Abstract
Germinal matrix hemorrhage (GMH) is a major cause of brain damage in prematurity and has long-lasting neurological implications. The development of brain inflammation contributes to brain injury, leading to a lifetime of neurologic deficits. PAR-1 and 4 receptors are involved with inflammatory pathways after brain hemorrhage in adult models of stroke, of which cyclooxygenase-2 (COX-2) is a potential mediator. We therefore hypothesized a role for PAR-1, 4/ COX-2 signaling following GMH. Postnatal day 7 Sprague-Dawley rats were subjected to GMH induction, which entailed stereotactic collagenase infusion into the ganglionic eminence. Animals were euthanized at two time points: 72 h (short-term) or 4 weeks (long-term). Short-term COX-2 expression was evaluated in the context of PAR-1 (SCH-79797) and PAR-4 (P4pal10) inhibition. Pups in the long-term group were administered the selective COX-2 inhibitor (NS-398); and the neurobehavioral and pathological examinations were performed 4 weeks later. Pharmacological PAR-1, 4 antagonism normalized COX-2 expression following GMH and reduced hydrocephalus. Early inhibition of COX-2 by NS-398 improved long-term neurobehavioral outcomes. COX-2 signaling plays an important role in brain injury following neonatal GMH, possibly through upstream PAR-1, 4 receptor mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stroke, experimental
- COX-2
- Neonatal rats
- Germinal matrix hemorrhage
- Hydrocephalus
- Neurological dysfunction
Introduction
Germinal matrix hemorrhage (GMH) is the most common neurological disease of premature infants, partly because this germinal region is most vulnerable to spontaneous bleeding within the first 3 days of preterm life [1]. Intracerebroventricular expansion partly contributes to long-term brain injury through mechanical compression of surrounding tissues [2–4]. Devastating outcomes include hydrocephalus, mental retardation, and cerebral palsy [1, 5, 6]. Current treatment modalities are largely ineffective, and GMH has been thus far not preventable [7].
Importantly, the blood constituent thrombin is an established factor in hydrocephalus formation [8–10], which binds and trans-activates a subfamily of G protein-coupled receptors named proteinase-activated receptors (specifically PAR-1 and PAR-4) [11], theoretically leading to increased COX-2 expression [12]. Therefore, we hypothesized that modulation of brain injury through thrombin, PAR-1,-4, and COX-2 could be an eventual strategy to help improve outcomes after GMH.
Methods
All studies, protocols, and procedures were approved by the Institutional Animal Care and Use Committee at Loma Linda University. Postnatal day 7 (P7) neonatal rats were subjected to stereotactic ganglionic eminence collagenase infusion. Groups were as follows: animals were euthanized at either of two time points 72 h (short-term) or 4 weeks (long-term). Short-term COX-2 expression was evaluated in the context of PAR-1 (SCH-79797) and PAR-4 (P4pal10) inhibition; pups in the long-term group were administered the selective COX-2 inhibitor (NS-398) as routinely performed [13].
Animal Surgeries
P7 Sprague-Dawley rat pups (14–19 g) were randomly allocated to either GMH or sham operation. A stereotactically guided, 0.3 U bacterial collagenase infusion model was used to model preterm right-sided ganglionic eminence bleeds [14–16]. Timed pregnant rats were purchased from Harlan Laboratories (Indianapolis, IN, USA), and pups of equally both genders were subjected to collagenase infusion [15]. Briefly, general anesthesia was obtained by using isoflurane (3 % in 30/70 % oxygen/medical air). Anesthetized pups were positioned prone, with heads secured onto the neonatal stereotactic frame (Kopf Instruments, Tujunga, CA, USA). The scalp was then sterilized (using betadine solution), and a small midline incision made to expose the bregma. Using a standard dental drill, a 1-mm cranial burr hole was made (bregma coordinates: 1.8 mm anterior, 1.5 mm lateral, 2.8 mm deep), through which a 26-gauge needle was lowered, and at this position, clostridial collagenase VII-S (0.3 U: Sigma, St. Louis, MO, USA) was infused at 0.25 μl/min into the right basal ganglion. Needles were left in place for 10 min after infusion to prevent backflow. Thereafter, the needle was slowly withdrawn at rate of 1 mm/min; burr holes were sealed with bone wax; and the scalp was sutured closed. All animals were allowed to recover under observation on a 37 °C warm heating blanket before being returned to their dams. Shams received all the above without collagenase infusion, as routinely performed [13].
