Abstract
The transduction of sound into nerve impulses requires an ionic environment that depends on a variety of ion transport processes in epithelial and endothelial cells of the cochlea. Specific ion transport functions occur in specific cell types that coordinate the production and maintenance of endolymph, which is the fluid in the lumen of the cochlear duct that supports the sensory transduction process. The critical nature of these ion transport processes is underscored by observations of hearing loss when ion transport mechanisms malfunction as a result of mutations, drug exposure, or hormonal imbalance. This chapter describes our basic understanding of salient ion transport processes and their regulation by hormones and other regulatory pathways.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Acid-base balance
- Aquaporin
- Calcium homeostasis
- Endolymph
- Hereditary deafness
- Hormone receptors
- Hormonal regulation
- Intrastrial fluid
- Outer sulcus cells
- Perilymph
- Potassium secretion
- Reissner’s membrane
- Sodium absorption
- Spiral prominence
- Stria vascularis
9.1 Introduction
The mechanosensory transduction of sound in the cochlea depends on large electrochemical gradients and an unusual composition of the luminal fluid in the inner ear. This chapter focuses on ion transport mechanisms that establish and maintain those gradients. Although the focus is on the cochlea, much of what we know is informed by closely analogous mechanisms in the vestibular labyrinth. As such, reference to knowledge of pertinent homeostatic mechanisms in the vestibular labyrinth is included in this narrative. A short review of epithelial transport principles and nomenclature is provided next.
9.1.1 Transepithelial Vectorial Transport Between Compartments
Epithelial cells form sheets or tubules within the body that thereby separate two fluid compartments. Examples include glands, kidney tubules, digestive intestinal tract, airways, and the inner ear. In addition to the passive structural role of the epithelial cells, they engage in highly specialized transepithelial solute and water transport that can occur via a transcellular and/or paracellular pathway. Epithelial cells produce vectorial transcellular movements of solutes by virtue of an asymmetric distribution of transport proteins in the apical (luminal or mucosal) plasma membrane and in the basolateral (abluminal or nutrient) plasma membrane. The apical membrane is often the site of “gatekeeper” transport proteins that control the overall rate of transepithelial transport. The rate of transport is regulated by a variety of hormonal receptor and second-messenger signal pathways (e.g., β-adrenergic receptors via cyclic AMP [cAMP], purinergic receptors via extracellular ATP, intracellular Ca2+ levels; see Sect. 9.5).
The paracellular pathway is also important for mediating transepithelial transport and consists primarily of the tight junction barrier between cells. This barrier consists of combinations of intracellular and membrane proteins with widely varying functional properties provided by myriad expression patterns of junctional gene products and by cellular control of the junction proteins (Turner et al. 2014). Solutes cross the tight junction barrier driven by electrical and/or chemical concentration gradients, and the transport rates are regulated by the selective properties of the junction, which in turn are under cellular control.
Net transepithelial fluid transport results in fluid secretion (basolateral to apical) or absorption (apical to basolateral). The driving force for fluid movement results from net solute transport and the resulting local osmotic pressure differences generated, and the primary pathways for water are through plasma membrane proteins, especially the class known as aquaporins (AQPs).
Proteins that mediate transport across the apical and basolateral membranes of epithelia are of three broad types: primary active transporters (also referred to as “pumps”), transporters, and channels. The pumps consume metabolic energy, usually in the form of ATP, and transform that energy into creation of transmembrane solute gradients. The most pervasive of these is the Na+, K+-ATPase (a.k.a. the Na+ pump) that pushes 2 K+ ions into the cytosol and pulls 3 Na+ ions out of the cytosol for each ATP cleaved. The energy from the ATP is then stored as an inward-directed Na+ concentration gradient that can be utilized by secondary active transporters to move other solutes across the apical and/or basolateral plasma membranes. An example of such transporters includes Na+/H+ exchangers in which the inward movement of Na+ drives the obligatory movement of acid (H+) out of the cell. The accumulation of cytosolic K+ by the action of the Na+, K+-ATPase often establishes a negative electrical potential difference mediated by membrane K+-selective channels, and this negative voltage can then drive electrogenic solute transport. Examples include efflux of negative ions such as Cl− through Cl−-selective ion channels and Ca2+ movements via coupled electrogenic Na+/Ca2+ exchange. The overall constellation of apical and basolateral transport activity must, of course, be coordinated and balanced to avoid catastrophic accumulation or depletion of any solutes in the cytosol during transepithelial secretion or absorption.
9.2 Fluid Composition
There are several fluid compartments in the inner ear that have distinct compositions and communication pathways among them. Blood vessels are adjacent to the perilymphatic space (see Sect. 9.2.1) and to the intrastrial space (see Sect. 9.2.3). The restricted communication between blood and perilymph is referred to as the blood-perilymph (or blood-labyrinth) barrier (see Sect. 9.2.1). The luminal compartment contains endolymph that provides the environment of the sensory stereocilia and enables the auditory transduction process. Perilymph is the extracellular fluid that surrounds the cochlear duct epithelium (Fig. 9.1, blue areas) and endolymph is the luminal fluid within the cochlear duct (Fig. 9.1, pink areas). The epithelial cell domains that form the cochlear duct and their locations are also shown (Fig. 9.1).
Diagram of a cross section of the cochlear duct. The luminal compartment, scala media, contains endolymph and is bounded by epithelial cells whose apical membranes are designated by the bold orange line around the scala media. The abluminal fluid, perilymph, is within the scala tympani, scala vestibuli, extracellular spaces of the spiral ligament, and spiral limbus and is in contact with the basolateral membranes of all the epithelial cells except the marginal cells of the stria vascularis. TM tectorial membrane, IHC inner hair cell, OHC outer hair cells, DC deiters cells, OS outer sulcus cells, SC spindle-shaped cells. Adapted from Marcus (2012), with permission
9.2.1 Perilymph and the Blood-Perilymph Barrier
The perilymphatic space is contiguous throughout the inner ear. The cochlear cross section displays two perilymphatic compartments, which locally may have small differences in ion composition (e.g., K+ is slightly higher in the scala vestibuli than in the scala tympani), but these “two compartments” are openly joined at the cochlear apex (helicotrema) and the perilymph diffuses rapidly between the two scalae through the spiral ligament of the lateral wall (Salt et al. 1991a, b). At the cochlear base, the scala tympani is bounded by the round window, with a connection to the cerebral spinal fluid via the cochlear aqueduct (Salt et al. 2003), but the scala vestibuli is openly continuous with the perilymph bathing the vestibular organs. The complexity of the perilymph-filled spaces and the fact that the perilymph and cerebrospinal fluid are under pressure compared with the atmosphere complicate the investigation of perilymph homeostasis (Salt et al. 2003).
The composition of the perilymph is closely similar to that of blood plasma and to cerebrospinal fluid, but the perilymph is not merely an ultrafiltrate of plasma or an extension of CSF. The differences can be seen in Table 9.1, and several studies have demonstrated a tightly regulated separation of perilymph from blood. This separation is termed the blood-perilymph barrier (Juhn et al. 1982), in analogy to the better characterized blood-brain barrier. The exchange of solutes between blood and perilymph has been proposed to be primarily localized at the vessels of the spiral limbus (Firbas et al. 1981). Glucose is known to be the primary fuel for inner ear metabolism, but it does not pass freely from the blood but rather is transferred via a regulated transendothelial facilitated transport pathway (Ferrary et al. 1987). Putative facilitated diffusion of glucose via the GLUT-1 transporter into the intrastrial space (see Sect. 9.2.3) may also occur from capillaries in the stria vascularis (Ando et al. 2008). Exchange of Ca2+ across the blood-perilymph barrier occurs at a remarkably slow rate, although the molecular basis of restricted transport across the barrier has not been identified (Juhn et al. 1982).
The blood-perilymph barrier can be compromised by systemic inflammation induced by lipopolysaccharides, similar to the blood-brain barrier (Hirose et al. 2014). By contrast, other insults to auditory function such as impulse noise, hypertension, and the ototoxic drugs cisplatin and gentamycin have not been found to cause compromise of the blood-perilymph barrier (Laurell et al. 2000, 2008; Mosnier et al. 2001).
9.2.2 Endolymph
Endolymph is a highly unusual extracellular fluid with its high K+, low Na+, and low Ca2+ concentrations. This composition is essential for the transduction of sound and acceleration into hearing and balance, respectively. The sensory cells employ apical mechanotransduction channels with very large single-channel conductance and that are permeable to all three of the above-named cations (Effertz et al. 2015; Corey, Ashmore, and Ó Maoiléidigh, Chap. 4). Endolymphatic Ca2+ concentration needs to be highly regulated due to the relatively high permeability of the mechanotransduction channel to Ca2+ over Na+ but also due to the Ca2+ dependence of channel ion selectivity, rectification, and conductance (reviewed in Effertz et al. 2015). In addition, the low level of endolymphatic Ca2+ prevents sensory cell Ca2+ loading.
The special energetic advantage of the radically different cation compositions of endolymph and perilymph was recognized by Ruediger Thalmann over 45 years ago (Thalmann 1971) and elaborated by others (Patuzzi 2011a). The basic principle has 2 components. (1) The transduction process in hair cells requires very little energy when the transduction current is carried by K+ ions (see below in this section) and therefore needs no dedicated vascular supply (although the lower turn has a single spiral vessel in the basilar membrane). (2) The high concentration of endolymphatic K+ and the extra electrical driving force produced by the stria vascularis requires a massive energy supply provided by the high density of blood capillaries, as embodied in the name of the tissue. This separation of energy production and consumption sites allows the pulsations of the blood in the stria to be physically dampened by the spongy spiral ligament attached to the rigid outer bony shell of the cochlea so that we are not overwhelmed by the sound of our circulation instead of hearing the important external sounds.
The mechanotransduction current is carried passively by K+ from endolymph through the apical cation channels in the hair cells, driven by the electrical potential difference between the endolymph and the hair cell cytosol. The low endolymphatic Na+ maintains osmotic balance against K+ and prevents Na+ loading of the sensory cells through entry via the nonselective cation transduction channels. K+ exits the basolateral side of the sensory cells passively through a high density of K+-selective channels, driven by the membrane voltage that is slightly above electrochemical equilibrium at this membrane.
9.2.3 Intrastrial Fluid
The basolateral membrane of the strial marginal cells is in contact with the intrastrial fluid, which is a microenvironment that couples marginal cell function and intermediate cell function. This interdependence of cell functions is described in Sect. 9.4.2 on the endocochlear potential (EP).
The composition of the intrastrial fluid is maintained by the adjacent cells, but the extensive and intimate capillary network in the stria appears to serve for metabolic gas exchange but not for solutes such as K+ (although some drugs such as furosemide pass through easily). This view derives from the observations that vascular perfusion with artificial blood devoid of K+ or glucose has a long-delayed effect on the metabolically labile EP generated by the stria vascularis (see Sect. 9.4.2), whereas perilymphatic perfusion of K+-free or glucose-free solution causes an immediate decline in EP (Wada et al. 1979; Kambayashi et al. 1982).
This exchange barrier at the strial capillaries provides a means to control drug delivery to the ear by synthesizing drug analogs with high or low permeability through this barrier. Loop diuretics of the furosemide family penetrate this barrier with tremendous ease but exchange with perilymph comparatively slowly. Vascular perfusion of furosemide causes a nearly instantaneous loss of EP, whereas perilymphatic perfusion of furosemide has a delay of more than 6 min due to the extremely low permeability of the basal cell tight junctions, which restrict access of the drug to the site of action at the basolateral membrane of strial marginal cells (see Sect. 9.4.1).
