Abstract
Prostate cancer is a substantial public health problem worldwide. It is the most common neoplasm among men and third-ranked cause of cancer death in Europe, with almost 400,000 cases and over 92,000 deaths. Beginning in the early to mid-1990s, the PSA-induced detection of a substantial number of early-stage prostate cancers brought about rapid increases in population-level incidence rates, initially across the higher-income countries of Northern, Western and Southern Europe. Prostate cancer incidence rates are on the increase in populations across all European regions, ranging from 3 to 10 % per annum. At the same time, mortality rates are uniformly in decline in 24 countries in Europe, with the only exception, the Baltic countries, where mortality rates are high and stable or rising.
This chapter seeks to describe the current profile of prostate cancer in Europe, compare recent trends in incidence and mortality and assess the factors that contribute to this evolving landscape. The epidemiology of prostate cancer and the prospects of prevention are reviewed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Prostate Cancer
- Prostate Cancer Screening
- Prostate Cancer Incidence
- Prostate Cancer Mortality
- National Mortality
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Malignant neoplasms of the prostate, hereafter referred to as prostate cancer (ICD-10 C61), usually originate in the glandular tissue. While these cancers, mainly adenocarcinomas, are often indolent, there is a subset of men who are diagnosed with highly malignant prostate cancers associated with poor prognosis. The disease poses a substantial public health burden worldwide and in Europe: it is the second most frequently diagnosed cancer and the fifth leading cause of cancer death among men globally, with an estimated 1.1 million new cases diagnosed and 307,000 deaths from the disease in 2012 [16]. Among European men, it is the most common neoplasm and third-ranked cause of cancer death, with almost 400,000 cases and over 92,000 deaths
Incidence rates of prostate cancer are heavily influenced by the diagnosis of latent cancers by serum prostate-specific antigen (PSA) testing of asymptomatic individuals, and by the detection of latent cancer in tissue removed during prostatectomy operations, or at autopsy. When PSA became commercially available in the mid-1980s in the USA and the late 1980s in Europe, the intensive use of the test by general practitioners and urologists as an early detection and diagnostic tool led to inflated incidence rates first in the USA [21] and within a few years in Greater Europe, notably in several Nordic countries [26].
During the early to mid-1990s, the detection of a substantial number of early-stage prostate cancers brought about rapid increases in population-level incidence rates across the higher-income countries of Northern, Western and Southern Europe. The extent to which prostate cancer incidence is now (as estimated in 2012) the leading form of cancer occurrence in men in these regions can be visibly grasped in Fig. 1. An East–west divide can be seen in Europe that combines differences in diagnostic intensity and the prominent cause of cancer in the region: in Central and Eastern Europe, PSA testing has been historically lower but male tobacco consumption higher and declining later, relative to elsewhere in Europe. Indeed, lung cancer remains the leading cancer in the eastern areas of Europe, prostate cancer in the west. In contrast, only in Sweden is prostate cancer the leading cause of cancer death, a country in which the male population did not take up the smoking habit like neighbouring countries; lung cancer ranks as the most important form of cancer death in men in all of the 39 remaining countries in Europe.
Most common type of cancer in 40 European countries, based on the frequency of new cases as estimated in 2012 (Source: GLOBOCAN (http://globocan.iarc.fr))
Trends in incidence and mortality are not static, however, and prostate cancer incidence rates are to a great extent dependent on GP and urologist practices with respect to PSA testing. Conversely, prostate cancer mortality rates tend to be a better marker of extended disease and case fatality than of early diagnosis of asymptomatic cancers. Moderate declines in mortality rates have provided critical evidence of the favourable effect of increased curative treatment, particularly of early-diagnosed prostate cancer, within the last two decades.
1.1 Aims of Chapter
The aims of this chapter are threefold: (i) to describe the current profile of prostate cancer in Europe, (ii) to compare and contrast how recent trends in incidence and mortality are changing and (iii) to assess the factors that contribute to this evolving landscape, with a focus on the epidemiology of prostate cancer, the underlying risk factors and prospects of prevention. This chapter begins with a brief exploration of the global statistics of prostate cancer, followed by a more thorough comparison of the incidence and mortality burden and rates across European countries by region, and within these populations over time.
1.2 Data Sources and Methods
In presenting recent geographic variations, national incidence and mortality estimates of prostate cancer were available by country, sex and age and extracted from GLOBOCAN database for the year 2012 (http://globocan.iarc.fr). Temporal comparisons make use of recorded incidence of the disease in 1975–2014 from national and regional population-based cancer registries of high quality complied in successive volumes of Cancer Incidence in Five Continents (http://ci5.iarc.fr) and in corresponding recorded mortality available nationally from the WHO mortality databank (http://www-dep.iarc.fr/WHOdb/WHOdb.htm); we obtained more recent data from published or online sources for a number of European populations, including the Nordic countries (http://ancr.nu) and the Netherlands (http://www.cijfersoverkanker.nl/). To enable comparison adjusted for the effects of differing age composition and population ageing over time, all incidence and mortality rates presented in this chapter are age-standardised to the world standard population [14], and are denoted ASR. In deciphering incidence and mortality trends over time, joinpoint regression models [25] were fitted to identify sudden linear changes in annual rates and to estimate the direction and magnitude of the slope within these distinct periods of time.
2 Prostate Cancer Incidence and Mortality
2.1 Global Patterns and Trends
By 2012, prostate cancer became the fourth most common cancer in the world, ranking third in importance in men, and the most frequent male cancer in 91 countries worldwide. While the estimated total annual number of 1.1 million cases represents about 15 % of all male cancers, it is a less prominent cause of cancer mortality, with just over 300,000 deaths estimated annually, or almost 7 % of male cancer deaths. The relatively low case fatality signifies many men are alive years after their initial diagnosis of prostate cancer – an estimated 3.9 million at 5 years in 2012 – making this by far the most prevalent form of cancer in men. Prostate cancer is also a cancer of the elderly, with three-quarters of a million cases diagnosed (68 %) in men aged 65 years or more.
