Abstract
Phytochelatins (PCs) are nonprotein cysteine-rich oligopeptides having the general structure of (γ-glutamyl-cysteinyl)n-glycine (n = 2–11). They are synthesized from the precursor glutathione (a reduced form, GSH) by the activity of phytochelatin synthase (PCS). The biosynthesis is stimulated by several heavy metals (HMs), especially Cd and metalloid As. PCs can bind to various HMs like Cd, As, Cu, Pb, Zn, and Ag, via their sulfhydryl (–SH) and carboxyl (–COOH) groups. The complexations become more stable and massive in vacuole where acid-labile sulfides (S2−) are incorporated to make the PCs–S–HMs conjugates. Both the thiols and S2− are originated from sulfate through a partially common energy-dependent metabolism (sulfur assimilation), which is again enhanced by Cd, besides essential metals (Co, Mg). To date, fundamental roles of PCs and also related iso-peptides such as hPCs in intracellular detoxification and/or transport of HMs are well demonstrated in various plants, especially in experiments targeting genes and enzymes for PC and GSH biosynthesis. However, how they function as a defense molecule in the oxidative stresses or other biological processes are still unknown or conceiving subtle problems. Some of the possible functions are highlighted in this chapter as tentative examples for further discussion: (1) PCs–S–HMs complex as a potent pool/stock of thiols or reducing powers to be reusable for further robustious responses by the tolerant plants against various abiotic and biotic stresses including oxidative stress and (2) PCs as a possible mediator for metal translocation or redistribution via phloem rather than xylem, regardless of a trait of “hyperaccumulator” for HMs in land plants. Apart from the positive roles of PCs in HM-tolerant plants, arguments still hot arise an issue (3) the roles of PCs, GSH, and other thiols as delicate barometer or indicators in the mineral and redox balance and/or homeostasis, in addition to their well-known functions being substrates and antidotes. In the absence of HMs, the levels of PCs are too minute to account for their sufficient bindings to the essential metals. Although GSH is ubiquitous and abundant, it is a multifunctional peptide that rapidly consumed or oxidized for numerous enzymic or nonenzymic antioxidants/redox systems as well as direct substrate for PCS. Eventually, importance of preservation of thiols and sulfide (S2−) as resource for reducing powers in sensitive sessile plants against various oxidative stresses is again emphasized in return for PCs in the HM-tolerant plants in metalliferous habitats.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Degradation of environmental quality due to metal and metalloid pollution has become a universal problem. Dispersions of untreated industrial and municipal wastes are widespread and create instability of natural equilibrium increasing the risk that the toxic pollutants would inflict their harmful effects upon the ecosystem and the individual organisms in the community. Constituting a diverse group of elements, heavy metals (HMs) having a density equal or greater than 4.0 or 5.0 g/cm3 vary in their chemical characteristics, biological functions, and toxicity (Chatterjee et al. 2007). Most of them are microelements essential and vital for plant growth and nutrition at optimum concentrations but toxic at excessive concentrations (i.e., supra-optimal concentration). The others are nonessential and just toxic even at low concentrations, simply called as “toxic HMs.” Among the representatives as shown in Table 1, Cd, Pb, Hg, and Ni are very toxic. As is a metalloid but often put in to a member of HMs for convenience mainly because of the highly poisonous chemical property. Those elements are actually listed up in the high rankings applied for the various toxic and hazardous chemicals, i.e., As, Pb, Hg are no.1–3, and Cd is no.7 of the list (CERCLA 2007).
The toxicity of HMs lead to interference with metabolism and other biological activities through the generation of reactive oxygen species (ROS) such as superoxide radical (O2 •−), hydroxyl radical (•OH), and hydrogen peroxide molecule (H2O2), in addition to their direct disruptive functions to the essential enzymes and other molecules (Prasad and Freitas 2003). HMs are divided into two groups (redox-active or -inactive) for the characteristics whether or not having a redox activity by itself to produce free radicals (Viehweger 2014). According to this category, Fe, Cu, Cr, Co, Mn, and V are redox-active, and most of the rests (including Cd, Zn, Ni, Pb, and As, as noted above) are redox-inactive like Al and Na in light metals (Hossain et al. 2012a; Shahid et al. 2014). The redox-active metals are directly involved in the redox reaction in cells resulting in the formation of O2 •− and •OH radicals, while the redox-inactive metals indirectly increase the levels of O2 •−, •OH, and H2O2 by inhibiting various enzyme activities directing towards ROS sequestrations (Hossain et al. 2012b; Shahid et al. 2014). After all HMs are responsible in activating ROS formation and causing strong oxidative stress in plant cells. In conjunction to this commonality, anti-oxidation systems and processes that resume the HMs-induced stresses have attracted attention of scientists and experts (Shahid et al. 2014). Various types of anti-oxidative systems operate using many types of molecules. These are named as the redox (reduction and oxidation) cycling molecules, quenching antioxidant molecules (low molecular substances with anti-oxidation powers), detoxification enzymes (high molecular proteins involved in sequestration of ROS using low molecular antioxidants or substrates), etc. In these systematic strategies, quantitatively abundant molecules used are small antioxidants such as GSH and ascorbic acid (AsA), which reach or exceed 1 mM order at the intracellular concentrations. These play a central role in the maintenance of redox status and the nutritional homeostasis by buffering or pooling of the reducing powers within the soluble organic matters that are usable for the respective cases and places in plants under HMs and/or oxidative stresses. Concurrently, biological roles of these and other antioxidation systems especially in relation to plant’s HM stress have been reviewed by several researchers (Shahid et al. 2014).
As measures to deduce the toxic HM ions within plant cells, vacuolar compartmentalization has been also suggested. However, traffic movements of HMs as an inert binding form from the outside to vacuole are necessary through the cytoplasm, the vital site of cells. Here, plant cells produce quite unique HMs-chelating thiol peptides named phytochelatins (PCs) (Grill et al. 1987; Rauser 1999; Inouhe 2005). These peptides were first recognized to be present in Cd-binding complexes in plants and yeast cells exposed to Cd as peptides having a function homologous to metallothioneins (MTs) in animals and other living organisms (Rauser 1999). It has been demonstrated that PCs are derivatives from glutathione (GSH), after all, not a protein unlike MTs (Rauser 1999; Inouhe 2005). The roles of PCs in HMs binding and detoxification have been demonstrated in various plants. However, their roles in the other biological processes and functions are still unknown in plants and other living organisms and now being very important questions to be addressed.
Further, mechanisms that appear independent from PCs and PC-related peptides have been reported from different aspects, i.e., plant species, HMs, and various molecular species involved in HM sequestration and/or transportation. Especially, regulation at transportation level can be useful as HMs defense mechanism by blocking the entrance via traffic channel or eliminating the toxic matter to outside of cells or plants. These defense mechanisms as well as intracellular PC- and antioxidant-mediated mechanisms are all required for normal life cycles of sessile plants that depend on the mineral uptakes and photosynthetic activities under different environmental conditions.
In this chapter, we summarize some information’s of HMs-induced oxidative stress in plants, especially focusing on the functions of PCs and other thiols. Thereafter, some pros and cons for their biological functions of them and later are taken for discussion about a possible benefit for the pooling of PCs, GSH, and S metabolites. All these topics are highlighted on what factors are best involved in the decrease in ROS evolution in cells.
2 Input and Impact of HMs
2.1 Route into Plant Cells from Environment
Natural or anthropogenic routes are the major source of Cd contamination in soil. Natural or edaphic stress factors may influence plants development, growth, or productivity due to alteration of concentrations of different bio-reactive metals (Schützendübel and Polle 2002; Chatterjee et al. 2011). Natural phenomenon like Cd-rich rock weathering can enhance natural mineral outcrops which in turn pollute the environment. While, burning of fossil fuels such as coal or oil and the incineration of municipal wastes, cement factories, and as a by-product of phosphate fertilizers are the major anthropogenic sources of the Cd in environment (Mengel et al. 2001; Chen 2005; Kirkby and Johnson 2008; Lux et al. 2011). The concentrations of Cd may be up to 40–300 nM in natural non-polluted soils; however, the concentration may increase with clay concentration up to 1 μg/g dry soil (Wagner 1993; Mengel et al. 2001; Inaba et al. 2005). Availability of Cd to plants is greater in acid soils and its solubility increases with exudates of roots (Mengel et al. 2001; Lux et al. 2011). Delivery of Cd to plant roots is dominated by a transpiration-driven mass-flow process of the soil solution (Sterckeman et al. 2004; Ingwersen and Streck 2005).
