Abstract
Allosteric modulators are molecules that interact with a site on a receptor which is distinct from the orthosteric recognition site for the endogenous ligand. By modifying the receptor conformation, they change the affinity and/or efficacy of agonists, but often have no intrinsic activity on their own. Because of this use-dependent mechanism, they are expected to have a much better side-effect profile than agonist drugs. The first positive GABA type B (GABAB) receptor modulators, CGP7930 and GS39783, have been described more than 10 years ago. They were discovered in a high-throughput screen using GTP(γ)35S assays, in which they enhanced both the affinity and the maximal effect of γ-aminobutyric acid (GABA), without having any agonist activity of their own. This positive modulation was subsequently confirmed in a number of different radioligand binding, biochemical and electrophysiological assay systems. The recombinant expression of engineered receptor constructs allowed to locate the site of action of these positive modulators to the seven-transmembrane domain of the GABAB2 subunit, through which they could to some extent directly activate the receptor in sufficiently sensitive assay systems. These early findings have fostered the search for other molecules acting in a similar way, and a number of positive GABAB receptor modulators, and also the first negative modulators, have been described in recent years. In vivo microdialysis experiments have demonstrated at the biochemical level that the mechanism of positive allosteric GABAB receptor modulation also applies in living animals. Behavioural experiments have confirmed that positive GABAB receptor modulators have a better side-effect profile than the therapeutically used agonist drug baclofen. Numerous studies have shown that these compounds show promising activity in animal models for anxiety, drug and alcohol abuse, pain, gastrointestinal indications and possibly more.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- GABAB receptor
- Allosteric modulators
- Affinity cooperativity
- Activation cooperativity
- Radioligand binding
- GTPγ35S binding
- Intracellular Ca2+ signalling
- Electrophysiology
- In vivo effects
- Clinical indications
1 Introduction: Principles of Allosteric Modulation
Allosteric receptor modulation is an appealing and proven principle in drug targeting (Bowery 2006; Conn et al. 2009; Changeux 2013). The concept of allosterism was introduced for the first time 50 years ago in a classic paper by Monod et al. (1965). Allosteric modulators are molecules that act at a site on a receptor (or enzyme) which is distinct from the “orthosteric” recognition site for the endogenous ligand (or substrate). Numerous recent articles (e.g. Hall 2000; Christopoulos and Kenakin 2002; Urwyler 2011; Christopoulos 2014) address the principles and theoretical aspects of allosteric drug action. By inducing conformational changes in the receptor protein, positive or negative modulators enhance or decrease, respectively, the affinity and/or the efficacy of orthosteric ligands (the constants γ and δ in the model by Hall 2000, Fig. 18.1). Although they often have no intrinsic effects on their own, it is basically possible that they also act as agonists or inverse agonists to various degrees through the allosteric site (intrinsic efficacy constant β in Fig. 18.1). Moreover, neutral allosteric ligands are “silent” (β = 1, γ = 1 and δ = 1), but they block the effects of allosteric modulators or agonists by competitive displacement from the allosteric site. The cooperativity constants γ (binding) and δ (activation/efficacy) in Fig. 18.1 are determined by the conformational changes induced by given chemical structures of the orthosteric and allosteric ligands; therefore, their interactions are “probe dependent”. Because the thermodynamic stability of the ternary complex between the receptor and its two ligands is independent of the way on which it is formed, cooperativity between allosteric and orthosteric ligands is reciprocal.
The allosteric two-state model . R inactive state of the receptor, R* active state of the receptor, A orthosteric ligand, B allosteric ligand, K binding constant of A, L receptor isomerization constant, M binding constant of B, α intrinsic efficacy of A, β intrinsic efficacy of B, γ binding cooperativity between A and B, δ activation cooperativity between A and B. Slightly modified after Hall (2000). Reproduced from reference Urwyler 2011, with permission
Benzodiazepines are an early generation of clinically successful drugs acting as allosteric receptor modulators. These sedative/anxiolytic agents enhance the function of the inhibitory ionotropic (chloride channel) GABA type A (GABAA) receptor , basically without stimulating it by themselves. While these positive allosteric modulators are clinically useful drugs , compounds acting directly at the γ-aminobutyric acid (GABA) recognition site (such as the agonist muscimol or the antagonist bicuculline) have prohibitive side-effects.
A more recent case of a clinically successful positive modulator drug is the “calcimimetic” agent cinacalcet, which enhances the sensitivity of the G protein-coupled calcium sensing receptor for its natural ligand, the Ca2+ ion (reviewed in Urwyler 2011). This example nicely illustrates the potential of drugs acting as allosteric modulators. The calcium sensing receptor is a key regulator of calcium homeostasis; it acts by monitoring extracellular calcium concentrations in blood plasma and various organs and by triggering tissue responses that restore these to normal when needed. There are several diseases in which malfunction of the calcium sensing receptor is critically involved. An important example is secondary hyperparathyroidism resulting from chronic renal failure, in which a downregulation of the parathyroid calcium sensing receptor and a concomitant change of the calcium set point towards higher concentrations are observed. This situation can be corrected with the positive allosteric modulator cinacalcet, which increases receptor affinity and thereby shifts the calcium response curve towards lower concentrations. On the other hand, orthosteric agonist drugs (difficult to find for a site recognizing the calcium ion!), which would persistently activate the receptor, would not be useful in targeting a receptor whose function is to monitor changes in calcium concentrations.
Allosteric modulators undoubtedly have numerous advantages over orthosteric ligands as therapeutically active molecules. Firstly, whereas an agonist drug will basically stimulate each of its receptors which it encounters, a positive allosteric modulator will typically only enhance the receptor activation by its endogenous ligand (for example, when and where a neurotransmitter is released). Thus, compared to an agonist, it acts much more in concert with the temporal and spatial organization of physiological receptor activation. For this reason, a positive allosteric modulator can be expected to have a much lower side-effect potential compared to agonists. In addition, if a positive allosteric modulator were to be administered concomitantly with a drug acting as an agonist, the dose of the latter could be substantially reduced, thereby minimizing putative off-target side-effects. Secondly, whereas persistent receptor activation by agonists often leads to receptor desensitization (and thereby tolerance development), positive allosteric modulators, again because of their use-dependent mechanism, may well have a lower potential for inducing receptor desensitization . Thirdly, whereas the recognition sites for the endogenous ligands usually remained well conserved between different receptor subtypes during evolution, allosteric binding sites in general appear more variable. For example, while it has proven to be virtually impossible to find subtype-selective orthosteric agonists for a given group of metabotropic glutamate receptors, highly selective allosteric modulators for particular subtypes have been described (for review see Urwyler 2011). As a last point, in those cases where it is inherently difficult to conceive organic molecules mimicking the endogenous ligand, allosteric modulators may offer an elegant solution to the problem. The calcium-sensing receptor discussed above is a nice example for such a situation.