Worldwide, recorded incidence is very high where health-seeking behaviour and health-care systems are advanced, and estimates of national incidence rates vary at least 25-fold (Fig. 2a). As a result of a substantial diagnosis of latent cancers through PSA testing of asymptomatic individuals, rates are often elevated in the high-income countries within Oceania, Northern America, and Western and Northern Europe, and low in many Asian populations, particularly in Southern Asia. Incidence rates are intermediate to high in many regions and countries in economic transition, where PSA testing is not likely to be highly prevalent, including the Caribbean, South America and Sub-Saharan Africa. A combination of genetic (ethnic) risk differences and environmental, dietary and lifestyle factors are at play, although the specific risk components are largely unknown. Clearly, rates are higher in populations where men of African-Caribbean origin is a key risk factor; in the USA, rates among blacks remain 35 % higher than those in whites.
(a) Global map of prostate cancer incidence in 184 countries, based on age-standardised rates (World). Source: GLOBOCAN (http://globocan.iarc.fr). (b) Global map of prostate cancer mortality in 184 countries, based on age-standardised rates (World) (Source: GLOBOCAN (http://globocan.iarc.fr))
With almost 60,000 new cases estimated in 2012, cancer of the prostate is the most frequently diagnosed cancer in Sub-Saharan African men, with the risk of developing prostate cancer before age 75 of 3.4 % (i.e. affecting almost 1 in 30 men) equivalent to the lifetime risks of breast (3.5 %) and cervical cancer (3.8 %) among women in the region [31]. While the disease is the most frequent neoplasm among men, there is a tenfold variation in prostate cancer incidence rates in Sub-Saharan countries with a cumulative risk ranging from 0.8 % in Ethiopia to greater than 8 % in the Republic of South Africa in 2012. Even in the latter country, rates are modest compared with those in men of African descent in the USA and Caribbean [16] [], although the incidence is markedly increasing in a number of African populations, for example, in Kampala [35] and in the black population of Harare [10].
Mortality rates are less affected by early diagnosis of asymptomatic disease, and although a better marker of underlying risk of extended prostate cancer, they are also heavily dependent on the treatment options available in a given country (Fig. 2b). Mortality rates are high in North America, Northern and Western Europe, Australia/New Zealand, but also in parts of Latin America (Brazil) and the Caribbean, and in much of Sub-Saharan Africa. Indeed of the 42 countries where prostate cancer is the leading cause of cancer death among men, 19 are in Sub-Saharan Africa, 13 in Central and South America and 9 are in the Caribbean. Mortality rates are low in most Asian populations and in North Africa.
Using data from population-based cancer registries, five distinct time trend patterns have been demonstrated in prostate cancer incidence globally according to age [38]. Notably, incidence rates have been observed to peak among men aged over 75 years in most high-income populations, reflecting declining PSA screening at older ages and diagnosis at younger ages. In contrast, rates for men aged 45–54 years have not clearly stabilised or declined in most populations, and PSA testing is not likely to fully explain the rapidly rising rates of early-onset prostate cancer. In fact, decreasing overall prostate cancer mortality rates during the last decade has been reported mainly for North America, Oceania, Western Europe and parts of Northern Europe, where PSA testing has been more intensively implemented. This contrasts with the rising prostate cancer mortality rates observed in Central and Eastern Europe, and in parts of Asia and Africa [9]. The declining mortality rates may suggest that treatment and possibly earlier diagnosis have had an impact, whereas the rising rates could reflect an increasing diagnosis of prostate cancer; in both instances, the contribution of a changing prevalence and distribution of the underlying risk factors cannot be discounted.
2.2 Current Patterns in Europe
As with a global exposition of prostate cancer, the interpretation of observed variations in incidence in Europe – including any elucidation of potential risk determinants – is hampered by likely differences in the prevalence of PSA testing. Understanding the equivalent rates of mortality is also difficult given multiple contributory factors: the advent of curative treatment at about the same time as the increasing utilisation of the PSA test, and underlying this, the changing prevalence of one more (largely unknown) determinants of the disease. Each of these may have contributed to the levels of prostate cancer mortality in a given European population.
Geographic Variations in Incidence and Mortality
With over 400,000 new cases of prostate cancer, the disease is the leading cause of cancer in men, ahead of lung and colorectal cancer in second and third place, respectively. The disease is responsible for 22 % of the 1.8 million cancer cases among European men in 2012 and ranks fourth most frequent cancer in both sexes. Figure 3a, b, respectively, map the prostate cancer incidence and mortality rates in 2012 in 40 European countries, while Fig. 4 compares the ranking of mortality versus incidence. Rates of incidence vary tenfold in Europe, with the highest rates (125–160 per 100,000) in Lithuania, France, each of the Nordic countries as well as Switzerland and Ireland. Rates are intermediate (100–125) in Austria, Germany, Italy and England and Wales, and low (<50) in the Eastern European countries of Poland, Belarus, the Russian Federation and Bulgaria.
