Abstract
The Gram-negative bacterium Helicobacter pylori is predominantly known for its tight association with peptic ulcer disease and gastric cancer development. However, recent epidemiological and experimental evidence suggests that chronic infection with H. pylori may at the same time be beneficial to the host by conferring protection against gastroesophageal diseases, asthma, other allergic disease manifestations and inflammatory bowel diseases (IBD). In this chapter, we summarize the epidemiological data that are available to date to support or refute a possible inverse correlation of H. pylori infection with various extragastric diseases. We further examine and discuss the experimental evidence, generated mostly in mouse models of allergic diseases and IBD, showing that these disorders fail to develop in the presence of H. pylori. The proposed mechanisms of the protective effects of H. pylori, which appear to involve the induction of regulatory T-cells (Tregs) with highly suppressive activity, are presented and explained.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Gastro-esophageal reflux disease
- Inflammatory bowel diseases
- Immunomodulation
- Allergic diseases
- Allergen-induced asthma
- Epidemiological studies
- Dendritic cells
- Regulatory T-cells
- Inflammasome
1 H. pylori in Gastric and Extragastric Health and Disease
Helicobacter pylori is a highly successful pathobiont of humans that infects roughly 50% of the world population. Since its discovery in the early 1980s (Marshall and Warren 1984), H. pylori has been linked to a variety of gastric and extragastric disease manifestations (Salama et al. 2013; Pritchard and Crabtree 2006; Cover and Blaser 2009). H. pylori resides exclusively in the human stomach, where it colonizes the mucus layer overlying the gastric mucosa; minor populations adhere to gastric epithelial cells and colonize the glands of both the antrum and the corpus of the stomach (Salama et al. 2013). H. pylori causes histologically evident gastritis (Marshall and Warren 1984), which remains asymptomatic in the majority of infected individuals (Cover and Blaser 2009). Chronic H. pylori infection can result in gastric and duodenal ulcers and is the single most important risk factor for the development of gastric adenocarcinoma and gastric B-cell lymphoma, the so-called mucosa-associated lymphoid tissue (MALT) lymphoma (Herrera and Parsonnet 2009; Parsonnet et al. 1991, 1997, 1994; Parsonnet and Isaacson 2004; Cover and Blaser 2009). Bacterial virulence factors, host genetic predisposition and environmental factors such as lifestyle and diet have all been linked to an individual carrier’s risk of developing disease (Pritchard and Crabtree 2006; Cover and Blaser 2009). H. pylori strains expressing virulence factors such as the cytotoxin-associated gene A (CagA) and the cag pathogenicity island (PAI) as well as toxic versions of the vacuolating cytotoxin (VacA) are more tightly associated with peptic ulcer disease and gastric cancer than Cag/VacA-negative strains (Cover and Blaser 2009; Pritchard and Crabtree 2006; Backert and Blaser 2016). More recently, the asymptomatic carrier state versus clinically evident disease has been attributed to H. pylori-specific T-cell responses: carriers with peptic ulcer disease are more likely to launch T-helper 1 (Th1)- and Th17-biased responses to H. pylori, whereas asymptomatic carriers exhibit regulatory T-cell (Treg)-predominant responses (Robinson et al. 2008). Similarly, asymptomatic children are more likely to generate Treg-dominated anti-Helicobacter responses than (symptomatic) adults (Harris et al. 2008; Serrano et al. 2013). The differential responses of young versus adult, experimentally infected mice, mirror the observations made in humans and have been linked to the development of Treg-mediated peripheral immune tolerance (Arnold et al. 2011c). As a consequence, neonatally infected mice are largely protected against the characteristic Th1- and Th17-driven gastric immunopathology that virulent strains elicit in mice and that is reminiscent of the gastric preneoplastic pathology of a minority of infected humans (Arnold et al. 2011c). A large body of evidence now suggests that H. pylori has both pathogenic and strong immunomodulatory properties, with the latter potentially conferring beneficial effects to the human host. Although the H. pylori field has been driven mostly by the quest to understand the pathogenic traits of H. pylori and especially its pro-carcinogenic activities, investigating the immunomodulatory and other protective properties of H. pylori may be equally worthwhile. H. pylori is an ancient member of the human gastric microbiota and has co-evolved with humans for at least 60,000 years (Linz et al. 2007). Most humans are colonized with the same strain for life in an asymptomatic fashion. The intimate co-existence between H. pylori and its human host provides a context in which host and bacteria may benefit from one another.
The prevalence of H. pylori infection has decreased dramatically in the twentieth century from >50 to ~10% (Blaser and Falkow 2009) and parallels that of other infectious diseases (Bach 2002). As a consequence, gastric cancer rates and the associated mortality have declined steadily in countries from which H. pylori has disappeared (Forman 2005). In the same time frame, the incidence of immunological disorders such as autoimmune diseases (e.g., multiple sclerosis and type I diabetes), inflammatory bowel diseases (IBDs), asthma and other allergies has strongly increased (Bach 2002; Eder et al. 2006). Esophageal diseases such as gastro-esophageal reflux diseases (GERD), Barrett’s esophagus and esophageal carcinoma are also increasingly common in Western societies from which H. pylori is disappearing (Pohl and Welch 2005). Whether the inverse trend of H. pylori prevalence and the prevalence of extragastric diseases is merely coincidental, or causally linked, has lately been the focus of increasingly sophisticated epidemiological and, to some extent, experimental research. This chapter aims to discuss the epidemiological and experimental evidence for (and against) a direct causal link between H. pylori and a variety of these extragastric diseases; we will begin by discussing the link to esophageal diseases, followed by dedicated sections on H. pylori and IBDs and allergies, respectively. Populations from various geographical areas of the world and from various age groups will be discussed separately wherever possible or necessary.
2 Esophageal Diseases and H. pylori
In the 1990s, several interventional trials implied that curing H. pylori infection in patients with duodenal ulcers might provoke reflux esophagitis (Labenz et al. 1997). Although many studies have since addressed the topic, the beneficial role of H. pylori in GERD and the serious sequelae Barrett’s esophagus (BE) and esophageal adenocarcinoma (EA) remains controversially discussed and requires further clinical and experimental confirmation. Overall, most but by far not all clinical, epidemiological and experimental literature supports a positive and preventive role for H. pylori in esophageal diseases. The term GERD on the one hand refers to several related disorders that include BE and EA, but is on the other hand also thought to be the clinical starting point of a pathogenic sequence that can result in BE and EA (Fig. 1a) (Shaheen and Ransohoff 2002). GERD is characterized by esophageal acid exposure that is caused by the reflux of gastric contents and the failure of the esophagus to clear by means of peristaltic contractions. The severity of the disease depends strongly on the pH of the refluxed gastric juice (Collen et al. 1994; Gardner et al. 2004). BE is characterized by the replacement of the stratified squamous epithelium with a metaplastic columnar epithelium and is most likely caused by chronic GERD. The characteristic secretion of bicarbonate and mucus by Barrett’s epithelium is believed to represent a protective adaptation against continued gastric acid exposure. Chronic acid exposure and the resulting inflammation appear to drive the sustained proliferation of BE cells, thus promoting the development of EA (Lao-Sirieix et al. 2008). The prevalence of BE is approximately 1–2% in patients subjected to endoscopy for any indication. This percentage increases up to 5–15% in patients with GERD symptoms. Strikingly, even though EA is a rare malignancy, the risk of EA among patients with BE is assumed to be 30- to 125-fold higher than that of the general population (Runge et al. 2015). Moreover, the prevalence of EA has increased dramatically in recent decades (Brown et al. 2008).
