Abstract
Acanthocephalans (spiny head worms) are a medium-sized phylum (about 1000 species have been described) of usually small (few mm to over 1m) vertebrate intestinal parasites. They are pseudocoelomates with bilateral symmetry and usually cylindrical bodies. The sexes are separate, with females usually larger than males. The body consists of a proboscis, neck, and trunk. The proboscis, neck, and internal organs connected with them (proboscis receptacle and lemnisci) form the fore-body. In some cases, the trunk may be divided into two parts of different shape: fore-trunk and hind-trunk. The proboscis is armed with recurved hooks. The hooks consist of two parts: blade (thorn) and root, both usually directed posteriorly. Hooks situated at the base of the proboscis (basal hooks) are usually rootless. The proboscis (usually retractable) may be invaginated into the proboscis receptacle. The latter contains a cerebral ganglion. Two lemnisci lie parallel to the proboscis receptacle. The trunk may be unarmed, or armed with spines. This armament is usually restricted to the anterior part of the trunk, but sometimes reaches the posterior end of the body. The genital pore may be subterminal or terminal. Spines surrounding the genital pore are often separated from the other ones by a bare zone. In such cases, the armament of the trunk is divided into somatic and genital spines. Ligaments (one or two) run along the trunk, and sexual organs are attached to them. The male reproductive system consists of 2 testes, cement glands (4–8 in number in Antarctic species), seminal ducts, cement ducts and reservoirs, Säfftigen’s pouch, penis and the copulatory (retracted or everted). The female reproductive system consists of ovarian balls, a uterine bell (an organ for selection of immature and mature eggs), a uterus, and a vagina, with a single or a double sphincter. Ovarian balls are enclosed in ligament sacs in juvenile females and are liberated during maturation. Eggs mature in the pseudocoelom of a female. In fact, in mature females these are not eggs, but the first larval stage (acanthors) enclosed in 3–4 envelopes. More correct terms are “shelled acanthors” and “embryophores”, but these are rarely used. Acanthocephalans have reduced the muscular, nervous, circulatory, and excretory systems and complete loss of the digestive system. Absorption and excretion take place through the tegument. The latter contains a system of canals known as the lacular system. The number and arrangement of main lacular canals are of fundamental value in the classification of higher taxa (classes). Excretion is by diffusion except in Oligacanthorhynchidae (with two protonephridial organs). The life cycles involve an arthropod (intermediate host) and a vertebrate definitive or paratenic host. Eggs are shed with the host’s faeces, when the definitive host, the appropriate intermediate host ingests them, and the acanthor is liberated and pierces the gut wall. In the arthropod body cavity, the acanthor develops into an acanthella and then into an infective cystacanth, which matures to adulthood in the gut of the definitive host, following ingestion of the infected arthropod (Amin 1987; Zdzitowiecki 1991).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
Acanthocephalans (spiny head worms) are a medium-sized phylum (about 1000 species have been described) of usually small (few mm to over 1m) vertebrate intestinal parasites. They are pseudocoelomates with bilateral symmetry and usually cylindrical bodies. The sexes are separate, with females usually larger than males. The body consists of a proboscis, neck, and trunk. The proboscis, neck, and internal organs connected with them (proboscis receptacle and lemnisci) form the fore-body. In some cases, the trunk may be divided into two parts of different shape: fore-trunk and hind-trunk. The proboscis is armed with recurved hooks. The hooks consist of two parts: blade (thorn) and root, both usually directed posteriorly. Hooks situated at the base of the proboscis (basal hooks) are usually rootless. The proboscis (usually retractable) may be invaginated into the proboscis receptacle. The latter contains a cerebral ganglion. Two lemnisci lie parallel to the proboscis receptacle. The trunk may be unarmed, or armed with spines. This armament is usually restricted to the anterior part of the trunk, but sometimes reaches the posterior end of the body. The genital pore may be subterminal or terminal. Spines surrounding the genital pore are often separated from the other ones by a bare zone. In such cases, the armament of the trunk is divided into somatic and genital spines. Ligaments (one or two) run along the trunk, and sexual organs are attached to them. The male reproductive system consists of 2 testes, cement glands (4–8 in number in Antarctic species), seminal ducts, cement ducts and reservoirs, Säfftigen’s pouch, penis and the copulatory bursa (retracted or everted). The female reproductive system consists of ovarian balls, a uterine bell (an organ for selection of immature and mature eggs), a uterus, and a vagina, with a single or a double sphincter. Ovarian balls are enclosed in ligament sacs in juvenile females and are liberated during maturation. Eggs mature in the pseudocoelom of a female. In fact, in mature females these are not eggs, but the first larval stage (acanthors) enclosed in 3–4 envelopes. More correct terms are “shelled acanthors” and “embryophores”, but these are rarely used. Acanthocephalans have reduced the muscular, nervous, circulatory, and excretory systems and complete loss of the digestive system. Absorption and excretion take place through the tegument. The latter contains a system of canals known as the lacular system. The number and arrangement of main lacular canals are of fundamental value in the classification of higher taxa (classes). Excretion is by diffusion except in Oligacanthorhynchidae (with two protonephridial organs). The life cycles involve an arthropod (intermediate host) and a vertebrate definitive or paratenic host. Eggs are shed with the host’s faeces, when the definitive host, the appropriate intermediate host ingests them, and the acanthor is liberated and pierces the gut wall. In the arthropod body cavity, the acanthor develops into an acanthella and then into an infective cystacanth, which matures to adulthood in the gut of the definitive host, following ingestion of the infected arthropod (Amin 1987; Zdzitowiecki 1991).
One of the present authors (Zdzitowiecki) published in 1991 the monograph of Antarctic Acanthocephala, this chapter contains new data of this parasites.
The phylum includes four classes: Archiacanthocephala, Eoacanthocephala, Palaeacanthocephala, and Polyacanthocephala
Representatives of two orders of Palaeacanthocephala (Echinorhynchida and Polymorphida) occur in notothenioid fishes (Zdzitowiecki 1991). Echinorhynchida use fishes as definitive hosts and occur in the lumen of the alimentary tract. Fishes become infected by feeding on crustaceans (intermediate hosts), or in cases of Polymorphida also small infected fishes which play a role as paratenic hosts of Polymorphida localized in cysts in the body cavity. Crustaceans of the order Amphipoda were recorded as intermediate hosts of two echinorhynchid species, Aspersentis megarhynchus (Linstow 1892) and Metacanthocephalus johnstoni Zdzitowiecki 1983, and three polymorphids, Corynosoma bullosum (Linstow 1892), C. hamanni (Linstow 1892), and C. pseudohamanni Zdzitowiecki, 1984, in Antarctica (Hoberg 1986; Zdzitowiecki 2001; Zdzitowiecki and Presler 2001; Laskowski et al. 2008). Definitive hosts of Antarctic polymorphids are marine mammals and birds. The infective stage, the cystacanth, is similar to the mature worm, but differs from the latter in the size of the trunk and degree of development of the sexual organs (Zdzitowiecki 1991). In cystacanths of the Polymorphidae Meyer, 1931 (with exceptions of Filicollis Lühe, 1911 and Profilicollis Meyer, 1931), the dimensions of the proboscis and the development and size of both the proboscis hooks and trunk spines are usually identical with those of adults. Cystacanths occur in intermediate and paratenic hosts in cysts and are contracted; this is especially so in that they have an introverted proboscis. Cystacanths should be collected alive, liberated from their cysts, and relaxed. Such material can be determined on the basis of most of the diagnostic morphological features useful for adults.
8.2 Checklist of the Antarctic and Sub-Antarctic Acanthocephala
-
Class Palaeacanthocephala
-
Order Echinorhynchida
-
Family Heteracanthocephalidae; Subfamily Aspersentinae
-
Genus Aspersentis
-
Species:
-
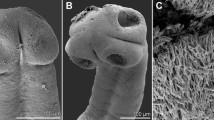
Aspersentis megarhynchus (von Linstow 1892) (Fig. 8.1)
Fig. 8.1 Aspersentis megarhynchus (Linstow 1892): adult male, proboscis; female body end; male cystacanth from Bovallia gigantea; advanced male acanthella from Hippomedon kergueleni
-
Aspersentis johni (Baylis 1929) (Fig. 8.2)
Fig. 8.2 Aspersentis johni (Baylis 1929): adult male, proboscis; female body end
-
Aspersentis zanclorhynchi (Johnston and Best 1937) Smales 1996
-
Family Arhythmacanthidae
-
Genus Heterosentis
-
Species:
-
Heterosentis heteracanthus Linstow 1896 (Fig. 8.3)
Fig. 8.3 Heterosentis heteracanthus (Linstow 1896): adult male, proboscis; female body end
-
Heterosentis hirsutus Pichelin and Cribb 1999
-
Heterosentis zdzitowieckii (Kumar 1992)
-
Genus Hypoechinorhynchus
-
Species:
-
Hypoechinorhynchus magellanicus Szidat 1950 (Fig. 8.4)
Fig. 8.4 Hypoechinorhynchus magellanicus (Szidat 1950): adult male, proboscis; arrangement of proboscis hooks; somatic spines of male
-
Family Echinorhynchidae Subfamily Echinorhynchinae
-
Genus Echinorhynchus
-
Species:
-
Echinorhynchus petrotschenkoi Rodjuk 1984 (Fig. 8.5)
Fig. 8.5 Echinorhynchus petrotschenkoi (Rodjuk 1984): adult female and male, proboscis
-
Echinorhynchus muraenolepisi Rodjuk 1984
-
Family Rhadinorhynchidae Subfamily Gorgorhynchinae
-
Genus Metacanthocephalus
-
Species:
-
Metacanthocephalus campbelli (Leiper and Atkinson 1914)
-
Metacanthocephalus dalmori Zdzitowiecki, 1983
-
Metacanthocephalus johnstoni Zdzitowiecki, 1983 (Fig. 8.6)
-
Metacanthocephalus rennicki (Leiper and Atkinson 1914)
-
Order Polymorphida
-
Family Polymorphidae
-
Genus Profilicollis
-
Species:
-
Profilicollis antarcticus Zdzitowiecki 1985 (Fig. 8.7)
Fig. 8.7 Profilicollis antarcticus Zdzitowiecki 1985: adult male and proboscis
-
Profilicollis novaezelandensis Brockerhoff and Smales, 2002
-
Genus Corynosoma
-
Species:
-
Corynosoma arctocephali Zdzitowiecki, 1984 (Fig. 8.8)
Fig. 8.8 Corynosoma bullosum (Linstow 1892): adult male and female; cystacanth from Waldeckia obesa
-
Corynosoma australe Johnston, 1937
-
Corynosoma beaglense Laskowski, Jeżewski, Zdzitowiecki, 2008 (Fig. 8.9)
-
Corynosoma bullosum (Linstow 1892) (Fig. 8.10)
Fig. 8.10 Corynosoma hamanni (Linstow 1892): adult male and female; female body end; cystacanth from Prostebbingia brevicornis
-
Corynosoma evae Zdzitowiecki, 1984 (Fig. 8.11)
-
Corynosoma gibsoni Zdzitowiecki, 1986 (Fig. 8.12)
-
Corynosoma hannae Zdzitowiecki, 1984
-
Corynosoma pseudohamanni Zdzitowiecki, 1984 (Fig. 8.14)
-
Corynosoma shackletoni Zdzitowiecki, 1978
-
Genus Andracantha
-
Species:
-
Andracantha baylisi (Zdzitowiecki 1986a, b, c, d, e, f, g) Zdzitowiecki, 1989 (Fig. 8.15)
-
Andracantha clavata (Goss 1940)
-
Genus: Bolbosoma
8.3 Representatives of Acanthocephalans Genera Occurring in Antarctica and Sub-Antarctica
(Zdzitowiecki 1991; Laskowski and Zdzitowiecki 2004, 2008; Laskowski et al. 2008, 2010)
Diagnosis: Trunk spined. Proboscis cylindrical, relatively short. Ventral proboscis hooks larger than dorsal. Proboscic receptacle double-walled, ganglion in its posterior half. Neck short. Cement glands in males pyriform, six in number, forming compact group. Vaginal sphincter in females double. Eggs with polar prolongations of middle envelope. Parasites of fishes.