Accumulation of HMs such as Ni, Pb, and Hg is also the result of several anthropogenic activities (Gupta et al. 2013a). Nonessential metals like Cd, As, Pb, and Hg present along with the essential one may also enter into the plant systems. Varied tactics are followed by plants in response to HMs toxicity, which include immobilization, exclusion, chelation, and compartmentalization of the metal ions, and expression of the general stress responses (Cobbett 2000). Several plants have been identified that possess the unique capability to live on toxic conditions at HMs contaminated sites and also been found to accumulate a considerable amount of such metals within their biomass (hyperaccumulators). Various studies have shown that natural hyperaccumulators like As hyperaccumulating fern species Pteris vittata (Gumaelius et al. 2004) and Ni hyperaccumulating species Thlaspi caerulescens (Freeman et al. 2004) can withstand higher amount of metal accumulation without having significant damage within cell system. Further studies on the conspicuous properties and functions of the hyperaccumulators will disclose the different and diverse mechanisms for HMs detoxification by plants (Inouhe et al. 2012).
2.2 Toxicity to Plant Cells
Biological impacts of HMs are different by the metal species, as well as plant species, their origins, growth stage, and condition. For example, Cd is very toxic for plants even at low concentrations and often interferes with other essential metals containing enzymes (enzymes of Zn, Fe, Cu, Mn, Mg, and Ca) by displacing these elements (Wagner 1993). Cd primarily damages photosystems and some other enzyme systems in plants. As noted earlier, Cd is a redox-inactive metal, like Zn or Ni, but usually accompanies an oxidative stress by causing a transient depletion of GSH and inhibition of antioxidant enzymes (Romero-Puertas et al. 1999). Thus, the strong and versatile phytotoxicity of Cd in growth, cell death, photosynthesis, and induced lignification, etc. are due to these direct and indirect effects (Hossain et al. 2012b). Ni causes inhibition of growth, chlorosis, necrosis, and flaccidity in plants, and this toxicity is also due to the generation of oxidative stress. Pb affects many processes of plants, causing a decrease in photosynthetic pigments, an increase in membrane permeability, and a disturbance of the mineral nutrition and affecting many enzyme activities. Hg is known to provoke oxidative stress in many plants accompanying overall increases in the antioxidant enzyme systems. Arsenic is not strictly a heavy metal since it is classified as a metalloid. However, it is an important poison, which induces toxicity in plants. Usually, As is present in two toxic inorganic forms, arsenate (AsO4 2−) and arsenite. Arsenate disrupts energy flows in cells and is taken up by plants through high-affinity phosphate transporters. Arsenite provokes toxicity by reacting with sulfhydryl groups of enzymes and tissue proteins and consequently resulted in inhibition of cellular function. Both forms of arsenic induce the formation of ROS leading to oxidative stress.
Apart from these toxic HMs, some others (microelements) are indispensable for living organisms at low doses, but exposure of plants above certain metal threshold concentrations, specific for each one, develops damaging effects linked to disturbances of the oxidative balance. Thus, contrary to other HMs reported above, Cu at an adequate concentration is strictly necessary for plants, since it serves as a cofactor of enzymes required for normal growth and development such as Cu/Zn-superoxide dismutase (Cu/Zn-SOD), cytochrome c, or plastocyanin. However, Cu at high concentrations causes multiple toxic effects in plants. Fe is also a key element in a large number of plant metabolic routes requiring a redox exchange. Although Fe is abundant in soils, its availability is depressed in alkaline soils provoking to plant Fe deficiency, which is a common nutritional disorder for many dicotyledonous species. However, an excess of Fe have also phytotoxic effects. Conclusive remarks with examples for the hazardous effects of HMs in plants are also shown in Table 1.
Almost all HMs are potently to be toxic if present in excess as free ions (e.g., Hg2+) or some organo-metallic forms (e.g., methyl-Hg) that are hydrophilic and hydrophobic, respectively. As mentioned above, free HM ions penetrated via root systems are the most probable xenobiotics for plants. Further considerations about the organic forms of HM contaminants are put aside in this chapter, while this will be a serious problem if the environmental pollution and contamination proceed and become more complex in the ecosystems during artificial activities.
2.3 ROS Production
Since pathways in which different ROS evolve are quite complex even in common plants under the influence of HMs, candidate pathways are shown here briefly (Fig. 1). ROS are produced during normal aerobic metabolisms, especially in chloroplast, mitochondria, peroxisome, apoplast (cell wall), and plasma membrane. HMs enhance the formations of ROS such as superoxide anion (O2 •−), hydroxyl radical (•OH), and H2O2 in those sites by inhibiting the enzymatic or nonenzymatic antioxidant systems (Shahid et al. 2014). The O2 •− is generated when oxygen molecule (O2) is reduced via electron transfer or energy transfer reactions. This radical is toxic but short-lived and readily converted to (H2O2) by the enzyme superoxide dismutase (SOD). The H2O2 molecule is weaker in toxicity than O2 •− but stable, long lasting, and permeable across membrane and hence also serves as inter/intracellular second signals for various oxidative stresses. These ROS are able to generate much toxic •OH radical, in the presence of redox-active HMs such as Fe and Cu. This radical is extremely reactive and causes strong oxidation damages in bio-membranes (usually known as lipid peroxidation reaction trigging a self-propagating chain reaction in membranes resulting in serious problems of the cell viability) and in other macromolecules including proteins, DNA, conjugated lipids, and photosynthetic pigments (Gechev et al. 2006; Hossain et al. 2012a, b). After all, ROS interact with HMs resulting in various damages in several cell sites and components (Shahid et al. 2014). ROS production is common in all living cells but the rate of production of ROS in chloroplasts is increased by influence of excessive light energy, and HM contamination is quite unique to plants. Typically, HM stress reduces photosynthesis rate and hence lead to increased production of ROS such as O2 •− and H2O2 (Takahashi and Murata 2008). These adverse effects of metal stress can be observed in several places of photosynthesis, including PS I, PS II, and carboxylating enzymes like RuBisCO and phosphoenol pyruvate carboxylase (PEPC) (Siedlecka and Baszynaski 1993; Hossain et al. 2012b). Usually PS II reaction center in the chlorophyll is mostly affected by metals like Cd that replaces Ca and Mn (Atal et al. 1991). Similarly, ROS generation is also evident in mitochondria at complex I and the ubiquinone (Q) zone (Blokhina and Fagerstedt 2006). Furthermore, several reports suggest that NADPH oxidase-dependent ROS induction can take place in response to Cd stress in Pisum sativum (Rodrıguez-Serrano et al. 2006), As stress in Arabidopsis thaliana (Gupta et al. 2013a), Pb stress in Vicia faba (Pourrut et al. 2008), Cd and Cu stress in A. thaliana (Remans et al. 2010), and Ni stress in wheat (Hao et al. 2006).
Reactive oxygen species (ROS) induced by stresses and the possible antioxidants and detoxifications enzymes involved in the ROS sequestration in plants. Different abiotic and biotic stresses including HMs and oxidative stresses induce ROS in different sites in cells of plant tissues. The antioxidants (mainly in water-soluble forms) and enzymes are collaborating to the ROS sequestration in cases and places under stresses. GSH is a multifunctional thiol peptide which is also an important precursor for PCs and other HM-binding substances
3 Mechanisms Against Heavy Metal Toxicity
3.1 Overview of Phytochelatin-Binding Defense Mechanism
3.1.1 Phytochelatins
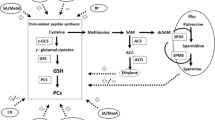
Because details for research history and topics of findings for PCs are available in reviews (Grill et al. 1987; Cobbett 2000; Inouhe 2005), the framework is shown briefly. PCs are nonprotein cysteine-rich oligopeptides having the general structure of (γ-glutamyl-cysteinyl)n-glycine (n = 2–11). They are synthesized from the precursor GSH by the activity of phytochelatin synthase (PCS), encoded in genes (PCS1, CAD1) isolated in 1999 at three laboratories (Ha et al. 1999; Vatamaniuk et al. 1999; Clemens et al. 1999). The PCs biosynthesis is stimulated by several HMs, especially Cd and metalloid As. PCs can bind to various HMs like Cd, As, Cu, Pb, Zn, and Ag, via their sulfhydryl (–SH) and carboxyl (–COOH) groups, and these complexations become more stable and massive in vacuole where acid-labile S2− are incorporated to make the PCs–S–HMs conjugates. Both the thiols and S2− are originated from sulfate through a partially common energy-dependent metabolism (sulfur assimilation), which is again enhanced by Cd, besides essential metals. Coordinative roles and functional linkages in these sulfur-containing compounds are expectable as discussed in detail later.
