overcome [117]. Current treatments for allergies consist of the use of medication such as steroids, adrenalin, and antihistamines [118]. Immunotherapy is also available to treat some forms of allergy by injecting an inactive form of the allergen to stimulate the production of specific antibodies by the adaptive immune system [119]. Even though immunotherapy provides a longer-lasting effect, it also represents an expensive alternative to pharmacological solutions.
Immunomodulatory peptides can modulate immune functions by enhancing lymphocyte proliferation, antibody synthesis, and cytokine regulation [120]. Moreover, immunomodulatory peptides may have the ability to reduce allergic reactions and enhance the mucosal immune system in the gastrointestinal tract. Immunomodulatory peptides isolated from human milk, rice, and soybean tryptic hydrolysates act to stimulate the innate immune system [121,122,123]. A review of literature reveals that the mechanism of action of immunomodulatory peptides is relatively non-specific, and this may be the reason why the exact mechanism and the in vivo destination of these peptides are still unknown. Figure 3 depicts the main mechanisms of action of anti-inflammatory peptides. The details of the anti-inflammatory peptides isolated from fish by-products are reported in Table 2.
Mechanisms of inflammation and potential sites of action of bioactive peptides. (Modified from Cicero et al. [124])
The main anti-inflammatory activity of bioactive peptides, as currently described in the literature, involves the up- and downregulation of signaling proteins (cytokines) such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) and adhesion molecules. Modification of the expression and translation of these components has the ability to reduce inflammation. Chalamaiah et al. (2018) have recently reviewed the area of immunomodulatory peptides from food protein hydrolysates [125]. Subhan et al. (2017) demonstrated that peptides from fish scale collagen could downregulate the expression of pro-inflammatory cytokines in vitro [126]. This suggests that peptides isolated from fish scale collagen had a beneficial effect in the control of inflammatory diseases.
As shown in Table 2, a number of peptides/hydrolysates derived from fish processing by-products are reported to possess anti-inflammatory capacity. For example, the tripeptide Pro-Ala-Tyr derived from an Atlantic salmon (Salmo salar) pectoral fin peptic hydrolysate exhibits anti-inflammatory activity via the downregulation of NO/iNOS and PGE2/COX-2 pathways by 64–75% and 45–48%, respectively, compared to the control group. The inhibition of the production of pro-inflammatory cytokines, TNF-α, IL-6, and IL-1β, has been also reported (Table 2) by Anh et al. [67].
5.3 Hypertension
The literature reports that dietary protein intake can contribute to reducing high blood pressure, coronary heart disease, and other infarctions [127]. Blood pressure is determined by measuring two values: systolic blood pressure (SBP), which measures the pressure in blood vessels when the heart beats, and diastolic blood pressure, which measures the pressure in blood vessels when the heart is at rest. The normal values for systolic and diastolic blood pressures are 120 and 80 mmHg, respectively. A range of mechanisms are involved in the control of blood pressure, including the secretion of specific hormones, modulation of blood volume, and secretion of nitric oxide by endothelial cells and the renin-angiotensin-aldosterone system (Fig. 4). ACE, for example, catalyzes the conversion of angiotensin I to angiotensin II, a hormone which leads to vasoconstriction and an increase in blood pressure [128]. Furthermore, ACE degrades the vasodilator molecule bradykinin. Consequently, ACE inhibitory agents can lower hypertension. Many studies have focused on the ability of fish protein hydrolysates/peptides to inhibit ACE [66, 89]. ACE inhibitory peptides are generally short sequences. Moreover, structural studies of the ACE active site using X-ray crystallography show a lid-like extension formed by the amino-terminal helix (α 1-3) that partially covers the active channel and leaves an opening of almost 3 Å for substrate and inhibitor access [129]. Wu et al. (2006) performed an in silico analysis of the ACE inhibitory activity of long (4–10 amino acids) and short (2–3 amino acids) peptides. The importance of the type of amino acid residue in the peptide sequence for ACE inhibitory activity was predicted [130]. It was concluded that the optimum amino acid residues for potent ACE inhibition starting from the C-terminus were Tyr and Cys in the first position; Trp, Met, and His in the second position; Leu, Ile, Val, and Met in the third position; and Trp in the fourth position. Blood pressure is highly regulated in vivo and involves mechanisms other than modulation of ACE activity. It is likely that bioactive peptides derived from fish protein hydrolysates also beneficially modulate these systems.
Mechanisms of blood pressure control and the potential action of bioactive peptides. (Modified from Cicero et al. [124])  : direct inhibition,
: direct inhibition,  : direct stimulation
: direct stimulation
Recently, research on nitric oxide synthase 3 (iNOS) suggested that stimulation of the production of nitric oxide (NO) in endothelial cells has a beneficial effect on blood pressure (Fig. 4) [131]. Ahn et al. (2015) isolated the tripeptide Pro-Ala-Tyr from an Atlantic salmon (Salmo salar) pectoral fin peptic hydrolysate and demonstrated that it possessed the ability to modulate the secretion of intracellular NO in vitro [67].
Several reports from in vivo studies using spontaneously hypertensive rats (SHRs) show hydrolysates/peptides derived from fish proteins having the ability to significantly reduce hypertension. Ko et al. (2016) identified ACE inhibitory peptides (Table 2) from hydrolysates of flounder fish (Paralichthys olivaceus) protein which were shown to have hypotensive effects in vivo [82]. The in vitro ACE IC50’s for two identified hexapeptides, Met-Glu-Val-Phe-Val-Pro and Val-Ser-Gln-Leu-Thr-Arg, was 79 and 105 μM, respectively. These peptides were found to reduce SBP in SHRs after 6 h oral administration (Table 2). Interestingly, the reduction in SBP value obtained with the Val-Ser-Gln-Leu-Thr-Arg-treated group was similar to the group treated with Captopril®, a synthetic drug inhibitor of ACE. As shown in Table 2, numerous peptides with ACE inhibitory activity have been derived from marine by-products. These include hydrolysates/peptides from Atlantic salmon, bluefin tuna, boarfish, leatherjacket, Pangasius catfish, rockfish, and skate.