9.3 Transport Epithelia in the Cochlea
There are a large number of cell types that border the cochlear duct. Each type makes specific contributions to the maintenance of endolymph composition and electrical potential, with each cell type able to secrete and/or absorb solutes through one or more transport pathways under different control mechanisms.
9.3.1 Stria Vascularis
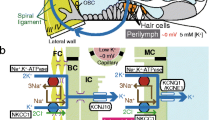
The stria vascularis is a complex epithelial structure on the cochlear lateral wall that (1) secretes K+ into endolymph and (2) generates the EP (Table 9.1). The high level of endolymphatic K+ provides the substrate for the transduction current through the sensory cells, and the EP provides a part of the driving force (in conjunction with the basolateral membrane voltage) for the flow of K+ that is modulated by sound. The cellular transport model by which the stria vascularis secretes potassium and generates the EP is shown in Fig. 9.2 and described in Sects. 9.4.1 and 9.4.2. Recall that all epithelial cells that produce a vectorial transport of substances do so by virtue of different membrane properties of their apical and basolateral membranes.
Diagram of a cell model of K+ transport from the perilymph by fibrocytes of the spiral ligament through the stria vascularis into the cochlear endolymph. The fibrocytes, basal cells, and intermediate cells form a syncytium through a dense network of gap junctions (yellow channel symbols), with K+ flow indicated (white arrows), although other small solutes also pass. Fibrocytes take up perilymphatic K+ via the Na+, K+-ATPase and Na+, K+, 2Cl− cotransporter; Cl− and Na+ recycle across that membrane back into perilymph, while K+ diffuses through gap junctions into the basal cells of stria vascularis and further into the intermediate cells. The high concentration of K+ in the intermediate cells creates a large voltage across its high density of KCNJ10 K+ channels (red channel symbols) in the membrane facing the intrastrial fluid space. Such a constellation of high cytosolic K+ concentration bounded by a highly K+-selective plasma membrane creates a negative resting membrane potential in “classical” examples of symmetrical cells such as nerve cell bodies. However, in this highly asymmetrical arrangement of cell membranes, the unusually low K+ concentration of the intrastrial space, coupled with the cytosol that is effectively clamped to “ground” (held near zero, the potential of perilymph) by the syncytium, the intermediate cells then push the intrastrial space voltage to +80 to +100 mV. The transepithelial voltage of the marginal cell layer is small, so that most of the intrastrial space voltage is observed in the scala media. The marginal cells remove K+ from the intrastrial space by the same transporter constellation as seen in the fibrocytes and secretes it into the endolymph through KCNQ1/KCNE1 K+ channels (red channel symbols) in the apical membrane. Adapted from Marcus, D. C. (2012). Acoustic transduction. In N. Sperelakis (Ed.), Cell Physiology Source Book: Essentials of Membrane Biophysics (pp. 649-668). San Diego, CA: Academic Press, with permission
The basal cell layer of the stria vascularis adjoins a bed of fibrocytes (spiral ligament) that is sponge-like and pervaded by perilymph. The basal cell layer forms a very tight barrier between the perilymph in the spiral ligament and the intrastrial fluid space. The basal cells form a syncytium via a high density of gap junctions with fibrocytes and the intermediate cells, which are responsible for generating the EP (see Sect. 9.4.2). Finally, a monolayer of strial marginal cells forms a barrier between the intrastrial fluid space and the endolymph. The marginal cells are joined to each other by tight junctions but have no gap junction connections among themselves. This barrier supports a large gradient in K+ concentration and electrical potential difference (Table 9.1; Fig. 9.2). The physiological significance of the functional independence of marginal cells is not known.
9.3.2 Reissner’s Membrane
Reissner’s membrane epithelium (Fig. 9.1) accounts for more boundary surface of the cochlear duct than any of the other epithelial cell domains. It contributes to the maintenance of endolymph composition by the absorption of Na+ mediated by epithelial Na+ channels in its apical membrane (see Sect. 9.4.5) and may provide acid/base control of endolymph via Ca2+/2H+ exchange mediated by a putative apical Ca2+-ATPase (see Sect. 9.4.7). The basolateral membrane expresses a Cl− channel (SLC26A7) that is associated with pH control in other epithelial cells (Kim et al. 2014). The apical membrane also expresses P2X2-nonselective cation channels that are activated by luminal nucleotides, which implies that Reissner’s membrane can contribute to absorption of Na+ and K+ under purinergic control (Lee and Marcus 2008; Morton-Jones et al. 2015; see Sect. 9.5.4.2).
9.3.3 Outer Sulcus, Spiral Prominence, and Strial Spindle Cells
These three epithelial cell types occur on the lateral wall and are contiguously adjacent to each other (Fig. 9.1). All of these cells express the Cl−/HCO3 − exchanger SLC26A4 and thereby contribute to the alkalinization of the endolymph (Wangemann et al. 2007). The outer sulcus epithelial cells have also been shown to absorb both K+ and Na+ via apical cation nonselective channels of two types, one of which is under purinergic control (Kim and Marcus 2011). It was also recently found that outer sulcus cells in the upper cochlear turns are involved with hormonal control of endolymph volume mediated by AQP translocation to the plasma membrane in response to hyperosmotic challenge or muscarinic agonist (see Sect. 9.5.2).
9.3.4 Spiral Limbus Epithelial Cells
The spiral limbus (Fig. 9.1) supports epithelial cells on its upper and lateral (inner sulcus) surfaces. Little is known about their respective transport functions.
9.4 Homeostasis
The cellular basis of ion and water transport in the cochlea and the ways in which these processes are regulated and coordinated among the many types of cells involved are presented here. The integration of these functions and their role in hearing have also been analyzed and discussed from molecular, pharmacological, and engineering perspectives (Patuzzi 2011a, b; Marcus 2012).
9.4.1 K+ Secretion by Strial Marginal Cell Epithelium
Active K+ secretion into the cochlear duct was first unambiguously demonstrated by radioactive K+ fluxes from either the perilymph or blood vessels (Konishi et al. 1978; Sterkers et al. 1982), even though earlier indirect evidence supported the proposition. These K+ fluxes were inhibited by drugs well characterized to block specific transport processes and by anoxia. The stria was taken to be the tissue source of secretion because the flux was nearly the same when the radiotracer was placed in either of the cochlear perilymphatic scalae; possible contributions by the spiral limbus were disregarded.
Another study demonstrated more directly that the stria vascularis secretes K+ (Wangemann et al. 1995a). A K+-selective self-referencing probe was positioned adjacent to the apical surface of the marginal cells, where an outward-directed K+ flux was detected and was inhibited by basolateral bumetanide (specific inhibitor of the Na+-K+-2Cl− cotransporter [NKCC]). In concert with additional pharmacological and electrophysiological experiments, a cell model that explains the underlying transport pathways at the molecular level was developed (Fig. 9.2). Briefly, K+ is taken up from the intrastrial fluid by the Na+, K+-ATPase (Na+ pump) and NKCC1, the former being a primary-active process that consumes cellular energy supplies and the latter a secondary-active process that utilizes the energy contained in the Na+ gradient established by the Na+-pump. This combined uptake mechanism, in concert with the small volume of the intrastrial space and the barrier to K+ between this space and the strial capillaries, yields the surprising result that the intrastrial space K+ concentration is reduced markedly below that in blood plasma, to about 1.2 mM (Takeuchi et al. 2000), which has important ramifications for normal and pathological function of the stria (see Sect. 9.4.2). The Na+ and Cl− that enter the cell at the basolateral membrane via NKCC1 are removed across the same membrane via the Na+ pump and Cl− channels, while the K+ accumulated in the cytosol is secreted across the apical membrane via K+-selective channels (Fig. 9.2).
The apical K+ channels are composed of α- and β-channel subunits (KCNQ1 and KCNE1, respectively), which are voltage-activated channels. They also occur in human cardiac myocytes, and a genetic disease due to mutations of this channel, Jervell and Lange-Nielsen syndrome, displays dysfunction of the heart and hearing (Shen et al. 1997; Marcus and Wangemann 2010). The critical contributions of the apical K+ channel and basolateral transport proteins of the model are supported by observations of collapsed scala media in gene knockout mice that lack either of the subunits of the channels or lack the cotransporter (Marcus 2012).
9.4.2 Production of Endocochlear Potential by Strial Intermediate and Basal Cells
The EP is functionally a transepithelial voltage that occurs across the monolayer of epithelial cells of the cochlear duct, including the sensory cells. However, it also occurs across the multilayered stria vascularis and originates predominantly from the basal cell-intermediate cell syncytial layer (see Sect. 9.3.1), in contrast to K+ secretion carried out by the marginal cell layer (see Sect. 9.4.1). Despite this separation of function, the two cell layers are operationally coupled via the K+ concentration of the intrastrial space.
The basal cell-intermediate cell layer maintains intracellular K+ at a high level by the uptake from perilymph by fibrocytes in the spiral ligament and diffusion via gap junctions into basal cells and intermediate cells (Kikuchi et al. 2000; Fig. 9.2). These cells generate a large potential difference between the intrastrial space and the spiral ligament (perilymph) fluid by virtue of its high expression of KCNJ10 K+ channels (also known as Kir4.1) in the intermediate cell membrane. The barrier between the intrastrial space and perilymph of the spiral ligament is formed by extremely tight junctions composed of claudin-11 proteins between the basal cells. The essential nature of these claudins was demonstrated by the collapse of the EP in claudin-11-null mice (Gow et al. 2004). The importance of the KCNJ10 channel to EP generation is supported by the observation that KCNJ10-knockout mice have no EP, but they maintain an elevated endolymphatic K+ concentration (Marcus et al. 2002). The basal and intermediate cells are thought to have a strongly depolarized membrane voltage with respect to the perilymph and a high K+ concentration (Fig. 9.2). This combination would create a canonical highly polarized intermediate cell membrane, made larger than in other K+-conductive cells due to the unusually low K+ concentration of the intrastrial space (see Sect. 9.4.1). This layer of cells thereby generates an intrastrial space voltage of 90 mV or more. Due to clamping of the basal/intermediate cell membrane voltage near zero, this potential difference of the intrastrial space is positive with respect to perilymph. The marginal cells contribute very little potential to the transepithelial difference, just as the physiologically analogous vestibular dark cells contribute little to the endolymphatic potential in the utricle, common crus, and ampullae. The vestibular dark cell epithelium is a K+-secretory monolayer without the intermediate cell/basal cell layer.
Although penetration of the stria with sharp double-barrel microelectrodes unavoidably results in some cellular damage, the profile of the potential difference with respect to the perilymph and the K+ concentration is consistent with the above view (Salt et al. 1987; Fig. 9.3). In the spiral ligament perilymph, the voltage is defined as zero and the K+ concentration is low. The electrode then enters a region where the K+ concentration rises but the voltage remains low, which is taken to indicate entry into the basal and intermediate cells. Further advance of the electrode into the stria leads to the observation of suddenly low K+ concentration that is accompanied by a high voltage, which is taken to indicate entry into the intrastrial space. Finally, the electrode traverses a barrier into a stable region of high K+ concentration and high voltage, which is taken to be traversal across the marginal cell layer into cochlear endolymph.