Animal Perfusion and Tissue Extraction
The animals were fatally anesthetized with isoflurane (≥5 %) followed by cardiovascular perfusion with ice-cold PBS for Western blot analyses. Forebrains were dissected and snap-frozen with liquid nitrogen and then stored in –80 °C freezer, awaiting quantification as routinely performed [13].
Western Blotting
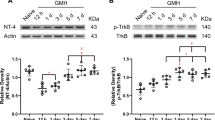
For the protein immunoblot [13], the concentration was determined using the DC protein assay (Bio-Rad, Hercules, CA, USA). The samples were then subjected to SDS-PAGE on 4–20 % gels, and then transferred to nitrocellulose membrane X 100 min at 100 V (Bio-Rad). Blotting membranes were incubated for 1 h with 5 % nonfat milk in Tris-buffered saline containing 0.1 % Tween 20, and these were then incubated overnight with the primary antibody, anti-COX2 (1:200; Cayman Chemical, Ann Arbor, MI, USA). Membranes were then incubated using secondary antibodies (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and processed with an ECL Plus kit (GE Healthcare and Life Science, Piscataway, NJ, USA). For an internal control, the same membrane was probed using an antibody against β-actin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) after being stripped. Relative densities of resultant protein immunoblot images were semiquantitatively analyzed by Image J software (4.0, Media Cybernetics, Silver Spring, MD, USA) as described elsewhere [17].
Neurological Deficits
All neurobehavior assessments were conducted in a blinded manner by experienced investigators [13–16]. Animals were assessed using a series of tests. Neurological deficit was quantified using a series of six tests measuring functional deficits (100 = severe, 50 = moderate, 0 = none): (1) proprioceptive limb placing, (2) lateral limb placement, (3) forelimb placement, (4) postural reflex, (5) back pressure toward edge, and (6) lateral pressure toward edge. These are routinely performed in brain-injured juvenile rats [18]. A T-maze was used to assess short-term (working) memory ability [19]; for each trial, rat were placed into the stem (40 cm × 10 cm) of the T-maze and allowed to explore until either the left or right path was chosen. Following a sequence of 10 trials, the rate of spontaneous alternation (0 % = none and 100 % = complete; alternations/trial) was recorded [18, 20].
Histological Slides
Animals were terminally anesthetized with isoflurane (≥5 %), followed by cardiovascular perfusion with ice-cold PBS and 10 % paraformaldehyde. Brains were removed and separated from surrounding tissues and post-fixed in 10 % paraformaldehyde and then 30 % sucrose (weight/volume) for total of 3 days. Histopathological pictographs used 10-μm thick coronal sections, caudally cut every 600 μm on a cryostat (Leica Microsystems LM3050S), then mounted and stained on poly-l-lysine-coated slides.
Statistical Analysis
Significance was based on <0.05. Data were statistically analyzed using one-way ANOVA, followed by Tukey post hoc test for significant analyses. Statistical analyses were performed using SigmaPlot version 10.0 for Windows.
Results
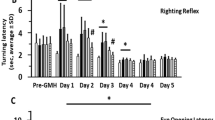
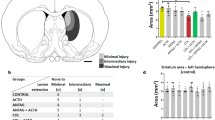
Early combined PAR-1 and PAR-4 signal inhibition reduced COX-2 expression (p < 0.05; Fig. 1) in a dose-responsive manner measured 72 h after collagenase infusion. Thereafter, in a separate cohort of animals, direct inhibition of COX-2 by NS-398 further reduced hydrocephalus (Fig. 2) and also improved long-term neurobehavioral outcome (p < 0.05; Fig. 3).
