Abstract
Enhanced recovery programs have repeatedly been shown to safely reduce perioperative morbidity and hospital length of stay for surgical patients, and they are being used across an increasing number of surgical specialties. For these programs to be successful, appropriate fluid management is essential throughout the whole perioperative period with the main aim being to maintain physiological normality for patients wherever possible. While excessive fluid administration increases the risk of harm through tissue edema and surgical ileus formation, insufficient fluid administration will result in end-organ failure. To minimize these risks and maintain a “zero-balanced” approach, patients should start surgery minimally dehydrated, be given fluids only to replace what is lost intraoperatively, and then converted to normal enteral intake again as soon as possible after the operation is finished. Good clinical assessment is essential throughout the perioperative period to evaluate how fluid-responsive the patient is at that time and whether they would benefit from further volume, or more inotropic support instead. Increasingly in mechanically ventilated patients, dynamic markers such as stroke volume variation have been shown to be the most effective way of doing this, although these measures do have a number of limitations that need careful consideration. Another approach is targeting fluid administration to a patient’s cardiac output—so-called “goal-directed therapy.” Again, there is good evidence that like enhanced recovery pathways, goal-directed therapy can also reduce perioperative morbidity and surgical patient’s length of stay. National guidelines currently recommend that every surgical patient should have an individualized fluid plan as part of their enhanced recovery program and that goal-directed therapy should be considered as part of this approach—particularly in either high-risk patients and/or more major surgical procedures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Enhanced
- Recovery
- Fluid
- Therapy
- Goal-directed
- Perioperative
- Length of stay
- Cardiac output
- Hemodynamic
- Postoperative complications prevention and control
-
1.
Enhanced recovery pathways are multidisciplinary care pathways that have repeatedly been shown to safely reduce postoperative morbidity and hospital length of stay.
-
2.
A “zero-balance” fluid approach is essential to a successful enhanced recovery approach and needs to be continued throughout the whole perioperative period.
-
3.
Preoperatively, patients should not be excessively starved or dehydrated, that is, avoid unnecessary mechanical bowel preparation, solids to 6 h pre-op, a carbohydrate drink, and clear fluids up to 2 h pre-op.
-
4.
A goal-directed approach should guide intraoperative fluid management in moderate- to high-risk cases with the aim of giving the least amount of fluid required to maintain optimal blood volume and cardiac output.
-
5.
Oral fluid should be encouraged and intravenous (IV) fluids discontinued as soon as possible postoperatively to minimize the risk of further complications. If an IV is needed post-op, beware of ongoing salt loading. Saline, Ringer’s lactate, or Hartmann’s are NOT maintenance fluids.
Introduction
In the late 1990s, professors Wilmore and Kehlet in Boston and Denmark developed a care pathway for colorectal patients undergoing major elective surgery with the aim of minimizing post-op morbidity and hospital length of stay. This pathway incorporated a number of different and wide-ranging interventions based on the best evidence available at that time [1, 2].
Similar “fast-track” pathways have since developed all over the world with various names. In the United Kingdom, this pathway is known simply as enhanced recovery, and since its first launch in the mid 2000s, enhanced recovery pathways have now become commonplace among many surgical specialties in most British hospitals [3]. While the strongest evidence base perhaps remains in colorectal surgery where the pathway has been running the longest, elective orthopedic joint replacements, major gynecological surgery, and urological teams were also quick to adopt similar schemes, and the evidence base in each of these specialties is now growing as well [3].
The Benefits of Enhanced Recovery Pathways
A number of different systematic reviews have now shown that fast-track surgery programs can successfully reduce length of stay in colorectal patients, even though mortality rates remain unchanged [4–8]. In 2010, a meta-analysis of six randomized control trials (RCTs), which used between four and nine different enhanced recovery pathway elements, showed that enhanced recovery pathways significantly reduced length of stay by at least 2 days and complication rates by 50 % [8]. A second independent systematic review in 2011 drew almost identical conclusions [7]. Despite encouraging earlier discharges, most studies suggest that enhanced recovery pathways do not result in increased numbers of readmissions at 30 days [6, 9].
Similar results are starting to be seen in other surgical specialties as well. A systematic review into enhanced recovery use in urological surgery in 2015 identified six studies and concluded that enhanced recovery reduced patient stay without increasing morbidity or mortality [10]. However, a similar Cochrane review into enhanced recovery use in gynecological oncology surgery, which was updated in 2014, failed to identify any RCTs that met their inclusion criteria [11]. Although, other nonrandomized control trials have shown similar decreases in length of stay with enhanced recovery use in gynecology as well. For example, an observational study in Sweden, published in 2014, showed that 17 % more women were discharged within 2 days of an abdominal hysterectomy immediately after the introduction of an enhanced recovery pathway [12]. Two recent systematic reviews have examined enhanced recovery use in upper gastrointestinal surgery; Gemmill et al. concluded after reviewing 18 eligible studies (including three RCTs) that enhanced recovery appeared safe and might reduce length of stay in patients undergoing surgery for both gastric and esophageal cancer, but the evidence base remained weak [13]. Meanwhile, Beamish et al. identified 14 studies (including nine RCTs), with a total of 1,676 patients with gastric cancer. They concluded that enhanced recovery pathways were safe, feasible, and cost-effective, with a nonsignificant trend toward reduced length of hospital stay [14].
The largest study to investigate the effect of enhanced recovery programs so far is a recently published three-year cross-specialty national audit conducted by the Enhanced Recovery Partnership Programme. Four surgical specialties were audited (colorectal, urology, orthopedic, and gynecology) in 61 British hospital trusts from 2009 to 2012, with a weak correlation seen between enhanced recovery pathway compliance and reduced length of stay in colorectal, orthopedic, and gynecological surgeries. The median lengths of stay in colorectal, orthopedic, and gynecological surgeries reduced by 2, 3, and 4 days, respectively, over this period, with no change in length of stay seen in gynecology [15].
Components of Enhanced Recovery Pathways
Kehlet’s initial pathway focused on minimizing the effects of the surgical stress response through improving analgesia, using short-acting anesthetics (or where possible regional anesthesia) and minimally invasive surgery, and encouraging early mobilization and nutrition [1, 2].
While the specific components of any particular enhanced recovery pathway vary between different hospitals and different surgical specialties, the majority of the interventions remain remarkably consistent, and fluid therapy is always one of the major components.
The requirements of what the UK Enhanced Recovery Partnership Programme specify should be included in a typical enhanced recovery pathway are shown in Table 13.1 [3, 16]. Fluid therapy is clearly mentioned (and highlighted) in each of the three stages of the table (preoperatively, intraoperatively, as well as postoperatively).
