Abstract
In this chapter we focus our attention on the enigmatic structural and functional roles of the major, non-bilayer lipid monogalactosyl-diacylglycerol (MGDG) in the thylakoid membrane. We give an overview on the state of the art on the role of MGDG and non-bilayer lipid phases in the xanthophyll cycles in different organisms. We also discuss data on the roles of MGDG and other lipid molecules found in crystal structures of different photosynthetic protein complexes and in lipid-protein assemblies, as well as in the self-assembly of the multilamellar membrane system. Comparison and critical evaluation of different membrane models – that take into account and capitalize on the special properties of non-bilayer lipids and/or non-bilayer lipid phases, and thus to smaller or larger extents deviate from the ‘standard’ Singer-Nicolson model – will conclude this review. With this chapter the authors hope to further stimulate the discussion about, what we think, is perhaps the most exciting question of membrane biophysics: the why and wherefore of non-bilayer lipids and lipid phases in, or in association with, bilayer biological membranes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bilayer phase
- Inverted hexagonal phase
- Light-harvesting complex
- Membrane models
- Merocyanine-540
- Non-lamellar lipid phases
- 31P-NMR
- Photosystem
- Xanthophyll cycle
- Zeaxanthin
Introduction
In oxygenic photosynthetic organisms the light reactions of photosynthesis occur in the thylakoid membrane, flattened lipid vesicles, which are densely packed with pigment-protein complexes and other constituents of the photosynthetic machinery. They contain the two photosystems: PSII (photosystem II or water-plastoquinone oxidoreductase) – responsible for the production of molecular oxygen, and PSI (photosystem I or plastocyanin:ferredoxin oxidoreductase) – responsible for the reduction of NADP to NADPH2, which is then used as reducing power to assimilate carbon dioxide. The two photochemical reaction centers are fed by excitation energy via their associated core antenna complexes and the main, peripheral light-harvesting antenna complexes, LHCII and LHCI, for PSII and PSI, respectively. The membranes also embed the cytochrome b6f complex and some further constituents of the electron transport system. The operation of the vectorial electron transport, linked to proton transport, generate the energized state of the thylakoid membrane: an electrochemical potential gradient, which consists of a transmembrane ΔpH and an electric potential gradient (ΔΨ) – which are utilized for the synthesis of the energy carrier molecule ATP with the aid of ATP-synthase.
The build-up of the energized state of thylakoid membranes, and thus the ATP synthesis, requires the separation of the two aqueous phases, the lumenal and stromal sides, and requires, in particular, the impermeability of membranes to most water-soluble molecules and to ions. This is warranted by the organization of thylakoid membranes as bilayers (Williams 1998), a requirement in all energy-converting membranes.
Given this strong restriction of the bilayer state of the functional membrane, for the first glance (and perhaps also for second and third glances), it is difficult to understand that the major lipid species in thylakoid membranes is the non-bilayer lipid monogalatosyl-diacylglycerol (MGDG), which constitutes about half of the total lipid content of thylakoid membranes. This is because, non-lamella-forming or non-bilayer lipids are not capable to self-assemble into bilayers in aqueous media under physiologically relevant conditions; instead, they are assembled into different non-lamellar or non-bilayer lipid phases (see section “Phase behaviour of thylakoid membrane lipids”). Only about the other half of the thylakoid membrane lipids, digalactolsyl-diacylglycerol (DGDG), sulfoquinosyl-diacylglycerol (SQDG) and phosphatidylglycerol (PG) are bilayer lipids (see section “Thylakoid Lipids”).
The problem is not limited to thylakoid membranes. All energy converting membranes are (and evidently must be, for their functioning) organized as bilayers. On the other hand, their dominant lipid species are non-bilayer lipids. This is most remarkable because the chemical composition of the major constituent lipids and proteins are quite different: mainly galactolipids and pigment-protein complexes in the thylakoid membranes; while e.g. the inner mitochondrial membranes are composed mainly of phosopholipids, phosphatidylcholine (PC) and phosphatidylethanolamine (PE), and cardiolipin (CL), and respiratory proteins. Only in small part they both contain essentially identical or similar protein complexes, the ATP-synthase and the cytochrome b6f/bc complex. Further, albeit at lower concentrations, apparently all biological membranes contain non-bilayer lipid species (Epand 1998). Hence, while probably all non-bilayer lipids participate in essential lipid-protein interactions, the general answer to their roles in lipid bilayers of biological membranes must be related to their non-lamella-forming property rather than in their chemical composition. Also, while the significance of specific lipid-protein interactions, e.g. between MGDG and the key protein complexes of the thylakoid membrane should not be ignored (cf. MGDG bound to protein complexes.), explanation must be offered for the behaviour of bulk lipids, about 60 % of the total thylakoid lipids, which are found in a fluid-like phase at room temperature (Páli et al. 2003 and references therein), i.e. which constitute the bulk bilayer membrane structure.
Because of the presence of non-bilayer lipids in the bulk phase and the absence of lateral heterogeneity on mesoscopic scale (Van Eerden et al. 2015), the bulk phase lipid mixture acquires a strong non-bilayer propensity (see also Garab et al. 2000). Several membrane models have been proposed in the past years to answer this enigmatic question, i.e. on the roles of non-bilayer lipids in the bilayer membranes, and on the possible physiological roles of non-bilayer lipid phases in, or in association with, the thylakoid membranes, and biomembranes in general. In the present chapter, while we will also outline recent experimental data on the role of MGDG and non-bilayer lipid phases in the xanthophyll cycle, as well as on bound MGDG and other lipid molecules found in different crystal structures, our attention will be focused on the above basic question. For many details, on the composition of thylakoid membranes, the phase behaviour of different isolated lipids and lipid mixtures, the physical mechanisms and physiological significances of phase transitions etc., the readers are referred to excellent reviews in the literature both on thylakoid membranes and on broader subjects, on non-bilayer lipids and lipid phases (Epand 1998; Sackman 1995; Seddon and Templer 1995; Selstam 1998; Williams 1998; Van den Brink-van der Laan et al. 2004; Vigh et al. 2005; Quinn 2012; Koynova and Tenchov 2013; Jouhet 2013; Boudière et al. 2014).
Thylakoid Lipids
Thylakoid membranes contain glycolipids, MGDG, DGDG and SQDG, and only one phospholipid, PG. The neutral galactolipids, DGDG and mainly MGDG, are the major lipid components and only smaller amounts of negatively charged lipids SQDG and PG contribute to the thylakoid membranes. This membrane composition is different from other types of eukaryotic and bacterial membranes, which are built up mainly from phospholipids and may also contain sphingolipids and sterols. Thus, the lipid composition of thylakoid membranes is quite unique; nevertheless, as pointed out in the Introduction, with their high non-bilayer lipid content they resemble the energy-converting mitochondrial and retinal membranes. As most biological membranes, thylakoids also consist of lipid bilayers and membrane-intrinsic proteins and protein complexes and some other compounds.
The galactolipid MGDG is the main thylakoid membrane lipid of higher plants and green algae, which accounts for about 40–55 % of the total membrane lipid content (Murata and Siegenthaler 1998; Goss and Wilhelm 2009; Lepetit et al. 2012). The second most abundant lipid is the other galactolipid, DGDG, which is normally found in a concentration of around 25–30 %. The negatively charged lipids, SQDG and PG, contribute with about 10–15 % to the total lipid content of the thylakoid membrane. This typical lipid composition results in a ratio of neutral to negatively charged lipids of around 4 (Goss et al. 2009; Lepetit et al. 2012). This composition also means that thylakoids, to a significant degree, are built up from the non-bilayer lipid MGDG. This lipid has been proposed to be ‘forced’ into the bilayer by proteins – as shown by the self-assembly of LHCII:MGDG membranes (Simidjiev et al. 2000). This, i.e. the formation of a bilayer membrane, composed of thylakoid lipid mixture and photosynthetic proteins, however, also depends on the nature of proteins. As it has been thoroughly documented, essentially the same thylakoid lipids, and the proteins that are involved in the phototransformation of protochlorophylls assemble into cubic, rather than lamellar phases (Selstam 1998).
In the absence of proteins, the thylakoid lipid mixture, under physiologically relevant conditions is not capable of forming lamellar phases; instead, it assumes different non-bilayer structures (Williams 1998). The non-bilayer propensity of MGDG-containing lipid mixtures strongly depends on the proportion of MGDG in the lipid mixture. For instance, in the binary mixture of MGDG and DMPC (dimyristoylphosphatidylcholine) it was found that lipid bilayers are formed only when the mole fraction of MGDG is ≤0.6 (Castro et al. 2007). The fatty acid composition of MGDG also strongly influences the non-bilayer propensity of lipid mixtures; in particular, polyunsaturated lipids facilitate the transition from bilayer to non-bilayer lipid structures (Van Eerden et al. 2015 and references therein).
The negatively charged PG is the only phospholipid of thylakoid membranes; although represented in relatively small amount, it plays a crucial role in the structure formation and function of photosynthetic complexes (Sato et al. 2000; Wada and Murata 2007; Domonkos et al. 2008; Sozer et al. 2011). It appears that specific PG species are needed for the proper performance of the cyanobacterial cellular processes since the PG deficient Synechocystis cells retailored the supplied artificial dioleoyl PG molecules, and created more natural molecular species (Laczko-Dobos et al. 2010).
SQDG , the other negatively charged lipid of the thylakoids in some cases could substitute for PG – apparently replacing its negative charge (Yu and Benning 2003; Benning et al. 1993; Güler et al. 1996). SQDG could contribute to the activity of PSII by associating with PSII and LHCII complexes (Sato et al. 1995; Minoda et al. 2002). It also had an impact on the assembly and the function of photosynthetic complexes (Kansy et al. 2014; Mizusawa and Wada 2012).
The galactolipids, DGDG and MGDG, are the dominant thylakoid lipids and they appear to be very important for functional photosynthesis. DGDG plays an important role in the stability and activity of PSII and PSI and also in the stabilization of LHCII trimers (Dörmann and Hölzl, Chap. 3). Changes in the ratio of MGDG per DGDG affected the membrane organization and the protein folding and insertion, which exerted an impact on the photosynthetic performance (Dörmann and Benning 2002; Kobayashi et al. 2013). Proteoliposome experiments have revealed that MGDG enhances the assembly and energetic coupling of LHCII to the core of PSII (Zhou et al. 2009). As suggested by the experiments of Wang et al. (2014), using MGDG synthase mutants, the two galactolipids play an important structural role in salt-stress response of vascular plants. MGDG deficiency also affected the photoprotection capability of plants, evidently via hindering the functioning of the xanthophyll-cycle (Aronsson et al. 2008) (cf. “Roles of MGDG in xanthophyll cycles”). Changes in the relative amounts of non-bilayer lipids are usually observed in plants in response to variations of the environmental conditions (Harwood 1998). Such alterations are of special physiological importance in photosynthetic organisms, which encounter a wide range of temperatures and other environmental stresses, such as drought and high light.
