Abstract
Increasing knowledge and growing concern about the elevated cost of inorganic fertilizers or chemical pesticides with their vast applications on various crop plants has raised interest in the alternative method of plant disease protection caused by plant parasitic nematodes. These alternative methods are not only cost-effective but also eco-friendly to the environment and human health. Among the various rhizospheric microorganisms, opportunistic fungi like Paecilomyces lilacinus, Pochonia chlamydosporia, and arbuscular mycorrhizal (AM) fungi have the potential to reduce the severity of diseases caused by plant parasitic nematodes and also improved the plant growth and biomass production. This chapter provides an overview on the biocontrol potential of opportunistic as well as AM fungi on the growth and development of various crop plants. The details about the interactions between these fungi and plant parasitic nematodes have been discussed. An overview of the recent cost-effective technologies used for the mass propagation of these beneficial rhizospheric microorganisms is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
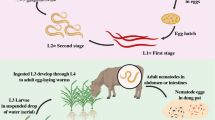
Nematodes are filiform roundworms belonging to phylum Nematoda commonly found in plants, animals, and soil. They have the ability to utilize the various organic sources for the production of energy (Akhtar and Panwar 2011). Some plant parasitic nematodes usually feed on plant cells by choosing and establishing a single feeding site known as sedentary feeders, while others are migratory feeders which means they move from site to site on the root and rarely feed on plant single cell. In general, the plant parasitic nematodes are documented as the utmost vicious pests for several economically important crops worldwide. Bowers et al. (1996) reported that the nematode had the ability to alter the root exudates in qualitative and quantitative fashion, which may influence the activity of beneficial and pathogenic microbes in the rhizosphere. The estimated average annual yield loss of various crops by plant parasitic nematodes is about 12.3 % (Sasser and Freckman 1987), but it varies from 8.8 to 14.6 % from developed to developing countries (Nicol et al. 2011; Palomares-Rius and Kikuchi 2013). Among the sedentary feeders, Meloidogyne species are predominant and are considered as the most damaging genera throughout the world. About 95 % of the total nematode populations are represented only by four major species such as M. incognita, M. javanica, M. arenaria, and M. hapla.
Suppression of plant diseases in the presence of a pathogen, suitable host plant, and favorable climatic conditions is known as soil suppressiveness (Mazzola et al. 2004; Weller et al. 2007). It is directly associated with the nature and fertility level of the soil and the types of soil microorganisms. However, the level of disease suppressiveness is directly proportional to the level of soil microbial activity, meaning the larger the active microbial biomass, the greater the soil capacity to use carbon, nutrients, and energy, thus lowering their availability to pathogens (Kumar et al. 2012). Any treatment to increase the microbial activity in the soil enhanced the suppression of pathogens by increasing competition for nutrients, but overall it is a very tough task to control all types of soilborne pathogens by suppressive soils. To control the diseases caused by plant parasitic nematodes, frequent use of chemical nematicides has been increased in the past few decades globally (Gupta and Dikshit 2010; Leng et al. 2011). But these chemical nematicides possess several toxic effects on the human health, soil microbiota, and environment. Thus, several cultural practices have been adopted for the management of nematodes, but gradually the annual losses observed in the quality and quantity of crop yields revealed that there is a decisive need to develop a new eco-friendly way to control the plant parasitic nematodes. In this regard, biological control strategies provide an alternative tool for management of plant parasitic nematodes over the conventional chemical control strategies (Mazzola 2007). The biological control of nematodes could be achieved either by managing the natural habitats to marmalade by increasing the activity of native fungi or by introducing new beneficial rhizospheric fungi or by the combination of both (Timper 2011). Nevertheless, the augmentation of the beneficial microorganisms in the agricultural fields and their potential benefits on the various crops is feasible through the adoption of various management practices such as reduced tillage, crop rotation, and lowering the micronutrient uses.
The rhizosphere is the immediate microenvironment surrounding the plant roots which provides novel environments for microbes due to change in increased levels of nutrients and intense microbial population (Giri et al. 2005; Gupta et al. 2012; Yadav et al. 2015). The rhizoplane and the surrounding rhizosphere soil are colonized and occupied by a wide range of microorganisms. Of the various microorganisms present, opportunistic fungi and arbuscular mycorrhizal (AM) fungi play a key role in the biocontrol of diseases caused by plant parasitic nematodes. Consequently, the plant parasitic nematode and beneficial rhizospheric fungi share common ecological niche and also influenced the plant growth and yield attributes in various means (Akhtar and Siddiqui 2008; Akhtar and Panwar 2011). Because of multifaceted nature, it is very hard to generalize the overall underground interaction processes taking place between the plant parasitic nematodes, opportunistic fungi, and AM fungi. The aim of this chapter is to provide an overview of the biocontrol potential of opportunistic as well as AM fungi on the growth and improvement of various crop plants and population of plant parasitic nematodes in different pathosystems. The chapter also focuses on the cost-effective technologies used for the mass propagation of opportunistic fungi and AM fungi and their ample application in the expansion of practical control system desired for the sustainable agricultural practices.
2 Opportunistic Fungi
Fungi have the immense miscellany in their metabolic pathways and offer numerous important classes of commercial compounds having nematicidal activity (Li et al. 2007; Anke 2010) and limit the nematode densities by the production of nematotoxic compounds due to their parasites and antagonistic or predatory actions between fungi and plant parasitic nematodes (Lopez-Llorca and Jansson 2007; Akhtar and Panwar 2011). Lopez-Llorca and Jansson (2007) found that the opportunistic fungi either directly parasitize the nematodes or secrete some nematicidal metabolites which may affect the viability of one or more stages of the nematode life cycle or having deleterious effects on reproductive structures of a nematode. The secondary reproductive stage of the nematode is highly susceptible against the opportunistic fungi. The obese females are highly prone to fungal attack similarly like the parasitism of egg masses. The opportunistic fungi when come in contact with nematode eggs grow more rapidly and parasitize the eggs during initial embryonic developmental stages. This may reduce the parasitic actions of nematode juveniles. Among the various known opportunistic fungi, P. lilacinus and P. chlamydosporia have been extensively studied by several previous researchers for their nematophagous knack and biocontrol potentiality (Khan et al. 2004; Kiewnick and Sikora 2006; Siddiqui and Akhtar 2009a, b; Akhtar and Panwar 2011; Azam et al. 2013).
