Abstract
This chapter has presented a number of approaches that may be effective in augmenting the limited repair capacity of the mammalian central nervous system (CNS). In comparison to the CNS, the regenerative capacity of the peripheral nervous system can be quite robust and a number of investigators have sought to identify differences in the hope of identifying molecular targets that may be exploited to enhance CNS regeneration. Another promising approach has been cell transplantation in order to replace lost cells within the damaged or diseased CNS. The choice of cell type will be critical in developing cell-based regeneration and repair strategies. For example, if stem cells (pluripotent or multipotent) are selected, the cells must be competent to generate specific cell types targeted for each disease. Furthermore, if pluripotent cells are selected, this increases the risk of teratoma formation. Cellular reprogramming or direct conversion of somatic cells into functional “induced” neuronal cell types may also be a feasible approach. However, there may be limitations on the number of cells that can be effectively generated using this methodology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- 3-D scaffolds
- Adipose stem cells
- Astrocyte
- Bioengineering
- Biomaterials
- Cellular reprogramming
- Embryonic stem cells
- Genetic engineering
- Glia
- Glial cell
- Induced pluripotent stem cells
- Mesenchymal stem cells
- Microglia
- Multipotent stem cells
- Neuron
- Neuroprotection
- Neuroregeneration
- Neurotrophic factors
- Oligodendrocyte
- Regeneration
- Schwann cell
- Tissue engineering
1 Introduction
The field of regenerative medicine in the context of the central nervous system (CNS) is focused on the ability to repair, replace and regenerate neural tissues, affected due to some disease condition, injury, or due to the aging process. The underlying goals are to develop therapies capable of restoring function of the CNS.
The nervous system is composed of two major parts, the central nervous system (CNS) and the peripheral nervous system (PNS). The major components of the vertebrate CNS include the brain, spinal cord, and retinas (and optic nerves). The CNS integrates sensory information from the environment as well as internally and is responsible for coordinating the organism’s activities and behaviors. The PNS consists of the ganglia and nerves outside of the CNS and a principal function is to connect the CNS with limbs and organs, serving as the communication relay. Disease or damage to the nervous system can lead to severe functional and behavioral deficits. The neuroregenerative capacity of the PNS and the CNS are quite different and can vary widely across species (Horner and Gage 2000). While regeneration of cut or damaged axons readily occurs in the mammalian PNS, as well as in the CNS of lower vertebrates such as frogs, newts and some fish, the adult mammalian CNS has limited capacity for regeneration.
A prominent difference in the regenerative capacity of the mammalian PNS versus the CNS is associated with the glial cells that form the myelin sheath on the axons, the Schwann cells and oligodendrocytes, respectively. The myelin sheath formed by these two glial cell types serves as an electrical insulator and facilitates the propagation of action potentials along the length of many axons throughout the nervous system. While there are a number of similarities between these two glial cell types, significant differences are clearly evident with respect to their role in neural regeneration (Martini et al. 2010). The Schwann cells of the PNS are derived from the neural crest and following peripheral nerve injury provide a conducive environment for axonal regeneration for peripheral neurons, as well as CNS neurons projecting through the PNS. Following peripheral nerve injury, proliferating Schwann cells produce and secrete a number of trophic factors with growth promoting activities including, nerve growth factor (NGF) , brain-derived neurotrophic factor (BDNF) , and glial cell line-derived neurotrophic factor (GDNF) (Bunge 1994; Johnson et al. 1988). These neurotrophic factors promote the survival of the injured neurons and are also involved in regulating gene expression programs involved in axon regeneration and formation of the growth cone (Reichardt 2006; Kwok et al. 2014). In addition, they produce extracellular matrix molecules (ECM) such as laminin and fibronectin that promote neurite outgrowth and also express integrin ECM receptors and numerous cell adhesion molecules including, neural cell adhesion molecules (NCAMs) and N-Cadherin (Thornton et al. 2005).
A principal reason for the absence of a prominent regenerative capacity in the adult CNS resides within the glial environment. The myelin producing cells of the CNS, the oligodendrocytes are derived from oligodendrocyte progenitor cells (OPCs) generated from neuroepithelial cells lining the ventricles and central canal of the brain and spinal cord, respectively. The limited regenerative capacity of the adult mammalian CNS is in large part due to the oligodendrocytes, along with astrocytes (another major glial cell component of the CNS), that produce a number of inhibitory molecules at the site of injury that suppress regeneration and the reestablishment of function. Lesions in the CNS result in the formation of a glial scar and the production of myelin-associated molecules that inhibit axon regrowth including chondroitin sulfate proteoglycans (CSPGs), Nogo-A, myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), ephrins (B3 and A3), semaphorins (4D, 5A, and 3F), and myelin glycolipid sulfatide (Giger et al. 2010; Silver et al. 2015). Identification of corresponding receptor systems for many of these molecules have demonstrated that the functional receptors mediate growth cone collapse and growth inhibition.
Advances in the fundamental understanding of neurodevelopment can provide insight in development of strategies for rescue and repair of the damaged and diseased nervous system. Experimental studies during early neural development as well as clinical observations suggest that when compared to the adult mammalian CNS, the immature CNS provides a more permissive environment such as that can support regeneration. For example, axonal regeneration across a lesion site can occur in fetal mouse and rat spinal cord. Results for successful spinal cord regeneration across a crush or transection in the neonatal opossum spinal cord (before 12 days postnatal) have led to reestablishment of synaptic connections and behavioral recovery (Saunders et al. 1998). During this permissive period of CNS regeneration there is a notable lack of myelin. Furthermore, the end of this “critical period” for CNS regeneration is marked by the appearance of neurite growth inhibiting molecules associated with the appearance of the oligodendrocytes (Varga et al. 1995). As such, a number of strategies to stimulate CNS regeneration have focused on neutralizing these inhibitory myelin oligodendrocyte-associate factors.
An additional limiting factor for successful axon regeneration appears to be a relatively poor intrinsic regenerative capacity within adult mammalian CNS neurons (Lu et al. 2014). Indeed, there is a difference in physiological neurite outgrowth during development compared to axon regeneration in mature neurons. One might surmise that axonal regeneration recapitulates axonogenesis during development since both processes require the formation of a growth cone for process extension. However, although intrinsic mechanisms play a significant role in axon outgrowth during development, extrinsic environmental cues are also essential in regulating this process and in determining target recognition (Kolodkin and Tessier-Lavigne 2011). Understanding how intrinsic and extrinsic cues regulate regeneration in the mature CNS has been a focus for regenerative medicine for many years (Fawcett et al. 2012; Harel and Strittmatter 2006). The earlier work of Aguayo and colleagues demonstrated that providing a permissive environment that of a peripheral nerve graft , supported the regeneration of some adult CNS axons (David and Aguayo 1981; Keirstead et al. 1989). Additional studies have demonstrated that, following lesions in the CNS, undamaged axons can sprout new neurites and form functional synaptic connections with considerable specificity within relatively localized regions. Furthermore, under the appropriate circumstances some severed CNS axons can regrow considerable distances (several centimeters) and form synaptic connections, however the majority of severed axons fail to regenerate. A number of recent studies have elucidated several molecular signaling pathways that are involved in regulating the ability for adult axonal CNS regeneration (Lu et al. 2014). The PTEN/mTOR pathway appears to be a general signaling pathway regulating axon regeneration (Park et al. 2009). The JAK/STAT and DLK/JNK pathways may also be involved in providing signals from the damaged or lesioned axon to the neuronal soma in order to initiate an injury response leading to regeneration. Identification of these as well as other molecular pathways may provide insight into mechanisms for promoting regeneration of adult CNS axons following injury or disease.
2 Development of Experimental Strategies to Regenerate and Repair the CNS
The lack of a robust regenerative capacity in the CNS, along with limited treatment options for patients with CNS injury or other neurodegenerative conditions has in part led to the development of the fields of regenerative medicine and CNS tissue engineering. A number of different strategies are actively being pursued to enhance CNS regeneration.
One approach takes into consideration the regenerative capacity of the PNS by grafting a segment of peripheral nerve into the CNS. Earlier studies by Aguayo and colleagues were among the first to demonstrate that CNS axons, those of the retinal ganglion cells (RGCs), were able to regenerate effectively through an autologous peripheral nerve graft (Keirstead et al. 1989). In these studies a segment of the optic nerve was removed and replaced by a peripheral nerve graft taken from the sciatic nerve. The other end of the sciatic nerve was then sutured into the normal visual target, the superior colliculus. Following regeneration of at least some RGC axons, electrophysiological recordings revealed light evoked activity in the superior colliculus. However, behavioral recovery was not demonstrated after regeneration of host projections. These early studies provided compelling evidence for the regenerative capacity of adult mammalian CNS axons, under the appropriate conditions. Thus, providing a regenerative conducive environment (in this case a grafted peripheral nerve containing Schwann cells) is an important consideration for development of CNS regenerative therapies.
2.1 CNS Regenerative Failure and the Glial Environment
Studies using animal models of CNS injury have provided excitement in the field of CNS regenerative medicine by modulating and boosting the intracellular signaling pathways involved in axon outgrowth as well as manipulating the extracellular barriers associated with the glial scar. A number of approaches have been employed to neutralize myelin-associated inhibitory factors that suppress CNS axon regeneration following injury. Approaches have included development of function-blocking antibodies, Nogo-receptor-blocking peptides and fusion proteins, as well as extensive in vivo studies generating knockout mice to regulate levels of expression of the myelin-associated inhibitory molecules. These strategies have been used in a number of experimental models including spinal cord injury, stroke, and autoimmune diseases. Studies conducted in adult non-human primates subjected to a unilateral spinal cord cervical lesion showed functional and behavioral recovery of some motor skills following intrathecal administration of a function blocking anti-Nogo A antibody (Freund et al. 2009). These strategies have been implemented in clinical trials for acute spinal cord injury (Abel et al. 2011) and ozanezumab, a humanized monoclonal antibody against Nogo-A has been used in amyotrophic lateral sclerosis (ALS) patients (Meininger et al. 2014).