Esophageal diseases and Helicobacter pylori. a Pathogenic sequence of GERD to BE to EA. GERD patients are more prone to develop BE due to increased esophageal acid exposure. BE patients in turn are characterized by an inflamed esophageal, fast-dividing columnar tissue, which subsequently may shift to EA. b In an inflamed H. pylori-infected stomach, acid production by parietal cells is likely inhibited by bacterial LPS and the virulence/persistence factor VacA. Gastric atrophy resulting from chronic inflammation further decreases acid levels and eventually results in achlorhydria. Consequently, the lower pH is believed to relieve gastric esophageal reflux symptoms and disease progression. This phenomenon is predominantly associated with CagA-positive H. pylori strains
Several epidemiological studies and meta-analyses have addressed a possible inverse correlation of H. pylori infection with the incidence of a broad range of non-malignant GERDs. In a first meta-analysis of European studies published in 2003, Raghunath et al. showed that H. pylori infection is significantly inversely linked to GERD as defined as abnormal esophageal pH or erosive esophagitis (Raghunath et al. 2003). However, significance was lost after removal of a single dominant outlier study, thereby challenging the reliability of the analysis (Kandulski and Malfertheiner 2014). Similarly, Nordenstedt et al. (2007) found that there was no negative association of GERD symptoms with H. pylori infection except in patients with reduced pepsinogen levels and gastric atrophy (Nordenstedt et al. 2007). In 2013, Rubenstein et al. confirmed the lack of association of GERD symptoms with H. pylori infection, irrespective of the CagA status of the colonizing strains. In contrast, this analysis found an inverse correlation of H. pylori with erosive esophagitis, especially in patients harboring CagA-positive strains (Rubenstein et al. 2014). This evidence was further supported by Korean and Japanese studies in which H. pylori could be negatively linked with the risk and severity of erosive esophagitis (Chung et al. 2011; Minatsuki et al. 2013). Numerous studies and meta-analyses have indicated a negative correlation of H. pylori infection with BE and EA. In a systemic review in 2007, Rokkas et al. (2007) found a lower prevalence of H. pylori in patients with either BE or EA, which was more significant for infections with CagA-positive strains (Rokkas et al. 2007). In 2012, Fischbach et al. arrived at a similar conclusion when investigating four methodologically comparable studies, and also documented a decreased risk of EA predominantly in patients infected with CagA-positive H. pylori strains (Fischbach et al. 2012). A similar result was obtained in a recent US-based case–control study (Rubenstein et al. 2014). In addition, Fischbach and co-workers recently confirmed a negative association of H. pylori with the risk of BE, which was particularly evident in participants with likely low gastric acidity due to corpus atrophy or anti-secretory drug use (Fischbach et al. 2014). The association of H. pylori infection with a decreased risk of BE and EA thus is rather consistent, but seems to generally be more pronounced in Eastern compared to Western countries (Thrift et al. 2012; Xie et al. 2013a, b). In a recent meta-analysis, Nie et al. (2014) speculated that CagA-positive strains might even have opposing associations with another esophageal malignancy, esophageal squamous cell carcinoma (ESCC) in Asian and non-Asian populations; however, these authors also found that CagA-positive H. pylori strains were associated with a decreased risk of EA in all populations, irrespective of geographical location (Nie et al. 2014). Numerous studies have addressed whether H. pylori eradication promotes the development of GERD or associated diseases. Several studies have reported an aggravation of symptoms of GERD or BE after eradication of the bacterium (Ahmed and Sechi 2005; Carroll et al. 2004; Tanaka et al. 2004; Fallone et al. 2000; Haruma 2004). Some literature even suggests that successful eradication serves as the starting point of GERD in certain cases (Nakajima and Hattori 2003; Sakata and Fujimoto 2005). This was particularly evident in Asian populations (Xie et al. 2013b; Cremonini et al. 2003). A recent study conducted in Japan reported that healthy asymptomatic H. pylori-infected individuals have a lower prevalence of reflux esophagitis than those subjected to eradication therapy (Minatsuki et al. 2013). Additionally, a study from Taiwan reported increased morbidity associated with reflux esophagitis upon eradication (Lee et al. 2013). On the other hand, two studies have failed to detect an effect of eradication therapy on the subsequent development of GERD, BE or similar (Saad et al. 2012; Laine and Sugg 2002). Vaira et al. (2003) even found an amelioration of GERD symptoms after H. pylori eradication. Two other meta-analyses similarly concluded that eradication does not influence the incidence of reflux symptoms or esophagitis (Qian et al. 2011; Yaghoobi et al. 2010). Possible explanations for the discrepancies have raised issues of ethnic differences and the reasons for why eradication therapy was prescribed in the first place (Iijima et al. 2015).
Little definitive mechanistic data are available to explain putative protective or detrimental effects of H. pylori on GERD, BE or EA. On the one hand, H. pylori has been implicated in raising gastric acid secretion by promoting the destruction of somatostatin-secreting D-cells in antrum-predominant gastritis, resulting in increased parietal cell mass, hyperchlorhydria and an aggravation of GERD symptoms (Kamada et al. 1998). On the other hand, pangastritis, which is mostly associated with CagA and VacAs1 bearing strains, results in the destruction of acid-secreting parietal cells, causing hypo- or achlorhydria due to gastric atrophy and an amelioration of GERD-associated symptoms (Ghoshal and Chourasia 2010). VacA is believed to directly disrupt the apical membrane–cytoskeletal interaction in parietal cells and thereby lower acid secretion (Wang et al. 2008). Bacterial lipopolysaccharides may also dampen esophageal acid exposure through the prostaglandin system and by inhibition of the enzymatic function of the H/K ATPase (Tsuji et al. 1992; Helmer et al. 2004). In a recent publication, Gall et al. reported in a BE cohort that infection with the bacterium was associated with a decreased incidence of genomic instability which predicts progression to EA. The authors also detected H. pylori at esophageal sites, which raise the possibility that the bacterium directly impacts the survival and proliferation of esophageal epithelial cells (Gall et al. 2015). When investigating the role of H. pylori in BE and EA formation in a rat model, Liu et al. (2011) found that the bacteria reduce BE severity when the infection site is restricted to the stomach, but promote an increase in inflammation and incidence of BE and EA when colonizing the esophagus (Liu et al. 2011).
Various host physiological and genetic determinants but also environmental and dietary factors have been implicated in playing a critical role in shaping the outcome of GERD diseases in the context of H. pylori infections (summarized in Fig. 1b) (Chourasia and Ghoshal 2008; Ghoshal and Chourasia 2010). Moreover, the difference in responsiveness between Eastern and Western countries has been explained by the fact that H. pylori infection does not lead to a significant change in gastric acid secretion in Europeans and Americans, whereas a strong decrease is observed in Asians (Iijima et al. 2015). However, the cellular and molecular mechanisms behind this finding remain to be elucidated.
In summary, epidemiological studies have repeatedly described a negative association between H. pylori infection and erosive esophagitis, BE and EA, but not between H. pylori and GERD symptoms. Infection with CagA-positive strains in particular appears to protect the distal esophagus by causing fundic gland atrophy and impairing gastric acid secretion. Although several early reports have suggested the development of erosive esophagitis after H. pylori eradication, more recent studies have failed to corroborate an important clinical impact on GERD of H. pylori eradication. As in many other disorders, the patient’s ethnic, genetic, physiological and pathological background as well as dietary habits appears to play a crucial role in shaping disease risk.
