Abstract
Compression of the left common iliac vein by the overlying right common iliac artery has become an increasingly common finding in the era of advanced imaging modalities, albeit with a benign course in most individuals. However, patients with significant vein compression are at risk of developing May-Thurner syndrome, defined as unilateral left leg swelling or acute iliofemoral deep venous thrombosis in conjunction with this venous compression. The development of symptoms is likely due to a combination of venous outflow obstruction and chronic intraluminal damage to the affected vein. Although identifiable by less invasive imaging, pathologic compression of the left iliac vein is best identified by venography with intravascular ultrasound, which has become the gold standard for diagnosis and is useful in guiding therapy. Iliocaval stenting has become the standard treatment for this condition and is associated with excellent patency rates and clinical outcomes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- May-Thurner syndrome
- Intraluminal webs
- Non-thrombotic May-Thurner syndrome
- Chronic venous occlusive disease
- Iliocaval stenting
- Catheter-directed thrombolysis
- Pharmacomechanical thrombectomy
-
1.
May-Thurner syndrome should be suspected in patients with unilateral venous symptoms specially left sided and with a negative venous ultrasound study.
-
2.
Intravascular ultrasound is the modality of choice to diagnose and guide the treatment of MTS.
-
3.
Iliac vein stenting is effective treatment for MTS with patency of 80–90% up to 5 years. Non-thrombotic lesions have superior patency compared to thrombotic lesions.
Introduction
Since its original description in the seminal 1957 manuscript by May and Thurner, May-Thurner syndrome (MTS) has become increasingly recognized as a pathophysiologic variant of normal anatomy in which the left common iliac vein and caval confluence are compressed by the overlying aortic bifurcation and right common iliac artery, leading to physiologic venous outflow obstruction, intraluminal venous wall abnormalities, and ultimately deep venous thrombosis due to these aberrations. While venous compression of the left common iliac vein is commonly seen as an incidental finding on contemporary axial imaging, most persons with this compression are asymptomatic and only a small percentage go on to develop symptoms associated with MTS. Of those that are affected, however, the symptoms can be significant and range from unilateral leg swelling to iliofemoral deep venous thrombosis . Both recognition of this entity and options for treatment have expanded in recent years, largely due to the role of endovascular therapy, which has virtually replaced open surgical options for this uncommon clinical entity .
Historical Perspective
In their landmark manuscript in 1957, Robert May and Josef Thurner not only confirmed earlier observations that lower extremity deep venous thrombosis occurred with a “sinistral,” or left-sided, predominance but also offered pathologic basis for this clinically observed phenomenon [1]. Over 100 years before this report, Virchow had first observed and reported that iliofemoral DVT was five times more likely to occur in the left leg than the right [2]. Additionally, a prior report by McMurrich in 1908 had described the presence of intraluminal webs in the iliac veins, noting them in 33 of 107 unselected autopsy specimens, and suggested this finding as an etiologic factor in deep venous thrombosis [3]. In “The Cause of the Predominantly Sinistral Occurrence of Thrombosis of the Pelvic Veins,” May and Thurner attributed this risk of left-sided laterality of deep venous thrombosis to “venous spurs” which resulted to as an inflammatory response to the chronic overlying pulsations from the right common iliac artery [1]. In 1967, Cockett further validated and expanded upon the findings in May and Thurner’s original manuscript with the observation that 65% of patients with iliofemoral deep venous thrombosis (DVT) had evidence of left common iliac vein (LCIV) compression on venography and that the left leg was affected in 83% of unilateral cases [4]. Importantly, he also noted multiple anatomical variations that accounted for compression of the LCIV, vena cava, left external iliac vein, and right common iliac vein—clinical entities that are even less common than May-Thurner syndrome but are being increasingly recognized due to modern imaging techniques. Most of these variations, as well as May-Thurner syndrome itself, remained poorly recognized and largely untreated for many decades, until contemporary imaging techniques demonstrated these findings with increasing frequency and endovascular therapy began offering effective treatment associated with minimal morbidity.
Prevalence
The incidence of patients presenting with unilateral leg swelling due to May-Thurner syndrome in the general population is unknown, in large part due to the uncommon nature of this condition. However, based on axial imaging of normal individuals or patients undergoing imaging for nonvascular reasons, there is growing evidence that asymptomatic compression of the left common iliac vein is quite common. In a study of consecutive patients undergoing computed tomography scans for non-venous complaints, left common iliac vein compression resulting in at least 25% luminal compromise was found to occur in 66%, while greater than 50% compression was seen in 24% [5].