2.2 Inflammation and CNS Regeneration
Most neurodegenerative diseases and CNS injury are accompanied by a local inflammatory response. Characteristics of the neuroinflammatory response include invasion of circulating immune cells (macrophages and lymphocytes) and induction of inflammatory mediators including a variety of cytokines and other reactive molecules. Since many of these molecular entities are produced locally around the site of injury or inflammation, they have become targets for possible therapeutic intervention. Neurons and glial cells (oligodendrocytes, astrocytes and microglia) can produce inflammatory mediators, as well as their respective cytokine receptors throughout the CNS. Their low levels of expression in the healthy brain suggest they may contribute to normal homeostatic functions within the CNS. Under pathological conditions, microglia and macrophages are recruited to the site of injury. Damaged cells and their associated debris serves to stimulate the resting microglia to transition into migratory macrophages that produce cytokines and trophic factors that can exert both damaging as well as protective effects on cells within the local environment. Differentiation of macrophages towards “classically” (M1) activated state can be neurotoxic and induce extensive retraction of damaged axons (Horn et al. 2008; Kigerl et al. 2009). As such, depletion of M1 macrophages appears to have neuroprotective effects in models of spinal cord injury. Interestingly, macrophages can also mediate sprouting of injured CNS axons likely through release of neurotrophic and growth factors and/or by indirectly activating glial cells within the scar region (Benowitz and Popovich 2011). Provision of neurotrophic factors by transplanted microglia and macrophages may help to explain their activity in promoting axon outgrowth in different models of spinal cord injury (Rapalino et al. 1998). Differentiation of macrophages towards the “alternatively” (M2) activated state has been associated with neuroprotection and enhancement of neurite outgrowth from damaged CNS neurons. Compared with the M1 phenotype, the M2 macrophages appear more proficient at CNS repair (Silver et al. 2015). Therapeutic applications using autologous macrophage transplantation appears promising in clinical trials (Jones et al. 2010). Methods that modify the local injury environment to favor the conversion of macrophages towards the M2 phenotype are likely to have benefits in CNS regeneration and offer additional interesting therapeutic targets.
2.3 Adult Neural Stem Cells and Endogenous Repair of the CNS
Many neurodegenerative diseases result in significant neuronal loss. Neuronal cell death is also frequently associated with severe neural insults, such as traumatic brain injury (TBI) , chronic traumatic encephalopathy (CTE) , stroke, and spinal cord injuries. Development of strategies to replace dead or dying neurons is likely to rely, in part, on important advances in understanding the fundamental mechanisms of endogenous neurogenesis. As such, a fundamental concept of regenerative medicine in the CNS is to develop strategies to improve neuronal survival and replace cells lost as a result of neurological disease or injury.
The discovery of neural stem cells within the adult mammalian brain has focused considerable interest into harnessing the endogenous capacity of these cells for CNS regeneration and repair. The original concept of adult neurogenesis in the mammalian CNS was quite controversial and was met with considerable skepticism. However, it is now well established that new neurons are generated throughout life in specific neurogenic regions of the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) (Altman and Das 1965; Gage et al. 1998; Lois and Alvarez-Buylla 1993; Reynolds and Weiss 1992; Aimone et al. 2014). Since the original reports a number of studies have demonstrated that adult neurogenesis in the DG plays a role in hippocampal-dependent learning and memory (Aimone et al. 2014), while adult neurogenesis in the SVZ results in production of new olfactory interneurons necessary for the normal function of the olfactory bulb and associated behaviors (Sakamoto et al. 2014). Considerable research efforts have elucidated mechanisms regulating proliferation and the differentiation of these adult stem cells into specific neural cell types. Furthermore, studies have also investigated adult neurogenesis in the context of neurodegenerative conditions such TBI and experimentally induced focal lesions (Perederiy et al. 2013; Sun 2016). Studies have demonstrated that different models of TBI significantly increased cell proliferation in the DG and SVZ of rodents (Sun 2016). However, due to a number of challenges including, difficulties in obtaining human brain tissue and cell birth dating studies, evidence of TBI-induced neurogenesis in the human brain is lacking. Nevertheless, mobilizing and augmenting endogenous adult neurogenesis is an attractive strategy towards repopulating the damaged or diseased CNS. Since the natural regenerative capacity of the adult CNS is quite limited, exogenous methods must be employed. With this type of strategy in mind, a number of studies have shown that different growth factors can enhance adult neurogenesis in vitro and in vivo in animal studies. Factors that have a demonstrated enhancement of neurogenesis and synaptic plasticity include, BDNF, basic fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), NGF, vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF) (Vivar et al. 2013). In addition to neurotrophic and growth factors, a number of drugs currently in clinical trials for treating neurological conditions, including TBI, enhance neurogenesis in animal studies. These include, erythropoietin, statins, and imipramine (Sun 2016; Xiong et al. 2013). However, it should be noted that in addition to an enhancement of neurogenesis, these exogenous agents in many cases also exert neuroprotection which may also contribute to enhanced recovery in behavioral and functional studies. Nevertheless, these findings should prove valuable in designing clinical strategies to improve the prospects of endogenous adult neural stem cell-based therapies.
3 Stem Cells for CNS Repair Strategies
The successful isolation of human embryonic stem cells and the development of induced pluripotent stem cell technologies is making it possible to recapitulate developmental processes, as well as modeling neurodegenerative conditions. Furthermore, the advent of cellular reprogramming technologies has made it possible to implement rational strategies to generate specific cell types with a goal of developing cell-based therapies for CNS disorders. Additionally, advances in biomaterials and in 3-D scaffold fabrication techniques is making it feasible to mimic the neural stem cell niche. In this section I provide an overview of approaches merging stem cells, scaffolds, drug delivery systems, gene therapy, cellular engineering and biomaterials to develop experimental regenerative strategies for the CNS (Mallapragada et al. 2015; Sandquist et al. 2016).
Current therapies targeted for CNS-related disorders often rely on the use of pharmacological-based methods, which in many cases are not without serious side effects. As such, the implementation of cell-based therapies has gained considerable interest. In recent decades the field of stem cell biology and cell transplantation has come to the forefront of biomedical research with aspirations of development of cures for various neurological disorders.
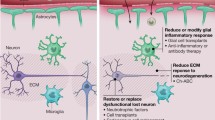
One approach is for transplanting cells that can replace the lost populations of neurons or glia (Fig. 47.1). Alternatively, transplanted cells may also provide trophic support via autocrine and paracrine signaling related to their cellular secretomes. An alternative approach involves harnessing the natural abilities for adult neurogenesis in order to generate new neurons. While another route takes advantage of bioengineering approaches , where cell-based approaches are combined with biomaterials, scaffolds and nanoneuromedicine concepts, in order to develop novel therapies that target neurological conditions.
Central nervous system regeneration and repair strategies. Injections and administration of therapeutic agents to enhance regeneration (neuroprotection, stimulating axon growth, neutralizing inhibitory factors). Stimulating endogenous neurogenesis. Cell-based therapies using stem cells (ESCs, iPSCs), neural stem cells, neurons and glial cell types. A variety of other somatic stem cells, as well as other cell types may be used for cellular therapies. Coupling of engineering approaches with cell-based therapies is another strategy for CNS regeneration
3.1 Pluripotent Stem Cells
Stem cells are defined by their potentially unlimited capacity for self-renew and ability to differentiate into multiple cell types. These properties are attractive to the field of regenerative medicine, which seeks to replace cells lost to neurodegenerative conditions, injury, or due to the natural aging process. Stem cells from a number of different sources are being used in neuroscience research in search of therapeutic treatment strategies targeting neurodegenerative diseases including Alzheimer’s, Parkinson’s, ALS and damage such as that from stroke as well as CTE. In general, stem cells are broadly categorized by their differentiation potential, from pluripotent cells, which are able to generate cells from all three primary germ layers (endoderm, mesoderm and ectoderm), to multipotent cells, which differentiate into a limited number of tissue specific cell types (Fig. 47.2).
Stem cell hierarchy and multipotency of neural and retinal stem cells. Pluripotent embryonic stem cells (ESC) are derived from the inner cell mass of a blastocyst (in humans the blastocyst stage is about 4–5 days post fertilization). Under appropriate culture conditions the ESCs can produce multipotent tissue specific stem cells from all three germ layers (ectoderm, mesoderm and endoderm). This illustration depicts the generation of differentiated cell types from multipotent neural and retinal stem cells. Multipotent neural stem cells generate the three major neural cell types present within the central nervous system (CNS): neurons, oligodendrocytes and astrocyte s. Multipotent retinal stem cells produce the seven major cell types found within the vertebrate retina which include: ganglion cells, horizontal cells, cone and rod photoreceptors, amacrine cells, bipolar cells and Müller glial cells. Arrows curling back onto the same cell represent the ability for self renewal, while reversed arrows represent possible reprogramming events (Source: Reprinted from Sandquist et al.(2016), Copyright 2016, with permission from Springer)
3.1.1 Embryonic Stem Cells
Thomson and colleagues (Thomson et al. 1998) were the first to report the isolation and characterization of pluripotent human embryonic stem cells (ESCs) isolated from the inner cell mass of the blastocyst. Considerable interest has focused on the potential of ESCs to differentiate into various neural cell fates and thus, they have become useful as a source of cells for CNS transplantation and tissue engineering. However, derivation of specific cell types in some cases requires complicated and expensive schemes to generate a relatively small proportion of the desired cells and hence considerable effort is being devoted to overcome these obstacles.
Differentiation conditions to drive ESCs into a number of different neural cell types have been elucidated and these cells tested for possible therapeutic benefits in a number of neurodegenerative conditions; including Alzheimer’s, Parkinson’s, and Huntington’s disease, spinal cord injury, TBI, stroke, as well as blinding ocular disease with varying success.
Generation of mesencephalic precursor cells and dopaminergic neurons derived from ESCs has been effective in recovering some motor function in models of Parkinson’s disease (Bjorklund et al. 2002; Kim et al. 2002; Kriks et al. 2011; Yang et al. 2008). Implantation of ESC-derived neural precursors has also improved behavioral deficits in models of Huntington’s disease (Song et al. 2007). In addition, transplantation of ESC-derived neural stem cells into the cortex of rodent models of Alzheimer’s disease has resulted in some improvements in cognitive function (Wang et al. 2006). Embryonic stem cell-derived neural stem cells and neurons have also provided functional improvements in models of stroke (Daadi et al. 2008; Yanagisawa et al. 2006) and epilepsy (Cunningham et al. 2014). Preclinical studies using human ESC-derived OPC transplants in a rat model of spinal cord injury demonstrated significant improvement in locomotor activity and histological examination of the injured spinal cords showed improved axon survival and extensive remyelination around the axons (Okamura et al. 2007). Clinical trials are underway utilizing human ESC-derived OPC transplants for acute spinal cord injury. Promising clinical trials for wet age-related macular degeneration and Stargardt’s macular dystrophy (Schwartz et al. 2012) are employing transplantation of retinal pigmented epithelial (RPE) cells derived from human ESCs. In this paradigm, the human ESCs are directed along an in vitro differentiation paradigm taking into consideration the normal developmental profile to generate RPE cells.
The stem cell debate: While ESCs offer hope for development of new therapies, their use in biomedical research is still hotly debated and raises considerable ethical and political concerns. Many believe that ESC research may lead to discoveries of new medical treatments for a number of debilitating neurological conditions that would potentially alleviate the suffering of thousands of patients. However, it still necessitates the destruction of a human embryo. This debate as to whether the potential benefits of human ES research outweigh ethical objections still remains a critical moral dilemma. The recent discovery of reprogramming somatic cells to pluripotent stem cells (see below) may serve as an alternative method of deriving stem cells with minimal ethical concerns. Nevertheless, many biomedical researchers strongly support the continued study of all stem cell types to determine similarities and differences and it still remains unclear which cell source will be the most useful for cell replacement therapies in the future.