The agonist molecule baclofen, so far the only marketed drug targeting the GABA type B (GABAB) receptor, is used since decades for the treatment of muscle spasticity in patients suffering from multiple sclerosis or spinal cord injury (see Chap. 17 of this book for a comprehensive review on baclofen pharmacology). Moreover, more recently several “off-label” clinical indications for baclofen have emerged, such as, e.g. gastroesophageal reflux disease (GERD) (see Chap. 16 of this book) or alcohol use disorder (see Chap. 15 of this book). However, in uses other than the treatment of muscle spasticity, the strong muscle relaxant properties of baclofen are an unwanted side-effect. Baclofen also has other important shortcomings, such as a short duration of action, a narrow therapeutic window and rapid development of tolerance to some of its effects (Vacher and Bettler 2003, see also Chap. 17 of this book). For these reasons, in the light of their advantages mentioned above, positive allosteric GABAB receptor modulators seem to have therapeutic potential possibly avoiding the shortcomings of baclofen. Although information on such molecules is only available from preclinical models so far, optimism for therapeutic efficacy in man is justified on the basis of the success of cinacalcet, since the calcium sensing receptor belongs to the same G protein-coupled receptor (GPCR) family C as the GABAB receptor. Cinacalcet may thus well have paved the way for GABAB receptor modulators .
2 Molecular and Cellular Pharmacology of Allosteric GABAB Receptor Modulators
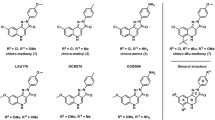
The first positive allosteric GABAB receptor modulators, CGP7930 and GS39783 (Fig. 18.2), were discovered and described at the beginning of this century (Urwyler et al. 2001, 2003). Since then, high-throughput screening and synthetic chemistry efforts have produced more such compounds, and allosteric GABAB receptor modulators have been the subject of extensive pharmacological characterization in vitro and in vivo. Several reviews have addressed this topic in recent years (e.g. Pin and Prézeau 2007; Urwyler 2011; Brown et al. 2015, see also Froestl 2010 for a patent survey). The chemistry of allosteric GABAB receptor modulators is covered by C. Mugnaini and F. Corelli in Chap. 3 of this book; it also contains the structures of modulators other than CGP7930 and GS39783.
Chemical structures of the prototypical positive allosteric GABAB receptor modulators CGP7930 (left) and GS39783 (right). See Chap. 3 of this book for the structures of other compounds acting in a similar way
CGP7930 and GS39783 , and a number of analogues thereof, were discovered in a high-throughput screening program based on a GTPγ35S binding assay using membranes from a Chinese hamster ovary (CHO) cell line expressing the two GABAB receptor subunits, and subsequently characterized in detail in vitro (Urwyler et al. 2001, 2003). CGP7930 and GS39783 at low micromolar concentrations enhanced GTPγ35S binding stimulated by GABA or baclofen, without having any effect by themselves. The two positive modulators had no effect on GABA-stimulated GTPγ35S binding in the presence of the competitive antagonist CGP56999A, confirming that their activity is dependent on concomitant stimulation of the orthosteric receptor site. Very similar results were obtained on native GABAB receptors in rat brain membranes (Urwyler et al. 2001, 2003) or in human post-mortem brain tissue (Olianas et al. 2005). Unexpectedly, CGP7930 and GS39783 not only enhanced the potency of GABA in the GTPγ35S assay (by a five- to tenfold), but also its maximal effect (by a 1.5- to 2-fold) (Fig. 18.3). This was a rather novel type of allosteric modulatory effect at the time, but is accounted for by recent theoretical receptor models (Hall 2000; Christopoulos and Kenakin 2002, see also Urwyler 2011).
Effects of CGP7930 in membranes from a recombinant Chinese hamster ovary (CHO) cell line expressing the GABAB receptor. In a GTPγ[35S] stimulation assay, CGP7930 enhances at the same time the potency and the maximal efficacy of GABA as an agonist at the GABAB receptor. GABA concentration–response curves were measured in the absence (filled square) and in the presence of 1 μM (open circle), 3 μM (filled diamond), 10 μM (triangle) and 30 μM (filled circle) CGP7930. Reproduced from reference Urwyler et al. (2001), with permission
Radioligand binding assays are a powerful tool to assess the binding cooperativity (affinity modulation) between two molecules interacting with distinct sites on the same receptor protein. The rate constants of association and dissociation of orthosteric ligands are sensitive to changes in receptor conformation induced by an allosteric ligand. In our experiments , 30 μM GS39783 unexpectedly reduced the rate of association of the agonist radioligand [3H]3-aminopropylphosphinic acid ([3H]APPA) to native GABAB receptors, but this effect was overcompensated by an even greater reduction in the rate of dissociation, resulting in a net increase of ligand affinity (Urwyler et al. 2003). GPCRs exist in a high agonist affinity (G protein-bound) and a low agonist affinity (uncoupled from the G protein) state. The agonist radioligand [3H]APPA labels only the high affinity state of the GABAB receptor. When saturation experiments were performed with this ligand using native GABAB receptors in rat brain membranes, 30 μM CGP7930 increased the agonist affinity by about a 2.5- to 3-fold, and also marginally but not significantly its maximal binding capacity (Urwyler et al. 2001). On the other hand, antagonist radioligands such as [3H]CGP62349 label both receptor states. Therefore, agonist displacement curves are biphasic, comprising a high- and a low-affinity component. CGP7930 and GS39783 (30 μM) enhanced the affinities for agonists of both components (Fig. 18.4), indicating that both the G protein-coupled and -uncoupled receptor forms are amenable to allosteric modulation. Moreover, the relative proportion of the high affinity component was increased, suggesting that the two positive modulators also enhance the interaction between receptor and G protein (Urwyler et al. 2003, 2004). This interpretation is in line with the slight increase in maximal agonist binding capacity observed earlier, the meaning of which was overlooked at the time.
Displacement of the competitive antagonist radioligand [3H]CGP62349 from native GABAB receptors in rat cortical membranes by GABA (squares), L-baclofen (circles) and APPA (triangles) in the absence (open symbols) and in the presence (filled symbols) of 30 μM GS39783. Reproduced from Urwyler et al. (2003), with permission
A complex situation was encountered when radioligand binding experiments were performed with recombinant GABAB receptor preparations. In membranes from CHO cells expressing the GABAB1 subunit only, GABA displaced the antagonist radioligand [3H]CGP62349 with low affinity, which was not affected by CGP7930 . On the other hand, in membranes from cells expressing both GABAB receptor subunits, biphasic displacement curves were observed with GABA. The low-affinity component comprised the major part (about 70 %) of the total binding, had a similar IC50 as in the GABAB1 monomer, was not influenced by CGP7930 and was therefore attributed to the strongly overexpressed GABAB1 subunit in these cells. However, the remaining part corresponding to dimeric GABAB receptors displayed a much higher affinity for GABA, which was increased by a 2.5-fold in the presence of 30 μM CGP7930. The binding assay was not sufficiently sensitive to further resolve this part into G protein-coupled and -uncoupled components. These results demonstrate that the GABAB2 subunit is essential for the modulatory action of CGP7930 and suggest that it harbours the modulator binding site (Urwyler et al. 2001).