Synonyms: A. austrinus Van Cleave 1929 , Rhadinorhynchus wheeleri Baylis 1929, Heteracanthocephalus hureaui Dollfus 1965.
Diagnosis (after Zdzitowiecki 1981): Proboscis hooks in 13–16 rows of 8–11. The largest hook is the third one counting from tip. Trunk spines conspicuous anteriorly (maximum length 35 μm), very small, and hardly visible at posterior trunk end. Lemnisci slightly longer than proboscis receptacle.
Male. Total dimensions 3.6–5.5 × 0.73–1.39 mm. Proboscis 0.47–0.63 × 0.20–0.31 mm. Maximum hook length 106–135 μm. Testes arranged in tandem to diagonally.
Female. Total dimensions 5.6–9.6 × 1.16–2.09 mm. Proboscis 0.51–0.73 × 0.29–0.35 mm. Maximum hook length 119–149 μm. Eggs 60–88 × 19–25 μm.
-
Suitable definitive hosts: fishes.
Nototheniidae: Notothenia acuta, N. coriiceps, N. cyanobrancha, N. rossii, Nototheniops mizops, Lindbergichthys nudifrons, Gobionotothen gibberifrons, Pagothenia bernacchii, P. hansoni, Trematomus newnesi; Bathydraconidae: Parachaenichthys charcoti, P. georgianus; Channichthyidae: Channichthys rhinoceratus, Chaenocephalus aceratus; Harpagiferidae: Harpagifer antarcticus.
Intermediate hosts: amphipods. Eusiridae: Bovallia gigantea; Gammarellidae: Gondogeneia antarctica; Ischyroceridae: Jassa ingens; Lysianassoidea: Hippomedon kergueleni and Orchomenella rotundifrons.
Habitat: Males mainly in posterior half of small intestine, females mainly in large intestine. Few specimens in other parts of intestine.
Biology and ecology: According to Zdzitowiecki and Rokosz (1986), Zdzitowiecki (1990b), Zdzitowiecki and White (1996), Zdzitowiecki and Presler (2001), Zdzitowiecki and Laskowski (2004), Laskowski and Zdzitowiecki (2010), Laskowski et al. (2012), the species is associated with the inshore (fiord) environment, where infections of fishes take place. N. coriiceps and juvenile specimens of N. rossii living in Admiralty Bay (the South Shetland Islands) and N. coriiceps caught in the coastal zone at Signy Island (South Orkney Islands) are massively infected (prevalence 100%, maximum intensity of infection 180, 91, and 81, respectively). Other fishes are much less infected. A. megarhynchus, the dominant echinorhynchid species in the Admiralty Bay and South Orkney Islands, was extremely rare at the Vernadsky Station (Argentine Islands). Only two N. coriiceps specimens of 93 examined were infected by one and 14 parasites (prevalence 2 %). Adult specimens of N. rossii living in the open sea are also less infected, while other fishes living in the open sea at South Shetland Islands and at South Georgia are uninfected. The parasite occurs in fishes during the whole year, but infections of N. coriiceps and N. rossii in Admiralty Bay are more numerous in winter than in summer (incomplete seasonality). Cystacanths of A. megarhynchus were found in four sub-coastal host species belonging to four families of Amphipoda in the Admiralty Bay.
Distribution: Circumpolar species not far from the Antarctic convergence in the Sub-Antarctic (Kerguelen subregion); South Shetland Islands, South Orkney Islands, South Georgia, Heard, Kerguelen, Crozet, Macquarie, Ob Bank, and Argentine Islands (Linstow 1892; Van Cleave 1929; Baylis 1929; Joyeux and Baer 1954; Edmonds 1955, 1957; Dollfus 1965; Szidat and Graefe 1967; Golvan 1969; Parukhin and Sysa 1975; Parukhin and Lyadov 1982; Hoogesteger and White 1981; Zdzitowiecki 1981, 1987, 1990a, b; Zdzitowiecki and Rokosz 1986; Zdzitowiecki and Laskowski 2004; Rodjuk 1985; Reimer 1987).
Diagnosis (after Laskowski and Zdzitowiecki 2004): Proboscis almost cylindrical, relatively narrow (length/width ratio 2.16–3.22:1, mean 2.78:1), widest anteriorly, curved towards ventral side. Hooks normally arranged in 14 rows, rarely in 13 or 15 rows, of 10–13 hooks, either in same number in all rows around proboscis or with difference of one hook in neighbouring rows. Ventral hooks (with exceptions of 2–4 posterior-most) much larger than dorsal hooks. Number of large hooks gradually decreases in lateral rows; not less than 3 dorsal rows exclusively contain small hooks. Dimensions of large hooks decrease posteriorly; distal or sub-distal hooks are longest. All small hooks of similar length and with roots (roots of small hooks are hardly visible and often unmeasurable); blades and roots directed posteriorly. Roots of small hooks have process directed anteriorly. Blades are longer than roots, larger in females than males. Neck unarmed curved towards ventral side. Anterior trunk armed with spines, maximum length of spines on ventral side c.30 μm, smaller on dorsal side. Conspicuous ventral spines extend posteriorly over 9.7–13.9 (11.9)% of trunk length in males, 7.6–13.3 (10.7)% in females. Smaller spines of various dimensions are visible more posteriorly, especially near posterior end of trunk and on ventral side just beyond large spines. Proboscis receptacle extends posteriorly beyond range of large spines. Lemnisci longer and narrower than proboscis receptacle.
Male. Total dimensions 4.03–6.21 × 0.518–0.912 mm. Proboscis 0.419–0.580 × 0.156–0.226 mm. Maximum length of ventral hook 77–101 μm. Trunk spindle-shaped. Testes oval, tandem to oblique in mid-length of trunk. Cement glands pear-shaped, 6 in number, forming compact group. Posterior end of trunk oval; genital aperture shifted slightly to dorsal side. When everted genital bursa is bell-like.
Female. Total dimensions 6.12–8.54 × 0.71–1.16 mm. Proboscis 0.49–0.66 × 0.18–0.28 mm. Maximum length of ventral hook 84–108 μm. Eggs 87–102 × 20–26 μm. Trunk spindle-shaped, more elongate than in males, with 2 lateral lobes invariably present at posterior end. Uterine bell obscured by eggs. Vaginal sphincter double. Genital aperture in concavity between lateral lobes. Total length of female genital system (uterine bell, uterus, and vagina) was measurable approximately in 2 cases and reached 1.5 mm in immature specimen and 2.1 mm in mature specimen. Mature eggs elongate, with polar prolongations of middle envelope.
-
Definitive host: fishes.
-
Nototheniidae: Patagonotothen longipes; Merlucciidae: Merluccius sp.; Channichthyidae: Champsocephalus esox.
-
Habitat: intestine, large intestine (rectum).
Biology and ecology: (after Laskowski and Zdzitowiecki 2004, 2009): The infection of the Patagonotothen longipes and Champsocephalus esox at Beagle Channel (eastern mouth of the Beagle Channel): prevalence 85 and 25%, maximum intensity 18 and 4 parasites in one fish, respectively.
Distribution: Beagle Channel, Magellanic subregion of the Sub-Antarctic waters off the Falkland Islands (Baylis 1929; Laskowski and Zdzitowiecki 2004).
The only other representative of Aspersentis occurring in notothenioids is A. megarhynchus (Linstow 1892). Features useful to distinguish A. johni from A. megarhynchus are: 10–13 vs. 7–11 proboscis hooks in each row, the maximum length of the ventral hooks 77–108 vs. 106–149 μm, a narrower proboscis with a length/width ratio of 2.16–3.22:1 (mean 2.78:1) vs. 1.66–2.27:1 (mean 2.015:1), an egg length of 87–102 vs. 60–88 μm, and an unusual form of the posterior extremity of females (the presence of a terminal concavity between two lateral lobes). Another representative is A. zanclorhynchi (Johnston and Best 1937) Smales 1996, synonym Echinorhynchus sensu lato from Zanclorhynchus spinifer (Zdzitowiecki 1986a).
-
Family Arhythmacanthidae Yamaguti, 1935
-
Genus Heterosentis Van Cleave 1931
Diagnosis: Trunk spined anteriorly. Proboscis relatively short, cylindrical to globular. Two to three types of hooks along proboscis. Proboscis receptacle double-walled. Ganglion at base of proboscis receptacle. Neck short. Cement glands in males pyriform, six in number, forming compact group. Vaginal sphincter in females single. Eggs with polar prolongations of middle envelope. Parasites of fishes.
Diagnosis (after Zdzitowiecki 1984a): Proboscis short, narrowed at base. Hooks in 10 rows of 3–5. One large distal hook and 2–4 rootless basal hooks in every row. Blade and root of distal hook similar in length. Lemnisci longer than proboscis receptacle.
Male. Total dimensions 3.6–4.5 × 0.43–0.58 mm. Proboscis 0.224–0.252 × 0.154–0.161 mm. Maximum length of distal hook 58–60 μm. Testes in tandem.
Female. Total dimensions 6.6 × 0.75 mm. Proboscis 0.264 × 0.195 mm. Maximum length of distal hook 76 μm.
-
Definitive hosts: fishes.
Atherinidae: Chirostoma microlepidotus; Nototheniidae: N. coriiceps, N. rossii, N. squamifrons, Gobionotothen gibberifrons, N. nybelini, Lindbergichthys nudifrons, Patagonotothen longipes, P. tessellata; Artedidraconidae: Artedidraco mirus; Bathydraconidae: Parachaenichthys georgianus: Channichthyidae: Champsocephalus esox.
-
Habitat: Mainly large intestine. Few specimens in posterior half of small intestine.
Biology and ecology: The species is rare in the Antarctic and it seems to be more frequent in fiords than in the open sea. Of the fish examined in the eastern mouth of the Beagle Channel, Patagonotothen longipes was the most infected (prevalence 50%, maximum intensity 25), P. tessellata and Champsocephalus esox were less infected (prevalence 15% and 10%, maximum intensity 17 and 1, respectively) (Laskowski and Zdzitowiecki 2009).
Distribution: Strait of Magellan (South America), Beagle Channel, South Shetland Islands, and South Georgia (Linstow 1896; Van Cleave 1931; Meyer 1931; Zdzitowiecki 1984a, 1986g, 1987, 1990b; Laskowski and Zdzitowiecki 2009).
-
Genus Hypoechinorhynchus Yamaguti, 1939
Diagnosis (after Pichelin and Cribb 1999) Hypoechinorhynchus have the characteristic abrupt transition from basal spines to apical hooks; they also possess longitudinal rows, which alternate in their possession of a middle spine. The middle and posterior spines are small, thin and without roots (or very reduced roots); the middle spine may be longer than the posterior spine. Each longitudinal row has at least one large hook with a root.