5.4 Type 2 Diabetes Mellitus
Type 2 diabetes mellitus (T2DM) is characterized by insulin deficiency caused by pancreatic β-cell dysfunction and insulin resistance [132], which arises from the fact that the pancreatic cells produce and release insulin, but the quantity released is insufficient. All these complications lead to hyperglycemia and many side effects such as atherosclerosis leading to heart attack, stroke, and organ failure. Type 1 diabetes, also named “insulin-dependent diabetes,” is a malfunction of the pancreatic cells that fail to produce and release insulin resulting from a cell-mediated autoimmune attack of pancreatic β-cells [133]. However, T2DM is the most common type of diabetes accounting for 90–95% of all diabetes cases [132]. The occurrence of T2DM has increased in developed countries and is associated with an unhealthy lifestyle and obesity which contributes to higher rates of morbidity as diabetic individuals generally have a higher risk of heart disease, kidney failure, blindness, and nerve and circulatory damage [132]. The global prevalence of diabetes was 415 million adults aged over 20 years in 2015 (8.8% of the adult population), and this is expected to increase to 642 million by 2040 (10.4% of the adult population) [134]. The countries with the highest incidences of diabetes in 2015 were China (109.6 million), India (69.2 million), the United States (29.3 million), Brazil (14.3 million), the Russian Federation (12.1 million), Mexico (11.5 million), Indonesia (10.0 million), Egypt (7.8 million), Japan (7.2 million), and Bangladesh (7.1 million). Several types of medication are currently employed for the management and control of T2DM. The most common treatments involve the use of Metformin® and Gliclazide® which decrease the release of hepatic glucose and increase insulin secretion, respectively. Both medications are on the World Health Organization list of essential medicines for the treatment of T2DM. Other treatments used in the management of the disease include the injection of glucagon-like peptide-1 analogues and the use of enzyme inhibitors which inhibit the activity of dipeptidyl peptidase-IV (DPP-IV), α-amylase, and α-glucosidase. These treatment approaches enhance the body’s response to reduce postprandial serum glucose levels. The enzymes targeted for inhibition are linked to the reduction of glucagon release and the stimulation of insulin synthesis, glucose absorption, and metabolism, as well as appetite reduction [135,136,137]. DPP-IV degrades two incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1). In the presence of glucose, the incretin system stimulates pancreatic β-cells to secrete insulin. Therefore, by inhibiting the action of DPP-IV, the incretin hormones are maintained at a stable level in the circulation and continue to stimulate insulin production [138]. Human in vivo studies have shown that the inhibition of DPP-IV leads to a reduction in glycated hemoglobin (hemoglobin A1c) [139] as well as an increase in circulating GLP-1 [140]. Consequently, this represents clear evidence of the blood glucose-lowering effect of DPP-IV inhibitors. Although DPP-IV inhibitors and GLP-1 analogues are interesting targets for blood glucose regulation, they represent distinct drug classes with different mechanisms of action, route of administration, and clinical efficiency. However, some synthetic drugs with DPP-IV inhibitory activity are now being used for the treatment of T2DM, such as sitagliptin (Merck & Co. as Januvia®, FDA approved in 2006), vildagliptin (Novartis as Galvus®, EU approved in 2007), and alogliptin (Takeda Pharmaceutical Company as Nesina®, FDA approved in 2013). While these drugs have proven to be efficient in the management of T2DM, side effects including postprandial hypoglycemia, nasopharyngitis, headache, nausea, heart failure, hypersensitivity, skin reaction, joint pain, and adverse cardiovascular effects have been associated with their use [141]. Therefore, the use of natural sources to produce DPP-IV inhibitors is being explored in order to reduce the side effects of antidiabetic drugs. Protein hydrolysates derived from fish processing by-products have been reported to have in vitro DPP-IV inhibitory activity, and numerous DPP-IV inhibitory peptides have been identified. These include Gly-Gly-Pro-Ala-Gly-Pro-Ala-Val (624.7 Da) and Gly-Pro-Val-Ala (342.4 Da) which can inhibit DPP-IV by 50% at a concentration (IC50 value) of 0.26 and 8.14 mg.mL−1, respectively [64]. Recent in vivo studies on protein hydrolysates derived from the underutilized fish blue whiting (Micromesistius poutassou) have shown the ability to lower glucose and increase insulin level in mice [77]. Another approach to regulate blood glucose is to stimulate the production of cholecystokinin (CKK) by enteroendocrine cells in the duodenum. The secretion of CCK is linked to gastric emptying, the stimulation of pancreatic secretion, and satiety [142]. Several in vitro and in vivo studies testing intact proteins and their hydrolysates or corresponding amino acid mixtures demonstrate this phenomenon. Sharara et al. (1993) have shown that protein intake stimulates CKK secretion postprandially in rats, whereas free amino acid intake had no effect [143]. Furthermore, Cudennec et al. (2008) have shown that protein hydrolysates derived from blue whiting can enhance the secretion of CKK and GLP-1 in vitro [144]. In vivo studies with the same hydrolysates showed an increase in CKK and GLP-1 levels in the plasma of rat [145] and in human [62]. Greco et al. (2017) in reviewing the effect of protein intake on appetite have shown that the effect observed depends on the protein source [146]. Madani et al. (2015) showed that feeding obese rats with sardine proteins resulted in reduced plasma glucose and reduced insulin resistance as well as higher plasma GLP-1 levels compared to the group fed with casein [147]. A list of DPP-IV inhibitory peptides and CKK-stimulating hydrolysates obtained from fish processing by-products and underutilized fish species is provided in Table 2. The in vitro DPP-IV inhibitory activity of two peptides, Gly-Pro-Ala-Glu and Gly-Pro-Gly-Ala, identified from Atlantic salmon skin hydrolysates had IC50 values of 49.6 and 41.9 μM, respectively. Furthermore, Gly-Pro-Val-Ala identified from an Atlantic salmon trimming protein hydrolysate possessed a DPP-IV IC50 value of 264.74 μM. It was noted that the differences in amino acid residues in the peptide sequence had a major impact on DPP-IV inhibitory activity. The peptide Ile-Pro-Ile which is a known DPP-IV inhibitor and found in many dietary proteins was reported to have an IC50 value of 3.2 μM [148]. It is possible that fish processing by-products could be a potential source of peptides with similar or higher levels of DPP-IV inhibitory activity.
