Abstract
Progesterone is a steroid hormone whose physiological effects can affect various systems, including reproductive, immune and cardiorespiratory systems. In fact, there are growing evidences proving that progesterone is potent respiratory stimulant with therapeutic value for sleep-disordered breathing. However there is no clear understanding of how progesterone mediates its stimulant respiratory effects and alleviates apnea. Mechanistically, it was demonstrated that this hormone elicits some of its respiratory effect via the classical mechanism of the nuclear progesterone receptor (nPR), a transcription factor belonging to the super family of steroid hormone receptors. Moreover, experimental results indicate that activation of alternative non-genomic (i.e. non-nuclear) signaling pathways such as the membrane progesterone receptors (mPR) could have a key role in the regulation of the respiratory control system. We provide preliminary results suggesting an important role of mPRβ on respiratory control and ventilatory response to hypoxia in adult female mice.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
30.1 Introduction
Breathing disorders such as obstructive sleep apnea, sudden infant death, and Rett syndromes show several sex differences in their prevalence, indicating that sex is primordial determinant of respiratory health and lending weight to the link of gonadal steroid hormones in respiratory control (Kapsimalis and Kryger 2002; Chahrour and Zoghbi 2007). Progesterone is a steroid synthetized primarily in the gonads, the adrenal glands and in the placenta, but progesterone is also synthetized de novo in the central and peripheral nervous system, and can thus be classified as a neurosteroid (Birzniece et al. 2006). Progesterone is well- known as a powerful respiratory stimulant with a potential therapeutic value for the treatment of apnea in adults (Shahar et al. 2003), and it has been suggested that it could also be used for the treatment of apnea in preterm neonates (Finer et al. 2006). However, progesterone is not clinically approved for respiratory disordered breathing, because experimental data are still scarce, and there is no clear mechanical studies explaining how progesterone influences the control of breathing and alleviates apnea. Here, we will briefly focus on progesterone receptors, to highlight that we lack critical knowledge on their relative contributions in the regulation of the respiratory control system. However, our preliminary results clearly indicate that these receptors could play an important role in regulation of breathing.
30.2 Respiratory Effects of Progesterone
Progesterone is a potent respiratory stimulant, (Dempsey et al. 1986). Medroxyprogesterone, an analogue of progesterone, increases minute ventilation and responsiveness to hypercapnia or hypoxia (Skatrud et al. 1978; Zwillich et al. 1978). In addition, menopause is a risk factor for sleep apnea, and hormone replacement with progesterone reduces sleep disordered breathing in post-menopausal women (Shahar et al. 2003). Furthermore, progesterone reduces the occurrence of apnea in newborn and adult rats (Yamazaki et al. 2005; Lefter et al. 2007). Progesterone acts at different levels of the respiratory control system, including areas in central nervous system (Bayliss et al. 1987) and in peripheral chemoreceptors (Hannhart et al. 1990; Joseph et al. 2012). The direct application of progesterone on the dorsal surface of the medulla, at the level of the Nucleus Tractus Solitarius (NTS), increases phrenic nerve activity (Bayliss et al. 1987), and the effects of progesterone on respiratory activity requires intact hypothalamic structures (Bayliss et al. 1990).
30.3 Molecular Mechanisms by Which Progesterone Stimulates Breathing
Progesterone exerts it biological effects by genomic and non-genomic mechanisms (Fig. 30.1). We recently reported that progesterone uses non-genomic mechanisms to increase VO2 and VCO2 (Marcouiller et al. 2014), most likely through the truncated form of the nuclear progesterone receptor (nPR) located on the external membrane of the mitochondria (Dai et al. 2013). Moreover, allopregnanolone, a neuroactive metabolite of progesterone, affects breathing in newborn rats and arterial baroreflex responses in adults (Ren and Greer 2006; Heesch 2011). Despite the multiple potential pathways by which progesterone acts, little is known about the role of these pathways in regulating respiration.
Potential mechanisms of action by which progesterone might produce its respiratory effects. Progesterone, either from systemic circulation or produced locally in the neuronal system, can bind and signal throughout the classical nuclear receptor, membrane progesterone receptor (mPRα to mPRξ), and the Progesterone receptor membrane component (Pgrmc) 1 and 2. In addition, allopregnanolone, a metabolite of progesterone, can bind GABAA receptor
30.3.1 Genomic Mechanisms
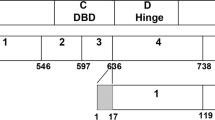
The nuclear progesterone receptor (nPR) belongs to the superfamily of the steroid hormone receptors. In the classical model of nPR activation, progesterone, a lipophilic molecule, diffuses through the cellular and nuclear membranes to bind to a nPR. When bound by progesterone, nPR undergoes conformational changes, dissociates from chaperone proteins, dimerizes, and directly interacts with specific response elements in the promoter regions of target genes through its DNA-binding domain (Mani 2006). The nPR gene (located on chromosome 11 in humans) encodes a single mRNA that, through alternative splicing, generates two major PR isoforms (nPR-A and nPR-B). These isoforms regulate the expression of different target genes and, therefore, different functions in cells (Mani 2006; Camacho-Arroyo et al. 2007; González-Flores et al. 2011). Several other isoforms have been identified. Because some of these isoforms have a defective DNA-binding domain and lack the nuclear localization signal, they are expected to be localized in the cytosol or on cell membranes, rather than in the nucleus, and activate the mitogen-activated protein kinase (MAPK) signaling cascade (Maller 2003; Brinton et al. 2008).