While it is now widely accepted that enhanced recovery pathways can safely reduce hospital length of stay, it remains controversial as to which elements in the enhanced recovery approach are most important in achieving this [3]. Even though a recent systematic review found no evidence that goal-directed fluid therapy impacts C-reactive protein (CRP) values [17], given that fluid management still varies greatly between different centers and different clinicians [18, 19], and different fluid protocols can greatly impact surgical complication rates (possibly by as much as nearly 50 % [20]), it seems reasonable to propose that optimal perioperative fluid management should be an essential component of any enhanced recovery protocol. The challenge is in defining what constitutes “optimal.”
Fluid Therapy in Enhanced Recovery: A “Zero-Balance” Approach
Perioperative fluid management is an important consideration throughout the whole surgical pathway, and optimal fluid management should be viewed as a continuum throughout the patient’s whole hospital admission. Suboptimal management at any point will not only lead to significantly longer hospital admissions, but also risks compromising the benefits conferred by other elements of the enhanced recovery package [21].
The overarching focus—as with many enhanced recovery elements—should be to always aim for as near physiological normality as possible. In the context of fluids, this can be thought of as avoiding dehydration and hypovolemia or fluid overload with their associated complications. Inadequate fluid administration results in insufficient perfusion pressures, reducing oxygen delivery and increasing anaerobic metabolism, which ultimately leads to cell death and end-organ failure [22]. One of the most common perioperative manifestations of this is probably acute kidney injury (AKI).
Conversely, excess fluid administration can have equally harmful consequences, raising hydrostatic pressures and increasing levels of atrial-natriuretic peptides, which damage the delicate glycocalyx layer of the vascular endothelium [22]. This renders blood vessels “leaky” and causes damaging tissue edema to develop in the interstitium, which again impairs tissue and organ oxygenation [22]. This interstitial edema, together with high salt loads from excess crystalloid infusion, can also lead to postoperative ileus and further increase patient’s length of stay [23]. See Fig. 13.1 [24].
Both “restrictive” and “liberal” fluid administration increase the risk of end-organ damage compared to a “zero-balance” approach through hypoperfusion or tissue edema generation, respectively (Adapted from [24])
Unfortunately, terminology in this area has often been confusing historically. “Liberal fluid therapy” was initially encouraged perioperatively to maintain a proposed “3rd space.” However, evidence to support this theoretical compartment has always been scarce, and as our understanding about glycocalyx damage and resulting interstitial edema (as explained previously) increased, consensus has shifted toward using a more “restrictive” approach to fluid administration [25]. Yet, the term “restrictive” suggests tending toward an equally damaging hypovolemic state. Recent reviews have since proposed abolishing the terms “restrictive” and “liberal” and instead using the phrase “zero-balance.” This avoids these misinterpretations and encourages an approach that simply replaces what is lost (e.g., insensible loss through ventilation and respiration, or volume loss from intraoperative hemorrhage) [21, 24].
“Zero-balance” is also the term that the American Society for Enhanced Recovery (ASER) has adopted in their guidelines on perioperative fluid management to encourage this approach [26]. Again, the ASER guidelines emphasize the importance of maintaining a zero-balance approach throughout the preoperative, intraoperative, and postoperative phases of surgery, but as different considerations arise at each of these different points, it might be helpful to consider how this approach should apply to each of these stages in turn.
Preoperative Fluid Management
The main aim with preoperative fluid management is to prevent patients from becoming dehydrated before the surgery even starts. It is clearly much easier to maintain a zero-balance approach intraoperatively if the patient starts their operation in a normal euvolemic state [21]. However, although this concept sounds straightforward, there are a surprising number of challenges to achieving this in practice.
While patients evidently need to avoid solid food for elective procedures to minimize the risk of aspiration on induction, increasingly international guidelines are recognizing the importance of not prolonging this fasting period for fluids further than 2 h prior to surgery. Cochrane reviews have shown that drinking clear fluids up until 2 h before surgery is not associated with an increased risk of aspiration or other complications in either adults or children. If anything, these reviews suggest that drinking clear fluids actually reduced adult gastric volumes and made the preoperative experience more comfortable for both adults and children [27, 28]. The European Society of Anaesthesiology also now encourages both adults and children to drink fluids up to 2 h preoperatively in its guidelines [29].
Many enhanced recovery protocols also encourage the avoidance of mechanical bowel preparation in many patients for similar reasons, although this is becoming more controversial. Mechanical bowel preparations have been shown to increase dehydration and decrease patient’s comfort, without reducing the risk of early postoperative complications in the majority of cases [30–32]. However, some surgeons think that mechanical bowel preparation does make certain procedures easier, particularly laparoscopic cases, and some recent evidence suggests that mechanical bowel preparation may significantly improve 10-year survival data in elective colorectal cancer cases [33, 34]. Overall though, avoiding mechanical bowel preparation currently remains an essential part of the enhanced recovery package because of the significant effects the preparation can have on preoperative hydration status.
As well as being well hydrated preoperatively, patients’ nutritional status should also be optimized prior to surgery using carbohydrate energy drinks. These drinks also decrease patient discomfort while waiting for surgery as well as decreasing postoperative insulin resistance through increasing insulin activity [35, 36]. They can safely be taken 2–3 h prior to surgery depending on the nutritional content [37].
Intraoperative Fluid Management
Again, as with most other enhanced recovery elements, the main aims of intraoperative fluid balance in enhanced recovery pathways should be to maintain physiological normality as much as possible, that is, to maintain euvolemia and minimize electrolyte disturbance. Successful preoperative fluid management should allow the patient to start surgery well hydrated, meaning that the main intraoperative aims are simply to replace ongoing losses without giving excess salt or water [21].
Insensible losses (e.g., perspiration or urine output) will make up a very small percentage of ongoing losses, and these will need replacing with a maintenance fluid regime, often using crystalloids. Direct measurements of intraoperative evaporative losses have shown this to normally be less than 1 ml/kg/h in normal conditions, and it is important to remember that giving fluids in excess of this rate can rapidly lead to harm and postoperative complications (such as ileus as explained earlier) [23, 38]. While acknowledging that more liberal fluid administrations of up to 20 or 30 ml/kg/h might confer some benefits in ambulatory patients (such as decreasing postoperative drowsiness, nausea, and pain), international guidelines recommend that maintenance fluids should be given at 1–2 ml/kg/h for all longer or more major operations [16, 39].