Considering the purported causative link between deep venous thrombosis and iliac venous compression or intraluminal webs and spurs, it is not surprising that patients with left iliofemoral deep venous thrombosis have a relatively high incidence of left iliac venous compression consistent with a diagnosis of May-Thurner syndrome [4, 6]. The true incidence of LCIV compression in iliofemoral DVT is likely underreported, as most patients with acute deep venous thrombosis historically have had this diagnosis confirmed by duplex ultrasonography without other imaging modalities. This notion is supported by at least one study of patients with left iliofemoral DVT, in which those patients who had more extensive imaging than duplex ultrasound alone were frequently found to have LCIV stenosis, occurring in up to 55% of cases [6]. Because venous compression syndromes can exist in both genders and all age groups, patients with extensive iliofemoral deep venous thrombosis, especially those with unprovoked thrombosis, generally warrant pelvic imaging to assess for the possibility of correctable causes of the deep venous thrombosis and rule out lesions putting them at heightened risk of recurrent thromboembolic events.
In addition to playing a direct role in unilateral left leg swelling and acute left iliofemoral deep venous thrombosis, iliac venous compression likely has an important role in the heterogenous group of patients with chronic venous disease. In a recent large series of 1000 patients with venous disease of all CEAP classifications who underwent intravascular ultrasound (IVUS) imaging, non-thrombotic compression of the common iliac vein was noted in 53% of cases and postthrombotic stenosis in 40% [7]. When looking specifically at patients with symptoms and physical exam findings consistent with postthrombotic syndrome in conjunction with deep and superficial axial vein reflux, there appears to be a high likelihood of iliac vein compression, and correction of this compression and other postthrombotic lesions may allow for resolution of symptoms of chronic venous disease. In 2010, Raju and colleagues reported a series of 504 patients with chronic venous insufficiency suffering from lipodermatosclerosis and venous ulcers who underwent iliac vein stenting for either iliac vein compression or postthrombotic lesions without concomitant treatment of superficial axial vein reflux. With iliac stenting alone, the ulcer healing rate and freedom from ulcer recurrence at 5 years in CEAP class 5 limbs were 54% and 88%, respectively, and symptom improvement occurred in 55% of patients presenting with leg swelling [8]. These data suggest that while the classic presentation of MTS with unilateral left leg swelling is an uncommon clinical entity, pathologic compression of the left common iliac vein occurs frequently across a wide spectrum of venous disease.
Pathophysiology
Although there are several anatomic variants of May-Thurner syndrome, this condition is traditionally defined as compression of the left common iliac vein against the fifth lumbar vertebral body by the right common iliac artery as the artery crosses in front of the vein. The exact point of maximal compression can vary from patient to patient, sometimes affecting solely the left common iliac vein but other times leading to significant compression of the caval confluence as well.
In addition to the venous flow abnormalities intuitively attributed to the significant compression of these structures, chronic compression can also lead to structural changes within the vein. Chronic pulsation of the artery is thought to cause a significant inflammatory response within the vein, ultimately leading to elastin and collagen deposition intraluminally and resulting in intimal fibrosis and the formation of venous spurs and webs as originally described by May, Thurner, and McMurrich [1, 3]. These pathologic changes can ultimately result in significant luminal narrowing leading to development of unilateral chronic leg swelling and contributing to venous thrombosis. External compression of the iliac vein is therefore a ubiquitous finding but not the only contributing factor.
Still, the clinical significance of MTS in chronic venous disease remains a matter of debate, as up to 50% of asymptomatic patients have findings of LCIV compression on axial imaging or IVUS. What makes these lesions symptomatic in some and silent in others is not well understood. MTS may be thought of as a “permissive” condition that predisposes a person to thrombosis when a “second hit” occurs, such as initiation of oral contraceptives, prolonged immobility, malignancy, or hypercoagulable state. The presence of intraluminal webs and external compression may contribute to increased morbidity from conditions such as new distal DVT, heart failure, saphenous vein valvular incompetence, cellulitis, and lymphedema. Recognition of the role that venous compression syndromes can play in any patient with leg swelling or iliofemoral DVT should therefore not be neglected.
Additional anatomic variants can produce compression at the IVC, right common iliac vein, and left external iliac vein by the right common iliac artery or left hypogastric artery as it crosses over the vein into the pelvis. Similarly, the right hypogastric artery can sometimes lead to pathologic venous compression of the right external iliac vein by the same mechanism.
Clinical Presentation
Previously considered to be primarily a disease of women and isolated to the left leg, more recent studies have found that venous compression syndromes occur in both men and women and can also involve the right leg. In one study of asymptomatic individuals, venous compressive lesions were found as frequently in men, but women were found to have higher degrees of stenosis [5]. In a large modern series of symptomatic patients with MTS without reflux, the female to male ratio was 4.7:1, with a left to right preponderance of 3:1 [9].
