Abstract
The preoptic-hypothalamo-hypophyseal system of sturgeons, located at the base of the brain, has a neurosecretory role exerted by hypophysiotropic neurons most of them located in the preoptic and hypothalamic periventricular region. The majority of those cells are of the cerebrospinal fluid-contacting type and exhibit short processes reaching the ventricular lumen. Moreover, the processes of those hypophysiotropic neurons course along the hypothalamic floor toward the hypophysis forming a preoptic-hypothalamo-hypophyseal tract. This chapter summarizes available data on the distribution of several hypophysiotropic factors, such as galanin, neurophysin, somatostatin, or gonadotropin-releasing hormone, in the preoptic-hypothalamo-hypophyseal system of sturgeons obtained by the use of immunohistochemical techniques. Immunoreactive neurons to those substances were found in the preoptic and hypothalamic nuclei, and immunoreactive fibers were observed along the preoptic-hypothalamo-hypophyseal tract and in the hypophysis, indicating their hypophysiotrophic role in the brain of sturgeons. Thus, most of the neuropeptides and neurohormones found in tetrapods are also present in sturgeons, suggesting that their common ancestors already possessed such regulatory systems. Unfortunately, because of the difficulty in approaching the physiology of sturgeons (size, cost, etc.), the number of experimental studies aiming at deciphering the roles of such neuropeptides and neurohormones is very limited, although we can speculate that part of the functions supported by these neurohormones would be similar.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 The Anatomy of the Preoptic Area and Hypothalamus of Sturgeons
Studies concerning the cytoarchitecture of the diencephalon of chondrosteans, and in particular of sturgeons, are scarce, and current knowledge concerning the organization of this encephalic region is based on anatomical studies carried out at the beginning of the nineteenth century by Johnston (1901) although it has been more recently analyzed in detail by Nieuwenhuys (1998), Rupp and Northcutt (1998), and Rustamov (2006a, b).
The diencephalon is a complex brain region bounded rostrally by the telencephalon and caudally by the mesencephalon, although there is a long-lasting controversy about where exactly are those boundaries. Briefly, there are two different models of forebrain organization based on developmental studies: the His-Herrick model which proposed that the origin of all brain regions is in a series of longitudinal columns separated by longitudinal sulci and divides the diencephalon into four longitudinally arranged zones, epithalamus, dorsal thalamus, ventral thalamus, and hypothalamus (Herrick 1910, 1933), and the neuromeric model which suggests that the origin of all brain regions is in a series of transverse regions (neuromeres) separated by vertical sulci and that the diencephalon arises from a rostral parencephalic neuromere but does not include the preoptic area and the hypothalamus which together with the telencephalon are considered part of the secondary prosencephalon (Puelles and Rubenstein 1993). Thus, as in chondrosteans there are no data on neither embryological nor gene expression which could delimitate diencephalic boundaries; most authors have organized the diencephalon of sturgeons as suggested by Braford and Northcutt (1983) for the diencephalon of ray-finned fishes. Braford and Northcutt (1983) follow the His-Herrick model and thus subdivide the diencephalon into the epithalamus, thalamus, and hypothalamus and include the entire preoptic region in the diencephalon. However, Nieuwenhuys (1998), although basically following His-Herrick model for the rest of the diencephalic regions, assigns the preoptic region to the telencephalon, following classic anatomical studies by Johnston (1901). In this review we will follow the organization suggested by Braford and Northcutt (1983) for the diencephalon of ray-finned fishes, i.e., we will include the entire preoptic region in the diencephalon.
1.1 Preoptic Area
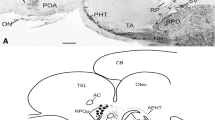
The preoptic area is the most rostral part of the diencephalon. It surrounds the preoptic recess of the third ventricle and lies between the anterior commissure rostrally and the optic chiasm caudally (Fig. 13.1a). This area is characterized by the periventricular position of most of its cells, containing three periventricular nuclei located around the preoptic recess: the parvocellular preoptic nucleus, the most rostral diencephalic nucleus with small cells arranged in a few rows parallel to the ventricular surface (Fig. 13.1b–f); the magnocellular preoptic nucleus, with large neurosecretory cells occupying a central position in the preoptic region dorsal to the rostral portion of the optic chiasm (Fig. 13.1f); and the suprachiasmatic nucleus, with small densely arranged cells situated dorsal to the optic chiasm. Moreover, there is a migrated nucleus, the entopeduncular nucleus, with small loosely arranged cells. In the parvocellular preoptic nucleus, Rupp and Northcutt (1998) distinguished an anterior and a posterior part. Along the ventral part of the anterior wall of the preoptic recess is located the organum vasculosum of the lamina terminalis (OVLT, Fig. 13.1d, e), one of the circumventricular organs present in all vertebrate groups.
Micrographs of hematoxylin-eosin-stained sagittal (a) and transverse (b–j) sections through the brain of the Siberian sturgeon Acipenser baerii. (a) Sagittal section through the entire brain of Acipenser. Lines with the letters at the top indicate the levels of the figures (b–j). (b) Section through the rostral preoptic area showing the small cells of the parvocellular preoptic nucleus (NPOp) arranged in a few rows parallel to the ventricular surface around the preoptic recess (PR). (c) Detail of figure (a) showing the NPOp. (d) Section through the caudal preoptic area showing the small cells of the NPOp and the large cells of the magnocellular preoptic nucleus (NPOm) which occupy a central position in the preoptic region dorsal to the rostral portion of the optic chiasm (OC). Abundant cells are located along the ventral part of the anterior wall of the preoptic recess, in the organum vasculosum of the terminal lamina (OVLT). (e) Detail of figure (d) showing the abundant cells in the NPOp and in the OVLT. (f) Section through the preoptic area at a more caudal level than figure (d) showing the NPOp and the NPOm (arrowheads). (g) Section through the rostral hypothalamus showing the inferior hypothalamic lobes (HL), which surround the hypothalamic lateral recesses (LR). Along the ventromedial walls of the lateral recesses, the third ventricle gives rise to the infundibulum (i) in which more rostral walls the anterior tuberal nucleus (NAT) is located. (h) Detail of the figure (g) showing the major part of hypothalamic neurons located in the ventricular surface around the infundibulum and the lateral recesses. (i) Section through the hypothalamus showing the position of the cells of the lateral tuberal nucleus (NLT) at rostral levels. (j) Section through the caudal hypothalamus showing the NLT surrounding the posterior recess (POR), the median eminence (ME), the hypophysis (H), and the saccus vasculosus (SV). Inset: coronet cells in the floor of the hypothalamus. Abbreviations: CB, corpus cerebelli; h, habenula; H, hypophysis; HL, hypothalamic lobes; i, infundibulum; LR, lateral hypothalamic recess; ME, median eminence; MO, medulla oblongata; NAT, anterior tuberal nucleus; NLT, lateral tuberal nucleus; NPOm, magnocellular preoptic nucleus; NPOp, parvocellular preoptic nucleus; OB, olfactory bulb; OC, optic chiasm; OT, optic tectum; OVLT, organum vasculosum of the terminal lamina; POR, posterior recess; PR, preoptic recess; PT, posterior tubercle; SV, saccus vasculosus; T, telencephalon; vc, valvula cerebelli. Scale bars: 2 mm (a); 1 mm (g); 500 μm (b, d, h); 200 μm (c, e, i–j); 100 μm (f); 50 μm (inset in j)
1.2 Hypothalamus
During brain ontogenesis, the hypothalamus differentiates relatively late in sturgeons showing the normal histological pattern of the adult at 90 days post-hatching and reaching a considerable size compared to other parts of the brain (Vázquez et al. 2002; Gómez et al. 2009). Due to its extremely enlarged ventricle, the hypothalamus forms the largest and the most ventral diencephalic structure in sturgeons (Figs. 13.1a and 13.2), and it comprises a large paired rostral region which is bordered dorsally by the preoptic area and by the posterior tubercle and a much smaller unpaired caudal portion which is continuous with the pituitary and the saccus vasculosus (Figs. 13.1a, g–j and 13.2). The major part of hypothalamic neurons of the sturgeons is located adjacent to the ventricular surface, with only a few migrated cells situated more laterally (Figs. 13.1g–j and 13.2a). Most of the neurons located in the periventricular zone are of the cerebrospinal fluid-contacting (CSF-C) type. Such neurons exhibit an apical dendrite that ends by a ventricular bulb (Fig. 13.1c, e–f). These CSF-C cells are really abundant in the hypothalamus of the sturgeons, but they represent a “primitive” character that tends to disappear in the course of evolution. Bony fish exhibit much less CSF-contacting cells.
(a) Detail of Fig. 13.1a showing a hematoxylin-eosin-stained sagittal section through the hypothalamus of the Siberian sturgeon Acipenser baerii. (b) Topography of the hypothalamo-hypophyseal neurosecretory system in Acipenseridae. Figure (b) was modified from Polenov and Garlov (1971). Abbreviations: i, infundibulum; Nh, neurohypophysis; OC, optic chiasm; PA, preoptic area; Pd, pars distalis of the hypophysis; Pi, pars intermedia of the hypophysis; PNCR, proximal neurosecretory contact region; POR, posterior recess; PR, preoptic recess; PT, posterior tubercle; SV, saccus vasculosus. Scale bar: 500 μm
In the rostral hypothalamus, the walls are rather thin and laterally evaginate to form the inferior or lateral hypothalamic lobes, which surround the hypothalamic lateral recesses, a pair of diverticula in the midrostral portion of the third ventricle (Fig. 13.1g–h). In sturgeons, these inferior hypothalamic lobes are much larger than in other chondrosteans and in other fish groups (Fig. 13.1g–h) (Nieuwenhuys 1998; Rupp and Northcutt 1998). Different nomenclatures for the diverse hypothalamic regions of sturgeons have been used in cytoarchitectonic studies. Thus, Nieuwenhuys (1998) divided the rostral hypothalamus of the shovelnose sturgeon (Scaphirhynchus) into two longitudinal periventricular regions: a hypothalamic dorsal part, extending along the dorsal and lateral walls of the lateral recesses, which corresponds to the dorsal and lateral parts of the periventricular hypothalamus of Acipenser (Fig. 13.1a, g–h, Rupp and Northcutt 1998), and an hypothalamic ventral part, extending along the ventromedial walls of the lateral recesses where the third ventricle gives rise to the infundibulum (Fig. 13.1a, g–i) in which more rostral walls the anterior tuberal nucleus is located (Fig. 13.1g–h). This ventral part of Scaphirhynchus corresponds to the ventral part of the periventricular hypothalamus in Acipenser (Rupp and Northcutt 1998).