Diagnosis (after Laskowski and Zdzitowiecki 2008): Trunk with antero-dorsal curvature. Proboscis spherical (length/width ratio 0.89–1.22:1) slightly curved towards ventral side. Proboscis armature: 40 hooks, including 15 large hooks with root > c .50% length of blade and 25 rootless basal spines. Large hooks arranged in 10 alternating rows of 1 and 2 hooks; anterior hook of each pair slightly smaller than other hooks; each single large hook is followed in same row by 2 spines; pairs of large hooks are followed by single spines. Ten single spines are present at base of proboscis between rows. This arrangement of proboscis armature could be also interpreted as 3 transverse rows of 5 large hooks in each and 3 transverse rows of basal spines containing 5, 10, and 10 spines, respectively. Unarmed neck slightly curved towards ventral side. Trunk cylindrical, armed anteriorly with loosely arranged small spines of 15–26 × 3–13 in size. Region of spination extends back 5.3–16.0% of trunk length. Proboscis receptacle double-walled, with ganglion at base. Lemnisci long, narrow, considerably longer than proboscis receptacle.
Male. Total length 5.90–7.81 mm. Proboscis 0.336–0.396 × 0.289–0.330 mm. Five large sub-apical hooks: blade length 109–138 μm, basal width 21–32 μm, root length c.60 μm in; posterior hook of pairs: blade length 132–170 μm, basal width 35–36 μm, root length 69–91 μm; single large hooks: blade length 145–184 μm, basal width 36–45 μm, root length 71–85 μm. Basal proboscis spines: blade length 37–94 μm, basal width 8–11 μm. Neck conical, 0.113–0.181 mm in length. Proboscis receptacle 0.875–1.072 × 0.237–0.318 mm. Length of lemnisci 1.800–2.137 mm. Trunk spindle-shaped, 5.435–7.230 × 1.187–1.387 mm; length/width ratio 4.58–5.21:1. Testes and cement glands form compact group far beyond proboscis receptacle. Testes more oblique than tandem; anterior testis 0.823–1.030 × 0.528–0.637 mm; posterior testis partly parallel with cement glands, 0.764–1.050 × 0.550–0.621 mm. Cement glands pear-shaped, 6 in number, form compact group. Säfftigen’s pouch 0.990–1.157 × 0.221–0.238 mm. Genital pore terminal.
Female. Total length 9.54 mm and 7.78 mm. Proboscis 0.364 × 0.409 mm and 0.399–0.375 mm. Five sub-apical hooks: blade length 122–124 μm, basal width 32 μm, 61 μm root length; posterior hooks of pairs: blade length 155–162 μm, 36 μm basal width, root length 81 μm; single large hooks: blade length 173–175 μm, basal width 45 μm, root length 85 μm. Basal proboscis spines: blade length 50–94 μm, basal width 10–21 μm. Neck conical, 211–213 μm in length. Proboscis receptacle 0.953 × 0.294 and 1.053 × 0.346. Lemnisci, uterine bell, and uterus obscured by eggs. Trunk spindle-shaped, 8.975–1.820 mm and 7.167–1.658 mm. Trunk length/width ratio 4.93:1 and 4.32:1. Genital pore terminal. Vaginal sphincter single, c. 160 × 150 μm. Eggs with polar prolongations of middle envelope, 71–86 × 16–22 μm, mean 76 × 19 μm.
-
Suitable definitive hosts: fishes. Nototheniidae: Eleginops maclovinus; Channichthyidae: Champsocephalus esox.
-
Habitat: Large intestine.
-
Biology and ecology: Not known.
Distribution: Beagle Channel, Ushuaia (Tierra del Fuego, South America) (Szidat 1950; 1965 Laskowski and Zdzitowiecki 2008; Laskowski and Zdzitowiecki 2009).
-
Family Echinorhynchidae Cobbold, 1876
-
Genus Echinorhynchus Zoega in Müller, 1776
Diagnosis: Trunk cylindrical, not spined. Proboscis cylindrical. Neck short. Proboscis receptacle double-walled. Ganglion at half of length of proboscis receptacle. Lemnisci claviform. Cement glands in males spherical or oval, six in number, arranged either in a compact group or in line along the trunk. Testes in tandem. Vaginal sphincter in females single. Eggs elongated, with long polar prolongations of middle envelope. Parasites of fishes.
Synonyms: Echinorhynchus sp. Kagei et Watanuki, 1975, E. nototheniae Zdzitowiecki, 1986, E. georgianus Rodjuk 1986.
Diagnosis (after Zdzitowiecki 1989b): Proboscis hooks arranged in 14–20 rows of 9/10–14/15, including 1–2 basal ones. Blades of hooks longer than roots. Proboscis receptacle a little longer than lemnisci.
Male. Total dimensions 6.3–13.6 × 0.51–1.34 mm. Proboscis 0.766–1.015 × 0.218–0.303 mm. Maximum hook length 63–85 μm. Testes oval. Cement glands arranged in principle along trunk, closely to each other. However, some cement glands can lie parallel forming one or two pairs.
Female. Total dimensions 12.2–30.0 × 0.75–1.48 mm. Proboscis 0.764–1.176 × 0.233–0.340 mm. Maximum hook length 64–87 μm. Eggs 89–121 × 19–25 μm.
Suitable definitive hosts: fishes. Muraenolepidae: Muraenolepis microps; Nototheniidae: Dissostichus eleginoides, Pagothenia bernacchii. Other (? unsuitable) definitive hosts: fishes. Nototheniidae: Dissostichus mawsoni, Notothenia coriiceps, Nototheniops nybelini, Pagothenia hansoni; Channichthyidae: Chaenocephalus aceratus, Cryodraco antarcticus.
-
Habitat: Small intestine.
Biology and ecology: The species is associated mainly with the open sea shelf environment (Zdzitowiecki 1990b). Prevalence of infection of the main definitive host, M. microps, at South Georgia 40%, maximum intensity 11.
Distribution: Probably circumpolar. Till now found at South Shetland Islands, South Georgia, and Syowa Station (Enderby Land) (Kagei and Watanuki 1975; Rodjuk 1984, 1986; Zdzitowiecki 1986d, g, 1989b, 1990b).
-
Family Rhadinorhynchidae Subfamily Gorgorhynchinae
-
Genus Metacanthocephalus Yamaguti, 1959
Diagnosis: Trunk not spined. Neck short. Proboscis cylindrical to ovoid. Ganglion in anterior half of proboscis receptacle. Proboscis receptacle double-walled. Lemnisci (in Antarctic species) longer than proboscis receptacle. Testes in tandem. Cement glands pyriform, eight in number, arranged in a compact group. Vaginal sphincter in females double. Eggs with polar prolongations of middle envelope. Parasites of fishes.
-
Metacanthocephalus johnstoni Zdzitowiecki, 1983 (Fig. 8.6)
-
Synonyms: Leptorhynchoides campbelli (1914) in Johnston and Best (1937) pro parte.
Diagnosis (after Zdzitowiecki 1983): Trunk oval or egg-shaped. Maximum width at half of its length. Proboscis cylindrical. Hooks arranged in 12–17 rows of 5–7/8 (usually 14–16 × 6–7). Blade of hook longer than root. Longest hook is the second or third one counting from base of proboscis.
Male. Total dimensions 3.60–7.37 × 0.60–2.10 mm. Proboscis 0.426–0.554 × 0.182–0.280 mm. Length: width ratio of proboscis 1.74–2.67: 1. Maximum hook length 71–86 μm.
Female. Total dimensions 6.06–8.66 × 1.94–3.22 mm. Proboscis 0.486–0.599 × 0.229–0.323 mm. Length: width ratio of proboscis 1.69–2.51: 1. Maximum hook length 79–96 μm. Length of female genital system 1.0–1.7 mm. Eggs 88–108 × 20–25 μm (mean 97 × 22 μm).
Suitable definitive hosts: fishes. Nototheniidae: Notothenia coriiceps, N. rossii, Gobionotothen gibberifrons, Lindbergichthys nudifrons, Pagothenia bernacchii, P. hansoni, Trematomus eulepidotus. T. newnesi; Bathydraconidae: Parachaenichthys georgianus.
-
Other definitive (? unsuitable) hosts: fishes. Channichthyidae: Champsocephalus gunnari.
-
Intermediate host: amphipods. Lysianassoidea: Cheirimedon femoratus.
-
Habitat: Mainly pyloric caeca and anterior half of small intestine. Few specimens occur more posteriorly in small and large intestine.
Biology and ecology: According to Zdzitowiecki 1986g, 1990b; Zdzitowiecki and Laskowski 2004; Laskowski et al. 2010, 2012, the species is associated with the inshore fiord environment and infections take place at a depth smaller than 50 m. N. coriiceps and juvenile N. rossii living in the Admiralty Bay (the South Shetland Islands) were heavily infected (prevalence 85 and 100%, maximum intensity 85 and 130 parasites in one fish). M. johnstoni infection of N. coriiceps were less abundant (prevalence was 74 % and maximum intensity 25). The species is rare in fishes living in the open sea at the South Shetland Islands and at South Georgia. It was found there almost exclusively in adults of N. rossii. The parasite occurs in fishes the whole year (lack of seasonality). Cystacanths of Metacanthocephalus johnstoni were found in the haemocoeloma of C. femoratus (5707 examined specimens) caught at the Galindez Island (Argentine Islands, Western Antarctica) with prevalence 0.51%. A total of 1416 specimens of Cheirimedon femoratus caught in the Admiralty Bay (South Shetland Islands) were found to be free of M. johnstoni
Distribution: The South Shetland Islands, South Georgia, Adelie Land, Argentine Islands (Johnston and Best 1937; Zdzitowiecki 1983; 1986g, 1987, 1990b; Zdzitowiecki and Laskowski 2004; Laskowski et al. 2007, 2010).
-
Order Polymorphida
-
Family Polymorphidae
-
Genus Profilicollis Meyer 1931
Diagnosis: Trunk cylindrical, spined in anterior half. Proboscis of both sexes spherical. Neck long. Proboscis receptacle long, double-walled. Lemnisci claviform. Testes in tandem. Cement glands tubular. Vaginal sphincter in females double. Eggs without polar prolongations of middle envelope. Parasites of birds.
Diagnosis (after Zdzitowiecki 1985): Proboscis spherical, wider than long. Hooks relatively small, arranged in 18–22 rows of 7–8/9. Anterior 2–3 hooks solid, with short blades and long roots directed posteriorly. Posterior 4–5 hooks with long blades directed posteriorly and short roots directed anteriorly. Neck very long, constituting 15–22% of total body length. Anterior 16–24% of trunk covered with small spines. Lemnisci reaching more posteriorly than proboscis receptacle.
Male. Total dimensions 14.1–21.1 × 2.0–3.0 mm. Proboscis 0.86–1.56 × 1.06–1.98 mm. Maximum hook length 71–74 μm. Neck length 2.1–4.0 mm. Four cement glands.
Female. One immature specimen was available. Total dimensions 11.1 × 1.2 mm. Proboscis 1.01 × 1.25 mm. Maximum hook length 80 μm. Neck length 2.46 mm. Genital pore subterminal. Eggs unknown.
-
Definitive hosts (probably unsuitable): birds (Chionis alba).
-
Habitat: Ileum and caecum.
-
Biology and ecology: Not known.
Synonyms: C. mirabilis Skryabin 1966, C. singularis Skryabin et Nikolsky, 1971 pro parte.
Diagnosis (after Zdzitowiecki 1986c): Proboscis hooks in 16 (rarely 17 or 18) rows of 10/11–14/15, including 2–3/4 rootless basal ones. Distal hook the longest one. Hind-trunk cylindrical, considerably longer than fore-trunk. Genital armature separated from somatic one. Lemnisci flat, shorter than proboscis receptacle.