5.5 Cancer
Cell division is a normal physiological event that occurs in tissues. Cell proliferation and cell death are highly regulated processes. Certain mutations in cellular DNA destabilize this process and can ultimately lead to cancer. The process that transforms normal cells into cancer cells is called carcinogenesis. It is characterized by a series of changes at cellular and genetic level that reprogram the cell into an uncontrolled division process leading to the formation of a tumor. This malignant mass can remain at a particular site or spread throughout the body via an angiogenesis process and metastasis diffusion.
Apoptosis is a form of programmed cell death and is one of the main mechanisms used in cancer treatment. As apoptosis does not enhance immune response or produce inflammation, it is a better method of treatment compared to classic chemical chemotherapies. Therefore, selective induction and modulation of apoptotic pathways in cancer cells represent a promising approach for cancer therapy [149]. In mammals, two major apoptosis signaling pathways are involved in the activation of cysteine proteases (caspases), the extrinsic death receptor, and the intrinsic mitochondrial pathways [150]. These interlinked pathways involve pro- and anti-apoptotic molecules that can trigger or regulate apoptosis. Therefore, the development of antiproliferative peptides that specifically target these pathways has become an interesting strategy for the development of anticancer therapies.
A large diversity of peptides with anticancer activity have been extracted from various marine organisms, mainly sessile animals, such as sponges, molluscs, and tunicates, which synthesize potent cytotoxic compounds to protect themselves against predators. These compounds are currently being exploited for cancer therapy. However, reports on the antiproliferative activity of peptides derived from fish protein hydrolysates are limited. Chalamaiah et al. (2018) reviewed the area of anticancer peptides from food protein hydrolysates [125]. Several studies have reported that free amino acids have diverse effects on different cancer cells [151, 152]. Cys promoted the proliferation of gastric and breast cancer cells. Asp and Arg stimulated the growth of breast cancer cells, while Glu induced apoptosis in gastric cancer cells. Ala showed an in vitro antiproliferative activity against gastric and breast cancer cells, while Pro and Lys showed an antiproliferative activity against prostate cancer cells. These reports suggest that the presence of specific amino acids in peptide sequences could modulate their activity against different cancer cell metabolic pathways. Furthermore, two peptides, Leu-Pro-His-Val-Leu-Thr-Pro-Glu-Ala-Gly-Ala-Thr and Pro-Thr-Ala-Glu-Gly-Gly-Val-Tyr-Met-Val-Thr, isolated from tuna protein hydrolysates possessed antiproliferative EC50 values of 8.1 and 8.8 μM on the human breast cancer cell line MCF-7 in vitro, respectively [80]. A list of the antiproliferative peptides obtained from half-fin anchovy, loach, and rohu by-products is provided in Table 2. To date, Alemán et al. (2011) have reported the highest antiproliferative EC50 value (0.13 mg/mL) for a squid gelatin hydrolysate using Esperase® on MCF-7 cell line [153].
5.6 Other Bioactivities
Other biological activities involving peptides which possess antiviral and antimicrobial activity have been reported in the Antarctic fish (Pleuronectes americanus) and small red scorpionfish (Scorpaena notate), respectively (Table 2). Mineral-binding peptides have been identified from Alaska pollock and seabass. Mineral-binding capacities are important in many metabolic processes, including nutrient absorption, cellular proliferation, energy production, and oxygen transport [68, 70, 88]. Anticoagulant activity had been reported in peptides extracted from yellowfin sole frame [93] (Table 2).
6 Bioavailability
As already outlined, bioactive peptides can be released from food proteins during food processing by fermentation or enzymatic hydrolysis as well as during normal gastrointestinal digestion. Research has proven that fish processing by-products are a potential source of bioactive peptides for the production of functional food ingredients [154]. However, more studies on the stability of the bioactive properties need to be carried out. In order to be active at the target site in the human body, the peptides must maintain their biological activity after passing through the gastrointestinal tract, be resistant to extreme pH values and the action of gastrointestinal enzymes. An in vitro simulated gastrointestinal digestion (SGID) approach is often carried out to determine the stability of bioactive peptides following in vitro incubation with gastrointestinal enzymes. The enzymes used are salivary amylase at pH 7.0 for the oral phase, pepsin at pH 3.0 to simulate the gastric system, and a pancreatic enzyme preparation composed of trypsin, chymotrypsin, elastase, lipase, and amylase at pH 7.0 to mimic the intestinal phase [155]. Hydrolysis of proteins by these enzymes can release bioactive peptides. This has been shown when using the SGID approach for the generation of bioactive peptides from different food sources such as cereals, dairy products, and fish [156,157,158,159]. Moreover, in vitro SGID has shown that the hydrolytic action of gastrointestinal enzymes has the potential to modulate the bioactive properties of peptides generated following hydrolysis using nonmammalian food-grade enzyme preparations [160]. For this reason, bioactive peptides may be tested using in vitro gastrointestinal digestion to assess their potential stability and bioavailability after ingestion. However, some bioactive peptides have shown resistance to further digestion by gastrointestinal enzymes, such as the ACE inhibitory peptide Leu-Leu-Pro from tilapia, which maintained its activity following incubation with pepsin, pancreatin, and α-chymotrypsin [161]. The permeability of biological membranes, which allow bioactive peptides to reach the