May-Thurner syndrome most commonly presents in the second to fourth decade of life and may manifest with either of two classic presentations: (1) unilateral leg swelling or (2) acute iliofemoral deep venous thrombosis. Roughly half of patients treated at our institution presented with chronic unilateral leg swelling. The severity of this swelling can range considerably, from barely noticeable asymmetry due to trace left leg edema to severe swelling involving the entire lower leg and thigh. Most patients presenting with unilateral leg swelling report a duration of symptoms of many months, but on careful questioning, many of these patients have been aware of asymmetry between their right and left legs for years, even noting lifelong differences in the way their shoes fit on the left and right feet. The degree of disability these symptoms cause is also quite variable, with some patients noting only cosmetic concerns but most reporting chronic symptoms of heaviness, aching, and vague discomfort that is typical of patients with venous reflux as well. In severe cases the swelling is associated with venous claudication, in which the leg becomes full or tight with exercise due to the venous outflow obstruction and resultant engorgement of left leg veins. Most patients will report the need to elevate the affected leg periodically to relieve symptoms, and many have previously been prescribed compression therapy at some point in their lives, but seldom have they complied with a full trial of daily stocking usage in our experience.
May-Thurner patients who present with acute deep venous iliofemoral thrombosis also tend to present between the second and fourth decade of life but also can present at older ages, especially in conjunction with another prothrombotic risk factor, such as oral contraceptive use or a period of prolonged immobility. In the acute presentation, accounting for between 18 and 49% of cases, patients typically present with sudden onset of leg pain, swelling, and edema [10], and the diagnosis of deep venous thrombosis is typically confirmed by duplex ultrasound. While the deep venous thrombosis in these cases can be isolated to the iliac venous system, most commonly patients with thrombotic MTS suffer relatively extensive deep venous thrombosis involving the iliac and femoral segments, and symptoms seem to be more severe than patients presenting with acute deep venous thrombosis in the absence of compression syndromes. Because disease occurs as a result of compression against the lumbar vertebrae, patients with scoliosis and dilated perimedullary veins should be suspected of having MTS [11]. Spontaneous iliac vein rupture and retroperitoneal hematoma have also been reported as a rare but life-threatening acute presentation of MTS.
While the classic presentation of May-Thurner syndrome includes either unilateral leg swelling or acute left iliofemoral deep venous thrombosis, there is growing evidence that a certain percentage of patients with chronic venous occlusive disease and postthrombotic syndrome are patients whose initial thrombotic event was due to May-Thurner compression of the iliac vein, and this diagnosis had gone unrecognized at the time. These patients may exhibit a range of clinical findings, including chronic leg pain and other symptoms associated with deep system valvular incompetence, varicose veins, recurrent superficial vein thrombophlebitis, lower leg hyperpigmentation, lipodermatosclerosis, and even venous ulceration (which usually localize to the “gaiter” distribution above the medial malleolus). While the contribution of May-Thurner compression of the left common iliac vein in these patients was previously thought to be insignificant, modern imaging and endovascular treatment options have led to an increased recognition of this diagnosis in this patient population. Patients with these conditions, especially when unilateral, should therefore undergo evaluation of the iliac system to assess for the contribution of these compression syndromes to their chronic venous disease.
Diagnostic Imaging
Appropriate diagnostic testing should be considered in all patients with unilateral leg swelling and/or unilateral DVT as routine history and physical exam cannot rule out May-Thurner pathology. Upon initial evaluation of patients presenting with unilateral leg swelling, duplex ultrasonography is also performed to rule out deep venous thrombosis, superficial or deep venous reflux, and other venous pathology. Ultrasound is significantly limited in the case of identifying iliac venous pathology, as the position of iliac veins deep in the pelvis makes them difficult to adequately visualize in most patients. However, evaluation of venous waveforms, including comparison between the right and left common femoral waveforms, can provide evidence of unilateral venous outflow obstruction. In cases of severe compression or occlusion of the left common iliac vein, continuous waveforms without respiratory phasicity can often be appreciated.
Axial computed tomographic venography (CTV) or magnetic resonance venography (MRV) is highly sensitive and specific for detecting venous compression syndromes and pelvic or lower extremity deep venous thrombosis. They also have the advantage of being noninvasive and operator independent. These modalities can rule out other intra-abdominal or pelvic pathology, such as malignant compression of the venous system, and can delineate congenital venous abnormalities that can mimic May-Thurner syndrome. These modalities are widely available and, in the case of MR venography, can be performed without the need for ionizing radiation. However, the static nature of these imaging techniques does not allow for assessment of the physiologic impact of iliac vein compression because they do not show real-time flow patterns.