The caudal portion of the hypothalamus is unpaired and surrounds the hypothalamic posterior recess, located in the caudal portion of the third ventricle, which displays small, laterally directed diverticula (Figs. 13.1a, j and 13.2). The caudoventral region of the posterior recess suffers narrowness in the most ventral region which leads to a cavity lined by a thin and strongly folded epithelium, named saccus vasculosus (SV). This structure whose function remains unclear is present in many jawed fish, including chondrosteans (Figs. 13.1a, j and 13.2). The epithelium forming the wall of this organ contains CSF-C cells, supporting cells, and numerous coronet cells, a highly specialized cell type exclusive to the saccus vasculosus of jawed fishes and which is probably glial in nature (Fig. 13.1j) (Arochena et al. 2004; Sueiro et al. 2007). Coronet cells are characterized by a round or pear-shaped perikarya with a short, thick apical process that protruded into the ventricle bearing a crown of globule-tipped cilia (inset in Fig. 13.1j). Unlike other fishes, coronet cells of sturgeons are not confined to the saccus vasculosus, and they are distributed over the entire floor of the hypothalamus and also on the preoptic recess (Kotrschal et al. 1983). The function of this organ remained intriguing for a long time, and it has been suggested to be involved in sensory and transport functions as well as secretory functions (e.g., liquor pressure perception, osmoregulation, calcium homeostasis, glucose loading, and transcellular ion exchange between blood vessels and CSF). However, recently, it has been suggested that the SV is implicated in the regulation of photoperiodism in fish (Nakane et al. 2013). However, we cannot exclude the possibility that the SV serves other physiological functions such as neuroendocrine, as suggested by the localization of thyroid-stimulating hormone in the coronet cells (Nakane et al. 2013) or the expression of brain aromatase shown in trout (Menuet et al. 2003). Additionally, the SV and the hypothalamus are connected to each other by afferent and efferent fiber systems (Yáñez et al. 1997; Sueiro et al. 2007).
In the dorsolateral and ventral periventricular walls of the posterior recess of Scaphirhynchus lies the lateral tuberal nucleus (Nieuwenhuys 1998), which corresponds to the caudal zone of the periventricular hypothalamus of Acipenser (Fig. 13.1i–j, Rupp and Northcutt 1998). Caudally, the dorsal area progressively forms the posterior tuberal nucleus in both sturgeon species (Nieuwenhuys 1998; Rupp and Northcutt 1998).
The hypothalamic organization described for sturgeons, characterized by laterally expanded inferior lobes and thin hypothalamic walls, also exists in chondrichthyans (Smeets et al. 1983), but not in other ray-finned fishes, so the lateral expansions in sharks and sturgeons must be viewed as independently evolved parallel features rather than as homologous structures as it was suggested by Northcutt (1995). However, in the sturgeons, as in all ray-finned fishes, the most conserved feature of the hypothalamus is the periventricular cellular zone which exhibits basically the same type of organization in other fish groups (Rustamov 2006b).
1.3 Hypothalamo-Hypophyseal Relationships
In sturgeons, as in all vertebrates, the hypophysis or pituitary gland is attached to the caudoventral region of the hypothalamus (Figs. 13.1a, g–j and 13.2). The hypophysis of sturgeons, flat and elongated in the rostral-caudal direction, is composed of the adenohypophysis located rostrally and the neurohypophysis located caudally close to the transition of the infundibulum to the saccus vasculosus. The adenohypophysis is the glandular part and is mainly composed of secretory cells, while the neurohypophysis represents the neural part of the gland and consists of neurosecretory terminals originating from the hypothalamus and other brain regions (Fig. 13.2) (Grandi and Chicca 2004). During brain ontogenesis, although the hypothalamus differentiates relatively late, the adenohypophysis is already evident on the floor of the diencephalon at hatching time but reaches the adult morphology at 5 months of age, while the neurohypophysis does not begin its development until 80 days post-hatching and does not reach the adult morphology before 9 months of age (Grandi and Chicca 2004; Gómez et al. 2009).
A comprehensive description of the hypothalamo-hypophyseal system was reported in the classical studies of Russian researchers (Sathyanesan and Chavin 1967; Polenov and Garlov 1971, 1973; Polenov et al. 1972, 1976, 1983, 1997; Polenov and Pavlovic 1978; Belenky et al. 1985). The adenohypophysis in sturgeons consists in a pars distalis and a pars intermedia, while the neurohypophysis is included in a neurointermediate lobe consisting in tubular rootlike processes of the bottom of the infundibular wall that penetrate among the cell cords of the pars intermedia (Fig. 13.2b). The distribution of the pituitary endocrine cells was studied by immunocytochemical techniques in the adenohypophysis of different sturgeon species, and seven types of endocrine cells were identified: the adrenocorticotropic, prolactin, growth hormone, gonadotropic and thyroid-stimulating hormone cells in the pars distalis, and the melanocyte-stimulating hormone and somatolactin cells in the pars intermedia (Hansen 1971; Hansen and Hansen 1975; Pelissero et al. 1988; Joss et al. 1990; Amemiya et al. 1999).
Moreover, in chondrosteans, the hypothalamic floor, located rostroventrally just dorsal to the pars distalis of the adenohypophysis, differentiates into a typical median eminence named proximal neurosecretory contact region (Fig. 13.2) (Polenov et al. 1976; Kotrschal et al. 1985). In the space between this region and the pars distalis, there are blood vessels that form part of a hypothalamo-hypophyseal portal system where numerous unmyelinated neurosecretory fibers form synaptic endings and, as in other vertebrates, discharge their products into the portal circulation. There are two types of those fibers that are also found among the processes of the neurohypophysis: Type A, peptidergic and originate in the preoptic area (magnocellular preoptic nucleus) and Type B, monoaminergic and presumably originate in the hypothalamus (lateral tuberal nucleus, Polenov et al. 1972). Thus, both the peptide and monoamine neurohormones along the way hypothalamic neurons regulate the activity of the glandular cells in the pars distalis (Polenov et al. 1976).
2 Hypophysiotropic Factors in the Preoptic-Hypothalamo-Hypophyseal System of Sturgeons
The presence of numerous cells and fibers containing different hypophysiotrophic factors, such as corticoliberin (Belenky et al. 1985), corticotropin-releasing factor (CRF, González et al. 1992), gonadotropin-releasing hormone (GnRH, Leprêtre et al. 1993; Amiya et al. 2011), neuropeptide Y (NPY, Chiba and Honma 1994; Amiya et al. 2011), methionine-enkephalin (Met-enk, Rodríguez-Moldes et al. 1997), melanin-concentrating hormone (MCH, Baker and Bird 2002), serotonin (5-HT, Adrio et al. 1999; Piñuela and Northcutt 2007), tyrosine hydroxylase (TH, Adrio et al. 2002), neurophysin (NPH, Adrio et al. 2005), galanin (GAL, Adrio et al. 2005; Amiya et al. 2011), substance P, dopamine and leucine-enkephalin (SP, DA, Leu-enk, Piñuela and Northcutt 2007), and somatostatin (SOM, Adrio et al. 2008), has been observed in the preoptic region and the rostral and caudal hypothalamus of the sturgeons by the use of immunohistochemical techniques. All these neuromodulators showed high concentrations and had a similar distribution in the hypothalamus of all the sturgeon species studied. Although some neurons immunoreactive to those factors were observed in nuclei located away from the ventricle, most of them were located in the periventricular region, and they were of the CSF-C type. These CSF-C cells containing those factors were observed both in the preoptic area and in the hypothalamus of all the sturgeon species studied, and they were very abundant in the parvocellular preoptic nucleus; in the dorsal, lateral, and ventrolateral walls of the lateral recesses (lateral and dorsal periventricular hypothalamus of Rupp and Northcutt 1998); and in the tuberal and caudal hypothalamus where they were located in the ventromedial walls of the infundibulum, corresponding to the position of the anterior and lateral tuberal nuclei (ventral periventricular hypothalamus of Rupp and Northcutt 1998). CSF-C cells are considered phylogenetically ancient in type and may play an important role in the neurotransmission and/or neuromodulation of neuroendocrine pathways in fish (Vigh-Teichmann et al. 1983). In fact, these hypothalamic CSF-C cells showed an intense staining to the different hypophysiotrophic factors studied in both the subventricular perikarya and ventricular processes.