The lipid composition of the thylakoid membranes of diatoms is different from that of higher plants and green algae, and MGDG may not be the main membrane lipid (Vieler et al. 2007; Goss and Wilhelm 2009; Goss et al. 2009; Lepetit et al. 2012). Diatom thylakoid membranes are strongly enriched in the negatively charged lipids, SQDG and PG, and high light illumination during the cultivation of the algal cells further increased the amount of the negatively charged lipids (Lepetit et al. 2012). Thus, the most abundant lipid in diatom thylakoids is the anionic lipid SQDG, which is found in equal or even higher concentrations than MGDG. The high amount of SQDG and PG in the diatom thylakoid membranes results in a ratio of neutral to negatively charged lipids of around 1 (Goss et al. 2009; Lepetit et al. 2012), whereas this ratio has a value between 3 and 4 in higher plants and green algae due to the high MGDG concentration. It has been proposed that the high amount of SQDG and PG in the diatom thylakoids has significant consequences for the structure of the diatom thylakoid membrane and for the function of the diatom xanthophyll cycle (see section “Roles of MGDG in xanthophyll cycles”). A recent model for the distribution of the membrane lipids and photosynthetic pigment-protein complexes (Lepetit et al. 2012) predicts an enrichment of MGDG in the inner membranes of the stacks of three thylakoid membranes that are typical for the diatom chloroplasts (for further details see section “Localization and operation of xanthophyll cycles in the native thylakoid membrane”).
Phase Behaviour of Thylakoid Membrane Lipids
The large amounts of the non-bilayer lipid MGDG in the thylakoid membranes lends its lipid mixture a high propensity to form non-lamellar (non-bilayer) phases. This propensity, as it has been thoroughly documented in the literature (for reviews see e.g. Epand 1998; Williams 1998) arises from the conical shape of the non-bilayer lipid molecules, as opposed to the cylindrical shapes of the lamella-forming lipids. For thylakoid membrane lipids, this is illustrated in Fig. 6.1, which shows the preferred, inverted hexagonal (HII) phase of MGDG in aqueous media, while DGDG, PG and SQDG spontaneously form lamellar phases.
(a) Structure formation of thylakoid lipid molecules. Small polar-headgroup containing lipid molecules possess a conical shape and thus form inverted hexagonal (HII) phases or cubic (bicontinuous) structures. Lipids that have similar headgroup and tail size look like cylinders and form lamellar phases, with no curvatures. (b) Simplified model of thylakoid membrane (a) containing a bilayer and membrane-embedded proteins, which via (b) local, transient non-bilayer structures extrudes lipids which, in turn, assemble into (c) cubic or (d) HII phases (From Garab et al. (2000) with permission)
Depending on their composition and the physico-chemical and external environmental conditions (e.g. ionic strength, pH, hydration state, and ambient temperature and drought stress, respectively), thylakoid membrane lipids in vitro and in vivo can form different lamellar and non-lamellar phases. For the phase behavior and transitions of different thylakoid lipids the reader is referred to the reviews by Williams (1998) and Selstam (1998); and for membrane lipids and phase transitions, in general, to the reviews of Sackman (1995), Seddon and Templer (1995), and Koynova and Tenchov (2013). These latter authors display the structure of six different lamellar phases, five micellar structures and six non-lamellar liquid-crystalline phases with various topologies, including the inverted hexagonal (HII) phase and some cubic phases of different space group symmetry, and show how these different phases are capable to transform into each other – a key feature of lipid membranes.
As concerns the detection of these different phases the most straightforward and most commonly used techniques are X-ray diffraction (or SAXS/WAXS small-angle/wide-angle X-ray scattering) (Tyler et al. 2015; Pan et al. 2015) and neutron diffraction (also measured as SANS, small-angle neutron scattering) (Deme et al. 2014). They detect the periodic structures and measure all lattice constants. Hence, these techniques can be used primarily on lipid aggregates, rather than on biological membranes, where diffractions may be smeared or the lattice-structures perturbed. Freeze fracture electron microscopy, which is often used in combination with SAXS (e.g. Csiszár et al. 2003), can more readily be used to detect e.g. HII phases even in thylakoid membrane preparations (Kirchhoff et al. 2007 and references therein). This, i.e. the detectability on biomembranes, is an important issue because, as already pointed out above, the phase behaviour of thylakoid membranes (and of biological membranes, in general) is largely influenced by their protein contents. The best example is the cubic phase of the prolamellar body of etiolated plastids, as opposed to the lamellar phase of thylakoids in mature chloroplasts – despite the virtually identical lipid composition in the two lipid phases (Selstam 1998).
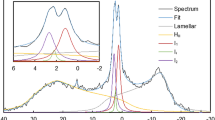
Another powerful and commonly used technique, which can be applied both for model and native systems, is 31P-NMR (Fig. 6.2) (Gruner 1985; Castro et al. 2007). The signature of 31P-NMR is based on the sensitivity of this measurement on the degree of diffusional freedom of the phospholipid molecule in the lipid phase. There is a ‘weakness’ of this technique, namely, that it can be used for phospholipids only – and PG is only a minor lipid in thylakoid membranes (see section “Thylakoid Lipids”). Nevertheless, as shown first by Haranczyk et al. (1995) it can be used for thylakoid membrane preparations. As demonstrated recently on model membranes composed of glycolipids and phospholipids, it readily detects the co-existence of lipid phases, a feature of special interest, which will be discussed in section “Membrane models using the properties of non-bilayer lipids” in conjunction with 31P-NMR data of Krumova et al. (2008a) obtained on intact thylakoid membranes.
31P-NMR signatures of different lipid phases (Based on NMR data of Cullis and de Kruijff (1979) with permission)
Non-bilayer Lipids and Lipid Phases: Facts and Hypotheses
The most widely established role of MGDG in the thylakoid membranes is in the operation of the xanthophyll cycle (XC) . This will be discussed in depth in section “Roles of MGDG in xanthophyll cycles” – focusing on molecular mechanisms and some open questions. Although the role of MGDG and the involvement of a non-bilayer lipid phase have been firmly established in this case, it evidently does not answer the why and wherefore of non-bilayer lipids in energy converting membranes; not even in thylakoid membranes, in general, since MGDG is present in cyanobacteria, the ancestors of chloroplast, but these prokaryotic thylakoids do not operate a XC.
With regard to other possible roles of MGDG, as the non-bilayer lipid of thylakoid membranes, and the phase behaviour of thylakoid lipids in vivo, the main boundary condition is that the thylakoid membranes (again, similar to all energy converting membranes) are densely packed with proteins, which constitute ∼80 % of the dry mass and likewise the lipid content is as low as ~20 % (Williams 1998). Accordingly, thylakoid membranes contain large protein domains, ordered arrays of protein complexes and supercomplexes separated by relatively narrow lipid ‘channels’ (Dekker and Boekema 2005; Kouril et al. 2013; Garab 2014; Tietz et al. 2015).
The high protein content of thylakoid membranes and the presence of extended ordered protein arrays in the stacked region (grana) determine the overall appearance of thylakoid membranes of higher plants and green algae. In particular, they self-assemble into stacks of flat membrane vesicles. Although it is generally agreed that non-bilayer lipids, because of their conical shape, tend to induce curvatures (Gruner 1985), this does not take effect on the overall shape of thylakoids – evidently the protein arrays span the membrane sheet (cf. Garab 2014). Further, recent data show that the extreme curvatures at the margins depend on the CURVATURE THYLAKOID1 (CURT1 ) protein family (Armbruster et al. 2013), rather than on the non-bilayer lipid MGDG.
As indicated by EPR measurements , somewhat more than half of the total thylakoid lipid content are found in a fluid-like phase, i.e. not bound to proteins and/or not found in their inner shell layer (cf. Páli et al. 2003; Lee 2003 and references therein). Conversely, a large fraction of the lipids are bound tightly or loosely to proteins. Indeed, as revealed by crystal structures, the membrane-intrinsic proteins bind a number of lipids – while it must also be realized that often only a fraction of them is resolved in the X-ray structure. These lipid-protein interactions are of interest from the point of view of protein functions – examples will be shown in section “MGDG bound to protein complexes”. More often, however, bound lipids do not appear to have a well discernible, specific role related to the structure and function of proteins – the majority of cases in section “MGDG bound to protein complexes”. In fact, as pointed out by Lee (2003), because the transmembrane domains of membrane-embedded proteins contain protrusions, fatty acyl chains of lipids fill the grooves. By this means, the permeability barrier of membranes can be maintained, which is of paramount importance with regard to the energization of thylakoid membranes.
Another fraction of the bound lipids are found in the immediate molecular environment of protein complexes but are dynamically exchanging with the bulk lipids. These lipids surrounding the membrane-intrinsic proteins, non-bilayer lipids like MGDG, in particular, might exert strong effects on the structure and function of proteins. Different models have been proposed which are ‘using’ the special features of MGDG and non-bilayer lipids in the bilayer membranes and complex membrane systems (see section “Membrane models using the properties of non-bilayer lipids”).
The loose association of non-bilayer lipids with proteins might play important roles. Zick et al. (2014) have demonstrated that the membrane fusions of yeast vacuoles, which had been known to be mediated by SNARE proteins, require the presence of three types of non-bilayer phospholipid molecules. This finding is perfectly in line with earlier data and models suggesting the crucial role of non-bilayer lipids in membrane fusion (Seddon and Templer 1995; Lohner 1996; Epand 1998), which is proposed to occur via the formation of local and temporal non-bilayer structures (Pomorski et al. 2014 and references therein).
As follows from previous arguments, the thylakoid membranes also contain areas occupied by ‘free’ lipids – which, in turn, can be directly exposed to water, sugars etc., factors that influence their phase behavior. In section “Membrane models using the properties of non-bilayer lipids”, a membrane model will be outlined, which is based on the experimentally proven co-existence of bilayer and non-bilayer lipid phases and their ‘communication’.