3 Inflammatory Bowel Diseases and H. pylori
Inflammatory bowel diseases (IBDs) are chronic relapsing disorders of increasing incidence that affect the gastrointestinal tract. The two main forms of IBD, Crohn’s disease and ulcerative colitis, are both characterized by intestinal inflammation and epithelial injury, but differ in terms of their clinical and histopathological features, suggesting that they represent independent clinical entities. In Crohn’s disease, inflammation is discontinuous and can affect any part of the gastrointestinal tract and all layers of the bowel wall. The transmural nature of inflammation accounts for the serious complications associated with Crohn’s disease such as fibrostenosis, abscesses and fistula formation. In contrast, ulcerative colitis is confined to the superficial layer of the mucosa and expands continuously from the rectum, with progressive inflammation and ulceration of the distal colon in more advanced forms. Histological features of IBD further include disruption of the intestinal epithelium with goblet cell depletion, decreased mucus production and hyperplasia (Xavier and Podolsky 2007). The precise etiology of IBD is unclear, but appears to involve a complex combination of host genetic, microbial and environmental factors. Chronic inflammation arises from an abnormal immune response against the microorganisms of the intestinal flora in genetically susceptible individuals and results in the breakdown of intestinal homeostasis (Maloy and Powrie 2011). Both dysregulated innate and adaptive immune pathways contribute to the chronic inflammatory response in patients with IBD (Geremia et al. 2014). Interestingly, both ulcerative colitis and Crohn’s disease patients have altered microbial communities, with reduced diversity in major phyla such as Firmicutes (including Clostridium) and Bacteroidetes (including Bacteroides fragilis), and increased numbers of adherent-invasive strains of the Enterobacteriaceae. These observations suggest a direct link between the presence of specific bacterial products and the maintenance of major anti-inflammatory pathways in the gut. Changes in the human microbiota composition arising from modern hygienic practices and diet have been proposed to account for the increasing incidence of IBD in Western societies (Baumgart et al. 2011). However, whether the dysbiosis observed in IBD patients represents a primary predisposing factor or results from the combination of other deficiencies is still unclear.
An inverse correlation between H. pylori infection and IBD in its various manifestations has long been suspected by gastroenterologists. The initial sporadic observations by clinicians were examined more systematically in a series of epidemiological studies initiated in the mid-1990s; of the roughly 10 studies published between 1994 and 2004, the vast majority found a lower seroprevalence of H. pylori infection in patients with IBDs relative to an age-matched control population. Crohn’s disease and ulcerative colitis patients were both less likely to be seropositive for H. pylori than healthy controls; Crohn’s disease patients had an even lower prevalence of H. pylori infection than ulcerative colitis patients (el-Omar et al. 1994; Halme et al. 1996; Parente et al. 1997). Later studies in which active H. pylori infection was detected by urea breath test rather than serum IgG or IgA also unequivocally confirmed a lower prevalence of H. pylori in IBD patients (Pearce et al. 2000; Pronai et al. 2004; Piodi et al. 2003). All early studies were conducted on European populations, in which IBDs are much more common than in other geographical areas of the world; however, several more recent studies have since confirmed the same trends in Asian and American populations (Wu et al. 2015; Sonnenberg and Genta 2016; Jin et al. 2013). A recent study of pediatric IBD patients confirmed the trend seen in adults, i.e., children with newly diagnosed Crohn’s disease or ulcerative colitis were significantly less likely to harbor H. pylori than non-IBD controls from the same general population (Roka et al. 2014). Several of the more recent studies have used multivariate logistic regression analyses to adjust for gender, ethnicity, age, income, ZIP code and other measures of socioeconomic status, but found the trends to hold true irrespective of these parameters (Sonnenberg and Genta 2016, 2012; Castano-Rodriguez et al. 2015). Moreover, studies that include very large numbers of subjects such as one analysis of the surgical pathology files of 65,515 patients (1061 IBD patients and 64,451 controls) confirm the low prevalence of H. pylori infection among patients with IBD (Sonnenberg and Genta 2012).
Several meta-analyses have been conducted on the topic in recent years. A meta-analysis of ten studies involving 1299 Asian IBD patients and 1817 controls showed infection rates of 24.9% in IBD patients relative to 48.3% in the controls, with a resulting pooled risk ratio of 0.48 for H. pylori infection in IBD patients (Wu et al. 2015). Other meta-analyses have reached similar conclusions: For example, a meta-analysis of 23 studies conducted in 2010 (5903 subjects in total) found that overall, 27.1% of IBD patients had evidence of infection with H. pylori compared to 40.9% of patients in the control group (relative risk of 0.64). A more recent meta-analysis of 33 eligible studies that included 4400 IBD patients and 4763 controls (the vast majority being non-Asian) found that 26.5% of IBD patients were H. pylori-positive, compared to 44.7% of individuals in the control group (risk ratio of 0.62) (Rokkas et al. 2015). In the most comprehensive meta-analysis to date, with data from 40 studies, Crohn’s disease and ulcerative colitis patients were evaluated together as well as separately (Castano-Rodriguez et al. 2015). The entire study population included 6130 patients with IBD and 74,659 non-IBD controls. The overall calculated risk ratio for H. pylori infection was 0.43; stratification by patient age revealed an even lower risk ratio for pediatric populations (0.24 relative to 0.45 in adults). Crohn’s disease patients had a lower risk ratio than ulcerative colitis patients (0.38 relative to 0.53), and Eastern populations had a lower risk ratio than Western populations (0.35 relative to 0.46) (Castano-Rodriguez et al. 2015). The same meta-analysis found a positive association between infection with enterohepatic Helicobacter species and Campylobacter species and IBD, suggesting that closely related bacteria can have vastly different effects on this disease group (Castano-Rodriguez et al. 2015). All meta-analyses and almost all original articles covering the topic thus consistently find a strong negative association between H. pylori colonization and IBD.
3.1 H. pylori and Host Factors Determine Protection Against IBDs
Several pieces of experimental evidence are now available to support a direct contribution of H. pylori to protection against the chronic intestinal inflammation that is the main histopathological hallmark of IBD. Several complementary models have been used to investigate protection against IBD by live H. pylori and purified components of the bacteria. For example, Higgins et al. (2010) examined the effects of H. pylori infection on Salmonella typhimurium-induced chronic colitis, a model for Crohn’s disease. H. pylori co-infection suppressed the Th17 response to S. typhimurium in the mouse cecum and reduced the histopathological symptoms associated with this model (Higgins et al. 2010). The beneficial effects could be linked to IL-10 production in the mesenteric lymph nodes (MLNs) of the co-infected animals (Higgins et al. 2010) and to H. pylori DNA, which contains a high ratio of immunoregulatory to immunostimulatory sequences, especially relative to other Gram-negative bacteria such as Escherichia coli (Luther et al. 2011). Oral administration of H. pylori DNA was sufficient to protect against the histopathological symptoms of dextran sodium sulfate (DSS)-induced colitis in acute and chronic models of the disease (Luther et al. 2011). Further work identified a specific immunoregulatory sequence, 5’-TTTAGGG, as being unique to H. pylori genomes and particularly active in suppressing DCs (Owyang et al. 2012). Work by the same group and others has revealed a role for TLR2 signaling in the suppression of DC activation, the induction of Treg-biased T-helper cell responses and protection against IBD (Sun et al. 2013; Koch et al. 2015). H. pylori expresses TLR2 ligands that dominate the bacteria’s interaction with DCs, and other innate and adaptive immune cell compartments, including B-cells (Rad et al. 2009; Sun et al. 2013; Sayi et al. 2011; Koch et al. 2015). TLR2 signaling thus presumably drives a tolerogenic response in DCs that directs Treg-biased responses to H. pylori antigens and suppresses T-effector responses to the bacteria (Koch et al. 2015). The host benefits from this regulatory response due to protection from gastric immunopathology even in the face of high-level colonization (Koch et al. 2015). Interestingly, responses to unrelated (bystander) T-cell antigens are suppressed as well, including allergen-specific and maybe autoantigen-specific immune responses (Koch et al. 2015; Oertli et al. 2012; Arnold et al. 2011a). This finding has been used to explain why H. pylori-infected individuals are less likely to develop allergic disease manifestations (Blaser et al. 2008; Chen and Blaser 2007, 2008; Reibman et al. 2008), celiac disease (Lebwohl et al. 2013) and possibly autoimmune diseases (Cook et al. 2015) (as well as IBD), as discussed below.