Recording of profiles for voltage (orange line) and K+ concentration ([K]; blue line) during penetration of the stria vascularis (SV). A region was found where the voltage with respect to the perilymph was highly positive but the K+ concentration was low; this region was interpreted to be the intrastrial space. SL spiral ligament, bc basal cell, ic intermediate cell, mc marginal cell, el penetrating electrode advanced from left to right. x-Axis, time/distance of electrode advancement. Adapted from Marcus (2012), with permission
The coupling of function of the two strial layers referred to above is illustrated by observations of the rapid decline in the EP during inhibition of marginal cell K+ secretion by pharmacological or metabolic block of basolateral K+ uptake (ouabain, bumetanide, anoxia) from the intrastrial space. The cessation of K+ uptake from the intrastrial space would lead to a rapid rise in K+ concentration due to the continued exit from intermediate cells through the KCNJ10 channels, leading to a rapid depolarization across these channels and the resulting collapse of the EP (Marcus and Wangemann 2010).
9.4.3 K+ Cycling
K+ is the main charge carrier that mediates the quiescent and stimulus-activated currents from the endolymph through the apical mechanosensitive ion channels into the cochlear and vestibular sensory cells. This current flow depolarizes the sensory cells, which leads to contraction of the motor protein prestin (SLC26A5) in cochlear outer hair cells (the substrate of stimulus amplification) and to Ca2+ influx into cochlear inner hair cells and vestibular hair cells (the signal for vesicular neurotransmitter release and activation of the sensory neurons). This K+ current into the sensory cells is balanced by an efflux of K+ via basolateral K+ channels into interstitial spaces that are continuous with the perilymph (Marcus and Wangemann 2010).
The molecular identities of the major K+ channels in hair cells that mediate K+ efflux have been established. Most prominently, these include the voltage-gated K+ channel KCNQ4 and the Ca2+-activated K+ channel KCNMA1 (Robbins 2001; Sakai et al. 2011). Several splice variants of KCNQ4 are expressed in the inner ear and mutations of this channel are associated with hearing loss (Gao et al. 2013). The K+ channel KCNQ4 associates with the β-subunit KCNE1 and possibly with other KCNE subunits that are expressed in hair cells (Strutz-Seebohm et al. 2006). K+ released from the sensory hair cells is likely at least partially recycled by uptake into spiral ligament fibrocytes, transferred into the stria vascularis, and secreted back into the endolymph.
Two pathways have been proposed to shuttle K+ from the base of the cochlear sensory cells toward the fibrocytes of the lateral wall, and both are likely to participate. The first is simple diffusion through the perilymph, and its involvement is supported by the observation of voltage gradients due to electric currents in the scala tympani (Zidanic and Brownell 1990). The second is diffusion through the gap junction-coupled epithelial cells located between the sensory cells and the fibrocytes of the lateral wall (Spicer and Schulte 1996). This intraepithelial pathway is posited to involve uptake of K+ into Deiters cells, dispersion among the cells along the basilar membrane and outer sulcus via gap junctions, and release of K+ from outer sulcus root cells into the interstitial space of the spiral ligament (Kikuchi et al. 2000). A comparable theory has also been advanced to account for diffusion from inner hair cells to the spiral limbus and release into the scala vestibuli, followed by diffusion through the perilymph to the suprastrial spiral ligament and back to the stria vascularis (Kikuchi et al. 2000). Deletion of expression of KCNK5 K+ channels in developmentally mature mice leads to profound deafness, consistent with an essential role in K+ cycling. These channels are normally expressed in Böttcher, Claudius, and outer sulcus root cells (Cazals et al. 2015).
The final step consists of K+ uptake by specialized fibrocytes in the spiral ligament and diffusional movement via the gap junctions GJB2 (Cx26) and GJB6 (Cx30) into the strial basal and intermediate cells, followed by supply to marginal cells and the endolymphatic space, as described (Sect. 9.4.1). Fibrocyte types II, IV, and V express Na+, K+-ATPase, the NKCC1 SLC12A2 and the Cl− channels CLCNKA and CLCNKB. The resemblance of this constellation of transporters to the basolateral membrane of strial marginal cells (Sect. 9.4.1) suggests that fibrocytes take up K+ from the perilymph, although functional data from fibrocytes are lacking (Marcus and Wangemann 2010). Despite the attractiveness of this hypothesis, a number of observations are not consistent with it, such as a known human deafness genotype that is associated with a mutation in a cochlear gap junction connexin that nonetheless is as permeable to K+ as the normal connexin, and are reviewed elsewhere (Mammano 2013).
9.4.4 K+ Buffering
K+ efflux from the basolateral membrane of sensory cells during acoustic stimulation was demonstrated to increase the adjacent perilymphatic K+ concentration (Johnstone et al. 1989). Elevated K+ in this region would be expected to depolarize the sensory cells, supporting cells, and nerve terminals, leading to hearing loss. It is thought that these increases in K+ are limited in magnitude by local K+-buffering mechanisms in neighboring supporting cells such as Deiters cells.
Deiters cells express basolateral K+ channels (Nenov et al. 1998), the K+, Cl− cotransporter isoform KCC4 (Boettger et al. 2002) and gap junctions that connect to neighboring supporting cells of the epithelial gap junction network (see Sect. 9.4.3). The importance of K+, Cl− cotransporters in the inner ear is underscored by the observation that KCC4-knockout mice are deaf (Boettger et al. 2002). These observations support the speculation that Deiters cells buffer extracellular K+ through the uptake of K+ at the base of the hair cells and expel it across a part of the membrane facing away from the sensory cell and/or move the K+ by diffusion from the Deiters cell to neighboring supporting cells via gap junctions. The former mechanism for buffering of extracellular K+ by Müller glial cells has been proposed to explain a similar phenomenon in the retina (Kofuji et al. 2002). Whether K+, Cl− cotransporters participate in K+ buffering as mediators of cellular K+ uptake or efflux awaits experimental determination (Marcus and Wangemann 2009).
9.4.5 Na+ Absorption
The cochlea regulates endolymphatic Na+ concentrations to low levels to maintain sensory cell function. The cation nonselective permeability of the mechanotransduction channel would otherwise allow elevated Na+ to flood the cytosol of the sensory cells, leading to cellular dysfunction and hearing loss (Shi et al. 2005). Pathologically elevated endolymphatic Na+ occurs during transient ischemic anoxia, which is a putative etiology of sudden hearing loss (Sellick and Johnstone 1972). Mutations underlying nonsyndromic autosomal recessive deafness (DFNA8/10) due to dysfunction of the Na+ transport regulatory gene TMPRSS3 is also expected to disrupt Na+ homeostasis (Guipponi et al. 2002). Furthermore, altered endolymphatic Na+ concentration has been proposed as a mechanism of premenstrual exacerbation of Ménière’s disease (Andrews and Honrubia 2010).
9.4.5.1 Na+ Absorption Pathways
Normal Na+ absorptive flux in the cochlea is only about 1% of the normal K+ secretory flux (Konishi et al. 1978), suggesting a lower requirement for metabolic support of Na+ absorption than for K+ secretion. This supposition is supported by the dense blood capillary network in the K+-secreting stria vascularis as compared with the avascular Reissner’s membrane and single-vessel metabolic supply of the outer sulcus and Claudius cells, which are all involved in Na+ absorption (Kim and Marcus 2011).
The cochlea absorbs Na+ from the endolymph via three types of apical ion channels expressed in several cell types. These cells all create an inward driving force for cations across the apical membrane by virtue of Na+, K+-ATPase in parallel with K+ channels in the basolateral membrane (Fig. 9.4b, c), which create an inward electrochemical driving force for Na+ (and K+) across the apical membrane. Reissner’s membrane and Claudius cells utilize the epithelial Na+ channel (ENaC) to mediate transport across the apical membrane from the endolymph into the cytosol (Yoo et al. 2012; Kim and Marcus 2011; Fig. 9.4c). ENaC transport is regulated by several signal pathways, but one prominent mechanism is the genomic control of channel numbers in the membrane via glucocorticoid signaling (see Sect. 9.5.5). Both outer sulcus cells and sensory hair cells mediate apical cation entry via nonselective cation channels (Kim and Marcus 2011; Fig. 9.4b). The outer sulcus absorption of K+ is referred to as parasensory K+ absorption (see Sect. 9.4.6). All of these cell types mediate the cellular entry of both Na+ and K+ via ionotropic purinergic receptors (see Sect. 9.5.4), which are activated under conditions such as noise (Kim and Marcus 2011).
Purinergic signaling in the cochlear epithelium. a Stria vascularis marginal cells (SMC). Activation of P2Y4 receptors in the apical membrane reduce secretory K+ flux via a G-protein (G) signal cascade. There are additional purinergic receptors on the basolateral side (not shown; Liu et al. 1995). b Outer sulcus cells (OSC). Activation of apical P2X2 receptors open the associated nonselective cation channels. Also shown are the apical purinergic-independent NSC channels; this model also applies to hair cells and the apical transduction channels. c Reissner’s membrane epithelial cells (RM). Activation of P2Y4 receptors (membrane location unknown) reduces epithelial Na+ channel (ENaC)-mediated Na+ absorption via a G signal cascade, and activation of apical P2X2 receptors open the associated nonselective cation channels. White arrows, direction of flow. Adapted from Marcus (2012), with permission
9.4.5.2 Na+ Secretion Pathways
The entry of Na+ into the endolymph has long been tacitly assumed to simply be via an undefined “leak” pathway. Although this view largely remains, it was found recently that an active H+ flux can emanate from the apical surface of stria vascularis under energetically favorable conditions and that a part of this flux is mediated by a Na+/H+-exchanger (Miyazaki et al. 2016). Na+/H+-exchangers are secondary active transporters that can operate in either direction depending on the net chemical driving force. The apical Na+/H+-exchanger in the stria vascularis likely operates close to equilibrium under normal steady-state conditions but can either secrete or absorb Na+ when away from equilibrium. In secretion mode, it may provide at least a part of the Na+ “leak” into endolymph.
9.4.6 Parasensory K+ Absorption
Auditory and vestibular transduction depend on the balance of secretion and absorption of cations by the epithelial cells bounding the endolymphatic spaces. Potassium is secreted by strial marginal cells in the cochlea (see Sect. 9.4.1), and there is both a quiescent and a stimulus-induced efflux of K+ from the endolymphatic space through the hair cells. Variations in the intensity and duration of acoustic stimuli would cause fluctuations in endolymph cation composition if there were no coordinated regulation of the rates of secretion and absorption. Secretion is under the control of several extracellular hormones and factors, including purinergic agonists (see Sect. 9.5.4). It has been shown that the outer sulcus epithelial cells provide a parasensory pathway in the cochlea that sustains an apical-to-basal transepithelial cation current and that this current is regulated by purinergic agonists via P2X2 purinergic receptors (Lee et al. 2001; Kim and Marcus 2011). P2X2 receptors are ligand-gated nonselective cation channels; therefore, the Na+- and K+-absorptive currents through the outer sulcus epithelial cells have both constitutively active and regulated components. The K+-mediated transduction current in hair cells is also accompanied by a Na+ flux because the transduction channels are nonselective cation channels (Fig. 9.4).