The majority of ongoing intraoperative losses, however, will be intravascular volume losses. For example, the patient could lose volume through blood loss or from compartmental fluid shifts, such as interstitial edema formation, secondary to the surgical inflammatory response [21, 22]. These losses will require replacement with equivalent volumes of similar fluids (e.g., blood loss should ideally be replaced with blood products, including platelets and clotting factors in the event of significant hemorrhage) [22].
While heavy blood loss may be easy to see if the suction equipment is rapidly filling in the operating theater, intercompartmental shifts may be much less obvious to either the surgeon or the anesthetist. If volume loss is suspected, then a “volume challenge” or “fluid challenge” should be used to see if there is any evidence of intravascular depletion, which might respond to further filling.
The fluid challenge remains one of the singlemost important tools for the anesthetist in assessing fluid responsiveness [39]. If the patient is fluid-deplete and can tolerate further fluids, then a small but rapid fluid bolus should increase preload enough to cause a measurable increase in stroke volume and therefore cardiac output. A positive response proves that the patient is “fluid (or volume) responsive” [39].
A typical fluid challenge would be 500 ml of fluid given rapidly over 5–10 min, and a fluid-responsive patient should increase their stroke volume by at least 10–15 % in response to this [21, 40, 41].
Another simple way of testing fluid responsiveness is with a passive leg raise (PLR), where the legs are lifted above the height of the heart. This generates a similar response to a traditional fluid challenge by increasing venous return (and preload) by moving blood out of the venous system in the legs [42]. While rarely of use during surgery, this maneuver has a place in the assessment of volume status following surgery.
However, it is essential to remember that “fluid responsiveness” and “hemodynamic instability” are not interchangeable or equivalent. Around half of all hemodynamically unstable critically ill patients were still not responding to fluid alone—they will not be “volume-responsive,” and they may also require treatments with vasopressors to increase systemic resistance, or inotropes to increase contractility [41]. Equally, a volume-responsive patient will not always be intravascularly fluid-deplete [21]. Patients in successful enhanced recovery pathways should normally be less fluid-responsive than other patients, as they more likely start their operation well hydrated [39].
The whole clinical picture should always be viewed in context when assessing for fluid responsiveness, and hence a good clinical assessment is vital for correct decisions on intraoperative fluid management (see later in chapter).
Postoperative Fluid Management
Postoperatively, patients should be encouraged to restart normal oral food and fluids as early as possible in enhanced recovery pathways, and intravenous fluids should be stopped as soon as this is achieved. Continuing intravenous fluids into the postoperative phase further increases the risk of developing postoperative ileus as explained earlier, particularly as patients’ ability to excrete and remove both sodium and chloride is reduced postoperatively [23]. For this reason, if fluids are required to continue postoperatively, then low-volume fluids with relatively low sodium contents should be considered—particularly, when most patients will already have been given an excess of sodium and chloride intraoperatively [21].
Continuing intravenous fluid postoperatively will also have negative consequences on other enhanced recovery pathway elements. One of the main focuses of enhanced recovery in the postoperative phase is to encourage early mobility, and patients are inherently less likely to mobilize if they are connected to intravenous fluid lines. Equally, catheters will also discourage patients from mobilizing, and should be removed as soon as possible [21]. Adequate analgesia is also important to maximize the chances of early mobilization, but laxatives may also be required to minimize constipation and urinary retention depending on the surgical procedure that has been performed.
Early oral intake also has independent surgical benefits. A systematic review has shown that early feeding significantly reduces the risk of postoperative infection and also independently reduces hospital length of stay. In addition, it may lower the risk of surgical anastomotic dehiscence, wound infection, pneumonia, intra-abdominal abscess, and mortality, although these did not reach statistical significance in the meta-analysis performed [43].
Clearly, how fluids are managed throughout the preoperative and intraoperative phases will impact how the postoperative phase is managed and how successful the enhanced recovery fluid regime will be overall. For example, failing to prevent preoperative dehydration would mean the patient would already start the intraoperative stage with a relative fluid deficit and require larger volumes of fluids intraoperatively, increasing the risk of ileus postoperatively and delaying the patient’s discharge.
The Need to Individualize Fluid Therapy
While a “zero-balance approach” will need to be applied throughout every patient’s perioperative pathway, the exact management cannot be completely protocolized in advance as it will always differ from patient to patient, from operation to operation, and in some cases between different anesthetic techniques [44].
In other words, an individualized zero-balance approach to fluids is required for each operation, which means that a way of continuously assessing and reassessing an individual patient’s fluid requirements throughout the whole perioperative period is essential.
Clinical Assessment of Fluid Status
Assessing a patient’s fluid status is an essential clinical skill that is taught from the very first days of medical school. There are a number of different physiological markers that clinicians are traditionally taught to use to monitor fluid status. Some examples of clinical signs and parameters that might suggest a patient is hypovolemic are as follows:
-
Heart rate above 90 (or n % > baseline)
-
Systolic blood pressure below 90 (or n % < baseline)
-
Urine output of less than 0.5 ml/kg/h
-
High lactate
-
Low central venous pressure
However, it is becoming increasingly clear that none of these markers are reliable indicators of an anesthetized patient’s fluid status [21]. Many are not specific. For example, a heart rate over 90 could be expected in any type of systemic inflammatory response (as per the “SIRS criteria”). Although SIRS was originally defined to identify sepsis, it could equally be a response to trauma, inflammation, or ischemia among other things—in fact, over 80 % of surgical intensive care patients would meet the SIRS criteria [45, 46].
Many of these markers are also not very sensitive for detecting changes in volume status, partly due to a confounding effect of the normal physiological response to systemic blood loss, which is constriction of the splanchnic circulation. Splanchnic vasoconstriction has a protective physiological effect by moving blood back into the systemic circulation and maintaining vital organ perfusion. However, because this means that the systemic circulation is relatively maintained, heart rate and blood pressure do not alter dramatically, even in the presence of large overall volume deficits. These variables only start to change when the volume deficit cannot be contained within the splanchnic circulation alone [21]. For example, in one study where young healthy volunteers gradually had 25 % of their total blood volume removed by phlebotomy over an hour, the only significant marker to change was gastric tonometry—a specific monitor of splanchnic perfusion. Heart rate and mean arterial blood pressures remained unchanged on average, despite this large volume loss [47].
Urine output is another measure that is often taught as being a good marker of volume status and a relatively simple way of approximating kidney function. However, urine output is often poorly recorded both intraoperatively and postoperatively, and intraoperative oliguria (i.e., urine output <0.5 ml/kg/h) is not predictive of developing acute kidney injury or of overall volume status in patients undergoing major noncardiac surgery [48].
