Abstract
Over 625 fungal species have been reported to cause infection in vertebrates. The fungal kingdom contains 167 orders, of which 40 (24%) were repeatedly cited in the medical literature. Recurrence indicates that these species have a certain predisposition to cause infection. In the present chapter, the different categories of pathogens and outbreak fungi are presented and discussed. Most emerging fungi concern infections that are non-transmissible; their frequency may show moderate increase due to changes of host conditions. Outbreaks may concern multiple infections from a common environmental source, known as sapronoses. When their life cycle has an invasive phase with adaptations to reside inside host tissue, the fungi are referred to as environmental pathogens. Host-to-host transmission occurs in zoophilic pathogens, which have no environmental phase. This kind of fungi is responsible for mycoses which may occur with changes in host factors and when naive host populations are exposed to novel fungal genotypes. The most dramatic transmissible mycoses are expected with a combination of host and fungal changes. Successful outbreak fungi are recognizable by low genetic diversity.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Opportunistic and Pathogenic Fungi
The animal body can be regarded as an extreme environment for fungal growth ; only a small fraction of the species known in the fungal kingdom is able to cause infection. When a fungus occupies an environmental niche but is able to survive in animal host tissue—because some of the factors needed in its environmental niche coincidentally enhance survival in the host—the infection is referred to as opportunistic . However, the infection is non-transmissible and the etiologic agent will finally die with the animal host at the end of its life span (Fig. 1.1). Infection is thus not profitable for the fungus: the infectious individuals will be lost for the fungal population and thus have a detrimental effect on the fitness of the species. For example, fungi belonging to Mucorales (Rhizopus, Lichtheimia, Saksenaea) are responsible for diseases with a severe morbidity, but do not propagate and are even difficult to culture from the host; their preferred habitat remains dead material such as foodstuffs , on which their sporulation is massive. Another common example is Aspergillus fumigatus , which is thermotolerant because of the conditions in its natural habitat of self-heated compost, and this factor also enables survival in immunocompromised patients or in birds whose internal body temperature (up to 43 °C) is much higher than in mammals. In general, decreased host immunity allows extended fungal growth and increases severity of the infection.
Diagram of possible life cycles . (a) Non-transmissible opportunist; (b) transmissible environmental pathogen, with infectious propagule distribution via the environment; (c) transmissible zoopathogen via contagious hosts; (d) sapronosis by opportunist, non-transmissible; (e) sapronosis by environmental pathogen, with infectious propagule distribution via the environment; (f) zoonosis by transmissible zoophilic pathogen via contagious hosts
A small fraction of the extant fungal species is adapted to the animal host, i.e., have an increased fitness when a living mammal host is used in their life cycle. The infection is transmissible , so that mutations acquired during infection can be passed on to next generations. In a zoophilic pathogen , this transmission takes place from host-to-host; the infected animal is contagious. For most transmissible fungi, the animal host may be used as a vehicle for fungal dispersal, but the main reservoir of the fungus remains environmental. Such fungi have a double life cycle: in the environment versus in the animal; the host is infected via propagules that were produced in the environment, and thus infected individuals are not contagious. We call such fungi environmental pathogens .
Of the estimated 1.5 million extant fungal species, only about 625 have been reported to cause infection in vertebrates. The fungal kingdom presently contains 167 orders, of which 40 (24%) are listed in the Atlas of Clinical Fungi (de Hoog et al. 2017) containing members that were repeatedly cited in the medical literature. Recurrence indicates that these species have a certain predisposition to cause infection. If we count the number of fungal genera within the 40 orders, the distribution of fungi that are predisposed to infection is even more skewed: of the approximately 18,000 described genera (Crous et al. 2014), only 206 (0.01%) are listed in the Atlas. Considering that only 45 genera, dispersed over 10 orders, belong to the relatively common infectious agents, it is obvious that vertebrate pathogenicity is an extremely rare ability of fungal species. These figures (i.e., the number of species involved) have not really changed with the event of hospitalized patient populations with severe immune disorders; just the frequency of infectious species has increased and some have become quite common. Thus, the popular statement of the “immunocompromised host as a living Petri dish,” suggesting that such patients can be infected by “any” fungus, is far from correct.