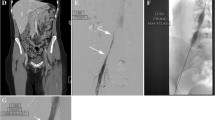
The authors therefore favor the use of detailed contrast venography in conjunction with intravascular ultrasound (IVUS) to reliably identify abnormal venous flow patterns and precisely pinpoint the location and degree of maximal compression. Venography in only an anteroposterior view will show flattening and widening of the left CIV but will not demonstrate an actual narrowing of the vein in the majority of cases. Therefore, venography in the left anterior oblique and cranial caudal views is necessary to visualize the narrowing. Venogram findings that suggest a pathological degree of compression of the left common iliac vein are those that suggest outflow obstruction at the level of the junction between the left common iliac vein and the caval confluence and include (1) contrast stagnation in the left iliac venous system compared to the right, (2) contralateral cross-filling to the right iliac venous system via hypogastric collateral system (Fig. 35.1), and (3) extensive retroperitoneal and pelvic collateralization (Fig. 35.2). Any of these findings suggest a pathophysiologic degree of compression of the left common iliac vein and tend to predict successful improvement or complete amelioration of symptoms following stenting. The best determinant of the degree of compression of the vein by the overlying artery is provided by IVUS, which allows for intraluminal measurement of vein diameters and cross-sectional area and allows for demonstrating the precise location of the area of maximal compression, and thus is helpful at guiding accurate stent placement. Some have proposed stenting of the left common iliac vein based on a finding of >50% reduction in cross-sectional area at the region of compression, although we believe optimal assurance of improvement with stenting occurs in patients with both this cross-sectional area reduction and the venogram findings described above. Venography and IVUS typically require only local anesthesia and should be done safely on an outpatient basis.
Pelvic venogram demonstrating brisk filling and washout on the right side but slow flow on the left related to compression (A—yellow rectangle). Contrast in the left iliac veins fills retrograde into paravertebral ascending network of veins (B). There is persistent contrast in the left CIV that is slowly washing through the collaterals back into the IVC beyond the lesion, while contrast washed immediately from the right iliac veins into the IVC (C)
Treatment
While MTS and its sequelae were historically treated with open surgical bypass procedures (albeit rarely), the current treatment choice is minimally invasive, venography- and IVUS-guided, endovascular stenting. Beginning with the development of lytic techniques in the early 1990s, the use of catheter-directed thrombolysis and venography for the treatment of iliofemoral DVT frequently identified compression of the left CIV and simultaneously offered the ability to treat the underlying conditions with iliocaval stenting. With the widespread adoption of advanced endovascular techniques, iliac vein stenting for symptomatic MTS leading to acute iliofemoral deep venous thrombosis, as well as a host of other chronic venous occlusive lesions , has now become standard practice in most centers.
Any treatment should be preceded by thorough history and physical exam that subsequently guides appropriate diagnostic imaging. Hypercoagulable workup should be done when any underlying coagulopathy may be suspected, as genetic thrombophilias can dictate anticoagulation management in some patients undergoing stenting for MTS.
For those patients with non-thrombotic MTS presenting with symptoms of mild unilateral left leg swelling, a trial period of daily compression stocking use, exercise program, weight loss, and other conservative measures is appropriate and should be considered first-line therapy after initial evaluation with duplex ultrasonography. Daily use of stockings can control many of the symptoms and may eliminate the need for stent implantation in compliant patients. Patients with severe or debilitating symptoms, or those that have failed a trial of compression therapy, should undergo venography and IVUS assessment of the iliac venous system, often with the intention of stenting at the same setting if appropriate.
Venography and IVUS can be performed under local anesthesia in an outpatient setting, although conscious sedation can be helpful if stenting is planned due to the associated back pain that often accompanies stent placement . Access for therapeutic interventions is guided by duplex ultrasound, which is used to identify a non-diseased segment of femoral or common femoral vein. For non-thrombotic MTS with unilateral leg swelling, antegrade duplex-guided access is generally performed at the common femoral vein, although patients with previously unrecognized deep venous thrombosis may have common femoral vein or femoral vein scarring that necessitates puncture of the femoral vein at the mid-thigh level or popliteal level. In anticipation of stent implantation, the puncture should be done below all diseased segments to allow for stenting of all diseased segments of vein, including across the inguinal ligament if necessary.
Initial venograms are obtained after venous access is achieved, observing for the venogram findings described above, including iliac vein contrast stagnation, extensive collateralization, and contralateral cross-filling to the right iliac circulation. Our practice includes selective catheterization of the contralateral right iliofemoral venous system via left femoral access, followed by simultaneous contrast injection within the diagnostic catheter in the right iliac circulation and the left femoral sheath to compare flow patterns bilaterally (Fig. 35.3). Next, wire access is established across the left common iliac vein into the inferior vena cava, and IVUS is used to assess cross-sectional area of the left iliac system and to identify the point of maximal compression of the left common iliac vein. If proceeding with stent implantation, the sheath is upsized to an appropriately large sheath for stent delivery (generally 10Fr for braided stainless steel stents), and the patient is anticoagulated with 100 units/kg of intravenous heparin.
