Abstract
AMPK is important in numerous physiological systems but plays a vital role in embryonic and placental development. The placenta is a unique organ that is the essential lifeline between the mother and baby during pregnancy and gestation. During placental development, oxygen concentrations are very low until cells differentiate to establish the appropriate lineages that take on new functions required for placental and embryonic survival. Balancing the oxygen regulatory environment with the demands for energy and need to maintain metabolism during this process places AMPK at the center of maintaining placental cellular homeostasis as it integrates and responds to numerous complex stimuli. AMPK plays a critical role in sensing metabolic and energy changes. Once activated, it turns on pathways that produce energy and shuts down catabolic processes. AMPK coordinates cell growth, differentiation, and nutrient transport to maintain cell survival. Appropriate regulation of AMPK is essential for normal placental and embryonic development, and its dysregulation may lead to pregnancy-associated disorders such as intrauterine growth restriction, placental insufficiency, or preeclampsia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 AMPK
AMP-activated protein kinase (AMPK) is a heterotrimeric, serine/threonine protein kinase that is present in almost every cell in the body and is composed of a catalytic alpha 1 (AMPKα1, PrkAA1) or alpha 2 (AMPKα2, PrkAA2) subunit, in combination with beta/gamma regulatory subunits (Viollet et al. 2003, 2009; Steinberg and Kemp 2009). AMPK is evolutionarily conserved and homologues have been identified in all eukaryotes, including protozoa (Steinberg and Kemp 2009; Viollet et al. 2009). Such a highly conserved gene and its subsequent signaling mechanisms highlight the essential role it plays in physiological homeostasis and cell survival.
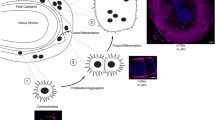
AMPK acts as a critical sensor of cellular energy, metabolism, and stress. It functions to coordinate cell growth, differentiation, and apoptosis as well as regulates autophagy and mitochondrial biogenesis to maintain cell survival (Mihaylova and Shaw 2011; Hardie 2011; Rafalski et al. 2012). It is activated by energy depletion and mitochondrial stress that can lead to mitophagy (Toyama et al. 2016; Kim et al. 2011). AMPK also directly activates the first step in the autophagy pathway and responds to low nutrient and metabolic status as a means to maintain cell survival (Egan et al. 2011). Under chronic stress, AMPK is capable of stimulating the production of new mitochondria to replace those lost or damaged (Toyama et al. 2016). AMPK is activated by hypoxia or when a decrease in energy levels occur, i.e., when there is an increased ratio of cellular AMP:ATP, and in response triggers signaling pathways to increase the cells’ production of its energy source, ATP (Evans et al 2006). Thus, AMPK activates energy-producing signaling pathways while inhibiting energy-consuming processes to maintain homeostasis (Fig. 4.1).
AMPK acts as a critical sensor of oxygen regulation, cellular energy, and metabolism. AMPK recognizes the decrease in energy as a reduction in ATP or elevation in AMP/ADP. In response to low-energy conditions, AMPK plays a major role in regulating mitochondrial respiration, autophagy, differentiation, nutrient transport, and cell proliferation to promote cell survival
2 AMPK in Development
AMPK has been shown to be important in numerous physiological systems; however, its vital role in placental and embryonic development has only recently begun to emerge (Carey et al. 2014). The AMPK catalytic subunits mediate energy utilization during development and gene knockout of AMPKα1 or AMPKα2 alone have shown distinct physiological phenotypes in mice, but are viable (Viollet et al. 2003, 2009). In contrast, AMPKα1/AMPKα2 double knockout mice are lethal at embryonic day 10.5 in utero and this signifies the importance of AMPK in development (Viollet et al. 2009). Recent reports indicate that AMPK is an important regulator and determinant of cell lineage during the differentiation of embryonic stem cells (Young et al. 2016; Louden et al. 2014; Vazquez-Martin et al. 2012); however, the role of AMPK in placental development has only recently come under study.