(a) European map of prostate cancer incidence in 40 countries, based on age-standardised rates (World) (Source: GLOBOCAN (http://globocan.iarc.fr)). (b) European map of prostate cancer mortality in 40 countries, based on age-standardised rates (World) (Source: GLOBOCAN (http://globocan.iarc.fr))
Bar chart of prostate cancer incidence versus mortality in 32 countries, based on age-standardised rates (Europe), sorted by mortality in descending order (Source: Cancer Incidence in Five Continents (http://ci5.iarc.fr), WHO mortality database (http://www-dep.iarc.fr/WHOdb/WHOdb.htm))
Approximately 92,000 deaths from prostate cancer were estimated to have occurred in 2012 in Europe, and thus the third-ranked cause of cancer death among men, after lung and colorectal cancer. In contrast to incidence, mortality rates vary only by a factor of 3, with some geographic differences observed. As with incidence, the highest mortality rates are seen in Lithuania, with their Baltic neighbours, Latvia and Estonia, ranked in second and third position. Rates are also relatively high (>25 per 100,000) in several Nordic countries (Denmark, Norway and Sweden), and in several Southern European countries (Slovenia, Croatia and Portugal) but moderate in several others (Spain, Italy and Greece); as with incidence, many of the lowest rates are seen in Central and Eastern European countries. The lowest rate is in Belarus, among the countries compared.
Clearly, there is little correlation in the present rates of prostate cancer incidence and mortality in Europe (Fig. 5). There is considerably more variability in incidence, and while the lowest and highest rates of both measures are, respectively, seen in Lithuania and Belarus, there are instances where incidence in a given country is relatively low and mortality relatively high (Latvia, Croatia), and vice versa (France). Figure 5 portrays the incidence rates in 3–5 year-periods (1983–87, 1993–97 and 2000–04) against mortality rates 5–10 years later (circa 1993, 2003, 2010). The correlation is reasonably strong between the two measures in the 1980s diagnostic era, with the mortality rates directly related to the prior level of incidence in a given population. That correlation appears to weaken over time, however, as one enters the era of PSA availability and its expanded use as a test in Europe, during the 1990s and early 2000s.
Scatterplot of prostate cancer incidence versus mortality rates for three recent periods (Source: Cancer Incidence in Five Continents (http://ci5.iarc.fr), WHO mortality database (http://www-dep.iarc.fr/WHOdb/WHOdb.htm))
2.3 Comparative Trends by European Region
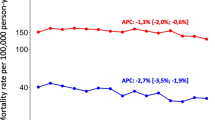
The incidence has increased rapidly over the past two decades, and rates are influenced by early diagnosis among asymptomatic individuals, and prior to the PSA testing era, detection of latent cancer in tissue removed during prostate surgery. Examining trends in prostate cancer incidence and mortality in 32 countries, the trends are presented for various years spanning 1975–2014 for 17 Northern and Western countries (Fig. 6a) and 15 Southern and European countries (Fig. 6b); the estimated annual percentage change is given.
(a) Line graphs of prostate cancer incidence versus mortality rates 1975–2014 in Northern and Western Europe. Circles: observed rates; Solid lines: trends based on Joinpoint regression (Source: Cancer Incidence in Five Continents (http://ci5.iarc.fr), WHO mortality database (http://www-dep.iarc.fr/WHOdb/WHOdb.htm)). (b) Line graphs of prostate cancer incidence versus mortality rates 1975–2014 in Southern and Eastern Europe. Circles: observed rates; Solid lines: trends based on Joinpoint regression (Source: Cancer Incidence in Five Continents (http://ci5.iarc.fr), WHO mortality database (http://www-dep.iarc.fr/WHOdb/WHOdb.htm))
Increasing trends in the incidence of prostate cancer have been observed in all countries from the mid-1970s through to the early 2000s, and for the period 1990–2004, the rate of increase ranged from 6 to 10 % on average per annum in France, Spain, Ireland, Italy, Slovenia, the Russian Federation and the Baltic countries (Estonia, Latvia, Lithuania) to 3–5 % in the remaining countries shown in Fig. 7. Notable are the uniform declines in prostate cancer incidence seen from the mid-2000s in almost all Northern and Western European countries, with the possible exception of countries in the Baltic region and the UK (Fig. 6a and Table 1). These recent decreases are not seen in any country within Southern or Eastern Europe, except in Italy (Fig. 6b, Table 1).
Average annual percentage changes in prostate cancer incidence (1990–2004) and mortality trends (1996-) based on the joinpoint regression, sorted in ascending order of mortality trends (Source: Cancer Incidence in Five Continents (http://ci5.iarc.fr), WHO mortality database (http://www-dep.iarc.fr/WHOdb/WHOdb.htm))
There appears to be little relation between the extent of the increases in prostate cancer incidence (as estimated from 1990) and the subsequent mortality declines (as estimated from 1996, Tables 1 and 2). National mortality declines in prostate cancer mortality were observed from 1996 in 19 of the 27 countries where both incidence and mortality measures are available (Fig. 7); these ranged from 2 to 3 % declines in Austria, France, Switzerland, Germany, the Netherlands, Finland, Spain and Norway to less than 1 % declines in Denmark and Slovakia. In contrast, increases in mortality of 0.5 % (Poland) through to 4 % (Lithuania) are seen in the remaining eight countries in the Baltic region, Southern or Eastern Europe. Below is a more detailed exposition of the trends by region.
Northern Europe
In the five Nordic countries, rates have been uniformly increasing during the 1990s (Fig. 6a and Table 1). Notable are the very recent declines in rates seen during period 2004–8, although incidence rates in Finland subsequently increased in 2008 following a short-term decline from 2005. Significant mortality declines of 2–3 % per annum are observed in all Nordic countries (Table 2), with the declines beginning in 1992 in Iceland, through to 1998 in Sweden (Table 3). The incidence has also been increasing in the UK and Ireland but unlike their Nordic counterparts, no recent incidence declines are seen. Significant annual declines in mortality of slightly over 1 % were observed in the constituent countries of the UK. – as early as 1992 in England and Wales (Table 3) – with mean declines of 2.1 % observed in Ireland (since 1997). The Baltic countries have a very different prostate cancer profile, with significantly increasing rates of both incidence and mortality observed in the last decades; these correspond to 3 % in Estonia and 4 % in Lithuania (Fig. 6b and Table 2). A suggestion of a stabilisation of mortality rates can be observed in Latvia from 2004.