TLR2 signaling is required for the H. pylori-induced production and secretion of IL-10 (Sun et al. 2013; Sayi et al. 2011), a well-studied cytokine with a plethora of anti-inflammatory and regulatory activities. It is also required for the priming of inflammasome activation, a critical event during the H. pylori/host interaction (Kim et al. 2013; Koch et al. 2015). H. pylori exclusively activates the NLRP3 inflammasome; in contrast, other cytoplasmic innate immune sensors such as AIM2, NLRP6 and NLRC4 do not contribute measurably to inflammasome and caspase-1 activation (Koch et al. 2015; Semper et al. 2014; Kim et al. 2013). NLRP3 inflammasome activation is preceded by a “priming” event, which allows cells to upregulate NLRP3 transcription in a TLR2-dependent manner (Kim et al. 2013; Koch et al. 2015). DCs lacking TLR2 are incapable of NLRP3 transcriptional activation and caspase-1 auto-proteolysis and activation, and therefore fail to process and secrete the caspase-1-dependent cytokines IL-1β and IL-18 (Kim et al. 2013; Koch et al. 2015). Both have critical roles in the H. pylori/host interaction, with IL-1β driving Th1- and Th17-polarized T-cell responses and H. pylori control, and IL-18 providing regulatory activity (Hitzler et al. 2012). The lack of mature IL-18 in particular recapitulates the phenotypes of TLR2 deficiency and NLRP3 deficiency: mice lacking either the cytokine or its receptor control H. pylori more efficiently due to unrestricted Th1 and Th17 responses, but suffer from severe infection-associated immunopathology (Hitzler et al. 2012; Oertli et al. 2012). The critical contribution of the TLR2/NLRP3/caspase-1/IL-18 signaling axis to immune tolerance induced by H. pylori hinted at a role of this pathway also in protection against IBD. Indeed, confirming earlier data, H. pylori protected effectively against DSS-induced colitis not only via its DNA as shown previously (Luther et al. 2011), but also in the context of experimental infection (Engler et al. 2015). Infection of mice during the neonatal period, when their predisposition to develop tolerance to foreign antigens is at its peak, alleviated DSS colitis symptoms later in life (Engler et al. 2015). The effect of live infection could be mimicked by regular doses of H. pylori extract, administered orally or intraperitoneally starting from the neonatal period onwards (Engler et al. 2015). The effects of live infection and extract treatment required NLRP3 and IL-18, and were attributed to the production of copious amounts of mucus in NLRP3/IL-18 proficient animals (Engler et al. 2015). Mucus production was detectable by endoscopic procedures as well as at the transcriptional level (the main intestinal mucin is Muc2, which was strongly upregulated upon infection or extract treatment) (Engler et al. 2015) and likely explains the resistance to barrier destruction by DSS that is the underlying cause of colitis in this model. Overall, there is now more and more convincing experimental evidence supporting a protective role of H. pylori on IBD development. Combined with the epidemiological data in humans documenting an inverse correlation of IBD risk with H. pylori prevalence, it appears likely that direct effects (via the regulatory activity of H. pylori DNA, NLRP3 ligands and potentially other immunomodulators) of H. pylori on immune cells, mainly DCs and Tregs, account for its beneficial effects (summarized in Fig. 2).
H. pylori in its relationship to intestinal diseases. H. pylori exclusively inhabits the gastric mucosa of humans. 10–20% of infected individuals will develop gastric infection-associated diseases, such as chronic gastritis and gastric ulcers, that are driven by pathogenic T-cells polarized to express Th1 and Th17 cytokines. The majority (greater than 80% of the infected population) will remain asymptomatic throughout life despite harboring high levels of H. pylori. Asymptomatic carriers mount a Treg-predominant response to the infection (upper inset); Tregs (in yellow) suppress Th1, Th17 and Th2 responses, both locally in the gastric mucosa and at other sites of the GI tract (shown here are the small and large intestine). H. pylori-induced Tregs are believed to contribute to the alleviation of colitis symptoms in models of inflammatory bowel disease (lower two insets). Treg- and DC-derived IL-10 contributes to H. pylori-specific immunomodulation
4 H. pylori and Allergic Disease Manifestations
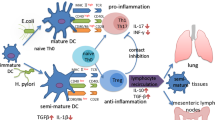
The severity and incidence of allergic asthma and other atopic diseases have increased dramatically in developed countries over the last decades. Allergic diseases thus follow two major trends that have dominated public health in developed countries since the second half of the twentieth century: The incidence of infectious diseases has declined sharply in that time frame, whereas immunological disorders such as multiple sclerosis (MS), type I diabetes, the aforementioned IBDs and allergies have dramatically increased in incidence over the same time period (Bach 2002). Numerous epidemiological studies have addressed, and demonstrated, an inverse association of H. pylori infection with asthma and other allergies with respiratory tract manifestations (Blaser et al. 2008; Chen and Blaser 2007, 2008; Reibman et al. 2008; Amberbir et al. 2011). This inverse association was particularly strong in children and adolescents and in individuals with early onset allergies and asthma (Blaser et al. 2008; Chen and Blaser 2007, 2008; Reibman et al. 2008; Amberbir et al. 2011). The chronic inflammatory skin disease atopic dermatitis/eczema has also been inversely linked to H. pylori infection in studies including over 3000 German school children and almost 2000 Japanese university students (Herbarth et al. 2007; Shiotani et al. 2008). Two meta-analyses have since been conducted that have investigated a possible inverse association of H. pylori with allergic asthma. Wang et al. (2013) retrieved 19 studies conducted up until 2012 (nine cross-sectional studies, seven case–control studies and three prospective cohort studies) and from these calculated a pooled OR for the association between asthma and H. pylori infection of 0.81. A second meta-analysis—also published in 2013—which included 14 studies involving 28,283 patients also found a significantly lower rate of H. pylori infection in the asthmatics than in the controls (OR = 0.84, P = 0.013) (Zhou et al. 2013). Following up on the various observational studies in human populations, mechanistic studies in experimental models have examined a possible protective effect of experimental H. pylori infection in animal models of allergic asthma. In a murine model of allergic asthma induced by ovalbumin or house dust mite antigen sensitization and challenge, H. pylori infection confers almost complete protection against the airway hyper-responsiveness, broncho-alveolar eosinophilia, lung inflammation and goblet cell metaplasia that are hallmarks of asthma in humans and mice (Arnold et al. 2011a). The protective effects are particularly pronounced in animals that have been experimentally infected during the neonatal period (Arnold et al. 2011a), i.e., at an age when humans typically contract the infection from their mothers (Weyermann et al. 2009). Asthma protection conferred by H. pylori is abolished by antibiotic eradication therapy prior to allergen challenge and depends critically on regulatory T-cells (Tregs, Fig. 3) (Arnold et al. 2011a). The systemic depletion of Tregs abrogates asthma protection, and conversely, pure populations of Tregs are sufficient to transfer protection from neonatally infected donors to naive recipients. These results are in line with earlier observations that neonatal infection with H. pylori induces Treg-mediated immune tolerance to the bacteria (Arnold et al. 2011c) and that mice can be actively “tolerized” against H. pylori by vaccination (Arnold et al. 2011b). Interestingly, the suppressive activity of Tregs in the asthma model depends on interleukin-18 proficiency of the donor (Oertli et al. 2012), which is reminiscent of the prerequisites of protection against chronic intestinal inflammation (see above). In the absence of IL-18 signaling, neonatal tolerance to the infection cannot be established; Tregs derived from IL-18−/− or IL-18R−/− donors are not protective against asthma (Oertli et al. 2012). Further work has shown that IL-18 is produced by DCs upon exposure to H. pylori infection (Oertli et al. 2012). IL-18 production by DCs appears to be required for H. pylori-specific tolerance. IL-18 proficiency is required both in DCs derived from bone marrow and DCs isolated immunomagnetically from mesenteric lymph nodes for the conversion of naive CD4+ T-cells into CD25+FoxP3+ Tregs (Oertli et al. 2012). In line with these observations, FoxP3+ Treg numbers in the MLNs of both infected IL-18−/− and IL-18R−/− mice are significantly lower than those of infected wild-type mice (Oertli et al. 2012).
