Abstract
The use of argon to degas molten aluminium is a widespread practice in aluminium casthouses, and the removal of the hydrogen contained in the metal by using this noble gas is a proven method with no downside effect on the metallurgical quality of the resulting metal. On the other hand, an increasing number of producers are looking for cost reduction opportunities, and nitrogen often comes as an alternative to replace argon to degas molten aluminium. However, some are questioning the degassing efficiency of this gas or the risk to deteriorate metal cleanliness. STAS Inc. has carried out a series of comparative tests, using argon and nitrogen in an ACD . Metallurgical measures for degassing and metal cleanliness have been taken to compare both gases. This paper presents the results obtained during this test campaign carried out in North America.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
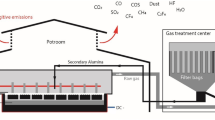
The main function of a degasser is to remove the dissolved hydrogen content in molten aluminium, while secondary functions are to remove alkali and alkaline earth metals as well as to improve metal cleanliness. Degassing occurs when an inert gas is injected into molten aluminium through one to several hollow shafts spinning at high speed (Fig. 1). The principle according to which gas is sheared into small bubbles into which hydrogen migrates and then rises to the surface has already been very well explained [1, 2]. When a reactive agent like chlorine gas (Cl2) or magnesium chlorine fluxes are injected with the inert gas, a reaction occurs with sodium and calcium, resulting in the flotation of the generated salt (NaCl, CaCl2). Also, injecting Cl2 or a flux changes the interfacial properties of the metal, which helps inclusions such as oxides, spinels and carbides to be removed by flotation [3, 4].
In theory, inert gases like argon (Ar) and nitrogen (N2) should provide the same degassing performances when a high purity gas is used. But in practice, most degassers are operated with argon, even though this gas is more expensive, for it is believed that N2 can deteriorate the metal cleanliness. Of the 245+ inline degassers sold by STAS to this day, more than 95% are operated with argon.
Upstream of the degasser, argon and nitrogen are often used in furnace treatment through Rotary Flux/Gas Injectors (RF/GI) (Fig. 2). But of the 155 RF/GI units sold by STAS to this day, approximately 80% are operated with N2, the remainder with argon. In an RF/GI, experience with customers have shown that the operation gas is chosen for economical rather than metallurgical reasons. However, it has also been argued that N2 could affect the metal cleanliness [5, 6] and produce wetter dross when used without a reactive agent like Cl2 or a flux. But as Cl2 or a flux is always used with nitrogen in a furnace, no significant difference in metal cleanliness has been demonstrated [7]. The presence of Cl2 or flux prevents the generation of hot and wet dross which could boost the production of aluminium nitrides (AlN). N2 can react with aluminium to produce AlN, but this reaction needs some time and a high temperature [5]. And when AlN is generated, this compound takes the form of solid inclusions that can have a deleterious effect on the casting. However, treatment in a furnace always involves some settling time to allow inclusions to float on the surface of the metal or to settle to the bottom of the furnace, which prevents most of them from moving downstream.
In a degasser, the picture is somewhat different since the metal residence time is much less and Cl2 or a flux are not always used with the gas. Doutre et al. compared Ar and N2 with the same amount of Cl2 and reported a decline in metal cleanliness [8]. Moreover, previous works from STAS’ customers to compare Ar and N2 in a box type degasser have shown the same efficiency in terms of hydrogen removal, but with a detrimental effect on the amount of inclusions (Fig. 3)—unfortunately, no details were available on the method, especially with respect to gas quality. The same works showed that N2 also seemed to produce a wetter dross, though this was not measured. Additionally, an analysis of metal cleanliness did not show any AlN but rather oxides with grain refiner.
In consideration of all the information stated above, STAS has decided to carry out some tests in an Aluminium Compact Degasser (ACD ) to clarify the results on the use of N2 when no chlorine gas or flux is used. The main objective of the tests was to compare Ar and N2 with regard to their degassing and inclusion removal efficiencies (including the formation of AlN).
Scope of Experiment
In early 2015, experiments were conducted in a sealed 6-rotor ACD commissioned in 2010. Since the degassing efficiency was achieved beyond expectations, only 5 of the 6 rotors were operated. The average metal flow was 460 kg/min, and 50 L/min of gas per rotor was injected (Ar or N2). The alloy cast was from the 3xxx series with low Mg concentration. The degassing efficiency was measured on 3 casts with Ar and 3 casts with N2, while the metal cleanliness was measured on 4 casts with Ar and 4 casts with N2.
Method
AlSCAN was the method used to measure the hydrogen concentration. Only one AlSCAN unit was available, and it was moved before and after the ACD . A PoDFA unit was used to measure the metal cleanliness. Each time, one sample was taken before and after the ACD, with another one taken after the filter. Since no Cl2 or flux was used, the alkali and alkaline earth metal removal was not measured.