Likewise, hourly monitoring of central venous pressure (i.e., the pressure recorded from either the right atrium or the superior vena cava) was routine in intensive care units all over the world 10 years ago. However, in 2008, a systematic review of 24 studies concluded that central venous pressure was actually a very poor predictor of which patients needed more fluid and also of an individual patient’s overall blood volume. The authors recommended that routine central venous pressure monitoring should no longer be performed perioperatively [49].
Transesophageal echocardiography has also been trialed as a method for assessing volume status. The principle seems particularly appealing as it allows direct visualization of the heart itself, and it is relatively simple to perform in an anesthetized patient. Yet, measurements of both right and left end-diastolic volumes are variable, and, as with many of the static variables described previously, have ultimately proved to not be helpful in assessing a patient’s fluid responsiveness [50, 51].
However, using ultrasound to measure the change in diameter of either the inferior or superior vena cava has been shown to be very predictive in assessing fluid responsiveness of patients undergoing positive pressure ventilation [51]. Measuring the change in diameter makes this a dynamic marker and, indeed, many other dynamic variables have also been shown to be useful in assessing fluid responsiveness, particularly in mechanically ventilated patients.
Using Dynamic Variables to Assess Fluid Status
Using dynamic indicators to assess fluid responsiveness has repeatedly been shown to be more effective than using static markers [39, 42, 50]. This conclusion was supported by the findings of a systematic review comparing static and dynamic indices. Many of the dynamic variables used in this review were related to pressure changes at different points of the respiratory cycle, for example, pulse pressure variation [50].
Pulse pressure variation (see Fig. 13.2) is produced by changes in venous return (cardiac preload) at different points in the respiratory cycle if the patient is mechanically ventilated. This is due to the right atrial pressure increasing during positive pressure inspiration and decreasing again in expiration. These pressure changes cause cyclical changes to venous return and to ventricular filling pressures [42]. These changes are also greater in volume-depleted and fluid-responsive patients, giving a very accurate measure of fluid responsiveness. Pulse pressure variation is relatively easy to visualize on a normal arterial pulse pressure trace too, making it a very useful measure of fluid responsiveness in mechanically ventilated patients with an arterial line in situ [52]. In general, a pulse pressure variation of at least 13 % will predict a 15 % or greater increase in cardiac output in response to a 500 ml bolus of crystalloid [42].
Similar cyclical changes can be seen in the oxygen plethysmography trace. These plethysmography variations have been shown to approximate well to the pulse pressure variations described earlier, with a plethysmography variation over 15 % accurately predicting a pulse pressure variation over 13 %. The big advantage of using plethysmography variation over pulse pressure variation is that it can be measured noninvasively through a normal oxygen saturation finger probe and no invasive lines are required [53, 54].
As the main determinant of pulse pressure is stroke volume, it is not surprising that similar variations in stroke volume are also seen in mechanically ventilated patients and can also be used as an accurate predictor of fluid responsiveness. Stroke volume variation can be measured using transesophageal echocardiography, and dedicated transesophageal Doppler probes are now recommended by the National Institute of Clinical Excellence and routinely used throughout the United Kingdom for this purpose [42, 50, 55].
Although being very accurate, all of these different variations rely on a number of assumptions. For instance, patients must be mechanically ventilated with normal intrathoracic and abdominal pressures to ensure these respiratory pressure changes cycle appropriately. The patient must also be in sinus rhythm, as other rhythms such as atrial fibrillation (with an irregular R-wave to R-wave time) will affect how the intrathoracic pressure cycles are transduced at the right atrium, and the ventricles and smaller tidal volumes will reduce these pressure changes, again altering the test’s predictive value significantly [21, 42, 52]. Some of these factors will cause pulse pressure variation to appear artificially large (false-positive), while others will cause a decrease in variation size despite no change in fluid responsiveness (false-negative). The acronym “LIMITS” offers one way of remembering these effects [56]. See Fig. 13.3 [56].
The “LIMITS” to using pulse-pressure variation monitoring, and whether a false-positive or false-negative result should be expected in each case (Adapted from [56])
Ultimately, both pulse pressure and oxygen plethysmography variations are simply less invasive ways of indirectly measuring stroke volume variation. As a successful fluid challenge is one that results in an increase in stroke volume (and therefore cardiac output) as explained earlier, fluid therapy should always be targeted to increase stroke volume and not thought of as reducing oxygen plethysmography or pulse pressure variation.
Whereas previously standard (static) ways of assessing fluid status left no clear end point, and it was often very challenging to know whether fluid administration had been beneficial or not, with dynamic assessment of stroke volume variation there is a very clear end goal to fluid therapy: a measurable increase in stroke volume and cardiac output [21, 41, 50].
This concept has led to whole new method of giving fluids known as “goal-directed fluid therapy,” which is increasingly being used in many enhanced recovery pathways around the world [21, 57].
Goal-Directed Fluid Therapy
Goal-directed fluid therapy can be defined as using fluids, vasopressors, and/or inotropic agents to increase cardiac output and therefore tissue oxygen delivery. Fluid administration would achieve this aim normally through increasing preload and consequently stroke volume as explained previously.
The concept was developed more than 30 years ago after the invention of the Swan–Ganz pulmonary artery catheter in the early 1970s allowed rapid changes in cardiac output to be measured for the first time [58, 59]. Soon after this, in 1978, Bland et al. proposed that oxygen delivery would be a useful therapeutic goal to target [60]. In 1988, Shoemaker et al. used a protocol with the pulmonary artery catheter to target increased oxygen delivery and showed that this significantly reduced mortality in high-risk surgical patients—a concept that we continue to use today [61].
Since then the pulmonary artery catheter has gone from being a common sight in most critical care units to now hardly being used at all. This is partly due to the many risks associated with its use, and also because a number of other reliable, less invasive, and less risky cardiac output monitors have since entered the market [62].
Different ways of monitoring cardiac output include everything from using routine monitoring to visualize changes in the stroke volume, pulse pressure, or oxygen plethysmography variations as described above, through to specially designed devices such as the esophageal Doppler mentioned earlier or lithium-dilution cardiac output monitoring devices such as the LiDCOrapid device (LiDCO, Cambridge, UK). This device injects a small bolus of lithium and uses this together with the arterial waveform trace to give a calibrated beat-by-beat estimate of cardiac output [55, 63, 64].
Goal-directed therapy approaches are now widely used in Australia, New Zealand, the United States of America, and particularly in the United Kingdom where the esophageal Doppler device remains the most common method of monitoring cardiac output changes [63].