Male. Total dimensions 9.6–13.4 × 1.4–2.0 mm. Proboscis 0.91–1.35 × 0.31–0.37 mm. Maximum hook length 89–117 μm. Fore-trunk constitutes 30–40% of trunk length. Somatic armature covers 33–54% of trunk length on ventral side. Number of genital spines circa 80–250, usually 100–200. Cement glands tubular.
Female. Total dimensions 13.6–19.7 × 1.8–2.8 mm. Proboscis 1.11–1.33 × 0.34–0.40 mm. Maximum hook length 0.099–1.120 mm. Fore-trunk constitutes 20–32% of trunk length. Somatic armature covers 28–38% of trunk length on ventral side. Number of genital spines 3–120, usually 20–50. Genital pore terminal. Eggs 107–125 × 35–39 μm.
Suitable definitive hosts: elephant seals (Mirounga leonina, M. angustirostris (?)). Unsuitable definitive hosts: seals (Hydrurga leptonyx, Leptonychotes weddelli, Lobodon arcinophagus); whales (Physeter catodon). Juvenile specimens also in intestine of birds (Phalacrocorax atriceps, Pygoscelis papua).
Paratenic hosts: fishes. Nototheniidae: Notothenia macrophthalma, N. coriiceps, N. rossii, N. squamifrons, N. nybelini, Nototheniops larseni, Gobionotothen gibberifrons, Lindbergichthys nudifrons, Dissostichus eleginoides, D. mawsoni, Pagothenia bernacchii, P. hansoni, Patagonotothen brevicauda guntheri; Artedidraconidae: Artedidraco mirus, Artedicraco sp.; Bathydraconidae: Parachaenichthys charcoti, P. georgianus; Channichthyidae: Chaenocephalus aceratus, Chionodraco rastrospinosus, Cryodraco antarcticus, Pseudochaenichthys georgianus; Macrouridae: Macrourus holotrachys; Muraenolepidae: Muraenolepis microps; Liparidae: Paraliparis sp.
Intermediate hosts: amphipods. Lysianassoidea: Waldeckia obesa; Eusiridae: Bovallia gigantea.
-
Habitat: Small and large intestine.
Biology and ecology: According to Zdzitowiecki (1986b, g, 1990b), infections of paratenic hosts take place mainly in the open sea shelf environment, deeper than 100 m. Predatory fishes living at the South Shetland Islands and at South Georgia are massively infected, up to one thousand cystacanths in one host specimen (D. eleginoides).
Cystacanths of C. bullosum were found in amphipods (intermediate hosts) in Admiralty Bay (Zdzitowiecki 2001b; Zdzitowiecki and Presler 2001).
Three elephant seals examined on King George Island (the South Shetland Islands) harboured 2520–3753 parasites per host. Five elephant seals examined in the maritime Antarctic were less infected (Nikolsky 1974).
-
Synonyms: C. singularis Skryabin et Nikolsky, 1971 pro parte.
Diagnosis (after Zdzitowiecki 1991): Proboscis hooks arranged in 19–22 rows of 10/11–13/14, including 3/4–4/5 rootless basal ones. Subdistal and prebasal hooks the longest ones. Fore-trunk and hind-trunk of similar length. Hind-trunk cylindrical. Somatic spines cover about 60% of trunk length on ventral side. Genital spines (if present) separated from somatic ones. Lemnisci flat, shorter than proboscis receptacle.
Male. Total dimensions 6.9–7.7 × 1.4–2.0 mm. Proboscis 0.728–0.878 × 0.284–0.343 mm. Maximum hook length 66–76 μm. Genital spines, circa 150 in number, arranged in 8–9 irregular circles. Cement glands pyriform.
Female. Total dimensions 7.7–9.6 × 1.8–2.7 mm. Proboscis 0.821–1.001 × 0.313–0.343 mm. Maximum hook length 71–86 μm. Genital spines present (1–100) or absent. Genital pore terminal. Eggs 126–159 × 38–47 μm.
Suitable definitive hosts: seals (Arctocephalus gazella, Hydrurga leptonyx). Unsuitable definitive hosts: seals (Lobodon carcinophagus). Juvenile specimens also in intestine of birds (Phalacrocorax atriceps). Paratenic hosts: fishes. Nototheniidae: Notothenia coriiceps, N. rossii, N. squamifrons, Lindbergichthys nudifrons, Dissostichus eleginoides, Patagonotothen brevicauda guntheri; Bathydraconidae: Parachaenichthys charcoti, P. georgianus; Channichthyidae: Chaenocephalus aceratus, Cryodraco antarcticus; Muraenolepidae: Muraenolepis microps.
-
Habitat: Mainly posterior half of ileum. Few specimens in jejunum and large intestine.
Biology and ecology: According to Zdzitowiecki (1986b, g, 1990b), infections of paratenic hosts take place mainly in the fiord environment. The species was probably very rare at the beginning of the twentieth century, because its main definitive hosts, A. gazella (Antarctic fur seal), was almost completely exterminated. Thus C. arctocephali was absent in samples of cystacanths from fishes caught at South Georgia in 1925–1928 (Baylis 1929; Zdzitowiecki 1987). The population of fur seal increased under protection and so did the parasite population. Now, C. arctocephali is abundant in fishes of the fiord environment in the same area (Zdzitowiecki 1987, 1990b), in Admiralty Bay (Laskowski et al. 2012), at the South Orkney Islands (Zdzitowiecki and White 1996), and at the Argentine Islands (Zdzitowiecki and Laskowski 2004).
Numerical data concerning the occurrence of C. arctocephali in definitive hosts are limited. Maximum intensity found till now in fur seal was 65 acanthocephalans. The most heavily infected paratenic hosts: N. rossii at South Georgia (prevalence 91 %, maximum intensity 84 cystacanths) and Notothenia coriiceps at the South Orkney Islands (prevalence 100 %, maximum intensity 36 cystacanths).
Distribution: The South Shetland Islands, South Georgia, Antarctic Peninsula, Argentine Islands, Ross Sea (probably its northern part) (Skryabin and Nikolsky 1971; Nikolsky 1974; Zdzitowiecki 1978, 1984b, 1986b, c, 1987, 1990b; Rodjuk 1985; Hoberg 1986; Zdzitowiecki and Laskowski 2004).
Synonyms: C. antarcticum (Rennie 1906), C. sipho Railliet et Henry, 1907, C. pacifica Nikolsky 1974.
Diagnosis (after Zdzitowiecki 1984c): Proboscis hooks arranged in 19–22 (usually 20) rows of 12/14–16, including 2/3–3/4 rootless basal ones. Subdistal (third to fifth) hooks the longest ones. Body shape depends from sex. Somatic and genital armature not separated. Lemnisci strongly folded, similar in length as proboscis receptacle.
Male. Total dimensions 5.2–7.1 × 1.7–2.5 mm. Proboscis 1.004–1.161 × 0.333–0.412 mm. Maximum hook length 77–98 μm. Fore-trunk constitutes 54–71 % of trunk length. Hind-trunk tapering posteriorly. Cement glands pyriform.
Female. Total dimensions 5.2–6.4 × 1.9–2.7 mm. Proboscis 1.072–1.278 × 0.339–0.410 mm. Maximum hook length 81–99 μm. Fore-trunk constitutes 59–80% of trunk length. Hind-trunk terminates with two lateral lobes. Slightly subterminal genital pore lies in concavity between lobes. Genital armature covers both lobes and ventral body side. Only narrow unarmed zone remains on mid-dorsal side at trunk end. Eggs 155–202 × 46–58 μm.
Suitable definitive hosts: seals (Hydrurga leptonyx, Leptonychotes weddelli). Unsuitable definitive hosts: seals (Lobodon carcinophagus). Juvenile specimens also in intestine of birds (Chionis alba, Phalacrocorax atriceps)
Paratenic hosts: fishes. Nototheniidae: Notothenia coriiceps, N. rossii, Dissostichus mawsoni, Pagothenia bernacchii, P. hansoni, Trematomus newnesi, T. bernacchii, Lindbergichthys nudifrons, Gobionotothen gibberifrons; Bathydraconidae: Parachaenichthys charcoti, P. georgianus; Channichthyidae: Chaenocephalus aceratus, Chionodraco rastrospinosus, Cryodraco antarcticus, Pseudochaenichthys georgianus; Harpagiferidae: Harpagifer antarcticus.
-
Intermediate host: amphipods. Eusiridae: Prostebbingia brevicornis.
-
Habitat: Pyloric part of stomach, duodenum, and anterior part of jejunum. Few specimens more posteriorly, in small and large intestine.
Biology and ecology: According to Zdzitowiecki (1986b, g, 1990b, Zdzitowiecki and White 1996, Zdzitowiecki and Presler 2001, Zdzitowiecki and Laskowski 2013, Laskowski and Zdzitowiecki 2010, Laskowski et al. 2012), infections of paratenic hosts take place in the fiord environment in the shallow water up to a depth of circa 50 m. Leopard seals, Weddell seals, and some paratenic hosts are massively infected, with up to several thousand parasites in one seal and over one hundred cystacanths in one fish. Probably all seals of both species mentioned above living in Admiralty Bay (the South Shetland Islands) are infected. N. coriiceps, N. rossii, and Ch. aceratus are the main paratenic hosts in this area (prevalence 96%, 100%, and 81%, maximum intensity 149, 166, and 123, respectively). C. hamanni found appears to be specific parasites of Prostebbingia brevicornis. Intermediate hosts occur mainly in sub-coastal waters (specimens examined were caught at the depth 5–15 m).
Distribution: Previous literature data concerning distribution and lists of hosts are partially doubtful and should be referred fully or partially to Corynosoma pseudohamanni. However, there are no doubts that the species occurs circumpolar: South Georgia, South Orkney Islands, South Shetland Islands, Antarctic Peninsula, Adelie Land, King George V Land, Argentine Islands, and maritime Antarctic (Linstow 1892; Rennie 1906; Railliet and Henry 1907; Baylis 1929; Johnston and Best 1937; Markowski 1971; Nikolsky 1974; Zdzitowiecki 1978, 1984c, 1986a, b, 1987, 1990b; Rodjuk 1985; Hoberg 1986; Zdzitowiecki and White 1996; Zdzitowiecki and Laskowski 2004). Doubtful data: Enderby Land, Ongul Island, McMurdo Sound, Kerguelen, Crozet and Heard islands, Lena, Skiff, and Ob banks (Leiper and Atkinson 1914, 1915; Edmonds 1957; Golvan 1959; Nickol and Holloway 1968; Holloway and Nickol 1970; Kamegai and Ichihara 1973; Holloway and Spence 1980; Parukhin and Lyadov 1982).
Synonyms: C. hamanni of various authors nec Linstow (1892) pro parte, C. antarcticum of Johnston and Best (1937) nec Rennie (1906) pro parte.
Diagnosis (after Zdzitowiecki 1984c): Proboscis hooks in 18–22 rows of 10/11–14, including 1–2/3 rootless basal ones. Subdistal (second to fourth) hooks the longest ones. Body shape depends from sex. Somatic and genital armature not separated. Lemnisci strongly folded, similar in length as proboscis receptacle.
Male. Body shape similar to that of C. hamanni. Total dimensions 4.8–6.2 × 1.4–1.8 mm. Proboscis 0.799–0.929 × 0.258–0.325 mm. Maximum hook length 67–79 μm. Fore-trunk constitutes 56–69% of trunk length. Hind-trunk slightly tapering posteriorly. Cement glands pyriform.
