Abstract
Many of the physiological responses that comprise the surgical stress response are known to promote cancer-signaling pathways. Tissue resection and exposure to the pharmaco-physiological stressors of anesthesia required for surgery activate local and systemic inflammatory cytokines, up-regulate cyclooxygenase with increased prostaglandin production, and increase adrenergic activity. The activation of neuro-hormonal pathways is increasingly linked with cancer propagation. Retrospective evidence suggests that the use of anesthetic techniques and adjuncts that modulate these pathways and commonly available to practicing anesthesiologists may benefit patients scheduled for cancer surgery. Minimising the inflammatory response, preventing perioperative immunosuppression, and optimizing fluid delivery may have oncological benefits (improved disease free survival, reduced postoperative complications with timely delivery of adjuvant therapies) that extend beyond enhanced postoperative recovery. This review will consider key components of local and systemic inflammatory response, relevant immune cell mediators, perioperative endothelial dysfunction, and relevant perioperative therapies specific to the care of the patient receiving cancer surgery.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Over the last decade focus has increasingly been placed on the role of anesthesiologists as perioperative physicians. This is, in part, due to the recognition that surgery results in a substantial physiological impact. More specifically, the role of the perioperative physician in improving long-term cancer outcomes is gaining increasing attention, as uncomplicated recovery from surgery is vital to ensuring an uninterrupted cancer journey that may include adjuvant therapy. Similarly, anaesthetic techniques and perioperative adjuncts may impact cancer-signaling pathways and thus impact cancer recurrence and survival. It is therefore essential that optimal management of the perioperative period in cancer patients considers preoperative optimization of modifiable risk factors and careful management of non-modifiable risk factors to ensure optimal recovery. This focus on the perioperative journey has led to improvements in risk stratification of patients, preoperative optimization (prehabilitation) programs, careful selection of anesthetic technique and perioperative adjuvants, enhanced recovery goals, and strategies to avoid ‘failure-to-rescue’ when complications do occur.
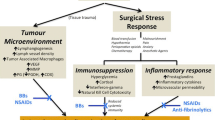
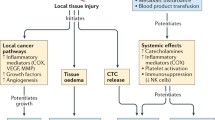
The surgical stress response involves physiological processes that are teleological remnants of our ancestral need to survive trauma, injury, and infection. This primordial axis, however, may be disadvantageous in the context of appropriate inflammatory response and resolution following the stress of cancer resection surgery in, most frequently, the elderly. The biological perturbation of surgical stress is underpinned by activation of the adrenergic-inflammatory pathway and associated immunosuppression: systemic release of catecholamines, local release of inflammatory mediators (interleukins, cytokines, prostaglandins) culminating in the activation of leukocytes, platelets and the endothelium. Platelet and neutrophil activation also triggers neutrophil extracellular trap (NET) formation within the sinusoids (measuring single cell diameter) of end organs such as the liver and lungs, with extracellular DNA strands that trap bacteria and parasites. While teleologically advantageous, unfortunately these NETs also trap circulating tumor cells that are known to be released into the circulation during cancer surgery [1]. Additionally, inflammatory mediators cleave the endothelial glycocalyx to facilitate an increase in capillary permeability and trans-capillary migration of leukocytes into the interstitial space, to facilitate removal of bacteria within the interstitial space. Prostaglandins mediate lymphodilation by signaling to lymphatic endothelial cells; and adrenergic signaling, via sympathetic activation, increases lymphatic contractility. As a consequence, lymphatic flow through the lymph nodes and lymphatic ducts of the parallel circulatory systems increase several fold under conditions of stress. While this is intended to mediate an immune response to infectious agents, it may unfortunately be the mechanism whereby minimal residual disease (MRD, cancer cells within the surgical field) enters the lymphatic system with regional dissemination of cancer following surgery or radiotherapy.
The perioperative period is further characterized by changes in T-lymphocyte, natural killer (NK) cell and monocyte function—resulting in temporary immunosuppression. The changes occur through the adrenergic-inflammatory effects of surgical stress, but also through the exposure to anesthetic agents, hypothermia, and blood transfusion. Endothelial dysfunction, a hallmark of many comorbid disease states, is triggered and/or exacerbated during the postoperative inflammatory period predisposing to tissue edema with increased risk of hypoperfusion and subsequent postoperative complications such as wound infection. Such complications lead to a protracted recovery period, delaying the delivery of adjuvant cancer therapies, and subsequently reducing long-term cancer survival.
Awareness of these perioperative factors has led to increasing emphasis on a ‘cancer anesthetic’ specifically focused on offsetting the perioperative stress response: avoiding adrenergic, inflammatory and immunosuppressive pathway activation during surgery. Fortunately, a number of anesthetic agents and perioperative adjuncts are available to help achieve this hitherto unrecognised role of improving outcomes following cancer surgery.
4.2 Importance of the Perioperative Period to Cancer Outcomes
The cornerstone of solid organ cancer treatment remains surgical excision. Unfortunately, for many patients, cancer progression (local recurrence or metastatic disease) following surgery is a frequent occurrence carrying significant mortality risk, for which a number of postulates have been proposed. First, despite optimal surgical techniques and apparent ‘clear margins’, MRD remains and progresses at the resection site. Secondly, mature isolated tumor cells remain in the interstitium following surgery and are transported using innate wound resolution (lymphatic) pathways [2, 3] leading to the clinical scenarios such as carcinoma-in-transit, regional lymph node recurrence, peritoneal carcinomatosis, and port site recurrence. Thirdly, perioperative iatrogenic displacement of blood-borne circulating tumor cells occurs [4, 5] leading to dissemination and seeding of epithelial mesenchymal transition-like or progenitor cancer cells [6,7,8,9,10,11,12,13], with potential entrapment in the sinusoids of the liver and lungs [1]. A number of studies have indicated a disadvantageous prognostic significance of circulating tumor cell release [9, 14, 15]. Lastly, immunoediting theory [16] suggests that pre-potential cancer cells (micrometastatic disease) held in ‘equilibrium’ in distant organs through active immunosurveillance, are postulated to, through a brief period of perioperative immunosuppression of endogenous innate immunity, ‘escape’ to form de novo malignancies [17, 18]. Removal of a primary tumor has been shown to increase growth rates of such distant micrometastatic disease [19, 20].
A number of animal studies have demonstrated that intervening in a simulated perioperative setting to offset inflammation and immune impairment have resulted in improved cancer outcomes [21,22,23,24]. This supports the theoretical framework that biological perturbation during the perioperative period, through systemic and local pro-cancerous processes, places a patient at an increased risk of cancer recurrence. As such, by limiting perioperative adrenergic-inflammatory activity, immunosuppression, and increased lymphatic flow through focused implementation of commonly used anesthetic techniques and adjuvants (favoring anti-adrenergic, anti-inflammatory, anti-angiogenic, anti-lymphangiogenic techniques) perioperative clinicians may provide additional benefit to plausibly limit cancer recurrence following surgery. Numerous randomised clinical trials are being conducted to translate these findings.
4.3 Perioperative Adrenergic-Inflammatory Processes
The first 48–72 h following a surgical procedure, hereafter the ‘perioperative period’, is characterized as a period during which physiological stress and pharmacologic agents modulate physiological derangements.
4.3.1 Surgical Stress Response
Patients presenting for cancer resection surgery present a unique challenge for anesthesiologists. Additional to the age-associated, co-morbid diseases of the commonly older patient, cancer itself imposes a physiological strain on patients through their disease (anemia, malnutrition), paraneoplastic syndromes, and psychological stress of a cancer diagnosis. Patients may be further exposed to the debilitating “double hit” [25] effect of combined neoadjuvant chemo-radiotherapy. This translates to a baseline level of impaired functional capacity, chronic inflammation, and immune deficiency even before approaching surgery. Patients’ abilities to respond to surgical stress are further compromised by the pre-existing state of malnourishment, deconditioning, and immunosuppression [26, 27]. Perioperatively, patients are then further exposed to psychological, physiological and immunological stress [28,29,30,31]. Historically, this has been referred to as the ‘surgical stress response’ that arises as a consequence of not only surgical excision, but also exposure to numerous perioperative events (Table 4.1). The stress response is characterized by impaired homeostasis of the neuroendocrine (hypothalamic-adrenocorticoid up-regulation), sympathetic nervous system, inflammation (cytokine and prostaglandin release), metabolism (hyperglycemia, protein breakdown), and host innate immune (natural killer cell and T-helper cell impairment) systems.