Western Europe
Increasing incidence rates are observed in all five countries since the mid-1980s, ranging from around 3 % per annum for the period 1990–2004 (Switzerland, the Netherlands) to almost 7 % (France). As seen in the Nordic countries, incidence rates have uniformly declined in Western Europe, with the decrease beginning during the period 2002–4 (Fig. 6a and Table 1). Some of the largest decreases in prostate cancer mortality in European men are seen in the region (Fig. 7), notably the close to 4 % rate declines in Austria and France, beginning in 2000 and 2003, respectively (Fig. 6b and Table 2).
Southern Europe
Incidence trends in the four Southern European countries are increasing rapidly, particularly in Italy, Slovenia and Spain where the mean annual increases are 6–7 % per annum from 1990 to 2004 (Fig. 6b and Table 1). The mortality trends showed more variability across the six countries examined, although decreasing rates are seen in all countries except Slovenia. Among the most impressive declines are the 3.4 % and 3.9 % per annum reductions in Spain 1998–2009 and Malta 1994–2011, respectively (Fig. 6b and Table 2).
Eastern Europe
Some of the largest rate increases in prostate cancer incidence are observed in the five Eastern European countries, including the Czech Republic and Russia, where the rates rose 9–10 % per year during the 2000s, although the increases have attenuated subsequently in very recent years (Fig. 6b and Table 1). In terms of mortality, there is greater variability; the long-term increases in the Russian Federation and Bulgaria of 2–3 % per annum contrast with the rapid declines of the same order of magnitude in Hungary (since 1996) and more recently in the Czech Republic and Slovakia (Fig. 6b and Table 2).
Lastly, Table 3 indicates the 24 countries where prostate cancer mortality rates have declined, the year the downturn began and the extent of the decrease per annum. The first declines in prostate cancer mortality rates were seen in France and Switzerland in 1990, while the latest are observed in Greece in 2007, but in most countries rates began to fall during the mid- to late-1990s. There was considerable variability in the timing and order of magnitude of the year-on-year decreases, varying from approximately 0.6 % in Austria (from 1992) to 4–8 % for the quite recent declines observed in the Czech Republic and Greece.
2.4 Key Determinants of the Cancer Burden
Towards one-quarter (22 %) of all cancers diagnosed in men in Europe today are cancers of the prostate, compared with 11 % estimated in 1995[5]. While the true impact of prostate cancer screening can be only evaluated indirectly, incidence rates are clearly heavily influenced by the radical changes in diagnostic capabilities and practice over the last decades. The increasing rates in European men can be partly attributed to TURP in the 1970s and 1980s, while the more marked upsurge in incidence over the last 15–20 years (as identified in many countries via the joinpoint analyses) can be largely attributed to the greater use of PSA testing and subsequent biopsy. The initial rise in PSA testing in the late 1980s, closely followed by increasing prostate cancer incidence rates, has been clearly demonstrated in the Nordic countries [24]; given the consistent observation of increases in incidence in European countries – ranging from 3 to 10 % per annum from the early to mid-1900s – it is likely that such practices have prevailed in all regions of Europe. Of note are the recent accelerations in the historically lower rates observed in Southern and Eastern Europe, including Croatia, Slovenia, the Czech Republic and Slovakia.
There is little correlation between incidence and mortality rates in different European populations, nor in the evolution in trends in the last 15 years. Where observable, the slow and steady increases in prostate cancer mortality in the 1970s and 1980s have been replaced uniformly by declining mortality rates that are now apparent in 24 countries in Europe, with only the Baltic countries, where mortality rates are stable or rising, the clear exception. The underlying reasons for the fall in mortality across Europe are likely to imitate those conjectured in the USA, at least in part; Brawley [5] has noted possible explanations for the rate declines since 1991 in the USA that include an effect of screening and treatment, changes in the attribution of cause of death, or improved treatment resulting in a genuine postponement of death for some men with metastatic disease. Ecologic studies have revealed that declines in prostate cancer mortality rates are seen too early to be solely attributed to PSA testing; some have postulated they may be the result of improving treatment of both localised and high-risk disease [18]. The extent to which underlying changes in the prevalence and distribution of risk factors contribute to these trends remains largely unexplored and unknown.
Still, incidence varies tenfold and detectable falls in incidence have occurred recently in many higher-income countries, particularly in Northern and Western Europe. The changing but persistent influence of PSA on incidence relates to the perceptions and practices of health-care professionals regarding its utility as a prognostic test as well as public awareness of the controversy surrounding prostate cancer screening; in France, public perceptions of screening have been observed to vary by age and socioeconomic status [20]. The evidence of the benefits and harms of screening have become increasingly evident, as has the question of whether PSA can reduce prostate cancer mortality via the European Randomised Study of Screening for Prostate Cancer (ERSPC) trial. Schroder et al. [32] have reported a 22 and 21 % risk reduction from PSA screening at 11 or 13 years of follow-up, respectively, although in absolute terms, one death from prostate cancer was prevented for every 781 men invited for screening at 13 years follow-up. With three-fifths of screen-detected cancers in the ERSPC trial classified as low risk, experts have stressed that decision-making must be informed by tools that are able to stratify risk of low or high grade cancers on biopsy; the extent to which the trial findings will influence PSA testing practices and PSA screening awareness in Europe will reveal itself in the temporal patterns of prostate cancer incidence in due course.