Female. Total dimensions 3.9–5.3 × 1.3–2.1 mm. Proboscis 0.804–1.001 × 0.300–0.325 mm. Maximum hook length 64–81 μm. Fore-trunk constitutes 67–85% of trunk length. Hind-trunk slightly tapering posteriorly, with rounded end. Genital pore terminal. Genital armature ends just before genital pore on ventral side. Spines spread at sides before genital pore, but they never occur on dorsal side of hind-trunk. Eggs 92–120 × 29–40 μm.
Suitable definitive hosts: seals (Leptonychotes weddelli, Hydrurga leptonyx, Lobodon carcinophagus). Unsuitable definitive hosts: seals (Arctocephalus gazella, Mirounga leonina). Juvenile specimens also in intestine of birds (Catharacta lonnbergi, Chionis alba, Larus dominicanus, Phalacrocorax atriceps).
Paratenic hosts: fishes. Nototheniidae: Notothenia coriiceps, N. rossii, N. nybelini, Lindbergichthys nudifrons, Gobionotothen gibberifrons, Dissostichus eleginoides, D. mawsoni, Pagothenia bernacchii, P. hansoni, Trematomus newnesi; Bathydraconidae: Parachaenichthys charcoti; Channichthyidae: Chaenocephalus aceratus, Champsocephalus gunnari, Chionodraco rastrospinosus, Cryodraco antarcticus, Gymnodraco acuticeps; Harpagiferidae: Harpagifer antarcticus. Probably also further species of fishes listed by Holloway and Spence (1980) as paratenic hosts of Corynosoma hamanni in McMurdo Sound: Nototheniidae: Pagothenia borchgrevinki, Trematomus centronotus; Zoarcidae: Lycodichthys dearborni.
-
Intermediate hosts: amphipods. Eusiridae: Pontogeneiella sp.; Lysianassoidea: Cheirimedon femoratus.
Biology and ecology: According to Zdzitowiecki (1986b, g, 1990b; Zdzitowiecki and White 1996; Zdzitowiecki and Presler 2001; Zdzitowiecki and Laskowski 2004; Laskowski et al. 2007), infections of paratenic hosts take place in the fiord environment, but a little deeper than in the case of Corynosoma hamanni, at a depth of up to 100 m. Probably all Weddell seals living in the Admiralty Bay (South Shetland Islands) are infected; intensities of the infection sometimes exceed one thousand parasites per seal. N. coriiceps, N. rossii, P. charcoti, and Ch. aceratus are the main paratenic hosts in the same area (prevalence 99.6–100%, maximum intensity 856, 106, 219, and 263, respectively). At the Vernadsky Station (Argentine Islands) and at the South Orkney Islands, N. coriiceps was also heavily infected (prevalence 99% and 100%, maximum intensity 421 and 23, respectively). Cystacanths in intermediate hosts (C. femoratus) were found in Admiralty Bay and at Vernadsky Station.
Distribution: Circum-Antarctic: Antarctic Peninsula, Argentine Islands, South Shetland Islands, southern coasts of Weddell Sea, McMurdo Sound, Adelie Land, King George V Land, Enderby Land, South Orkney Islands, Ross Sea. Part of the material was originally referred to C. hamanni. It is here referred to C. pseudohamanni based on morphological data contained in papers of various authors (Leiper and Atkinson 1915; Johnston and Best 1937; Edmonds 1957; Golvan 1959; Nickol and Holloway 1968; Holloway and Nickol 1970; Holloway and Spence 1980; Zdzitowiecki 1978, 1984c, 1986a, b, 1990b; Hoberg 1986; Zdzitowiecki and White 1996; Zdzitowiecki and Laskowski 2004; Laskowski and Zdzitowiecki 2005, 2010). C. pseudohamanni is the only representative of the genus Corynosoma occurring without any doubts within the Antarctic Circle. The species was absent in the large sample of fishes examined at South Georgia (Zdzitowiecki 1990b).
-
Corynosoma beaglense Laskowski, Jeżewski, Zdzitowiecki, 2008 (Fig. 8.12)
Diagnosis (after Laskowski et al. 2008): Only juvenile specimens (cystacanths) of Corynosoma beaglense were found in Champsocephalus esox in Beagle Channel. It has an almost cylindrical proboscis (length 0.52–0.56 mm); a proboscis hook formula of 16 rows of 9/10–10/11, including 4–4/5 basal hooks; distal hooks shorter than the prebasal hooks; a fore-trunk not separated from the hind-trunk by a constriction; somatic spines contiguous with the genital spines on the ventral side of the trunk of the male and covering the entire length of the ventral side of the female trunk, and the presence of genital spines surrounding the terminal genital pore of the male.
Male. Total length approx. 2.6 mm. Proboscis 0.530 × 0.212 mm. Distal hook length 50 μm; prebasal hook length 56 μm. Neck retracted into trunk, c. 0.210 mm in width. Trunk 1.89 × 0.61 mm. Genital pore surrounded by genital spines (max. length 29 μm) contiguous with somatic spines (max. length 37 μm). Proboscis receptacle 746 × 239 μm. Lemnisci 0.502–0.209 and 0.458–0.206 mm. Testes oval, arranged diagonally at end of proboscis receptacle, 0.185 × 0.136 mm and 0.184 × 0.128 mm. Cement-glands elongate, pear-shaped, just posterior to testes, 6 in number. Säfftigen’s pouch club-shaped.
Female. Total length 2.79 mm and 2.53 mm. Proboscis 560 × 210 μm and 521 × 209 μm. Distal hook length 48 μm and 51 μm; prebasal hook length 52 μm and 63 μm. Neck wider than long, 269 × 367 μm and 271 × 307 μm. Trunk 1.98 × 0.93 mm and 1.76 9 0.72 mm; whole ventral side covered with somatic spines with max. length 37 μm. Proboscis receptacle 794 × 298 μm and 741 × 202 μm. Lemnisci 382–657 × 210–263 μm. Length of reproductive organs (from anterior end of uterine bell to genital pore) 588 μm in one case. Vaginal sphincter double, 79 × 77 μm and 76 × 64 μm.
The definitive host of this species is unknown. C. beaglense is similar to two Sub-Antarctic parasites of birds, Andracantha baylisi and C. clavatum Goss, 1940, in the shape of the trunk, neck, and proboscis, as well as the proboscis armature. It differs from them in the lack of a zone of small somatic spines between two zones of large spines (a generic feature), the somatic spines on the male contiguous with the genital spines, the somatic spines on females extending to the posterior extremity, a smaller proboscis, shorter hooks, and the distal hooks shorter than the prebasal hooks.
-
Corynosoma evae Zdzitowiecki, 1984 (Fig. 8.13)
Diagnosis (after Zdzitowiecki 1984b): Proboscis hooks in 20–24 rows of 11/12–13, including 3–4 rootless basal ones. Prebasal hook the longest, stout. Fore-trunk constitutes 55–64% of total trunk length. Hind-trunk cylindrical. Somatic armature covers 61–69% of trunk length on ventral side. Genital spines (if present) separated from somatic ones. Lemnisci flat, shorter than proboscis receptacle.
Male. Total dimensions 3.5–4.6 × 1.1–1.5 mm. Proboscis 0.633–0.719 × 0.257–0.296 mm. Maximum hook length 57–63 μm. Genital spines arranged in 4 irregular rows, 40–60 in number. Cement glands pyriform.
Female. Total dimensions 4.3–5.2 × 1.1–1.9 mm. Proboscis 0.612–0.788 × 0.254–0.337 mm. Maximum hook length 61–73 μm. Genital spines absent. Genital pore terminal. Eggs 103–127 × 34–43 μm.
-
Suitable definitive hosts: seals (Hydrurga leptonyx, Otaria flavescens).
-
Paratenic hosts: fishes. Bathydraconidae: Parachaenichthys georgianus; Nototheniidae: Patagonotothen longipes; Channichthyidae: Champsocephalus esox.
-
Habitat: Ileum.
-
Biology and ecology: Not known.
Distribution: The South Shetland Islands, South Georgia, Falkland Islands, Beagle Channel (Zdzitowiecki 1984b, 1986e; Laskowski and Zdzitowiecki 2009; Laskowski et al. 2007). It is probably rather a Sub-Antarctic than an Antarctic species. The present authors did not find cystacanths in the large sample of fishes examined at the South Shetland Islands, one cystacanth was found at South Georgia and 10 cystacanths were found in Beagle Channel. Cystacanths found by Reimer (1987) in fishes at the South Shetland Islands and South Georgia were probably wrongly determined and should be referred to C. arctocephali.
Description: All investigated specimens (five females) were partly contracted, with the proboscis, neck, and anterior part of the trunk retracted, and the proboscis partly invaginated. Total length of not contracted specimens, if attains about 6.2–6.7 mm (length of trunk about 4.6–5.2 mm). The maximum width of dilated fore-trunk 2.02–2.24 mm, width of the hind-trunk 0.66–1.01 mm. The fore-trunk is about twice as long as the hind-trunk. Approximate length of the proboscis (measured only in one specimen by adding the length of invaginated part to the length of non-invaginated part) about 1.2–1.3 mm. Width of the proboscis 0.39–0.42 mm. Hooks arranged in 19–20 rows, number of hooks per row exceeding 10 (the most probably 15), basal hooks with reduced roots 3–4 in number. The largest are the hooks situated just before the basal ones. Maximum length of the blade 100–119 μm. Neck impossible to observe. Somatic armature covers about 3/4 of the trunk at the ventral side, partly laterally. The anterior most genital spines are 40–91 μm distant from the body end. Width of the unarmed zone between somatic and genital spines 0.30–0.81 mm. Maximum dimensions of the somatic spines 65 × 15 μm, of the genital spines 72 × 24 μm. Dimensions of the proboscis receptacle about 1.7 × 0.5–0.6 mm. Lemnisci not visible, screened by embryophores. The genital duct, observed only in one specimen, measures 1.4 mm. The vagina is provided with double sphincter. Genital opening terminal. Dimensions of mature embryophores, measured inside the body, through the body wall, in three specimens 155–188 × 43–56 μm.
Females of C. gibsoni sp. n. are similar to C. hamanni (Linstow 1892) in respect of proboscis length and embryophore dimensions (cf. Zdzitowiecki 1984b) but differ from the latter by the presence of an unarmed zone separating somatic and genital armature, as well as by the shape of the posterior part of the trunk, especially its posterior tip. All other representatives of the genus Corynosoma have smaller embryophores (cf. Golvan 1959; Zdzitowiecki 1984a, b). Of these, C. arctocephali. Zdzitowiecki, 1984, the most similar in embryophore dimensions, has a shorter proboscis, smaller hooks, longer hind-trunk, shorter range of somatic armature and greater distance between somatic and genital armature.
-
Genus Andracantha Schmidt, 1975
Diagnosis: Proboscis cylindrical. Neck conspicuous. Fore-trunk forming bulb, connected with neck by short segment similar in width as neck. Hind-trunk tapering posteriorly. Conspicuous somatic spines arranged in two circular fields separated from each other by either a bare zone or a zone covered with smaller spines. Genital spines separated from somatic ones, present at least on some specimens of both sexes. Proboscis receptacle double-walled. Testes parallel. Cement glands tubular or pyriform, six or eight in number. Vaginal sphincter in females double. Eggs with or without polar prolongations of middle envelope. Parasites of birds. Paratenic hosts: fishes.
-
Andracantha baylisi (Zdzitowiecki 1986a, b, c, d, e, f, g) (Fig. 8.15)
-
Synonyms: Corynosoma sp. Zdzitowiecki 1985.
