Abstract
The genus Bromus (Poaceae) consists of approximately 150–160 species that occur in temperate regions worldwide. Members of section Genea in Bromus are native to Eurasia, and most are invasive in North America (NA). Most environmentally and economically important among these species is Bromus tectorum, which along with Bromus rubens and B. diandrus are some of the most destructive invasive grasses in the Western United States (USA). Members of section Genea form a polyploid series. Molecular data suggest a reticulate speciation pattern (a netlike pattern of diversification) for the polyploid members of Genea. Seed set, floral characteristics, sex allocation patterns, and genetic markers reveal that B. tectorum is predominately self-pollinating. Historical records and genetic data indicate that B. tectorum entered repeatedly into NA and that its current distribution can be attributed to multiple phenomena, including direct introduction from its native range and multidirectional range expansion. Across invasive populations of B. tectorum, overall genetic diversity (e.g., allelic richness and number of polymorphic loci) is lower compared to the diversity among native populations. Conversely, invasive populations of B. tectorum exhibit, on average, higher within-population genetic diversity than found in native populations, suggesting that multiple introductions have partially offset founder effects within invasive populations. Additionally, invasive populations appear to be genetic admixtures composed of two or more independently derived native genotypes. These admixtures have produced invasive populations with generally less genetic differentiation than native populations. The genetic consequences of multiple introductions have implications for the management of invasive populations of B. tectorum, especially in Western NA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Classical biological control
- Enzyme electrophoresis (allozymes)
- Founder effects
- Genetic admixture
- Herbarium specimens
- Multiple introductions
- Multidirectional range expansion
- Reticulate speciation pattern
- Self-pollinating mating system
- Source populations
1 Introduction
Some members of the Poaceae rank among the worst plant invaders, especially in steppe, shrub-steppe, and savannas (Holm et al. 1977; Mack 1989); furthermore, some invasive grasses have become prominent where the forest canopy has been removed, including low-elevation coniferous forest (Sumners and Archibold 2007; D’Antonio et al. 2011). The array of grasses that have become invasive (or at least extensively naturalized) defies straightforward taxonomic classification , but multiple species in Avena, Brachiaria, Eragrostis, Nassella, Pennisetum, and Poa have become major pests in their new ranges (Holm et al. 1977; Parsons and Cuthbertson 1992; Bromilow 1995). Whether outcompeting native species or causing devastating alterations of the fire cycle within invaded communities (D’Antonio and Vitousek 1992), these grass immigrants collectively cause enormous environmental and economic damage worldwide (Pimentel et al. 2005). Among any list of destructive grasses , few genera match the ecological breadth and resulting spread seen for many members of the genus Bromus L. (Germino et al. 2015; Brooks et al. 2015).
The introduction and range expansion of species in the genus Bromus (brome-grasses) across Western North America (NA) provide abundant examples of the negative consequences of plant invaders . For example, Bromus rubens L. (red brome) is the dominant invasive brome-grass in the Southwestern United States (USA) (Beatley 1966; Brooks 1999a, b; Salo 2005), and Bromus diandrus Roth. (ripgut brome) and Bromus hordeaceus L. (soft brome or chess) are invasive in California, especially the Central Valley (Dyer and Rice 1999; Gerlach and Rice 2003; Keeley et al. 2006; Brooks et al. 2015). In addition, the invasion of B. tectorum L. in sagebrush-steppe habitats of the Intermountain West (the Columbia Basin, especially the Snake River Plains, Basin and Range Province, and the Colorado Plateau) has contributed to the loss of native species diversity and community structure, modified ecosystem processes, such as nutrient cycling and productivity patterns, and altered disturbance regimes, especially the frequency, extent, and intensity of rangeland fires (Stewart and Hull 1949; Hulbert 1955; D’Antonio and Vitousek 1992; Knaap 1996; Sperry et al. 2006; Chambers et al. 2007; Mack 2011; Germino et al. 2015).
Three of the four species listed above, B. diandrus, B. rubens, and B. tectorum, as well as several other brome-grasses invasive in NA, occur in section Genea of the genus Bromus (Sales 1993, 1994). Consequently, we begin this chapter by describing evolutionary relationships among species in section Genea, which includes a polyploid series with diploid, tetraploid, hexaploid, and octoploid species. Bromus tectorum (cheatgrass, downy brome) will however be our main focus, given its enormous impacts as an invasive species in temperate grasslands in NA . We (1) describe the mating system of B. tectorum, based on seed set, floral characteristics, sex allocation patterns, and genetic markers, (2) present historical and genetic data to reconstruct the introduction and pattern of range expansion of B. tectorum in NA, and (3) assess the genetic consequences of these events by summarizing studies describing the level and structure of genetic diversity within and among native and invasive populations of B. tectorum. The results discussed here indicate that invasive populations of B. tectorum throughout much of NA have, on average, higher levels of genetic diversity than native populations, due to multiple introductions. Furthermore, the genetic consequences of multiple introductions have implications for the management of invasive populations of B. tectorum, especially in Western NA.
2 Evolutionary Relationships among Species in Section Genea
A context for assessing life history traits, ecology, and the potential for invasiveness of B. tectorum , and other species within section Genea, is provided by considering the species’ evolutionary history , phylogenetic relationships , and patterns of speciation (see Prinzing et al. 2002 for an interesting discussion of these issues). Such an assessment is particularly useful for the study of diploid species within this section and their roles in the origins of the polyploid taxa of this section.
Within the Poaceae , Bromus is classified in the tribe Bromeae, which is part of subfamily Pooideae (Catalan et al. 1997; Kellog 2001; Bouchenak-Khelladi et al. 2008; Schneider et al. 2009; Atkinson and Brown 2015). The genus Bromus consists of approximately 150–160 annual and perennial species; these species collectively have a worldwide distribution (Smith 1970; Acedo and Liamas 2001; Fortune et al. 2008). Smith (1970) assigned the species of Bromus to six sections, Bromus, Ceratochloa (P. Beauv.) Griseb., Genea Dumort., Neobromus Shear., Nevskiella V. Krecz and Vvevd., and Pnigma Dumort. Species within section Genea are hypothesized to have originated in the Middle East and the Mediterranean Region (Sales 1993, 1994), where they likely evolved concomitantly with the emergence of agriculture and human disturbance (Balfourier et al. 1998). In addition, these species exhibit much morphological variation, phenotypic plasticity, and polyploidy (Sales 1993, 1994). Seven species that form a polyploid series are recognized in section Genea: the diploids B. fasciculatus Presl., B. sterilis L., and B. tectorum, the tetraploids B. madritensis L. and B. rubens, the hexaploid B. rigidus Roth., and the octoploid B. diandrus Roth. (Smith 1970; Sales 1993, 1994; Acedo and Liamas 2001; Fortune et al. 2008). These species are native to Eurasia; all are invasive in the USA, with the exception of B. fasciculatus.
Fortune et al. (2008) elegantly assessed evolutionary relationships and patterns of speciation among these seven species. DNA sequence data from three gene regions were employed: the low copy nuclear gene Waxy , nuclear ribosomal DNA (rDNA) regions ITS1 and ITS2, and the chloroplast spacer regions trnT-trnL and trnL-trnF . Results from nuclear ITS regions and chloroplast DNA indicate that section Genea is monophyletic . The phylogenetic tree based on nucleotide sequence data from Waxy however indicates that section Genea is paraphyletic because this clade also includes species from section Bromus (Fortune et al. 2008). Two of the diploid species within section Genea, B. sterilis and B. tectorum, are sister species (i.e., they are more closely related to each other than any other species in this section); B. fasciculatus is more distantly related. Based on chloroplast DNA data, B. fasciculatus appears to have served as the maternal parent for the hybridization that produced the tetraploids B. madritensis and B. rubens, whereas the paternal parents for the two tetraploids appear to be B. sterilis and B. tectorum, respectively (Fig. 4.1). The hexaploid B. rigidus may have originated from a hybridization event involving B. rubens and an unknown diploid species, which served as the maternal parent (Fig. 4.1). The octoploid B. diandrus appears to have originated from a crossing event involving B. rigidus and B. sterilis as the maternal and paternal parents, respectively (Fig. 4.1).
Depiction of hypothesized origins of the polyploid species in section Genea of the genus Bromus, reproduced from Fortune et al. (2008). Molecular markers used in this analysis are listed in the table and include chlorotype (chloroplast spacer regions trnT-trnL and trnL-trnF), ITS (nuclear rDNA regions ITS1 and ITS2), and four Waxy (a low copy nuclear gene) nucleotide sequences. The table provides the identity of the genomes (F, S, T, and X) detected in the polyploid taxa based on different hybridization events. F = B. fasciculatus, S = B. sterilis, T = B. tectorum, and X = an unknown diploid species. The maternal parent in each cross is indicated (female symbol). See the text for a summary of the results shown in this figure
The above summarized results point to the reticulate (i.e., a netlike pattern of diversification) origins of the polyploid species in section Genea (Fortune et al. 2008). In addition, these data indicate that all seven species in this section have unique evolutionary histories. Thus, all four polyploid species in section Genea (B. madritensis, B. rubens, B. rigidus, and B. diandrus) are appropriately recognized as distinct, separate species. With the exception of B. fasciculatus, which is listed as endangered on Mediterranean islands (Fortune et al. 2008), the remaining six species in section Genea are considered weeds across much of their native range (Smith 1980). Traits that contributed to their weediness in the native range likely served as preadaptations (sensu Futuyma 2005), contributing to their establishment and subsequent invasions, especially in Western NA.
3 Mating Systems
In his classic paper “ The evolution of weeds ,” Baker (1974) described the characteristics of the “ideal weed,” and whereas no weed species exhibits all these characteristics, B. tectorum displays many. We concentrate here on the fifth characteristic in Baker’s list , which describes a weed’s potential mating system , “Self-compatible but not completely autogamous or apomictic.” The mating system (how much outcrossing versus self-pollination occurs) has a large influence on the biology and genetics of plant populations . The outcome of reproduction is directly associated with the mating system; plants with high rates of self-pollination (hereafter referred to as selfing ) typically have greater seed set compared with plants with an outcrossing mating system (Harmon-Threatt et al. 2009; Eckert et al. 2010). In addition, plants with a selfing mating system have lower levels of gene flow, through the movement of pollen, within and among populations. Thus, plant mating systems govern the spatial and temporal fluctuation of genotypes within and among populations: populations with high rates of selfing typically contain few individuals with heterozygous genotypes and many individuals with homozygous genotypes, compared to populations with high rates of outcrossing (Stebbins 1957; Allard et al. 1968; Barrett et al. 2008). Furthermore, species with high rates of selfing have lower levels of genetic diversity within populations and higher levels of genetic differentiation among populations, compared to species with high rates of outcrossing (Brown and Burdon 1987; Slatkin and Barton 1989; Ellstrand and Elam 1993; Sork et al. 1999).