3.1.2 Induced Pluripotent Stem Cells
The development of induced pluripotent stem cell (iPSC) technology was pioneered by S. Yamanaka and colleagues (Takahashi et al. 2007; Takahashi and Yamanaka 2006) who demonstrated that adult cells can be converted to pluripotent stem cells, thus removing many of the ethical barriers associated with ESC research. Induced pluripotent stem cells are created by genetically reprogramming adult cells into an “embryonic-like” pluripotent state by forced expression of genes and factors involved in maintaining pluripotency. Induced pluripotent stem cells hold significant promise in the field of regenerative medicine as they have become useful tools for drug development, for modeling diseases and have considerable appeal to generate patient specific-pluripotent stem cells (Fig. 47.3). Furthermore, since iPSCs have the ability of self-renewal, they can propagate indefinitely, as well as differentiate into a variety of cell types in the body (including, neurons, glia, heart, pancreatic, liver cells, etc.), thus representing a single source of cells that could potentially be used to replace cells lost to disease or damage. These unlimited supplies of patient-specific cells could be used to generate autologous transplants, thus reducing graft-host immune rejection. The iPSC technology has recently advanced to the stage of clinical trials for retinal degenerative conditions.
Stem cell research. Recent advances in stem cell biology have revolutionized research opportunities in drug discovery . The ability to isolate and generate stem cell and somatic cell lines associated with specific diseases is providing effective in vitro models for pre-clinical testing. Stem cell research is also providing powerful tools to help decipher the molecular mechanisms of gene regulation and development. Another important goal of stem cell research is to elucidate the pathways for generating specific cell types that can be used for cell transplantation to treat a variety of degenerative diseases and injuries (Source: Reprinted from Sandquist et al.(2016), Copyright 2016, with permission from Springer)
The field of stem cell biology has taken advantage of the normal developmental process of neural pattern formation of the CNS. Understanding and elucidating normal development has provided a rational strategy to differentiate pluripotent stem cells into specific neural cell types. Neuronal differentiation from iPSCs (as well as ESCs) is commonly achieved through a developmental profile that combines embryoid body cultures, retinoic acid stimulation and activation, and Sonic Hedgehog pathway agonists. Using specific factors that establish morphogenic gradients for patterning neural tissue, transcriptional regulation of modular patterns of CNS development has been key to differentiating pluripotent stem cells into neural precursors (Zhou et al. 2010), OPCs (Wang et al. 2013), and subsequently differentiating these progenitors and precursors into dopaminergic neurons (Kriks et al. 2011), cortical neurons (Shi et al. 2012), motor neurons (Chambers et al. 2009; Karumbayaram et al. 2009) as well as retinal cells (Hirami et al. 2009). In addition, identification of synthetic small molecules has also been useful for neural differentiation by activating signaling pathways that promote differentiation into specific cell lineages (Skalova et al. 2015).
A number of studies have successfully directed differentiation of iPSCs into specific neuronal cell types for therapeutic applications (Lindvall 2012; Lindvall et al. 2012). Transplant of iPSC-derived dopaminergic neurons into animal models used for the study of Parkinson’s disease have shown functional benefits (Kriks et al. 2011; Wernig et al. 2008). Re-myelination provided by OPCs derived from iPSCs has provided functional improvement in congenital hypomyelination disorder (Wang et al. 2013). Additionally, iPSC-derived neural progenitors transplanted into animal models of multiple sclerosis have ameliorated clinical features (Laterza et al. 2013). In animal models used to study ALS, iPSC-derived neural stem cell transplants has resulted in improved neuromuscular function and significantly increased life span (Nizzardo et al. 2014). Neural stem cells from iPSCs have also shown benefits for stroke (Yuan et al. 2013). A number of studies employing iPSC-derived neural precursor transplants in models of spinal cord injury have resulted in functional improvements as well (Romanyuk et al. 2014).
The development and differentiation of iPSCs and ESCs into specific neural cell types is often a very complicated, time consuming and expensive process. Furthermore, an inherent attribute of pluripotent stem cells is their ability for teratoma formation. To avoid this complication, a number of studies have used strategies to predifferentiate the stem cells and also selectively remove any remaining pluripotent cells prior to transplantation (Brederlau et al. 2006; Dihne et al. 2006; Doi et al. 2012; Erdo et al. 2003; Wernig et al. 2008).
Another approach is to avoid these developmental complications by direct induction of somatic cells to neural cells, skipping a pluripotent state (Fig. 47.4). Direct conversion has been achieved by forced expression of genes for differentiated neurons and by reprogramming somatic cells to a partially pluripotent state followed by culture with appropriate growth factors associated with neuronal differentiation (Matsui et al. 2014). A number of neuronal subtypes have been generated using these methods and have included, cholinergic neurons (Liu et al. 2013), dopaminergic neurons (Caiazzo et al. 2011; Pfiesterer et al. 2011), and motor neurons (Son et al. 2011).
Stem cell sources for CNS transplantation . Left side of illustration: Pluripotent hESCs isolated from the inner cell mass of a blastocyst are differentiated towards neural stem cells. Isolation of fetal CNS derived neural stem cells. IPSCs generated by reprogramming of somatic cells isolated from the patient. Direct conversion of somatic cells into neurons and/or glial cells. Right side of illustration: Different sources of MSCs for transplantation (BM-MSCs, UC-MSCs, and Adipose stem cells). Examples of other cell sources include OECs and OPCs. Arrows directed away from the patient indicate potential autologous cell sources. Arrows directed toward the patient indicate cell sources for transplantation to the CNS. Abbreviations: hESCs human embryonic stem cells, iPSCs induced pluripotent stem cells, OECs olfactory ensheathing cells, MSCs mesenchymal stem cells, BM-MSCs bone marrow-derived mesenchymal stem cells, hESCs human embryonic stem cells, iPSCs, induced pluripotent stem cells, OECs olfactory ensheathing cells, UC-MSC umbilical cord mesenchymal stem cells, OPCs oligodendrocyte progenitor cells
Human iPSCs have been praised as potential replacements for human ESCs. Although ESCs and iPSCs possess many similarities in differentiation potential, some studies suggest there are differences in gene expression and thus questions the equivalence of these two cell types (Ghosh et al. 2010; Narsinh et al. 2011). Comparison between different iPSC lines, as well as between different ESC lines has revealed differences in gene expression profiles, implicating differences in regulatory control (Chin et al. 2009; Wilson et al. 2009).
4 Multipotent Stem Cells for CNS Repair Strategies
Multipotent stem cells have the ability of self-renewal and can give rise to other cell types, but with more limited differentiation capacity when compared to pluripotent stem cells. Also referred to as somatic stem cells, multipotent stem cells tend to be tissue specific and are essentially committed to produce a particular set of cell types under normal conditions. Multipotent stem cells have been isolated and characterized from many organs and tissues, and are found in juvenile as well as in adult animals. In general, they appear to function to replenish cells in the various tissues throughout the life of the organism. Because of this cell replacement capacity, adult stem cells are of considerable interest in the field of regenerative medicine. Multipotent stem cells isolated from a number of different tissues have been used for CNS regeneration and repair strategies and include cells isolated from: brain, retina, bone marrow, adipose tissue, teeth, and skin (Fig. 47.4).
An important challenge has been to identify conditions or “factors” that trigger multipotent stem cells to generate specific cell types from that of their original tissue. Furthermore, it appears that at least some multipotent stem cells possess greater “plasticity” than originally thought, as they can be coaxed to go beyond original lineage boundaries for producing other cell types. However, the ability to isolate large quantities of somatic stem cells and to maintain them in a laboratory setting in order to generate therapeutic quantities of cells remains a significant issue. Although a number of challenges must be overcome before multipotent stem cells can be considered for routine use in the CNS, their potential benefits are numerous and they hold promise for treating neurodegenerative diseases and CNS injury (Fig. 47.4).
4.1 Neural Stem Cells
Neural stem cells are self-renewing multipotent stem cells that can differentiate into neurons, astrocytes and oligodendrocytes (Fig. 47.2). They have been isolated from the developing and adult CNS and have been produced in vitro through the differentiation of pluripotent stem cells (ESCs and iPSCs). Although significant progress has been made in understanding the biology of neural stem cells, much remains to be elucidated regarding identification of factors and conditions that regulate their fate.
Neural stem cells possess a number of characteristics that make them ideal vectors for CNS rescue and repair. Once isolated, they can be maintained long-term in vitro. In some cases neural stem cells isolated from the neonatal and adult mammalian brain have been maintained in culture as free-floating neurospheres in the presence of EGF and/or FGF-2 (Reynolds and Weiss 1992). Neural stem cells have also been isolated from the adult hippocampus and maintained in monolayer cultures grown on laminin or other ECM substrates in the presence of growth factors (Gage et al. 1998; Palmer et al. 1995). In addition, neural stem cells have also been isolated from the adult spinal cord (Weiss et al. 1996) and adult retinal stem cells from the pigmented ciliary margin (Tropepe et al. 2000).
Novel therapeutic applications are being developed that call for replacement of specific cell types, such as dopaminergic neurons for Parkinson’s disease, oligodendrocytes to treat spinal cord injury, and generating photoreceptors and RPE for blinding ocular disorders. One approach to manipulate neural stem cell fate has been through addition of neurotrophic and growth factors in vitro as well as in vivo. A number of different trophic factors, have been identified that can influence gene expression and ultimately neural stem cell differentiation potential. For example, as mentioned earlier, the mitogenic growth factors EGF and FGF-2 are important for maintenance and proliferation of neural stem cells. In addition, EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells (Doetsch 2003). Leukemia Inhibitory Factor (LIF) has been shown to support neural stem cell self-renewal in the adult brain (Bauer 2009; Bauer and Patterson 2006). A number of other studies have also demonstrated that multiple classes of trophic factors and growth factors, individually and especially in combinations, are also required for cell survival, avoidance of programmed cell death and for differentiation of neural stem cells and glial progenitors (Gallo and Deneen 2014; Urban and Guillemot 2014). Epigenetic regulation of transcription as a means of manipulating stem cell fate is a very active area of biomedical research (Fan et al. 2005). Micro-RNAs are an additional means to regulate transcription/translation and to influence neural stem cell fate (Hsieh et al. 2004; Kuwabara et al. 2004). The discovery of neural stem cells in the adult brain as well as retina has encouraged research into their role during neurogenesis in the normal mature nervous system and following traumatic injury. Gaining a more thorough understanding of adult neurogenesis can contribute greatly to our knowledge of neurodegenerative diseases and to development of novel treatment platforms.