circulation, depends on many factors such as peptide molecular mass and chemical stability and hydrophobicity. The transport of peptides through the gastrointestinal tract and the intestinal cell barrier is mediated via three main transport mechanisms. These mechanisms are schematically represented in Fig. 5. These consist of (A) active transport via the PepT1 carrier, which transports di- and tripeptides coupled with a proton pump, (B) endocytosis-exocytosis which transports basic and hydrophobic peptides via endocytosis vesicles, and (C) tight junction paracellular diffusion, which transports intact oligopeptides through tight junction pores [154]. However, active transport via PepT1 and passive paracellular diffusion are more efficient routes than endocytosis-exocytosis-mediated transport as peptides may be hydrolyzed after endocytosis by intracellular enzymes into amino acids before reaching the bloodstream. Furthermore, larger peptides and single amino acids have been shown to be less easily absorbed by gastrointestinal cells than short peptides (i.e., containing two to six amino acids) [162]. Transfer across the gastrointestinal membrane also depends on the amino acid sequence of the peptide [163]. Bioactive peptides isolated from fish have been reported to be resistant to the gastrointestinal digestion process and to be able to pass through intestinal membranes to reach the bloodstream. For example, in vivo studies in hypertensive rats have shown that the antihypertensive effects of bioactive peptides derived from fish, such as salmon, sardine, sole, tuna, and Alaska pollock, remain stable after passage through the digestion and assimilation processes. For example, Hou et al. (2016) showed that Pro-Thr-Gly-Ala-Asp-Tyr derived from tryptic hydrolysates of Alaska pollock frame could significantly enhance the humoral, cellular, and non-specific immune system in immunosuppressed mice [164]. This indicates that this peptide was resistant to digestion and was able to pass into the bloodstream. However, the stability of bioactive peptides can be enhanced via several strategies which have been developed by the pharmaceutical industry. Among these, encapsulation and structural modification of peptides at C- and/or N- terminal residues, including glycosylation and alkylation, have been shown to improve the bioavailability of peptides. Furthermore, peptides containing Thr, Glu, Phe, and His amino acids seem to be absorbed significantly faster compared to their free amino acid mixture equivalent [163]. The presence of a high percentage of Hyp and Pro amino acids also seems to improve resistance to hydrolysis by gastrointestinal enzymes [165]. Some of these approaches have been used to improve the bioavailability of fish-derived bioactive peptides. For example, the encapsulation of rainbow trout peptides in biopolymer-coated nanoliposomes was an efficient technique to maintain their antioxidant capacity [166].
Peptide transport mechanisms across intestinal cells. (Modified from Harnedy and FitzGerald [154])
7 Conclusion
The study of fish protein for the generation of bioactive peptides has increased in the last few years, and fish processing by-products as well as underutilized fish species have been identified as potential sources for bioactive peptides. However, even though several studies with regard to the extraction and hydrolysis of proteins from fish and fish by-products, as well as the purification, characterization, and identification of bioactive peptides, have been carried out, more research is required to fully exploit and deliver their potential to consumers. While interesting studies on the use of fish processing by-products as functional food ingredients have been carried out, more research is needed in addressing the large-scale production of these products, their bioavailability, compatibility with different food matrices, long-term stability, and in vivo efficiency. Furthermore, it is necessary to determine the mechanisms by which peptides and hydrolysates can mediate their physiological effects. More nutrikinetic and metabolomic studies are required in order to understand the relationship between the dose administered and physiological effect. Marketing and economic studies are also required to establish consumer needs and preferences. Finally, in vivo validation studies are required to generate health promoting claims acceptable for international food safety agency approval.
References
Teh LCL, Sumaila UR (2013) Contribution of marine fisheries to worldwide employment. Fish Fish 14:77–88
FAO (2016) The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. FAO, Rome
Chalamaiah M, Dinesh Kumar B, Hemalatha R et al (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135:3020–3038
Commission Delegated (20 October 2014) Regulation (EU) No 1392/2014 – establishing a discard plan for certain small pelagic fisheries in the Mediterranean Sea, Brussels
García-Moreno PJ, Pérez-Gálvez R, Espejo-Carpio FJ et al (2017) Functional, bioactive and antigenicity properties of blue whiting protein hydrolysates: effect of enzymatic treatment and degree of hydrolysis. J Sci Food Agric 97:299–308
Neves AC, Harnedy PA, O’Keeffe MB et al (2017) Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem 218:396–405
Sukkhown P, Jangchud K, Lorjaroenphon Y et al (2018) Flavored-functional protein hydrolysates from enzymatic hydrolysis of dried squid by-products: effect of drying method. Food Hydrocoll 76:103–112
Ettelaie R, Zengin A, Lishchuk SV (2017) Novel food grade dispersants: review of recent progress. Curr Opin Colloid Interface Sci 28:46–55
Lafarga T, Hayes M (2017) Bioactive protein hydrolysates in the functional food ingredient industry: overcoming current challenges. Food Rev Int 33:217–246
Sidhu KS (2003) Health benefits and potential risks related to consumption of fish or fish oil. Regul Toxicol Pharmacol 38:336–344
Ross A, Vincent A, Savolainen OI et al (2017) Dietary protein sources beyond proteins and amino acids – a comparative study of the small molecular weight components of meat and fish using metabolomics. FASEB J 31:652.613