Allosteric modulation by GS39783 is not observed with the Drosophila melanogaster GABAB receptor (Dupuis et al. 2006). However, co-expression of the Drosophila GABAB1 subunit together with rat GABAB2 gave functional receptors positively modulated by GS39783 in GTPγ35S binding assays. Further data obtained with chimeric Drosophila melanogaster/rat GABAB2 subunit constructs and point mutations demonstrated a critical role of the GABAB2 transmembrane region for positive modulation. Of particular interest was the finding that in a construct containing two key amino acid substitutions in transmembrane domain VI, GS39783 directly activated the rat GABAB2 subunit even in the absence of GABAB1, in contrast to wild-type GABAB2 (Dupuis et al. 2006). Binet et al. (2004), using a sensitive phosphoinositol turnover assay, have found that CGP7930 activated the wild-type GABAB receptor on its own as a partial agonist with low efficacy. Moreover, it could also directly activate GABAB2 expressed alone, as well as a truncated construct in which GABAB2 was deprived of its extracellular domain (Binet et al. 2004). Taken together, these results demonstrate that CGP7930 and GS39783 not only act as positive allosteric GABAB receptor modulators, but they are also the first compounds shown to be able to directly activate the GABAB2 receptor subunit, via its heptahelical domain. Such “allosteric agonism ” is accounted for (intrinsic efficacy constant β in Fig. 18.1) and allowed by the theoretical model proposed by Hall (2000, see also Urwyler 2011 for more explanations).
The model shown in Fig. 18.1 is basically applicable to all kinds of orthosteric ligands, independently of their intrinsic properties. This means that partial or full agonists, inverse agonists, as well as competitive antagonists, should be equally amenable to modulation by allosteric drugs. In fact, in radioligand binding assays the affinities of a number of competitive GABAB receptor antagonists were found to be decreased in the presence of 30 μM CGP7930 or GS39783 (Urwyler et al. 2005). In a GTPγ35S assay, the maximal response of the partial agonist CGP47656 was increased by approximately fourfold by the two modulators, compared to only 1.5- to 2-fold increases found with GABA. Interestingly, in the same study (Urwyler et al. 2005) it was found that the two compounds CGP35348 and 2-hydroxy-saclofen, previously believed to be neutral or “silent” competitive antagonists, did not stimulate GTPγ35S binding on their own, but became partial agonists in the presence of 30 μM CGP7930 or GS39783. Apparently the modulators amplified hidden, marginal agonistic effects of these two compounds. Partial agonistic properties of CGP35348 and 2-hydroxy-saclofen were also seen in seemingly more sensitive cyclic AMP (cAMP) measurements and were further enhanced by 10 μM CGP7930 and GS39783 (Urwyler et al. 2005).
Matsushita et al. (2010) have looked at the mechanisms of GABAB receptor activation in a novel way. They fused each GABAB receptor subunit with either Cerulean or enhanced yellow fluorescent protein at intracellular loops and measured changes in fluorescence resonance energy transfer (FRET) after agonist application. FRET decreases were observed between GABAB1a loop 2 and GABAB2 loops 1 or 2. These FRET decreases were more pronounced when 3 or 10 μM GABA were applied together with CGP7930 (100 μM); the allosteric modulator alone had no effect. In contrast, intrasubunit constructs labelled with Cerulean at the C terminus and enhanced yellow fluorescent protein at the intracellular loop 1 of either subunit did not reveal any FRET change upon application of GABA, with or without CGP7930. The authors proposed a model according to which GABAB receptor activation would result in the widening of a cleft between the two receptor subunits, leaving the configuration of the transmembrane domains of each subunit unchanged. CGP7930 would act by binding in the cleft, thereby further widening it. However, this model seems difficult to reconcile with the view that CGP7930 acts through the GABAB2 subunit in the transmembrane domain as discussed above.
Whereas radioligand binding and GTPγ35S stimulation assays are mostly performed on membrane preparations, the two prototypical positive GABAB receptor modulators were also tested in a number of biochemical and electrophysiological experiments in cellular or intact tissue preparations. CGP7930 (10 μM) and GS39783 (10 μM) enhanced the potency of GABA to inhibit adenylyl cyclase activity in a recombinant GABAB receptor expressing CHO cell line (Urwyler et al. 2005). This experimental system was highly sensitive, apparently due to a substantial degree of receptor reserve, the potency of GABA being considerably higher than in GTPγ35S assays. This high sensitivity again allowed to detect a low degree of partial agonistic activity of CGP7930 and GS39783 on their own, via the allosteric site.
Masharina et al. (2012) have developed an elegant GABAB receptor-based biosensor to determine concentrations of GABA and synthetic GABAB receptor ligands on the surface of living cells, using a FRET readout. In their hands, 30 μM CGP7930 increased the receptor affinity for GABA, and thereby the sensitivity of the biosensor, by a threefold.
In Xenopus laevis oocytes which were injected with mRNA for the two GABAB receptor subunits and for inwardly rectifying (Kir3) potassium channels, the application of 0.3 μM GABA elicited potassium currents which were enhanced in the presence of 30 μM CGP7930 or 3 μM GS39783 (Urwyler et al. 2001, 2003). In the case of CGP7930, this effect was observed with both GABAB receptor isoforms GABAB(1a/2) and GABAB(1b/2). No potassium currents were produced by either of the modulators alone. In human embryonic kidney (HEK293) cells transiently co-transfected with GABAB receptors and a chimeric G-protein coupling them to the phospholipase C pathway, the application of GABA elicited a transient increase in intracellular cytosolic calcium concentration. Both modulators enhanced that signal with EC50-values in the low micromolar range, depending on the GABA concentrations used, with lower EC50s at higher GABA concentrations. This is a good example of the reciprocity of allosteric modulation mentioned in the Introduction. In the case of GS39783 , this effect was again observed with both receptor isoforms, and the modulator not only increased the potency, but also the maximal efficacy of GABA. No intrinsic agonist activity was observed with either CGP7930 or GS39783 in this assay system (Urwyler et al. 2001, 2003). GABAergic effects on intracellular calcium were also observed in a neuronal network preparation. R(−)-baclofen reduces the frequency of synchronized intracellular calcium oscillations in mouse or rat cortical neurons in primary culture. Both CGP7930 (0.3 μM) and GS39783 (1 and 3 μM) further reduced the calcium oscillation frequency in the presence of baclofen, but not on their own (Urwyler et al. 2001; Gjoni and Urwyler 2008). In a hippocampal slice preparation, two consecutive stimulations of afferent pathways result in inhibition of the second population response as a consequence of the activity of GABAergic interneurons. Activation of presynaptic GABAB receptors will inhibit GABA release from these interneurons and thereby reverse paired pulse inhibition. This is what was observed with 10 μM GS39783 applied alone, like with baclofen (Urwyler et al. 2003). The effects of both compounds were counteracted by the competitive antagonist CGP55845A , indicating that the reversal of paired pulse inhibition by GS39783 was due to a potentiation of the activity of endogenous GABA rather than a direct activation of presynaptic GABAB receptors. On a similar line, Chen et al. (2006) have found that CGP7930 enhances the inhibitory effects of baclofen on synaptic inhibition in the CA1 area of the hippocampus. Also, CGP7930 enhanced the inhibitory effect of baclofen on dopamine neuron activity in rat midbrain slices (Chen et al. 2005).