9.4.7 Ca2+ Balance
Endolymphatic Ca2+ concentration is unusually low for an extracellular fluid (see Table 9.1) and its level is apparently maintained at its set point by a balanced secretion-absorption system. Ca2+ is secreted into the endolymph by the plasma membrane Ca2+-ATPase PMCA2, which is located in the stereocilia of hair cells and also in Reissner’s membrane (Furuta et al. 1998; Chen et al. 2011). Function of this protein was supported by the demonstration of PMCA activity on the apical membranes of Reissner’s membrane (Yoshihara and Igarashi 1987) and stereocilia of hair cells (Yamoah et al. 1998). Mutation or deletion of the gene for PMCA2 leads to a reduced level of endolymphatic Ca2+ (Wood et al. 2004) and to deafness (Kozel et al. 1998).
A Ca2+-absorptive system is expressed in the cochlea, and its gatekeeper apical channels, TRPV5 and TRPV6, are located in the inner and outer sulcus cells and have also been observed in the marginal cells (Yamauchi et al. 2010). The basic cell model components for Ca2+ absorption consist of the TRPV5 and TRPV6 epithelial Ca2+ channels in the apical membrane, cytosolic Ca2+ buffer, and basolateral Na+/Ca2+ exchanger and Ca2+-ATPase (Hoenderop et al. 2005). Ca2+ enters the cell from endolymph via the apical Ca2+ channels and is bound by the Ca2+ buffers. The bound Ca2+ diffuses across the cell to the basolateral membrane where the Ca2+ is released from the buffer and removed from the cell across the basolateral membrane by the combined action of the Ca2+-ATPase and Na+/Ca2+ exchanger. Surprisingly, Ca2+-ATPase activity was observed at the apical membrane of the outer sulcus cells as well as at the lateral membrane (Yoshihara and Igarashi 1987), although it is conceivable that the apical staining was in the subapical vesicles. The TRPV5 and TRPV6 transport system in the semicircular canal epithelium was found to be strongly inhibited by acidic shifts in the luminal pH (Nakaya et al. 2007) and stimulated by binding of vitamin D to its nuclear receptor (Yamauchi et al. 2005). Expression of all of these genes was also observed in the cochlea (Yamauchi et al. 2005, 2010).
9.4.8 Acid-Base Balance
Endolymphatic acid-base balance is also a secretion-absorption system. H+ secretion from the stria vascularis has been observed with self-referencing pH electrodes (Miyazaki et al. 2016). In addition, HCO3 − secretion occurs via the Cl−/HCO3 − exchanger pendrin (SLC26A4), which is expressed in the apical membrane of strial spindle cells, the spiral prominence, and outer sulcus epithelial cells (Wangemann 2013). Pendrin, and likely other HCO3 − transporters, maintain endolymph alkaline with respect to perilymph (Table 9.1). As expected, adult Slc26a4-mutant mice have acidic endolymphatic pH (Wangemann et al. 2007). Further studies with conditional expression of Slc26a4 point to a more complex acid-base regulatory system (Choi et al. 2011) that is under continuing investigation.
9.4.9 Water Movement: Aquaporins
The volume flow of water across biological membranes is always driven by osmotic differences across membranes and thereby follows active and passive movements of solutes, including ions. In other words, there are no known active “water pump” transporters. Diffusive water flow across biological membranes is mediated by a number of different proteins, but most attention has been paid to the 13 mammalian isoforms of AQP (reviewed in Hosoi 2016). Some AQPs are located in epithelial cells but others are in mesenchymal, vascular, or neural cells. Much interest has focused on epithelial cells in the cochlea (including stria vascularis and outer sulcus) and on the epithelium of the endolymphatic sac, an organ devoid of sensory cells that is involved in fluid absorption from endolymph. The expression and purported functions of AQPs in the inner ear was recently reviewed (Eckhard et al. 2012).
Movements of water across epithelia require a pathway in both the apical and basolateral membranes. These pathways may be mediated by different constellations of AQPs, some of which are constitutively expressed and others which are under hormonal control of translocation between cytosol and plasma membrane. AQP isoforms detected in inner ear tissues include AQP1, AQP4, and AQP5 in the cochlea (Li and Verkman 2001) and AQP2 in the endolymphatic sac (Maekawa et al. 2010). Two prominent systems of regulated AQP-mediated water movement in the inner ear are mediated by AQP5 under muscarinic control (see Sects. 9.3.3 and 9.5.2) and by AQP2 under vasopressin control (see Sect. 9.5.3).
In addition to hormonal control of some AQPs, the isoforms are also characterized by their permeabilities to water and glycerol. Glycerol-permeable AQPs (aquaglyceroporins) include AQP3 and AQP8; others carry water but are impermeable to glycerol (AQP1, AQP2, AQP4, AQP5; Verkman and Mitra 2000). Another distinguishing characteristic is their sensitivity to inhibition by mercury compounds (mercurial-sensitive AQPs include AQP1, AQP2, and AQP3 but not AQP4; Verkman and Mitra 2000). Dependence of hearing on the expression of AQPs was tested in mice with individually knocked out AQP1, AQP3, AQP4, and AQP5. Auditory brainstem response to click stimulus was unaffected by the knockout of AQP1, AQP3, or AQP5. A significant hearing loss was observed in AQP4-null mice (Li and Verkman 2001).
9.5 Hormonal Regulation
In a changing environment, all cells respond to varying levels of systemic and local hormones, osmotic strength, and extracellular K+ concentration. These extracellular influences modulate cell function via interaction with effector molecules in the plasma membrane (e.g., ionotropic receptors) or via intracellular signals (e.g., via metabotropic receptors and second-messenger signal molecules such as intracellular Ca2+ concentration, pH, and organic molecule signal pathways). These signal pathways modulate the rates of K+ secretion, Na+ and Ca2+ absorption, and water transport in the cochlea.
Homeostatic mechanisms in the inner ear occur in the absence of innervation of the stria vascularis and of the other extrasensory cells in the cochlea. Nonetheless, marginal cells contain receptors coupled to ion transport for several local or systemic hormones, including catecholamines, ATP, muscarinic agonists, and adrenocorticosteroid hormones. The sources of agonists for these receptors are systemic and/or paracrine rather than neural.
9.5.1 β-Adrenergic Receptors
Stimulation of β-adrenergic receptors in the stria vascularis leads to an increase in K+ secretion, as represented by the transepithelial short-circuit current. The involvement of these receptors was established by determination of the potency order of specific antagonists and by demonstration of the presence of transcripts in cochlear tissue (Wangemann 2002). β-Adrenergic receptors are commonly coupled via G proteins to adenylate cyclase. Indeed, in addition to receptor stimulation, an increase in strial cytosolic cAMP via direct stimulation of adenylate cyclase, by perfusion of a membrane-permeable cAMP analog, or by inhibition of phosphodiesterases that catalyze the breakdown of cAMP all lead to an increase of short-circuit current.
9.5.2 Muscarinic Receptors
Cholinergic signaling often occurs in functional units that are stimulated by activation of β-adrenergic receptors and inhibited by cholinergic receptors. Consistent with that constellation, activation of β-adrenergic receptors stimulates K+ secretion, while activation of the (cholinergic) muscarinic receptors M3 and/or M4 on the basolateral membrane of strial marginal cells inhibit K+ secretion. The source of the muscarinic agonists is not clear; however, they are likely arriving by the circulation from remote organs or from local paracrine release (Wangemann 2002). It is conceivable that acetylcholine levels would increase in the perilymph under conditions that overwhelm acetylcholinesterase activity in the synaptic clefts beneath the cochlear sensory cells and be carried by local blood vessels to the strial marginal cells (Hoya et al. 2001; Wangemann 2002).
Muscarinic receptors were recently found to also regulate water movements through AQP water channels expressed in outer sulcus cells of the upper cochlear turns (see Sect. 9.3.3). The isoform AQP4 is constitutively expressed in the basolateral membrane, and the isoform AQP5 is translocated into the apical plasma membrane in response to muscarinic agonists (Eckhard et al. 2015). The muscarinic control of AQP in outer sulcus cells is therefore poised to control transepithelial water flux in the cochlea, suggesting that one etiology of Ménière’s disease could be dysfunction of this system. It has also been posited that the M3 receptors in the outer sulcus cells are constitutively activated in the absence of agonist, as found in an expression system study (Eckhard et al. 2012). This scenario would fit with findings of parasensory cation absorption by these cells (Kim and Marcus 2011; see Sect. 9.4.6); AQP5 and AQP4 in the apical and basolateral membranes could provide a pathway for reabsorptive water flux that accompanies the cation flux.
9.5.3 Vasopressin Control of Aquaporin 2
Transepithelial water fluxes across kidney tubules and across some other epithelia occur through AQP water channels (see Sect. 9.4.9) and the primary mode of regulation is via translocation of AQP2 between the cytosol and plasma membrane in response to vasopressin V2-receptor activation and the resultant downstream cAMP-protein kinase A (PKA) signaling (Brown et al. 2012). A long-standing interest in the possible role of V2/AQP2 in cochlear volume homeostasis has been pursued by many investigators who have searched for evidence of involvement of this system in endolymph homeostasis and in the formation of cochlear hydrops in Ménière’s disease.
The literature has been difficult to evaluate and integrate due to (1) uneven quality and resolution of expression studies, with widely conflicting results; (2) “expression” often defined only at the transcript level; (3) comparison of systemic versus local administration of receptor agonists and antagonists; and (4) the apparently blinding drive to support and adopt the kidney model. There have been varying degrees of stringency applied to the evaluation of antibody staining (the severity of the problem has been described; Delpire 2015), the selection of target tissues to stain, and often the exclusion of functional measures of hormonal signaling. Some of these conflicts in the literature have been reviewed by others (Eckhard et al. 2012; Takumida et al. 2012). Investigations that utilize systemically administered agents may result in effects either directly at the intended inner ear cellular targets or indirectly on the ear via alterations in the levels of hormones and other substances in the blood that then subsequently act in the inner ear, thereby confounding interpretations of drug actions. Nonetheless, we present here some of the most salient studies.
9.5.3.1 Kidney
In the kidney, key aspects of the V2/AQP2 system include binding of the agonist vasopressin (or antidiuretic hormone [ADH]) to the V2 receptor, followed by activation of the Gs protein, stimulation of adenylyl cyclase, increase in cytosolic cAMP, activation of PKA, and translocation and insertion of AQP2 into the apical cell membrane. Vasopressin thereby increases apical membrane water permeability through elevated numbers of AQP2 water channels in that membrane, which transports water in concert with constitutively expressed AQP3 and/or AQP4 in the basolateral membrane (Brown et al. 2012). This vasopressin-regulated system is a highly tempting story to adopt for the inner ear and could provide a basis for understanding the endolymphatic hydrops that often (but not always) accompanies the defining symptoms of Ménière’s disease and would thereby be a possible drug target for treatment of Ménière’s disease.
9.5.3.2 Cochlea
Several reports support the notion of this system contributing to the homeostasis of the cochlea at a local site. V2 receptors and AQP2 protein expression were both observed by fluorescence and immuno-gold immunolocalization in the basal cells of the stria vascularis; AQP2 was at the perilymph/spiral ligament side of the cells, while the V2 receptor and AQP3 were expressed at the intrastrial membrane (Nishioka et al. 2010). The two faces of the basal cells are separated by a barrier of tight junctions composed of claudin-11 (see Sect. 9.4.2). Expression of AQP2 in the basal cells was also shown by another group (Takumida et al. 2012) but was not detected in another study (Li and Verkman 2001).
