Abstract
Chicken has been selected for higher production performance over the years and are highly sensitive to changes in their environment. The average global temperature has increased over the century and is further expected to rise. In open house rearing system chicken is vulnerable to this increasing environmental temperature and may experience thermal stress. Heat shock proteins (HSP) are highly conserved family of proteins playing important role in normal cellular physiology and cytoprotection against different stressors including heat stress. In chicken levels of different members of HSP family are increased in almost all the tissues in response to heat stress. This increased HSP level protects cellular proteins from heat stress induced damage. Efforts to overcome the heat stress conditions in chicken have lead to development of thermal manipulation protocols whereby epigenetic modifications are introduced. Through epigenetic adaptation the birds acquire protection against the adverse effects of heat stress. This chapter discusses the findings on cellular HSP responses to heat stress and the thermal manipulation strategy to overcome heat stress in chicken.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The global mean surface temperature has increased by 0.85 °C over the period (1880–2012) and by the end of this century it is further expected to increase 0.3 to 4.8 °C under different scenarios (IPCC 2014). The optimum temperature range for production for broiler and layer chicken is 18 to 22 °C (Charles 2002; Aengwanich and Simaraks 2004) with slight variations on either side due to differences in age, sex and line/strain. In the tropics and sub tropics chicken are generally reared in open-sided houses. This makes chicken more vulnerable to high environmental temperature during summer and is likely to experience heat stress. This is a challenging situation for the commercial poultry industry in terms of sustaining production and animal welfare.
Chicken are homeothermic and the body temperature is maintained in the range of 41 to 42 °C. Chicken are more sensitive to high ambient temperatures due to lack of sweat glands and high body temperature. Furthermore, the fast growing commercial broilers and layers producing high number of eggs generate metabolic heat that adds to the already stressful situation during summer. Birds selected for fast growth exhibit a reduced thermoregulatory capacity in comparison to layers and are therefore more susceptible to heat stress and related problems (Mitchell et al. 2005). During heat stress evaporative cooling is an important mechanism for body temperature control. In birds evaporative cooling involves evaporative mechanisms involving skin and respiratory system. The gradient between body surface and air moisture determines the rate of evaporative cooling. When high environmental temperature combined with high relative humidity prevails the evaporative cooling mechanism is impeded. Thus the combined effects of high ambient temperature with high humidity results in hyperthermia leading to decreased food consumption, growth rate, feed efficiency, total egg production, egg quality and survivability (Mashaly et al. 2004; Rajkumar et al. 2011; Xie et al. 2014). The performance of birds gets affected when the ambient temperature increases and approaches the upper critical temperature and above this point the body temperature increases until it reaches the lethal stage. Under heat stress conditions birds try to adapt themselves by increasing peripheral blood flow that helps in dissipating excess body heat, decreases feed intake so as to reduce heat increment and increases panting to facilitate evaporative cooling (Daghir 2008). A variety of cellular structures and metabolic processes are damaged by heat stress and the extent of damage is based on magnitude and duration of heat exposure.
All organisms respond to heat stress by inducing a set of proteins called as Heat Shock Proteins (HSP). The HSP are synthesized in cells in response to different types of stressors. Ferruccio Ritossa (1962) reported chromosomal puffing pattern and higher transcriptional activity in Drosophila salivary glands after exposure to heat. Later the transcriptional activity was discovered to be synthesis of heat induced polypeptides and named as heat shock proteins (Ashburner and Bonner 1979). HSP are highly conserved proteins consisting of several families found in all living organisms (Lindquist and Craig 1988). The HSP act as molecular chaperons that help in protein folding and assembly, assist in restoring the native state of protein, regulate degradation of protein and in translocation across membranes (Hartl and Hayer-Hartl 2002). HSP synthesis up-regulation under different stress conditions is an adaptive phenomenon resulting in improved tolerance. There exists a relationship between thermo tolerance and HSP synthesis in organisms (Parsell and Lindquist 1994).
2 Heat Stress
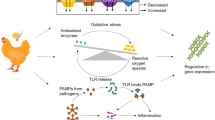
Lee (1965) has defined stress as “the magnitude of forces external to the bodily system which tend to displace that system from its resting or ground state”. Stress may be caused by many factors including climate in which birds are reared. The ambient temperature range within which birds do not use additional energy to maintain the body temperature is known as thermoneutral zone. Weather conditions above this thermoneutral zone for prolonged time results in heat stress. Heat stress is a result of interaction between ambient temperature and relative humidity and in birds this interaction effect is complex (Yahav et al. 1995). In birds, excess body heat is dissipated through conduction, convection, radiation and water evaporation through respiratory tract. The physiological events that occur during the period of heat stress are described in Fig. 6.1.