Pangestuti R, Kim S-K (2017) Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar Drugs 15:67
Felix M, Romero A, Rustad T et al (2017) Physicochemical, microstructure and bioactive characterization of gels made from crayfish protein. Food Hydrocoll 63:429–436
Vidotti RM, Viegas EMM, Carneiro DJ (2003) Amino acid composition of processed fish silage using different raw materials. Anim Feed Sci Technol 105:199–204
Villamil O, Váquiro H, Solanilla JF (2017) Fish viscera protein hydrolysates: production, potential applications and functional and bioactive properties. Food Chem 224:160–171
Friedman M (1996) Nutritional value of proteins from different food sources. A review. J Agric Food Chem 44:6–29
Venugopal V (2009) Seafood proteins: functional properties and protein supplements. In: Venugopal V (ed) Marine products for healthcare: functional and bioactive nutraceutical compounds from the ocean. CRC Press, Boca Raton, pp 51–102
Bechtel PJ (1986) Muscle development and contractile proteins. In: Muscle as food. Academic, San Diego, pp 1–35
Lowey S, Risby D (1971) Light chains from fast and slow muscle myosins. Nature 234:81–85
Gordon AM, Homsher E, Regnier M (2000) Regulation of contraction in striated muscle. Physiol Rev 80:853–924
Lanier T, Yongsawatdigul J, Carvajal-Rondanelli P (2013) Surimi gelation chemistry. In: Park J (ed) Surimi and surimi seafood, 3rd edn. CRC Press, Boca Raton, pp 101–140
Kim S-K, Mendis E (2006) Bioactive compounds from marine processing byproducts – a review. Food Res Int 39:383–393
Pearson AM, Young RB (1989) The connective tissues: collagen, elastin, and ground substance. In: Pearson AM (ed) Muscle and meat biochemistry. Academic, San Diego, pp 338–390
Kristinsson HG, Lanier TC, Halldorsdottir SM et al (2013) Fish protein isolate by pH shift. In: Park J (ed) Surimi and surimi seafood, 3rd edn. CRC Press, Boca Raton, pp 169–192
Hayes M, Mora L, Hussey K et al (2016) Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innovative Food Sci Emerg Technol 37:253–260
Park J, Graves D, Draves R et al (2013) Manufacture of surimi. In: Park J (ed) Surimi and surimi seafood, 3rd edn. CRC Press, Boca Raton, pp 55–100
Nguyen E, Jones O, Kim YHB et al (2017) Impact of microwave-assisted enzymatic hydrolysis on functional and antioxidant properties of rainbow trout Oncorhynchus mykiss by-products. Fish Sci 83:317–331
Auwal SM, Zarei M, Abdul-Hamid A et al (2017) Optimization of bromelain-aided production of angiotensin I-converting enzyme inhibitory hydrolysates from stone fish using response surface methodology. Mar Drugs 15:104
Salampessy J, Reddy N, Phillips M et al (2017) Isolation and characterization of nutraceutically potential ACE-inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT Food Sci Technol 80:430–436
Klomklao S, Benjakul S (2017) Utilization of tuna processing byproducts: protein hydrolysate from skipjack tuna (Katsuwonus pelamis) viscera. J Food Process Preserv 41:e12970
Venkatesan J, Anil S, Kim S-K et al (2017) Marine fish proteins and peptides for cosmeceuticals: a review. Mar Drugs 15:143
Cermeño M, FitzGerald RJ, O’Brien NM (2016) In vitro antioxidant and immunomodulatory activity of transglutaminase-treated sodium caseinate hydrolysates. Int Dairy J 63:107–114
Jeewanthi RKC, Lee N-K, Paik H-D (2015) Improved functional characteristics of whey protein hydrolysates in food industry. Korean J Food Sci Anim Resour 35:350–359
Adler-Nissen J (1976) Enzymatic hydrolysis of proteins for increased solubility. J Agric Food Chem 24:1090–1093
Pacheco-Aguilar R, Mazorra-Manzano MA, Ramírez-Suárez JC (2008) Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem 109:782–789
Guérard F, Decourcelle N, Sabourin C et al (2010) Recent developments of marine ingredients for food and nutraceutical applications: a review. J Sci Halieut Aquat 2:21–27
Chéret R, Delbarre-Ladrat C, de Lamballerie-Anton M et al (2007) Calpain and cathepsin activities in post mortem fish and meat muscles. Food Chem 101:1474–1479
Busconi L, Folco EJ, Martone C et al (1984) Identification of two alkaline proteases and a trypsin inhibitor from muscle of white croaker (Micropogon opercularis). FEBS Lett 176:211–214
Li Q, Zhang L, Lu H et al (2017) Comparison of postmortem changes in ATP-related compounds, protein degradation and endogenous enzyme activity of white muscle and dark muscle from common carp (Cyprinus carpio) stored at 4 °C. LWT Food Sci Technol 78:317–324
Kleekayai T, Harnedy PA, O’Keeffe MB et al (2015) Extraction of antioxidant and ACE inhibitory peptides from Thai traditional fermented shrimp pastes. Food Chem 176:441–447
Wenno MR, Suprayitno E, Aulanni’am Aulanni’am H (2016) Identification and molecular interaction, mechanism and angiotensin converting enzyme inhibitory peptide from Bakasang (fermented Skipjack tuna (Katsuwonus pelamis)). Int J PharmTech Res 9:591–598
Mouritsen OG, Duelund L, Calleja G et al (2017) Flavour of fermented fish, insect, game, and pea sauces: garum revisited. Int J Gastron Food Sci 9:16–28
Kumar S, Nayak BB (2015) Health benefits of fermented fish. In: Prakash Tamang J (ed) Health Benefits of Fermented Foods and Beverages. CRC Press, Boca Raton, FL, 475–488
Skåra T, Axelsson L, Stefánsson G et al (2015) Fermented and ripened fish products in the northern European countries. J Ethnic Foods 2:18–24
Wu H-C, Chen H-M, Shiau C-Y (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber australasicus). Food Res Int 36:949–957
Wisuthiphaet N, Kongruang S, Chamcheun C (2015) Production of fish protein hydrolysates by acid and enzymatic hydrolysis. J Med Bioeng 4:466–470
Beddows CG (1997) Fermented fish and fish products. In: Wood BJB (ed) Microbiology of fermented foods. Springer, Boston, pp 416–440
Korhonen H, Pihlanto A (2006) Bioactive peptides: production and functionality. Int Dairy J 16:945–960
Udenigwe CC, Aluko RE (2012) Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci 77:R11–R24
Bouglé D, Bouhallab S (2017) Dietary bioactive peptides: human studies. Crit Rev Food Sci Nutr 57:335–343