PCs and structurally PC-related peptides have been described in various plants and other organisms. Initially, PCs with different degrees of polymerization were reported from more than 300 species of plants and other organisms (Grill et al. 1989; Gekeler et al. 1989). In angiosperms, more than 23 species of monocotyledonous plants and 90 species of dicotyledonous plants were tested, and all of the plants were shown to produce PCs after Cd treatments (Gekeler et al. 1989). The PC synthesis was also confirmed either in suspension cultures or differentiated plant seedlings of various higher plants as well as in the lower plants that belong to groups of mosses or ferns (Gekeler et al. 1989). Such ubiquitous occurrences of PC peptides with the same structures among plant kingdom strongly suggest significant role of PCs as common metabolites in plants, while their physiological roles in the absence of heavy metals are still in open question at present, as described later.
Fundamental roles of PCs in intracellular detoxification are well demonstrated in various plants but especially for Cd (and As). This metal might be nonessential for most living organisms as so far known; nevertheless, why most plants have the most favorite response with PCs to this metal remains as an interesting question. Recent molecular phylogenetics approaches have started disclosing the ubiquitous roles of widespread PCS enzymes and genes in various organisms, which would have been diverged, specified, or converged during more than hundred billions of years of evolutionally time-span. However, evidences still show very low levels of PCs produced in the ancient type of plants like bryophyte as compared with their substantial levels in the some group of fungi (yeast), green algae, and various species of vascular plants (Hayashi et al. 1991; Mehra and Winge 1990; Inouhe et al. 1996; Murasugi 2008). Besides Cd, in response to other HMs stress in plants, PCs may play a significant role in detoxification in higher plants (Cobbett and Goldsbrough 2002) and make a complex, as immobilized metals are less toxic than the free ions. Synthesis and emergence of these metal-binding peptides in plants indicate HMs contamination under various environments (Gupta et al. 2002, 2004). It has been reported that in plants, PCs–HMs complexes form during detoxification process against a wide range metal ions, like Cd, Pb, As, Ag, Hg or Zn, Cu, Ni (Maitani et al. 1996; Mehra et al. 1996; Rauser 1999; Ha et al. 1999; Manara 2012). More direct evidence for the role of PCs against HMs contamination was presented through study on isolated PC-deficient cad1 mutants in heavy metal stress condition. Indeed, such a mutant of A. thaliana was more sensitive towards Cd and arsenate (AsO4 2−) than wild-type plants; however, no considerable difference was found for the others like Zn, selenite (SeO3 2−), and Ni (Ha et al. 1999). A PCS-deficient mutant of Schizosaccharomyces pombe showed moderate sensitivity to Cu and Hg and modestly to Ag (Maitani et al. 1996; Ha et al. 1999; Manara 2012). Further evidence for Cu-induced triggering of PCs biosynthesis in Cu tolerance has been shown in Cu-tolerant species Mimulus guttatus. In contrast, a differential tolerance was reported in Silene vulgaris, on exposing root tips to Cu; both the Cu-tolerant and Cu-sensitive ecotypes produced comparable quantity of PCs. It is also manifested that PC–Cu complexes are comparatively transient and relatively poorly sequestered to the vacuole (Schat and Kalff 1992; De Knecht et al. 1994; Cobbett and Goldsbrough 2002). However, in plant Rubia tinctorum, exposure to different heavy metals leads to the formation of PC-metal complexes in the roots. Heavy metals ions like Ag, As, Cd, Cu, Hg, and Pb were appeared most effective in stimulation of PCs, though, PC complexes known in vivo were with Cd, Ag, and Cu ions (Maitani et al. 1996; Cobbett and Goldsbrough 2002). Moreover, the key role of PCs against heavy metal stress in plants and detoxification of different heavy metals has been corroborated in many studies. However, why such a change appears in the contribution of PCs to tolerance and detoxification against different HMs is still not fully understood.
3.1.2 Variation in Phytochelatins: Homo- and Iso-phytochelatins
PCs and structurally PC-related peptides have been described in various plants and other organisms. Such a ubiquitous occurrence of the PC peptides with the similar structures among plant kingdom strongly suggests their significant roles as primary metabolites common in the plants. However, here are some exceptional cases for the ubiquity of PCs in some restricted plants and yeast, i.e., some diversity is known for the molecules. PC peptides have Gly in the C-terminal end in general. The presence of some des-Gly variants of PCs in Cd-binding complexes was reported in S. pombe (Hayashi et al. 1991) and Candida glabrata (Mehra and Winge 1990). They have a structure of (γ-Glu-Cys)n. Similar peptides were not abundant in many higher plants but its substantial level can be found in Zea mays roots treated with Cd ions. Furthermore, four other PC-related peptides were discovered from plant sources. They have different amino acid residues at the C-terminal end of (γ-Glu-Cys)n peptides, Ala, Ser, Glu, or Gln. The (γ-Glu-Cys)n-Ala peptides first isolated from plants belonging to Fabaceae (Phaseoleae) are called homo-phytochelatins (hPCs) because they are synthesized from homo-glutathione (hGSH) with the structure of γ-Glu-Cys-Ala. Some other variants of those peptides have been also detected in maize and other plants and named as iso-phytochelatins (iso-PCs), which have the structures of (γ-Glu-Cys)n–Ser, (γ-Glu-Cys)n–Glu, or (γ-Glu-Cys)n–Gln (Cobbett 2000; Rea 2012). Biological roles of these variant peptides have not been well understood for a long time; however, their biochemical functions as thiol peptides are assumed to be basically equivalent to that of PCs. They also have common pathways in metabolisms, at least, some enzymes and precursors such as (γ-Glu-Cys) dipeptidyl units, or glutamate (Glu, E) and cysteine (Cys, C), except glycine (Gly, E), for biosynthesis. Amount and distribution of these PC-related peptides may differ in different plant species; as for example, cells of A. thaliana are capable of synthesizing different PCs and iso-PCs (Ducruix et al. 2006). Synthesis of iso-PCs typically depends upon the availability of Gly or GSH synthetase in the cells that helps to switch over to synthesize the peptide (as, e.g., synthesis of dipeptide γ-glutamyl cysteine (γ-EC) when plant comes under stress (Ducruix et al. 2006; Rea 2012). The appearance of the mixture of PCs and iso- PCs such as (γ-Glu-Cys)n–Ser, (γ-Glu-Cys)n–Glu, or (γ-Glu-Cys)n–Gln, and (γ-Glu-Cys)n has been conceivably demonstrated as characteristic of Poaceae family (grasses) under Cd stress by several workers (Klapheck et al. 1994; Cobbett and Goldsbrough 2002) and also under As stress (Zhang et al. 2010; Duan et al. 2011; Batista et al. 2014). As induced hPCs and other variants PCs in Lotus japonicus (Ramos et al. 2008). However, it is suggested that for the PCS1 and hPCS enzymes, hGSH is a good acceptor, but a poor donor, of γ-EC units. Purified AtPCS1 and LjPCS1 were activated (in decreasing order) by Cd, Zn, Cu, and Fe, but not by Co or Ni, in the presence of 5 mM GSH and 50 mM metal ions. Activation of both enzymes by Fe was proven by the complete inhibition of PC synthesis by the Fe-specific chelator, desferrioxamine. Arabidopsis and Lotus plants accumulated hPCs only in response to a large excess of Cu and Zn, but to a much lower extent than did with Cd, indicating that hPC synthesis may not significantly contribute in vivo to Cu, Zn, and Fe detoxification.