4.2 Non-neural Adult Stem Cells
It is well established that endogenous CNS stem cells, as well as those derived through differentiation of ESCs or iPSCs, can generate neurons, astrocytes , and oligodendrocytes. However, since neural stem cells are difficult to harvest from the adult brain, other stem cell sources are being evaluated as alternatives for cell-based therapies for neurological disorders. A number of somatic stem cell sources have been investigated for potential therapeutic applications in the CNS, including mesenchymal stem cells (MSCs) , umbilical cord (blood) stem cells, adipose stem cells, and dental pulp stem cells, all sources that normally do not generate neural cell types (Fig. 47.4). In most cases, these non-neural adult stem cells appear to be capable of inducing endogenous neurogenesis. In addition to their neurogenic influence, these cell types may also have trophic effects that exert neuroprotective benefits when used to treat neurodegenerative conditions. However, evidence that they in fact differentiate into specific neural phenotypes is limited (Abraham and Verfaillie 2012; Stewart and Przyborski 2002).
This section will focus on MSCs as they have been isolated from a number of different tissue sources, including bone marrow, adipose tissue, liver, umbilical cord, and dental pulp tissues (Ding et al. 2011). They have the ability to differentiate into osteocytes, chondrocytes, myoblasts and adipocytes (Prockop 1997). Mesenchymal stem cells are emerging as particularly strong candidates for CNS cellular therapies (Fig. 47.4) due to a number of advantages. First, they are readily isolated from a number of tissue sources using well-established procedures. Second, they are easily maintained and expanded in culture and can be engineered to produce neurotrophic growth factors for long-term delivery of neuroprotective substances to the injured CNS (Harper et al. 2011; Levkovitch-Verbin et al. 2010; Sasaki et al. 2009). Third, MSCs can be isolated from the patient and therefore potentially serve as an autologous cell source. In addition, because MSCs express intermediate to low or negligible levels of MHC Class I or MHC Class II antigens, respectively, they are potentially suitable for use in allogeneic transplantation procedures and may thus avoid or minimize the need for aggressive immunosuppressive therapy (Aggarwal and Pittenger 2005; Le Blanc et al. 2003). Fourth, unlike pluripotent stem cells, MSCs have not been reported to form teratomas following transplantation. Another important advantage is that there are few if any moral objections associated with the isolation and use of this adult stem cell population.
Mesenchymal stem cells have been used to treat CNS diseases in a number of preclinical models used to study Parkinson’s disease, cerebral ischemia, TBI, retinal degeneration, and spinal cord injury. Benefits that have been reported include reduced lesion size, enhanced neuronal survival, axonal regeneration, and improved functional outcomes (Harper et al. 2011; Johnson et al. 2011; Kocsis 2009; Kurozumi et al. 2005; Abraham et al. 2009; Sasaki et al. 2009; Zhao et al. 2002; Lin et al. 2011). Although considerable optimism is associated with the use of MSCs for treatment of CNS neurological conditions, many challenges remain that must be overcome before their widespread clinical application.
In this section we have presented some examples of adult, non-neural stem cells that have been, or are currently, being evaluated for possible neural plasticity and neuroregenerative properties. While it is clear that true neural stem cells have the capacity to generate CNS neurons and glia, there is only limited evidence that non-neural stem cells generate CNS neural progeny. Nevertheless, many sources of non-neural adult stem cells, including bone marrow, adipose tissue, dental pulp and skin, appear to possess unique qualities that make them useful for consideration in cell-based therapies for CNS repair and regeneration. In some cases these cells appear to stimulate endogenous neurogenesis and in many cases can also provide neuroprotection when transplanted into the diseased or damaged brain, spinal cord, or retina.
4.3 Cellular Reprogramming Strategies
The biotechnological advancement of the ability to generate iPSCs has brought tremendous excitement to the field off regenerative medicine (Takahashi et al. 2007; Takahashi and Yamanaka 2006). However, the methodology is not without limitations and the reprogramming technology is sometimes inefficient, variable, time intensive and expensive. Furthermore, the pluripotent nature of the iPSCs can result in genetic instability, and when transplanted into animal models, the iPSCs may be tumorigenic. Therefore, it is important to develop alternative (or complementary) approaches for cellular reprogramming.
4.3.1 Directed Differentiation Towards Specific Cell Types
Cellular reprogramming is a strategy used to convert fully differentiated somatic cell types with defined functions, into another cell type. As a result, these reprogrammed cells display different characteristics and functions not normally associated with their original phenotype. In developing reprogramming strategies clues are often collected from normal embryonic or early developmental processes. For example, normal embryonic cellular development is guided by an assortment of specific extracellular soluble factors, gradients of chemical cues and cell-to-cell interactions that ultimately lead to induction and activation of specific combinations of lineage-determining transcription factor pathways. By focusing on key developmental pathways and cell differentiation factors it has been possible to directly convert somatic cells from one fate/lineage to another using a small molecules approach (Zhang et al. 2012). This approach has identified a number of small molecules that serve to maintain self-renewal and to induce and/or facilitate cellular reprogramming (Zhang et al. 2012). Recently, epigenetic reprogramming of skin-derived fibroblasts into neural cells was achieved using a small molecules approach (Thoma et al. 2014). In this approach fibroblasts were initially subjected to conditions that enhance reversion towards a more “primitive” state. Under these conditions the cells were more susceptible to cell-fate changes, a process often referred to as transdifferentiation (Tursun et al. 2011). Potent neural inducing factors were then used to drive the cells towards proliferating neural progenitor cells, and finally the cells were induced towards specific neural cell fates. In this study the selection of small molecules was guided by developmental signaling molecules known to be involved in generation of the particular cell types. This gene-free approach for cellular reprogramming is likely to have applicability beyond generation of peripheral glial cells and may become an important strategy to generate CNS cell types as well (Thoma et al. 2014).
The direct conversion of somatic cells into neural stem/progenitor cells, as well as neurons has been demonstrated using viral delivery of transcription factors. Vierbuchen and colleagues (Vierbuchen et al. 2010; Vierbuchen and Wernig 2012) demonstrated that fibroblasts can be reprogrammed into functioning neurons using combinatorial expression of neural-lineage-specific transcription factors. They identified a combination of three factors, Ascl1, Brn2 (also called Pou3f2) and Myt1l, which were sufficient to convert mouse embryonic and postnatal fibroblasts into functional neurons in vitro. Recently, the direct conversion of mouse fibroblasts into induced dopaminergic precursors was achieved by ectopic expression of a set of transcription factors that directly reprogram somatic cells into neuronal lineage-restricted progenitors (Tian et al. 2015). Following transplantation into a mouse Parkinson’s disease model (MPTP-lesioned mice), the induced precursors differentiated into dopaminergic neurons and were found to alleviate some motor deficits. Using direct reprogramming strategies to convert somatic cells into neurons or neuronal precursors has important applications for studies of neural development, patient-specific neurological disease modeling and CNS regenerative medicine.
4.3.2 Ex Vivo Gene Therapy Approaches for CNS Neuroprotection
Regeneration and repair strategies for the CNS should comprise a multi-factorial approach addressing a number of relevant issues, including optimization of survival and function of remaining CNS elements and modulation of trophic influences to promote neuroregeneration. Neurotrophic/growth factors are important candidates to augment neurorepair. Neurotrophic factors, including NGF, BDNF, and GDNF are essential during neuronal development and plasticity, and also can prevent apoptosis, enhance neuronal survival and facilitate axon regeneration. A number of approaches have been used for delivery of trophic factors to the damaged CNS. These have included direct bolus injections of the factors, incorporation into biodegradable polymer microparticles that slowly release the trophic factors, and gene therapy approaches. While each of these methodologies has advantages, a number of disadvantages necessitate the need to develop alternative strategies. Transplantation of genetically engineered cells can deliver therapeutic factors to the target site in the CNS and produce therapeutic effects at lower doses than are required with other means of delivery. In addition, a cell-based delivery system provides a potentially long-term delivery source.
Ex vivo gene transfer to a variety of different somatic cell types, including neural stem cells, MSCs, Schwann cells, and fibroblasts, prior to transplantation holds promise as cellular platforms for delivery of therapeutic factors. A number of viral vectors are available for ex vivo gene delivery, including, adeno-associated viral, adenoviral, retroviral and lentiviral vectors, each with their own advantages and disadvantages (Hendriks et al. 2004). A number of in vitro studies have demonstrated proof of concept for use of genetically engineered cells for delivery of trophic factors for neuroprotective strategies. Fibroblasts and MSCs engineered to secrete BDNF (Frim et al. 1994) enhanced the survival of retinal ganglion cells (RGCs) (Castillo et al. 1994) and also provided neuroprotection in a retinal cell line (RGC-5) when exposed to toxic cellular stressors such as glutamate or hydrogen peroxide (Harper et al. 2009), respectively. Mesenchymal stem cells engineered for delivery of BDNF have been transplanted into the striatum of a mouse model of Huntington’s disease and resulted in a decrease in behavioral symptoms typically associated with the disease (Dey et al. 2010). In addition, Harper and colleagues transplanted neurotrophic factor-engineered MSCs to deliver a constant, low level of BDNF and demonstrated that this approach had potential for functional and structural neuroprotection in an experimental rat model used to study glaucoma (Harper et al. 2011). Olfactory ensheathing cells (OECs) genetically modified to secrete GDNF were effective in promoting spinal cord repair. Another study demonstrated that human neural progenitor cells (hNPC) can be genetically modified to release GDNF using an inducible promoter system (Behrstock et al. 2006). When transplanted into the striatum of mice the engineered cells migrated within the striatum, released physiologically relevant levels of GDNF, and enhanced host dopamine neuron survival and fiber outgrowth. Furthermore, these cells were found to survive and release GDNF for up to 3 months following transplantation into the aged monkey brain. These studies demonstrated the genetically modified hNPCs may be considered a safe and powerful cellular platform for delivering therapeutic factors to specific targets within the CNS for diseases such as Parkinson’s.
Stem cells can be used to obtain a more thorough understanding of the complex events occurring during animal and human development. Gaining a more complete understanding of the genetic and molecular controls regulating developmental processes will likely yield information about how neurological diseases arise, and may provide novel strategies and targets for therapy (Fig. 47.3). Furthermore, stem cells (pluripotent and multipotent) and their derivatives are proving to be useful as model cellular systems for drug discovery and for toxicological bioassays (Fig. 47.3). This is particularly important for preclinical testing and verification of drug efficacy. Perhaps an especially significant application is for development and implementation of cell-based transplantation therapies (Fig. 47.3). Stem cells have the ability to generate specific cell types, providing a potentially renewable source of replacement cells that may be used to treat a variety of neurodegenerative conditions. Coupling stem cell biology with biocompatible materials provides a powerful toolbox for development and implementation of experimental strategies for CNS regenerative medicine.
