Abstract
Nanoparticles have specific physicochemical properties different to bulk materials of the same composition and such properties make them very attractive for commercial and medical applications. Mucoadhesive nanoparticulate dosage forms are designed to enable prolonged retention of these nanoparticles at the site of application, providing a controlled drug release for improved therapeutic outcome. Moreover, drug delivery across the mucosa bypasses the first-pass hepatic metabolism and avoids the degradation by gastrointestinal enzymes. However, like most new technologies, there is a rising debate concerning the possible transmucosal side effects resulting from the use of particles at the nano level. In fact, these nanoparticles on entering the body, deposit in several organs and may cause adverse biological reactions by modifying the physiochemical properties of living matter. Several investigators have found nanoparticles responsible for toxicity in different organs. In addition, the toxicity of nanoparticles also depends on whether they are persistent or cleared from the different organs of entry and whether the host can raise an effective response to sequester or dispose of the particles. In contrast to many efforts aimed at exploiting desirable properties of nanoparticles for medicine, there are limited attempts to evaluate potentially undesirable effects of these particles when administered intentionally for medical purposes. This chapter focuses on the overview of the mucosal systems, fate of nanoparticles, mechanism of nanoparticle’s toxicity and the various toxicity issues associated with nanoparticles through mucosal routes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
3.1.1 Overview of Mucosal System
A mucous membrane is an epithelial tissue layer lining various cavity of the body and surrounds internal organs. It is of ectodermal origin and is incessant with the skin at various body openings such as the eyes, ears, inside the mouth, inside the nose, the urogenital tract, digestive tract, and respiratory tract. Mucous membranes are moist due to the presence of glands which secrete a thick fluid known as mucous. It serves many functions like lubrication for the passage of objects, maintenance of a hydrated layer over the epithelium, a barrier to pathogens and noxious substances and as a permeable gel layer for the exchange of gases and nutrients with the underlying epithelium. Mucous is primarily composed of water (95%), but also contains salts, lipids such as fatty acids, phospholipids and cholesterol, proteins which help a defensive purpose with lysozyme, immunoglobulins, defensins and growth factors. However, the glycoprotein mucin is the main component that is responsible for its viscous and elastic gel-like properties. Mucins are large, extracellular glycoproteins with molecular weights ranging from 0.5 to 20 kDa. Both membrane bound mucins, and secreted mucins share many common features. They both are highly glycosylated consisting of 80% carbohydrates primarily N-acetylgalactosamine, N-acetylglucosamine, fucose, galactose, and sialic acid (N-acetylneuraminic acid) and traces of mannose and sulphate. Mucin has been difficult to characterize, due to its large molecular weight, high polydispersity and high degree of glycosylation. The conformation of mucin depends on various factors such as pH and ionic strength though sugars also play important role for maintaining the extended conformation of mucin [1].
3.1.1.1 Mucous and Pharmacology
The physical state of the mucous, change in the concentration of mucin, and the strong dependence of its physicochemical properties on factors such as ionic strength and pH play a significant role in many diseases. For example, many bacteria possess specific adhesins that specifically bind to mucous which helps them to reside within the mucus. This includes pathogenic strains of Helicobacter, Pseudomonas, Streptococcus and Pneumococcus. Helicobacter pylori particularly resides in the mucus layer of the stomach, and is a common cause of ulcers [2, 3]. Some parasitic organisms also secret their own layers of mucus to escape the immune system. Secondly, overproduction of mucus is involved in cystic fibrosis, bronchitis, asthma and in middle ear infections, and mucus gels serve as the matrix in which gallstones are nucleated and grow. Whilst, mucus underproduction is present in dry eye syndromes and in some forms of ulcer disease. Various drug delivery systems based on mucoadhesive interactions like polyelectrolytic interactions (chitosans, poly-acrylic acid, etc.), hydrogen bonds (hydrogels), and disulphide binding (thiomers) have been optimized to increase the residence time. Development of nanoparticles for mucosal DNA vaccines and gene therapy are also being underway [4].
3.1.2 Brief Discussion on Mucosal Routes of Exposure
Currently many of the treatments depend on systemically administered therapies which treat the diseased sites but are toxic to healthy tissues limiting treatment efficiency due to patient noncompliance [5]. The advent of micro-and nano-delivery technologies combined with the non-invasive administration brings new confidence for the treatment of disease. Micro-and nano-delivery technologies overcomes the problem of drugs and genes poor solubility, protect drugs from acid degradation or enzymatic degradation, increase blood circulation, reduce plasma clearance, escape the reticuloendothelial system uptake, and achieve higher cellular interaction. Micro- and nano-carriers must increase retention time in the mucus to improve the diffusion across the mucus barrier, which is a challenge in drug delivery. Positively or negatively charged nanocarriers could prolong the retention time in the mucus by binding forces with negatively or positively charged mucin glycoproteins. Mucoadhesive drug delivery systems in the past have been formulated as powders, compacts, sprays, semisolids, or films [6, 7]. For example, compacts have been used for drug delivery to the oral cavity, and powders and nanoparticles have been used to facilitate drug administration to the nasal mucosa. Recently oral strips were developed for tongue or buccal cavity. Transmucosal routes covered in the review are discussed as follows:
3.1.2.1 Ocular Mucosa
Drug administration to the eye is a challenge because of several clearance mechanisms (tear production, tear flow, and blinking) that protect the eye from harmful agents. The mucus layer, 40 mm, which is secreted by the goblet cells onto the eye surface, is intimately associated with the glycocalyx of the corneal/conjunctival epithelial cells. The mucus layer is very sensitive to hydration and forms a gel-layer with viscoelastic rheological properties. It protects the epithelia from damage and enables movements of the eyelids. The mucus gel entraps bacteria, cell debris, and foreign bodies, forming “mucous threads” consisting of thick fibres arranged in bundles. Due to the composition, physicochemical properties and structure of the tear film, various factors impact the ocular mucoadhesion. Very few ophthalmic formulations containing bioadhesives or penetration enhancers are commercially available in the market. The use of bioadhesives considerably extends the corneal retention time, whereas the absorption promoters increase the rate and amount of drug transport. Combining the two approaches will promise an increase in the bioavailability [8, 9].
3.1.2.2 Nasal Mucosa
The nasal mucous membrane lines the nasal cavities having area of approximately 150 cm2 with highly dense vascular network and relatively permeable membrane structure, and is adherent to the periosteum or perichondrium of the nasal conchae [10]. From the nasal cavity, it is continuous with the conjunctiva through the nasolacrimal and lacrimal ducts; and with the frontal, ethmoidal, sphenoidal, and maxillary sinuses, through the several openings in the meatuses. The mucus layer is 5–20 mm thick and is divided into two layers, where the outer layer has a high viscosity and a gel-like character, while the layer closest to the cells has a lower viscosity enabling the cilia to move. The turnover time for mucus is usually given as 10–15 min, but it is affected by both environmental conditions and diseases [11]. Nasal mucoadhesive drug delivery has been under active investigation for controlled release dosage forms to deliver drugs directly to the CNS bypassing the BBB. Drugs administered intranasally can travel along the olfactory and trigeminal nerves to reach many regions within the CNS and achieve brain targeting. Although, nasal route of administration has gained substantial interest, it is limited by the rapid mucociliary clearance, resulting in a limited contact period allowed for drug absorption through the nasal mucosa [12].