3.1.3 Glutathione and Homo-glutathione
GSH is a direct precursor for PC synthesis but itself a very multifunctional metabolite and antioxidant important for metal tolerance and many other biological processes. GSH synthesis consists of two steps of energy-dependent processes that can occur in the cytosol or in the cell organelle like chloroplasts and mitochondria (Zechmann and Müller 2010). First step is an ATP-dependent rate-limiting reaction catalyzed by γ-EC synthetase (EC 6.3.2.2) producing γ-EC from glutamate and cysteine. Second step is an addition of glycine to γ-EC by GSH synthetase (EC 6.3.2.3) activity (Noctor et al. 2012). Both enzymes (named as GSH1 and GSH2, respectively) are encoded by single genes with alternate transcription initiation sites, and GSH1 is exclusively localized in the plastids, whereas GSH2, albeit also present in the chloroplasts, is to a large extent, a cytosolic protein. Thus, the compartmentalization of GSH synthesis functionally links the different cellular compartments and may provide a platform for intracellular redox signaling (Wachter et al. 2005). These and other data lead to the view that the synthesis of γ-EC is restricted to the plastid but that GSH synthesis can also occur in the cytosol using γ-EC, transported from plastids (Wachter et al. 2005; Noctor et al. 2011). In general, GSH synthetase expression and activity increased concurrently with that of γ-EC synthetase, both of which are otherwise indispensable for early developmental stages in plants. GSH-deficit Arabidopsis resulted in increased sensitivity to Cd (Sengupta et al. 2012). The γ-EC synthetase is a rate-limiting enzyme for GSH synthesis (Noctor and Foyer 1998) whose activity is elevated by the presence of metal ions like Cd2+ and repressed by the treatment with buthionine sulfoximine (BSO), a specific inhibitor of this enzyme (Grill et al. 1987; Scheller et al. 1987). GSH plays also an important role in Pb detoxification in Sedum alfredii, under stress conditions, where PCs are absent, and chelated Pb, in conjunction with PCs synthesis and complexation, reduces stress in Pb-tolerant plants (Gupta et al. 2010, 2013b). Likewise, other reports suggested that Vigna radiata under a long-term stress with water deficit condition showed a decrease in both γ-ECS activity and its transcript levels in roots but with higher mRNA levels during the recovery period (Zagorchev et al. 2013). Homo-glutathione (hGSH) has antioxidant activity and serves functions in the transport of reduced sulfur and as direct substrate for hPC synthesis in legumes, as GSH does for PCs in these and other plants (Sobrino-Plata et al. 2009; Zagorchev et al. 2013). If the PCS or hPCS activities are same or samely reduced, these tripeptide levels become important factors that control the major antioxidative reactions for HMs and ROS sequestration. Here, the γ-ECS or similar enzyme has been shown to be involved in hGSH synthesis, while still unknown for the other iso-peptides. Whereas PC synthases (PCSs) are categorized as the γ-EC dipeptidyltranspeptidase (EC 2.3.2.15) that adds a γ-EC-unit of GSH to PCs or another GSH in vitro (Grill et al. 1989; Loeffler et al. 1989), which has been reported to be effective for the formations of hPCs and other iso-PCs (Ramos et al. 2008). Biochemical functions of hGSH and GSH are therefore basically similar if the metabolic or enzymic backgrounds are fulfilled in plants. It was shown that hGSH is an important regulator of root nodule formation, symbiotic interactions and nitrogen fixation in legumes (Zagorchev et al. 2013). Furthermore, their levels, distributions, and redox balances change differently in specific plants in response to different stress or hormone treatments and the developmental stages of the plants (Becana et al. 2010; Clemente et al. 2012; Zagorchev et al. 2013). The biological and evolutionary importance of hGSH and those for substitutions or deletion of the C-terminal amino acid in GSH to form other isotypes in different plants or organ sites await further investigations.
3.2 Other Mechanisms
3.2.1 Transport
Metal ions are vital for life and therefore maintenance of homeostasis of those ions is tremendously important (Fig. 2). Loss of homeostatic balance of elements may create severe metabolic and physiological dysfunction leading to death or severe illness of the plants. The homeostatic maintenance is a highly regulated process integrating uptake, storage, and secretion, where a number of transporters and antiporters proteins are involved. Inhibition in essential nutrient will decrease the plant vitality and its ability to cope with (metal) stress (Huang et al. 2008). Precise activation of metal-responsive genes to counteract the stress through the synthesis of proteins and signaling molecules related to stress takes place (Maksymiec 2007). Physiological transport of nutrients like Ca, Fe, Mg, Mn, Co, and Zn is unique in plants and some of these are inhibited by HMs. For example, Cd competes with these essential nutrients during transportation through transmembrane energy-dependent nutrient transporters and ion channels (Clemens et al. 1998; Curie et al. 2000; Thomine et al. 2000; Papoyan and Kochian 2004). Cortical tissues of the root help entering metal ions and usually become accumulated in the roots. It gets into the xylem by apoplastic and/or symplastic pathway and further transported to shoots. During the journey, the metal may be complexed by a number of ligands such as organic acids and/or PCs. Here, plant roots have the ability either to exclude and/or chelate or sequester Cd and other HMs from the plant tissues (Cataldo and Wildung 1983; Salt et al. 1995).
Impact and route of HM in three ideal types of plants. (1) Hyperaccumulators absorb HMs from roots and transport them to shoots via xylem transport, where various kinds of HM-transports have critical roles. In shoots (and roots), special detoxification/sequestration mechanisms operate. (2) Plants that developed the HM exclusion mechanisms at roots can be useful in agricultural purpose as safety products for other organisms. (3) HM-sensitive plant will be good biological/biochemical index or markers against HMs contaminations
HMs-hyperaccumulator plant ecotypes include the Cd-hyperaccumulators such as Noccaea caerulescens (J&C Presl.) FK Mey, Phytolacca americana L., and A. halleri (L.) O’Kane and Al-Shehbazsetc (Lux et al. 2011). These plants also have the defensive mechanism through the production of Cd-chelators (such as organic acids, etc., as described below) other than PCs at the root zone that confining the entry of Cd to the xylem to prevent the metal accumulation in shoot tissues (Liu et al. 2010; Lux et al. 2011). Cd accumulation in shoot of species of the Caryophyllales and Lamiales was much higher than other species (Broadley et al. 2001). In general, Cd concentrations are mostly (but not always) higher in roots than in shoots, indicating that transportation of Cd to the xylem and phloem is limited in most plants and lowest in seeds, fruits, and tubers (Seregin and Kozhevnikova 2008; Conn and Gilliham 2010). Absorption of HMs in higher plants is a critical issue, where especially rhizosphere region interacts with HMs (Wenzel et al. 2003). They are usually cotransported in the form of cation across the plasma membrane (Manara 2012). Reports suggest that plant roots primarily secrete exudates in its surrounding soil matrix that helps in the chelation of unwanted metals to prevent transportation inside the cell (Marschner 1995). For example, histidine (His) and citrate (CA) are secreted as root exudates to prevent the Ni uptake from the soil (Salt et al. 2000). Pectic sites and a number of extra cellular carbohydrate molecules present on the cell wall play an important role for immobilization of toxic heavy metal ions (Manara 2012). However, HM homeostasis is mainly maintained by transporters present on the plasma membrane. Typical examples of these transporters are the ZIP, the HMA, the YSL, the NRAMP, the CDF, and the CAX families (Williams et al. 2000; Guerinot 2000; Hossain et al. 2012a, b; Sochia and Guerinot 2014), as shown below briefly.
-
1.
The ZIP (zinc-regulated transporter/iron-regulated transporter [ZRT/IRT1]-related protein) family transporters are well characterized for divalent metal uptake, which consist
sof eight transmembrane domains with similar topology at N- and C-termini exposed to apoplast also containing a histidine-rich domain supposed to involve in specific metal binding (Guerinot 2000; Nishida et al. 2008). ZIP protein gets activated in response to Fe or Zn loading. In A. thaliana, IRT1, the founding member of ZIP family, was the first reported transporter in root cells and has an important role in Fe uptake from the soil (Vert et al. 2002). IRT1 can also transport Mn, Zn, and Cd (Korshunova et al. 1999). AtIRT1 in yeast enhanced the Ni-uptake activity (Nishida et al. 2011). Furthermore, AtZIP4 proteins, expressed in roots and shoots, are involved in Zn transport and also helpsin Cd uptake from soil into the root cells and Cd transport from root to shoot (Krämer et al. 2007). -
2.
The HMAs family transporters (P1B-type ATPases that belong to P-type ATPase superfamily) efflux various metal cations across biological membranes (Axelsen and Palmgren 2001). They are basically internal transporters to load Cd and Zn metals into the xylem from the surrounding tissues and act as an efflux pump. The HMAs were categorized as both monovalent and divalent cation transporters (Baxter et al. 2003; Krämer et al. 2007). In A. thaliana, AtHMA3 transporter helps in sequestration of a wider range of HMs, and its overexpression increases the tolerance to HMs like Cd, Pb, Co, and Zn (Morel et al. 2009; Manara 2012). In ABC transporter family, AtPDR8 was discovered in the plasma membrane of A. thaliana root hairs and epidermal cells that helps in effluxing of Cd and Pb from plasma membrane (Kobae et al. 2006; Kim et al. 2007).
-
3.