Conclusion
Translational stroke studies, in particular those involving animal modeling, are greatly needed to safely integrate basic preclinical investigations ahead of eventual clinical applications [21–25]. This study therefore investigated the value of modulating thrombin–PAR-1 and PAR-4 with reversing COX-2 upregulation, as well as the effect of direct COX-2 inhibition on post-hemorrhagic hydrocephalus and on neurological deficits. In prior studies, others hypothesized that hydrocephalus mechanisms involved increased production of infiltrating extracellular matrix (ECM) proteins throughout the cerebroventricular system and that these would lead to the obstruction of CSF outflow [1, 2, 10, 14, 15, 26–30]. Our data suggest that thrombin-induced PAR-1, -4 stimulation could upregulate harmful signaling, exacerbating inflammatory signaling (i.e., COX-2 mediated) upstream of ECM dysregulation [1, 8, 12, 14, 15, 31–34]. Thus, we hypothesized that thrombin binding to PAR-1, -4 receptors could consequently upregulate COX-2 protein. Furthermore, we investigated inhibition of PAR-1, -4 using a combined treatment with SCH79797 (PAR-1 antagonist) and p4pal10 (PAR-4 antagonist), which also significantly improved COX-2 after 72 h. Next, we asked whether directly inhibiting COX-2 following GMH could circumvent long-term negative outcomes. Our findings demonstrated that vehicle-treated animals had significantly worsened outcomes compared with shams, and treatment with NS398 (COX-2 inhibitor) significantly improved not only neuropathology but also and neurological ability. Therefore, by decreasing the early inflammatory COX-2 signaling pathway, we improved long-term outcome in juvenile animals. In summary, this study is the first to show that normalization of thrombin–PAR-1, -4 signals positively affect early COX-2 expression levels and improve long-term outcomes following collagenase infusion-mediated GMH.
References
Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67:1–8
Aquilina K, Chakkarapani E, Love S, Thoresen M (2011) Neonatal rat model of intraventricular haemorrhage and post-haemorrhagic ventricular dilatation with long-term survival into adulthood. Neuropathol Appl Neurobiol 37:156–165
Chen Q, Zhang J, Guo J, Tang J, Tao Y, Li L, Feng H, Chen Z (2014) Chronic hydrocephalus and perihematomal tissue injury developed in a rat model of intracerebral hemorrhage with ventricular extension. Transl Stroke Res. doi:10.1007/s12975-014-0367-5
Zhao J, Chen Z, Xi G, Keep RF, Hua Y (2014) Deferoxamine attenuates acute hydrocephalus after traumatic brain injury in rats. Transl Stroke Res 5:586–594
Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B (2010) Annual summary of vital statistics: 2007. Pediatrics 125:4–15
Uria-Avellanal C, Robertson NJ (2014) Na(+)/H(+) exchangers and intracellular pH in perinatal brain injury. Transl Stroke Res 5:79–98
Whitelaw A (2001) Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol 6:135–146
Gao F, Liu F, Chen Z, Hua Y, Keep RF, Xi G (2014) Hydrocephalus after intraventricular hemorrhage: the role of thrombin. J Cereb Blood Flow Metab 34:489–494
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y (2014) Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res 5:472–475
Siler DA, Gonzalez JA, Wang RK, Cetas JS, Alkayed NJ (2014) Intracisternal administration of tissue plasminogen activator improves cerebrospinal fluid flow and cortical perfusion after subarachnoid hemorrhage in mice. Transl Stroke Res 5:227–237
Kataoka H, Hamilton JR, McKemy DD, Camerer E, Zheng YW, Cheng A, Griffin C, Coughlin SR (2003) Protease-activated receptors 1 and 4 mediate thrombin signaling in endothelial cells. Blood 102:3224–3231
Lo HM, Chen CL, Tsai YJ, Wu PH, Wu WB (2009) Thrombin induces cyclooxygenase-2 expression and prostaglandin E2 release via PAR1 activation and ERK1/2- and p38 MAPK-dependent pathway in murine macrophages. J Cell Biochem 108:1143–1152
Lekic T, Rolland W, Hartman R, Kamper J, Suzuki H, Tang J, Zhang JH (2011) Characterization of the brain injury, neurobehavioral profiles, and histopathology in a rat model of cerebellar hemorrhage. Exp Neurol 227:96–103