Gastric H. pylori colonization protects against allergic asthma. Despite exclusively colonizing the gastric mucosa, H. pylori has robust systemic effects on T-cell responses in other organs. The H. pylori persistence factors γ-glutamyl-transpeptidase (GGT) and vacuolating cytotoxin (VacA) promote chronic infection by tolerizing DCs and thereby promoting local Treg differentiation (lower inset). H. pylori-induced Tregs and DC/Treg-derived IL-10 are required for the suppression of allergen-specific Th2 and Th17 responses in the lung (upper inset). Children and young adults are more likely than older hosts of H. pylori to benefit from the infection in terms of their individual allergy risk
Pro-IL-18 is processed by caspase-1 to yield the mature cytokine. Several recent publications have identified the NLRP3 inflammasome as the predominant type of inflammasome to become activated upon H. pylori exposure of murine DCs (Semper et al. 2014; Kim et al. 2013; Koch et al. 2015). TLR2 proficiency was found to be a clear prerequisite of NLRP3 inflammasome activation, as TLR2−/− DCs failed to activate caspase-1 and secrete caspase-1-dependent cytokines (Koch et al. 2015). The available evidence thus points to a critical role of the TLR2/NLRP3/caspase-1/IL-18 axis in H. pylori-specific immune modulation, with TLR2 signaling leading to the transcriptional activation of NLRP3, which then assembles with pro-caspase-1 and the adaptor protein ASC to form the functional NLRP3 inflammasome, auto-proteolytically activate caspase-1 and process the caspase-1-dependent cytokines IL-18 and IL-1β (Koch and Muller 2015). Accordingly, TLR2-deficient mice are not protected against allergic asthma induced by house dust mite allergen (Koch et al. 2015).
Several H. pylori determinants have been implicated in immune tolerance, the differentiation and function of suppressive Tregs, and the protection against allergic disease manifestations. In particular, the persistence factors and H. pylori immunomodulators vacuolating cytotoxin (VacA) and γ-glutamyl-transpeptidase (GGT) are known to be required for persistent high-level colonization on the one hand, and protection against allergic asthma on the other hand (Fig. 3) (Oertli et al. 2013). VacA- or GGT-deficient mutants fail to colonize at wild-type levels, which correlates with higher numbers of Th1 and Th17 cells, higher expression of IFN-γ and IL-17 by restimulated mesenteric lymph node (MLN) single cell preparations, and lower numbers of FoxP3+ CD25+ regulatory T-cells in MLNs (Oertli et al. 2013). VacA in particular appears to bias T helper cell responses toward Tregs, which could be attributed to VacA’s effects on DCs. DCs that were immunomagnetically purified based on their CD11c expression from the MLNs of wild-type-infected mice induced FoxP3 and CD25 expression in co-cultured naive CD4+ T-cells ex vivo (Oertli et al. 2013). This was not observed with DCs from uninfected animals or from mice infected with a vacA mutant (Oertli et al. 2013). Interestingly, both GGT and VacA can be administered to mice in purified form and confer a level of protection against allergen-induced asthma that is comparable to the live infection (Engler et al. 2014). Administration of several doses of VacA protects efficiently against allergen-induced asthma, especially if the protein is provided in the neonatal tolerance window (Engler et al. 2014). This time frame constitutes a period in both mice and humans in which immune tolerance to antigens is readily established; therefore, it is perhaps not surprising that VacA acts most potently during this time. Active tolerization against allergens using VacA requires its interaction with DCs (Fig. 3), as mouse strains lacking IL-10 expression in the DC compartment cannot be tolerized with VacA (Engler et al. 2014). Administration of several doses of VacA protects efficiently against allergen-induced asthma, especially if the protein is provided in the neonatal tolerance window (Engler et al. 2014).
In conclusion, substantial epidemiological evidence is available to support the idea that H. pylori is not just a pathogen, but in its function as a normal, ancient member of the gastric microbiota may also contribute to esophageal health, protection against allergies and IBDs, and possibly against auto-immune diseases although the evidence toward this end remains less well documented. There is hope that the immunomodulatory properties of H. pylori can be separated from its pathogenic properties to enable its future exploitation for therapeutic purposes in one or more of these disease areas.
5 Concluding Remarks
Controlling the epidemic increase in allergic, chronic inflammatory and auto-immune diseases is by many accounts one of the great public health challenges of this century. An overly “sterile” life style, exaggerated use of antibiotics in childhood and many other sanitary and behavioral practices common in developed countries are known to contribute to the rise in incidence in these “immunological” disorders (Bach 2002). The gradual disappearance of our normal, “ancestral” microbiota has been blamed by some investigators for this trend (Blaser and Falkow 2009). Epidemiological and experimental data point to a special role of H. pylori, a dominant component of the normal flora until half a century ago, in this context due to its strong immunomodulatory capacity. More work is clearly required to gain a detailed understanding of the mechanistic basis of H. pylori-specific immune tolerance and identify the H. pylori factors involved in immunomodulation, until it will be possible to harness the immunomodulatory properties of H. pylori for the purpose of H. pylori-specific tolerization against asthma and allergies, IBDs and possibly auto-immune diseases.
References
Ahmed N, Sechi LA (2005) Helicobacter pylori and gastroduodenal pathology: new threats of the old friend. Ann Clin Microbiol Antimicrobials 4:1. doi:10.1186/1476-0711-4-1
Amberbir A, Medhin G, Erku W, Alem A, Simms R, Robinson K, Fogarty A, Britton J, Venn A, Davey G (2011) Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy. doi:10.1111/j.1365-2222.2011.03831.x
Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Müller A (2011a) Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 121:3088–3093. doi:45041 [pii] 10.1172/JCI45041
Arnold IC, Hitzler I, Engler D, Oertli M, Agger EM, Müller A (2011b) The C-Terminally Encoded, MHC Class II-Restricted T Cell Antigenicity of the Helicobacter pylori Virulence Factor CagA Promotes Gastric Preneoplasia. J Immunol 186(11):6165–6172. doi:jimmunol.1003472 [pii] 10.4049/jimmunol.1003472
Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Müller A (2011c) Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 140(1):199–209. doi:S0016-5085(10)00956-X [pii] 10.1053/j.gastro.2010.06.047
Bach JF (2002) The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 347(12):911–920. doi:10.1056/NEJMra020100347/12/911 [pii]
Backert S, Blaser MJ (2016) The role of CagA in the gastric biology of Helicobacter pylori. Cancer Res 76(14):4028–4031. doi:10.1158/0008-5472.CAN-16-1680