Oligopeptide transporters (OPTs) are a group of membrane-localized proteins. The OPT proteins belong to a small gene family in plants, named as the YSL (yellow stripe-like) subfamily, taken its name from the maize Yellow stripe 1 protein (ZmYS1), and are involved in uptake of Fe by transporting Fe(III)-phytosiderophore complexes (Curie et al. 2000). Heavy metal ions like Fe, Zn, Cu, Ni, and to a lesser extent Mn and Cd are transported by ZmYS1 transporter (Schaaf et al. 2004). Based on sequence similarity with maize gene, eight presumed YSL transporters have been identified in A. thaliana (Colangelo and Guerinot 2006). AtYSL1 is expressed in the leaf xylem parenchyma, in pollen, and in young siliques, whereas AtYSL2 is expressed in shoot and root vascular tissues and is present in the lateral plasma membrane, steady with a role in the lateral movement of metals into the veins (DiDonato et al. 2004; Schaaf et al. 2004).
-
4.
Metal Tolerance Proteins (MTPs) are metal efflux transporters in plants that belongs to CDF (cation diffusion facilitator) transporter family involved in the pumping divalent metal cations like Zn, Cd, Co, Fe, Ni, and Mn and transportation from the cytoplasm to the vacuole (Nies 1992; Krämer et al. 2007; Peiter et al. 2007; Montanini et al. 2007; Manara 2012). CDF transporters consist of six transmembrane domains, a C-terminal cation efflux domain, and a histidine-rich region between transmembrane domains IV and V (Mäser et al. 2001) which probably act as a sensor for heavy metal concentration (Kawachi et al. 2008).
-
5.
Natural resistance-associated macrophage protein (NRAMP) transporters such as AtNRAMP3 or AtNRAMP4 are localized in the tonoplast and help in the transport of Fe from the vacuole (Thomine et al. 2003; Lanquar et al. 2005). Overexpression of AtNRAMP3 increases Cd sensitivity and prevents the accumulation of Mn, indicating a possible role in the homeostasis of metals other than Fe (Thomine et al. 2003).
-
6.
The CAX (cation exchanger) proteins are one of five transporter families that constitute the Ca/cation antiporters (CaCA) superfamily (Shigaki et al. 2006; Emery et al. 2012). The CAX family members were first identified as Ca transporters but later it was revealed that they are capable of transporting more kinds of HMs. Typical CAX proteins contain 11 transmembrane domains. They facilitate the redistribution of cations across a membrane using electrochemical energy generated by a proton pump in order to maintain optimal ionic concentrations in the cell (Socha and Guerinot 2014).
Among these, the HM transporters which are involved in transport to vacuole or in exclusion at plasma membrane are effective in reducing HMs levels in the cytological active compartments including cytoplasm and plasmids in the cells, which reduce the toxicity exerted by HMs as free radicals or indirect inducers of ROS in the sites. There are strongly convincing proofs that many hyperaccumulator plants for various HMs are prevailed for these transportation mechanisms via xylem transport systems rather than their special detoxification mechanisms in the cells. For details about the respective functions of the transports in hyperaccumulators or HM-tolerant plants, see recent reviews cited above and others (Hossain et al. 2012b; Socha and Guerinot 2014).
3.2.2 Redox Enzymes
Antioxidant system in plants is an intrinsic defense mechanism that regulates ROS levels according to the cellular requirements at a certain period. This system is actually governed under the catalytic activities by the several cooperative enzymes, named redox enzymes or detoxification enzymes (Fig. 1), which involve superoxide dismutase (SOD; EC 1.15.1.1), monodehydroascorbate reductase (MDHAR; EC 1.6.5.4), ascorbate peroxidase (APX; EC 1.11.1.11), catalase (CAT; EC 1.11.1.6), glutathione peroxidase (GPX; EC 1.11.1.9), dehydroascorbate reductase (DHAR; EC 1.8.5.1), and glutathione reductase (GR; EC 1.6.4.2). These enzymes are regulated under HM stresses and hence consequently participate in the mechanism of protection against oxidative stress mediated by HMs. Glutathione S-transferase (GST; EC 2.5.1.18) that catalyzes the GSH-dependent conjunction with various types of substrate molecules to form thioether bond between them also contribute to detoxification of xenobiotics by conjugation reactions (Sherratt and Hayes 2001). There are many reports that support the positive effects of HM on the enzymic defense mechanisms expressed prior to or simultaneously with the enhanced tolerance characteristics to the stress. The water-soluble compounds such as AsA and GSH are used central substrates (Hossain et al. 2012b), but little is known for the role of PCs and h-PCs in the enzymic antioxidant systems at present. Gene expressions related with stress responses to quench directly ROS and develop further tolerance appear to be mediated by GSH and its oxidates (GSSG). In addition, abiotic stress tolerance through the glyoxalase pathway is widely been reported which consists of glyoxalase I (Gly I; lactoylglutathionelyase; EC 4.4.1.5) and glyoxalase II (Gly II; hydroxy-acylglutathione hydrolase; EC 3.1.2.6) (Hossain et al. 2009).
As mentioned earlier, ROS generation is evident in chloroplast, mitochondria, peroxisome, and apoplast adjacent to membrane. Here, several reports suggest that NADPH oxidase-dependent ROS induction can take place in response to Cd stress in P. sativum (Rodrıguez-Serrano et al. 2006), As stress in A. thaliana (Gupta et al. 2013a), Pb stress in V. faba (Pourrut et al. 2008), Cd and Cu stress in A. thaliana (Remans et al. 2010), and Ni stress in wheat (Hao et al. 2006). There is no evidence that cytoplasmic PCs have a role preventing the ROS induction at plasma membrane-associated ROS formation or at apoplast. However, it is acceptable that cell-wall-associated peroxidase catalyzes formation of membrane-permeable H2O2 in apoplast and then makes it possible to interact with cytosolic PCs and other thiol peptides. It is interesting that living with the appropriate concentration level of ROS like H2O2 can promote plant development and support resistance to environmental stressors by controlling the expression of genes and redox signaling (Neill et al. 2002). However, direct interactions between PCs and ROS that result in induction of any HM-tolerant mechanisms are not yet demonstrated; therefore, their actions might be independent but be synergistic.
3.2.3 Sulfur Assimilation
Sulfur (S) is an essential and ubiquitous element involved in a large number of vital biochemical and physiological processes. It is also responsible for developing hypersensitivity to HMs. Earlier studies on transgenic plants revealed that excessive PC levels helps the plant to accumulate more amounts of HMs without enhancing tolerance conferring HM-hypersensitivity (Lee et al. 2003; Pomponi et al. 2006; Manara 2012). Sulfur is known for its catalytic or electrochemical properties and having a capacity to react with a broad spectrum of agents, like cytotoxic electrophilic organic xenobiotics, HMs, and free radicals. GSH and PCs biosynthesis is highly regulated and coordinates to meet the demand for Cys consuming activities, which indirectly explain the overall S demand by plants. Sulfur requirement by plants vary under the diverse environmental conditions, biotic and abiotic stresses including HMs (Rausch and Wachter 2005). It has been previously observed that the withdrawal of S from the growing medium dramatically decreases the levels of S, Cys, and GSH in plant tissues (Lappartient and Touraine 1996; Lappartient et al. 1999; Saito 2004; Nocito et al. 2007). In addition, importance of PCs in homeostasis of metals, antioxidant property, and also in S metabolism was suggested (Dietz et al. 1999; Cobbett 2000). Furthermore, when more stable and massive Cd-PCs conjugates are formed in vacuole under Cd stress, large quantity of S2− is incorporated to the complexes. All of these thiols and S2− are originated through the common energy-dependent S assimilation using sulfate taken by roots. These processes are summarized in Fig. 3 (top), as a series of five process termed as A to E for convenience, where several transporters and/or enzymes with some intermediates as the key factors interconnecting functions among S-containing substances are also shown. Simultaneous or cooperative stimulation of these processes may result in total increase in the level of S-containing compounds as well as the total reducing power in the plants. The diversity of the components accumulated in the tissues also increases. Therefore, gross activation of the processes by HMs or other stressors may contribute to the stress tolerance mechanisms in many plants. However, it is important to note that such influences on the process or component are quite different in case and place, as shown in Fig. 3 (bottom) as tentative examples. Furthermore, it is already evident that diverse species of S-containing substances have diverse functions in plants. Briefly, Cys is the first organic product of S assimilation in plants and is notably used for synthesis of proteins (amino acids) directly or after converting to methionine (Leustek et al. 2000; Saito 2004). These amino acids are thought to be an important sink for reduced S, as well known as for GSH (Noctor et al. 2011). Such a supply or increased sink for reduced S in plants has been positively correlated with resistance to some pathogens, a phenomenon termed S-induced resistance (SIR) by some researchers (Bloem et al. 2007). Here, tissue contents of GSH or precursors are thought to be the factors linking S nutrition to the responses of plants to fungal and viral infection (Noctor et al. 2011).