Furthermore, the basal processes of these CSF-C neurosecretory cells coursed ventrolaterally toward the external surface and along the hypothalamic floor toward the hypophysis forming a preoptic-hypothalamo-hypophyseal tract, where fibers immunoreactive to neuropeptides (corticoliberin (Belenky et al. 1985), CRF (González et al. 1992), GnRH (Leprêtre et al. 1993), NPY (Chiba and Honma 1994), Met-enk (Rodríguez-Moldes et al. 1997), MCH (Baker and Bird 2002), GAL and NPH (Adrio et al. 2005), SOM (Adrio et al. 2008), and Leu-enk (Adrio, unpublished results) were observed in different sturgeon species. Some of these fibers innervate the proximal neurosecretory contact region, and others coursed more caudally in the thin floor of the hypothalamus and the hypophyseal stalk toward the neurointermediate lobe of the neurohypophysis, where they appear close to glandular cells of the pars intermedia. Although the origin of the hypothalamus-hypophyseal projections in the sturgeon has not been studied experimentally, these fibers could arise, at least in part, from neurosecretory neurons observed in the preoptic and/or the hypothalamic region, such as those of the anterior and lateral tuberal nuclei, as previously reported in the hypothalamus of teleosts (Holmqvist and Ekström 1995). In fact, the preoptic nuclei and the lateral tuberal nucleus were described in Acipenser fulvescens as neurosecretory nuclei by Sathyanesan and Chavin (1967) who used Gomori’s aldehyde fuchsin method for neurosecretion (a classical marker of the hypothalamo-hypophyseal neurosecretory system), and, more recently, cells immunoreactive to neurophysin (NPH-ir) were described in the preoptic (magnocellular preoptic nucleus, Figs. 13.3b, c and 13.4k, l) and hypothalamic (anterior and lateral tuberal nuclei, Figs. 13.3d–f and 13.5e) areas in Acipenser baerii suggesting that both regions contain neurosecretory cells related with the hypothalamo-hypophyseal system (Adrio et al. 2005). Therefore, the presence of different hypophysiotrophic factors in those nuclei appears general to fishes (see references in Adrio et al. 2005).
Schematic drawings of transverse sections (a–h) through the preoptic region and hypothalamus of Acipenser baerii (from rostral to caudal) showing the distribution of neurons (solid circles) and fibers (dotted areas) immunoreactive to galanin (GAL), serotonin (5-HT), tyrosine hydroxylase (TH), or somatostatin (SOM). On the left, neurons (solid circles) and fibers (dotted areas) immunoreactive to neurophysin (NPH) are shown, and the main anatomical regions are also indicated. The levels of the sections are indicated in a lateral view of the brain on the top. Correspondence with photomicrographs in other figures is indicated by boxed areas. Schematic drawings were modified from Adrio et al. (1999, 2002, 2005, 2008). Abbreviations: CB, corpus cerebelli; CP, choroid plexus; Dd, dorsal part of the dorsal telencephalon; Dl, lateral part of the dorsal telencephalon; FR, fasciculus retroflexus; h, habenula; H, hypophysis; HL, hypothalamic lobes; i, infundibulum; III, third ventricle; IIIn, oculomotor nucleus; IV, fourth ventricle; IVn, trochlear nucleus; LR, lateral hypothalamic recess; ME, median eminence; MLF, medial longitudinal fascicle; MO, medulla oblongata; MPT, medial nucleus of the posterior tubercle; NAT, anterior tuberal nucleus; NIL, neurointermediate lobe of the hypophysis; NLT, lateral tuberal nucleus; NPOm, magnocellular preoptic nucleus; NPOp, parvocellular preoptic nucleus; NPT, posterior tuberal nucleus; NRP, posterior recess nucleus; OB, olfactory bulb; OC, optic chiasm; OT, optic tectum; OVLT, organum vasculosum of the terminal lamina; P, pineal organ; PC, posterior commissure; Pd, pars distalis of the hypophysis; Pi, pars intermedia of the hypophysis; POR, posterior recess; PR, preoptic recess; Pt, pretectum; PT, posterior tubercle; SV, saccus vasculosus; T, telencephalon; Td, dorsal thalamus; TG, mesencephalic tegmentum; tl, torus longitudinalis; TPp, periventricular nucleus of the posterior tubercle; vc, valvula cerebelli; VM, ventromedial thalamic nucleus. Scale bars = 1 mm (lateral view), 500 μm (sections)
Transverse sections through the preoptic region of Acipenser. (a–e) Sections at the rostral level of the preoptic region showing CSF-C cells immunoreactive to GAL (a–b), 5-HT (c), TH (d) or SOM (e) in the parvocellular preoptic nucleus (NPOp, note the typical apical dendrite of these CSF-C cells, arrowheads in b–e) and the dense GAL-ir innervation in the ventrolateral region (a, asterisk). (f–l) Sections at the caudal level of the preoptic region showing abundant CSF-C cells immunoreactive to GAL (f–g), 5-HT (h), TH (i), or SOM (j) in the parvocellular preoptic nucleus (NPOp) and to NPH in the rostral (k) and caudal (l) magnocellular preoptic nucleus (NPOm). In the NPOm not all NPH-ir cells were of the CSF-C type (arrows in k–l). Note GAL-ir fibers in the organum vasculosum of the lamina terminalis (f, OVLT) and the dense GAL-ir innervation in the ventrolateral region (f, asterisk). The levels of the sections correspond to those of Fig. 13.3a (a–e), 13.3b (f–k), and 13.3c ((l)). Abbreviations: NPOm, magnocellular preoptic nucleus; NPOp, parvocellular preoptic nucleus; OVLT, organum vasculosum of the terminal lamina; PR, preoptic recess. Scale bars = 500 μm (f), 250 μm (a), 100 μm (b–c, g–i, k–l), 50 μm (d–e, j)
Transverse sections through the rostral hypothalamus of Acipenser. (a–b) Sections showing GAL-ir CSF-C cells in the anterior tuberal nucleus (NAT). The basal process of these GAL-ir cells appeared to end in the median eminence (asterisk) close to the pars distalis of the hypophysis (Pd). (c) Section showing abundant 5-HT-ir fibers that innervate the NAT and scarce 5-HT-ir fibers in the median eminence (asterisk). (d) While TH-ir fibers are very abundant in the median eminence (asterisk), only a few innervate the NAT. (e) Detail of scarce NPH-ir CSF-C cells in the NAT (arrows) and abundant NPH-ir fibers in the median eminence (asterisk). (f) Detail of SOM-ir CSF-C cells in the NAT. Note the abundant SOM-ir fibers in the median eminence (asterisk) and the presence of SOM-ir cells in the Pd (arrowheads). The level of the sections corresponds to that of Fig. 13.3e. Abbreviations: i, infundibulum; NAT, anterior tuberal nucleus; Pd, pars distalis of the hypophysis. Scale bars = 200 μm (a), 100 μm (b-f)
In addition, the presence of cells immunoreactive to hypophysiotrophic factors such as CRF (González et al. 1992), NPY (Chiba and Honma 1994), Met-enk (Rodríguez-Moldes et al. 1997), MCH (Baker and Bird 2002), and SOM (Adrio et al. 2008), in the pars distalis of the sturgeon, suggests that these factors are released to the blood in the adenohypophysis and have peripheral functions. Alternatively, some of these factors could have local actions in the pituitary.
Therefore, the hypothalamus of the sturgeons contains neurosecretory cells related to the hypophysis and most likely acting as hypophysiotropic factors modulating the release of adenohypophyseal hormones. However, the hypophysis-related CSF-C neuronal groups of the sturgeon hypothalamus are heterogeneous in terms of their neurochemical content, and, therefore, different types of CSF-C cells could play different roles in neurosecretion and neurotransmission (Adrio et al. 2005).
2.1 Galaninergic System
Galanin is a 29-amino acid peptide widely distributed in the central nervous system of vertebrates, and the hypothalamus is particularly rich in galanin-synthesizing neurons and nerve processes. Depending on the destination of galanin in nerve terminals, this neuropeptide may function as a neuromodulator/neurotransmitter when it innervates other neurons or as a hypophysiotropic messenger when it is released into the hypothalamo-hypophyseal portal circulation and reaches the anterior pituitary (Merchenthaler et al. 2013). The presence of galaninergic structures in the preoptic-hypothalamic regions seems to be highly conserved among vertebrates. Thus, the location of most of the galanin-immunoreactive (GAL-ir) cell bodies in the preoptic-hypothalamic area and the high galaninergic innervation observed in the hypophysis of all of the vertebrates studied so far (fish, amphibians, reptiles, birds, and mammals; see Adrio et al. (2005) and Mensah et al. (2010) for a review), and the observation that galanin may directly influence hormone release from the pituitary gland (Murakami et al. 1987; Maiter et al. 1990; López et al. 1991; Rao et al. 1996), has led to consider it as a hypophysiotropic peptide in both mammal and nonmammalian vertebrates, including humans (see Mechenthaler (2008), Merchenthaler et al. (2013) and Mensah et al. (2010) for a review). Moreover, galanin expression appears to be modulated by gonadal steroids both in mammals (Park et al. 1997; Rugarn et al. 1999; Shen et al. 1999; Scheffen et al. 2003; Splett et al. 2003) and in teleosts (Olivereau and Olivereau 1991b), which suggests that this peptide is involved in sexual and reproductive behavior. Therefore, fish galanin is implicated in the regulation of various physiological functions, such as feeding, growth, reproduction, or hormone release from the pituitary gland and gonads, and has an important role in the neuroendocrine integration of those functions in fishes (see Mensah et al. (2010) for a review).