MGDG Bound to Protein Complexes
Importance of MGDG in LHCII Structures
The crystal structure of LHCII shows the existence of one PG and one DGDG molecule per LHCII monomer (Liu et al. 2004). The main thylakoid membrane lipid MGDG is not observed as intrinsic LHCII lipid in the crystal structures. However, LHCII preparations obtained by solubilisation of the thylakoid membrane, followed by protein separation, contain significant amounts of this galactolipid (Schaller et al. 2010). The close association between MGDG and LHCII also appears to be important in the higher order, multilamellar, onion-like structures adopted by LHCII, somewhat similar to those in the grana (Simidjiev et al. 1998). Addition of DGDG to the isolated LHCII induced the formation of stacked unilamellar vesicles, while in the presence of externally added PG and SQDG lamellar sheets were observed (Simidjiev et al. 1998), suggesting specific roles of each of the lipid species (cf. also Zhou et al. 2009). The structural flexibility of lamellar aggregates of LHCII enriched with MGDG exhibited the most prominent structural flexibility, which manifested in intense light-induced reversible changes in their macrodomain-associated CD signals (Simidjiev et al. 1998). MGDG and DGDG have also been shown to play crucial roles in the structurally flexible multibilayer LHCII-containing macro-assemblies (Janik et al. 2013).
Recent measurements have investigated the influence of purified lipids on the isolated, lipid-depleted and highly aggregated LHCII (Schaller et al. 2011). Addition of MGDG (and DGDG) induced a modification of the disorganized structures of the lipid-depleted LHCII and supported the aggregated state of the complex. In contrast, the negatively charged lipids SQDG and PG exerted a strong disaggregating effect on the isolated LHCII. LHCII disaggregation was suppressed under a high proton concentration and in the presence of cations (Mg2+). This suggested that the negative charge of the anionic lipids in conjunction with negatively charged domains of the LHCII proteins was responsible for the disaggregation. Additional measurements by photon correlation spectroscopy, which were used to gain information about the size of the LHCII aggregates, showed that in the presence of MGDG (and DGDG) an increased number of LHCII aggregates with large particle sizes in the μm-range were formed. This observation is in line with results that reported the establishment of LHCII macrodomains upon the interaction with MGDG (Simidjiev et al. 2000, see above). The incubation with the negatively charged lipids, on the other hand, led to much smaller LHCII particles (around 40 nm in the case of PG) with a homogeneous distribution (Schaller et al. 2011).
Association of MGDG with the Light-Harvesting Complexes of Diatoms
The peripheral antenna system of the diatom PSII core complex is composed of the so-called fucoxanthin chlorophyll protein (FCP) complexes (for recent review, see Büchel 2015). Depending on the detergent used for the preparation of isolated FCP complexes, i.e. dodecylmaltoside or Triton X-100, these antenna systems can be isolated with a shield of MGDG molecules (Lepetit et al. 2010, see also Dörmann and Hölzl Chap. 2) or in a form that contains high amounts of surrounding SQDG and PG molecules (Schaller et al. 2014). For the latter FCP preparation from the diatom P. tricornutum it was shown that it was less sensitive to Mg2+ and low pH than LHCII, which was characterized by lower amounts of SQDG and a higher concentration of MGDG. High MgCl2 concentrations and pH-values below pH 6 induced significant changes in the spectroscopic features of LHCII, and were accompanied by a strong aggregation, which was also visible as a pellet after centrifugation on a sucrose cushion. The FCP enriched in SQDG and PG responded with less pronounced changes in the absorption and fluorescence spectra to low pH and Mg2+ incubation, and did not show a visible pellet after incubation with either low pH or high Mg2+ concentrations. Only the combined action of Mg2+ and pH 5 led to FCP aggregates of a size that could be pelleted by centrifugation. The decreased sensitivity of the SQDG- and PG-containing FCP to aggregation at high Mg2+ and low pH is in line with other data that show that neutral galactolipids, and especially MGDG, are able to stabilize or induce the formation of large, ordered aggregates of light-harvesting complexes (Simidjiev et al. 1998, 2000). Another study on the lipid dependence of FCP aggregation employed artificial lipid membranes, i.e. liposomes, to incorporate isolated FCP complexes of the diatom C. meneghiniana (Gundermann and Büchel 2012). The lipid composition of the liposomes was comparable to the lipid composition of the diatom thylakoid membrane, which was shown to contain significantly higher concentrations of the negatively charged lipids SQDG and PG and a reduced amount of the neutral galactolipids MGDG and DGDG (Goss et al. 2009; Lepetit et al. 2012, see Wada Chap. 2). In this study (Gundermann and Büchel 2012) it was shown that high lipid concentrations in general reduced the ability of FCP complexes to form aggregates. Reduction of the lipid per protein ratio led to an aggregation of FCP complexes and a quenching of Chl a fluorescence. Additionally, FCP complexes at low lipid concentrations were sensitive to low pH.
Roles of MGDG in the Structure and Function of PSII
As revealed by the crystal structures of the PSII core complex of the cyanobacterium T. elongatus (Guskov et al. 2009; Kern and Guskov 2011) a PSII monomer binds 11 molecules of MGDG. In addition, seven DGDG, five SQDG and two PG are found within the PSII core complex. In contrast to the negatively charged lipids SQDG and PG, whose headgroups are only oriented towards the lumenal side, the headgroups of the MGDG molecules are oriented towards both the cytoplasmic and lumenal side of the thylakoid membrane (Guskov et al. 2009). Umena and co-workers identified six MGDG, five DGDG, four SQDG and five PG molecules in the crystal structure of oxygen-evolving PSII at a resolution of 1.9 Å of T. vulcanus (Umena et al. 2011). The MGDG and DGDG molecules, except one MGDG molecule are located on the lumenal side, while the headgroups of SQDG and PG molecules were located at the cytoplasmic surface of the thylakoid membrane. Together with SQDG, MGDG shows an enrichment at the monomer-monomer interface of the PSII dimer, where 14 lipids are found (Guskov et al. 2009; Kern and Guskov 2011). Six of these 14 lipids are arranged in such a way that they form contact with protein subunits from both monomers, six other lipids mediate interactions between a protein subunit of one monomer and a lipid of the other monomer. The lipids mediating the dimerization of PSII core complexes show an enrichment of MGDG and 8 of the 14 lipids located at the interface are MGDG molecules. The enrichment of MGDG at the monomer-monomer interface points to an important role of MGDG in PSII dimerization. This was recently supported by experiments where isolated monomeric PSII core complexes were incubated with a surplus of purified MGDG (Kansy et al. 2014). Size exclusion chromatography showed that after the incubation with MGDG the complete fraction of PSII monomers had been transformed into dimeric PSII core complexes. The ability of MGDG to induce dimerization of PSII monomers was in sharp contrast to the effect of the negatively charged lipids SQDG and PG. Incubation with the anionic lipids led to further destabilization of the monomeric PSII by the dissociation of the inner antenna protein CP43 and part of the D1 protein of the reaction center. A second important cluster of lipids within the PSII structure is found at the PSII acceptor side and is composed of eight lipids (Loll et al. 2007; Guskov et al. 2009; Kern and Guskov 2011). These lipids are located close to the QB binding site and form a lipid bilayer within the PSII core complex. The cluster of lipids is thought to act as a transfer pathway, which targets QB molecules to their PSII binding site at the D1 protein. Alternative mechanisms for the entry and exit of QB molecules have been proposed (Kern and Guskov 2011). In addition, the lipids also serve as a storage pool for additional QB (plastoquinone) molecules. Interestingly, the area around QA, which is tightly bound to the D2 protein, is dominated by MGDG whereas the QB region is enriched in the negatively charged lipids SQDG and PG (Kern and Guskov 2011). It has been proposed that different lipids within the vicinity of the two quinone sites modulate the properties of QA and QB in different ways without the need to change the protein environment. With respect to lipid-protein interactions at the donor side of PSII it seems that MGDG does not play a crucial role. Three DGDG molecules are located in the area of OEC (Guskov et al. 2009) and it has been shown that depletion of DGDG has a negative influence on the functionality of water splitting (Reifarth et al. 1997; Steffen et al. 2005). MGDG is most probably also not involved in the interaction between the inner antenna proteins CP43 and CP47 with the PSII reaction center. In this case, PG seems to play a major role in this interaction via two PG molecules located at the interface between CP43 and the D1/D2 heterodimer (Guskov et al. 2009; Kern and Guskov 2011). Depletion of PG in mutant strains of Synechocystis sp. PCC6803 led to the dissociation of CP43 from the PSII core complex (Laczko-Dobos et al. 2008).
With regard to the influence of lipids on the functionality of PSII a recent study (Kansy et al. 2014) has shown that neutral galactolipids have a tendency to stabilize the PSII electron transport. MGDG and DGDG molecules are involved in the maintenance of the flexibility and assembly of PSII even under stress conditions (Loll et al. 2005; Mizusawa and Wada 2012; Wang et al. (2014). It should be also noted that lipase treatment of isolated PSII leads to the degradation of only half of the bound MGDG molecules (Leng et al. 2008), which might indicate that the other half are more strongly bound to the protein subunits and therefore protected from the lipase. Conversely, about half of the MGDG molecules are found in the bulk lipid phase. The lipase treatment did not induce significant changes in the function of PSII, although, the strongly bound MGDG molecules may play more important role in the structure and function of PSII, as it was suggested by Mizusawa and Wada (2012). This finding is in good agreement with reports showing that phase separation of lipids with non-bilayer propensity from the thylakoid membrane, upon the addition of cosolutes, does not inhibit PSII but increases its thermal stability (Williams et al. 1992).
MGDG Bound to Cytochrome b6f
Kurisu and co-workers identified two phosphatidylcholine molecules in the cytochrome b6f monomers of Mastogocladus laminosus (Kurisu et al. 2003), while Stroebel and co-workers found one SQDG and two other lipid, most probably MGDG, molecules at the lumenal side in the crystalized cytochrome b6f of Chlamydomonas reinhardtii (Stroebel et al. 2003). Investigations using MGDG Langmuir monolayer and isolated cytochrome b6f indicated specific interaction between MGDG and cytochrome b6f that could be attributed to hydrogen bonding between the galactose headgroup of MGDG and the protein (Georgiev et al. 2012). Characterization of transgenic M18 tobacco plants containing reduced MGDG content by 53 % indicated that MGDG deficiency reduces the amount of cytochrome b6f in the mutant plants, which may be due to unstable integration of the complex into the membrane (Wu et al. 2013). The lowered MGDG content also could be the reason of the inefficient intersystem electron transport in the mutant plants. In vitro experiments also indicated that MGDG efficiently stimulates the electron transfer activity of cytochrome b6f (Yan et al. 2000).