2.5 Caveats in Interpretation
There are several points of caution we should note in the above analysis linked to the availability and quality of the data sources and the methods applied. GLOBOCAN was utilised to present cancer incidence and mortality maps for 2012 worldwide and for Europe. These are estimates that rely upon the best available data on cancer incidence and mortality in a given country. In Europe, the methods used to estimate national rates involve projections of recent trends, where annual data are available prior to 2012 [17]. Incidence data derive from population-based cancer registries which may cover national populations or subnational areas; estimates in France, Spain and Italy are all based on national estimates based on regional rather than national coverage, for example. An aggregation of regional registry datasets was required, assuming that the pertaining cancer registries collectively represented national patterns and trends. Where no recorded incidence data were available or when they were considered to be lacking sufficient quality, as was the case in nine countries in Europe including Greece, Hungary and Romania, modelled estimates were derived by applying available national mortality to regional data from other countries. In Europe, almost all countries have national mortality data through death registration systems compiled in the WHO mortality database, the exceptions being Bosnia Herzegovina and Montenegro.
To further compare patterns and trends in prostate cancer in Europe, we focussed on 32 countries, predominantly with high quality incidence and mortality, the former measure based mainly on registries included in the recent volumes of the Cancer Incidence in Five Continents (CI5) series. Those compiled in these volumes have been assessed as having high quality incidence data following a peer-reviewed assessment of their comparability, completeness and accuracy; yet for a number of countries – including Germany, Italy and Spain – regional registries are used to convey national profiles. These regional proxies may be more or less representative in certain countries than others. Given the difficulties in interpreting contemporary rates of prostate cancer incidence and mortality in Europe, comparative data on PSA use, treatment modalities and stage information may have provided insight, but were not available.
One methodological shortcoming is the use of joinpoint regression [23]. Quantification of the trends within linear segments can be unduly influenced by the last data points, while joinpoints and arbitrary slopes are sometimes identified by the regression where the underlying data are subject to substantial random variation. The technique is, however, particularly suitable for prostate cancer, permitting, in this chapter, quantification of the rather abrupt linear trends in incidence and mortality in Europe over time.
3 Epidemiology and the Prospects for Prevention
This chapter closes with a review of the epidemiology of prostate cancer and by extension, the potential to reduce the burden via removal or reduction of the causes of the disease through primary prevention strategies. The first thing to note is that, for a disease as prevalent and incident as prostate cancer, relatively little is known about its exact aetiology. Convincing evidence has been produced for only a few risk factors: ageing, genetic predisposition, ethnicity and body fatness. Numerous scientific papers have suggested a long list of other risk factors, of which those most intensely investigated will be reported in this section. Results of these studies are quite inconsistent which makes any definitive conclusions difficult. Apart from the general problems in observational studies on risk factors for disease, in prostate cancer the definition of the disease is arbitrary. Because of the large impact of PSA testing on prostate cancer incidence and the differences between indolent and potentially lethal prostate cancers, epidemiological studies should preferably study the latter subgroup of tumours in order to validly identify risk factors for the disease [21].
Ageing
The most well-known risk factor for prostate cancer is ageing, as evidenced by the age-specific incidence rates in the previous paragraphs. Prostate cancer is rarely diagnosed before the age of 45. In most western communities the peak in the incidence rates lies between 65 and 75 years of age. In a recent review of postmortem studies, the estimated mean cancer prevalence in men who died from other causes increased in a nonlinear fashion from 5 % (95 % CI: 3–8 %) at age <30 years to 59 % (95 % CI: 48–71 %) by age >79 years [3]. This underlines one of the greatest dilemmas in prostate cancer diagnostics nowadays: most men who have prostate cancer will die with the disease, not from the disease. The pivotal issue of research in prostate cancer is the identification of discriminative tests that can accurately predict invalidating and lethal prostate cancer.
Family History and Genetics
Besides age, a positive family history of prostate cancer is the most well-established risk factor for prostate cancer. First-degree relatives of affected men carry a two- to threefold increased risk of being diagnosed with the disease themselves. It is estimated that 5–10 % of prostate cancers have a true genetic cause. But the identification of the genes underlying these Mendelian forms of prostate cancer has appeared to be much more problematic than in, for example, breast cancer. Apparently, familial prostate cancer is a far more heterogeneous disease with contributions from many more genetic loci than familial breast cancer [28]. Mutations in the few high-penetrance genes are so rare that testing in families with hereditary prostate cancer, that is, families with three or more first-degree relatives (or 2 first-degree relatives of young age) with prostate cancer [8] is not useful, possibly with the exception of two genes: BRCA2 and HOXB13. Male carriers of a BRCA2 mutation have a two- to sixfold increased risk of prostate cancer, occurring earlier in life and with a more aggressive phenotype. The G84E (rs138213197) mutation in HOXB13 is something like a middle-penetrance mutation with a quite high population frequency of about 0.1–1.3 % and a fairly high risk ratio of 3.5–7 for prostate cancer [21, 25]. More and more clinical genetics centres around the world are starting to test for these genes in men at increased prostate cancer risk.
In addition to the handful of high-penetrance genes, since 2007, genome-wide association studies have identified approximately 100 low-penetrance genetic polymorphisms (single nucleotide polymorphisms – SNPs) that are associated with an increased risk of prostate cancer [28]. Some of these SNPs are in or near genes, for example, the HNF1B gene, the KLK3 gene (PSA) and the MSMB gene, but many if not most are in intergenic regions with unknown functions. The 8q24 region is a good example of the latter type, containing multiple SNPs that are significantly associated with prostate cancer and other cancer types. Because of the design of the GWAS studies, the prevalence of these SNPs in the population is high. The direct consequence, however, is that their effect is weak: typically, odds ratios of 1.1–1.3 are found. Using combinations of SNPs, polygenic risk scores are being developed to aid in predicting the individual risk of prostate cancer. With such scores, it is possible to discriminate men with a very high or a very low risk Table 4 [1]. The problem, however, is that the proportion of men with a clinically relevant increased risk is still quite small while all men have to be genotyped to identify this small group. The challenge is how to counsel the men who are not in the highest risk category. Nevertheless, at some point in the near future, such polygenic risk scores will probably be used to individualise population screening programmes for prostate cancer.
