Abstract
As ultraviolet (UV) radiation is naturally and ubiquitously emitted by the sun, almost everyone is exposed to it on a daily basis, and it is necessary for normal physiological function. Human exposure to solar UV radiation thus has important health implications. The generation of reactive oxygen species (ROS) by UV radiation is one of the mechanisms through which UV light can manifest its possible detrimental effects on health. When an imbalance develops due to ROS generation exceeding the body’s antioxidant defence mechanisms, oxidative stress can develop. Oxidative stress can lead to cellular damage (e.g. lipid peroxidation and DNA fragmentation), apoptosis and cell death. Broadly UV can induce ROS by affecting the cellular components directly or by means of photosensitization mechanisms. More specifically UV light can induce ROS by affecting the enzyme catalase and up-regulating nitric oxide synthase (NOS) synthesis. It may also cause a decrease in protein kinase C (PKC) expression leading to increased ROS production. UVR is capable of modifying DNA and other chromophores resulting in elevated ROS levels. The effects of raised ROS levels can vary based on the intracellular oxidant status of the cell. It is therefore important to protect yourself against the potentially harmful effects of UV light as it can lead to pathological UV-induced ROS production.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
As ultraviolet (UV) radiation is naturally emitted by the sun and thus regarded as ubiquitous, almost everyone is exposed to it on a daily basis. Human exposure to solar ultraviolet radiation has important health implications. Suitable amounts of UV radiation exposure has beneficial effects, however many studies have demonstrated evidence in support of harm associated with overexposure to UV. Adequate exposure is vital for UV-induced vitamin D synthesis, while a number of health effects (e.g. skin cancer, malignant melanoma) have been identified as a result of excess exposure.
The generation of reactive oxygen species (ROS) by UV radiation is one of the mechanisms through which UV light can manifest its detrimental effects on health. ROS are free radicals and can be defined as an unstable chemical species possessing an unpaired electron. When an imbalance develops due to ROS generation exceeding the body’s antioxidant defence mechanisms, oxidative stress can develop. Oxidative stress can lead to cellular damage (e.g. lipid peroxidation and DNA fragmentation), apoptosis and cell death [22].
Due to industrialization there is a dramatic increase in chlorofluorocarbons (CFCs), with resulting loss of stratospheric ozone. This situation subsequently can lead to increased levels of specially UVC radiation reaching the earth’s surface (which otherwise cannot) and therefore growing human exposure to UV radiation. The burden on human health is more noticeable with increased UV radiation induced pathologies and thus the need to explore this phenomenon in more detail. The chapter aims to provide a detailed relationship between UV and ROS as well as the associated burden.
2 Ultraviolet Light
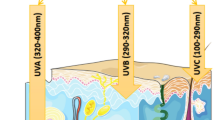
Ultraviolet (UV) light is part of the solar emissions spectrum which falls between the electromagnetic radiation spectrum of X-rays and visible light with wavelengths ranging from 100 nm to 400 nm. Based on their wavelengths, UV light can be subdivided into several categories with three bands, namely UVA, UVB and UVC [3, 15]. Environmental and dermatological photobiologists commonly use slightly different divisions, which are more closely associated with the biological effect of the different wavelengths [19].
-
UVA 320–400 nm: The most commonly encountered UV light as it passes through the atmospheric ozone with little change. Initially UVA cause pigment darkening (tanning) followed by sunburn when over exposed. UVA is necessary for Vitamin D production in humans but over exposure could result in epidermal hardening, immune system suppression and formation of cataracts. UVA is vastly used in the cosmetic industry (sunbeds or tanning booths).
-
UVB 290–320 nm: UVB is the key factor in photochemical damage to cellular DNA. UVB is also essential for production of Vitamin D in humans; however, over exposure may hold harmful effects to the human body. These harmful effects include sunburn, cataracts as well as the initiation of the carcinogenic process in the skin.
