Abstract
Mesenchymal stem cells (MSC) are multipotent stem cells with a broad well-described immunosuppressive potential. They are able to modulate both the innate and the adaptive immune response. Particularly, MSC are able to regulate the phenotype and function of macrophages that are critical for different biological processes including wound healing, inflammation, pathogenesis of several autoimmune diseases, and tumor growth. These multifunctional roles of macrophages are due to their high plasticity, which enable them to adopt different phenotypes such as a pro-inflammatory M1 and anti-inflammatory M2 phenotype. MSC promote macrophage differentiation toward an M2-like phenotype with a high tissue remodeling potential and anti-inflammatory activity but also a pro-tumorigenic function. MSC regulatory effect on macrophages is mediated through the secretion of different immunomodulatory molecules such as PGE2, IL1RA, and IL-6. Moreover, the presence of macrophages in damaged tissue and inflammation is essential for MSC to exert their therapeutic function. In this chapter, we discuss how the interplay between macrophages and MSC mutually modulates their phenotypes and functions, orchestrates tissue repair, and controls inflammation during autoimmunity and tumor growth.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Mesenchymal Stem Cell
- Macrophage Polarization
- Promote Tumor Progression
- Macrophage Phenotype
- Mesenchymal Stem Cell Transplantation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
- Arg1:
-
Arginase 1
- ASCs:
-
Adipose-derived mesenchymal stem cells
- CIA:
-
Collagen-induced arthritis
- COX-2:
-
Cyclooxygenase type 2
- CXCR4:
-
Chemokine receptor type 4
- ET-1:
-
Endothelin-1
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- KCs:
-
Kupffer cells
- LPS:
-
Lipopolysaccharides
- M1:
-
Macrophages type 1
- M2a:
-
Macrophages type 2A
- M2b:
-
Macrophages type 2B
- M2c:
-
Macrophages type 2C
- M-CSF:
-
Macrophage colony-stimulating factor 1
- MSC:
-
Mesenchymal stem cells
- NFκβ:
-
Nuclear factor kappa-beta
- NK:
-
Natural killer
- PD-1:
-
Pathway of cell death-1
- PDL1:
-
Pathway of cell death ligand-1
- PGE2:
-
Prostaglandin E2
- RA:
-
Rheumatoid arthritis
- RANK-L:
-
Receptor activator of NFκ-β ligand
- SFD-1:
-
Stromal cell-derived factor 1
- TAMs:
-
Tumor-associated macrophages
- TGF-β1:
-
Transforming growth factor β1
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor alpha
- TSG-6:
-
TNF-α-stimulated gene 6
- VEGF:
-
Vascular endothelial growth factor
- Ym1:
-
Chitinase-like 3
1 Introduction
Mesenchymal stem cells (MSC) are adult multipotent stromal cells widely studied for their regenerative and immunomodulatory properties (Jackson et al. 2012; Le Blanc and Ringden 2007; Djouad et al. 2006, 2009; Ruiz et al. 2016). The therapeutic effect of MSC in mouse experimental disease models has been shown to be associated with their role in tissue maintenance or regeneration, support for hematopoiesis, stimulation of angiogenesis, and modulation of the immune response (Arminan et al. 2010; Kim et al. 2012; Singer and Caplan 2011). In an inflammatory environment, MSC are able to interact with a broad range of immune cells via the secretion of several paracrine factors such as transforming growth factor (TGF)-β1, IL-6, and PGE-2 (Djouad et al. 2007; English et al. 2009) but also through cell–cell contact via Jagged/Notch or PD-1/PD-L1 pathways (Liotta et al. 2008; Luz-Crawford et al. 2012; Cahill et al. 2015). As a consequence of this interaction, MSC interact with and inhibit the function of immune effector cells inducing regulatory cell functions (Luz-Crawford et al. 2013; Glenn and Whartenby 2014). Here, we focus on the dialogue between MSC and macrophages, which results in the generation of MSC-educated macrophages and their role in (1) tissue repair, (2) immune tolerance, and (3) tumor growth.