Among different heat loss mechanisms blood flow redistribution and passive water loss from respiratory system are important. Water loss during prolonged heat stress condition results in dehydration and decreased blood volume. This decreased blood volume alters the venous return and blood circulation to upper respiratory tract culminating in hyperthermia and finally death. The physiological changes due to heat stress are accompanied by damaging changes at cellular level. There is significant increase in induction of different members of heat shock protein family during heat stress. The different events occurring at cellular level during heat stress are:
-
Increase in membrane liquidity
-
Destabilization of membrane components
-
Cytoskeleton modification
-
Mitochondrial swelling
-
Endoplasmic reticulum and golgi apparatus fragmentation
-
Nucleolus disintegration
-
Formation of electron dense granules – stress bodies – in nucleus
-
Decrease in cell viability and cell death (depending on heat stress intensity, type of cells and stage of cell cycle)(Velichko et al. 2013)
Birds respond differently to acute and chronic heat stress. Plasma metabolites are primarily disturbed in acute heat stress and tissue damage occurs in chronic heat stress (Xie et al. 2015). Acute heat stress results in increase in body temperature and increase in respiration rate to 235 breaths/min from 21.7 breaths/min observed during thermoneutral conditions (Mitchell and Siegel 1973). The capillary blood flow to exterior of body increases whereas to the internal organs it decreases (Wolfenson et al. 1981). Though heat stress affects the production in both layers and broilers the effect is more pronounced in commercial broilers and broiler breeders because of their heavy body. The huge muscle mass of broilers posses challenge in excess heat dissipation during heat stress conditions resulting in higher core body temperature and fatality. The magnitude of heat tolerance is genetically variable and related to the growth potential of the bird (Settar et al. 1999).
2.1 Heat Shock Proteins
Heat shock proteins are expressed in cells constitutively or induced by different stimuli such as heat, chemical etc. HSP are classified into six families based on molecular weights namely HSP100, HSP90, HSP70, HSP40, small HSP and chaperonins (De Maio and Vazquez 2013). Apart from the molecular chaperone activity small HSP are also implicated in other cellular functions such as stress tolerance, protein folding, cytoskeletal integrity and cell cycle (Bakthisaran et al. 2015). HSP are also found in the extracellular environment, secreted actively or passively, where they may act as stress signals and stimulates immune cells (De Maio and Vazquez 2013). HSP90 is the most abundant soluble protein comprising 1–2% of total cell proteins and under stress conditions it is upregulated (Csermely et al. 1998). The cytoplasmic HSP90 is found in two forms namely inducible HSP90α and constitutive HSP90β. In mammalian cells HSP27 expression has been shown to be correlated with thermotolerance (Landry et al. 1989).
2.2 Heat Stress and HSP
Natural incubation conditions may not be uniform as hens regularly move in search of food and there may be non-uniform nest insulation. Heat stress may affect the developing embryos and induct HSP during natural incubation. Reports indicate HSP induction in chick embryos after exposure to high incubation temperature for various durations. Chicken embryo fibroblasts exposed to 45 °C was observed to increase the synthesis of three proteins (MW 22,000, 76,000 and 95,000 daltons), the level was exceeding the synthesis of cell structural proteins. The level of these proteins returned back to normal after 8 h (Kelley and Schlesinger 1978). High temperature increasing some HSP expression in chicken embryonic cells was reported by Edington and Hightower (1990). Exposing chicken embryonic cells to 44 °C for 30 minutes caused two to three fold increases in HSP23, HSP71 and HSP88 proteins expression. Similarly, exposing developing chick embryos to higher incubation temperature than normal increased different HSP gene and protein expressions (Gabriel et al. 2002; Vinoth et al. 2015).
The testes in birds are located inside the body with an internal temperature range of 40–41.7 °C and spermatogenesis is efficient even at this temperature. This is because of HSPA2 gene in bird testes that provides an adaptive advantage over the mammals (Padhi et al. 2016). However, the internal location of testes also pre-disposes it to heat stress induced hyperthermia that affects spermatogenic process. The impact of high ambient temperature on semen quality and fertility is severe in broiler males having best semen quality index (Karaca et al. 2002). Acute heat stress (38 °C and 55% RH for 4 h) in Taiwanese roosters caused upregulation of genes belonging to HSP family (HSP70, HSP90AA1, HSPB8, HSPA5, HSPA8, HSPB1, HSPA4) in the testes (Wang et al. 2013). However, in a subsequent report it was shown that acute heat stressed rooster testes had decreased expression of HSP90α and HSP25 proteins that are involved in protein folding (Wang et al. 2014). Besides the action of preventing aggregation of misfolded proteins and protein repair HSP70 has important role in spermatogenesis. The molecular chaperone HSPA2 is a member of the HSP70 family. It has also been shown to play important role in spermatogenesis and male fertility. In response to heat stress HSP25, HSPD1, HSPA5 and HSPA2 gene expression in chicken testes is upregulated (Wang et al. 2015). The genes HSPD1 and HSPA5 are involved in apoptotic process. Thus higher expression of these two genes indicates occurrence of apoptosis in testicular cells. There is difference in gene expression of the HSPD1 in testis of heat stressed broiler and layer type chicken (Wang et al. 2015). Chicken testes subjected to heat shock (46 °C for 2 h) had increased expression and polyadenylation of HSP70 and was suggested that this might contribute to development of thermotolerance (Mezquita et al. 1998). HSP70 is expressed constitutively in chicken testes and the expression increased when subjected to heat shock at 44 °C or 46 °C (Mezquita et al. 2001). Taiwanese country chicken subjected to heat stress conditions for six weeks had higher sperm HSP70 concentration, however, semen samples kept at 41 °C had decreased or no change in HSP70 concentration depending on the semen diluent used (Yan 2001). The differing result of HSP70 in this experiment may be due to difference between in vitro and in vivo conditions. During heat stress the increase in HSP expression is an indirect indication of disturbance in testes physiology and consequent heat stress related fertility problems.