The placenta is a unique organ that is generated within the mother’s uterus only during pregnancy and is essential for embryonic development and survival. The placenta originates from specialized cells of the developing embryo (trophoblasts) and serves as the lifeline between the mother and developing baby to carry out functions that the embryo/fetus cannot. The placenta functions to facilitate the uptake and delivery of nutrients and aids in the elimination of wastes. The placenta is also important in mediating the exchange of critical gases, such as oxygen and carbon dioxide, via the mother’s blood supply and produces hormones necessary to sustain pregnancy. In addition, the placenta provides an immunoprivileged site that not only provides a barrier against viral infection but also prevents the rejection of the fetus as a foreign body.
Placental development is regulated by a critical balance between stem cell proliferation and the differentiation of trophoblasts into distinct placental cell lineages. The mouse placenta, which shares many attributes and functions with the human placenta, is made up of three lineages and numerous cellular subtypes that are important for trophoblast invasion, stem cell/progenitor growth, and nutrient transport (Watson and Cross 2005). Labyrinthine cells are responsible for nutrient transport and the exchange of gases and waste at the maternal fetal interface (Natale et al. 2006; Jansson and Powell 2013; Lager and Powell 2012). As proper placental transport is necessary for a healthy pregnancy and to allow for normal fetal development, alterations in AMPK signaling and trophoblast differentiation could impair fetal viability and result in pregnancy-associated disorders.
3 Pathophysiology of Pregnancy-Associated Disorders
Abnormal placental development is known to be associated with a number of pregnancy-associated disorders including intrauterine growth restriction (IUGR), placental insufficiency, and preeclampsia (Caniggia et al. 2000; Chaddha et al. 2004; Red-Horse et al. 2004). In humans, alterations in the AMPKα1 genotype are associated with birth weight, oxygen regulation, and metabolic homeostasis and may be implicated in the pathophysiology of IUGR and high altitude adaptation (Bigham et al. 2014). Furthermore, AMPK is present in placental tissue in humans and mice, was increased under low oxygen or hypoxic conditions, and facilitated maternal uterine artery blood flow (Skeffington et al. 2016). These results suggest that AMPK provides a protective role by sensing and rescuing cellular energy depletion to maintain maternal/fetal blood flow and prevent placental insufficiency. The role of AMPK in the pathogenesis of preeclampsia has been reported using a reduced uteroplacental perfusion pressure (RUPP) rat model (Banek et al. 2013). RUPP mice have an angiogenic imbalance and develop high blood pressure. Administration of an AMPK activator, AICAR, was able to prevent the development of hypertension and normalize angiogenesis (Banek et al. 2013). These results suggest that reduced levels of AMPK can lead to significant pathological conditions during pregnancy and indicate that decreasing oxidative stress and maintaining proper metabolic and energy levels are critical to normal placental development and fetal outcome, as well as maternal well-being.
4 AMPK in Placental Progenitor Cells and Differentiation
Trophoblast cells experience environmental changes as they divide and migrate, such as increasing oxygen concentrations and increased metabolic demands, which leads to corresponding changes in gene regulation. These changes in gene expression determine placental progenitor differentiation into the appropriate cell lineages at the proper time during development. Recent reports have suggested that placental stem cell proliferation and differentiation can be regulated by AMPK (Carey et al. 2014). To examine the effect of AMPK inhibition, we created a small interfering RNA (siRNA) and short hairpin RNA (shRNA) capable of targeting both catalytic isoforms of AMPKα1/2. Causing significant knockdown of both AMPKα isoforms in mouse and human cells resulted in a significant reduction in AMPK activity (Tangeman et al. 2012). The major metabolic and energy demands on the placenta would occur in labyrinthine nutrient-transporting cells (Jansson and Powell 2013). Therefore, we examined the effects of AMPK knockdown in the mouse labyrinthine trophoblast progenitor cell line, SM10 (Selesniemi et al. 2005a, b). Inhibition of AMPK results in the loss of appropriate placental differentiation with significant alterations in cell morphology, inhibition of cell growth, and reduced nutrient transport (Carey et al. 2014). Our results are supported by two studies in blastocysts and early trophoblast stem cells that show that AMPK is important in controlling trophoblast stem cell (TS) differentiation (Xie et al. 2013; Zhong et al. 2010). While these studies considered AMPK signaling in the context of a stress response and the manner in which trophoblast cells may respond to changes in their microenvironment, these findings could also be considered in the context of normal development and differentiation. As trophoblasts differentiate, their glycolytic need and mitochondrial respiratory capacity change in concert with their energy requirements and ability to produce ATP. AMPK could play a key role in controlling metabolic homeostasis in placental trophoblasts and its dysregulation could therefore negatively impact differentiation of these placental progenitor cells. Interestingly, mitochondrial DNA has been shown to be increased in human IUGR placentas and was inversely correlated with oxygen levels (Cetin and Alvino 2009). This could be due to a compensatory mechanism for a hypoxic environment or a metabolic response to reduced nutrient transport.