2 The Interplay Between Mesenchymal Stem Cells and Macrophages in Tissue Repair
Macrophages are major players during both inflammatory and tissue repair processes. They are one of the first immune cells to arrive to the injured site in order to avoid any microbial infection and to phagocyte the remaining debris of the injured tissue (Mantovani et al. 2013; Chazaud 2014). In particular, the inflammatory subset of macrophages referred to as M1 macrophages are the first to be found in the damaged tissue. M1 macrophages are activated by LPS and IFN-γ and express high levels of the co-stimulatory molecules, inducible nitric oxide synthase (iNOS) and pro-inflammatory cytokines such as TNF-α, and low levels of Ym1 (Novak and Koh 2013). M1 macrophages induce cell proliferation to replenish the damage area and its deficiency during the process of tissue repair (Wynn and Vannella 2016). In a second wave of macrophage recruitment, another subtype of macrophages referred to as alternatively activated M2 are dominant. Upon induction with IL-4, macrophages can adopt an M2a macrophage phenotype expressing low levels of co-stimulatory molecules together with high Ym1 and Arginase-1 (Arg-1) activities, high CD206 expression, and VEGF production. All together these factors released by M2a macrophages are known to exert wound healing/pro-fibrotic functions. M2b macrophages producing pro-inflammatory cytokines are induced by immune complexes while M2c also known as an anti-inflammatory subtype of macrophages releasing high levels of IL-10 are induced by IL-10 and TGF-β1 (Novak and Koh 2013). M2 macrophages are involved in the resolution of inflammation promoting the survival, proliferation, and differentiation of the remaining or recruited progenitor cells at the site of injury. Interestingly, MSC interact with and impact on macrophage functions. Indeed, in vitro, while MSC inhibit M1 markers such as TNF-α and iNOS, they promote the differentiation of macrophages toward M2 phenotype expressing IL-10, CD206, and Arg1 (Abumaree et al. 2013; Maggini et al. 2010). These latter results are in line with observations from an in vivo model of myocardial infarction, revealing that infiltrated macrophages in the heart of mice treated with MSC express higher levels of Arg1 and lower levels of pro-inflammatory M1 markers compared to macrophages in the mice that did not receive MSC treatment (Dayan et al. 2011).
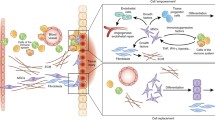
Moreover, it has been also demonstrated that the injection of MSC in a cardiac failure model significantly increases the local recruitment of macrophages, accelerating cardiac muscle repair (Wang et al. 2015). Additionally, macrophages are critical for MSC to exert their therapeutic function since the depletion of macrophages using the lipoclodronate solution significantly decreased the therapeutic effect of MSC on cardiac regeneration. This effect was associated with the inhibition of an enhanced angiogenesis observed after MSC transplantation and occurring in the presence of macrophages (Wang et al. 2015). In another experimental model of acute kidney ischemia (AKI), MSC injection improved regeneration of the kidney promoting the switch from inflammatory M1 macrophages into an anti-inflammatory M2 phenotype. Indeed, this switch was associated with an increase in the matrix metalloproteinase (MMP)-9 activity in ischemic kidneys, which contributed to a reduction of total collagen I, and a subsequent decrease of fibrosis (Wise et al. 2014). Finally, in an experimental model of diabetes, the administration of MSC significantly increased the recruitment of macrophages with mainly a M2 phenotype. This recruitment of macrophages depends on the CXCR4/Stromal cell-derived factor (SFD)-1 axis that promotes beta cell replication and regeneration reducing diabetes progression (Cao et al. 2014). All together these data demonstrate that macrophages play a critical role in the therapeutic effect of MSC in tissue regeneration through the capacity of MSC to stimulate the migration and recruitment of M2-like macrophages into the site of damage, promoting angiogenesis and tissue remodeling (Fig. 4.1).
MSC regulate macrophage immunological fate and their functions. MSC are able to induce M2-like macrophages that increase wound healing resolution. In response to inflammation, MSC induce the generation of “M2-educated macrophages” that mainly secrete IL-10 which will reduce autoimmune disease progression. In tumors, TAM macrophages will increase the survival and progression of tumor growth
3 The Interplay Between Mesenchymal Stem Cells and Macrophages in Immune Tolerance
Macrophages are one of the main players in early stages of inflammation by playing several functions such as antigen presentation and the secretion of pro-inflammatory factors. However, the persistence of macrophage pro-inflammatory activity was shown to be associated with the development of chronic inflammatory diseases. In contrast, tissue homeostasis depends on the capacity of macrophages to adopt different phenotypes in response to different mediators promoting macrophage reprograming from pro-inflammatory M1 into an anti-inflammatory M2. This plasticity is critical for the resolution of inflammation (Jou et al. 2013).