Diagnosis (after Zdzitowiecki 1985, 1989a; Laskowski et al. 2008): Proboscis almost cylindrical slightly dilated just beyond mid-length, with length/width ratio 2.69–2.92:1. Hooks arranged in 16 rows of 9/10–10/11, including 5–5/6 rooted ones and 4/5 basal ones with reduced roots. Anterior hooks gradually increase from apex in blade width and root length, but distal-most hook is longest by far. Blades of anterior 4–5 hooks longer than roots; blade of prebasal hook shorter than root. Area of basal hooks constitutes 33–39% of proboscis length. Neck trapezoid, may be longer or shorter than wide, curved towards ventral side. Fore-trunk not separated from hind-trunk by constriction. Short anterior part of trunk similar in width to neck, then trunk dilates greatly before tapering posteriorly. Anterior 36–40% of trunk length covered with somatic spines (max. length 48 μm), which are arranged in 2 densely spined zones separated by zone of smaller, loosely arranged spines. Anterior zone of large spines constitutes 12–22% of length of whole armature area, zone of minute spines 36–42% and posterior zone of large spines 39–46% (measured along ventral side of trunk). Approximately 20–30 genital spines (max. length 21 μm) present at posterior extremity of trunk. Genital pore terminal, surrounded by genital spines in both sexes. Proboscis receptacle double-walled, extending to level of posterior zone of larger somatic spines. Lemnisci flat, rounded to ellipsoid, shorter than proboscis receptacle.
Male. Only juvenile specimens from paratenic hosts were available. Total dimensions 3.66–5.0 × 1.12–1.74 mm. Proboscis 0.823–0.920 × 0.290–0.350 mm. Distal hook length 107–119 μm, prebasal hook length 79–95 μm. Neck length 0.404–0.407 × 0.373–0.461 mm. Trunk 2.485–2.975 × 1.133–1.351 mm. Proboscis receptacle 1.257–1.411 × 0.275–0.378 mm. Lemnisci 0.646–0.930 × 0.341–0.464 mm. Testes parallel, at end of proboscis receptacle, 0.219–0.272 × 0.120–0.192 mm. Cement glands elongate, pear-shaped, 6 in number. Säfftigen’s pouch club-shaped.
Female. Total dimensions of adult specimens about 5–5.7 × 1.60–1.86 mm. Proboscis 0.820–0.970 × 0.240–0.380 mm. Distal hook length 119–136 μm. Prebasal hook length 92–119 μm. Genital spines present (1–20 in number) or absent. Genital pore terminal. Eggs with polar prolongations of middle envelope, 81–101 × 27–30 μm.
Total dimensions of juvenile specimens 4.0–5.7 × 1.31–1.86 mm. Proboscis 0.820–0.970 × 0.240–0.380 mm. Distal hook length 104–136 μm. Prebasal hook length 89–119 μm. Neck 0.421–0.461 μm. Trunk 2.714 × 1.091 mm. Genital spines present (1–20 in number) or absent. Genital pore terminal. Eggs with polar prolongations of middle envelope, 81–101 × 27–30 μm.
-
Suitable definitive hosts: birds (Chionis alba, Phalacrocorax albiventer).
-
Paratenic hosts: fishes. Nototheniidae: Notothenia rossii, Patagonotothen longipes; Bathydraconidae: Parachaenichthys georgianus; Channichthyidae: Chaenocephalus aceratus, Champsocephalus esox
-
Habitat: Intestine.
Biology and ecology: Cystacanths are present, though rare, in fishes at South Georgia and in Beagle Channel. Thus, the life cycle is completed in this area.
Distribution: Western Antarctic and Sub-Antarctic: the South Shetland Islands, South Georgia, Patagonia, Beagle Channel. The only specimen found in the definitive host (Chionis alba) on King George Island (South Shetland Islands) probably arrived from another area, as cystacanths of the species were not found in fishes at the South Shetland Islands. Six out of 290 notothenioid fishes examined at South Georgia housed few cystacanths (1–2 specimens per host) (Zdzitowiecki 1985, 1986f, 1989a, 1990b; Laskowski et al. 2007; Laskowski and Zdzitowiecki 2009).
-
Genus Bolbosoma Porta, 1908
Diagnosis: Proboscis cylindrical or conical. Neck short. Fore-trunk consists of short conical anterior part, large bulb, and narrow part beyond bulb. Hind-trunk cylindrical. Somatic spines present on prebulbar part of fore-trunk and usually on bulb. Genital spines absent. Proboscis receptacle double-walled. Testes in tandem. Cement glands tubular. Vaginal sphincter in females double. Eggs with polar prolongations of middle envelope. Parasites of mammals, mainly whales. Intermediate hosts – crustaceans (till now found only in euphausiids). Fishes may play a role as paratenic hosts.
-
Bolbosoma brevicolle (Maim 1867) (Fig. 8.16)
-
Synonyms: B. paramuschiri Skryabin, 1959.
Diagnosis (according Zdzitowiecki 1991): Proboscis hooks arranged in 20–22 rows of 7 (rarely 6 or 8), including one small basal hook, which may be rooted or not. Subdistal (second) hook the longest one. Somatic spines arranged in 20 irregular circles, covering the whole prebulbar part of fore-trunk and reaching beyond half of length of bulb. Anterior spines small, posterior spines two to three times longer. Hind-trunk constituting 74–84% of trunk length. Lemnisci very long, filiform, as long as trunk. Proboscis receptacle ends inside bulb.
Male. Total length 23–32 mm. Bulb 2.3–3.1 × 1.9–2.3 mm. Hind-trunk width 1.70–2.75 mm. Proboscis 0.51–0.57 × 0.42–0.51 mm. Anterior spines 40–60 × 15–32 μm. Posterior spines 100–160 × 60–90 μm. Testes in tandem, oblique, not separated.
Female. Total length 21–38 mm. Bulb 2.5–2.8 × 2.0–2.65 mm. Hind-trunk width 2.0–3.3 mm. Proboscis 0.54–0.60 × 0.45–0.52 mm. Maximum hook length 113 μm. Anterior spines 60–80 × 20–30 μm. Posterior spines 95–120 × 48–75 μm. Eggs 118–131 × 25–29 μm (mean 124 × 26 μm).
-
Suitable definitive hosts: whales (Balaenoptera musculus).
-
Unsuitable definitive hosts: whales (Balaenoptera borealis).
-
Other suitable and unsuitable definitive hosts: whales (Balaenoptera acutorostrata, B. physalus, Eubalaena glacialis sieboldi, Physeter catodon).
-
Habitat: Intestine.
Biology and ecology: The species is abundant in blue whales (B. musculus) at South Georgia.
Distribution: Cosmopolitan, including the Antarctic: environs of the South Shetland Islands and South Georgia (Baylis 1929; Petrotschenko 1958; Yamaguti 1963; Zdzitowiecki 1986a).
Key to the classes of acanthocephala
(After Amin 1987, modified)
1a Main longitudinal lacular canals lateral. Nuclei of lemnisci and cement glands and hypodermal nuclei fragmented. Ligament sacs in females single, not persistent. Proboscis receptacle double-walled. Definitive hosts: fishes, amphibians, reptiles, birds and mammals. Intermediate hosts: crustaceans | Class Palaeacanthocephala* |
1b Main longitudinal lacular canals dorsal and ventral, or only dorsal. Nuclei of lemnisci and cement glands and/or hypodermal nuclei not fragmented, usually giant. Ligament sacs in females double, persistent. Proboscis receptacle single-walled, complex, or absent | 2 |
2a(lb) Protonephridia present or absent. Trunk not spined. Proboscis receptacle absent or single-walled. Cement glands separate, pyriform. Eggs usually oval, thick-shelled. Definitive hosts: birds and mammals. Intermediate hosts: insects, rarely myriapods | Class Archiacanthocephala |
2b Protonephridia absent. Trunk spined or not. Proboscis receptacle single-walled. Cement glands elongate to tubular, or syncytial. Eggs variable. Definitive hosts: fishes, amphibians, and reptiles. Intermediate hosts: probably crustaceans | 3 |
3a(2b) Trunk spined. Proboscis claviform, with numerous longitudinal rows of hooks. Cement glands separate, elongate pyriform to tubular. Eggs oval, with radial sculpturings at right angles to surface. Definitive hosts: fishes and Crocodilia. Intermediate hosts unknown, probably crustaceans | Class Polyacanthocephala |
3b Trunk spined or not. Proboscis usually small, with few radially arranged hooks. Cement gland single, syncytial. Eggs variably shaped, but not like those of Polyacanthocephala. Definitive hosts: fishes and occasionally amphibians and reptiles. Intermediate hosts: crustaceans | Class Eoacanthocephala |
Key to the orders, families, subfamilies, genera, and species of the antarctic acanthocephala (palaeacanthocephala)
(After Zdzitowiecki 1991, modified)
la Mature stage parasite of fishes. Trunk armed or not. (Order Echinorhynchida) | 2 |
lb Mature stage parasite of mammals and birds. Fishes are paratenic hosts of many species. Trunk armed. (Order Polymorphida, family Polymorphidae, subfamily Polymorphinae) | |
2a(1a) Anterior part of trunk armed with spines | 3 |
2b Trunk unarmed | 5 |
3a(2a) Proboscis cylindrical, slightly dilated subterminally. Ventral proboscis hooks larger than dorsal. Vulvar sphincter in females double | (Family Heteracanthocephalidae, subfamily Aspersentinae) |
Aspersentis megarhynchus | |
3b Proboscis globular. Ventral proboscis hooks not different from dorsal. 2–3 types of hooks arranged along proboscis. Vulvar sphincter in females single | (Family Arhythmacanthidae, subfamily Arhythmacanthinae, genus Heterosentis) 4 |
3c Proboscis relatively short, cylindrical to globular, armed with ten basal spines between 10 rows with rooted hooks (one or two) and basal spines (one or two). Parasites of fishes (Family Arhythmacanthidae, genus Hypoechinorhynchus) | Hypoechinorhynchus magellanicus |
4a(3b) Proboscis hooks arranged in 10 longitudinal rows. One large and 2–4 small hooks in row. Blade and root of large hook similar in length | Heterosentis heteracanthus |
4b Proboscis hooks arranged in circa 15 rows. Probably 1-2 large and 1–3 small hooks in row. Blade of large hook considerably longer than root | Heterosentis magellanicus |
5a(2b) Parasite of Zanclorhynchus spinifer at Macquarie Island. Proboscis circa 1 mm long, armed with 14–16 rows of hooks, circa 10–12 in row | Echinorhynchus zanclorhynchi |
5b Parasites of other Antarctic and Sub-Antarctic fishes | 6 |
6a(5b) Eight pyriform cement glands arranged in compact group in males. Vulvar sphincter in females double | (Family Rhadinorhynchidae, subfamily Gorgorhynchinae, genus Metacanthocephalus) 7 |