Immunohistochemical studies on the presence of galanin in the brain of nonmammalian vertebrates mainly used fish as the model organism. In fact, the distribution of GAL-ir cells and fibers was studied in the central nervous system of cyclostomes, elasmobranchs, chondrosteans, and teleosts (see references in Mensah et al. 2010), and the majority of the galaninergic neurons were located in the preoptic-hypothalamic regions in all fish species studied. Thus, the highest density of GAL-ir neurons, most of them CSF-C cells, was observed in the preoptic area and the hypothalamus of the sturgeon brain (Adrio et al. 2005). In the periventricular region, GAL-ir cells extended along the parvocellular preoptic nucleus bordering the preoptic recess (Figs. 13.3a, b and 13.4a, b, f, g); the lateral, ventrolateral, and dorsal walls of the lateral recesses (Fig. 13.3d–f); along the laterodorsal walls of the posterior recess (posterior recess nucleus, Fig. 13.3g); and in the ventromedial walls of the infundibulum corresponding to the position of the anterior (Figs. 13.3d, e and 13.5a, b) and lateral (Figs. 13.3f, g and 13.6a, b) tuberal nuclei (Adrio et al. 2005). The basal processes of these GAL-ir cells coursed ventrolaterally toward the external surface and along the ventral hypothalamus probably contributing to the dense plexus of GAL-ir fibers observed at the level of the median eminence (Figs. 13.3d–g, 13.5a, b and 13.6a–c) and more caudally in the neurointermediate lobe of the hypophysis (Figs. 13.3h and 13.7a, b) (Adrio et al. 2005). These GAL-ir fibers projecting from the preoptic-hypothalamic region onto the pituitary observed in the sturgeon are well characterized in fish (Cornbrooks and Parsons 1991a, b; Moons et al. 1991; Olivereau and Olivereau 1991a; Anglade et al. 1994; Power et al. 1996; Rodríguez et al. 2003; Rodríguez Díaz et al. 2011) and mammals (Ch'ng et al. 1985; Arai et al. 1990; Gai et al. 1990).
Transverse sections through the caudal hypothalamus of Acipenser. (a) Section through the tuberal region showing abundant GAL-ir CSF-C cells in the lateral tuberal nucleus (NLT) and GAL-ir fibers coursing in the hypothalamic floor toward the neurohypophysis (arrows). (b) Section through the caudal hypothalamus showing some GAL-ir CSF-C cells in the NLT (empty arrowheads) and GAL-ir fibers coursing in the hypothalamic floor toward the neurohypophysis (arrows). Note also the typical thick apical process of coronet cells, which were not GAL-ir (arrowheads). (c) Detail of GAL-ir fibers coursing in the hypothalamic floor toward the neurohypophysis. Note that coronet cells were not GAL-ir (asterisk). (d) Detail of 5-HT-ir CSF-C cells in the NLT and dense serotoninergic innervation in the median eminence (ME). (e) Detail of TH-ir CSF-C cells in the NLT. Note the very long apical dendrites exhibited by these cells (arrowheads). (f) Detail of TH-ir fibers coursing in the hypothalamic floor toward the neurohypophysis. (g) SOM-ir CSF-C cells in the NLT and SOM-ir cells in the pars distalis of the hypophysis (arrowheads). Note the dense SOM-ir fibers in lateral regions and in the ME. (h) Detail of very scarce SOM-ir fibers coursing in the hypothalamic floor toward the neurohypophysis. Coronet cells were not SOM-ir (asterisk). The level of the sections corresponds to that of Fig. 13.3g. Abbreviations: ME, median eminence; NLT, lateral tuberal nucleus; POR, posterior recess. Scale bars = 100 μm (a-b, d-f), 50 μm (c, h)
Transverse sections through the caudal region of the hypophysis of Acipenser showing fibers immunoreactive to GAL (a–b), 5-HT (c), TH (d), NPH (e), or SOM (f) in the neurointermediate lobe (NIL) of the neurohypophysis near the glandular cells of the pars intermedia (Pi) of the adenohypophysis. The level of the sections corresponds to that of Fig. 13.3g. Scale bars = 100 μm (a, d, e), 50 μm (c, f), 25 μm (b)
Numerous studies in mammals indicate that galanin is functionally related to other neuroactive substances, and the activity of GAL-ir neurons is regulated by afferents containing different neuropeptides, catecholamines, and indolamines and by hormones acting via their corresponding membrane or nuclear receptors (see Merchenthaler 2010 for a review).
Anatomical relations between galanin and other hypophysiotrophic factors, such as NPH, 5-HT, and catecholamines (Adrio et al. 2005) or NPY (Amiya et al. 2011), were studied in the sturgeon brain. GAL, NPH, 5-HT, and catecholamines (TH) are related in the hypophysiotrophic nuclei, such as preoptic (Figs. 13.3a, b and 13.4a–d, f–l) and tuberal nuclei (Figs. 13.3d–g, 13.5a–e and 13.6a–f) and hypophysis (Figs. 13.3g, h and 13.7a–e) (Adrio et al. 1999, 2002, 2005). These cell populations are clearly overlapped in the sturgeon (Figs. 13.3, 13.4, 13.5, 13.6 and 13.7), but double immunolabeling experiments which have compared the location of GAL-ir neurons with that of these NPH-ir neurons have revealed no coexistence of GAL and NPH in preoptic and tuberal cells of Acipenser, although GAL-ir buttons are surrounding NPH-ir cells in the NPOp (see Fig. 9A–C already published in Adrio et al. 2005). However, the comparison of the location of GAL-ir neurons with that of these 5HT-ir or TH-ir neurons shows partial codistribution in some cells of the NPOp (see Figs. 9D–F and I already published in Adrio et al. 2005), but not in the hypothalamus, although in the rodent brain most of the GAL-ir neurons located in the hypothalamic arcuate nucleus also contained TH (Melander et al. 1986) or GAL-ir fibers made direct contact on TH-ir neuronal cell bodies of that nucleus (Kageyama et al. 2008; Merchenthaler et al. 2013). As far as we know, there are no studies that evidence a functional or anatomical relationship of galaninergic, serotoninergic, and catecholaminergic cells of the vertebrate preoptic/hypothalamic region, although it has been demonstrated that galanin modulates the metabolism of serotonin and dopamine in several brain areas of mammals (Fuxe et al. 1988; Jansson et al. 1989) and catecholaminergic fibers regulate the activity of GAL-ir hypothalamic neurons in rodents and humans (Merchenthaler et al. 2013). Therefore, the relationship between galaninergic, serotoninergic, and catecholaminergic cells observed in the preoptic and tuberal areas of the sturgeon could represent a primitive condition, but more studies in different vertebrate species will be necessary to prove this.
Galanin and NPY are among the most abundant neuropeptides in the hypothalamus of vertebrates, and their role in the regulation of the secretory activity of the anterior pituitary has been well established (see Merchenthaler et al. 2010 for a review). Although in the sturgeon hypothalamus both neuropeptides have a similar distribution (Chiba and Honma 1994; Adrio et al. 2005), the study of the interaction between galanin and NPY did not show neurons containing both neuropeptides, but NPY-ir fibers in close contact with GAL-ir neurons of the anterior tuberal nucleus were observed (Amiya et al. 2011). These results suggest the existence of reciprocal connections between the NPY-ir and GAL-ir neurons in the brain of the sturgeon which may mediate effects of NPY on neuronal systems innervated by galanin and therefore may play a pivotal role in the regulation of reproduction, growth, energy, and metabolism, as it was suggested for teleosts (Volkoff et al. 2005; Amano et al. 2009) and mammals (Horvath et al. 1996; Takenoya et al. 2002; Merchenthaler et al. 2010) where numerous NPY-ir nerve terminals also surrounded the majority of the GAL-ir neurons in the hypothalamus.
Finally, a possible coexistence of GAL and other neuroactive substances in nerve terminals in the median eminence and in the neurohypophysis must also be considered taking in account that GAL-ir, NPH-ir, 5-HT-ir, TH-ir, and NPY-ir cell populations are overlapped in hypophysiotrophic nuclei of the sturgeon and which basal processes coursed ventrolaterally and along the hypothalamic floor toward the pituitary (Figs. 13.3g, h, 13.4a, f, 13.5a–e, 13.6a–e, f and 13.7a–e) (Chiba and Honma 1994; Adrio et al. 1999, 2002, 2005; Amiya et al. 2011). There are no data about this possible colocalization which will indicate an interaction of those substances in the neuroendocrine system of chondrosteans. For instance, the presence of GAL-ir and NPY-ir fibers in the sturgeon hypothalamic floor and the median eminence (Chiba and Honma 1994; Adrio et al. 2005) could indicate that both neuropeptides play roles in the neuronal circuitry regulating the secretion of hormones and have a similar effect on the hypothalamo-pituitary-gonadal axis as it was reported in mammals (see Merchenthaler et al. 2010 for a review).
2.2 Somatostatinergic System
Somatostatin (SOM) is a neuropeptide that is widely distributed in the central nervous system of vertebrates. In mammals, two major forms of SOM (SOM-14 and SOM-28) are produced by tissue-dependent processing of the same precursor protein (Bohlen et al. 1980; Patzelt et al. 1980; Schindler et al. 1996). The primary structure of SOM-14 has been strongly conserved during evolution, although different molecular forms with similar biological activity have been found (Tostivint et al. 2004). Two isoforms of SOM-14 (SS1, which is identical to mammalian S14, and SS2, which is a variant with one amino acid changed ([Pro2] S14)) have been characterized in sturgeon (Nishii et al. 1995; Kim et al. 2000; Li et al. 2009). Moreover, two SOM precursors, which are encoded by two distinct genes, have been characterized in sturgeons: PSS1, which generates SOM-14, and PSS2, which gives rise to the [Pro2] SOM-14 variant (Trabucchi et al. 2002). In situ hybridization studies have demonstrated that the two SOM precursors (PSS1 and PSS2) are differentially expressed in numerous regions of the sturgeon brain (Trabucchi et al. 2002).