Nevertheless, the number of known infectious agents is growing. This is largely due to developments in awareness and diagnostics and particularly in molecular taxonomic approaches which enable species distinction with much larger resolution. Also next-generation sequencing improves our knowledge of commensals on the skin and the intestinal microbiome, with concomitant increase of the number of known human- or animal -associated fungi.
2 Epidemic and Epizootic Expansions
A small number of opportunistic and pathogenic species or species groups tend to occur in the form of outbreaks, epidemics (in humans), or epizootics (in animals) (Fisher et al. 2012). Fungal epidemics have already been reported in the early twentieth century. Beurmann and Gougerot (1912) described an expansion of sporotrichosis in France, caused by Sporothrix schenckii. Zhang et al. (2015) demonstrated that each closely related species of Sporothrix has a consistent pattern of outbreaks. Common-source outbreaks are usually reported, which die out when the environmental conditions no longer support growth of the fungus. An epidemic of sporotrichosis by S. schenckii among miners in South Africa involved more than 3000 cases and subsequently disappeared when the wood that was used in the mines was treated with preservatives (Govender et al. 2015). When nonliving biological material is the source of infection, the epidemic is referred to as a sapronosis . Infected vertebrates do not spread the fungus, and thus sapronoses are caused by non-transmissible opportunistic fungi (Fig. 1.1). Sapronotic outbreaks are linked to a common source of infection , and prevention thus requires physical removal of the infective material.
The epidemiological pattern is fundamentally different when fungal pathogens are transmissible between animals including humans. The agent of chytridiomycosis causing global frog decline, Batrachochytrium dendrobatidis, efficiently utilizes the host for its dispersal (Chap. 14). The fungus produces zoosporangia in frog skin, which release massive amounts of zoospores into the environment and contaminate new susceptible hosts. The time of transmission is short, and the environmental phase does not require growth of the thallus but just zoospore dispersal. Transmission can thus be nearly direct, from host-to-host, maximally with a short intermittent phase of motile spores in water. Contagious individuals potentially infect multiple individuals, and hence direct transmission often leads to exponentially expanding epizootics (Fig. 1.1). The fungus completes its life cycle on the host and is thus considered as a pathogen. This kind of mycosis is transmitted directly between living hosts. According to the official definition from the World Health Organization, zoonoses are diseases and infections that are naturally transmitted between vertebrate animals and humans and vice versa. Among transmissible fungal pathogens, a few species should be considered as zoonotic: Sporothrix brasiliensis (from cats) (Chap. 10) and some species of dermatophytes, e.g., Microsporum canis (from cats), Trichophyton verrucosum (from cattle), and T. benhamiae (from guinea pigs) (Chap. 3).
Environmental pathogens have a double life cycle, combining characteristics of both groups above. Part of their life cycle is completed in the environment, while they also have a reservoir in the vertebrate host. Infection takes place by propagules from the environment, and expansions are thus classified as sapronoses. After infection and completion of a pathogenic life cycle, fungal cells should be able to escape from the dead animal body to return to the environmental habitat—although this has rarely been proven. When sapronoses are caused by environmental pathogens, where the environmental habitat is part of the fungus’ natural life cycle, the source will be more difficult to eradicate.
3 Expansion Due to Changing Host Factors
In the literature, many fungi are attributed as “emerging,” which refers to a prevalence of the infection increasing significantly above the baseline. In most cases, this is triggered by opportunity. Several immunologically naive patient populations have emerged. Host changes in human populations concern, for example, novel medical technologies allowing patients with low immunity, socioeconomic changes, and emerging immune and metabolic diseases. Transplant recipients, patients with chronic diabetes, and those with various long-term chemotherapy are such novelties. These are potentially infected by a gamut of infectious opportunists , such as members of Mucorales, non-albicans Candida, and various Aspergillus and Fusarium species. Most of these fungi—with the exception of Fusarium which simply seems to have been neglected (Al-Hatmi et al. 2016)—were already known as agents of disease since the nineteenth century, but their incidence has increased due to the expanding populations of susceptible hosts (Fig. 1.2a). Environmental pathogens respond to host changes in a similar way. As endemic or enzootic fungi with a narrowly defined environmental reservoir, their global frequency is low, and their increase, e.g., taking advantage of the AIDS pandemic in humans or FIV/FeLV infection in cats, remains moderate (Fig. 1.2b).