Exposure of chicken to acute heat stress of 40 °C for duration spanning 1 and 1.5 h resulted in higher HSP70 gene expression in brain. There was no difference in HSP70 gene expression between native chickens but the commercial layer chicken had higher expression (Tamzil et al. 2013). The susceptibility of commercial layers to acute heat stress might be due to higher production performance. Furthermore, HSP70 gene expression during heat stress was influenced by HSP70 genotype of the chicken. Chicken having certain HSP70 genotype was found to be heat tolerant and acute heat stress had no negative effect on growth performance and egg production (Liang et al. 2016).
Heat stress causes variety of changes in broiler gastrointestinal tract such as alteration in intestinal microflora, intestinal injury and intestinal barrier integrity impairment (Quinteiro-Filho et al. 2010; Song et al. 2014). In the process of heat dissipation blood flow is diverted to the periphery of the body and the functions of internal organs such as intestine get adversely affected. Furthermore, the susceptibility to heat stress differs with the region of the intestine. Broilers subjected to heat stress had upregulated expression of HSP70 and HSP90 mRNA expression in jejunum and ileum, however, the response was more pronounced in ileum than in jejunum. The HSP mRNA expression was unaltered in duodenum and colon (Varasteh et al. 2015). At the protein level only HSP70 and not HSP90 was increased in jejunum and ileum.
Hypothalamus is crucial for detection and regulation of body temperature. Taiwan broiler type country chicken subjected to acute heat stress (38 °C for 2 h) had upregulated HSPB1 and HSP25 through the recovery period indicating their essential role in the hypothalamus (Tu et al. 2016). HSP70 gene expression increased but expression of HSP90α and HSP90β were unaltered in brain of heat stressed chicken (Mahmoud et al. 2004; Zhen et al. 2006; Zhang et al. 2014). Furthermore, this induced expression of HSP70 mRNA in brain differed with the HSP70 genotype of the chicken (Zhen et al. 2006). HSP70 protein expression increases in brain and liver of heat stressed but not in heat acclimated broilers (Guerreiro et al. 2004).
Liver is highly metabolically active organ and therefore, comparatively is more susceptible to heat stress than other organs. Acute heat stress increased HSP90α, HSP90β, HSP70 and HSP60 expressions in broiler liver and HSP60 expression gradually decreased at 3 h of exposure (Gabriel et al. 1996; Mahmoud et al. 2004; Zhen et al. 2006; Yu and Bao 2008; Yu and Bao 2009; Yan et al. 2009; Zhang et al. 2014). Broilers subjected to chronic heat stress for 3 or 6 weeks had higher rectal temperature and up-regulated liver HSP70 mRNA expression (Givisiez et al. 1999; Zuo et al. 2015). RNA-seq technology was applied to study the liver transcriptome response to acute and chronic heat stress and found that broilers differentially expressed more genes than heat resistant Fayoumi line (Lan et al. 2016). Gene expression response to acute heat stress was stronger compared to chronic heat stress in broilers. Furthermore, this response differed between the chicken lines studied. Network analysis of differentially expressed genes indicated HSP having numerous connections with immune related genes. Furthermore, the secreted HSP tend to attract immune cells (Shevtsov and Multhoff 2016). In another study using RNA seq method, chicken hepatocarcinoma cell line maintained at 43 °C for 2.5 h had several HSP upregulated with HSP25 having the highest expression (Sun et al. 2015). Liver of laying broiler breeder had increased HSP70 expression during acute heat stress and HSP90 expression during chronic heat stress (Xie et al. 2014).
HSP70 and HSP90 gene expression in muscle were higher during acute heat stress but not in chronic heat stress (Zhen et al. 2006; Xie et al. 2014; Zhang et al. 2014). Similarly acute heat stress caused increase in HSP90 and HSP70 protein and mRNA expression in kidney of broilers (Yu and Bao 2008; Yu and Bao 2009).