2.1 Paecilomyces lilacinus
Paecilomyces lilacinus (Thom) Samson is a mutual Hyphomycetes and is ubiquitously distributed especially in warmer climates (Samson 1974). It is encompassed in the group of frequently tested biocontrol agents against the plant parasitic nematodes (Brand et al. 2010; Pau et al. 2012; Azam et al. 2013). It is basically a saprophyte but could also compete for extensive range of substrates (Holland et al. 2003; Pau et al. 2012).
Jatala (1986) reported that P. lilacinus infects eggs and females of plant parasitic nematodes and destroyed the embryo within 5 days under laboratory conditions. He found that the infection of nematode eggs starts in a gelatinous matrix with the development of fungal hyphae which latter surrounds the entire nematode eggs. The colonization of nematode eggs occurred through the diffusion of egg cuticle by the fungal hyphal network by enzymatic or mechanical actions. His experiments clearly indicated that P. lilacinus grow well between 15 and 30 °C. It also had the adaptability to grow in a wide range of soil pH which made it a pretty modest organism in most of the cultivated fields. The suppression of plant parasitic nematode by P. lilacinus is ascribed by disintegration of the embryo, inhibition of hatching, and parasitism of adult females (Fig. 11.1). However, after establishment of P. lilacinus in soil, it grows faster and spread rapidly within a short span in the introduced area as dominant species. Moreover, the production of secondary metabolites such as chitinases, leucinotoxins, and proteases has also been associated with P. lilacinus infection (Park et al. 2004).
Cross section of tomato root infected with root-knot nematode; (a) showing presence of nematode, egg masses, abnormal phloem, and abnormal xylem in the cortical region; (b) showing conidia of P. lilacinus surrounding the nematode eggs and egg masses; (c) disruption of eggs and egg masses by P. lilacinus hyphae; (d) complete disintegration of nematode eggs by P. lilacinus hyphae
2.2 Pochonia chlamydosporia
Pochonia chlamydosporia (Goddard) Zare and Gams is a well-known nematophagous fungus and ubiquitously distributed in all parts of the world. It is naturally occurring as a facultative parasite of females, eggs, cyst, and plant parasitic nematodes (Lopez-Llorca et al. 2008; Manzanilla-Lopez et al. 2013). In the rhizosphere, this fungus could settle the host root as endophytes preferably with the plants belonging to families Gramineae and Solanaceae and provide numerous benefits to host plant defense against the soilborne pathogens (Macia-Vicente et al. 2009a, b). P. chlamydosporia have been extensively studied for its biocontrol potential against plant parasitic nematodes (Kerry and Hirsch 2011; Manzanilla-Lopez et al. 2013). The efficacy of this potential biological fungus against the plant parasitic nematode is affected by three major factors: (1) the amount of fungus in the rhizosphere, (2) the rate of development of eggs in the egg masses, and (3) the size of galls in which female nematode develops.
The population of P. chlamydosporia could be identified on the basis of position and shape of conidia, the plethora of dictyo-chlamydospores, and the development of conidia either in heads or chains (Zare and Gams 2004). P. chlamydosporia infects the nematode eggs through the expansion of aspersoria at the tip or lateral position of hyphae, which encompasses tightly to the surface of eggshells (Fig. 11.2), and finally penetrated into eggshells by the formation of an infection peg (Holland et al. 1999). A postinfection bulb leads to the expansion of mycelia within the eggs that caused almost the complete devastation of their contents (Tikhonov et al. 2002; Esteves et al. 2009a). Khan et al. (2004) reported that the eggshells and juvenile cuticles both have been physically disrupted, and the fungal hyphae willingly multiplied inside the eggs and juveniles due to enzymatic activity and biosynthesis of diffusible toxic metabolites. P. chlamydosporia are reported to secrete serine, protease, and chitinase responsible for the major structural changes inside the nematode eggs which may result in the disintegration of lipid and vitelline layers. Application of P. chlamydosporia as soil inoculants could reduce the natural nematode population up to 90 % under field condition (Bordallo et al. 2002), but the fungus differs in virulence toward nematode competence to colonize the root and production of chlamydospore (Bordallo et al. 2002; Yang et al. 2007; Macia-Vicente et al. 2009a, b). All these specific features make P. chlamydosporia a successful biocontrol agent under different pathosystems (van Damme et al. 2005; Rumbos et al. 2006; Esteves et al. 2009b).
Classical and electron microscopic images of root-knot nematode infected by P. chlamydosporia; (a) egg of a nematode infected by P. chlamydosporia hyphae; (b) complete disintegration of nematode egg by P. chlamydosporia hyphae; (c) electron microscopic view of P. lilacinus hyphae covering the nematode egg; (d) disruption of nematode egg by P. chlamydosporia hyphae
3 Arbuscular Mycorrhizal Fungi
Arbuscular mycorrhizal (AM) fungi are the key components of soil microbial populations with ubiquitous distribution in almost all the agroclimatic conditions of the world and form symbiosis with most of the land plants, in any kind of terrestrial ecosystem (Akhtar and Siddiqui 2008). Currently, AM fungi have been cited in the phylum Glomeromycota (Redecker and Raab 2006), and over 200 morphospecies of Glomeromycota have been described (Schüßler 2008). AM fungi have been categorized on the basis of extra-radical mycelium and branched haustoria-like structure within the cortical cells, termed as arbuscules. These arbuscules are the core sites for the nutrient exchange (Fig. 11.3), where the fungi supply water and nutrients like N and P to plants and in turn receive carbon from plants (Bonfante and Genre 2010).