Diagram of path for PCs, GSH, Cys, and S2−. Top, examples for via root viz shoot circulate paths. (1) AST68 (SO4 2− transporter), (2) APS1 (ATP sulfurylase), (3) APR (APS reductase), (4) Sulfite reductase, (5) SAT (O-acetylserine (thiol) lyase), (6) Cys transporter, (7) γEC synthetase, (8) γEC transporter, (9) GSH synthetase, (10) GSH transporter, (11) PC synthase, (12) PC transporter, (13) PC-Cd-S complexation. Bottom, ideal changes in the S and thiol pools as affected by representative HMs. The data are tentative ones and never cover all cases or previously reported respective evidence
Besides their roles as the major storage and transport forms of reduced S, the importance of GSH and Cys have been implicated in the regulation of S metabolism (Kopriva and Rennenberg 2004). GSH inhibits sulfate uptake and S assimilation by repressing the activities and expressions of several functional proteins mediating the earlier steps of the process, such as sulfate transporter (AST), ATP sulfurylase (APS1), and adenosine 5′-phosphosulfate reductase (APR) in plants (Lappartient et al. 1999; Leustek 2002; Noctor et al. 2011). In most studies, the increased concentrations of Cys in response to oxidative stresses have been reported together with increased GSH concentrations, leading to the conclusion that Cys is mainly needed for the biosynthesis of S-rich compounds with antistress activity, such as GSH and stress-related proteins. However, it is generally thought that at concentrations above 50 μM, Cys is toxic for plants (Meyer and Hell 2005). Cys is a potent chelator of heavy metals ions, but the formed Cys-metal complexes can trigger the Fenton reaction, thereby producing the highly toxic •OH radical, and free Cys is often irreversibly oxidized to different by-products. Sulfide is more toxic than sulfate but stabilized in the complex such as Cd-S-PCs. GSH is quickly turning over but the balance of it and its oxidized form (GSSG) are strictly regulated under enzymic redox systems. Therefore, like a general rule for stock chemicals, more stable S-containing substances (or ones under more strict control) may play a central role as a stable state S-donors in plants. Cd-S-PC complex may be a potential stable S donor as well as other S-containing storage proteins or polysaccharides.
3.2.4 Other Mechanisms: Hypothetical View
The detoxification and sequestration mechanisms driven by PCs may be restricted to the HMs and ROS invaded or occurring in the cell compartments in hyperaccumulator plants or tolerant plants grown under naturally metalliferous or artificially contaminated environments. Here, it is important to note that the direct precursor of PCs is GSH, which is multifunctional and thus required for the many other biochemical processes in response to or not to various HMs. In these plants, by blocking or inefficiency of or inefficiency of HMs and other oxidants to the synthesis of PC peptides and other S-containing oligo-/poly- peptides, can increase the small organic and inorganic S-substances such as S2− or Cys, and thus GSH. This can be mimicked by the case if the final S-conjugated products are stored or immobilized in a certain compartment apart from cytoplasm. These increased S-substances are alone or in combination, capable of increasing the binding and quenching of the free radicals of HMs and ROS more. Furthermore, S can make more stable complex with PCs-Cd, and the core Cd-S formed inside of the PC-Cd-S complexes can act as the semi-conductance micro-devise to control electron flow or exchange between two or more different molecules in cytosol or protein complexes on the bio-membrane, while a direct association of PC peptides to other biopolymer/oligomer has not yet been proven. PC has been associated with many important functions in plants against HM ions, including intracellular binding, detoxification, transport, following compartmentalization in vacuole, and also as a substantial coordinator for the distinguished characteristics of several plant-hyperaccumulators of different HM species. However, relatively less attention has been paid on their potential roles in defense mechanism against ROS induced by various abiotic or biotic oxidative stresses, other than by HMs. This is mainly due to the smaller contents of PCs (less than 0.01 mM) than the other antioxidants under normal condition with little contaminated HMs. Intracellular levels of total PCs rise drastically up to 1–10 mM and 0.1–0.5 mM orders as maximum so far reported, when exposed to 0.2–0.8 mM of metal Cd and 0.02–0.5 mM of metalloid As respectively; hence, the detoxification and sequestration mechanisms driven by PCs may be restricted to the HMs and ROS invaded or occurring in the cell compartments in hyperaccumulator plants or tolerant plants grown under naturally metalliferous or artificially contaminated environments. However, more interestingly, PC-HM complex has been detected in phloem sap rather than xylem sap, indicating that PC-functioning site is again intracellular well-reduced state or symplastic place but not oxidative places to produce ROS like apoplastic sites with high oxygen pressure and organelle having active electron transport system. Further assessments are needed but phloem loading and transport will be the attractive performance stage as nutritional PCs.
4 Conclusion and Future Prospective
PCs and related thiol peptides have been associated with many important functions in plants against HM ions, including intracellular binding, detoxification, transport, following compartmentalization in vacuole, and also as a substantial coordinator for the distinguished characteristics of several plant-hyperaccumulators of different HM species. However, relatively less attention has been paid on their potential roles in defense mechanism against ROS induced by HMs and/or other various abiotic or biotic stresses. This is mainly due to the trace levels of PCs (generally less than 10 μM) constitutively maintained in control plants and their trivial changes after either the treatment with HMs besides Cd or the influence of the other abiotic/biotic stresses, where instead evoked are drastic change and activation of the other antioxidants and redox systems. However, we need further consideration for the potential roles of PCs in the plants growing or habituated on the grounds with artificially or naturally HM-dense conditions, as core or polymeric absorbent for nutritional elements and especially S-coordinated substances, resulting also in a dominant storage bank for thio-mediated reduction power sources. As linking to a phenomenon SIR or a concept GSH/thiol pools, it can be urged for our reconsideration that these stocks will play an important role in the maintenance or increase of the robustious characteristics of the plants against various oxidative stresses under biotic and abiotic occasions with no further excess biochemical or metabolic costs. Potential roles of PCs as the key peptidic thiol and reducing agents in the interconnection to other antioxidant compounds and components, such as LWM soluble thiols, AsA, sugars and sugar alcohols, proline and other amino acids and compatible solutes, as well as membrane and cell-wall-associated redox cycle systems will be highlighted in future.
