Abstract
Iron plays an important role in brain injury after intracerebral hemorrhage (ICH). Our previous study found minocycline reduces iron overload after ICH. The present study examined the effects of minocycline on the subacute brain injury induced by iron. Rats had an intracaudate injection of 50 μl of saline, iron, or iron + minocycline. All the animals were euthanized at day 3. Rat brains were used for immunohistochemistry (n = 5–6 per each group) and Western blotting assay (n = 4). Brain swelling, blood-brain barrier (BBB) disruption, and iron-handling proteins were measured. We found that intracerebral injection of iron resulted in brain swelling, BBB disruption, and brain iron-handling protein upregulation (p < 0.05). The co-injection of minocycline with iron significantly reduced iron-induced brain swelling (n = 5, p < 0.01). Albumin, a marker of BBB disruption, was measured by Western blot analysis. Minocycline significantly decreased albumin protein levels in the ipsilateral basal ganglia (p < 0.01). Iron-handling protein levels in the brain, including ceruloplasmin and transferrin, were reduced in the minocycline co-injected animals. In conclusion, the present study suggests that minocycline attenuates brain swelling and BBB disruption via an iron-chelation mechanism.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Intracerebral hemorrhage (ICH) accounts for 10–15 % of all strokes, but it results in a disproportionately high morbidity and mortality [9, 10]. Experiments have indicated that clot lysis and iron play an important role in ICH-induced brain injury. Iron overload occurs in the brain after ICH in rats. Iron chelators like deferoxamine can reduce brain edema and improve neurological function in experimental models of ICH [4, 8, 19, 20].
A number of proteins, including ferritin, transferrin (Tf), transferrin receptor (TfR), ceruloplasmin (Cp), and heme oxygenase 1 (HO-1), are involved in maintaining brain iron homeostasis. Ferritin, a cytosolic heterodimeric protein that assembles as a 24-subunit sphere, has a dual function of iron detoxification and iron reserve [11]. Tf and TfR are involved in the transport of iron across biological membranes. Tf is the main source of iron for neurons, which express high levels of TfR. Cp is a ferroxidase necessary for the oxidation of Fe2+ to Fe3+ and subsequent binding of iron to transferrin. HO-1 is involved in the degradation of heme, which results in the production of iron (as well as biliverdin and carbon monoxide).
Minocycline, a second generation of tetracycline-based molecule, is a potent inhibitor of microglia activation [16]. It has been shown to be beneficial in several stroke models [2], presumably due to its anti-inflammatory effect. However, minocycline also has strong iron-chelating activity [3] and a previous study demonstrated it can attenuate iron neurotoxicity in cortical cell cultures, whereas two other inhibitors of microglial activation, doxycycline and macrophage/microglia inhibitory factor (MIF), were ineffective [1]. Our previous study found minocycline attenuates iron-induced brain injury, in vivo at least in part due to chelation of iron [23]. The current study further examined the effects of minocycline on the subacute brain injury induced by iron.
Materials and Methods
Animal Preparation and Intracerebral Injection
The protocols for these animal studies were approved by the University of Michigan Committee on the Use and Care of Animals. Male Sprague-Dawley rats from Charles River Laboratories (weight 275–300 g) were used in this study. Septic precautions were utilized in all surgical procedures and body temperature was maintained at 37.5 °C using a feedback-controlled heating pad. Rats were anesthetized with pentobarbital (50 mg/kg, intraperitoneally (IP)) and the right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. Blood from the catheter was used to determine pH, PaO2, PaCO2, hematocrit, and glucose. The animals were positioned in a stereotactic frame (Kopf Instruments). Minocycline was purchased from Sigma (St. Louis, MO, USA). Fifty microliters of saline, FeCl2 (0.5 mM), or FeCl2 mixed with minocycline (0.5 mM) was injected into the right caudate through a 26-gauge needle at a rate of 10 μl per minute using a microinfusion pump (Harvard Apparatus Inc.). The coordinates were 0.2 mm anterior to the bregma and 5.5 mm ventral and 4.0 mm lateral to midline. After intracerebral infusion, the needle was removed and the skin incision closed with suture.
Experiment Groups
Rats had an intracaudate injection of 50 μl of saline, FeCl2 (0.5 mM), or FeCl2 (0.5 mM) + minocycline (0.5 mM) and were euthanized at 72 h. Rat brains were used for immunohistochemistry (n = 5–6 per group) and Western blotting assay (n = 4 for each group).