3.1 Mating System of Bromus tectorum: Seed Set, Floral Characteristics, and Sex Allocation Pattern
McKone (1985, 1987, 1989) compared the mating systems of five Bromus species: B. inermis Leyss., B. latiglumis (Shear) Hitchc., B. ciliatus L., B. kalmii A. Gray, and B. tectorum. These grasses encompass a range of characteristics; they include species that are native and introduced in NA, perennials and annuals, rhizomatous and non-rhizomatous species, and species with diploid and polyploid cytotypes (see McKone 1985). In order to determine self-fertility, McKone (1985) measured the seed set of bagged inflorescences of all five species. He also documented floral characteristics , including the manner in which the florets of each species opened and the extent to which anthers were exserted. Both the bagged and open-pollinated inflorescences of B. tectorum had the highest seed set (McKone 1985). These results are consistent with previous reports for highly selfing plant species (Stebbins 1957) and indicate that B. tectorum is self-compatible and highly selfing. In addition, McKone (1985) made observations on floral characteristics of all five Bromus species and found that the florets of B. tectorum “…rarely (if ever) opened…” (p. 1336), and he “…never found anthers completely exserted from the florets” (p. 1335). These results indicate that B. tectorum produces cleistogamous flowers (i.e., they rarely open), which is associated with the production of progeny almost exclusively through selfing (see Campbell et al. 1983).
Sex allocation theory predicts equal paternal (pollen production) and maternal investment (seed production) for hermaphroditic plants (Charlesworth and Charlesworth 1981; Brunet 1992; Campbell 2000). McKone (1987) determined the relative paternal and maternal investment among the same five Bromus species listed above by measuring the ratio of pollen to seed production. He also determined the species’ absolute paternal and maternal investment by measuring the caloric energy and mineral content (nitrogen, phosphorous, potassium, magnesium, and calcium) of their reproductive structures. Of the five species, B. tectorum has the shortest anther length (0.65 mm), smallest pollen diameter (32.43 μm), least volume of pollen per floret (cm3 × 10−6), intermediate seed weight (1.99 mg), largest value for percent seed set per floret (79.0 %), and smallest ln (ratio of pollen volume to seed weight per floret) (2.28), compared with values for the obligate outcrosser B. inermis (4.02 mm, 41.61 μm, 424.1 cm3 × 10−6, 2.32 mg, 29.9 %, and 6.31, respectively) (McKone 1987). Estimates of sex allocation based on caloric energy and mineral content show that the paternal investment of B. tectorum was < 2 %, compared to approximately 50 % for B. inermis. Additionally, the cleistogamous flowers of B. tectorum produce less pollen relative to number of seeds produced, especially compared to B. inermis (McKone 1989). Bromus tectorum invests little in pollen production, results that are consistent with the ratio of paternal to maternal investment expected for a highly selfing plant species (Brunet 1992; Campbell 2000).
3.2 Mating System of Bromus tectorum: Genetic Markers and Outcrossing Rates
A species’ mating system can also be estimated using molecular markers that are expressed through codominance, such as allozymes ( enzyme electrophoresis) or microsatellite DNA (Avise 2004; Schlotterer 2004). Using codominant genetic markers , individuals with homozygous and heterozygous genotypes can be clearly identified based on their banding or chromatogram phenotypes (i.e., patterns). Two methods are commonly used to estimate mating system parameters using molecular data: progeny array analysis and Wright’s Coefficient of Inbreeding (Wright 1931; Clegg 1980; Brown 1990; Dudash and Fenster 2001). With progeny array analyses , the genotypes of 10–20 seeds from a maternal plant are determined; mating system parameters ( outcrossing rate t and selfing rate s) are estimated from these data, typically using a mixed mating system model that assumes both outcrossing and selfing are occurring (Ritland 2002). The Coefficient of Inbreeding , F, can be calculated from population genetic data based on the ratio of mean observed heterozygosity (H obs) to expected mean heterozygosity (H exp), using the equation F = 1 − H obs/H exp (Wright 1931; Dudash and Fenster 2001). The outcrossing rate is calculated as t f = (1 – F)/(1 + F), and the selfing rate is calculated as t s = 1 − t f.
Along with our students, we have assessed the genetic diversity within and among populations of B. tectorum, using enzyme electrophoresis (Novak et al. 1991; Novak and Mack 1993; Bartlett et al. 2002; Valliant et al. 2007; Schachner et al. 2008; Huttanus et al. 2011; Pawlak et al. 2015). To date, we have analyzed 10,150 individuals from 312 naturalized or invasive populations in NA and 1730 individuals from 51 native populations in Eurasia (Table 4.1), with the genetic diversity of each individual assayed at 25 putative enzyme loci . Heterozygous individuals have been detected (reported as H obs values) within populations from the Central USA, the mid-continent USA, California and the American Southwest, Eastern Canada, Western Canada, Europe, and Southwest Asia (Table 4.1).
Using data from these population genetic surveys , we have estimated mating system parameters ( outcrossing rate , t f, and selfing rate, t s) for populations of B. tectorum, employing Wright’s Coefficient of Inbreeding (Table 4.2). These results indicate that for six of the seven regions analyzed, the average outcrossing rate per population ranged from 0.11 % to 1.17 %, and the average selfing rate per population ranged from 98.83 % to 99.89 % (Table 4.2). Estimates of the mating system parameters for populations from Southwest Asia are in striking contrast; populations from this region had an outcrossing rate of 25 % and a selfing rate of 75 %. This outcome probably arose because low-frequency alleles were only d etected in individuals with heterozygous genotypes in several populations in Southwest Asia (Novak and Mack 1993); this result increased the value of H obs relative to that of H exp.
The estimated outcrossing rates (t) published by Meyer et al. (2013) are remarkably similar to the rates reported for six of the seven regions listed in Table 4.2. Meyer et al. (2013) estimated outcrossing rates for four “wild” populations of B. tectorum from the Western USA, which ranged from 0.0027 to 0.0133 (i.e., 0.27 % to 1.33 %, with selfing rates that ranged from 98.67 % to 99.73 %). Similar results (t = 0.0082, or 0.82 %) were obtained with analysis of individuals grown in a common garden (Meyer et al. 2013). The values of H obs reported in Table 4.2 are generally consistent with observed heterozygosity values previously reported for B. tectorum by Ramakrishnan et al. (2006), Kao et al. (2008), Leger et al. (2009), and Scott et al. (2010). In contrast, the high levels of observed heterozygosity reported by Ashley and Longland (2007, 2009) in two of four populations of B. tectorum from Northern Nevada are inconsistent with the values reported by other investigators. For instance, using seven microsatellite DNA loci, Ashley and Longland (2009) report an observed heterozygosity value of 0.06 for the Peavine Mountain population . This value is approximately 35- to 54-fold larger than values reported by Meyer et al. (2013) for the Lower Peavine (0.0017) and Upper Peavine (0.0011) populations, even though Meyer et al. (2013) used a more polymorphic marker system , 91–93 single nucleotide polymorphisms (SNPs) . This wide discrepancy among these results for populations of B. tectorum from a similar locality (Peavine Mountain) remains unresolved.
The preponderance of data indicates that B. tectorum re production is predominantly through selfing (as reported by McKone 1985); outcrossing rarely occurs within populations from NA. High selfing rates also appear to occur in European populations of B. tectorum (Table 4.2); consequently, this trait may form a preadaptation that has contributed to the establishment and invasion of B. tectorum in NA. Rare outcrossing events in populations that are comprised of genetic admixtures may generate novel, recombinant genotype s (Novak 2007). Such events may indicate post-immigration evolution (Maron et al. 2004; Lavergne and Molofsky 2007; Schierenbeck and Ellstrand 2009; Xu et al. 2010). Reproductive plasticity in B. tectorum (see Meyer et al. 2013) could in turn enhance the species’ invasion in NA (Huttanus et al. 2011). Whether or not post-immigration evolution is occurring within populations of invasive spec ies is not solely an intriguing theoretical question; it also holds important implications for the management of invasion s (Allendorf and Lundquist 2003; Sakai et al. 2001; Muller-Scharer et al. 2004; Le Roux and Wieczorek 2009).
4 Biological Invasions: Propagule Pressure , Preadaptation, and Post-immigration Evolution
Invasive species can have profoundly negative ecological, conservation, economic, and health consequences (D’Antonio and Vitousek 1992; Mack et al. 2000; Sala et al. 2000; Pimentel et al. 2005; Mack and Smith 2011) and have consequently been a focus of much deserved research. Identifying which species will become invasive and which communities are susceptible to invasion however remains elusive (Novak 2007), and our ability to predict which introduced species will become established (and potentially invasive) will likely be facilitated by gaining a better understanding of the invasion process (Estoup and Guillemaud 2010). Consequently, propagule pressure , defined as the number of individuals transported to and introduced into a new range (Lockwood et al. 2005), has emerged as one predictor of naturalization and the likelihood of invasion (Kolar and Lodge 2001; Lockwood et al. 2005; Colautti and MacIsaac 2004; von Holle and Simberloff 2005; Colautti et al. 2006; Simberloff 2009; Ricciardi et al. 2011). Simberloff (2009) described three components of propagule pressure : (1) propagule size, the number of founder individuals in a propagule; (2) propagule number, the rate at which propagules arrive per unit time; and (3) both propagule size and propagule number. Humans’ pervasive role as dispersal agents certainly increases the probability of high propagule pressure. With high propagule pressure, alien species are buffered from negative stochastic events during all phases of an invasion (Mack 2000), and large founder populations or multiple introduction events or both could characterize the release of an alien species in a new range.