5 Future Directions
While the importance of AMPK in placental differentiation and pregnancy-associated disorders has only recently become evident, many questions remain. The role of mitochondria and their response to the energy demands of trophoblast differentiation and maintenance of homeostasis have not been reported. Furthermore, examining the interactions AMPK encounters and the signaling in response to a low oxygen environment are of great interest as they represent the in vivo scenario that occurs during pregnancy as the placenta develops. The comparative response of AMPK to chronic and high-dose stress compared to low stress levels that may allow for developmental adaptation may provide valuable information as to how placental plasticity may be involved and at what point plasticity may be lost or beyond adaptation (Mansouri et al. 2012).
Analysis of genes capable of interacting with or in response to AMPK levels to mediate differentiation may provide new information on the regulatory controls mediating placental development (Zhong et al. 2010; Selesniemi et al. 2016). Determining the signal transduction pathways that govern the hypoxic response as they relate to the control of cellular metabolism and nutrient transport during development should provide new findings about the integrated network necessary to keep cells alive and maintain metabolism in the very low oxygen state in which the placenta develops and eventually undergoes differentiation.
While gene knockout studies can be highly valuable in determining the function of genes, they suffer from the ability to distinguish whether the critical function is in the embryo or placenta, as both are targeted by this technique. Studies directed at examining placental-specific gene expression of AMPK using Cre-lox technology, Crisper/Cas, and/or lentiviral blastocyst transduction would provide novel insights into understanding AMPK function (Kaufman et al. 2014; Okada et al. 2007). Placental transgenesis studies would accelerate our understanding of the role of placental AMPK and pregnancy-associated disorders and provide new possibilities for therapeutic advances.
References
Banek CT, Bauer AJ, Needham KM, Dreyer HC, Gilbert JS (2013) AICAR administration ameliorates hypertension and angiogenic imbalance in a model of preeclampsia in the rat. Am J Physiol Heart Circ Physiol 304:H1159–H1165
Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, Moore LG (2014) Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics 46:687–697
Caniggia I, Winter J, Lye SJ, Post M (2000) Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 21(Suppl A):S25–S30
Carey EAK, Albers RE, Doliboa SR, Hughes M, Wyatt CN, Natale DRC, Brown TL (2014) AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem Cells Dev 23:2921–2930
Cetin I, Alvino G (2009) Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30(Suppl A):S77–S82
Chaddha V, Viero S, Huppertz B, Kingdom J (2004) Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med 9:357–369
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461
Evans AM, Hardie DG, Galione A, Peers C, Kumar P, Wyatt CN (2006) AMP-activated protein kinase couples mitochondrial inhibition by hypoxia to cell-specific Ca2+ signalling mechanisms in oxygen-sensing cells. Novartis Found Symp 272:234–252
Hardie DG (2011) AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25:1895–1908
Jansson T, Powell TL (2013) Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol 56:591–601
Kaufman MR, Albers RE, Keoni C, Kulkarni-Datar K, Natale DR, Brown TL (2014) Important aspects of placental-specific gene transfer. Theriogenology 82:1043–1048
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Lager S, Powell TL (2012) Regulation of nutrient transport across the placenta. J Pregnancy 2012:179827
Louden ED, Luzzo KM, Jimenez PT, Chi T, Chi M, Moley KH (2014) TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev 27:31–39
Mansouri L, Xie Y, Rappolee DA (2012) Adaptive and pathogenic responses to stress by stem cells during development. Cells 1:1197–1224
