Abstract
West Africa is the only region in the world where six out of seven mycobacterial lineages of human importance are endemic. In particular, two evolutionary ancient lineages, Mycobacterium africanum West Africa 1 (MTBC Lineage 5) and M. africanum West Africa 2 (MTBC Lineage 6) are of interest as they cause up to 40% of all pulmonary TB cases in some West African countries. Although these M. africanum lineages are closely related to M. tuberculosis sensu stricto lineages, they differ significantly in respect to biology, epidemiology and in their potential to cause disease in humans. Most importantly the M. africanum lineages are exclusive to West Africa. Although the exact mechanisms underlying this geographical restriction are still not understood, it is increasingly suspected that this is due to an adaptation of the bacteria to West African host populations. In this chapter, we summarize the geographical distribution of the M. africanum lineages within the region, describe biological and clinical differences and the consequent implications for TB control in West Africa. We also try to shed light on the geographical restriction, based on recently published analyses on whole genomes of M. africanum isolates.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 General Introduction and History
The pathogen Mycobacterium africanum (Maf) was first isolated from an expectorate of a Senegalese patient with pulmonary tuberculosis (TB), and described by Castets and colleagues (1968) as a member of the tuberculosis causing mycobacteria with phenotypic characteristics that place it in an intermediate position between M. tuberculosis (Mtb) and M. bovis (David et al. 1978). Later, Meissner described similar mycobacterial isolates in Ghana in 1969 (Meissner and Schroder 1969) and related organisms were also isolated in Democratic Republic of Congo and Uganda (Sticht-Groh et al. 1994), and subsequently from other African countries (Haas et al. 1997; Frothingham et al. 1999). However, in contrast to Mtb and M. bovis, Maf strains isolated from the different countries showed higher degrees of variability in their phenotypic attributes (Haas et al. 1997; Kato-Maeda et al. 2001). This heterogeneity of Maf complicated its unequivocal species identification that could have led to the misclassification of clinical strains. Prat and colleagues concluded and speculated that the biochemical heterogeneity observed among Maf strains represents a continuous spectrum linking the classical human and bovine variants of Mtb (Prat et al. 1974). This made other scientists question the wisdom in the separation of the three pathogens into three species (Pattyn et al. 1970), and suggested its designation as subspecies or biovar status using different classification approaches including Adansonian classification (Tsukamura 1967, 1976; David et al. 1978); DNA homology (Bradley 1972; Baess 1979) and immune-diffusion analysis (Stanford and Grange 1974). Grange in UK in 1979 (Grange 1979) therefore described them all as Mtb with designation as human, bovine or African strains, and Tsukamura (1976) was the first to refer to them as part of the ‘M. tuberculosis complex’ (MTBC).
Based on a battery of biochemical assays (Table 6.1), results of which varied with geographical origin, Maf was divided into two major subgroups: Maf subtype I originating from West Africa and Maf subtype II from East Africa (David et al. 1978; Vasconcellos et al. 2010), with Maf subtype I more closely related to M. bovis, whereas subtype II more closely resembling Mtb (Table 6.1). However, based on modern molecular analysis, we now know that Maf constitutes two distinct lineages that branched at an early stage in evolution of the MTBC from the “modern” Mtb lineages common to America, Europe and Asia (see Chap. 1) (Smith et al. 2009; Galagan 2014).
Early comparative genomic analysis also indicated that Maf subtype I strains from West African countries were characterized by the presence of low or medium numbers of IS6110 bands and different spoligotype signatures (absence of spacers 7 through 12 and 37–39) from the subtype II (see Chap. 3) (de Jong et al. 2009; Streicher et al. 2007). Moreover, they showed a characteristic genomic deletion (RD9) and distinct gyrB DNA sequences that permitted their clear distinction from Mtb (Sola et al. 2003; Parsons et al. 2002). RD12 and RD4 are further genomic regions, intact in MAF I yet absent in M. bovis that allow their differentiation. Maf strains from Uganda (subtype II/ East African) on the contrary showed high IS6110 copy numbers, spoligotype pattern similar to that of Mtb (absence of spacers 33 through 36), and identical gyrB sequence, presence of the RD9 region, and the Mtb specific deletion TbD1 (Brosch et al. 2002; Niemann et al. 2002).
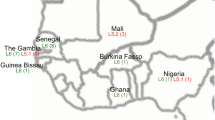
Based on the above studies and others, Maf type II (East African clade) has been reclassified as Mtb genotype “Uganda”, a sublineage of Mtb Lineage 4 (also known as the Euro-American lineage) (Sola et al. 2003; Mostowy et al. 2004). The ‘true’ Maf is therefore Maf subtype 1 (West African clade), which has further been sub-divided into two separate lineages that are genetically diverse; i.e. Maf West African type 1 (Maf1) or Lineage 5, characterized by the deletion of RD711, prevalent around the Eastern part of West Africa (Gulf of Guinea) (Gagneux et al. 2006), and Maf West African type 2 (Maf2) or Lineage 6 characterized by the deletion of RD702, (Gagneux et al. 2006; Mostowy et al. 2004) prevalent in Western West Africa; with countries in the middle harboring both variants. Within the subsequent sections of this chapter, ‘Maf’ will refer only to what was previously known as Maf subtype I including both MTBC Lineage 5 and 6.
6.2 Epidemiology of Maf
6.2.1 Prevalence and Geographical Distribution of Maf in Humans
Though Maf has sporadically been identified in other regions of the world, the pathogen is almost exclusively restricted to West-Africa, where a prevalence of more than 50% among smear-positive TB cases has been indicated in distinct countries and time periods (Huet et al. 1971; Kallenius et al. 1999; de Jong et al. 2010). Maf1 has been isolated as far East as the Democratic Republic of Congo (Hermans-Boveroulle et al. 1965) and used to cause 56% of TB in Cameroon (Huet et al. 1971). Biochemical speciation at that time did not permit distinction between Maf1 and Maf2, although the present distribution suggests that all 56% were Maf1. The distribution of Maf1 and Maf2 overlap in the central part of West Africa or middle of West Africa, particularly Ghana, Benin (Affolabi et al. 2009), Sierra Leone and Ivory Coast (de Jong et al. 2010). Countries outside Western Africa where cases have been occasionally reported include: England (Grange and Yates 1989; Brown et al. 2010), France (Frottier et al. 1990; Grosset et al. 1974), Spain (Perez-de Pedro et al. 2008; Isea-Pena et al. 2012), Germany (Jungbluth et al. 1978; Schroder 1982), Italy (Garzelli et al. 2010), Denmark (Bek et al. 2010), California, USA (Desmond et al. 2004) and Columbia (Hurtado et al. 2016) from both pulmonary and extra-pulmonary sources. While most of the identified TB cases carrying Maf within these reports were African migrants, a few of the studies also identified other nationalities (Schroder 1982; Desmond et al. 2004). For example, the study of Desmond that retrospectively analysed an isolate collection of the California State using spoligotyping and IS6110 restriction length polymorphism, identified 2/5 Maf isolates from a Vietnamese- and a US born African American patient, neither of whom had any travel history to Africa (Desmond et al. 2004), suggesting that transmission had occurred outside of Africa.
The prevalence of Maf estimated by various studies in West Africa showed that actual species and sublineage prevalence varies from country to country, region within a country, as well as period of sampling with figures ranging between 3% and 56%. During a national survey of primary resistance carried out from May 1995 to May 1996 in Côte d’Ivoire, using randomized cluster sputum sampling, 17 Maf were identified among 321 (5.3%) mycobacterial isolates and Maf was isolated from four regional centers (Dosso et al. 1999). In Nigeria, Ibadan (17%) and Nnewi (14%) both showed medium prevalence, while in Abuja, only 8% of all TB was due to Maf1 infection (Lawson et al. 2012). In a countrywide study in Senegal from the 1970s, the prevalence of Maf ranged from 10% in Casamance to 46% near the Senegal river (Diop et al. 1976). Likewise, in the southern sector of Ghana, a prevalence of 20% have been reported as compared to 26% in the north central sector.
There are some indications that Maf probably is being replaced by variants of Mtb. Based on phenotypic identification a study by Huet et al. conducted over 40 years ago, reported that 56% of cases of TB were due to Maf strains in the West and South regions of Cameroon (Huet et al. 1971). A reduced prevalence of 9% was however observed between 1997 and 1998, (Niobe-Eyangoh et al. 2003) and a recent publication in 2013 suggested that Maf1 has almost disappeared from Cameroon (Koro et al. 2013). Of the total 565 MTBC strains analysed, only 19 were identified as Maf, representing 3.3% of MTBC strains. The authors concluded that their findings showed a real regression of Maf compared to the 9% described in the 1990s (P = 0.0001), giving the impression that Maf is gradually being out-competed by the Cameroon sublineage of Lineage 4 Mtb. To confirm that the decline of Maf in Cameroon is not due to identification bias, the study conducted by Niobe-Eyango et al. used the same phenotypic assays as done by Huet et al., as well as molecular methods; both approaches gave almost the same prevalence (9%) of Maf. Similar trends have been reported in Guinea Bissau; in 2011 Groenheit et al. reported a declining prevalence from 52% in 1994 to 38% in 2008 (Groenheit et al. 2011).