3.1.2.3 Oral Mucosa
Drug delivery through the oral mucosa (buccal and sublingual) has gained significant attention due to its convenient accessibility. The total surface area of the oral cavity is approximately 100 cm2, of which the buccal mucosa represents approximately one-third. The epithelium of the oral mucosa consists of a stratified squamous epithelium, the thickness of which varies depending on the site. In the buccal region, the epithelium is around 40–50 cells thick, whereas it is somewhat thinner in the sublingual area. The mucus in the oral cavity is secreted by salivary glands as a component of the saliva and is adsorbed to the surface of the oral mucosa, forming a 0.1-0.7mm thick layer. Sublingual mucosa is more permeable than buccal mucosa, but sublingual administration is difficult for formulations intended to act over a long period of time [11]. Drug delivery through the oral mucosa offers several advantages over other drug delivery systems including bypassing hepatic first-pass metabolism, increasing the bioavailability of drugs, improved patient compliance, excellent accessibility, unidirectional drug flux, and improved permeability [13]. The oral cavity has been used as a site for local and systemic drug delivery in different dosage forms like adhesive gels, tablets, films, patches, ointments, mouth washes, and pastes.
3.1.2.4 Pulmonary Mucosa
The bronchial wall is made up of mucosa, lamina propria, smooth muscle, and submucosa with interspersed cartilage. Submucosal glands are found in the normal human bronchial tree in airways with cartilage in the wall. The glands lie between the epithelium and plates of cartilage and between, and occasionally external to, the plates of cartilage. The secretory tubules in bronchial tree arise directly from the collecting duct and are usually branched. At the end of each, a cluster of short tubules is found. Two types of secretory cells have been recognized in the bronchial submucosal glands lining the tubules, the mucous and serous. Mucous cells line each secretory tubule and its main branches, from the collecting duct to the distal cluster of short tubules. The mucous tubules comprise only columnar mucous cells with goblet cells and basal cells. The density of goblet cells progressively decreases from the periphery and disappears at the level of terminal bronchioles. The presence of mucin, water, and electrolytes contributes to the solubility of bronchial secretions while the cilia present in the luminal facet of epithelial cells are responsible for the rhythmic upward movement of bronchial secretions within the lung to the pharynx [14, 15].
3.1.2.5 Rectal and Vaginal Mucosa
The geometry and morphology of the lower colorectal canal vary with location, and exhibit both macroscopic and microscopic features. On the macroscopic scale, rectal folds create creases and canyons on the mucosal surface. The large folds are found in the relatively short anal canal, which is about 2–4 cm long, ending in the anal verge and contains a stratified squamous epithelium. The rectum has crypts on its surface with a thin columnar epithelium having 40–120 μm in diameter and up to about 1 mm in depth. The characteristic length for transport into the mucosal tissue is only about 1 mm, i.e. the thickness of the mucosa.
The human vaginal walls are lined with stratified squamous epithelium containing numerous folds, or rugae, which permit for distension and increased surface area for absorption. Due to the intra-abdominal pressure that collapses the rugae, high internal surface area, and tortuosity of the vaginal canal, to achieve adequate distribution of a vaginal product is a challenge. Cervicovaginal mucus (CVM) serves as a physical barrier to protect the vagina against infection in addition to the epithelium. Mucus produced at the cervix bathes and coats the vaginal walls, mix with vaginal epithelial cells and vaginal transudate. The CVM is composed mostly of water (~90 to 95%) with gel-forming glycoproteins, lipids, soluble proteins, enzymes, and various immune factors. However, ovulatory mucus is produced in more copious amounts, thus facilitating clearance and impeding drug absorption [10, 11].
3.2 Fate of Nanoparticles via Transmucosal Route
Nanoparticles (NPs), when administered via transmucosal route aid in efficient delivery of the drug without eliciting pain. Drug absorption is higher through a mucosal surface as compared to transdermal delivery due to the absence of stratum corneum. Mucosal surfaces are usually rich in blood supply, providing the means for rapid drug transport to the systemic circulation and avoiding degradation by first-pass hepatic metabolism. However, mucus acts as a barrier to diffusion of lipophilic drugs that interact with the glycoproteins and lipids in the mucus [16]. Nanoparticle (NP) research has proved that NPs cross mucosal barriers and undergo cellular uptake. Properties such as size, surface charge, shape, hydrophobicity, surface chemistry, and protein and ligand conjugates affect the phenomena [17].
3.2.1 Molecular and Cellular Interactions
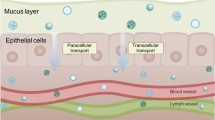
Following administration, NPs interact with the mucosal membranes in the vicinity and are either internalised into the cell or remain attached to the mucosal lining and are eliminated without any further activity. After internalisation, NPs release their contents inside the cell and are then subjected to degradation. Figure 3.1 represents the pathways followed by Nanoparticles following administration via transmucosal route.
3.2.1.1 Pathways of Internalisation
Internalization occurs through intracellular, paracellular, and transcellular pathways [18].
-
(a)
Intracellular endocytosis
Intracellular endocytosis is of two types i.e. pinocytosis and phagocytosis.
Pinocytosis is the ingestion of liquid into a cell by the budding of small vesicles from the cell membrane. Pinocytosis is further divided into Clathrin mediated, Caveolae mediated and macropinocytosis [17]. Clathrin-mediated endocytosis involves clathrin-coated vesicle formation in the presence of adaptor and accessory proteins. Signalling of the NP on the cell surface, aligns surface proteins and aids to begin clathrin-coating on the inner membrane of the cell. An adaptor protein, Epsin, helps pit formation and accessory protein dynamin (GTPase) affects vesicle formation. Thus, a clathrin-coated vesicle with a size of 100–150 nm is formed due to polymerization of the coat complex. The NP containing clathrin-coated vesicle then internally detaches from the donor membrane. Once within the cell, clathrin and adaptor proteins uncoat to allow fusing of the vesicle within the cell to release the endocytosed NPs [19].
In case of Caveole mediated pathway, NPs signalling induces actin reorganization and dynamin recruitment from the cytosol to stimulate membrane invagination and vesicle budding. The caveolae membrane then fuses into the acceptor compartment and releases its contents [17].
Macropinocytosis proceeds by forming protrusions due to actin polymerization from the cell membrane, which then encapsulates the substance to be internalized and once again fuses back with the cell membrane causing internalisation [17].
Phagocytosis is the ingestion of material by phagocytes and amoeboid protozoans. It involves cell surface recognition followed by sequential instigation of receptors leading to internalization by encircling it into triggered cup-shaped cell membrane deformations forming a phagosome [18].
-
(b)
Transcellular endocytosis
In this pathway, the NPs to be transported bind to the cell membrane receptors and form a complex. Membrane invagination is then followed by internalization. The endocytosed vesicle is then converted into a transcytotic vesicle to prevent typical endosome degradation. The transcytotic vesicle is then transported to the other end of the cell, where the vesicle membrane fuses with the cell membrane and the content of the vesicle is secreted externally [20].
-
(c)
Paracellular endocytosis
Paracellular delivery of hydrophilic drugs occurs through the intercellular space between adjacent cells via tight junctions (TJs).
3.2.1.2 Protein Binding to NPs
It has been established through a study by Fleishcher and Payne [21] that extracellular serum proteins present in blood get adsorbed onto the surface of NPs, forming a “protein corona”. When polystyrene NPs functionalized with either amine or carboxylate groups were prepared, serum proteins got adsorbed onto the surface of both the NPs. Bovine serum albumin (BSA)–NP complexes formed from anionic NPs bonded with albumin receptors on the cell surface. BSA–NP complexes formed from cationic NPs were redirected to scavenger receptors. This observation that similar NPs with identical protein corona compositions were bound to different cellular receptors suggested that a difference in the structure of the adsorbed protein may be responsible for the differences in cellular binding of the protein–NP complexes. Similar results were obtained for anionic quantum dots and colloidal gold nanospheres. Protein corona remained bound to the NP throughout the endocytic uptake and transport, however, the NP itself altered the structure of the adsorbed protein [21].
3.2.1.3 Degradation
Particles generally end intracellularly in endosomes or lysosomes followed by degradation. Chemical characteristics such as surface charge determine the fate of NPs in cells.