Due to their unique ability and adaptability in different agroclimatic conditions, the AM fungi improved plant health by the acquisition of essential mineral nutrient and water from soil and enhanced production of growth regulations, tolerance toward various abiotic conditions, and mutualistic relationship with additional rhizospheric microorganisms existing in the same ecological niche (Akhtar and Siddiqui 2008; Akhtar 2011).
4 Efficacy and Biocontrol Strategies of Beneficial Rhizospheric Fungi
Persistence of plant parasitic nematodes is the most serious problem worldwide because they nourish and multiply their population on live host plants and also actively migrate inside the plants and aerial parts or in the rhizosphere. Among all the available options, chemical control has been extensively used against the plant parasitic nematode, due to its nonselective nature. However, use of chemicals to control plant parasitic nematodes has been restricted in many countries due to their environmental toxicity and ability to leach into the soil. They may cause the hazardous effect on the soil microbial flora and fauna as well as on the environment (Akhtar 1997). In the beginning, most of the fumigants were effectively used to control the plant parasitic nematodes due to their nematicidal properties, but later the detection of their remains in soil, water, and edible crops has caused awareness among the global scientific community concerned about the safety of human health and the environment (Alphey et al. 1988). Methyl bromide was the first fumigant which was widely used against the pathogens causing soilborne diseases, but it has been now banned and completely withdrawn from the market by imposing an international agreement in most of countries worrying about the environment safety (Oka et al. 2000).
Nowadays, several control measures such as the use of green manure, organic or inorganic soil amendments, crop rotation, resistant variety cultivation, unplanted treatment, and biological control have been used to limit the population of plant parasitic nematodes in the soil. But, unfortunately, all these control methods have led to limited success (Barker and Koenning 1998). Integrated pest management provides a working methodology for pest management in sustainable agricultural systems. With the increasing cost of inorganic fertilizers and the environmental and human health hazards associated with the use of pesticides, opportunistic and AM fungi may provide a more suitable and environmentally acceptable alternative for sustainable agriculture. Several comprehensive reviews have been published time to time exploring the possibilities of using AM fungi (Barea et al. 2005; Akhtar and Siddiqui 2008; Smith and Read 2008; Akhtar and Panwar 2011) and opportunistic fungi in the biocontrol of plant diseases (Atkins et al. 2005; Hildalgo-Diaz and Kerry 2008). We have summarized some recently published results of interaction studies between opportunistic fungi, AM fungi, and plant parasitic nematodes in tabular forms (Tables 11.1, 11.2, and 11.3).
5 Mass Propagation Strategies of Opportunistic Fungi and AM Fungi
5.1 Mass Production of Opportunistic Fungi
Several media have been extensively used for the mass production of opportunistic fungi. For the mass production of P. lilacinus potato dextrose broth (Rangaswami 1972), Richard’s medium, 10 % molasses (Rangaswami 1972), and semi-selective medium (Mitchell et al. 1987) can be used. The highest mycelium weight and spore production were achieved by using the semi-selective medium followed by 10 % molasses medium (Prabhu et al. 2008). Corn meal agar and potato dextrose agar media have also been used for the mass production of P. lilacinus (Robl et al. 2009). Similarly, the mass production of Pochonia spp. was achieved by using shrimp agar medium (Moosavi et al. 2010). Besides this wheat, bran and barley grain were also used for the mass production of Pochonia spp. (de Leij and Kerry 1991; Crump and Irving 1992). For the large-scale commercial production, liquid fermentation method is generally used because of difficulties to improve spore production on solid medium (Khan and Anwer 2011).
5.2 Mass Production of AM Fungi
AM fungi have the unique ability to improve the uptake of water and mineral nutrients from the soil and also to guard the plants against the pathogen attack (Smith and Read 2008). AM fungi also scavenge the available P through their extra-radical hyphae and upsurge the secretion of various amino acids (such as serine and isoleucine) and defense-related proteins (Akhtar and Siddiqui 2008; Akhtar et al. 2011), which augments their importance toward the modern and profitable agronomic practices. Due to their obligate nature, the AM fungi could not be cultured in vitro, which may limit the mass production of AM fungal propagules. In the conventional method of propagation, the AM fungi are propagated through the pot or pan culture usually with single spore culture, swiftly spread on the substrate, and finally colonize the root of host plants (Akhtar and Abdullah 2014). This method is quite useful for the production of clean fungal inoculum with high potentiality in a short span of time. Similarly, aeroponic culture systems allow the production of cleaner spores and enable even nourishment of AM fungi-colonized plants (Jarstfer and Sylvia 1999). Propagation of any AM fungal strains on root-organ cultur e permitted the propagation of monoxenic strains that could be used either directly as inoculum or as a starter inoculum for the mass production of AM fungi. A very simple and low-cost technique of single spore pot culture has been developed by Panwar et al. (2007). It permits undistributed growth of the mutualistic partners and visualization of germinating AM fungal spores and their mass multiplication. Moreover, the mass production of AM fungal inoculum requires control and optimization of both host growth and fungal development. The microscopic sizes of AM fungi, together with the complex identification processes, also contribute to the drawbacks of inoculum propagation.
Nevertheless in vitro bulk production of AM fungal inoculum is a promising approach, offering clean, viable, contamination-free fungal propagules. The cost of in vitro inoculum may appear expensive compared to the greenhouse-propagated fungal inoculum, but its use as starting inoculums is a warranty of purity (Akhtar and Abdullah 2014). The main purpose of this cultural method is to provide pure, clean, and reliable material as starter inoculum for the fundamental and applied research. There were several reports which indicate that mycorrhizologists were able to produce 25 spores/ml in 4 months’ incubation time (Chabot et al. 1992), while the other workers claimed for the production of 3250 spores/ml in 7 months (Douds 2002). Recently another work justifies the production of more than 2400 spore/100 g of soil after 120 days from single spore culture (Panwar et al. 2007).