Predicted emergence of species . (a) Opportunist with rather high frequency, increase relative to growth of susceptible populations; (b) environmental pathogen, relatively frequent in endemic area, in equilibrium with its habitat, increase relative to growth of susceptible populations; (c) pathogen developing novelty by which it increases the number of susceptible hosts; (d) zoophilic pathogen developing novelty, confronted with naive host populations which leads to epidemic expansion, expected decrease when susceptible hosts are no longer available
4 Expansion Due to Fungal Novelties
In addition to changes in opportunity, also fungal populations change perpetually in their genetic makeup. For instance, virulent genotypes may be novel, or at least the hosts are confronted with fungal genotypes to which they lacked resistance (Fig. 1.2c). Low genetic diversity was at the basis of the Vancouver outbreak of Cryptococcus gattii (Kidd et al. 2004), and the Oregon outbreak concerned a single highly virulent genotype (Byrnes et al. 2010) (Chap. 12). Emerging fungal infections in humans and other vertebrates often take us by surprise. Bat white-nose disease suddenly killed thousands of bats in the USA and was caused by a species that was known in Europe and to which European bats were resistant (Chap. 13). The fungus appeared extremely successful in susceptible American bat populations, likely owing to physiological and behavioral differences between new- and old-world bats. The causative agent , Pseudogymnoascus destructans, was described only in 2009 (Gargas et al. 2009) and the fungus seemed to appear out of the blue. Several of the species that cause large epidemics or epizootics have only recently been described; first cases were often recorded only a few decades ago. One of the examples is Aspergillus felis causing infections in cats in Australia (Barrs et al. 2007) (Chap. 15). One might suppose that this is a fallacy due to insufficient awareness and inadequate diagnostics in the past, but in large collections of, for example, archived Sporothrix material, the recent outbreak fungus Sporothrix brasiliensis was not detected (Rodrigues et al. 2014). The frog disease agent Batrachochytrium dendrobatidis was described only in 1999 (Longcore et al. 1999) with its newly introduced counterpart B. salamandrivorans killing salamanders in 2013 (Martel et al. 2013) (Chap. 14). These pathogens have likely been present in low abundance and were undetected until the encounter of a virulent genotype and susceptible host found a match, which was thought to have occurred due to trade-facilitated intercontinental movements. Both species have low genetic diversity suggesting clonal expansion (Morehouse et al. 2003; Fisher et al. 2012). Over a larger sampling area, there is genetic diversity, but local frog mortality is caused by single clones (Morgan et al. 2007). Fastest population expansion is predicted to occur when virulent genotypes of pathogens match with a novel window of opportunity (Fig. 1.2). An extra window of opportunity for frog disease has been suggested to be climate change, weakening frog populations (Clare et al. 2016). The combined changes in fungus and host allow asymptotic expansion of the pathogen, finally leading to decline because susceptible hosts have become rare (Fig. 1.2d). This may account for the wavelike pattern of lethargic crab disease along the Brazilian coast (Ávila et al. 2012) (Chap. 11).
The emergence of sporotrichosis is still largely unexplained. Cat-transmitted Sporothrix brasiliensis shows epizootic and epidemic expansion in Southeast Brazil since 1990 (Ortiz Sanchotene et al. 2015) (Chap. 10). The species is one of two near-clonal entities that have evolved from Sporothrix schenckii (Rodrigues et al. 2014; Moussa et al. 2017). In the ancestral species, both cat and plant transmission are documented. The clones have specialized in either one of these modes of transmission: both are traumatically inoculated into the skin, but S. brasiliensis enters via cat scratches, whereas S. globosa infection stems from trauma caused by sharp plant debris or thorns. Sporothrix globosa infection is seasonal (Yu et al. 2013), whereas the more virulent S. brasiliensis replicates asymptotically to become overabundant, outcompeting the ancestral species, S. schenckii (de Araujo et al. 2015). The remarkable difference in transmission mode, with large consequences for evolutionary success and public health is observed at a very small phylogenetic distance (Moussa et al. 2017).