Mihaylova MM, Shaw RJ (2011) The AMPK signaling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13:1016–1023
Natale DR, Starovic M, Cross JC (2006) Phenotypic analysis of the mouse placenta. Methods Mol Med 121:275–293
Okada Y, Ueshin Y, Isotani A, Saito-Fujita T, Nakashima H, Kimura K, Mizoguchi A, Oh-Hora M, Mori Y, Ogata M, Oshima RG, Okabe M, Ikawa M (2007) Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol 25:233–237
Rafalski VA, Mancini E, Brunet A (2012) Energy metabolism and energy-sensing pathways in mammalian embryonic and adult stem cell fate. J Cell Sci 125:5597–5608
Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ (2004) Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 114:744–745
Selesniemi KL, Reedy MA, Gultice AD, Brown TL (2005a) Identification of committed placental stem cell lines for studies of differentiation. Stem Cells Dev 14:535–547
Selesniemi K, Reedy M, Gultice A, Guilbert LJ, Brown TL (2005b) Transforming growth factor-beta induces differentiation of the labyrinthine trophoblast stem cell line SM10. Stem Cells Dev 14:697–711
Selesniemi KL, Albers RE, Brown TL (2016) Id2 mediates differentiation of labyrinthine placental progenitor cell line, SM10. Stem Cells Dev. ePub
Skeffington KL, Higgins JS, Mahmoud AD, Evans AM, Sferruzzi-Perri AN, Fowden AL, Yung HW, Burton GJ, Giussani DA, Moore LG (2016) Hypoxia, AMPK activation and uterine artery vasoreactivity. J Physiol 594:1357–1369
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89:1025–1078
Tangeman L, Wyatt CN, Brown TL (2012) Knockdown of AMP-activated protein kinase alpha 1 and alpha 2 catalytic subunits. J RNAi Gene Silencing 8:470–478
Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Losón OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ (2016) Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351:275–281
Vazquez-Martin A, Vellon L, Quirós PM, Cufí S, Ruiz de Galarreta E, Oliveras-Ferraros C, Martin AG, Martin-Castillo B, López-Otín C, Menendez JA (2012) Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle 11:974–989
Viollet B, Andreelli F, Jørgensen SB, Perrin C, Flamez D, Mu J, Wojtaszewski JF, Schuit FC, Birnbaum M, Richter E, Burcelin R, Vaulont S (2003) Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans Part 1:216–219
Viollet B, Athea Y, Mounier R, Guigas B, Zarrinpashneh E, Horman S, Lantier L, Hebrard S, Devin-Leclerc J, Beauloye C, Foretz M, Andreelli F, Ventura-Clapier R, Bertrand L (2009) AMPK: lessons from transgenic and knockout animals. Front Biosci 14:19–44
Watson ED, Cross JC (2005) Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 20:180–193
Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA (2013) Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells. Stem Cells Dev 22:1564–1575
Young NP, Kamireddy A, Van Nostrand JL, Eichner LJ, Shokhirev MN, Dayn Y, Shaw RJ (2016) AMPK governs lineage specification through Tfeb-dependent regulation of lysosomes. Genes Dev 30:535–552
Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, Puscheck EE, Rappolee DA (2010) Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction 140:921–930
Acknowledgements
We would like to thank Dr. David Natale (University of California San Diego) for critical reading of the manuscript and helpful input. This work was supported in part by a grant from the National Institutes of Health NICHD-R01 HD059969 (TLB) and The Wright State University Endowment for Research on Pregnancy Associated Disorders (www.wright.edu/give/pregnancyassociateddisorders) (TLB).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kaufman, M.R., Brown, T.L. (2016). AMPK and Placental Progenitor Cells. In: Cordero, M., Viollet, B. (eds) AMP-activated Protein Kinase. Experientia Supplementum, vol 107. Springer, Cham. https://doi.org/10.1007/978-3-319-43589-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-43589-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43587-9
Online ISBN: 978-3-319-43589-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)