HSP60, HSP70 and HSP90 mRNA expressions were increased in broiler heart after 2 h of heat exposure and subsequently expression declined except for HSP90 which expression again increased at 10 h of heat exposure. However, the corresponding proteins were increased in concentration and remained till 10 h post exposure (Yu and Bao 2008; Yu et al. 2008; Yu and Bao 2009) indicating a prolonged adverse effect of heat exposure. One hour exposure of broilers to high environmental temperature (40/41 °C) caused increase in expressions of HSP90α, HSP90β and HSP70 in heart (Mahmoud et al. 2004; Zhang et al. 2014). Apart from heart, HSP90 and HSP70 proteins were detected in the broiler heart blood vessels by immunolocalization (Yu et al. 2008; Yu and Bao 2009). In another study, during chronic heat stress HSP70 mRNA expression was upregulated and during acute heat stress HSP90 mRNA expression upregulated in heart of laying broiler breeders (Xie et al. 2014). The difference in the expression pattern among the reports may be due to the age difference of the birds used in the experiments. In vitro and in vivo high temperature exposure induced higher levels of HSP 23, 70 and 90 expressions in broiler chickens peripheral leukocytes (Wang and Edens 1998). Layer chicken subjected to long term moderate heat stress had higher HSP70 protein concentration in circulating mononuclear blood cells but the response to heat stress was not uniform at different ages studied (Maak et al. 2003). Exposing chicken macrophage-line HD11 cells to 45 °C for 2 h caused increase in mRNA expression of chaperone genes HSP25, HSPA2 and HSPH1 (Slawinska et al. 2016). The manifold expressed small HSP (HSP25) is ATP independent chaperone that bind to unfolded proteins and other HSP engage with these proteins to bring back to their original structure. Acute heat stress increases HSP90α, HSP90β and HSP70 expression in broiler spleen (Mahmoud et al. 2004). The body temperature of heat stressed young White Leghorn chickens were positively correlated with HSP70 expression in heart and liver (Mahmoud et al. 2003). High ambient temperature severely affects egg production and quality (Rozenboim et al. 2007). Apart from reduced feed intake and altered physiological parameters of the hen (Darre and Harrison 1987) change in gene expressions of small ovarian follicles in the laying hens was reported. HSP25 gene expression was upregulated indicating damage to proteins of follicle cells (Cheng et al. 2015). There is 70–80% fall in capillary blood flow to large ovarian follicles and infundibulum in heat stressed laying hens (Wolfenson et al. 1981). Collectively, low blood flow and ovarian follicle cellular damage may be the reasons for reduction in egg production in laying hens.
The mRNA expression of HSP70 in tissues during heat stress differs depending on the breed of chicken, some were more tolerant to high ambient temperature than others (Zhang et al. 2014). Even the basal expression of HSP70 mRNA in liver and muscle differs with the HSP70 genotype of the chicken, expression being higher in heterozygous genotypes than that of homozygous genotypes (Zhen et al. 2006). A global gene expression study using microarray in acute heat exposed broiler chicken revealed differentially expressed genes including HSPH1 and HSP25 in brain, liver and leg muscle (Luo et al. 2014). These HSP genes were reported to be part of different functional clusters that were related to the effects of heat stress.
A clear difference in HSP gene expressions was found between the tissues. This might be due to the functional or metabolic status of the tissues. Likewise a difference in HSP gene expression was found between the chicken lines. The duration of heat exposure, acute or chronic, differentially affected HSP gene expressions. HSP are expressed in almost all vital organs studied in response to heat stress in chicken. The functional relationship between the HSP expression and other systems/process vis-à-vis production in chicken will be helpful to address the production and welfare of chicken during heat stress conditions.
3 Epigenetics and Thermal Manipulation
The term epigenetics was introduced by Conrad Waddington (1942) and defined it as “the branch of biology which studies the causal interactions between genes and their products which bring the phenotype into being.” However, at present there is lack in clear definition of the term (Deans and Maggert 2015). It is now accepted that apart from information contained in DNA sequence the overall phenotype is also determined by epigenetic information. The epigenome is generated by different epigenetic processes such as DNA methylation, chromatin remodeling, post translational histone modifications, regulation of gene expression by non-coding RNA’s instability of genome and modification of animal phenotype by other forces (Triantaphyllopoulos et al. 2016). Epidemiological studies in humans and genetic studies in animals have revealed that epigenetic marks, in addition to the DNA sequence, may be transmitted across generations and influence the offspring phenotype (Jablonka 2009; Nätt et al. 2012).
Body temperature regulation system, similar to most functional systems, during embryogenesis develops from open loop control systems into closed control systems (Dӧrner 1974). The preoptic anterior hypothalamic area contains thermosensitive neurons that receive and integrate thermal signals from the body and induces thermoregulatory responses so as to maintain relatively constant core body temperature. In prenatal or early post-hatching ontogeny, during critical developmental phases lifelong epigenetic adaptation takes place (Tzschentke and Plagemann 2006). Thus by thermal manipulation (TM) the ‘set point’ can be altered resulting in change in the threshold for heat production as well as heat loss of an animal. In chicken, TM can be carried out during egg incubation (pre-hatching) and post hatch period. In the process of thermal manipulations during embryogenesis three factors should be considered namely the critical phase, the temperature level and duration of exposure (Yahav 2015). TM carried from 7th day of egg incubation till 18th day of incubation and during first 10 days post hatch has been shown to produce beneficial effects (reduced stress and lower mortality) when broiler type chicken are raised under hot environmental conditions. Higher temperature during incubation may result in poor hatchability (own unpublished data; Yahav et al. 2004). The hypothesis underlying TM is that cells exposed to non lethal higher temperatures activate cellular stress responses that confer thermotolerance and resistance to future heat stress related damages. Epigenetic modifications such as DNA methylation and histone modifications in developing chicken embryos were found to be relatively stable after hatching, indicating these alterations may play critical role in development of chick embryos and neonates (Li et al. 2015).