5 Recent Outbreaks and Epizootics
Candida albicans , commonly causing mucocutaneous infections in humans, is transmitted directly, from patient to patient, e.g., from mother to child. The host is niche as well as the reservoir of the fungus. The species has an advantage of infecting the vertebrate host and can thus be interpreted as a zoophilic pathogen (Fig. 1.1). Carriage by the healthy host is mostly non-symptomatic, the fungus then maintaining as a commensal (Achkar and Fries 2010) and every new host may be contagious. With only slight impairment of innate or acquired immune systems of the host, C. albicans infections become symptomatic. With host-to-host transmission, there is no common source of contamination, as opposed to sapronoses. In the susceptible population of hospitalized patients, other Saccharomycetales are emerging, such as the fluconazole-resistant Candida auris (Chowdhary et al. 2016). The species has now been reported globally from bloodstream infections in severely compromised patients. It often occurs in the form of local outbreaks (Schelenz et al. 2016). Its mode of infection is as yet unknown, but host-to-host transmission seems an option.
Emergomyces africanus is a novel human pathogen involved in an outbreak of disseminated disease in HIV-positive individuals in South Africa (Kenyon et al. 2014; Dukik et al. 2017). Its origin is unknown; probably the fungus is an environmental pathogen and might occur in animal populations. The genus Emergomyces contains a number of species which since recently were repeatedly involved in human infection, while related species reside fairly commonly in the lungs of small rodents (e.g., Emmonsia crescens) (Borman et al. 2009, Hubálek et al. 1998) (Chap. 7), infection of humans being coincidental (Dot et al. 2009). Screening of wild animals is required to understand, and to possibly predict, host jumps to humans. Humans are likely non-optimal hosts in most species, and relations between animal hosts may be quite complicated (Vilela et al. 2016). Jiang et al. (2018) noted that small temperature differences of dimorphic switch in Emergomyces and Blastomyces species may explain predilections for different host animals and thus may be a driver of new directions in the evolution of these fungi. Ecological fitting (Araujo et al. 2015) via the sloppy fitness space of the not-yet-suitable host might be the optimal model to describe the sympatric evolutionary processes in these fungi.
Most Emergomyces species and other members of Ajellomycetaceae such as Paracoccidioides (Chap. 6) are environmental pathogens with a double life cycle (Fig. 1.3), i.e., living permanently in the environment but having a certain advantage (increased fitness) if an animal host is used in any stage of the life cycle. The host may be regarded as a vehicle, which enhances dispersal, optimizing the distribution of the fungus in the environmental niche. For example, Histoplasma capsulatum is found on sheltered animal droppings, e.g., bat feces in caves or bird roosting sites (Rocha-Silva et al. 2014). Often the resident animals are asymptomatically colonized (Naiff et al. 1996), return to the cave, and ultimately die there (Chap. 5). Thus, there is no efficient vehicle of dispersal, but susceptible visitors of the cave after contamination and infection are likely to die elsewhere, enabling further dispersal of the pathogen. The fungus thus has a strategy to reach optimal fitness. A common-source outbreak is concerned with the number of infections corresponding with the number of susceptible individuals entering the contaminated site; thus usually no epidemic or epizootic expansion is noted. Hosts are not contagious, but are infected by environmental propagules; the infections are sapronoses.
In the main common-source, sapronotic outbreaks of environmental pathogens such as Histoplasma, the role of the susceptible host in fungal expansion is rather insignificant. The size of the inoculum is proportional to the presence of the fungus in its environmental niche. Outbreaks of Coccidioides immitis are related to weather conditions and subsequent dust storms that lead to massive inhalation of propagules (Valdivia et al. 2006) (Chap. 4). Over time, the fungal prevalence remains more or less the same, though with fluctuations. Coccidioides is hypothesized to form hyphae from cells located in host tissue after its death, to colonize a new environmental site (Lewis et al. 2015). The infection is controlled primarily by acquired cellular immunity and therefore tends to expand with an increasing number of AIDS patients (Rempe et al. 2007).