Roles of MGDG in the Structure and Function of PSI
One MGDG molecule and three PG molecules were identified in the PSI complex of T. elongatus by X-ray crystallography at 2.5 Å resolution (Jordan et al. 2001). The MGDG molecule and two of the PG molecules are bound to PsaA and PsaB (Jordan et al. 2001; Domonkos et al. 2008). One of the PG molecule and the MGDG located symmetrically to each other in the complex may have an important role in the photochemical reaction center of PSI (Mizusawa and Wada 2012). Investigation of the dgd1 mutant of Arabidopsis thaliana indicated that MGDG could have a role in the stability of the PSI complex (Guo et al. 2005; see also Krumova et al. 2010). Recently in the architecture of the PSI-LHCI supercomplex six PG, three MGDG and one DGDG molecules were assigned (Qin et al. 2015). Two of the MGDG molecules were identified as cofactors in the four Lhca subunits and the third MGDG was found in PsaG.
Roles of MGDG in Xanthophyll Cycles
Xanthophyll Cycles of Higher Plants and Algae
The xanthophyll cycles (XC) of higher plants and algae are important photoprotective mechanisms, which help to prevent an overexcitation of the photosynthetic apparatus during illumination with high light intensities (for recent reviews see Goss and Jakob 2010; Lavaud and Goss 2014; Goss and Lepetit 2015). The operation of the XCs is associated with the thylakoid membrane and strongly depends on the presence of MGDG (see sections “Role of MGDG in the solubilisation of xanthophyll cycle pigments”, “Importance of the MGDG-dependent non-bilayer phases for the activity of xanthophyll cycles”, and “Localization and operation of xanthophyll cycles in the native thylakoid membrane”). The conversion of special xanthophyll molecules in the XCs plays a key role in switching LHCII from a light-harvesting state into a state where the excessive excitation energy is dissipated as heat (reviewed by Horton and Ruban 2005; Jahns and Holzwarth 2012; Niyogi and Truong 2013; Domonkos et al. 2013). This is made possible by a structural change, i.e. an aggregation of the antenna complexes. The enhanced heat dissipation from the aggregated LHCII is visible as a strong quenching of the chlorophyll (Chl) a fluorescence (Krause and Jahns 2004), termed non-photochemical quenching of Chl a fluorescence (NPQ) (Demmig-Adams et al. Eds. 2014).
The main XCs that are presently known are the violaxanthin, antheraxanthin, zeaxanthin (VAZ) cycle of higher plants, green and brown algae, and the diadinoxathin, diatoxanthin (DD/Dt) cycle of diatoms, haptophytes and dinophytes (for recent review see Goss and Jakob 2010; Lavaud and Goss 2014; Goss and Lepetit 2015). Both cycles consist of a forward reaction that is taking place during illumination with high light intensities and a back reaction that is observed during low light or dark periods that follow the high light phase. In the VAZ cycle V, which contains two epoxy-groups, is de-epoxidized in two steps to the epoxy-free Z with A as intermediate (Yamamoto et al. 1962; Hager 1967a). The forward reaction of the DD/Dt cycle comprises only one de-epoxidation step from DD to Dt since DD contains only one epoxy-group (Hager and Stransky 1970; Stransky and Hager 1970). The back reaction of the cycle reintroduces the epoxy group into Z or Dt and converts these molecules back to V or DD, respectively (Hager 1967b; Hager and Stransky 1970; Stransky and Hager 1970). The forward reaction is triggered by the enzymes V or DD de-epoxidase (VDE, DDE). These enzymes are water-soluble and located in the thylakoid lumen in their inactive form (Hager and Holocher 1994; Arnoux et al. 2009; Saga et al. 2010). After their activation, which is induced by the decrease of the lumenal pH during photosynthetic electron transport and possibly includes a dimerization (Arnoux et al. 2009; Saga et al. 2010), the enzymes bind to the thylakoid membrane (see Chap. 2.2.4) and convert the respective epoxy-xanthophylls (Hager and Holocher 1994; Schaller et al. 2010). Both enzymes have a pH-optimum at around pH 5 (VDE, pH 5.2; DDE, pH 5.5) and use ascorbate as cosubstrate, i.e. as electron donor for the reduction of the epoxy-group (Hager 1969; Pfündel et al. 1994; Jakob et al. 2001). The back reaction of the cycles is catalysed by the Z or Dt epoxidase (ZEP, DEP). These enzymes are most probably peripheral membrane proteins located at the stromal side of the thylakoid membrane (Schaller et al. 2012). Recent results have indicated that DEP might contain an additional membrane spanning domain (Coesel et al. 2008). Both enzymes have a broad pH-optimum in the neutral to slightly basic pH-range and use O2 and NADPH+H+ as cosubstrates to reintroduce the epoxy-group (Hager 1975; Siefermann and Yamamoto 1975; Büch et al. 1995). The de-epoxidases as well as the epoxidases belong to the lipocalin family of proteins (Hieber et al. 2000; Grzyb et al. 2006), a protein family that mainly binds small hydrophobic substrates. The catalytic site is composed of eight β-strands which form a hydrophobic barrel-like structure. The binding of the hydrophobic xanthophylls takes place in the central cavity of the barrel and is realized by a penetration of the catalytic site into MGDG-enriched regions of the thylakoid membrane where the XC pigments are located (see sections “Role of MGDG in the solubilisation of xanthophyll cycle pigments”, “Importance of the MGDG-dependent non-bilayer phases for the activity of xanthophyll cycles”, and “Localization and operation of xanthophyll cycles in the native thylakoid membrane”).
Role of MGDG in the Solubilisation of Xanthophyll Cycle Pigments
In the thylakoid membrane a part of the XC pigments is bound to the antenna proteins of PSII and PSI whereas another part is localized in the lipid phase of the membrane. In higher plants and green algae the majority of the VAZ cycle pigments are located in LHCII and the minor Chl a/b binding proteins of the PSII antenna, CP29, CP26 and CP24 (Bassi et al. 1993; Ruban et al. 1994; Goss et al. 1997). In diatoms the DD/Dt cycle pigments are bound to the fucoxanthin chlorophyll proteins (FCP) that form the peripheral antenna complex for both PSII and PSI (Büchel 2003; Lavaud et al. 2003; Lepetit et al. 2007). Data of Lepetit et al. (2008) have indicated that special FCP proteins which are associated with PSI, i.e. form a PSI specific antenna, are enriched in DD/Dt cycle pigments. In addition to the XC pigments bound to the respective antenna proteins a part of the hydrophobic xanthophylls are freely located in the lipid phase of the membrane. The existence of free Z in the thylakoid membrane of higher plants was proposed to account for the anti-oxidative function of the de-epoxidized XC pigments against the harmful action of reactive oxygen species (ROS) (Havaux and Niyogi 1999). More recent results obtained with isolated LHCII of higher plants have shown that part of the total pool of VAZ cycle pigments is bound to the LHCII apoproteins whereas another part of the VAZ pool is localized in a lipid shield purified with the trimeric LHCII (Schaller et al. 2010). This lipid shield consisted mainly of MGDG molecules and it was proposed that in the native thylakoid membrane an MGDG phase is surrounding the antenna complexes and serves to solubilise the VAZ cycle pigments. In diatoms a comparable separation of DD/Dt cycle pigments between protein bound and lipid solubilised pigments has been detected (Lepetit et al. 2010). Especially in high-light cultivated diatom cells a large pool of DD/Dt cycle pigments was found. Since the additional high-light synthesized DD/Dt pigments were unable to participate in the process of NPQ (Schumann et al. 2007) it was hypothesized that these pigments are not bound to the FCP complexes. Later, comparing isolated FCP complexes from high-light and low-light cultures, it could be shown that the additional DD/Dt synthesized during high-light cultivation has the same spectroscopic features as DD/Dt dissolved in purified MGDG (Lepetit et al. 2010). This observation together with the finding that the isolated FCP complexes were enriched in MGDG led to the conclusion that especially in high-light cultivated diatoms a significant part of the total XC pigment pool is located in an MGDG phase surrounding the antenna complexes. This observation is in line with the finding that, similar to Z in higher plants, the lipid-dissolved Dt has an antioxidative function (Lepetit et al. 2010).
The localization of XC pigments in MGDG phases of the thylakoid membrane is in line with in vitro experiments with isolated XC pigments, isolated de-epoxidases and different membrane lipids. The first studies were conducted by Yamamoto and Higashi (1978), who found that MGDG is able to enhance the de-epoxidation of V to Z, and concluded that one of the functions of MGDG is the solubilisation of the XC pigments. More recent experiments have shown that non-bilayer lipids which form inverted hexagonal structures (HII phases) in aqueous media, i.e. MGDG and PE, are much better suited to solubilise the VAZ and DD/Dt cycle pigments than lipids which form bilayer structures, i.e. DGDG, SQDG or PC (Goss et al. 2005). In addition, it was demonstrated that the XC pigments are preferentially located in MGDG or PE phases in liposome systems composed of non-bilayer and bilayer lipids that served as a model for the native membrane (Goss et al. 2007). This, together with the data on the isolated LHCII and FCP complexes indicate that a large part of the free XC pigments is localized within MGDG phases in the native thylakoid membrane of higher plants and diatoms. Furthermore, the experiments (Goss et al. 2005, 2007) showed that solubilisation of V or DD is essential for the operation of the VAZ and DD/Dt cycles, respectively. Without solubilisation of the xanthophylls in MGDG the pigments form aggregates in an aqueous medium which cannot be converted by the enzymes VDE and DDE, respectively.
Importance of the MGDG-Dependent Non-bilayer Phases for the Activity of Xanthophyll Cycles
The higher solubilisation capacity of the non-bilayer lipids is one of the two main functions of these lipids with respect to xanthophyll cycling (see section “Role of MGDG in the solubilisation of xanthophyll cycle pigments”). The second main function is the provision of a non-bilayer phase , possibly inverted hexagonal phase, itself which has first been observed by Latowski et al. (2002, 2004). Later, it has been demonstrated that bilayer lipids are able to completely solubilise the XC pigments V and DD, albeit at a significantly higher lipid concentrations (Goss et al. 2005). However, despite the complete solubilisation of the pigments no or only a very minor conversion of V to Z and DD to Dt have been observed in lipid bilayer systems (Goss et al. 2005). The situation is comparable in liposome system consisting of bilayer and non-bilayer lipids, where despite the complete pigment solubilisation a certain percentage of non-bilayer lipids are needed before xanthophyll de-epoxidation can be observed (Goss et al. 2007). These results have implied that not only the efficient solubilisation of the xanthophyll but also the three-dimensional lipid structure is important for the activity of the XC enzymes VDE and DDE. It has been proposed that only a non-bilayer phase allows the penetration of the enzyme into the hydrophobic membrane interior where the XC pigments are located (Goss et al. 2007). The penetration has to be deep enough to allow the catalytic site of the enzyme, i.e. the central cavity of the barrel-like structure (see section “Xanthophyll cycles of higher plants and algae”) to come into contact with its substrate. Furthermore, it has been suggested that the penetration of VDE or DDE into the lipid phase is made possible by a reduced surface tension in the non-bilayer phase due to the reduced size of the headgroup of MGDG (Goss et al. 2007, for a review concerning lipids and the lateral pressure profile see Van den Brink-van der Laan et al. 2004). In contrast, bilayer lipids like DGDG are characterized by a comparable size of the headgroup area and the area which is occupied by the fatty acid chains; leading to a rather tightly closed surface of the lipid bilayer (Van den Brink-van der Laan et al. 2004).