Baumgart DC, Bernstein CN, Abbas Z, Colombel JF, Day AS, D’Haens G, Dotan I, Goh KL, Hibi T, Kozarek RA, Quigley EM, Reinisch W, Sands BE, Sollano JD, Steinhart AH, Steinwurz F, Vatn MH, Yamamoto-Furusho JK (2011) IBD Around the world: comparing the epidemiology, diagnosis, and treatment: proceedings of the World Digestive Health Day 2010—inflammatory bowel disease task force meeting. Inflamm Bowel Dis 17(2):639–644. doi:10.1002/ibd.21409
Blaser MJ, Chen Y, Reibman J (2008) Does Helicobacter pylori protect against asthma and allergy? Gut 57(5):561–567
Blaser MJ, Falkow S (2009) What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 7(12):887–894. doi:nrmicro2245 [pii] 10.1038/nrmicro2245
Brown LM, Devesa SS, Chow WH (2008) Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 100(16):1184–1187. doi:10.1093/jnci/djn211
Carroll IM, Khan AA, Ahmed N (2004) Revisiting the pestilence of Helicobacter pylori: insights into geographical genomics and pathogen evolution. Infect Genet Evol: J Mol Epidemiol Evol Genet Infect Dis 4(2):81–90. doi:10.1016/j.meegid.2004.01.006
Castano-Rodriguez N, Kaakoush NO, Lee WS, Mitchell HM (2015) Dual role of Helicobacter and Campylobacter species in IBD: a systematic review and meta-analysis. Gut. doi:gutjnl-2015–310545 [pii] 10.1136/gutjnl-2015-310545
Chen Y, Blaser MJ (2007) Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 167(8):821–827
Chen Y, Blaser MJ (2008) Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 198(4):553–560
Chourasia D, Ghoshal UC (2008) Pathogenesis of gastro-oesophageal reflux disease: what role do Helicobacter pylori and host genetic factors play? Trop Gastroenterol: Official J Dig Dis Found 29(1):13–19
Chung SJ, Lim SH, Choi J, Kim D, Kim YS, Park MJ, Yim JY, Kim JS, Cho SH, Jung HC, Song IS (2011) Helicobacter pylori serology inversely correlated with the risk and severity of reflux esophagitis in Helicobacter pylori endemic area: a matched case-control study of 5616 health check-up Koreans. J Neurogastroenterol Motil 17(3):267–273. doi:10.5056/jnm.2011.17.3.267
Collen MJ, Johnson DA, Sheridan MJ (1994) Basal acid output and gastric acid hypersecretion in gastroesophageal reflux disease. Correlation with ranitidine therapy. Dig Dis Sci 39(2):410–417
Cook KW, Crooks J, Hussain K, O’Brien K, Braitch M, Kareem H, Constantinescu CS, Robinson K, Gran B (2015) Helicobacter pylori infection reduces disease severity in an experimental model of multiple sclerosis. Front Microbiol 6:52. doi:10.3389/fmicb.2015.00052
Cover TL, Blaser MJ (2009) Helicobacter pylori in health and disease. Gastroenterology 136(6):1863–1873. doi:S0016-5085(09)00339-4 [pii] 10.1053/j.gastro.2009.01.073
Cremonini F, Di Caro S, Delgado-Aros S, Sepulveda A, Gasbarrini G, Gasbarrini A, Camilleri M (2003) Meta-analysis: the relationship between Helicobacter pylori infection and gastro-oesophageal reflux disease. Aliment Pharmacol Ther 18(3):279–289
Eder W, Ege MJ, von Mutius E (2006) The asthma epidemic. N Engl J Med 355(21):2226–2235
el-Omar E, Penman I, Cruikshank G, Dover S, Banerjee S, Williams C, McColl KE (1994) Low prevalence of Helicobacter pylori in inflammatory bowel disease: association with sulphasalazine. Gut 35(10):1385–1388
Engler DB, Leonardi I, Hartung ML, Kyburz A, Spath S, Becher B, Rogler G, Muller A (2015) Helicobacter pylori-specific protection against inflammatory bowel disease requires the NLRP3 inflammasome and IL-18. Inflamm Bowel Dis 21(4):854–861. doi:10.1097/MIB.0000000000000318
Engler DB, Reuter S, van Wijck Y, Urban S, Kyburz A, Maxeiner J, Martin H, Yogev N, Waisman A, Gerhard M, Cover TL, Taube C, Muller A (2014) Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc Natl Acad Sci U S A 111 (32):11810–11815. doi:1410579111 [pii] 10.1073/pnas.1410579111
Fallone CA, Barkun AN, Friedman G, Mayrand S, Loo V, Beech R, Best L, Joseph L (2000) Is Helicobacter pylori eradication associated with gastroesophageal reflux disease? Am J Gastroenterol 95(4):914–920. doi:10.1111/j.1572-0241.2000.01929.x
Fischbach LA, Graham DY, Kramer JR, Rugge M, Verstovsek G, Parente P, Alsarraj A, Fitzgerald S, Shaib Y, Abraham NS, Kolpachi A, Gupta S, Vela MF, Velez M, Cole R, Anand B, El Serag HB (2014) Association between Helicobacter pylori and Barrett’s esophagus: a case-control study. Am J Gastroenterol 109(3):357–368. doi:10.1038/ajg.2013.443
Fischbach LA, Nordenstedt H, Kramer JR, Gandhi S, Dick-Onuoha S, Lewis A, El-Serag HB (2012) The association between Barrett’s esophagus and Helicobacter pylori infection: a meta-analysis. Helicobacter 17(3):163–175. doi:10.1111/j.1523-5378.2011.00931.x
Forman D (2005) Re: the role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97(13):1013–1014; author reply 1014. doi:97/13/1013 [pii] 10.1093/jnci/dji180
Gall A, Fero J, McCoy C, Claywell BC, Sanchez CA, Blount PL, Li X, Vaughan TL, Matsen FA, Reid BJ, Salama NR (2015) Bacterial composition of the human upper gastrointestinal tract microbiome is dynamic and associated with genomic instability in a Barrett’s esophagus cohort. PLoS ONE 10(6):e0129055. doi:10.1371/journal.pone.0129055
Gardner JD, Sloan S, Robinson M, Miner PB Jr (2004) Frequency analyses of gastric pH in control and gastro-oesophageal reflux disease subjects treated with a proton-pump inhibitor. Aliment Pharmacol Ther 20(11–12):1381–1386. doi:10.1111/j.1365-2036.2004.02279.x
Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A (2014) Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev 13(1):3–10. doi:10.1016/j.autrev.2013.06.004
Ghoshal UC, Chourasia D (2010) Gastroesophageal reflux disease and Helicobacter pylori: what may be the relationship? J Neurogastroenterol Motil 16(3):243–250. doi:10.5056/jnm.2010.16.3.243
Halme L, Rautelin H, Leidenius M, Kosunen TU (1996) Inverse correlation between Helicobacter pylori infection and inflammatory bowel disease. J Clin Pathol 49(1):65–67
Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, Pena A, Rollan A, Viviani P, Guiraldes E, Schmitz JM, Lorenz RG, Novak L, Smythies LE, Smith PD (2008) Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134(2):491–499. doi:S0016-5085(07)01996-8 [pii] 10.1053/j.gastro.2007.11.006
Haruma K (2004) Review article: influence of Helicobacter pylori on gastro-oesophageal reflux disease in Japan. Aliment Pharmacol Ther 20(Suppl 8):40–44. doi:10.1111/j.1365-2036.2004.02228.x
Helmer KS, West SD, Vilela R, Chang L, Cui Y, Kone BC, Mercer DW (2004) Lipopolysaccharide-induced changes in rat gastric H/K-ATPase expression. Ann Surg 239(4):501–509
Herbarth O, Bauer M, Fritz GJ, Herbarth P, Rolle-Kampczyk U, Krumbiegel P, Richter M, Richter T (2007) Helicobacter pylori colonisation and eczema. J Epidemiol Community Health 61(7):638–640. doi:61/7/638 [pii] 10.1136/jech.2006.046706
Herrera V, Parsonnet J (2009) Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect 15(11):971–976. doi:CLM3031 [pii] 10.1111/j.1469-0691.2009.03031.x
Higgins PD, Johnson LA, Luther J, Zhang M, Sauder KL, Blanco LP, Kao JY (2010) Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: Mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis. doi:10.1002/ibd.21489
Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, Muller A (2012) Caspase-1 Has Both Proinflammatory and Regulatory Properties in Helicobacter Infections, Which Are Differentially Mediated by Its Substrates IL-1beta and IL-18. J Immunol 188(8):3594–3602. doi:jimmunol.1103212 [pii] 10.4049/jimmunol.1103212
Iijima K, Koike T, Shimosegawa T (2015) Reflux esophagitis triggered after Helicobacter pylori eradication: a noteworthy demerit of eradication therapy among the Japanese? Front Microbiol 6:566. doi:10.3389/fmicb.2015.00566