Propagule pressure also has genetic consequences (Simberloff 2009; Huttanus et al. 2011): with high propagule pressure (e.g., multiple introductions ), the overall genetic and phenotypic diversity of a species would likely be increased in its new range, and the potential for severe founder effects would likely decrease (Novak and Mack 2005; Wares et al. 2005; Durka et al. 2005; Lavergne and Molofsky 2007; Dlugosch and Parker 2008). In addition, high propagule pressure may lead to the formation of introduced populations that are admixtures , i.e., introduced populations containing the genetic information of multiple native populations (Kolbe et al. 2007). Propagule pressure can also influence the likelihood of an invasion through the introduction of preadapted genotypes and by increasing the potential for post-immigration evolution.
Preadaptation during invasion involves the chance sampling of genotypes (and phenotypes) in one environment and their subsequent release into a new environment where they contribute to fitness (Futuyma 2005). Increased propagule pressure increases the likelihood of preadapted genotypes arriving in a new range, thus increasing the potential for invasion. The introduction of preadapted genotypes is likely to be the main mechanism leading to the invasion of some plant species, especially species that exhibit uniparental reproduction such as apomixis, vegetative reproduction, and selfing . The introduction of preadapted genotypes is implicated in the invasions of Capsella bursa-pastoris (Neuffer and Hurka 1999) in North America, the colonization of Hypericum canariense (Dlugosch and Parker 2007) in the Canary Islands, and the invasion of Chondrilla juncea (Gaskin et al. 2013) across three continents.
Post-immigration evolution is now widely recognized as an important contributor to invasion. Post-immigration evolution results from multiple introductions ; the descendants conjointly produce genetic admixtures , outcrossing or hybridization among individuals with different genotypes, genetic reshuffling or recombination, and generation of novel genotypes, leading to subsequent n atural selection (Lee 2002; Cox 2004; Facon et al. 2006; Novak 2007; Suarez and Tsutsui 2008; Prentis et al. 2008; Schierenbeck and Ellstrand 2009). Post-immigration evolution has been demonstrated repeatedly (e.g., Brown and Marshall 1981; Sexton et al. 2002; Leger and Rice 2003; Maron et al. 2004; Blair and Wolfe 2004; Brown and Eckert 2004; Lavergne and Molofsky 2007; Cano et al. 2008; Xu et al. 2010). Sufficient genetic diversity (especially additive genetic variance) within introduced populations is a prerequisite for post-immigration adaptive evolution. High propagule pressure increases the likelihood for high levels of genetic diversity and can set the stage for an increase in invasiveness through post-immigration evolution (Novak and Mack 2005; Wares et al. 2005). The multiple facets of evolution in an invasion suggest that it may be difficult to predict whether an invasion will occur because invasions unfold over widely varying periods of time (Novak 2007).
5 Introduction of B. tectorum in North America: Historical Information and Genetic Data
As pointed out above, assessing the propagule pressure of a biological invasion requires the number of founder individuals (founder population size), the rate at which propagules arrive (the number of discrete founder events), or both (Lockwood et al. 2005; Simberloff 2009). Such detailed demographic information is usually unavailable, especially for accidentally introduced plants, such as B. tectorum. As a result, attempts to determine patterns of introduction and range expansion for an invasive species (its propagule pressure) are almost always constructed retrospectively (Mack 2005). These investigations require multiple sources of data, including historical records and genetic analyses (Estoup and Guillemaud 2010; Estoup et al. 2010; Lombaert et al. 2011; Novak 2011; Gaskin et al. 2013).
We have attempted to reconstruct the introduction history and the concatenation in the range expansion for B. tectorum into and across NA by combining reliable historical sources (herbarium specimens and published accounts) with the results of molecular genetic analyses that employ enzyme electrophoresis (allozymes) (Novak et al. 1991, 1993; Novak and Mack 1993, 2001; Bartlett et al. 2002; Valliant et al. 2007; Schachner et al. 2008; Huttanus et al. 2011; Pawlak et al. 2015). Other studies assessing the genetic diversity of B. tectorum in NA using molecular markers either have analyzed only 2–10 populations from a limited geographic area (1–2 states) (Ramakrishnan et al. 2004; Ashley and Longland 2007, 2009; Kao et al. 2008; Leger et al. 2009; Scott et al. 2010) or have analyzed larger numbers of populations from across a portion of Western NA (Ramakrishnan et al. 2006; Merrill et al. 2012). Our reconstruction has been assembled through the analysis of 10,150 individuals from 312 populations distributed across NA (the USA and Canada). The main results of these studies are summarized here.
The earliest record of B. tectorum in NA of which we are aware is from Lancaster County, Pennsylvania , where the grass was collected no later than 1790 (Muhlenberg 1793). Unfortunately, Muhlenberg’s identification cannot be verified because his herbarium specimen has been lost, although he probably employed a European voucher specimen with which to identify the grass (Bartlett et al. 2002). The species was not reported again in the Eastern USA until a specimen was collected approx. 80 km east of Lancaster in West Chester, Pennsylvania, in 1859. This gap in detection could have arisen because populations of the plant were small and overlooked or the populations Muhlenberg saw went extinct and the species’ detection in the nineteenth century stems from reintroductions. In any event, the frequency of its collection in the Eastern USA increased markedly after 1860 (see Table 7, Bartlett et al. 2002). Although the geographic spread of these records (1860–1880) suggests that multiple introductions occurred, these data alone do not demonstrate this scenario.
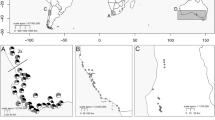
We determined the distribution of multilocus genotypes (MLGs ) and genetic diversity among 38 populations of B. tectorum from the Eastern USA to assess the grass’ pattern of introduction and regional spread (Bartlett et al. 2002). All 25 populations sampled at localities extending from Maine to Delaware are fixed for what we have termed the Most Common Genotype (MCG ) (Fig. 4.2). The MCG occurs most frequently in populations from the native and introduced ranges of B. tectorum and refers to the most common combination of alleles at the 25 scored loci (Novak et al. 1991; Novak and Mack 1993; Bartlett et al. 2002; Valliant et al. 2007; Schachner et al. 2008; Huttanus et al. 2011; Pawlak et al. 2015). The Pgm-1a & Pgm-2a MLG occurs in four Eastern US populations included in this analysis, and we also detected the Mdh-2b & Mdh-3b MLG in two populations (Fig. 4.2). Consequently, Eastern US populations of B. tectorum appear to have arisen from a minimum of two to three separate introductions. Additional introductions may have taken place but remain undetected because of the preponderance of the MCG in East Coast populations . The distribution of B. tectorum across NA suggests that the grass could have spread westward with European settlers. Alternatively, the species’ occurrence throughout NA may stem from separate introductions. These two hypotheses were tested through genetic analysis of populations of B. tectorum across the USA and Canada.
Multilocus genotypes for 38 populations of Bromus tectorum in the Eastern USA . Pie diagrams illustrate the proportions of each multilocus genotype in each population. (Sizes of the pie diagrams and any offset segments here and in Figs. 4.3–4.8 vary only to enhance legibility.) Lines accompanying pie diagrams indicate locations of the populations. Numbers are population designations from Bartlett et al. (2002)
The earliest collections of B. tectorum in the Central USA were made in the late 1880s and early 1890s (Mack 2011), 20–30 years after collections of the plant were being made with increasing frequency in the Eastern USA (Bartlett et al. 2002). If the species arrived in the Central USA with settlers that migrated westward, we predict that the same MLGs detected in Eastern US populations (or a subset of these genotypes) would also be detected among Central US populations. Alternatively, if the species arrived in this region through direct introduction(s) from the native range or from another region in NA, we would detect MLGs not found in the Eastern USA.
We analyzed 60 populations of B. tectorum from the Central USA using enzyme electrophoresis (Huttanus et al. 2011); six MLGs were detected (Fig. 4.3). Three of these genotypes (MCG, Pgm-1a & Pgm-2a, and Mdh-2b & Mdh-3b) match genotypes detected within Eastern US populations. This finding supports the hypothesis that B. tectorum was introduced into the Central USA as settlers from the East traveled westward. The MCG occurs in high frequency in the Central USA , the Pgm-1a & Pgm-2a MLG was detected in 34 of 60 populations, and the Mdh-2b & Mdh-3b genotype occurred at low frequency (four of 60 populations).
Three other MLGs were also detected at low frequency in populations in the Central USA : Got-4c, Pgm-1ab & Pgm-2ab, and Pgm-1a, Pgm-2a, Mdh-2b & Mdh-3b (Fig. 4.3). Only a single individual from the Valley Head, Alabama population displayed the Pgm-1ab & Pgm-2ab genotype; as a heterozygous genotype, it appears to be the product of a rare outcrossing event (Huttanus et al. 2011). The Pgm-1a, Pgm-2a, Mdh-2b & Mdh-3b MLG was detected in three populations and also occurs in a single individual collected in Martin, South Dakota (Fig. 4.4). This MLG may be a novel, recombinant genotype that arose in situ wherever plants with the Pgm-1a & Pgm-2a and Mdh-2b & Mdh-3b MLGs co-occurred (Huttanus et al. 2011). Alternatively, this genotype may have been introduced directly into the USA (e.g., near Daylight/Earle, Indiana, where it occurs at its highest frequency among sampled populations) and subsequently spread. This MLG has not been reported among native populations analyzed thus far (Novak and Mack 1993, 2001); its detection in the native range would be unlikely if indeed it is a novel, recombinant genotype. Only analysis of additional native populations will allow us to resolve this issue.
The occurrence of the Got-4c MLG in three populations in the Central USA is intriguing because this genotype was undetected among Eastern US populations (Fig. 4.2); the closest US population with this genotype is far to the west in Laramie, Wyoming (Fig. 4.4). Consequently, at least two scenarios explain the occurrence of the Got-4c MLG in the Central USA. It may have been introduced directly from the native range. Alternatively, the Got-4c genotype may have spread from Eastern Canada , where it has been detected in four of 13 populations in Southern Ontario (Fig. 4.5). Great Lakes commerce and shipping between the Central USA and Southeastern Canada predates 1800, and B. tectorum may have been dispersed by this traffic (Huttanus et al. 2011). This same traffic may provide an alternative explanation for the occurrence of the Pgm-1a & Pgm-2a MLG in the Central USA ; this genotype was detected in nine of 13 populations from Southern Ontario (Fig. 4.5). Thirty-two of the 60 populations (53.3 %) from the Central USA are genetically polymorphic (i.e., genetic admixtures ).