Numerous immunohistochemical studies have demonstrated the wide distribution of SOM in the central nervous system of many vertebrate taxa, including fishes (see references in Adrio et al. 2008 and Coveñas et al. 2011). SOM-immunoreactive (SOM-ir) cells and fibers are widely distributed throughout the central nervous system of the Siberian sturgeon (Acipenser baerii) where most SOM-ir cells were found in the preoptic area and hypothalamus as observed in the brain of other fish and tetrapods, mainly at the level of hypothalamic regions, where the majority of these SOM-ir cells in the sturgeon were CSF-C, as previously reported in the hypothalamus of several fish and amphibians (see references in Adrio et al. 2008). Thus, in the periventricular region, SOM-ir CSF-C cells were found in the parvocellular preoptic nucleus bordering the preoptic recess (Figs. 13.3a, b and 13.4e, j), the lateral walls of the lateral recesses (Figs. 13.3d–f), along the laterodorsal walls of the posterior recess (posterior recess nucleus, Fig. 13.3g), and in the anterior and lateral tuberal nuclei (Figs. 13.3d–h, 13.5f and 13.6g) (Adrio et al. 2008). The evolutionarily conserved expression pattern of SOM suggests that, at least in the preoptic area and hypothalamus, this peptide serves a basic function that was already present in ancestral vertebrates. Moreover, the abundance of SOM-ir CSF-C cells observed in the brain of Acipenser (Figs. 13.4e, j, 13.5f and 13.6g) (Adrio et al. 2008) suggests that SOM may act as a neurotransmitter of neuroendocrine pathways, as reported in fish (Vigh-Teichmann et al. 1983), or as a regulator of neural circuits related to cerebrospinal fluid homeostasis as observed in amphibians (Mathieu et al. 2004). SOM-ir adenohypophysial cells were also observed in the pars distalis of the hypophysis of A. baerii, which suggests that SOM is released to the blood in the adenohypophysis, and has peripheral functions (Figs. 13.3e, f, 13.5f and 13.6g) (Adrio et al. 2008).
In situ hybridization studies have demonstrated that mRNAs of the two SOM precursors (PSS1 and PSS2) are differentially expressed in neurons of the brain of the white sturgeon (Trabucchi et al. 2002). Thus, both precursors are expressed in cells of some hypothalamic regions, but only PSS1 mRNA is expressed in the preoptic region (Trabucchi et al. 2002), so observations in A. baerii with immunohistochemical techniques indicate that the used antiserum by Adrio et al. (2008) probably only reveals the SS1 isoform in the Siberian sturgeon brain.
Abundant SOM-ir fibers, presumably arising from preoptic and tuberal cells, coursed along the hypothalamic floor toward the median eminence, where SOM-ir innervation was very dense (Figs. 13.3d–g, 13.5f and 13.6g, h), and some of them reaching the neurointermediate lobe of the neurohypophysis (Figs. 13.3h and 13.7f) (Adrio et al. 2008). The presence of SOM in the hypothalamo-hypophyseal system of Acipenser (Adrio et al. 2008) indicates that this peptide has a role in the regulation of secretion of pituitary hormones, as reported in other vertebrates (Eigler and Ben-Shlomo 2014) including teleosts, in which the projection of SOM-ir cells to the pituitary was related to the control of prolactin (Grau et al. 1985), growth hormone (Marchant et al. 1989; Lin et al. 1993), or adrenocorticotropic hormone (Langhorne 1986) secretion. In fact, the distribution of SOM-ir elements observed in the hypothalamus of A. baerii (Adrio et al. 2008) is similar to the distribution of hypophysiotrophic neurons containing other peptides in the hypothalamus of other sturgeon species (CRF: González et al. 1992; GnRH: Leprêtre et al. 1993; NPY: Chiba and Honma 1994; MCH: Baker and Bird 2002; GAL: Adrio et al. 2005), which again suggests a hypophysiotrophic role for this peptide.
Finally, and as it was indicated previously for galanin, several neuropeptides, such as somatostatin, often exert their actions together with catecholamines and other classical neurotransmitters. In fact, the overlapping between distributions of SOM-ir cells and fibers (Figs. 13.3d–h, 13.5f, 13.6g, h and 13.7f) (Adrio et al. 2008) and catecholaminergic cells and fibers (Figs. 13.3d–h, 13.5d, 13.6e and 13.7d) (Adrio et al. 2002) in the hypothalamus and the median eminence of Acipenser suggests interaction between these systems which could influence each other’s functions, as has been shown in mammals (Ibata et al. 1982; Sakanaka et al. 1990) and amphibians (González et al. 2003).
2.3 Gonadotropin Releasing-Hormone System
Gonadotropin-releasing hormone (GnRH) plays a major role in growth regulation in fish and is one of the key regulators of the reproduction in all vertebrates and some invertebrates, since it acts in the brain-pituitary-gonad axis inducing synthesis and release of gonadotropins in the pituitary. Gonadotropins in turn stimulate synthesis of the steroid hormones in gonads, and some of these steroids, besides regulating steroidogenesis and gametogenesis, also exert feedback regulation upon the hypothalamus and/or pituitary gland in order to complete the reproduction cycle as it was reported in teleosts (Weltzien et al. 2004; Kim et al. 2006; Chang et al. 2009; Taranger et al. 2010; Hildahl et al. 2011).
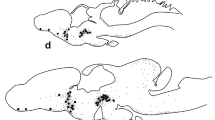
Using a combination of HPLC and radioimmunoassay, two variants of gonadotropin-releasing hormone (GnRH) have been identified in the brain of the white sturgeon (Acipenser transmontanus) and other phylogenetically ancient bony fish (Sherwood et al. 1991). One of these forms corresponds to the mammalian decapeptide (mGnRH; pGlu-His-Trp-Ser-His-Gly-Trp-Tyr-Pro-Gly-NH2) and the other to chicken GnRH-II (cGnRH-II; pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2). The presence of these two forms was confirmed in other sturgeon species such as the Siberian (Acipenser baerii, Leprêtre et al. 1993), the Russian (Acipenser gueldenstaedtii, Lescheid et al. 1995), the Beluga (Huso huso, Gharaei et al. 2010), and the Chinese sturgeon (Acipenser sinensis, Yue et al. 2013). Only two studies examined the distribution of the GnRH neurons in the brain of sturgeons revealed by immunohistochemical techniques (Leprêtre et al. 1993; Amiya et al. 2011). Cell bodies mGnRH-immunoreactive (mGnRH-ir) were observed in the olfactory nerves and bulbs, the telencephalon, the preoptic region, and the mediobasal hypothalamus (Fig. 13.8) (Leprêtre et al. 1993; Amiya et al. 2011). In the preoptic area, mGnRH-ir cells were located in the parvocellular preoptic nucleus and the organum vasculosum of the terminal lamina (Fig. 13.8a, b) and in the anterior and lateral tuberal nuclei in the hypothalamus (Fig. 13.8d, e). Most of these mGnRH-ir neurons were of the CSF-C type and exhibited short processes reaching the ventricular lumen (Fig. 13.8a, b, d, e). All these cell bodies were observed along mGnRH-ir fiber tracts that could be followed from the olfactory nerve to the hypothalamo-pituitary interface. Thus, numerous mGnRH-ir fibers were observed in all brain regions, in particular in the anterior brain (Fig. 13.8a–e), although mGnRH-ir fibers were not observed in the anterior lobe of the pituitary, but a few were seen to enter the neurointermediate lobe (Fig. 13.8f), similar to the situation existing in teleosts (Kah et al. 1986, 1991). In contrast, cGnRH-II was more abundant in the posterior brain, although a few fibers could be detected in the preoptic region and the hypothalamus (Fig. 13.8d, e). The only cGnRH-II-positive cells, which were negative for mGnRH, were consistently observed in the midbrain located close to the nucleus of the medial longitudinal fasciculus (Fig. 13.8f), similar to what has been reported in many teleosts or even tetrapods (see references in Leprêtre et al. 1993). These observations suggest that in sturgeons, mGnRH has a hypophysiotrophic role regulating the release of gonadotropin and also functions as a neuromodulator, whereas cGnRH-II has only neuromodulatory functions (Leprêtre et al. 1993) as it was reported for salmon GnRH (sGnRH) and cGnRH-II in the masu salmon (Amano et al. 1991).
Schematic drawings of transverse sections through the preoptic region (a–c) and hypothalamus (d–f) of Acipenser baerii (from rostral to caudal) showing the distribution of neurons (solid circles) and fibers (dotted areas) immunoreactive to mammalian (mGnRH) and chicken (cGnRH-II) gonadotropin-releasing hormone. At the left, anatomical regions are summarily indicated. The levels of the sections are indicated in a lateral view of the brain on the top. Schematic drawings were modified from Leprêtre et al. (1993). Abbreviations: CP, choroid plexus; Dd, dorsal part of the dorsal telencephalon; Dl, lateral part of the dorsal telencephalon; FR, fasciculus retroflexus; h, habenula; H, hypophysis; HL, hypothalamic lobes; Hyp, hypothalamus; i, infundibulum; III, third ventricle; IV, fourth ventricle; IVn, trochlear nucleus; LR, lateral hypothalamic recess; ME, median eminence; MLF, medial longitudinal fascicle; MPT, medial nucleus of the posterior tubercle; NAT, anterior tuberal nucleus; NIL, neurointermediate lobe of the hypophysis; NLT, lateral tuberal nucleus; NPOp, parvocellular preoptic nucleus; OB, olfactory bulb; OC, optic chiasm; OT, optic tectum; OVLT, organum vasculosum of the terminal lamina; P, pineal organ; PC, posterior commissure; Pd, pars distalis of the hypophysis; POR, posterior recess; PR, preoptic recess; SV, saccus vasculosus; T, telencephalon; Td, dorsal thalamus; tl, torus longitudinalis; TPp, periventricular nucleus of the posterior tubercle; vc, valvula cerebelli; VM, ventromedial thalamic nucleus. Scale bars = 1 mm (lateral view), 500 μm (sections)
Despite the fact that the sturgeon specimens used in Leprêtre et al. (1993) and Amiya et al. (2011) studies were immature, both reported the presence of an overall distribution of mGnRH in the brain that was very close similar to that of the distribution of salmon GnRH in teleosts (Kah et al. 1986, 1991; Oka and Ichikawa 1990; Amano et al. 1991), which suggests that this GnRH system is established early during development as shown in other vertebrate species and is probably activated by increasing level of sex steroids at the time of puberty.