Diagnostic venogram technique for evaluation asymmetry in venous flow patterns between the right and left iliac veins. Left common femoral access with selective cannulation of the contralateral right iliac system (A) allows comparison of flow rates by simultaneous contrast injection through the catheter in the right iliac system (A) and the left femoral sheath (B). While there is some flattening and widening of the left common iliac vein (B, red arrows) consistent with compression of the vein, there were symmetric flow patterns in both iliac systems and absence of significant pelvic collaterals in this patient
Choosing the proper stent size is based upon IVUS diameter measurements of the compressed vein and also the proximal vein segment, which is an important anchor point for the stent. Oversizing 10–20% is appropriate, and undersizing should be avoided as it may lead to stent migration or embolization. In patients with isolated compression of the distal left CIV and no evidence of postthrombotic scarring, stents are placed from the normal-appearing proximal segment of the left common iliac vein to the caval confluence. It is critical to extend the stent at least 1–2 cm beyond the point of maximal compression, as determined by IVUS. This generally includes extension of the stent into the inferior vena cava by at least 1 cm, which is rarely of any consequence to the flow through the right iliac system. Our group exclusively uses braided stainless steel stents, typically in diameters of 16–20 mm, for iliac vein stenting. These stents perform well in this location but have reduced radial force at the ends, thus the requirement to extend the stent into the vena cava (Fig. 35.4). While there are self-expanding nitinol stents specifically designed for venous stenting under investigation in the USA, these are not yet commercially available. In patients found to have postthrombotic scarring of portions of the iliac or common femoral veins, it is generally recommended that all diseased areas be stented to avoid stent thrombosis due to poor venous blood flow. Following stent implantation, balloon angioplasty is used to help stent expansion and achieve adequate wall apposition (Fig. 35.5). Completion venography and IVUS imaging should be performed subsequently to evaluate luminal gain and stent apposition to the vein wall (Fig. 35.6).
In thrombotic May-Thurner patients presenting with acute iliofemoral DVT, lysis or pharmacomechanical thrombectomy is required prior to treatment of the underlying venous compression pathology. Venous access in these cases is typically via the popliteal vein with the patient in the prone position to access the venous system below the lower extent of the thrombus burden. For patients with acute deep venous thrombosis and symptoms of less than 1-week duration, an attempt of a single-session clearance of the thrombus with pharmacomechanical thrombectomy is reasonable, generally utilizing the AngioJet system (Boston Scientific, Minneapolis, MN) in the “power pulse” mode in which the thrombus is laced with 10 mg of tissue plasminogen activator, followed by aspiration of the lysed thrombus after a 10–20-min dwell time. For patients with a longer interval between initial symptom onset and treatment, we have noted less success with single-session thrombus clearance attempts and therefore recommend overnight catheter-directed thrombolysis , typically at tissue plasminogen activator drip rates of 0.5–1.0 mg/h. Following clearance of thrombus, venographic and IVUS evaluation for May-Thurner compression of the left common iliac vein is nearly identical to that discussed for non-thrombotic May-Thurner patients , with the caveat that these patients are more likely to have additional postthrombotic occlusive lesions, and these lesions from the common femoral vein (even below the inguinal ligament) up to the caval confluence should be stented if they are flow limiting (Figs. 35.7 and 35.8).
Anticoagulation with heparin is performed intra-procedurally, with activated clotting times (ACT) of 250–300 desired prior to stent implantation. Postoperatively, aspirin 81 mg daily and clopidogrel 75 mg daily are prescribed to all stented patients for a period of 3 months, at which point we favor single antiplatelet therapy with aspirin alone. In patients with a history of hypercoagulable state or those treated with lysis for acute DVT before correction of the MTS lesions, appropriate systemic anticoagulation is continued according to national guidelines. Oral opioid analgesics and muscle relaxants are prescribed perioperatively for pain control, as oftentimes patients complain of lower back pain within the first 1–2 weeks after stent implantation.
Perioperative complications are infrequent , with the most common being back pain that can be managed with oral analgesics and muscle relaxants as noted above. Access site complications, including puncture site hematomas, may occur in the setting of full anticoagulation and antiplatelet therapy, although these are uncommon complications that require no treatment in most cases. The rate of complications has been reported to be as low as 0.3% in large series of iliac vein stenting [12].
Post-intervention, patients are imaged with duplex US to confirm patency of the iliac venous system within 2 weeks, as our experience suggests that patients that lose patency tend to do so in the early postoperative period due to technical factors, and if these are identified early, the patient can undergo lysis and correction of the inciting issue. Thereafter, patients are followed at 6 months and then yearly with duplex ultrasonography and assessment of residual symptoms. Patients generally experience significant improvement shortly after stenting, but ongoing clinical improvement can continue to be seen for up to 1 year.
Outcomes Following Iliac Vein Stenting for May-Thurner Syndrome
The paradigm shift toward endovascular treatment of May-Turner patients is now a decade and a half old, with a growing library of evidence to support not only acute endovascular thrombus clearance strategies in thrombotic May-Thurner syndrome but also the effectiveness of stenting as a definitive treatment of iliofemoral compression.
