Abstract
The kinetochore is a large protein complex, which is assembled at the centromere of a chromosome to ensure faithful chromosome segregation during M-phase. The centromere in most eukaryotes is epigenetically specified by DNA sequence-independent mechanisms. The constitutive centromere-associated network (CCAN) is a subcomplex in the kinetochore that localizes to the centromere throughout the cell cycle. The CCAN has interfaces bound to the centromeric chromatin and the spindle microtubule-binding complex; therefore, it functions as a foundation of kinetochore formation. Here, we summarize recent progress in our understanding of the structure and organization of the CCAN. We also discuss an additional role of the CCAN in the maintenance of centromere position and dynamic reorganization of the CCAN.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Constitutive Centromere-associated Network (CCAN)
- Ndc80 Complex
- Chicken DT40 Cells
- Kinetochore Assembly
- Human Artificial Chromosomes (HAC)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
One of the distinguishing features of living organisms is self-replication. To maintain the continuity of life, genetic materials have to be faithfully inherited by successive generations. In the early 1900s, Walter Sutton and Theodor Boveri independently suggested that chromosomes carry genetic materials, using insect germ cells and echinoderm embryos, respectively (Sutton 1902, 1903; Boveri 1904). Their “chromosome theory” explained the mechanism underlying Mendel’s laws that were rediscovered at the same time (de Vries 1900; Tschermak 1900; Correns 1900; Birchler 2015). Thereafter, the chromosome theory was experimentally verified by Thomas Morgan Hunt based on fly genetics (Morgan 1915). These findings led to the next important question: how were the chromosomes correctly segregated into daughter cells? A clue to answering this question was described by Walter Flemming decades before the theory was established (Flemming 1882). He found primary constrictions on chromosomes, where the mitotic spindle attached to deliver these chromosomes into daughter cells (Flemming 1882).
In 1936, Cyril Darlington defined the term “centromere” (Darlington 1936). Centromeres are now known as regions on chromosomes where a macro proteinaceous complex, called the kinetochore, is assembled to connect centromeres with spindle microtubules during mitosis (Cheeseman and Desai 2008; McKinley and Cheeseman 2016; Pesenti et al. 2016; Nagpal and Fukagawa 2016). The kinetochore also contributes to correct chromosome segregation by producing a signal upon incorrect attachment of kinetochores with microtubules (Foley and Kapoor 2013; London and Biggins 2014; Stukenberg and Burke 2015; Musacchio 2015).
The kinetochore in vertebrates is made-up of more than 100 proteins (Tipton et al. 2012). These proteins are divided into sub-protein complexes. The constitutive centromere-associated network (CCAN) is one of the major subcomplexes in the kinetochore (Cheeseman and Desai 2008; Perpelescu and Fukagawa 2011; Takeuchi and Fukagawa 2012). The CCAN proteins constitutively localize to the centromeres throughout the cell cycle and form a foundation for kinetochore assembly. Another major subcomplex is the KMN (the KNL1, the Ndc80 and the Mis12 complexes) network, which is recruited to the CCAN during M-phase (Cheeseman et al. 2006; Cheeseman and Desai 2008; Varma and Salmon 2012; Foley and Kapoor 2013; Nagpal et al. 2015). The CCAN is associated with centromere chromatin and the KMN network binds directly to the spindle microtubule; therefore, the kinetochore effectively mediates the interaction between the chromosomes and the microtubules.
The centromere is specified at a particular position on a chromosome in many species and the kinetochore is formed within the centromeric region. In this chapter, we introduce centromere specification and then describe and discuss the CCAN structure and function and its dynamic regulations for kinetochore assembly.
2 Centromere
2.1 Centromere Organization
The centromere is a genome region, where the kinetochore is assembled (Fig. 1a). Although the centromere is crucial for faithful chromosome segregation, genome organization of centromeres is diverse among various species. The budding yeast, Saccharomyces cerevisiae, has simple and small centromeres, which are defined by a 125-basepair-specific DNA sequence in each chromosome (Fig. 1b) (Hegemann and Fleig 1993; Pluta et al. 1995; Clarke 1998). The short budding yeast centromere DNA is sufficient to assemble the kinetochore for efficient chromosome segregation (Hegemann and Fleig 1993). The sequence-dependent centromere in the budding yeast is known as a point centromere, which is bound to a single microtubule through the kinetochore (Pluta et al. 1995).
Centromere structure and organization. a A schematic representation of vertebrate chromosome. The centromere is a specific genomic region where the kinetochore is assembled to establish a microtubule-binding interface for faithful chromosome segregation. The centromeres are found on sites where constriction is formed in chromosomes during mitosis. b The point centromere in budding yeast, Saccharomyces cerevisiae (top). It is specified by a 125-bp sequence, which contains centromere DNA elements (CDE) I, II, and III. The short DNA motif is occupied with a nucleosome, which contains a centromere-specific histone H3 variant, CENP-A (Ces4 in Saccharomyces cerevisiae). Bottom shows the regional centromere. It stretches over large regions that comprise repetitive sequences in most species (e.g., alpha-satellite repeats DNA in human). Although the repetitive sequences facilitate centromere formation, the position of a regional centromere is specified by the CENP-A nucleosome, which is an epigenetic marker of the centromere
In contrast to the budding yeast, the majority of other organisms, with the exception of Caenorhabditis elegans and some insects that have holocentromeres, have a regional centromere, which spans a much larger chromosomal region (several kilobasepairs to megabasepairs) (Fukagawa and Earnshaw 2014a; Kursel and Malik 2016). The regional centromeres typically have repetitive DNA sequences and are bound to multiple microtubules (Fukagawa and Earnshaw 2014a; Kursel and Malik 2016). Human centromeres span hundreds of kilobasepairs to several megabasepairs and consist of arrays of alpha-satellite DNA repeats (Fig. 1b) (Aldrup-Macdonald and Sullivan 2014). The biological significance of the repetitive DNA for centromere function is controversial. Human artificial chromosomes (HACs), which have the alpha-satellite DNA repeats from human centromeres, are efficiently generated and stably maintained in human cells (Harrington et al. 1997; Ikeno et al. 1998). Although the alpha-satellite repeats promote the functional kinetochore assembly in the experimental condition of the HAC formation (Ohzeki et al. 2002), observations in several species suggest that the repetitive sequences are not always necessary to specify centromere regions on chromosomes. Species in which this was observed include horse (Wade et al. 2009; Piras et al. 2010), chicken (Shang et al. 2010), and orangutan (Locke et al. 2011).
A neocentromere is a newly formed centromere within a non-centromeric locus on a chromosome (Fig. 2) (Marshall et al. 2008; Fukagawa and Earnshaw 2014b). The first human neocentromere, which lacks the alpha-satellite repeats, was discovered in 1993 (Voullaire et al. 1993). Since then, over 90 cases of human neocentromeres have been reported (Marshall et al. 2008). Discovery of these neocentromeres supports the idea that repetitive sequences are not essential for centromere specification. Neocentromeres can be formed naturally on various DNA sequences on chromosomes upon disruption or inactivation of a native centromere. The neocentromere formation process was experimentally reproduced using genetic engineering to remove native centromeres in fungi and chicken DT40 cells (Fig. 3a) (Ishii et al. 2008; Ketel et al. 2009; Shang et al. 2013). Based on these observations, the locus for the regional centromeres does not seem to be specified with genetic marks, such as particular DNA sequences. This suggests that epigenetic marks play a key role in centromere specification.
CENP-A exists in active centromeres. A neocentromere is a newly formed centromere at non-centromeric region, when the native centromere is inactivated or disrupted. The repetitive sequence is not necessary for the neocentromere formation, indicating that the regional centromere is specified by sequence-independent epigenetic mechanisms. CENP-A is found in active neocentromeres, but not in inactive centromeres, which contain the repetitive DNA sequences, in dicentric chromosomes
The experimental systems to generate neocentromeres artificially. Because efficiency of natural neocentromere formation is very low, the experimental systems, which generate neocentromeres artificially, help to understand mechanisms of neocentromere formation. a The inducible centromere removal system by which the native centromere flanked with loxP sequences are excised by Cre recombinase and neocentromeres are formed on non-centromere loci. The system is applied to fungi and chicken DT40 cells. b Neocentromeres are also induced by ectopic CENP-A nucleosome deposition on non-centromeric region. Artificial tethering of CENP-A chaperones (HJURP in vertebrate and CAL1 in Drosophila) using lacO/LacI system deposits the CENP-A nucleosomes on a non-centromere locus. Kinetochore proteins are recruited to the ectopic site to form a functional kinetochore. The artificial kinetochore is functional, because the artificial kinetochore is replaceable in the native centromere (see also Fig. 5a)
2.2 CENP-A Is a Critical Epigenetic Mark for Centromere Specification
The insight of centromere specification in the regional centromere was derived from the discovery of centromere proteins. Centromere protein (CENP)-A was originally identified as an antigen for anti-centromere autoimmune sera from patients with CREST syndrome (Earnshaw and Rothfield 1985). CENP-A is a centromere-specific histone H3 variant that forms a nucleosome, replacing the canonical histone H3 (Fig. 1b) (Palmer et al. 1987; Yoda et al. 2000). CENP-A orthologs are found in most eukaryotes, including the budding yeast, which have the point centromere (Stoler et al. 1995; Buchwitz et al. 1999; Takahashi et al. 2000; Blower and Karpen 2001; Talbert et al. 2002). CENP-A null mice exhibit early embryonic lethality (Howman et al. 2000) and inactivation or depletion of CENP-A in most organisms causes chromosome mis-segregation during M-phase (Stoler et al. 1995; Howman et al. 2000; Takahashi et al. 2000; Oegema et al. 2001; Blower and Karpen 2001; Goshima et al. 2003; Regnier et al. 2005), indicating that CENP-A is an essential gene for faithful chromosome segregation.
CENP-A is only localized onto the active centromere in human dicentric chromosomes. Dicentric chromosomes have two centromeres on a chromosome—one is an active centromere and the other is inactive (Fig. 2) (Earnshaw and Rothfield 1985). CENP-A never localizes on the inactive centromere, regardless of the presence of the repetitive alpha-satellite DNA (Fig. 2) (Warburton et al. 1997). These observations lead to the idea that CENP-A is an epigenetic mark for specification of active centromeres.