In the brain of the Siberian sturgeon, reciprocal connections were reported between the NPY and GnRH neurons (Amiya et al. 2011). NPY-ir profiles were observed in close contact with GnRH-ir cell bodies in the preoptic area, while NPY-ir cell bodies were contacted by GnRH-ir fibers. This suggests that NPY and GnRH neural activities are reciprocally regulated in the brain of sturgeons (Amiya et al. 2011).
Conclusions
In conclusion, the hypothalamus of sturgeons is characterized by the importance of a very large ventricle and the presence of a majority of CSF-C cells most likely representing a basal character that can be also observed in teleosts such as the eel. In early teleosts, such as the zebrafish, the hypothalamic ventricle is not so large, although CSF-C cells are also very abundant. On another hand, most of the neuropeptides and neurohormones found in tetrapods are present in sturgeons, suggesting that their common ancestors, before the split between sarcopterygians and actinopterygians, already possessed such regulatory systems. Unfortunately, because of the difficulty in approaching the physiology of sturgeons (size, cost, etc.), the number of experimental studies aiming at deciphering the roles of such neuropeptides and neurohormones is very limited, although we can speculate that part of the functions supported by these neurohormones would be similar.
References
Adrio F, Anadón R, Rodríguez-Moldes I (1999) Distribution of serotonin (5HT)-immunoreactive structures in the central nervous system of two chondrostean species (Acipenser baeri and Huso huso). J Comp Neurol 407:333–348
Adrio F, Anadón R, Rodríguez-Moldes I (2002) Distribution of tyrosine hydroxylase (TH) and dopamine beta-hydroxylase (DBH) immunoreactivity in the central nervous system of two chondrostean fishes (Acipenser baeri and Huso huso). J Comp Neurol 448:280–297
Adrio F, Anadón R, Rodríguez-Moldes I (2008) Distribution of somatostatin immunoreactive neurons and fibres in the central nervous system of a chondrostean, the Siberian sturgeon (Acipenser baeri). Brain Res 1209:92–104
Adrio F, Rodríguez MA, Rodríguez-Moldes I (2005) Distribution of galanin-like immunoreactivity in the brain of the Siberian sturgeon (Acipenser baeri). J Comp Neurol 487:54–74
Amano M, Amiya N, Hiramatsu M, Tomioka T, Oka Y (2009) Interaction between neuropeptide Y immunoreactive neurons and galanin immunoreactive neurons in the brain of the masu salmon, Oncorhynchus masou. Neurosci Lett 462:33–38
Amano M, Oka Y, Aida K, Okumoto N, Kawashima S, Hasegawa Y (1991) Immunocytochemical demonstration of salmon GnRH and chicken GnRH-II in the brain of the Masu salmon. J Comp Neurol 314:587–597
Amemiya Y, Sogabe Y, Nozaki M, Takahashi A, Kawauchi H (1999) Somatolactin in the white sturgeon and African lungfish and its evolutionary significance. Gen Comp Endocrinol 114:181–190
Amiya N, Amano M, Tabuchi A, Oka Y (2011) Anatomical relations between neuropeptide Y, galanin, and gonadotropin-releasing hormone in the brain of chondrostean, the Siberian sturgeon Acipenser baeri. Neurosci Lett 503:87–92
Anglade I, Wang Y, Jensen J, Tramu G, Kah O, Conlon JM (1994) Characterization of trout galanin and its distribution in trout brain and pituitary. J Comp Neurol 350:63–74
Arai R, Onteniente B, Trembleau A, Landry M, Calas A (1990) Hypothalamic galanin-immunoreactive neurons projecting to the posterior lobe of the rat pituitary: a combined retrograde tracing and immunohistochemical study. J Comp Neurol 299:405–420
Arochena M, Anadón R, Díaz-Regueira SM (2004) Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J Comp Neurol 469:413–436
Baker BI, Bird DJ (2002) Neuronal organization of the melanin-concentrating hormone system in primitive actinopterygians: evolutionary changes leading to teleosts. J Comp Neurol 442:99–114
Belenky MA, Kuzik VV, Chernigovskaya EV, Polenov AL (1985) The hypothalamo-hypophysial system in Acipenseriade. X. Corticoliberin-like immunoreacticity in the hypothalamus and hypophysis of Acipenser ruthenus L. Gen Comp Endocrinol 60:20–26
Bohlen P, Brazeau P, Benoit R, Ling N, Esch F, Guillemin R (1980) Isolation and amino acid composition of two somatostatin-like peptides from ovine hypothalamus: somatostatin-28 and somatostatin-25. Biochem Biophys Res Commun 96:725–734
Braford MR Jr, Northcutt RG (1983) Organization of the diencephalon and pretectum of the ray-finned fishes. In: Davis RE, Northcutt RG (eds) Neurobiology. Higher brain areas and functions, vol 2. University of Michigan Press, Ann Arbor, pp 117–164
Chang JP, Johnson JD, Sawisky GR, Grey CL, Mitchell G, Booth M, Volk MM, Parks SK, Thompson E, Goss GG, Klausen C, Habibi HR (2009) Signal transduction in multifactorial neuroendocrine control of gonadotropin secretion and synthesis in teleosts-studies on the goldfish model. Gen Comp Endocrinol 161:42–52
Chiba A, Honma Y (1994) Neuropeptide Y-immunoreactive structures in the telencephalon and diencephalon of the white sturgeon, Acipenser transmontanus, with special regard to the hypothalamo-hypophyseal system. Arch Histol Cytol 57:77–86
Ch'ng JL, Christofides ND, Anand P, Gibson SJ, Allen YS, Su HC, Tatemoto K, Morrison JF, Polak JM, Bloom SR (1985) Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience 16:343–354
Cornbrooks EB, Parsons RL (1991a) Sexually dimorphic distribution of a galanin-like peptide in the central nervous system of the teleost fish Poecilia latipinna. J Comp Neurol 304:639–657
Cornbrooks EB, Parsons RL (1991b) Source of sexually dimorphic galanin-like immunoreactive projections in the teleost fish Poecilia latipinna. J Comp Neurol 304:658–665
Coveñas R, Mangas A, Medina LE, Sánchez ML, Aguilar LA, Díaz-Cabiale Z, Narváez JA (2011) Mapping of somatostatin-28 (1-12) in the alpaca diencephalon. J Chem Neuroanat 42:89–98
Eigler T, Ben-Shlomo A (2014) Somatostatin system: molecular mechanisms regulating anterior pituitary hormones. J Mol Endocrinol 53:R1–R19
Fuxe K, Ogren SO, Jansson A, Cintra A, Harfstrand A, Agnati LF (1988) Intraventricular injections of galanin reduces 5-HT metabolism in the ventral limbic cortex, the hippocampal formation and the frontoparietal cortex of the male rat. Acta Physiol Scand 133:579–581
Gai WP, Geffen LB, Blessing WW (1990) Galanin immunoreactive neurons in the human hypothalamus: colocalization with vasopressin-containing neurons. J Comp Neurol 298:265–280
Gharaei A, Mahboudi F, Esmaili-Sari A, Edalat R, Adeli A, Keyvanshokooh S (2010) Molecular cloning of cDNA of mammalian and chicken II gonadotropin-releasing hormones (mGnRH and cGnRH-II) in the beluga (Huso huso) and the disruptive effect of methylmercury on gene expression. Fish Physiol Biochem 36:803–817
Gómez A, Durán E, Ocaña FM, Jiménez-Moya F, Broglio C, Domezain A, Salas C, Rodríguez F (2009) Observations on the brain development of the sturgeon Acipenser naccarii. In: Carmona R, Domezain A, García-Gallego M, Hernando JA, Rodríguez F, Ruiz-Rejón M (eds) Biology, conservation and sustainable development of sturgeons, Fish & fisheries series, vol 29. Springer, Netherlands, pp 155–174
González GC, Belenky MA, Polenov AL, Lederis K (1992) Comparative localization of corticotropin and corticotropin releasing factor-like peptides in the brain and hypophysis of a primitive vertebrate, the sturgeon Acipenser ruthenus L. J Neurocytol 21:885–896
González A, Moreno N, Morona R, López JM (2003) Somatostatin-like immunoreactivity in the brain of the urodele amphibian Pleurodeles waltl. Colocalization with catecholamines and nitric oxide. Brain Res 965:246–258
Grandi G, Chicca M (2004) Early development of the pituitary gland in Acipenser nacarii (Chondrostei, Acipenseriformes): an immunocytochemical study. Anat Embryol 208:311–321
Grau EG, Nishioka RS, Young G, Bern HA (1985) Somatostatin-like immunoreactivity in the pituitary and brain of three teleosts fish species: somatostatin as a potential regulator of prolactin cell function. Gen Comp Endocrinol 59:350–357
Hansen GH (1971) On the structure and vascularization of the pituitary gland in some primitive actinopterygians (Acipenser, Polyodon, Calamoichthys, Polypterus, Lepisosteus and Amia). Biol Skr 18:1–64
Hansen GH, Hansen BL (1975) Inmmunohistochemical localization of growth hormone and prolactin in the pituitary gland of Acipenser güldenstaedti Brandt (Chondrostei). Acta Zool 56:29–41