5 Stem Cells and Bioengineering for CNS Repair Strategies
Materials fabricated from natural and synthetic polymers have been successfully used for fabrication of biomimetic 3-D scaffolding environments . A central goal of biomimetics in the context of CNS regenerative medicine is to imitate and model the extracellular matrix (ECM) and cellular microenvironment that support the growth and differentiation of native and transplanted cells. When developing materials and constructs for 3-D-scaffolds there are a number of important considerations regarding material selection, including biocompatibility, biodegradability, biological activity, mechanical properties, surface chemistry, cytotoxicity and trophic/growth factor binding capabilities (Dhandayuthapani et al. 2011; Sell et al. 2010; Zhu and Marchant 2011; Marti et al. 2012). Materials that are biodegradable, with chemistries permitting tunable degradation rates, and displaying mechanical and structural properties favoring cell adhesion, growth and proliferation will be important to neural regeneration applications. This section provides a brief survey of potential stem cell-based biomaterial applications for CNS repair and regeneration. Recent review articles provide greater detail about specific biomaterials and applications in tissue engineering and CNS regeneration (Tam et al. 2014; Mallapragada et al. 2015; Sandquist et al. 2016).
A number of bioactive molecules have been used to enhance plasticity, stimulate neurogenesis, provide neuroprotection and promote CNS regeneration following neurological insults. Neurotrophins are a family of bioactive molecules that play a role in growth and survival of developing as well as maintenance of mature neurons. The neurotrophin family of trophic factors includes NGF, BDNF and Neurotrophin-3 and 4 (NT-3, NT-4). Other neurotrophic factors include CNTF, the GDNF family of ligands (GDNF, Nurturin, artemin and persephin), transforming growth factor β family, interleukin-6-related cytokines, FGF family members, as well as a number of other inductive signaling molecules involved in neural patterning (Reichardt 2006). Development of nano/microparticle systems capable of delivering and releasing such therapeutic molecules has revolutionized the field of drug delivery. Natural and synthetic polymers have been used to fabricate particulate systems for drug release due to their advantages, including improved drug stability, optimal encapsulation capacity, lower toxicity, fewer administration time points, ability to incorporate hydrophobic as well as hydrophilic drugs, sustained drug release capabilities, cellular uptake potential and ability to cross the blood–brain barrier (BBB) (Gelperina et al. 2005; De Jong and Borm 2008; Singh and Lillard 2009). The encapsulation of biologically active agents within biodegradable particulate systems has provided an effective means of overcoming numerous challenges encountered when developing strategies for drug and gene delivery, stem cell differentiation, imaging of live cells and encapsulated cell delivery systems for therapeutic proteins (Mudshinge et al. 2011; Norizadeh-Abbariki et al. 2014; Brustle et al. 2015; Ilie et al. 2012; Lee et al. 2012; Wang et al. 2010).
A number of studies have investigated delivery of neurotrophic growth factors including NGF, BDNF, CNTF and GDNF-encapsulated in poly-lactic co-glycolic acid (PLGA) microparticles as a therapeutic approach in animal models used for study of neurodegenerative conditions, including Alzheimer’s, Parkinson’s disease and retinal degeneration (Garbayo et al. 2009; Andrieu-Soler et al. 2005; Jollivet et al. 2004a, b; Péan et al. 2000; Grozdanic et al. 2010; Kyhn et al. 2009). Delivery of these trophic factors resulted in functional improvements and tissue regeneration, likely resulting from neuroprotective qualities associated with these factors. As a combination strategy, microparticles have also been incorporated into polymer or hydrogel scaffolds for better localization, sustained release and better clearance when implanted in vivo (Burdick et al. 2006). Other studies have encapsulated cells along with growth factors within biofunctionalized polymer scaffolds as a promising strategy for neural regeneration (Wang et al. 2012).
A number of synthetic and natural polymers have been used to develop scaffolding platforms for organizing and delivering cells to the CNS. Nano and microfiber systems [such as poly(L-lactic acid) (PLLA), poly(D,L-lactide-co-glycolide) (PLGA), poly(ɛ-caprolactone) (PCL) etc.] have been extensively used to direct neural regeneration. Yang and colleagues produced aligned fibrous scaffolds using an electrospinning technique and found that the aligned nanofibers improved neurite outgrowth and enhanced differentiation of neural stem cells (Yang et al. 2005). Others have also used nano/microfiber systems to direct neurite growth and cellular alignment (Subramanian et al. 2011). In addition to providing physical alignment cues for cell growth, these types of scaffolds have also been functionalized and loaded with neurotrophic factors to promote neural regeneration (Chew et al. 2005).
Different types of scaffolds including films, nanotubes, gels, and porous materials have been used in developing neural regeneration strategies (Spivey et al. 2012; McCreedy and Sakiyama-Elbert 2012). Polymer-based porous films loaded with neurotrophic factors have been used to create gradients and surface modification used to create nano/micropatterns as conduits for neural regeneration (Tang et al. 2013; Kim et al. 2015). Scaffold systems bearing surface patterning have been used to provide topographic guidance cues to create regenerative platforms for a variety of cells, including astrocytes, neural stem cells, MSCs and Schwann cells (Roberts et al. 2014; Ho et al. 2015; McMurtrey 2014; Houchin-Ray et al. 2007; Rutkowski et al. 2004; Recknor et al. 2006; Sharma et al. 2016). Coupling stem cells and cellular reprogramming, along with 3-D scaffolds (i.e., nerve regeneration conduits) is a significant strategy to facilitate nerve regeneration. In many cases these bioengineering approaches have been effective in promoting peripheral nerve regeneration. However, with appropriate modifications these approaches will likely have significant benefits when applied to CNS regeneration as well.
6 Summary
The complexity and accessibility of the CNS has been a limitation in development of effective therapeutic interventions promoting neural regeneration. In addition, the environment of the damaged or diseased CNS is generally somewhat hostile and does not support extensive regeneration. The use of stem cells and/or neuroprotective factors coupled with biomaterials may provide a powerful approach to overcome many of these limitations.
This chapter has presented a number of approaches that may be effective in augmenting the limited repair capacity of the mammalian CNS. In comparison to the CNS, the regenerative capacity of the PNS can be quite robust and a number of investigators have sought to identify differences in the hope of identifying molecular targets that may be exploited to enhance CNS regeneration. Another promising approach has been cell transplantation in order to replace lost cells within the damaged or diseased CNS. The choice of cell type will be critical in developing cell-based regeneration and repair strategies. For example, if stem cells (pluripotent or multipotent) are selected, the cells must be competent to generate specific cell types targeted for each disease. Furthermore, if pluripotent cells are selected, this increases the risk of teratoma formation. Cellular reprogramming or direct conversion of somatic cells into functional “induced” neuronal cell types may also be a feasible approach. However, there may be limitations on the number of cells that can be effectively generated using this methodology.
Once cells have been carefully and rigorously characterized in vitro they must be tested in vivo in preclinical animal models that best model the disease/condition. In vivo the transplanted cells must survive long-term at multiple CNS regions, integrate into existing host circuitry (for neurons), receive appropriate and specific regulatory inputs and elaborating axons that grow and form specific and functional synaptic contacts. Moreover, the transplanted cells must induce a clear long-term functional benefit. While established models may continue to prove beneficial, the generation of new genetic models, toxin-induced lesion models, or surgically induced lesion models should also be considered.
For cell transplantation, not only is choice of cell type important, but also the dose (number of cells) and location of cell transplants will need to be carefully considered. In addition to possible cell replacement, cell transplantation may also mediate repair and recovery through neuroprotection via release of neurotrophic growth factors, as well as the modification of the inflammatory environment. The successful outcome of cell transplants will also depend critically on the timing of the transplant in relation to the optimal stage of the disease at which the patient is likely to benefit the most from the therapy. It is important that preclinical studies also demonstrate protection of remaining neural elements using strategies that might be employed in future patients. Although histological evidence for cellular protection will be important, this must be accompanied with significant functional and behavioral improvements. To be clinically viable as a treatment option for CNS neurological conditions, the therapy must induce substantial amelioration of the neurological deficits without inducing significant deleterious side effects.
The combination of cellular-based therapies along with bioengineering approaches is an extremely powerful approach for regenerative medicine. Stem cell bioengineering provides a means of manipulating the molecular, physical and cellular environment in order to enhance regeneration and repair of the CNS. Understanding and mimicking the complexity of the local environment, composed of a multitude of soluble and surface-associated signaling molecules, cell-to-cell contacts, cell-to-ECM, and local mechanical/physical cues will be important in regulating not only cell fate, but also cell behavior.
As the field of regenerative medicine in the CNS moves toward the future, it is apparent that systems-level approaches will help guide the field of stem cell bioengineering and the development of effective therapeutics. Understanding regulatory networks as well as the continued elucidation of neural connectomes will be essential for development of novel and effective neurotherapeutics for CNS regeneration and repair.
7 Review Questions
-
1.
A prominent difference in the regenerative capacity of the mammalian PNS versus the CNS is associated with the:
-
(a)
Neurons
-
(b)
Glial cell types
-
(c)
Stem cells
-
(a)
-
2.
Stem cells are characterized by which of the following features?
-
(a)
unlimited capacity for self-renew
-
(b)
ability to differentiate into multiple cell types
-
(c)
ability to differentiate into neurons only
-
(d)
a and b only
-
(e)
a and c only
-
(a)
-
3.
A CNS neural stem cell can differentiate into which of the following cell types?
-
(a)
neurons, muscle, and skin cells
-
(b)
neurons, astrocytes and oligodendrocytes
-
(c)
astrocytes, oligodendrocytes, and Schwann cells
-
(d)
Schwann cells, motor neurons and microglia
-
(a)
-
4.
What group of proteins plays a key role in controlling the program of developmental changes?
-
(a)
motor proteins
-
(b)
transporter proteins
-
(c)
transcription factors
-
(d)
synaptic proteins
-
(e)
restriction endonucleases
-
(a)
-
5.
A pluripotent stem cell is capable of:
-
(a)
generating skin cells, but not nerve cells
-
(b)
generating epidermal cells, but not mesoderm or endodermal cells
-
(c)
generating cell types from skin and brain, but not muscle
-
(d)
generating muscle and intestinal cells, but not neurons
-
(e)
generating neurons, skin, muscle and lung cells.
-
(a)
-
6.
Adult neural stem cells have been isolated from:
-
(a)
muscle tissue and pancreatic tissue
-
(b)
the hippocampus and the subventricular zone
-
(c)
the pons and the superchiasmatic nucleus of the thalamus
-
(d)
lewy bodies and neurofibrillary tangles
-
(a)
-
7.
Which cell type produces factors that actively inhibit CNS regeneration?