In contrast, two recent studies covering several regions of Nigeria identified a high Maf prevalence, ranging from 14% to 33% (Lawson et al. 2012; Thumamo et al. 2012), and detected active foci of recent Maf transmission in 2009–2010 (Lawson et al. 2012), which is consistent with the prevalence of 37% Maf recorded in Benin (Gehre et al. 2013a). Similarly, various studies conducted in Ghana spanning periods of about two decades using either phenotypic or genotypic assays have recorded a prevalence rate of not less than 20% (Lawn et al. 2001; Addo et al. 2007; Yeboah-Manu et al. 2011; Asante-Poku et al. 2015). Also in the Gambia, two independent studies reported in 2008 and 2013 indicated that the prevalence of Maf2 was consistently around 38%. Among isolates from 552 TB cases the prevalence of Maf 2 (n = 223) was 40%, (Gehre et al. 2013a), while the previous study conducted in the same country by de Jong et al. in 2008 recorded a prevalence of 39%. Other country prevalence values and year of report are indicated in Table 6.2. While some researchers have postulated that Maf may be more prevalent in rural West Africa, within our work in Ghana, where both Maf lineages are prevalent we did not observe this. We however, did find statistical association of Maf with an ethnic group (the Ewe ethnic group, one of the native tribes of coastal West Africa) driven by Lineage 5 (Asante-Poku et al. 2015, 2016).
6.2.2 Isolation of Maf from Animals
The phylogenetic proximity of Maf to the animal strains of the MTBC as well as its restricted geographic distribution, gave the indication that the pathogen may have an animal reservoir in West Africa. Different phylogenetic analyses of MTBC strains have always shown Maf, especially Maf2, is closer to animal adapted strains compared to other lineages of Mtb (de Jong et al. 2010). For example an MTBC strain isolated from a wild chimpanzee in Côte d’Ivoire that was shown by comparative genomic and phylogenomic analyses to belong to a new lineage of MTBC was most closely related to Maf2 (i.e. MTBC Lineage 6) and shares 32 SNPs with this lineage (Coscolla et al. 2013). Two other animal-associated members of the MTBC are known to cluster with lineage 6 rather than with the other classical animal-adapted lineages: M. mungi and the dassie bacillus, which infect African mongooses and hyraxes, respectively (Cousins et al. 1994; Alexander et al. 2010). One of the 32 SNPs shared between the chimpanzee bacillus genome and the Maf also occurred in M. mungi or dassie bacillus (Coscolla et al. 2013). Similar to Maf, the dassie bacillus was shown to lack the regions RD7, RD8, RD9, and RD10 (Mostowy et al. 2004). Moreover, the association of Maf2 with HIV infection suggests that the ratio of latent/active Maf2 infection is greater than for Mtb infections, maintenance of which could again indicate an animal reservoir sustaining zoonotic infection in humans.
However so far, no animal reservoir has been identified for Maf based on extensive skin testing and abattoir surveys in Senegal, Gambia, Guinea and Guinea Bissau not identifying mycobacterial infection and lesion (Unger et al. 2003). Nevertheless, Maf has been isolated from diverse animal species (Hart and Sutherland 1977; Thorel 1980a; Alfredsen and Saxegaard 1992). A study conducted in North-Central Nigeria, identified 1/5 Maf mycobacterial isolates from 400 unpasteurized milk (Cadmus et al. 2006), and in Ghana, Asante-Poku et al. identified 1/6 mycobacterial isolates obtained from 17 lesions of a cow carcass by spoligotyping as Maf (Asante-Poku et al. 2014). Other studies that have isolated Maf from cattle have been reported in Bangladesh (Rahim et al. 2007) and Bavaria (Weber et al. 1998). Furthermore, Maf has also been isolated from pigs during an outbreak of TB in pigs and cows in 1992, where lesions were found to be similar to those caused by M. bovis and M. avium (Alfredsen and Saxegaard 1992). Among wild animals, disseminated TB disease was observed in an adult female hyrax (Procavia capensis) imported from United Arab Emirates and held in captivity at a zoo in Croatia, and confirmed by genotyping to be caused by Maf (Gudan et al. 2008). Thorel et al. also identified isolates in the Veterinary Research Centre in France (Thorel 1980b).
6.3 Genomic Structure of Maf
Until recently, mycobacterial lineages were defined based on a phylogenetic classification system that considered the absence or presence of genomic deletions, namely regions of differences (RDs, see Chap. 1) (Tsolaki et al. 2004). These original classification systems revealed that the two Maf lineages, together with the animal strains, belong to a group of evolutionary ancient mycobacterial lineages that share a common deletion of RD9. Maf1 has a further deletion in RD711, while Maf2 can be identified by a deletion of RD702 (de Jong et al. 2005). Using spoligotyping, Maf1 is characterised by a loss of spacers 8–12 and 37–39, while in Maf2 spacers 7–9 and spacer 39 are missing (de Jong et al. 2010). With the increased availability of whole genome sequencing data, lineage defining SNPs for either of the Maf lineages were identified and developed into SNP assays for rapid speciation (Stucki et al. 2012).
Although no in-depth phylogenetic studies were conducted to date, spoligotyping in combination with 12-loci MIRU-VNTR genotyping of Maf1 isolates from several West African countries (Sierra Leone, Nigeria, Benin) suggested the presence of at least ten sub-lienages within the Maf1 lineage, which differ in their capacity to spread within the human population (Gehre et al. 2013a). For Maf2, no comparable data is currently available.
6.4 Genomic Diversity and Implications for Epidemiological Differences Between Maf and Other MTBC Lineages
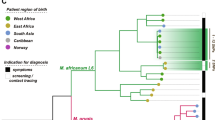
So far, only few whole genome sequencing studies of Maf isolates have been performed (Bentley et al. 2012; Gehre et al. 2013b; Winglee et al. 2016). Besides the reference genome for Maf2 (Bentley et al. 2012) and a study on a limited number of Maf2 isolates (Gehre et al. 2013b), a third sequencing study (the most comprehensive of all) on 24 Maf2 and 2 Maf1 isolates was recently published (Winglee et al. 2016). No reference genome for Maf1 is available at the moment, although efforts are ongoing and the present preliminary findings (Winglee et al. 2016) need to be confirmed in future large-scale studies. For Maf2, all studies described similar key observations. When compared to Mtb genomes, Maf2 is characterised by increased genome erosion due to a larger number of pseudogenes (Bentley et al. 2012). The above-mentioned decrease of Maf prevalence could be due to a replacement of Maf isolates by rapidly spreading Mtb isolates in West Africa. It was speculated that a loss of functional genes in the Maf genome and therefore a loss of fitness of Maf when compared to Mtb may ultimately lead to an inability of Maf to compete with newly introduced Mtb isolates in the region. If this was the case, one would expect that the genetic diversity of rapidly spreading Mtb lineages would be higher than in the slowly vanishing, decreasing Maf populations (Winglee et al. 2016). However, available sequencing data did not find any differences in genetic diversity between Maf and Mtb isolates, and therefore did not detect a genetic bottleneck indicative of a selective sweep that would have occurred during exerted selective pressure on Maf isolates following introduction of Mtb lineages (Winglee et al. 2016). Similarly, no quantitative difference in mutations of virulence genes between the Maf and Mtb lineages was found that could explain a loss of fitness and competitiveness. Therefore, the exact interaction between the various MTBC lineages and the reason for the geographical restriction of Maf to West Africa is still unknown. One suggested scenario based on sequence data was that while Mtb isolates may be able to establish infection independent of host ethnicity, Maf appears to be particularly well adapted to West African hosts. As Winglee et al. described, Mtb is one of a few bacterial species capable of Vitamin B12 synthesis essential for bacterial survival during Vitamin B12 limiting conditions within the host (Winglee et al. 2016). DNA sequence data however revealed that Maf2 isolates lost the capacity to synthesize Vitamin B12. As West Africans are speculated to have higher Vitamin B12 levels than other ethnicities, the auxotroph Maf will still encounter sufficient Vitamin B12 amounts for bacterial survival within these West African hosts, while the lack of available Vitamin B12 in other host ethnicities outside the region limits Maf growth. This hypothesis is supported by a recent publication investigating Maf infections in the US that found a strong association between Maf infection and patients born in West Africa or of West African ancestry (Sharma et al. 2016).