At present it is not clear to what extent non-bilayer phases exist in the native thylakoid membrane of higher plants and diatoms and where these phases may be located. It has been proposed that non-bilayer phases may be established within the membrane (Jahns et al. 2009) or that these phases are excluded from the membrane but remain in tight local and functional association with the membrane bilayer (Garab et al. 2000; Goss et al. 2007, see also section “Localization and operation of xanthophyll cycles in the native thylakoid membrane”). Data on the interaction between LHCII and MGDG (Simidjiev et al. 1998, 2000), which show that this interaction forces MGDG into the membrane bilayer, suggest a co-existence and interaction of bilayer and non-bilayer phases in the native membrane (for further arguments, see section “Membrane models using the properties of non-bilayer lipids”).
Localization and Operation of Xanthophyll Cycles in the Native Thylakoid Membrane
Recent models of NPQ predict a dissociation and aggregation of LHCII and FCP complexes from the PSII core complex in higher plants and diatoms, respectively (Miloslavina et al. 2009; Holzwarth et al. 2009; Lavaud and Goss 2014; Goss and Lepetit 2015). This dissociation and aggregation leads to the formation of the so-called quenching site Q1 during high-light illumination of plants or algal cells. Since both LHCII and FCPs have been shown to be surrounded by an MGDG shield that incorporates the VAZ or DD/Dt cycle pigments (Schaller et al. 2010; Lepetit et al. 2010, see sections “Role of MGDG in the solubilisation of xanthophyll cycle pigments”, and “Importance of the MGDG-dependent non-bilayer phases for the activity of xanthophyll cycles”) it has been proposed that the aggregation of antenna complexes leads to a dissociation of MGDG from the complexes (Goss et al. 2007). The dissociation of a higher amount of MGDG molecules is thought to result in an increased non-bilayer phase, possibly an inverted hexagonal phase, in the thylakoids. With respect to the operation of the XC (Fig. 6.3) it has been proposed that after the activation of the VDE and DDE the enzymes bind to the non-bilayer lipid phase, which contains the free XC pigments (Goss et al. 2007; Schaller et al. 2010; Goss and Lepetit 2015). These pigments can exist as free pigments in the lipid phase of the membrane or can detach from the LHCII/FCP during illumination and diffuse into the non-bilayer phase. The de-epoxidases can then penetrate into the hydrophobic core of the non-bilayer phase, where they gain access to V or DD. The conversion results in the generation of Z in higher plants and Dt in diatoms. It is reasonable to believe that the establishment of the non-bilayer phase takes place in the vicinity of the light-harvesting complexes because the de-epoxidized XC pigments have to rebind to the antenna proteins to participate in the process of NPQ (Horton and Ruban 2005; Lavaud and Goss 2014; Goss and Lepetit 2015). This reasoning is supported by the finding that the de-epoxidized DD/Dt cycle pigment Dt exhibits a decreased solubility in MGDG compared to the epoxidized DD thus facilitating the rebinding of Dt to the antenna apoproteins (Goss et al. 2007).
Model for the MGDG dependence of xanthophyll de-epoxidation in thylakoid membranes. High-light illumination of thylakoid membranes of higher plants that contain the VAZ cycle leads to a disconnection and aggregation of LHCII. The MGDG molecules surrounding the LHCII dissociate and form non-bilayer phases. The VAZ cycle pigment V is disconnected from its binding site at the LHCII apoproteins and diffuses into the non-bilayer phase. The non-bilayer phase acts as an attraction site for the enzyme VDE, which forms a dimer upon activation by the pH-drop of the thylakoid lumen. The non-bilayer phase with its reduced surface tension allows the penetration of the enzyme and its catalytic site into the hydrophobic area of the lipid phase, where it gains access to the hydrophobic V which is preferentially solubilised in MGDG. After the conversion of V to Z, Z rebinds to the LHCII, possibly due to a reduced solubility in the non-bilayer phase compared to V. Z bound to the LHCII increases NPQ and thus the protection against an excess of excitation energy under high-light illumination
With respect to the possible localization of the non-bilayer phase in diatom thylakoids it has to be taken into account that the diatom membrane is not differentiated into grana and stroma thylakoids as in higher plants (for a review see Garab and Mustárdy 1999) but shows regular stacks of three membranes (Gibbs 1962, 1970). Based on the finding that MGDG forms a lipid shield around the FCP complexes, which incorporates the free DD/Dt cycle pigments (Lepetit et al. 2010), and the observation that the negatively charged lipid SQDG, which is present in high concentration in the diatom membranes, inhibits the de-epoxidation of DD (Goss et al. 2009) a model for the lipid and protein arrangement in the thylakoids has been presented (Lepetit et al. 2012). This model predicts that in the inner membranes of the stacks of three PSII with its peripheral antenna complexes is located. Since the FCP complexes are surrounded by an MGDG phase, MGDG may also be enriched in the inner membranes. The enrichment of MGDG in the inner membranes, together with an aggregation of FCP complexes, may lead to the formation of non-bilayer structures in the inner membranes, which then represent an attraction site for the enzyme DDE and support the efficient conversion of DD to Dt. Finally, it has been demonstrated that the efficiency of the VAZ cycle is hindered in mutants deficient in MGDG, which displayed reduced XC activity and contained less Z after a high-light illumination compared to the wild type (Aronsson et al. 2008). The lower Z concentration resulted in a decreased capacity for NPQ and thus a higher photoinhibition of PSII. The authors ascribed the reduced Z concentration to a higher conductivity of the thylakoid membrane for protons, resulting in a decreased activation of the VDE. However, taking into account the results described in sections above, it is likely that MGDG deficiency had an impact on the formation and extent of non-bilayer phases in the thylakoids, thus affecting both the solubilisation of V and the activity of VDE.
Membrane Models Using the Properties of Non-bilayer Lipids
The ‘standard’, fluid mosaic membrane model of Singer and Nicolson (1972) describes the biological membranes as a two-dimensional liquid, a bilayer, in which lipid and protein molecules diffuse easily. Although the existence and phase behaviour of non-bilayer lipids were known from early works of Luzzati (1968), the proposed model provides no hint on their possible roles (the phase behaviour of MGDG was described a year later by Shipley et al. (1973)). In the past more than four decades the standard model has been refined many times and by many research groups in order to accommodate special features, such as the asymmetry of the two membrane leaflets, protein-lipid interactions, lipid rafts, membrane curvature etc. (https://en.wikipedia.org/wiki/Fluid_mosaic_model). In the following sections, focusing on the roles of non-bilayer lipids and possible occurrence of non-lamellar lipid phases, two different approaches, refined models will be briefly discussed, noting that they appear to us as mutually non-exclusive models. Nonetheless, they differ from each other in the proposed localization and roles of non-bilayer lipids and non-lamellar lipid phases in relation to the bilayer membrane. A common, important feature in both approaches is that the appearance of non-bilayer phases is restricted only locally and transiently in the bilayer membranes, e.g. to allow membrane fusion.
Models Focusing on the Role of Non-bilayer Lipids in the Bilayer Membrane
In a series of systematic works Ben de Kruijff, Antoinette Killian and coworkers elaborated a model, which might be named as “lateral pressure bilayer membrane model ”. This is based on the special geometry of non-bilayer lipids, i.e. their conical shapes, which thus, when incorporated in the bilayer will cause a frustration, and exert higher lateral pressure on the membrane-embedded proteins than the cylindrical bilayer lipids (de Kruijff 1997). The model explains several important features of biomembranes and properties of membrane-intrinsic and peripheral proteins (reviewed by van den Brink-van der Laan et al. 2004). In particular, it is pointed out that in order to understand the properties of a protein, the lateral pressure profile, which largely depends on the presence of non-bilayer lipids, must be understood. Their experimental data also suggest that non-bilayer lipids facilitate membrane binding of peripheral membrane proteins via changes in the lateral pressure profiles (for further details see the above cited review). In thylakoid membranes, the regulation of the MGDG/DGDG ratio might be correlated with the lateral pressure – which evidently affects the structural flexibility of membranes (see section “Importance of MGDG in LHCII structures”); this might be of high physiological significance in light adaptation and stress tolerance mechanisms.
The flexible surface model (FSM) proposed by Brown (2012) also focuses on lipid-protein interactions in the membrane. It challenges the standard model, to a large extent via assigned structural roles of non-bilayer lipid molecules in the bilayer membrane and roles of curvature forces. The FSM model also offers explanation on a number of earlier experimental results on membranes enriched in non-bilayer lipids. It emphasizes that the “polymorphism of membrane lipids is connected with their spontaneous curvature (or molecular packing)” and points out that both curvature matching and hydrophobic thickness matching must be taken into account. This concept would probably help the construction of theoretical models on lipid:LHCII assemblies and eventually of the bilayer thylakoid membranes.
A Model Proposing the Co-existence and Close Association of Bilayer and Non-bilayer Lipid Phases
The model presented here can be considered as a refined model proposed earlier by Garab et al. (2000). It focuses on the properties of bulk lipids, i.e. which are found in fluid-like phase and constitute at least half of the total lipid content of thylakoid membranes; this bulk lipid mixture, on mesoscopic scale, displays no lateral heterogeneity (see Introduction). The model described below on thylakoid membranes is thought to be applicable, mutatis mutandis, for other energy-converting membranes and might be adapted to other biological membranes with substantial amounts of non-bilayer lipids and thus lending a significant degree of non-bilayer propensity to their lipids. The model is based on several premises, which will be outlined below.
Although it might look trivial, we (here and below, GG and his coworkers) proposed that the most important property of non-bilayer lipids, what we should take into account in the first place, is that, if the lipids are in surplus they segregate from the bilayer (Garab et al. 2000). This offered an explanation on the apparently constant and high protein to lipid ratio in the thylakoids and in all energy-converting membranes. This self-regulatory (physical) mechanism prevents the ‘dilution’ of membranes, which otherwise could easily occur in the absence of strict synchronization of lipid and protein syntheses. The dilution of membranes could easily exert devastating effects on the protein macro-assemblies and the entire, highly organized membrane system (Garab 2014).