-
(a)
dorsal root ganglion neurons
-
(b)
Schwann cells
-
(c)
spinal reticular neurons
-
(d)
oligodendrocytes
-
(a)
-
8.
Myelin producing cells of the CNS are the:
-
(a)
dorsal root ganglion neurons
-
(b)
Schwann cells
-
(c)
spinal reticular neurons
-
(d)
oligodendrocytes
-
(a)
-
9.
Myelin producing cells of the PNS are the:
-
(a)
dorsal root ganglion neurons
-
(b)
Schwann cells
-
(c)
spinal reticular neurons
-
(d)
oligodendrocytes
-
(a)
-
10.
Which of the following are members of the neurotrophin family?
-
(a)
CNS, PNS and ESC
-
(b)
NCAM, N-cadherin, and laminin
-
(c)
NGF, BDNF and NT-3
-
(d)
IL-6, CNTF and FGF-2
-
(a)
-
11.
Which of the following can be involved in cell-cell or cell-ECM interactions?
-
(a)
PLLA, PLGA and PCL
-
(b)
NCAM, N-cadherin, and laminin
-
(c)
NGF, BDNF and NT-3
-
(d)
IL-6, CNTF and FGF-2
-
(a)
-
12.
Which of the following are myelin-associated molecules that inhibit axon regrowth?
-
(a)
integrin receptors, neural cell adhesion molecule, and N-Cadherin
-
(b)
PTEN/mTOR, JAK/STAT, and DLK/JNK
-
(c)
fibroblast growth factor-2 and epidermal growth factor
-
(d)
chondroitin sulfate proteoglycans, Nogo-A, myelin-associated glycoprotein, and oligodendrocyte-myelin glycoprotein
-
(a)
-
13.
Generating dopaminergic neurons would likely be a very important consideration when developing stem cell-based therapies to treat which disease?
-
(a)
glaucoma
-
(b)
spinal muscular atrophy
-
(c)
Parkinson’s disease
-
(d)
macular degeneration
-
(a)
-
14.
Bone marrow-derived mesenchymal stem cells normally differentiate into which of the following cell types?
-
(a)
islet cells, Schwann cells, and macrophages
-
(b)
osteocytes, chondrocytes, and adipocytes
-
(c)
neurons, astrocytes, and oligodendrocytes
-
(d)
pigment cells, skin cells, and dorsal root ganglion cells
-
(a)
-
15.
In designing an in vitro system to isolate and characterize neural stem cells which factors might you select to promote cell proliferation?
-
(a)
NGF and/or BDNF
-
(b)
CNTF and/or GDNF
-
(c)
CSPG and/or LIF
-
(d)
EGF and/or FGF-2
-
(a)
-
16.
Integrin receptors typically bind …
-
(a)
neurotrophins
-
(b)
ECM molecules
-
(c)
myelin glycoproteins
-
(d)
Nogo-A, but not Nogo-B
-
(a)
-
17.
Characteristics of the neuroinflammatory response include invasion of circulating …
-
(a)
immune cells (oligos and astrocytes)
-
(b)
MSCs
-
(c)
immune cells (macrophages and lymphocytes)
-
(d)
iPSCs
-
(a)
-
18.
Adult neurogenesis in the SVZ results in production of new …
-
(a)
granule cells
-
(b)
photoreceptor cells
-
(c)
retinal pigment epithelial cells
-
(d)
olfactory interneurons
-
(a)
-
19.
Formation of teratomas is a potential risk associated with transplantation of …
-
(a)
MSCs
-
(b)
ESCs
-
(c)
neurons
-
(d)
astrocytes
-
(a)
-
20.
In theory, pluripotent cells are able to generate cells from which primary germ layers?
-
(a)
endoderm, mesoderm and ectoderm
-
(b)
periderm, endoderm and meristem
-
(c)
mesencephalon, diencephalon and metencephalon
-
(a)
References
Abel R, Baron HC, Casha S, Harms J, Hurlbert J, Kucher K, Maier D, Thietje R, Weidner N, Curt A (2011) Therapeutic anti-Nogo-A antibodies in acute spinal cord injury: safety and pharmacokinetic data from an ongoing first-in-human trial. In: The International Spinal Cord Society (ed) International conference on spinal cord medicine and rehabilitation. The International Spinal Cord Society, Washington, DC
Abraham R, Verfaillie CM (2012) Neural differentiation and support of neuroregeneration of non-neural adult stem cells. Prog Brain Res 201:17–34. doi:10.1016/B978-0-444-59544-7.00002-0
Abraham S, Eroshenko N, Rao RR (2009) Role of bioinspired polymers in determination of pluripotent stem cell fate. Regen Med 4(4):561–578. doi:10.2217/Rme.09.23
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822. doi:10.1182/blood-2004-04-1559
Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94(4):991–1026. doi:10.1152/physrev.00004.2014
Altman J, Das GD (1965) Post-natal origin of microneurones in the rat brain. Nature 207(5000):953–956
Andrieu-Soler C, Aubert-Pouessel A, Doat M, Picaud S, Halhal M, Simonutti M, Venier-Julienne MC, Benoit JP, Behar-Cohen F (2005) Intravitreous injection of PLGA microspheres encapsulating GDNF promotes the survival of photoreceptors in the rd1/rd1 mouse. Mol Vis 11:1002–1011
Bauer S (2009) Cytokine control of adult neural stem cells. Ann N Y Acad Sci 1153:48–56
Bauer S, Patterson PH (2006) Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 26(46):12089–12099
Behrstock S, Ebert A, McHugh J, Vosberg S, Moore J, Schneider B, Capowski E, Hei D, Kordower J, Aebischer P, Svendsen CN (2006) Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther 13(5):379–388. doi:10.1038/sj.gt.3302679
Benowitz LI, Popovich PG (2011) Inflammation and axon regeneration. Curr Opin Neurol 24(6):577–583. doi:10.1097/WCO.0b013e32834c208d
Bjorklund LM, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O (2002) Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A 99(4):2344–2349. doi:10.1073/pnas.022438099
Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY (2006) Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells 24(6):1433–1440. doi:10.1634/stemcells.2005-0393
Brustle I, Simmet T, Nienhaus GU, Landfester K, Mailander V (2015) Hematopoietic and mesenchymal stem cells: polymeric nanoparticle uptake and lineage differentiation. Beilstein J Nanotech 6:383–395. doi:10.3762/bjnano.6.38
Bunge RP (1994) The role of the Schwann cell in trophic support and regeneration. J Neurol 242(1 Suppl 1):S19–S21
Burdick JA, Ward M, Liang E, Young MJ, Langer R (2006) Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials 27(3):452–459
Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476(7359):224–227. doi:10.1038/nature10284
Castillo B, Delcerro M, Breakefield XO, Frim DM, Barnstable CJ, Dean DO, Bohn MC (1994) Retinal ganglion-cell survival is promoted by genetically-modified astrocytes designed to secrete brain-derived neurotrophic factor (Bdnf). Brain Res 647(1):30–36. doi:10.1016/0006-8993(94)91395-1
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27(3):275–280. doi:10.1038/nbt.1529
Chew SY, Wen J, Yim EKF, Leong KW (2005) Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules 6(4):2017–2024. doi:10.1021/bm0501149
Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5(1):111–123. doi:10.1016/j.stem.2009.06.008
Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, Bolshakov VY, Chung S (2014) hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15(5):559–573. doi:10.1016/j.stem.2014.10.006
Daadi MM, Maag AL, Steinberg GK (2008) Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One 3(2), e1644. doi:10.1371/journal.pone.0001644
David S, Aguayo AJ (1981) Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214(4523):931–933
De Jong WH, Borm PJA (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 3(2):133–149
Dey ND, Bombard MC, Roland BP, Davidson S, Lu M, Rossignol J, Sandstrom MI, Skeel RL, Lescaudron L, Dunbar GL (2010) Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav Brain Res 214(2):193–200. doi:10.1016/j.bbr.2010.05.023
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011) Polymeric scaffolds in tissue engineering application: a review. Int J Polym Sci 2011:1–19. doi:10.1155/2011/290602
Dihne M, Bernreuther C, Hagel C, Wesche KO, Schachner M (2006) Embryonic stem cell-derived neuronally committed precursor cells with reduced teratoma formation after transplantation into the lesioned adult mouse brain. Stem Cells 24(6):1458–1466. doi:10.1634/stemcells.2005-0413
Ding DC, Shyu WC, Lin SZ (2011) Mesenchymal stem cells. Cell Transplant 20(1):5–14. doi:10.3727/096368910X
Doetsch F (2003) A niche for adult neural stem cells. Curr Opin Genet Dev 13(5):543–550
Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J (2012) Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells 30(5):935–945. doi:10.1002/stem.1060
Erdo F, Buhrle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Focking M, Kustermann E, Kolossov E, Hescheler J, Hossmann KA, Trapp T (2003) Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab 23(7):780–785. doi:10.1097/01.WCB.0000071886.63724.FB
Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, ten Hoeve J, Shuai K, Sun YE (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132(15):3345–3356. doi:10.1242/dev.01912
Fawcett JW, Schwab ME, Montani L, Brazda N, Muller HW (2012) Defeating inhibition of regeneration by scar and myelin components. Handb Clin Neurol 109:503–522. doi:10.1016/B978-0-444-52137-8.00031-0
Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, Rouiller EM (2009) Anti-Nogo-A antibody treatment promotes recovery of manual dexterity after unilateral cervical lesion in adult primates—re-examination and extension of behavioral data. Eur J Neurosci 29(5):983–996. doi:10.1111/j.1460-9568.2009.06642.x
Frim DM, Uhler TA, Galpern WR, Beal MF, Breakefield XO, Isacson O (1994) Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc Natl Acad Sci U S A 91(11):5104–5108
Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J (1998) Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol 36(2):249–266
Gallo V, Deneen B (2014) Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83(2):283–308. doi:10.1016/j.neuron.2014.06.010
Garbayo E, Montero-Menei CN, Ansorena E, Lanciego JL, Aymerich MS, Blanco-Prieto MJ (2009) Effective GDNF brain delivery using microspheres—a promising strategy for Parkinson’s disease. J Control Release 135(2):119–126. doi:10.1016/j.jconrel.2008.12.010
Gelperina S, Kisich K, Iseman MD, Heifets L (2005) The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med 172(12):1487–1490. doi:10.1164/rccm.200504-613PP
Ghosh Z, Wilson KD, Wu Y, Hu S, Quertermous T, Wu JC (2010) Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One 5(2), e8975. doi:10.1371/journal.pone.0008975
Giger RJ, Hollis ER 2nd, Tuszynski MH (2010) Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol 2(7):a001867. doi:10.1101/cshperspect.a001867
Grozdanic SD, Lazic T, Kuehn MH, Harper MM, Kardon RH, Kwon YH, Lavik EB, Sakaguchi DS (2010) Exogenous modulation of intrinsic optic nerve neuroprotective activity. Graefes Arch Clin Exp Ophthalmol 248(8):1105–1116. doi:10.1007/s00417-010-1336-7
Harel NY, Strittmatter SM (2006) Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci 7(8):603–616. doi:10.1038/nrn1957