3.1 HSP in Thermal Manipulated Chicken
Pre-natal thermal manipulated coloured broilers reared and slaughtered at high ambient temperature had lower HSP gene and protein expressions in different tissues (Rajkumar et al. 2015; Rajkumar et al. 2017; Vinoth et al. 2015). There was breed differences, Naked Neck chicken had no or variable effect of TM in these reports. This may be due to lesser feather coverage in Naked Neck chicken that helped in dissipating excess body heat than the coloured broiler used in the experiment. The lower HSP genes expression in TM birds were analysed by luciferase reporter gene assay and found to be due to methylation at several sites in the promoter regions of these genes (Vinoth 2016). Furthermore, in the same study difference in overall methylation frequency of HSP70 due to TM between two breeds of chicken was observed. Heat conditioning of 5–7 day old chicks on subsequent thermal challenge at 42nd day had decreased HSP 27, 70 and 90 expressions in heart, liver and lung tissues (Yahav et al. 1997; Vinoth et al. 2016). It is clear from these reports that TM during incubation or at early age confers thermotolerance during adulthood and this thermotolerance is through lowered body temperature during stress period. Furthermore, lower HSP levels in thermotolerant birds indicate lesser damage to the cellular structures. Few reports state that adult chicken that were heat conditioned as chicks or thermal manipulated during incubation when heat stressed later had higher HSP gene expressions (Wang and Edens 1998; Al-Zhgoul et al. 2013). One possibility for the differences in HSP expression in stressed adult birds after TM during embryonic development may be due to different protocols or birds used. Most of the TM reports are in broiler chicken since this protocol may be expected to be more beneficial to broilers because of their higher body mass and therefore comparatively greater predisposition to heat stress than layer chicken. A single report indicated beneficial effect of TM in layer breeders where TM manipulated birds had lower HSP 70 and HSP 27 gene expression in spermatozoa under hot ambient condition (Shanmugam et al. 2015). There is correlation between body temperature and induction of HSP, however, it was opined that the HSP response to early age TM was not part of long-term mechanism of this strategy (Yahav 2009). HSP response in acquisition of thermotolerance is only a part of the TM strategy and efforts in understanding other molecular mechanisms playing role are in progress. Peri-natal TM affects the development of certain tissues/organs in the adult stage. For example, in brain two genes (R-Ras3 and brain derived neurotropic factor) were found to be expressed higher in TM birds (Yahav 2009). Proteomic analysis revealed higher expression of UMP-CMP kinase enzyme in heat stressed TM birds (Vinoth 2016). This enzyme is involved in both denovo and salvage pathways of nucleosides and catalyses the synthesis of UTP, CTP and dCTP (Pasti et al. 2003).
4 Conclusions
Climate change and resultant increase in environmental temperature posses challenges in sustaining commercial chicken production and welfare. Heat stress causes reduction in production performance of both layer and broiler chicken. It is well established that in response to heat stress HSP expression is increased as a cellular mechanism to protect different protein against damage. During heat stress higher HSP expressions could be observed in almost all important body organs of chicken. Recent research is pointing to the advantages of epigenetic adaptation through thermal manipulation strategy. However, this strategy has to be fine-tuned for the temperature level and embryonic developmental stage so that the critical phases of different body control systems are beneficially modified. Furthermore, the interaction between HSP and other molecular systems needs detailed study for widespread commercial exploitation of thermal manipulation strategies in chicken.
Abbreviations
- CO2 :
-
Carbon dioxide
- HSP:
-
Heat shock protein
- RH:
-
Relative humidity
- TM:
-
Thermal manipulation
References
Aengwanich, W., & Simaraks, S. (2004). Pathology of heart, lung, liver and kidney in broilers under chronic heat stress. Songklanakarin. Journal of Science and Technology, 26, 417–424.
Al-Zhgoul, M. B., Dalab, A. E., Ababneh, M. M., Jawasreh, K. I., Al Busadah, K. A., & Ismail, Z. B. (2013). Thermal manipulation during chicken embryogenesis results in enhanced Hsp70 gene expression and the acquisition of thermotolerance. Research in Veterinary Science, 95, 502–507.
Ashburner, M., & Bonner, J. J. (1979). The induction of gene activity in drosophila by heat shock. Cell, 17, 241–254.
Bakthisaran, R., Tangirala, R., & Rao, C. M. (2015). Small heat shock proteins: Role in cellular functions and pathology. Biochimica et Biophysica Acta – Proteins and Proteomics, 1854, 291–319.
Charles, D. R. (2002). Responses to the thermal environment. In D. A. Charles & A. W. Walker (Eds.), Poultry environment problems, a guide to solutions (pp. 1–16). Nottingham: Nottingham University Press.
Cheng, C. Y., Tu, W. L., Wang, S. H., Tang, P. C., Chen, C. F., Chen, H.-H., et al. (2015). Annotation of differential gene expression in small yellow follicles of a broiler-type strain of Taiwan country chickens in response to acute heat stress. PLoS One, 10, e0143418.
Csermely, P., Schnaider, T., Soti, C., Prohászka, Z., & Nardai, G. (1998). The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacology & Therapeutics, 79, 129–168.
Daghir, N. J. (2008). Poultry production in hot climates (p. 49). Cambridge: CABI.
Darre, M. J., & Harrison, P. C. (1987). Heart rate, blood pressure, cardiac output, and total peripheral resistance of single comb white leghorn hens during an acute exposure to 35C ambient temperature. Poultry Science, 66, 541–547.
De Maio, A., & Vazquez, D. (2013). Extracellular heat shock proteins: A new location, a new function. Extracellular heat shock proteins: A new location, a new function. Shock, 40, 239–246.
Deans, C., & Maggert, K. A. (2015). What do you mean, “epigenetic”? Genetics, 199, 887–896.