The idea that centromeres are specified epigenetically but not genetically is further supported by artificial kinetochore assembly on chromosome arms using a lacO/LacI system in which LacI-fused centromere proteins are tethered on a lacO array on non-centrometric region in a chromosome arm (Fig. 3b). Targeting the CENP-A-specific chaperones, HJURP and CAL1 in vertebrates and Drosophila melanogaster respectively, to their non-centromeric region induces CENP-A deposition and kinetochore assembly on the targeting sites (Fig. 3b) (Barnhart et al. 2011; Hori et al. 2013; Chen et al. 2014). Once the artificial CENP-A chromatin is established, the CENP-A and kinetochore proteins are maintained without the LacI-fused CENP-A chaperone (Fig. 3b) (Barnhart et al. 2011; Hori et al. 2013; Chen et al. 2014). The artificially CENP-A-deposited chromatin forms a functional active centromere, because the induced artificial kinetochore can be replaced with an endogenous centromere in chicken DT40 cells (Hori et al. 2013). These tethering experiments (Fig. 3b), combined with the centromere removal experiments (Fig. 3a) provide evidence that CENP-A is an epigenetic mark for centromere specification independent of DNA sequence. Similarly, ectopic Drosophila CENP-A, CID, localization to non-centromeric region via overexpression or using the lacO/LacI system induces centromeres with the CENP-A nucleosomes (Heun et al. 2006; Mendiburo et al. 2011).
In human cells, LacI-fused CENP-A array on the lacO repeat is likely to induce nucleosomes with the LacI-fused CENP-A into the tethered sites. However, it is inefficient, possibly as a result of an indirect consequence of recruitment of HJURP or other kinetochore proteins onto the high-density LacI-CENP-A array. In fact, overexpression of CENP-A results in CENP-A misincorporation into non-centromere chromosome loci, but does not cause ectopic centromere formation in human cells (Van Hooser et al. 2001; Gascoigne et al. 2011). This suggests that additional regulation may be involved in the formation of active centromeres in CENP-A-incorporated chromatin. Indeed, we recently demonstrated that histone H4 Lys20 is mono-methylated specifically in the CENP-A nucleosome in the centromeric region and that this methylation is essential for kinetochore assembly (Hori et al. 2014). It is possible that additional modifications occur in CENP-A-containing chromatin (Blower et al. 2002; Sullivan and Karpen 2004; Bergmann et al. 2011; Bailey et al. 2016; Shang et al. 2016), and that the combination of such modifications would function as additional epigenetic marks for centromere specification and kinetochore assembly.
3 CCAN Organization
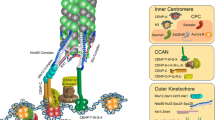
Once a centromere is specified by CENP-A, additional proteins are assembled on a centromere region to form a functional kinetochore. Extensive genetic and biochemical approaches have identified kinetochore proteins in vertebrate cells. Among them, CCAN proteins are constitutively localized to centromeres throughout the cell cycle (Cheeseman and Desai 2008; Perpelescu and Fukagawa 2011; Nagpal and Fukagawa 2016). The CCAN is composed of at least 16 proteins in vertebrates (CENP-C, -H, -I, -K, -L, -M, -N, -O, -P, -Q, -R, -S, -T, -U, -W, -X). These proteins make subfunctional complexes, which interact together to form the entire CCAN assembly (Nagpal and Fukagawa 2016). Since the CCAN is bound to the CENP-A chromatin and the KMN network is bound to the spindle microtubules (Pesenti et al. 2016; Nagpal and Fukagawa 2016), the CCAN forms a base of the kinetochore to link between the centromere and microtubules.
3.1 CCAN Subcomplexes
3.1.1 CENP-C
Like CENP-A, CENP-C was originally identified as an antigen for the autoimmune sera from patients with CREST syndrome (Earnshaw and Rothfield 1985). Using electron microscopy, CENP-C was the first protein found to localize in the inner kinetochore (Saitoh et al. 1992). CENP-C homologs are found in most organisms, including the budding yeast, and their depletion causes chromosome misalignment (Saitoh et al. 1992; Brown et al. 1993; Tomkiel et al. 1994; Brown 1995; Meluh and Koshland 1995; Fukagawa and Brown 1997; Fukagawa et al. 1999; Moore and Roth 2001; Holland et al. 2005; Heeger et al. 2005). Although entire sequence homology between yeast and vertebrate CENP-Cs is not high, several domains, which are predicted to be functionally important domains, are highly conserved (Brown 1995; Meluh and Koshland 1995; Fukagawa and Brown 1997; Moore and Roth 2001; Holland et al. 2005; Heeger et al. 2005; Milks et al. 2009; Carroll et al. 2010; Przewloka et al. 2011; Screpanti et al. 2011; Kato et al. 2013; Klare et al. 2015; Nagpal et al. 2015). The N-terminal region of CENP-C is one such conserved domain. It binds to the Mis12 complex, which is a member of the KMN network (see Fig. 5) (Przewloka et al. 2011; Screpanti et al. 2011). Ectopic targeting of the CENP-C N-terminus, including the Mis12 complex-binding domain into a non-centromere locus, induces formation of the functional kinetochore, which does not contain other CCAN proteins (see Fig. 5b, c) (Hori et al. 2013). The KMN network directly binds to microtubules (Cheeseman and Desai 2008); therefore, CENP-C connects with microtubules through association with the KMN network. The central domain and the C-terminal region of CENP-C, which are also conserved, have been shown to associate with the CENP-A nucleosomes (see Fig. 5) (Carroll et al. 2010; Kato et al. 2013; Falk et al. 2015, 2016). This interaction reshapes the CENP-A nucleosome structure and results in stabilization within the centromere (Falk et al. 2015, 2016). These conserved domains make CENP-C an important bridge molecule between the centromeric chromatin and the microtubules. Another functional domain, which is also conserved among vertebrates, is found in the middle region (also known as PEST rich domain) of CENP-C (Klare et al. 2015; Nagpal et al. 2015). This domain is critical for interaction with other CCAN proteins such as CENP-H and CENP-L/N (Klare et al. 2015; Nagpal et al. 2015; McKinley et al. 2015). CENP-C is required for localization of other CCAN members into the centromere in human cells (Klare et al. 2015; McKinley et al. 2015). This leads to the idea that CENP-C is a blueprint for kinetochore assembly (Klare et al. 2015). However, CENP-C does not simply stand at the hierarchical top for kinetochore assembly in chicken DT40 cells, because CENP-C depletion does not result in the complete loss of other CCAN proteins (Fukagawa et al. 2001; Kwon et al. 2007; Hori et al. 2008a). In addition, our data suggest that interaction between CENP-C and CENP-H and the CENP-L/N is not a simple linear pathway (Fukagawa et al. 2001; Kwon et al. 2007; Nagpal et al. 2015); rather, this appears to be a complex and dynamic process (see Sect. 4). This idea is also supported by a recent study in human cells (McKinley et al. 2015). Therefore, although CENP-C plays a central role in CCAN organization, we believe that CCAN is formed through a complex process (Fig. 4).
3.1.2 CENP-H/I/K/M
CENP-H has been identified as a coiled-coil protein, which has been shown to constitutively localize to the centromere in mouse cells (Sugata et al. 1999) and thereafter human and chicken homologs were identified (Sugata et al. 2000; Fukagawa et al. 2001). CENP-I was cloned from chicken cells using a homologous region of fission yeast centromere protein Mis6 (Saitoh et al. 1997), and the interaction between CENP-H and CENP-I was shown by a two-hybrid assay (Nishihashi et al. 2002).
We performed immunoprecipitation experiments with CENP-H and CENP-I to identify additional centromere-associated proteins and found CENP-K and -M in chicken and human cells (Okada et al. 2006). CENP-H, -I, -K, and -M were also identified as CENP-A chromatin-associated proteins in human cells (Obuse et al. 2004; Foltz et al. 2006). CENP-M is proposed to be a pseudo GTPase, based on crystal structure analysis (Basilico et al. 2014), but its detailed function in centromeres remains unknown.
Biochemical and functional experiments suggest that CENP-H, -I, -K and -M form a complex, which is essential for chromosomal alignment and segregation, as well as cell viability (Nishihashi et al. 2002; Foltz et al. 2006; Okada et al. 2006; Izuta et al. 2006; Basilico et al. 2014). Depletion of each factor disrupts the centromere localization of other factors in the complex, suggesting that their localization is interdependent (Nishihashi et al. 2002; Foltz et al. 2006; Okada et al. 2006; Izuta et al. 2006; Basilico et al. 2014; McKinley et al. 2015). Consistent with this observation, these proteins interact together to form a complex in vivo, which can be reconstituted with recombinant proteins (Nishihashi et al. 2002; Foltz et al. 2006; Okada et al. 2006; Izuta et al. 2006; Basilico et al. 2014; McKinley et al. 2015). The CENP-H/I/K/M complex interacts with other subcomplexes of the CCAN (Basilico et al. 2014; McKinley et al. 2015; Weir et al. 2016); therefore, one of its functions could be maintenance of CCAN integrity (Fig. 4).
3.1.3 CENP-L/N
CENP-L and -N were also identified as interacting proteins of CENP-H and -I in chicken cells (Okada et al. 2006) and as CENP-A chromatin-associated proteins in human cells (Obuse et al. 2004; Foltz et al. 2006). Depletion of these proteins results in chromosome misalignment (Foltz et al. 2006; Okada et al. 2006; Izuta et al. 2006; McClelland et al. 2007). The CENP-N N-terminus directly binds to the CENP-A nucleosome (Carroll et al. 2009; McKinley et al. 2015). CENP-L forms a heterodimer with CENP-N through association with the C-terminus of CENP-N (Carroll et al. 2009; Nagpal et al. 2015) and the recombinant CENP-L/N dimer interacts with the CENP-H/I/K/M subcomplex in vitro (McKinley et al. 2015; Weir et al. 2016). The CENP-N needs to interact with other CCAN members to be localized to the centromere in vivo, despite its direct interaction with the CENP-A nucleosomes in vitro (Carroll et al. 2009).
3.1.4 CENP-O/P/Q/R/U
Extensive proteomic analysis identified additional CCAN members. The members of the CENP-O/P/Q/R/U subcomplex were co-purified with CENP-H and -I or the CENP-A chromatin (Obuse et al. 2004; Foltz et al. 2006; Okada et al. 2006). CENP-U was originally identified as a constitutive centromere protein called CENP-50 (Minoshima et al. 2005). In contrast to other CCAN subcomplexes, the members of the CENP-O/P/Q/R/U are not essential for cell viability in chicken DT40 cells, although disruption of the members (except for CENP-R) causes cell cycle delay and deficiency in recovery from spindle damages (Hori et al. 2008b). CENP-R appears to be downstream of other members, because centromere localization of those proteins is independent of CENP-R (Hori et al. 2008b). Nonetheless, in vitro biochemical experiments clearly show that these five proteins form a complex (Hori et al. 2008b; McKinley et al. 2015). The members of this subcomplex (except for CENP-R) are conserved as the COMA (Ctf19-Okp1-Mcm21-Ame1) complex in the budding yeast (De Wulf et al. 2003). In contrast to the vertebrate cells, the COMA complex is essential in the budding yeast (De Wulf et al. 2003).