Herrick CJ (1910) The morphology of the forebrain in amphibia and reptilia. J Comp Neurol 20:413–547
Herrick CJ (1933) Morphogenesis of the brain. J Morphol 54:233–258
Hildahl J, Sandvik GK, Edvardsen RB, Fagernes C, Norberg B, Haug TM, Weltzien FA (2011) Identification and gene expression analysis of three GnRH genes in female Atlantic cod during puberty provides insight into GnRH variant gene loss in fish. Gen Comp Endocrinol 172:458–467
Holmqvist BI, Ekström P (1995) Hypophysiotropic systems in the brain of the Atlantic salmon. Neuronal innervation of the pituitary and the origin of pituitary dopamine and nonapeptides identified by means of combined carbocyanine tract tracing and immunocytochemistry. J Chem Neuroanat 8:125–145
Horvath TL, Naftolin F, Leranth C, Sahu A, Kalra SP (1996) Morphological and pharmacological evidence for neuropeptide Y-galanin interaction in the rat hypothalamus. Endocrinology 137:3069–3078
Ibata Y, Fukui K, Obata HL, Tanaka M, Hisa Y, Sano Y, Ishigami T, Imagawa K, Sin S (1982) Postnatal ontogeny of catecholamine and somatostatin neuron systems in the median eminence of the rat as revealed by a colocalization technique. Brain Res Bull 9:407–415
Jansson A, Fuxe K, Eneroth P, Agnati L (1989) Centrally administered galanin reduces dopamine utilization in the median eminence and increases dopamine utilization in the medial neostriatum of the male rat. Acta Physiol Scand 135:199–200
Johnston JB (1901) The brain of Acipenser. A contribution to the morphology of the vertebrate brain. Zool Jahrb Abt Anat Ontog 15:59–260
Joss JMP, Dores RM, Crim JW, Beshaw M (1990) Immunocytochemical location of pituitary cells containing ACTH, α-MSH, and β-endorphin in Acipenser transmontanus, Lepisosteus spatula, and Amia calva. Gen Comp Endocrinol 78:459–468
Kageyama H, Takenoya F, Hori Y, Yoshida T, Shioda S (2008) Morphological interaction between galanin-like peptide- and dopamine-containing neurons in the rat arcuate nucleus. Regul Pept 145:165–168
Kah O, Breton B, Dulka JG, Nunez-Rodriguez J, Peter RE, Corigan A, Rivier JJ, Vale WW (1986) A reinvestigation of the Gn-RH (gonadotropin-releasing hormone) systems in the goldfish brain using antibodies to salmon Gn-RH. Cell Tissue Res 244:327–337
Kah O, Zanuy S, Mañanós E, Anglade I, Carrillo M (1991) Distribution of salmon gonadotrophin releasing-hormone in the brain and pituitary of the sea bass (Dicentrarchus labrax). An immunocytochemical and immunoenzymoassay study. Cell Tissue Res 266:129–136
Kim JB, Gadsboll V, Whittaker J, Barton BA, Conlon JM (2000) Gastroenteropancreatic hormones (insulin, glucagon, somatostatin, and multiple forms of PYY) from the pallid sturgeon, Scaphirhynchus albus (Acipenseriformes). Gen Comp Endocrinol 120:353–363
Kim J, Hayton WL, Schultz IR (2006) Modeling the brain–pituitary–gonad axis in salmon. Mar Environ Res 62(Suppl):S426–S432
Kotrschal K, Krautgartner WD, Adam H (1983) Crown cells in the diencephalon of Acipenser ruthenus (Acipenseridae, Chondrostei). J Hirnforsch 24:655–657
Kotrschal K, Krautgartner WD, Adam H (1985) Distribution of aminergic neurons in the brain of the sterlet, Acipenser ruthenus (Chondrostei, Actinopterygii). J Hirnforsch 26:65–72
Langhorne P (1986) Somatostatin stimulates ACTH release in brown trout (Salmo trutta L.) Gen Comp Endocrinol 61:71–75
Leprêtre E, Anglade I, Williot P, Vandesande F, Tramu G, Kah O (1993) Comparative distribution of mammalian GnRH (gonadotrophin-releasing hormone) and chicken GnRH-II in the brain of the immature Siberian sturgeon (Acipenser baeri). J Comp Neurol 337:568–583
Lescheid DW, Powell JF, Fischer WH, Park M, Craig A, Bukovskaya O, Barannikova IA, Sherwood NM (1995) Mammalian gonadotropin-releasing hormone (GnRH) identified by primary structure in Russian sturgeon, Acipenser gueldenstaedti. Regul Pept 55:299–309
Li CJ, Wei QW, Zhou L, Cao H, Zhang Y, Gui JF (2009) Molecular and expression characterization of two somatostatin genes in the Chinese sturgeon, Acipenser sinensis. Comp Biochem Physiol A Mol Integr Physiol 154:127–134
Lin XW, Lin HR, Meter RE (1993) Growth hormone and gonadotropin secretion in the common carp (Cyprinus carpio L.): in vitro interactions of gonadotropin-releasing hormone, somatostatin, and the dopamine agonist apomorphine. Gen Comp Endocrinol 89:62–71
López FJ, Merchenthaler I, Ching M, Wisniewski MG, Negro-Vilar A (1991) Galanin: a hypothalamic-hypophysiotropic hormone modulating reproductive functions. Proc Natl Acad Sci U S A 88:4508–4512
Maiter DM, Hooi SC, Koenig JI, Martin JB (1990) Galanin is a physiological regulator of spontaneous pulsatile secretion of growth hormone in the male rat. Endocrinology 126:1216–1222
Marchant TA, Dulka JG, Peter RE (1989) Relationship between serum growth hormone levels and the brain and pituitary content of immunoreactive somatostatin in the goldfish, Carassius auratus L. Gen Comp Endocrinol 73:458–468
Mathieu M, Bruzzone F, Chartrel N, Serra GP, Spiga S, Vallarino M, Vaudry H (2004) Somatostatin in the brain of the cave salamander, Hydromantes genei (Amphibia, Plethodontidae): immunohistochemical localization and biochemical characterization. J Comp Neurol 475:163–176
Mechenthaler I (2008) Galanin and the neuroendocrine axes. Cell Mol Life Sci 65:1826–1835
Melander T, Hökfelt T, Rökaeus A (1986) Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol 248:475–517
Mensah ET, Volkoff H, Unniappan S (2010) Galanin systems in non-mammalian vertebrates with special focus on fishes. EXS 102:243–262
Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O (2003) Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: comparison with estrogen receptor alpha. J Comp Neurol 462:180–193
Merchenthaler I (2010) Galanin and the neuroendocrine axes. In: Hökfelt T (ed) Galanin. Springer, Basel, pp 71–86
Merchenthaler I, Rotoli G, Grignol G, Dudas B (2010) Intimate associations between the neuropeptide Y system and the galanin-immunoreactive neurons in the human diencephalon. Neuroscience 170:839–845
Merchenthaler I, Rotoli G, Peroski M, Grignol G, Dudas B (2013) Catecholaminergic system innervates galanin-immunoreactive neurons in the human diencephalon. Neuroscience 238:327–334
Moons L, Batten TF, Vandesande F (1991) Autoradiographic distribution of galanin binding sites in the brain and pituitary of the sea bass (Dicentrarchus labrax). Neurosci Lett 123:49–52
Murakami Y, Kato Y, Koshiyama H, Inoue T, Yanaihara N, Imura H (1987) Galanin stimulates growth hormone (GH) secretion via GH-releasing factor (GRF) in conscious rats. Eur J Pharmacol 136:415–418
Nakane Y, Ikegami K, Iigo M, Ono H, Takeda K, Takahashi D, Uesaka M, Kimijima M, Hashimoto R, Arai N, Suga T, Kosuge K, Abe T, Maeda R, Senga T, Amiya N, Azuma T, Amano M, Abe H, Yamamoto N, Yoshimura T (2013) The saccus vasculosus of fish is a sensor of seasonal changes in day length. Nat Commun 4:2108
Nieuwenhuys R (1998) Chondrostean fishes. In: Nieuwenhuys R, Ten Donkelaar HJ, Nicholson C (eds) The central nervous system of vertebrates, vol 1. Springer, Berlin, pp 701–757
Nishii M, Movérus B, Bukovskaya OS, Takahashi A, Kawauchi H (1995) Isolation and characterization of (Pro2)somatostatin-14 and melanotropins from Russian sturgeon, Acipenser gueldenstaedti Brandt. Gen Comp Endocrinol 99:6–12
Northcutt RG (1995) The forebrain of gnathostomes: in search of a morphotype. Brain Behav Evol 46:275–318
Oka Y, Ichikawa M (1990) Gonadotropin-releasing hormone (GnRH) immunoreactive system in the brain of the dwarf gourami (Colisa lalia) as revealed by light microscopic immunocytochemistry using a monoclonal antibody to common amino acid sequence of GnRH. J Comp Neurol 300:511–522
Olivereau M, Olivereau JM (1991a) Immunocytochemical localization of a galanin-like peptidergic system in the brain and pituitary of some teleost fish. Histochemistry 96:343–354
Olivereau M, Olivereau JM (1991b) Galanin-like immunoreactivity is increased in the brain of estradiol- and methyltestosterone-treated eels. Histochemistry 96:487–497
Park JJ, Baum MJ, Tobet SA (1997) Sex difference and steroidal stimulation of galanin immunoreactivity in the ferret’s dorsal preoptic area/anterior hypothalamus. J Comp Neurol 389:277–288