6b Six spherical or ovoid cement glands usually arranged along trunk of males. Vulvar sphincter in females single | (Family Echinorhynchidae, subfamily Echinorhynchinae, genus Echinorhynchus) 10 |
7a(6a) Trunk cylindrical, slightly dilated anteriorly. Eggs longer than 100 μm | 8 |
7b Trunk elongate, oval with maximum width at mid-body. Mean length of eggs smaller than 100 μm | 9 |
8a(7a) Proboscis cylindrical, 0.54–0.68 mm long. Hooks in 13–15 rows of 8–10. Length of eggs 110–150 μm | Metacanthocephalus campbelli |
8b Proboscis ovoid to cylindrical, 0.30–0.44 mm long. Hooks in 11–16 rows of 4–6 (usually 5). Length of eggs 100–120 μm | Metacanthocephalus dalmori |
9a(7b) Proboscis 0.43–0.60 mm long. Hooks in 12–17 rows of 5–8 (usually 6–7). Length of eggs 80–110 μm (mean 97 μm) | Metacanthocephalus johnstoni |
9b Proboscis 0.30–0.42 mm long. Hooks in 12–13 rows of 5–7 (usually 6). Length of eggs 80–90 μm | Metacanthocephalus rennicki |
10a(6b) Proboscis hooks in 14–20 rows. Length of eggs 90–120 μm | Echinorhynchus petrotschenkoi |
l0b Proboscis hooks in 12 rows. Length of eggs 70–100 μm | Echinorhynchus muraenolepisi |
lla (lb) Proboscis spherical. Neck very long and narrow. Trunk without anterior dilatation. Parasite of birds | Profilicollis antarcticus |
11b Proboscis cylindrical or conical. Trunk dilated anteriorly. Parasites of birds and mammals | 12 |
12a (llb) Proboscis cylindrical. Fore-trunk forming bulb not separated from hind-trunk. Parasites of seals and birds (males of some species may be found in whales) | 13 |
12b Proboscis conical, rarely cylindrical. Fore-trunk forming bulb separated from hind-trunk by constriction. Parasites of whales | (Genus Bolbosoma) 23 |
13a(l2a) Somatic armature divided into anterior and posterior fields. Genital armature present or absent in specimens of both sexes. Parasites of birds, mainly cormorant | (Genus Andracantha) 14 |
13b Somatic armature not divided. Genital spines present in all males and usually in females. Parasites of seals and penguins | (Genus Corynosoma) 15 |
14a(l3a) Length of proboscis 0.82–0.97 mm. Distal proboscis hooks longer than prebasal. Length of eggs 90–100 μm. The species occurs in western Antarctic and Sub-Antarctic | Andracantha baylisi |
14c Length of proboscis 0.63–0.75 mm. Distal proboscis hooks shorter than prebasal. Length of eggs 70–80 μm. The species occurs in environs of South Australia, New Zealand, and Kerguelen | Andracantha clavata |
15a(l3b) Somatic and genital armature connected on ventral side of trunk | 16 |
15b Genital armature separated from somatic or absent in females | 20 |
16a(l5a) Lemnisci flat | 17 |
16b Lemnisci consist of many irregular folds | 19 |
17a(16a) Proboscis ovoid to cylindrical, 0.88–1.12 mm long. Length of largest hooks 130–160 μm. Parasite of penguins | Corynosoma shackletoni |
17b(16a) Proboscis almost cylindrical, dilated just posterior to mid-length, 0.52–0.56 mm long, shorter than proboscis receptacle | Corynosoma beaglense |
17c Proboscis cylindrical, dilated before base, shorter than 0.75 mm. Largest hooks shorter than 90 μm. Parasites of seals | 18 |
18a(17b) Proboscis hooks in 16–18 rows of 11–15, including 2–4 rootless basal hooks. Genital pore in females subterminal | Corynosoma australe |
18b Proboscis hooks in 22 rows of 12–13, including 4–6 rootless basal hooks. Genital pore in females terminal | Corynosoma hannae |
19a(l6b) Proboscis longer than 1 mm. Number of proboscis hooks in row 12–16 (usually 14–15). Genital pore in females on the bottom of the hollow between two lateral folds. Length of eggs 160–200 μm | Corynosoma hamanni |
19b Proboscis shorter than 1 mm. Number of proboscis hooks in row 10–14 (usually 12–13). Genital pore in females terminal. Length of eggs 90–120 μm | Corynosoma pseudohamanni |
20a(l5b) Number of rows of proboscis hooks 15–18 | 21 |
20b Number of rows of proboscis hooks 19–24 | 22 |
21a(20a) Hind-trunk cylindrical, considerably longer than dilated fore-trunk. Proboscis longer than 0.9 mm. Cement glands in males tubular. Length of eggs 110–130 μm. Parasite of elephant seals | Corynosoma bullosum |
21b Hind-trunk cylindrical, a little shorter than dilated fore-trunk. Proboscis shorter than 0.75 mm. Cement glands in males pyriform. Length of eggs 70–80 μm. Parasite of fur seals and leopard seals | Corynosoma australe |
22a(20b) Length of proboscis 0.7–1.0 mm. Genital spines in males arranged in 8–9 circles, circa 150 in number. Genital spines in females present or absent. Length of eggs 130–160 μm | Corynosoma arctocephali |
22b Length of proboscis 0.6–0.8 mm. Genital spines in males arranged in 4 circles, circa 40–60 in number. Genital spines in females absent. Length of eggs 100–130 μm | Corynosoma evae |
23a(12b) Total length circa 20 mm. Fore-trunk spines arranged in 6–10 circles before bulb. Lemnisci short, flat | Bolbosoma balaenae |
23b Total length less than 7 mm. Somatic spines cover anterior part of fore-trunk, including bulb, arranged in at least 15 circles. Lemnisci very long, filiform | 24 |
24a(23b) Proboscis hooks usually in 19–22 (rarely 23 or 24) rows of usually 6–7 (rarely 5 or 8) | 25 |
24b Proboscis hooks in 24–27 rows of 7–8 | 26 |
25a(24a) Total length 11–25 mm. Fore-trunk spines arranged in circa 15 circles. Length of eggs 130–170 μm. Parasite of sei whales of southern hemisphere | Bolbosoma turbinella australis |
25b Total length 21–38 mm. Fore-trunk spines arranged in circa 20 circles. Length of eggs 120–130 μm. Parasite of blue whales and fin whales | Bolbosoma brevicolle |
26a(24b) Total length 60–64 mm. Length of eggs 110–140 μm | Bolbosoma hamiltoni |
26b Total length 16–39 mm. Length of eggs 90–120 μm | Bolbosoma tuberculata |
References
Amin OM (1987) Key to the families and subfamilies of Acanthocephala, with the erection of a new class (Polyacanthocephala) and a new order (Polyacanthorhynchida). J Parasitol 73:1216–1219
Baylis HA (1929) Parasitic Nematoda and Acanthocephala collected in 1925–1927. Discov Rep 1:541–560
Dollfus RP (1965) Acanthocephale d’un téléostéen du genre Notothenia Richardson des Kerguelen (Mission Jean-Claude Hureau, 1963–1964). Bull Mus Natn Hist Nat Paris Ser 2(36):641–646
Edmonds SJ (1955) Acanthocephala collected by the Australian national Antarctic research expedition on heard island and Macquarie island during 1948–50. Trans R Soc S Aust 78:141–144
Edmonds SJ (1957) Acanthocephala. Rep BANZ Antarct Res Expect Ser B 6:91–98
Golvan YJ (1959) Acanthocephales du genre Corynosoma Ltihe, 1904, parasites de mammiferes d’Alaska et de Midway. Ann Parasit Hum Comp 34:288–321
Golvan YJ (1969) Systematique des acanthocephales (Acanthocephala Rudolphi 1801). Premiere partie: l’ordre des Palaeacanthocephala Meyer 1931, premier fascicule: la super-famille des Echinorhynchoidea (Cobbold 1876) Golvan et Houin 1963. Mem Mus Natn Hist Nat Paris Ser A Zool 57:1–373
Hoberg EP (1986) Aspects of ecology and biogeography of Acanthocephala in Antarctic seabirds. Ann Parasitol Hum Comp 61:199–214
Holloway HL Jr, Nickol BB (1970) Morphology of the trunk of Corynosoma hamanni (Acanthocephala: Polymorphidae). 1. Morph 130:151–161
Holloway HL Jr, Spence JA (1980) Ecology of animal parasites in McMurdo Sound. Antarctica Comp Phys Ecol 5:262–284
Hoogesteger JN, White MG (1981) Notes on parasite infestation of inshore fish at Signy Island, South Orkney Islands. Br Antarct Surv Bull 54:23–31
Johnston TH, Best EW (1937) Acanthocephala. Aust. Antarct. Exped., 1911–14. Sci Rep Ser C 10(2):1–20
Joyeux C, Baer JG (1954) Cestodes et Acanthocephales recoltes par M. Patrice PAULIAN aux iles Kerguelen et Amsterdam. Mem Lnst Scient Madagascar Ser A 9:23–40
Kagei N, Watanuki T (1975) On the parasites of fishes from the Antarctic ocean. Antarctic Res 54:84–93
Kamegai S, Ichihara A (1973) Parasitic helminths of Antarctic animals (2). Corynosoma hamanni (Linstow, 1892) from Weddell seal, Leptonychotes weddelli, caught on the Ongul Isl. Antarctic Res Bull Meguro Parasit 7:26–27
Kumar P. (1992) Arhythmacanthus zdzitowieckii, new species (Acanthocephala: Arhythmacanthidae) from estuarine fish, Clariasbatrachus of Chilka lake, Orissa, India. Pak. J. Zool. 24: 143–144
Laskowski Z, Zdzitowiecki K (2004) New morphological data on a sub-Antarctic acanthocephalan, Aspersentis johni (Baylis, 1929) (Palaeacanthocephala: Heteracanthocephalidae). Syst Parasitol 59:39–44
Laskowski Z, Zdzitowiecki K (2005) The helminth fauna of some notothenioid fishes collected from the shelf of Argentine Islands, West Antarctica. Pol Polar Res 26:315–324
Laskowski Z, Rocka A, Zdzitowiecki K, Ghigliotti L, Pisano E (2005) New data on the occurrence of internal parasitic worms in the Gymnodraco acuticeps and Cygnodraco mawsoni (Bathydraconidae) fish in the Ross Sea, Antarctica. Pol Polar Res 26:37–40
Laskowski Z, Rocka A, Zdzitowiecki K, Ozouf-Costaz C (2007) Occurrence of endoparasitic worms in dusky notothen, Trematomus newnesi (Actinopterygii Nototheniidae), at Adelie Land, Antarctica. Pol Polar Res 28:37–42
Laskowski Z, Zdzitowiecki K (2008) New morphological data on the acanthocephalan Hypoechinorhynchus magellanicus Szidat, 1950 (Palaeacanthocephala: Arhythmacanthidae). Syst Parasitol 69:179–183
Laskowski Z, Jeżewski W, Zdzitowiecki K (2008) Cystacanths of Acanthocephala in notothenioid fish from the Beagle Channel (sub-Antarctica). Syst Parasitol 70:107–117
Laskowski Z, Zdzitowiecki K (2009) Occurrence of acanthocephalans in notothenioid fishes in the Beagle Channel (Magellanic sub-region, sub-Antarctic). Pol Polar Res 30:179–186
Laskowski Z, Zdzitowiecki K (2010) Contribution to the knowledge of the infection with Acanthocephala of a predatory Antarctic ice-fish Chaenocephalus aceratus. Pol Polar Res 31:303–308
Laskowski Z, Jeżewski W, Zdzitowiecki K (2010) New data on the occurrence of Acanthocephala in Antarctic Amphipoda. Acta Parasitologica 55:161–166
Laskowski Z, Korczak-Abshire M, Zdzitowiecki K (2012) Changes in acanthocephalan infection of the Antarctic fish Notothenia coriiceps in Admiralty Bay, King George Island, over 29 years. Pol Polar Res 33:99–108