It has been well described that MSC are able to educate tissue-resident macrophages in order to diminish local inflammation (Eggenhofer and Hoogduijn 2012). In the first in vitro approach, Kim and collaborators demonstrated that MSC were able to educate macrophages after 3 days of coculture of activated M1-like macrophages with MSC (Kim and Hematti 2009). These “educated” macrophages produce low levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12p70 and display a higher capacity to produce IL-10 and IL-12p40 after LPS stimulation (Kim and Hematti 2009). In addition, adipose-derived MSC (ASCs) have been shown to induce M2-like macrophage phenotype independently of cell-to-cell contact when treated with either LPS or the serum of patients with acute respiratory distress syndrome (Hu et al. 2016). In another report, authors used ASC-conditioned medium to educate macrophages for chronic colitis treatment. Indeed, systemic infusion of such MSC-educated macrophages inhibited colitis in mice and reduced mortality protecting against sepsis (Anderson et al. 2013). In rheumatoid arthritis (RA), an autoimmune and inflammatory disease, macrophages are among the main players of disease progression (Udalova et al. 2016). They are significantly increased in both the synovium and the adjacent tissues (Janossy et al. 1981; Udalova et al. 2016). Indeed, macrophages in the synovial membranes of patients with RA have been described as the main initiators of T-cell infiltration and activation in an antigen-dependent manner (Janossy et al. 1981). A large body of studies has demonstrated that in RA patients, as compared to healthy individuals, there is an imbalance between pro-inflammatory M1 secreting TNF-α and anti-inflammatory M2c secreting IL-10 macrophages in favor of the first macrophage subtype (Kennedy et al. 2011; Ye et al. 2014). Moreover, in healthy individuals there is a balance between osteoclasts and osteoblasts (bone resorption versus bone regeneration) that is completely lost in RA patients. Indeed, the production of pro-inflammatory cytokines such as IL-6 and TNF-α will stimulate the secretion of receptor activator of nuclear factor-κB ligand (RANKL) and the macrophage colony-stimulating factor 1 (M-CSF) by synovial fibroblasts, which are critical for osteoclast formation through the fusion of myeloid precursors of monocytes and macrophages (Hamilton et al. 1993; Shigeyama et al. 2000; Teitelbaum 2000). In this context, MSC have shown promising results in the treatment of arthritis. For example, ASCs significantly improve the collagen-induced arthritis (CIA) in murine model mainly through their capacity to inhibit RANK-induced osteoclastogenesis (Gonzalez et al. 2009; Garimella et al. 2015). In addition to their capacity to prevent osteoclast formation, MSC also participate in the regulation of the phenotypic switch from a pro-inflammatory M1-like to an IL-10 producing M2-like macrophage subset (Abumaree et al. 2013). In another model of liver transplantation, the MSC were able to reprogram Kupffer cells (KCs) that are resident hepatic macrophages that control innate liver immunity (You et al. 2015). Similar to typical macrophages, they can display different phenotypes, depending on the stimuli they receive, to promote hepatic immune tolerance (Movita et al. 2012). Several studies have demonstrated that the negative or positive outcome in liver injury will strictly depend on the phenotype of KCs (Movita et al. 2012; Ahsan et al. 2013; Akamatsu et al. 2003). In this context, MSC were able to induce the switch of KCs from a M1 phenotype into an M2 phenotype, which significantly contributed to liver allograft tolerance (You et al. 2015). In a sepsis experimental model, the infusion of MSC has been shown to polarize pro-inflammatory macrophages into an anti-inflammatory phenotype resulting in an improvement of survival. However, when macrophages were depleted using either the lipoclodronate or specific antibodies against IL-10, the therapeutic effect of MSC was completely lost (Nemeth et al. 2009).