Depletion of the members of the CENP-O/P/Q/R/U does not dramatically change the centromere localization of other CCAN proteins in vertebrate cells, as those cells are viable; however, some proteins are subtly reduced in cells with depletion of the CENP-O/P/Q/R/U complex (Hori et al. 2008b). In contrast, centromere localization of CENP-O/P/Q/R/U is completely abolished in CENP-H- or CENP-I-knockout cells, indicating that centromere recruitment of the CENP-O/P/Q/R/U depends on CENP-H and -I (Okada et al. 2006; Izuta et al. 2006; Hori et al. 2008b). Although it has been shown that CENP-Q and -U directly interact with microtubules (Amaro et al. 2010; Hua et al. 2011), the significance of this interaction and the functional role of the subcomplex for kinetochore assembly is largely unknown.
3.1.5 CENP-T/S/W/X
CENP-T was originally identified as a protein that interacts with CENP-A chromatin, whereas CENP-S was found as a CENP-M- and -U- associated protein (Foltz et al. 2006; Izuta et al. 2006). Subsequent immunopurification of CENP-T and -S identified CENP-W and -X as their binding proteins, respectively (Hori et al. 2008a; Amano et al. 2009). Although CENP-S and -X constitutively localize to centromeres throughout the cell cycle (Amano et al. 2009), they also interact with Fanconi Anemia M, which is involved in the response to and repair of DNA damage (Singh et al. 2010; Yan et al. 2010). CENP-T, -W, -S and -X have a histone fold domain (HFD) (Hori et al. 2008a; Nishino et al. 2012), which distinguishes them from other CCAN members. Biochemical and structural analysis revealed that CENP-T and -W form a heterodimer by binding with their HFD (Nishino et al. 2012). The CENP-T/W heterodimer has DNA-binding activity, which requires their HFDs in vitro (Nishino et al. 2012; Takeuchi et al. 2014). This DNA-binding ability is essential for the CENP-T/W to localize to the centromere (Nishino et al. 2012; Takeuchi et al. 2014).
CENP-S and -X form a heterotetramer (Nishino et al. 2012). Strikingly, when CENP-T/W heterodimer and CENP-S/X heterotetramer are mixed, a dimer part of CENP-S/X is replaced with the CENP-T/W heterodimer to form the CENP-T/W/S/X heterotetramer, which has a nucleosome-like structure (Nishino et al. 2012). The nucleosome-like complex binds to 80–100 bp of DNA and introduces positive supercoils into DNA in vitro, whereas canonical histone nucleosome induces negative supercoils (Takeuchi et al. 2014). These data suggest that the DNA-binding of the CENP-T/W/S/X complex might contribute to a distinct feature of centromeric chromatin. Centromere localization of the CENP-T/W depends on the CENP-H/I/K/M subcomplex proteins in human cells (Basilico et al. 2014; McKinley et al. 2015), although CENP-T/W is localized in CENP-H-depleted chicken DT40 cells despite a reduction in their levels (Hori et al. 2008a). These findings suggest that the CENP-H/I/K/M is likely to support centromere localization of the CENP-T/W/S/X subcomplex in addition to its direct DNA-binding activity.
Although the CENP-T/W and the CENP-S/X form the complex, the CENP-T/W is essential for cell viability, whereas the CENP-S/X is not (Hori et al. 2008a; Amano et al. 2009; Nishino et al. 2012). CENP-S- or -X-deficient cells show mild mitotic defects as observed in knockout cells for CENP-O complex proteins (Amano et al. 2009). This suggests that, although the CENP-T/W can be recruited onto the centromere without the CENP-S/X to form a scaffold in the kinetochore, the CENP-S/X is required for proper kinetochore formation and to support proper mitotic progression (Amano et al. 2009).
CENP-W, -S, and -X are small proteins (~100 aa) and entire regions of these proteins make up the HFD. In contrast, CENP-T has the HFD in its C-terminus, but contains a long N-terminal region (~500 aa). Interestingly, the CENP-T N-terminus directly binds to the Ndc80 complex, which is a key microtubule-binding complex, through phosphorylation of CENP-T (see Fig. 5) (Gascoigne et al. 2011; Nishino et al. 2013; Rago et al. 2015). Since CENP-T associates with centromeric DNA and the Ndc80 complex, which binds to the microtubules, in its C- and N-termini, respectively, CENP-T would function as a bridge between chromatin and microtubules (Gascoigne et al. 2011; Nishino et al. 2012, 2013; Rago et al. 2015). Recent studies showed that the budding yeast CENP-T homolog Cnn1 has similar functions (Bock et al. 2012; Schleiffer et al. 2012).
An experimental design for generation of a functional artificial kinetochore. a A schematic representation of the experimental procedure in chicken DT40 cells. A protein of interest fused with LacI is tethered onto the lacO array inserted in a non-centromeric region of a Z chromosome. The native centromere flanked by loxP sites is removed by Cre recombinase. Functionality of the artificial kinetochore can be tested by cell viability. b Schematic representation of the domain organization of CENP-C and -T. c CENP-C N-terminal fragment including the Mis12 complex (Mis12C)-binding domain and the conserved middle region recruits the KMN (KNL1, Mis12, and Ndc80 complexes) network and induces functional kinetochore formation on a non-centromeric region (left). CENP-T N-terminus including Ndc80 complex (Ndc80C)-binding domain but not histone fold domain also induces functional kinetochore formation (right). Given the fragments can establish the functional kinetochores without other CCAN members, each N-terminus of CENP-C and -T is sufficient to form a functional kinetochore. This suggests that the CCAN could set two independent-pathways to recruit the KMN network, which binds to microtubules, in the kinetochore
As discussed below, ectopic localization of the CENP-T N-terminus into a non-centromere locus recruits the Ndc80 complex and induces a functional artificial kinetochore on the targeting site in chicken DT40 cells (Hori et al. 2013). Interestingly, other CCAN proteins, including CENP-C, are not detected in the CENP-T-derived artificial kinetochore (Hori et al. 2013). Considering that the CENP-C N-terminus, which recruits the Ndc80 complex through interaction with the Mis12 complex, also establishes a functional kinetochore without other CCAN proteins on it (Hori et al. 2013), CENP-T would make a pathway to recruit the Ndc80 complex independently of CENP-C in the CCAN. This implies the existence of two independent parallel pathways to recruit the Ndc80 complex to kinetochores (Hori et al. 2013; Nishino et al. 2013; Kim and Yu 2015; Rago et al. 2015).
3.2 CCAN Organization and Functions
3.2.1 CCAN Interaction
The 16 CCAN proteins are assembled on the centromere, where they interact together in both inter and intra subcomplexes (Fig. 4). The complicated features of their interaction network and their interdependency for centromere localization was characterized via extensive knockdown and conditional knockout studies (Fukagawa et al. 2001; Foltz et al. 2006; Okada et al. 2006; Izuta et al. 2006; Kwon et al. 2007; Hori et al. 2008a, b; Amano et al. 2009; Basilico et al. 2014; Klare et al. 2015; McKinley et al. 2015). In addition, biochemical reconstitution of the CCAN with recombinant proteins complements our understanding of the molecular interaction in the CCAN (Nishino et al. 2012, 2013; Basilico et al. 2014; Klare et al. 2015; Nagpal et al. 2015; McKinley et al. 2015; Weir et al. 2016). As described above, CENP-C is a key factor for the CCAN localization to the centromere, because CENP-C has multiple functional domains (see Fig. 5b) (Milks et al. 2009; Carroll et al. 2010; Przewloka et al. 2011; Screpanti et al. 2011; Hori et al. 2013; Kato et al. 2013; Klare et al. 2015; Nagpal et al. 2015), which include the KMN network-binding, the DNA-binding, and the CENP-A nucleosome binding domains, as well as domains to interact with other CCAN subunits.
Centromere localization of most CCAN proteins depends on CENP-C in human cells (Klare et al. 2015; McKinley et al. 2015). CENP-C binds to the CENP-H/I/K/M and the CENP-L/N through its middle region (Klare et al. 2015; McKinley et al. 2015; Weir et al. 2016). Although CENP-C is required for centromeric localization of the CENP-T/W/S/X in human cells (Klare et al. 2015; McKinley et al. 2015), the interaction is indirect through the CENP-H/I/K/M sub-complex, because the CENP-T/W/S/X does not directly bind to CENP-C in vitro (McKinley et al. 2015) and the CENP-T/W requires the CENP-H/I/K/M for its centromeric localization (Basilico et al. 2014; McKinley et al. 2015). In addition to playing an important role in CCAN organization, CENP- C binds both the KMN network and the CENP-A nucleosome; therefore, CENP-C is proposed to be a central component of the kinetochore assembly (Klare et al. 2015; Weir et al. 2016).
However, when CENP-C was conditionally knocked-out in chicken DT40 cells, the CENP-H/I/K/M and -T/W/S/X remained on the kinetochores despite a slight reduction of their levels (Hori et al. 2008a). In addition, dependency of CENP-C localization on the CENP-H/I/K/M varies between interphase and M-phase in both human cells and chicken DT40 cells (Fukagawa et al. 2001; Kwon et al. 2007; Nagpal et al. 2015; McKinley et al. 2015) (see in Sect. 4). This suggests that the CCAN assembly is not mediated by a simple linear pathway, but rather a complicated meshwork among the subcomplexes with multiple binding interfaces (Fig. 4).
3.2.2 The CCAN as a Bridge Between Centromere and Microtubule
The kinetochore is assembled on the centromeric chromatin. One of the key functions of CCAN is generation of a basement to build the kinetochore on the CENP-A-containing chromatin. CENP-C and -N have been shown to directly interact with the CENP-A nucleosome (Carroll et al. 2009, 2010; Kato et al. 2013; Nagpal et al. 2015; McKinley et al. 2015). These bindings to CENP-A nucleosomes might trigger the formation of CCAN on the centromeric chromatin (Fig. 6). Although centromeric localization of the CENP-T/W/S/X depends on other CCAN subunits (Hori et al. 2008a; Basilico et al. 2014; McKinley et al. 2015), it has its own DNA-binding activity through their HFD, which is essential for its centromere localization (Hori et al. 2008a; Nishino et al. 2012). Thus, the CENP-T/W/S/X might recognize a specific structure of centromeric chromatin attributed to the CENP-A nucleosomes possibly with CENP-C and -N binding (Fig. 6). Once the CENP-T/W/S/X is targeted into centromeric chromatin, it could contribute to formation of its specific features. Understanding how the CENP-T/W/S/X complex recognizes the centromere to localize there specifically is an important issue. While a recent study suggested that FACT, a histone chaperone, might bring the CENP–T/W/S/X to the centromere in human cells (Prendergast et al. 2016), further studies are needed to clarify this issue.