4.3.2 Local Inflammation
Skin incision inevitably results in tissue inflammation and lymphatic dilation, and an innate response that promotes wound healing [32]. Tissue healing is dependent on a localized inflammatory response characterized by vasodilation, local release of growth factors, angiogenesis and dilation of lymphatic channels. These mediators that co-ordinate the process of wound healing are also directly linked with the inflammatory processes of the tumor microenvironment [33]. The release of local angiogenic growth factors and inhibition of angiostatin and endostatin secretion may facilitate local tumor cell escape to develop malignancy [34, 35].
As acute inflammatory mediators in tissue, prostaglandins and vascular endothelial growth factors (VEGF) facilitate angiogenic and lymphangiogenic processes [36]. Lymphatic dilation is a key component of cellular repair [37, 38]. However, locally released prostaglandins and VEGF are also key components of cancer invasion [39, 40], and high VEGF expression is associated with accelerated cancer progression and more aggressive disease [41,42,43,44]. These cytokines are up-regulated in response to surgical trauma [45]. Given their role in cancer pathways, in the presence of residual disease, exaggerated up-regulation of prostaglandins and VEGF is hence postulated to be disadvantageous [46,47,48,49].
Leukocyte invasion of healing tissue is an appropriate component of wound healing that includes the recruitment of blood-borne monocytes [50]. Of note, peri-incisional wound inflammation shifts macrophages to the M2 sub-type, which is associated with an immunosuppressed tumor microenvironment and cancer progression [51]. M2 macrophages up-regulate stromal cyclooxygenase (COX), matrix metalloproteinase (MMP) and VEGF expression—all mediators of cancer progression [52].
4.3.3 Immune Cells Relevant to Perioperative Period
In brief, perioperative immune suppression can be considered by examining the pathophysiological changes occurring in three distinct leukocyte cell types.
4.3.3.1 Macrophages
As components of host innate immunity cells, phagocytic macrophages are sub-classified into classically activated (M1) and alternatively activated (M2) lineages [52].
-
M1 macrophages have a key role in the localized stimulation of T-helper lymphocytes. M1 differentiated macrophages secrete cytotoxic superoxide anions and free radicals.
-
M2 macrophages are classically induced by pro-inflammatory states. Conceivably, this teleological development was rooted in the need for lymphangiogenic processes and resolution of pathogen associated wound trauma. M2 macrophages are also increasingly considered as Tumor Associated Macrophages (TAMs) that promote a localized immunosuppressed environment facilitating tumor growth [53]. TAMs are integral to the process of lymphatic vessel formation and tumor invasion [48]. TAM presence is used as a prognostic marker of cancer outcome [54].
4.3.3.2 CD4+−Th1 ‘Helper’ Lymphocytes
T-helper lymphocytes will differentiate to sub-types (to Th1 or Th2) based on their exposure to a number of cytokines and interleukins (e.g. IL-2, IL-4, IL-10) [55].
-
Progenitor T-lymphocytes differentiate to Th1 under the influence of IL-2. Th1 cells can be considered as anti-tumor effector cells and, with M1 macrophages, facilitate the activation of CD8+ cytotoxic T-lymphocytes as well as natural killer (NK) cells [56]. Immunoediting theory and host immunosurveillance are strongly based on the role of Th1 lymphocytes to co-ordinate macrophage based antigen-presenting cells and enhance tumor surveillance [16].
-
Also facilitating an immunosuppressed tumor microenvironment are Th2 lymphocytes that assist in tumor progression [57, 58]. Progenitor Th2 lymphocytes differentiate under the influence of IL-4 and IL-10, and favor non-cellular immunity to actively inhibit NK and cytotoxic T-lymphocytes. Th2 cells can be broadly considered to promote tumor growth and metastasis. Th2 cells dominate Th1 cells after severe injury such as surgical stress [59].
4.3.3.3 Natural Killer Cells
-
Representing 8–16% of peripheral blood mononuclear cells, NK cells may be considered key anti-tumor effector cells and vital components of host immunosurveillance and tumor cell destruction [60, 61]. A hallmark of the perioperative period and response to surgical inflammation is the suppression of NK cells. Volatile anesthesia agents will also suppress NK cells [62]. Natural killer cells function synergistically to potentiate cytotoxic T-lymphocytes [63]. Poor cancer outcomes are associated in patients with poor NK cell function and cytotoxicity [64,65,66].
4.3.3.4 Neutrophils
-
As the most abundant of the circulating leukocytes, neutrophils play a key role in the acute inflammatory response. The role of neutrophils in the cancer context is complex. In the tumor microenvironment, neutrophil secretion of VEGF and matrix metalloproteinases facilitate cancer invasion, and immature neutrophils (myeloid-derived suppressor cells) promote localized immunosuppression.
-
Neutrophil activation and subsequent expulsion of DNA (chromatin) is a key step in the formation of Neutrophil-Extracellular Traps (NETs). Teleologically advantageous in the trapping of pathogens following tissue trauma to decrease bacteremia, NET formation within the sinusoids of end organs such as the liver and lungs also traps circulating tumor cells released during surgery. This process may initiate micrometastasis and is associated with a reduction in disease free survival [1].
-
A recent systematic review found a perioperative elevated neutrophil-lymphocyte ratio associated with a reduction in recurrence free survival following surgery for resection of solid tumor [67].
4.3.3.5 Platelets
-
Platelets are vital in the first response to tissue injury initiating primary thrombosis and endothelial activation. Platelet activation is likely to be a co-factor in the formation of NETs [1].
-
In controlling inflammatory processes in the tumor microenvironment, platelet release of pro-inflammatory hormones and cytokines can potentiate cancer progression [68].
-
Two retrospective studies have found a perioperative elevated platelet-lymphocyte ratio associated with a reduction in recurrence free survival following surgery for resection of solid tumor [69, 70].
4.3.3.6 Perioperative, Clinical Significance of Immune Cells
Coincident with the inflammatory response to surgical stress are changes in patients’ immune cell profile [17]. Broadly, the perioperative physiological and pharmacological stressors lead to impairment of the innate immune system and a shift from a patient’s capacity to optimally recognize and destroy cancer cells. Surgical stress induces a shift of T-lymphocyte differentiation from a Th1 to Th2 dominance [71] together with direct inhibition of NK and cytotoxic T-lymphocyte proliferation [72]. This differential state is partly influenced by circulating cytokines and catecholamines [55, 60]. Interestingly, non-invasive surgical techniques, likely through a reduction in inflammatory burden, reduce perioperative immune suppression (and Th2 dominance) [71, 73]. The catecholamine surge associated with perioperative stress may reduce the Th1/Th2 lymphocyte ratio [74,75,76] and has been shown to further depress the impaired Th1 lymphocyte activity reported in cancer patients [73]. Sympathetic nervous system activity and circulating noradrenaline facilitates macrophages towards an M2 sub-type [77]. This polarization is partially mediated by beta-2 adrenergic receptors on the macrophage cell surface, and may account for the suggestion that non-selective beta-blockers have a protective effect against cancer progression [78,79,80].
NK cells and NK cell cytotoxicity is significantly depressed for 24–72 h following exposure to surgical stress [81, 82]. Specifically, suppression of NK cell number and activity has been reported following lung, breast, and colorectal surgery [83]. As vital anti-tumor effector cells, NK cells are normally active in the presence of Th1 cells and in response to IFN-gamma [84]. The reduction in perioperative NK cell activity may be due to the surgical stress response—specifically through increased circulating epinephrine and cortisol, or through a reduction in IFN-gamma [85, 86]. Overall, these perioperative changes promote an immunological state less competent to manage residual disease or circulating cancer cells and has been implicated in cancer recurrence and metastatic disease [87].