The mechanism by which MSC modulate macrophage polarization is still under investigation. However, it has been demonstrated that MSC treated with TNF-α, the main pro-inflammatory cytokine produced by M1-like macrophages, significantly increase the secretion of anti-inflammatory molecules such as TNF-α-stimulated gene 6 protein (TSG-6) (Torihashi et al. 2015). Recently, it has been reported that intravenous administration of MSC prompts the generation of M2 alveolar macrophages that will induce immune tolerance (Ko et al. 2016). Moreover, prostaglandin E2 (PGE2) through the upregulation of Cox2 expression as well as other components of the arachidonic acid pathway reprograms macrophages into a M2-like phenotype (Nemeth et al. 2009). TSG-6 prevents the Toll-like receptor 2 (TLR2) signaling in macrophages via CD44, which will inhibit NF-κB and decrease macrophage inflammatory response (Choi et al. 2011), and PGE2 promotes the polarization of macrophages toward an M2-like phenotype (Uccelli and de Rosbo 2015). Others and we have demonstrated that the production of IL-1 receptor antagonist (IL-1RA) by MSC plays a critical role in the modulation of macrophage phenotype promoting their differentiation toward an M2-like phenotype (Ortiz et al. 2007; Luz-Crawford et al. 2015). It has been proposed that in vivo the beneficial effect of MSC is initiated in the lung where MSC migrate after intravenous administration. This was associated with an enhanced polarization of macrophages toward an M2-like phenotype resulting in the increased IL-10 levels in the lung in response to MSC injection. Moreover, the protective role of MSC on hepatic injury was significantly decreased upon administration of an anti-IL10 neutralizing antibody (Lee et al. 2015). All together these data suggest that the therapeutic effect of MSC in autoimmune disorders is associated with the generation of M2-like macrophages that increase IL-10 production to dampen pathogenic inflammation.
4 Mesenchymal Stem Cells Promote Tumor Progression Through Macrophages
Macrophages are one of the most represented leukocyte population within solid tumors. Indeed, their role in tumor cell growth depends on the phenotype acquired by macrophages in the tumor microenvironment (Lamagna et al. 2006). Anti-tumorigenic-activated M1-like macrophages are able to stimulate activation of resting NK cells and recruitment of pro-inflammatory T cells into the tumor, while tumor-associated macrophages (TAMs) are alternatively activated M2-like macrophages that stimulate anti-inflammatory responses exerting pro-tumorigenic functions (Fig. 4.1) (Solinas et al. 2009; Wong et al. 2009). Because it has been shown that the deficiency in M1 macrophage polarization significantly increases tumor progression (Kondo et al. 2016), one of the main targets for cancer therapy is the modulation of macrophage polarization in the tumor microenvironment from a pro-tumorigenic M2-like to an anti-tumorigenic M1-like macrophage phenotype. In this context, MSC have been shown to promote tumor progression by increasing the generation of M2-like macrophages, calling into question the use of MSC to treat tumors (Jia et al. 2016). In line with this study, Yamada and collaborators have shown that MSC infusion significantly favors tumor progression by controlling macrophage differentiation and function (Yamada et al. 2016). Indeed, MSC induce generation of a particular M2-like macrophage subset able to inhibit the cytotoxic activity of both NK and CD8+ T cells by reducing the expression of NKp44, CD69, and CD25 markers, and production of IFN-γ, and by inducing generation of T regulatory cells, which will lead to an improvement of tumor growth (Mathew et al. 2016). Furthermore, MSC secreting VEGF and ET-1 will significantly promote tumor progression by increasing the number of M2-like macrophages within tumors inducing a tolerogenic environment and promoting tumor angiogenesis (Yamada et al. 2016). Interestingly, the cross talk between MSC and macrophages also favors the tumor to escape from immune surveillance since M1-like macrophages enhance the capacity of MSC to promote tumor growth in vivo. Moreover, primed MSC produce significantly higher levels of iNOS and MCP1 as compared to unstimulated MSC, which increases recruitment of macrophages to the tumor sites. Furthermore, IL-6 secreted by stimulated MSC polarizes infiltrated macrophages into an M2-like phenotype. Thus, in the presence of anti-tumorigenic M1-like macrophages in the tumor microenvironment, MSC seems to act as sensors and switchers of inflammation accelerating tumor progression (Ren et al. 2012).