-
UVC 220–290 nm: UVC is almost completely absorbed by the atmospheric ozone and has little effect on human health. Germicidal lamps emit UVC in order to kill microbes. Humans get exposed to UVC accidentally may cause corneal burns and snow blindness. UVC is absorbed by the dead outer layer of the dermis thus exposure may cause severe pain but clears up within a few days [3, 15].
2.1 The Global Solar UV Index (UVI)
The global solar UV index [28] provides a description of the level of solar UV radiation at the Earth’s surface [19]. The values are reported as a number from zero upwards; the higher the number the greater the risk for UV induced damage. The UV index (UVI) can be used as a guideline and educational tool to determine the risk for potential UV damage to the skin and eye when exposed to solar UV radiation. This could be a valuable tool to inform individuals of the increased risk of skin cancers associated with excessive UV radiation exposure, and to encourage individuals to adopt protective measures [19].
UVI is presented usually as the maximum UV radiation levels on a given day, which occurs around solar noon. Geographical location plays a determining factor but none the less, solar noon takes place between local noon and 2 pm [19].
3 Sources of UV Light
Humans are exposed to UV radiation through outdoor sun exposure or due to artificial sources.
3.1 Outdoor
Outdoor exposure by the sun is either through deliberate activities (sun tanning) or as a result of recreational or occupational activities.
3.2 Artificial Sources
Exposure includes sources from medical and cosmetic treatments. The following sources are major contributors to artificial UV exposure:
-
Phototherapy/ sunbeds: They expose human skin to UVA and some UVB radiation. Black light, which is also referred to as a UV-A light, is predominately used by tanning booths and phototherapies
-
Medical: Exposure will depend upon treatment type but, include a majority of diagnostic and treatment apparatus.
-
Industrial/ commercial: Arc welding is a potential source of UV exposure which can cause severe damage to the eyes and skin.
-
Lighting: Fluorescent lamps emit minor amount of UV light which only contributes a small percentage to the total yearly exposure [3].
4 Tissue Exposure to UV
Vitamin D binds to steroid vitamin D receptors in the body. These receptors are directly involved in proliferation and differentiation of cells. The vitamin D dependent renewal process takes place in the keratinocytes. Keratinocytes accounts for a large portion of the cells in the epidermis.
Vitamin D is able to regulate the low resting levels of cell signalling components such as ROS by means of controlling the expression of these components. Therefore, vitamin D is vital in the replenishment of new cells for the skin surface. Vitamin D production is dependent on the amount of melanin in the skin [4, 5]. The amount of melanin the skin produces depends how fair or dark the skin colour is and the Vitamin D production is dependent on the amount of melanin in the skin. Darker skins have more melanin which allows less UVB to enter the skin. Due to less UVB entering the skin, less vitamin D is produced; therefore dark skinned individuals require more sun exposure.
These factors make it difficult to determine the amount of sun exposure time one requires in order to get adequate vitamin D and avoid damage. A good rule of thumb for sun exposure is to expose your skin for half the time before it turns pink. For fair skinned individuals it could be only a few minutes, but darker skinned person requires a longer exposure time [9].
A small amount of UV radiation is required for the production of vitamin D in humans. The amount of vitamin D one gets from exposing the bare skin to the sun depends on the intensity of the sun’s UV rays reaching the earth’s surface which is influenced by a number of different factors [19]. Vitamin D’s role in cell proliferation and differentiation could possibly have a protective role against UV-induced ROS damage.
Prolonged human exposure to solar UV radiation may result in acute and chronic health effects on the skin, eye and immune system. Sunburn and tanning are the best known acute effects of excessive UV radiation exposure; in the long term, UV radiation-induced degenerative changes in cells, fibrous tissue and blood vessels lead to premature skin ageing. UV radiation can also cause inflammatory reactions of the eye, such as photokeratitis [19].