9.5.3.3 Endolymphatic Sac
Despite the incomplete and conflicting studies referred to in Sect. 9.5.3, some investigations of the V2/AQP2 system in the endolymphatic sac are more compelling than those in the cochlea. One conclusion from this body of work is that (1) the V2 receptor is located primarily in the basolateral membrane of the ribosome-rich cells in the endolymphatic sac; (2) in the absence of V2-receptor activation, the AQP2 water channels are localized primarily in intracellular vesicles and possibly in the apical membrane; and (3) the density of AQP2 channels in the basolateral membrane is increased by activation of the V2 receptors and subsequent translocation of the AQP2-containing vesicles. The most attractive hypothesis is that increased levels of vasopressin reduce transepithelial water permeability by removing gatekeeper AQP2 from the apical membrane of ribosomal-rich cells in the endolymphatic sac, which reduces absorption of fluid from the endolymph, leading to endolymphatic hydrops in the face of continued secretion in the cochlea.
This hypothesis is supported at several levels by the following observations.
-
Importantly, two lines of evidence are consistent with the presence of AQP2 channels in the apical membrane that undergo endocytosis and translocation on activation of the V2 receptors (Kumagami et al. 1998; Maekawa et al. 2010). An acute primary culture of the rat endolymphatic sac maintained two types of cells with the appearance of ribosomal-rich and mitochondria-rich cells of the native sac. Functional V2 receptors were shown to inhibit endocytosis at the apical membrane in the cultured cells and to cause cochlear hydrops in guinea pigs chronically exposed to a V2 agonist (Kumagami et al. 1998).
-
The correlation between Ménière’s disease and elevated plasma vasopressin levels has been established by some teams (Eckhard et al. 2012), but see exception below in this section.
-
Clinically relevant levels of vasopressin during chronic (1-week) infusion in guinea pigs were found to create significant cochlear hydrops compared with the control animals that received only saline infusions (Takeda et al. 2000).
If this hypothesis is correct, it implies a striking difference in the signal pathways in the kidney and endolymphatic sac. Activation of V2 receptors in the kidney lead to insertion of AQP2 in the apical membrane and consequent stimulation of transepithelial water flux, while in the endolymphatic sac, it leads to endocytosis of apical AQP2 and the consequent inhibition of transepithelial water flux. Problematic for this hypothesis is that (1) the obligatory correspondence of endolymphatic hydrops to Ménière’s disease has been disputed (Merchant et al. 2005; Foster and Breeze 2013) and (2) some research teams found no elevation in plasma vasopressin in Ménière’s patients (Eckhard et al. 2012).
9.5.4 Purinergic Signaling
Purinergic signaling in the cochlea is well-known, and evidence has been found to support a complete autocrine and/or paracrine signaling cycle (Lee and Marcus 2008). This includes observation of a local source of agonist, expression of purinergic receptors in the plasma membrane of inner ear epithelial cells, and termination of signaling. The signal pathway is apparently stimulated by stressful conditions such as noise. The level of endolymphatic ATP was observed to increase significantly after a short exposure to noise (Muñoz et al. 2001). Increases in endolymphatic ATP were correlated with decreases in the EP and in the input resistance of the cochlear duct, consistent with a protective effect on the sensory cells of purinergic signaling during noise exposure (Thorne et al. 2004).
9.5.4.1 Purinergic Agonist
The sources of purinergic agonist have been investigated, and it has been proposed that ATP is released from strial marginal cells into the endolymph via subapical membrane vesicles (Muñoz et al. 2001) and from nonsensory supporting cells in the organ of Corti mediated by connexin hemichannels (Anselmi et al. 2008). The involvement of pannexin-1 hemichannels in ATP release has also been proposed (Chen et al. 2015). Purinergic signaling in the cochlea is terminated through the breakdown of agonist catalyzed by ectonucleotidases (Vlajkovic et al. 2004). Effective concentrations of extracellular ATP on purinergic receptors are orders of magnitude less than those of intracellular ATP in metabolic reactions.
9.5.4.2 Purinergic Receptors
Electrophysiological and pharmacological investigations have demonstrated purinergic signaling in several cell types of the inner ear. Both ionotropic (P2X receptor) and metabotropic (P2Y receptor) types are expressed and control specific physiological processes.
Reissner’s membrane, outer sulcus cells, and vestibular transitional epithelial cells express ionotropic P2X2 receptors in the apical membrane (Lee et al. 2001; Housley et al. 2013; Fig. 9.4b, c) that likely mediate a protective response to noise. P2X receptors are agonist-gated nonselective cation channels in the plasma membrane. As such, activation opens conductive pathways in the epithelial barrier and leads to a gated parasensory shunting of K+ currents away from the sensory cells in overstimulated conditions. Inner and outer hair cells in the cochlea also express ionotropic P2X2 receptors in the apical membrane (Housley et al. 2013) (Fig. 9.4b) that likely mediate a protective response to noise by shunting the transduction channel in the same cell membrane.
Marginal cells in the stria vascularis secrete K+ under the control of apical P2Y4 receptors (Fig. 9.4a). Activation of these receptors reduces K+ secretion and the EP primarily via the diacylglycerol (DAG)-protein kinase C (PKC) branch of a G protein signal pathway (Lee and Marcus 2008), thereby contributing to the protective response to noise through reduction of the driving force for auditory transduction. Claudius cells utilize P2Y receptors to control transepithelial current, although the membrane location and electrogenic transport pathway coupled to the receptors are not known (Yoo et al. 2012). Activation of basolateral P2Y2 receptors in semicircular canal duct cells produced complex changes in transepithelial current and resistance (Pondugula et al. 2010). No electrical response to the apical purinergic agonists ATP or UTP was observed in this epithelium.
It was also reported that activation of P2Y2 and/or P2Y4 receptors, expressed on the apical membrane of Hensen, Böttcher, and Claudius cells of developing rats, by nanomolar levels of ATP initiates a complex pattern of Ca2+ waves through the coupled epithelial layer in the developing cochlea (Mammano 2013). Purinergic signaling thus plays a significant role in hearing through the regulation of endolymphatic ion composition and transduction currents (Housley et al. 2013).
9.5.5 Steroid Hormones
Steroid regulation of ion transport is exemplified by Reissner’s membrane in the cochlea and by both saccular and semicircular canal duct epithelia in the vestibular system. These epithelia absorb Na+ via highly Na+-selective apical channels (ENaC) that are upregulated in expression and function specifically by glucocorticoids and not by mineralocorticoids (Kim and Marcus 2011). The signal pathway is illustrated in Fig. 9.5.
Schematic diagram of glucocorticoid-regulated Na+ absorption in mouse Reissner’s membrane and rat semicircular canal duct epithelial cells. Inactive forms of glucocorticoid (GC) are activated by 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1), which then increases ENaC expression via the glucocorticoid receptor (GR)-serum glucocorticoid-regulated kinase 1 (SGK1)-neural precursor cell-expressed developmentally downregulated 4-2 (Nedd4-2) pathway. WNK4 was also suggested to be involved in the glucocorticoid-regulated Na+ transport pathway in Reissner’s membrane. Glucocorticoid-stimulated Na+ absorption is positively regulated by phosphatidylinositol 3-kinase (PI3K) and negatively regulated by protein kinase C (PKC) in semicircular canal epithelial cells. The rate of Na+ absorption is stimulated by glucocorticoid activation of GR in mouse saccule; however, the mechanism of glucocorticoid-enhanced Na+ absorption via ENaC was not molecularly defined. White arrows, direction of flow. Adapted from Kim and Marcus (2011), with permission
The corticosteroids diffuse into the target cells where the active form of glucocorticoid is increased by enzymatic action transforming inactive forms of glucocorticoid to the active form via 11β-hydroxysteroid dehydrogenase (HSD1). The active glucocorticoid binds to the receptor in the cytoplasm that then translocates into the nucleus where the agonist-receptor complex promotes expression of several proteins involved in the stimulation of Na+ absorption. The stimulated signal pathway involves the increase in ENaC expression at the cell surface via SGK1 and Nedd4-2 through reduction of channel retrieval from the plasma membrane (Kim and Marcus 2011).
Binding sites for both glucocorticosteroid (ten Cate et al. 1993) and mineralocorticosteroid (Yao and Rarey 1996) have been demonstrated in the stria vascularis. Both corticoids control the activity of Na+, K+-ATPase in the stria vascularis (adrenalectomy reduced activity 60% and systemic administration of either the glucocorticosteroid dexamethasone or the mineralocorticosteroid aldosterone restored activity; Curtis et al. 1993), and the increase in Na+, K+-ATPase expression with aldosterone was not dependent on major changes in blood plasma cation concentration (ten Cate et al. 1994). However, despite the strong dependence of the EP on strial Na+, K+-ATPase, a reduction in adrenocorticosteroids by adrenalectomy did not significantly reduce the EP in the presence or absence of strong acoustic stimulation (Ma et al. 1995). In addition, glucocorticoid hormones alone apparently do not stimulate the formation of Na+, K+-ATPase in the inner ear because no difference in the level of antibody binding to Na+, K+-ATPase was observed in glucocorticoid receptor-knockout mice (Erichsen et al. 1998).
9.5.6 Basolateral K+ Concentration and Apical pH
Changes in extracellular ion composition, such as K+ and H+, can exert powerful control over ion transport by cells that are neighbors to the cells that produce those changes. In that regard, we may think of the controlling ions as paracrine-signaling hormones. The concentration of K+ in the perilymph that surrounds the sensory cells and their nerve synapses is known to increase on sensory stimulation (see Sects. 9.4.3 and 9.4.4), and the K+ thus exuded from the sensory cells is known to “recycle” to the stria vascularis in the cochlea and to the vestibular dark cells where it is pumped back into endolymph (see Sects. 9.4.3 and 9.4.4). The rate of basolateral uptake of K+ in strial marginal cells and vestibular dark cells is exquisitely sensitive to the level of extracellular K+ at that membrane so that small increases supplied from the sensory cells would be expected to be avidly taken up and secreted across the apical (endolymphatic) membrane (Wangemann et al. 1996). This K+ sensitivity is believed to play a significant role in regulating the recirculation of K+ back into the endolymph (Wangemann et al. 1996).
The endolymphatic pH is regulated by several transport processes (see Sect. 9.4.8), and this in turn exerts control over the activity of at least two key transporters. The gatekeeper K+ channel KCNQ1/KCNE1 in the apical membrane of strial marginal cells and vestibular dark cells is stimulated by acidification of endolymph (Wangemann et al. 1995b; Heitzmann et al. 2007), which thereby influences the rate of secretion of K+ (see Sect. 9.4.1). The endolymphatic Ca2+ concentration in both the cochlea and the vestibular labyrinth is thought to be partially controlled by absorption through epithelial Ca2+ channels (see Sect. 9.4.7), and those channels have been shown to be strongly inhibited by acidic endolymphatic pH (Nakaya et al. 2007).
9.6 Genetic Diseases
Genetic factors underlie more than 50% of the cases of prelingual hearing impairment (Oonk et al. 2015). Genetic causes of hearing loss are classified and identified in several ways. Clinical presentations that are exclusively hearing related are “nonsyndromic” (70% of cases) and those with pathologies also in other organs are “syndromic” (30% of cases). The chromosomal loci of nonsyndromic hereditary DeaFNess are designated with DFN numbers, such as DFNA1 or DFNB1. The A and B refer to autosomal-dominant (roughly 20% of the nonsyndromic cases) and autosomal-recessive (roughly 80%) hereditary transmission of hearing loss (Bayazit and Yilmaz 2006). Cases that are not classified A or B (about 1–2%) are mitochondrial, X- or Y-linked inheritance (Oonk et al. 2015). Gene expression profiles for causative genes and the locations of the corresponding proteins involved in hereditary hearing loss are collected in a recent review (Nishio et al. 2015) and databases are maintained online (e.g., http://hereditaryhearingloss.org).