6.5 Growth Requirement, Morphology and Biochemical Characteristics of Maf
Mycobacteriological laboratory methods have traditionally utilized a series of phenotypical tests based on growth rate, colony morphology, growth requirements and other biochemical properties to separate the classical members of the MTBC. In the past, Maf strains were generally identified by default, having initally ruled-out both Mtb and M. bovis by biochemical tests (Hoffner et al. 1993; Grange and Yates 1989). Maf prefers minimal oxygen (microaerophilic) and produces dysgonic colonies compared to the abundant growth (eugonic colonies) exhibited by Mtb. Similar to M. bovis and M. microti, Maf is unable to use glycerol as a sole carbon source, thus to improve isolation of Maf, solid medium are normally supplemented with 0.4–1.0% pyruvate for primary culture; this culture medium has shown a better recovery rate (Stonebrink 1958; Wayne and Kubica 1986), which has been attributed to the lack of pyruvate kinase activity. A SNP in pykA seems to affect the organization at the active site of the pyruvate kinase enzyme, including association with the co-factor (Mg2+) and substrate [ADP/ATP] and phosphoenolpyruvate binding (Munoz and Ponce 2003).
Most Maf isolates also show catalase activity; in one study it was found that all Maf isolates analysed had catalase activity less than or equal to 10 mm (height of bubbles above agar) at 22 °C, and all were negative for catalase activity at 68 °C (Frothingham et al. 1999). Like M. bovis, Maf is nitrate negative, with a variable reaction to accumulation of niacin, between negative and a weak production of niacin. Comparison of the narGHJI promoter revealed that at nucleotide −215 prior to the start codon of narG, Maf has a cytosine residue, compared to Mtb, which carries a thymine residue (Stermann et al. 2003). Furthermore, Maf are susceptible to thiophene-2-carboxylic acid hydrazide (TCH) (5 mg/l) and pyrazinamide (PZA, positive pyrazinamidase activity), although, like Mtb, resistance to PZA can develop, typically reported among multi-drug resistant cases (Frothingham et al. 1999; Homolka et al. 2010). Very importantly, Maf grows more slowly than Mtb. From the first descriptions of Maf, the time to detection on solid medium was known to be longer when compared to Mtb (Grange and Yates 1989; Castets et al. 1968). Gehre et al. compared the growth rates of Mtb and Maf isolates in The Gambia in two liquid culture systems on primary isolation and found Maf to grow significantly more slowly than Mtb (Gehre et al. 2013a). This observation was confirmed in growth experiments under defined laboratory conditions.
6.6 Diagnostic Implications
The two Maf lineages are phylogenetically related yet distinct from the remaining Mtb lineages. Consequently, differences in the bacterial physiology were observed that not only impact on the direct detection of bacteria in the laboratory but also lead to clinical differences that need to be considered during patient diagnosis. As most of culture media and diagnostics were traditionally optimized using Mtb isolates and Mtb infected patients, the sensitivity in detecting Maf is not known for most of the commonly used assays. While clinical researchers in West Africa are slowly progressing in evaluating diagnostic assay performance for Maf2, data on Maf1 is still lacking.
A key first step of laboratory TB diagnostics is microscopy of sputa, with or without Xpert MTB/RIF, and culture on solid or liquid media for patients who need phenotypic drug susceptibility performed. As mentioned above, Maf2 isolates cannot metabolize glycerol and therefore require pyruvate-supplemented media to grow in vitro (Keating et al. 2005). This finding was demonstrated in traditional biochemical growth experiments, and therefore it was recommended to add pyruvate to Loewenstein-Jensen slopes, which should be commonly done in Maf-endemic West African countries. However, the compositions of other solid media such as Kudoh/Ogawa slopes (Palaci et al. 2013; Jaspe et al. 2009; Kothadia and Sengupta 1982) or the recently described MOD9 plates (Asmar et al. 2015) as well as liquid culture systems such as the widely used BactecMGIT960 were never evaluated for their suitability for Maf culture. Similarly, as previous publications described a deficiency of Maf2 in vitamin B metabolism (Bentley et al. 2012; Winglee et al. 2016), non egg-based media (eggs contain vitamin B) supplemented with vitamin B might result in higher yields of Maf. Also, the impact that the preferable microaerophilic growth of Maf has on automated culture systems is not fully understood, as the majority of culture techniques are either under aerobic growth conditions or, in the case of the Bactec MGIT 960, use oxygen depletion as a proxy for bacterial proliferation. As a microaerophilic organism, Maf2 might consume less oxygen during growth.
Another factor that could influence Maf laboratory diagnosis is the pre-defined length of a 42 day incubation period for TB diagnosis of primary isolation from sputa, or a 12 day incubation period for phenotypic drug-susceptibility testing using the Bactec MGIT 960. As the doubling time of Maf2 was described to be significantly longer (24.12 h) compared to that of MTB (20.16 h) (Gehre et al. 2013b), it is conceivable that incubation periods in Maf endemic countries need to be extended. Therefore, in one of the first publications by Castets et al., an extended incubation period of 90 days for isolates from West Africa was already recommended (Castets et al. 1968), yet never implemented in modern diagnostic laboratory algorithms.
To confirm that growing bacteria in positive cultures are indeed members of the MTBC, lateral flow rapid tests are widely used around the world. These rapid tests detect the secreted MPT64 antigen common to all members of the MTBC (Bekmurzayeva et al. 2013; Jiang et al. 2014; Wang et al. 2007). However, unpublished data from The Gambia demonstrated that Maf2 isolates express the mpt64 gene at 2.5-fold lower levels than Mtb. Indeed, during the 10 day test window, recommended by most manufacturers, in which positive MGIT tubes should be processed, the sensitivity of the test in correctly identifying Maf2 isolates as members of the MTBC ranged from only 78% to 84% for day 1 to day 10 after MGIT positivity, respectively. Although no evaluation was performed in Maf1 isolates to date, genomic analysis of the mpt64 gene in Maf1 already demonstrated a lineage-defining nsSNP that could impair the functionality of rapid tests in the Maf1 lineage as well (Gagneux and Small 2007).
To detect whether a patient’s immune system was previously exposed to MTBC bacteria, ELISPOT assays are performed. For these assays, PBMCs are stimulated with various antigens, for instance ESAT-6, and the amount of T-cells responding to ESAT-6 stimulation is assessed. However, when comparing Mtb and Maf2, it was found that the sensitivity of ESAT-6 ELISPOT assay in Maf2 infected patients’ blood was significantly lower than in patients infected with Mtb (de Jong et al. 2006). However, no difference in responsiveness towards the other two ELISPOT antigens PPD and CFP-10 was observed (de Jong et al. 2006). It was concluded that Maf2 induces a somewhat attenuated anti-ESAT-6 immune response, possibly due to impaired ESAT-6 synthesis or secretion, therefore limiting the use of ESAT-6 ELISPOT tests in Maf endemic areas.
Taken together, various examples show that commonly used diagnostics might have a reduced performance when applied to Maf, due to underlying physiological differences of the bacteria. Therefore, it is strongly recommended that all novel diagnostic assays need to be re-evaluated for their performance in correctly detecting Maf isolates, including Maf1.
6.7 Biological Implications
Besides diagnostic implications, a solid understanding of the differences and similarities between Mtb sensu stricto and Maf lineages is essential, as numerous potential drug or vaccine targets are designed to interfere with biological mechanisms identified in Mtb sensu stricto laboratory strains or clinical isolates. However, to assure success of any therapeutic intervention it is imperative that it targets mechanisms equally conserved between Mtb and Maf. Fulfilling this task becomes increasingly more complex, when considering novel research findings on the microbiology and immunology of Maf, which to date have hinted at the distinct niche adaptation by these lineages.