6 Conclusions
The present chapter provides an overview on the interactions between opportunistic fungi, AM fungi, and plant parasitic nematodes. Use of opportunistic and AM fungi will not only reduce the load of nematicides in agricultural practices but also increase the plant vigor through the uptake of essential mineral nutrients and also reduce the nematode buildup in the plant and soil. Moreover, use of these biocontrol agents has an eco-friendly approach toward the environment as well as human health. The protection of nematode diseases by the application of these biocontrol agents is a complex process which may depend upon the molecular interactions between hosts, biocontrol agents, and pathogenic microorganisms. Application of single or mixed inoculum of opportunistic fungi, AM fungi were found to be effective in controlling the nematode diseases under greenhouse, pot, and field conditions in various agroclimatic conditions. An overview of the recent cost-effective technologies used for the mass propagation of these beneficial rhizospheric microorganisms is discussed. The success of mass propagation of indigenous biocontrol agents depends upon its selective nature toward edaphic, environment, and other rhizospheric biota, but it is still a challenge to develop these biocontrol agents in the sustainable agricultural practices to understand real underground mechanisms involved between the host, biocontrol agents, and pathogenic microorganisms.
References
Abd-El-Khair H, El-Nagdi WMA (2014) Field application of biocontrol agents for controlling fungal root rot and root-knot nematode in potato. Arch Phytopathol Plant Protect 47:1218–1230
Akhtar M (1997) Current options in integrated management of plant-parasitic nematodes. Integr Pest Manag Rev 2:187–197
Akhtar MS (2011) Biocontrol of root-rot disease complex of chickpea by arbuscular mycorrhizal fungi and other phosphate solubilizing microorganisms. Lambert Academic, Saarbrücken
Akhtar MS, Abdullah SNA (2014) Mass production techniques of arbuscular mycorrhizal fungi: major advantages and disadvantages: a review. Biosci Biotechnol Res Asia 11:1199–1204
Akhtar MS, Panwar J (2011) Arbuscular mycorrhizal fungi and opportunistic fungi: efficient root symbionts for the management of plant parasitic nematodes. Adv Sci Eng Med 3:165–175
Akhtar MS, Panwar J (2013) Efficacy of root-associated fungi and the growth of Pisum sativum (Arkil) and reproduction of root-knot nematode Meloidogyne incognita. J Basic Microbiol 53:318–326
Akhtar MS, Siddiqui ZA (2006) Effects of phosphate solubilizing microorganisms on the growth and root-rot disease complex of chickpea. Mycol Phytopathol 40:246–254
Akhtar MS, Siddiqui ZA (2007) Biocontrol of a chickpea root-rot disease complex with Glomus intraradices, Pseudomonas putida and Paenibacillus polymyxa. Aust Plant Pathol 36:175–180
Akhtar MS, Siddiqui ZA (2008) Arbuscular mycorrhizal fungi as potential bioprotectants against plant pathogens. In: Siddiqui ZA, Akhtar MS, Futai K (eds) Mycorrhizae: sustainable agriculture and forestry. Springer, Dordrecht, pp 61–98
Akhtar MS, Siddiqui ZA (2009) Effects of phosphate solubilizing microorganisms and Rhizobium sp. on the growth, nodulation, yield and root-rot disease complex of chickpea under field condition. Afr J Biotechnol 8:3479–3488
Akhtar MS, Siddiqui ZA, Wiemken A (2011) Arbuscular mycorrhizal fungi and Rhizobium to control plant fungal diseases. In: Lichtfouse E (ed) Alternative farming systems, biotechnology, drought stress and ecological fertilisation, vol 6, Sustainable agriculture reviews. Springer, Dordrecht, pp 263–292
Alphey TJW, Robertson WM, Lyon GD (1988) Rishitin, a natural plant product with nematicidal activity. Rev Nematol 11:399–404
Aminuzzaman FM, Xie HY, Duan WJ, Sun BD, Liu XZ (2013) Isolation of nematophagous fungi from eggs and females of Meloidogyne spp. and evaluation of their biological control potential. Biocontrol Sci Technol 23:170–182
Anastasiadis IA, Giannakou IO, Prophetou-Athanasiadou DA, Gowen SR (2008) The combined effect of the application of a biocontrol agent Paecilomyces lilacinus, with various practices for the control of root-knot. Crop Prot 27:352–361
Anjos ECT, Cavalcante UMT, Gonçalves DMC, Pedrosa EMR, dos Santos VF, Maia LC (2010) Interactions between an arbuscular mycorrhizal fungus (Scutellospora heterogama) and the root-knot nematode (Meloidogyne incognita) on sweet passion fruit (Passiflora alata). Braz Arch Biol Technol 53:801–809
Anke H (2010) Insecticidal and nematicidal metabolites from fungi. In: Hotrichter M (ed) The mycota: industrial applications, 2nd edn. Springer, Berlin
Atkins SD, Clark IM, Pande S, Hirsch PR, Kerry BK (2005) The use of real-time PCR and species-specific primers for the identification and monitoring of Paecilomyces lilacinus. FEMS Microbiol Ecol 51:257–264
Azam T, Akhtar MS, Hisamuddin A (2013) Histological interactions of Paecilomyces lilacinus with root-knot nematode Meloidogyne incognita and their effect on the growth of tomato. Adv Sci Eng 5:335–341