Patzelt C, Tager HS, Carroll RJ, Steiner DE (1980) Identification of prosomatostatin in pancreatic islets. Proc Natl Acad Sci U S A 77:2410–2414
Pelissero C, Núñez-Rodríguez J, Le Menn F, Kah O (1988) Immunohistochemical investigation of the pituitary of the sturgeon (Acipenser baeri, Chondrostei). Fish Phisiol Biochem 5:109–119
Piñuela C, Northcutt RG (2007) Immunohistochemical organization of the forebrain in the white sturgeon, Acipenser transmontanus. Brain Behav Evol 69:229–253
Polenov AL, Belenky MA, Garlov PE, Konstantinova MS (1976) The hypothalamo-hypophysial system in Acipenseriade. VI. The proximal neurosecretory contact region. Cell Tissue Res 170:129–144
Polenov AL, Efimova NA, Konstantinova MS, Senchik YI, Yakovleva IV (1983) The hypothalamo-hypophysial system in Acipenseriade. IX. Formation of monoaminergic neurosecretory cells in the preoptic nucleus region during early ontogeny. Cell Tissue Res 232:651–667
Polenov AL, Garlov PE (1971) The hypothalamo-hypophysial system in Acipenseriade. I. Ultrastructural organization of large neurosecretory terminals (herring bodies) and axoventricular contacts. Z Zellforsch 116:349–374
Polenov AL, Garlov PE (1973) The hypothalamo-hypophysial system in Acipenseriade. III. The neurohypophysis of Acipenser güldenstädti Brandt and Acipenser stellatus Pallas. Z Zellforsch 136:461–477
Polenov AL, Garlov PE, Konstantinova MS, Belenky MA (1972) The hypothalamo-hypophysial system in Acipenseriade. II. Adrenergic structures of the hypophysial neurointermediate complex. Z Zellforsch 128:470–481
Polenov AL, Kuzik VV, Danilova OA (1997) The hypothalamo-hypophysial system in Acipenseriade. XI. Morphological and immunohistochemical analysis of nonapeptidergic and cortociliberin-immunoreactive elements in hypophysectomized starlet (Acipenser ruthenus L.) Gen Comp Endocrinol 105:314–322
Polenov AL, Pavlovic M (1978) The hypothalamo-hypophysial system in Acipenseriade. VII. The functional morphology of the peptidergic neurosecretory cells in the preoptic nucleus of the sturgeon, Acipenser güldenstädti Brandt. A quantitative study. Cell Tissue Res 186:559–570
Power DM, Canario AV, Ingleton PM (1996) Somatotropin release-inhibiting factor and galanin innervation in the hypothalamus and pituitary of seabream (Sparus aurata). Gen Comp Endocrinol 101:264–274
Puelles L, Rubenstein JLR (1993) Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. TINS 16:472–479
Rao PD, Murthy CK, Cook H, Peter RE (1996) Sexual dimorphism of galanin-like immunoreactivity in the brain and pituitary of goldfish, Carassius auratus. J Chem Neuroanat 10:119–135
Rodríguez MA, Anadón R, Rodríguez-Moldes I (2003) Development of galanin-like immunoreactivity in the brain of the brown trout (Salmo trutta fario), with some observations on sexual dimorphism. J Comp Neurol 465:263–285
Rodríguez Díaz MA, Candal E, Santos-Durán GN, Adrio F, Rodríguez-Moldes I (2011) Comparative analysis of met-enkephalin, galanin and GABA immunoreactivity in the developing trout preoptic-hypophyseal system. Gen Comp Endocrinol 173:148–158
Rodríguez-Moldes I, Candal E, Huesa G, Adrio F, Anadón R (1997) Distribución de neuronas inmunorreactivas a la Met-encefalina en el SNC del esturión. Rev Neurol 25:1800
Rugarn O, Theodorsson A, Hammar M, Theodorsson E (1999) Effects of estradiol, progesterone, and norethisterone on regional concentrations of galanin in the rat brain. Peptides 20:743–748
Rupp B, Northcutt RG (1998) The diencephalon and pretectum of the white sturgeon (Acipenser transmontanus): a cytoarchitectonic study. Brain Behav Evol 51:239–262
Rustamov EK (2006a) Organization of diencephalon of the sturgeons. Preoptic area. J Evol Biochem Physiol 42:195–207
Rustamov EK (2006b) Organization of hypothalamic area of diencephalon in sturgeons. J Evol Biochem Physiol 42:342–353
Sakanaka M, Magari S, Inoue N (1990) Somatostatin co-localizes with tyrosine hydroxylase in the nerve cells of discrete hypothalamic regions in rats. Brain Res 516:313–317
Sathyanesan AG, Chavin W (1967) Hypothalamo-hypophyseal neurosecretory system in the primitive actinopterygian fishes (Holostei and Chondrostei). Acta Anat (Basel) 68:284–299
Scheffen JR, Splett CL, Desotelle JA, Bauer-Dantoin AC (2003) Testosterone-dependent effects of galanin on pituitary luteinizing hormone secretion in male rats. Biol Reprod 68:363–369
Schindler M, Humphrey PP, Emson PC (1996) Somatostatin receptors in the central nervous system. Prog Neurobiol 50:9–47
Shen ES, Hardenburg JL, Meade EH, Arey BJ, Merchenthaler I, López FJ (1999) Estradiol induces galanin gene expression in the pituitary of the mouse in an estrogen receptor alpha-dependent manner. Endocrinology 140:2628–2631
Sherwood NM, Doroshov S, Lance V (1991) Gonadotropin-releasing hormone (GnRH) in bony fish that are phylogenetically ancient: reedfish (Calamoichthys calabaricus), sturgeon (Acipenser transmontanus), and alligator gar (Lepisosteus spatula). Gen Comp Endocrinol 84:44–57
Smeets WJAJ, Nieuwenhuys R, Roberts BL (1983) The central nervous system of cartilaginous fishes. Structure and functional correlations. Springer Verlag, New York
Splett CL, Scheffen JR, Desotelle JA, Plamann V, Bauer-Dantoin AC (2003) Galanin enhancement of gonadotropin-releasing hormone-stimulated luteinizing hormone secretion in female rats is estrogen dependent. Endocrinology 144:484–490
Sueiro C, Carrera I, Ferreiro S, Molist P, Adrio F, Anadón R, Rodríguez-Moldes I (2007) New insights on Saccus vasculosus evolution: a developmental and immunohistochemical study in elasmobranchs. Brain Behav Evol 70:187–204
Takenoya F, Funahashi H, Matsumoto H, Ohtaki T, Katoh S, Kageyama H, Suzuki R, Takeuchi M, Shioda S (2002) Galanin-like peptide is co-localized with alpha-melanocyte stimulating hormone but not with neuropeptide Y in the rat brain. Neurosci Lett 331:119–122
Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, Weltzien FA, Dufour S, Karlsen O, Norberg B, Andersson E, Hansen T (2010) Control of puberty in farmed fish. Gen Comp Endocrinol 165:483–515
Tostivint H, Trabucchi M, Vallarino M, Conlon JM, Lihrmann I, Vaudry H (2004) Molecular evolution of somatostatin genes. In: Patel YC (ed) Somatostatin endocrine updates. Kluwer Academic, Dordrecht
Trabucchi M, Tostivint H, Lihrmann I, Sollars C, Vallarino M, Dores RM, Vaudry H (2002) Polygenic expression of somatostatin in the sturgeon Acipenser transmontanus: molecular cloning and distribution of the mRNAs encoding two somatostatin precursors. J Comp Neurol 443:332–345
Vázquez M, Rodríguez F, Domezain A, Salas C (2002) Development of the brain of the sturgeon Acipenser nacarii. J Appl Ichthyol 18:275–279
Vigh-Teichmann I, Vigh B, Korf HW, Oksche A (1983) CSF-contacting and other somatostatin-immunoreactive neurons in the brains of Anguilla anguilla, Phoxinus phoxinus and Salmo gairdneri (Teleostei). Cell Tissue Res 233:319–334
Volkoff H, Canosa LF, Unniappan S, Cerdá-Reverter JM, Bernier NJ, Kelly SP, Peter RE (2005) Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol 142:3–19
Weltzien FA, Andersson E, Andersen O, Shalchian-Tabrizi K, Norberg B (2004) The brain–pituitary–gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comp Biochem Physiol A Mol Integr Physiol 137:447–477
Yáñez J, Rodríguez M, Pérez S, Adrio F, Rodríguez-Moldes I, Manso MJ, Anadón R (1997) The neuronal system of the saccus vasculosus of trout (Salmo trutta fario and Oncorhynchus mykiss): an immunocytochemical and nerve tracing study. Cell Tissue Res 288:497–507
Yue H, Ye H, Chen X, Cao H, Li C (2013) Molecular cloning of cDNA of gonadotropin-releasing hormones in the Chinese sturgeon (Acipenser sinensis) and the effect of 17β-estradiol on gene expression. Comp Biochem Physiol A Mol Integr Physiol 166:529–537
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kah, O., Adrio, F. (2018). Chemical Neuroanatomy of the Hypothalamo-Hypophyseal System in Sturgeons. In: Williot, P., Nonnotte, G., Vizziano-Cantonnet, D., Chebanov, M. (eds) The Siberian Sturgeon (Acipenser baerii, Brandt, 1869) Volume 1 - Biology. Springer, Cham. https://doi.org/10.1007/978-3-319-61664-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-61664-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61662-9
Online ISBN: 978-3-319-61664-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)