Recently, it has been shown that the prevalence of low-penetrance SNPs is about the same, or a little bit higher, in patients from hereditary prostate cancer families as in patients from the general population [13]. This may be interpreted as evidence that the clustering of such SNPs rather than high-penetrance genes may cause a clustering of patients in families. The alternative explanation is, however, that so-called hereditary prostate cancer families are not strongly genetically determined but merely the result of increased awareness and PSA testing of men in such families. The finding that prostate cancer patients in these families have a better prognosis than patients from the general population supports this alternative explanation [12]. This emphasises the importance of considering the aggressiveness and method of diagnosis of prostate cancers in families before deciding that unaffected men in these families should be tested in order to avoid overdiagnosis.
Ethnicity
As shown in the previous section on incidence, enormous differences in prostate cancer incidence exist between ethnic populations. The lowest incidence is found in men of Asian descent, whereas men who live in North America and Northern Europe have a very high prostate cancer risk. Particularly men of African-American heritage have a very high risk of prostate cancer. Ethnic differences are most probably caused by a combination of genetic factors, exposure to environmental risk factors and factors related to health-seeking behaviour. This is illustrated most clearly by the results of migration studies, which looked at prostate cancer incidence trends in Asian men (low incidence) who migrated to the USA (high incidence); prostate cancer incidence in these men increased markedly and significantly, but to a level that was intermediate between the incidence in the Japanese and the original American population [11]. A similar phenomenon was found for Japanese men who emigrated to Brazil [20].
Diet
It has long been thought that diet is an important factor in the development and progression of prostate cancer. And it probably is, considering the observation that second and following generation migrants adopt the risks of their new countries, combined with the fact that there are no other lifestyle factors that can easily explain this observation. The paradox here is that the strongest evidence for the role of diet comes from the weakest study designs, such as migrant studies. Designs that are supposedly stronger such as prospective cohort studies and randomised trials have yielded inconsistent results. A clear example of this is the SELECT trial (Selenium and Vitamin E Cancer Prevention Trial) [25]. This large prospective trial, in which 31,000 men were included, studied the effect of vitamin E, selenium, and the combination of both vs. placebo. No effect on prostate cancer incidence was found for administering selenium, either alone or in combination. This refuted the result found in the Nutrition Prevention of Cancer (NPS) trial [15], which observed a 50 % reduction in prostate cancer incidence in men randomised to selenium supplements. The Continuous Update Program of the World Cancer Research Fund brings expert nutritional epidemiologists together from around the globe and continuously reviews the literature on diet and cancer in a meticulous way. It concluded in 2014 that there is no diet or nutritional factor that is convincingly or probably associated with prostate cancer [36] (http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/prostate-cancer). On the contrary, the CUP project concludes that there is strong evidence that beta-carotene, either through food or supplements, is unlikely to have a substantial risk on the risk of prostate cancer. So, the numerous studies on dietary fats, red and processed meat, vitamin E, selenium, lycopene, cruciferous vegetables, green tea, tomato products and many other nutritional factors have not resulted in any clarity about the role of diet in prostate cancer. The recent report [36] specifically concludes that:
-
The evidence that a higher consumption of dairy products increases the risk of prostate cancer is limited.
-
The evidence that diets high in calcium increase the risk of prostate cancer is limited.
-
The evidence that low plasma alpha-tocopherol concentration (vitamin E) increases the risk of prostate cancer is limited.
-
The evidence that low plasma (blood) selenium concentrations increases risk of prostate cancer is limited.
One has to question, however, whether the best designs to study aetiology are really the best designs in the field of nutritional epidemiology. For example, most randomised trials on supplements and cohort studies on nutritional factors start with study populations over 50 years of age. If diet has its most important effect in puberty or even earlier in childhood or pre-conception, these designs will not be able to validly assess any effect. Other problems have to do with misclassification of food intake over the years, variable within-person eating habits, arbitrary dosages of interventions in trials and so forth. Possibly, the weakest study designs (ecological migrant studies) are the best when it comes to nutritional epidemiology. Unfortunately, these designs cannot come up with any specific conclusion beyond typical diets in certain parts of the world.
Body Fatness
In its 2014 report on prostate cancer, the World Cancer Research Fund concludes that greater body fatness (marked by BMI, waist circumference and waist-hip ratio) is probably a cause of advanced prostate cancer. In a meta-analysis of 23 studies (N = 11,149) on advanced prostate cancer, a statistically significant 8 % increased risk was found per 5 kg/m2 increase in body mass index (BMI) [36]. A meta-analysis of four studies on waist circumference (N = 1,781) showed a statistically significant 12 % increased risk per 10 cm and a meta-analysis of 4 studies on waist-hip ratio resulted in a significant 15 % higher risk per 0.1 unit increase. It is not entirely clear what the mechanism is behind this association. Obesity influences the levels of quite a few hormones and growth factors such as insulin and leptin, which can promote the growth of cancer cells. In men, obesity is associated with lower testosterone levels, although the importance of this is not really clear. Serum testosterone levels do not seem to have a strong effect on prostate cancer risk but because it is essential for differentiation of prostate epithelium, decreased levels may facilitate the growth of a less differentiated, aggressive prostate cancer phenotype. Obesity is also associated with a low-grade chronic inflammatory state which can promote cancer development. Obese adipose tissue is characterised by macrophage infiltration, an important source of inflammation. Fat cells produce pro-inflammatory factors, leading to elevated concentrations of circulating TNF-alpha, IL-6 and CRP.