GABAB receptors couple to multiple intracellular pathways, either directly or via “crosstalk” with other G protein-coupled receptors. Onali et al. (2003) have studied the allosteric effects of CGP7930 on native GABAB receptors in membrane preparations from different rat brain regions. In membranes from olfactory bulb and frontal cortex, GABAB receptor activation enhances basal and corticotropin-releasing hormone-stimulated adenylyl cyclase activity via the β/γ-subunits of Gi/o-proteins. In both cases, CGP7930 at concentrations in the range from 10 to 100 μM enhanced the stimulatory effects on cAMP formation produced by baclofen or GABA, without having any agonistic effect on its own. It increased both the potencies and maximal effects of the two agonists. On the other hand, when inhibition of forskolin- or Ca2+/calmodulin-stimulated adenylyl cyclase (in frontal cortex or striatum and cerebellum, respectively) was measured, CGP7930 increased the potency of baclofen or GABA with little or no effect on the maximal inhibition. These results indicate that signalling via both the α- and β/γ-subunits of the Gi/o-proteins coupled to GABAB receptors is enhanced by CGP7930.
Mannoury la Cour et al. (2008) used an immunocapture scintillation proximity assay to investigate the coupling of GABAB receptors to different G-protein subtypes. GABA and (R)-baclofen stimulated GTPγ35S binding to Gαo and Gαi1/3, but not to Gαq and Gαs/olf in membranes from rat cortex, hippocampus and cerebellum. CGP7930 and GS39783 did not stimulate GTPγ35S binding on their own, but they enhanced agonist potencies and maximal efficacy for the stimulation of GTPγ35S binding to Gαo, but not to Gαi1/3, in all three brain regions. On the other hand, in a recombinant human embryonic kidney (HEK) cell line expressing GABAB receptors, the modulators enhanced the potency, but not the maximal efficacy, of GABA to stimulate GTPγ35S binding to Gαi1/3. This G-protein subtype might have been a limiting factor in both native and recombinant assay systems.
In cultured cerebellar granule neurons, GABA and baclofen induce ERK1/2 phosphorylation (Tu et al. 2007). This effect was also found with 50 μM CGP7930 applied on its own, this assay system thus giving another example of allosteric agonism by this compound. Again using cerebellar granule neurons, research from the same group (Tu et al. 2010) later demonstrated interesting neuroprotective effects of CGP7930 . Using potassium deprivation to induce apoptosis in these neurons, they observed that baclofen (30 μM) and CGP7930 (3–30 μM) on its own significantly decreased the number of apoptotic neurons. This neuroprotection seemed to follow a complex pathway involving GABAB/insulin-like growth factor 1 receptor transactivation (“crosstalk”) and ultimately leading to inhibition of caspase-3 activity (Tu et al. 2010).
Receptor desensitization is a mechanism often involved in the development of tolerance upon chronic drug treatment. Rapid development of tolerance is indeed one of the major problems in the clinical use of baclofen, at least for some indications (Vacher and Bettler 2003). Because of their use-dependent mechanism of action, positive allosteric modulators can be expected to induce less receptor desensitization than orthosteric agonists. We tested this hypothesis by measuring different functional responses after continuous exposure of native or recombinant GABAB receptors on the one hand to desensitizing agonist concentrations and, on the other hand, to a combination of a low agonist concentration and GS39783 that activated the receptor to the same extent (Gjoni and Urwyler 2008). In our recombinant GABAB receptor expressing CHO cell line, we observed a decrease of the potency of GABA to inhibit adenylyl cyclase activity after pre-exposure to a saturating concentration (100 μM) of GABA. On the other hand, no such desensitization was seen after pre-exposure to a low GABA concentration (0.3 μM) in combination with 10 μM GS39783. We made similar observations in primary neuronal cultures for baclofen-induced inhibition of spontaneous Ca2+ oscillations (Gjoni and Urwyler 2008). Thus it seems that the change in receptor conformation induced by GS39783 would only enhance functional GABAB receptor-mediated responses, but not its desensitization pathway—a finding reminiscent of the phenomenon of “agonist-directed trafficking”. However, because of the probe-dependence of allosteric effects mentioned above, the outcome in such experiments might be different with an allosteric modulator other than GS39783 .
In this context, it is also of interest to note that cAMP measurements in a recombinant cell line revealed that GS39783 became an allosteric agonist at desensitized GABAB receptors in which the activation mechanisms of the receptor have apparently undergone fundamental changes (Gjoni and Urwyler 2009).
The prototypical positive allosteric GABAB receptor modulators CGP7930 and GS39783 have of course fostered the search for more compounds with such properties. The extracellular Ca2+ sensing receptor is allosterically modulated by amino acids and arylalkylamines. For this reason, Kerr et al. (2002) and Kerr and Ong (2003) have examined the effects of such compounds on GABAB receptor-mediated responses. They concluded that several amino acids, dipeptides and arylalkylamines such as fendiline are allosteric modulators because they enhanced baclofen-induced field potentials in rat neocortical slices, without having an effect on their own. However, in our radioligand binding , GTPγ35S stimulation and intracellular Ca2+ signalling assays, these compounds were completely inactive (Urwyler et al. 2004). It therefore appears that the observations made in brain tissue slices are due to “downstream” effects, rather than allosteric mechanisms. Later on, Kerr et al. (2006, 2007) have made a series of derivatives of CGP7930, none of which, however, surpassed the lead compound in terms of potency and efficacy.
In an effort to obtain allosteric GABAB receptor modulators devoid of the genotoxic potential of GS39783, Guery et al. (2007) made a number of derivatives thereof lacking the nitro group. The most active molecule from their series is BHF177, with a potency (EC50 = 1.7 μM) and cooperative effects in the GTPγ35S assay similar to those of the lead compound.
The compound rac-BHFF (Malherbe et al. 2008) acted in a way similar to CGP7930 , but with higher potency (EC50 = 234 nM) at recombinant GABAB receptors. In GTPγ35S and intracellular calcium mobilization assays, it enhanced both the potency and maximal effects of GABA. However, unlike CGP7930, it stimulated GTPγ35S binding also in the absence of GABA (allosteric agonism). (+)-BHFF was more potent than (−)-BHFF, the first described case of enantioselectivity at the allosteric GABAB receptor binding site. In the FRET-biosensor setup by Masharina et al. (2012), rac-BHFF at 10 μM increased the affinity of GABA by a ninefold and was thus more potent than CGP7930. Because rac-BHFF is quickly hydrolysed in vivo, Malherbe et al. (2008) also made its more stable lactam analogue BHFI, which has similar activity as a positive allosteric GABAB receptor modulator. The more recently described positive modulator ADX71943 (Kalinichev et al. 2014a) also has good, submicromolar potency in intracellular calcium mobilization and GTPγ35S binding assays .