4.3.4 Inflammatory Mediators of the Surgical Stress Response
This stress response to surgery is characterized by up-regulation of a number of acute phase physiological pathways. With surgical incision, the hypothalamic-pituitary-adrenal axis is immediately activated and sympathetic up-regulation leads to suppressed cell mediated immunity [88]. Raised catecholamine levels are a feature of the perioperative period [89, 90], through activation of neural sympathetic outflow and adrenal medulla adrenaline and noradrenaline release [91]. The up-regulation of the sympathetic nervous system is likely to begin prior to surgical incision through anxiety, fear and hypothermia [92, 93]. Catecholamine levels remain elevated for at least 24 h following surgery [90].
In health, prostaglandins are vital to the maintenance of the cellular microenvironment: fluid permeability, endothelial maintenance, and lymphatic flow modification [36, 94]. Surgery and associated tissue trauma release prostaglandins locally and into the systemic circulation [45]. Cyclooxygenase (cox) exists in two isoforms—cox1 and (inducible) cox2. The latter enzyme’s activity is greatly increased in the setting of active inflammation and is a focus for perioperative stress response strategies. Cox activity is difficult to measure due to the instability of its key product prostaglandin-E, though prostaglandins appear to be elevated for up to 48 h following minor surgery [45]. Cerebrospinal fluid prostaglandin E2 levels elevate in response to surgery [95]. Co-incident with the rise in prostaglandins following tissue trauma, inflammatory cytokines (IL-1, IL-6 and IL-8) remain elevated for up to 48 h [96,97,98,99].
Because of the implicit involvement of the sympathetic nervous system, prostaglandins and subsequent up-regulation of systemic cytokines, a focus of research has been the perioperative blockade of these pathways to modulate the surgical stress response and improve patients’ outcomes.
4.3.5 Microcirculation Changes and Endothelial Dysfunction
Endothelial dysfunction results in impairment of the microcirculation with a loss of the endothelium’s key physiological tendency toward vasodilation, fibrinolysis, and anti-aggregation. Perioperative inflammatory response results in the endothelium undergoing a change in its phenotype from a baseline quiescent state to an activated, or pathological dysfunctional state characterized by loss of the glycol-polysaccharide ‘glycocalyx’ layer. The set point of the endothelium reflects the balance between the underlying chronic health of the endothelium, acute exacerbating triggers such as inflammation and oxidative stress, and the ‘regenerative’ ability of the bone marrow that releases endothelial progenitor cells into the peripheral circulation [100, 101].
To maintain physiological microcirculation, the bioavailability of key mediators such as nitric oxide is crucial, otherwise endothelial dysfunction will result in vasoconstriction, pro-inflammatory, and pro-thrombotic changes. In a perioperative setting, microcirculatory changes as described above serve as an appropriate adaptive physiologic response to acute stressors like surgical trauma. Furthermore, elderly patients presenting for cancer surgery often have underlying vascular disease based on comorbid risk factors such as diabetes mellitus, hypertension, hyperlipidemia, and obesity that result in clinically unapparent but underlying endothelial dysfunction [102].
The pro-inflammatory and pro-oxidant milieu resulting from surgical trauma further injures the endothelium and is ubiquitous in the perioperative period [103,104,105]. Plausibly, the loss of glycocalyx, increased extravasation, and subsequent tissue edema is disadvantageous in cancer surgery, given the presence of circulating tumor cells and residual tumor cells in the interstitium whose removal is dependent on lymphatic processes.
A temporal link exists between acute systemic inflammatory load and acute deterioration in endothelial function. In human volunteers, a pro-inflammatory cytokine challenge resulted in a transient loss of endothelial vasodilator function, recovery taking up to seven days [106]. Interestingly, this process is reversible. Studies indicating that removal of the inflammatory source (a 6 month aggressive treatment for chronic periodontitis) [107] or though anti-inflammatory strategies (hydrocortisone or high dose aspirin) [106, 108] improve endothelial vasodilator function. Hu et al. found patients receiving a laparotomy, when compared with less invasive laparoscopic surgery, had greater and more prolonged deterioration in endothelial dysfunction for up to seven postoperative days [105]. A deterioration in endothelial-dependent vasodilation occurs in the first 24–48 h following surgical treatment [107], which reflects an important clinical correlation: the peak incidence of postoperative myocardial infarction occurs at 48 h following surgery, when flow stagnation and increased thrombogenicity manifest [109].
Patients undergoing major cancer surgery are at substantial risk for postoperative morbidity, with 30–60% of patients developing complications [110, 111]. The risk of postoperative complications may relate to perioperative endothelial dysfunction: impaired vascular homeostasis and reduced tissue (organ) perfusion. Research has hence focused on both the measurement of endothelial-dependent vascular function and the prevention of its dysregulation in order to minimize the risk for perioperative cardiovascular complications [112]. Measurement tools include characterization of endothelial vasodilatory function (e.g. assess vascular function through endothelial dependent vasodilation), quantification of vascular damage (e.g. measurement of endothelial, thrombogenic and inflammatory biomarkers) and levels of denuded circulating endothelial cells or endothelial microparticles. In addition, analyzing the endothelial regenerative capacity via endothelial progenitor cell (EPC), a key stem cell line for endothelial repair, has been a recent focus of clinical research [113]. In patients with metabolic syndrome, EPC levels decrease following surgery—a potential correlate with the postoperative morbidity seen in these patients [102]. To prevent endothelial damage mobilization, proliferation, survival and homing of EPCs is important, and microcirculatory impairment is an early pathogenic event in end-organ damage (cardiomyopathy, nephropathy, retinopathy, and neuropathy) [102].
It appears the fundamental determinant for endothelial dysfunction is activation of inflammatory pathways (such as the surgical stress response). The same processes, exacerbated specifically by neutrophil-platelet activation, lead to subsequent NET formation [1]. As such, maintaining endothelial integrity to prevent complications related to surgery or circulating tumor cell entrapment by NETs may be an important strategy in the perioperative care of the cancer patient. This is especially important when one considers that surgical morbidity results in significant prolonged hospital stay, with a substantial delay in return to intended oncologic therapy (RIOT). Reduced RIOT has been attributed to surgical complications and shown to increase risk for poor oncologic outcome in breast, liver, and pancreatic cancer surgery [114, 115].
4.4 Modifying Inflammatory Response and Preventing Endothelial Dysfunction
4.4.1 Appropriate Fluid Delivery
Given the susceptibility of the endothelial glycocalyx to inflammatory insult, with consequent increased permeability and lymphatic flow, it is crucial that anesthetic techniques for cancer surgery incorporate a strong anti-inflammatory strategy. Further consideration should also be given to judicious goal-directed fluid therapy as fluid overload may result in beta natriuretic peptide release which, in turn, cleaves the endothelial glycocalyx [116].
A plausible link with increased lymphatic flow and residual cancer cells impacting on cancer recurrence through residual cells in lymph nodes has been described [117]. Prevention of tissue edema through optimal, goal directed fluid delivery would likely reduce lymphatic flow and is also known to reduce postoperative complications [118]. The extent to which optimal perioperative fluid delivery and lymphatic flow reduction impacts upon a timely return to intended oncologic (adjuvant) therapy (i.e. RIOT) or long-term oncological outcomes requires further study within the setting of adequately powered prospective studies.
4.4.2 Regional Anesthesia
Epidurals have been extensively investigated as a means of reducing perioperative opioid requirements following intra-cavity surgery [119]. Additional benefits from the use of epidural anesthesia have been the reduction in neural sympathetic outflow and circulating catecholamines, and subsequent reduction in the perioperative stress response. Neuraxial analgesia have been shown to reduce cytokine assessed inflammatory response through cancer surgery [99], preserve endothelial function [120], and possibly reduce lymphatic flow [121].