Jin X, Chen YP, Chen SH, Xiang Z (2013) Association between Helicobacter pylori infection and ulcerative colitis–a case control study from China. Int J Med Sci 10(11):1479–1484. doi:10.7150/ijms.6934ijmsv10p1479 [pii]
Kamada T, Haruma K, Kawaguchi H, Yoshihara M, Sumii K, Kajiyama G (1998) The association between antral G and D cells and mucosal inflammation, atrophy, and Helicobacter pylori infection in subjects with normal mucosa, chronic gastritis, and duodenal ulcer. Am J Gastroenterol 93(5):748–752. doi:10.1111/j.1572-0241.1998.218_a.x
Kandulski A, Malfertheiner P (2014) Helicobacter pylori and gastroesophageal reflux disease. Curr Opin Gastroenterol 30(4):402–407. doi:10.1097/MOG.0000000000000085
Kim DJ, Park JH, Franchi L, Backert S, Nunez G (2013) The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori-infected dendritic cells. Eur J Immunol 43:2650–2658. doi:10.1002/eji.201243281
Koch KN, Hartung ML, Urban S, Kyburz A, Bahlmann AS, Lind J, Backert S, Taube C, Muller A (2015) Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J Clin Invest 125(8):3297–3302. doi:79337 [pii] 10.1172/JCI79337
Koch KN, Muller A (2015) Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes 6(6):382–387. doi:10.1080/19490976.2015.1105427
Labenz J, Blum AL, Bayerdorffer E, Meining A, Stolte M, Borsch G (1997) Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology 112(5):1442–1447
Laine L, Sugg J (2002) Effect of Helicobacter pylori eradication on development of erosive esophagitis and gastroesophageal reflux disease symptoms: a post hoc analysis of eight double blind prospective studies. Am J Gastroenterol 97(12):2992–2997. doi:10.1111/j.1572-0241.2002.07116.x
Lao-Sirieix P, Corovic A, Jankowski J, Lowe A, Triadafilopoulos G, Fitzgerald RC (2008) Physiological and molecular analysis of acid loading mechanisms in squamous and columnar-lined esophagus. Dis Esophagus: Official J Int Soc Dis Esophagus/ISDE 21(6):529–538. doi:10.1111/j.1442-2050.2007.00807.x
Lebwohl B, Blaser MJ, Ludvigsson JF, Green PH, Rundle A, Sonnenberg A, Genta RM (2013) Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am J Epidemiol 178(12):1721–1730. doi:kwt234 [pii] 10.1093/aje/kwt234
Lee YC, Chen TH, Chiu HM, Shun CT, Chiang H, Liu TY, Wu MS, Lin JT (2013) The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 62(5):676–682. doi:10.1136/gutjnl-2012-302240
Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW, Yamaoka Y, Graham DY, Perez-Trallero E, Wadstrom T, Suerbaum S, Achtman M (2007) An African origin for the intimate association between humans and Helicobacter pylori. Nature 445(7130):915–918. doi:nature05562 [pii] 10.1038/nature05562
Liu FX, Wang WH, Wang J, Li J, Gao PP (2011) Effect of Helicobacter pylori infection on Barrett’s esophagus and esophageal adenocarcinoma formation in a rat model of chronic gastroesophageal reflux. Helicobacter 16(1):66–77. doi:10.1111/j.1523-5378.2010.00811.x
Luther J, Owyang SY, Takeuchi T, Cole TS, Zhang M, Liu M, Erb-Downward J, Rubenstein JH, Chen CC, Pierzchala AV, Paul JA, Kao JY (2011) Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut 60(11):1479–1486. doi:gut.2010.220087 [pii] 10.1136/gut.2010.220087
Maloy KJ, Powrie F (2011) Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474(7351):298–306. doi:10.1038/nature10208
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1(8390):1311–1315
Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, Sakaguchi Y, Nakayama C, Konno-Shimizu M, Matsuda R, Mochizuki S, Asada-Hirayama I, Tsuji Y, Kodashima S, Ono S, Niimi K, Mitsushima T, Koike K (2013) Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS ONE 8(7):e69891. doi:10.1371/journal.pone.0069891
Nakajima S, Hattori T (2003) Active and inactive gastroesophageal reflux diseases related to Helicobacter pylori therapy. Helicobacter 8(4):279–293
Nie S, Chen T, Yang X, Huai P, Lu M (2014) Association of Helicobacter pylori infection with esophageal adenocarcinoma and squamous cell carcinoma: a meta-analysis. Dis Esophagus: Official J Int Soc Dis Esophagus/ISDE 27(7):645–653. doi:10.1111/dote.12194
Nordenstedt H, Nilsson M, Johnsen R, Lagergren J, Hveem K (2007) Helicobacter pylori infection and gastroesophageal reflux in a population-based study (The HUNT Study). Helicobacter 12(1):16–22. doi:10.1111/j.1523-5378.2007.00466.x
Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Muller A (2013) Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A 110(8):3047–3052. doi:1211248110 [pii] 10.1073/pnas.1211248110
Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C, Quiding-Jarbrink M, Muller A (2012) DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest 122(3):1082–1096. doi:61029 [pii] 10.1172/JCI61029
Owyang SY, Luther J, Owyang CC, Zhang M, Kao JY (2012) Helicobacter pylori DNA’s anti-inflammatory effect on experimental colitis. Gut Microbes 3(2):168–171. doi:19181 [pii] 10.4161/gmic.19181
Parente F, Molteni P, Bollani S, Maconi G, Vago L, Duca PG, Rembacken B, Axon AT, Bianchi Porro G (1997) Prevalence of Helicobacter pylori infection and related upper gastrointestinal lesions in patients with inflammatory bowel diseases. A cross-sectional study with matching. Scand J Gastroenterol 32(11):1140–1146
Parsonnet J, Friedman GD, Orentreich N, Vogelman H (1997) Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut 40(3):297–301
Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK (1991) Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 325(16):1127–1131
Parsonnet J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, Orentreich N, Vogelman JH, Friedman GD (1994) Helicobacter pylori infection and gastric lymphoma. N Engl J Med 330(18):1267–1271
Parsonnet J, Isaacson PG (2004) Bacterial infection and MALT lymphoma. N Engl J Med 350(3):213–215
Pearce CB, Duncan HD, Timmis L, Green JR (2000) Assessment of the prevalence of infection with Helicobacter pylori in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 12(4):439–443
Piodi LP, Bardella M, Rocchia C, Cesana BM, Baldassarri A, Quatrini M (2003) Possible protective effect of 5-aminosalicylic acid on Helicobacter pylori infection in patients with inflammatory bowel disease. J Clin Gastroenterol 36(1):22–25
Pohl H, Welch HG (2005) The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97(2):142–146. doi:97/2/142 [pii] 10.1093/jnci/dji024
Pritchard DM, Crabtree JE (2006) Helicobacter pylori and gastric cancer. Curr Opin Gastroenterol 22(6):620–625. doi:10.1097/01.mog.0000245539.50765.f600001574-200611000-00007 [pii]
Pronai L, Schandl L, Orosz Z, Magyar P, Tulassay Z (2004) Lower prevalence of Helicobacter pylori infection in patients with inflammatory bowel disease but not with chronic obstructive pulmonary disease—antibiotic use in the history does not play a significant role. Helicobacter 9(3):278–283. doi:10.1111/j.1083-4389.2004.00223.xHEL223 [pii]
Qian B, Ma S, Shang L, Qian J, Zhang G (2011) Effects of Helicobacter pylori eradication on gastroesophageal reflux disease. Helicobacter 16(4):255–265. doi:10.1111/j.1523-5378.2011.00846.x
Rad R, Ballhorn W, Voland P, Eisenacher K, Mages J, Rad L, Ferstl R, Lang R, Wagner H, Schmid RM, Bauer S, Prinz C, Kirschning CJ, Krug A (2009) Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology 136(7):2247–2257. doi:S0016-5085(09)00352-7 [pii] 10.1053/j.gastro.2009.02.066