Talaromyces marneffei is an unrelated environmental pathogen. The species occurs in soil and has a reservoir in large rodents in Southeast Asia but found a susceptible new host due to the human AIDS pandemic. Dormancy inside living pulmonary tissue of the animal requires resistance to innate immunity and control by acquired cellular immunity (Cooper and Vanittanakom 2008), and hence impairment of T-cell function provides an obvious portal for dissemination. Also, this species is overwhelmingly clonal (Fisher et al. 2005) despite local sexuality. Populations are geographically substructured with likely adaptation to different host species (Henk et al. 2012). The environmental habitat of the agent of bat white-nose syndrome (WNS), Pseudogymnoascus destructans causing bat decline in northeastern USA (Blehert et al. 2009), is still unknown (Chap. 13) (Rajkumar et al. 2011). The virulent clone causing the bat near extinction presumably already pre-existed in Europe, where bats had found a balance toward infection without causing significant problems. However, when reaching susceptible bat populations in the USA, the fungus was able to expand exponentially (Leopardi et al. 2015).
Recent outbreaks have also been caused by dermatophytes, which are keratinophilic fungi with a gradual adaptation to living tissues (Chap. 3). Anthropophilic dermatophytes are highly specialized species with niche and reservoir on human skin. Transmission takes place from human-to-human, or via propagules which can survive in the environment but do not form assimilative thalli, comparable to Batrachochytrium. Zoophilic dermatophytes are adapted to animal skin, with different level of host-specificity. Trichophyton equinum is isolated from horses only, whereas Microsporum canis can be detected in a wide range of animals (and sometimes in humans). Zoophilic dermatophytes are mostly carried in animal fur, which is often in close contact with soil and plant material of the host’s burrow. Furred animal thus may be infected directly from their nest-mates but also from their environment. Carriage in the fur is often asymptomatic, infection only taking place in susceptible nest-mates, e.g., juveniles, or when a non-suitable host is coincidentally infected, such as a predator or a human. Geophilic dermatophytes have a double life cycle with elaborate sexual phases that are produced in soil. Sexuality in anthropophilic dermatophytes is not known to exist or concerns somatic cell fusion at most. A gradual loss of sexuality in dermatophytes with advancing adaptation to mammalian hosts has been observed (de Hoog et al. 2016). Clonal reproduction is often prevalent (Gräser et al. 2006), which is underlined by the fact that potential mating partners having either high mobility group (HMG) or α-box transcription factors can be phenotypically different (Symoens et al. 2013).
6 Location of Outbreak Fungi in the Fungal Kingdom
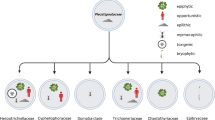
Opportunism and pathogenicity are polyphyletic in the fungal kingdom (Fig. 1.4). Pathogenicity was defined in an ecological sense above as having advantage of the use of a vertebrate host. This is the case in species of Batrachochytrium (order Chytridiales, Chytridiomycota), Cryptococcus (Tremellales, Basidiomycota), dermatophytes and some systemic dimorphic fungi (Onygenales, Ascomycota), Talaromyces and Pseudogymnoascus (Eurotiales, Ascomycota), and perhaps Sporothrix (Ophiostomatales, Ascomycota). If we compare this with criteria of transmission (host-to-host versus host-environment-host), we observe that Batrachochytrium, Candida, Pneumocystis, and anthropophilic (and some zoophilic) dermatophytes lack assimilative thalli in the environment and thus hosts are principally contagious, while Cryptococcus, geophilic dermatophytes, and systemic dimorphic fungi are environmental pathogens. Judging from PCR data, Talaromyces seems to occur in the environment (Pryce-Miller et al. 2008), suggesting that T. marneffei is also an environmental pathogen that is amplified by infection in bamboo rat hosts. Sporothrix species are somewhat outside these categories: S. brasiliensis is transmitted by cats and the fungus is able to produce a large amount of infective material in feline tissues (which is rarely the case in other mammalian hosts).