Multilocus genotypes for 51 populations of Bromus tectorum in Canada . This figure does not include nine population of B. tectorum from central British Columbia (see Fig. 4.8). These nine populations were omitted only to increase the readability of the figure. Pie diagrams illustrate the proportions of each multilocus genotype in each population. (See caption for Fig. 4.2 for further explanation of the pie diagrams.) Data are from Valliant et al. (2007)
The earliest herbarium specimens of B. tectorum from the northern portion of the mid-continent USA were collected in the 1890s, whereas the earliest specimens from the southern portion of this vast region were collected in the 1920s. These sizeable differences in the apparent timing of the entry of B. tectorum in the mid-continent USA may reflect different patterns of introduction, patterns that can also be detected through genetic analysis. Among the 54 mid-continent US populations of B. tectorum analyzed by Schachner et al. (2008), nine MLGs were detected: the MCG, Pgm-1a & Pgm-2a, Mdh-2b & Mdh-3b, Got-4c, Got-4d, Tpi-1b, Pgm-1a, Pgm-2a, Mdh-2b & Mdh-3b, Pgm-1ab & Pgm-2ab, and Mdh-2ab & Mdh-3ab (Fig. 4.4). Approximately one-half of the populations in the mid-continent USA (26 of 54) are genetic admixtures , i.e., they consist of two or more genotypes previously detected among native Eurasian populations (Novak and Mack 1993). The MCG occurs at high frequency (52 of 54 populations) in the mid-continent USA . The Pgm-1a & Pgm-2a MLG occurs in 24 of 54 populations from the mid-continent USA: 17 of these populations occur in the eight states in the region’s southern portion. Only three MLGs (MCG, Pgm-1a & Pgm-2a, and Pgm-1ab & Pgm-2ab) were detected in the eight states in this southern portion, whereas all nine MLGs found within the mid-continent USA were detected in populations from the eight states in the region’s northern portion (Fig. 4.4). These patterns of genotype distributions indicate that events leading to the establishment of B. tectorum in the northern and southern portions of the mid-continent USA arose via different scenarios.
The MCG is clearly prominent within the 26 widespread populations we sampled in the northern portion of the mid-continent USA . The Pgm-1a & Pgm-2a MLG is a distant second in prominence with detection in seven populations (Fig. 4.4). Other MLGs however have more restricted distributions . For instance, the Mdh-2b & Mdh-3b MLG, the Got-4d, and the Tpi-1b genotypes were found in two populations each. The Got-4c genotype was detected in a single population. All populations with these MLGs are located in either South Dakota or Eastern Wyoming . The seemingly scattered occurrence of populations with these MLGs indicates additional sampling is warranted to improve estimates of the regional genetic diversity of populations . In addition, field experimentation with B. tectorum populations could be conducted to determine whether this genetic diversity has contributed to greater invasiveness.
Five heterozygous individuals were encountered within three populations in the mid-continent USA (Fig. 4.4). The Pgm-1ab & Pgm-2ab heterozygous MLG was detected in two individuals from the Oskaloosa, Kansas, population and one individual from the Martin, South Dakota, population. Two individuals from the Colfax, North Dakota, population had the Mdh-2ab & Mdh-3ab genotype. These heterozygous individuals were the product of outcrossing events. The Pgm-1a, Pgm-2a, Mdh-2b & Mdh-3b genotype was observed in a single individual from the Martin, South Dakota population, and as discussed above, several mechanisms potentially explain its occurrence.
Several non-mutually exclusive scenarios potentially explain the occurrence of different MLGs among B. tectorum populations in the mid-continent USA, especially those in the region’s northern portion. European settlers migrating from the east may have dispersed some of these genotypes (e.g., the MCG, Pgm-1a & Pgm-2a, and Mdh-2b & Mdh-3b), some genotypes (e.g., Got-4c, Got-4d, and Tpi-1b) may have arrived from the Western USA, where they also occur (Fig. 4.6), and some may have been introduced directly from the native range. Ascertaining the role, if any, of these scenarios in the regional occurrence of genotypes will require a molecular marker with greater resolving power (e.g., microsatellite DNA or SNPs) than allozymes (Avise 2004; Schlotterer 2004; Estoup et al. 2010; Lombaert et al. 2011).
Multilocus genotypes for 40 populations of Bromus tectorum in the Western USA . Pie diagrams illustrate the proportions of each multilocus genotype in each population. (See caption for Fig. 4.2 for further explanation of the pie diagrams.) Numbers are the population designations from Novak et al. (1991)
The collection history of B. tectorum in the Intermountain West of NA is well documented (Mack 1981). The grass was first collected at Spences Bridge, British Columbia, in 1889; Ritzville, Washington, in 1893; Provo, Utah, in 1894; Pullman, Washington, in 1897, Eastern Colorado in 1897, Boise, Idaho, in 1899, and the Klamath Valley of Oregon in 1902. Similar to the introduction of B. tectorum in the Eastern USA, the chronology in the West suggests multiple introductions . Analysis of 40 populations from this region (which includes 26 populations from the Intermountain West and 14 populations from Nevada and California, see Table 4.1), using enzyme electrophoresis largely confirms this hypothesis (Novak et al. 1991).
Seven MLGs were identified among Western US populations : the MCG, Pgm-1a & Pgm-2a, Mdh-2b & Mdh-3b, Got-4c, Got-4d, Pgi-2b, and Got-4c, Mdh-2b & Mdh-3b (Fig. 4.6). Unlike the prominence of genotypes elsewhere in the USA, the MCG and the Got-4c genotype are equally common in the West. The Got-4c genotype occurs in 34 of 40 populations and is the only genotype (i.e., it is “fixed”) in six of these populations. The MCG occurs in 33 of 40 populations and is also fixed in six populations. Other MLGs occur at lower frequency : Mdh-2b & Mdh-3b occurs in six populations (four from Eastern Washington and two from Northern Nevada); Pgm-1a & Pgm-2a was detected in two populations (Gunnison, Colorado, and Tonasket, Washington), and Got-4d was detected in the populations from Provo, Utah, and Dubois, Idaho (Fig. 4.6). The Got-4c, Mdh-2b & Mdh-3b MLG was found only in the population at Emigrant Pass, Nevada, and appears to be a novel, recombinant genotype. The Pgi-2b MLG was detected in three populations near Reno, Nevada, and among several individuals in the population at Truckee, California (Fig. 4.6). In addition, this genotype has been also found at low frequency in seven populations from California and the American Southwest (the Southwestern USA) (Fig. 4.7). No heterozygous individuals were detected among the individuals from the 40 mostly Intermountain West populations we analyzed (Table 4.1). Among these 40 populations, most (28) were genetically polymorphic (i.e., genetic admixtures ).
Several scenarios potentially explain the occurrence of different MLGs among B. tectorum populations from the Western USA. The Got-4d genotype may have been introduced through long distance dispersal into Provo, Utah, an early collection site for B. tectorum in the West (Mack 1981). Other genotypes may have arrived from elsewhere in NA. For instance, the occurrence of plants with the Pgm-1a & Pgm-2a genotype at Gunnison, Colorado, may be the result of dispersal from mid-continent US populations, and the occurrence of this genotype in a population at Tonasket, Washington, may reflect spread from populations in nearby British Columbia. The Pgm-1a & Pgm-2a MLG has a high frequency of occurrence within populations in the southern portion of the mid-continent USA (Fig. 4.4) and in central British Columbia (Fig. 4.8).
Multilocus genotypes for 25 populations of Bromus tectorum in British Columbia . This figure includes nine population of B. tectorum from central British Columbia previously reported in Novak et al. (1991). Pie diagrams illustrate the proportions of each multilocus genotype in each population. (See caption for Fig. 4.2 for further explanation of the pie diagrams.) Data are from Valliant et al. (2007)
The earliest collection of B. tectorum in California of which we are aware was made in Siskiyou County in 1899 (Pawlak et al. 2015). Over the next 20 years, other collections were made in northern counties in California (Siskiyou, Shasta, and Humboldt). The conspicuous absence of any collection records well into the twentieth century in the San Francisco Bay area, a locale of intense plant collecting, strongly suggests that ships arriving in San Francisco Bay played no role in its introduction (Pawlak et al. 2015). The earliest collections in Southern California occurred in 1902 in Santa Cruz, Santa Barbara, and Ventura counties. Earliest collections in both northern and southern parts of Nevada were made in the early 1900s (Pawlak et al. 2015). The earliest collection in Arizona was in Coconino County in 1901.The collection record of B. tectorum in the rest of the American Southwest is exceptionally sparse, especially for Colorado and New Mexico. As indicated above, the earliest collection in Utah (1894) occurred in Provo.
Ten MLGs were detected among the 60 populations of B. tectorum from California and the American Southwest analyzed by Pawlak et al. (2015). Two MLGs are conspicuous in California and the American Southwest; the MCG occurs in 57 of 60 populations and is predominant in many, whereas the Got-4c genotype has a high frequency of occurrence within populations in Northern California and Southern Arizona (Fig. 4.6). The Pgi-2b genotype occurs at relatively low frequency in seven populations, three from Northern California, two from Nevada, and two from Utah. The Pgm-1a & Pgm-2a and Mdh-2b & Mdh-3b MLGs sporadically occur across the region, within three and four populations, respectively. Two populations in southeastern Colorado (Olathe and Hermosa) contain the Tpi-1b MLG. The only heterozygous individual we detected in this region (Tpi-1ab) was collected at Hermosa, Colorado. Three MLGs believed to be novel, recombinant genotypes may have arisen in situ among regional populations: Mdh-2b, Mdh-3b & Got-4c and Tpi-1b & Got-4c occur in populations in Southern Arizona (Turkey Flat and Mount Lemmon, respectively), and the Pgi-2b & Got-4c MLG was detected in a population from Northern California (Orleans).