An organization of the vertebrate kinetochore. The CCAN constitutively localizes to the centromere throughout the cell cycle and functions as a foundation to create a linkage between the centromere and microtubules in the kinetochore. CENP-C and the CENP-L/N subcomplex make direct interactions with the CENP-A nucleosomes. The CENP-T/W/S/X subcomplex has DNA-binding activity via its histone fold domains that form a nucleosome-like structure; therefore, the CCAN would interact with the centromere through three interfaces, CENP-C, the CENP-L/N and the CENP-T/W/S/X. In M-phase, the KMN network, which binds to microtubules, is recruited onto the CCAN. CENP-C and -T recruit the KMN network via their N-termini, independently. This implies existence of two pathways from the centromere to the microtubules, because both CENP-C and -T would be bound to the centromere through direct binding to the CENP-A nucleosome and DNA, respectively
The CCAN also functions as a platform to recruit the KMN network, which is directly bound to microtubules, onto the kinetochore (Fig. 6) (Cheeseman and Desai 2008; Przewloka and Glover 2009; Varma and Salmon 2012; Foley and Kapoor 2013). CENP-C is one of the scaffolds in the CCAN, because it recruits the Mis12 complex, a subcomplex of the KMN network, onto the CCAN via the conserved N-terminus of CENP-C (Fig. 6) (Screpanti et al. 2011; Hori et al. 2013; Kim and Yu 2015; Rago et al. 2015). Direct interaction of the Mis12 complex with the conserved N-terminus has been demonstrated with biochemical reconstitution and structural analysis (Przewloka et al. 2011; Screpanti et al. 2011; Petrovic et al. 2016).
CENP-T also provides another scaffold for microtubule-binding (Fig. 6). The disordered N-terminus of CENP-T directly binds the Ndc80 complex (Gascoigne et al. 2011; Hori et al. 2013; Nishino et al. 2013; Rago et al. 2015). Structural studies revealed that phosphorylation on the CENP-T N-terminus by Cdk1 stabilized its binding to the Ndc80 complex (Nishino et al. 2013). When the Ndc80 complex-binding domain in CENP-T is disrupted, Ndc80 recruitment is prevented, resulting in cell death (Nishino et al. 2013). Together with the CENP-A nucleosomes and DNA-binding ability of CENP-C and -T, respectively, these results indicate that CENP-T together with CENP-C create the Ndc80 complex-binding platforms to bridge the centromere and the microtubules (Fig. 6).
When the CENP-C N-terminus and the CENP-T N-terminus are tethered onto a non-centromeric region using the lacO/LacI system, both fragments recruit the KMN network and induce the functional artificial kinetochores (Gascoigne et al. 2011; Hori et al. 2013). Importantly, both the CENP-C- and the CENP-T-derived kinetochores contain none of the other CCAN members, nor CENP-A (Hori et al. 2013). This indicates that CENP-C and -T have the ability to make a functional kinetochore independently. Presumably, the two pathways could form an independent parallel pathway to recruit the Ndc80 complex on the CCAN in the native kinetochore (Hori et al. 2013; Nishino et al. 2013; Kim and Yu 2015; Rago et al. 2015). If so, other challenging questions arise: why do the two pathways to recruit the Ndc80 complex exist in the native kinetochore? How do the two pathways coordinate with each other in the kinetochore assembly?
3.2.3 In Vitro Reconstitution of the CCAN
Recently, all subcomplexes of the vertebrate CCAN and the KMN network have been reconstituted with recombinant proteins (Hori et al. 2008b; Screpanti et al. 2011; Nishino et al. 2012, 2013; Basilico et al. 2014; Nagpal et al. 2015; McKinley et al. 2015; Weir et al. 2016). Using these as building blocks, interaction of the CCAN and the KMN subcomplexes was assembled in vitro (Weir et al. 2016). Combined with cross link mass spectrometry analysis (Basilico et al. 2014; Weir et al. 2016), the complicated meshwork of the CCAN and the KMN network that has multiple binding interfaces among the subunits have been revealed.
The most recent efforts have successfully assembled the large part of the CCAN in vitro; this includes CENP-C and the CENP-H/I/KM and the CENP-L/N subcomplexes (Weir et al. 2016). This reconstituted CCAN is bound to the CENP-A nucleosome through CENP-C and -N (Weir et al. 2016), as previously shown by genetic assay and in vitro binding studies (Carroll et al. 2009, 2010; Kato et al. 2013; Nagpal et al. 2015; McKinley et al. 2015). Since CENP-C has the KMN-binding activity in its N-terminus, the reconstituted CCAN binds the KMN network and established microtubule-binding via the KMN network (Weir et al. 2016). The in vitro assembly of linkage from the CENP-A nucleosome to microtubules through the CCAN and the KMN network is a seminal work for reconstitution of the functional vertebrate kinetochores in the future.
The current reconstituted kinetochore misses another essential subcomplex, the CENP-T/W/S/X. The structural analyses and ectopic tethering experiments in various systems showed that the CENP-T/W/S/X is also directly bound to the Ndc80 complex to establish microtubule-binding on the kinetochore as well as DNA-binding activity through the HFD (Hori et al. 2008a, 2013; Gascoigne et al. 2011; Nishino et al. 2012, 2013); therefore, to understand the entire kinetochore structure, it is interesting to include the CENP-T/W/S/X in the reconstituted complexes. The reconstituted whole kinetochore would allow us to understand how the CCAN utilizes the two pathways from the centromere to the microtubules. In fact, a functional kinetochore, which is assembled onto the point kinetochore, has been purified from S. cerevisiae (Akiyoshi et al. 2010). The purified kinetochore includes the CCAN and the KMN network as well as other kinetochore factors (Akiyoshi et al. 2010). The biophysics studies with the purified yeast kinetochores provided a great deal of mechanistic insight into the kinetochore regulation (Akiyoshi et al. 2010; Miller et al. 2016). Future studies with the reconstituted vertebrate kinetochore will unveil new molecular insight into functions of the kinetochore assembled on the regional centromere.
3.2.4 The CCAN-Dependent Stabilization of Centromere Position
It has been shown that the centromeric CENP-A chromatin can move its location in horse fibroblasts (Purgato et al. 2015). Recently, we demonstrated a new role of CCAN in the stabilization of centromere position through centromere movement suppression (Fig. 7) (Hori et al. 2017). One of unique features of chicken DT40 cells is that they have non-repetitive centromeres in chromosomes 5, 27, and Z (Shang et al. 2010). This attribute allows us to examine precise centromere position and size in the chromosomes by chromatin immunoprecipitation using anti-CENP-A antibody combined with deep-sequencing (ChIP-Seq). The extensive CENP-A ChIP-Seq from DT40 cells found evidence that centromere position on the chromosomes could move during many passages (Hori et al. 2017). Centromere size of the Z chromosome from a laboratory stock was about 50 kbp (Hori et al. 2017). In contrast, isolated clones from the laboratory stock as a parental line had smaller centromeres of about 30 kbp on the chromosome Z. This suggests that the 50 kbp centromere size in the parental cell line is made from a mixture of cells with various centromere positions (Hori et al. 2017). Interestingly, although the repetitive DNA in the centromere is thought to contribute to centromere position specification, the centromere drift was also found in a repetitive centromere on chromosome 1 (Hori et al. 2017).
A model for stabilization of centromere position by the complete CCAN structure. ChIP-Seq analyses with anti-CENP-A antibody using DT40 cells have demonstrated that the centromeres (chromatin region associated with the CENP-A nucleosomes) are mobile, although the centromeres scarcely move in the wild type cells. In contrast, conditional knockout of CENP-S and -U, which are the nonessential CCAN subunits in DT40 cells, increases frequency of the centromere drift due to incomplete structure of CCAN in these knockout cells. The complete CCAN organization stabilizes the centromere position
However, as seen with the smaller sized centromere in the freshly isolated lines, the centromere barely moves during the relative short-term culture, which is at least 2–3 weeks (Hori et al. 2017). Strikingly, the frequent centromere drift was found in knockout of the nonessential CCAN proteins, CENP-U or -S (Hori et al. 2008b; Amano et al. 2009), even during the short-term culture, suggesting that depletion of those CENPs increased mobility of the centromere (Fig. 7) (Hori et al. 2017). Considering that depletion of CENP-U or -S destabilized interaction among other CCAN proteins in the kinetochore, the intact CCAN organization may be required for stable centromere positioning (Fig. 7) (Hori et al. 2017). In other words, when cells exhibit stress or damage in their CCAN, centromere position moves during the cell cycle, presumably resulting in deleterious effects. This may occur because the centromere and kinetochore formation could be affected by the chromatin environment, such as histone modifications and transcription levels (Bergmann et al. 2012), and transcription is suppressed in a neocentromere in chicken DT40 cells (Shang et al. 2013).
4 Dynamic Rearrangement of CCAN Organization
4.1 Reorganization of the CCAN During the Cell Cycle
Given that the CCAN is constitutively localized to the centromere throughout the cell cycle and that the recombinant CCAN subcomplexes are successfully reconstituted, one might think the CCAN could form stable interaction networks on the centromere. However, this is not the case. The CCAN interaction appears to be dynamically reorganized along the cell cycle progression (Fukagawa et al. 2001; Kwon et al. 2007; Nagpal et al. 2015; McKinley et al. 2015).
It has been demonstrated that CENP-C changes dependency on CENP-H/K for its kinetochore localization during the cell cycle in chicken DT40 cells (Fukagawa et al. 2001; Kwon et al. 2007). Although recombinant CENP-C interacts with the CENP-H/I/K/M subcomplex in vitro (McKinley et al. 2015; Weir et al. 2016), CENP-C remains bound to the mitotic centromere in CENP-H- or -K-deficient chicken DT40 cells (Kwon et al. 2007; Hori et al. 2008a), suggesting that CENP-C binds to the centromere independently of the CENP-H/I/K/M in M-phase. However, when CENP-H or -K is depleted, CENP-C is dissociated from the centromere in interphase cells (Fukagawa et al. 2001; Kwon et al. 2007). In contrast, CENP-H localization is not completely abolished in CENP-C-deficient DT40 cells (Fukagawa et al. 2001; Hori et al. 2008a). Similar CENP-C regulation is also found in human cells (McKinley et al. 2015) using the auxin-inducible degron system by which a target protein can be rapidly degraded after auxin treatment (Nishimura et al. 2009). CENP-C localization relies on CENP-I, a member of the CENP-H/I/K/M as well as CENP-L in interphase cells (McKinley et al. 2015). However, when cells enter into M-phase, CENP-C binds to the kinetochore independently of CENP-I and -L (McKinley et al. 2015). It is worth mentioning that CENP-C is required for centromeric localization of all other subcomplexes of the CCAN in human mitotic cells (McKinley et al. 2015).