In summary, cellular interactions between MSC and immune effectors, in particular macrophages, in the tumor microenvironment play a pivotal role in the establishment of tumor immune escape.
5 Conclusions
The dialogue between MSC and macrophages has a critical role for their phenotype and function. In the context of wound healing, MSC induce generation of an M2-like phenotype which will control the resolution of inflammation and promote angiogenesis and tissue repair. In inflammatory disease models, MSC inhibit pro-inflammatory M1-like macrophages promoting M2-like phenotype that will reduce autoimmune disease progression. However, in tumor, MSC will support the anti-inflammatory microenvironment by generating TAMs with pro-tumorigenic growth activities. In conclusion, M2-like macrophages induced by MSC improve tissue repair, inhibit inflammation, and support tumor growth. Thus, the specific mechanisms by which MSC are able to interact with macrophages have to be clearly defined to ensure safe clinical use of MSC.
References
Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA (2013) Human placental mesenchymal stem cells (pMSC) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev 9(5):620–641. doi:10.1007/s12015-013-9455-2
Ahsan MH, Gill AF, Alvarez X, Lackner AA, Veazey RS (2013) Kinetics of liver macrophages (Kupffer cells) in SIV-infected macaques. Virology 446(1–2):77–85. doi:10.1016/j.virol.2013.07.026
Akamatsu Y, Ohkohchi N, Doi H, Satomi S (2003) Effect of elimination of donor Kupffer cells and/or recipient macrophages on acute rejection in liver transplantation. Hepato-Gastroenterology 50(52):1105–1110
Anderson P, Souza-Moreira L, Morell M, Caro M, O’Valle F, Gonzalez-Rey E, Delgado M (2013) Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 62(8):1131–1141. doi:10.1136/gutjnl-2012-302152
Arminan A, Gandia C, Garcia-Verdugo JM, Lledo E, Trigueros C, Ruiz-Sauri A, Minana MD, Solves P, Paya R, Montero JA, Sepulveda P (2010) Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J Am Coll Cardiol 55(20):2244–2253. doi:10.1016/j.jacc.2009.08.092
Cahill EF, Tobin LM, Carty F, Mahon BP, English K (2015) Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Therapy 6:19. doi:10.1186/s13287-015-0021-5
Cao X, Han ZB, Zhao H, Liu Q (2014) Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int J Biochem Cell Biol 53:372–379. doi:10.1016/j.biocel.2014.06.003
Chazaud B (2014) Macrophages: supportive cells for tissue repair and regeneration. Immunobiology 219(3):172–178. doi:10.1016/j.imbio.2013.09.001
Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ (2011) Anti-inflammatory protein TSG-6 secreted by activated MSC attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 118(2):330–338. doi:10.1182/blood-2010-12-327353
Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A (2011) Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol 106(6):1299–1310. doi:10.1007/s00395-011-0221-9
Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C (2009) Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 5(7):392–399. doi:10.1038/nrrheum.2009.104
Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D (2007) Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells 25(8):2025–2032. doi:10.1634/stemcells.2006-0548
Djouad F, Mrugala D, Noel D, Jorgensen C (2006) Engineered mesenchymal stem cells for cartilage repair. Regen Med 1(4):529–537. doi:10.2217/17460751.1.4.529
Eggenhofer E, Hoogduijn MJ (2012) Mesenchymal stem cell-educated macrophages. Transplant Res 1(1):12. doi:10.1186/2047-1440-1-12
English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP (2009) Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 156(1):149–160. doi:10.1111/j.1365-2249.2009.03874.x
Garimella MG, Kour S, Piprode V, Mittal M, Kumar A, Rani L, Pote ST, Mishra GC, Chattopadhyay N, Wani MR (2015) Adipose-derived mesenchymal stem cells prevent systemic bone loss in collagen-induced arthritis. J Immunol 195(11):5136–5148. doi:10.4049/jimmunol.1500332
Glenn JD, Whartenby KA (2014) Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cells 6(5):526–539. doi:10.4252/wjsc.v6.i5.526
Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M (2009) Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 60(4):1006–1019. doi:10.1002/art.24405