Barea JM, Pozo MJ, Azcon R, Azcon-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Barker KR, Koenning SR (1998) Developing sustainable systems for nematode management. Annu Rev Phytopathol 36:165–205
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat Commun 1:1–11
Bordallo JJ, Lopez-Llorca LV, Jansson HB, Salinas J, Persmark L, Asensio L (2002) Effects of egg-parasitic and nematode-trapping fungi on plant roots. New Phytol 154:491–499
Bowers JH, Nameth ST, Riedal RM, Rowe RC (1996) Infection sand colonization of potato roots by Verticillium dahliae as infected by Pratylenchus penetrans and P. crenatus. Phytopathology 86:614–621
Brand D, Soccol CR, Sabu A, Roussos S (2010) Production of fungal biological control agents through solid state fermentation: a case study on Paecilomyces lilacinus against root-knot nematodes. Micol Apl Int 22:31–48
Carneiro RMDG, Hidalgo-Díaz L, Martins I, de Souza Silva KFA, de Sousa MG, Tigano MS (2011) Effect of nematophagous fungi on reproduction of Meloidogyne enterolobii on guava (Psidium guajava) plants. Nematology 13:721–728
Chabot S, Becard G, Piche Y (1992) Life cycle of Glomus intraradices in root organ culture. Mycologia 84:315–321
Crump DH, Irving F (1992) Selection of isolates and methods of culturing Verticillium chlamydosporium and its efficacy as a biocontrol agent of beet and potato cyst nematodes. Nematologica 38:367–374
Dallemole-Giaretta R, Freitas LG, Lopes EA, Pereira OL, Zooca RJF, Ferrazb S (2012) Screening of Pochonia chlamydosporia Brazilian isolates as biocontrol agents of Meloidogyne javanica. Crop Prot 42:102–107
de Leij FAAM, Kerry BR (1991) The nematophagous fungus Verticillium chlamydosporium as a potential biological control agent for Meloidogyne arenaria. Rev Nematol 14:157–164
Dhawan SC, Singh S (2009) Compatibility of Pochonia chlamydosporia with nematicide and neem cake against root-knot nematode, Meloidogyne incognita infesting okra. Indian J Nematol 39:85–89
Dhawan SC, Singh S (2011) Biomanagement of root-knot nematode, Meloidogyne incognita by egg parasitic fungus, (Pochonia chlamydosporia) on okra. Veg Sci 38:128–134
Douds DDJ (2002) Increased spore production by Glomus intraradices in the split-plate monoxenic culture system by repeated harvest, gel replacement, and resupply of glucose to the mycorrhiza. Mycorrhiza 12:163–167
El-Shanshoury AR, El-Sayed SA, Mahmoud YAG, Khalefa DM (2005) Evaluation of Pochonia chlamydosporia, Paecilomyces lilacinus and Arthrobotrys dactyloide as biocontrol agents for Meloidogyne incognita under greenhouse condition. Pak J Biol Sci 8:1511–1516
Escudero N, Lopez-Llorca LV (2012) Effects on plant growth and root-knot nematode infection of an endophytic GFP transformant of the nematophagous fungus Pochonia chlamydosporia. Symbiosis 57:33–42
Esfahani MN, Pour BA (2006) The effect of Paecilomyces lilacinus on the pathogenesis of Meloidogyne javanica and tomato plant growth parameters. Iran Agric Res 24:67–76
Esteves I, Peteira B, Atkins SD, Magan N, Kerry BR (2009a) Production of extracellular enzymes by different isolates of Pochonia chlamydosporia. Mycol Res 113:867–876
Esteves I, Peteira B, Powers S, Magan N, Kerry B (2009b) Effects of osmotic and matric potential on growth and accumulation of endogenous reserves in three isolates of Pochonia chlamydosporia. Biocontrol Sci Technol 19:185–199
Ganaie MA, Khan T (2010) Biocontrol potential of Paecilomyces lilacinus on pathogenesis of Meloidogyne javanica infecting tomato plant. Eur J Appl Sci 2:80–84
Giri B, Giang PH, Kumari RAP, Oelmuller R, Varma A (2005) Mycorrhizosphere: strategies and function. In: Buscot F, Varma A (eds) Microorganisms in soil: roles in genesis and function, vol 3, Soil biology. Springer, Berlin
Goswami BK, Pandey RK, Rathour KS, Bhattacharya C, Singh L (2006) Integrated application of some compatible biocontrol agents along with mustard oil seed cake and furadan on Meloidogyne incognita infecting tomato plants. J Zhej Uni Sci 7:873–875
Gupta S, Dikshit AK (2010) Biopesticides: an ecofriendly approach for pest control. J Biopest 3:186–188
Gupta G, Panwar J, Akhtar MS, Jha PN (2012) Endophytic nitrogen-fixing bacteria as biofertilizer. In: Lichtfouse E (ed) Sustainable agriculture reviews, vol 11. Springer, Dordrecht, pp 183–221
Hildalgo-Diaz L, Kerry BR (2008) Integrated management and biocontrol of vegetable and grain crops nematodes. In: Ciancio A, Mukerji KG (eds) Integrated management and biocontrol of vegetable and grain crops nematodes. Springer, Dordrecht, pp 29–49
Holland RJ, Williams KL, Khan A (1999) Infection of Meloidogyne javanica by Paecilomyces lilacinus. Nematology 1:131–139
Holland RJ, Williams KL, Nevalainen KMH (2003) Paecilomyces lilacinus strain Bioact251 is not a plant endophyte. Aust Plant Pathol 32:473–478
Jaizme-Vega MC, Rodríguez-Romero AS, Barroso Nunez LA (2006) Effect of the combined inoculation of arbuscular mycorrhizal fungi and plant-growth promoting rhizobacteria on papaya (Carica papaya L.) infected with the root-knot nematode Meloidogyne incognita. Fruits 61:1–7
Jalaluddin M, Hajra NB, Firoza K, Shahina F (2008) Effect of Glomus callosum, Meloidogyne incognita and soil moisture on growth and yield of sunflower. Pak J Bot 40:391–396