5 UV Light Induced Reactive Oxygen Species
Human skin provides continuous protection against chemicals, radiation and infection. The skin is composed of different layers namely the epidermis (composed of mainly dead cells), the dermis and subcutaneous tissue. The epidermis is constantly renewed and is separated from the dermis by a layer of continuously dividing cells namely keratinocytes and melanocytes. Melanin pigment is produced by melanocytes, which is the precursor of melanoma [17], and is transferred to the neighbouring keratinocytes. Keratinocytes create a highly effective physical barrier; they accumulate melanin pigments as they mature, and epidermal melanin functions to potently block UV penetration into the skin. A third cell type is the Langerhans cells which are present under the stratum corneum. They play a role in immunological reactions of the skin and their actions are highly sensitive to UV. The dermis contains collagen fibres which assists in the skins’ elasticity and provides supportive strength. Collagen fibres are broken down by high levels of UV which leads to premature aging as skin loses its elasticity [3, 12].
As the human skin is the largest organ and covers the body’s whole surface area it is prone to continuous UV light exposure [11]. Melanin synthesis is stimulated by sun exposure [11], artificial sources [3] and inflammation. This results in post inflammatory hyperpigmentation. Epidermal melanocytes are thus susceptible to ROS which is induced through excessive sun exposure [11]. If homeostasis is disrupted by the increased production of ROS this could possibly drive the process of malignant transformation of cells [11, 21].
5.1 What Are Reactive Oxygen Species?
Free radicals are defined as molecules containing one or more unpaired electrons in their electron orbitals. Electrons are considered to be more stable when paired free radicals are generally more reactive than non-radical species [13]. Radicals can combine their unpaired electrons by forming a covalent bond. Radical interactions with non-radicals often involve the radical donating its electron (a reducing action) or it could accept an electron from the non-radical molecule (an oxidising action) or it could simply join onto a non-radical [13]. This results in the non-radical becoming a radical and can have various physiological repercussions.
ROS is the umbrella term used to describe oxygen derived free radicals as well as non-radicals such as hydrogen peroxide. Organisms that are dependent on the reduction of oxygen for energy, aerobic organisms, are the most susceptible to the potentially damaging actions of ROS that are released during this process [6] (Table 2.1).
ROS is constitutively produced in the body and sources of ROS can either be exogenous or endogenous.
Endogenous ROS are predominantly produced by the mitochondria in the cell, as this is where oxygen is reduced for ATP production by the addition of four electrons to produce water as the product. The largest portion of oxygen is reduced in the electron transport chain (ETC). No remaining intermediates are produced. However approximately 5% of oxygen is reduced by the univalent pathway thereby leading to free radical production. ROS producing capabilities are often dependent on the composition of the mitochondrial membrane, the species of animal and the age of the animal [6].
Most reactions that take place in the mitochondrial ETC consist of redox reactions. These reactions involve the exchange and movement of electrons, with the enzyme cytochrome oxidase being the only enzyme involved in a reaction that uses oxygen. It has been found that the redox reactions in the ETC ‘leak’ electrons and subsequently generate superoxide (O2 .-) with the majority of the ROS formation taking place at complex III and to a lesser extent at complex I. ROS is also formed in the endoplasmic reticulum (ER) and peroxisomes of the cell. Extracellular or exogenous ROS sources are vast and are responsible for a large percentage of ROS present in the body. Common air pollutants and industrial contaminants, exhaust fumes and the smoke generated by cigarettes have been implicated in ROS generated in the body. ROS generated in this manner has been found to both directly and indirectly cause O2 .- formation and various types of nitric oxide derivatives, either by direct contact with the skin or by inhalation. Some drugs, narcotic substances and anesthetizing gases are also thought to contribute to ROS production. Certain food and alcohol consumption have also been implicated in ROS formation. Other environmental agents and non-genotoxic carcinogens such as gamma irradiation can also induce ROS formation [6].
5.2 UV – Light Induced ROS
Even exposure to the ultraviolet light spectrum can indirectly lead to the production of a variety of ROS including O2 .-, singlet oxygen, hydroxyl radicals and hydrogen peroxide through various mechanisms [17] (Fig. 2.1).