Epidural analgesia inhibits neural sympathetic activity and the catecholamine rise of surgical incision both in animal models [122] and in patients receiving major surgery [89, 90]. Preventing the adrenaline surge maintains lymphocyte numbers, activity and the Th1/Th2 ratio to preserve cell-mediated immunity [91, 123]. This may occur through the preservation of Th1 cell number, and maintenance of interferon (IFN)-gamma levels [123, 124] crucial to adaptive immune defense and anti-tumorigenic cell-mediated immunity [91, 96, 124,125,126,128]. Regional anesthesia reduces other markers of the surgical stress response such as elevated cortisol and hyperglycemia [97, 129]. As a strategy to improve clinical outcomes, epidural analgesia’s reduction in the surgical stress response has been shown to improve post-operative morbidity in surgical sub-populations [91, 119, 130].
Specific to cancer surgery, retrospective studies have found an association between perioperative epidural analgesia and improvements in long-term cancer recurrence [130,131,133]. A mooted explanation for this is the reduced catecholamine levels and β-adrenergic activity following neuraxial analgesia [90, 134]. Numerous studies are currently recruiting patients to prospective trials examining the influence of perioperative analgesia with a primary endpoint of cancer recurrence and survival (NCT01318161, NCT00684229, NCT02801409).
4.4.3 Beta-Blockers
An alternate means of minimising the sympathetic nervous system component of the surgical stress response is through the use of beta-blocker medication. In animal models, limiting the stress response of surgery through beta-blockade has led to improved cancer outcomes.
When modelling the surgical stress response in animals, prevention of β-adrenergic activation through the use of beta-blockers increased in NK cell number and activity with resultant improvements in cancer outcomes [21, 22]. The peak period of immunosuppression occurs 24 h following incision, partially induced by unregulated sympathetic hyperactivity [135]. Also during this time frame is a reduction in NK cell cytotoxicity, Th1 and B cell decline and a rise in IL-10. The use of peri-incision selective β(1)-blockade prevents immunosuppression, presumably through reduction in sympathetic nervous system activity [136].
There are few published trials demonstrating the utility of beta-blockers to offset the immunosuppressive component of the surgical stress response. Small trials have shown that beta-blockers preserve NK cell cytotoxicity [85, 86, 96]. One study prospectively examined the effect of placebo or perioperative atenolol (a non-selective beta-blocker) in patients receiving abdominal surgery [137]. While no difference in adrenaline or noradrenaline levels were observed, β-blockade modified the stress and inflammatory response as indicated by faster recovery from anesthesia, reduced pain, and reduced opioid requirement.
Traditionally, trials examining perioperative beta-blockade have focused on its role in preventing post-operative cardiovascular events such as myocardial infarction and stroke. Investigators hypothesize that improvements seen through the use of beta-blockers would be mediated through limiting of the surgical stress response [138, 139]. The largest of these trials is the 8351 patient ‘POISE’ study which found that perioperative administration of the non-selective beta-blocker metoprolol led to a reduction in post-operative myocardial infarction (176 [4.2%] vs. 239 [5.7%] patients; 0.73, 0.60–0.89; p = 0.002) [140]. However, this occurred at the cost of excess deaths in the metoprolol group compared with the placebo group (129 [3.1%] vs. 97 [2.3%] patients; 1.33, 1.03–1.74; p = 0.0317) due to an increased rate of post-operative hypotension and stroke. While the increased risk of stroke may be specific to metoprolol rather than to all beta-blockers, caution must be used in their perioperative administration [141].
A number of retrospective studies have found an association between non-selective beta-blocker use and improved cancer outcomes [142, 143]. These studies have arisen in the setting of patients simultaneously treated with beta-blocker anti-hypertensive medication coincidentally with their cancer diagnosis.
The use of non-selective beta-blockers has appeal due to increased mechanistic evidence of β-adrenergic signaling in tumor progression, macrophage recruitment and metastasis in animal models. The perioperative period is dominated by a period of intense catecholamine activity. Modification of β-adrenergic activity and reduction in surgical stress through beta-blocker use may improve patients’ cancer outcomes through regulation of the pathogenic behavior of residual disease and preservation of host immune responses. No study to date has considered the role of perioperatively commenced beta-blockers and improvement in patients’ cancer outcomes.
4.4.4 Non-steroidal Anti-inflammatory Agents (NSAID)
Due to the increased tissue expression of cyclooxygenase and prostaglandin production in the perioperative period, the use of selective (cox2) NSAID agents is an appealing strategy to minimize the surgical inflammatory response. Surgical stress response can be partially suppressed through NSAID administration. In non-cancer surgery, a rise in systemic and wound prostaglandin levels was partially inhibited through the use of NSAIDs [45, 144]. However, in cancer surgery a single dose of diclofenac during surgery was unable to suppress post-operative PGE2 rise [31]. Following cardiac surgery, NSAIDs have been shown to suppress the inflammation (IL-6, IL-8) and potentiate anti-inflammatory cytokines (IL-10) [145]. A number of trials have demonstrated that, either through a reduction in surgical stress response or opioid related side effects, patients receiving perioperative NSAIDs have improved pain control and improved clinical outcomes [145,146,148].
The role of NSAIDs in minimising stress response has been demonstrated in a number of animal models where attempts to model the ‘perioperative’ period in animals has arisen through an interest in off-setting the inflammatory and immunological changes associated with surgery that are cancer promoting [21, 22, 149, 150]. Perioperative NK cell suppression induced through sham laparotomy is prevented through the use of single or multiple doses of NSAID agents [151]; in multiple murine studies such interventions have been shown to reduce cancer growth [21, 150, 152]. In animals, cox2 specific agents (etodolac) have been shown to be particularly efficacious in preventing melanoma [22] and lung cancer growth in both the surgical [153] and non-surgical setting [153,154,155,157].
The successful demonstration of NSAIDs’ improvement in tumor progression in animal models is probably a consequence of the vital role prostaglandins have in cancer progression. At the cancer cell-stroma interface, tumor cells utilize prostaglandins to achieve growth and metastasis via newly-formed lymphatic channels and blood vessels [157,158,159,160,162]. The perioperative up-regulation of prostaglandins and VEGF, and consequent facilitation of lymphatic and vascular channel dilation, provides an ideal conduit for iatrogenic tumor cell dissemination in the post-surgical period. NSAIDs have been shown to have an integral role in reversing prostaglandin-mediated lymphangiogenesis and lymphatic dilation that ultimately lead to reduced tumor dissemination and metastasis [47, 87, 163, 164].
Investigators have questioned whether NSAID administration in the perioperative period of cancer surgery may impact on patients’ long-term cancer outcomes. It has been observed that in humans, tumors with high cox expression by breast [165], lung [166] and cervical [167] cancers are associated with poor prognosis. These findings, combined with animal evidence led to a number of trials prospectively analysing whether NSAIDs might impact cancer outcomes. Selective cox2 inhibitors prevent colon cancer progression from adenomas [168, 169]. Cox2 inhibitor ‘chemoprophylaxis’ in ex-smokers at high risk of cancer development resulted in reduced lung cancer biomarkers with subsequent clinical benefit [170, 171]. Cohort studies support a beneficial role of cox2 inhibitors in patients with lung cancer [172]. Subsequent trials of cox2 inhibitors added to chemotherapy regimens in advanced lung cancer have not consistently shown survival benefit [172,173,174,175,177]. Hence, the role for cox2 inhibitors in the prevention of cancer appears to be in early chemoprophylaxis against cancer development rather than cox2 inhibition in established disease [178]. These observations have led investigators to research the benefit of administering NSAIDs during the period of cancer surgery—a pro-inflammatory, immunosuppressed period of low volume disease.