Many of the most prevalent types of sensorineural hearing loss with hereditary etiology arise from the dysfunction of cells that are responsible for ionic homeostasis of the endolymph. These include (1) DFNB1 caused by mutations of GJB2 (connexin-26) and/or GJB6 (connexin-30) gap junction genes, (2) DFNB4 (Pendred syndrome) caused by mutations in SLC26A4, a Cl−/HCO3 − transporter that is essential for normal development of the inner ear, and (3) Jervell and Lange-Nielsen syndrome, which results from mutations of the KCNQ1/KCNE1 K+ channel in either of these subunits.
DFNB1 is the most common DFNB and can occur as either syndromic or nonsyndromic deafness (Jagger and Forge 2015). Gap junctions composed of CX26 homomers, CX30 homomers, and C26/CX30 heteromers connect epithelial and connective tissue cells in the cochlea and vestibular labyrinth in patterns that support multiple critical transport processes among cells (Jagger and Forge 2015). The neuroepithelial sensory cells, however, have no gap junction connections to neighboring cells.
Multiple functions of connexins in the ear have been proposed. These include shuttling K+ from the basolateral side of sensory cells during acoustic stimulation back to the stria vascularis for secretion into the endolymph (see Sect. 9.4.3); the transfer of essential second messengers such as inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] and Ca2+ among the coupled cells; guidance of nutrients and hormones (e.g., glucose and locally produced thyroid hormone) to target cells (Wangemann et al. 2009; Jagger and Forge 2015); and paracrine signaling via connexins expressed in a single-cell membrane (hemichannels). Hemichannels of connexins are thought to participate in purinergic signaling in the ear by providing a route for ATP release from cells (Jagger and Forge 2015). Hemichannels can open when the extracellular fluid has a low divalent cation concentration. The apical membranes of cochlear epithelial cells face the endolymph, which contains only about 20 µM Ca2+ and Mg2+ each, thus providing a permissive environment. The mechanisms by which gap junctions participate in cochlear function are far from fully understood and continue to draw considerable attention.
Pendred syndrome results from mutations of the Cl−/HCO3 − exchanger coded by SLC26A4 and accounts for about 7.5% (varies by population) of cases of hereditary deafness. Deletion of this gene in mice results in an acidic shift of endolymphatic pH, as expected from the loss of a HCO3 −-secretory transporter (see Sect. 9.4.8). The effects of pendrin mutation, however, go far beyond this relatively obvious consequence and are described by Wangemann (2013) and Ito et al. (2014). A clinical diagnostic parameter for Pendred syndrome is an enlarged vestibular aqueduct and an endolymphatic hydrops throughout the inner ear. An enlarged vestibular aqueduct is readily observed in MRI scans, and the syndromic condition is hypothyroidism in humans (but not mice); although pendrin-knockout mice are euthyroid, there appears to be a local hypothyroidism in the cochlea (Wangemann et al. 2009).
Jervell and Lange-Nielsen syndrome (JLNS) is “syndromic” because the affected K+ channel plays a prominent role in human cardiac function as well as providing the sole route of K+ efflux from strial marginal cells and vestibular dark cells. Dysfunction of this channel results in a reduced volume of endolymph and concentration of K+, and the EP is virtually abolished (Marcus et al. 2002). Patients with dysfunctional mutations of these K+-channel subunits are profoundly deaf in both ears and their QTc EKG interval is associated with tachyarrhythmias (Tranebjaerg et al. 2014). Parents of a child with JLNS are usually heterozygotes and the inheritance is autosomal recessive.
The identification of genes underlying many of the clinically defined hereditary deafness types (Nishio et al. 2015) suggests that gene therapy may be a successful treatment option. This approach requires vectors that can hold the required amount of genetic information, can enter the desired target cells, and can stimulate gene expression over the necessary time period. There has been intense work on each of these aspects, with varied results (Chien et al. 2015). Hearing loss that originates from gene mutations whose expression is critical for function during only relatively short time periods have the highest potential to be effective. The most severe effects of Pendred syndrome originate from a lack of normal expression in the ear during a 2-week period during development (Choi et al. 2011), which thereby offers a clear window of opportunity for gene therapy.
9.7 Free Radical Stress and Mitochondrial Dysfunction
Free radical stress that exceeds the capacity of defense mechanisms has been implicated in the inner ear pathogenesis of genetic (Pendred syndrome; see Sect. 9.6) and acquired (acoustic trauma, drug-induced ototoxicity, and age-related hearing loss) origins. Free radical stress originates largely from mitochondrial activity, but other mitochondrial actions have also been implicated in the cellular causes of these cochlear pathologies (Bottger and Schacht 2013). The stria vascularis is prone to free radical stress due to its high O2-dependent metabolic activity and dense vascular system, and, indeed, EP generation over the KCNJ10 K+ channel in intermediate cells is highly sensitive to local levels of free radical stress (Singh and Wangemann 2008). Hair cells are also susceptible to free radical stress even though they function at a lower level of metabolic activity because they produce a lower content of antioxidants (Bottger and Schacht 2013). In addition to free radical stress, mitochondrial modes of dysfunction include aberrant calcium regulation, mitochondrial DNA deletions, and the targeting of mitochondrial ribosomes (Bottger and Schacht 2013). A combined therapeutic strategy of administration of free radical scavengers and cochlear vasodilators has been proposed for the treatment of age-related hearing loss (Alvarado et al. 2015), but the widely variable results in the field has led to a measure of uncertainty in the general applicability of the proposal (Bottger and Schacht 2013).
9.8 Summary
Regulated solute and water transport in the cochlea is essential for hearing. This chapter describes the cellular mechanisms of transport in the different epithelial cells of the cochlear duct that produce and maintain the cochlear fluid composition in the face of local and systemic perturbations. Epithelial cells produce net secretory and/or absorptive fluxes by virtue of gene expression of specific transport proteins and asymmetrical placement in the apical and basolateral cell membranes. Transport is coordinated across the two membrane surfaces, and the net rate is further up- and downregulated by extracellular, intracellular, and transmembrane signals. Many genetic disorders that result in hearing loss relate to solute and water transport and their regulatory pathways.
Specific directions for future investigation include experimentally testing the hypotheses concerning (1) cellular mechanisms of putative K+ buffering and cycling in the cochlea, (2) the putative involvement of AQPs and hormonal signaling in the volume control of cochlear fluids, and (3) the ways in which the cochlea, vestibular organs, and endolymphatic sac communicate during development, as alluded to in this chapter. Furthermore, a rapidly expanding arsenal of techniques to manipulate inner ear genes and their modulation promises an exciting future of discovery and increased understanding of the importance of ion homeostasis in hearing and balance.
References
Alvarado, J. C., Fuentes-Santamaria, V., Melgar-Rojas, P., Valero, M. L., Gabaldón-Ull, M. C., Miller, J. M., & Juiz, J. M. (2015). Synergistic effects of free radical scavengers and cochlear vasodilators: A new otoprotective strategy for age-related hearing loss. Frontiers in Aging Neuroscience, 7, 1–7.
Ando, M., Edamatsu, M., Fukuizumi, S., & Takeuchi, S. (2008). Cellular localization of facilitated glucose transporter 1 (GLUT-1) in the cochlear stria vascularis: Its possible contribution to the transcellular glucose pathway. Cell and Tissue Research, 331, 763–769.
Andrews, J. C., & Honrubia, V. (2010). Premenstrual exacerbation of Meniere’s disease revisited. Otolaryngologic Clinics of North America, 43, 1029–1040.
Anselmi, F., Hernandez, V. H., Crispino, G., Seydel, A., Ortolano, S., Roper, S. D., Kessaris, N., Richardson, W., Rickheit, G., Filippov, M. A., Monyer, H., & Mammano, F. (2008). ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proceedings of the National Academy of Sciences of the United States of America, 105, 18770–18775.
Bayazit, Y. A., & Yilmaz, M. (2006). An overview of hereditary hearing loss. ORL Journal for Oto-Rhino Laryngology and Related Specialties, 68, 57–63.
Boettger, T., Hubner, C. A., Maier, H., Rust, M. B., Beck, F. X., & Jentsch, T. J. (2002). Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature, 416, 874–878.
Bottger, E. C., & Schacht, J. (2013). The mitochondrion: A perpetrator of acquired hearing loss. Hearing Research, 303, 12–19.
Brown, D., Bouley, R., Paunescu, T. G., Breton, S., & Lu, H. A. J. (2012). New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells. American Journal of Physiology - Cell Physiology, 302, C1421–C1433.
Cazals, Y., Bevengut, M., Zanella, S., Brocard, F., Barhanin, J., & Gestreau, C. (2015). KCNK5 channels mostly expressed in cochlear outer sulcus cells are indispensable for hearing. Nature Communications, 6, 8780.
Chen, J., Zhu, Y., Liang, C., Chen, J., & Zhao, H.-B. (2015). Pannexin1 channels dominate ATP release in the cochlea ensuring endocochlear potential and auditory receptor potential generation and hearing. Scientific Reports, 5, 10762.
Chen, Q., Chu, H., Wu, X., Cui, Y., Chen, J., Li, J., Zhou, L., Xiong, H., Wang, Y., & Li, Z. (2011). The expression of plasma membrane Ca2+-ATPase isoform 2 and its splice variants at sites A and C in the neonatal rat cochlea. International Journal of Pediatric Otorhinolaryngology, 75, 196–201.
Chien, W. W., Monzack, E. L., McDougald, D. S., & Cunningham, L. L. (2015). Gene therapy for sensorineural hearing loss. Ear and Hearing, 36, 1–7.
Choi, B. Y., Kim, H. M., Ito, T., Lee, K. Y., Li, X., Monahan, K., Wen, Y., Wilson, E., Kurima, K., Saunders, T. L., Petralia, R. S., Wangemann, P., Friedman, T. B., & Griffith, A. J. (2011). Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. Journal of Clinical Investigation, 121, 4516–4525.
Curtis, L. M., ten Cate, W. J., & Rarey, K. E. (1993). Dynamics of Na, K-ATPase sites in lateral cochlear wall tissues of the rat. European Archives of Oto-Rhino-Laryngology, 250, 265–270.
Delpire, E. (2015). Research antibodies: Do not use them to stain your reputation. American Journal of Physiology-Cell Physiology, 309, C707–C708.
Eckhard, A., Gleiser, C., Arnold, H., Rask-Andersen, H., Kumagami, H., Müller, M., Hirt, B., & Löwenheim, H. (2012). Water channel proteins in the inner ear and their link to hearing impairment and deafness. Molecular Aspects of Medicine, 33, 612–637.
Eckhard, A., Dos, S. A., Liu, W., Bassiouni, M., Arnold, H., Gleiser, C., Hirt, B., Harteneck, C., Müller, M., Rask-Andersen, H., & Löwenheim, H. (2015). Regulation of the perilymphatic-endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Pflügers Archiv - European Journal of Physiology, 467, 2571–2588.