Currently, the largest outbreaks, i.e., with the largest degree of acceleration, are Batrachochytrium, Cryptococcus, Pseudogymnoascus, and Sporothrix. Given the expansion of Cryptococcus in Southern Africa, it might be expected that Emergomyces africanus, which also occurs in HIV-positive individuals , might expand in the near future. In contrast, species like Candida albicans or Pneumocystis spp. are intimately associated with specific hosts, responding to transient host susceptibilities, but on average remain with comparable frequencies in animal or human populations. Talaromyces marneffei initially stabilized due to the control of the HIV pandemic as a result of HAART therapy but now emerges in patients with other comorbidities (Chan et al. 2016) and in otherwise healthy individuals (Ye et al. 2015). The emergence of anthropophilic dermatophytes is strongly associated with human socioeconomic changes. In preindustrial societies in developmental transition, a shift is observed from anthropophilic to zoophilic dermatophytes, resulting from increasing hygiene to the growing habit in urban settings to live closely with companion animals. This process was observed in Europe half a century ago (Mantovani and Morganti 1977) and currently takes place in China (Zhan et al. 2015). To a certain extent, the dermatophyte species reflect the popularity of pets, including dogs, cats, and more frequently rabbits and small rodents (Nenoff et al. 2014).
References
Achkar JM, Fries BC (2010) Candida infections of the genitourinary tract. Clin Microbiol Rev 23:253–273
Al-Hatmi AMS, Meis JF, de Hoog GS (2016) Fusarium: molecular diversity and intrinsic drug resistance. PLoS Pathog 12(4):e1005464. https://doi.org/10.1371/journal.ppat.1005464
de Araujo ML, Rodrigues AM, Fernandes GF, de Camargo ZP, de Hoog GS (2015) Human sporotrichosis beyond the epidemic front reveals classical transmission types in Espírito Santo, Brazil. Mycoses 58(8):485–590
Araujo SBL, Braga MP, Brooks DR, Agosta SJ, Hoberg EP, von Hartenthal FW, Boeger WA (2015) Understanding host-switching by ecological fitting. PLoS One 10:e0139225
Ávila RA, Mancera PFA, Estevac L, Pied MR, Ferreira CP (2012) Traveling waves in the lethargic crab disease. Appl Math Comput 218:9898–9910
Barrs VR, Beatty JA, Lingard AE, Malik R, Krockenberger MB, Martin P, O’Brien C, Angles JM, Dowden M, Halliday C (2007) Feline sino-orbital aspergillosis: an emerging clinical syndrome? Aust Vet J 85:N23
Beurmann L, Gougerot H (1912) Les Sporotrichose. Librairie Felix Alcan, Paris
Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Coleman JTH, Darling SR, Gargas A, Niver R, Okoniewski JC, Rudd RJ, Stone WB (2009) Bat white-nose syndrome: an emerging fungal pathogen? Science 323(5911):227
Borman AM, Simpson VR, Palmer MD, Linton CJ, Johnson EM (2009) Adiaspiromycosis due to Emmonsia crescens is widespread in native British mammals. Mycopathologia 68:153–163
Byrnes EJ, Li WJ, Lewit Y, Ma HS, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J (2010) Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the Northwest United States. PLoS Pathog 6(4):e1000850. https://doi.org/10.1371/journal.ppat.1000850
Chan JFW, Lau SKP, Yuen KY, Woo PCY (2016) Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 5(3):e19
Clare F, Halder JB, Daniel O, Bielby J et al (2016) Climate forcing of an emerging pathogenic fungus across a montane multihost community. Philos Trans R Soc Lond B Biol Sci 371(1709):20150454. https://doi.org/10.1098/rstb.2015.0454
Cooper CR, Vanittanakom N (2008) Insights into the pathogenicity of Penicillium marneffei. Future Microbiol 3:43–55
Chowdhary A, Voss A, Meis JF (2016) Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J Hosp Infect 94:209–212