In the case of macromolecular therapeutics, following intracellular uptake, the contents of the endocytic vesicle are delivered to lysosomes for degradation. Although a therapeutic agent encapsulated in NPs are less susceptible to degradation in the endo-lysosomal compartment, the relatively faster degradation of NPs under acidic conditions in the endo-lysosomal compartment may result in the release of the therapeutic agent, which could then degrade quite rapidly. Thus, NPs are expected to be efficiently internalized into the cells and then deliver their payload into the cytoplasmic compartment rather than be retained in the degradative environment of endo-lysosomal compartment.
A study by Panyam et al. [22], showed that (PLGA) poly (lactic-co-glycolic acid) NPs were internalized through clathrin depended endocytosis. Following their uptake, NPs were localized in the early, recycling endosomes and late endosomes and lysosomes. It was summarised that NPs are either recycled back to the surface from the early endosomes or are transported to the secondary endosomes and lysosomes from which the NPs escape into the cytosol. The early endocytic vesicles possess physiological pH wherein NPs have a net negative charge and hence are repelled by the negatively charged endosomal membrane. The secondary endosomes and lysosomes are predominantly acidic, with pH values ranging from 4–5. In this pH, NPs have a net cationic potential and hence interact with the negatively charged membrane leading to their escape into cytoplasmic compartment. NPs do not open up the endo-lysosomal vesicles but are released by localized destabilization of the endo-lysosomal membrane at the point of contact with NP, followed by extrusion of the NP through the membrane. PLGA NPs are cationic only in the endosomal compartment and do not destabilize the lysosomes. After their escape, NPs deliver their payload in the cytoplasm at a slow rate, leading to a sustained therapeutic effect. Because NPs are biodegradable and biocompatible and are capable of sustained intracellular delivery of multiple classes of cargoes, they are a suitable system for intracytoplasmic delivery of drugs, proteins, or genes [22].
3.3 Mechanism of Toxicity at Cellular and Molecular Level
Nanotoxicity or NP related toxicity implies toxic effects of NPs which are uncommon and not seen with larger particles on the biological system. The significance of nanotoxicity is such that even when the NPs made up of inert materials like gold or silver, they are highly active owing to their nanosized dimension. Nanotoxicity pursues the level or extent to which these properties may cause any threat to environment and living beings. It also intends to quantitatively determine the severity and regularity of toxic effects of the exposure of the NPs on the organism.
The impetus for designing a nano drug delivery system is to reduce the toxicity of a drug and to increase its bioavailability as well as biocompatibility. On the other hand, their exceptional properties like surface area to volume ratio, particle size, solubility, surface coating and shape or structure may pose additional risks to the patients [23]. Toxic manifestations observed with in vitro models are hardly relatable to the effects seen in vivo. Though major entry routes as well as recognised targets have been identified, intense research is still required to demonstrate the pathway and mechanism of toxicity of NPs in the body [24].
It is in general consent that NPs display toxicity through varied mechanisms and can affect in allergy, fibrosis, organ failure, neurotoxicity, hepatological toxicities, nephrotoxicities, haematological toxicities, splenic toxicities, and pulmonary toxicities, among others.
3.3.1 Physiochemical Properties of Nanoparticle and Their Toxic Effects
-
(a)
Particle size and surface area: Particle size and surface area of NPs play an important role in their interaction with biological molecules or system. Interestingly, particle size is inversely proportional to the surface area relative to the volume, means decrease in size leads to an increase in surface area to volume ratio. Several biological mechanisms including phagocytosis, endocytosis and passive diffusion as well as endocytic processing (antigen presentation on MHC class molecules) are dependent on size of the material. One of the prime mechanism for toxicity is generation of reactive oxygen species, these free radicals have been known for hazardous impact on biological molecules like DNA, lipids, proteins etc.
Furthermore, surface area also results in some toxic manifestation, i.e. increase in surface area leads to extensive interaction with biomolecules that root more oxidation and DNA damage abilities as compared to larger particles of same mass [25].
-
(b)
Particle shape and aspect ratio: Particle shape dependent toxicity is related to NPs made of gold, silver, carbon nanotube, nickel, titanium etc. Endocytosis and phagocytosis processes are mostly influenced by this property. It has been shown that spherical particles are more prone to endocytosis than any other particle shapes [25]. Studies also report that particle shape can affect the cellular level e.g. blocking of K+ channel by rod shaped SWNTs were two to three times more efficient than spherical C60 fullerenes [26]. Also in another study nanorod ZnO was found to me more cytotoxic than spherical ZnO [27]
Similarly, greater the aspect ratio more will be the toxicity of NPs. It has been observed that asbestos particles which are <2 μm in size caused asbestosis, <5 μm caused mesothelioma and 10 μm caused lung carcinoma [28]. TiO2 nanofibres having length of 15 mm were more toxic than fibres having length 5 mm, here former induced more inflammatory response by alveolar macrophages in mice than later one. Similarly, in case of Carbon Nanotubes (CNTs), long MWCNTs caused inflammatory response in mice abdominal cavity whereas small MWCNTs did not cause any inflammation at all [29].
-
(c)
Surface charge: Surface charge of NPs also play an important role in toxicity. Rather, they have an even greater impact on the biological system. Surface charge on the particles dictate various interactions such as plasma protein binding, selective absorption, blood brain barrier integrity and membrane permeability. Mammalian cell membranes possess negative charge on their surface, thereby promoting association of cationic particles with the cells to a greater extent as compared to the negative or neutral particles. However, higher cationic charge leads to the severe toxicity via haemolysis and platelet aggregation [25]. Positively charged silica NPs have been shown to induce more reactive oxygen species than neutral and negatively charged silica NPs [30].
-
(d)
Crystalline structure: Besides the three parameters contributing substantially towards toxicity, crystalline structure may also be responsible for nanotoxicity. Studies have claimed that anatase form of TiO2 NPs induce higher lipid peroxidation and oxidative DNA damage in presence of light compared to their rutile form [31].
-
(e)
Aggregation: Particle aggregation also imparts toxicity. Aggregation is mostly dependent on the surface charge, size and composition. Aggregation of NPs are mostly seen in the case of CNTs, where it has been observed that aggregated CNTs have more cytotoxic effects than dispersed ones [32].
-
(f)
Surface coating: Surface coating eventually alters the physiochemical properties of NPs such as surface charge, magnetic, electric, optical and chemical properties. These changes may lead to varied interactions with biomolecules that result into significant toxicity of NPs. It was well acknowledged that the existence of ozone, oxygen radicals along with heavy metals on nanoparticle surface leads to the formation of ROS that induces inflammation in cells.
However, in most cases surface coating could also be employed to abate the toxicity of NP. For example, coating is very essential in the case of quantum dots to render them nontoxic since their metallic core is hydrophobic and composed of heavy toxic metals like cadmium.
While these factors mainly contribute to the toxicity of NPs, concentration is the principle factor dictating the toxicity of macroparticles [25].
3.3.2 Mechanism of Toxicity of NPs
Toxic scenario of NPs in organisms and environment are well established. Unique physiochemical properties of NPs pave way for their application in several fields. On the other hand, they also increase the risk of their exposure to humans and environment.
The mechanisms due to which these properties result in toxicity are discussed in the following section.
-
(a)
Reactive Oxygen Species (ROS) or Oxidative stress production: ROS are oxygen containing chemically reactive species that have important roles in cell signalling and homeostasis. They are generally formed as a natural by-product of oxygen metabolism. ROS are generated intrinsically as well as extrinsically within the cell, the pool of ROS constitutes of oxidative species including superoxide anion (O2 −), hydroxyl radical (OH−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and hypochlorous acid (HOCl), hypochlorite ion (−OCl−) [33].
NADPH oxidase (NOX) complexes in cell membranes, mitochondria, peroxisomes, and endoplasmic reticulum are responsible for endogenous production of ROS. Whereas, exogenous ROS can be formed from tobacco, pollutants, smoke, engineered NPs, drugs, xenobiotics, or radiation.