Another positive allosteric modulator which was found in a high-throughput screening campaign is the compound CMPPE (Perdonà et al. 2011). In GTPγ35S assays in a recombinant cell line expressing the human GABAB receptor or in rat cortical membranes, it enhanced the stimulation produced by a low concentration of GABA in a way seemingly similar to that of CGP7930 and GS39783. However, unlike the two prototypical modulators, CMPPE displayed a high degree of allosteric agonism, stimulating GTPγ35S binding on its own to a maximal level approximately the same as that produced by GABA. It thus seems difficult to distinguish to which degree the GABA-enhancing effects of CMPPE were truly of cooperative nature or rather due to additivity of agonism through the orthosteric and allosteric sites. On the other hand, when CMPPE (1 μM) was tested on currents through inwardly rectifying potassium channels in rat hippocampal neurons, it was found, like GS39783, to enhance currents produced by baclofen but did not evoke any current when applied alone up to 10 μM.
Interestingly, the two compounds COR627 and COR628 were identified by a virtual screening protocol using a pharmacophore model based on previously described positive allosteric GABAB receptor modulators (Castelli et al. 2012). In rat cortical membranes, both compounds enhanced GABA- or baclofen-stimulated GTPγ35S binding at low micromolar concentrations, without any agonist effect when given alone. However, unlike the observations made with CGP7930 and GS39783, the allosteric effect of these compounds consisted almost solely in binding cooperativity, i.e. an increase in the potency of GABA. The maximal efficacy of GABA was only little (with COR627) or not at all (with COR628) increased by these two compounds. In radioligand binding experiments (displacement of an antagonist radioligand by GABA in rat cortical membranes), both compounds enhanced the affinities of GABA for both high- and low-affinity sites. Unlike CGP7930 and GS39783, the two COR compounds did not increase the relative proportion of the high agonist affinity component, suggesting that they do not facilitate the coupling between the receptor and its G-protein. COR627 and COR628 then served as a starting point for the synthesis of a whole series of 2-(acylamino)thiophene derivatives (Mugnaini et al. 2013). These molecules had a profile in vitro similar to that of the two COR compounds. Although their potency in vitro was lower compared to the reference compound GS39783, a few among them showed good effectiveness in vivo .
Until recently, no negative allosteric GABAB receptor modulators have been known. From a synthetic program based on the scaffold of CGP7930, Chen et al. (2014) obtained three compounds which were found to inhibit GABA-induced inositol phosphate 3 (IP3) production in HEK cells overexpressing GABAB receptors together with a Gq-protein. They did so by decreasing the maximal effect of GABA without changing its EC50, thus acting as non-competitive antagonists. For one of the compounds it was shown in a radioligand binding assay that it did not bind to the orthosteric receptor site, thus confirming that it acted as an allosteric modulator with negative efficacy cooperativity. Furthermore, these compounds not only inhibited baclofen-induced ERK1/2 phosphorylation in HEK293 cells overexpressing GABAB receptors, but they also blocked the stimulation of this same pathway by CGP7930 , which had been previously shown to be an allosteric agonist in this assay system (see above, Tu et al. 2007). Most likely the compounds inhibited the effect of baclofen by negative modulation, whereas the stimulation by CGP7930 may well have been blocked by a competitive mechanism at the allosteric site, in the light of the structural similarity between the positive and negative modulators. It would be interesting to see how these negative modulators behave in membrane-based assays (GTPγ35S and radioligand binding ), in particular whether their lack of binding cooperativity could be confirmed.
Taken together, screening and synthetic chemistry efforts have yielded so far a number of interesting allosteric GABAB receptor modulators displaying binding and/or efficacy cooperativity toward orthosteric agonists in different ways, and with different intrinsic agonist activity. The two prototypical modulators CGP7930 and GS39783 , along with COR627 and COR628 , seem to be among those exerting the lowest degree of allosteric agonism and might therefore be considered the allosteric GABAB receptor modulators acting mostly through binding and/or activation cooperativity .
3 Effects of Positive Allosteric GABAB Receptor Modulators In Vivo
To address the question of whether allosteric GABAB receptor modulators act in the same way in a living organism, we have carried out an in vivo microdialysis study measuring cyclic AMP formation in the brain of freely moving rats (Gjoni et al. 2006). Orally applied GS39783 dose dependently inhibited cAMP formation in rat striatum only in conjunction with a threshold concentration of locally administered baclofen (Fig.18.5). On its own, GS39783 at the doses tested was inactive, suggesting a sub-threshold endogenous GABA-tone. The inhibition of forskolin-stimulated cAMP production by GS39783 and baclofen was reversed by a competitive GABAB receptor antagonist. Thus, just as the early in vitro GTPγ35S experiments, this study at the biochemical-mechanistic level in vivo demonstrated that the effects of GS39783 are dependent on the concomitant activation of the orthosteric agonist site.
GS39783 enhances the inhibition of cAMP formation by the GABAB agonist baclofen in vivo. cAMP concentrations were measured in rat brain striatum by in vivo microdialysis. Adenylyl cyclase was stimulated by two consecutive administrations of a water-soluble forskolin analogue through the dialysis probe. Drugs were administered before the second stimulation, and drug effects were calculated as the ratio between the areas under the two cAMP peaks (S2/S1). Baclofen was administered through the dialysis probe at a threshold concentration (1 μM), which by itself did not evoke a detectable inhibition of cAMP formation (S2/S1 = 1). However, when the positive allosteric modulator GS39783 was administered orally in addition to 1 μM baclofen, a dose-dependent and significant (asterisk) inhibition of cAMP production was observed. This inhibition was significantly (+) reverted by coapplication of the competitive antagonist CGP56999A , indicating the dependence of the effects of GS39783 on the presence of an orthosteric agonist. GS39783 alone had no effect (not shown). Reproduced from reference Gjoni et al. (2006), with permission
The agonist baclofen has long been the almost only available tool compound to investigate the role of GABAB receptors in behavioural processes. However, baclofen induces sedation, hypothermia and muscle relaxation, which may seriously interfere with behavioural observations in animals. The effectiveness of CGP7930 in vivo has in fact been demonstrated by its ability to enhance the sedative/hypnotic effects (loss of righting reflex) of threshold doses of the GABAB receptor agonists baclofen and γ-hydroxybutyrate (GHB) in DBA mice (Carai et al. 2004). Conversely, at the doses tested CGP7930 did not induce any loss of righting reflex when administered alone. Similar results were obtained with COR627 and COR628 (Castelli et al. 2012). Koek et al. (2010) have also observed that CGP7930 and rac-BHFF enhanced baclofen- and GHB-induced loss of righting reflex, but not hypothermia. These results have fostered the interest in testing allosteric modulators, which are potentially devoid of the unwanted effects of GABAB receptor agonists such as baclofen, in behavioural paradigms. Numerous such studies have been performed in recent years; many of them are covered in separate chapters in this book and are therefore only briefly mentioned here, mainly highlighting the impact of some mechanistic aspects on the in vivo situation.