In the central nervous system, nPR is localized in the preoptic, paraventricular, ventromedial, dorsomedial, and arcuate hypothalamic nuclei and more importantly, in brainstem areas involved in respiratory control, such as the NTS (the major site of peripheral chemoreceptors integration in the brainstem), the motor nuclei of the Xth and XIIth cranial nerves, and the locus coeruleus (Romeo et al. 2005; Helena et al. 2006; Brinton et al. 2008). In the peripheral nervous system, nPR immunostaining is localized in cells in the carotid bodies of adults, newborn and fetal rats (Joseph et al. 2006). Thus, localization of nPR in these areas of the peripheral and central nervous system are highly suggestive that nPR are involved in respiratory control. Indeed, intraperitoneal injection of progesterone decreases the frequency of apneic episodes recorded during sleep (identified by behavioral criteria) by 50 % in 14-week-old male rats and by almost 80 % in 26- week-old rats. This effect is abolished when mifepristone, an nPR antagonist, is injected 1.5 h before progesterone injection (Yamazaki et al. 2005). Moreover, in anesthetized cats, i.v. progesterone administration enhances phrenic nerve activity, this effect is not elicited by other steroids, and is blocked by pre-treatment with mifepristone suggesting that it is mediated by nPR. Progesterone also enhances the peripheral chemoreceptor response to hypoxia (Hannhart et al. 1990) and enhances ventilatory activity by decreasing the synthesis of dopamine, an inhibitory neurotransmitter in peripheral chemoreceptors (Joseph et al. 2002); nPR-A signaling regulates the expression of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis (González-Flores et al. 2011). Our recent data suggest that nPR is an important modulator of respiratory control during sleep and in chemoreflex sensitivity. Specifically, adult female mice in which nPR is deleted (PRKO mice) have a higher frequency of sighs and post-sigh apneas during non-REM sleep and reduced responses to hypercapnia after chronic treatment with administration of progesterone (Marcouiller et al. 2014)
30.3.2 Non-genomic Mechanisms
Activation of the nuclear progesterone receptor is expected to induce measurable physiological responses coinciding with de novo protein synthesis (within 30–45 min). However, steroid- induced effects may occur rapidly (within 2–3 min); these effects are likely initiated by cell surface receptors (Revelli et al. 1998). Based on these data, the search for membrane progesterone receptors (mPR) has been of great interest, culminating in the discovery of a gene and a corresponding protein with the characteristics of a fully functional mPR in sea trout ovaries (Zhu et al. 2003b). Structural and phylogenetic analyses have revealed the presence of similar genes in other species, including humans and mice (Zhu et al. 2003a), and three distinct mPR genes (mPRα, mPRβ, and mPR\( \gamma \)) have been originally identified. The family of mPR include five different members (mPRα, mPRβ, mPR\( \gamma \), mPRδ and mPRξ) (Singh et al. 2013), which belong to the progestin and adipoQ receptor (PAQR) family. mPR proteins are located in the plasma membrane and have seven transmembrane domains with extracellular and intracellular terminals that respectively confer selective progesterone binding and activation of intracellular Gi proteins. When bound by progesterone, mPRα inhibits cAMP production to activate MAPK, which in turns activates the extracellular signal-regulated kinase (Erk1 and Erk2) signaling pathway. mPRα is predominantly expressed in reproductive tissues, mPRβ in the brain, and mPR\( \gamma \) in the kidneys (Zhu et al. 2003b). In mice, mPRα and mPRβ proteins and mRNA are expressed in the spinal cord with a distinct staining pattern that likely underlies the important trophic and protective effects of progesterone at this level (Labombarda et al. 2010). In rats, the mRNAs for mPRα and mPRβ are expressed in the cortex and thalamic nuclei (Intlekofer and Petersen 2011).