Dörner, G. (1974). Environment-dependent brain differentiation and fundamental processes of life. Acta Biologica et Medica Germanica, 33, 129–148.
Edington, B. V., & Hightower, L. E. (1990). Induction of a chicken small heat shock (stress) protein: Evidence of multilevel posttranscriptional regulation. Molecular and Cellular Biology, 10, 4886–4898.
Gabriel, J. E., Ferro, J. A., Stefani, R. M. P., Ferro, M. I. T., Gomes, S. L., & Macari, M. (1996). Effect of acute heat stress on heat shock protein 70 messenger RNA and on heat shock protein expression in the liver of broilers. British Poultry Science, 37, 443–449.
Gabriel, J. E., da Mota, A. F., Boleli, I. C., Macari, M., & Coutinho, L. L. (2002). Effect of moderate and severe heat stress on avian embryonic hsp70 gene expression. Growth, Development, and Aging, 66, 27–33.
Givisiez, P. E., Ferro, J. A., Ferro, M. I., Kronka, S. N., Decuypere, E., & Macari, M. (1999). Hepatic concentration of heat shock protein 70 kD (Hsp70) in broilers subjected to different thermal treatments. British Poultry Science, 40, 292–296.
Guerreiro, E. N., Giachetto, P. F., Givisiez, P. E. N., et al. (2004). Brain and hepatic Hsp70 protein levels in heat-acclimated broiler chickens during heat stress. Braz ilian Journal of Poultry Science, 6, 201–206.
Hartl, F. U., & Hayer-Hartl, M. (2002). Molecular chaperones in the cytosol: From nascent chain to folded protein. Science, 295, 1852–1858.
IPCC. (2014). Climate change 2014: Synthesis report. In Core Writing Team, R. K. Pachauri, & L. A. Meyer (Eds.), Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Geneva: IPCC.
Jablonka, E. (2009). Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. The Quarterly Review of Biology, 84, 131–176.
Karaca, A. G., Parker, H. M., Yeatman, J. B., & Mcdaniel, C. D. (2002). The effects of heat stress and sperm quality classification on broiler breeder male fertility and semen ion concentrations. British Poultry Science, 43, 621–628.
Kelley, P. M., & Schlesinger, M. J. (1978). The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell, 15, 1277–1286.
Lan, X., Hsieh, J. C. F., Schmidt, C. J., Zhu, Q., & Lamont, S. J. (2016). Liver transcriptome response to hyperthermic stress in three distinct chicken lines. BMC Genomics, 17, 955.
Landry, J., Chrétien, P., Lambert, H., Hickey, E., & Weber, L. A. (1989). Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. The Journal of Cell Biology, 109, 7–15.
Lee, D. H. K. (1965). Climatic stress indices for domestic animals. International Journal of Biometeorology, 9, 29–35.
Li, C., Guo, S., Zhang, M., Gao, J., & Guo, Y. (2015). DNA methylation and histone modification patterns during the late embryonic and early postnatal development of chickens. Poultry Science, 94, 706–721.
Liang, H. M., Lin, D. Y., Hsuuw, Y. D., et al. (2016). Association of heat shock protein 70 gene polymorphisms with acute thermal tolerance, growth, and egg production traits of native chickens in Taiwan. Archives Animal Breeding, 59, 173–181.
Lindquist, S., & Craig, E. A. (1988). The heat shock proteins. Annual Review of Genetics, 22, 631–677.
Luo, Q. B., Song, X. Y., Ji, C. L., Zhang, X. Q., & Zhang, D. X. (2014). Exploring the molecular mechanism of acute heat stress exposure in broiler chickens using gene expression profiling. Gene, 546, 200–205.
Maak, S., Melesse, A., Schmidt, R., Schneider, F., & Von Lengerken, G. (2003). Effect of long-term heat exposure on peripheral concentrations of heat shock protein 70 (Hsp70) and hormones in laying hens with different genotypes. British Poultry Science, 44, 133–138.
Mahmoud, K. Z., Edens, F. W., Eisen, E. J., & Havenstein, G. B. (2003). Effect of ascorbic acid and acute heat exposure on heat shock protein 70 expression by young white leghorn chickens. Comparative Biochemistry and Physiology, Part C Toxicology and Pharmacology, 136, 329–335.
Mahmoud, K. Z., Edens, F. W., Eisen, E. J., & Havenstein, G. B. (2004). The effect of dietary phosphorus on heat shock protein mRNAs during acute heat stress in male broiler chickens (Gallus Gallus). Comparative Biochemistry and Physiology, Part C Toxicology and Pharmacology, 137, 11–18.
Mashaly, M., Hendricks, G. L., Kalama, M. A., Gehad, A. E., Abbas, A. O., & Patterson, P. H. (2004). Effect of heat stress on production parameters and immune response of commercial laying hens. Poultry Science, 83, 889–894.
Mezquita, B., Mezquita, C., & Mezquita, J. (1998). Marked differences between avian and mammalian testicular cells in the heat shock induction and polyadenylation of Hsp70 and ubiquitin transcripts. FEBS Letters, 436, 382–386.
Mezquita, B., Mezquita, J., Durfort, M., & Mezquita, C. (2001). Constitutive and heat-shock induced expression of Hsp70 mRNA during chicken testicular development and regression. Journal of Cellular Biochemistry, 82, 480–490.