Effertz, T., Scharr, A. L., & Ricci, A. J. (2015). The how and why of identifying the hair cell mechano-electrical transduction channel. Pflügers Archiv - European Journal of Physiology, 467, 73–84.
Erichsen, S., Stierna, P., Bagger-Sjoback, D., Curtis, L. M., Rarey, K. E., Schmid, W., & Hultcrantz, M. (1998). Distribution of Na, K-ATPase is normal in the inner ear of a mouse with a null mutation of the glucocorticoid receptor. Hearing Research, 124, 146–154.
Ferrary, E., Sterkers, O., Saumon, G., Tran Ba Huy, P., & Amiel C. (1987). Facilitated transfer of glucose from blood into perilymph in the rat cochlea. American Journal of Physiology - Renal Physiology, 253, F59–F65.
Firbas, W., Gruber, H., & Wicke, W. (1981). The blood vessels of the limbus spiralis. Archives of Oto-Rhino-Laryngology, 232, 131–137.
Foster, C. A., & Breeze, R. E. (2013). The Meniere attack: An ischemia/reperfusion disorder of inner ear sensory tissues. Medical Hypotheses, 81, 1108–1115.
Furuta, H., Luo, L., Hepler, K., & Ryan, A. F. (1998). Evidence for differential regulation of calcium by outer versus inner hair cells: Plasma membrane Ca-ATPase gene expression. Hearing Research, 123, 10–26.
Gao, Y., Yechikov, S., Vazquez, A. E., Chen, D., & Nie, L. (2013). Impaired surface expression and conductance of the KCNQ4 channel lead to sensorineural hearing loss. Journal of Cellular and Molecular Medicine, 17, 889–900.
Gow, A., Davies, C., Southwood, C. M., Frolenkov, G., Chrustowski, M., Ng, L., Yamauchi, D., Marcus, D. C., & Kachar, B. (2004). Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. The Journal of Neuroscience, 24, 7051–7062.
Guipponi, M., Vuagniaux, G., Wattenhofer, M., Shibuya, K., Vazquez, M., Dougherty, L., Scamuffa, N., Guida, E., Okui, M., Rossier, C., Hancock, M., Buchet, K., Reymond, A., Hummler, E., Marzella, P. L., Kudoh, J., Shimizu, N., Scott, H. S., Antonarakis, S., & Rossier, B. C. (2002). The transmembrane serine protease (TMPRSS3) mutated in deafness DFNB8/10 activates the epithelial sodium channel (ENaC) in vitro. Human Molecular Genetics, 11, 2829–2836.
Heitzmann, D., Koren, V., Wagner, M., Sterner, C., Reichold, M., Tegtmeier, I., Volk, T., & Warth, R. (2007). KCNE beta subunits determine pH sensitivity of KCNQ1 potassium channels. Cellular Physiology and Biochemistry, 19, 21–32.
Hirose, K., Hartsock, J. J., Johnson, S., Santi, P., & Salt, A. N. (2014). Systemic lipopolysaccharide compromises the blood-labyrinth barrier and increases entry of serum fluorescein into the perilymph. Journal of the Association for Research in Otolaryngology, 15, 707–719.
Hoenderop, J. G., Nilius, B., & Bindels, R. J. (2005). Calcium absorption across epithelia. Physiological Reviews, 85, 373–422.
Hosoi, K. (2016). Physiological role of aquaporin 5 in salivary glands. Pflügers Archiv - European Journal of Physiology, 468, 519–539.
Housley, G. D., Morton-Jones, R., Vlajkovic, S. M., Telang, R. S., Paramananthasivam, V., Tadros, S. F., Wong, A. C. Y., Froud, K. E., Cederholm, J. M. E. Sivakumaran, Y., Snguanwongchai, P., Khakh, B. S., Cockayne, D. A., Thorne, P. R., & Ryan, A. F. (2013). ATP-gated ion channels mediate adaptation to elevated sound levels. Proceedings of the National Academy of Sciences of the United States of America, 110, 7494–7499.
Hoya, N., Ogawa, K., Inoue, Y., Takiguchi, Y., & Kanzaki, J. (2001). The glutamate receptor agonist, AMPA, induces acetylcholine release in guinea pig cochlea; A microdialysis study. Neuroscience Letters, 311, 206–208.
Ito, T., Li, X., Kurima, K., Choi, B. Y., Wangemann, P., & Griffith, A. J. (2014). Slc26a4-insufficiency causes fluctuating hearing loss and stria vascularis dysfunction. Neurobiology of Disease, 66, 53–65.
Jagger, D. J., & Forge, A. (2015). Connexins and gap junctions in the inner ear—it’s not just about K+ recycling. Cell and Tissue Research, 360, 633–644.
Johnstone, B. M., Patuzzi, R., Syka, J., & Sykova, E. (1989). Stimulus-related potassium changes in the organ of Corti of guinea-pig. The Journal of Physiology, 408, 77–92.
Juhn, S. K., Rybak, L. P., & Fowlks, W. L. (1982). Transport characteristics of the blood-perilymph barrier. American Journal of Otolaryngology, 3, 392–396.
Kambayashi, J., Kobayashi, T., DeMott, J. E., Marcus, N. Y., Thalmann, I., & Thalmann, R. (1982). Effect of substrate-free vascular perfusion upon cochlear potentials and glycogen of the stria vascularis. Hearing Research, 6, 223–240.
Kikuchi, T., Kimura, R. S., Paul, D. L., Takasaka, T., & Adams, J. C. (2000). Gap junction systems in the mammalian cochlea. Brain Research Reviews, 32, 163–166.
Kim, K. X., Sanneman, J. D., Kim, H. M., Harbidge, D. G., Xu, J., Soleimani, M., Wangemann, P., & Marcus, D. C. (2014). Slc26a7 chloride channel activity and localization in mouse Reissner’s membrane epithelium. PLoS ONE, 9, e97191.
Kim, S. H., & Marcus, D. C. (2011). Regulation of sodium transport in the inner ear. Hearing Research, 280, 21–29.
Kofuji, P., Biedermann, B., Siddharthan, V., Raap, M., Iandiev, I., Milenkovic, I., Thomzig, A., Veh, R. W., Bringmann, A., & Reichenbach, A. (2002). Kir potassium channel subunit expression in retinal glial cells: Implications for spatial potassium buffering. Glia, 39, 292–303.
Konishi, T., Hamrick, P. E., & Walsh, P. J. (1978). Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Oto-Laryngologica, 86, 22–34.
Kozel, P. J., Friedman, R. A., Erway, L. C., Yamoah, E. N., Liu, L. H., Riddle, T., Duffy, J. J., Doetschman, T., Miller, M. L., Cardell, E. L., & Shull, G. E. (1998). Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. Journal of Biological Chemistry, 273, 18693–18696.
Kumagami, H., Loewenheim, H., Beitz, E., Wild, K., Schwartz, H., Yamashita, K., Schultz, J., Paysan, J., Zenner, H.-P., & Ruppersberg, J. P. (1998). The effect of anti-diuretic hormone on the endolymphatic sac of the inner ear. Pflügers Archiv - European Journal of Physiology, 436, 970–975.
Laurell, G., Viberg, A., Teixeira, M., Sterkers, O., & Ferrary, E. (2000). Blood-perilymph barrier and ototoxicity: An in vivo study in the rat. Acta Oto-Laryngologica, 120, 796–803.
Laurell, G. F., Teixeira, M., Duan, M., Sterkers, O., & Ferrary, E. (2008). Intact blood-perilymph barrier in the rat after impulse noise trauma. Acta Oto-Laryngologica, 128, 608–612.
Lee, J. H., & Marcus, D. C. (2008). Purinergic signaling in the inner ear. Hearing Research, 235, 1–7.
Lee, J. H., Chiba, T., & Marcus, D. C. (2001). P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. The Journal of Neuroscience, 21, 9168–9174.
Li, J., & Verkman, A. S. (2001). Impaired hearing in mice lacking aquaporin-4 water channels. Journal of Biological Chemistry, 276, 31233–31237.
Liu, J., Kozakura, K., & Marcus, D. C. (1995). Evidence for purinergic receptors in vestibular dark cell and strial marginal cell epithelia of the gerbil. Auditory Neuroscience, 1, 331–340.
Ma, Y. L., Gerhardt, K. J., Curtis, L. M., Rybak, L. P., Whitworth, C., & Rarey, K. E. (1995). Combined effects of adrenalectomy and noise exposure on compound action potentials, endocochlear potentials and endolymphatic potassium concentrations. Hearing Research, 91, 79–86.
Maekawa, C., Kitahara, T., Kizawa, K., Okazaki, S., Kamakura, T., Horii, A., Imai, T., Doi, K., Inohara, H., & Kiyama, H. (2010). Expression and translocation of aquaporin-2 in the endolymphatic sac in patients with Meniere’s disease. Journal of Neuroendocrinology, 22, 1157–1164.
Mammano, F. (2013). ATP-dependent intercellular Ca2+ signaling in the developing cochlea: Facts, fantasies and perspectives. Seminars in Cell and Developmental Biology, 24, 31–39.
Marcus, D. C. (2012). Acoustic transduction. In N. Sperelakis (Ed.), Cell physiology source book: Essentials of membrane biophysics (pp. 649–668). San Diego, CA: Academic Press.
Marcus, D. C., & Wangemann, P. (2009). Cochlear and vestibular function and dysfunction. In F. J. Alvarez-Leefmans, & E. Delpire (Eds.), Physiology and Pathology of Chloride Transporters and Channels in the Nervous System: From Molecules to Diseases (pp. 425–437). New York: Elsevier.
Marcus, D. C., & Wangemann, P. (2010). Inner ear fluid homeostasis. In P. A. Fuchs (Ed.), The Oxford Handbook of Auditory Science: The Ear (pp. 213–230). Oxford, UK: Oxford University Press.
Marcus, D. C., Wu, T., Wangemann, P., & Kofuji, P. (2002). KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. American Journal of Physiology - Cell Physiology, 282, C403–C407.
Merchant, S. M., Adams, J. C., & Nadol, J. B. (2005). Meniere’s syndrome: Are symptoms caused by endolymphatic hydrops? The Registry, 12, 1–7.
Miyazaki, H., Wangemann, P., & Marcus, D. C. (2016). The gastric H, K-ATPase in stria vascularis contributes to pH regulation of cochlear endolymph but not to K secretion. BMC Physiology, 17(1), 1.
Morton-Jones, R. T., Vlajkovic, S. M., Thorne, P. R., Cockayne, D. A., Ryan, A. F., & Housley, G. D. (2015). Properties of ATP-gated ion channels assembled from P2X2 subunits in mouse cochlear Reissner’s membrane epithelial cells. Purinergic Signalling, 11, 551–560.
Mosnier, I., Teixeira, M., Loiseau, A., Fernandes, I., Sterkers, O., Amiel, C., & Ferrary, E. (2001). Effects of acute and chronic hypertension on the labyrinthine barriers in rat. Hearing Research, 151, 227–236.
Muñoz, D. J., Kendrick, I. S., Rassam, M., & Thorne, P. R. (2001). Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Oto-Laryngologica, 121, 10–15.
Nakaya, K., Harbidge, D. G., Wangemann, P., Schultz, B. D., Green, E. D., Wall, S. M., & Marcus, D. C. (2007). Lack of pendrin HCO3 − transport elevates vestibular endolymphatic [Ca2+] by inhibition of acid-sensitive TRPV5 and TRPV6 channels. American Journal of Physiology - Renal Physiology, 292, F1314–F1321.