Previous genomic studies already suggested a different lifestyle of Maf from Mtb. For instance, besides the overall gene erosion outlined above, fundamental genetic changes occurred in genes associated with lipid cata– and metabolism in Maf. These include, amongst others, an accumulation of nsSNPs in lipid transporters of the mmpL gene family (Gehre et al. 2013b) as well as frameshift mutations in genes synthesising phenolic glycolipids (Malaga et al. 2008) and genes responsible for the synthesis of sulfolipids, DATs and PATs regulated by a mutated phoP/R system (Broset et al. 2015; Gonzalo-Asensio et al. 2014). Confirming this, the phenotypic lipid profile of Maf dramatically differs from Mtb. It was shown that it is essential for Mtb to adjust its lipid catabolism in response to hypoxic, intracellular survival within the human host cell, as lipids are the major intracellular carbon source in the absence of sugars (Niederweis 2008). A key regulon dosR/Rv0081 was recently described in Mtb to orchestrate the adaptation to hypoxic conditions experienced during anaerobic growth within the human macrophage or granuloma (Galagan et al. 2013). The fact that Maf might already have acquired fundamental genetic changes in lipid metabolism, ion transport and genes essential for intracellular survival (Gehre et al. 2013b) might indicate alternative survival strategies. Unpublished preliminary mRNA expression data from The Gambia shows that the expression of genes belonging to the dosR regulon is significantly downregulated in Maf isolates when compared to Mtb. Although speculation at this point, it is conceivable that Maf does not rely on rapid dosR/Rv0081 switching between aerobic and anaerobic growth to the same degree as Mtb. This could be a consequence of increased adaptation to a more persistent, anaerobic lifestyle and explain the fact that patients infected with Maf progress to disease significantly slower when compared to Mtb (de Jong et al. 2008). Mtb outnumbering Maf in several traditionally Maf-endemic West African countries, as mentioned earlier, may be the consequence of this slower progression of Maf. As an accelerated spread of bacteria within the human population, as observed for Mtb, requires the capacity to rapidly switch between aerobic and anaerobic growth due to faster infection-reinfection cycles between hosts, Maf might have lost its capacity for rapid spread as a sacrifice to its preferred anaerobic growth niche.
Another important virulence factor of Mtb is the early secreted antigen 6 kDa (ESAT-6), implicated in the mycobacterial translocation from the phagolysosome to the cytosol (van der Wel et al. 2007) of the macrophage during intracellular survival. The above mentioned reduced sensitivity of ESAT-6 ELISPOTs for the detection of Maf2 (de Jong et al. 2006) was a first indication of a potential lower overall ESAT-6 synthesis or secretion defect of Maf2 isolates. The exact mechanism is still elusive, and, while one publication attributes the overall reduced ESAT-6 immunogenicity of Maf2 to a relative secretion defect of ESAT-6 in Maf2 when compared to Mtb (Bold et al. 2012), other studies suggest a more complex mechanism that leads to reduced synthesis of the ESAT-6 peptide (Gonzalo-Asensio et al. 2014). For instance, it was demonstrated that ESAT-6 expression is directly linked to the expression of the espACD operon, which itself is regulated by the phoP/R regulatory system (Gonzalo-Asensio et al. 2014). Maf2 isolates possess mutations in the phoR gene, which, when transformed into an Mtb background, will lead to an abolishment of ESAT-6 production and a dramatically altered lipid profile. Despite the presence of this deleterious phoR mutation, ESAT-6 synthesis is not fully ceased in Maf2 indicating the presence of a Maf2-specific compensatory mechanism that restores ESAT-6 synthesis. Although it does not fully restore ESAT-6 secretions to the levels of wildtype Mtb, the rescue mechanism could be associated with a gain of espACD operon function due to the loss of the RD8 region in Maf2 isolates (Gonzalo-Asensio et al. 2014).
As shown, despite the fact that both Maf and Mtb cause TB, they significantly differ, amongst many others, in two crucial virulence mechanisms: the dosR/Rv0081 regulon and phoP/R and ESAT-6 regulation, rendering these pathways less suitable drug or vaccine targets. Therefore, identifying common biological mechanisms between Mtb and Maf isolates is essential for drug and vaccine design. Failing to do so risks selecting for Maf isolates in West Africa by the implementation of drugs/vaccines specific only to Mtb targets, yet the outlined microbiological differences might already reveal potential for vaccine escape variants that could emerge in Mtb isolates in the future, similar to their natural evolution in Maf.
6.8 Clinical Differences Between Maf and Mtb
Unlike its close relative M. bovis, Maf is sensitive to pyrazinamide. As treatment outcomes on regular Category 1 therapy (Cat 1; 2 months of isoniazid, rifampicin, ethambutol and pyrazinamide, followed by 4 months of isoniazid and rifampicin), do not differ between Maf and Mtb patients (de Jong et al. 2007), there is no clinical urgency in diagnosing TB infection as due to Maf vs Mtb. Several studies found lower rates of drug resistance in Maf than in Mtb isolates, and/or an underrepresentation of Maf among retreatment patients, suggesting that Maf may be more effectively treated by Cat 1. Given the complexities of clinical trials for TB, specific regimen shortening studies for Maf infected patients however seem not warranted.
The underrepresentation of Maf among retreatment cases could also be linked to its lower progression to disease, observed in contacts exposed to Maf2 infected index cases. While the skin test conversion rate was the same as in contacts exposed to Mtb, the contacts exposed to Maf2 were significantly less likely to develop TB during the 2-year follow-up period relative to Mtb exposed contacts, suggesting that transmission rates are similar, yet the rate of progression to active disease is lower in Maf2 (de Jong et al. 2008).
Studies from Ghana (unpublished data) and Gambia (de Jong et al. 2005) have identified an association of Maf2 with HIV infection. In Ghana, Maf1 was not associated with HIV infection (unpublished data) and an older study from Ghana, in which Maf1 and Maf2 were analysed together, also did not identify such an association (Meyer et al. 2008). In Gambia, Maf2 is also associated with patients with lower BMIs than Mtb. It is however unclear whether Maf2 preferentially causes disease in underweight persons, or causes more immune stimulation of e.g. TNFa, associated with increased weight loss, or causes more indolent disease for which patients present later, after more weight loss has taken place.
While the frequency of extrapulmonary disease has not been systematically studied in Africa, where diagnostic testing facilities for non-pulmonary TB disease are limited, a recent study from the US, in which MAF1 and MAF2 were analysed together, found Maf to be more common in extrapulmonary disease (Sharma et al. 2016). Whether this is related to the microaerophilic adaptation of Maf is unclear. The extrapulmonary predilection of M. bovis, ascribed to the additional gastro-intestinal route of transmission, is based on similar mechanisms as Maf, also remains to be investigated.
6.9 Future Research Questions
As outlined above, understanding fundamental biological processes in any of the two Maf lineages will inform research about pathogenicity, virulence and drug/vaccine design of the MTBC. Although research on Maf2 is slowly increasing, major efforts need to be made in investigating Maf1 biology. Knowledge about both lineages will allow optimisation of diagnostic algorithms and targeted intervention strategies.
Identifying the various differences of the molecular interactions between Maf and the human host, might give further insights into overall virulence of MTBC bacteria. Similarly, studying the regulatory strategy of Maf2 to compensate for a reduced activity of the dosR/Rv0081 regulon will reveal novel mycobacterial strategies to adjust to microaerophilic growth, persistence and latency. On an epidemiological level, insights into the causes and consequences of the geographical restriction of Maf isolates to certain regions within West Africa will reveal important clues on sub-regional or even intercontinental TB transmission mechanisms. Moreover, the predilection for a weakened host (especially for Maf2, Lineage 6) suggests a relatively larger reservoir of latent infection relative to that of ‘modern’ Mtb. As mycobacterial DNA to date has not been isolated from persons with latent TB infection, and antigens specific for Maf2 relative to Mtb proved insufficiently sensitive nor specific (Hill et al. 2005), the only imperfect proxy for latent infection available to date is whether a person was exposed to an index case with Maf vs Mtb. However, novel phylogenetic modelling techniques may be able to estimate the total mycobacterial population size based on the genomes obtained from patients with active disease, and thus test the size of the relative latent reservoirs (see Chap. 15). Taken together, carefully comparing these ancient West African MTBC lineages with modern Mtb lineages will not only result in optimised vaccine and treatment strategies but also in the design of novel transmission blocking interventions.