Raju and Neglen have published widely on iliofemoral stenting for a broad range of obstructive venous lesions, beginning with a publication on their early experience in 2000, establishing the technique’s safety and good short-term outcomes. The study of 77 patients showed a technical success rate of 97% and primary and secondary patency rates of 82% and 92%, respectively, at 1 year [13]. Followed further to an average of 30 months by the same group, a cohort of 610 limbs stented for nonmalignant obstructive lesions of the iliofemoral and caval venous system had an overall primary patency rate of 67%, assisted primary patency rate of 89%, and secondary cumulative primary patency rate of 93% at 6 years [12].
In congruence with the publication of these encouraging results, Baron and colleagues first described iliac vein stenting as a safe and effective method of treating May-Thurner syndrome specifically [14]. Since that time, multiple studies have demonstrated the efficacy of endovascular intervention in May-Thurner syndrome, establishing percutaneous stenting as the primary mode of treatment for these patients. A series of 36 patients undergoing iliac venous stenting from the Cleveland Clinic in 2011, in fact, showed that patients stented for a diagnosis of May-Thurner syndrome had higher patency rates than those stented for malignant compression, thrombophilia, or other causes of iliac vein disease. In the 15 patients for whom May-Thurner syndrome was identified as the etiology of iliac vein obstruction, there was 100% primary patency at 24 months compared to 78% for the entire group [15].
A relatively large retrospective review from the University of Pittsburg again demonstrated favorable stent patency rates and further demonstrated persistent symptom resolution in May-Thurner syndrome patients treated endovascularly. Seventy patients (77 interventions) with May-Thurner syndrome were evaluated as two separate groups: postthrombotic patients (56 interventions) and de novo leg swelling patients without acute DVT (21 interventions). At a median follow-up of approximately 2 years, symptom resolution persisted in 93% of patients in the postthrombotic group and 96% of patients in the de novo leg swelling group . Primary and secondary patency was 91% and 98% at 3 years in postthrombotic patients and 91% and 91% in de novo leg swelling patients. In both groups, patients experienced symptomatic relief that mirrored stent patency, underlying the importance of long-term stent patency for durable treatment [16].
Further analysis of endovascular treatment in cases presenting without acute DVT reveals high technical success rates and few complications; however, permanent symptomatic relief may be variable between patients. A study of 34 patients from the University of Chicago in 2016 showed a 100% technical success rate with no major complications and 100% stent patency at 1 year. Even with these results, only 62% of patients with edema and 88% of patients with pelvic pain experienced clinical improvement. Two patients with edema, who had initially reported improvement, subsequently returned to their baseline level of swelling within 1 month [17]. Dr. Raju published similar results as part of a larger series, in which 196 patients underwent stenting for chronic venous disease attributed to non-thrombotic May-Thurner physiology . Stent patency at 5 years was 82%, and significant improvement of pain and swelling was 78% and 55%, respectively [8]. While other series have reported higher response rates in patients treated for non-thrombotic May-Thurner syndrome, this data certainly suggests that clinical outcomes can be variable and may be dependent on patient presentation and symptom chronicity, although the overall results of endovascular treatment of May-Thurner syndrome appear quite favorable.
As discussed previously, in the case of thrombotic MTS presenting with acute iliofemoral DVT, patients must first undergo acute thrombus removal. Although no studies have compared catheter-directed thrombolysis (CDT) versus pharmacomechanical thrombectomy (PMT) specifically in the setting of MTS, both techniques have been shown to have similar rates of thrombus removal. In a 2006 study of 98 interventions at Baylor College of Medicine, complete thrombus removal occurred in 70% and 75% of the CDT and PMT groups, respectively. Minor access site complications were observed in two patients in both groups. Notably, CDT was associated with longer ICU stay and greater overall cost to the hospital, and thus PMT may have some potential advantages over CDT, at least in patients whose duration between symptom onset and interventional management is short [18].
Following thrombus removal and stenting in the thrombotic May-Thurner syndrome patients, recent studies demonstrate both long-term stent patency and symptom relief. A 2014 study of 61 patients showed one 1- and 6-month and 1-, 2-, 3-, and 5-year primary patency rates to be 96.7%, 95.1%, 91.8%, 90.2%, 88.5%, and 85.2%, respectively. Stent occlusion occurred on average 1 year after the procedure. Although the incidence of postthrombotic syndrome was 11.5%, no patients reported venous claudication, discoloration, varicosities, or ulcerations, and only 4 (6.5%) patients reported persistent limb swelling [19]. Similar results were reported in a series of 51 patients who underwent thrombolysis and iliac vein stenting following acute DVT due to MTS. Primary patency was 84.3% after 2 years, and at a median follow-up of 16 months, only 8% of patients had recurrent thrombotic occlusions [20]. Postthrombotic syndrome and recurrence of thrombo-occlusive disease certainly remain a concern in patients treated endovascularly, as recurrent thrombosis may range from 4% to 11% [19,20,21]. However, the great majority of these patients, between 81 and 92%, have complete or partial symptomatic relief of their lower extremity edema [16, 19, 22].