References
Atal N, Saradhi PP, Mohanty P (1991) Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: analysis of electron transport activities and changes in fluorescence yield. Plant Cell Physiol 32:943–951
Axelsen KB, Palmgren MG (2001) Inventory of the superfamily of P-Type ion pumps in Arabidopsis. Plant Physiol 126:696–706
Batista BL, Nigar M, Mestrot A, Rocha BA, Júnior FB, Price AH, Raab A, Feldmann J (2014) Identification and quantification of phytochelatins in roots of rice to long-term exposure: evidence of individual role on arsenic accumulation and translocation. J Exp Bot 65:1467–1479
Baxter I, Tchieu J, Sussman MR, Boutry M, Palmgren MG, Gribskov M, Harper JF, Axelsen KB (2003) Genomic comparison of P-Type ATPase ion pumps in Arabidopsis and rice. Plant Physiol 132:618–628
Becana M, Matamoros MA, Udvardi M, Dalton DA (2010) Recent insights into antioxidant defenses of legume root nodules. New Phytol 188:960–976
Bloem E, Haneklaus S, Salac I, Wichkenhauser P, Schnug E (2007) Facts and fiction about sulfur metabolism in relation to plant-pathogen interaction. Plant Biol 9:596–607
Blokhina O, Fagerstedt K (2006) Oxidative stress and antioxidant defenses in plants. In: Singh KK (ed) Oxidative stress, disease and cancer. Imperial College Press, London
Broadley MR, Willey NJ, Wilkins JC, Baker AJM, Mead A, White PJ (2001) Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytol 152:9–27
Cataldo DA, Wildung RE (1983) The role of soil and plant metabolic processes in controlling trace element behavior and bioavailability to animals. Sci Total Environ 28:159–168
CERCLA (2007) Priority list of hazardous substances. http://www.atsdr.cdc.gov/spl/supportdocs/appendix-d.pdf
Chatterjee S, Chattopadhyay B, Mukhopadhyay SK (2007) Sequestration and localization of metals in two common wetland plants of contaminated east Calcutta wetlands: a Ramsar Site in India. Land Contam Reclamat 15:437–452
Chatterjee S, Chetia M, Singh L, Chattopadhyay B, Datta S, Mukhopadhyay SK (2011) A study on the phytoaccumulation of waste elements in wetland plants of a Ramsar site in India. Environ Monit Assess 178:361–371
Chen A (2005) Long distance transport of phytochelatins in Arabidopsis and the isolation and characterization of cadmium tolerant mutants in Arabidopsis. PhD thesis, UC San Diego Electronic Theses and Dissertations. http://escholarship.org/uc/item/0fm285zm
Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI (1998) The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci USA 95:12043–12048
Clemens S, Kim EJ, Neumann D, Schroeder JI (1999) Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J 18:3325–3333
Clemente MR, Bustos-Sanmamed P, Loscos J, James EK, Pérez-Rontomé C, Navascués J, Gay M, Becana M (2012) Thiol synthetases of legumes: immunogold localization and differential gene regulation by phytohormones. J Exp Bot 63:3923–3934
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Cobbett C, Goldsbrough P (2002) Phytochelatin and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Colangelo EP, Guerinot ML (2006) Put the metal to the petal: metal uptake and transport throughout plants. Curr Opin Plant Biol 9:322–330
Conn S, Gilliham M (2010) Comparative physiology of elemental distributions in plants. Ann Bot 105:1081–1102
Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347:749–755
De Knecht JA, van Dillen M, Koevoets PLM, Schat H, Verkleij JAC, Ernst WHO (1994) Phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris: chain length distribution and sulfide incorporation. Plant Physiol 104:255–261
DiDonato RJ Jr, Roberts LA, Sanderson T, Eisley RB, Walker EL (2004) Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine–metal complexes. Plant J 39:403–414
Dietz KJ, Baier M, Krämer U (1999) Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants: from molecules to ecosystems. Springer, Heidelberg
Duan GL, Hu Y, Liu WJ, Kneer R, Zhao FJ, Zhu YG (2011) Evidence for a role of phytochelatins in regulating arsenic accumulation in rice grain. Environ Exp Bot 71:416–421
Ducruix C, Junot C, Fiévet JB, Villiers F, Ezan E, Bourguignon J (2006) New insights into the regulation of phytochelatin biosynthesis in A. thaliana cells from metabolite profiling analyses. Biochimie 88:1733–1742
Emery L, Whelan S, Hirschi KD, Pittman JK (2012) Protein phylogenetic analysis of Ca(2+)/cation Antiporters and insights into their evolution in plants. Front Plant Sci 3:1
Freeman JL, Persans MW, Nieman K, Albrecht C, Peer W, Pickering IJ, Salt DE (2004) Increased glutathione biosynthesis plays a role in nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Cell 16:2176–2191
Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 11:1091–1101
Gekeler W, Grill E, Winnacker EL, Zenk MH (1989) Survey of the plant kingdom for the ability to bind heavy metals through phytochelatins. Z Naturforsh 44:361–369
Grill E, Winnacker EL, Zenk MH (1987) Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84:439–443
Grill E, Loffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86:6838–6842
Guerinot ML (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465:190–198
Gumaelius L, Lahner B, Salt DE, Banks JA (2004) Arsenic hyperaccumulation in gametophytes of Pteris vittata: a new model system for analysis of arsenic hyperaccumulation. Plant Physiol 136:3198–3208
Gupta DK, Tohoyama H, Joho M, Inouhe M (2002) Possible roles of phytochelatins and glutathione metabolism in cadmium tolerance in chickpea roots. J Plant Res 115:429–437
Gupta DK, Tohoyama H, Joho M, Inouhe M (2004) Changes in the levels of phytochelatins and related metal binding peptides in chickpea seedlings exposed to arsenic and different heavy metal ions. J Plant Res 117:253–256
Gupta DK, Huang HG, Yang XE, Razafindrabe BHN, Inouhe M (2010) The detoxification of lead in Sedum alfredii H. is not related with phytochelatins but the glutathione. J Hazard Mater 177:437–444
Gupta DK, Inouhe M, Rodríguez-Serrano M, Romero-Puerta MC, Sandalio LM (2013a) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90:1987–1996
Gupta DK, Huang HG, Corpas FJ (2013b) Lead tolerance in plants: strategies for phytoremediation. Environ Sci Pollut Res 20:2150–2161
Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O’Connell MJ, Goldsbrough PB, Cobbett CS (1999) Phytochelatin synthase genes from Arabidopsis and the yeast, Schizosaccharomyces pombe. Plant Cell 11:1153–1164
Hao F, Wang X, Chen J (2006) Involvement of plasma membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedling. Plant Sci 170:151–158
Hayashi Y, Nakagawa CW, Mutoh N, Isobe M, Goto T (1991) Two pathways in the biosynthesis of cadystins (gEC)nG in the cell-free system of the fission yeast. Biochem Cell Biol 69:115–121
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Hossain MA, Hossain MD, Rohman MM, da Silva JAT, Fujita M (2012a) Onion major compounds (flavonoids, organosulfurs) and highly expressed glutathione-related enzymes: possible physiological interaction, gene cloning and abiotic stress response. In: Aguirre CB, Jaramillo LM (eds) Onion consumption and health. Nova, New York
Hossain MA, Piyatida P, Teixeira da Silva JA, Fujita M (2012b) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and Methylglyoxal and in heavy metal chelation. J Bot 872875:37
Huang HG, Li TX, Tian S, Gupta DK, Zhang X, Yang XE (2008) Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Bioresour Technol 99:6088–6096
Inaba T, Kobayashi E, Suwazono Y, Uetani M, Oishi M, Nakagawa H, Nogawa K (2005) Estimation of cumulative cadmium intake causing Itai-itai disease. Toxicol Lett 15:192–201
Ingwersen J, Streck T (2005) A regional-scale study on the crop uptake of cadmium from sandy soils: measurement and modeling. J Environ Qual 34:1026–1035
Inouhe M (2005) Phytochelatins. Braz J Plant Physiol 17:65–78
Inouhe M, Sumiyoshi M, Tohoyama H, Joho M (1996) Resistance to cadmium ions and formation of a cadmium-binding complex in various wild-type yeasts. Plant Cell Physiol 37:341–346
Inouhe M, Huang HG, Chaudhary SK, Gupta DK (2012) Heavy metal bindings and its interactions with thiol peptides and other biological ligands in plant cells. In: Gupta DK, Sandalio LM (eds) Metal toxicity in plants: perception, signaling and remediation. Springer, Heidelberg
Kawachi M, Kobae Y, Mimura T, Maeshima M (2008) Deletion of a histidine-rich loop of AtMTP1, a vacuolar Zn2+/H+ antiporter of Arabidopsis thaliana, stimulates the transport activity. J Biol Chem 283:8374–8383
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218
Kirkby EA, Johnson AE (2008) Soil and fertilizer phosphorus in relation to crop nutrition. In: White PJ, Hammond JP (eds) The ecophysiology of plant-phosphorus interactions. Springer, Dordrecht
Klapheck S, Fliegner W, Zimmer I (1994) Hydroxymethyl-phytochelatins [(gamma-glutamylcysteine)n-serine] are metal-induced peptides of the Poaceae. Plant Physiol 4:1325–1332
Kobae Y, Sekino T, Yoshioka H, Nakagawa T, Martinoia E, Maeshima M (2006) Loss of AtPDR8, a plasma membrane ABC transporter of Arabidopsis thaliana, causes hypersensitive cell death upon pathogen infection. Plant Cell Physiol 47:309–318
Kopriva S, Rennenberg H (2004) Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolisms. J Exp Bot 55:1831–1842
Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB (1999) The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate range. Plant Mol Biol 40:37–44
Krämer U, Talke IN, Hanikenne M (2007) Transition metal transport. FEBS Lett 581:2263–2272
Lanquar V, Lelièvre F, Bolte S, Hamès C, Alcon C, Neumann D, Vansuyt G, Curie C, Schröder A, Krämer U, Barbier-Brygoo H, Thomine S (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J 24:4041–4051
Lappartient AG, Touraine B (1996) Demand-driven control of root ATP sulfurylase activity and SO42- uptake in intact canola. Plant Physiol 111:147–157