Enzymes like catalase, glutathione peroxidase, super oxide dismutase, peroxiredoxins as well as light are responsible for ROS generation. Apart from the deleterious effects of ROS, they also have some positive effects such as programmed cell death i.e. apoptosis and induction of host defence mechanism.
The harmful effects on biomolecules are often seen in the form of:
-
(i)
DNA or RNA: Single and double stranded breaks in DNA or RNA
-
(ii)
Proteins: Oxidation of amino acids
-
(iii)
Lipids: Oxidation of polyunsaturated fatty acids i.e. lipid peroxidation
-
(iv)
Enzymes: Inactivation by oxidation of co-factors
NPs may generate ROS via three mechanisms; particle-cell interaction, active redox cycling on surface of NPs (especially in case of transition metals) and oxidative groups functionalized on NPs [23]. Overproduction of ROS leads to the activation of interleukins, cytokines, kinase and tumour necrosis factors which eventually cause the proinflammatory response.
Eom and Choi [34] reported an elevated ROS production when Jurkat T cells that were exposed to AgNPs in contrast to the unexposed ones [34]. Moreover, numerous researchers have established single stranded DNA damage caused due to TiO2, Carbon black and diesel exhaust particles [31, 35, 36].
-
(i)
-
(b)
Apoptosis: It is programmed cell death that occurs in multicellular organisms. This process involves various events such as chromosome condensation, nuclear fragmentation, cell shrinkage, DNA and mRNA decay etc. Apoptosis can be initiated by an intrinsic pathway (cell kills itself because it senses cell stress) as well as an extrinsic pathway (cell kills itself because of signals from other cells). In case of NPs, they are interacting with macrophages which causes the activation extrinsic pathway of apoptosis. Extrinsic pathway is activated via two signals:
-
(i)
Tumour Necrosis Factor (TNF) signal: TNF-α is a cytokine produced extensively by activated macrophages. Human cells have two receptors for it i.e. TNFR1 and TNFR2. Binding of TNF-α to these receptors leads to the activation of caspases.
-
(ii)
First Apoptosis Signal (FAS): FAS, a transmembrane protein belonging to the TNF family, binds to the FAS ligand. Interaction between them results in the formation of the death-inducing signaling complex (DISC), which contains, caspase-8 and caspase-10. In some cases, caspase-8 is directly activated by interacting with foreign materials and subsequently activate further caspases.
Eom and Choi [34] also reported that 39% of Jurkat T cells underwent apoptosis when exposed to the AgNPs [34]. Zno NPs also triggered cell death via Caspase mediated apoptosis as reported by Wilhelmi et al. [37]. Similarly, high concentration of FeO NPs led to the 35–40% apoptosis [38].
-
(i)
-
(c)
Genotoxicity: Nanogenotoxicity is a new term that has emerged in the field of nanotechnology. This term highlighted NP induced genotoxicity and carcinogenesis. Literature showed that, long term inflammation and oxidative stress present in a cell ultimately leads to DNA damage. Continuous production of ROS causes mutagenesis (due to the oxidation, hydrolysis and deamination of nucleic acid bases, substitution etc.) and carcinogenesis (due to the gene deletion, insertion etc.)
The results reported by Eom and Choi also indicate that DNA damage would have resulted into release of the DNA damage marker protein, p-H2AX which increased drastically after the exposure of AgNPs [34].
-
(d)
Cell surface interaction with proteins: It is well known that NPs, especially heavy metal NPs tend to interact with proteins and amino acids. These interactions cause the formation of protein corona, protein unfolding, and altered protein function, which finally culminate into protein damage or non-functional. Similarly, interaction of NPs with protein molecules such as serum albumin, human blood protein haemoglobin (Hb), and cytoskeletal proteins result in protein conformational changes or protein damage.
Silica NPs significantly influence the unfolding of RNase, where the enzyme is less stable on NPs surface than in free solution [39].
-
(e)
Mitochondrial interaction: Mitochondria is the powerhouse of cell, which provides the energy for vital cell functions. Several toxicological studies shown that NPs have identified mitochondria as a potentially relevant target organelle.
Mitochondrial interaction of NPs lead to following consequences:
-
(i)
Disturbance in proton gradient pumps, voltage-gated channels
-
(ii)
Change in mitochondrial outer membrane permeability, which releases cytochrome C (key event in intrinsic pathway of apoptosis)
-
(iii)
Mitochondrial DNA damage, also releases cytochrome C (results into the necrosis)
Similarly, mitochondrial DNA encodes many structural proteins involved in various pathways. DNA mutagenesis causes the production of defective proteins, thus hampering the pathways like ATP synthesis, Electron Transfer Chain, oxidative phosphorylation etc. [33].
Cationic polystyrene NPs induced mitochondrial damage which eventually resulted into cell death [40]. Ning et al. [41] asserted that ultrafine particles are much potent for induction of mitochondrial damage [41].
-
(i)
-
(f)
Interaction with cellular signalling pathway: NPs are inclined to induce the signalling cascade pathways via particle induced release of cytokines, particle to cell interactions and binding to the several cellular receptors as a ligand [33]. Signalling pathways which are activated through NPs; for e.g. MAPK, ERK, EGFR etc. are described in detail in the following section:
-
(i)
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): NF-κB is a group of proteins that controls transcription of DNA, cytokine production, cell proliferation, apoptosis, inflammation and cell survival. These proteins are also responsible for the activation of defence mechanisms as well as cellular responses to stimuli such as stress, cytokine, heavy metals, ultraviolet radiation. ROS activate NF-B via the release of IBs resulting in the nuclear translocation of NF-B [42].
ZnO and CdS NPs were found to induce toxicity via ROS-dependent NF-B activation [43].
-
(ii)
Activator protein-1 (AP-1): Activator protein-1 is a transcription factor that regulates gene expression in response to a stimuli; including stress, oxidants, bacterial and viral infection, growth factors, and cytokines. AP-1 regulates cell proliferation, cell differentiation, and apoptosis. This gene is activated via phosphorylation of protooncogene c-jun. Cr, Ni, and Fe NPs have been shown to activate AP-1 via ROS generation [44].
-
(iii)
Mitogen-activated protein kinase (MAPK): MAPK is a type of serine/threonine-specific protein kinase. It is involved in various cellular responses to a stimuli; such as proinflammatory cytokines, osmotic stress, heat shock and mitogens. It regulates cell proliferation, cell differentiation, apoptosis, gene expression, cell survival and mitosis. MAPK consist of growth factor regulated extracellular signal-related kinases (ERK) and the stress-activated MAPK, p38MAPK and c-jun NH2-terminal kinases (JNK). MAPK activation is based on the oxidative modification of MAPK signalling proteins (e.g., RTK and MAP3 K). The concentration and kinetics of ROS production and cellular antioxidant pool are mostly important for activation of MAPK signalling pathway. Silver NPs tend to activate JNK pathway and apoptosis whereas CeO2 NPs trigger p38 MAPK signalling in broncho alveolar cells [45].
-
(iv)
Protein tyrosine phosphatases (PTP): PTP is a tyrosine kinase that regulates the phosphorylation of various signalling molecules involved in signal transduction cascades. Signal transduction cascade pathways are involved in oncogenic transformation, mitosis, cell growth and cell differentiation. PTP is highly susceptible to oxidative stress in the form of free radicals and H2O2 [42]. Zn2+ and V4+ NPs are critical in redox regulation of PTP via the inhibition of MAPK and EGFR [46].
-
(v)
Epidermal growth factor receptor (EGFR): It acts as an extracellular protein ligand for various members of epidermal growth factor family (EGF family). EGFR dimerization induces intracellular protein-tyrosine kinase activity which results into auto phosphorylation. This auto phosphorylation initiates further downstream signalling pathway such as MAPK, Akt and JNK leading to DNA synthesis and cell proliferation [46].