A number of retrospective studies have found an association between perioperatively administered NSAIDs and cancer outcomes following breast cancer surgery [178,179,180,182]. NSAIDS appear to impact on the first peak of the bimodal recurrence pattern observed in breast cancer patients following surgery [183]. Prospective randomized studies of 2 week preoperative courses of the cox2 inhibitor celecoxib found improvements in the tumor microenvironment (increased tumor apoptosis, VEGF suppression, reduced lymphangiogenesis) in bladder [184], prostate [185], and oesophageal [186] cancers. There is a paucity of evidence regarding long-term outcomes following the randomized intervention of a perioperative NSAID. The largest study to date is the follow-up of a 1500 patient randomized trial of aspirin in patients receiving gastroesophagectomy; the investigators found a 10% reduction (51% aspirin, 41% placebo) in 5-year survival from the use of perioperative aspirin [187].
As such, NSAIDs (in particular cox2 inhibitors) have a role in reducing pain and the stress response to surgery, have clear anti-cancer properties, and in animal ‘perioperative’ models of cancer prevent overt tumor development. Preliminary evidence of specific anti-cancer benefit from NSAIDs use in the perioperative period is plausible, given the conditions of low volume disease and a pro-inflammatory state.
4.4.5 Total Intravenous Anesthesia (TIVA)
A number of preclinical in vitro and in vivo studies indicate differing effects of anesthesia agents on both inflammatory pathways and cancer cells. In the majority of cases, general anesthesia is administered via techniques of intravenous or inhalational (volatile) agents. Anesthesia agent-specific effects have already been identified with regard to postoperative outcomes; TIVA, using propofol, is used for the prevention of post operative nausea and vomiting in high risk patients [188].
In murine studies, propofol has been identified as a prostaglandin E suppressant through inhibition of cox in both murine studies [189, 190] and human monocytes [191]. Clinically, propofol appears to have protective influence on endothelial inflammatory mediator release by reducing IL-1, IL-6 and IL-8 when compared with sevoflurane (volatile) based anesthesia [192]. These findings have been confirmed in studies examining serial plasma levels of cytokines following open cholecystectomy [193].
The role of propofol as the choice anesthesia agent specific for cancer surgery has been mooted due to its properties as a cox inhibitor [194]. Furthermore, in murine studies, propofol appears to act as an immune enhancer and has been shown to have anti-tumor properties [195]. A large 11,345 patient retrospective study recently published found an association between volatile anesthesia, compared with TIVA, and reduced survival after propensity matching: hazard ratio 1.59 (95% Confidence Interval 1.30–1.95) [196]. The apparent benefit for patients that appeared in the 12 months following surgery indicates that TIVA may have a role in modifying the perioperative stress response and medium-term morbidity. Alternatively, the benefit may lie in avoidance of volatiles, which may be tumor promoting by activating biological pathways (e.g. HIF-1 alpha) that could be tumorigenic. The impact of TIVA on cancer recurrence rates was not examined.
4.4.6 Lidocaine
The use of systemic lidocaine has been studied extensively in the perioperative setting for its role in improving short-term patient outcomes and inhibition of the surgical stress response. Lidocaine acts through blockade of the voltage-gated sodium channel in the neuronal cell membrane. It is postulated that it is through a systemic reduction in neuronal signaling that profound analgesic benefits are achieved for patients in the post-operative period—in particular following abdominal surgery in which neuraxial analgesia is not implemented [197]. Compared with opioid based analgesia, the addition of systemic lidocaine therapy reduces inflammation (IL-6, IL-8), markers of immune function (complement activation, CD11b) following laparotomy that led to a reduction in opioid consumption and improvements in clinical outcomes including patients’ earlier discharge from hospital [198]. Furthermore, lidocaine clearly suppressed pro-inflammatory behavior (IL1, IL6 secretion) of peripheral blood mononuclear cells in the peri-surgical setting [199]. Improvements in non-oncologic clinical outcomes from perioperative lidocaine use have been confirmed in other trials of colon resection surgery [200, 201].
Additionally, a number of studies have demonstrated key anti-proliferative properties of local anesthetics, in particular lidocaine. In a number of cancer cell lines, lidocaine promotes cancer cell apoptosis [202, 203] and anti-proliferation of mesenchymal stem cells [204]. In the clinical setting, epidural lidocaine is associated with a reduction in cancer recurrence rates following radical prostatectomy surgery [131]. It is difficult to extrapolate whether lidocaine’s benefit (if any) is observed due to systemic exposure of the drug, associated reduction in inflammatory response, or to a reduction in opioid and anesthesia requirements [205].
4.5 Conclusion
The expansion of our understanding of the pathophysiological processes involved in cancer progression, and the awareness that many of these processes are temporarily activated through the perioperative period, has led researchers and anesthesiologists to view the provision of care at this time to be tailored specifically for the patient receiving cancer resection surgery.
Furthermore, retrospective evidence suggests that specific anesthetic approaches and adjuvants may beneficially impact not only recovery time, but also facilitate more rapid RIOT, potentially increasing disease free survival. A ‘cancer anesthetic’ appears to be increasingly defined as one that focuses on anti-adrenergic and anti-inflammatory strategies that reduce cytokine production and prevent endothelial dysfunction: intravenous anesthesia, goal directed fluid therapy, cox2 inhibitors, neuraxial anesthesia and potentially perioperative beta-blockade. A number of prospective trials are currently recruiting patients; the results of these studies will more effectively guide practice.
References
Tohme S et al (2016) Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res 76(6):1367–1380
Camara O et al (2006) Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol 4:67
Tvedskov TF et al (2012) Iatrogenic displacement of tumor cells to the sentinel node after surgical excision in primary breast cancer. Breast Cancer Res Treat 131(1):223–229
Akiyoshi S et al (2013) Laparoscopic surgery minimizes the surgical manipulation of isolated tumor cells leading to decreased metastasis compared to open surgery for colorectal cancer. Surg Today 43(1):20–25
Eguchi H et al (2009) Presence of minute cancer cell dissemination in peritoneal lavage fluid detected by reverse transcription PCR is an independent prognostic factor in patients with resectable pancreatic cancer. Surgery 146(5):888–895
Ge MJ et al (2006) Observation of circulating tumour cells in patients with non-small cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in perioperative period. J Cancer Res Clin Oncol 132(4):248–256
Katoh H et al (2009) Prognostic significance of peritoneal tumour cells identified at surgery for colorectal cancer. Br J Surg 96(7):769–777
Li J et al (2005) [Detection of blood dissemination during the operation of lung cancer and its significance]. Zhonghua wai ke za zhi [Chin J Surg] 43(2):76–79
Rolle A et al (2005) Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: a preliminary report. World J Surg Oncol 3(1):18
Sawabata N et al (2007) Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg 55(5):189–192
Temesi R et al (2012) Impact of positive intraabdominal lavage cytology on the long-term prognosis of colorectal cancer patients. World J Surg 36(11):2714–2721
Tsakok T et al (2012) Washout after lobectomy: is water more effective than normal saline in preventing local recurrence? Interact Cardiovasc Thorac Surg 14(2):200–204
Weitz J, Herfarth C (2001) Surgical strategies and minimal residual disease detection. Semin Surg Oncol 20(4):329–333
Ma X-L et al (2012) Meta-analysis of circulating tumor cells as a prognostic marker in lung cancer. Asian Pac J Cancer Prev 13(4):1137–1144
Yamashita JI et al (2000) Detection of circulating tumor cells in patients with non-small cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg 119(5):899–905