Overall, despite differences in treatment modalities and underlying disease in the published literature, both thrombotic and non-thrombotic patients appear to benefit from high stent patency rates and a high degree of symptom amelioration following iliac vein stenting. A summary of stent patency in the largest studies of endovascular intervention for MTS is presented in Table 35.1 [16, 17, 19,20,21,22,23,24].
When looking at the differences between patients with thrombotic and non-thrombotic May-Thurner syndrome, a few common themes emerge in the published literature. While both groups do well with endovascular treatment, stent patency rates tend to be lower and recurrent thromboembolic events tend to be higher in the thrombotic May-Thurner patients compared to those non-thrombotic May-Thurner patients presenting with leg swelling. Considering that a significant proportion of thrombotic May-Thurner patients have additional contributing factors to their thromboembolic events, this finding is not surprising. Hypercoagulability is not an uncommon diagnosis in this patient population, and it is reasonable to consider that inappropriate management of this risk factor will leave these patients at greater risk of thrombotic complications [15,16,17]. Additionally, patients who suffer a deep venous thrombosis are sometimes left with postthrombotic lesions in the iliocaval and infrainguinal femoral venous circulation, and these residual lesions indicate a larger volume of disease that may portend toward recurrent events and loss of patency.
When looking specifically at complete resolution of leg swelling, data in the literature are somewhat inconsistent but seem to suggest that patients with thrombotic MTS may have a higher likelihood of complete symptom resolution after treatment compared to non-thrombotic patients. In the studies detailed above, between 55% and 62% of non-thrombotic May-Thurner patients presenting with leg swelling had complete resolution of swelling following intervention. Conversely, 81–92% of patients who presented with acute iliofemoral DVT had resolution of their lower extremity swelling. While some of these differences could be explained by differing patient expectations and definitions of success, it is also not surprising that complete resolution of swelling is more likely in patients whose swelling is sudden and related to acute thrombotic occlusion of the iliocaval and femoral veins, compared to the non-thrombotic May-Thurner patients who generally have suffered years of chronic venous hypertension in the affected leg.
One special circumstance worth considering is the pregnant patient with MTS. The displacement of the pelvic anatomy by the gravid uterus during pregnancy can exacerbate iliac vein compression, thus leading to worsening left leg swelling and predisposing to venous thrombosis while simultaneously presenting unique challenges to treatment. Pregnant women with unilateral leg swelling thought to be due to MTS are almost always treated conservatively with stenting deferred until after delivery if swelling persists. On the contrary, pregnant patients with MTS who develop acute deep venous thrombosis obviously require treatment of their DVT, and traditionally these patients are managed with anticoagulation (with fractionated heparin) with or without IVC filter placement, as pregnancy is a relative contraindication to the use of pharmacologic catheter-directed thrombolysis due to the concern for placental abruption. However, these patients are at significant risk for developing postthrombotic sequela and generally tend to be quite symptomatic from their venous thrombosis. Although no large retrospective data has yet been published, there is limited evidence for the safe of use of pharmacomechanical catheter-directed thrombolysis in this patient population [25, 26]. The largest study of 11 patients with extensive iliofemoral DVT and persistent pain and edema on anticoagulation reported 100% successful thrombolysis and rapid clinical improvement without any pregnancy or postpartum-related complications. Thrombolysis was done with a combination of catheter-directed and pharmacomechanical techniques, and patients were not stented until the postpartum period. At a mean follow-up of 1.3 years, 85% of patients had normal Villalta scores [27]. Undoubtedly, further studies are still required to establish the role of thrombolysis and/or stenting in pregnant patients with presentation of thrombotic May-Thurner syndrome.
Conclusion
May-Thurner syndrome is an increasingly recognized clinical entity resulting in chronic unilateral leg swelling in non-thrombotic patients and acute iliofemoral deep venous thrombosis in thrombotic patients. Chronic left common iliac vein compression by the overlying right common iliac artery is the hallmark of this syndrome and is likely a contributing pathologic factor in many patients with chronic venous disease not formally diagnosed with May-Thurner syndrome. Increasing awareness of these conditions, coupled with advancements in endovascular technology and techniques, has allowed for improvement in the quality of life for many patients with this disease process. The published results of iliac venous stenting for MTS are quite favorable, with high stent patency rates and significant symptom resolution in most patients.
References
May R, Thurner J. The cause of the predominately sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419–27.
Virchow R. Uber die Erweiterung kleiner Gefasse. Arch Pathol Anat. 1851;3:427–62.