In pigeons discriminating baclofen from saline, CGP7930 and rac-BHFF enhanced the discriminative stimulus effects of baclofen (but not of GHB), but produced only a partial baclofen-appropriate responding by themselves (Koek et al. 2012). In the latter regard, rac-BHFF was more effective than CGP7930, a finding possibly related to the fact that rac-BHFF has a higher intrinsic agonist efficacy at the GABAB receptor. In pigeons which were, conversely, trained to discriminate rac-BHFF from its vehicle, the discriminative stimulus produced by rac-BHFF was not mimicked by baclofen and not antagonized by the competitive antagonist CGP35348 (Koek et al. 2013). On the other hand, it was attenuated by CGP7930 , suggesting that rac-BHFF produces its effects by directly activating the GABAB2 receptor subunit. For more details on GABAB receptor-mediated mechanisms in drug discrimination paradigms, see Chap. 9 of this book.
Cryan et al. (2004) studied the effects of GS39783 in various rodent models for anxiety and depression. Treatment with GS39783 produced anxiolytic-like activity in the light-dark box and elevated zero maze tests, but no effect of GS39783 was found in the forced swim test for antidepressant activity. Importantly, the positive modulator did not show any of the side-effects known to be associated with the use of baclofen or benzodiazepines. A similar profile was later reported for CGP7930, which showed anxiolytic-like activity in different mouse models without inducing motor impairment or hypothermia (Jacobson and Cryan 2008). The role of the GABAB receptor and its pharmacology in psychiatric indications is covered in detail in Chap. 12 of this book.
GABAB receptors seem to play an important role in drug addiction (Filip and Frankowska 2008). Baclofen reduces the consumption of different drugs of abuse in laboratory animals and seemingly also in humans, an effect most likely due to the fact that it inhibits nicotine-, cocaine- and opiate-induced release of dopamine in the nucleus accumbens, which is believed to mediate the rewarding effects of drugs of abuse. Xi et al. (2003) and Amantea et al. (2004) have reported that on repeated administration of cocaine or nicotine, respectively, to rats, GABAB receptor density in the nucleus accumbens was not altered. However, the level of G-protein coupling to the receptor, assessed by the stimulation of GTPγ35S binding by baclofen, was reduced. It is exactly in this type of situation that positive allosteric modulators are expected to be of benefit, because at least some precisely act by enhancing the efficiency of the coupling of the GABAB receptor to its G-proteins. Lhuillier et al. (2007) have aligned behavioural effects of short- and long-term cocaine administration with several biochemical correlates. GS39783 counteracted the increase in both locomotion and in striatal expression of the immediate early gene c-fos produced by acutely administered cocaine. After long-term cocaine treatment, GS39783 somewhat attenuated behavioural sensitization and at the same time reduced the upregulation of cAMP-response-element-binding protein (CREB) and dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) and the accumulation of the transcription factor ΔFosB in the nucleus accumbens and/or dorsal striatum. These findings strongly suggest that the positive GABAB receptor modulator prevents long-term adaptive changes in dopaminergic signalling pathways induced by chronic cocaine intake. In a similar study, it was shown that GS39783 blocked the rewarding effects of nicotine in rats; at the same time, GS39783 inhibited nicotine-induced accumulation of ΔFosB in the nucleus accumbens of these animals (Mombereau et al. 2007). Numerous behavioural studies have been performed in recent years to investigate the role of GABAB receptors in alcohol and drug use disorder. They have been reviewed recently by Filip et al. (2015) and are covered in detail in Chaps. 14 and 15 of this book. The compound ADX71441 is the first positive allosteric GABAB receptor modulator to be tested clinically, after it has been found to reduce voluntary alcohol consumption in two animal models of binge-like drinking and of long-term excessive drinking, respectively (Hwa et al. 2014).
Systemic CGP7930 , like baclofen, has analgesic properties in a mechanically evoked visceral pain model (Brusberg et al. 2009). Both drugs reduced colorectal distension-induced visceromotor and cardiovascular responses in conscious rats. GABAB receptors might therefore be a promising target for the treatment of painful gastrointestinal disorders, such as irritable bowel syndrome. Peripheral GABAB receptors are localized on nerve endings innervating different regions of the gastrointestinal tract. Allosteric modulators devoid of blood–brain barrier permeability would selectively target these peripheral receptors. One such compound might be ADX71943 , which also showed analgesic effects in peripheral pain models (acetic acid-induced writhing, formalin tests), but was suggested to have a fully peripheral activity profile based on its lack of anxiolytic-like activity (marble burying, elevated plus maze tests) despite reaching high concentrations in plasma (Kalinichev et al. 2014a). Also, ADX71943 had no effect on body temperature, muscle relaxation (rotarod test) and spontaneous locomotor activity. Another clinical indication calling for a drug acting only peripherally is overactive bladder syndrome , in animal models for which ADX71441 proved to be effective (Kalinichev et al. 2014b).
4 Outlook: Therapeutic Perspectives
Allosteric GABAB receptor modulators have come a long way since their first description in 2001. High-throughput screening and synthetic chemistry efforts have yielded a variety of structural classes of compounds acting through this mechanism. Most of these have served as research tools, while only few compounds have reached a development stage close to clinical trials. The optimization of such molecules in terms of potency, selectivity, in vivo efficacy and desired brain permeability (depending on peripheral or central site of action, respectively) still remains challenging for pharmacologists and medicinal chemists. On the other hand, based on extensive testing in a large number of animal models in vivo, it appears that allosteric GABAB receptor modulators hold their promise of a better side-effect profile compared to the agonist baclofen, because of their use-dependent mechanism.
The clinical indications for which a therapy with allosteric GABAB receptor modulators might be successful include anxiety, epilepsy, pain, drug abuse, gastrointestinal disorders, overactive bladder syndrome and possibly more. The GABAB receptor agonist baclofen has been in clinical use as an antispastic agent for decades. However, its strong muscle-relaxant property precludes its therapeutic application in other indications, in which it would be an unwanted side-effect. On the other hand, the fact that allosteric modulators are devoid of this side-effect in animal experiments means that spasticity will likely not be a potential indication for such compounds. It thus seems that GABAB receptor agonists and positive modulators might well cover complementary sets of clinical indications. The first results from clinical trials should become available in a not too distant future .
References
Amantea, D., Tessari, M., & Bowery, N. G. (2004). Reduced G-protein coupling to the GABAB receptor in the nucleus accumbens and the medial prefrontal cortex of the rat after chronic treatment with nicotine. Neuroscience Letters, 355, 161–164.
Binet, V., Brajon, C., Le Corre, L., Acher, F., Pin, J. P., & Prézeau, L. (2004). The heptahelical domain of GABAB2 is activated directly by CGP7930, a positive allosteric modulator of the GABAB receptor. Journal of Biological Chemistry, 279, 29085–29091.