The collection record and genetic analysis of B. tectorum populations from California and the American Southwest indicate that the introduction and regional spread of the grass were rapid and multidirectional and resulted in 31 of 60 (52 %) populations being comprised of genetic admixtures (i.e., polymorphic at one or more loci). For instance, the high frequency of Got-4c MLG in Northern California (Fig. 4.7) likely arose through the spread of B. tectorum southward from the Pacific Northwest; evidence for this pathway is provided by the high frequency of the Got-4c genotype among populations in Oregon (Fig. 4.6). The Got-4c MLG also has a high frequency of occurrence within populations in the mountains of Southern Arizona (Fig. 4.7). Postfire reseeding can inadvertently introduce nonnative plant species (Keeley et al. 2006). Perhaps B. tectorum with the Got-4c genotype arrived in Southern Arizona by this mode: forests on Mount Lemmon were extensively burned in 2003, and B. tectorum was first detected on the mountain 2 years later (L.A. Brigham, personal communication) (Pawlak et al. 2015).
In contrast, the MCG and other genotypes appear to have spread into California and the American Southwest from populations farther east. Finally, the Pgi-2b MLG has only been detected in 11 populations from California, Nevada, and Utah (Figs. 4.6 and 4.7). These are the only populations of B. tectorum in NA with this MLG, and the restricted distribution of this genotype likely stems from direct introduction from the native range, followed by dispersal (Pawlak et al. 2015).
The collection history of B. tectorum in Canada was described by Valliant et al. (2007). The oldest reported herbarium specimen for the grass in Canada was collected in Kingston, Ontario, in 1886 (see Fig. 4.3, Valliant et al. 2007). Other early collection sites for B. tectorum in Southern Ontario are ports along the Great Lakes. In Western Canada, B. tectorum was first collected in 1889 from an irrigated field near Spences Bridge, British Columbia, a site more than 3000 km west of Southern Ontario. A second pre-1900 collection of the grass in Western Canada was made on Vancouver Island. Additional collecting of B. tectorum in Southern Ontario in the early twentieth century appears to have lagged, while the grass was collected with increasing frequency in the arid interior of British Columbia (the Okanagan Valley) between 1901 and 1925 (Valliant et al. 2007).
As described earlier, this distinct bicoastal pattern of collection is consistent with multiple introductions for the grass. This hypothesis was tested with an enzyme electrophoresis analysis of 60 B. tectorum populations from across Canada (Valliant et al. 2007). In Eastern Canada, the MCG and the Pgm-1a & Pgm-2a MLG occur commonly, and two populations in Southern Ontario (Port Colborne and Alliston) contain individuals with the heterozygous genotype Pgm-1ab & Pgm-2ab (Fig. 4.5). The Got-4c genotype occurs in four populations in Southern Ontario. Although the frequency of this genotype in Eastern Canada is low (Fig. 4.5), it is higher than its frequency in populations in the eastern third of the USA (see Figs. 4.2 and 4.3). As observed in Eastern Canada , the MCG and the Pgm-1a & Pgm-2a genotype are commonly found within populations in Western Canada (Fig. 4.5). The Pgm-1a & Pgm-2a genotype was detected in populations from Manitoba, Saskatchewan, Alberta, and British Columbia. But its highest frequency (seven populations) occurs in central British Columbia (Fig. 4.8). Several genotypes were found in populations in Western Canada: the Tpi-1b MLG in two populations in British Columbia (Golden and Osoyoos) and the Tpi-1ab heterozygous genotype in a population at Osoyoos (Fig. 4.8). In Western Canada, the Got-4d genotype has been detected only in a population at Waterton, Alberta. In the Western USA, this same genotype had been previously reported from populations in Provo, Utah, and Dubois, Idaho (Fig. 4.6) (Novak et al. 1991, 1993). The area around Waterton, Alberta, was settled by people from Utah at the end of the nineteenth century, and their route to Alberta took them near Dubois, Idaho (Valliant et al. 2007). Whether B. tectorum with this genotype may have been spread from Provo, Utah, via Dubois, Idaho, to Waterton, Alberta, by these settlers remains an intriguing hypothesis.
6 Source Populations
Although the patterns described above are consistent with multiple introductions , definitive test of such introductions requires detecting the same genotype within native populations. We have conducted an enzyme electrophoresis analysis of 1730 individuals from 51 native population of B. tectorum in Eurasia (Novak and Mack 1993), and these data allow us to assess the species’ pattern of introduction into NA and identify source populations (or regions) for such introductions. We are not aware of any other published reports that have assessed and compared the genetic diversity (and the distribution of genotypes) of native and invasive populations of B. tectorum using the same molecular marker system.
For B. tectorum, many of the multilocus genotypes observed in NA are widely distributed across the continent. In contrast, the same genotypes often have restricted geographic distribution among native, Eurasian populations (Novak and Mack 1993). The exception to this general pattern is the MCG, which occurs across much of the species’ native range. If native genotypes have restricted geographic distributions (i.e., geographically structured), accurate identification of the geographic origins of an invasion is facilitated (Novak and Mack 2001; Novak 2011). The Pgm-1a & Pgm-2a MLG has been detected in the native range at Bratislava, Slovakia, and Vac, Hungary (Novak and Mack 1993, 2001). The Got-4c MLG occurs in some populations in Eastern NA and has its highest prominence in Western NA, whereas in the native range this genotype is known only from Libochovice, Czech Republic, and Bayreuth, Germany. In the native range, we have detected the Got-4d genotype in a single population in Vienna, Austria. In Western NA, the Pgi-2b genotype has been detected in 11 populations from California, Nevada, and Utah; in the native range, this genotype occurs in populations in France, Spain, and Morocco. In contrast, no published accounts report the Mdh-2b & Mdh-3b and Tpi-1b genotypes among native Eurasian populations. Based on these data, the geographic origins of the invasion of B. tectorum in NA appear to be among populations from Central Europe and the western Mediterranean region (Novak and Mack 1993, 2001). Identification of the sources for other genotypes in NA will require analysis of additional native populations and perhaps a different genetic marker.
7 Genetic Diversity within and among Populations of B. tectorum
If the above listed demographic data are unobtainable, propagule pressure can be estimated with molecular data (Ficetola et al. 2008; Ross and Shoemaker 2008; Goncalves da Silva et al. 2010; Huttanus et al. 2011). Huttanus et al. (2011) lists four patterns of genetic diversity that may reflect high propagule pressure: (1) the number of multilocus genotypes of a species across its new range would likely be large; (2) genetic admixtures , if propagule pressure results in the establishment of two or more independently derived native genotypes within invasive populations; (3) similar levels of genetic diversity within native and introduced populations and little evidence for founder effects ; and (4) if genetic admixtures are common, introduced populations will have similar, or even less, genetic structure than native populations.
The detection of seven homozygous, nonrecombinant multilocus genotypes among NA populations of B. tectorum indicates that multiple introductions have occurred, which in turn likely reflect moderate propagule pressure . In addition, 156 of the 312 (50 %) NA populations we have analyzed are genetically diverse, the products of either direct introduction events or dispersal of genotypes among populations. These populations are genetic admixtures .
The genetic diversity of a species, or group of populations, can be hierarchically partitioned across (among) and within populations, and the amount of diversity at these two levels can be compared to assess the genetic consequences of introduction and range expa nsion (Novak and Mack 2005). At the 25 scored loci, NA populations of B. tectorum have lower allelic richness (33 vs. 43) and fewer polymorphic loci (7 vs. 13) than native populations (Novak and Mack 1993, 2005), indicating that NA populations arose through the loss of genetic diversity by founder effects . Despite reduced genetic diversity across invasive populations, the amount of genetic diversity detected, on average, within populations from all regions in NA except one (Eastern USA) is higher than the diversity within populations in either of the major regions we denote in the grass’s native range (Table 4.1). Thus, at the within-population level, populati ons of B. tectorum from NA possess more genetic diversity than native populations.
Values of G ST for the populations of B. tectorum from all regions of NA (G ST = 0.241–0.582) are lower than the values for either European populations (G ST = 0.656) or populations from Southwest Asia (G ST = 0.735) (Table 4.1). These data indicate that the genetic structure among populations in NA is less than the genetic structure of populations from both regions in the native range, a likely outcome of multiple introductions . The propagule pressure associated with the intro duction of B. tectorum into NA from genetically diverse source populations and subsequent dispersal events appears to ha ve largely offset the reduction in genetic diversity associated with transcontinental m i gration.
8 Management Implications
The evolutionary history of species within section Genea, including B. tectorum (Sales 1993, 1994; Balfourier et al. 1998), serves as a guide to management of brome-grasses and B. tectorum in particular. First, disturbances (e.g., livestock grazing, off-road vehicle use) should be minimized in habitats within which Bromus species do not yet occur or are not yet abundant. Although the link between disturbance and invasion is well known (Hobbs and Huenneke 1992; Hierro et al. 2006), the information presented here places the relationship between the occurrence of these species and disturbance in an evolutionary context.
Results of the genetic analysis of B. tectorum across NA reveal the importance of multiple introductions of MLGs from different native source populations to the subsequent spread of these genotypes among NA populations. These events have resulted in genetic admixtures : one-half (156 of 312) of NA populations are comprised of genotypes from different native populations. This diversity complicates the management of B. tectorum in NA because no one control prescription is likely to be effective for all genotypes, especially in all habitats. Consequently, we advocate measures that reduce propagule pressure (i.e., reduce or eliminate additional introduction events). These efforts should certain ly include prevention through border inspections as well as early detection and rapid response, and eradication while the infested area remains small (Mack et al. 2000; Wittenberg and Cock 2005). In addition, gene flow should be deliberately limited (i.e., reduce propagule dispersal) among populations of B. tectorum; gene flow can produce populations with increased genetic diversity and bring together genotypes that have never co-occurred in the same population.
We also believe that the management of B. tectorum in NA can be enhanced by assessing the performance (survival, growth and reproduction) of plants with different MLGs across an array of environmental conditions. Based on this information, optimal habitat conditions for specific MLGs may be identified. If a stringent regime for genetic monitoring of B. tectorum populations were implemented (see below), land managers could specifically target certain MLGs for control because of the ability of plants with these genotypes to dominate a site. Such information on the performance of different MLGs could also be used to predict changes in the distribution and abundance of B. tectorum driven by climate change (see Hufft and Zelikova 2015).