Nenov, A. P., Chen, C., & Bobbin, R. P. (1998). Outward rectifying potassium currents are the dominant voltage activated currents present in Deiters’ cells. Hearing Research, 123, 168–182.
Nishio, S. Y., Hattori, M., Moteki, H., Tsukada, K., Tsukada, K., Miyagawa, M., Naito, T., Yoshimura, H., Iwasa, Y., Mori, K., Shima, Y, Sakuma, N., & Usami, S. (2015). Gene expression profiles of the cochlea and vestibular endorgans: Localization and function of genes causing deafness. The Annals of Otology, Rhinology and Laryngology, 124 Suppl. 1, 6S–48S.
Nishioka, R., Takeda, T., Kakigi, A., Okada, T., Takebayashi, S., Taguchi, D., Nishimura, M., & Hyodo, M. (2010). Expression of aquaporins and vasopressin type 2 receptor in the stria vascularis of the cochlea. Hearing Research, 260, 11–19.
Oonk, A. M., Huygen, P. L., Kunst, H. P., Kremer, H., & Pennings, R. J. E. (2015). Features of autosomal recessive nonsyndromic hearing impairment: A review to serve as a reference. Clinical Otolaryngology, 41, 487–497. doi:10.1111/coa.12567.
Patuzzi, R. (2011a). Ion flow in cochlear hair cells and the regulation of hearing sensitivity. Hearing Research, 280, 3–20.
Patuzzi, R. (2011b). Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hearing Research, 277, 4–19.
Pondugula, S. R., Raveendran, N. N., & Marcus, D. C. (2010). Ion transport regulation by P2Y receptors, protein kinase C and phosphatidylinositol 3-kinase within the semicircular canal duct epithelium. BMC Research Notes, 3, 100.
Robbins, J. (2001). KCNQ potassium channels: Physiology, pathophysiology, and pharmacology. Pharmacology and Therapeutics, 90, 1–19.
Sakai, Y., Harvey, M., & Sokolowski, B. (2011). Identification and quantification of full-length BK channel variants in the developing mouse cochlea. Journal of Neuroscience Research, 89, 1747–1760.
Salt, A. N., Melichar, I., & Thalmann, R. (1987). Mechanisms of endocochlear potential generation by stria vascularis. The Laryngoscope, 97, 984–991.
Salt, A. N., Ohyama, K., & Thalmann, R. (1991a). Radial communication between the perilymphatic scalae of the cochlea. I: Estimation by tracer perfusion. Hearing Research, 56, 29–36.
Salt, A. N., Ohyama, K., & Thalmann, R. (1991b). Radial communication between the perilymphatic scalae of the cochlea. II: Estimation by bolus injection of tracer into the sealed cochlea. Hearing Research, 56, 37–43.
Salt, A. N., Kellner, C., & Hale, S. (2003). Contamination of perilymph sampled from the basal cochlear turn with cerebrospinal fluid. Hearing Research, 182, 24–33.
Sellick, P. M., & Johnstone, B. M. (1972). Changes in cochlear endolymph Na+ concentration measured with Na+ specific microelectrodes. Pflügers Archiv - European Journal of Physiology, 336, 11–20.
Shen, Z., Marcus, D. C., Sunose, H., Chiba, T., & Wangemann, P. (1997). IsK channel in strial marginal cells: Voltage-dependence, ion-selectivity, inhibition by 293B and sensitivity to clofilium. Auditory Neuroscience, 3, 215–230.
Shi, X., Gillespie, P. G., & Nuttall, A. L. (2005). Na+ influx triggers bleb formation on inner hair cells. American Journal of Physiology - Cell Physiology, 288, C1332–C1341.
Singh, R., & Wangemann, P. (2008). Free radical stress-mediated loss of Kcnj10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. American Journal of Physiology - Renal Physiology, 294, F139–F148.
Spicer, S. S., & Schulte, B. A. (1996). The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hearing Research, 100, 80–100.
Sterkers, O., Saumon, G., Tran Ba, H. P., & Amiel, C. (1982). K, Cl, and H2O entry in endolymph, perilymph, and cerebrospinal fluid of the rat. American Journal of Physiology - Renal Physiology, 243, F173–F180.
Strutz-Seebohm, N., Seebohm, G., Fedorenko, O., Baltaev, R., Engel, J., Knirsch, M., & Lang, F. (2006). Functional coassembly of KCNQ4 with KCNE-β-subunits in Xenopus oocytes. Cellular Physiology and Biochemistry, 18, 57–66.
Takeda, T., Takeda, S., Kitano, H., Okada, T., & Kakigi, A. (2000). Endolymphatic hydrops induced by chronic administration of vasopressin. Hearing Research, 140, 1–6.
Takeuchi, S., Ando, M., & Kakigi, A. (2000). Mechanism generating endocochlear potential: Role played by intermediate cells in stria vascularis. Biophysical Journal, 79, 2572–2582.
Takumida, M., Kakigi, A., Egami, N., Nishioka, R., & Anniko, M. (2012). Localization of aquaporins 1, 2, and 3 and vasopressin type 2 receptor in the mouse inner ear. Acta Oto-Laryngologica, 132, 807–813.
ten Cate, W. J., Curtis, L. M., Small, G. M., & Rarey, K. E. (1993). Localization of glucocorticoid receptors and glucocorticoid receptor mRNAs in the rat cochlea. The Laryngoscope, 103, 865–871.
ten Cate, W. J., Curtis, L. M., & Rarey, K. E. (1994). Effects of low-sodium, high-potassium dietary intake on cochlear lateral wall Na+, K+-ATPase. European Archives of Oto-Rhino-Laryngology, 251, 6–11.
Thalmann, R. (1971). Metabolic features of auditory and vestibular systems. The Laryngoscope, 81, 1245–1260.
Thorne, P. R., Muñoz, D. J., & Housley, G. D. (2004). Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the guinea pig. Journal of the Association for Research in Otolaryngology, 5, 58–65.
Tranebjaerg, L., Samson, R. A., & Green, G. E. (2014). Jervell and Lange-Nielsen syndrome. In R. A. Pagon, M. P. Adam, H. H. Ardinger, S. E. Wallace, H. C. Mefford, & K. Stephens (Eds.), GeneReviews. Available at https://www.ncbi.nlm.nih.gov/books/NBK1405/.
Turner, J. R., Buschmann, M. M., Romero-Calvo, I., Sailer, A., & Shen, L. (2014). The role of molecular remodeling in differential regulation of tight junction permeability. Seminars in Cell and Developmental Biology, 36, 204–212.
Verkman, A. S., & Mitra, A. K. (2000). Structure and function of aquaporin water channels. American Journal of Physiology - Renal Physiology, 278, F13–F28.
Vlajkovic, S. M., Housley, G. D., Muñoz, D. J., Robson, S. C., Sévigny, J., Wang, C. J. H., & Thorne, P. R. (2004). Noise exposure induces up-regulation of ecto-nucleoside triphosphate diphosphohydrolases 1 and 2 in rat cochlea. Neuroscience, 126, 763–773.
Wada, J., Kambayashi, J., Marcus, D. C., & Thalmann, R. (1979). Vascular perfusion of the cochlea: Effect of potassium-free and rubidium-substituted media. Archives of Oto-Rhino-Laryngology, 225, 79–81.
Wangemann, P. (2002). Adrenergic and muscarinic control of cochlear endolymph production. Advances in Oto-Rhino-Laryngology, 59, 42–50.
Wangemann, P. (2013). Mouse models for pendrin-associated loss of cochlear and vestibular function. Cellular Physiology and Biochemistry, 32, 157–165.
Wangemann, P., Liu, J., & Marcus, D. C. (1995a). Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hearing Research, 84, 19–29.
Wangemann, P., Liu, J., & Shiga, N. (1995b). The pH-sensitivity of transepithelial K+ transport in vestibular dark cells. Journal of Membrane Biology, 147, 255–262.
Wangemann, P., Shen, Z., & Liu, J. (1996). K+-induced stimulation of K+ secretion involves activation of the IsK channel in vestibular dark cells. Hearing Research, 100, 201–210.
Wangemann, P., Nakaya, K., Wu, T., Maganti, R. J., Itza, E. M., Sanneman, J. D., Harbidge, D. G., Billings, S., & Marcus, D. C. (2007). Loss of cochlear HCO3 − secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. American Journal of Physiology - Renal Physiology, 292, F1345–F1353.
Wangemann, P., Kim, H. M., Billings, S., Nakaya, K., Li, X., Singh, R., Sharlin, D. S., Forrest, D., Marcus, D. C., & Fong, P. (2009). Developmental delays consistent with cochlear hypothyroidism contribute to failure to develop hearing in mice lacking Slc26a4/pendrin expression. American Journal of Physiology - Renal Physiology, 297, F1435–F1447.
Wood, J. D., Muchinsky, S. J., Filoteo, A. G., Penniston, J. T., & Tempel, B. L. (2004). Low endolymph calcium concentrations in deafwaddler 2J mice suggest that PMCA2 contributes to endolymph calcium maintenance. Journal of the Association for Research in Otolaryngology, 5, 99–110.
Yamauchi, D., Raveendran, N. N., Pondugula, S. R., Kampalli, S. B., Singh, R., Wangemann, P., & Marcus, D. C. (2005). Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochemical and Biophysical Research Communications, 331, 1353–1357.
Yamauchi, D., Nakaya, K., Raveendran, N. N., Harbidge, D. G., Singh, R., Wangemann, P., & Marcus, D. C. (2010). Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiology, 10, 1.
Yamoah, E. N., Lumpkin, E. A., Dumont, R. A., Smith, P. J., Hudspeth, A. J., & Gillespie, P. G. (1998). Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. The Journal of Neuroscience, 18, 610–624.
Yao, X. F., & Rarey, K. E. (1996). Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Oto-Laryngologica, 116, 493–496.
Yoo, J. C., Kim, H. Y., Han, K. H., Oh, S. H., Chang, S. O., Marcus, D. C., & Lee, J. H. (2012). Na+ absorption by Claudius’ cells is regulated by purinergic signaling in the cochlea. Acta Oto-Laryngologica, 132 Suppl. 1, S103–S108.
Yoshihara, T., & Igarashi, M. (1987). Cytochemical localization of Ca++-ATPase activity in the lateral cochlear wall of the guinea pig. Archives of Oto-Rhino-Laryngology, 243, 395–400.
Zidanic, M., & Brownell, W. E. (1990). Fine structure of the intracochlear potential field. I. The silent current. Biophysical Journal, 57, 1253–1268.
Acknowledgements
This work was supported by Grant R01-DC012151 from the National Institute on Deafness and Other Communication Diseases, National Institutes of Health, and by the Kansas State University College of Veterinary Medicine.
Compliance with Ethics Requirements Philine Wangemann declares that she has no conflict of interest. Daniel C. Marcus declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Wangemann, P., Marcus, D.C. (2017). Ion and Fluid Homeostasis in the Cochlea. In: Manley, G., Gummer, A., Popper, A., Fay, R. (eds) Understanding the Cochlea. Springer Handbook of Auditory Research, vol 62. Springer, Cham. https://doi.org/10.1007/978-3-319-52073-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-52073-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52071-1
Online ISBN: 978-3-319-52073-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)