Crous PW, Giraldo A, Hawksworth DL, Robert V, Kirk PM, Guarro J, Robbertse B et al (2014) The genera of fungi: fixing the application of type species of generic names. IMA Fungus 5(1):141–160
De Hoog GS, Guarro J, Gené J, Figueras MJ (2017) Atlas of clinical fungi. E-edition 4.1.4. Westerdijk Fungal Biodiversity Institute/Universitat Rovira I Virgili, Utrecht/Reus
De Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, Kubsch C, Stielow JB, Göker M, Rezaei-Matehkolaei A, Mirhendi H, Gräser Y (2016) Towards a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 82:5–31
Dot J-M, Debourgogne A, Champigneulle J, Salles Y, Brizion M, Puyhardy JM, Collomb J, Plénat F, Machouart M (2009) Molecular diagnosis of disseminated adiaspiromycosis due to Emmonsia crescens. J Clin Microbiol 47:1269–1273
Dukik K, Muñoz JF, Jiang Y, Feng P et al (2017) Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 60(5):296–309. https://doi.org/10.1111/myc.12601
Fisher MC, Hanage WP, de Hoog GS, Johnson E et al (2005) Low effective dispersal of asexual genotypes in heterogeneous landscapes by the endemic pathogen Penicillium marneffei. PLoS Pathog 1(2):e20
Fisher MC, Henk DA, Briggs CJ, Brownstein JS et al (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484(7393):186–194
Gargas A, Trest MT, Christense M, Volk TJ, Blehert DS (2009) Geomyces destructans sp.nov., associated with bat white-nose syndrome. Mycotaxon 108:147–154
Govender NP, Maphanga TG, Zulu TG, Patel J, Walaza S, Jacobs C, Ebonwu JI, Ntuli S, Naicker SD, Thomas J (2015) An outbreak of lymphocutaneous sporotrichosis among mine-workers in South Africa. PLoS Negl Trop Dis 9(9):e0004096. https://doi.org/10.1371/journal.pntd.0004096
Gräser Y, de Hoog GS, Summerbell RC (2006) Dermatophytes: recognizing species of clonal fungi. Med Mycol 44:199–209
Henk DA, Shahar-Golan R, Ranjana DK et al (2012) Clonality despite sex, the evolution of sexual neighborhoods in the pathogenic fungus Penicillium marneffei. PLoS Pathog 8(10):e1002851. https://doi.org/10.1371/journal.ppat.1002851
Hubálek Z, Nesvadbova J, Halouzka J (1998) Emmonsiosis of rodents in an agroecosystem. Med Mycol 36:387–390
Jiang Y, Dukik K, Muñoz J, Sigler L, Schwartz IS et al (2018) Phylogeny, ecology and taxonomy of systemic pathogens in Ajellomycetaceae (Onygenales). Fungal Divers (in press)
Kenyon C, Corcoran C, Govender NP (2014) An Emmonsia species causing disseminated infection in South Africa. New Engl J Med 370:283–284
Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, MacDougall L, Boekhout T, Kwon-Chung KF, Meyer W (2004) A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A 101:17258–17263
Leopardi S, Blake D, Puechmaille SJ (2015) White-nose syndrome fungus introduced from Europe to North America. Curr Biol 25(6):R217–R219. https://doi.org/10.1016/j.cub.2015.01.047
Lewis ERG, Bowers JR, Barker BM (2015) Dust devil: the life and times of the fungus that causes valley fever. PLoS Pathog 11(5):e1004762
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227
Mantovani A, Morganti L (1977) Dermatophytozoonoses in Italy. Vet Res Comm 1:171–177
Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F (2013) Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci U S A 110:15325–15329
Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE (2003) Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12:395–403
Morgan JAT, Vredenburg VT, Rachowicz LJ, Knapp RA et al (2007) Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A 104(34):13845–13850
Moussa TAA, Kadasa NMS, Al Zahrani HS, Ahmed SA, Feng P, Gerrits van den Ende AHG, Zhang Y, Kano R, Li F, Li S, Yang S, Bilin D, Rossato L, Dolatabadi S, de Hoog GS (2017) Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J Med Microbiol 66(5):560–569