Jo C, Khan FF, Khan MI et al (2017) Marine bioactive peptides: types, structures, and physiological functions. Food Rev Int 33:44–61
Wilkins E, Wilson L, Wickramasinghe K et al (2017) European cardiovascular disease statistics 2017. European Heart Network, Brussels
Zielińska E, Baraniak B, Karaś M (2017) Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 9:970
Nongonierma AB, Lalmahomed M, Paolella S et al (2017) Milk protein isolate (MPI) as a source of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem 231:202–211
Vieira EF, da Silva DD, Carmo H et al (2017) Protective ability against oxidative stress of brewers’ spent grain protein hydrolysates. Food Chem 228:602–609
Mao X, Bai L, Fan X et al (2017) Anti-proliferation peptides from protein hydrolysates of Pyropia haitanensis. J Appl Phycol 29:1623–1633
Nongonierma AB, Hennemann M, Paolella S et al (2017) Generation of wheat gluten hydrolysates with dipeptidyl peptidase IV (DPP-IV) inhibitory properties. Food Funct 8:2249–2257
Nongonierma AB, Paolella S, Mudgil P et al (2017) Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J Funct Foods 34:49–58
Mojica L, Luna-Vital DA, González de Mejía E (2017) Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J Sci Food Agric 97:2401–2410
Zhou D-Y, Liu Z-Y, Zhao J et al (2017) Antarctic krill (Euphausia superba) protein hydrolysates stimulate cholecystokinin release in STC-1 cells and its signaling mechanism. J Food Process Preserv 41:e12903
Flaim C, Kob M, Di Pierro AM et al (2017) Effects of a whey proteins supplementation on oxidative stress, body composition and glucose metabolism among overweight people affected by diabetes mellitus or impaired fasting glucose: a pilot study. J Nutr Biochem 50:95–102
Nobile V, Duclos E, Michelotti A et al (2016) Supplementation with a fish protein hydrolysate (Micromesistius poutassou): effects on body weight, body composition, and CCK/GLP-1 secretion. Food Nutr Res 60:29857
Poprac P, Jomova K, Simunkova M et al (2016) Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci 38:592–607
Neves AC, Harnedy PA, O’Keeffe MB et al (2017) Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res Int 100:112–120
Li-Chan ECY, Hunag S-L, Jao C-L et al (2012) Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem 60:973–978
Gu R-Z, Li C-Y, Liu W-Y et al (2011) Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res Int 44:1536–1540
Ahn C-B, Cho Y-S, Je J-Y (2015) Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem 168:151–156
Jung W-K, Karawita R, Heo S-J et al (2006) Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem 41:2097–2100
Hou H, Fan Y, Li B et al (2012) Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem 134:821–828
Guo L, Harnedy PA, O’Keeffe MB et al (2015) Fractionation and identification of Alaska pollock skin collagen-derived mineral chelating peptides. Food Chem 173:536–542
Nikoo M, Benjakul S, Ehsani A et al (2014) Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J Funct Foods 7:609–620
Lee S-H, Qian Z-J, Kim S-K (2010) A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem 118:96–102
Je J-Y, Qian Z-J, Byun H-G et al (2007) Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem 42:840–846
Chi C-F, Wang B, Hu F-Y et al (2015) Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res Int 73:124–129
Egerton S, Culloty S, Whooley J et al (2017) Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem 245:698–706
Rochelle HDL, Courois E, Cudennec B et al (2015) Fish protein hydrolysate having a satietogenic activity, nutraceutical and pharmacological compositions comprising such a hydrolysate and method for obtaining same. Compagnie des Pêches Saint Malo Santé, Museum National D’Histoire Naturelle. US Patent 14/085,350, 22 Jan 2015
Harnedy PA, Parthsarathy V, McLaughlin CM et al (2018) Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J Funct Foods 40:137–145
Song R, Wei R, Zhang B et al (2011) Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Mar Drugs 9:1142–1156
You L, Zhao M, Liu RH et al (2011) Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J Agric Food Chem 59:7948–7953
Hsu K-C, Li-Chan ECY, Jao C-L (2011) Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem 126:617–622
Huang S-L, Jao C-L, Ho K-P et al (2012) Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121
Ko J-Y, Kang N, Lee J-H et al (2016) Angiotensin I-converting enzyme inhibitory peptides from an enzymatic hydrolysate of flounder fish (Paralichthys olivaceus) muscle as a potent anti-hypertensive agent. Process Biochem 51:535–541
Mahmoodani F, Ghassem M, Babji AS et al (2014) ACE inhibitory activity of pangasius catfish (Pangasius sutchi) skin and bone gelatin hydrolysate. J Food Sci Technol 51:1847–1856
Kim HJ, Park KH, Shin JH et al (2011) Antioxidant and ACE inhibiting activities of the rockfish Sebastes hubbsi skin gelatin hydrolysates produced by sequential two-step enzymatic hydrolysis. Fish Aquat Sci 14:1–10
Chalamaiah M, Hemalatha R, Jyothirmayi T et al (2014) Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Res Int 62:1054–1061
Yang J-I, Tang J-Y, Liu Y-S et al (2016) Roe protein hydrolysates of Giant Grouper (Epinephelus lanceolatus) inhibit cell proliferation of oral cancer cells involving apoptosis and oxidative stress. Biomed Res Int 2016:12
Jang HL, Liceaga AM, Yoon KY (2017) Isolation and characteristics of anti-inflammatory peptides from enzymatic hydrolysates of sandfish (Arctoscopus japonicus) protein. J Aquat Food Prod Technol 26:234–244
Senphan T, Benjakul S (2014) Antioxidative activities of hydrolysates from seabass skin prepared using protease from hepatopancreas of Pacific white shrimp. J Funct Foods 6:147–156
Ngo D-H, Ryu B, Kim S-K (2014) Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem 143:246–255
Aissaoui N, Chobert J-M, Haertlé T et al (2017) Purification and biochemical characterization of a neutral serine protease from Trichoderma harzianum. Use in antibacterial peptide production from a fish by-product hydrolysate. Appl Biochem Biotechnol 182:831–845