-
(vi)
Src family kinase: Src family belongs to a non-receptor tyrosine kinases family, which is involved in regulation of cell growth, cell differentiation and oncogenic transformation. Oxidative stress is responsible for activation of this family, which later on triggers the signal transduction pathway. Src family kinases interact with many membranes, cytosolic and nuclear proteins by phosphorylation of tyrosine [42]. Low dose of Chromium NPs induced cell death via ROS dependent Src Kinase [47].
-
(i)
3.4 Toxicological Aspects of Nanoparticles via Different Transmucosal Routes
3.4.1 Ocular Mucosa-
In perceiving the advantages of new advances in nanobioadhesives for enhancing topical ocular delivery, the other side of the coin must also be considered. The toxicity literature in this area of research is not as robust as other fields, because most publications focus on the discovery and development of new therapeutic agents. It comes as no surprise that the same properties that make nanosystems attractive for drug delivery applications, may confer reactivity in biological systems and lead to toxicity. For topically ocular administered nanosystems, aggregation and tissue accumulation must be considered. Nanosystem aggregation may block cell metabolism and could impair tissue function. For example, blockage of the lachrymal drainage punctum and decreased tear film recycling can occur due to aggregation of topically applied nanosystems on the ocular surface. Furthermore, indiscriminate ocular nanosystem accumulation results in distortion of the ocular tissue architecture leading to altered function. A very important consideration of toxic effects with nanosystems is, that it may be attributable to actual approaches that are directed to enhance ocular drug bioavailability; the presence of high concentration of the loaded drug in a non-target tissue.
Chitosan has some unique and important characteristics like being mucoadhesive and cationic. The positively charged chitosan NPs bind to the negatively charged surface of the cornea. Prow et al. [48] evaluated Chitosan, PCEP (poly{[(cholesteryl oxocarbonylamido ethyl) methyl bis(ethylene) ammonium iodide] ethyl phosphate}), and magnetic NPs (MNPs) for the safe gene delivery in the eye. Rabbits were administered with NPs either intravitreally (IV) or subretinally (SR) and sacrificed 7 days later. Eyes were grossly evaluated for retinal pigment epithelium abnormalities, retinal degeneration, and inflammation. IV chitosan showed inflammation in 12/13 eyes, whereas IV PCEP and IV MNPs were not inflammatory and did not induce retinal pathology. Acute inflammation and polymorphonuclear cell infiltrates resulted with injection of these nanoparticle formulations which were grossly visible in the eye cup after 7 days. Histological examination confirmed massive numbers of immune cells at the site of injection. It is possible that the hyalocytes, the sentinel immune cells of the vitreous, are particularly sensitive to polysaccharides leading to the inflammation [48].
Guo et al. [49] synthesized and evaluated a series of positively charged phospholipids and cholesterols as membrane components for liposomes. Selected liposome preparations formulated with these synthetic lipid materials were found to be non-cytotoxic in vitro by using a cell growth inhibition assay, whereas liposomes containing positively charged components (stearylamine and cetyltrimethylammonium bromide) showed considerable cytotoxicity. Their investigations thus, indicate a specific adhesion of the cationic liposomes to the surface of mucosal tissues owing to the presence of negative charge [49].
3.4.2 Nasal Mucosa-
Nasal mucoadhesive drug delivery system offers the advantage of higher residence time as compared to the normal dosage forms. Mucoadhesive polymers are being used to increase the residence time of formulation within the nasal cavity; the polymers possibly interact with the epithelial tight junctions to facilitate drug absorption by some histopathological alterations of the nasal mucosa and effect on ciliary beating. Hence it becomes necessary to evaluate the toxicological effects of such nano systems on the structural alterations of the nasal environment.
Hackenberg et al. [50] evaluated the toxic effect of repeated exposure of ZnO-NPs in three-dimensional (3D) mini organ cultures (MOCs) of human nasal mucosa determined by trypan blue exclusion and caspase-3 activity, respectively. MOCs were exposed once, twice, or three times to 0.1 or 5 μg/ml of ZnO-NPs for 1 h. per exposure and then evaluated for cytotoxicity and genotoxicity. DNA fragmentation augmented after 24 h of regeneration at both concentrations of ZnO-NPs. In contrast, DNA damage induced by the positive control, methyl methanesulfonate, was significantly reduced after 24-h regeneration. Thus, results suggest that repetitive exposure to low concentrations of ZnO-NPs results in persistent DNA damage. Various mechanisms responsible for ZnO-NPs related genotoxicity were proposed by the authors based on the study, such as direct interaction of particles with the DNA in the nucleus, ROS generation, or influence of dissolved zinc ions to DNA damage [50].
Genter et al. [51] assessed the distribution and toxic potential of 25-nm Silver NPs (AgNPs) (100 or 500 mg/kg) following intranasal (IN) exposure in adult male C57BL/6J mice. Histopathology of selected organs was performed, and tissue reduced glutathione (GSH) levels were measured after 1 or 7 days as an indicator of oxidative stress. Aggregated AgNPs were found in the spleen, lung, kidney, and nasal airway by routine light microscopy. Autometallography revealed AgNPs distributed in olfactory bulb and the lateral brain ventricles. Elevated tissue GSH levels was observed in nasal epithelia (both doses at 1 day, 500 mg/kg at 7 days) and blood (500 mg/kg at 7 days). Therefore, intranasal administration of AgNPs permits systemic distribution, produces oxidative stress in the nose and in blood, and develops macrophage-mediated erythrocyte destruction in the spleen [51].
3.4.3 Oral Buccal Mucosa
The buccal area appears to be an attractive site for administration of drugs due to the smooth and relatively immobile surface, good accessibility, the evasion of possible degradation in the gastrointestinal tract, and no first-pass metabolism in the liver. However, continuous salivation and swallowing may lead to a very short residence time in the oral cavity [4]. To overcome this problem, novel bioadhesive dosage forms have been developed; such as bioadhesive tablets, patches, bioadhesive gels and ointments, and medicated chewing gums. Smistad et al. [52] evaluated the toxicity of liposomal formulations on the human buccal cell line TR146. The main objective of this paper was to identify important liposomal formulation factors influencing the toxicity on cells in the buccal region, and identifying significant interactions between the formulation variables. Positively charged liposomes were shown to be toxic compared to the negatively charged liposomes. Diacyl-TAP (l,2-diacyl-3-trimethylammonium propane) was less toxic than SA, and DPPC was less toxic than DMPC. The amount of negatively charged component, the liposome size, and the total lipid concentration did not affect the toxicity within the experimental room. The most toxic combination in this study was egg-PC/positively charged lipid (20 mol%) [52].
Klemetsrud et al. [53] also investigated the in vitro toxicity, mucoadhesive potential and impact on cell permeability of polymer coated liposomes intended for oral use in TR146 cell line. The following polymers were tested: chitosan, low-methoxylated pectin (LM-pectin), high-methoxylated pectin (HM-pectin), amidated pectin (AM-pectin), Eudragit, poly(N-isopropylacrylamide-co-methacrylic acid) (p(NIPAAM-co-MAA)), hydrophobically modified hydroxyethyl cellulose (HM-HEC), and hydrophobically modified ethyl hydroxyethyl cellulose (HM-EHEC). All the systems, except with chitosan, exhibited no significant effect on cell viability and permeability at the considered concentrations. At the lowest chitosan concentration (0.05%) there was no sign of cell toxicity. However, there was a significant reduction of cell viability (65%) when the polymer concentration was increased to 0.1%. At even higher concentrations (≥0.25%), the cell viability was around 9%; representing an almost complete cell death. No significant reductions in the cell viability were observed for the other polymers [53].