The landscape level management of B. tectorum in NA by herbicides, tillage, and grazing has been largely ineffective (Mack 2011); consequently, a classical biological control program, which involves foreign exploration for natural enemies, such as insects or fungi, should be considered. Although use of classical biological control for invasive grasses has been viewed as problematic (McFadyen 1998), this method is gaining more support for particularly noxious grasses. For instance, biological control agents have been sought for Spartina alterniflora Loisel. in t he USA (Grevstad et al. 2003; Fisher et al. 2005) and Chilean needle grass (Nassella neesiana (Trin. and Rupr.) Barkworth in Australia and New Zealand (Anderson et al. 2010, 2011).
The information summarized in this chapter (source populations, reconstructing the introduction and range expansion of B. tectorum in its new range, and an assessment of the genetic diversity of invasive populations) is requisite for development of a classical biological control program (Roderick and Navajas 2003; Novak and Sforza 2008; Gaskin et al. 2011). For instance, the overall genetic diversity of B. tectorum in NA is low compared with native populations of the grass; consequently, as suggested by Muller-Scharer et al. (2004), only a few highly specialized control agents may be necessary. In such cases, control agents would probably exper ience fast population buildup and spread as they attack the target species. With multiple introductions , genotypes from several native source populations have been detected across NA, and under these circumstances, agents from different portions of the native range would be required for control (Burdon and Marshall 1981). Furthermore, several control agents may be needed within the same locality (Muller-Scharer et al. 2004), given the high frequency of genetic admixtures among invasive populations of B. tectorum. Finally, the geographic origins for the invasion of B. tectorum into NA appear to be in Central Europe and the western Mediterranean region. The search for biological control agents that are both specific and effective should be conducted w ithin these two regions (Goolsby et al. 2006; Gaskin et al. 2011).
9 Research Needs
The composite picture that emerges f rom the genetic analysis of B. tectorum summarized here is that multiple introductions into NA occurred, and range expansion of the grass across the continent was rapid and multidirectional. Future research should analyze both native and invasive populations of B. tectorum using genetic markers with higher resolving power than enzyme electrophoresis (e.g., microsatellite DNA, SNPs) to more precisely determine the number of introductions that have occurred in NA and the location of source populations within the native range. The genetic markers listed above are considered to be neutral to selection; thus care should be exercised when attempting to infer the adaptive significance of these data (Avise 2004). These markers can be instead used more reliably to infer demographic processes (e.g., dispersal and colonization events and the effective population size), detect population admixture, estimate mating system parameters, and assess parental (paternity) and kinship relationships (Schlotterer 2004).
Alternatively, the amount of genetic diversity within populations, especially the level of allelic richness within populations, is associated with a species’ evolutionary potential (Fisher 1958; Van Kleunen et al. 2000; Vergeer et al. 2003). To more accurately estimate evolutionary potential of B. tectorum, efforts need to be made to determine more precisely the amount of single-locus genetic diversity (measured using genetic markers), quantitative (ecologically important) trait variation (e.g., ecophysiological traits), and phenotypic plasticity within a nd among native and invasive populations of the grass within the same experimental design. For example, this approach was utilized by Lavergne and Molofsky (2007) to show that invasive populations of Phalaris arundinacea L. possess higher single-locus allelic diversity, quantitative trait variability, and phenotypic plasticity, compared with native populations; these features increased the evolutionary potential that contributed to this species’ invasion in NA.
Outcrossing events, although rare, have nonetheless led to the production of heterozygous individuals within populations of B. tectorum in several regions; legacies of such outcrossing events are novel, recombinant genotypes within populations in the Central USA, mid-continent USA, and California and the American Southwest. These findings suggest that post-immigration evolution is taking place, even within populations of this highly selfing species. Thus, future efforts should be taken to evaluate the extent to which these events influence the invasion of B. tectorum by monitoring populations using a genetic approach (see Novak and Rausch 2009). This genetic approach might first focus on marginal habitats or recently invaded sites, as described in Kao et al. (2008).
Future research could assess how the occurrence of heterozygous individuals and recombinant genotypes influence quantitative trait variation and phenotypic plasticity within invasive populations of B. tectorum. In the future, this effort could incorporate genomics and proteomics approaches that include DNA sequence analysis, quantitative trait loci (QTL) mapping, and microarray analysis of transcriptional regulation (Basu et al. 2004; Hudson 2008; Stinchcombe and Hoekstra 2008; Nadeau and Jiggins 2010; Ekblom and Galindo 2011). In addition, assessing the role of epigenetic variation and differentiation in the invasio n of B. tectorum may prove especially important because epigenetic mechanisms can result in phenotypic trait variability, even in the absence of genetic variation (Bossdorf et al. 2008; Richards et al. 2010, 2012). Finally, the manner in which single-locus genetic diversity, quantitative trait variation, phenotype plasticity, and epigenetics, singly and in combination, influences the invasion of B. tectorum in the USA under global climate change will need to be assessed (Reusch and Wood 2007; Salamin et al. 2010; Nicotra et al. 2010) if this most noxious member of Bromus section Genea is to be curbed.
References
Acedo C, Liamas F (2001) Variation of morphological characters of lemma and palea in the genus Bromus (Poaceae). Ann Bot Fenn 38:1–14
Allard RW, Jain SK, Workman PL (1968) The genetics of inbreeding populations. Adv Genet 14:55–131
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Anderson FE, Barton J, McLaren D (2010) Studies to assess the suitability of Uromyces pencanus as a biological control agents for Nassella neesiana (Poaceae) in Australia and New Zealand. Australas Plant Pathol 39:69–78
Anderson FE, Diaz ML, Barton J et al (2011) Exploring the life cycles of three South American rusts that have potential as biological control agent of the stipoid grass Nassella neesiana in Australia. Fungal Biol 115:370–380
Ashley MC, Longland WS (2007) Microsatellite evidence of facultative outcrossing in cheatgrass (Bromus tectorum): implications for the evolution of invasiveness. Plant Species Biol 22:197–204
Ashley MC, Longland WS (2009) Assessing cheatgrass (Bromus tectorum) genetic diversity and population structure using RAPD and microsatellite molecular markers. Western N Am Nat 69:63–74
Atkinson SY, Brown CS (2015) Attributes that confer invasiveness and impacts across the large genus Bromus – lessons from the Bromus REEnet database. In: Germino MJ, Chambers JC, Brown CS (eds) Exotic brome-grasses in arid and semiarid ecosystems of the Western US: causes, consequences, and management implications. Springer, New York, NY (Chapter 6)
Avise JC (2004) Molecular markers, natural history, and evolution. Sinauer, Sunderland, MA
Baker HG (1974) The evolution of weeds. Annu Rev Ecol Syst 5:1–24
Balfourier F, Charmet G, Ravel C (1998) Genetic differentiation within and between natural populations of perennial and annual ryegrass (Lolium perenne and L. rigidum). Heredity 81:100–110
Barrett SCH, Colautti RI, Eckert CG (2008) Plant reproductive systems and evolution during biological invasion. Mol Ecol 17:373–383
Bartlett E, Novak SJ, Mack RN (2002) Genetic variation in Bromus tectorum (Poaceae): differentiation in the eastern United States. Am J Bot 89:602–612
Basu C, Halfhill MD, Mueller TC et al (2004) Weed genomics: new tools to understand weed biology. Trends Plant Sci 9:391–398
Beatley JC (1966) Ecological status of introduced brome grasses (Bromus spp.) in desert vegetation of southern Nevada. Ecology 47:548–554
Blair AC, Wolfe LM (2004) The evolution of an invasive plant: an experimental study with Silene latifolia. Ecology 85:3035–3042
Bossdorf O, Richards CL, Pigliucci M (2008) Epigenetics for ecologists. Ecol Lett 11:106–115
Bouchenak-Khelladi Y, Salamin N, Saolainen V et al (2008) Large multigene phylogenetic trees of the grasses (Poaceae): progress towards complete tribal and generic level sampling. Mol Phylogenet Evol 47:488–505
Bromilow C (1995) Problem plants of South Africa. Briza Press, Arcadia, South Africa
Brooks ML (1999a) Habitat invasibility and dominance by alien annual plants in the western Mojave Desert. Biol Invasions 1:325–337
Brooks ML (1999b) Alien annual grasses and fire in the Mojave Desert. Madrono 46:13–19
Brooks ML, Belnap J, Brown CS et al (2015) Exotic annual Bromus invasions - comparisons among species and ecoregions in the Western United States. In: Germino MJ, Chambers JC, Brown CS (eds) Exotic brome-grasses in arid and semiarid ecosystems of the Western US: causes, consequences, and management implications. Springer, New York, NY (Chapter 2)
Brown AHD (1990) Genetic characteristics of plant mating systems. In: Brown AHD, Glegg MT, Kahler AL et al (eds) Plant population genetics, breeding and genetic resources. Sinauer, Sunderland, MA, pp 145–162
Brown AHD, Burdon JJ (1987) Mating systems and colonizing success in plants. In: Gray AJ, Crawley MJ, Edwards PJ (eds) Colonization, succession and stability. The 26th symposium of the British Ecological Society held jointly with the Linnean Society of London. Blackwell, Oxford, pp 115–131
Brown AHD, Marshall DR (1981) Evolutionary changes accompanying colonization in plants. In: Scudder GG, Reveal JL (eds) Evolution today. Hunt Institute of Botanical Documents, Carnegie-Mellon University Press, Pittsburgh, PA, pp 351–363