References
Addo K, Owusu-Darko K, Yeboah-Manu D, Caulley P, Minamikawa M, Bonsu F, Leinhardt C, Akpedonu P, Ofori-Adjei D (2007) Mycobacterial species causing pulmonary tuberculosis at the Korle-Bu Teaching Hospital, Accra, Ghana. Ghana Med J 41(2):52–57
Affolabi D, Anyo G, Faihun F, Sanoussi N, Shamputa IC, Rigouts L, Kestens L, Anagonou S, Portaels F (2009) First molecular epidemiological study of tuberculosis in Benin. Int J Tuberc Lung Dis 13(3):317–322
Alexander KA, Laver PN, Michel AL, Williams M, van Helden PD, Warren RM, Gey van Pittius NC (2010) Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg Infect Dis 16(8):1296–1299. doi:10.3201/eid1608.100314
Alfredsen S, Saxegaard F (1992) An outbreak of tuberculosis in pigs and cattle caused by Mycobacterium africanum. Vet Rec 131(3):51–53
Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, Blattner W (2013) Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One 8(5):e63170. doi:10.1371/journal.pone.0063170PONE-D-13-06198. [pii]
Asante-Poku A, Aning KG, Boi-Kikimoto B, Yeboah-Manu D (2014) Prevalence of bovine tuberculosis in a dairy cattle farm and a research farm in Ghana. Onderstepoort J Vet Res 81(2):E1–E6
Asante-Poku A, Yeboah-Manu D, Otchere ID, Aboagye SY, Stucki D, Hattendorf J, Borrell S, Feldmann J, Danso E, Gagneux S (2015) Mycobacterium africanum is associated with patient ethnicity in Ghana. PLoS Negl Trop Dis 9(1):e3370. doi:10.1371/journal.pntd.0003370PNTD-D-14-01295. [pii]
Asante-Poku A, Otchere ID, Osei-Wusu S, Sarpong E, Baddoo A, Forson A, Laryea C, Borrell S, Bonsu F, Hattendorf J, Ahorlu C, Koram KA, Gagneux S, Yeboah-Manu D (2016) Molecular epidemiology of Mycobacterium africanum in Ghana. BMC Infect Dis 16(1):385. doi:10.1186/s12879-016-1725-6
Asmar S, Chatellier S, Mirande C, van Belkum A, Canard I, Raoult D, Drancourt M (2015) A novel solid medium for culturing Mycobacterium tuberculosis Isolates from clinical specimens. J Clin Microbiol 53(8):2566–2569. JCM.01149-15 [pii]. doi:10.1128/JCM.01149-15
Assam JPA, Beng Vé P, Cho-Ngwa F, Toukam M, Ngoh AAI, Kitavi M, Nzuki I, Nyonka JN, Tata E, Tedom JC, Skilton RA, Pelle R, Titanji VPK (2013) Mycobacterium tuberculosis is the causative agent of tuberculosis in the southern ecological zones of Cameroon, as shown by genetic analysis. BMC Infect Dis 13:431
Baess I (1979) Deoxyribonucleic acid relatedness among species of slowly-growing mycobacteria. Acta Pathol Microbiol Scand B 87(4):221–226
Bedrossian N, Hamze M, Rahmo AK, Jurjus A, Saliba J, Dabboussi F, Karam W (2013) Mycobacterium tuberculosis spoligotypes circulating in the Lebanese population: a retrospective study. East Mediterr Health J 19(2):119–124
Bek D, Kjeldsen MK, Kamper-Jorgensen Z, Hansen NF, Rasmussen EM (2010) Tuberculosis caused by Mycobacterium africanum. Ugeskr Laeger 172(7):549–550. VP12080608 [pii]
Bekmurzayeva A, Sypabekova M, Kanayeva D (2013) Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis (Edinb) 93(4):381–388. S1472-9792(13)00082-6 [pii]. doi:10.1016/j.tube.2013.03.003
Bentley SD, Comas I, Bryant JM, Walker D, Smith NH, Harris SR, Thurston S, Gagneux S, Wood J, Antonio M, Quail MA, Gehre F, Adegbola RA, Parkhill J, de Jong BC (2012) The genome of Mycobacterium africanum West African 2 reveals a lineage-specific locus and genome erosion common to the M. tuberculosis complex. PLoS Negl Trop Dis 6(2):e1552. doi:10.1371/journal.pntd.0001552PNTD-D-11-01108. [pii]
Blouin Y, Hauck Y, Soler C, Fabre M, Vong R, Dehan C, Cazajous G, Massoure PL, Kraemer P, Jenkins A, Garnotel E, Pourcel C, Vergnaud G (2012) Significance of the identification in the Horn of Africa of an exceptionally deep branching Mycobacterium tuberculosis clade. PLoS One 7(12):e52841. doi:10.1371/journal.pone.0052841PONE-D-12-22563. [pii]
Bold TD, Davis DC, Penberthy KK, Cox LM, Ernst JD, de Jong BC (2012) Impaired fitness of Mycobacterium africanum despite secretion of ESAT-6. J Infect Dis 205(6):984–990. jir883 [pii]. doi:10.1093/infdis/jir883
Bradley SG (1972) Reassociation of deoxyribonucleic acid from selected mycobacteria with that from Mycobacterium bovis and Mycobacterium farcinica. Am Rev Respir Dis 106:122–124
Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, Garnier T, Gutierrez C, Hewinson G, Kremer K, Parsons LM, Pym AS, Samper S, van Soolingen D, Cole ST (2002) A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99:3684–3689. doi:10.1073/pnas.052548299
Broset E, Martin C, Gonzalo-Asensio J (2015) Evolutionary landscape of the Mycobacterium tuberculosis complex from the viewpoint of PhoPR: implications for virulence regulation and application to vaccine development. MBio 6(5):e01289–e01215. mBio.01289-15 [pii]. doi:10.1128/mBio.01289-15
Brown T, Nikolayevskyy V, Velji P, Drobniewski F (2010) Associations between Mycobacterium tuberculosis strains and phenotypes. Emerg Infect Dis 16(2):272–280. doi:10.3201/eid1602.091032
Cadmus S, Palmer S, Okker M, Dale J, Gover K, Smith N, Jahans K, Hewinson RG, Gordon SV (2006) Molecular analysis of human and bovine tubercle bacilli from a local setting in Nigeria. J Clin Microbiol 44(1):29–34. 44/1/29 [pii]. doi:10.1128/JCM.44.1.29-34.2006
Cadmus SI, Jenkins AO, Godfroid J, Osinusi K, Adewole IF, Murphy RL, Taiwo BO (2009) Mycobacterium tuberculosis and Mycobacterium africanum in stools from children attending an immunization clinic in Ibadan, Nigeria. Int J Infect Dis 13(6):740–744. S1201-9712(09)00006-X [pii]. doi:10.1016/j.ijid.2008.11.016
Castets M, Boisvert H, Grumbach F, Brunel M, Rist N (1968) Tuberculosis bacilli of the African type: preliminary note. Rev Tuberc Pneumol (Paris) 32(2):179–184
Chavadi S, Wooff E, Coldham NG, Sritharan M, Hewinson RG, Gordon SV, Wheeler PR (2009) Global effects of inactivation of the pyruvate kinase gene in the Mycobacterium tuberculosis complex. J Bacteriol 191(24):7545–7553
Collins CH, Yates MD, Grange JM (1982) Subdivision of Mycobacterium tuberculosis into five variants for epidemiological purposes: methods and nomenclature. J Hyg (Lond) 89(2):235–242
Coscolla M, Lewin A, Metzger S, Maetz-Rennsing K, Calvignac-Spencer S, Nitsche A, Dabrowski PW, Radonic A, Niemann S, Parkhill J, Couacy-Hymann E, Feldman J, Comas I, Boesch C, Gagneux S, Leendertz FH (2013) Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg Infect Dis 19(6):969–976. doi:10.3201/eid1906.121012
Cousins D, Peet R, Gaynor W, Williams S, Gow B (1994) Tuberculosis in imported hyrax (Procavia capensis) caused by an unusual variant belonging to the Mycobacterium tuberculosis complex. Vet Microbiol 42:135. doi:10.1016/0378-1135(94)90013-2
David HL, Jahan M, Jumin A, Grandry J, Lehman EH (1978) Numerical taxonomy analysis of Mycobacterium africanum. Int J Syst Bacteriol 28(4):467–472
de Jong BC, Hill PC, Brookes RH, Otu JK, Peterson KL, Small PM, Adegbola RA (2005) Mycobacterium africanum: a new opportunistic pathogen in HIV infection? AIDS 19(15):1714–1715
de Jong BC, Hill PC, Brookes RH, Gagneux S, Jeffries DJ, Otu JK, Donkor SA, Fox A, McAdam KP, Small PM, Adegbola RA (2006) Mycobacterium africanum elicits an attenuated T cell response to early secreted antigenic target, 6 kDa, in patients with tuberculosis and their household contacts. J Infect Dis 193(9):1279–1286. JID35711 [pii]. doi:10.1086/502977
de Jong B, Hill P, Aiken A, Jeffries D, Onipede A, Small P, Adegbola R, Corrah T (2007) Clinical presentation and outcome of tuberculosis patients infected by M. africanum versus M. tuberculosis. Int J Tuberc Lung Dis 11:450