Lappartient AG, Vidmar JJ, Leustek T, Glass AMD, Touraine B (1999) Inter-organ signalling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J 18:89–95
Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS (2003) Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiol 131:656–663
Leustek T, Martin MN, Bich JN, Davies JP (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol 51:141–165
Leustek T (2002) Sulfate metabolism. In: Arabidopsis book. doi:10.1199/tab.0017
Liu F, Tang Y, Du R, Yang H, Wu Q, Qiu R (2010) Root for aging for zinc and cadmium requirement in the Zn/Cd hyperaccumulator plant Sedum alfredii. Plant Soil 327:365–375
Loeffler S, Hochberger A, Grill E, Winnacker EL, Zenk MH (1989) Termination of the phytochelatin synthase reaction through sequestration of heavy metals by the reaction product. FEBS Lett 258:42–46
Lux A, Martinka M, Vaculık M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62:21–37
Maitani T, Kubota H, Sato K, Yamada T (1996) The composition of metals bound to class III metallothionein (phytochelatins and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiol 110:1145–1150
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed) Plants and heavy metals, Springer briefs in biometals. Springer, New York. doi:10.1007/978-94-007-4441-7_2
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126:1646–1667
Mehra RK, Winge DR (1990) Metal ion resistance in fungi: molecular mechanisms and their regulated expression. J Cell Biochem 45:1–11
Mehra RK, Tran K, Scott GW, Mulchandani P, Sani SS (1996) Ag(I)-binding to phytochelatins. J Inorg Biochem 61:125–142
Mengel K, Kirkby EA, Kosegarten H, Appel T (2001) Principles of plant nutrition. Kluwer, Dordrecht
Meyer AJ, Hell R (2005) Glutathione homeostasis and redox regulation by sulfhydryl groups. Photosynth Res 86:435–457
Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M (2007) Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8:107
Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P (2009) AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol 149:894–904
Murasugi A (2008) Cadystin (phytochelatin) synthesis induced by cadmium and resulted formation of cadmium sulphide nanoparticles in Schizosaccharomyces pombe. Curr Top Biotechnol 4:65–73
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Nies DH (1992) Resistance to cadmium, cobalt, zinc, and nickel in microbes. Plasmid 27:17–28
Nishida S, Mizuno T, Obata H (2008) Involvement of histidine-rich domain of ZIP family transporter TjZNT1 in metal ion specificity. Plant Physiol Biochem 46:601–606
Nishida S, Tsuzuki C, Kato A, Aisu A, Yoshida J, Mizuno T (2011) AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol 52:1433–1442
Nocito FF, Lancilli C, Giacomini B, Sacchi GA (2007) Sulfur metabolism and cadmium stress in higher plants. Plant Stress 1:142–156, © Global Science Books (downloaded on 21-2-2013 from: http://globalsciencebooks.info/JournalsSup/images/Sample/PS_1(2)142-156.pdf)
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Queval G, Mhamdi A, Chaouch S, Foyer CH (2011) Glutathione. The Arabidopsis book. American Society of Plant Biologists, Rockville, MD. doi:10.1199/tab.0142, e0142
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Papoyan A, Kochian LV (2004) Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance: characterization of a novel heavy metal transporting ATPase. Plant Physiol 136:3814–3823
Peiter E, Montanini B, Gobert A, Pedas P, Husted S, Maathuis FJM, Blaudez D, Chalot M, Sanders D (2007) A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc Natl Acad Sci USA 104:8532–8537
Pomponi M, Censi V, Di Girolamo V, De Paolis A, Di Toppi LS, Aromolo R, Costantino P, Cardarelli M (2006) Overexpression of Arabidopsis phytochelatin synthase in tobacco plants enhances Cd2+ tolerance and accumulation but not translocation to the shoot. Planta 223:180–190
Pourrut B, Perchet G, Silvestre J, Cecchi M, Guiresse M, Pinelli E (2008) Potential role of NADPH-oxidase in early steps of lead-induced oxidative burst in Vicia faba roots. J Plant Physiol 165:571–579
Prasad MNV, Freitas HMO (2003) Metal hyperaccumulation in plants-biodiversity prospecting for phytoremediation technology. Electron J Biotechol 6:285–321
Ramos J, Naya L, Gay M, Abián J, Becana M (2008) Functional characterization of an unusual phytochelatin synthase, LjPCS3, of Lotus japonicus. Plant Physiol 148:536–545
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10:503–509
Rauser WE (1999) Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin and metallothioneins. Cell Biochem Biophys 31:19–48
Rea PA (2012) Phytochelatin synthase: of a protease a peptide polymerase made. Physiol Plant 145:154–164
Remans T, Opdenakker K, Smeets K, Mathijsen D, Vangronsveld J, Cuypers A (2010) Metal-specific and NADPH oxidase dependent changes in lipoxygenase and NADPH oxidase gene expression in Arabidopsis thaliana exposed to cadmium or excess copper. Funct Plant Biol 37:532–544
Rodrıguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gomez M, Del Rio LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Romero-Puertas MC, McCarthy I, Sandalio LM, Palma JM, Corpas FJ, Gómez M, del Río LA (1999) Cadmium toxicity and oxidative metabolism of pea leaf peroxisomes. Free Radic Res 31(Suppl 1):25–31
Saito K (2004) Sulfur assimilatory metabolism: the long and smelling road. Plant Physiol 136:2443–2450
Salt DE, Prince RC, Pickering IJ, Raskin I (1995) Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol 109:1427–1433
Salt DE, Kato N, Krämer U, Smith RD, Raskin I (2000) The role of root exudates in nickel hyperaccumulation and tolerance in accumulator and nonaccumulator species of Thlaspi. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soils and waters. CRC, Boca Raton, FL
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279:9091–9096
Schat H, Kalff MMA (1992) Are phytochelatins involved in differential metal tolerance or do they merely reflect metal-imposed strain? Plant Physiol 99:1475–1480
Scheller HV, Huang B, Hatch E, Goldsbrough PB (1987) Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol 85:1031–1035
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Sengupta D, Ramesh G, Mudalkar S, Kumar KRR, Kirti PB, Reddy AR (2012) Molecular cloning and characterization of γ-glutamyl cysteine synthetase (VrγECS) from roots of Vigna radiate (L.) Wilczek under progressive drought stress and recovery. Plant Mol Biol Rep 30:894–903
Seregin IV, Kozhevnikova AD (2008) Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ J Plant Physiol 55:1–22
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinel E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol 232:1–44
Sherratt PJ, Hayes JD (2001) Glutathione S-transferases. In: Ioannides C (ed) Enzyme systems that metabolise drugs and other xenobiotics. Willey, New York
Shigaki T, Rees I, Nakhleh L, Hirschi KD (2006) Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J Mol Evol 63:815–825
Siedlecka A, Baszynaski T (1993) Inhibition of electron flow around photosystem I in chloroplasts of Cd-treated maize plants is due to Cd-induced iron deficiency. Physiol Plant 87:199–202
Sobrino-Plata J, Ortega-Villasante C, Flores-Cáceres ML, Escobar C, Del Campo FF, Hernández LE (2009) Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77:946–954
Socha AL, Guerinot ML (2014) Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front Plant Sci 5:106
Sterckeman T, Perriguey J, Caël M, Schwartz C, Morel JL (2004) Applying a mechanistic model to cadmium uptake by Zea mays and Thlaspi caerulescens: consequences for the assessment of the soil quantity and quality factors. Plant Soil 262:289–302
Takahashi S, Murata N (2008) How do environmental stresses accelerate photo inhibition? Trends Plant Sci 13:178–182
Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporters family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA 97:4991–4996
Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multi specific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695
Vatamaniuk OK, Mari S, Lu YP, Rea PA (1999) AtPCS1, a phytochelatins synthase from Arabidopsis: isolation and in vitro reconstitution. Proc Natl Acad Sci USA 96:7110–7115
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Viehweger K (2014) How plants cope with heavy metals. Bot Stud 55:35
Wachter A, Wolf S, Steininger H, Bogs J, Rausch T (2005) Differential targeting of GSH1 and GSH2 is achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J 41:15–30
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Wenzel WW, Bunkowski M, Puschenreiter M, Horak O (2003) Rhizosphere characteristics of indigenously growing nickel hyperaccumulator and excluder plants on serpentine soil. Environ Pollut 123:131–138
Williams LE, Pittman JK, Hall JL (2000) Emerging mechanisms for heavy metal transport in plants. Biochim Biophys Acta 77803:1–23
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zechmann B, Müller M (2010) Subcellular compartmentation of glutathione in dicotyledonous plants. Protoplasma 246:15–24
Zhang J, Zhao QZ, Duan GL, Huan YC (2010) Influence of sulphur on arsenic accumulation and metabolism in rice seedlings. Environ Exp Bot 72:34–40
Acknowledgments
The authors apologize for the many colleagues who are not referenced in this work due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Inouhe, M. et al. (2015). General Roles of Phytochelatins and Other Peptides in Plant Defense Mechanisms Against Oxidative Stress/Primary and Secondary Damages Induced by Heavy Metals. In: Gupta, D., Palma, J., Corpas, F. (eds) Reactive Oxygen Species and Oxidative Damage in Plants Under Stress. Springer, Cham. https://doi.org/10.1007/978-3-319-20421-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-20421-5_9
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-20420-8
Online ISBN: 978-3-319-20421-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)