Jarstfer AG, Sylvia DM (1999) Aeroponic culture of VAM fungi. In: Varma A, Hock B (eds) Mycorrhiza: structure, function, molecular biology and biotechnology, 2nd edn. Springer, Berlin, pp 427–441
Jatala P (1986) Biological control of plant parasitic nematodes. Annu Rev Phytopathol 24:453–489
Kannan R, Veeravel R (2008) Effect of an ovi-parasitic fungus Paecilomyces lilacinus Samson on Meloidogyne incognita in tomato. Ann Plant Prot Sci 16:466–470
Kannan R, Veeravel R (2012) Effect of different dose and application methods of Paecilomyces lilacinus (Thom.) Samson against root-knot nematode, Meloidogyne incognita (Kofoid and White) Chitwood in okra. J Agric Sci 4:119–127
Kantharaju V, Krishnappa K, Ravichardra NG, Karuna K (2005) Management of root-knot nematode, Meloidogyne incognita on tomato by using indigenous isolates of AM fungus, Glomus fasciculatum. Indian J Nematol 35:32–36
Kerry BR, Hirsch PR (2011) Ecology of Pochonia chlamydosporia in the rhizosphere at the population, whole organisms and molecular scales. In: Davies K, Spiegel Y (eds) Biological control of plant parasitic nematodes building coherence between microbial ecology and molecular mechanisms. Progress in biological control, vol 11. Springer, Dordrecht, pp 71–182
Khalil MS (2013) The potential of five eco-biorational products on the reproduction of root-knot nematode and plant growth. ESci J Plant Pathol 2:4–91
Khalil MSEH, Allam AFG, Barakat AST (2012) Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under greenhouse conditions. J Plant Protect Res 50:47–52
Khan MR, Anwer MA (2011) Fungal bioinoculants for plant disease management. In: Ahmad I, Ahmad F, Pichtel J (eds) Microbes and microbial technology: agriculture and environmental applications. Springer, Dordrecht, pp 447–488
Khan A, Williams KL, Nevalainen HKM (2004) Effects of Paecilomyces lilacinus protease and chitinase on the eggshell structures and hatching of Meloidogyne javanica juveniles. Biol Contr 31:346–352
Khan MR, Khan SM, Mohide F (2005a) Root-knot nematode problem of some winter ornamental plants and its biomanagement. J Nematol 37:198–206
Khan MR, Mohiddin FA, Khan SM, Khan B (2005b) Effect of seed treatment with certain biopesticides on root-knot of chickpea. Nematol Medit 33:107–112
Kiewnick S, Sikora RA (2006) Biological control of root-knot nematode Meloidogyne incognita by Paecilomyces lilacinus strain 251. Biol Contr 38:179–187
Kiewnick S, Neumann S, Sikora RA, Frey JE (2011) Effect of Meloidogyne incognita inoculum density and application rate of Paecilomyces lilacinus strain 251 on biocontrol efficacy and colonization of egg masses analyzed by real-time quantitative PCR. Phytopathology 101:105–112
Kumar P, Satyajeet Khare S, Dubey RC (2012) Diversity of Bacilli from disease suppressive soil and their role in plant growth promotion and yield enhancement. New York Sci J 5:90–111
Leng P, Zhang Z, Guangtang P, Zhao M (2011) Applications and development trends in biopesticides. Afr J Biotechnol 10:19864–19873
Li GK, Zhang J, Xu JD, Liu Y (2007) Nematicidal substances from fungi. Recent Pat Biotechnol 1:1–22
Lopez-Llorca LV, Jansson HB (2007) Fungal parasites of invertebrates: multimodal biocontrol agents. In: Robson GD, van West P, Gadd GM (eds) Exploitation of fungi. Cambridge University Press, Cambridge, pp 310–335
Lopez-Llorca LV, Macia-Vicente JG, Jansson HB (2008) Mode of action and interactions of nematophagous fungi. In: Ciancio A, Mukerji KG (eds) Integrated management and biocontrol of vegetable and grains crops nematodes. Springer, Dordrecht, pp 51–76
Macia-Vicente JG, Jansson HB, Talbot NJ, Lopez-Llorca LV (2009a) Real-time PCR quantification and live-cell imaging of endophytic colonization of barley (Hordeum vulgare) roots by Fusarium equiseti and Pochonia chlamydosporia. New Phytol 182:213–228
Macia-Vicente JG, Rosso LC, Ciancio A, Jansson HB, Lopez-Llorca LV (2009b) Colonisation of barley roots by endophytic Fusarium equiseti and Pochonia chlamydosporia: effects on plant growth and disease. Ann Appl Biol 155:391–401
Manzanilla-Lopez RH, Esteves I, Finetti-Sialer MM, Hirsch PR, Ward E, Devonshire J, Hidalgo-Diaz L (2013) Pochonia chlamydosporia: advances and challenges to improve its performance as a biological control agent of sedentary endo-parasitic nematodes. J Nematol 45:1–7
Mazzola M (2007) Manipulation of rhizosphere bacterial communities to induce suppressive soils. J Nematol 39:213–220
Mazzola M, Funnell DL, Raaijmakers JM (2004) Wheat cultivar-specific selection of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas species from resident soil populations. Microbial Ecol 48:338–348
Mishra KK, Dwivedi S, Pandre PK (2014) Evaluation of fungal bio-agents on plant growth and M. incognita infestation on chickpea. Chem Mater Res 6:135–139
Mitchell DJ, Kannwischer-Mitchell ME, Dickson DW (1987) A semi selective medium for the isolation of Paecilomyces lilacinus from soil. J Nematol 19:255–256
Moosavi MR, Zare R, Zamanizadeh HR, Fatemy S (2010) Pathogenicity of Pochonia species on eggs of Meloidogyne javanica. J Invertebr Pathol 104:125–133