de Jong BC, Hill PC, Aiken A, Awine T, Antonio M, Adetifa IM, Jackson-Sillah DJ, Fox A, Deriemer K, Gagneux S, Borgdorff MW, McAdam KP, Corrah T, Small PM, Adegbola RA (2008) Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis 198(7):1037–1043. doi:10.1086/591504 [doi]
de Jong B, Antonio M, Awine T, Ogungbemi K, de Jong Y, Gagneux S, De Riemer K, Zozio T, Rastogi N, Borgdorff M, Hill P, Adegbola R (2009) Use of spoligotyping and large sequence polymorphisms to study the population structure of the Mycobacterium tuberculosis complex in a cohort study of consecutive smear-positive tuberculosis cases in The Gambia. J Clin Microbiol 47:994. doi:10.1128/jcm.01216-08
de Jong BC, Antonio M, Gagneux S (2010) Mycobacterium africanum—review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis 4(9):e744
Desmond E, Ahmed AT, Probert WS, Ely J, Jang Y, Sanders CA, Lin SY, Flood J (2004) Mycobacterium africanum cases, California. Emerg Infect Dis 10(5):921–923. doi:10.3201/eid1005.030016
Diop S, de Medeiros D, de Medeiros G, Baylet R, Sankale M (1976) Incidence and geographic distribution of Mycobacterium africanum in Senegal. Bull Soc Med Afr Noire Lang Fr 21(1):50–56
Dosso M, Bonard D, Msellati P, Bamba A, Doulhourou C, Vincent V, Peyre M, Traore M, Koffi K, Coulibaly IM (1999) Primary resistance to antituberculosis drugs: a national survey conducted in Cote d’Ivoire in 1995–1996. Ivoirian Study Group on Tuberculosis Resistance. Int J Tuberc Lung Dis 3(9):805–809
Frothingham R, Strickland PL, Bretzel G, Ramaswamy S, Musser JM, Williams DL (1999) Phenotypic and genotypic characterization of Mycobacterium africanum isolates from West Africa. J Clin Microbiol 37(6):1921–1926
Frottier J, Eliaszewicz M, Arlet V, Gaudillat C (1990) Infections caused by Mycobacterium africanum. Bull Acad Natl Med 174(1):29–33. discussion 34–25
Gagneux S, Small PM (2007) Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7(5):328–337. S1473-3099(07)70108-1 [pii]. doi:10.1016/S1473-3099(07)70108-1
Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong BC, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM (2006) Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103(8):2869–2873. 0511240103 [pii]. doi:10.1073/pnas.0511240103
Galagan JE (2014) Genomic insights into tuberculosis. Nat Rev Genet 15(5):307–320. nrg3664 [pii]. doi:10.1038/nrg3664 [doi]
Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu WH, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf HJ, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SH, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK (2013) The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499(7457):178–183. nature12337 [pii]. doi:10.1038/nature12337
Garzelli C, Lari N, Cuccu B, Tortoli E, Rindi L (2010) Impact of immigration on tuberculosis in a low-incidence area of Italy: a molecular epidemiological approach. Clin Microbiol Infect 16(11):1691–1697. S1198-743X(14)60567-X [pii]. doi:10.1111/j.1469-0691.2009.03149.x
Gehre F, Antonio M, Faihun F, Odoun M, Uwizeye C, de Rijk P, de Jong BC, Affolabi D (2013a) The first phylogeographic population structure and analysis of transmission dynamics of M. africanum West African 1 – combining molecular data from Benin, Nigeria and Sierra Leone. PLoS One 8(10):e77000. doi:10.1371/journal.pone.0077000PONE-D-13-26514. [pii]
Gehre F, Otu J, DeRiemer K, de Sessions PF, Hibberd ML, Mulders W, Corrah T, de Jong BC, Antonio M (2013b) Deciphering the growth behaviour of Mycobacterium africanum. PLoS Negl Trop Dis 7(5):e2220. doi:10.1371/journal.pntd.0002220PNTD-D-12-01634. [pii]
Godreuil S, Torrea G, Terru D, Chevenet F, Diagbouga S, Supply P, Van de Perre P, Carriere C, Banuls AL (2007) First molecular epidemiology study of Mycobacterium tuberculosis in Burkina Faso. J Clin Microbiol 45(3):921–927. JCM.01918-06 [pii]. doi:10.1128/JCM.01918-06
Gomgnimbou MK, Refregier G, Diagbouga SP, Adama S, Kabore A, Ouiminga A, Sola C (2012) Spoligotyping of Mycobacterium africanum, Burkina Faso. Emerg Infect Dis 18(1):117–119. doi:10.3201/eid1801.110275
Gonzalo-Asensio J, Malaga W, Pawlik A, Astarie-Dequeker C, Passemar C, Moreau F, Laval F, Daffe M, Martin C, Brosch R, Guilhot C (2014) Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci U S A 111(31):11491–11496. 1406693111 [pii]. doi:10.1073/pnas.1406693111
Goyal M, Lawn S, Afful B, Acheampong JW, Griffin G, Shaw R (1999) Spoligotyping in molecular epidemiology of tuberculosis in Ghana. J Infect 38:171–175
Grange JM (1979) Tuberculosis: the changing tubercle. Br J Hosp Med 22(6):540–548
Grange JM, Yates MD (1989) Incidence and nature of human tuberculosis due to Mycobacterium africanum in South-East England: 1977–87. Epidemiol Infect 103(1):127–132
Groenheit R, Ghebremichael S, Svensson J, Rabna P, Colombatti R, Riccardi F, Couvin D, Hill V, Rastogi N, Koivula T, Kallenius G (2011) The Guinea-Bissau family of Mycobacterium tuberculosis complex revisited. PLoS One 6(4):e18601. doi:10.1371/journal.pone.0018601
Grosset J, Sangare S, Rist N, Meyer L (1974) Cultural and biochemical characteristics of tubercle bacilli isolated from 230 cases of tuberculosis in Mali. Bull Int Union Tuberc 49:177–187
Gudan A, Artukovic B, Cvetnic Z, Spicic S, Beck A, Hohsteter M, Naglic T, Bata I, Grabarevic Z (2008) Disseminated tuberculosis in hyrax (Procavia capensis) caused by Mycobacterium africanum. J Zoo Wildl Med 39(3):386–391. doi:10.1638/06-041.1
Haas WH, Bretzel G, Amthor B, Schilke K, Krommes G, Rusch-Gerdes S, Sticht-Groh V, Bremer HJ (1997) Comparison of DNA fingerprint patterns of isolates of Mycobacterium africanum from east and west Africa. J Clin Microbiol 35(3):663–666
Hart PD, Sutherland I (1977) BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J 2(6082):293–295
Hermans-Boveroulle MT, Pattyn SR, Gatti F (1965) Study of human strains of Mycobacterium tuberculosis Isolated in Leopoldville. Ann Soc Belge de Med Trop 45(5):531–540
Hill PC, Jackson-Sillah D, Fox A, Franken KL, Lugos MD, Jeffries DJ, Donkor SA, Hammond AS, Adegbola RA, Ottenhoff TH, Klein MR, Brookes RH (2005) ESAT-6/CFP-10 fusion protein and peptides for optimal diagnosis of mycobacterium tuberculosis infection by ex vivo enzyme-linked immunospot assay in the Gambia. J Clin Microbiol 43(5):2070–2074. 43/5/2070 [pii]. doi:10.1128/JCM.43.5.2070-2074.2005
Hoffner SE, Svenson SB, Norberg R, Dias F, Ghebremichael S, Kallenius G (1993) Biochemical heterogeneity of Mycobacterium tuberculosis complex isolates in Guinea-Bissau. J Clin Microbiol 31(8):2215–2217
Homolka S, Post E, Oberhauser B, George A, Westman L, Dafae F, Rüsch-Gerdes S, Niemann S (2008) High genetic diversity among Mycobacterium tuberculosis complex strains from Sierra Leone. BMC Microbiol 8:103. doi:10.1186/1471-2180-8-103
Homolka S, Meyer CG, Hillemann D, Owusu-Dabo E, Adjei O, Horstmann RD, Browne EN, Chinbuah A, Osei I, Gyapong J, Kubica T, Ruesch-Gerdes S, Niemann S (2010) Unequal distribution of resistance-conferring mutations among Mycobacterium tuberculosis and Mycobacterium africanum strains from Ghana. Int J Med Microbiol 300(7):489–495. S1438-4221(10)00050-0 [pii]. doi:10.1016/j.ijmm.2010.04.019 [doi]
Huet M, Rist N, Boube G, Potier D (1971) Bacteriological study of tuberculosis in Cameroon. Rev Tuberc Pneumol (Paris) 35(4):413–426