Manaenko A, Lekic T, Barnhart M, Hartman R, Zhang JH (2014) Inhibition of transforming growth factor-beta attenuates brain injury and neurological deficits in a rat model of germinal matrix hemorrhage. Stroke 45:828–834
Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, Altay O, Tang J, Zhang JH (2012) Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol 236:69–78
Leitzke AS, Rolland WB, Krafft PR, Lekic T, Klebe D, Flores JJ, Van Allen NR, Applegate RL 2nd, Zhang JH (2013) Isoflurane post-treatment ameliorates GMH- induced brain injury in neonatal rats. Stroke 44:3587–3590
Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH (2004) Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab 24:1133–1145
Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH (2010) Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med 38:572–578
Hughes RN (2004) The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 28:497–505
Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH (2009) Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res 1270:131–139
Tso MK, Macdonald RL (2014) Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res 5:174–189
Marbacher S, Nevzati E, Croci D, Erhardt S, Muroi C, Jakob SM, Fandino J (2014) The rabbit shunt model of subarachnoid haemorrhage. Transl Stroke Res 5:669–680
Pluta RM, Bacher J, Skopets B, Hoffmann V (2014) A non-human primate model of aneurismal subarachnoid hemorrhage (SAH). Transl Stroke Res 5:681–691
Zhang YP, Cai J, Shields LB, Liu N, Xu XM, Shields CB (2014) Traumatic brain injury using mouse models. Transl Stroke Res 5:454–471
Wada K, Makino H, Shimada K, Shikata F, Kuwabara A, Hashimoto T (2014) Translational research using a mouse model of intracranial aneurysm. Transl Stroke Res 5:248–251
Strahle J, Garton HL, Maher C, Muraszko K, Keep R, Xi G (2012) Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res 3:25–38
Crews L, Wyss-Coray T, Masliah E (2004) Insights into the pathogenesis of hydrocephalus from transgenic and experimental animal models. Brain Pathol 14:312–316
Sorensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T (2014) miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res 5:711–718
Yamauchi T, Saito H, Ito M, Shichinohe H, Houkin K, Kuroda S (2014) Platelet lysate and granulocyte-colony stimulating factor serve safe and accelerated expansion of human bone marrow stromal cells for stroke therapy. Transl Stroke Res 5:701–710
Khanna A, Kahle KT, Walcott BP, Gerzanich V, Simard JM (2014) Disruption of ion homeostasis in the neurogliovascular unit underlies the pathogenesis of ischemic cerebral edema. Transl Stroke Res 5:3–16
Jiang X, Zhu S, Panetti TS, Bromberg ME (2008) Formation of tissue factor- factor VIIa-factor Xa complex induces activation of the mTOR pathway which regulates migration of human breast cancer cells. Thromb Haemost 100:127–133
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G (2014) Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 34:1070–1075
Reuter B, Rodemer C, Grudzenski S, Meairs S, Bugert P, Hennerici MG, Fatar M (2014) Effect of simvastatin on MMPs and TIMPs in human brain endothelial cells and experimental stroke. Transl Stroke Res. doi:10.1007/s12975-014-0381-7
Badaut J, Bix GJ (2014) Vascular neural network phenotypic transformation after traumatic injury: potential role in long-term sequelae. Transl Stroke Res 5:394–406
Acknowledgment
This study was partially supported by National Institutes of Health grant RO1 NS078755 (Dr. Zhang).
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lekic, T. et al. (2016). Cyclooxygenase-2 Inhibition Provides Lasting Protection Following Germinal Matrix Hemorrhage in Premature Infant Rats. In: Applegate, R., Chen, G., Feng, H., Zhang, J. (eds) Brain Edema XVI. Acta Neurochirurgica Supplement, vol 121. Springer, Cham. https://doi.org/10.1007/978-3-319-18497-5_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-18497-5_36
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18496-8
Online ISBN: 978-3-319-18497-5
eBook Packages: MedicineMedicine (R0)