UV radiation is able to mediate damage to cellular components in two ways:
The first mechanism is by the direct absorption of incident rays by the cell and its components. This results in the formation of an excited state of the components and subsequent chemical reactions.
The second mechanisms are by means of photosensitization. Incident rays are absorbed by endogenous or exogenous photosensitizers such as bilirubin. This results in an excitation of the sensitizers to their triplicate states. The excited photosensitizers exert their effects by two mechanisms. Type I photochemical reactions involve electron transfer and the process of hydrogen abstraction to form free radicals. Type II photochemical reactions involve the transfer of energy with O2 to yield reactive state singlet oxygen (1O2) [20, 22]
5.2.1 Catalase
The first mechanism that will be discussed is the UV induced ROS by the enzyme catalase. Catalase is known to be able to degrade hydrogen peroxide via the reaction 2H2O2 →2H2O + O2 through a process known as catalytic activity. The enzyme catalase is also capable of exhibiting peroxidatic activity when low concentrations of hydrogen peroxide (H2O2) are present. However, it was observed that UVB light caused a marked increase in ROS in keratinocytes, more so in glutathione depleted cells. Upon further experimentation it was found that the oxidant generating protein was catalase [14].
This is thought to be a result of the ability of the short wave UV (Likely UVC) light to alter the H2O2 binding site on the catalase enzyme, thus allowing for water molecules to access the heme iron. This essentially allows for the water molecules to act as a source for the generation of protons. These protons are then able to interact with diatomic oxygen to generate ROS such as reactive peroxides. The activity of the charge relay network of the enzyme was also found to be of importance on the effect that UV light mediates on catalase. The effects of UVB light on catalase were found to be pH-sensitive and oxygen dependent, this and the intracellular oxidant status influences whether the catalase activity in response to UV light is cytotoxic or protective [14].
5.2.2 Nitric Oxide Synthase
UVB has also been implicated in the up-regulation of membrane-bound nitric oxide synthase (NOS) in human keratinocytes, thereby resulting in increased production of NO [10]. While UVA has been shown by [2] to generate increased superoxide levels at moderate UVR levels in real time, O2 .- is able to inactivate iron-sulphur proteins leading to release of the reducing ferrous iron, thereby resulting in further production of ROS [23]. The exact mechanisms as to how UV causes these effects are not fully elucidated, however the simultaneous production of O2 .- and NO within proximity of each other can lead to the spontaneous generation of peroxynitrite, a highly reactive free radical [10].
5.2.3 Signalling Pathways
Various signaling pathways are activated by UV-mediated ROS generation, especially in the pathophysiology of skin diseases. MAPKs have been shown to be a target of oxidative stress such the oxidative stress induced by solar UV radiation, the UV radiation influences the pathways in a manner that closely resembles ROS [7, 16]. The ROS produced lead to the activation of the MAPKs such as ERK and JNK. These MAPKs play a pivotal role in the recruitment of factors that lead to the downstream activation of transcription factor AP-1. The factor p38 and the inhibitory kappa kinase activation are vital in the process of transcriptional activation of NF-kB. UVA irradiation of the fibroblast cells in the skin result in the release of labile iron, which is implicated in the activation of NF-kB [7, 24]. AP-1 and NF-kB play essential roles in the regulation of a diverse array of genes involved in processes such as the cell cycle, proliferation and apoptosis [7].