Mitchell, B. W., & Siegel, H. S. (1973). Physiological response of chickens to heat stress measured by radio telemetry. Poultry Science, 52, 1111–1119.
Mitchell, M.A., Sandercock, D.A., Macleod, M.G. and Hunter, R.R. (2005). Thermoregulatory and metabolic heat production responses during acute heat stress in genetically improved broiler chickens. Proceedings of the International Poultry Scientific Forum (Southern Poultry Science Society) Atlanta, Georgia, USA, p. 110.
Nätt, D., Rubin, C., Wright, D., Johnsson, M., Beltéky, J., Andersson, L., & Jensen, P. (2012). Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics, 13, 59.
Padhi, A., Ghaly, M. M., & Ma, L. (2016). Testis-enriched heat shock protein A2 (HSPA2): Adaptive advantages of the birds with internal testes over the mammals with testicular descent. Scientific Reports, 6, 18770.
Parsell, D. A., & Lindquist, S. (1994). Heat shock proteins and stress tolerance. Cold Spring Harbor Monograph Archive, 26, 457–494.
Pasti, C., Gallois-Montbrun, S., Munier-Lehmann, H., Veron, M., Gilles, A., & Deville-Bonne, D. (2003). Reaction of human UMP-CMP kinase with natural and analog substrates. European Journal of Biochemistry, 270, 1784–1790.
Quinteiro-Filho, W. M., Ribeiro, A., Ferraz-de-Paula, V., et al. (2010). Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science, 89, 1905–1914.
Rajkumar, U., Reddy, M. R., Rama Rao, S. V., Radhika, K., & Shanmugam, M. (2011). Evaluation of growth, carcass, immune competence, stress parameters in naked neck chicken and their normal siblings under tropical winter and summer temperatures. Asian-Australasian Journal of Animal Sciences, 24, 509–516.
Rajkumar, U., Vinoth, A., Shanmugam, M., Rajaravindra, K. S., & Rama Rao, S. V. (2015). Effect of embryonic thermal exposure on heat shock proteins (Hsps) gene expression and serum T3 concentration in coloured broiler populations. Animal Biotechnology, 26, 260–267.
Rajkumar, U., Vinoth, A., Shanmugam, M., Rajaravindra, K. S., & Rama Rao, S. V. (2017). Effect of increased incubation temperature on Hsp 90 and 60 gene expressions in coloured broiler chickens. Journal of Applied Animal Research, 45, 298–303.
Ritossa, F. M. (1962). A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia, 18, 571–573.
Rozenboim, I., Tako, E., Gal-Garber, O., Proudman, J. A., & Uni, Z. (2007). The effect of heat stress on ovarian function of laying hens. Poultry Science, 86, 1760–1765.
Settar, P., Yalçin, S., Türkmut, L., Ozkan, S., & Cahanar, A. (1999). Season by genotype interaction related to broiler growth rate and heat tolerance. Poultry Science, 78, 1353–1358.
Shanmugam, M., Vinoth, A., Rajaravindra, K. S., & Rajkumar, U. (2015). Thermal manipulation during embryogenesis improves certain semen parameters in layer breeder chicken during hot climatic conditions. Animal Reproduction Science, 161, 112–118.
Shevtsov, M., & Multhoff, G. (2016). Heat shock protein-peptide and HSP-based immunotherapies for the treatment of cancer. Frontiers in Immunology, 7, 171.
Slawinska, A., Hsieh, J. C., Schmidt, C. J., & Lamont, S. J. (2016). Heat stress and lipopolysaccharide stimulation of chicken macrophage-like cell line activates expression of distinct sets of genes. PLoS One, 11, e0164575.
Song, J., Xiao, K., Ke, Y. L., et al. (2014). Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poultry Science, 93, 581–588.
Sun, L., Lamont, S. J., Cooksey, A. M., et al. (2015). Transcriptome response to heat stress in a chicken hepatocellular carcinoma cell line. Cell Stress & Chaperones, 20, 939–950.
Tamzil, M. H., Noor, R. R., Hardjosworo, P. S., Manalu, W., & Sumantri, C. (2013). Acute heat stress responses of three lines of chickens with different heat shock protein (HSP)-70 genotypes. International Journal of Poultry Science, 12, 264–272.
Triantaphyllopoulos, K. A., Ikonomopoulos, I., & Bannister, A. J. (2016). Epigenetics and inheritance of phenotype variation in livestock. Epigenetics & Chromatin, 9, 31.
Tu, W. L., Cheng, C. Y., Wang, S. H., et al. (2016). Profiling of differential gene expression in the hypothalamus of broiler-type Taiwan country chickens in response to acute heat stress. Theriogenology, 85, 483–494.
Tzschentke, B., & Plagemann, A. (2006). Imprinting and critical periods in early development. World’s Poultry Science Journal, 62, 626–637.
Varasteh, S., Braber, S., Akbari, P., Garssen, J., & Fink-Gremmels, J. (2015). Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One, 10, e0138975.
Velichko, A. K., Markova, E. N., Petrova, N. V., Razin, S. V., & Kantidze, O. L. (2013). Mechanisms of heat shock response in mammals. Cellular and Molecular Life Sciences, 70, 4229–4241.