Leiper RT, Atkinson EL (1914) Helminthes of the British Antarctic Expedition, 1910–1913. Proc Zool Soc Lond 1:222–226
Leiper RT, Atkinson EL (1915) Parasitic worms with a note on a freeliving nematode. Nat Hist Rep Br Antarct Terra Nova Exped Zool 2:19–60
Linstow O (1892) Helminthen von Stidgeorgien. Nach der Ausbeute der deutschen Station von 1882–1883. Jb Hamb Wiss Anst 9:59–77
Linstow O (1896) Nemathelminthen. Hamburger Magalhaensische Sammelreise. L. Friedrichsen u. Co. Hamburg, p 22
Markowski S (1971) On some species of parasitic worms in the “Discovery” collections obtained in the years 1925–36. Bull Brit Mus (Nat Hist) Zool 21:53–65
Meyer A (1931) Die Stellung des Genus Heterosentis van Cleave 1931 in Acanthocephalensystem. Zool Anz 94:258–265
Nickol BB, Holloway HL Jr (1968) Morphology of the presoma of Corynosoma hamanni (Acanthocephala: Polymorphidae). J Morph 124:217–226
Nikolsky OR (1974) [Acanthocephalan fauna of 105 pinnipeds of the Pacific Ocean sector of the marine Antarctic]. lzv. tikhookean nauchnoissled. Inst Ryb Khoz Okeanogr 88:101–106 [In Russian]
Parukhin AM, Lyadov VN (1982) Helminth fauna of food Nototheniidae fishes from Kerguelen subregion. Ekol Morya Kiev 10:49–57 [In Russian]
Parukhin AM, Sysa VN (1975) [The incidence of infection in Notothenioidei fish in Sub-Antarctic waters]. Problemy Parazitologii. Materialy nauchnojj konferencii parazitologov USSR, 2, Kiev, “Naukova Dumka”, pp 97–99 [In Russian]
Petrotschenko VI (1956) [Acanthocephala of domestic and wild animals]. Tom I. lzdatelstvo Akademii Nauk SSSR, Moskva, p 435 [In Russian]
Petrotschenko VI (1958) [Acanthocephala of domestic and wild animals]. Tom II. Izdatelstvo Akademii Nauk SSSR, Moskva, p 458 [In Russian]
Pichelin S, Cribb TH (1999) A review of the Arhythmacanthidae (Acanthocephala) with a description of Heterosentis hirsutus n.sp. from Cnidoglanis macrocephala (Plotosidae) in Australia. Parasite 6:293–302
Railliet A, Henry A (1907) Nemathelminthes parasites. Exped. antarctique française, 1903–1905. Masson et Cie, Paris, p 15
Reimer LW (1987) Helminthen von Fischen der Antarktis. Fischerei-Forschung Rostock 25:36–40
Rennie J (1906) “Scotia” Collections. On Echinorhynchus antarcticus n. sp., and its Allies. Proc R Soc Edinburgh 26:437–446
Rodjuk GN (1984) New representatives of the genus Metechinorhynchus (Acanthocephala), parasites of fishes of the western Antarctic. Zool Zh 63:1893–1896 [In Russian]
Rodjuk GN (1985) Parasitic Fauna of the Fishes of the Atlantic Part of the Antarctic (South Georgia Island and South Shetland Isles). In: Hargis Jr WJ (ed) Parasitology and pathology of marine organisms of the World Ocean. NOAA Tech. Rep. NMFS 25, pp 31–32
Rodjuk GN (1986) New species of Acanthocephala of the genus Echinorhynchus (Echinorhynchidae) from the southwestern Atlantic. Parazitologiya 20:224–227 [In Russian]
Zdzitowiecki, Rokosz B (1986) Prevalence of acanthocephalans in fishes of South Shetlands (Antarctic). II. Aspersentis austrinus Van Cleave, 1929 and remarks on the validity of Heteracanthocephalus hureaui Dollfus, 1965. Acta Parasit Pol 30:161–171
Skryabin AS (1966) [New corinosome Corynosoma mirabilis n. sp. – a parasite of the sperm whale]. In: Delyamure SL (ed) [Helminth fauna of animals of southern seas]. Izd. “Naukova Dumka”, Kiev, pp 10–12 [In Russian]
Skryabin AS (1970) A new species, Bolbosoma tuberculata sp.n. (Polymorphydae Meyer, 1931), a parasite of whales. Parazitologiya 4:334–337 [In Russian]
Skryabin AS (1972) On morphological differences between acanthocephals Bolbosoma turbinella (Diesing, 1851) (fam. Polymorphidae) from the northern and southern hemispheres. Parazitologiya 6:57–64 [In Russian]
Skryabin AS, Nikolsky OR (l971) [Corynosoma singularis sp. nov. (family Polymorphidae) – a parasite of marine mammals of the Antarctic]. Nauch Dokl Wyss Skholy Biol Nauki (1971):7–9 [In Russian]
Smales LR (1996) A redescription of Aspersentis zanclorhynchi (Johnston and Best, 1937) comb. nov. (Heteracanthocephalidae: Acanthocephala). Trans R Soc S Aust 120:167–171
Szidat L (1950) Los Parasitos del Robalo (Eleginops maclovinus (Cuv. & Val.)). Prim. Congr. nac. de pesquerias Marit. e Indust., Mar del Plata, 1949, vol 2, Buenos Aires, pp 235–270
Szidat L (1965) Estudios sobre la fauna de panisitos de peces antarcticos. I – Los panisitos de Notothenia neglecta Nybelin. Servicio de Hidrografia Naval, Secretaria de Marina, Republica Argentina, Publico H 910, Buenos Aires, p 84
Szidat L, Graefe G (1967) Estudios sobre la fauna de panisitos de peces antarcticos. II – Los parasitos de Parachaenichthys charcoti. Servicio de Hidrografia Naval, Armada Argentina, Republica Argentina, Publico H 911, Buenos Aires, p 27
Van Cleave HJ (1929) New Genus and new Species of Acanthocephala from the Antarctic. Ann Mag Nat Hist 10(4):229–232
Van Cleave HJ (1931) Heterosentis, a new Genus of Acanthocephala. Zool Anz 93:144–146
Yamaguti S (1963) Systema Helminthum. Volume V. Acanthocephala. Interscience Publishers, New York/London, p 423
Zdzitowiecki K (1978) On the occurrence of juvenile acanthocephalans of the genus Corynosoma Lühe, 1904 in fishes off South Georgia and South Shetland Islands (the Antarctic). Acta Ichthyologica et Piscatoria 8:111–127
Zdzitowiecki K (1981) Redescription of Aspersentis austrinus Van Cleave, 1929 (Acanthocephala). Acta Parasit Pol 28:73–83
Zdzitowiecki K (1983) Antarctic acanthocephalans of the genus Metacanthocephalus. Acta Parasit Pol 28:417–437
Zdzitowiecki K (1984a) Description of Heterosentis heteracanthus (Linstow, 1896) (Acanthocephala, Arythmacanthidae) from Antarctic fishes, and remarks on the taxonomic status of Heterosentis Van Cleave, 1931. Acta Parasit Pol 29:111–115
Zdzitowiecki K (1984b) Some Antarctic acanthocephalans of the genus Corynosoma parasitizing Pinnipedia, with descriptions of three new species. Acta Parasit Pol 29:359–377
Zdzitowiecki K (1984c) Redescription of Corynosoma hamanni (Linstow, 1892) and description of C. pseudohamanni sp. n. (Acanthocephala) from the environs of South Shetlands (Antarctic). Acta Parasit Pol 29:379–393
Zdzitowiecki K (1985) Acanthocephalans of birds from South Shetlands (Antarctic). Acta Parasit Pol 30:11–24.*
Zdzitowiecki K (1986a) Acanthocephala of the Antarctic. Pol Polar Res 7:79–117.*
Zdzitowiecki K (1986b) Prevalence of acanthocephalans in fishes of South Shetlands (Antarctic). I. Juvenile Corynosoma spp. Acta Parasit pol 30:143–160
Zdzitowiecki K (1986c) A contribution to the knowledge of morphology of Corynosoma bullosum (Linstow, 1892) (Acanthocephala). Acta parasit Pol 30:225–232
Zdzitowiecki K (1986d) Echinorhynchus nototheniae sp. n. (Acanthocephala) from nototheniid fishes from the environs of South Shetlands (Antarctic). Acta Parasit Pol 31:23–27
Zdzitowiecki K (1986e) Corynosoma gibsoni sp. n., a parasite of Otaria flavescens (Shaw, 1800) from the Falkland Islands and a note on the occurrence of C. evae Zdzitowiecki, 1984. Acta Parasit Pol 31:29–32.*
Zdzitowiecki K (1986f) Redescription of Corynosoma tunitae (Weiss, 1914) and description of C. baylisi sp. n. (Acanthocephala, Polymorphidae), parasites of piscivorous birds. Acta Parasit Pol 31:117–123
Zdzitowiecki K (1986g) Prevalence of acanthocephalans in fishes of South Shetlands (Antarctic). III. Metacanthocephalus johnstoni Zdzitowiecki, 1983, M. dalmori Zdzitowiecki, 1983 and notes on other species; general conclusions. Acta Parasit Pol 31:125–141
Zdzitowiecki K (1987) Acanthocephalans of marine fishes in the regions of South Georgia and South Orkneys (Antarctic). Acta Parasit Pol 31:211–217
Zdzitowiecki K (1989a) New data on the morphology and distribution of two acanthocephalans, Andracantha baylisi (Zdzitowiecki, 1986) comb. n. and Corynosoma australe Johnston, 1937. Acta Parasit Pol 34:167–172
Zdzitowiecki K (1989b) A redescription of Echinorhynchus petrotschenkoi (Rodjuk, 1984) comb. n. (Acanthocephala). Acta Parasit Pol 34:173–180
Zdzitowiecki K (1990a) Reexamination of five Antarctic and Subantarctic digenean and acanthocephalan species from Professor Szidat’s collection. Acta Parasit Pol 35:31–36
Zdzitowiecki K (1990b) Occurrence of acanthocephalans in the open sea fishes off South Shetlands and South Georgia (Antarctic). Acta Parasit Pol 35:131–142
Zdzitowiecki K, White MG (1996) Acanthocephalan infection of inshore fishes at the South Orkney Islands. Antarct Sci 8:273–276
Zdzitowiecki K (1991) Antarctic Acanthocephala. In: Wägele JW, Sieg J (eds) Synopses of the Antarctic benthos, vol 3, Koeltz Scientific Books, Koenigstein pp 1–116
Zdzitowiecki K (2001) Acanthocephala occurring in intermediate hosts, amphipods, in Admiralty Bay (South Shetland Islands, Antarctica). Acta Parasit 46:202–207
Zdzitowiecki K, Presler P (2001) Occurrence of Acanthocephala in intermediate hosts, Amphipoda, in Admiralty Bay, South Shetland Islands, Antarctica. Pol Polar Res 22:205–212
Zdzitowiecki K, Laskowski Z (2004) Helminths of an antarctic fish, Notothenia coriiceps, from the Vernadsky Station (Western Antarctica) in comparison with Admiralty bay (South Shetland Islands). Helminthologia 41:201–207
Zdzitowiecki K, Laskowski Z (2013) New data on the occurrence of Acanthocephala in some fish in Admiralty Bay (South Shetland Islands). Acta Parasitol 58:547–550. doi:10.2478/s11686-013-0175-1
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Laskowski, Z., Zdzitowiecki, K. (2017). Acanthocephalans in Sub-Antarctic and Antarctic. In: Klimpel, S., Kuhn, T., Mehlhorn, H. (eds) Biodiversity and Evolution of Parasitic Life in the Southern Ocean. Parasitology Research Monographs, vol 9. Springer, Cham. https://doi.org/10.1007/978-3-319-46343-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-46343-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46342-1
Online ISBN: 978-3-319-46343-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)