Bowery, N. G. (Ed.). (2006). Allosteric receptor modulation in drug targeting. London: Taylor & Francis.
Brown, K. M., Roy, K. K., Hockerman, G. H., Doerksen, R. J., & Colby, D. A. (2015). Activation of the γ-aminobutyric acid type B (GABAB) receptor by agonists and positive allosteric modulators. Journal of Medicinal Chemistry, 58, 6336–6347.
Brusberg, M., Ravnefjord, A., Martinsson, R., Larsson, H., Martinez, V., & Lindström, E. (2009). The GABAB receptor agonist, baclofen, and the positive allosteric modulator, CGP7930, inhibit visceral pain-related responses to colorectal distension in rats. Neuropharmacology, 56, 362–367.
Carai, M. A. M., Colombo, G., Froestl, W., & Gessa, G. L. (2004). In vivo effectiveness of CGP7930, a positive allosteric modulator of the GABAB receptor. European Journal of Pharmacology, 504, 213–216.
Castelli, M. P., Casu, A., Casti, P., Lobina, C., Carai, M. A. M., Colombo, G., et al. (2012). Characterization of COR627 and COR628, two novel positive allosteric modulators of the GABAB receptor. Journal of Pharmacology and Experimental Therapeutics, 340, 529–538.
Changeux, J. P. (2013). The concept of allosteric interaction and its consequences for the chemistry of the brain. Journal of Biological Chemistry, 288, 26969–26986.
Chen, L. H., Sun, B., Zhang, Y., Xu, T. J., Xia, Z. X., Liu, J. F., et al. (2014). Discovery of a negative allosteric modulator of GABAB receptors. ACS Medicinal Chemistry Letters, 5, 742–747.
Chen, Y., Menendez-Roche, N., & Sher, E. (2006). Differential modulation by the GABAB receptor allosteric potentiator 2,6-di-tert-butyl-4-(3-hydroxy-2,2dimethylpropyl)-phenol (CGP7930) of synaptic transmission in the rat hippocampal CA1 area. Journal of Pharmacology and Experimental Therapeutics, 317, 1170–1177.
Chen, Y., Phillips, K., Minton, G., & Sher, E. (2005). GABAB receptor modulators potentiate baclofen-induced depression of dopamine neuron activity in the rat ventral tegmental area. British Journal of Pharmacology, 144, 926–932.
Christopoulos, A. (2014). Advances in G protein-coupled receptor allostery: From function to structure. Molecular Pharmacology, 86, 463–478.
Christopoulos, A., & Kenakin, T. (2002). G protein-coupled receptor allosterism and complexing. Pharmacological Reviews, 54, 323–374.
Conn, P. J., Christopoulos, A., & Lindsley, C. W. (2009). Allosteric modulators of GPCRs: A novel approach for the treatment of CNS disorders. Nature Reviews Drug Discovery, 8, 41–54.
Cryan, J. F., Kelly, P. H., Chaperon, F., Gentsch, C., Mombereau, C., Lingenhoehl, K., et al. (2004). Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N-dicyclopentyl-2methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): Anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. Journal of Pharmacology and Experimental Therapeutics, 310, 952–963.
Dupuis, D. S., Relkovic, D., Lhuillier, L., Mosbacher, J., & Kaupmann, K. (2006). Point mutations in the transmembrane region of GABAB2 facilitate activation by the positive modulator N,N-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6diamine (GS39783) in the absence of the GABAB1 subunit. Molecular Pharmacology, 70, 2027–2036.
Filip, M., & Frankowska, M. (2008). GABAB receptors in drug addiction. Pharmacological Reports, 60, 755–770.
Filip, M., Frankowska, M., Sadakierska-Chudy, A., Suder, A., Szumiec, L., Mierzejewski, P., et al. (2015). GABAB receptors as a therapeutic strategy in substance use disorders: Focus on positive allosteric modulators. Neuropharmacology, 88, 36–47.
Froestl, W. (2010). Novel GABAB receptor positive modulators: A patent survey. Expert Opinion on Therapeutic Patents, 20, 1007–1017.
Gjoni, T., Desrayaud, S., Imobersteg, S., & Urwyler, S. (2006). The positive allosteric modulator GS39783 enhances GABAB receptor-mediated inhibition of cyclic AMP formation in rat striatum in vivo. Journal of Neurochemistry, 96, 1416–1422.
Gjoni, T., & Urwyler, S. (2008). Receptor activation involving positive allosteric modulation, unlike full agonism, does not result in GABAB receptor desensitization. Neuropharmacology, 55, 1293–1299.
Gjoni, T., & Urwyler, S. (2009). Changes in the properties of allosteric and orthosteric GABAB receptor ligands after a continuous, desensitizing agonist pretreatment. European Journal of Pharmacology, 603, 37–41.
Guery, S., Floersheim, P., Kaupmann, K., & Froestl, W. (2007). Syntheses and optimization of new GS39783 analogues as positive allosteric modulators of GABAB receptors. Bioorganic and Medicinal Chemistry Letters, 17, 6206–6211.
Hall, D. A. (2000). Modeling the functional effects of allosteric modulators at pharmacological receptors: An extension of the two-state model of receptor activation. Molecular Pharmacology, 58, 1412–1423.
Hwa, L. S., Kalinichev, M., Haddouk, H., Poli, S., & Miczek, K. A. (2014). Reduction of excessive alcohol drinking by a novel GABAB receptor positive allosteric modulator ADX71441 in mice. Psychopharmacology, 231, 333–343.
Jacobson, L. H., & Cryan, J. F. (2008). Evaluation of the anxiolytic-like profile of the GABAB receptor positive modulator CGP7930 in rodents. Neuropharmacology, 54, 854–862.
Kalinichev, M., Donovan-Rodriguez, T., Girard, F., Riguet, E., Rouillier, M., Bournique, B., et al. (2014a). Evaluation of peripheral versus central effects of GABAB receptor activation using a novel, positive allosteric modulator of the GABAB receptor ADX71943, a pharmacological tool compound with a fully peripheral activity profile. British Journal of Pharmacology, 171, 4941–4954.
Kalinichev, M., Palea, S., Haddouk, H., Royer-Urios, I., Guilloteau, V., Lluel, P., et al. (2014b). ADX71441, a novel, potent and selective positive allosteric modulator of the GABAB receptor, shows efficacy in rodent models of overactive bladder. British Journal of Pharmacology, 171, 995–1006.
Kerr, D. I., Khalafy, J., Ong, J., Perkins, M. V., Prager, R. H., Puspawati, N. M., et al. (2006). Synthesis and biological activity of allosteric modulators of GABAB receptors, part 2. 3-(2,6-Bis-tert-butyl-4-hydroxyphenyl)propanols. Australian Journal of Chemistry, 59, 457–462.