5.2.4 Protein Kinase C
UV irradiation was found to have an effect on protein kinase C (PKC) in murine fibroblasts. Several different isoforms of this serine/threonine kinase exist and have been implicated in UV-induced signal transduction pathways [8]. Low doses of UV exposure result in the adhesion of cultured fibroblasts to the collagen matrices by means of PKC isoform activation and integrins. PKC α has also been found to be irreversibly inhibited in UVA irradiated cells while PKC ε is known to act as an endogenous photosensitizer that plays a role in inducing UV-induced cutaneous damage. The accumulation of ROS associated with skin aging is thought to be linked to UV radiations effect on PKC. In a study done by Bossi et al. [8] it was observed that UVA irradiation causes an increase in ROS levels and lipid peroxidation in both young and aging fibroblasts; however basal ROS levels were much higher in the aged cell population and they also exhibited a much slower response to UV irradiation. A decline in PKC δ expression in these fibroblasts was also observed to be closely linked to the translocation of PKCδ to the nucleus due to the exposure to UV light. At the same time it also resulted in an increase in ROS production. This observation is suggestive of the role that PKC δ may have in regulating ROS production in ROS-induced states and lean towards the idea that loss of PKC δ expression can cause the activation and elevation of ROS levels [8]. It was also found that PKC α expression post UV irradiation is not linked to the elevation in ROS levels, regardless of increased PKC α expression following UV irradiation.
5.2.5 DNA Damage
Exposure of cells to UVA, UVB or UVC is capable of causing 8-oxo-7-8-dihydro-2′-deoxyguanosine (8-oxodGuo). This is an oxidatively modified DNA base that is often used as a marker of possible oxidative DNA damage, both in vivo and in vitro in calf thymus DNA as well as cultured cells [28]. Various ROS have been implicated in the UV-induced oxidation of 8-oxodGuo. Singlet oxygen (1O2) is proposed to be the only source of ROS involved in UVC-induced production of 8-oxodGuo. While for UVA and UVB 1O2 is a large source of the ROS involved. Both HO2 and hydroxyl radicals (.OH) were also found to play a significant role as well. Surprisingly it was observed that the O2 .-, usually a highly reactive free radical, was not involved in UV-induced oxidative damage in these cells [28]. These observations were also noted in a study conducted by Wei et al. [27] where they confirmed the role of 1O2 in oxidative DNA damage induced by UV exposure.
5.3 Biochemical Actions of UV in the Skin
Chromophores absorb UV energy which allows for biochemical reactions in human skin [26]. They are molecules that absorb certain wavelengths of visible light and transmit to others. Chromophores could either be an exogenous agent or endogenous compound [1]. Cutaneous chromophores include DNA, urocanic acid, aromatic amino acids, retinoids, carotenoids, bilirubin, flavins, haemoglobin, melanin and NAD(P)H [26]. Chromophores may be damaged directly or they can act as photosensitizers. This results in the generation of ROS in the presence of molecular oxygen [1].
If the epidermis is exposed to direct UV radiation from the sun it can lead to oxidative stress through activating the enzyme NADPH oxidase or by promoting lipid peroxidation. When molecular oxygen is reduced to O2 .- the production of ROS is initiated. This process can be enzymatic through NADPH oxidase or xanthine oxidase catalysed reactions or non-enzymatic. Glutathione is recycled by glutathione reductase to the detriment of NADPH, which is recycled by glucose 6- phosphate dehydrogenase after glucose has been phosphorylated by hexokinase [26].
UV radiation also causes oxidative stress in the skin by inducing the release of mediators of inflammation. Leukocytes produce the radical anion O2 .-, catalysed by the enzyme NADPH oxidase. O2 .- then undergoes dismutation to oxygen and H2O2, catalysed by superoxide dismutase. Myeloperoxidase then converts H2O2 to hypochlorite. Another pathway for oxidative stress caused by UV is the removal of a proton and an electron from lipid molecules which produces lipid radicals. Lipid radicals can interact with molecular oxygen giving rise to lipid peroxidase and new lipid molecules [26].
6 UV, ROS and Pathophysiological Skin Effects
The best known effect of excessive UV radiation exposure is erythema, which is reddening of the skin due to sunburn. Chronic exposure to UV radiation can cause a series of degenerative skin conditions.