Raghunath A, Hungin AP, Wooff D, Childs S (2003) Prevalence of Helicobacter pylori in patients with gastro-oesophageal reflux disease: systematic review. BMJ 326(7392):737. doi:10.1136/bmj.326.7392.737
Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, Blaser MJ (2008) Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS ONE 3(12):e4060
Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, Zaitoun AM, Atherton JC (2008) Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut 57(10):1375–1385. doi:gut.2007.137539 [pii] 10.1136/gut.2007.137539
Roka K, Roubani A, Stefanaki K, Panayotou I, Roma E, Chouliaras G (2014) The prevalence of Helicobacter pylori gastritis in newly diagnosed children with inflammatory bowel disease. Helicobacter 19(5):400–405. doi:10.1111/hel.12141
Rokkas T, Gisbert JP, Niv Y, O’Morain C (2015) The association between Helicobacter pylori infection and inflammatory bowel disease based on meta-analysis. United European Gastroenterol J 3(6):539–550. doi:10.1177/205064061558088910.1177_2050640615580889 [pii]
Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G (2007) Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol 5(12):1413–1417, 1417 e1411–1412. doi:10.1016/j.cgh.2007.08.010
Rubenstein JH, Inadomi JM, Scheiman J, Schoenfeld P, Appelman H, Zhang M, Metko V, Kao JY (2014) Association between Helicobacter pylori and Barrett’s esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol 12(2):239–245. doi:10.1016/j.cgh.2013.08.029
Runge TM, Abrams JA, Shaheen NJ (2015) Epidemiology of Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am 44(2):203–231. doi:10.1016/j.gtc.2015.02.001
Saad AM, Choudhary A, Bechtold ML (2012) Effect of Helicobacter pylori treatment on gastroesophageal reflux disease (GERD): meta-analysis of randomized controlled trials. Scand J Gastroenterol 47(2):129–135. doi:10.3109/00365521.2011.648955
Sakata H, Fujimoto K (2005) Barrett’s esophagus and Helicobacter pylori. Nihon Rinsho 63(8):1383–1386
Salama NR, Hartung ML, Muller A (2013) Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol 11(6):385–399. doi:nrmicro3016 [pii] 10.1038/nrmicro3016
Sayi A, Kohler E, Toller IM, Flavell RA, Muller W, Roers A, Müller A (2011) TLR-2-activated B cells suppress Helicobacter-induced preneoplastic gastric immunopathology by inducing T regulatory-1 cells. J Immunol 186(2):878–890. doi:jimmunol.1002269 [pii] 10.4049/jimmunol.1002269
Semper RP, Mejias-Luque R, Gross C, Anderl F, Muller A, Vieth M, Busch DH, Prazeres da Costa C, Ruland J, Gross O, Gerhard M (2014) Helicobacter pylori-Induced IL-1beta Secretion in Innate Immune Cells Is Regulated by the NLRP3 Inflammasome and Requires the Cag Pathogenicity Island. J Immunol 193:3566–3576. doi:jimmunol.1400362 [pii] 10.4049/jimmunol.1400362
Serrano C, Wright SW, Bimczok D, Shaffer CL, Cover TL, Venegas A, Salazar MG, Smythies LE, Harris PR, Smith PD (2013) Downregulated Th17 responses are associated with reduced gastritis in Helicobacter pylori-infected children. Mucosal Immunol 6(5):950–959. doi:mi2012133 [pii] 10.1038/mi.2012.133
Shaheen N, Ransohoff DF (2002) Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA 287(15):1972–1981
Shiotani A, Miyanishi T, Kamada T, Haruma K (2008) Helicobacter pylori infection and allergic diseases: epidemiological study in Japanese university students. J Gastroenterol Hepatol 23(7 Pt 2):e29–33. doi:JGH5107 [pii] 10.1111/j.1440-1746.2007.05107.x
Sonnenberg A, Genta RM (2012) Low prevalence of Helicobacter pylori infection among patients with inflammatory bowel disease. Aliment Pharmacol Ther 35(4):469–476. doi:10.1111/j.1365-2036.2011.04969.x
Sonnenberg A, Genta RM (2016) Inverse Association Between Helicobacter pylori Gastritis and Microscopic Colitis. Inflamm Bowel Dis 22(1):182–186. doi:10.1097/MIB.0000000000000595
Sun X, Zhang M, El-Zataari M, Owyang SY, Eaton KA, Liu M, Chang YM, Zou W, Kao JY (2013) TLR2 mediates Helicobacter pylori-induced tolerogenic immune response in mice. PLoS ONE 8(9):e74595. doi:10.1371/journal.pone.0074595PONE-D-13-08235 [pii]
Tanaka I, Tatsumi Y, Kodama T, Kato K, Fujita S, Mitsufuji S, Kashima K (2004) Effect of Helicobacter pylori eradication on gastroesophageal function. J Gastroenterol Hepatol 19(3):251–257
Thrift AP, Pandeya N, Smith KJ, Green AC, Hayward NK, Webb PM, Whiteman DC (2012) Helicobacter pylori infection and the risks of Barrett’s oesophagus: a population-based case-control study. Int J Cancer 130(10):2407–2416. doi:10.1002/ijc.26242
Tsuji K, Uehara A, Okumura T, Taniguchi Y, Kitamori S, Takasugi Y, Namiki M (1992) The gastric antisecretory action of lipopolysaccharide is blocked by indomethacin. Eur J Pharmacol 210(2):213–215
Vaira D, Vakil N, Rugge M, Gatta L, Ricci C, Menegatti M, Leandro G, Holton J, Russo VM, Miglioli M (2003) Effect of Helicobacter pylori eradication on development of dyspeptic and reflux disease in healthy asymptomatic subjects. Gut 52(11):1543–1547
Wang F, Xia P, Wu F, Wang D, Wang W, Ward T, Liu Y, Aikhionbare F, Guo Z, Powell M, Liu B, Bi F, Shaw A, Zhu Z, Elmoselhi A, Fan D, Cover TL, Ding X, Yao X (2008) Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J Biol Chem 283(39):26714–26725. doi:10.1074/jbc.M800527200
Wang Q, Yu C, Sun Y (2013) The association between asthma and Helicobacter pylori: a meta-analysis. Helicobacter 18(1):41–53. doi:10.1111/hel.12012
Weyermann M, Rothenbacher D, Brenner H (2009) Acquisition of Helicobacter pylori infection in early childhood: independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol 104(1):182–189. doi:ajg200861 [pii] 10.1038/ajg.2008.61
Wu XW, Ji HZ, Yang MF, Wu L, Wang FY (2015) Helicobacter pylori infection and inflammatory bowel disease in Asians: a meta-analysis. World J Gastroenterol 21(15):4750–4756. doi:10.3748/wjg.v21.i15.4750
Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448(7152):427–434. doi:10.1038/nature06005
Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, Shao L, Zou DH, Yu XM, Mao WM (2013a) Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol 19(36):6098–6107. doi:10.3748/wjg.v19.i36.6098
Xie T, Cui X, Zheng H, Chen D, He L, Jiang B (2013b) Meta-analysis: eradication of Helicobacter pylori infection is associated with the development of endoscopic gastroesophageal reflux disease. Eur J Gastroenterol Hepatol 25(10):1195–1205. doi:10.1097/MEG.0b013e328363e2c7
Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH (2010) Is there an increased risk of GERD after Helicobacter pylori eradication?: a meta-analysis. Am J Gastroenterol 105(5):1007–1013; quiz 1006, 1014. doi:10.1038/ajg.2009.734
Zhou X, Wu J, Zhang G (2013) Association between Helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol 25(4):460–468. doi:10.1097/MEG.0b013e32835c280a
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kyburz, A., Müller, A. (2017). Helicobacter pylori and Extragastric Diseases. In: Tegtmeyer, N., Backert, S. (eds) Molecular Pathogenesis and Signal Transduction by Helicobacter pylori. Current Topics in Microbiology and Immunology, vol 400. Springer, Cham. https://doi.org/10.1007/978-3-319-50520-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-50520-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50519-0
Online ISBN: 978-3-319-50520-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)