Adult Attained Height
In a meta-analysis of 34 studies (N = 79,387), the WCRF report found a statistically significant 4 % increased risk per 5 cm taller height: RR 1.04 (95 % CI 1.03–1.05). Adult height is related to the rate of growth during foetal life and childhood. Health and nutrition status in the neonatal period and childhood may impact on the age of sexual maturity. Resulting effects on circulating levels of growth factors, insulin, and other endocrine or tissue specific mediators may influence cancer risk.
Diabetes
Most data on the association between diabetes and prostate cancer come from studies on diabetes type 2. The results from epidemiological studies are somewhat inconsistent but, overall, there seems to be a reduced risk [30]. This contradicts the finding that body fatness is a risk factor for prostate cancer. Because the link between diabetes type 2 and prostate cancer is mainly observed in studies from the PSA era, diabetes is known to decrease the serum PSA value, and the association is stronger for low-grade than for high-grade prostate cancer; it is possible that the association is caused by detection bias. In addition, it is extremely difficult to disentangle the effects of diabetes and its treatment.
In a recent cohort study using five nationwide registers of persons with type 1 diabetes (Australia, Denmark, Finland, Scotland and Sweden), 553 prostate cancers were diagnosed among 2 million male person-years of follow-up. A reduced risk of prostate cancer was found (HR = 0.56; 95 % CI 0.51–0.61) [7].
Androgens
Because the function of the prostate is so dependent on androgens and because hormonal treatment is used in metastasised prostate cancer, it has long been believed that having higher levels of testosterone in the blood may increase the risk of prostate cancer. And indeed, clinical trials with 5-alpha reductase inhibitors (5-ARIs), the Prostate Cancer Prevention Trial (PCPT), in which men were treated with finasteride 5 mg daily or placebo for 7 years, and the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial, in which patients were treated with dutasteride 0.5 mg daily or placebo for 4 years [2, 34] suggested a decrease in risk (see Chapter 2 by Bertrand Tombal). However, the results of these trials may have been influenced by several factors such as end-of-study biopsies. In the non-trial situation, a link between androgens and prostate cancer development is not clear [31]. Recently, a large prospective study from Finland, Sweden and Norway confirmed the absence of an association between prediagnostic serum testosterone levels and prostate cancer development [27]. More research is needed to clarify the link between diabetes and prostate cancer.
Vasectomy
Several recent meta-analyses of the association between vasectomy and prostate cancer have concluded that there is no link between the two (e.g. [37]). US-based studies found a positive association (RR = 1.54) but non-USA studies did not (RR = 0.74). Probably, some studies that did find a positive association have suffered from bias due to differences in health-seeking behaviour by vasectomised and non-vasectomised men.
Aspirin
There is some evidence in the literature that aspirin and other NSAIDS slightly reduce the risk of prostate cancer. However, a recent analysis of the Health Professionals Follow-up Study among 48,000 men did not find any effect of regular aspirin use on prostate cancer risk [6].
Physical Activity
It is not clear whether being more physically active reduces the risk of prostate cancer. A review and meta-analysis of 43 studies did report a decreased risk (pooled RR = 0.90; 95 % CI 0.84–0.95) but because many low-quality studies were included, a definitive conclusion is impossible [26].
Prostatitis
Despite the fact that a definitive causative infectious agent or agents has yet to be identified, accumulating evidence both in human studies and in animal models indicate that infections may contribute to potentially tumour-promoting chronic prostatic inflammation [33].
In conclusion, because ageing, genetic predisposition and ethnicity are not modifiable, until harder evidence becomes available on other suspected risk factors, maintaining a healthy weight is the only lifestyle factor that can lower the risk of prostate cancer.
References
Al Olama AA. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46(10):1103–9.
Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–202.
Bell KJ, Del Mar C, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137:1749–57.
Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Antoni S, Soerjomataram I, Forman D. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060–71.
Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012(45):152–6.
Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, Spiegelman D, Fuchs CS, Giovannucci EL, Chan AT. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–9. doi:10.1001/jamaoncol.2015.6396.
Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson AM, Ljung R, Wild SH, Kerssens JJ, Harding JL, Magliano DJ, Gudbjörnsdottir S, Diabetes and Cancer Research Consortium. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59(5):980–8. [Epub ahead of print].
Carter BS, Bova GS, Beaty TH, et al. Hereditary prostate cancer: epidemiologic and clinical features. J Urol. 1993;150(3):797–802.
Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–92.
Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013;133(3):721–9.
Cook LS, Goldoft M, Schwartz SM, et al. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J Urol. 1999;161(1):152–5.
Cremers RG, Aben KK, Van Oort IM, Sedelaar JP, Vasen HF, Vermeulen SH, Kiemeney LA. The clinical phenotype of hereditary versus sporadic prostate cancer: HPC definition revisited. Prostate. 2016;76(10):897–904. doi:10.1002/pros.23179. [Epub ahead of print].
Cremers RG, Galesloot TE, Aben KK, van Oort IM, Vasen HF, Vermeulen SH, Kiemeney LA. Known susceptibility SNPs for sporadic prostate cancer show a similar association with “hereditary” prostate cancer. Prostate. 2015;75(5):474–83.
Doll R, Payne P, Waterhouse J. Cancer incidence in five continents: a technical report. New York: UICC/Springer; 1966.
Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91(7):608–12.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Feletto E, Bang A, Cole-Clark D, Chalasani V, Rasiah K, Smith DP. An examination of prostate cancer trends in Australia, England, Canada and USA: Is the Australian death rate too high? World J Urol. 2015 Nov;33(11):1677-87. doi:10.1007/s00345-015-1514-7. PubMed PMID: 25698456; PubMed Central PMCID:PMC4617845.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403.