Kerr, D. I., Khalafy, J., Ong, J., Prager, R. H., & Rimaz, M. (2007). Synthesis and biological activity of allosteric modulators of GABAB receptors part 3. 3-(2,6-Bis-iso-propyl-4-hydroxyphenyl)propanols. Journal of the Brazilian Chemical Society, 18, 721–727.
Kerr, D. I., & Ong, J. (2003). Potentiation of metabotropic GABAB receptors by L-aminoacids and dipeptides in rat neocortex. European Journal of Pharmacology, 468, 103–108.
Kerr, D. I., Ong, J., Puspawati, N. M., & Prager, R. H. (2002). Arylalkylamines are a novel class of positive allosteric modulators at GABAB receptors in rat neocortex. European Journal of Pharmacology, 51, 69–77.
Koek, W., Cheng, K., & Rice, K. C. (2013). Discriminative stimulus effects of the GABAB receptor-positive modulator rac-BHFF: Comparison with GABAB receptor agonists and drugs of abuse. Journal of Pharmacology and Experimental Therapeutics, 344, 553–560.
Koek, W., France, C. P., Cheng, K., & Rice, K. C. (2010). GABAB receptor-positive modulators: Enhancement of GABAB receptor agonist effects in vivo. Journal of Pharmacology and Experimental Therapeutics, 335, 163–171.
Koek, W., France, C. P., Cheng, K., & Rice, K. C. (2012). Effects of the GABAB receptor-positive modulators CGP7930 and rac-BHFF in baclofen- and γ-hydroxybutyrate-discriminating pigeons. Journal of Pharmacology and Experimental Therapeutics, 341, 369–376.
Lhuillier, L., Mombereau, C., Cryan, J. F., & Kaupmann, K. (2007). GABAB receptor positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology, 32, 388–398.
Malherbe, P., Masciadri, R., Norcross, R. D., Knoflach, F., Kratzeisen, C., Zenner, M. T., et al. (2008). Characterization of (R,S)-5,7-di-tert-butyl-3-hydroxy-3-trifluoromethyl-3H-benzofuran-2-one as a positive allosteric modulator of GABAB receptors. British Journal of Pharmacology, 154, 797–811.
Mannoury la Cour, C., Herbelles, C., Pasteau, V., de Nanteuil, G., & Millan, M. J. (2008). Influence of positive allosteric modulators on GABAB receptor coupling in rat brain: A scintillation proximity assay characterisation of G protein subtypes. Journal of Neurochemistry, 105, 308–323.
Masharina, A., Reymond, L., Maurel, D., Umezawa, K., & Johnsson, K. (2012). A fluorescent sensor for GABA and synthetic GABAB receptor ligands. Journal of the American Chemical Society, 134, 19026–19034.
Matsushita, S., Nakata, H., Kubo, Y., & Tateyama, M. (2010). Ligand-induced rearrangements of the GABAB receptor revealed by fluorescence resonance energy transfer. Journal of Biological Chemistry, 285, 10291–10299.
Mombereau, C., Lhuillier, L., Kaupmann, K., & Cryan, J. F. (2007). GABAB receptor positive modulation-induced blockade of the rewarding properties of nicotine is associated with a reduction in nucleus accumbens ΔFosB accumulation. Journal of Pharmacology and Experimental Therapeutics, 321, 172–177.
Monod, J., Wyman, J., & Changeux, J. P. (1965). On the nature of allosteric transition. Journal of Molecular Biology, 12, 88–118.
Mugnaini, C., Pedani, V., Casu, A., Lobina, C., Casti, A., Maccioni, P., et al. (2013). Synthesis and pharmacological characterization of 2-(acylamino)-thiophene derivatives as metabolically stable, orally effective, positive allosteric modulators of the GABAB receptor. Journal of Medicinal Chemistry, 56, 3620–3635.
Olianas, M. C., Ambu, R., Garau, L., & Onali, P. (2005). Allosteric modulation of GABAB receptor function in human frontal cortex. Neurochemistry International, 46, 149–158.
Onali, P., Mascia, F. M., & Olianas, M. C. (2003). Positive regulation of GABAB receptors dually coupled to cyclic AMP by the allosteric agent CGP7930. European Journal of Pharmacology, 471, 77–84.
Perdonà, E., Costantini, V. J. A., Tessari, M., Martinelli, P., Carignani, C., Valerio, E., et al. (2011). In vitro and in vivo characterization of the novel GABAB receptor positive allosteric modulator, 2-{1-[2-(4-chlorophenyl)-5-methylpyrazolo[1,5-a]pyrimidin-7-yl]-2-piperidinyl}ethanol (CMPPE). Neuropharmacology, 61, 957–966.
Pin, J. P., & Prézeau, L. (2007). Allosteric modulators of GABAB receptors: Mechanism of action and therapeutic perspective. Current Neuropharmacology, 5, 195–201.
Tu, H., Rondard, P., Xu, C., Bertaso, F., Cao, F., Zhang, X., et al. (2007). Dominant role of GABAB2 and Gβγ for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cellular Signalling, 19, 1996–2002.
Tu, H., Xu, C., Zhang, W., Liu, Q., Rondard, P., Pin, J. P., et al. (2010). GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. Journal of Neuroscience, 30, 749–759.
Urwyler, S. (2011). Allosteric modulation of family C G-protein-coupled receptors: From molecular insights to therapeutic perspectives. Pharmacological Reviews, 63, 59–126.
Urwyler, S., Gjoni, T., Kaupmann, K., Pozza, M. F., & Mosbacher, J. (2004). Selected amino acids, dipeptides and arylalkylamine derivatives do not act as allosteric modulators at GABAB receptors. European Journal of Pharmacology, 483, 147–153.
Urwyler, S., Gjoni, T., Koljatić, J., & Dupuis, D. S. (2005). Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: Effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology, 48, 343–353.
Urwyler, S., Mosbacher, J., Lingenhoehl, K., Heid, J., Hofstetter, K., Froestl, W., et al. (2001). Positive allosteric modulation of native and recombinant γ-aminobutyric acidB receptors by 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethylpropyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Molecular Pharmacology, 60, 963–971.
Urwyler, S., Pozza, M. F., Lingenhoehl, K., Mosbacher, J., Lampert, C., Froestl, W., et al. (2003). N,N′Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: Novel allosteric enhancers of γ-aminobutyric acidB receptor function. Journal of Pharmacology and Experimental Therapeutics, 307, 322–330.
Vacher, C. M., & Bettler, B. (2003). GABAB receptors as potential therapeutic targets. Current Drug Targets: CNS & Neurological Disorders, 2, 248–259.
Xi, Z. X., Ramamoorthy, S., Shen, H., Lake, R., Samuvel, D. J., & Kalivas, P. W. (2003). GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. Journal of Neuroscience, 23, 3498–3505.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Urwyler, S. (2016). Allosteric Modulators: The New Generation of GABAB Receptor Ligands. In: Colombo, G. (eds) GABAB Receptor. The Receptors, vol 29. Humana Press, Cham. https://doi.org/10.1007/978-3-319-46044-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-46044-4_18
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-46042-0
Online ISBN: 978-3-319-46044-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)