The changes in localization dependency of CENP-C on the CENP-H/I/K/M and CENP-L/N during cell cycle progression suggest that CENP-C could alter its binding partners in the CCAN (Fig. 8). Indeed, when the CENP-C C-terminus, which includes the conserved CENP-C motif and the dimerization domain (Fig. 5b), was expressed in CENP-C-deficient DT40 cells, the fragment was targeted onto the kinetochore in M-phase but not in interphase cells (Nagpal et al. 2015). In contrast, a CENP-C N-terminal fragment, which contained the conserved middle region (Fig. 5b), was localized to the kinetochore restrictively in interphase but not in M-phase (Nagpal et al. 2015). Biochemical studies showed that the middle region and the C-terminal fragment of CENP-C are bound to the CENP-H/I/K/M and -L/N subcomplexes and the CENP-A nucleosomes, respectively (Kato et al. 2013; Klare et al. 2015; Nagpal et al. 2015; McKinley et al. 2015). These observations suggest that CENP-C would change its major interacting domains with the kinetochore between interphase and M-phase (Fig. 8). Since CENP-C has multiple functional domains (Brown 1995; Meluh and Koshland 1995; Fukagawa and Brown 1997; Moore and Roth 2001; Holland et al. 2005; Heeger et al. 2005; Milks et al. 2009; Carroll et al. 2010; Przewloka et al. 2011; Screpanti et al. 2011; Kato et al. 2013; Klare et al. 2015; Nagpal et al. 2015), another domain might also contribute to CENP-C-targeting onto the kinetochore. Nevertheless, CENP-C could dynamically alter its kinetochore-binding mode via cell cycle-dependent regulations (Fig. 8) (Nagpal et al. 2015; Nagpal and Fukagawa 2016).
A model for reorganization of the CCAN during the cell cycle progression. The CCAN constitutively localizes to the centromere during the cell cycle. However, interaction network among its subunits appears to be dynamically reorganized along cell cycle progression. In interphase, centromeric localization of CENP-C depends on the CENP-L/N and -H/I/K/M subcomplexes. In contrast, when cells enter into M-phase, CENP-C is localized to the centromere independently of the CENP-L/N and -H/I/K/M subcomplexes probably through its direct binding to the CENP-A nucleosome. This suggests that CENP-C switches its major interaction interfaces during the cell cycle
Although the significance of cell cycle-dependent CCAN reorganization has not been elucidated yet, it might be related to fine localization of the CCAN subunits in kinetochores. In fact, electron microscopy observation suggests that the CCAN subunits change their distribution in the kinetochore when tension is applied to the kinetochore from microtubules during M-phase (Suzuki et al. 2011). Consistent with this observation, a super-resolution microscopy analysis also shows that distance between CENP-A and the KMN network is stretched when the tension is applied during M-phase (Wan et al. 2009), suggesting that in order to resist the tension generated from microtubules, the CCAN might form an elastic and strong interaction meshwork (Suzuki et al. 2014). The structural rearrangement of the CCAN could be important for proper M-phase progression, because the rearrangement in the kinetochore is proposed to play a role in silencing of the spindle assembly checkpoint (Maresca and Salmon 2009; Uchida et al. 2009). Following M-phase completion, newly synthesized CENP-A is deposited onto the centromere during early G1 phase (Jansen et al. 2007; Dunleavy et al. 2009; Foltz et al. 2009). It is also suggested that new CCAN proteins are likely to be recruited during interphase (Hemmerich et al. 2008; Prendergast et al. 2011). Studies using fluorescence recovery after photobleaching assays suggest that the CCAN subunits are dynamically incorporated into the centromere during interphase but not during M-phase (Hemmerich et al. 2008; Prendergast et al. 2011). The CCAN rearrangement in interphase might convert the CCAN meshwork to a more open structure to facilitate the targeting of the new centromere protein to the kinetochore.
4.2 CCAN Organization During Development
The CCAN displays different regulation and function when viewed from a developmental point of view. The depletion of subunits of the CENP-O/P/Q/R/U sub-complex did not affect cell viability in chicken DT40 cells despite a slight mitotic defect (Hori et al. 2008b). In contrast, CENP-U null mice died during early embryo development (Kagawa et al. 2014). The CENP-U+/− intercross never gave birth to homozygous CENP-U−/− mice, rather the CENP-U null mice died after late gastrulation stage (E7.5) (Kagawa et al. 2014). However, mouse embryonic fibroblast cells isolated from CENP-U null mice were viable with mild mitotic defects as seen in the CENP-U-deficient DT40 cells (Kagawa et al. 2014). Interestingly, when CENP-U was depleted in mouse embryonic stem (mES) cells, the mES cells died after showing chromosome segregation errors (Kagawa et al. 2014). Despite the cell-proliferation defects in the CENP-U null mES, CENP-H localization into the kinetochore was not affected as seen in the CENP-U-deficient DT40 cells (Kagawa et al. 2014). This suggests that although the CCAN composition in mES cells is similar to that in DT 40 cells, the CCAN without CENP-U is unfunctional in the undifferentiated mES cells or early embryonic cells (Hori et al. 2008b; Kagawa et al. 2014).
CENP-U depletion appeared to have an effect on the CCAN meshwork formation and stability of the centromere position in DT40 cells (Hori et al. 2008b, 2017). Spindle assembly checkpoint may be weaker in mES cells than in somatic cells; therefore, mES cells could fail chromosome segregation more often with the impaired CENP-U-deficient CCAN (Kagawa et al. 2014). An alternate possibility is that the difference in pericentromeric heterochromatin (PCH) between differentiated and undifferentiated cells is responsible. It has been shown that a transcription factor, NANOG (Chambers et al. 2003; Mitsui et al. 2003), which is a key pluripotency factor, establishes and maintains open PCH structure in mES cells (Novo et al. 2016). Because PCH is thought to be involved in faithful chromosome segregation and centromere stability (Peters et al. 2001; Yamagishi et al. 2008), open and active forms of PCH combined with the impaired CCAN might cause cell death in undifferentiated cells with depletion of CENP-U.
5 Conclusion
Since establishment of the chromosome theory from studies with various systems including echinoderm embryos, insect germ lines, and plant cells (Benson 2001; Satzinger 2008), our understanding of the molecular mechanisms responsible for the correct segregation of chromosomes to the next generations has been accelerated. These large efforts revealed the basic structure of the CCAN organization. Although reconstitution of the CCAN assembly in vitro is a key step in building a functional recombinant kinetochore for further biochemical and biophysics studies, this structure might show only one aspect of the CCAN structure as a snapshot. Indeed, accumulating data suggest that dynamic remodeling occurs in the CCAN organization during the cell cycle. How the CCAN organization is remodeled and what is the significance of the remodeling are future questions to be addressed combining various experimental approaches including genetics, cell biology, molecular biology, genome science, biochemistry, structural biology, and biophysics.
Most of the CCAN members are found in both yeasts and vertebrates; therefore, the CCAN looks to be a conserved structure among various species (Przewloka and Glover 2009). However, the CCAN organization has been dynamically rewired during evolution. For example, CENP-C is the sole CCAN component in D. melanogaster (Drinnenberg et al. 2014, 2016). CENP-C could make a platform of kinetochore assembly in the fly. On the other hand, the silkworm, Bombyx mori, lacks in CENP-C (Drinnenberg et al. 2016). Since most of the KMN network subunits exist in B. mori, the silkworm should have another scaffold to the KMN other than CENP-C. Moreover, B. mori has holocentromeres (Murakami and Imai 1974), a type of centromere, which are extended through entire chromosomes (Dernburg 2001). In fact, the holocentromere is found in many insects or plants as well as C. elegans (Albertson and Thomson 1982; Drinnenberg et al. 2014). The CCAN could adapt to be assembled on the holocentric chromatin in those species.
Centromere structures and CCAN organizations are highly diverse among species. Studies on molecular functions and regulatory mechanisms of the kinetochore in various species in addition to the model systems should lead us to a comprehensive understanding of the chromosome segregation, similar to when the chromosome theory was established.