Dunn GP, Old LJ, Schreiber RD (2004) The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21(2):137–148
Kurosawa S, Kato M (2008) Anesthetics, immune cells, and immune responses. J Anesth 22(3):263–277
Retsky M et al (2013) Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem 20(33):4163–4176
Fisher B, Gunduz N, Saffer EA (1983) Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res 43(4):1488–1492
Gunduz N, Fisher B, Saffer EA (1979) Effect of surgical removal on the growth and kinetics of residual tumor. Cancer Res 39(10):3861–3865
Benish M et al (2008) Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol 15(7):2042–2052
Glasner A et al (2010) Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol 184(5):2449–2457
Goldfarb Y et al (2012) Fish oil attenuates surgery-induced immunosuppression, limits post-operative metastatic dissemination and increases long-term recurrence-free survival in rodents inoculated with cancer cells. Clin Nutr 31(3):396–404
Wada H et al (2007) Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology 106(3):499–506
Jones LW et al (2009) Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 10(6):598–605
Fearon KC et al (2013) Patient optimization for gastrointestinal cancer surgery. Br J Surg 100(1):15–27
Marik PE, Zaloga GP (2010) Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr 34(4):378–386
Colvin LA, Fallon MT, Buggy DJ (2012) Cancer biology, analgesics, and anaesthetics: is there a link? Br J Anaesth 109(2):140–143
Heaney A, Buggy DJ (2012) Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth 109(Suppl 1):i17–i28
Kavanagh T, Buggy DJ (2012) Can anaesthetic technique effect postoperative outcome? Curr Opin Anaesthesiol 25(2):185–198
O’Riain SC et al (2005) Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth Analg 100(1):244–249
Huggenberger R et al (2011) An important role of lymphatic vessel activation in limiting acute inflammation. Blood 117(17):4667–4678
Heinrich EL et al (2012) The inflammatory tumor microenvironment, epithelial mesenchymal transition and lung carcinogenesis. Cancer Microenviron 5(1):5–18
O’Reilly MS et al (1994) Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79(2):315–328
O’Reilly MS et al (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88(2):277–285
Kalish BT et al (2013) The growing role of eicosanoids in tissue regeneration, repair, and wound healing. Prostaglandins Other Lipid Mediat 104–105:130–138
Greco KV et al (2006) Lymphatic regeneration across an incisional wound: inhibition by dexamethasone and aspirin, and acceleration by a micronized purified flavonoid fraction. Eur J Pharmacol 551(1–3):131–142
Narayan K, Cliff WJ (1981) In vivo morphology and ultrastructure of thyroid autografts in rabbit ear chambers. Q J Exp Physiol 66(3):237–252
Ferrandina G et al (2002) Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol 20(4):973–981
Zhang R et al (2009) [Expression of P53, COX2 and CD44V6 in early-stage squamous carcinoma of cervix with lymph vascular space invasion positive and negative and its relationship with prognosis]. Zhonghua Yi Xue Za Zhi 89(47):3341–3345
Gou H-F et al (2011) Expressions of COX-2 and VEGF-C in gastric cancer: correlations with lymphangiogenesis and prognostic implications. J Exp Clin Cancer Res 30:14
Hashimoto I et al (2001) Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br J Cancer 85(1):93–97
Kopfstein L et al (2007) Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol 170(4):1348–1361
Lutgendorf SK et al (2009) Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun 23(2):176–183
Zhao H et al (2011) Comparison of different loading dose of celecoxib on postoperative anti-inflammation and analgesia in patients undergoing endoscopic nasal surgery-200 mg is equivalent to 400 mg. Pain Med 12(8):1267–1275
Alessandri G et al (1987) Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res 47(16):4243–4247
Karnezis T, Shayan R, Caesar C et al (2012) VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21(2):181–195
Schoppmann SF et al (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 161(3):947–956
Stacker SA, Achen MG (2008) From anti-angiogenesis to anti-lymphangiogenesis: emerging trends in cancer therapy. Lymphat Res Biol 6(3–4):165–172
Viswanathan K, Dhabhar FS (2005) Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci U S A 102(16):5808–5813
Harvey NL, Gordon EJ (2012) Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vasc cell 4(1):15
Hao N-B et al (2012) Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012:948098
Solinas G et al (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073
Medrek C et al (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306
Kurosawa S (2012) Anesthesia in patients with cancer disorders. Curr Opin Anaesthesiol 25(3):376–384
Powrie F, Coffman RL (1993) Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today 14(6):270–274
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437
Mack VE et al (1996) Dominance of T-helper 2-type cytokines after severe injury. Arch Surg 131(12):1303–1308. Discussion 1308–9
Kurosawa S et al (1993) Early appearance and activation of natural killer cells in tumor-infiltrating lymphoid cells during tumor development. Eur J Immunol 23(5):1029–1033
Kurosawa S et al (1995) Early-appearing tumour-infiltrating natural killer cells play a crucial role in the generation of anti-tumour T lymphocytes. Immunology 85(2):338–346
Huitink JM et al (2010) Volatile anesthetics modulate gene expression in breast and brain tumor cells. Anesth Analg 111(6):1411–1415
Kos FJ, Engleman EG (1996) Immune regulation: a critical link between NK cells and CTLs. Immunol Today 17(4):174–176
Fujisawa T, Yamaguchi Y (1997) Autologous tumor killing activity as a prognostic factor in primary resected nonsmall cell carcinoma of the lung. Cancer 79(3):474–481
Koda K et al (1997) Preoperative natural killer cell activity: correlation with distant metastases in curatively research colorectal carcinomas. Int Surg 82(2):190–193
Takeuchi H et al (2001) Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol 96(2):574–578
Paramanathan A, Saxena A, Morris DL (2014) A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 23(1):31–39
Sharma D et al (2014) Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol 229(8):1005–1015
Goh BKP et al (2016) Blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios are independent prognostic factors for surgically resected gastrointestinal stromal tumors. Surgery 159(4):1146–1156
Que Y et al (2015) Preoperative platelet-lymphocyte ratio is superior to neutrophil-lymphocyte ratio as a prognostic factor for soft-tissue sarcoma. BMC Cancer 15:648
Evans C et al (2009) Impact of surgery on immunologic function: comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am J Surg 197(2):238–245
Kuroda E, Yamashita U (2003) Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in Th1 activation in Th2-dominant BALB/c mice. J Immunol 170(2):757–764
Ishikawa M et al (2009) Perioperative immune responses in cancer patients undergoing digestive surgeries. World J Surg Oncol 7:7
Elenkov IJ, Chrousos GP (2002) Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 966:290–303
Greenfeld K et al (2007) Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun 21(4):503–513
Vallejo R et al (2003) Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol 22(2):139–146
Grailer JJ et al (2014) Induction of M2 regulatory macrophages through the β2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6(5):607–618
Horowitz M et al (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 12:213–226
Sloan EK et al (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res 70(18):7042–7052
Watkins JL et al (2015) Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer 121(19):3444–3451
Cata JP et al (2013) Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth 25(4):255–262
Nosotti M et al (2011) Leukocyte subsets dynamics following open pulmonary lobectomy for lung cancer: a prospective, observational study. Interact Cardiovasc Thorac Surg 13(3):262–266
Ramirez MF et al (2015) Innate immune function after breast, lung, and colorectal cancer surgery. J Surg Res 194(1):185–193
Gajewski TF, Schreiber H, Fu Y-X (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14(10):1014–1022
Madden KS, Sanders VM, Felten DL (1995) Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol 35:417–448
Robertson MJ, Ritz J (1990) Biology and clinical relevance of human natural killer cells. Blood 76(12):2421–2438
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22(2):231–237
Shakhar G, Ben-Eliyahu S (2003) Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann Surg Oncol 10(8):972–992