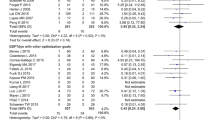
In 2012, a Cochrane systematic review of 31 trials with a total of more than 5,000 patients concluded that goal-directed fluid therapy significantly reduced morbidity in elective surgery. The rates of acute kidney injury, respiratory complications, and wound infections were all significantly reduced when goal-directed fluid therapy was used; the length of hospital stay was also 1 day shorter on average, and overall, fewer patients suffered complications. The review found no evidence to suggest any potential harm through using goal-directed therapy, and although there were hints of a possible downward trend in 28-day mortality, this did not reach significance (28 day mortality = 7/100 in control, 6/100 in GDFT arm, RR = 0.81, CI 0.65–1.00) [65].
Overall, these conclusions were very similar to the effects seen in studies looking at the benefits of enhanced recovery pathways as described earlier—both interventions have been shown to significantly reduce morbidity and length of stay in surgical patients, but have not been shown to significantly impact the mortality of these patients [8, 65].
Goal-Directed Fluid Therapy in Enhanced Recovery Protocols
A number of recent studies have specifically tried to assess the benefits of using goal-directed fluid therapy within an enhanced recovery protocol, but overall the results have been mixed.
In 2012, Brandstrup et al. randomized 150 elective colorectal patients in an enhanced recovery pathway to receive either fluid therapy guided by an esophageal Doppler or to receive a zero-balance fluid therapy approach. No significant difference between the two groups was seen at 30 days for major or minor complications, mortality, or hospital length of stay [66].
Srinivasa et al. conducted a similar trial in 2013 in 85 elective colorectal patients who were all part of an enhanced recovery protocol that included a preoperative drink and avoidance of prolonged fasting. Half of the patients received “restrictive fluid management” (maximum 1,500 ml intraoperatively) and the other half received goal-directed therapy. Again, median lengths of stay and number of complications were almost identical between both groups (6 vs 5 days, and 26 vs 27 developed complications). Interestingly, the amount of intraoperative fluids administered to each group was also similar: 1,994 ml in the goal-directed fluid group compared to 1,614 ml on average in the restrictive group, and there was no clinically relevant difference in the hemodynamic variables [67].
In 2014, Phan et al. again randomized 100 elective colorectal patients so that 50 received a restrictive approach and 50 received fluids based on esophageal Doppler guidance. All 100 patients otherwise followed an identical enhanced recovery protocol. Again, there was no difference in the median length of stay (6 days in the restrictive group compared to 6.5 in the goal-directed group, p = 0.421) or rate of complications (52 % in restrictive compared to 60 % in goal-directed, p = 0.42). In this case, the two groups did receive a statistically different amount of fluid intraoperatively, but both still received relatively small overall volumes (1,500 ml in the restrictive compared to 2,190 ml in the goal-directed group, p = 0.008) [68].
In a similar randomized trial in 2015, Lai et al. looked at 220 enhanced recovery patients having either rectal resections or cystectomy with ileal conduit operations and again randomized them to receive either goal-directed fluid therapy using colloid fluids guided by the LiDCO rapid or a relatively liberal control group. Interestingly, this group also stratified their samples by preoperative fitness using cardiopulmonary exercise testing. Despite the goal-directed (intervention) group receiving an average of 956 ml more Gelofusine (B. Braun, Melsungen, Germany), no significant differences were seen in either mean length of stay (9.6 days control to 11.8 days intervention, p = 0.091) or postoperative complication rates (48.6 % control vs 50.5 % intervention, p = 0.717). There was also no statistical association between stroke volume and aerobic fitness to either length of stay or complication rate [69].
These four relatively small studies might not have shown any significant benefit to using goal-directed fluid therapy in enhanced recovery protocols, but, possibly more importantly, again none suggested any harm in using this approach either. Perhaps more interesting is the similarities in the amounts of fluid that were administered intraoperatively between the three studies, and particularly how much smaller these values are (in not only the intervention but also the control groups) compared to similar studies conducted before enhanced recovery after surgery pathways were introduced [21].
The goal-directed protocol study by Srinivasa in 2013 was actually identical to that used in 2006 by Noblett et al., where all of their patients received bowel preparation and were also fasted for longer. The total fluids given in the 2006 study were 3,638 ml in the goal-directed group and 3,834 ml in the control group—more than double the amount given to the control (restrictive) group in the study by Srinivasa (2013), with an enhanced recovery protocol. Unlike the 2013 study, the patients of Noblett et al. were also much more fluid-responsive as surgery started, and their cardiac indexes increased significantly throughout the operation (from average 3.2 l/min to 3.8 l/min) in response to filling. Overall, the complications (2 % vs 15 %, p = 0.04) and average lengths of stay (7 days vs 9 days, p = 0.005) in the 2006 goal-directed group were significantly lower than the liberally treated controls. Together, these results really highlight the significant impact enhanced recovery pathways have had on surgical outcomes in just an 8-year period and emphasize the importance of a zero-balance fluid management approach in this improvement [21, 67, 70].
So, where does this leave goal-directed fluid therapy in enhanced recovery protocols? In 2014, a large multicenter randomized control trial, Optimisation of Cardiovascular Management to Improve Surgical Outcome (OPTIMISE), conducted by Pearse et al., reported on the effect of using goal-directed fluid therapy together with an inotrope (dopexamine) on high-risk patients undergoing major abdominal surgery during and up to 6 h after the procedure [71]. All 734 patients followed some form of enhanced recovery protocol, making it the largest goal-directed fluid therapy trial in enhanced recovery to date. Their primary outcome was a composite score of predefined moderate or major postoperative complications and mortality at 30 days. Again, the intervention arm failed to show a significant reduction in the combined morbidity and mortality outcome, but in this larger study there was a clear trend toward benefit with the intervention (intervention group rate 36.6 % vs control group rate 43.4 %, p = 0.07, 95 % confidence interval 0.71–1.01). Interestingly, for the first time the OPTIMISE study also suggested a trend toward a lower mortality at 180 days through using a goal-directed approach, although again this was not statistically significant (180 day mortality rates of 7.7 % with intervention compared to 11.6 % in control, p = 0.08). The trial had been powered to recruit more than 1,000 patients, and had this initial target been reached, then may be these two end points would have reached significance, but that remains unknown [71].
The OPTIMISE authors went on to update the earlier 2012 Cochrane systematic review with their new results as well as seven other smaller trials that had been published in the intervening period, taking the total number of studies reviewed up to 38. This new meta-analysis showed that using a cardiac output-directed hemodynamic therapy algorithm (goal-directed fluid therapy) did significantly reduce complication rates in surgical patients [71].