Naiff RD, Barrett TV, Naiff MDF, de Lima Ferreira LC, Arias JR (1996) New records of Histoplasma capsulatum from wild animals in the Brazilian Amazon. Rev Inst Med Trop Sao Paulo 38:273–277
Nenoff P, Uhrlaß S, Krüger C, Erhard M, Hipler U-C, Seyfarth F et al (2014) Trichophyton species of Arthroderma benhamiae – a new infectious agent in dermatology. J Dtsch Dermatol Ges 12(7):571–581
Ortiz Sanchotene K, Martins Madrid I, Baracy Klafke G, Bergamashi M, Della Terra PP, Rodrigues AM, de Camargo ZP, Orzechowski Xavier M (2015) Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses 58:652–658
Pryce-Miller E, Aanensen D, Vanittanakom N, Fisher MC (2008) Environmental detection of Penicillium marneffei and growth in soil microcosms in competition with Talaromyces stipitatus. Fungal Ecol 1:49–56
Rajkumar SS, Li X, Rudd RJ et al (2011) Clonal genotype of Geomyces destructans among bats with white nose syndrome, New York, USA. Emerg Infect Dis 17:1273–1276
Rempe S, Sachdev M, Bhakta R, Pineda-Roman M, Vaz A, Carlson R (2007) Coccidioides immitis fungemia: clinical features and survival in 33 adult patients. Heart Lung 36:64–71
Rocha-Silva F, Figueiredo SM, Silveira TT, Assunção CB, Campolina SS, Pena-Barbosa JP, Rotondo A, Caligiorne RB (2014) Histoplasmosis outbreak in Tamboril cave-Minas Gerais state, Brazil. Med Mycol Case Rep 4:1–4
Rodrigues AM, de Hoog GS, Zhang Y, de Camargo ZP (2014) Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg Microbes Infect 3:e32
Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC (2016) First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. eCollection 2016
Symoens F, Jousson O, Packeu A, Fratti M, Staib P, Mignon B, Monod M (2013) The dermatophyte species Arthroderma benhamiae: intraspecies variability and mating behaviour. J Med Microbiol 62:377–385
Valdivia L, Nix D, Wright M, Lindberg E, Fagan T, Lieberman D, Stoffer TP, Ampel NM, Galgiani JN (2006) Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis 12:958–962
Vilela R, Bossart GD, St. Leger JA, Dalton LM et al (2016) Cutaneous granulomas in dolphins caused by novel uncultivated Paracoccidioides brasiliensis. Emerg Infect Dis 22:2063–2069
Yu X, Wan Z, Zhang Z, Li F, Li R, Liu X (2013) Phenotypic and molecular identification of Sporothrix isolates of clinical origin in Northeast China. Mycopathologia 176:67–74
Ye F, Luo Q, Zhou Y, Xie J, Zeng Q, Chen G, Su D, Chen R (2015) Disseminated penicilliosis marneffei in immunocompetent patients: a report of two cases. Indian J Med Microbiol 33(1):161–165. https://doi.org/10.4103/0255-0857.148433
Zhan P, Geng C, Li Z, Jin Y, Jiang Q, Tao L, Luo Y, Xiong L, Wu S, Li D, Liu W, de Hoog GS (2015) Evolution of tinea capitis in the Nanchang area, Southern China: a 50-year survey (1965–2014). Mycoses 58:261–266
Zhang Y, Hagen F, Stielow B, Rodrigues AM, Samerpitak K, Zhou X, Feng P, Yang L, Chen M, Deng S, Li S, Liao W, Li R, Li F, Meis JF, Guarro J, Teixeira M, Al-Zahrani HS, Pires de Camargo Z, Zhang L, de Hoog GS (2015) Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia 35:1–20
Acknowledgments
The authors are indebted to Matthew Fisher for constructive discussions and comments on the text.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
de Hoog, G.S., A. Ahmed, S., Danesi, P., Guillot, J., Gräser, Y. (2018). Distribution of Pathogens and Outbreak Fungi in the Fungal Kingdom. In: Seyedmousavi, S., de Hoog, G., Guillot, J., Verweij, P. (eds) Emerging and Epizootic Fungal Infections in Animals. Springer, Cham. https://doi.org/10.1007/978-3-319-72093-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-72093-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72091-3
Online ISBN: 978-3-319-72093-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)








 = environmental pathogen.
= environmental pathogen.  = zoophilic pathogen
= zoophilic pathogen