Brown JS, Eckert CG (2004) Evolutionary increase in sexual and clonal reproductive capacity during biological invasion in an aquatic plant Butomus umbellatus. Am J Bot 92:495–502
Brunet J (1992) Sex allocation in hermaphroditic plants. Trends Ecol Evol 3:79–84
Burdon JJ, Marshall DR (1981) Biological control and the reproductive mode of weeds. J Appl Ecol 18:649–658
Campbell CS, Quinn JA, Cheplick GP et al (1983) Cleistogamy in grasses. Annu Rev Ecol Syst 14:411–441
Campbell DR (2000) Experimental tests of sex-allocation theory in plants. Trends Ecol Evol 15:227–232
Cano L, Escarre J, Fleck I et al (2008) Increased fitness and plasticity of an invasive species in its introduced range: a study using Senecio pterophorus. J Ecol 96:468–476
Catalan P, Kellogg EA, Olmstead RG (1997) Phylogeny of Poaceae subfamily Pooideae based on chloroplast ndhF gene sequences. Mol Phylogenet Evol 8:150–166
Chambers JC, Roundy BA, Blank RR et al (2007) What makes Great Basin sagebrush steppe ecosystems invasible by Bromus tectorum. Ecol Monogr 77:117–145
Charlesworth D, Charlesworth B (1981) Allocation of resources to male and female functions in hermaphrodites. Biol J Linn Soc 15:57–74
Clegg MT (1980) Measuring plant mating systems. BioScience 30:814–818
Colautti RI, MacIsaac HJ (2004) A neutral terminology to define “invasive” species. Divers Distrib 10:135–141
Colautti RI, Grigorovich IA, MacIsaac HJ (2006) Propagule pressure: a null model for biological invasions. Biol Invasions 8:1023–1037
Cox GW (2004) Alien species and evolution: the evolutionary ecology of exotic plants, animals, microbes, and interacting native species. Island Press, Washington, DC
D’Antonio CM, Vitousek PM (1992) Biological invasion by exotic grasses, the grass/fire cycle and global change. Annu Rev Ecol Syst 23:63–87
D’Antonio CM, Hughes RF, Tunison JT (2011) Long-term impacts of invasive grasses and subsequent fire in seasonally dry Hawaiian woodlands. Ecol Appl 21:1617–1628
Dlugosch KM, Parker IM (2007) Molecular and quantitative trait variation across the native range of the invasive species Hypericum canariense: evidence for ancient patterns of colonization via pre-adaptation? Mol Ecol 16:4269–4283
Dlugosch KM, Parker IM (2008) Founder events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Dudash MR, Fenster CB (2001) The role of breeding system and inbreeding depression in the maintenance of an outcrossing mating strategy in Silene virginica (Caryophyllaceae). Am J Bot 88:1953–1959
Durka W, Bossdorf O, Prati D et al (2005) Molecular evidence for multiple introductions of garlic mustard (Alliaria petiolata, Brassicaceae) to North America. Mol Ecol 14:1697–1706
Dyer AR, Rice KJ (1999) Effects of competition on resource availability and growth of a California bunchgrass. Ecology 80:2697–2710
Ekblom R, Galindo J (2011) Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 107:1–15
Eckert CG, Kalisz S, Geber MA et al (2010) Plant mating systems in a changing world. Trends Ecol Evol 25:35–43
Ellstrand NC, Elam DR (1993) Population genetic consequences of small population size: implications for plant conservation. Annu Rev Ecol Syst 24:217–242
Estoup A, Guillemaud T (2010) Reconstructing routes of invasion using genetic data: why, how and so what? Mol Ecol 19:4113–4130
Estoup A, Baird SJE, Ray N et al (2010) Combining genetic, historical and geographical data to reconstruct the dynamics of bioinvasions: application to the cane toad Bufo marinus. Mol Ecol Resour 10:886–901
Facon B, Genton BJ, Shykoff J et al (2006) A general eco-evolutionary framework for understanding bioinvasions. Trends Ecol Evol 21:130–135
Ficetola GF, Bonin A, Miaud C (2008) Population genetics reveals origin and number of founders in a biological invasion. Mol Ecol 17:773–782
Fisher AJ, DiTomasso JM, Gordon TR (2005) Intraspecific groups of Claviceps purpurea associated with grass species in Willapa Bay, Washington, and the prospects for biological control of invasive Spartina alterniflora. Biol Control 34:170–179
Fisher RA (1958) The genetical theory of natural selection. Dover, New York, NY
Fortune PM, Pourtau N, Viron N et al (2008) Molecular phylogeny and reticulate origins of the polyploidy Bromus species from section Genea (Poaceae). Am J Bot 95:454–464
Futuyma DJ (2005) Evolution. Sinauer, Sunderland, MA
Gaskin JF, Bon M-C, Cock MJW et al (2011) Applying molecular-based approaches to classical biological control of weeds. Biol Control 58:1–21
Gaskin JF, Schwarzlander M, Kinter CL et al (2013) Propagule pressure, genetic structure, and geographic origins of Chondrilla juncea (Asteraceae): an apomictic invader of three continents. Am J Bot 100:1871–1882
Gerlach JD, Rice KJ (2003) Testing life history correlates of invasiveness using congeneric plant species. Ecol Appl 13:167–179
Germino MJ, Belnap J, Stark JM et al (2015) Ecosystem impacts of exotic annual invaders in the genus Bromus. In: Germino MJ, Chambers JC, Brown CS (eds) Exotic brome-grasses in arid and semiarid ecosystems of the Western US: causes, consequences, and management implications. Springer, New York, NY (Chapter 3)
Goncalves da Silva A, Eberhard JR, Wright TF et al (2010) Genetic evidence for high propagule pressure and long-distance dispersal in monk parakeet (Myiopsitta monachus) invasive populations. Mol Ecol 19:3336–3350
Goolsby JA, De Barro PJ, Makinson JR et al (2006) Matching the origins of an invasive weed for selection of a herbivore haplotype for a biological control programme. Mol Ecol 15:287–297
Grevstad FS, Strong DR, Garcia-Rossi D et al (2003) Biological control of Spartina alterniflora in Willapa Bay, Washington using the planthopper Prokelisia marginata: agent specificity and early results. Biol Control 27:32–42
Harmon-Threatt AN, Burns JH, Shemyakina LA et al (2009) Breeding system and pollination ecology of introduced plants compared to their native relatives. Am J Bot 96:1544–1550
Hierro JL, Villarreal D, Eren O et al (2006) Disturbance facilitates invasion: the effects are stronger abroad than at home. Am Nat 168:144–156
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion: implications for conservation. Conserv Biol 6:324–337
Holm LG, Plucknett DL, Pancho JV et al (1977) The world’s worst weeds: distribution and biology. University Press of Hawaii, Honolulu, HI
Hudson ME (2008) Sequencing breakthroughs for genomic ecology and evolutionary biology. Mol Ecol Res 8:3–17
Hufft RA, Zelikova TJ (2015) Ecological Genetics, Local Adaptation, and Phenotypic Plasticity in Bromus tectorum in the Context of a Changing Climate. In: Germino MJ, Chambers JC, Brown CS (eds) Exotic brome-grasses in arid and semiarid ecosystems of the Western US: causes, consequences, and management implications. Springer, New York, NY (Chapter 5)Hulbert LC (1955) Ecological studies of Bromus tectorum and other annual bromegrasses. Ecol Monogr 25:181–213
Huttanus TD, Mack RN, Novak SJ (2011) Propagule pressure and introduction pathways of Bromus tectorum (cheatgrass; Poaceae) in the Central United States. Int J Plant Sci 172:783–794
Kao RH, Brown CS, Hufbauer RA (2008) High phenotypic and molecular variation in downy brome (Bromus tectorum). Invasive Plant Sci Manag 1:216–225
Keeley JE, Allen CD, Betancourt J et al (2006) A 21st century perspective on postfire seeding. J Forestry 104:103–110
Kellog EA (2001) Evolutionary history of grasses. Plant Physiol 125:1198–1205
Knaap PA (1996) Cheatgrass (Bromus tectorum L.) dominance in the Great Basin desert: history, persistence, and influence of human activities. Glob Environ Chang 6:37–52
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204
Kolbe JJ, Larson A, Losos JB (2007) Differential admixture shapes morphological variation among invasive populations of the lizard Anolis sagrei. Mol Ecol 16:1579–1591
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci U S A 104:3883–3888
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Leger EA, Rice KJ (2003) Invasive California poppies (Eschscholzia californica Cham.) grow larger than native individuals under reduced competition. Ecol Lett 6:257–264
Leger EA, Espeland EK, Merrill KR et al (2009) Genetic variation and local adaptation at a cheatgrass (Bromus tectorum) invasion edge in western Nevada. Mol Ecol 18:4366–4379
Le Roux J, Wieczorek AM (2009) Molecular systematics and population genetics of biological invasions: towards a better understanding of invasive species management. Ann Appl Biol 154:1–17
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lombaert E, Guillemaud T, Thomas CE et al (2011) Inferring the origin of populations introduced from a genetically structured native range by approximate Bayesian computation: case study of the invasive ladybird Harmonia axyridis. Mol Ecol 20:4654–4670
Mack RN (1981) Invasion of Bromus tectorum L. into western North America: an ecological chronicle. Agro-Ecosystems 7:145–165
Mack RN (1989) Temperate grasslands vulnerable to plant invasion: characteristics and consequences. In: Drake J (ed) Biological invasions: a global perspective. Wiley, New York, NY, pp 155–179
Mack RN (2000) Cultivation fosters plant naturalization by reducing environmental stochasticity. Biol Invasions 2:111–122
Mack RN (2005) Assessing biotic invasions in time and space: the second imperative. In: Mooney HA, Mack RN, McNeely JA et al (eds) Invasive alien species: a new synthesis. Island Press, Washington, DC, pp 179–208
Mack RN (2011) Fifty years of “Waging war on cheatgrass”: research advances, while meaningful control languishes. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton. Wiley-Blackwell, Oxford, pp 253–265
Mack RN, Smith MC (2011) Invasive plants as catalysts for the spread of human parasites. NeoBiota 9:13–29
Mack RN, Simberloff D, Lonsdale WM et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Maron JL, Montserrat V, Bommarco R et al (2004) Rapid evolution of an invasive plant. Ecol Monogr 74:261–280
McFadyen REC (1998) Biological control of weeds. Annu Rev Entomol 43:369–393
McKone MJ (1985) Reproductive biology of several bromegrasses (Bromus): breeding system, pattern of fruit maturation, and seed set. Am J Bot 72:1334–1339
McKone MJ (1987) Sex allocation and outcrossing rate: a test of theoretical predictions using bromegrasses (Bromus). Evolution 41:591–598
McKone MJ (1989) Intraspecific variation in pollen yield in bromegrass (Poaceae; Bromus). Am J Bot 76:231–237