Brain Swelling Measurements
Rats were anesthetized and underwent intracardiac perfusion with 4 % paraformaldehyde in 0.1 mol/l (pH 7.4) phosphate-buffered saline (PBS). The brains were removed and kept in 4 % paraformaldehyde for 12 h, then immersed in 30 % sucrose for 3–4 days at 4 °C. Brains were then placed in embedding OCT compound and sectioned on a cryostat (18-μm thick). Coronal sections at the blood injection site were stained with hematoxylin and eosin (H&E) and then were scanned. The bilateral caudate were outlined for area measurement using Image J (National Institutes of Health). All measurements were repeated three times and the mean value was used. Brain swelling was determined as (ipsilateral area/contralateral area) × 100 %.
Immunohistochemistry
Immunohistochemistry was performed as previously described [5, 7]. Primary antibodies were polyclonal rabbit anti-human ferritin IgG (DACO; 1:400 dilution). Normal rabbit IgG was used as negative control.
Western Blot Analysis
Western blot analysis was performed as previously described [7]. The primary antibodies were polyclonal goat anti-mouse albumin antibody (1:10,000 dilution; Bethyl Laboratories Inc. Montgomery, TX), rabbit polyclonal HO-1 antibody (1:2000 dilution; Assay Designs/Stressgen, Farmingdale, NY), polyclonal rabbit anti-human Tf (1:2000 dilution; Dako, Carpinteria, CA), monoclonal mouse anti-human TfR (1:2000 dilution; Invitrogen, Grand Island, NY), and sheep anti-ceruloplasmin antibody (1:2000 dilution; Abcam, Cambridge, MA). The antigen-antibody complexes were observed with the ECL system and exposed to Kodak X-OMAT film. The membranes were then stripped and reprobed with antibody against β-actin. The relative densities of bands were analyzed with NIH Image J [24].
Statistical Analysis
All the data in this study are presented as mean ± SD. Data were analyzed by one-way analysis of variance (ANOVA). A level of p < 0.05 was considered statistically significant.
Results
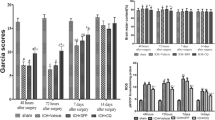
Brain swelling (ipsilateral caudate area as a percentage of contralateral area) was determined on H&E-stained coronal sections at 72 h after injection. FeCl2 resulted in swelling of the ipsilateral caudate. Minocycline co-injection significantly reduced that swelling (104.2 % ± 3.1 % vs 112.7 % ± 5.3 % in FeCl2 group, n = 5, p < 0.01, Fig. 1a)
(a) Coronal gross hematoxylin and eosin stained sections and the bar graph demonstrating ipsilateral caudate size expressed as a percentage of the contralateral side 72 h after injection of saline, FeCl2, or FeCl2 + minocycline (MC; 0.5 mM). Values are expressed as means ± SD; n = 5, #p < 0.01, compared with FeCl2 group. (b) The albumin levels in the ipsilateral basal ganglia 72 h after injection of saline, FeCl2, or FeCl2 + MC (0.5 mM). Equal amounts 25 μg of protein were loaded. Values are means ± SD; n = 4, #p < 0.01, compared with FeCl2 group
Brain albumin, a marker of BBB disruption, was measured by Western blot analysis. Albumin levels in the ipsilateral basal ganglia were markedly increased after FeCl2 injection (Fig. 1b). This increase was greatly reduced by co-injection of minocycline (1946 ± 1122 vs 6973 ± 1481 pixels in FeCl2 alone group, n = 4, p < 0.01, Fig. 1b).
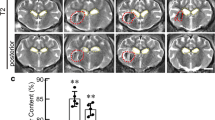
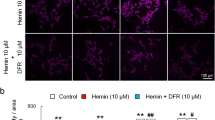
The protein level of heme oxygense-1 (HO-1) in the ipsilateral basal ganglia was significantly increased by FeCl2 injection. The HO-1 levels in FeCl2 + minocycline group (2489 ± 2022 pixels) were significantly lower than that in the FeCl2 group (7668 ± 1467 pixels, n = 4, p < 0.05, Fig. 2a). Also, the immunoreactivity for ferritin (an iron storage protein) was upregulated after FeCl2 injection. Ferritin-positive cells were fewer in minocycline-treated animals (510 ± 41 vs 905 ± 107 cells/mm2 in FeCl2 group, n = 5, p < 0.01, Fig. 2b).