Photoaging is a consequence of exposure to UVA and UVB. Abnormal elastic fibres in the dermis and a decrease in collagen fibres are distinct characteristics of photo aged skin. Photoaged skin displays an increased degradation of collagen and elastic fibres in the dermis which is caused by an increase in proteolytic activation and abnormal Extra Cellular Matrix (ECM) turnover. UV rays can also contribute to the generation of ROS that stimulates the inflammatory process in the skin. This causes reduced levels of natural enzymatic and non-enzymatic antioxidant defence mechanisms of the photoaged skin as well as an increased neutrophil infiltration into skin and increased inflammation [3].
UVA mainly drives the production of ROS. UVA has the ability to penetrate the deep dermal layer, inducing changes and driving the process and progression of photoaging. Once UVA penetrates the skin it is absorbed by cellular chromophores which comprise of molecules like riboflavins, melanin, bilirubin, but DNA is not included. The absorption of photons/energy results in the excitation of chromophores, referred to as the singlet exited state. The photon/energized molecule then falls back to the ground state and emits either heat or fluorescence or second an intersystem crossing leading to a triplet excited state. The triplet state could react with both DNA and molecular oxygen which can induce changes in DNA or lead to ROS production. Photoaging could also drive DNA damage, particularly mtDNA damage. ROS production in mitochondrial DNA increases as UV radiation causes a well-recognized 4977 base pair long deletion of the mtDNA [25].
UVB has the ability to penetrate the epidermis but not the deeper dermal layer. Damage is thus limited to keratinocytes and melanocytes in the epidermal layer. However, UVA passes the through the epidermal layer inducing damage which leads to damage in the deeper dermal tissue [25].
7 Conclusion
Humans are exposed to UV light on a daily basis, with the skin bearing the brunt of this exposure due to its large surface area. The constant UV radiation is imperative for normal physiological function. However over-exposure to UV light is known to have detrimental physiological effects. It is believed that these effects are a result of an increase in ROS in the cells. UV light can induce ROS in various ways, such as affecting the enzyme catalase and up-regulating NOS synthesis. UV radiation may also cause a decrease in PKC expression leading to increased ROS production. It can also modify DNA and other chromophores resulting in elevated ROS levels. The effects of raised ROS levels can vary based on the intracellular oxidant status of the cell. However, it is still important to protect yourself against the potentially harmful effects of UV light as it can lead to pathological UV-induced ROS production.
References
Ainbinder D, Touitou E (2010) Skin photodamage prevention: state of the art and new prospects. In: Textbook of aging skin. Springer, Berlin
Aitken GR, Henderson JR, Chang SC, Mcneil CJ, Birch-Machin MA (2007) Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin Exp Dermatol 32:722–727
Belkin M, Césarini J, Diffey B, Hietanen M, Kojima M, Mariutti G, Mckinlay A, Repacholi M, Roy C, Rubenstein R (1994) Protection against exposure to ultraviolet radiation. World Health Organization/United Nations Environment Programme, Geneva
Berger, U., Wilson P, Mcclelland RA, Colston K, Haussler MR, Pike JW, Coombes RC (1988) Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab 67(3):607–613
Berridge MJ (1988) Vitamin D, reactive oxygen species and calcium signalling in ageing and disease. LID - 10.1098/rstb.2015.0434 [doi] LID - 20150434 [pii]
Bhattacharyya S, Saha J (2015) Tumour, oxidative stress and host T cell response: cementing the dominance. Scand J Immunol 82(6):477–488
Bickers DR, Athar M (2006) Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol 126:2565–2575
Bossi O, Gartsbein M, Leitges M, Kuroki T, Grossman S, Tennenbaum T (2008) UV irradiation increases ROS production via PKCδ signaling in primary murine fibroblasts. J Cell Biochem 105:194–207