McMurrich JP. The occurrence of congenital adhesions in the common iliac veins and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci. 1908;135:342–6.
Cockett FB, Thomas ML, Negus D. Iliac vein compression –its relation to iliofemoral thrombosis and the post-thrombotic syndrome. Br Med J. 1967;2:14–9.
Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937–43.
Fraser DG, Moody AR, Martel A, Morgan PS. Re-evaluation of iliac compression syndrome using magnetic resonance imaging in patients with acute deep venous thromboses. J Vasc Surg. 2004;40(4):604–11.
Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136–43; discussion 144. PubMed PMID: 16828437.
Raju S, Darcey R. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51(2):401–8; discussion 408.
Birn J, Vedantham S. May–Thurner syndrome and other obstructive iliac vein lesions: meaning, myth, and mystery. Vasc Med. 2015;20:74–83.
Lee KH, Han H, Kee KJ, Yoon CS, Kim SH, Won JY. Mechanical thrombectomy of acute iliofemoral deep vein thrombosis with use of an arrow-Trerotola percutaneous thrombectomy device. J Vasc Interv Radiol. 2006;17(3):487–95.
Mousa AY, AbuRahma AF. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013;27(7):984–95.
Neglen P, Hollis KC, Oliver J, Raju S. Stenting of the venous outflow in chronic venous disease: long term stent-related outcome, clinical and hemodynamic result. J Vasc Surg. 2007;46(5):979–90.
Neglen P, Raju S. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcomes. J Endovasc Ther. 2000;7(2):79–91.
Baron HC, Shams J, Wayne M. Illiac vein compression syndrome: a new method of treatment. Am Surg. 2000;66(7):653–5.
Titus JM, Moise MA, Bena J, Lyden SP. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53(3):706–12.
Hager ES, You T, Tahara R, Dillavou E. Outcomes of endovascular intervention for May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(3):270–5.
Ahmed O, Ng J, Patel M, Ward T. Endovascular stent placement for May-Thurner syndrome in the absence of acute deep vein thrombosis. J Vasc Interv Radiol. 2016;27(2):167–73.
Lin PH, Zhou W, Dardik A, Mussa F. Catherer-directed thrombolysis versus pharmacomechanical thrombectomy for the treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192(6):782–8.
Xue GH, Hunag XZ, Ye M, Liang M. Catheter-directed thrombolysis and stenting in the treatment of iliac vein compression syndrome with acute iliofemoral deep vein thrombosis: outcome and follow-up. Ann Vasc Surg. 2014;28(4):957–63.
Park JY, Ahn JH, Jeon YS, Cho SG. Iliac vein stenting as a durable option for residual stenosis after catheter-directed thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May-Thurner syndrome. Phlebology. 2014;29(7):461–70.
Zhu QH, Zhou CY, Chen Y, Wang J. Percutaneous manual aspiration thrombectomy followed by stenting for iliac vein compression syndrome with secondary acute isolated iliofemoral deep vein thrombosis: a prospective study of single-session endovascular protocol. Eur J Vasc Endovasc Surg. 2014;47(1):68–74.
Liu Z, Gao N, Shen L, Yang J. Endovascular treatment for symptomatic iliac vein syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg. 2014;28(3):695–704.
Igari K, Kudo T, Toyofuku T, Jibiki M. Surgical thrombectomy and simultaneous stenting for deep venous thrombosis caused by iliac vein compression syndrome (May-Thurner syndrome). Ann Thorac Cardiovasc Surg. 2014;20(6):995–1000.
Goldman RE, Arendt VA, Kothary N, Kuo WT. Endovascular management of May-Thurner syndrome in adolescents: a single-center experience. J Vasc Interv Radiol. 2016.
DeStephano CC, Werner EF, Holly BP, Lessne ML. Diagnosis and management of iliac vein thrombosis in pregnancy resulting from May-Thurner syndrome. J Perinatol. 2014;34(7):566–8.
Bloom AL, Farkas A, Kalish Y, Elchalal U. Pharmacomechanical catheter-directed thrombolysis for pregnancy related iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2015;26(7):992–1000.
Herrera S, Comerota AJ, Thakur S, Sunderji S. Managing iliofemoral deep venous thrombosis of pregnancy with a strategy of thrombus removal is safe and avoids post thrombotic morbidity. J Vasc Surg. 2014;59(2):456–64.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
DeRubertis, B., Patel, R. (2018). May-Thurner Syndrome: Diagnosis and Management. In: Chaar, C. (eds) Current Management of Venous Diseases . Springer, Cham. https://doi.org/10.1007/978-3-319-65226-9_35
Download citation
DOI: https://doi.org/10.1007/978-3-319-65226-9_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65225-2
Online ISBN: 978-3-319-65226-9
eBook Packages: MedicineMedicine (R0)