Hurtado UA, Solano JS, Rodriguez A, Robledo J, Rouzaud F (2016) Draft genome sequence of a Mycobacterium africanum clinical isolate from Antioquia, Colombia. Genome Announc 4(3):e00486. 4/3/e00486-16 [pii]. doi:10.1128/genomeA.00486-16
Isea-Pena MC, Brezmes-Valdivieso MF, Gonzalez-Velasco MC, Lezcano-Carrera MA, Lopez-Urrutia-Lorente L, Martin-Casabona N, Monforte-Cirac ML, Palacios JJ, Penedo-Pallares A, Ramirez-Rosales A, Sanchez-Silos R, Tortola-Fernandez T, Vinuelas-Bayon J, Vitoria-Agreda A, Esteban J (2012) Mycobacterium africanum, an emerging disease in high-income countries? Int J Tuberc Lung Dis 16(10):1400–1404. doi:10.5588/ijtld.12.0142
Jaspe RC, Rojas YM, Flores LA, Sofia Toro E, Takiff H, de Waard JH (2009) Evaluation of the Kudoh swab method for the culturing of Mycobacterium tuberculosis in rural areas. Tropical Med Int Health 14(4):468–471. TMI2236 [pii]. doi:10.1111/j.1365-3156.2009.02236.x
Jiang Y, Liu H, Wan K (2014) MPT64 polymorphisms of Mycobacterium tuberculosis strains suggest ongoing immune evasion. Tuberculosis (Edinb) 94(6):712–714. S1472-9792(14)20573-7 [pii]. doi:10.1016/j.tube.2014.08.013
Jungbluth H, Fink H, Reusch F (1978) Tuberculous infection caused by Myco. africanum in black africans resident in the German Federal Republic (author’s transl). Prax Klin Pneumol 32(5):306–309
Kallenius G, Koivula T, Ghebremichael S, Hoffner SE, Norberg R, Svensson E, Dias F, Marklund BI, Svenson SB (1999) Evolution and clonal traits of Mycobacterium tuberculosis complex in Guinea-Bissau. J Clin Microbiol 37(12):3872–3878
Kato-Maeda M, Bifani PJ, Kreiswirth BN, Small PM (2001) The nature and consequence of genetic variability within Mycobacterium tuberculosis. J Clin Invest 107(5):533–537. doi:10.1172/JCI11426
Keating LA, Wheeler PR, Mansoor H, Inwald JK, Dale J, Hewinson RG, Gordon SV (2005) The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth. Mol Microbiol 56(1):163–174. MMI4524 [pii]. doi:10.1111/j.1365-2958.2005.04524.x
Koro FK, Simo YK, Piam FF, Noeske J, Gutierrez C, Kuaban C, Eyangoh SI (2013) Population dynamics of tuberculous bacilli in Cameroon as assessed by spoligotyping. J Clin Microbiol 51(1):299–302
Kothadia SN, Sengupta SR (1982) Evaluation of swab culture method of Kudoh and Kudoh for cultivation of M. tuberculosis. Indian J Med Sci 36(1):9–11
Kuaban C, Um Boock A, Noeske J, Bekang F, Eyangoh S (2014) Mycobacterium tuberculosis complex strains and drug susceptibility in a cattle-rearing region of Cameroon. Int J Tuberc Lung Dis 18(1):34–38. doi:10.5588/ijtld.13.0333
Lawn SD, Frimpong EH, Al-Ghusein H, Acheampong JW, Uttley AH, Butcher PD, Griffin GE (2001) Pulmonary tuberculosis in Kumasi, Ghana: presentation, drug resistance, molecular epidemiology and outcome of treatment. West Afr J Med 20(2):92–97
Lawson L, Zhang J, Gomgnimbou MK, Abdurrahman ST, Le Moullec S, Mohamed F, Uzoewulu GN, Sogaolu OM, Goh KS, Emenyonu N, Refrégier G, Cuevas LE, Sola C (2012) A molecular epidemiological and genetic diversity study of tuberculosis in Ibadan, Nnewi and Abuja, Nigeria. PLoS One 7(6):e38409
Leao SC, Martin A, Mejia GI, Palomino JC, Robledo J, Telles MAS, Portaels F (2004) Practical handbook for the phenotypic and genotypic identification of mycobacteria. Vanden BROELLE, Brugges
Ledru S, Cauchoix B, Yameogo M, Zoubga A, Lamande-Chiron J, Portaels F, Chiron JP (1996) Impact of short-course therapy on tuberculosis drug resistance in South-West Burkina Faso. Tuber Lung Dis 77(5):429–436. S0962-8479(96)90116-1 [pii]
Malaga W, Constant P, Euphrasie D, Cataldi A, Daffe M, Reyrat JM, Guilhot C (2008) Deciphering the genetic bases of the structural diversity of phenolic glycolipids in strains of the Mycobacterium tuberculosis complex. J Biol Chem 283(22):15177–15184. M710275200 [pii]. doi:10.1074/jbc.M710275200
Meissner G, Schroder K (1969) The so-called African mycobacteria strains from the tropical West Africa. Zentralbl Bakteriol Orig 211(1):69–81
Meyer CG, Scarisbrick G, Niemann S, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, Kubica T, Rusch-Gerdes S, Thye T, Horstmann RD (2008) Pulmonary tuberculosis: virulence of Mycobacterium africanum and relevance in HIV co-infection. Tuberculosis (Edinb) 88(5):482–489. S1472-9792(08)00056-5 [pii]. doi:10.1016/j.tube.2008.05.004
Mostowy S, Onipede A, Gagneux S, Niemann S, Kremer K, Desmond EP, Kato-Maeda M, Behr M (2004) Genomic analysis distinguishes Mycobacterium africanum. J Clin Microbiol 42(8):3594–3599. doi:10.1128/JCM.42.8.3594-3599.200442/8/3594. [pii]
Munoz ME, Ponce E (2003) Pyruvate kinase: current status of regulatory and functional properties. Comp Biochem Physiol B Biochem Mol Biol 135:197–218
Niederweis M (2008) Nutrient acquisition by mycobacteria. Microbiology 154(Pt 3):679–692. 154/3/679 [pii]. doi:10.1099/mic.0.2007/012872-0
Niemann S, Richter E, Rusch-Gerdes S (2000) Differentiation among members of the Mycobacterium tuberculosis complex by molecular and biochemical features: evidence for two pyrazinamide-susceptible subtypes of M. bovis. J Clin Microbiol 38(1):152–157
Niemann S, Rüsch-Gerdes S, Joloba ML, Whalen CC, Guwatudde D, Ellner JJ, Eisenach K, Fumokong N, Johnson JL, Aisu T, Mugerwa RD, Okwera A, Schwander SK (2002) Mycobacterium africanum subtype II is associated with two distinct genotypes and is a major cause of human tuberculosis in Kampala, Uganda. J Clin Microbiol 40(9):3398–3405
Niobe-Eyangoh SN, Kuaban C, Sorlin P, Cunin P, Thonnon J, Sola C, Rastogi N, Vincent V, Gutierrez MC (2003) Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon. J Clin Microbiol 41(6):2547–2553
Nuru A, Mamo G, Worku A, Admasu A, Medhin G, Pieper R, Ameni G (2015) Genetic diversity of Mycobacterium tuberculosis complex isolated from tuberculosis patients in Bahir Dar City and its surroundings, Northwest Ethiopia. Biomed Res Int 2015:174732. doi:10.1155/2015/174732
Ontsira Ngoyi EN, Obengui, Taty Taty R, Koumba EL, Ngala P, Ossibi Ibara RB (2014) Mycobacterial species repartition: experience of the Antituberculosis Center in Pointe Noire (Republic of Congo). Bull Soc Pathol Exot 107(5):342–345. doi:10.1007/s13149-014-0395-4 [doi]
Palaci M, Peres RL, Maia R, Cunha EA, Ribeiro MO, Lecco R, de Souza RC, Ferro ESRR, Vinhas SA, Dietze R, Vianna S, de Morais CG (2013) Contribution of the Ogawa-Kudoh swab culture method to the diagnosis of pulmonary tuberculosis in Brazil. Int J Tuberc Lung Dis 17(6):782–786. doi:10.5588/ijtld.12.0500
Parsons L, Brosch R, Cole S, Somoskövi A, Loder A, Bretzel G, Van Soolingen D, Hale Y, Salfinger M (2002) Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J Clin Microbiol 40:2339. doi:10.1128/jcm.40.7.2339-2345.2002
Pattyn SR, Portaels F, Spanoghe L, Magos J (1970) Further studies on African strains of Mycobacterium tuberculosis: comparison with M. bovis and M. microti. Ann Soc Belges Med Trop Parasitol Mycol 50(2):211–227
Perez-de Pedro I, Bermudez P, Artero I, Jimenez MS (2008) Orchiepididymitis due to Mycobacterium africanum. Enferm Infecc Microbiol Clin 26(9):600–602
Prat R, Rist N, Dumitrescu N, Mugabushaka A, Clavel S, Duponchel C (1974) Special characteristics of the cultures of tubercle bacilli isolated in Ruanda. Bull Int Union Tuberc 49:53–62
Rahim Z, Mollers M, te Koppele-Vije A, de Beer J, Zaman K, Matin MA, Kamal M, Raquib R, van Soolingen D, Baqi MA, Heilmann FG, van der Zanden AG (2007) Characterization of Mycobacterium africanum subtype I among cows in a dairy farm in Bangladesh using spoligotyping. Southeast Asian J Trop Med Public Health 38(4):706–713