References
Akiyoshi B, Sarangapani KK, Powers AF, Nelson CR, Reichow SL, Arellano-Santoyo H, Gonen T, Ranish JA, Asbury CL, Biggins S (2010) Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468:576–579. doi:10.1038/nature09594
Albertson DG, Thomson JN (1982) The kinetochores of Caenorhabditis elegans. Chromosoma. doi:10.1007/BF00292267
Aldrup-Macdonald ME, Sullivan BA (2014) The past, present, and future of human centromere genomics. Genes (Basel) 5:33–50. doi:10.3390/genes5010033
Amano M, Suzuki A, Hori T, Backer C, Okawa K, Cheeseman IM, Fukagawa T (2009) The CENP-S complex is essential for the stable assembly of outer kinetochore structure. J Cell Biol 186:173–182. doi:10.1083/jcb.200903100
Amaro AC, Samora CP, Holtackers R, Wang E, Kingston IJ, Alonso M, Lampson M, McAinsh AD, Meraldi P (2010) Molecular control of kinetochore-microtubule dynamics and chromosome oscillations. Nat Cell Biol 12:319–329. doi:10.1038/ncb2033
Bailey AO, Panchenko T, Shabanowitz J, Lehman SM, Bai DL, Hunt DF, Black BE, Foltz DR (2016) Identification of the post-translational modifications present in centromeric chromatin. Mol Cell Proteomics 15:918–931. doi:10.1074/mcp.M115.053710
Barnhart MC, Kuich PHJL, Stellfox ME, Ward JA, Bassett EA, Black BE, Foltz DR (2011) HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol 194:229–243. doi:10.1083/jcb.201012017
Basilico F, Maffini S, Weir JR, Prumbaum D, Rojas AM, Zimniak T, De Antoni A, Jeganathan S, Voss B, van Gerwen S, Krenn V, Massimiliano L, Valencia A, Vetter IR, Herzog F, Raunser S, Pasqualato S, Musacchio A (2014) The pseudo GTPase CENP-M drives human kinetochore assembly. Elife 3:e02978. doi:10.7554/eLife.02978
Benson KR (2001) T.H. Morgan’s resistance to the chromosome theory. Nat Rev Genet 2:469–474. doi:10.1038/35076532
Bergmann JH, Martins NMC, Larionov V, Masumoto H, Earnshaw WC (2012) HACking the centromere chromatin code: insights from human artificial chromosomes. Chromosome Res 20:505–519. doi:10.1007/s10577-012-9293-0
Bergmann JH, Rodríguez MG, Martins NMC, Kimura H, Kelly DA, Masumoto H, Larionov V, Jansen LET, Earnshaw WC (2011) Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J 30:328–340. doi:10.1038/emboj.2010.329
Birchler JA (2015) Mendel, mechanism, models, marketing, and more. Cell 163:9–11. doi:10.1016/j.cell.2015.09.008
Blower MD, Karpen GH (2001) The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat Cell Biol 3:730–739. doi:10.1038/35087045
Blower MD, Sullivan BA, Karpen GH (2002) Conserved organization of centromeric chromatin in flies and humans. Dev Cell 2:319–330
Bock LJ, Pagliuca C, Kobayashi N, Grove RA, Oku Y, Shrestha K, Alfieri C, Golfieri C, Oldani A, Dal Maschio M, Bermejo R, Hazbun TR, Tanaka TU, De Wulf P (2012) Cnn1 inhibits the interactions between the KMN complexes of the yeast kinetochore. Nat Cell Biol 14:614–624. doi:10.1038/ncb2495
Boveri T (1904) Ergebnisse über die Konstitution der chromatischen Substanz des Zellkerns. Verlag von Gustav Fischer, Jena
Brown MT (1995) Sequence similarities between the yeast chromosome segregation protein Mif2 and the mammalian centromere protein CENP-C. Gene 160:111–116. doi:10.1016/0378-1119(95)00163-Z
Brown MT, Goetsch L, Hartwell LH (1993) MIF2 is required for mitotic spindle integrity during anaphase spindle elongation in Saccharomyces cerevisiae. J Cell Biol 123:387–403
Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S (1999) A histone-H3-like protein in C. elegans. Nature 401:547–548. doi:10.1038/44062
Carroll CW, Milks KJ, Straight AF (2010) Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol 189:1143–1155. doi:10.1083/jcb.201001013
Carroll CW, Silva MCC, Godek KM, Jansen LET, Straight AF (2009) Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol 11:896–902. doi:10.1038/ncb1899
Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655. doi:10.1016/S0092-8674(03)00392-1
Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997. doi:10.1016/j.cell.2006.09.039
Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9:33–46. doi:10.1038/nrm2310
Chen C-C, Dechassa ML, Bettini E, Ledoux MB, Belisario C, Heun P, Luger K, Mellone BG (2014) CAL1 is the Drosophila CENP-A assembly factor. J Cell Biol 204:313–329. doi:10.1083/jcb.201305036
Clarke L (1998) Centromeres: proteins, protein complexes, and repeated domains at centromeres of simple eukaryotes. Curr Opin Genet Dev 8:212–218. doi:10.1016/S0959-437X(98)80143-3
Correns CE (1900) G. Mendel’s Regel über das Verhalten der Nachkommenschaft der Rassenbastarde. Berichte der Deutsche Botanischen 18:158–168. doi:10.1111/j.1438-8677.1900.tb04893.x
Darlington CD (1936) The external mechanics of the chromosomes. Proc R Soc Lond 121:264–319. doi:10.2307/82059
de Vries H (1900) Sur la loi de disjonction des hybrides. Comptes rendus de l’Académie des Sciences 130:845–847
De Wulf P, McAinsh AD, Sorger PK (2003) Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev 17:2902–2921. doi:10.1101/gad.1144403
Dernburg AF (2001) Here, there, and everywhere. J Cell Biol 153:F33–F38. doi:10.1083/jcb.153.6.F33
Drinnenberg IA, deYoung D, Henikoff S, Malik HS (2014) Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife 3:2104–2119. doi:10.7554/eLife.03676
Drinnenberg IA, Henikoff S, Malik HS (2016) Evolutionary turnover of kinetochore proteins: a Ship of Theseus? Trends Cell Biol 26:498–510. doi:10.1016/j.tcb.2016.01.005
Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G (2009) HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell 137:485–497. doi:10.1016/j.cell.2009.02.040
Earnshaw WC, Rothfield N (1985) Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91:313–321
Falk SJ, Guo LY, Sekulic N, Smoak EM, Mani T, Logsdon GA, Gupta K, Jansen LET, Van Duyne GD, Vinogradov SA, Lampson MA, Black BE (2015) Chromosomes. CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science 348:699–703. doi:10.1126/science.1259308
Falk SJ, Lee J, Sekulic N, Sennett MA, Lee T-H, Black BE (2016) CENP-C directs a structural transition of CENP-A nucleosomes mainly through sliding of DNA gyres. Nat Struct Mol Biol 23:204–208. doi:10.1038/nsmb.3175
Flemming W (1882) Zellsubstanz, kern und zelltheilung. F.C.W. Vogel, Leipzig
Foley EA, Kapoor TM (2013) Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 14:25–37. doi:10.1038/nrm3494
Foltz DR, Jansen LET, Bailey AO, Yates JR, Bassett EA, Wood S, Black BE, Cleveland DW (2009) Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell 137:472–484. doi:10.1016/j.cell.2009.02.039
Foltz DR, Jansen LET, Black BE, Bailey AO, Yates JR, Cleveland DW (2006) The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol 8:458–469. doi:10.1038/ncb1397
Fukagawa T, Brown W (1997) Efficient conditional mutation of the vertebrate CENP-C gene. Hum Mol Genet. doi:10.1093/hmg/6.13.2301
Fukagawa T, Earnshaw WC (2014a) The centromere: chromatin foundation for the kinetochore machinery. Dev Cell 30:496–508. doi:10.1016/j.devcel.2014.08.016
Fukagawa T, Earnshaw WC (2014b) Neocentromeres. Curr Biol 24:R946–R947. doi:10.1016/j.cub.2014.08.032
Fukagawa T, Mikami Y, Nishihashi A, Regnier V, Haraguchi T, Hiraoka Y, Sugata N, Todokoro K, Brown W, Ikemura T (2001) CENP-H, a constitutive centromere component, is required for centromere targeting of CENP-C in vertebrate cells. EMBO J 20:4603–4617. doi:10.1093/emboj/20.16.4603
Fukagawa T, Pendon C, Morris J, Brown W (1999) CENP-C is necessary but not sufficient to induce formation of a functional centromere. EMBO J 18:4196–4209. doi:10.1093/emboj/18.15.4196
Gascoigne KE, Takeuchi K, Suzuki A, Hori T, Fukagawa T, Cheeseman IM (2011) Induced ectopic kinetochore assembly bypasses the requirement for CENP-A nucleosomes. Cell 145:410–422. doi:10.1016/j.cell.2011.03.031
Goshima G, Kiyomitsu T, Yoda K, Yanagida M (2003) Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J Cell Biol 160:25–39. doi:10.1083/jcb.200210005
Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF (1997) Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet 15:345–355. doi:10.1038/ng0497-345
Heeger S, Leismann O, Schittenhelm R, Schraidt O, Heidmann S, Lehner CF (2005) Genetic interactions of separase regulatory subunits reveal the diverged Drosophila Cenp-C homolog. Genes Dev 19:2041–2053. doi:10.1101/gad.347805
Hegemann JH, Fleig UN (1993) The centromere of budding yeast. BioEssays 15:451–460. doi:10.1002/bies.950150704
Hemmerich P, Weidtkamp-Peters S, Hoischen C, Schmiedeberg L, Erliandri I, Diekmann S (2008) Dynamics of inner kinetochore assembly and maintenance in living cells. J Cell Biol 180:1101–1114. doi:10.1083/jcb.200710052
Heun P, Erhardt S, Blower MD, Weiss S, Skora AD, Karpen GH (2006) Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell 10:303–315. doi:10.1016/j.devcel.2006.01.014
Holland S, Ioannou D, Haines S, Brown WRA (2005) Comparison of Dam tagging and chromatin immunoprecipitation as tools for the identification of the binding sites for S. pombe CENP-C. Chromosome Res 13:73–83. doi:10.1007/s10577-005-7062-z
Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwen BF, Shang W-H, Suzuki E, Okawa K, Cheeseman IM, Fukagawa T (2008a) CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135:1039–1052. doi:10.1016/j.cell.2008.10.019
Hori T, Kagawa N, Toyoda A, Fujiyama A, Misu S, Monma N, Makino F, Ikeo K, Fukagawa T (2017) Constitutive centromere-associated network controls centromere drift in vertebrate cells. J Cell Biol 216:101–113. doi:10.1083/jcb.201605001
Hori T, Okada M, Maenaka K, Fukagawa T (2008b) CENP-O class proteins form a stable complex and are required for proper kinetochore function. Mol Biol Cell 19:843–854. doi:10.1091/mbc.E07-06-0556
Hori T, Shang W-H, Takeuchi K, Fukagawa T (2013) The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol 200:45–60. doi:10.1083/jcb.201210106
Hori T, Shang W-H, Toyoda A, Misu S, Monma N, Ikeo K, Molina O, Vargiu G, Fujiyama A, Kimura H, Earnshaw WC, Fukagawa T (2014) Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly. Dev Cell 29:740–749. doi:10.1016/j.devcel.2014.05.001
Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KH (2000) Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci USA 97:1148–1153
Hua S, Wang Z, Jiang K, Huang Y, Ward T, Zhao L, Dou Z, Yao X (2011) CENP-U cooperates with Hec1 to orchestrate kinetochore-microtubule attachment. J Biol Chem 286:1627–1638. doi:10.1074/jbc.M110.174946
Ikeno M, Grimes B, Okazaki T, Nakano M, Saitoh K, Hoshino H, McGill NI, Cooke H, Masumoto H (1998) Construction of YAC-based mammalian artificial chromosomes. Nat Biotechnol 16:431–439. doi:10.1038/nbt0598-431
Ishii K, Ogiyama Y, Chikashige Y, Soejima S, Masuda F, Kakuma T, Hiraoka Y, Takahashi K (2008) Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science 321:1088–1091. doi:10.1126/science.1158699
Izuta H, Ikeno M, Suzuki N, Tomonaga T, Nozaki N, Obuse C, Kisu Y, Goshima N, Nomura F, Nomura N, Yoda K (2006) Comprehensive analysis of the ICEN (interphase centromere complex) components enriched in the CENP-A chromatin of human cells. Genes Cells 11:673–684. doi:10.1111/j.1365-2443.2006.00969.x
Jansen LET, Black BE, Foltz DR, Cleveland DW (2007) Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol 176:795–805. doi:10.1083/jcb.200701066
Kagawa N, Hori T, Hoki Y, Hosoya O, Tsutsui K, Saga Y, Sado T, Fukagawa T (2014) The CENP-O complex requirement varies among different cell types. Chromosome Res 22:293–303. doi:10.1007/s10577-014-9404-1
Kato H, Jiang J, Zhou B-R, Rozendaal M, Feng H, Ghirlando R, Xiao TS, Straight AF, Bai Y (2013) A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science 340:1110–1113. doi:10.1126/science.1235532
Ketel C, Wang HSW, McClellan M, Bouchonville K, Selmecki A, Lahav T, Gerami-Nejad M, Berman J (2009) Neocentromeres form efficiently at multiple possible loci in Candida albicans. PLoS Genet 5:e1000400. doi:10.1371/journal.pgen.1000400
Kim S, Yu H (2015) Multiple assembly mechanisms anchor the KMN spindle checkpoint platform at human mitotic kinetochores. J Cell Biol 208:181–196. doi:10.1083/jcb.201407074
Klare K, Weir JR, Basilico F, Zimniak T, Massimiliano L, Ludwigs N, Herzog F, Musacchio A (2015) CENP-C is a blueprint for constitutive centromere-associated network assembly within human kinetochores. J Cell Biol 210:11–22. doi:10.1083/jcb.201412028
Kursel LE, Malik HS (2016) Centromeres. Curr Biol 26:R487–R490. doi:10.1016/j.cub.2016.05.031
Kwon M-S, Hori T, Okada M, Fukagawa T (2007) CENP-C is involved in chromosome segregation, mitotic checkpoint function, and kinetochore assembly. Mol Biol Cell 18:2155–2168. doi:10.1091/mbc.E07-01-0045
Locke DP, Hillier LW, Warren WC, Worley KC, Nazareth LV, Muzny DM, Yang S-P, Wang Z, Chinwalla AT, Minx P, Mitreva M, Cook L, Delehaunty KD, Fronick C, Schmidt H, Fulton LA, Fulton RS, Nelson JO, Magrini V, Pohl C, Graves TA, Markovic C, Cree A, Dinh HH, Hume J, Kovar CL, Fowler GR, Lunter G, Meader S, Heger A, Ponting CP, Marques-Bonet T, Alkan C, Chen L, Cheng Z, Kidd JM, Eichler EE, White S, Searle S, Vilella AJ, Chen Y, Flicek P, Ma J, Raney B, Suh B, Burhans R, Herrero J, Haussler D, Faria R, Fernando O, Darré F, Farré D, Gazave E, Oliva M, Navarro A, Roberto R, Capozzi O, Archidiacono N, Valle Della G, Purgato S, Rocchi M, Konkel MK, Walker JA, Ullmer B, Batzer MA, Smit AFA, Hubley R, Casola C, Schrider DR, Hahn MW, Quesada V, Puente XS, Ordoñez GR, López-Otín C, Vinar T, Brejova B, Ratan A, Harris RS, Miller W, Kosiol C, Lawson HA, Taliwal V, Martins AL, Siepel A, Roychoudhury A, Ma X, Degenhardt J, Bustamante CD, Gutenkunst RN, Mailund T, Dutheil JY, Hobolth A, Schierup MH, Ryder OA, Yoshinaga Y, de Jong PJ, Weinstock GM, Rogers J, Mardis ER, Gibbs RA, Wilson RK (2011) Comparative and demographic analysis of orang-utan genomes. Nature 469:529–533. doi:10.1038/nature09687
London N, Biggins S (2014) Signalling dynamics in the spindle checkpoint response. Nat Rev Mol Cell Biol 15:736–747. doi:10.1038/nrm3888
Maresca TJ, Salmon ED (2009) Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol 184:373–381. doi:10.1083/jcb.200808130
Marshall OJ, Chueh AC, Wong LH, Choo KHA (2008) Neocentromeres: new insights into centromere structure, disease development, and karyotype evolution. Am J Hum Genet 82:261–282. doi:10.1016/j.ajhg.2007.11.009
McClelland SE, Borusu S, Amaro AC, Winter JR, Belwal M, McAinsh AD, Meraldi P (2007) The CENP-A NAC/CAD kinetochore complex controls chromosome congression and spindle bipolarity. EMBO J 26:5033–5047. doi:10.1038/sj.emboj.7601927
McKinley KL, Cheeseman IM (2016) The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol 17:16–29
McKinley KL, Sekulic N, Guo LY, Tsinman T, Black BE, Cheeseman IM (2015) The CENP-L-N complex forms a critical node in an integrated meshwork of interactions at the centromere-kinetochore interface. Mol Cell 60:886–898. doi:10.1016/j.molcel.2015.10.027
Meluh PB, Koshland D (1995) Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the Mammalian centromere protein CENP-C. Mol Biol Cell 6:793–807. doi:10.1091/mbc.6.7.793
Mendiburo MJ, Padeken J, Fülöp S, Schepers A, Heun P (2011) Drosophila CENH3 is sufficient for centromere formation. Science 334:686–690. doi:10.1126/science.1206880
Milks KJ, Moree B, Straight AF (2009) Dissection of CENP-C-directed centromere and kinetochore assembly. Mol Biol Cell 20:4246–4255. doi:10.1091/mbc.E09-05-0378
Miller MP, Asbury CL, Biggins S (2016) A TOG protein confers tension sensitivity to kinetochore-microtubule attachments. Cell 165:1428–1439. doi:10.1016/j.cell.2016.04.030
Minoshima Y, Hori T, Okada M, Kimura H, Haraguchi T, Hiraoka Y, Bao Y-C, Kawashima T, Kitamura T, Fukagawa T (2005) The constitutive centromere component CENP-50 is required for recovery from spindle damage. Mol Cell Biol 25:10315–10328. doi:10.1128/MCB.25.23.10315-10328.2005
Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642. doi:10.1016/S0092-8674(03)00393-3
Moore LL, Roth MB (2001) Hcp-4, a Cenp-C–Like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J Cell Biol 153:1199–1208. doi:10.1083/jcb.153.6.1199
Morgan TH (1915) The mechanism of Mendelian heredity. Holt
Murakami A, Imai HT (1974) Cytological evidence for holocentric chromosomes of the silkworms, Bombyx mori and B. mandarina, (Bombycidae, Lepidoptera). Chromosoma 47:167–178. doi:10.1007/BF00331804
Musacchio A (2015) The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol 25:R1002–R1018. doi:10.1016/j.cub.2015.08.051
Nagpal H, Fukagawa T (2016) Kinetochore assembly and function through the cell cycle. Chromosoma 125:645–659. doi:10.1007/s00412-016-0608-3
Nagpal H, Hori T, Furukawa A, Sugase K, Osakabe A, Kurumizaka H, Fukagawa T (2015) Dynamic changes in CCAN organization through CENP-C during cell-cycle progression. Mol Biol Cell 26:3768–3776. doi:10.1091/mbc.E15-07-0531
Nishihashi A, Haraguchi T, Hiraoka Y, Ikemura T, Regnier V, Dodson H, Earnshaw WC, Fukagawa T (2002) CENP-I is essential for centromere function in vertebrate cells. Dev Cell 2:463–476
Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M (2009) An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods 6:917–922. doi:10.1038/nmeth.1401
Nishino T, Rago F, Hori T, Tomii K, Cheeseman IM, Fukagawa T (2013) CENP-T provides a structural platform for outer kinetochore assembly. EMBO J 32:424–436. doi:10.1038/emboj.2012.348
Nishino T, Takeuchi K, Gascoigne KE, Suzuki A, Hori T, Oyama T, Morikawa K, Cheeseman IM, Fukagawa T (2012) CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell 148:487–501. doi:10.1016/j.cell.2011.11.061
Novo CL, Tang C, Ahmed K, Djuric U, Fussner E, Mullin NP, Morgan NP, Hayre J, Sienerth AR, Elderkin S, Nishinakamura R, Chambers I, Ellis J, Bazett-Jones DP, Rugg-Gunn PJ (2016) The pluripotency factor Nanog regulates pericentromeric heterochromatin organization in mouse embryonic stem cells. Genes Dev 30:1101–1115. doi:10.1101/gad.275685.115
Obuse C, Yang H, Nozaki N, Goto S, Okazaki T, Yoda K (2004) Proteomics analysis of the centromere complex from HeLa interphase cells: UV-damaged DNA binding protein 1 (DDB-1) is a component of the CEN-complex, while BMI-1 is transiently co-localized with the centromeric region in interphase. Genes Cells 9:105–120
Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA (2001) Functional analysis of kinetochore assembly in Caenorhabditis elegans. J Cell Biol 153:1209–1226
Ohzeki J-I, Nakano M, Okada T, Masumoto H (2002) CENP-B box is required for de novo centromere chromatin assembly on human alphoid DNA. J Cell Biol 159:765–775. doi:10.1083/jcb.200207112
Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR, Desai A, Fukagawa T (2006) The CENP-H–I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol 8:446–457. doi:10.1038/ncb1396
Palmer DK, O’Day K, Wener MH, Andrews BS, Margolis RL (1987) A 17-kD centromere protein (CENP-A) copurifies with nucleosome core particles and with histones. J Cell Biol 104:805–815
Perpelescu M, Fukagawa T (2011) The ABCs of CENPs. Chromosoma 120:425–446. doi:10.1007/s00412-011-0330-0
Pesenti ME, Weir JR, Musacchio A (2016) Progress in the structural and functional characterization of kinetochores. Curr Opin Struct Biol 37:152–163. doi:10.1016/j.sbi.2016.03.003
Peters AHFM, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schöfer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T (2001) Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107:323–337. doi:10.1016/S0092-8674(01)00542-6
Petrovic A, Keller J, Liu Y, Overlack K, John J, Dimitrova YN, Jenni S, van Gerwen S, Stege P, Wohlgemuth S, Rombaut P, Herzog F, Harrison SC, Vetter IR, Musacchio A (2016) Structure of the MIS12 complex and molecular basis of its interaction with CENP-C at human kinetochores. Cell 167(1028–1040):e15. doi:10.1016/j.cell.2016.10.005
Piras FM, Nergadze SG, Magnani E, Bertoni L, Attolini C, Khoriauli L, Raimondi E, Giulotto E (2010) Uncoupling of satellite DNA and centromeric function in the genus Equus. PLoS Genet 6:e1000845. doi:10.1371/journal.pgen.1000845
Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC (1995) The centromere: hub of chromosomal activities. Science 270:1591–1594