Hamilton JA, Filonzi EL, Ianches G (1993) Regulation of macrophage colony-stimulating factor (M-CSF) production in cultured human synovial fibroblasts. Growth Factors 9(2):157–165
Hu Y, Qin C, Zheng G, Lai D, Tao H, Zhang Y, Qiu G, Ge M, Huang L, Chen L, Cheng B, Shu Q, Xu J (2016) Mesenchymal stem cell-educated macrophages ameliorate LPS-induced systemic response. Mediat Inflamm 2016:3735452. doi:10.1155/2016/3735452
Jackson WM, Nesti LJ, Tuan RS (2012) Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 1(1):44–50. doi:10.5966/sctm.2011-0024
Janossy G, Panayi G, Duke O, Bofill M, Poulter LW, Goldstein G (1981) Rheumatoid arthritis: a disease of T-lymphocyte/macrophage immunoregulation. Lancet 2(8251):839–842
Jia XH, Feng GW, Wang ZL, Du Y, Shen C, Hui H, Peng D, Li ZJ, Kong DL, Tian J (2016) Activation of mesenchymal stem cells by macrophages promotes tumor progression through immune suppressive effects. Oncotarget 7(15):20934-20944. doi:10.18632/oncotarget.8064
Jou IM, Lin CF, Tsai KJ, Wei SJ (2013) Macrophage-mediated inflammatory disorders. Mediators Inflamm 2013:316482. doi:10.1155/2013/316482
Kennedy A, Fearon U, Veale DJ, Godson C (2011) Macrophages in synovial inflammation. Front Immunol 2:52. doi:10.3389/fimmu.2011.00052
Kim J, Hematti P (2009) Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37(12):1445–1453. doi:10.1016/j.exphem.2009.09.004
Kim SW, Lee DW, Yu LH, Zhang HZ, Kim CE, Kim JM, Park TH, Cha KS, Seo SY, Roh MS, Lee KC, Jung JS, Kim MH (2012) Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model. Cardiovasc Res 95(4):495–506. doi:10.1093/cvr/cvs224
Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, Choi H, Prockop DJ, Oh JY (2016) Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci USA 113(1):158–163. doi:10.1073/pnas.1522905113
Kondo T, Tsunematsu T, Yamada A, Arakaki R, Saito M, Otsuka K, Kujiraoka S, Ushio A, Kurosawa M, Kudo Y, Ishimaru N (2016) Acceleration of tumor growth due to dysfunction in M1 macrophages and enhanced angiogenesis in an animal model of autoimmune disease. Lab Invest 96(4):468–480. doi:10.1038/labinvest.2015.166
Lamagna C, Aurrand-Lions M, Imhof BA (2006) Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 80(4):705–713. doi:10.1189/jlb.1105656
Le Blanc K, Ringden O (2007) Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med 262(5):509–525. doi:10.1111/j.1365-2796.2007.01844.x
Lee KC, Lin HC, Huang YH, Hung SC (2015) Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J Hepatol 63(6):1405–1412. doi:10.1016/j.jhep.2015.07.035
Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, Santarlasci V, Consoloni L, Angelotti ML, Romagnani P, Parronchi P, Krampera M, Maggi E, Romagnani S, Annunziato F (2008) Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells 26(1):279–289. doi:10.1634/stemcells.2007-0454
Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noel D (2015) Mesenchymal stem cell-derived IL1RA promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. doi:10.1002/stem.2254
Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G, Noel D, Jorgensen C, Figueroa F, Djouad F, Carrion F (2013) Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Therapy 4(3):65. doi:10.1186/scrt216
Luz-Crawford P, Noel D, Fernandez X, Khoury M, Figueroa F, Carrion F, Jorgensen C, Djouad F (2012) Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One 7(9):e45272. doi:10.1371/journal.pone.0045272
Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, Geffner JR (2010) Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One 5(2):e9252. doi:10.1371/journal.pone.0009252
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M (2013) Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229(2):176–185. doi:10.1002/path.4133
Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, Vinta A, Buckanovich RJ, di Magliano MP (2016) Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia 18(3):142–151. doi:10.1016/j.neo.2016.01.005
Movita D, Kreefft K, Biesta P, van Oudenaren A, Leenen PJ, Janssen HL, Boonstra A (2012) Kupffer cells express a unique combination of phenotypic and functional characteristics compared with splenic and peritoneal macrophages. J Leukoc Biol 92(4):723–733. doi:10.1189/jlb.1111566