(a) Heme oxygenase-1 (HO-1) levels in the ipsilateral basal ganglia 72 h after injection of saline, FeCl2, or FeCl2+ minocycline (MC; 0.5 mM). Values are means ± SD; n = 4, *p < 0.05, compared with FeCl2. (b) Ferritin immunoreactivity in the ipsilateral basal ganglia 72 h after injection of saline, FeCl2, or FeCl2 + MC (0.5 mM). Values are means ± SD; n = 6, #p < 0.01, compared with FeCl2. Scale bar = 20 μm
Ceruloplasmin (CP) is involved in iron metabolism by oxidizing ferrous iron to ferric iron. Brain CP levels in the ipsilateral basal ganglia were significantly increased by FeCl2 injection. This upregulation was blocked by co-injection of minocycline (2184 ± 675 vs 6629 ± 1123 pixels in FeCl2 group, n = 4, p < 0.01, Fig. 3a). Tf, through binding to its receptor (TfR), is involved in the transport of iron into cells. Compared with saline control, both Tf and TfR protein levels were significantly higher in the ipsilateral basal ganglia after FeCl2 injection. The co-injection of minocycline reduced that upregulation (Tf: 5574 ± 589 vs 7742 ± 1428 pixels in FeCl2 group, p < 0.05, Fig. 3b; TfR: 3851 ± 861 vs 6702 ± 312 pixels in FeCl2 group, p < 0.01, Fig. 3c)
Discussion
Brain iron overload occurs after ICH and can have detrimental effects [4, 5]. Intracerebral infusion of iron causes brain edema, whereas deferoxamine, an iron chelator, attenuates ICH-induced brain injury in animals, which suggests that iron may contribute to brain injury after ICH [4, 6, 19]. The current study indicates that minocycline can reduce iron-induced brain injury (brain swelling, BBB disruption) and alter the expression of iron-handling proteins.
Minocycline, a tetracycline derivative, is a potent inhibitor of microglia activation [15, 16]. In vivo, minocycline reduced perihematomal brain edema, neurological deficits, and brain atrophy [18]. To date, the primary CNS mechanism implicated in minocycline neuroprotection is via a highly potent inhibitory effect on microglial activation [12, 21]. On the other hand, minocycline is a strong iron-chelator [1, 3, 23]. Recent evidence in vitro has shown that minocycline can attenuate iron neurotoxicity in cortical cell cultures. Cortical cultures treated with 10 μM ferrous sulfate for 24 h sustained loss of most neurons and an increase in malondialdehyde. Minocycline prevented this injury, with near-complete protection at 30 μM. Two other inhibitors of microglial activation, doxycycline and MIF, were ineffective. Oxidation of isolated culture membranes by iron was also inhibited by minocycline [1, 3]. Minocycline can attenuate this iron-induced brain edema, DNA damage, and neuronal death, but MIF, a microglia inhibitor, had no effect [23]. In the current study, minocycline reduced iron-induced brain swelling. This confirms the ability to reduce iron neurotoxicity and indicates that the effects of minocycline on ICH-induced brain injury may at least in part be related to the effects of this drug on iron overload [23].
Multiple forms of edema are present after ICH, but the main form is vasogenic. In the present study, we found that BBB disruption occurred after intracerebral injection of ferrous iron and that minocycline greatly reduced that disruption. Preservation of BBB function by minocycline is, therefore, likely a major contributor to the reduced iron-induced brain swelling found with this drug.
Further evidence for the impact of minocycline on brain iron comes from the effects on iron handling proteins. A number of those proteins (ferritin, Tf, TfR, and CP) were markedly increased 72 h after FeCl2 injection. That upregulation was significantly reduced by coinjection of minocycline. The iron chelation effect of minocycline may reduce the induction of these proteins.
Ferritin, a naturally occurring iron chelator, is involved in maintaining brain iron homeostasis. Ferritin consists of a heavy (FTH) subunit that catalyzes the rapid oxidation of ferrous to ferric iron and a light (FTL) subunit that may be involved in the nucleation of the iron core within the protein shell. Thus, ferritin has a dual function of iron detoxification and iron reserve [17], and the brain can produce ferritin. Tf and TfR are involved in the transport of iron across biological membranes. Brain endothelial cells express TfR and it is involved in transporting iron from blood to brain. However, one report indicates that there is rapid efflux of Tf from brain to blood across the BBB [22], suggesting that Tf and TfR could contribute to iron clearance when there is brain iron overload. Cp is the major copper transport protein in plasma and catalyzes the conversion of toxic ferrous iron to the safer ferric iron. Elevated brain Cp levels have been observed in patients with neurodegenerative conditions, including Alzheimer’s, Parkinson’s, and Huntington’s diseases [14]. The upregulation of these iron handling proteins in brain in the setting of iron overload (FeCl2 injection or ICH) may have important protective functions. By chelating iron, minocycline may fulfill some of the same functions (iron detoxification), but its effect on other functions (iron distribution/clearance) need to be investigated.