Karnjanapratum S, Benjakul S, O’Callaghan YC et al (2016) Purification and identification of antioxidant peptides from gelatin hydrolysates of unicorn leatherjacket skin. Ital J Food Sci 29:158–170
Vilas Boas LCP, de Lima LMP, Migliolo L et al (2017) Linear antimicrobial peptides with activity against Herpes simplex virus 1 and Aichi virus. Pept Sci 108:e22871
Rajapakse N, Jung W-K, Mendis E et al (2005) A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci 76:2607–2619
Wang L, Dong C, Li X et al (2017) Anticancer potential of bioactive peptides from animal sources. Oncol Rep 38:637–651
Ishak NH, Sarbon NM (2017) A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. https://doi.org/10.1007/s11947-017-1940-1
Karoud W, Sila A, Krichen F et al (2017) Characterization, surface properties and biological activities of protein hydrolysates obtained from hake (Merluccius merluccius) heads. Waste Biomass Valorization. https://doi.org/10.1007/s12649-017-0069-9
Yesmine BH, Antoine B, da Silva Ortência Leocádia NG et al (2017) Identification of ACE inhibitory cryptides in Tilapia protein hydrolysate by UPLC–MS/MS coupled to database analysis. J Chromatogr B 1052:43–50
Gauthier SF, Vachon C, Savoie L (1986) Enzymatic conditions of an in vitro method to study protein digestion. J Food Sci 51:960–964
Fekete S, Veuthey J-L, Guillarme D (2012) New trends in reversed-phase liquid chromatographic separations of therapeutic peptides and proteins: theory and applications. J Pharm Biomed Anal 69:9–27
Lemieux L, Piot J-M, Guillochon D et al (1991) Study of the efficiency of a mobile phase used in size-exclusion HPLC for the separation of peptides from a casein hydrolysate according to their hydrodynamic volume. Chromatographia 32:499–504
Ghassem M, Arihara K, Mohammadi S et al (2017) Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct 8:2046–2052
WHO (2014) Global status report on noncommunicable diseases 2014. World Health Organization, Geneva
Brieger K, Schiavone S, Miller FJ et al (2012) Reactive oxygen species: from health to disease. Swiss Med Wkly 142:w13659
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1:441–445
Barbour JA, Turner N (2014) Mitochondrial stress signaling promotes cellular adaptations. Int J Cell Biol 2014:156020
Sae-Leaw T, Karnjanapratum S, O’Callaghan YC et al (2017) Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J Food Biochem 41:e12350
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53:1841–1856
Hernández-Ledesma B, Dávalos A, Bartolomé B et al (2005) Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J Agric Food Chem 53:588–593
Murase H, Nagao A, Terao J (1993) Antioxidant and emulsifying activity of N-(long-chain-acyl) histidine and N-(long-chain-acyl) carnosine. J Agric Food Chem 41:1601–1604
Park P-J, Jung W-K, Nam K-S et al (2001) Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J Am Oil Chem Soc 78:651–656
Husain Z, Schwartz RA (2013) Food allergy update: more than a peanut of a problem. Int J Dermatol 52:286–294
Pawankar R (2014) Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J 7:12–14
Kawai T, Akira S (2006) Innate immune recognition of viral infection. Nat Immunol 7:131–137
Le Y, Zhou Y, Iribarren P et al (2004) Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol 1:95–104
Martin P, Leibovich SJ (2005) Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15:599–607
Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449:819–826
Andersen MH, Schrama D, Thor Straten P et al (2006) Cytotoxic T cells. J Invest Dermatol 126:32–41
Portnoy JM, Van Osdol T, Williams PB (2004) Evidence-based strategies for treatment of allergic rhinitis. Curr Allergy Asthma Rep 4:439–446
Meltzer EO (2017) Sublingual immunotherapy: a guide for primary care. J Fam Pract 66:S58–S58
Reyes-Díaz A, González-Córdova AF, Hernández-Mendoza A et al (2017) Immunomodulation by hydrolysates and peptides derived from milk proteins. Int J Dairy Technol. https://doi.org/10.1111/1471-0307.12421
Tsuruki T, Kishi K, Takahashi M et al (2003) Soymetide, an immunostimulating peptide derived from soybean β-conglycinin, is an fMLP agonist. FEBS Lett 540:206–210
Jaziri Mh, Migliore-Samour D, Casabianca-Pignède M-R et al (1992) Specific binding sites on human phagocytic blood cells for Gly-Leu-Phe and Val-Glu-Pro-Ile-Pro-Tyr, immunostimulating peptides from human milk proteins. Biochim Biophys Acta 1160:251–261
Takahashi M, Moriguchi S, Ikeno M et al (1996) Studies on the ileum-contracting mechanisms and identification as a complement C3a receptor agonist of oryzatensin, a bioactive peptide derived from rice albumin. Peptides 17:5–12
Cicero AFG, Fogacci F, Colletti A (2017) Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br J Pharmacol 174:1378–1394
Chalamaiah M, Yu W, Wu J (2018) Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: a review. Food Chem 245:205–222
Subhan F, Kang HY, Lim Y et al (2017) Fish scale collagen peptides protect against CoCl2/TNF-α-induced cytotoxicity and inflammation via inhibition of ROS, MAPK, and NF-κB pathways in HaCaT cells. Oxid Med Cell Longev. https://doi.org/10.1155/2017/9703609
Elango R, Laviano A (2017) Protein and amino acids: key players in modulating health and disease. Curr Opin Clin Nutr Metab Care 20:69–70
Li G-H, Le G-W, Shi Y-H et al (2004) Angiotensin I–converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res 24:469–486
Natesh R, Schwager SLU, Sturrock ED et al (2003) Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature 421:551–554
Wu J, Aluko RE, Nakai S (2006) Structural requirements of angiotensin I-converting enzyme inhibitory peptides: quantitative structure−activity relationship study of di- and tripeptides. J Agric Food Chem 54:732–738
de Oliveira-Sales EB, Nishi EE, Boim MA et al (2010) Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C wistar rat model. Am J Hypertens 23:708–715
Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. Lancet 389:2239–2251
Eisenbarth GS (2007) Update in type 1 diabetes. J Clin Endocrinol Metab 92:2403–2407
IDF (2015) IDF diabetes atlas. IDF, Brussels