As already mentioned (section “Thylakoid Lipids”), we have shown, by using purified MGDG and LHCII that non-bilayer lipids can be forced into the bilayer, apparently in a frustrated state (Simidjiev et al. 1998, 2000). These experimental findings suggested to us that non-bilayer lipids can be trafficking between the non-bilayer phase and the bilayer (Garab et al. 2000). In other terms we assumed that non-bilayer lipids, via this putative dynamic exchange mechanism, contribute to the structural flexibility of thylakoid membranes. It must be stressed, however, that with this assumption we hypothesized that the two phases , the bilayer phase and a non-bilayer phase, co-exist in the thylakoids; further, the non-bilayer lipids (lipid mixtures with high non-bilayer propensity) are hypothesized to enter the membrane not only occasionally (as examples have shown), or become excluded from the membrane not only under special conditions (known in the literature), but dynamically.
To test these hypotheses , first, experimental evidence had to be found that the bilayer and non-bilayer lipid phases co-exist in a way which allows their interaction. Although EM data showed the presence of non-bilayer lipid phases in mature chloroplasts, they, in lipid droplets, were remotely located from the thylakoid membranes, allowing no interaction between the two structures (see Williams 1998, Garab et al. 2000 and references therein). Thus, we turned to the technique of 31P-NMR and performed experiments on isolated intact thylakoid membranes (Krumova et al. 2008a). Earlier, using Tris-washed thylakoids, which, as we also showed, severely affects the organization of thylakoid membranes, Haranczyk et al. (1995) reported on the appearance of HII phases in the sample. Our experiments provided clear evidence that in intact thylakoid membranes at low (but still physiological) temperatures the lamellar phase co-existed with a non-bilayer phase (Krumova et al. 2008a) (Fig. 6.4a). Surprisingly, however, the NMR signature of the lamellar phase disappeared already around 20 °C, while we had convincing experimental evidences that the bilayer structure was maintained (Fig. 6.4b). These experimental findings could only be attributed to the interconnection of the two lipid phases – the bilayer membrane and a non-bilayer lipid phase thought to be located primarily in the lumenal aqueous phase. (The lipids that were expelled from the membranes on the stromal side could easily detach in the absence of the native stroma liquid, which was replaced by a buffered medium.) Figure 6.4c shows the irreversible heat-induced formation of the isotropic and non-bilayer phases at 0 and ~4 ppm, respectively. Additional experiments, using the fluorescent lipid probe Merocyanine-540 corroborated the NMR data by showing complex, temperature-dependent phase behaviour of lipids, which could not be accounted for by the sole, bilayer phase (Krumova et al. 2008b). These experiments also revealed that at elevated temperature the lipids tend to be found in a lipid phase that could be assigned to a non-bilayer phase. Further, using a DGDG-deficient Arabidopsis mutant, Krumova et al. (2010) have also shown that the substantially increased relative abundance of MGDG brings about significant alterations in the overall organization of the thylakoid membranes and decreased thermal stabilities and an enhanced lipid extrusion from the bilayer membrane of the mutant thylakoids.
31P-NMR spectrum of thylakoid membranes at 7 °C. (a) The positions (in ppm) of the different resonances are denoted by arrows and are assigned to the lamellar (L) (bilayer) phase, isotropic structures and a non-bilayer lipid structure. (b) 31P-NMR spectra of thylakoid membranes at different temperatures, as indicated, and, for comparison, (c) the spectra at 7 °C obtained before and after heating of the sample to 49 °C. The dashed lines indicate the positions of the different resonances (Based on figures from Krumova et al. (2008a) with permission)
These data are explained within the frameworks of a simplified model shown in Fig. 6.5 illustrating that the thylakoid lumen contains a non-bilayer lipid phase, that is associated with the bilayer phase and the two phases form a continuum. Because of the narrow space the extruded lipids in the aqueous phase will not be able to assume a periodic structure, such as a ‘true’ HII but will nevertheless be distinguishable from the bilayer phase. The model also points to the proposed roles of VDE and other lipocalin proteins in the lumen, which are capable of binding lipid molecules (see section ‘‘Roles of MGDG in xanthophyll cycles’’). These proteins might be capable of mediating between the two phases. Recent molecular dynamics simulations on two adjacent bilayers that contain plant lipid mixtures have “demonstrated that the thylakoid membrane is close to the formation of an inverted hexagonal phase”. Further, the simulations revealed that these thylakoid model lipid membranes have a strong, hydration-dependent propensity to the formation of stalk, the main intermediate between bilayer and hexagonal phases (Van Eerden et al. 2015). These findings are fully consistent with our model, which nevertheless requires further rigorous experimental and molecular dynamics tests.
Simplified thylakoid membrane model illustrating that the bilayer and non-bilayer lipid phases are closely associated with each other and interact both with membrane proteins (LHCII) and lumenal proteins (VDE/lipocalin) (The model is based on the experimental findings of Krumova et al. 2008a, b. Drawing, courtesy of Dr. Sashka B. Krumova)
References
Armbruster U, Labs M, Pribil M, Viola S, Xu W, Scharfenberg M, Hertle AP, Rojahn U, Jensen PE, Rappaport F, Joliot P, Dörmann P, Wanner G, Leister D (2013) Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. Plant Cell 25:2661–2678
Arnoux P, Morosinotto T, Saga G, Bassi R, Pignol D (2009) A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21:2036–2044
Aronsson H, Schöttler MA, Kelly AA, Sundqvist C, Dörmann P, Karim S, Jarvis P (2008) Monogalactosyldiacylglycerol deficiency in Arabidopsis affects pigment composition in the prolamellar body and impairs thylakoid membrane energization and photoprotection in leaves. Plant Physiol 148:580–592
Bassi R, Pineau B, Dainese P, Marquardt J (1993) Carotenoid-binding proteins of photosystem II. Eur J Biochem 212:297–303
Benning C, Beatty JT, Prince RC, Somerville CR (1993) The sulfolipid sulfoquinovosyldiacylglycerol is not required for photosynthetic electron transport in Rhodobacter sphaeroides but enhances growth under phosphate limitation. Proc Natl Acad Sci U S A 90:1561–1565
Boudière L, Michaud M, Petroutsos D, Rébeillé F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J, Block MA, Maréchal E (2014) Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta 1837:470–480
Brown MF (2012) Curvature forces in membrane lipid-protein interactions. Biochemistry 51:9782–9795
Büch K, Stransky H, Hager A (1995) FAD is a further essential cofactor of the NAD(P)H and O2-dependent zeaxanthin-epoxidase. FEBS Lett 376:45–48
Büchel C (2003) Fucoxanthin-chlorophyll proteins in diatoms: 18 and 19 kDa subunits assemble into different oligomeric states. Biochemistry 42:13027–13034
Büchel C (2015) Evolution and function of light harvesting proteins. J Plant Physiol 172:62–75
Castro V, Dvinskikh SV, Widmalm G, Sandström D, Maliniak A (2007) NMR studies of membranes composed of glycolipids and phospholipids. Biochim Biophys Acta 1768:2432–2437
Coesel S, Obornik M, Varela J, Falciatore A, Bowler C (2008) Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 3, e2896
Csiszár A, Klumpp E, Bóta A, Szegedi K (2003) Effect of 2,4-dichlorophenol on DPCC/water liposomes studied by X-ray and freez-facture electron microscopy. Chem Phys Lipids 126:155–166
Cullis PR, de Kruijff B (1979) Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta 559:399–420
de Kruijff B (1997) Biomembranes. Lipids beyond the bilayer. Nature 386:129–130
Dekker JP, Boekema EJ (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochim Biophys Acta 1706:12–39
Demé B, Cataye C, Block MA, Maréchal E, Jouhet J (2014) Contribution of galactoglycerolipids to the 3-dimensional architecture of thylakoids. FASEB J 28:3373–3383
Demmig-Adams B, Adams WW III, Garab G, Govindjee (eds) (2014) Non-photochemical quenching and thermal energy dissipation in plants, algae and cyanobacteria, Advances in Photosynthesis and Respiration. Springer, Dordrecht
Domonkos I, Laczko-Dobos H, Gombos Z (2008) Lipid-assisted protein-protein interactions that support photosynthetic and other cellular activities. Prog Lipid Res 47:422–435
Domonkos I, Kis M, Gombos Z, Ughy B (2013) Carotenoids, versatile components of oxygenic photosynthesis. Prog Lipid Res 52:539–561
Dörmann P, Benning C (2002) Galactolipids rule in seed plants. Trends Plant Sci 7:112–118
Epand RM (1998) Lipid polymorphism and protein–lipid interactions. Biochim Biophys Acta 1376:353–368
Garab G (2014) Hierarchical organization and structural flexibility of thylakoid membranes. Biochim Biophys Acta 1837:481–494
Garab G, Mustardy L (1999) Role of LHCII-containing macrodomains in the structure, function and dynamics of grana. Aust J Plant Physiol 27:649–658
Garab G, Lohner K, Laggner P, Farkas T (2000) Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis. Trends Plant Sci 5:489–494
Georgiev GA, Ivanov SI, Jordanova A, Tsanova A, Getov V, Dimitrov M, Lalchev Z (2012) Interaction of monogalactosyldiacylglycerol with cytochrome b6f complex in surface films. Biochem Biophys Res Commun 419:648–651
Gibbs SP (1962) The ultrastructure of the pyrenoids of algae, exclusive of the green algae. J Ultra Mol Struct R 7:247–261
Gibbs SP (1970) The comparative ultrastructure of the algal chloroplast. Ann NY Acad Sci 175:454–473
Goss R, Jakob T (2010) Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth Res 106:103–122
Goss R, Lepetit B (2015) Biodiversity of NPQ. J Plant Physiol 172:13–32
Goss R, Wilhelm C (2009) Lipids in algae, lichens and mosses. In: Wada H, Murata N, Govindjee (eds) Lipids in photosynthesis: essential and regulatory functions. Springer, Dordrecht, pp 117–137