Harper MM, Adamson L, Blits B, Bunge MB, Grozdanic SD, Sakaguchi DS (2009) Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp Eye Res 89:538–548
Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, Kardon RH, Sakaguchi DS (2011) Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci 52(7):4506–4515. doi:10.1167/iovs.11-7346
Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J (2004) Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res 146:451–476
Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M (2009) Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett 458(3):126–131
Ho D, Zou J, Chen X, Munshi A, Smith NM, Agarwal V, Hodgetts SI, Plant GW, Bakker AJ, Harvey AR, Luzinov I, Iyer KS (2015) Hierarchical patterning of multifunctional conducting polymer nanoparticles as a bionic platform for topographic contact guidance. ACS Nano 9(2):1767–1774. doi:10.1021/nn506607x
Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J (2008) Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28(38):9330–9341. doi:10.1523/JNEUROSCI.2488-08.2008
Horner PJ, Gage FH (2000) Regenerating the damaged central nervous system. Nature 407(6807):963–970. doi:10.1038/35039559
Houchin-Ray T, Swift LA, Jang JH, Shea LD (2007) Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials 28(16):2603–2611. doi:10.1016/j.biomaterials.2007.01.042
Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH (2004) Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A 101(47):16659–16664. doi:10.1073/pnas.0407643101
Ilie I, Ilie R, Mocan T, Bartos D, Mocan L (2012) Influence of nanomaterials on stem cell differentiation: designing an appropriate nanobiointerface. Int J Nanomedicine 7:3011–3025. doi:10.2147/Ijn.S29975
Johnson EM Jr, Taniuchi M, DiStefano PS (1988) Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci 11(7):299–304
Johnson TV, Bull ND, Martin KR (2011) Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res 93(2):196–203. doi:10.1016/j.exer.2010.05.016
Jollivet C, Aubert-Pouessel A, Clavreul A, Venier-Julienne M-C, Montero-Menei CN, Benoit J-P, Menei P (2004a) Long-term effect of intra-striatal glial cell line-derived neurotrophic factor-releasing microspheres in a partial rat model of Parkinson’s disease. Neuroscience letters 356(3):207–210. doi:10.1016/j.neulet.2003.11.051
Jollivet C, Aubert-Pouessel A, Clavreul A, Venier-Julienne M-C, Remy S, Montero-Menei CN, Benoit J-P, Menei P (2004b) Striatal implantation of GDNF releasing biodegradable microspheres promotes recovery of motor function in a partial model of Parkinson’s disease. Biomaterials 25(5):933–942. doi:10.1016/S0142-9612(03)00601-X
Jones LA, Lammertse DP, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Poonian D, Betz RR, Knoller N, Heary RF, Choudhri TF, Jenkins AL 3rd, Falci SP, Snyder DA (2010) A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal Cord 48(11):798–807. doi:10.1038/sc.2010.29
Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE (2009) Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells 27(4):806–811
Keirstead SA, Rasminsky M, Fukuda Y, Carter DA, Aguayo AJ, Vidal-Sanz M (1989) Electrophysiologic responses in hamster superior colliculus evoked by regenerating retinal axons. Science 246(4927):255–257
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29(43):13435–13444. doi:10.1523/JNEUROSCI.3257-09.2009
Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418:50–56
Kim S-E, Harker EC, De Leon AC, Advincula RC, Pokorski JK (2015) Coextruded, aligned, and gradient-modified poly(epsilon-caprolactone) fibers as platforms for neural growth. Biomacromolecules 16(3):860–867. doi:10.1021/bm501767x
Kocsis JD (2009) Neuroprotection and immunomodulation by cell transplantation are becoming central themes in potential therapeutic approaches for cell-based therapies. Neurosci Lett 456(3):99. doi:10.1016/j.neulet.2009.04.008
Kolodkin AL, Tessier-Lavigne M (2011) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3(6). doi:10.1101/cshperspect.a001727
Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480(7378):547–551. doi:10.1038/nature10648
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H (2005) Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther 11(1):96–104
Kuwabara T, Hsieh J, Nakashima K, Taira K, Gage FH (2004) A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 116(6):779–793
Kwok JC, Yang S, Fawcett JW (2014) Neural ECM in regeneration and rehabilitation. Prog Brain Res 214:179–192. doi:10.1016/B978-0-444-63486-3.00008-6
Kyhn MV, Klassen H, Johansson UE, Warfvinge K, Lavik E, Kiilgaard JF, Prause JU, Scherfig E, Young M, la Cour M (2009) Delayed administration of glial cell line-derived neurotrophic factor (GDNF) protects retinal ganglion cells in a pig model of acute retinal ischemia. Experimental eye research. Exp Eye Res 89(6):1012–1020. doi:10.1016/j.exer.2009.08.014
Laterza C, Merlini A, De Feo D, Ruffini F, Menon R, Onorati M, Fredrickx E, Muzio L, Lombardo A, Comi G, Quattrini A, Taveggia C, Farina C, Cattaneo E, Martino G (2013) iPSC-derived neural precursors exert a neuroprotective role in immune-mediated demyelination via the secretion of LIF. Nat Commun 4:2597. doi:10.1038/ncomms3597
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31(10):890–896
Lee JM, Kim BS, Lee H, Im GI (2012) In vivo tracking of mesechymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther 20(7):1434–1442. doi:10.1038/mt.2012.60
Levkovitch-Verbin H, Sadan O, Vander S, Rosner M, Barhum Y, Melamed E, Offen D, Melamed S (2010) Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci 51(12):6394–6400. doi:10.1167/iovs.09-4310
Lin H, Liu F, Zhang C, Zhang Z, Kong Z, Zhang X, Hoffman RM (2011) Characterization of nerve conduits seeded with neurons and Schwann cells derived from hair follicle neural crest stem cells. Tissue Eng Part A 17(13–14):1691–1698. doi:10.1089/ten.TEA.2010.0514
Lindvall O (2012) Dopaminergic neurons for Parkinson’s therapy. Nat Biotechnol 30(1):56–58. doi:10.1038/nbt.2077
Lindvall O, Barker RA, Brustle O, Isacson O, Svendsen CN (2012) Clinical translation of stem cells in neurodegenerative disorders. Cell Stem Cell 10(2):151–155. doi:10.1016/j.stem.2012.01.009
Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang CL (2013) Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun 4:2183. doi:10.1038/ncomms3183
Lois C, Alvarez-Buylla A (1993) Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A 90(5):2074–2077
Lu Y, Belin S, He Z (2014) Signaling regulations of neuronal regenerative ability. Curr Opin Neurobiol 27:135–142. doi:10.1016/j.conb.2014.03.007
Mallapragada SK, Brenza TM, McMillan JM, Narasimhan B, Sakaguchi DS, Sharma AD, Zbarska S, Gendelman HE (2015) Enabling nanomaterial, nanofabrication and cellular technologies for nanoneuromedicines. Nanomedicine (Lond) 11(3):715–729. doi:10.1016/j.nano.2014.12.013
Marti M, Sakaguchi DS, Mallapragada S (2012) Neural tissue engineering startegies. In: Stolz JF (ed) Regenerative medicine and cell therapy. Ios Press, Washington, DC. doi:10.3233/978-1-61499-076-5-275
Martini R, Groh J, Bartsch U (2010) Comparative biology of Schwann cells and oligodendrocytes. In: Armati P, Mathey E (eds) The biology of oligodendrocytes. Cambridge University Press, Cambridge, pp 19–48
Matsui T, Akamatsu W, Nakamura M, Okano H (2014) Regeneration of the damaged central nervous system through reprogramming technology: basic concepts and potential application for cell replacement therapy. Exp Neurol 260:12–18. doi:10.1016/j.expneurol.2012.09.016
McCreedy DA, Sakiyama-Elbert SE (2012) Combination therapies in the CNS: engineering the environment. Neurosci Lett 519(2):115–121. doi:10.1016/j.neulet.2012.02.025
McMurtrey RJ (2014) Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J Neural Eng 11(6):066009. doi:10.1088/1741-2560/11/6/066009
Meininger V, Pradat PF, Corse A, Al-Sarraj S, Rix Brooks B, Caress JB, Cudkowicz M, Kolb SJ, Lange D, Leigh PN, Meyer T, Milleri S, Morrison KE, Orrell RW, Peters G, Rothstein JD, Shefner J, Lavrov A, Williams N, Overend P, Price J, Bates S, Bullman J, Krull D, Berges A, Abila B, Meno-Tetang G, Wurthner J (2014) Safety, pharmacokinetic, and functional effects of the nogo-a monoclonal antibody in amyotrophic lateral sclerosis: a randomized, first-in-human clinical trial. PLoS One 9(5), e97803. doi:10.1371/journal.pone.0097803
Mudshinge SR, Deore AB, Patil S, Bhalgat CM (2011) Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J 19(3):129–141. doi:10.1016/j.jsps.2011.04.001
Narsinh KH, Plews J, Wu JC (2011) Comparison of human induced pluripotent and embryonic stem cells: fraternal or identical twins? Mol Ther 19(4):635–638. doi:10.1038/mt.2011.41
Nizzardo M, Simone C, Rizzo F, Ruggieri M, Salani S, Riboldi G, Faravelli I, Zanetta C, Bresolin N, Comi GP, Corti S (2014) Minimally invasive transplantation of iPSC-derived ALDHhiSSCloVLA4+ neural stem cells effectively improves the phenotype of an amyotrophic lateral sclerosis model. Hum Mol Genet 23(2):342–354. doi:10.1093/hmg/ddt425
Norizadeh-Abbariki T, Mashinchian O, Shokrgozar MA, Haghighipour N, Sen T, Mahmoudi M (2014) Superparamagnetic nanoparticles direct differentiation of embryonic stem cells into skeletal muscle cells. J Biomater Tissue Eng 4(7):579–585. doi:10.1166/jbt.2014.1205
Okamura RM, Lebkowski J, Au M, Priest CA, Denham J, Majumdar AS (2007) Immunological properties of human embryonic stem cell-derived oligodendrocyte progenitor cells. J Neuroimmunol 192(1–2):134–144. doi:10.1016/j.jneuroim.2007.09.030
Palmer TD, Ray J, Gage FH (1995) FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6(5):474–486
Park KK, Hu Y, Muhling J, Pollett MA, Dallimore EJ, Turnley AM, Cui Q, Harvey AR (2009) Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol Cell Neurosci 41(3):313–324. doi:10.1016/j.mcn.2009.04.002
Péan J-M, Menei P, Morel O, Montero-Menei CN, Benoit J-P (2000) Intraseptal implantation of NGF-releasing microspheres promote the survival of axotomized cholinergic neurons. Biomaterials 21(20):2097–2101. doi:10.1016/S0142-9612(00)00141-1
Perederiy JV, Luikart BW, Washburn EK, Schnell E, Westbrook GL (2013) Neural injury alters proliferation and integration of adult-generated neurons in the dentate gyrus. J Neurosci 33(11):4754–4767. doi:10.1523/JNEUROSCI.4785-12.2013
Pfiesterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M (2011) Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 25:10343–10348
Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276(5309):71–74
Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M (1998) Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med 4(7):814–821
Recknor JB, Sakaguchi DS, Mallapragada SK (2006) Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials 27(22):4098–4108
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361(1473):1545–1564. doi:10.1098/rstb.2006.1894
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255(5052):1707–1710
Roberts MJ, Leach MK, Bedewy M, Meshot ER, Copic D, Corey JM, Hart AJ (2014) Growth of primary motor neurons on horizontally aligned carbon nanotube thin films and striped patterns. J Neural Eng 11(3):036013. doi:10.1088/1741-2560/11/3/036013
Romanyuk N, Amemori T, Turnovcova K, Prochazka P, Onteniente B, Sykova E, Jendelova P (2014) Beneficial effect of human induced pluripotent stem cell-derived neural precursors in spinal cord injury repair. Cell Transplant 24(9):1781–1797. doi:10.3727/096368914X684042
Rutkowski GE, Miller CA, Jeftinija S, Mallapragada SK (2004) Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J Neural Eng 1(3):151–157
Sakamoto M, Ieki N, Miyoshi G, Mochimaru D, Miyachi H, Imura T, Yamaguchi M, Fishell G, Mori K, Kageyama R, Imayoshi I (2014) Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning. J Neurosci 34(17):5788–5799. doi:10.1523/JNEUROSCI.0674-14.2014
Sandquist EJ, Uz M, Sharma AD, Patel BB, Mallapragada SK, Sakaguchi DS (2016) Stem cells, bioengineering and 3-D scaffolds for nervous system repair and regeneration. In: Zhang LG, Kaplan D (eds) Neural engineering: from advanced biomaterials to 3D fabrication techniques. Springer, New York
Sasaki M, Radtke C, Tan AM, Zhao P, Hamada H, Houkin K, Honmou O, Kocsis JD (2009) BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci 29(47):14932–14941. doi:10.1523/JNEUROSCI.2769-09.2009
Saunders NR, Kitchener P, Knott GW, Nicholls JG, Potter A, Smith TJ (1998) Development of walking, swimming and neuronal connections after complete spinal cord transection in the neonatal opossum, Monodelphis domestica. J Neurosci 18(1):339–355
Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379(9817):713–720. doi:10.1016/s0140-6736(12)60028-2
Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL (2010) The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers 2(4):522–553. doi:10.3390/polym2040522
Sharma AD, Zbarska S, Petersen EM, Marti ME, Mallapragada SK, Sakaguchi DS (2016) Oriented growth and transdifferentiation of mesenchymal stem cells towards a Schwann cell fate on micropatterned substrates. J Biosci Bioeng 121(3):325–335. doi:10.1016/j.jbiosc.2015.07.006
Shi Y, Kirwan P, Livesey FJ (2012) Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc 7(10):1836–1846. doi:10.1038/nprot.2012.116
Silver J, Schwab ME, Popovich PG (2015) Central nervous system regenerative failure: role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb Perspect Biol 7(3):a020602. doi:10.1101/cshperspect.a020602
Singh R, Lillard JW Jr (2009) Nanoparticle-based targeted drug delivery. Exp Mol Pathol 86(3):215–223. doi:10.1016/j.yexmp.2008.12.004
Skalova S, Svadlakova T, Shaikh Qureshi WM, Dev K, Mokry J (2015) Induced pluripotent stem cells and their use in cardiac and neural regenerative medicine. Int J Mol Sci 16(2):4043–4067. doi:10.3390/ijms16024043
Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K (2011) Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9(3):205–218. doi:10.1016/j.stem.2011.07.014
Song J, Lee ST, Kang W, Park JE, Chu K, Lee SE, Hwang T, Chung H, Kim M (2007) Human embryonic stem cell-derived neural precursor transplants attenuate apomorphine-induced rotational behavior in rats with unilateral quinolinic acid lesions. Neurosci Lett 423(1):58–61. doi:10.1016/j.neulet.2007.05.066
Spivey EC, Khaing ZZ, Shear JB, Schmidt CE (2012) The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials 33(17):4264–4276. doi:10.1016/j.biomaterials.2012.02.043
Stewart R, Przyborski S (2002) Non-neural adult stem cells: tools for brain repair? Bioessays 24(8):708–713. doi:10.1002/bies.10124
Subramanian A, Krishnan UM, Sethuraman S (2011) Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed Mater 6(2):025004. doi:10.1088/1748-6041/6/2/025004
Sun D (2016) Endogenous neurogenic cell response in the mature mammalian brain following traumatic injury. Exp Neurol 275(Pt 3):405–410. doi:10.1016/j.expneurol.2015.04.017
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. doi:10.1016/j.cell.2006.07.024
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS (2014) Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology 39(1):169–188. doi:10.1038/npp.2013.237
Tang S, Zhu J, Xu Y, Xiang AP, Jiang MH, Quan D (2013) The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats. Biomaterials 34(29):7086–7096. doi:10.1016/j.biomaterials.2013.05.080
Thoma EC, Merkl C, Heckel T, Haab R, Knoflach F, Nowaczyk C, Flint N, Jagasia R, Jensen Zoffmann S, Truong HH, Petitjean P, Jessberger S, Graf M, Iacone R (2014) Chemical conversion of human fibroblasts into functional Schwann cells. Stem Cell Rep 3(4):539–547. doi:10.1016/j.stemcr.2014.07.014
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Thornton MR, Mantovani C, Birchall MA, Terenghi G (2005) Quantification of N-CAM and N-cadherin expression in axotomized and crushed rat sciatic nerve. J Anat 206(1):69–78. doi:10.1111/j.0021-8782.2005.00369.x
Tian C, Li Y, Huang Y, Wang Y, Chen D, Liu J, Deng X, Sun L, Anderson K, Qi X, Li Y, Mosley RL, Chen X, Huang J, Zheng JC (2015) Selective generation of dopaminergic precursors from mouse fibroblasts by direct lineage conversion. Sci Rep 5:12622. doi:10.1038/srep12622
Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D (2000) Retinal stem cells in the adult mammalian eye. Science 287(5460):2032–2036
Tursun B, Patel T, Kratsios P, Hobert O (2011) Direct conversion of C. elegans germ cells into specific neuron types. Science 331(6015):304–308. doi:10.1126/science.1199082
Urban N, Guillemot F (2014) Neurogenesis in the embryonic and adult brain: same regulators, different roles. Front Cell Neurosci 8:396. doi:10.3389/fncel.2014.00396
Varga ZM, Bandtlow CE, Erulkar SD, Schwab ME, Nicholls JG (1995) The critical period for repair of CNS of neonatal opossum (Monodelphis domestica) in culture: correlation with development of glial cells, myelin and growth-inhibitory molecules. Eur J Neurosci 7(10):2119–2129
Vierbuchen T, Wernig M (2012) Molecular roadblocks for cellular reprogramming. Mol Cell 47(6):827–838. doi:10.1016/j.molcel.2012.09.008
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463(7284):1035–1041. doi:10.1038/nature08797
Vivar C, Potter MC, van Praag H (2013) All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 15:189–210. doi:10.1007/7854_2012_220
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S (2006) Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest 53:61–69
Wang HC, Brown J, Alayon H, Stuck BE (2010) Transplantation of quantum dot-labelled bone marrow-derived stem cells into the vitreous of mice with laser-induced retinal injury: survival, integration and differentiation. Vision Res 50(7):665–673. doi:10.1016/j.visres.2009.09.003
Wang T-Y, Forsythe JS, Nisbet DR, Parish CL (2012) Promoting engraftment of transplanted neural stem cells/progenitors using biofunctionalised electrospun scaffolds. Biomaterials 33(36):9188–9197. doi:10.1016/j.biomaterials.2012.09.013
Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA (2013) Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12(2):252–264. doi:10.1016/j.stem.2012.12.002
Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16(23):7599–7609
Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A 105(15):5856–5861
Wilson KD, Venkatasubrahmanyam S, Jia F, Sun N, Butte AJ, Wu JC (2009) MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev 18(5):749–758. doi:10.1089/scd.2008.0247
Xiong Y, Mahmood A, Chopp M (2013) Animal models of traumatic brain injury. Nat Rev 14(2):128–142. doi:10.1038/nrn3407
Yanagisawa D, Qi M, Kim DH, Kitamura Y, Inden M, Tsuchiya D, Takata K, Taniguchi T, Yoshimoto K, Shimohama S, Akaike A, Sumi S, Inoue K (2006) Improvement of focal ischemia-induced rat dopaminergic dysfunction by striatal transplantation of mouse embryonic stem cells. Neurosci Lett 407(1):74–79. doi:10.1016/j.neulet.2006.08.007
Yang F, Murugan R, Wang S, Ramakrishna S (2005) Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26(15):2603–2610. doi:10.1016/j.biomaterials.2004.06.051
Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC (2008) Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells 26(1):55–63. doi:10.1634/stemcells.2007-0494
Yuan T, Liao W, Feng NH, Lou YL, Niu X, Zhang AJ, Wang Y, Deng ZF (2013) Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurologic function in a rat model of middle cerebral artery occlusion. Stem Cell Res Ther 4:73–83
Zhang Y, Li W, Laurent T, Ding S (2012) Small molecules, big roles—the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci 125(Pt 23):5609–5620. doi:10.1242/jcs.096032
Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC (2002) Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol 174(1):11–20
Zhou J, Su P, Li D, Tsang S, Duan E, Wang F (2010) High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor beta superfamily receptors. Stem Cells 28(10):1741–1750. doi:10.1002/stem.504
Zhu J, Marchant RE (2011) Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 8(5):607–626. doi:10.1586/erd.11.27
Acknowledgements
Illustrations prepared by Iowa State University undergraduates: Wooheock Kwon, L. Roy, H. Sinsel and R. Rossiter (L. Roy, H. Sinsel and R. Rossiter were undergraduates in the Biological and Pre-Medical Illustration Program (BPMI) at Iowa State University).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sakaguchi, D.S. (2017). Regenerative and Repair Strategies for the Central Nervous System. In: Ikezu, T., Gendelman, H. (eds) Neuroimmune Pharmacology. Springer, Cham. https://doi.org/10.1007/978-3-319-44022-4_47
Download citation
DOI: https://doi.org/10.1007/978-3-319-44022-4_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44020-0
Online ISBN: 978-3-319-44022-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)