Vinoth, A. (2016). Effect of thermal manipulation on heat shock proteins in chicken-An attempt for epigenetic modulation. Ph.D thesis.Department of Industrial Biotechnology, Bharathidasan University, Tiruchirappalli, India.
Vinoth, A., Thirunalasundari, T., Shanmugam, M., & Rajkumar, U. (2016). Effect of early age thermal conditioning on expression of heat shock proteins in liver tissue and biochemical stress indicators in colored broiler chicken. European Journal of Experimental Biology, 6, 53–63.
Vinoth, A., Thirunalasundari, T., Tharian, J. A., Shanmugam, M., & Rajkumar, U. (2015). Effect of thermal manipulation during embryogenesis on liver heat shock protein expression in chronic heat stressed coloured broiler chickens. Journal of Thermal Biology, 53, 162–171.
Waddington, C. H. (1942). The epigenotype. Endeavour, 1, 18–20.
Wang, S., & Edens, F. W. (1998). Heat conditioning induces heat shock proteins in broiler chickens and turkey poults. Poultry Science, 77, 1636–1645.
Wang, S. H., Cheng, C. Y., Chen, C. J., et al. (2014). Changes in protein expression in testes of L2 strain Taiwan country chickens in response to acute heat stress. Theriogenology, 82, 80–94.
Wang, S. H., Cheng, C. Y., Tang, P. C., et al. (2013). Differential gene expressions in testes of L2 strain Taiwan country chicken in response to acute heat stress. Theriogenology, 79, 374–382.
Wang, S. H., Cheng, C. Y., Tang, P. C., et al. (2015). Acute heat stress induces differential gene expressions in the testes of a broiler-type strain of Taiwan country chickens. PLoS One, 10, e0125816.
Wolfenson, D., Frei, Y. F., Snapir, N., & Berman, A. (1981). Heat stress effects on capillary blood flow and its redistribution in the laying hen. Pflügers Archiv, 390, 86–93.
Xie, J., Tang, L., Lu, L., et al. (2014). Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus Gallus). PLoS One, 9, e102204.
Xie, J., Tang, L., Lu, L., et al. (2015). Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poultry Science, 94, 1635–1644.
Yahav, S. (2009). Alleviating heat stress in domestic fowl – different strategies. World’s Poultry Science Journal, 65, 719–732.
Yahav, S. (2015). Regulation of body temperature - strategies and mechanisms. In Sturkie's avian physiology. Edited by Scanes, C. Elsevier Publications, Chapter 37, pp. 869–905.
Yahav, S., Collin, A., Shinder, D., & Picard, M. (2004). Thermal manipulations during broiler chick's embryogenesis - the effect of timing and temperature. Poultry Science, 83, 1959–1963.
Yahav, S., Goldfeld, S., Plavnik, I., & Hurwitz, S. (1995). Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. Journal of Thermal Biologico, 20, 245–253.
Yahav, S., Shamay, A., Horev, G., Bar-Ilan, D., Genina, O., & Friedman-Einat, M. (1997). Effect of acquisition of improved thermotolerance on the induction of heat shock proteins in broiler chickens. Poultry Science, 76, 1428–1434.
Yan, J., Bao, E., & Yu, J. (2009). Heat shock protein 60 expression in heart, liver and kidney of broilers exposed to high temperature. Research in Veterinary Science, 86, 533–538.
Yan, Q.C. (2001). Effect of temperature on semen characteristics and sperm heat shock protein 70 in males of Taiwan country chicken. Master thesis. Department of animal science, Taichung: National Chung Hsing University
Yu, J., & Bao, E. (2008). Effect of acute heat stress on heat shock protein 70 and its corresponding mrna expression in the heart, liver, and kidney of broilers. Asian-Australas Journal of Animal Science, 21, 1116–1126.
Yu, J., & Bao, E. (2009). Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. British Poultry Science, 50, 504–511.
Yu, J., Bao, E., Yan, J., & Lei, L. (2008). Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress & Chaperones, 13, 327–335.
Zhang, W. W., Kong, L. N., Zhang, X. Q., & Luo, Q. B. (2014). Alteration of HSF3 and HSP70 mRNA expression in the tissues of two chicken breeds during acute heat stress. Genetics and Molecular Research, 13, 9787–9794.
Zhen, F. S., Du, H. L., Xu, H. P., Luo, Q. B., & Zhang, X. Q. (2006). Tissue and allelic-specific expression of hsp70 gene in chickens: Basal and heat-stress-induced mRNA level quantified with real-time reverse transcriptase polymerase chain reaction. British Poultry Science, 47, 449–455.
Zuo, J., Xu, M., Abdullahi, Y. A., Ma, L., Zhang, Z., & Feng, D. (2015). Constant heat stress reduces skeletal muscle protein deposition in broilers. Journal of the Science of Food and Agriculture, 95, 429–436.
Acknowledgements
This work was supported by Indian Council of Agricultural Research under National Initiative on Climate Resilient Agriculture (NICRA) project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Murugesan, S., Ullengala, R., Amirthalingam, V. (2017). Heat Shock Protein and Thermal Stress in Chicken. In: Asea, A., Kaur, P. (eds) Heat Shock Proteins in Veterinary Medicine and Sciences. Heat Shock Proteins, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-319-73377-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-73377-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73376-0
Online ISBN: 978-3-319-73377-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)