Merrill KR, Meyer SE, Coleman CE (2012) Population genetic analysis of Bromus tectorum (Poaceae) indicates recent range expansion may be facilitated by specialist genotypes. Am J Bot 99:529–537
Meyer SE, Ghimire S, Decker S et al (2013) Ghost of outcrossing past in downy brome, an inbreeding annual grass. J Hered 104:476–490
Muhlenberg H (1793) Index Florae Lancastriensis. Trans Am Philos Soc Philadel 3:157–184
Muller-Scharer H, Schaffner U, Steinger T (2004) Evolution in invasive plants: implications for biological control. Trends Ecol Evol 19:417–422
Nadeau NJ, Jiggins CD (2010) A golden age of evolutionary genetics? Genomic studies of adaptation in natural populations. Trends Genet 26:484–492
Neuffer B, Hurka H (1999) Colonization history and introduction dynamics of Capsella bursa-pastoris (Brassicaceae) in North America: isozymes and quantitative traits. Mol Ecol 8:1667–1681
Nicotra AB, Atkin OIK, Bonser SP et al (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15:684–692
Novak SJ (2007) The role of evolution in the invasion process. Proc Natl Acad Sci U S A 104:3671–3672
Novak SJ (2011) Geographic origins and introduction dynamics. In: Simberloff D, Rejmanek M (eds) Encyclopedia of biological invasions. University of California Press, Berkeley, CA, pp 273–280
Novak SJ, Mack RN (1993) Genetic variation in Bromus tectorum (Poaceae): comparison between native and introduced populations. Heredity 71:167–176
Novak SJ, Mack RN (2001) Tracing plant introduction and spread: genetic evidence from Bromus tectorum (Cheatgrass). BioScience 51:114–122
Novak SJ, Mack RN (2005) Genetic bottlenecks in alien plant species: influence of mating systems and introduction dynamics. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution and biogeography. Sinauer, Sunderland, MA, pp 201–228
Novak SJ, Rausch JH (2009) Use of field surveys, distributional data and genetic analyses to monitor alien species: Taeniatherum caput-medusae as an example of the approach. NeoBiota 8:169–182
Novak SJ, Sforza R (2008) Genetic analysis of native and introduced populations of Taeniatherum caput-medusae (Poaceae): implications for biological control. In: Julien MH, Sforza R, Bon M-C et al (eds) Proceedings of the XII international symposium on biological control of weeds. CAB International, Wallingford, pp 422–428
Novak SJ, Mack RN, Soltis DE (1991) Genetic variation in Bromus tectorum (Poaceae): population differentiation in its North American range. Am J Bot 78:1150–1161
Novak SJ, Mack RN, Soltis PS (1993) Genetic variation in Bromus tectorum (Poaceae): introduction dynamics in North America. Can J Bot 71:1441–1448
Parsons WT, Cuthbertson EG (1992) Noxious weeds of Australia. Inkata Press, Melbourne
Pawlak AR, Mack RN, Busch JW et al (2015) Invasion of Bromus tectorum (L.) into California and the American Southwest: rapid, multi-directional and genetically diverse. Biol Invasions 17(1):287–306
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Prentis PJ, Wilson JRU, Dormontt EE et al (2008) Adaptive evolution in invasive species. Trends Ecol Evol 13:288–294
Prinzing A, Durka W, Klolz S et al (2002) Which species become aliens? Evol Ecol Res 4:385–405
Ramakrishnan AP, Meyer SE, Waters J et al (2004) Correlation between molecular markers and adaptively significant genetic variation in Bromus tectorum. (Poaceae), an inbreeding annual grass. Am J Bot 91:797–803
Ramakrishnan AP, Meyer SE, Fairbanks DJ et al (2006) Ecological significance of microsatellite variation in western North American populations of Bromus tectorum. Plant Species Biol 21:61–73
Reusch TB, Wood TE (2007) Molecular ecology of global change. Mol Ecol 16:3973–3992
Ricciardi A, Jones L, Kestrup ǺM et al (2011) Expanding the propagule pressure concept to understand the impact of biological invasions. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton. Wiley-Blackwell, Oxford, pp 225–235
Richards CL, Bossdorf O, Pigliucci M (2010) What role does heritable epigenetic variation play in phenotypic evolution? BioScience 60:232–237
Richards CL, Schrey AW, Pigliucci M (2012) Invasion of diverse habitats by few Japanese knotweed genotypes is correlated with epigenetic differentiation. Ecol Lett 15:1016–1025
Ritland K (2002) Extensions of models for the estimation of mating systems using independent loci. Heredity 88:221–228
Roderick GK, Navajas M (2003) Genes in new environment: genetics and evolution in biological control. Nat Genet 4:889–899
Ross KG, Shoemaker DD (2008) Estimation of the number of founders of an invasive pest insect population: the fire ant Solenopsis invicta in the USA. Proc R Soc B 275:2231–2240
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Sala OE, Chapin FS III, Armesto JJ et al (2000) Global diversity scenarios for the year 2100. Science 287:1770–1774
Salamin N, Wuest RO, Lavergne S et al (2010) Assessing rapid evolution in a changing environment. Trends Ecol Evol 25:692–698
Sales F (1993) Taxonomy and nomenclature of Bromus sect. Genea. Edinb J Bot 50:1–31
Sales F (1994) Evolutionary tendencies in some annual species of Bromus (Bromus L. section Genea Dum. (Poaceae)). Bot J Linn Soc 115:197–210
Salo LF (2005) Red brome (Bromus rubens subsp. madritensis) in North America: possible modes for early introduction, subsequent spread. Biol Invasions 7:165–180
Schachner LJ, Mack RN, Novak SJ (2008) Bromus tectorum (Poaceae) in midcontinental United States: population genetic analysis of an ongoing invasion. Am J Bot 95:1584–1595
Schlotterer C (2004) The evolution of genetic markers—just a matter of fashion? Nat Rev Genet 5:63–69
Scott JW, Meyer SE, Merrill KR et al (2010) Local population differentiation in Bromus tectorum L. in relationship to habitat-specific selection regimes. Evol Ecol 24:1061–1080
Schierenbeck KA, Ellstrand NC (2009) Hybridization and the evolution of invasiveness of plants and other organisms. Biol Invasions 11:1093–1105
Schneider J, Doring E, Hilu KW et al (2009) Phylogenetic structure of the grass subfamily Pooideae based on comparison of plastid matK gene – 3′ trnK exon and nuclear ITS sequences. Taxon 58:405–424
Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12:1651–1660
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Slatkin M, Barton NH (1989) A comparison of three indirect methods for estimating average levels of gene flow. Evolution 43:1349–1368
Smith PM (1970) Taxonomy and nomenclature of the brome-grasses (Bromus L. s.l.). Notes from the Royal Botanical Garden Edinburgh 30: 361–375
Smith PM (1980) Bromus L. In: Tutin TG, Heywood VH, Burges NA et al (eds) Florae europaea, vol 5. Cambridge University Press, Cambridge, pp 182–189
Sork VL, Nason J, Campbell DR (1999) Landscape approaches to historical and contemporary gene flow in plants. Trends Ecol Evol 14:219–224
Sperry LJ, Belknap J, Evans RD (2006) Bromus tectorum invasion alters nitrogen dynamics in undisturbed arid grassland ecosystems. Ecology 87:603–615
Stebbins GL (1957) Self-fertilization and population variability in higher plants. Am Nat 91:337–354
Stewart G, Hull AC (1949) Cheatgrass (Bromus tectorum L.)—an ecological intruder in southern Idaho. Ecology 30:58–74
Stinchcombe JR, Hoekstra HE (2008) Combining population genomics and quantitative genetics: finding the genes underlying ecologically important traits. Heredity 100:158–170
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Sumners WH, Archibold OW (2007) Exotic plant species in the southern boreal forest of Saskatchewan. For Ecol Manag 251:156–163
Valliant MT, Mack RN, Novak SJ (2007) Introduction history and population genetics of the invasive grass Bromus tectorum (Poaceae) in Canada. Am J Bot 94:1156–1169
Van Kleunen M, Fischer M, Schmidt B (2000) Clonal integration in Ranunculus reptans: by-product or adaptation? J Evol Biol 13:237–248
Vergeer P, Rengelink R, Ouborg NJ et al (2003) Effects of population size and genetic variation on the response of Succisa pratensis to eutrophication and acidification. J Ecol 91:600–609
von Holle B, Simberloff D (2005) Ecological resistance to biological invasions overwhelmed by propagule pressure. Ecology 86:3212–3218
Wares JP, Hughes AR, Grosberg RK (2005) Mechanisms that drive evolutionary change: insights from species introductions and invasions. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution and biogeography. Sinauer, Sunderland, MA, pp 229–257
Wittenberg R, Cock MJW (2005) Best practices for the prevention and management of invasive alien species. In: Mooney HA, Mack RN, McNeely JA et al (eds) Invasive alien species, a new synthesis. Island Press, Washington, DC, pp 209–232
Wright S (1931) Evolution in Mendelian populations. Genetics 16:97–159
Xu C-Y, Julien MH, Fatemi M et al (2010) Phenotypic divergence during the invasion of Phyla canescens in Australia and France: evidence for selection-driven evolution. Ecol Lett 13:32–44
Acknowledgments
It would not have possible to conduct the research summarized in this chapter without the assistance of countless people who over the years collected population samples of B. tectorum around the world. We thank them all. In addition, we thank our graduate students at Washington State University and Boise State University who have played a major role in many of these studies: Elizabeth Bartlett, Morgan Valliant, Lauren Schachner, Temsha Huttanus, and Angela Pawlak. We gratefully thank Rich Scott for his work on the figures (maps) presented in this chapter, and we especially thank him for his interest in this research over the years. SJN completed portions of this chapter while on sabbatical leave at and visiting the European Biological Control Laboratory, USDA-ARS, Montferrier-sur-Lez, France. He is extremely grateful to the personnel of this laboratory, especially Rene Francois Henri Sforza, Daniel Strickman, and Kim Hoelmer, for the opportunity to work at the lab.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Novak, S.J., Mack, R.N. (2016). Mating System, Introduction and Genetic Diversity of Bromus tectorum in North America, the Most Notorious Product of Evolution Within Bromus Section Genea . In: Germino, M., Chambers, J., Brown, C. (eds) Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US. Springer Series on Environmental Management. Springer, Cham. https://doi.org/10.1007/978-3-319-24930-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-24930-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24928-5
Online ISBN: 978-3-319-24930-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)