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15(1):42–49. doi:10.1038/nm.1905
Novak ML, Koh TJ (2013) Macrophage phenotypes during tissue repair. J Leukoc Biol 93(6):875–881. doi:10.1189/jlb.1012512
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG (2007) Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104(26):11002–11007. doi:10.1073/pnas.0704421104
Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B, Wen T, Han Y, Rabson AB, Tischfield JA, Shao C, Shi Y (2012) CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell 11(6):812–824. doi:10.1016/j.stem.2012.08.013
Ruiz M, Cosenza S, Maumus M, Jorgensen C, Noel D (2016) Therapeutic application of mesenchymal stem cells in osteoarthritis. Expert Opin Biol Ther 16(1):33–42. doi:10.1517/14712598.2016.1093108
Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S (2000) Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum 43(11):2523–2530. doi:10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z
Singer NG, Caplan AI (2011) Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6:457–478. doi:10.1146/annurev-pathol-011110-130230
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073. doi:10.1189/jlb.0609385
Teitelbaum SL (2000) Osteoclasts, integrins, and osteoporosis. J Bone Miner Metab 18(6):344–349
Torihashi S, Ho M, Kawakubo Y, Komatsu K, Nagai M, Hirayama Y, Kawabata Y, Takenaka-Ninagawa N, Wanachewin O, Zhuo L, Kimata K (2015) Acute and temporal expression of tumor necrosis factor (TNF)-alpha-stimulated gene 6 product, TSG6, in mesenchymal stem cells creates microenvironments required for their successful transplantation into muscle tissue. J Biol Chem 290(37):22771–22781. doi:10.1074/jbc.M114.629774
Uccelli A, de Rosbo NK (2015) The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci 1351:114–126. doi:10.1111/nyas.12815
Udalova IA, Mantovani A, Feldmann M (2016) Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol 12(8):472–485. doi:10.1038/nrrheum.2016.91
Wang M, Zhang G, Wang Y, Liu T, Zhang Y, An Y, Li Y (2015) Crosstalk of mesenchymal stem cells and macrophages promotes cardiac muscle repair. Int J Biochem Cell Biol 58:53–61. doi:10.1016/j.biocel.2014.11.003
Wise AF, Williams TM, Kiewiet MB, Payne NL, Siatskas C, Samuel CS, Ricardo SD (2014) Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol 306(10):F1222–F1235. doi:10.1152/ajprenal.00675.2013
Wong CP, Bray TM, Ho E (2009) Induction of proinflammatory response in prostate cancer epithelial cells by activated macrophages. Cancer Lett 276(1):38–46. doi:10.1016/j.canlet.2008.10.025
Wynn TA, Vannella KM (2016) Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462. doi:10.1016/j.immuni.2016.02.015
Yamada K, Uchiyama A, Uehara A, Perera B, Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, Ishikawa O, Motegi S (2016) MFG-E8 drives melanoma growth by stimulating mesenchymal stromal cell-induced angiogenesis and M2 polarization of tumor-associated macrophages. Cancer Res 76(14):4283–4292. doi:10.1158/0008-5472.CAN-15-2812
Ye L, Wen Z, Li Y, Chen B, Yu T, Liu L, Zhang J, Ma Y, Xiao S, Ding L, Li L, Huang Z (2014) Interleukin-10 attenuation of collagen-induced arthritis is associated with suppression of interleukin-17 and retinoid-related orphan receptor gammat production in macrophages and repression of classically activated macrophages. Arthritis Res Therapy 16(2):R96. doi:10.1186/ar4544
You Y, Zhang J, Gong J, Chen Y, Li Y, Yang K, Liu Z (2015) Mesenchymal stromal cell-dependent reprogramming of Kupffer cells is mediated by TNF-alpha and PGE2 and is crucial for liver transplant tolerance. Immunol Res 62(3):292–305. doi:10.1007/s12026-015-8660-2
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Luz-Crawford, P., Jorgensen, C., Djouad, F. (2017). Mesenchymal Stem Cells Direct the Immunological Fate of Macrophages. In: Kloc, M. (eds) Macrophages. Results and Problems in Cell Differentiation, vol 62. Springer, Cham. https://doi.org/10.1007/978-3-319-54090-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-54090-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54089-4
Online ISBN: 978-3-319-54090-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)