Teubl et al. [54] investigated interactions of three different titanium dioxide (TiO2) particles (i.e. NM 100, NM 101 and NM 105) with oral tissues. Physicochemical properties were addressed in relevant media, and particle penetration was investigated with an ex vivo model using porcine mucosa. Although TiO2 particles formed large aggregates once dispersed in media, 10–50% remained in the nanoscale range, rapidly interacting with the mucus layer and infecting the epithelium. NM 100 and NM 105 were found in both the upper part and the lower part of the buccal mucosa, while NM 101 (smallest particle sizes) only penetrated the upper parts. Transport studies revealed that TiO2 NPs were found in vesicles, as well as freely distributed in the cytoplasm. However, NM 105 triggered the production of reactive oxygen species [54].
3.4.4 Pulmonary Mucosa-
Pulmonary route serves as a non-invasive approach for systemic delivery of therapeutic actives like drug, proteins and peptides. Larger surface area of lungs, rich blood supply and a thin mucous layer make the pulmonary route popular for efficient systemic delivery. This route is an effective alternative for the delivery of those therapeutic actives that show less bioavailability via oral route, especially in the case of proteins and peptides. Specialized devices such as dry powder inhaler, metered dose inhaler and nebulizer have been fabricated to facilitate pulmonary delivery by ensuring deep lung deposition. The only significant formulation based parameter to be considered when designing a dosage form for pulmonary delivery is the particle size, the ideal size being 1–5 μ. Besides the use of polymeric or lipidic NPs for relief for pulmonary diseases, human beings are also inadvertently exposed to inorganic NPs. Metal NPs such as iron, silica, nickel, carbon etc. are inhaled into the body via upper respiratory tract in the work place environment, which is a common event [55]. In these conditions, lung primarily acts as a site of accumulation and long term exposure of NPs. Once they enter into the interstitial spaces, they are rapidly taken up by alveolar cells and induce toxic effects. Occupational exposure of these NPs causes hazardous and harmful effects on human being leading to cancer, asthma, fibrosis, and pneumonitis etc. Naturally occurring NPs are well tolerated or adapted by human and the environment. However, the unintentional and intentional inhalation and accumulation of NPs pose a serious threat to human as well as environment [56].
Coating of metallic NPs with polysaccharides can overcome the drawbacks by increasing stability and biocompatibility, improving size distributions and introducing chemical groups that allow for further functionalization of the NPs. Worthington et al. [57] have demonstrated that chitosan coating reduced the toxicity of copper NPs significantly after 24 and 52 h and the generation of ROS. Conversely, inflammatory response of mice exposed to chitosan coated NPs, measured using the number of WBC and cytokines/chemokines in the bronchoalveolar fluid was shown to increase, as was the concentration of copper ions. These results suggest that coating of metal NPs with mucoadhesive polysaccharides (e.g. chitosan) could increase their potential for use in controlled release of copper ions to cells, but will result in a higher inflammatory response if administered via the lung [57]. Tables 3.1 and 3.2 summarises the other in vivo and in vitro research studies pertaining to the possible toxic effects of NPs via pulmonary route.
3.4.5 Rectal/Vaginal Mucosa-
Nanotechnology based drug delivery systems may be an interesting option to advance in the field of microbicides and have been advocated for the delivery of promising microbicide drug candidates such as dapivirine. Among other advantages, nanosystems may be able to enhance drug/virus interaction, penetrate the mucosa, target HIV-susceptible cells, and provide an effective and durable drug barrier along the epithelial lining when administered by the route. In the case of vaginal and rectal administration, only a few studies explored this possibility thus resulting in a substantial lack of data supporting the potential value of nanotechnology-based drug delivery systems. However early methods for assessing toxic effects of NPs are insufficient to detect these toxicities. There is also a paucity of data regarding excipients, components often assumed to be non-toxic on the basis of past experience with commercial vaginal products.
The first report of microbicide toxicity was by Phillips and Zacharopoulos [76] who found that rectal application of N-9 caused rapid exfoliation of sheets of epithelial cells and failed to protect mice against rectal transmission of HSV-2 [76]. das Neves J et al. [77] reported the preparation and characterization of drug-loaded poly ε-caprolactone (PCL), poly(ethylene oxide) (PEO), cetyl trimethylammonium bromide (CTAB) NPs of dapivirine, as well as their influence on the permeation and retention in cervicovaginal and colorectal cell monolayer models, and pig vaginal and rectal mucosa at the cell and tissue level. Dapivirine-loaded NPs of around 175–200 nm and different surface properties were successfully prepared and were readily taken up by different anogenital epithelial cells. NPs were shown able to modulate differently the permeability and monolayer/tissue retention kinetics of dapivirine. PEO-PCL NPs reduced the permeability of dapivirine, while CTAB-PCL NPs increased the diffusion of the drug across studied models. Further, toxicity results in pig vaginal and rectal mucosa showed unacceptable toxicity with CTAB-PCL NPs as compared to free dapivirine where it did not show any toxicity issues [77]. The other toxicological cases of the NP drug delivery via vaginal route are discussed in Table 3.3.
3.5 Summary and Future Prospects
Nanomaterials are endowed with unique properties, which are responsible for their toxicological manifestations. This had led to concerns over the use of such materials, inspite of their widespread applications. Moreover, rapid developments are viewed in the field of nanotechnology every day, which will only further complicate the toxicological profile of nanomaterials. This necessitates development of a strategy to evaluate the toxic effects of the upcoming nanomaterials and their products before they are being brought into mainstream use. The toxicity of nanomaterials depends on the basic chemistry of interaction of the material with the molecular pathways in an organism. A thorough investigation on the molecular interaction and alteration in the biochemical machinery of an organism upon contact with a nanomaterial is a prerequisite for designing safe and sustainable nanomaterials. There is a multitude of research literature now available on the toxicity of nanomaterials to various model organisms from human to ecological models. But majority of the reports focus on acute high dose exposures. Research on the toxicity of other chemicals has shown that dose of a chemical can have a tremendous impact on the pathways that are affected within the organism. Overall, it appears from the literature that many of the current categories of available nanomaterials are not acutely toxic but are most likely to have toxic implications following long-term low dose exposures. There is a significant gap regarding these types of potential impacts.
The most common pathways investigated in nanotoxicity experiments are related to oxidative stress, yet oxidative stress can be a temporary and natural response to an insult without a negative outcome. Currently, a few individual biomarkers are being explored in this capacity and they are often limited to pathways involved in oxidative stress, which is known to be a complicated biomarker. There are a multitude of other potential non-oxidative stress mechanisms that may be triggered in response to sub-lethal exposures of nanoparticles. Testing these materials at low concentrations will allow the development of biomarkers for evaluating nanomaterial toxicity. Also, the ‘unique’ properties demand newer concepts and methodologies to understand and foresee the size (and shape)-specific interaction of nanoparticles within the human body. Recent research has led scientists to identify some discrete physicochemical characteristics of nanoparticles that required on priority basis to assess their toxicological potential. These include particle size and surface area in relevant media the route of administration, physical form, degree of aggregation, surface characteristics, sample purity, etc. Such assessment in the case of nanoparticles poses an additional challenge, since the characteristics of nanoparticles largely depend on their form and chemical composition; both exhibiting a dynamic nature before, during and after administration into in vitro or in vivo systems.
Also, there is a pressing need for the guidance and documentation of nanotoxicity which all researchers come across. The documentation will serve as a reference for further studies involving nanoparticles and nanocarriers. To our understanding, at present data obtained from diverse nanotoxicity testings are neither comparable nor reliable, robust or reproducible. It is a daunting task to extract reliable data from the huge amount of studies in total and to come to comprehensive and valid conclusions that allow us to safely implement nanotechnology in medical and other sophisticated applications. In addition, the strict utilization and realization of validated and standardized intelligent testing policies should become second nature to all ‘nano-scientists/toxicologists’.
Second, nanomaterials clearly demonstrate the need for alternatives assessment methods to consider the intrinsic exposure potential as part of the comparative assessment process because there are distinct physicochemical properties as well as use characteristics that will distinguish from the bulk material. In this respect the role of the peer-reviewing process of manuscripts should be to rigorously demand and enforce these high standards. All studies that address effects of nanomaterials should be reviewed by experts in the field of nanotoxicology. Moreover, these experts ought to closely stick to the given recommendations.