Mukhtar T, Hussain MA, Kayani MZ (2013) Biocontrol potential of Pasteuria penetrans, Pochonia chlamydosporia, Paecilomyces lilacinus and Trichoderma harzianum against Meloidogyne incognita in okra. Phytopathol Medit 52:66–76
Nicol JM, Turner SJ, Coyne DL, den Nijs L, Hockland S, Maafi ZT (2011) Current nematodes threats to world agriculture. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 21–43
Oclarit EL, Cumagun CJR (2009) Evaluation of efficacy of Paecilomyces lilacinus as biological control agent of Meloidogyne incognita attacking tomato. J Plant Protect Res 49:337–340
Odeyemi IS, Afolami SO, Sosanya OS (2010) Effect of Glomus mosseae (arbuscular mycorrhizal fungus) on host-parasite relationship of Meloidogyne incognita (Southern root-knot nematode) on four improved cowpea varieties. J Plant Protect Res 50:320–325
Odeyemi IS, Afolamia SO, Adekoyejoa AB (2013) Integration of Glomus mosseae with Chromolaena odorata powder for suppression of Meloidogyne incognita on maize (Zea mays L.). Arch Phytopathol Plant Protect 46:1589–1597
Oka Y, Koltai H, Bar-Eyal M, Mor M, Sharon E, Chet I, Spiegel Y (2000) New strategies for the control of plant-parasitic nematodes. Pest Manag Sci 56:983–988
Palomares-Rius JE, Kikuchi T (2013) Omics fields of study related to plant-parasitic nematodes. J Integr Omics 3:1–10
Pandey R (2005) Field application of bio-organics in the management of Meloidogyne incognita in Mentha arvensis. Nematol Medit 33:51–54
Panwar J, Tarafdar JC, Yadav RS, Saini VK, Aseri GK, Vyas A (2007) Technique for visual demonstration of germinating AM spore and their multiplication in pots. J Plant Nutri Soil Sci 170:659–663
Park JO, Hargreaves JR, McConville EJ, Stirling GR, Ghisalberti EL, Sivasithamparam K (2004) Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson. Lett Appl Microbiol 38:271–276
Pau CG, Leong CTS, Wong SK, Eng L, Jiwan M, Kundat FR, Aziz ZFBA, Ahmed OH, Majid NM (2012) Isolation of indigenous strains of Paecilomyces lilacinus with antagonistic activity against Meloidogyne incognita. Int J Agric Biol 14:197–203
Podestá GS, Freitas LG, Dallemole-Giaretta R, Zooca RJF, Caixeta LB, Ferraz S (2013) Meloidogyne javanica control by Pochonia chlamydosporia, Gracilibacillus dipsosauri and soil conditioner in tomato. Summa Phytopathol 39:122–125
Prabhu S, Kumar S, Subramanian S (2008) Mass production and commercial formulation of Paecilomyces lilacinus. Madra Agric J 95:415–417
Prakob W, Nguen-Hom J, Jaimasit P, Silapapongpri S, Thanunchai J, Chaisuk P (2009) Biological control of lettuce root-knot disease by the used of Pseudomonas aeruginosa, Bacillus subtilis and Paecilomyces lilacinus. J Agric Technol 5:179–191
Rangaswami G (1972) Disease of crop plants in India. Prentice Hall, New Delhi, p 504
Rao MS, Sowmya DS, Chaya MK, Kumar RM, Rathnamma K, Gavaskar J, Priti K, Ramachandran N (2012) Management of nematode induced wilt disease complex in capsicum using Pseudomonas fluorescens and Paecilomyces lilacinus. Nematol Medit 40:101–105
Ravindra H, Sehgal M, Pawan AS, Archana BS, Shruti SA, Narasimhamurty HB (2014) Eco-friendly management of root-knot nematodes using acacia compost and bioagents in brinjal. Pak J Nematol 32:33–38
Redecker D, Raab P (2006) Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 98:885–895
Reimann S, Hauschild R, Hildebrandt U, Sikora RA (2008) Interrelationships between Rhizobium etli G12 and Glomus intraradices and multitrophic effects in the biological control of the root-knot nematode Meloidogyne incognita on tomato. J Plant Dis Protect 115:108–113
Robl D, Sung LB, Novakovich JH, Marangoni PRD, Zawadneak MAC, Dalzoto PR, Gabardo J, Pimentel IC (2009) Spore production in Paecilomyces lilacinus (Thom.) Samson strains on agro-industrial residues. Braz J Microbiol 40:296–300
Rodríguez Romero AS, Jaizme-Vega MC (2005) Effect of the arbuscular mycorrhizal fungus Glomus manihotis on the root-knot nematode, Meloidogyne javanica in banana. Nematol Medit 33:217–221
Rumbos C, Reimann S, Kiewnick S, Sikora RA (2006) Interactions of Paecilomyces lilacinus strain 251 with the mycorrhizal fungus Glomus intraradices: implications for Meloidogyne incognita control on tomato. Biocontrol Sci Technol 16:981–986
Samson RA (1974) Paecilomyces and some allied Hyphomycetes. Study Mycol 6:1–119
Sasser JN, Freckman DW (1987) A world perspective on nematology: the role of the society. In: Veech JA, Dickson DW (eds) Vistas on nematology. Society of Nematologists, Hyattsville, pp 7–14
Schüßler A (2008) Glomeromycota species list. http://www.lrz-muenchen.de/~schuessler/amphylo/. Assessed on June 2014
Serfoji P, Smithra P, Saravanan K, Durai Raj K (2013) Management of Meloidogyne incognita on tobacco through Glomus aggregatum and oil cakes. Int J Pharma Biol Arch 4:451–454
Sharf R, Shiekh H, Abbasi S, Akhtar A, Robab MI (2014) Interaction between Meloidogyne incognita and Pochonia chlamydosporia and their effects on the growth of Phaseolus vulgaris. Arch Phytopathol Protect 47:622–630
Sharma P, Pandey R (2009) Biological control of root-knot nematode; Meloidogyne incognita in the medicinal plant; Withania somnifera and the effect of biocontrol agents on plant growth. Afr J Agric Res 4:564–567