Thomas MC, Paldánius PM, Ayyagari R et al (2016) Systematic literature review of DPP-4 inhibitors in patients with type 2 diabetes mellitus and renal impairment. Diabetes Ther 7:439–454
Mahmood N (2016) A review of α-amylase inhibitors on weight loss and glycemic control in pathological state such as obesity and diabetes. Comp Clin Pathol 25:1253–1264
Zhang L, Chen Q, Li L et al (2016) Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: a systematic review and meta-analysis. Sci Rep 6:32649
Lacroix IME, Li-Chan ECY (2016) Food-derived dipeptidyl-peptidase IV inhibitors as a potential approach for glycemic regulation – current knowledge and future research considerations. Trends Food Sci Technol 54:1–16
Stoimenis D, Karagiannis T, Katsoula A et al (2017) Once-weekly dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Expert Opin Pharmacother 18:843–851
Lovshin JA (2017) Glucagon-like peptide-1 receptor agonists: a class update for treating type 2 diabetes. Can J Diabetes 41:524–535
Gourgari E, Aroda VR, Wilhelm EE et al (2017) A comprehensive review of the FDA-approved labels of diabetes drugs: indications, safety, and emerging cardiovascular safety data. J Diabetes Complications 31:1719–1727
Nishi T, Hara H, Tomita F (2003) Soybean β-conglycinin peptone suppresses food intake and gastric emptying by increasing plasma cholecystokinin levels in rats. J Nutr 133:352–357
Sharara AI, Bouras EP, Misukonis MA et al (1993) Evidence for indirect dietary regulation of cholecystokinin release in rats. Am J Physiol Gastrointest Liver Physiol 265:G107–G112
Cudennec B, Ravallec-Plé R, Courois E et al (2008) Peptides from fish and crustacean by-products hydrolysates stimulate cholecystokinin release in STC-1 cells. Food Chem 111:970–975
Cudennec B, Fouchereau-Peron M, Ferry F et al (2012) In vitro and in vivo evidence for a satiating effect of fish protein hydrolysate obtained from blue whiting (Micromesistius poutassou) muscle. J Funct Foods 4:271–277
Greco E, Winquist A, Lee T et al (2017) The role of source of protein in regulation of food intake, satiety, body weight and body composition. J Nutr Health Food Eng 6:00223
Madani Z, Sener A, Malaisse WJ et al (2015) Sardine protein diet increases plasma glucagon-like peptide-1 levels and prevents tissue oxidative stress in rats fed a high-fructose diet. Mol Med Rep 12:7017–7026
Umezawa H, Aoyagi T, Ogawa K et al (1984) Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J Antibiot 37:422–425
Chinembiri T, du Plessis L, Gerber M et al (2014) Review of natural compounds for potential skin cancer treatment. Molecules 19:11679
Creagh EM (2014) Caspase crosstalk: integration of apoptotic and innate immune signalling pathways. Trends Immunol 35:631–640
Roomi MW, Shanker N, Niedzwiecki A et al (2015) Induction of apoptosis in the human prostate cancer cell line DU-145 by a novel micronutrient formulation. Open J Apoptosis 4:11
Ding G-F, Huang F-F, Yang Z-S et al (2011) Anticancer activity of an oligopeptide isolated from hydrolysates of Sepia ink. Chin J Nat Med 9:151–155
Alemán A, Pérez-Santín E, Bordenave-Juchereau S et al (2011) Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res Int 44:1044–1051
Harnedy PA, Fitzgerald RJ (2013) Bioactive proteins and peptides from macroalgae, fish, shellfish and marine processing waste. In: Kim S-K (ed) Marine proteins and peptides. Wiley, Chichester, pp 5–39
Minekus M, Alminger M, Alvito P et al (2014) A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct 5:1113–1124
Liu Y, Pischetsrieder M (2017) Identification and relative quantification of bioactive peptides sequentially released during simulated gastrointestinal digestion of commercial kefir. J Agric Food Chem 65:1865–1873
Vilcacundo R, Martínez-Villaluenga C, Hernández-Ledesma B (2017) Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J Funct Foods 35:531–539
Phongthai S, D’Amico S, Schoenlechner R et al (2018) Fractionation and antioxidant properties of rice bran protein hydrolysates stimulated by in vitro gastrointestinal digestion. Food Chem 240:156–164
Nieva-Echevarria B, Jacobsen C, García Moreno PJ et al (2016) Evaluation of the antioxidant activity in food model system of fish peptides released during simulated gastrointestinal digestion. Paper presented at the 14th Euro Fed Lipid Congress, Ghent, 18–21 Sept 2016
Sanchón J, Fernández-Tomé S, Miralles B et al (2018) Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem 239:486–494
Toopcham T, Mes JJ, Wichers HJ et al (2017) Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem 220:190–197
Grimble GK, Keohane PP, Higgins BE et al (1986) Effect of peptide chain length on amino acid and nitrogen absorption from two lactalbumin hydrolysates in the normal human jejunum. Clin Sci 71:65–69
Keohane PP, Grimble GK, Brown B et al (1985) Influence of protein composition and hydrolysis method on intestinal absorption of protein in man. Gut 26:907–913
Hou H, Fan Y, Wang S et al (2016) Immunomodulatory activity of Alaska pollock hydrolysates obtained by glutamic acid biosensor – artificial neural network and the identification of its active central fragment. J Funct Foods 24:37–47
FitzGerald RJ, Meisel H (2000) Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br J Nutr 84:33–37
Ramezanzade L, Hosseini SF, Nikkhah M (2017) Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem 234:220–229
Acknowledgments
Aurélien V. Le Gouic and Pádraigín A. Harnedy were funded by the Department of Agriculture, Food and the Marine, Ireland, under grant issue 14/F/873 and 13/F/467, respectively.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Le Gouic, A.V., Harnedy, P.A., FitzGerald, R.J. (2019). Bioactive Peptides from Fish Protein By-Products. In: Mérillon, JM., Ramawat, K.G. (eds) Bioactive Molecules in Food. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-78030-6_29
Download citation
DOI: https://doi.org/10.1007/978-3-319-78030-6_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-78029-0
Online ISBN: 978-3-319-78030-6
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics