Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disorder, characterized by polyarticular inflammation causing progressive joint damage and disability. The mechanisms underlying its pathogenesis involve activation of innate and adaptive immunity, microvascular endothelial cell activation, and inflammatory infiltration of lymphocytes and monocytes into the synovium. Spinal involvement in RA is not typical; when it occurs, the main radiological features are (1) atlantoaxial subluxation (AAS), which is the most typical form of cervical spine involvement; (2) cranial settling—also known as basilar impression, atlantoaxial impaction or superior migration of the odontoid—which is the most severe form of associated spinal instability; and (3) subaxial subluxation. A combination of these alterations may occur. Synovitis is characterized by infiltration of innate and adaptive immune cells; joint destruction is a consequence of activation of synovial fibroblasts, which acquire aggressive, inflammatory, invasive features, associated with increased chondrocyte catabolism and synovial osteoclastogenesis.
Neck pain is the most frequent symptom of spinal involvement in RA; it occurs in 40–80% of patients and is mostly localized at the craniocervical junction. Other symptoms—caused by compression of neural structures such as the greater occipital nerve (at C2), the nucleus of the spinal trigeminal tract and the greater auricular nerve—are occipital neuralgia, facial pain and ear pain, respectively. Irritation of the lesser occipital nerve (at C1) can cause pain in the suboccipital region. Sometimes patients may complain of a sensation of their head falling down with flexion, weakness, reduced endurance, loss of ability, gait alterations, paraesthesias or other symptoms due to cord and medullary compression, and upper or lower motor neuron signs, or both. Surgical management of RA remains a challenging field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Craniovertebral junction
- Rheumatoid arthritis
- Cervical spine
- Inflammation
- Transnasal decompression
- Transoral decompression
- Instrumentation and fusion procedures
- Atlantoaxial dislocation
- Atlantoaxial instability
- Atlantoaxial synovitis
- Basilar invagination
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder, characterized by polyarticular inflammation causing progressive joint damage and disability. The mechanisms underlying its pathogenesis involve activation of innate and adaptive immunity, microvascular endothelial cell activation and inflammatory infiltration of lymphocytes and monocytes into the synovium. The final consequence of these immunological processes is development of synovial hypertrophy with pannus formation, which finally leads to erosion of articular cartilage and subchondral bone [1].
RA is a chronic, symmetrical, erosive disease, mainly involving small joints in the hands and feet. However, cervical spine involvement may also be present in a significant number of patients, sometimes in early disease. Cervical spine involvement, however, is more commonly a finding of long-standing RA, observed in over half of all patients after a mean of 10 years of the disease [2, 3].
The prevalence of cervical spine abnormalities in RA is estimated to be between 17% and 88% [4, 5]. The breadth of this range is related to variability in populations (as observed in several retrospective studies), use of different classifications of the disease and evolving medical therapies for the disease [6]. Almost 1% of the general population in Europe and in the USA is affected by RA, and approximately 10% of these patients develop significant cervical spine involvement [7].
Cervical spine involvement in RA is related to the presence of peripheral erosions, the use of corticosteroids and previous joint surgery, which are considered independent risk factors for development of serious cervical spine abnormalities. Beyond inflammatory involvement of cervical spine related to RA, patients can develop age-related degenerative alterations (known as cervical spondylosis), which also affect the general population [8].
Anatomy and Physiopathology
The cervical spine can be separated into two different parts: the upper tract (C1 and C2, with the atlantoaxial, atlanto-odontoid and atlanto-occipital joints) and the lower tract (C3–C7, with uncovertebral and facet joints present at each level). The upper cervical spine is mainly involved in rotational movements of the neck, whereas the lower tract is involved in flexion–extension movements. Like the rest of the spine, the cervical spine has the function of guaranteeing protection of the neural structures contained in the spinal canal and allowing both physiological stability and movement capacity between the vertebrae through the joints connecting them, which are powered by various muscle attachments. The occiput–C1 and C1–C2 articulations lack intervertebral discs, consisting exclusively of synovial joints. Thanks to these peculiar anatomical features, the atlas and axis allow increased mobility of the cervical spine. The atlas, without a vertebral body, supports the head though lateral articulations with the occipital condyles. The superior articular facet of the atlas receives occipital condyles at the base of the skull, whereas the inferior articular facet stands upon the axis; articulation between the atlas and axis is permitted by vertical projections of the odontoid process, which assumes a position between the lateral masses of C1. The joints are stabilized by different ligaments, such as the transverse ligament, the alar ligaments and the accessory atlantoaxial ligaments [9, 10]. The integrity of the transverse ligament prevents anterior subluxation of the atlas, particularly during neck flexion. In RA, this ligament is often compromised because of its involvement in the inflammatory process of the synovial articulation of the dens. Whole rupture of the transverse ligament causes only 4–5 mm anterior subluxation of the atlas if the secondary stabilizers are not damaged. In RA, the stability of the atlantoaxial joint is definitely compromised as a result of impairment of the secondary stabilizers, and it can be further compromised by possible erosions of the odontoid process. Atlantoaxial instability (most frequently anterior) is often due to loss of ligament support caused by development of erosive pannus at the C1–C2 level and bone destruction (Fig. 1). This process leads to damage to the ligamentous complex that usually stabilizes the atlas in the axis, especially damage to the transverse ligament, but also to the articular capsular joint of C1–C2.
The weight of the head and insufficient mobility of the thoracic spine create dynamic forces that worsen the situation, further compromising the ligamentous stabilizers, causing fracture of an impaired dens, or both.
The most frequent clinicopathological presentations of spinal involvement in RA are (1) atlantoaxial subluxation (AAS), which is the most common manifestation of cervical damage; (2) cranial settling—also known as basilar impression, atlantoaxial impaction or superior migration of the odontoid—which is the most severe form of spinal involvement in RA; and (3) subaxial subluxation (SAS). A combination of these alterations may occur [11, 12].
In AAS, the normal <3 mm range of the anterior atlantodental interval (ADI) is extended and that of the posterior atlantodental interval (PADI)—the space between the posterior border of the dens and the anterior aspect of the posterior arch of C1—is decreased, inducing compression of the upper spinal cord [12]. Winfield et al. [13] observed anterior atlantoaxial dislocation (AAD) in 12% of RA patients during a follow-up period of 7 years. In autopsy studies, the rate changed from 11% to 46% [9]. Anterior AAD accounts for 75% of all cases of AAD. According to evidence from experimental studies, disruption of the transverse ligament alone can cause slipping of C1 anteriorly over C2. When the transverse ligament is the only one compromised, the maximum displacement is about 5 mm, but it increases to 6.5–10 mm and 7 mm when the alar or atlantoaxial ligaments, respectively, are also compromised, and 12 mm when all three ligaments are damaged [10, 14].
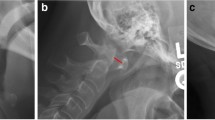
In 20% of cases of AAD, C1 is shifted laterally [15], causing an abnormal head posture. Lateral AAD is the result of unilateral or asymmetrical involvement of the lateral atlantoaxial joint. When no more than 1 mm of subchondral bone on the lateral mass of C1 or the articular process of C2 is lost, C1 shifts laterally by 2.5 mm. When the loss of bone is more than 1 mm, the shift can reach 5 mm and is limited by contact between the lateral mass of C1 and the dens. At the same time, the lateral mass of C1 is in contact with C2, shifting and tilting C1. An anteroposterior open-mouth x-ray permits diagnosis, evidencing involvement of either or both of the C1 and C2 joints with a major than 2 mm displacement of C1 on C2 and tilting of C1 on C2. The degree of compromise of the lateral mass of C2 determines the extent of the tilting [15]. Rotatory dislocation is caused by unilateral C1–C2 joint damage with impairment of the transverse ligament. Dislocation is well demonstrated by an open-mouth projection scan, which is considered the best x-ray projection because it shows lateral displacement of the dens, asymmetry of the C1 lateral masses with respect to the dens and abnormal lateral mass geometry (Fig. 2a). A rarer manifestation is posterior atlantoaxial subluxation, which is usually caused by a fracture of the dens and carries a greater risk of cord injury than AAS (6–7%) [16]. The dens damage caused by the pannus underlies posterior subluxation, subsequent to posterior slippage of C1. The result is upward movement of the anterior arch of C1 and downward inclination of the posterior arch until it is located in front of the spinous process of C2. The erosion of the occipitus–C1 and C1–C2 joints causes cranial settling, with displacement of the odontoid in the foramen magnum. This can cause brainstem compression and possibly death [17].
(a) Traditional X-ray measurements investigating the C1–C2 relationship. ADI anterior atlantodental interval, PADI posterior atlantodental interval (left) and rotatory subluxation (right, red arrow). (b) Radiological criteria for cranial settling. Chamberlain line: findings are considered positive if the apex of the odontoid is 3 mm above a line from the posterior edge of the hard palate to the opisthion (the posterior rim of the foramen magnum). McGregor line: findings are considered positive if the apex of the odontoid is >4.5 mm above a line drawn from the posterior hard palate to the most inferior point on the occipital curve. McRae line (1953): findings are positive if the tip of the odontoid extends above a line drawn from the basion (the anterior rim of the foramen magnum) to the opisthion
Cranial settling is observed in 4–35% of RA patients and is responsible for 20% of all AAD cases [15, 18]. It is caused by erosion of the occipital condyles, the superior articular processes of C2 and the lateral masses of C1. The collapse of the compromised joints is followed by settling of the skull on the upper cervical spine.
Subaxial subluxation can cause several abnormalities in the cervical spine, ranging from spondylolisthesis to cervical kyphosis and/or a “staircase” deformity due to the destruction of the facet joints as well as the uncovertebral joint. Plain X-rays permit us to visualize subaxial cervical spine abnormalities. Spinal cord compression is rarer than with upper cervical spine lesions but it can result in more severe lesions [19].
Pathogenesis
The pathogenesis of RA is heterogeneous and is characterized by activation of innate and adaptive immunity. The presence of autoantibodies is a negative prognostic factor and is related to more severe clinical manifestations, erosive disease and increased mortality [20, 21]. The increased mortality seems to be probably related to the presence of immune complexes between autoantibodies against citrullinated peptides (ACPAs) and citrulline-containing antigens, leading to complement activation [22]. The lung seems to be the main tissue in which the immune response is activated, through formation of ACPAs, which is increased by smoking. Several studies have demonstrated an association between smoking and the presence of shared citrullinated peptides in both lung and synovial tissue biopsies [23]. ACPAs themselves have a pathogenetic role, triggering innate immunity by directly stimulating macrophages (e.g. by binding to toll-like receptors through the bound antigen, by Fc-receptor engagement, or both). Recent studies have suggested that ACPAs could promote activation of osteoclasts through development of immune complex and Fc-receptor involvement or probably by ligating membrane citrullinated vimentin [24], therefore directly causing bone loss. In RA, activation of immune responses leads to development of leucocyte infiltration in synovial membranes, causing synovial hypertrophy, which clinically occurs with joint swelling. The cellular composition of synovitis in RA is heterogeneous, including innate immune cells (e.g. monocytes, dendritic cells, mast cells and innate lymphoid cells) and adaptive immune cells (e.g. T-helper-1 and T-helper-17 cells, B cells, plasmablasts and plasma cells) [24]. Joint destruction is a consequence of activation of synovial fibroblasts, which acquire aggressive inflammatory and invasive features, associated with increased chondrocyte catabolism and synovial osteoclastogenesis [25, 26]. Ultrasound-guided biopsies of small joints and detailed molecular analyses (in particular, transcriptomic analyses) have demonstrated the presence of different subtypes of synovial inflammatory infiltrates—namely, myeloid-dominant, lymphocytic-dominant and fibroid-dominant infiltrates—providing new insights into pathogenetic processes, which could have significant therapeutically implications [27].
The mediators of inflammation that are mainly implicated in progression of RA are chemokines, cytokines, growth factors and metalloproteinases. The immune response is characterized by activation and attraction of inflammatory cells from peripheral blood to the site of inflammation and by subsequent proliferation of synoviocytes. Tumour necrosis factor (TNF), interleukin 6 and probably granulocyte–monocyte colony-stimulating factor play central roles in the development and maintenance of the inflammatory process [28].
Activated fibroblasts—in synergy with inflammatory infiltrates composed of activated T and B cells, monocytes and macrophages—finally act by triggering osteoclast activation through the receptor RANKL (receptor activator of nuclear factor κB ligand, expressed on T and B lymphocytes and fibroblasts), which interacts with its cognate receptor, RANK, expressed on preosteoclasts, macrophages and dendritic cells [29, 30]. Activation of osteoclasts induces the appearance of joint erosions by release of proteolytic enzymes (matrix metalloproteinases). The cartilage matrix is degraded by matrix metalloproteinases and aggrecanases. The proteases can consequently infiltrate and damage articular cartilage, subchondral bone, tendons and ligaments, ultimately leading to instability and subluxation of all of the joints involved, including the cervical spine.
Clinical Manifestations
Cervical spine involvement in RA is often a silent condition. Even in the presence of severe cervical spine damage, many patients may be asymptomatic. Beyond RA-related inflammation in the cervical spine, this site can also be compromised by degenerative age-related disease. Both conditions occur with almost the same symptomatology, characterized by neck pain, myelopathy and radiculopathy. Neck pain is the most frequent symptom reported by patients with spinal involvement in RA; it occurs in 40–80% of patients [31], mostly localized at the craniocervical junction. Occipital headaches are often present as well. Compression of neural structures—such as the greater occipital nerve (in C2), the nucleus of the spinal trigeminal tract and the greater auricular nerve—can cause occipital neuralgia, facial pain and ear pain, respectively. Irritation of the lesser occipital nerve (in C1) can also cause pain in the suboccipital region. Sometimes patients may complain of a feeling of their head falling down with flexion and with a clunking sensation, weakness, reduced endurance and dexterity, gait alterations, paraesthesias [12, 32] or other symptoms due to cord and medullary compression, and upper or lower motor neuron signs, or both.
Axial pain originates from involvement of various components of the vertebral column and is due to damage of the bone and cartilage in the neck or deformities resulting in misalignment.
Myelopathy describes a constellation of symptoms usually caused by compression of the brainstem or spinal cord; earlier symptoms can worsen coordination of hand movements and cause disturbances in balance, a sensation of heaviness in the lower extremities and gait disorders. Other clinical manifestations may be difficulty in execution of fine motor skills—such as buttoning a shirt or inserting a key into a lock—or a progressive change in handwriting [33].
Neck movements, particularly flexion, can cause the occurrence of electric shock sensations in the torso and extremities (Lhermitte’s sign), or either alone. Vertebrobasilar insufficiency or mechanical compression of the cervicomedullary junction can cause symptoms of tinnitus, vertigo, visual alterations, disturbances of equilibrium, diplopia and dysphagia; the most severe manifestations of upper cervical spine involvement, caused by vertebrobasilar insufficiency, are stroke or sudden death, which are rarely reported [12, 34].
A complete history and physical examination can highlight bowel and bladder disturbances, movement disabilities, balance and coordination alterations, and/or difficulties in manual dexterity. Physical examination findings can differ significantly and have been demonstrated to be variable in patients with cord compression. Beside Lhermitte’s sign, suggestive signs of myelopathy are Hoffman’s sign, an inverted brachioradialis reflex, hyperreflexia, continuous clonus or more than five beats of clonus, a positive Romberg sign and/or a Babinski sign, as well as the occurrence of dysdiadochokinesia, dysmetria or problems with the heel to shin test and/or the tandem gait test. These signs/symptoms can be associated with various levels of motor and/or sensory alterations in the upper and/or lower extremities. A complete neurological examination should be performed, including all of the aforementioned provocative tests. Unfortunately, in patients with RA, severe joint involvement may sometimes prevent a thorough neurological examination from being done, delaying diagnosis and making it more difficult [35].
Diagnosis
Patients with RA, even asymptomatic ones, should undergo an X-ray evaluation in lateral, anteroposterior (AP) and open-mouth odontoid views, and in lateral flexion–extension dynamic projections, to assess cervical spine involvement [12, 36, 37]. Radiographic alterations related to early cervical spine involvement are most commonly represented by odontoid erosions, disc narrowing, and atlantoaxial and subaxial subluxation [38, 39]. X-ray evaluation permits clear identification of bone alignment, quality and deformities, but it has some limitations for identification of bony erosions, the craniovertebral junction (CVJ) and cervicothoracic junctions (because of superimposition of the cranial base structures and of the glenohumeral joints) and soft tissue changes such as pannus and spinal cord compression. In the presence of radiographic alterations in cervical spine involvement and/or in cases of neurological symptoms or cervical pain, computed tomography (CT) scans and/or magnetic resonance imaging (MRI) of the cervical spine are mandatory [11, 40].
CT scanning with multiplanar reconstruction represents the gold-standard method for clear visualization of bone changes (erosions, anatomy, ankylosis and spondylosis) but has limitations for assessment of soft tissues, the spinal cord and nerve roots [11, 41]. MRI evaluation is the most sensitive method for evaluation of cervical spine involvement in RA, especially because it allows better visualization of soft tissue and neural elements. It should be performed in all patients with suspected or confirmed radiographic signs of cervical spine involvement and in patients complaining of neurological symptoms [42, 43] (Fig. 1). Both CT scanning and MRI are fundamental for surgical planning.
As reported above, traditional X-ray evaluation, considering the C1–C2 relationship, includes the ADI; a distance of <3 mm is considered normal. However, patients with RA, even asymptomatic ones, frequently have measurements of 5 mm or even up to 10 mm [39]. In this regard, it has been demonstrated that the PADI is a better predictor of paralysis and recovery. The PADI, in particular, estimates the greater amount of space available for the upper cervical spinal cord [39, 43] (Fig. 2a). The cervical spinal cord occupies 10 mm of the canal diameter; additionally, it needs 1 mm for the dura and 1 mm for the cerebrospinal fluid (CSF) anterior to the cord, and the same posteriorly, for a total of 14 mm. Compression of the cord occurs when the available space is <14 mm. X-ray evaluations should include both PADI and ADI measurements obtained in flexion and extension. Boden et al. [39] observed a high rate of neurological recovery, after fusion and stabilization, in patients with a PADI >14 mm, whereas a PADI <10 mm was related to a worse clinical outcome. However, neither the ADI nor the PADI can be used to assess cord compression caused by soft tissues, such as pannus development in the retro-odontoid space. Thus, spinal cord compression can be present even in the absence of abnormalities in plain X-ray measurements.
The possibility of rotatory AAS should be considered in the presence of asymmetry or lateral displacement of the atlas on the axis by >2 mm in an open-mouth view or in cases of asymmetrical collapse of the lateral atlas mass [44]. Fracture of the dens may be another sign of lateral displacement. A CT scan is the method of choice for confirmation of the diagnosis. Diagnosis of cranial settling through X-ray evaluation can often be very difficult because osseous structures of the cranial base are superimposed upon the landmarks, particularly in the cervical spine [44]. Diagnosis of cranial settling may be further complicated by the presence of erosions of the dens. Plain X-rays generally allow evaluation of the extent of cranial settling in relation to several parameters based on different anatomical landmarks (Fig. 2). Various parameters are used to assess cranial settling with plain X-rays, including McRae’s line, McGregor’s line, the Ranawat index, the Redlund-Johnell value and the Clark stations. The measurements most commonly used are the Ranawat index [45] and the Redlund-Johnell value [46]. Lateral scanning allows clear visualization of the anatomical landmarks used for these measurements (Fig. 2b). An altered Ranawat index most likely matches settling at the C1–C2 level, and the Redlund-Johnell value shows occiput–C2 changes. In 1989, Clark et al. [47] described a method involving identification of three equidistant stations of the odontoid process of the axis. Usually the superior third of the odontoid is at the same level as, or close to, the anterior ring of the atlas. With mild cranial settling, the middle third of the odontoid process is level with the anterior ring of the atlas (station II). With severe cranial settling, the inferior third of the odontoid process is level with the anterior ring of the atlas (station III) [48]. In addition, these authors recommended considering as positive the results of screening for basilar impression when at least one of the following three criteria is positive: the Clark stations, the Redlund-Johnell value or the Ranawat index. Use of these criteria in combination increased the sensitivity to 94% and the negative predictive value to 91%. In the presence of a positive result on at least one of the three criteria, a more detailed study with CT scanning or MRI is mandatory. However, MRI has replaced CT scanning as the evaluation of choice in clinical evaluation because of its greater sensitivity in identifying inflammatory alterations in the joints and synovial changes, especially at the early stages of the disease. MRI allows better visualization of soft tissue, neural structures (the spinal cord and nerve roots) and the contents of the epidural space, providing further information for assessment of spinal cord compression [49].
Through triplanar images, MRI permits clear visualization of facet subluxations, joint damage and dens dislocation [50], giving precise information about CVJ relationships.
MRI is also suggested for assessment of the cervicomedullary angle: in two studies, patients with an angle <135° had a diagnosis of cranial settling and myelopathy [51, 52]. In addition, MRI alterations can be useful for prognosis: T1-weighted spinal cord signal changes are associated with a poor clinical status and also a poor final postoperative outcome [52]. MRI is also useful to evaluate pannus regression after surgical treatment.
Since pannus formation is probably related to articular hypermobility, fusion of the affected joint can lead to pannus regression (especially in cases imaged with contrast enhancement) [53]. Dynamic MRI has been used with the patient in flexed or extended positions and in the traditional neutral position. Roca et al. recommend performing functional MRI in a flexed position in patients with RA with suspected cervical subluxation when routine MRI findings in the neutral position are normal [54]. Other authors have suggested performing functional MRI as a preoperative examination [55].
Management
The goals of RA treatment are symptom relief and a reduction in the disease activity rate. Good control of disease activity is considered to be the primary non-surgical treatment of cervical spine involvement in RA. However, prolonged high-dosage use of corticosteroids represents an independent risk factor for cervical subluxation in RA [56,57,58,59]. Moreover, an elevated incidence of SAS has also been reported in patients not affected by RA, and it is directly associated with the duration of corticosteroid exposure.
In a clinical series of 67 patients it was demonstrated that treatment with disease-modifying antirheumatic drugs (DMARDs) was correlated with a greatly decreased incidence of cervical spine involvement: reductions of 9% in the rate of atlantoaxial subluxation, 4% in the rate of basilar invagination and 2% in the rate of subaxial subluxation were reported [60]. Combination therapy with different DMARDs might further reduce the rates of cervical spine involvement. No cases of atlantoaxial subluxation or basilar invagination were observed in a study of 195 patients with early RA treated with more DMARDs during a follow-up period of 2 years, and only 3.5% of these patients were reported to have subaxial subluxation [61]. These findings suggest that biological DMARDs might be more useful for preventing de novo cervical spine involvement than for slowing the progression of pre-existing pathology [62].
Conservative treatment is the preferred therapeutic strategy in RA patients with cervical spine involvement but without neurological deficits. When neurological symptoms and signs are present, surgical treatment should be considered, evaluating different factors such as age, the severity of the disease and the patient’s general condition. Properly administered anaesthesia is crucial, and surgical treatment should be performed at centres with expertise in treating CVJ abnormalities. Nevertheless, surgical management of RA is a stimulating field. There clearly has been a reduction in cases of mutilating RA involving the CVJ, thanks to steady improvement in surgical procedures.
A successful surgical procedure requires a great deal of expertise to achieve stable decompression of the CVJ. Therefore, a skilled preoperative evaluation, an appropriate choice of surgical procedure, and use of suitable haemostatic and sealant devices and stabilization instruments are required to achieve the best functional result and avoid surgical complications [63, 64].
Enlarged transoral approaches—despite being associated with greater morbidity—are helpful when severe basilar invagination, cranial extension of the lesion or limited jaw mobility are present.
The introduction of endoscopy in transoral surgery and the endoscopic transnasal approach have strongly improved the outcome of RA patients with rapidly progressive myelopathic symptoms. For those patients with chronic symptoms, CVJ fixation procedures seem to be more suitable both for stabilization and for secondary progressive pannus reabsorption [62, 65,66,67,68].
Compliance with Ethical Standards
No financial support was received for this work.
Competing Interests
The authors declare that they have no competing interests.
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–38.
Garrod AE. A treatise on rheumatism and rheumatoid arthritis. London: Griffin’s Medical Series; 1890.
Cha TD, An HS. Cervical spine manifestations in patients with inflammatory arthritis. Nat Rev Rheumatol. 2013;9:423–32.
Agarwal AK, Peppelman WC Jr, Kraus DR, Eisenbeis CH Jr. The cervical spine in rheumatoid arthritis. BMJ. 1993;306:79–80.
Halla JT, Hardin JG, Vitek J, Alarcon GS. Involvement of the cervical spine in rheumatoid arthritis. Arthritis Rheum. 1989;32:652–9.
Reiter MF, Boden SD. Inflammatory disorders of the cervical spine. Spine. 1998;23:2755–66.
Kauppi MJ, Neva MH, Laiho K, Kautiainen H, Luukkainen R, Karjalainen A, et al. Rheumatoid atlantoaxial subluxation can be prevented by intensive use of traditional disease modifying antirheumatic drugs. J Rheumatol. 2009;36(2):273–8.
Del Grande M, Del Grande F, Carrino J, Bingham CO III, Louie GH. Cervical spine involvement early in the course of rheumatoid arthritis. Semin Arthritis Rheum. 2014;43:738–44.
Bland J, Boushey D. Anatomy and physiology of the cervical spine. Semin Arthritis Rheum. 1990;20:1–20.
La Caffinière JY, Seringe R, Roy-Camille R. Étude physiopathologique des lésions ligamentaires graves dans les traumatismes de la charnière occipito-rachidienne. Rev Chir Orthop. 1972;58:11–9.
Krauss WE, Bledsoe JM, Clarke MJ, Nottmeier EW, Pichelmann MA. Rheumatoid arthritis of the craniovertebral junction. Neurosurgery. 2010;66:83–95.
Wasserman BR, Moskovich R, Razi AE. Rheumatoid arthritis of the cervical spine—clinical considerations. Bull NYU Hosp Jt Dis. 2011;69:136–48.
Winfield J, Young A, Williams P, Corbett M. Prospective study of the radiologic changes in hands, feet, and cervical spine in adult rheumatoid disease. Ann Rheum Dis. 1983;42:613–8.
Bouchaud-Chabot A, Lioté F. Cervical spine involvement in rheumatoid arthritis. A review. Joint Bone Spine. 2002;69:141–54.
Lipson S. Rheumatoid arthritis of the cervical spine. Clin Orthop. 1984;182:143–9.
Lipson S. Cervical myelopathy and posterior atlanto-axial subluxations in patients with rheumatoid arthritis. J Bone Joint Surg. 1985;67A:593–7.
Menezes AH, VanGilder JC, Clark CR, El-Khoury G. Odontoid upward migration in rheumatoid arthritis. An analysis of 45 patients with “cranial settling”. J Neurosurg. 1985;63(1):500–9.
Cabot A, Becker A. The cervical spine in rheumatoid arthritis. Clin Orthop. 1978;131:130–40.
Seignon B, Tellart-Chaudeur MO, Gougeon J. Les lesions destructrices du rachis cervical moyen et inférieur au cours de la polyarthrite rhumatoïde. Sem Hôp Paris. 1975;51:1157–66.
Gonzalez A, Icen M, Kremers HM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35:1009–14.
van Gaalen FA, van Aken J, Huizinga TW, et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 2004;50:2113–21.
Anquetil F, Clavel C, Offer G, Serre G, Sebbag M. IgM and IgA rheumatoid factors purified from rheumatoid arthritis sera boost the Fc receptor– and complement-dependent effector functions of the disease-specific anti-citrullinated protein autoantibodies. J Immunol. 2015;194:3664–74.
Reynisdottir G, Olsen H, Joshua V, et al. Signs of immune activation and local inflammation are present in the bronchial tissue of patients with untreated early rheumatoid arthritis. Ann Rheum Dis. 2016;75(9):1722–7.
Harre U, Georgess D, Bang H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Investig. 2012;122:1791–802.
Smolen JS, Aletaha D, Koeller M, Weisman M, Emery P. New therapies for the treatment of rheumatoid arthritis. Lancet. 2007;370:1861–74.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Humby F, Kelly S, Hands R, et al. Use of ultrasound-guided small joint biopsy to evaluate the histopathologic response to rheumatoid arthritis therapy: recommendations for application to clinical trials. Arthritis Rheumatol. 2015;67:2601–10.
Feldmann M, Maini SR. Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev. 2008;223:7–19.
Pettit AR, Ji H, von Stechow D, et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol. 2001;159:1689–99.
Redlich K, Hayer S, Ricci R, et al. Osteoclasts are essential for TNF-α-mediated joint destruction. J Clin Investig. 2002;110:1419–27.
Rawlins BA, Girardi FP, Boachie-Adjei O. Rheumatoid arthritis of the cervical spine. Rheum Dis Clin North Am. 1998;24:55–65.
Dreyer SJ, Boden SD. Natural history of rheumatoid arthritis of the cervical spine. Clin Orthop Relat Res. 1999;366:98–106.
Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–307.
Blom M, Creemers MC, Kievit W, Lemmens JA, van Riel PL. Long-term follow-up of the cervical spine with conventional radiographs in patients with rheumatoid arthritis. Scand J Rheumatol. 2013;42:281–8.
Kim HJ, Nemani VM, Riew KD, Brasington R. Cervical spine disease in rheumatoid arthritis: incidence, manifestations, and therapy. Curr Rheumatol Rep. 2015;17(2):9.
Yurube T, Sumi M, Nishida K, et al. Accelerated development of cervical spine instabilities in rheumatoid arthritis: a prospective minimum 5-year cohort study. PLoS One. 2014;18:e88970.
Zikou AK, Alamanos Y, Argyropoulou MI, Tsifetaki N, Tsampoulas C, Voulgari PV, et al. Radiological cervical spine involvement in patients with rheumatoid arthritis: a cross sectional study. J Rheumatol. 2005;32:801–6.
Kwek TK, Lew TW, Thoo FL. The role of preoperative cervical spine X-rays in rheumatoid arthritis. Anaesth Intensive Care. 1998;26(6):636–41.
Boden SD, Dodge LD, Bohlman HH, Rechtine GR. Rheumatoid arthritis of the cervical spine. A long-term analysis with predictors of paralysis and recovery. J Bone Joint Surg Am. 1993;75(9):1282–97.
Ahn JK, Hwang JW, Oh JM, Lee J, Lee YS, Jeon CH, et al. Risk factors for development and progression of atlantoaxial subluxation in Korean patients with rheumatoid arthritis. Rheumatol Int. 2011;31:1363–8.
Sugita S, Chikuda H, Kadono Y, Ohtsu H, Takeshita K, Nishino J, et al. Clinical characteristics of rheumatoid arthritis patients undergoing cervical spine surgery: an analysis of National Database of Rheumatic Diseases in Japan. BMC Musculoskelet Disord. 2014;15:203.
Zoli A, Priolo F, Galossi A, Altomonte L, Di Gregorio F, Cerase A, et al. Craniocervical junction involvement in rheumatoid arthritis: a clinical and radiological study. J Rheumatol. 2000;27:1178–82.
Joaquim AF, Ghizoni E, Tedeschi H, Appenzeller S, Riew KD. Radiological evaluation of cervical spine involvement in rheumatoid arthritis. Neurosurg Focus. 2015;38:1–7.
Aggarwal A, Kulshreshtha A, Chaturvedi V, Misra R. Cervical spine involvement in rheumatoid arthritis: prevalence and relationship with overall disease severity. J Assoc Physicians India. 1996;44:468–71.
Ranawat CS, O’Leary P, Pellicci P, et al. Cervical spine fusion in rheumatoid arthritis. J Bone Joint Surg Am. 1979;61:1003–10.
Redlund-Johnell I, Pettersson H. Radiographic measurements of the cranio-vertebral region. Designed for evaluation of abnormalities in rheumatoid arthritis. Acta Radiol Diagn (Stockh). 1984;25(1):23–8.
Clark CR, Goetz DD, Menezes AH. Arthrodesis of the cervical spine in rheumatoid arthritis. J Bone Joint Surg Am. 1989;71:381–92.
Riew KD, Hilibrand AS, Palumbo MA, Sethi N, Bohlman HH. Diagnosing basilar invagination in the rheumatoid patient. The reliability of radiographic criteria. J Bone Joint Surg Am. 2001;83-A:194–200.
Tehranzadeh J, Ashikyan O, Dascalos J. Magnetic resonance imaging in early detection of rheumatoid arthritis. Semin Musculoskelet Radiol. 2003;7:79–94.
Stiskal MA, Neuhold A, Szolar DH, Saeed M, Czerny C, Leeb B, et al. Rheumatoid arthritis of the craniocervical region by MR imaging: detection and characterization. Am J Roentgenol. 1995;165:585–92.
Bundschuh C, Modic MT, Kearney F, Morris R, Deal C. Rheumatoid arthritis of the cervical spine: surface-coil MR imaging. AJR Am J Roentgenol. 1988;151:181–7.
Reijnierse M, Breedveld FC, Kroon HM, Hansen B, Pope TL, Bloem JL. Are magnetic resonance flexion views useful in evaluating the cervical spine of patients with rheumatoid arthritis? Skeletal Radiol. 2000;29:85–9.
Kroft LJ, Reijnierse M, Kloppenburg M, Verbist BM, Bloem JL, van Buchem MA. Rheumatoid arthritis: epidural enhancement as an underestimated cause of subaxial cervical spinal stenosis. Radiology. 2004;231:57–63.
Roca A, Bernreuter WK, Alarcon GS. Functional magnetic resonance imaging should be included in the evaluation the cervical spine in patients with rheumatoid arthritis. J Rheumatol. 1993;20(9):1485–8.
Weissman BN, Aliabadi P, Weinfeld MS, et al. Prognostic features of atlantoaxial subluxation in rheumatoid arthritis patients. Radiology. 1982;144(4):745–51.
Lourie H, Stewart WA. Spontaneous atlantoaxial dislocation. A complication of rheumatoid disease. N Engl J Med. 1961;265:677–81.
Mathews JA. Atlanto-axial subluxation in rheumatoid arthritis. A 5-year follow-up study. Ann Rheum Dis. 1974;33:526–31.
Rasker JJ, Cosh JA. Radiological study of cervical spine and hand in patients with rheumatoid arthritis of 15 years’ duration: an assessment of the effects of corticosteroid treatment. Ann Rheum Dis. 1978;37:529–35.
Yonezawa T, Tsuji H, Matsui H, Hirano N. Subaxial lesions in rheumatoid arthritis. Radiographic factors suggestive of lower cervical myelopathy. Spine (Phila Pa 1976). 1995;20:208–15.
Paimela L, Laasonen L, Kankaanpaa E, Leirisalo-Repo M. Progression of cervical spine changes in patients with early rheumatoid arthritis. J Rheumatol. 1997;24:1280–4.
Neva MH, et al. Combination drug therapy retards the development of rheumatoid atlantoaxial subluxations. Arthritis Rheum. 2000;43:2397–401.
Kaito T, et al. Effect of biological agents on cervical spine lesions in rheumatoid arthritis. Spine. 2012;37:1742–6.
Graziano F, Maugeri R, Basile L, Meccio F, Iacopino DG. Aulogous fibrin sealant (Vivostat®) in the neurosurgical practice: part II: vertebro-spinal procedure. Surg Neurol Int. 2016;7(Suppl 3):S77–82.
Maugeri R, Giammalva GR, Graziano F, Iacopino DG. May autologue fibrin glue alone enhance ossification? An unexpected spinal fusion. World Neurosurg. 2016;95:611–2.
Visocchi M, Doglietto F, Della Pepa GM, et al. Endoscope-assisted microsurgical transoral approach to the anterior craniovertebral junction compressive pathologies. Eur Spine J. 2011;20:1518–25.
Visocchi M, Signorelli F, Liao C, Rigante M, Paludetti G, Barbagallo G, et al. Endoscopic endonasal approach for craniovertebral junction pathologies: myth and truth in clinical series and personal experience. World Neurosurg. 2017;101:122–9. https://doi.org/10.1016/j.wneu.2017.01.099.
Visocchi M, Pietrini D, Tufo T, Fernandez E, Di Rocco C. Preoperative irreducible C1–C2 dislocations: intraoperative reduction and posterior fixation. The always posterior strategy. Acta Neurochir. 2009;151(5):551–9.
Visocchi M, Iacopino DG, Signorelli F, Olivi A, Maugeri R. Walk the line. The surgical highways to the craniovertebral junction in endoscopic approaches: a historical perspective. World Neurosurg. 2018;110:544–57. https://doi.org/10.1016/j.wneu.2017.06.125.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ferrante, A. et al. (2019). The Craniovertebral Junction in Rheumatoid Arthritis: State of the Art. In: Visocchi, M. (eds) New Trends in Craniovertebral Junction Surgery. Acta Neurochirurgica Supplement, vol 125. Springer, Cham. https://doi.org/10.1007/978-3-319-62515-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-62515-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62514-0
Online ISBN: 978-3-319-62515-7
eBook Packages: MedicineMedicine (R0)