Abbreviations
- AgNPs:
-
Silver nanoparticles
- AP-1:
-
Activator protein-1
- ARE:
-
Antioxidant-response element
- ATP:
-
Adenosine triphosphate
- BBB:
-
Blood brain barrier
- BSA:
-
Bovine serum albumin
- CBF:
-
Ciliary beat frequency
- CeO2 :
-
Cerium dioxide
- CNS:
-
Central nervous system
- CNTs:
-
Carbon nanotubes
- CRP:
-
C-reactive protein
- CTAB:
-
Cetyltrimethylammonium bromide
- CuO:
-
Copper oxide or cupric oxide
- CVM:
-
Cervicovaginal mucus
- DCFH-DA:
-
2,7-dichlorofluorescein diacetate
- DISC:
-
Death-inducing signalling complex
- DMPC:
-
1,2 Dimyristoyl-sn-glycero-3-phosphocholine
- DNA:
-
Deoxyribonucleic acid
- DPPC:
-
Dipalmitoylphosphatidylcholine
- Egg-PC:
-
Egg-phosphatidylcholine
- ELISA:
-
Enzyme-linked immunosorbent assay
- ERK:
-
Extracellular signal-related kinases
- FAS:
-
First apoptosis signal
- Fe2O3:
-
Ferric oxide
- FeO:
-
Iron oxide or ferrous oxide
- GFR:
-
Epidermal growth factor receptor
- GIT:
-
Gastrointestinal tract
- GRO-alpha:
-
Growth-regulated oncogene-alpha
- GSH:
-
Glutathione (reduced)
- H2O2:
-
Hydrogen peroxide
- Hb:
-
Hemoglobin
- HM-EHEC:
-
Hydrophobically modified ethyl hydroxyethyl cellulose
- HM-HEC:
-
Hydrophobically modified hydroxyethyl cellulose
- HPLC:
-
High pressure liquid chromato-graphy
- HSV:
-
Herpes simplex virus
- IFN:
-
Interferon
- IL:
-
Interleukins
- JNK:
-
c-jun NH2-terminal kinases
- KD:
-
Kilo Dalton
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinase
- MgO:
-
Magnesium oxide
- MHC:
-
Major histocompatibility complex
- MNPs:
-
Magnetic nanoparticles
- MOCs:
-
Mini organ cultures
- mRNA:
-
Messenger ribonucleic acid
- MTT:
-
3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide
- MWCNTs:
-
Multi walled carbon nanotubes
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NiO:
-
Nickel oxide
- NPs:
-
Nanoparticles
- Nrf2:
-
LNT induced NF-E2 p45-related factor 2
- PbAc:
-
Lead acetate
- PCEP:
-
(poly{[(cholesteryl oxocarbonylamido ethyl) methyl bis(ethylene) ammonium iodide] ethyl phosphate})
- PCL:
-
Poly ε-caprolactone
- PCR:
-
Polymerase chain reaction
- PEG:
-
Polyethylene glycol
- PEO:
-
Poly(ethylene oxide)
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PTP:
-
Protein tyrosine phosphatases
- QD:
-
Quantum dot
- qPCR:
-
Quantitative polymerase chain reaction
- RNA:
-
Ribonucleic acid
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- SA:
-
Stearic acid
- SiO2:
-
Silica dioxide
- SOD:
-
Super oxide dismutase
- SWCNTs:
-
Single walled carbon nanotubes
- TAP:
-
Diacyl-TAP (l,2-diacyl-3-trimethy-lammonium propane
- TiO2:
-
Titanium dioxide
- TJs:
-
Tight junctions
- TNF:
-
Tumor necrosis factor
- WST-1:
-
Water-soluble tetrazolium salts
- ZnO:
-
Zinc oxide
References
Dekker J, Rossen JW, Büller HA et al (2002) The MUC family: an obituary. Trends Biochem Sci 27:126–131
Scharfman A, Lamblin G, Roussel P (1995) Interactions between human respiratory mucins and pathogens. Biochem Soc Trans 23:836–839
Cone RA (2009) Barrier properties of mucus. Adv Drug Deliv Rev 61:75–85
Rathbone MJ, Tucker IG (1993) Mechanisms, barriers and pathways of oral mucosal drug permeation. Adv Drug Deliv Rev 12:41–60
Wang Y-Y, Lai SK, So C et al (2011) Mucoadhesive nanoparticles may disrupt the protective human mucus barrier by altering its microstructure. PLoS One 6(6):e21547
Patel VF, Liu F, Brown MB (2011) Advances in oral transmucosal drug delivery. J Control Release 153:106–116
Sosnik A, Neves JD, Sarmento B (2014) Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog Polym Sci 39:2030–2075
Ludwig A (2005) The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 57:1595–1639
Kaur IP, Smitha R (2002) Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm 28:353–369
Donnelly R, Shaikh R, Singh TR et al (2011) Mucoadhesive drug delivery systems. J Pharm Bioallied Sci 3:89
Edsman K, Hägerström H (2005) Pharmaceutical applications of mucoadhesion for the non-oral routes. J Pharm Pharmacol 57:3–22
Ugwoke M, Agu R, Verbeke N et al (2005) Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev 57:1640–1665
Hoogstraate JA, Wertz PW, Wertz PW (1998) Drug delivery via the buccal mucosa. Pharm Sci Technol Today 1:309–316
Rhodin JA (1966) Ultrastructure and function of the human tracheal mucosa. Am Rev Respir Dis 93(3P2):1–15
Meyrick B, Sturgess JM, Reid L (1969) A reconstruction of the duct system and secretory tubules of the human bronchial submucosal gland. Thorax 24:729–736
Sigurdsson HH, Kirch J, Lehr C-M (2013) Mucus as a barrier to lipophilic drugs. Int J Pharm 453:56–64
Murugan K, Choonara YE, Kumar P et al (2015) Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int J Nanomedicine 10:2191
Kumari S, Mg S, Mayor S (2010) Endocytosis unplugged: multiple ways to enter the cell. Cell Res 20:256–275
Xiang S, Tong H, Shi Q et al (2012) Uptake mechanisms of non-viral gene delivery. J Control Release 158:371–378
Ferrati S, Mcconnell KI, Mack AC et al (2014) Cellular communication via nanoparticle-transporting biovesicles. Nanomedicine 9:581–592
Fleischer CC, Payne CK (2014) Nanoparticle–cell interactions: molecular structure of the protein corona and cellular outcomes. Acc Chem Res 47:2651–2659
Panyam J (2002) Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J 16:1217–1226
Aydın A, Sipahi H, Charehsaz M (2012) Nanoparticles toxicity and their routes of exposures. In: Recent advances in novel drug carrier systems. InTechOpen. Edited by Ali Demir Sezer.