It is still difficult to draw definite conclusions from these findings however. Using a single composite mortality and morbidity outcome might have limited the significance of the results given that no previous study has shown a significant difference in mortality to date. It also remains unclear whether the main benefit was from using a goal-directed approach to fluid administration, inotropic support, or both (OPTIMISE is one of the first studies to combine this approach in the intervention arm). And finally, although this study significantly improves the quality of the data in the updated Cochrane meta-analysis, most of the other studies in this review remain small single-center studies reporting their outcomes in different ways. Many of these studies are also more than 10 years old now and predate enhanced recovery pathways [71].
Ultimately though, goal-directed therapy has repeatedly been shown to be a safe intervention. Better fluid management through enhanced recovery pathways or through goal-directed approaches has both been shown to independently reduce postoperative complications. A goal-directed fluid approach may also add extra benefit to enhanced recovery protocols, particularly in higher risk patients, although this will require a large and well-powered clinical trial to answer conclusively [21, 69, 71, 72].
The biggest benefit to using a goal-directed approach is almost certainly in patients whose preoperative fluid status has not been optimized successfully and will start surgery fluid-responsive. As it is difficult to predict which patients will fall into this group, one suggestion is to use goal-directed therapy in all patients to ensure those patients that would benefit from goal-directed fluid therapy will still receive it [21]. In certain operations where large amounts of blood loss or significant intercompartmental fluid shifts are to be expected, it seems logical that targeting fluid therapy based on cardiac output monitoring should be seen as best practice [57].
Currently, consensus UK guidelines recommend using an individualized fluid plan with a zero-balance approach in all enhanced recovery patients. They also emphasize that some patients will benefit from cardiac output optimization through a goal-directed approach, with higher risk patients having higher risk operations most likely to gain [16, 21]. See Fig. 13.4 [21]. The UK Enhanced Recovery Consensus statement specifically recommends the use of intraoperative fluid management technology (such as the esophageal Doppler) in any operation, with any of the following features [16]:
The fluid management approach used should depend on both patient and surgical risk factors, with goal-directed fluid therapy (GDFT) indicated in higher risk cases (Adapted from [21])
-
Major surgery with a 30-day mortality rate of >1 %
-
Major surgery with anticipated blood loss of >500 ml
-
Major intra-abdominal surgery
-
Intermediate surgery (30-day mortality >0.5 %) in high-risk patients (e.g., age > 80, history of left ventricular failure or previous ischemic heart disease or stroke)
-
Unexpected blood loss requiring >2 l of fluid replacement
-
Patients with evidence of ongoing hypovolemia or tissue hypoperfusion (e.g., persistent lactic acidosis)
Fluid Choice
Choosing which intravenous fluid to use is also vitally important to a successful enhanced recovery pathway. In general, all intravenous fluids fall into one of just three categories:
-
1.
Crystalloids
-
2.
Colloids
-
3.
Blood products
Crystalloid solutions are mixed with electrolyte or glucose ions; for example, water is mixed with sodium chloride ions to make up saline solutions. They are best used to replace insensible losses (which are often mixed with electrolyte disturbances; e.g., sweating causes salt and water loss). Some can also be used as resuscitation fluids as they will also influence hemodynamic status—the exceptions are dextrose-based solutions as cellular glucose uptake is so rapid that no significant hemodynamic effect is seen. Crystalloids can be classified based on their constituent ions and their osmolality; see examples in Table 13.2 [22, 39, 73].
Colloid solutions, on the other hand, are solutions mixed with macromolecular solutes instead of electrolyte ions. Examples include starch, gelatin, or dextran solutions. These solutes exert an osmotic pressure across the glycocalyx endothelial wall and due to their particle size are thought to remain inside the intravascular space longer (and therefore exert a long-lasting hemodynamic effect) than crystalloid solutions [22, 39].
Blood products consist of individual blood constituents such as red cells, platelets, fresh frozen plasma (FFP), or clotting factor mixes.
Which fluid type is the “best type” of fluid remains hotly debated. Ideally, fluid losses should be replaced with fluids with a similar composition in an aim to keep physiological normality [22]. For example, blood loss should be replaced with blood products wherever possible, such as packed red cells as well as with platelets and other clotting factors if the blood loss is significant.
Insensible losses (such as through perspiration and respiration) should be replaced with balanced crystalloid solutions, and 0.9 % saline solutions (including some colloids that are mixed with 0.9 % solutions) should be avoided wherever possible. There are very few studies that show their administration to result in clinical benefit, and they have frequently been shown to cause a hyperchloremic acidosis through excess sodium and chloride administration, which may be harmful [21]. However, while Hartmann’s is a balanced crystalloid solution, simply repeatedly giving Hartmann’s alone will still result in an exceptionally high sodium load and a potassium shortage [18].
The British Consensus Guidelines on Intravenous Fluid Therapy for Adult Surgical Patients (GIFTASUP) advise that patients receive the following to meet their minimum daily maintenance requirements [73]:
-
1–2 mmol/kg of sodium
-
1 mmol/kg of potassium
-
30 ml/kg water
Consequently, maintenance fluid regimes should aim to replace the above, and ideally at a rate of less than 2 ml/kg/h (including any drug infusions) according to the consensus statement from the British Enhanced Recovery Partnership [16, 73]. Where intravenous fluids do need to be continued postoperatively, these guidelines strongly recommend using a low-sodium crystalloid solution (e.g., 0.18 % sodium/4 % dextrose with potassium) to minimize the risk of developing postoperative ileus from excessive sodium administration [16, 21].
In terms of replacing other volume losses, most goal-directed fluid studies have used colloid boluses. This is because colloid boluses are thought to increase stroke volume and blood pressure more (and also more quickly) than the same volume of a crystalloid solution, due to colloids being less likely to leak across the glycocalyx and out of intravascular space as rapidly as crystalloid solutions [21, 22].
The Colloids Versus Crystalloids for the Resuscitation of the Critically Ill (CRISTAL) trial (a large, multicenter randomized control trial comparing crystalloids and colloids for resuscitation of hypovolemic shock) showed a significant reduction in 90-day mortality in the colloid group, suggesting benefit in using colloid boluses in fluid-responsive patients to replace volume loss [74]. However, at least two other large randomized trials have recently suggested that using starch-based fluids in critical care patients is associated with an increased risk of kidney injury or the need for renal replacement therapy, throwing this perceived survival benefit into question [75, 76].