Heme oxygenases (HO) are key enzymes for the degradation of heme. HO-1, also called heat shock protein 32, is induced by a variety of stimuli. The biological significance of HO-1 upregulation is still uncertain. HO-1 upregulation increases free redox active iron production. Our results demonstrated that ferrous upregulated HO-1 protein levels in the brain and co-injection with minocycline reduced the upregulation of HO-1. HO-1 upregulation and reactive iron accumulation are associated with oxidative stress [13], which can be attenuated by minocycline.
In summary, systemic minocycline can alleviate iron-induced subacute brain injury. It also has a marked effect on the expression of iron-handling proteins in the brain.
References
Chen-Roetling J, Chen L, Regan RF (2009) Minocycline attenuates iron neurotoxicity in cortical cell cultures. Biochem Biophys Res Commun 386:322–326
Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC (2006) Minocycline for short-term neuroprotection. Pharmacotherapy 26:515–521
Grenier D, Huot MP, Mayrand D (2000) Iron-chelating activity of tetracyclines and its impact on the susceptibility of Actinobacillus actinomycetemcomitans to these antibiotics. Antimicrob Agents Chemother 44:763–766
Hua Y, Keep RF, Hoff JT, Xi G (2007) Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke 38:759–762
Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G (2006) Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg 104:305–312
Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT (2002) Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg 96:287–293
Jin H, Xi G, Keep RF, Wu J, Hua Y (2013) DARPP-32 to quantify intracerebral hemorrhage-induced neuronal death in basal Ganglia. Transl Stroke Res 4:130–134
Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G (2004) Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg 100:672–678
Pandey AS, Xi G (2014) Intracerebral hemorrhage: a multimodality approach to improving outcome. Transl Stroke Res 5:313–315
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344:1450–1460
Salvador GA (2010) Iron in neuronal function and dysfunction. Biofactors 36:103–110
Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W (2005) Minocycline as a neuroprotective agent. Neuroscientist 11:308–322
Suttner DM, Dennery PA (1999) Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 13:1800–1809
Texel SJ, Xu X, Harris ZL (2008) Ceruloplasmin in neurodegenerative diseases. Biochem Soc Trans 36:1277–1281
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580–2588
Tikka TM, Koistinaho JE (2001) Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol 166:7527–7533
Vidal R, Miravalle L, Gao X, Barbeito AG, Baraibar MA, Hekmatyar SK, Widel M, Bansal N, Delisle MB, Ghetti B (2008) Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J Neurosci 28:60–67
Wu J, Yang S, Xi G, Fu G, Keep RF, Hua Y (2009) Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol Res 31:183–188
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5:53–63
Xiong XY, Wang J, Qian ZM, Yang QW (2014) Iron and intracerebral hemorrhage: from mechanism to translation. Transl Stroke Res 5:429–441
Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A 95:15769–15774
Zhang Y, Pardridge WM (2001) Rapid transferrin efflux from brain to blood across the blood-brain barrier. J Neurochem 76:1597–1600
Zhao F, Hua Y, He Y, Keep RF, Xi G (2011) Minocycline-induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke J Cereb Circ 42:3587–3593
Zhao J, Chen Z, Xi G, Keep RF, Hua Y (2014) Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl Stroke Res 5:586–594
Acknowledgment
This study was supported by grants NS-073595, NS-079157, and NS-084049 from the National Institutes of Health (NIH), and 973 Program-2014CB541600 and NSFC81200891 from National Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zhao, F., Xi, G., Liu, W., Keep, R.F., Hua, Y. (2016). Minocycline Attenuates Iron-Induced Brain Injury. In: Applegate, R., Chen, G., Feng, H., Zhang, J. (eds) Brain Edema XVI. Acta Neurochirurgica Supplement, vol 121. Springer, Cham. https://doi.org/10.1007/978-3-319-18497-5_62
Download citation
DOI: https://doi.org/10.1007/978-3-319-18497-5_62
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18496-8
Online ISBN: 978-3-319-18497-5
eBook Packages: MedicineMedicine (R0)