Elsaesser A, Howard CV (2012) Toxicology of nanoparticles. Adv Drug Deliv Rev 64:129–137
Gatoo MA, Naseem S, Arfat MY et al (2014) Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int 2014(498420):1–8
Park KH, Chhowalla M, Iqbal Z et al (2003) Single-walled carbon nanotubes are a new class of ion channel blockers. J Biol Chem 278:50212–50216
Hsiao IL, Huang YJ (2011) Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci Total Environ 409:1219–1228
Lippmann M (1990) Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect 88:311–317
Poland CA, Duffin R, Kinloch I et al (2008) Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3:423–428
Bhattacharjee S, Haan LHJD, Evers NM et al (2010) Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part Fibre Toxicol 7:25
Gurr JR, Wang AS, Chen CH et al (2005) Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 213:66–73
Wick P, Manser P, Limbach L et al (2007) The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett 168:121–131
Unfried K, Albrecht C, Klotz LO et al (2007) Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology 1:52–71
Eom H-J, Choi J (2010) p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ Sci Technol 44:8337–8342
Carero ADP, Hoet P, Verschaeve L et al (2001) Genotoxic effects of carbon black particles, diesel exhaust particles, and urban air particulates and their extracts on a human alveolar epithelial cell line (A549) and a human monocytic cell line (THP-1). Environ Mol Mutagen 37:155–163
Dybdahl M, Risom L, Bornholdt J et al (2004) Inflammatory and genotoxic effects of diesel particles in vitro and in vivo. Mutat Res 562:119–131
Wilhelmi V, Fischer U, Weighardt H et al (2013) Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox-and Nrf2-independent manner. PLoS One 8(6):e65704
Naqvi S, Samim M, Abdin M et al (2010) Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine 5:983
Shang W, Nuffer JH, Dordick JS et al (2007) Unfolding of ribonuclease A on silica nanoparticle surfaces. Nano Lett 7:1991–1995
Xia T, Kovochich M, Brant J et al (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807
Li N, Sioutas C, Cho A et al (2002) Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111:455–460
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013:1–15
Pujalté I, Passagne I, Brouillaud B et al (2011) Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part Fibre Toxicol 8:10
Poljak-Blazi M, Jaganjac M, Mustapic M et al (2009) Acute immunomodulatory effects of iron polyisomaltosate in rats. Immunobiology 214:121–128
Park E-J, Choi J, Park Y-K et al (2008) Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 245:90–100
Kim Y-M, Reed W, Wu W et al (2006) Zn 2+−induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am J Phys Lung Cell Mol Phys 290(5):L1028–L1035
Balamurugan K, Rajaram R, Ramasami T et al (2002) Chromium(III)-induced apoptosis of lymphocytes: death decision by ROS and Src-family tyrosine kinases. Free Radic Biol Med 33:1622–1640
Prow TW, Bhutto I, Kim SY et al (2008) Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine 4:340–349
Guo L, Sarris A, Levy M (1989) A safe bioadhesive liposomal formulation for ophthalmic applications. Invest Ophthalmol Vis Sci (Suppl) 29:439
Hackenberg S, Zimmermann F-Z, Scherzed A et al (2011) Repetitive exposure to zinc oxide nanoparticles induces dna damage in human nasal mucosa mini organ cultures. Environ Mol Mutagen 52:582–589
Genter MB, Newman NC, Shertzer HG et al (2012) Distribution and systemic effects of intranasally administered 25 nm silver nanoparticles in adult mice. Toxicol Pathol 40(7):1004–1013
Smistad G, Jacobsen J, Sande SA (2007) Multivariate toxicity screening of liposomal formulations on a human buccal cell line. Int J Pharm 330(1):14–22
Klemetsrud T, Kjoniksen AL, Hiorth M et al (2016) Polymer coated liposomes for use in the oral cavity–a study of the in vitro toxicity, effect on cell permeability and interaction with mucin. J Liposome Res:1–12
Teubl BJ, Leitinger G, Schneider M et al (2015) The buccal mucosa as a route for TiO2 nanoparticle uptake. Nanotoxicology 9(2):253–261
Li JJ, Muralikrishnan S, Ng CT et al (2010) Nanoparticle-induced pulmonary toxicity. Exp Biol Med 235(9):1025–1033
Magaye R, Zhao J, Bowman L et al (2012) Genotoxicity and carcinogenicity of cobalt-, nickel-and copper-based nanoparticles. Exp Ther Med 4(4):551–561
Worthington KL, Adamcakova-Dodd A, Wongrakpanich A et al (2013) Chitosan coating of copper nanoparticles reduces in vitro toxicity and increases inflammation in the lung. Nanotechnology 24(39):395101
Salvi SS, Nordenhall C, Blomberg A et al (2000) Acute exposure to diesel exhaust increases IL-8 and GRO-α production in healthy human airways. Am J Respir Crit Care Med 161(2):550–557
Sadeghi L, Yousefi BV, Espanani H (2015) Toxic effects of the Fe2O3 nanoparticles on the liver and lung tissue. Bratisl Lek Listy 116(6):373–378
Muller J, Huaux F, Moreau N et al (2005) Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol 207(3):221–231
Sung JH, Ji JH, Yoon JU et al (2008) Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal Toxicol 20(6):567–574
Seiffert J, Hussain F, Wiegman C et al (2015) Pulmonary toxicity of instilled silver nanoparticles: influence of size, coating and rat strain. PLoS One 10(3):e0119726
Liu R, Yin L, Pu Y et al (2009) Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intra-tracheal instillation in rats. Prog Nat Sci 19(5):573–579
Cao Z, Fang Y, Lu Y et al (2016) Exposure to nickel oxide nanoparticles induces pulmonary inflammation through NLRP3 inflammasome activation in rats. Int J Nanomedicine 11:3331
Morimoto Y, Izumi H, Yoshiura Y et al (2015) Pulmonary toxicity of well-dispersed cerium oxide nanoparticles following intratracheal instillation and inhalation. J Nanopart Res 17(11):442
Zhang H, Ji Z, Xia T et al (2012) Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6:4349–4368
Konduru N, Keller J, Ma-Hock L et al (2014) Biokinetics and effects of barium sulfate nanoparticles. Part Fibre Toxicol 11(1):55
Rossi EM, Pylkkänen L, Koivisto AJ et al (2009) Airway exposure to silica-coated TiO2 nanoparticles induces pulmonary neutrophilia in mice. Toxicol Sci 113(2):422–433
Bonvallot V, Baeza-Squiban A, Baulig A et al (2001) Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol 25(4):515–521
Tamaoki J, Isono K, Takeyama K et al (2004) Ultrafine carbon black particles stimulate proliferation of human airway epithelium via EGF receptor-mediated signaling pathway. Am J Phys Lung Cell Mol Phys 287(6):L1127–L1133
Lin W, Huang YW, Zhou X-D et al (2006) In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol 217(3):252–259
Ahamed M, Akhtar MJ, Siddiqui MA et al (2011) Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 283(2):101–108
Fahmy B, Cormier SA (2009) Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol in Vitro 23(7):1365–1371
Ahamed M, Siddiqui MA, Akhtar MJ et al (2010) Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem Biophys Res Commun 396(2):578–583
Arul Prakash F, Dushendra Babu G, Lavanya M et al (2011) Toxicity studies of aluminium oxide nanoparticles in cell lines. Int J Nanotechnol Appl 5(2):99–107
Phillips DM, Zacharopoulos VR (1998) Nonoxynol-9 enhances rectal infection by herpes simplex virus in mice. Contraception 57(5):341–348
das Neves J, Araujo F, Andrade F et al (2013) In vitro and ex vivo evaluation of polymeric nanoparticles for vaginal and rectal delivery of the anti-HIV drug dapivirine. Mol Pharm 10(7):2793–2807
Chen D, Yang Z (2015) Tissue toxicity following the vaginal administration of nanosilver particles in rabbits. Regen Biomater 2(4):261–265
Ensign LM, Tang BC, Wang YY et al (2012) Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med 4(138):138ra179–138ra179
Yang M, Yu T, Wang YY et al (2014) Vaginal delivery of paclitaxel via nanoparticles with non-mucoadhesive surfaces suppresses cervical tumor growth. Adv Healthcare Mat 3(7):1044–1052
Jallouk AP, Moley KH, Omurtag K et al (2014) Nanoparticle incorporation of melittin reduces sperm and vaginal epithelium cytotoxicity. PLoS One 9(4):e95411
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Talkar, S., Dhoble, S., Majumdar, A., Patravale, V. (2018). Transmucosal Nanoparticles: Toxicological Overview. In: Saquib, Q., Faisal, M., Al-Khedhairy, A., Alatar, A. (eds) Cellular and Molecular Toxicology of Nanoparticles. Advances in Experimental Medicine and Biology, vol 1048. Springer, Cham. https://doi.org/10.1007/978-3-319-72041-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-72041-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72040-1
Online ISBN: 978-3-319-72041-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)