The pioneer study conducted by Pascual et al. (2002) showed that progesterone is able to restore the decreased transmission of afferent signals in the NTS during hypoxia, which could explain the stimulatory effect of progesterone in response to this stimulus. Because these effects required between 2 and 3 min to occur, it was suggested that a non-genomic mechanism of action was involved (Pascual et al. 2002). Based on these preliminary data, we sought to determine the expression of mPRβ and mPRα in the brainstem of adult female mice by immunohistochemistry (see (Labombarda et al. 2010), and found staining in the NTS, and the motor nuclei of the Xth and XIIth cranial nerves (Fig. 30.2). We have tested the functional role of mPRβ in adult female mice that were treated for 14 days with an intra-cerebro-ventricular infusion of a specific siRNA against mPRβ (0.04 mg/day; Stealth Select RNAi™, Life Technologies, Burlington, ON, Canada) in the IVth ventricle, to abolish its expression in central areas of respiratory control. Preliminary results indicate that the deletion of mPRβ induces a depression of the ventilatory response to hypoxia as shown in Fig. 30.3. Interestingly, while hypoxic exposure induced a rapid increase of respiratory frequency in control mice, we observed a mean delay of 4.5 ± 1.1 min before any observable response occurred in mice treated with the siRNA against mPRβ. The efficiency of the knock-down of mPRβ has been verified by immunohistochemistry, showing a wide-spread absence of staining in the brainstem (not shown). These preliminary results support a key role for mPRβ, in respiratory control in adult mice.
Immunohistochemistry for mPRα and mPRβ in the dorsal region of the adult mouse brainstem. (a) Neuroanatomical drawing at bregma – 7.76 mm (from Franklin and Paxinos) and corresponding staining at this level (100, 200, or 400×). (b) Neuroanatomical drawing at bregma – 7.08 mm and corresponding staining (100, 200, or 400×). Note the staining for mPRα in cell bodies on the NTS (SolM, SolC) and XIIthMN (12 N) at both bregma levels, and for mPRβ on neurites in the NTS. (c) Sagittal view (lateral 0.12 mm) of the brainstem; coronal planes at bregma −7.76 and −7.08 mm. CC: central canal, SolM, SolC, SolG, SolDL, SollM, SolV,… subdivision of the NTS. 12 N: hypoglos-sal nucleus, 10 N: vagal nucleus. Scale bar at 100× = 100 μm; at 200× = 50 μm
Example of respiratory recordings (Flow – ml/s, and frequency: fR – breaths/min) under normoxia and in response to hypoxia in control adult female mice (a) and after infusion of a specific siRNA against mPRβ (b). Note the delay of the response in the treated mice. The lower traces show for each animal a typical breathing pattern during the onset of hypoxic exposure (time scale: 1 s)
30.4 Conclusion
With growing awareness of the potent respiratory effects of progesterone, further experiments are required to elucidate its mechanisms of action with the perspective to develop novel pharmacological approaches for the treatment of respiratory disordered due to unstable respiratory control system. Specific knockdown of PR in-–vivo with siRNA infusion, or knock-out models appear as promising tools to elucidate the relative contributions of the different members from the large PR family.
References
Bayliss DA, Millhorn DE, Gallman EA et al (1987) Progesterone stimulates respiration through a central nervous system steroid receptor-mediated mechanism in cat. Proc Natl Acad Sci 84(21):7788–7792
Bayliss DA, Cidlowski JA, Millhorn DE (1990) The stimulation of respiration by progesterone in ovariectomized cat is mediated by an estrogen-dependent hypothalamic mechanism requiring gene expression. Endocrinology 126(1):519–527
Birzniece V, Bäckström T, Johansson I-M et al (2006) Neuroactive steroid effects on cognitive functions with a focus on the serotonin and GABA systems. Brain Res Rev 51(2):212–239
Brinton RD, Thompson RF, Foy MR et al (2008) Progesterone receptors: form and function in brain. Front Neuroendocrinol 29(2):313–339
Camacho-Arroyo I, Gonzalez-Arenas A, Gonzalez-Moran G (2007) Ontogenic variations in the content and distribution of progesterone receptor isoforms in the reproductive tract and brain of chicks. Comp Biochem Physiol A Mol Integr Physiol 146(4):644–652
Chahrour M, Zoghbi HY (2007) The story of Rett syndrome: from clinic to neurobiology. Neuron 56(3):422–437
Dai Q, Shah AA, Garde RV et al (2013) A truncated progesterone receptor (PR-M) localizes to the mitochondrion and controls cellular respiration. Mol Endocrinol 27(5):741–753
Dempsey JA, Olson EB, Skatrud JB (1986) Hormones and neurochemicals in the regulation of breathing. In: Handbook of physiology: the respiratory system. Control of breathing: section 3, vol II, part 1, chapter 7. American Physiological Society, Bethesda, pp 181–221