Goss R, Richter M, Wild A (1997) Pigment composition of PS II pigment protein complexes purified by anion exchange chromatography. Identification of xanthophyll cycle pigment binding proteins. J Plant Physiol 151:115–119
Goss R, Lohr M, Latowski D, Grzyb J, Vieler A, Wilhelm C, Strzalka K (2005) Role of hexagonal structure-forming lipids in diadinoxanthin and violaxanthin solubilization and de-epoxidation. Biochemistry 44:4028–4036
Goss R, Latowski D, Grzyb J, Vieler A, Lohr M, Wilhelm C, Strzalka K (2007) Lipid dependence of diadinoxanthin solubilization and de-epoxidation in artificial membrane systems resembling the lipid composition of the natural thylakoid membrane. Biochim Biophys Acta 1768:67–75
Goss R, Nerlich J, Lepetit B, Schaller S, Vieler A, Wilhelm C (2009) The lipid dependence of diadinoxanthin de-epoxidation presents new evidence for a macrodomain organization of the diatom thylakoid membrane. J Plant Physiol 166:1839–1854
Gruner SM (1985) Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A 82:3665–3669
Grzyb J, Latowski D, Strzalka K (2006) Lipocalins-a family portrait. J Plant Physiol 163:895–915
Güler S, Seeliger A, Härtel H, Renger G, Benning C (1996) A null mutant of Synechococcus sp. PCC7942 deficient in the sulfolipid sulfoquinovosyl diacylglycerol. J Biol Chem 271:7501–7507
Gundermann K, Büchel C (2012) Factors determining the fluorescence yield of fucoxanthin-chlorophyll complexes (FCP) involved in non-photochemical quenching in diatoms. Biochim Biophys Acta 1817:1044–1052
Guo J, Zhang Z, Bi Y, Yang W, Xu Y, Zhang L (2005) Decreased stability of photosystem I in dgd1 mutant of Arabidopsis thaliana. FEBS Lett 579:3619–3624
Guskov A, Kern J, Gabdulkhakov A, Broser M, Zouni A, Saenger W (2009) Cyanobacterial photosystem II at 2.9-angstrom resolution and the role of quinones, lipids, channels and chloride. Nat Struct Mol Biol 16:334–342
Hager A (1967a) Untersuchungen über die lichtinduzierten, reversiblen Xanthophyllumwandlungen an Chlorella und Spinacia oleracea. Planta 74:148–172
Hager A (1967b) Untersuchungen über die Rückreaktionen im Xanthophyll-Cyclus bei Chlorella, Spinacia und Taxus. Planta 76:138–148
Hager A (1969) Lichtbedingte pH-Erniedrigung in einem Chloroplasten-Kompartiment als Ursache der enzymatischen Violaxanthin-Zeaxanthin-Umwandlung: Beziehungen zur Photophosphorylierung. Planta 89:224–243
Hager A (1975) Die reversiblen, lichtabhängigen Xanthophyllumwandlungen im Chloroplasten. Ber Deutsch Bot Ges 88:27–44
Hager A, Holocher K (1994) Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta 192:581–589
Hager A, Stransky H (1970) Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. Archiv Mikrobiol 73:77–89
Harańczyk H, Strzałka K, Dietrich W, Blicharski JS (1995) 31P-NMR observation of the temperature and glycerol induced non-lamellar phase formation in wheat thylakoid membranes. J Biol Phys 21:125–139
Harwood JL (1998) Involvement of chloroplast lipids in the reaction of plants submitted to stress. In: Siegenthaler P-A, Murata N (eds) Advances in photosynthesis. Lipids in photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 287–302
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci U S A 96:8762–8767
Hieber AD, Bugos RC, Yamamoto HY (2000) Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin epoxidase. Biochim Biophys Acta 1482:84–91
Holzwarth AR, Miloslavina Y, Nilkens M, Jahns P (2009) Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem Phys Lett 483:262–267
Horton P, Ruban AV (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56:365–373
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Jakob T, Goss R, Wilhelm C (2001) Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum. J Plant Physiol 158:383–390
Janik E, Bednarska J, Zubik M, Puzio M, Luchowski R, Grudzinski W, Mazur R, Garstka M, Maksymiec W, Kulik A, Dietler G, Gruszecki WI (2013) Molecular architecture of plant thylakoids under physiological and light stress conditions: a study of lipid-light-harvesting complex II model membranes. Plant Cell 25:2155–2170
Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411:909–917
Jouhet J (2013) Importance of the hexagonal lipid phase in biological membrane organization. Front Plant Sci 4:494. doi:10.3389/fpls.2013.00494. eCollection 2013
Kansy M, Wilhelm C, Goss R (2014) Influence of thylakoid membrane lipids on the structure and function of the plant photosystem II core complex. Planta 240:781–796
Kern J, Guskov A (2011) Lipids in photosystem II: multifunctional cofactors. J Photochem Photobiol B Biology 104:19–34
Kirchhoff H, Haase W, Wegner S, Danielsson R, Ackermann R, Albertsson PA (2007) Low-light-induced formation of semicrystalline photosystem II arrays in higher plant chloroplasts. Biochemistry 46:11169–11176
Kobayashi K, Narise T, Sonoike K, Hashimoto H, Sato N, Kondo M, Nishimura M, Sato M, Toyooka K, Sugimoto K, Wada H, Masuda T, Ohta H (2013) Role of galactolipid biosynthesis incoordinated development of photosynthetic complexes and thylakoid membranes during chloroplast biogenesis in Arabidopsis. Plant J 73:250–261
Kouril R, Wientjes E, Bultema JB, Croce R, Boekema EJ (2013) High-light vs. low-light: effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim Biophys Acta 1827:411–419
Koynova R, Tenchov B (2013) Recent patents on nonlamellar liquid crystalline lipid phases in drug delivery. Recent Pat Drug Deliv Formul 7:165–173
Krause GH, Jahns P (2004) Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching. Characterization and function. In: Papageorgiou GC, Govindjee (eds) Chlorophyll fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 463–495
Krumova SB, Dijkema C, de Waard P, Van As H, Garab G, van Amerongen H (2008a) Phase behaviour of phosphatidylglycerol in spinach thylakoid membranes as revealed by 31P-NMR. Biochim Biophys Acta 1778:997–1003
Krumova SB, Koehorst RB, Bóta A, Páli T, van Hoek A, Garab G, van Amerongen H (2008b) Temperature dependence of the lipid packing in thylakoid membranes studied by time- and spectrally resolved fluorescence of Merocyanine 540. Biochim Biophys Acta 1778:2823–2833
Krumova SB, Laptenok SP, Kovács L, Tóth T, van Hoek A, Garab G, van Amerongen H (2010) Digalactosyl-diacylglycerol-deficiency lowers the thermal stability of thylakoid membranes. Photosynth Res 105:229–242
Kurisu G, Zhang H, Smith JL, Cramer WA (2003) Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science 302:1009–1014
Laczko-Dobos H, Ughy B, Toth SZ, Komenda J, Zsiros O, Domonkos I, Parducz A, Bogos B, Komura M, Itoh S, Gombos Z (2008) Role of phosphatidylglycerol in the function and assembly of Photosystem II reaction center, studied in a cdsA-inactivated PAL mutant strain of Synechocystis sp. PCC6803 that lacks phycobilisomes. Biochim Biophys Acta 1777:1184–1194
Laczko-Dobos H, Frycák P, Ughy B, Domonkos I, Wada H, Prokai L, Gombos Z (2010) Remodeling of phosphatidylglycerol in Synechocystis PCC6803. Biochim Biophys Acta 1801:163–170
Latowski D, Kruk J, Burda K, Skrzynecka-Jaskier M, Kostecka-Gugala A, Strzalka K (2002) Kinetics of violaxanthin de-epoxidation by violaxanthin de-epoxidase, a xanthophyll cycle enzyme, is regulated by membrane fluidity in model lipid bilayers. Eur J Biochem 269:4656–4665
Latowski D, Akerlund H-E, Strzalka K (2004) Violaxanthin de-epoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity. Biochemistry 43:4417–4420
Lavaud J, Goss R (2014) The peculiar features of non-photochemical fluorescence quenching in diatoms and brown algae. In: Demmig-Adams B, Adams WW III, Garab G, Govindjee (eds) Non-photochemical quenching and thermal energy dissipation in plants, algae and cyanobacteria, Advances in Photosynthesis and Respiration. Springer, Dordrecht, pp 421–443
Lavaud J, Rousseau B, Etienne A (2003) Enrichment of the light-harvesting complex in diadinoxanthin and implications for the nonphotochemical fluorescence quenching in diatoms. Biochemistry 42:5802–5808
Lee AG (2003) Lipid–protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 1612:1–40
Leng J, Sakurai I, Wada H, Shen JR (2008) Effect of phospholipase and lipase treatments on photosystem II core dimer from a thermophilic cyanobacterium. Photosynth Res 98:469–478
Lepetit B, Volke D, Szabo M, Hoffmann R, Garab G, Wilhelm C, Goss R (2007) Spectroscopic and molecular characterization of the oligomeric antenna of the diatom Phaeodactylum tricornutum. Biochemistry 46:9813–9822
Lepetit B, Volke D, Szabo M, Hoffmann R, Garab G, Wilhelm C, Goss R (2008) The oligomeric antenna of the diatom P. tricornutum – localization of diadinoxanthin cycle pigments. In: Allen JF, Gantt E, Golbeck JH, Osmond B (eds) Photosynthesis. Energy from the sun. Springer, Dordrecht, pp 277–280
Lepetit B, Volke D, Gilbert M, Wilhelm C, Goss R (2010) Evidence for the existence of one antenna-associated, lipid-dissolved, and two protein-bound pools of diadinoxanthin cycle pigments in diatoms. Plant Physiol 154:1905–1920
Lepetit B, Goss R, Jakob T, Wilhelm C (2012) Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth Res 111:245–257
Liu ZF, Yan HC, Wang KB, Kuang KY, Zhang JP, Gui LL, An XM, Chang WR (2004) Crystal structure of spinach major light-harvesting complex at 2.72 angstrom resolution. Nature 428:287–292
Lohner K (1996) Is the high propensity of ethanolamine plasmalogens to form non-lamellar lipid structures manifested in the properties of biomembranes? Chem Phys Lipids 81:167–184
Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Toward complete cofactor arrangement in the 3.0 A resolution structure of photosystem II. Nature 438:1040–1044
Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2007) Lipids in photosystem II: interactions with protein and cofactors. Biochim Biophys Acta 1767:509–519
Luzzati V (1968) X-ray diffraction studies of lipid–water systems. In: Chapman D (ed) Biological membranes. Academic, New York, pp 71–123
Miloslavina Y, Grouneva I, Lambrev PH, Lepetit B, Goss R, Wilhelm C, Holzwarth AR (2009) Ultrafast fluorescence study on the location and mechanism of non-photochemical quenching in diatoms. Biochim Biophys Acta 1787:1189–1197
Minoda A, Sato N, Nozaki H, Okada K, Takahashi H, Sonoike K, Tsuzuki M (2002) Role of sulfoquinovosyl diacylglycerol for the maintenance of photosystem II in Chlamydomonas reinhardtii. Eur J Biochem 269:2353–2358