Shreenivasa KR, Krishnappa K, Ravichandra NG (2007) Interaction effects of arbuscular mycorrhizal fungus Glomus fasciculatum and root-knot nematode, Meloidogyne incognita on growth and phosphorous uptake of tomato. Karn J Agric Sci 20:57–61
Siddiqui ZA, Akhtar MS (2007) Effects of AM fungi and organic fertilizers on the reproduction of the nematode Meloidogyne incognita and on the growth and water loss of tomato. Biol Fertil Soils 43:603–609
Siddiqui ZA, Akhtar MS (2008a) Synergistic effects of antagonistic fungi and a plant growth promoting rhizobacterium, an arbuscular mycorrhizal fungus, or composted cow manure on populations of Meloidogyne incognita and growth of tomato. Biocontrol Sci Technol 18:279–290
Siddiqui ZA, Akhtar MS (2008b) Effects of fertilizers, AM fungus and plant growth promoting rhizobacterium on the growth of tomato and on the reproduction of root-knot nematode Meloidogyne incognita. J Plant Inter 3:263–271
Siddiqui ZA, Akhtar MS (2009a) Effect of plant growth promoting rhizobacteria, nematode parasitic fungi and root-nodule bacterium on root-knot nematodes Meloidogyne javanica and growth of chickpea. Biocontrol Sci Technol 19:511–521
Siddiqui ZA, Akhtar MS (2009b) Effects of antagonistic fungi and plant growth-promoting rhizobacteria on growth of tomato and reproduction of the root-knot nematode, Meloidogyne incognita. Aust Plant Pathol 38:22–28
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Soliman AS, Shawky SM, Omar MNA (2011) Efficiency of bioagents in controlling root-knot nematode on Acacia plants in Egypt. American-Eurasian J Agric Environ Sci 10:223–229
Sundararaju P, Kiruthika P (2009) Effect of biocontrol agent, Paecilomyces lilacinus along with neemcake and botanicals for the management of Meloidogyne incognita on banana. Indian J Nematol 39:201–206
Tahseen Q, Clark IM, Atkins SD, Hirsch PR, Kerry BR (2005) Impact of the nematophagous fungus Pochonia chlamydosporia on nematode and microbial populations. Commun Agric Appl Biol Sci 70:81–86
Tikhonov VE, Lopez-Llorca LV, Salinas J, Jansson HB (2002) Purification and characterization of chitinases from the nematophagous fungi Verticillium chlamydosporium and V. suchlasporium. Fun Gen Biol 35:67–78
Timper P (2011) Utilization of biological control for managing plant-parasitic nematodes. In: Davies K, Spiegel Y (eds) Biological control of plant-parasitic nematodes: building coherence between microbial ecology and molecular mechanisms. Springer, New York, pp 259–289
Udo IA, Uguru MI, Ogbuji RO (2013) Pathogenicity of Meloidogyne incognita race 1 on tomato as influenced by different arbuscular mycorrhizal fungi and bioformulated Paecilomyces lilacinus in a dysteric cambisol soil. J Plant Protect Res 53:71–78
Usman A, Siddiqui MA (2012) Effect of some fungal strains for the management of root-knot nematode (Meloidogyne incognita) on eggplant (Solanum melongena). J Agric Technol 8:213–218
van Damme V, Hoedekie A, Viaene N (2005) Long-term efficacy of Pochonia chlamydosporia for the management of Meloidogyne javanica for glasshouse crops. Nematology 7:727–736
Viggiano JR, de Freitas LG, Lopes EA (2014) Use of Pochonia chlamydosporia to control Meloidogyne javanica in cucumber. Biol Control 69:72–77
Weller DM, Landa BB, Mavrodi OV, Schroeder KL, De la Fuente L, Bankhead SB, Molar RA, Bonsall RF, Mavrodi DV, Thomashow LS (2007) Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol 9:4–20
Yadav K, Akhtar MS, Panwar J (2015) Rhizospheric plant microbe interactions: key factor to soil fertility and plant nutrition. In: Arora NK (ed) Plant microbe symbiosis: applied facets. Springer, India, pp 127–145
Yang J, Tian B, Liang L, Zhang K (2007) Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl Microbiol Biotechnol 75:21–31
Yang J, Loffredo A, Borneman J, Becker JO (2012) Biocontrol efficacy among strains of Pochonia chlamydosporia obtained from a root-knot nematode suppressive soil. J Nematol 44:67–71
Zare R, Gams W (2004) A monograph of Verticillium section Prostrata. Rostaniha 3:1–188
Zhang L, Zhang J, Christie P, Li X (2008) Pre-inoculation with arbuscular mycorrhizal fungi suppresses root knot nematode (Meloidogyne incognita) on cucumber (Cucumis sativus). Biol Fertil Soils 45:205–211
Zhang L, Zhang J, Christie P, Li X (2009) Effect of inoculation with the arbuscular mycorrhizal fungus Glomus intraradices on the root-knot nematode Meloidogyne incognita in cucumber. J Plant Nutr 32:967–979
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Akhtar, M.S., Panwar, J., Abdullah, S.N.A., Siddiqui, Y., Swamy, M.K., Ashkani, S. (2015). Biocontrol of Plant Parasitic Nematodes by Fungi: Efficacy and Control Strategies. In: Meghvansi, M., Varma, A. (eds) Organic Amendments and Soil Suppressiveness in Plant Disease Management. Soil Biology, vol 46. Springer, Cham. https://doi.org/10.1007/978-3-319-23075-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-23075-7_11
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23074-0
Online ISBN: 978-3-319-23075-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)