Eisinger F, Morère JF, Touboul C, Pivot X, Coscas Y, Blay JY, Lhomel C, Viguier J. Prostate cancer screening: contrasting trends. Cancer Causes Control. 2015 Jun;26(6):949-52. doi: 10.1007/s10552-015-0573-9. PubMed PMID: 25822574.
Gudmundsson J, Sulem P, Gudbjartsson DF, Masson G, Agnarsson BA, Benediktsdottir KR, Sigurdsson A, Magnusson OT, Gudjonsson SA, Magnusdottir DN, Johannsdottir H, Helgadottir HT, Stacey SN, Jonasdottir A, Olafsdottir SB, Thorleifsson G, Jonasson JG, Tryggvadottir L, Navarrete S, Fuertes F, Helfand BT, Hu Q, Csiki IE, Mates IN, Jinga V, Aben KK, van Oort IM, Vermeulen SH, Donovan JL, Hamdy FC, Ng CF, Chiu PK, Lau KM, Ng MC, Gulcher JR, Kong A, Catalona WJ, Mayordomo JI, Einarsson GV, Barkardottir RB, Jonsson E, Mates D, Neal DE, Kiemeney LA, Thorsteinsdottir U, Rafnar T, Stefansson K. A study based on whole-genome sequencing yields a rare variant at 8q24 associated with prostate cancer. Nat Genet. 2012;44(12):1326–9. doi:10.1038/ng.2437. Epub 2012 Oct 28.
Hankey BF, Feuer EJ, Clegg LX, Hayes RB, Legler JM, Prorok PC, et al. Cancer surveillance series: interpreting trends in prostate cancer--part I: evidence of the effects of screening in recent prostate cancer incidence. J Natl Cancer Inst. 1999;91(12):1017–24.
Iwasaki M, Mameri CP, Hamada GS, et al. Secular trends in cancer mortality among Japanese immigrants in the state of Sao Paulo, Brazil, 1979–2001. Eur J Cancer Prev. 2008;17(1):1–8.
Jahn JL, Giovannucci EL, Stampfer MJ. The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int J Cancer. 2015;137(12):2795–802. doi:10.1002/ijc.29408. Epub 2015 Jan 8. Review.
Karlsson R, Aly M, Clements M, Zheng L, Adolfsson J, Xu J, Grönberg H, Wiklund F. A population-based assessment of germline HOXB13 G84E mutation and prostate cancer risk. Eur Urol. 2014;65:169–76.
Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51.
Kvåle R, Auvinen A, Adami HO, Klint A, Hernes E, Møller B, Pukkala E, Storm HH, Tryggvadottir L, Tretli S, Wahlqvist R, Weiderpass E, Bray F. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99(24):1881–7.
Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51.
Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, Ge J, An R, Zhao Y. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60:1029–44.
Lumme S, Tenkanen L, Langseth H, Gislefoss R, Hakama M, Stattin P, Hallmans G, Adlercreutz H, Saikku P, Stenman UH, Tuohimaa P, Luostarinen T, Dillner J. Longitudinal biobanks-based study on the joint effects of infections, nutrition and hormones on risk of prostate cancer. Acta Oncol. 2016;15:1–7. [Epub ahead of print].
Lynch HT, Kosoko-Lasaki O, Leslie SW, Rendell M, Shaw T, Snyder C, D’Amico AV, Buxbaum S, Isaacs WB, Loeb S, Moul JW, Powell I. Screening for familial and hereditary prostate cancer. Int J Cancer. 2016;138:2579–91.
Parkin DM, Bray F, Ferlay J, Jemal A. Cancer in Africa 2012. Cancer Epidemiol Biomarkers Prev. 2014;23(6):953–66. doi:10.1158/1055-9965.EPI-14-0281.
Pierce BL. Why are diabetics at reduced risk for prostate cancer? A review of the epidemiologic evidence. Urol Oncol. 2012;30:735–43.
Roddam AW, Allen NE, Appleby P, Key TJ, Endogenous Hormones and Prostate Cancer Collaborative Group. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–83.
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, Kwiatkowski M, Lujan M, Määttänen L, Lilja H, Denis LJ, Recker F, Paez A, Bangma CH, Carlsson S, Puliti D, Villers A, Rebillard X, Hakama M, Stenman UH, Kujala P, Taari K, Aus G, Huber A, Van der Kwast TH, Van Schaik RH, de Koning HJ, Moss SM,Auvinen A, ERSPC Investigators. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–35.
Sfanos KS, Isaacs WB, De Marzo AM. Infections and inflammation in prostate cancer. Am J Clin Exp Urol. 2013;1(1):3–11.
Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–24.
Wabinga HR, Nambooze S, Amulen PM, Okello C, Mbus L, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda 1991–2010. Int J Cancer. 2014;135(2):432–9. doi:10.1002/ijc.28661. Epub 2014 Feb 27.
WCRF. http://www.wcrf.org/sites/default/files/Prostate-Cancer-2014-Report.pdf. 2014.
Zhang XL, Yan JJ, Pan SH, Pan JG, Ying XR, Zhang GF. Vasectomy and the risk of prostate cancer: a meta-analysis of cohort studies. Int J Clin Exp Med. 2015;8(10):17977–85. eCollection 2015.
Zhou CK, Check DP, Lortet-Tieulent J, Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB, Devesa SS. Prostate cancer incidence in 43 populations worldwide: an analysis of time trends overall and by age group. Int J Cancer. 2016;138(6):1388–400.
Acknowledgements
We thank Mathieu Laversanne for development of the tables and figures included in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bray, F., Kiemeney, L.A. (2017). Epidemiology of Prostate Cancer in Europe: Patterns, Trends and Determinants. In: Bolla, M., van Poppel, H. (eds) Management of Prostate Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-42769-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-42769-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42768-3
Online ISBN: 978-3-319-42769-0
eBook Packages: MedicineMedicine (R0)