Mizusawa N, Wada H (2012) The role of lipids in photosystem II. Biochim Biophys Acta 1817:194–208
Murata N, Siegenthaler PA (1998) Lipids in photosynthesis: an overview. In: Siegenthaler PA, Murata N (eds) Lipids in photosynthesis: structure, function and genetics. Kluwer Academic Publishers, Dordrecht, pp 1–20
Niyogi KK, Truong TB (2013) Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol 16:307–314
Páli T, Garab G, Horvath LI, Kóta Z (2003) Functional significance of the lipid-protein interface in photosynthetic membranes. Cell Mol Life Sci 60:1591–1606
Pan J, Cheng X, Sharp M, Ho CS, Khadka N, Katsaras J (2015) Structural and mechanical properties of cardiolipin lipid bilayers determined using neutron spin echo, small angle neutron and X-ray scattering, and molecular dynamics simulations. Soft Matter 11:130–138
Pfündel EE, Renganathan M, Gilmore AM, Yamamoto HY, Dilley RA (1994) Intrathylakoid pH in isolated Pea chloroplasts as probed by violaxanthin deepoxidation. Plant Physiol 106:1647–1658
Pomorski TG, Nylander T, Cárdenas M (2014) Model cell membranes: discerning lipid and protein contributions in shaping the cell. Adv Colloid Interface Sci 205:207–220
Qin X, Suga M, Kuang T, Shen JR (2015) Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348:989–995
Quinn PJ (2012) Lipid-lipid interactions in bilayer membranes: married couples and casual liaisons. Prog Lipid Res 51:179–198
Reifarth F, Christen G, Seeliger AG, Dormann P, Benning C, Renger G (1997) Modification of the water oxidizing complex in leaves of the dgd1 mutant of Arabidopsis thaliana deficient in the galactolipid digalactosyldiacylglycerol. Biochemistry 36:11769–11776
Ruban AV, Young AJ, Horton P (1994) The effects of illumination on the xanthophyll composition of the photosystem II light-harvesting complexes of spinach thylakoid membranes. Plant Physiol 104:227–234
Sackman E (1995) Biological membranes architecture and function. In: Lipowsky R, Sackmann E (eds) Structure and dynamics of membranes. Elsevier, Amsterdam, pp 1–65
Saga G, Giorgetti A, Fufezan C, Giacometti GM, Bassi R, Morosinotto T (2010) Mutation analysis of violaxanthin de-epoxidase indentifies substrate-binding sites and residues involved in catalysis. J Biol Chem 285:23763–23770
Sato N, Sonoike K, Tsuzuki M, Kwaguchi A (1995) Impaired photosystem II in a mutant of Chlamydomonas reinhardtii defective in sulfoquinovosyl diacylglycerol. Eur J Biochem 234:16–23
Sato N, Hagio M, Wada H, Tsuzuki M (2000) Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc Natl Acad Sci U S A 97:10655–10660
Schaller S, Latowski D, Jemiola-Rzeminska M, Wilhelm C, Strzalka K, Goss R (2010) The main thylakoid membrane lipid monogalactosyldiacylglycerol (MGDG) promotes the de-epoxidation of violaxanthin associated with the light-harvesting complex of photosystem II (LHCII). Biochim Biophys Acta 1797:414–424
Schaller S, Latowski D, Jemioła-Rzemioska M, Dawood A, Wilhelm C, Strzałka K, Goss R (2011) Regulation of LHCII aggregation by different thylakoid membrane lipids. Biochim Biophys Acta 1807:326–335
Schaller S, Latowski D, Jemiola-Rzeminska M, Quaas T, Wilhelm C, Strzalka K, Goss R (2012) The investigation of violaxanthin de-epoxidation in the primitive green alga Mantoniella squamata (Prasinophyceae) indicates mechanistic differences in xanthophyll conversion to higher plants. Phycologia 51:359–370
Schaller S, Richter K, Wilhelm C, Goss R (2014) Influence of pH, Mg2+, and lipid composition on the aggregation state of the diatom FCP in comparison to the LHCII of vascular plants. Photosynth Res 119:305–317
Schumann A, Goss R, Jakob T, Wilhelm C (2007) Investigation of the quenching efficiency of diatoxanthin in cells of Phaeodactylum tricornutum (Bacillariophyceae) with different pool sizes of xanthophyll cycle pigments. Phycologia 46:113–117
Seddon JM, Templer RH (1995) Polymorphism of lipid–water system. In: Lipowsky R, Sackman E (eds) Structure and dynamics of membranes. Elsevier, Amsterdam, pp 97–161
Selstam E (1998) Development of thylakoid membranes with respect to lipids. In: Siegenthaler PA, Murata N (eds) Lipids in photosynthesis: structure, function and genetics. Kluwer Academic Publishers, Dordrecht, pp 209–224
Shipley GG, Green JP, Nichols BW (1973) The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim Biophys Acta 311:531–544
Siefermann D, Yamamoto H (1975) NADPH and oxygen-dependent epoxidation of zeaxanthin in isolated chloroplasts. Biochem Biopharm Res Co 62:456–461
Simidjiev I, Barzda V, Mustárdy L, Garab G (1998) Role of thylakoid lipids in the structural flexibility of lamellar aggregates of the isolated light-harvesting chlorophyll a/b complex of photosystem II. Biochemistry 37:4169–4173
Simidjiev I, Stoylova S, Amenitsch H, Javorfi T, Mustardy L, Laggner P, Holzenburg A, Garab G (2000) Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci U S A 97:1473–1476
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Sozer O, Kis M, Gombos Z, Ughy B (2011) Proteins, glycerolipids and carotenoids in the functional photosystem II architecture. Front Biosci (Landmark Ed) 16:619–643
Steffen R, Kelly AA, Huyer J, Dormann P, Renger G (2005) Investigations on the reaction pattern of photosystem II in leaves from Arabidopsis thaliana wild type plants and mutants with genetically modified lipid content. Biochemistry 44:3134–3142
Stransky H, Hager A (1970) Das Carotinoidmuster und die Verbreitung des lichtinduzierten Xanthophyllzyklus in verschiedenen Algenklassen. II: Xanthophyceae. Arch Microbiol 71:164–190
Stroebel D, Choquet Y, Popot JL, Picot D (2003) An atypical haem in the cytochrome b(6)f complex. Nature 426:413–418
Tietz S, Puthiyaveetil S, Enlow HM, Yarbrough R, Wood M, Semchonok DA, Lowry T, Li Z, Jahns P, Boekema EJ, Lenhert S, Niyogi KK, Kirchhoff H (2015) Functional implications of photosystem II crystal formation in photosynthetic membranes. J Biol Chem 290:14091–14106
Tyler AI, Law RV, Seddon JM (2015) X-ray diffraction of lipid model membranes. Methods Mol Biol 1232:199–225
Umena Y, Kawakami K, Shen JR, Kamaiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
van den Brink-van der Laan E, Killian JA, de Kruijff B (2004) Non-bilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666:275–288
Van Eerden FJ, de Jong DH, de Vries AH, Wassenaar TA, Marrink SJ (2015) Characterization of thylakoid lipid membranes from cyanobacteria and higher plants by molecular dynamics simulations. Biochim Biophys Acta 1848:1319–1330
Vieler A, Süß R, Wilhelm C, Schiller J (2007) The lipid composition of two different algae (Chlamydomonas reinhardtii, Chlorophyceae and Cyclotella meneghiniana, Bacillariophyceae) investigated by MALDI-TOF MS and TLC. Chem Phys Lipids 150:143–155
Vigh L, Escribá PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horváth I, Harwood JL (2005) The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res 44:303–344
Wada H, Murata N (2007) The essential role of phosphatidylglycerol in photosynthesis. Photosynth Res 92:205–215
Wang S, Uddin MI, Tanaka K, Yin L, Shi Z, Qi Y, Mano J, Matsui K, Shimomura N, Sakaki T, Deng X, Zhang S (2014) Maintenance of chloroplast structure and function by overexpression of the rice MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE gene leads to enhanced salt tolerance in tobacco. Plant Physiol 165:1144–1155
Williams WP (1998) The physical properties of thylakoid membrane lipids and their relation to photosynthesis. In: Siegenthaler PA, Murata N (eds) Advances in photosynthesis. Lipids in photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 103–118
Williams WP, Brain APR, Dominy PJ (1992) Induction of non-bilayer lipid phase separation in chloroplast thylakoid membranes by compatible co-solutes and its relation to the thermal stability of Photosystem II. Biochim Biophys Acta 1099:137–144
Wu W, Ping W, Wu H, Li M, Gu D, Xu Y (2013) Monogalactosyldiacylglycerol deficiency in tobacco inhibits the cytochrome b6f-mediated intersystem electron transport process and affects the photostability oft he photosystem II apparatus. Biochim Biophys Acta 1827:709–722
Yamamoto HY, Higashi RM (1978) Violaxanthin de-epoxidase-lipid-composition and substrate-specificity. Arch of Biochem Biophys 190:514–522
Yamamoto H, Nakayama T, Chichester C (1962) Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys 97:168–173
Yan JS, Mao DZ, Chen H, Kuang TY, Li LB (2000) Effects of membrane lipids on the electron transfer activity of cytochrome b6f complex from spinach. Acta Bot Sin 42:1267–1270
Yu B, Benning C (2003) Anionic lipids are required for chloroplast structure and function in Arabidopsis. Plant J 36:762–770
Zhou F, Liu S, Hu Z, Kuang T, Paulsen H, Yang C (2009) Effect of monogalactosyldiacylglycerol on the interaction between photosystem II core complex and its antenna complexes in liposomes of thylakoid lipids. Photosynth Res 99:185–193
Zick M, Stroupe C, Orr A, Douville D, Wickner WT (2014) Membranes linked by trans-SNARE complexes require lipids prone to non-bilayer structure for progression to fusion. eLife. doi:10.7554/eLife.01879
Acknowledgements
The authors are indebted to Dr. Sashka B. Krumova for fruitful discussions and for the drawing of the simplified membrane model outline in section “A model proposing the co-existence and close association of bilayer and non-bilayer lipid phases”. Financial supports from OTKA (K112688) and TÁMOP (422D-15/1/KONV-2015-0024) to GG and from the DFG (Go 818/7-1) to RG are also acknowledged. Susann Schaller-Laudel is acknowledged for the composition of Fig. 6.3. The authors thank Profs. Lászlo Vígh and Kazimierz Strzalka for inspiring discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Garab, G., Ughy, B., Goss, R. (2016). Role of MGDG and Non-bilayer Lipid Phases in the Structure and Dynamics of Chloroplast Thylakoid Membranes. In: Nakamura, Y., Li-Beisson, Y. (eds) Lipids in Plant and Algae Development. Subcellular Biochemistry, vol 86. Springer, Cham. https://doi.org/10.1007/978-3-319-25979-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-25979-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25977-2
Online ISBN: 978-3-319-25979-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)