Moore CM et al (1994) Effects of extradural anaesthesia on interleukin-6 and acute phase response to surgery. Br J Anaesth 72(3):272–279
Moore CM et al (1995) Hormonal effects of thoracic extradural analgesia for cardiac surgery. Br J Anaesth 75(4):387–393
Ahlers O et al (2008) Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth 101(6):781–787
Khadke VV, Khadke SV, Khare A (2012) Oral propranolol—efficacy and comparison of two doses for peri-operative anxiolysis. J Indian Med Assoc 110(7):457–460
Mavridou P et al (2013) Patient’s anxiety and fear of anesthesia: effect of gender, age, education, and previous experience of anesthesia. A survey of 400 patients. J Anesth 27(1):104–108
Salvemini D, Kim SF, Mollace V (2013) Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol 304(7):R473–R487
Buvanendran A et al (2006) Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology 104(3):403–410
Dong H, Zhang Y, Xi H (2012) The effects of epidural anaesthesia and analgesia on natural killer cell cytotoxicity and cytokine response in patients with epithelial ovarian cancer undergoing radical resection. J Int Med Res 40(5):1822–1829
Fant F et al (2013) Thoracic epidural analgesia inhibits the neuro-hormonal but not the acute inflammatory stress response after radical retropubic prostatectomy. Br J Anaesth 110(5):747–757
Frick VO et al (2012) Thoracotomy procedures effect cytokine levels after thoracoabdominal esophagectomy. Oncol Rep 27(1):258–264
Moselli NM et al (2011) Intraoperative epidural analgesia prevents the early proinflammatory response to surgical trauma. Results from a prospective randomized clinical trial of intraoperative epidural versus general analgesia. Ann Surg Oncol 18(10):2722–2731
Asahara T et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967
Hill JM et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348(7):593–600
Avogaro A et al (2011) Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34(Suppl 2):S285–S290
Chello M et al (2005) Effects of atorvastatin on arterial endothelial function in coronary bypass surgery. Eur J Cardiothorac Surg 28(6):805–810
Clapp BR et al (2004) Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res 64(1):172–178
Hu YJ et al (2013) Impact of non-cardiovascular surgery on reactive hyperaemia and arterial endothelial function. Clin Exp Pharmacol Physiol 40(7):466–472
Bhagat K, Vallance P (1997) Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation 96(9):3042–3047
Tonetti MS et al (2007) Treatment of periodontitis and endothelial function. N Engl J Med 356(9):911–920
Farb MG et al (2014) Cyclooxygenase inhibition improves endothelial vasomotor dysfunction of visceral adipose arterioles in human obesity. Obesity 22(2):349–355
Biccard BM, Rodseth RN (2010) The pathophysiology of peri-operative myocardial infarction. Anaesthesia 65(7):733–741
Duque JL et al (1997) Early complications in surgical treatment of lung cancer: a prospective, multicenter study. Grupo Cooperativo de Carcinoma Broncogénico de la Sociedad Española de Neumología y Cirugía Torácica. Ann Thorac Surg 63(4):944–950
Licker M et al (2002) Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest 121(6):1890–1897
Deanfield JE, Halcox JP, Rabelink TJ (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115(10):1285–1295
Gokce N (2011) Clinical assessment of endothelial function: ready for prime time? Circ Cardiovasc Imaging 4(4):348–350
Aloia TA et al (2014) Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol 110(2):107–114
Gagliato Dde M et al (2014) Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol 32(8):735–744
Marik PE, Lemson J (2014) Fluid responsiveness: an evolution of our understanding. Br J Anaesth 112(4):617–620
Gottschalk A et al (2012) Can regional anaesthesia for lymph-node dissection improve the prognosis in malignant melanoma? Br J Anaesth 109(2):253–259
Pearse RM et al (2014) Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 311(21):2181–2190
Rigg JRA et al (2002) Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 359(9314):1276–1282
Lauer S et al (2009) Thoracic epidural anesthesia time-dependently modulates pulmonary endothelial dysfunction in septic rats. Critical Care 13(4):R109
Hiller JG, et al (2016) Neuraxial Anesthesia Reduces Lymphatic Flow: Proof-of-Concept in First In-Human Study. Anesth Analg 123:1325–7
Enigk F et al (2014) Thoracic epidural anesthesia decreases endotoxin-induced endothelial injury. BMC Anesthesiol 14:23
Zhou D et al (2012) Effects of anesthetic methods on preserving anti-tumor T-helper polarization following hepatectomy. World J Gastroenterol 18(24):3089–3098
Nowarski R et al (2013) Innate immune cells in inflammation and cancer. Cancer Immunol Res 1(2):77–84
Kawasaki T et al (2007) Effects of epidural anaesthesia on surgical stress-induced immunosuppression during upper abdominal surgery. Br J Anaesth 98(2):196–203
Rao VSR et al (2006) Potential prognostic and therapeutic roles for cytokines in breast cancer (Review). Oncol Rep 15(1):179–185
Xu YJ et al (2014) Effect of thoracic epidural anaesthesia on serum vascular endothelial growth factor C and cytokines in patients undergoing anaesthesia and surgery for colon cancer. Br J Anaesth 113 Suppl 1:i49–55
Yokoyama M et al (2005) The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesth Analg 101(5):1521–1527
Volk T et al (2003) Stress induced IL-10 does not seem to be essential for early monocyte deactivation following cardiac surgery. Cytokine 24(6):237–243
Tønnesen E, Wahlgreen C (1988) Influence of extradural and general anaesthesia on natural killer cell activity and lymphocyte subpopulations in patients undergoing hysterectomy. Br J Anaesth 60(5):500–507
Biki B et al (2008) Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology 109(2):180–187
de Oliveira GSJ et al (2011) Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg Anesth Pain Med 36(3):271–277
Hiller J et al (2010) A retrospective observational study examining the admission arterial to end-tidal carbon dioxide gradient in intubated major trauma patients. Anaesth Intensive Care 38(2):302–306
Riedel BJ, Wright IG (1997) Epidural anesthesia in coronary artery bypass grafting surgery. Curr Opin Cardiol 12(6):515–521
Nelson CJ, Lysle DT (1998) Severity, time, and beta-adrenergic receptor involvement in surgery-induced immune alterations. J Surg Res 80(2):115–122
Woiciechowsky C et al (1998) Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med 4(7):808–813
Zaugg M et al (1999) Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 91(6):1674–1686
Blessberger H et al (2014) Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst Rev 9:CD004476
Mostafaie K, Bedenis R, Harrington D (2015) Beta-adrenergic blockers for perioperative cardiac risk reduction in people undergoing vascular surgery. Cochrane Database Syst Rev 1:CD006342
POISE Study Group et al (2008) Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 371(9627):1839–1847
Ashes C et al (2013) Selective β1-antagonism with bisoprolol is associated with fewer postoperative strokes than atenolol or metoprolol: a single-center cohort study of 44,092 consecutive patients. Anesthesiology 119(4):777–787
Melhem-Bertrandt A et al (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol 29(19):2645–2652
Powe DG et al (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 1(7):628–638
Zhu Y et al (2014) Effect of perioperative parecoxib on postoperative pain and local inflammation factors PGE2 and IL-6 for total knee arthroplasty: a randomized, double-blind, placebo-controlled study. Eur J Orthop Surg Traumatol 24(3):395–401
Wu Q et al (2013) The efficacy of parecoxib on systemic inflammatory response associated with cardiopulmonary bypass during cardiac surgery. Br J Clin Pharmacol 75(3):769–778
Ng A, Smith G, Davidson AC (2003) Analgesic effects of parecoxib following total abdominal hysterectomy. Br J Anaesth 90(6):746–749
Riest G et al (2008) Preventive effects of perioperative parecoxib on post-discectomy pain. Br J Anaesth 100(2):256–262
Wattchow DA et al (2009) Clinical trial: the impact of cyclooxygenase inhibitors on gastrointestinal recovery after major surgery—a randomized double blind controlled trial of celecoxib or diclofenac vs. placebo. Aliment Pharmacol Ther 30(10):987–998
Bar-Yosef S et al (2001) Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology 94(6):1066–1073
Melamed R et al (2005) Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun 19(2):114–126
Yakar I et al (2003) Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol 10(4):469–479