Chen TC, Lu Z, Holick MF (2010) Photobiology of vitamin D. Vitamin D. Springer, Berlin
Deliconstantinos G, Villiotou V, Stavrides JC (1996) Alterations of nitric oxide synthase and xanthine oxidase activities of human keratinocytes by ultraviolet B radiation: potential role for peroxynitrite in skin inflammation. Biochem Pharmacol 51:1727–1738
Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel-Malek ZA (2014) Melanocytes as instigators and victims of oxidative stress. J Investig Dermatol 134:1512–1518
D’orazio J, Jarrett S, Amaro-Ortiz A, Scott T (2013) UV radiation and the skin. Int J Mol Sci 14:12222–12248
Halliwell B (1991) Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 91:S14–S22
Heck DE, Vetrano AM, Mariano TM, Laskin JD (2003) UVB light stimulates production of reactive oxygen species unexpected role for catalase. J Biol Chem 278:22432–22436
Hussein A, Elhassaneen Y (2014) Natural dye from red onion skins and applied in dyeing cotton fabrics for the production of women’s headwear resistance to ultraviolet radiation (UVR). J Am Sci 10(3):129–139
Kim AL, Labasi JM, Zhu Y, Tang X, Mcclure K, Gabel CA (2005) Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J Invest Dermatol 124:1318–1325
Kimeswenger S, Schwarz A, Födinger D, Müller S, Pehamberger H, Schwarz T, Jantschitsch C (2016) Infrared A radiation promotes survival of human melanocytes carrying ultraviolet radiation-induced DNA damage. Exp Dermatol 25:447–452
Liu S, Mizu H, Yamauchi H (2010) Photoinflammatory responses to UV-irradiated ketoprofen mediated by the induction of ROS generation, enhancement of cyclooxygenase-2 expression, and regulation of multiple signaling pathways. Free Radic Biol Med 48:772–780
Lucas R, Mcmichael T, Smith W, Armstrong B (2006) Solar ultraviolet radiation. Assessing the environmental burden of disease at national and local levels, Environmental burden of disease series, vol 13. World Health Organization, Geneva
Pattison DI, Davies MJ (2006) Actions of ultraviolet light on cellular structures. EXS 96:131–157
Picardo M, Grammatico P, Roccella F, Roccella M, Grandinetti M, Del Porto G, Passi S (1996) Imbalance in the antioxidant pool in melanoma cells and normal melanocytes from patients with melanoma. J Investig Dermatol 107:322–326
Poljšak B, Dahmane R (2012) Free radicals and extrinsic skin aging. Dermatol Res Prac, pp. 1–4
Raha S, Robinson BH (2000) Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci 25:502–508
Reelfs O, Tyrrell RM, Pourzand C (2004) Ultravioleta radiation-induced immediate iron release is a key modulator of the activation of NF-kappaB in human skin fibroblasts. J Invest Dermatol 122:1440–1447
Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K (2015). Oxidative stress in aging human skin. Biomol Ther 5:545–589
Sorg O, Antille C, Saurat J-H (2004) Retinoids, other topical vitamins, and antioxidants. Basic Clin Dermatol 28:89–116
Wei H, Cai Q, Rahn R, Zhang X (1997) Singlet oxygen involvement in ultraviolet (254 nm) radiation-induced formation of 8-hydroxy-deoxyguanosine in DNA. Free Radic Biol Med 23:148–154
World Health Organization (2002) Global Solar UV Index: A Practical Guide. WHO, Geneva
Zhang X, Rosenstein BS, Wang Y, Lebwohl M, Wei H (1997) Identification of possible reactive oxygen species involved in ultraviolet radiation-induced oxidative DNA damage. Free Radic Biol Med 23:980–985
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
de Jager, T.L., Cockrell, A.E., Du Plessis, S.S. (2017). Ultraviolet Light Induced Generation of Reactive Oxygen Species. In: Ahmad, S. (eds) Ultraviolet Light in Human Health, Diseases and Environment. Advances in Experimental Medicine and Biology, vol 996. Springer, Cham. https://doi.org/10.1007/978-3-319-56017-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-56017-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56016-8
Online ISBN: 978-3-319-56017-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)