Prendergast L, Müller S, Liu Y, Huang H, Dingli F, Loew D, Vassias I, Patel DJ, Sullivan KF, Almouzni G (2016) The CENP-T/-W complex is a binding partner of the histone chaperone FACT. Genes Dev 30:1313–1326. doi:10.1101/gad.275073.115
Prendergast L, van Vuuren C, Kaczmarczyk A, Doering V, Hellwig D, Quinn N, Hoischen C, Diekmann S, Sullivan KF (2011) Premitotic assembly of human CENPs -T and -W switches centromeric chromatin to a mitotic state. PLoS Biol 9:e1001082. doi:10.1371/journal.pbio.1001082
Przewloka MR, Glover DM (2009) The kinetochore and the centromere: a working long distance relationship. Annu Rev Genet 43:439–465. doi:10.1146/annurev-genet-102108-134310
Przewloka MR, Venkei Z, Bolanos-Garcia VM, Debski J, Dadlez M, Glover DM (2011) CENP-C is a structural platform for kinetochore assembly. Curr Biol 21:399–405. doi:10.1016/j.cub.2011.02.005
Purgato S, Belloni E, Piras FM, Zoli M, Badiale C, Cerutti F, Mazzagatti A, Perini G, Valle Della G, Nergadze SG, Sullivan KF, Raimondi E, Rocchi M, Giulotto E (2015) Centromere sliding on a mammalian chromosome. Chromosoma 124:277–287. doi:10.1007/s00412-014-0493-6
Rago F, Gascoigne KE, Cheeseman IM (2015) Distinct organization and regulation of the outer kinetochore KMN network downstream of CENP-C and CENP-T. Curr Biol 25:671–677. doi:10.1016/j.cub.2015.01.059
Regnier V, Vagnarelli P, Fukagawa T, Zerjal T, Burns E, Trouche D, Earnshaw W, Brown W (2005) CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol Cell Biol 25:3967–3981. doi:10.1128/MCB.25.10.3967-3981.2005
Saitoh H, Tomkiel J, Cooke CA, Ratrie H, Maurer M, Rothfield NF, Earnshaw WC (1992) CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70:115–125
Saitoh S, Takahashi K, Yanagida M (1997) Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell 90:131–143. doi:10.1016/S0092-8674(00)80320-7
Satzinger H (2008) Theodor and Marcella Boveri: chromosomes and cytoplasm in heredity and development. Nat Rev Genet 9(3):231–238
Schleiffer A, Maier M, Litos G, Lampert F, Hornung P, Mechtler K, Westermann S (2012) CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat Cell Biol 14:604–613. doi:10.1038/ncb2493
Screpanti E, De Antoni A, Alushin GM, Petrovic A, Melis T, Nogales E, Musacchio A (2011) Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr Biol 21:391–398. doi:10.1016/j.cub.2010.12.039
Shang W-H, Hori T, Martins NMC, Toyoda A, Misu S, Monma N, Hiratani I, Maeshima K, Ikeo K, Fujiyama A, Kimura H, Earnshaw WC, Fukagawa T (2013) Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev Cell 24:635–648. doi:10.1016/j.devcel.2013.02.009
Shang W-H, Hori T, Toyoda A, Kato J, Popendorf K, Sakakibara Y, Fujiyama A, Fukagawa T (2010) Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res 20:1219–1228. doi:10.1101/gr.106245.110
Shang W-H, Hori T, Westhorpe FG, Godek KM, Toyoda A, Misu S, Monma N, Ikeo K, Carroll CW, Takami Y, Fujiyama A, Kimura H, Straight AF, Fukagawa T (2016) Acetylation of histone H4 lysine 5 and 12 is required for CENP-A deposition into centromeres. Nat Commun 7:13465. doi:10.1038/ncomms13465
Singh TR, Saro D, Ali AM, Zheng X-F, Du C-H, Killen MW, Sachpatzidis A, Wahengbam K, Pierce AJ, Xiong Y, Sung P, Meetei AR (2010) MHF1-MHF2, a histone-fold-containing protein complex, participates in the Fanconi anemia pathway via FANCM. Mol Cell 37:879–886. doi:10.1016/j.molcel.2010.01.036
Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M (1995) A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev 9:573–586
Stukenberg PT, Burke DJ (2015) Connecting the microtubule attachment status of each kinetochore to cell cycle arrest through the spindle assembly checkpoint. Chromosoma 124:463–480. doi:10.1007/s00412-015-0515-z
Sugata N, Li S, Earnshaw WC, Yen TJ, Yoda K, Masumoto H, Munekata E, Warburton PE, Todokoro K (2000) Human CENP-H multimers colocalize with CENP-A and CENP-C at active centromere–kinetochore complexes. Hum Mol Genet 9:2919–2926
Sugata N, Munekata E, Todokoro K (1999) Characterization of a novel kinetochore protein, CENP-H. J Biol Chem 274:27343–27346
Sullivan BA, Karpen GH (2004) Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol 11:1076–1083. doi:10.1038/nsmb845
Sutton WS (1902) On the morphology of the chromosome group in Brachystola magna. Biol Bull 4:24–39
Sutton WS (1903) The chromosomes in heredity. Biol Bull 4:231–250
Suzuki A, Badger BL, Wan X, DeLuca JG, Salmon ED (2014) The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper intrakinetochore stretch. Dev Cell 30:717–730. doi:10.1016/j.devcel.2014.08.003
Suzuki A, Hori T, Nishino T, Usukura J, Miyagi A, Morikawa K, Fukagawa T (2011) Spindle microtubules generate tension-dependent changes in the distribution of inner kinetochore proteins. J Cell Biol 193:125–140. doi:10.1083/jcb.201012050
Takahashi K, Chen ES, Yanagida M (2000) Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science 288:2215–2219
Takeuchi K, Fukagawa T (2012) Molecular architecture of vertebrate kinetochores. Exp Cell Res 318:1367–1374. doi:10.1016/j.yexcr.2012.02.016
Takeuchi K, Nishino T, Mayanagi K, Horikoshi N, Osakabe A, Tachiwana H, Hori T, Kurumizaka H, Fukagawa T (2014) The centromeric nucleosome-like CENP-T-W-S-X complex induces positive supercoils into DNA. Nucleic Acids Res 42:1644–1655. doi:10.1093/nar/gkt1124
Talbert PB, Masuelli R, Tyagi AP, Comai L, Henikoff S (2002) Centromeric localization and adaptive evolution of an Arabidopsis histone H3 variant. Plant Cell 14:1053–1066. doi:10.1105/tpc.010425
Tipton AR, Wang K, Oladimeji P, Sufi S, Gu Z, Liu S-T (2012) Identification of novel mitosis regulators through data mining with human centromere/kinetochore proteins as group queries. BMC Cell Biol 13(1):15. doi:10.1186/1471-2121-13-15
Tomkiel J, Cooke CA, Saitoh H, Bernat RL, Earnshaw WC (1994) CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J Cell Biol 125:531–545
Tschermak E (1900) Über künstliche Kreuzung bei Pisum sativum. Berichte der Deutsche Botanischen Gessellschaft 28:232–239
Uchida KSK, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T (2009) Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol 184:383–390. doi:10.1083/jcb.200811028
Van Hooser AA, Ouspenski II, Gregson HC, Starr DA, Yen TJ, Goldberg ML, Yokomori K, Earnshaw WC, Sullivan KF, Brinkley BR (2001) Specification of kinetochore-forming chromatin by the histone H3 variant CENP-A. J Cell Sci 114:3529–3542. doi:10.1007/BF00332792
Varma D, Salmon ED (2012) The KMN protein network—chief conductors of the kinetochore orchestra. J Cell Sci 125:5927–5936. doi:10.1242/jcs.093724
Voullaire LE, Slater HR, Petrovic V, Choo KH (1993) A functional marker centromere with no detectable alpha-satellite, satellite III, or CENP-B protein: activation of a latent centromere? Am J Hum Genet 52:1153–1163
Wade CM, Giulotto E, Sigurdsson S, Zoli M, Gnerre S, Imsland F, Lear TL, Adelson DL, Bailey E, Bellone RR, Blöcker H, Distl O, Edgar RC, Garber M, Leeb T, Mauceli E, MacLeod JN, Penedo MCT, Raison JM, Sharpe T, Vogel J, Andersson L, Antczak DF, Biagi T, Binns MM, Chowdhary BP, Coleman SJ, Valle Della G, Fryc S, Guérin G, Hasegawa T, Hill EW, Jurka J, Kiialainen A, Lindgren G, Liu J, Magnani E, Mickelson JR, Murray J, Nergadze SG, Onofrio R, Pedroni S, Piras MF, Raudsepp T, Rocchi M, Røed KH, Ryder OA, Searle S, Skow L, Swinburne JE, Syvänen AC, Tozaki T, Valberg SJ, Vaudin M, White JR, Zody MC, Broad institute genome sequencing platform, broad institute whole genome assembly team, Lander ES, Lindblad-Toh K (2009) Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 326:865–867. doi:10.1126/science.1178158
Wan X, O’Quinn RP, Pierce HL, Joglekar AP, Gall WE, DeLuca JG, Carroll CW, Liu S-T, Yen TJ, McEwen BF, Stukenberg PT, Desai A, Salmon ED (2009) Protein architecture of the human kinetochore microtubule attachment site. Cell 137:672–684. doi:10.1016/j.cell.2009.03.035
Warburton PE, Cooke CA, Bourassa S, Vafa O, Sullivan BA, Stetten G, Gimelli G, Warburton D, Tyler-Smith C, Sullivan KF, Poirier GG, Earnshaw WC (1997) Immunolocalization of CENP-A suggests a distinct nucleosome structure at the inner kinetochore plate of active centromeres. Curr Biol 7:901–904
Weir JR, Faesen AC, Klare K, Petrovic A, Basilico F, Fischböck J, Pentakota S, Keller J, Pesenti ME, Pan D, Vogt D, Wohlgemuth S, Herzog F, Musacchio A (2016) Insights from biochemical reconstitution into the architecture of human kinetochores. Nature 537:249–253. doi:10.1038/nature19333
Yamagishi Y, Sakuno T, Shimura M, Watanabe Y (2008) Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455:251–255. doi:10.1038/nature07217
Yan Z, Delannoy M, Ling C, Daee D, Osman F, Muniandy PA, Shen X, Oostra AB, Du H, Steltenpool J, Lin T, Schuster B, Décaillet C, Stasiak A, Stasiak AZ, Stone S, Hoatlin ME, Schindler D, Woodcock CL, Joenje H, Sen R, de Winter JP, Li L, Seidman MM, Whitby MC, Myung K, Constantinou A, Wang W (2010) A histone-fold complex and FANCM form a conserved DNA-remodeling complex to maintain genome stability. Mol Cell 37:865–878. doi:10.1016/j.molcel.2010.01.039
Yoda K, Ando S, Morishita S, Houmura K, Hashimoto K, Takeyasu K, Okazaki T (2000) Human centromere protein A (CENP-A) can replace histone H3 in nucleosome reconstitution in vitro. PNAS 97:7266–7271. doi:10.1073/pnas.130189697
Acknowledgements
The authors thank Tetsuya Hori and other members in the Fukagawa Lab for helpful discussions. This work is supported by JSPS KAKENHI Grant Numbers P25221106, JP16H06279, and JP15H05972 to TF and JP16K18491 to MH.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Hara, M., Fukagawa, T. (2017). Critical Foundation of the Kinetochore: The Constitutive Centromere-Associated Network (CCAN). In: Black, B. (eds) Centromeres and Kinetochores. Progress in Molecular and Subcellular Biology, vol 56. Springer, Cham. https://doi.org/10.1007/978-3-319-58592-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-58592-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58591-8
Online ISBN: 978-3-319-58592-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)