Particular attention was paid to gas purity, for the presence of as low as 1% of humidity could be very detrimental to the degassing efficiency. A certificate of analysis showed a purity over 99.999% for both nitrogen and argon .
Results and Discussion
Degassing
Figure 4 shows the AlSCAN results for the tests. The degassing efficiency is the same for argon and nitrogen, as expected. Overall, the efficiency rate is really good, averaging 77%. One test was carried out with only 4 rotors in operation but still showed very good efficiency.
Metal Cleanliness
Figure 5 shows a graph of the PoDFA value for the total concentration of inclusions without grain refiner. Grain refiner is not counted as an inclusion, since it is added on purpose. Besides, samples before the ACD were taken before the addition of grain refiner.
Carbides constitute most of the inclusions , averaging 86% of total inclusions before the degasser (the remainder comprising oxides and spinels). According to the PoDFA analysis, no AlN was detected in any of the samples after the degasser, even when N2 was injected. But with further Scanning Electron Microscope (SEM) and spectrometer (EDS) analysis, some nitrogen structures combined with spinels were identified. These were very thin structures which could not be quantified. But overall, the amount of spinels was very low in all the samples analysed. And the spinels were mostly removed by the ACD (see Fig. 6), while the rest was removed by the filter.
On average, the inclusion removal efficiency with an ACD is 85% when Ar is injected and 60% when N2 is injected. During the tests, both gases removed magnesium oxides and spinels adequately and with similar efficiency. But while most of the carbides were removed when Ar was injected, N2 seemed to slightly struggle with the former. Figure 7 compares carbide removal between N2 and Ar.
The amount of dross was not measured during the tests, but it appears that Ar and N2 produce approximately the same type and amount of dross. Figure 8 shows the typical appearance of the dross after treatment with Ar and N2.
Conclusion
At this stage, our investigation has led us to conclude that nitrogen can replace argon , since degassing with high purity N2 or Ar provided the same efficiency for the alloy tested. Thus, the use of nitrogen instead of argon, the former being less expensive than the latter, could bring important cost reductions in the operation of a casthouse. However, further trials will be required to confirm this result with other alloys, but for now the results seem conclusive with respect to the 3xxx series alloys.
In terms of inclusion removal, the results are positive with both gases, even when no Cl2 or flux is injected. With N2, a lower removal efficiency was achieved, but no aluminium nitrides were generated.
To ensure excellent metallurgical results at the lowest operation and maintenance costs, the ACD /Aluminium Compact Degasser® already tops the list of available technologies on the market. And the possibility of using nitrogen is another cost-saving option that could be beneficial for many users.
Future Work
To conclude on a larger use of nitrogen , we believe the following would prove helpful:
-
More tests with different concentrations and types of alloys.
-
More tests when Cl2 or a flux are injected.
-
Measuring weight and composition of dross when performing further tests.
References
G.K. Sigworth, “Gas fluxing of molten aluminum, part 1: hydrogen removal”. Light Metals, 641–649 (1999)
G.K. Sigworth, T.A. Engh, Chemical and kinetic factors related to hydrogen removal from aluminum. Metall. Trans. B 13B, 447–460 (1982)
P. Le Brun, “Hydrogen removal efficiency of in-line degassing units”, in Light Metals 2002, ed. By W. Schneider, TMS (The Minerals, Metals & Materials Society, 2002), pp. 869–875
R.R. Roy, T.A. Utigard, C. Dupuis, “Inclusion removal kinetics during chlorine fluxing of molten aluminum”. Light Metals, 871–875 (1998)
L.C.B. Martins, C.T. Keller, S. Shivkumar, “Chemical factors affecting the separation of inclusions from molten aluminum”, in Proceedings of 3rd International Conference on Molten Aluminum Processing (Amer. Foudryman’s Society, 1992), pp. 79–91
A.R. Anderson, “Rotary impeller degassing: practical observations”. Trans. AFS, 533–536 (1987)
G. Béland, C. Dupuis, G. Riverin, R. Desmeules, L. Rose, “Rotary flux injection: chlorine free technique for furnace preparation”. Light Metals, 843–847 (1998)
D. Doutre, B. Gariépy, J.P. Martin, G. Dubé, “Aluminium cleanliness and monitoring: methods and applications in process development and quality control”. Light Metals, 296–304 (1985)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Tremblay, É., Maltais, B. (2017). The Use of Nitrogen to Degas Molten Aluminium—Comparison of Metallurgical Results with Argon and Nitrogen Used in an ACDtm . In: Ratvik, A. (eds) Light Metals 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-51541-0_176
Download citation
DOI: https://doi.org/10.1007/978-3-319-51541-0_176
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51540-3
Online ISBN: 978-3-319-51541-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)