Backhus LM et al (2006) Perioperative cyclooxygenase 2 inhibition to reduce tumor cell adhesion and metastatic potential of circulating tumor cells in non-small cell lung cancer. J Thorac Cardiovasc Surg 132(2):297–303
Goldfarb Y et al (2011) Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 253(4):798–810
Diperna CA et al (2003) Cyclooxygenase-2 inhibition decreases primary and metastatic tumor burden in a murine model of orthotopic lung adenocarcinoma. J Thorac Cardiovasc Surg 126(4):1129–1133
Qadri SSA et al (2005) Surgically induced accelerated local and distant tumor growth is significantly attenuated by selective COX-2 inhibition. Ann Thorac Surg 79(3):990–995. Discussion 990–5
Tanaka T et al (2005) Treatment of lung cancer using clinically relevant oral doses of the cyclooxygenase-2 inhibitor rofecoxib: potential value as adjuvant therapy after surgery. Ann Surg 241(1):168–178
Zhang M et al (2011) Inhibitory effect of celecoxib in lung carcinoma by regulation of cyclooxygenase-2/cytosolic phospholipase A2 and peroxisome proliferator-activated receptor gamma. Mol Cell Biochem 355(1–2):233–240
Iwata C et al (2007) Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res 67(21):10181–10189
Ruan D, So S-P (2014) Prostaglandin E2 produced by inducible COX-2 and mPGES-1 promoting cancer cell proliferation in vitro and in vivo. Life Sci 116(1):43–50
Timoshenko AV et al (2006) COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer 94(8):1154–1163
Ungefroren H et al (2011) Interaction of tumor cells with the microenvironment. Cell Commun Signal 9:18
Xin X et al (2012) Targeting COX-2 and EP4 to control tumor growth, angiogenesis, lymphangiogenesis and metastasis to the lungs and lymph nodes in a breast cancer model. Lab Invest 92(8):1115–1128
Karnezis T, Shayan R, Fox S et al (2012) The connection between lymphangiogenic signalling and prostaglandin biology: a missing link in the metastatic pathway. Oncotarget 3(8):893–906
Roche-Nagle G et al (2004) Antimetastatic activity of a cyclooxygenase-2 inhibitor. Br J Cancer 91(2):359–365
Costa C et al (2002) Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol 55(6):429–434
Khuri FR et al (2001) Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res 7(4):861–867
Liu H et al (2011) COX-2 expression is correlated with VEGF-C, lymphangiogenesis and lymph node metastasis in human cervical cancer. Microvasc Res 82(2):131–140
Bertagnolli MM et al (2009) Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res 2(4):310–321
Steinbach G et al (2000) The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 342(26):1946–1952
Mao JT et al (2006) Celecoxib decreases Ki-67 proliferative index in active smokers. Clin Cancer Res 12(1):314–320
Mao JT et al (2011) Lung cancer chemoprevention with celecoxib in former smokers. Cancer Prev Res 4(7):984–993
Slatore CG et al (2009) Association of nonsteroidal anti-inflammatory drugs with lung cancer: results from a large cohort study. Cancer Epidemiol Biomarkers Prev 18(4):1203–1207
Altorki NK et al (2003) Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol 21(14):2645–2650
Edelman MJ et al (2008) Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy—Cancer and Leukemia Group B Trial 30203. J Clin Oncol 26(6):848–855
Groen HJM et al (2011) Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non-small-cell lung cancer: the NVALT-4 study. J Clin Oncol 29(32):4320–4326
Koch A et al (2011) Effect of celecoxib on survival in patients with advanced non-small cell lung cancer: a double blind randomised clinical phase III trial (CYCLUS study) by the Swedish Lung Cancer Study Group. Eur J Cancer 47(10):1546–1555
Mutter R et al (2009) A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res 15(6):2158–2165
Khuri FR (2011) The dawn of a revolution in personalized lung cancer prevention. Cancer Prev Res 4(7):949–953
Forget P et al (2010) Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg 110(6):1630–1635
Forget P et al (2014) Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 113(Suppl 1):i82–i87
Retsky M, Demicheli R et al (2012) Promising development from translational or perhaps anti-translational research in breast cancer. Clin Transl Med 1(1):17
Retsky M, Rogers R et al (2012) NSAID analgesic ketorolac used perioperatively may suppress early breast cancer relapse: particular relevance to triple negative subgroup. Breast Cancer Res Treat 134(2):881–888
Demicheli R et al (2008) Recurrence dynamics does not depend on the recurrence site. Breast Cancer Res 10(5):R83
Dhawan D et al (2010) Effects of short-term celecoxib treatment in patients with invasive transitional cell carcinoma of the urinary bladder. Mol Cancer Ther 9(5):1371–1377
Sooriakumaran P et al (2009) A randomized controlled trial investigating the effects of celecoxib in patients with localized prostate cancer. Anticancer Res 29(5):1483–1488
Liu J-F et al (2008) The effects of a COX-2 inhibitor meloxicam on squamous cell carcinoma of the esophagus in vivo. Int J Cancer 122(7):1639–1644
Liu J-F et al (2009) A preliminary study on the postoperative survival of patients given aspirin after resection for squamous cell carcinoma of the esophagus or adenocarcinoma of the cardia. Ann Surg Oncol 16(5):1397–1402
Gan TJ et al (2003) Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg 97(1):62–71
Inada T, Hirota K, Shingu K (2015) Intravenous anesthetic propofol suppresses prostaglandin E2 and cysteinyl leukotriene production and reduces edema formation in arachidonic acid-induced ear inflammation. J Immunotoxicol 12(3):261–265
Inada T, Kubo K, Ueshima H et al (2011) Intravenous anesthetic propofol suppresses prostaglandin E2 production in murine dendritic cells. J Immunotoxicol 8(4):359–366
Kambara T et al (2009) Propofol suppresses prostaglandin E(2) production in human peripheral monocytes. Immunopharmacol Immunotoxicol 31(1):117–126
Wakabayashi S et al (2014) Effects of anesthesia with sevoflurane and propofol on the cytokine/chemokine production at the airway epithelium during esophagectomy. Int J Mol Med 34(1):137–144
Ke JJ et al (2008) A comparison of the effect of total intravenous anaesthesia with propofol and remifentanil and inhalational anaesthesia with isoflurane on the release of pro- and anti-inflammatory cytokines in patients undergoing open cholecystectomy. Anaesth Intensive Care 36(1):74–78
Inada T, Kubo K, Shingu K (2011) Possible link between cyclooxygenase-inhibiting and antitumor properties of propofol. J Anesth 25(4):569–575
Kushida A, Inada T, Shingu K (2007) Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol 29(3–4):477–486
Wigmore TJ, Mohammed K, Jhanji S (2016) Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 124(1):69–79
Brinkrolf P, Hahnenkamp K (2014) Systemic lidocaine in surgical procedures: effects beyond sodium channel blockade. Curr Opin Anaesthesiol 27(4):420–425
Herroeder S et al (2007) Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 246(2):192–200
Yardeni IZ et al (2009) The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg 109(5):1464–1469
Swenson BR et al (2010) Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: a randomized clinical trial. Reg Anesth Pain Med 35(4):370–376
Wongyingsinn M et al (2011) Intravenous lidocaine versus thoracic epidural analgesia: a randomized controlled trial in patients undergoing laparoscopic colorectal surgery using an enhanced recovery program. Reg Anesth Pain Med 36(3):241–248
Chang Y-C et al (2014) Local anesthetics induce apoptosis in human breast tumor cells. Anesth Analg 118(1):116–124
Piegeler T et al (2012) Antimetastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology 117(3):548–559
Lucchinetti E et al (2012) Antiproliferative effects of local anesthetics on mesenchymal stem cells: potential implications for tumor spreading and wound healing. Anesthesiology 116(4):841–856
Fraser SP, Foo I, Djamgoz MBA (2014) Local anaesthetic use in cancer surgery and disease recurrence: role of voltage-gated sodium channels? Br J Anaesth 113(6):899–902
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Hiller, J., Schier, R., Riedel, B. (2017). Perioperative Biologic Perturbation and Cancer Surgery: Targeting the Adrenergic-Inflammatory Response and Microcirculatory Dysregulation. In: Retsky, M., Demicheli, R. (eds) Perioperative Inflammation as Triggering Origin of Metastasis Development. Springer, Cham. https://doi.org/10.1007/978-3-319-57943-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-57943-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57942-9
Online ISBN: 978-3-319-57943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)