Sangare L, Diande S, Kouanda S, Dingtoumda BI, Mourfou A, Ouedraogo F, Sawadogo I, Nebie B, Gueye A, Sawadogo LT, Traore AS (2010) Mycobacterium tuberculosis drug-resistance in previously treated patients in Ouagadougou, Burkina Faso. Ann Afr Med 9(1):15–19. AnnAfrMed_2010_9_1_15_62619 [pii]. doi:10.4103/1596-3519.62619
Schroder KH (1982) Occurence of M. africanum in the Federal Republic of Germany (author’s transl). Zentralbl Bakteriol Mikrobiol Hyg A 251(3):341–344
Sharma A, Bloss E, Heilig CM, Click ES (2016) Tuberculosis caused by Mycobacterium africanum, United States, 2004–2013. Emerg Infect Dis 22(3):396–403. doi:10.3201/eid2203.151505
Simonet F, Menard M, Le Mao G, Albert JP (1989) Action antituberculeuse au Burkina Faso L’experience du service de Pneumo-phtisiologie de l’Hospital National Sanon Souro de Bobo-Dioulaso. Societe’ Sante’ Industrie Servoice, Paris
Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV (2009) Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat Rev Micro 7(7):537–544
Sola C, Rastogi N, Gutierrez MC, Vincent V, Brosch R, Parsons L (2003) Is Mycobacterium africanum subtype II (Uganda I and Uganda II) a genetically well-defined subspecies of the Mycobacterium tuberculosis complex? J Clin Microbiol 41(3):1345–1346. author reply 1346–1348
Stanford JL, Grange JM (1974) The meaning and structure of species as applied to mycobacteria. Tubercle 55:143–152
Stermann M, Bohrssen A, Diephaus C, Maass S, Bange FC (2003) Polymorphic nucleotide within the promoter of nitrate reductase (NarGHJI) is specific for Mycobacterium tuberculosis. J Clin Microbiol 41(7):3252–3259
Sticht-Groh V, Bretzel G, Rüsch-Gerdes S, Bwire S, Kawuma HJS (1994) M. africanum strains isolated in East-Africa, Uganda. Tubercle and Lung Dis 75:46. doi:10.1016/0962-8479(94)90853-2
Stonebrink B (1958) The use of a pyruvate containing egg medium in the culture of isoniazid resistant strains of Mycobacterium tuberculosis var. hominis. Acta Tuberc Scandia 35:67–80
Streicher EM, Victor TC, van der Spuy G, Sola C, Rastogi N, van Helden PD, Warren RM (2007) Spoligotype signatures in the Mycobacterium tuberculosis complex. J Clin Microbiol 45(1):237–240. JCM.01429-06 [pii]. doi:10.1128/JCM.01429-06
Stucki D, Malla B, Hostettler S, Huna T, Feldmann J, Yeboah-Manu D, Borrell S, Fenner L, Comas I, Coscolla M, Gagneux S (2012) Two new rapid SNP-typing methods for classifying Mycobacterium tuberculosis complex into the main phylogenetic lineages. PLoS One 7(7):e41253. doi:10.1371/journal.pone.0041253PONE-D-12-11965. [pii]
Thorel MF (1980a) Isolation of Mycobacterium africanum from monkeys. Tubercle 61(2):101–104
Thorel MF (1980b) Mycobacteria identified in a centre for veterinary research between 1973 and 1979 (author’s transl). Ann Microbiol (Paris) 131(1):61–69
Thumamo BP, Asuquo AE, Abia-Bassey LN, Lawson L, Hill V, Zozio T, Emenyonu N, Eko FO, Rastogi N (2012) Molecular epidemiology and genetic diversity of Mycobacterium tuberculosis complex in the Cross River State, Nigeria. Infect Genet Evol 12(4):671–677. S1567-1348(11)00290-5 [pii]. doi:10.1016/j.meegid.2011.08.011
Tsolaki AG, Hirsh AE, DeRiemer K, Enciso JA, Wong MZ, Hannan M, Goguet de la Salmoniere YO, Aman K, Kato-Maeda M, Small PM (2004) Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc Natl Acad Sci U S A 101(14):4865–4870. doi:10.1073/pnas.03056341010305634101. [pii]
Tsukamura M (1967) Identification of mycobacteria. Tubercle 48(4):311–338
Tsukamura M (1976) Numerical classification of slowly growing mycobacteria. Int J Syst Bacteriol 26:409–420
Ueyama M, Chikamatsu K, Aono A, Murase Y, Kuse N, Morimoto K, Okumura M, Yoshiyama T, Ogata H, Yoshimori K, Kudoh S, Azuma A, Gemma A, Mitarai S (2014) Sub-speciation of Mycobacterium tuberculosis complex from tuberculosis patients in Japan. Tuberculosis (Edinb) 94(1):15–19. S1472-9792(13)00166-2 [pii]. doi:10.1016/j.tube.2013.09.006
Unger F, Münstermann S, Goumou A, Apia CN, Konte M (2003) Risk associated with Mycobacterium bovis infections detected in selected study herds and slaughter cattle in 4 countries of West Africa. Animal Health Working Paper 1. ITC (International Trypanotolerance Centre), Banjul, The Gambia
Uzoewulu GN, Lawson L, Nnanna IS, Rastogi N, Goyal M (2016) Genetic diversity of Mycobacterium tuberculosis complex strains isolated from patients with pulmonary tuberculosis in Anambra State, Nigeria. Int J Mycobacteriology 5(1):74–79. doi:http://dx.doi.org/10.1016/j.ijmyco.2015.06.008
Van Der Groen G, Pattyn SR (1975) Comparison of Mycobacterium africanum, M. tuberculosis and M. bovis by their utilization of carbon and nitrogen sources. Ann Soc Belg Med Trop 55(6):647–651
van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ (2007) M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129(7):1287–1298. S0092-8674(07)00782-9 [pii]. doi:10.1016/j.cell.2007.05.059
Vasconcellos SEG, Huard RC, Niemann S, Kremer K, Santos AR, Suffys PN, Ho JL (2010) Distinct genotypic profiles of the two major clades of Mycobacterium africanum. BMC Infect Dis 10(1):1–16. doi:10.1186/1471-2334-10-80
Wang Z, Potter BM, Gray AM, Sacksteder KA, Geisbrecht BV, Laity JH (2007) The solution structure of antigen MPT64 from Mycobacterium tuberculosis defines a new family of beta-grasp proteins. J Mol Biol 366(2):375–381. S0022-2836(06)01579-8 [pii]. doi:10.1016/j.jmb.2006.11.039
Wayne LG, Kubica GP (1986) The Mycobacteria. In: Snears PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2, 7th edn. William &Wilkins Co, Baltimore, pp 1436–1457
Waziri NE, Cadmus S, Nguku P, Fawole O, Owolodun OA, Waziri H, Ibrahim L, Biya O, Gidado S, Badung S, Kumbish P, Nsubuga P (2014) Factors associated with tuberculosis among patients attending a treatment centre in Zaria, North-west Nigeria, 2010. Pan Afr Med J 18(Suppl 1):5. doi:10.11694/pamj.supp.2014.18.1.4189PAMJ-SUPP-18-1-05. [pii]
Weber A, Reischl U, Naumann L (1998) Demonstration of Mycobacterium africanum in a bull from North Bavaria. Berl Munch Tierarztl Wochenschr 111(1):6–8
Winglee K, Manson McGuire A, Maiga M, Abeel T, Shea T, Desjardins CA, Diarra B, Baya B, Sanogo M, Diallo S, Earl AM, Bishai WR (2016) Whole genome sequencing of Mycobacterium africanum strains from Mali provides insights into the mechanisms of geographic restriction. PLoS Negl Trop Dis 10 (1):e0004332. 10.1371/journal.pntd.0004332PNTD-D-15-01035 [pii]
Yeboah-Manu D, Asante-Poku A, Bodmer T, Stucki D, Koram K, Bonsu F, Pluschke G, Gagneux S (2011) Genotypic diversity and drug susceptibility patterns among M. tuberculosis complex isolates from South-Western Ghana. PLoS One 6 (7):e21906. 10.1371/journal.pone.0021906PONE-D-10-05029 [pii]
Zink AR, Sola C, Reischl U, Grabner W, Rastogi N, Wolf H, Nerlich AG (2003) Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping. J Clin Microbiol 41(1):359–367
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Yeboah-Manu, D., de Jong, B.C., Gehre, F. (2017). The Biology and Epidemiology of Mycobacterium africanum . In: Gagneux, S. (eds) Strain Variation in the Mycobacterium tuberculosis Complex: Its Role in Biology, Epidemiology and Control. Advances in Experimental Medicine and Biology, vol 1019. Springer, Cham. https://doi.org/10.1007/978-3-319-64371-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-64371-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64369-4
Online ISBN: 978-3-319-64371-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)