However, both of these trials specifically looked at critically ill patients and many of whom had already been fluid-resuscitated prior to randomization. There is no evidence in the literature currently to suggest that using starch solutions perioperatively in surgical patients to treat a blood volume deficit increases the risk of adverse renal events [77]. Perioperative patients, who are normally fit and well at the start of surgery, constitute a very different physiological cohort to shocked patients on intensive care, and it may be that this extra risk of renal damage is due to damage to the glycocalyx as a result of the systemic inflammatory changes seen in shock [21]. In perioperative patients with preexisting renal impairment, however, it is probably still sensible to avoid using synthetic colloid solutions, and again the decision of whether to use crystalloid or colloid solutions will need to be made on a case-by-case basis as part of each individualized fluid plan [21].
Conclusion
Enhanced recovery pathways offer significant benefits to patients in terms of reducing morbidity and also length of stay after elective surgery, and they are gradually being used in more and more surgical specialties [15]. Rather than suggesting radical new treatments, enhanced recovery methodology emphasizes and focuses around doing simple things well, and its success is principally focused on aiming to maintain “physiological normality” as much as possible perioperatively [3]. Fluid management is a central component to the success of this approach, and the focus should be to achieve a zero-balance approach throughout the perioperative period [21]. Minimizing preoperative dehydration through shortening fasting times and avoiding mechanical bowel preparation is as important to this process as encouraging oral intake and avoiding excessive intravenous crystalloid administration postoperatively [21]. Good clinical assessment is essential throughout this process, and intraoperatively, this should focus on using dynamic markers where possible, and a goal-directed approach targeting increased cardiac output is recommended in all high-risk patients and major surgical cases [16, 42]. Losses should be replaced with similar fluids, and fluid challenges are a particularly useful tool for the anesthetist in assessing a patient’s fluid responsiveness [39]. Ultimately, the approach to giving fluids should be considered the same as the approach with any other pharmacological drug—they should be given at the correct time and in the correct dose, only given when indicated, and due care must be paid to the potential adverse effects they can cause in overdose [78].
References
Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–41.
Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ. 2001;322(7284):473–6.
Grocott MPW, Martin DS, Mythen MG. Enhanced recovery pathways as a way to reduce surgical morbidity. [Miscellaneous Article]. Curr Opin Crit Care. 2012;18(4):385–92.
Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta-analysis1. Colorectal Dis. 2009;11(4):344–53.
Wind J, Polle SW, Fung Kon Jin PHP, Dejong CHC, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93(7):800–9.
Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. 2009;24(10):1119–31.
Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149(6):830–40.
Varadhan KK, Neal KR, Dejong CHC, Fearon KCH, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29(4):434–40.
Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2011 [cited 2015 Oct 15]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD007635.pub2/abstract.
Di Rollo D, Mohammed A, Rawlinson A, Douglas-Moore J, Beatty J. Enhanced recovery protocols in urological surgery: a systematic review. Can J Urol. 2015;22(3):7817–23.
Lu D, Wang X, Shi G. Perioperative enhanced recovery programmes for gynaecological cancer patients. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2015 [cited 2015 Oct 15]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD008239.pub4/abstract.
Wijk L, Franzen K, Ljungqvist O, Nilsson K. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet Gynecol Scand. 2014;93(8):749–56.
Gemmill E, Humes D, Catton J. Systematic review of enhanced recovery after gastro-oesophageal cancer surgery. Ann R Coll Surg Engl. 2015;97(3):173–9.
Beamish AJ, Chan DSY, Blake PA, Karran A, Lewis WG. Systematic review and meta-analysis of enhanced recovery programmes in gastric cancer surgery. Int J Surg. 2015;19:46–54.
Simpson JC, Moonesinghe SR, Grocott MPW, Kuper M, McMeeking A, Oliver CM, et al. Enhanced recovery from surgery in the UK: an audit of the enhanced recovery partnership programme 2009–2012. Br J Anaesth. 2015;115(4):560–8.
Mythen MG, Swart M, Acheson N, Crawford R, Jones K, Kuper M, et al. Perioperative fluid management: consensus statement from the enhanced recovery partnership. Periop Med. 2012;1:2.
Watt DG, McSorley ST, Horgan PG, McMillan DC. Enhanced recovery after surgery: which components, if any, impact on the systemic inflammatory response following colorectal surgery? Medicine (Baltimore). 2015;94(36), e1286.
Minto G, Mythen MG. Perioperative fluid management: science, art or random chaos? Br J Anaesth. 2015;114(5):717–21.
Lilot M, Ehrenfeld JM, Lee C, Harrington B, Cannesson M, Rinehart J. Variability in practice and factors predictive of total crystalloid administration during abdominal surgery: retrospective two-centre analysis. Br J Anaesth. 2015;114(5):767–76.
Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens. Ann Surg. 2003;238(5):641–8.
Miller TE, Roche AM, Mythen M. Fluid management and goal-directed therapy as an adjunct to enhanced recovery after surgery (ERAS). Can J Anesth Can Anesth. 2014;62(2):158–68.
Edwards MR, Mythen MG. Fluid therapy in critical illness. Extrem Physiol Med. 2014;3:16.
Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet. 2002;359(9320):1812–8.
Miller TE, Raghunathan K, Gan TJ. State-of-the-art fluid management in the operating room. Best Pract Res Clin Anaesthesiol. 2014;28(3):261–73.
Jacob M, Chappell D, Rehm M. The “third space”–– fact or fiction? Best Pract Res Clin Anaesthesiol. 2009;23(2):145–57.
American Society for Enhanced Recovery (ASER). http://aserhq.org/Accessed. 19 Apr 2016.
Brady MC, Kinn S, Ness V, O’Rourke K, Randhawa N, Stuart P. Preoperative fasting for preventing perioperative complications in children. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2009 [cited 2015 Oct 17]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005285.pub2/abstract.
Brady MC, Kinn S, Stuart P, Ness V. Preoperative fasting for adults to prevent perioperative complications. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2003 [cited 2015 Oct 17]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004423/abstract.
Smith I, Kranke P, Murat I, Smith A, O’Sullivan G, Søreide E, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28(8):556–69.
Holte K, Nielsen KG, Madsen JL, Kehlet H. Physiologic effects of bowel preparation. Dis Colon Rectum. 2004;47(8):1397–402.
Jung B, Lannerstad O, Påhlman L, Arodell M, Unosson M, Nilsson E. Preoperative mechanical preparation of the colon: the patient’s experience. BMC Surg. 2007;7:5.
Jung B, Påhlman L, Nyström P-O, Nilsson E. Mechanical Bowel Preparation Study Group. Multicentre randomized clinical trial of mechanical bowel preparation in elective colonic resection. Br J Surg. 2007;94(6):689–95.