Finer NN, Higgins R, Kattwinkel J et al (2006) Summary proceedings from the apnea-of-prematurity group. Pediatrics 117(Suppl 1):S47–S51
González-Flores O, Gómora-Arrati P, García-Juárez M et al (2011) Progesterone receptor isoforms differentially regulate the expression of tryptophan and tyrosine hydroxylase and glutamic acid decarboxylase in the rat hypothalamus. Neurochem Int 59(5):671–676
Hannhart B, Pickett CK, Moore LG (1990) Effects of estrogen and progesterone on carotid body neural output responsiveness to hypoxia. J Appl Physiol 68(5):1909–1916
Heesch CM (2011) Neurosteroid modulation of arterial baroreflex function in the rostral ventrolateral medulla. Auton Neurosci 161(1):28–33
Helena CVV, de Oliveira PM, Sanvitto GL et al (2006) Changes in α-estradiol receptor and progesterone receptor expression in the locus coeruleus and preoptic area throughout the rat estrous cycle. J Endocrinol 188(2):155–165
Intlekofer KA, Petersen SL (2011) Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience 172:55–65
Joseph V, Soliz J, Soria R et al (2002) Dopaminergic metabolism in carotid bodies and high-altitude acclimatization in female rats. Am J Physiol Regul Integr Comp Physiol 282(3):R765–R773
Joseph V, Doan VD, Morency CE et al (2006) Expression of sex-steroid receptors and steroidogenic enzymes in the carotid body of adult and newborn male rats. Brain Res 1073–1074:71–82
Joseph V, Niane LM, Bairam A (2012) Antagonism of progesterone receptor suppresses carotid body responses to hypoxia and nicotine in rat pups. Neuroscience 207:103–109
Kapsimalis F, Kryger MH (2002) Gender and obstructive sleep apnea syndrome, part 2: mechanisms. Sleep 25(5):499–506
Labombarda F, Meffre D, Delespierre B et al (2010) Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 166(1):94–106
Lefter R, Morency C-E, Joseph V (2007) Progesterone increases hypoxic ventilatory response and reduces apneas in newborn rats. Respir Physiol Neurobiol 156(1):9–16
Maller JL (2003) Signal transduction. Fishing at the cell surface. Science 300(5619):594–595
Mani SK (2006) Signaling mechanisms in progesterone––neurotransmitter interactions. Neuroscience 138(3):773–781
Marcouiller F, Boukari R, Laouafa S et al (2014) The nuclear progesterone receptor reduces post-sigh apneas during sleep and increases the ventilatory response to hypercapnia in adult female mice. PLoS One 9(6), e100421
Pascual O, Morin-Surun MP, Barna B et al (2002) Progesterone reverses the neuronal responses to hypoxia in rat nucleus tractus solitarius in vitro. J Physiol 544(2):511–520
Ren J, Greer JJ (2006) Neurosteroid modulation of respiratory rhythm in rats during the perinatal period. J Physiol 574(2):535–546
Revelli A, Massobrio M, Tesarik J (1998) Nongenomic actions of steroid hormones in reproductive tissues 1. Endocr Rev 19(1):3–17
Romeo RD, Bellani R, McEwen BS (2005) Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress 8(4):265–271
Shahar E, Redline S, Young T et al (2003) Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 167(9):1186–1192
Singh M, Su C, Ng S (2013) Non-genomic mechanisms of progesterone action in the brain. Front Neurosci 7
Skatrud JB, Dempsey JA, Kaiser DG (1978) Ventilatory response to medroxyprogesterone acetate in normal subjects: time course and mechanism. J Appl Physiol Respir Environ Exerc Physiol 44(6):939–944
Yamazaki H, Haji A, Ohi Y et al (2005) Effects of progesterone on apneic events during behaviorally defined sleep in male rats. Life Sci 78(4):383–388
Zhu Y, Bond J, Thomas P (2003a) Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci 100(5):2237–2242
Zhu Y, Rice CD, Pang Y et al (2003b) Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci 100(5):2231–2236
Zwillich CW, Natalino MR, Sutton FD et al (1978) Effects of progesterone on chemosensitivity in normal men. J Lab Clin Med 92(2):262–269
Acknowledgements
Studies founded by a grant from the Canadian Institute for Health Research to VJ (MOP-102715). The authors acknowledge the work of Karim Habbal for immunohistological preparation of brainstem slices.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Boukari, R., Marcouiller, F., Joseph, V. (2015). Relative Contribution of Nuclear and Membrane Progesterone Receptors in Respiratory Control. In: Peers, C., Kumar, P., Wyatt, C., Gauda, E., Nurse, C., Prabhakar, N. (eds) Arterial Chemoreceptors in Physiology and Pathophysiology. Advances in Experimental Medicine and Biology, vol 860. Springer, Cham. https://doi.org/10.1007/978-3-319-18440-1_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-18440-1_30
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-18439-5
Online ISBN: 978-3-319-18440-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)