Mahajna A, Krausz M, Rosin D, Shabtai M, Hershko D, Ayalon A, et al. Bowel preparation is associated with spillage of bowel contents in colorectal surgery. Dis Colon Rectum. 2005;48(8):1626–31.
Collin Å, Jung B, Nilsson E, Påhlman L, Folkesson J. Impact of mechanical bowel preparation on survival after colonic cancer resection. Br J Surg. 2014;101(12):1594–600.
Hausel J, Nygren J, Lagerkranser M, Hellström PM, Hammarqvist F, Almström C, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344–50.
Svanfeldt M, Thorell A, Hausel J, Soop M, Nygren J, Ljungqvist O. Effect of “preoperative” oral carbohydrate treatment on insulin action––a randomised cross-over unblinded study in healthy subjects. Clin Nutr (Edinb, Scotl). 2005;24(5):815–21.
Lobo DN, Hendry PO, Rodrigues G, Marciani L, Totman JJ, Wright JW, et al. Gastric emptying of three liquid oral preoperative metabolic preconditioning regimens measured by magnetic resonance imaging in healthy adult volunteers: a randomised double-blind, crossover study. Clin Nutr (Edinb, Scotl). 2009;28(6):636–41.
Lamke LO, Nilsson GE, Reithner HL. Water loss by evaporation from the abdominal cavity during surgery. Acta Chir Scand. 1977;143(5):279–84.
Navarro LHC, Bloomstone JA, Auler JOC, Cannesson M, Rocca GD, Gan TJ, et al. Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioper Med [Internet]. 2015 Apr 10 [cited 2015 Oct 17];4. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4403901/.
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162(1):134–8.
Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth. 2014;112(4):617–20.
Pinsky MR. Hemodynamic evaluation and monitoring in the ICU. Chest. 2007;132(6):2020–9.
Lewis SJ, Egger M, Sylvester PA, Thomas S. Early enteral feeding versus “nil by mouth” after gastrointestinal surgery: systematic review and meta-analysis of controlled trials. BMJ. 2001;323(7316):773–6.
Yeager MP, Spence BC. Perioperative fluid management: current consensus and controversies. Semin Dial. 2006;19(6):472–9.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26 Suppl 1:S64–74.
Hamilton-Davies C, Mythen MG, Salmon JB, Jacobson D, Shukla A, Webb AR. Comparison of commonly used clinical indicators of hypovolaemia with gastrointestinal tonometry. Intensive Care Med. 1997;23(3):276–81.
Kheterpal S, Tremper KK, Englesbe MJ, O’Reilly M, Shanks AM, Fetterman DM, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107(6):892–902.
Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness?: a systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–8.
Michard F, Teboul J-L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–8.
Levitov A, Marik PE. Echocardiographic assessment of preload responsiveness in critically ill patients. Cardiol Res Pract [Internet]. 2012 [cited 2015 Oct 25];2012. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3171766/.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642–7.
Cannesson M, Besnard C, Durand PG, Bohé J, Jacques D. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care. 2005;9(5):R562–8.
Natalini G, Rosano A, Taranto M, Faggian B, Vittorielli E, Bernardini A. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg. 2006;103(6):1478–84.
National Institute for Health and Care Excellence. CardioQ-ODM (oesophageal Doppler monitor) [MTG3] [Internet]. London: National Institute for Health and Care Excellence; 2011 [cited 2013 Nov 24]. Available from: http://www.nice.org.uk/.
Michard F, Chemla D, Teboul J-L. Applicability of pulse pressure variation: how many shades of grey? Crit Care [Internet]. 2015 [cited 2015 Nov 8];19(1). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4372274/.
Cannesson M, Kain ZN. The role of perioperative goal-directed therapy in the era of enhanced recovery after surgery and perioperative surgical home. J Cardiothorac Vasc Anesth. 2014;28(6):1633–4.
Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(9):447–51.
Ganz W, Tamura K, Marcus HS, Donoso R, Yoshida S, Swan HJ. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971;44(2):181–95.
Bland R, Shoemaker WC, Shabot MM. Physiologic monitoring goals for the critically ill patient. Surg Gynecol Obstet. 1978;147(6):833–41.
Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176–86.
Marik PE. Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care. 2013;3:38.
Srinivasa S, Kahokehr A, Soop M, Taylor M, Hill AG. Goal-directed fluid therapy––a survey of anaesthetists in the UK, USA, Australia and New Zealand. BMC Anesthesiol. 2013;13:5.
Wiles MD, Whiteley WJ, Moran CG, Moppett IK. The use of LiDCO based fluid management in patients undergoing hip fracture surgery under spinal anaesthesia: Neck of femur optimisation therapy––targeted stroke volume (NOTTS): study protocol for a randomized controlled trial. Trials. 2011;12:213.
Grocott MPW, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev. 2012;11, CD004082.
Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109(2):191–9.
Srinivasa S, Taylor MHG, Singh PP, Yu T-C, Soop M, Hill AG. Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg. 2013;100(1):66–74.
Phan TD, D’Souza B, Rattray MJ, Johnston MJ, Cowie BS. A randomised controlled trial of fluid restriction compared to oesophageal Doppler-guided goal-directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesth Intensive Care. 2014;42(6):752–60.
Lai CW, Starkie T, Creanor S, Struthers RA, Portch D, Erasmus PD, et al. Randomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitness. Br J Anaesth. 2015;115(4):578–89.
Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93(9):1069–76.
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output–guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–90.
Pearse RM. The whole truth and nothing but the truth: the need for full reporting of randomised trials. Periop Med. 2015;4(1):7.
GIFTASUP [Internet]. [cited 2015 Oct 28]. Available from: http://www.bapen.org.uk/professionals/education-research-and-science/bapen-principles-of-good-nutritional-practice/giftasup?showall=1&limitstart=.
Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declère AD, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: The CRISTAL randomized trial. JAMA. 2013;310(17):1809–17.
Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367(20):1901–11.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367(2):124–34.
Van Der Linden P, James M, Mythen M, Weiskopf RB. Safety of modern starches used during surgery. Anesth Analg. 2013;116(1):35–48.
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(13):1243–51.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cumpstey, A.F., Grocott, M.P.W., Mythen, M.(.G. (2016). Fluid Management and Its Role in Enhanced Recovery. In: Farag, E., Kurz, A. (eds) Perioperative Fluid Management. Springer, Cham. https://doi.org/10.1007/978-3-319-39141-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-39141-0_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39139-7
Online ISBN: 978-3-319-39141-0
eBook Packages: MedicineMedicine (R0)