Abstract
Different types of central venous catheters (CVCs) have been used in clinical practice to improve the quality of life of chronically and critically ill patients. Unfortunately, indwelling devices are usually associated with microbial biofilms and eventually lead to catheter-related bloodstream infections (CLABSIs).
An estimated 250,000–400,000 CLABSIs occur every year in the United States, at a rate of 1.5 per 1,000 CVC days and a mortality rate of 12–25 %. The annual cost of caring for patients with CLABSIs ranges from 296 million to 2.3 billion dollars.
Biofilm formation occurs on biotic and abiotic surfaces in the clinical setting. Extensive studies have been conducted to understand biofilm formation, including different biofilm developmental stages, biofilm matrix compositions, quorum-sensing regulated biofilm formation, biofilm dispersal (and its clinical implications), and multi-species biofilms that are relevant to polymicrobial infections.
When microbes form a matured biofilm within human hosts through medical devices such as CVCs, the infection becomes resistant to antibiotic treatment and can develop into a chronic condition. For that reason, many techniques have been used to prevent the formation of biofilm by targeting different stages of biofilm maturation. Other methods have been used to diagnose and treat established cases of CLABSI.
Catheter removal is the conventional management of catheter associated bacteremia; however, the procedure itself carries a relatively high risk of mechanical complications. Salvaging the catheter can help to minimize these complications.
In this article, we provide an overview of microbial biofilm formation; describe the involvement of various genetic determinants, adhesion proteins, organelles, mechanism(s) of biofilm formation, polymicrobial infections, and biofilm-associated infections on indwelling intravascular catheters; and describe the diagnosis, management, and prevention of catheter-related bloodstream infections.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Central Venous Catheter
- Extracellular Polymeric Substance
- Cystic Fibrosis Patient
- Bloodstream Infection
- Peripherally Insert Central Catheter
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
Central venous access is an essential medical procedure in the care of chronically and critically ill patients. An estimated 8 % of hospitalized patients require a central venous catheter (CVC) to be placed during the course of their hospitalization (Ruesch et al. 2002). Each year, more than five million CVCs are inserted in the United States (Ruesch et al. 2002; McGee and Gould 2003).
Different types of CVCs are widely used. We review the most commonly used types of CVCs below:
-
Non-tunneled CVCs: These are the most common type of CVCs used for temporary access to the central circulation. Non-tunneled CVCs are available in different lengths (15–30 cm) and different materials (e.g., polyurethane, silicone). Non-tunneled CVCs can be single, double, or even triple lumen. CVCs for longer term infusion are supplied with valve mechanisms to decrease the backflow of the blood and thus possibly decrease the rate of infection and catheter thrombotic occlusion. The peripherally inserted central catheter (PICC) is another type of CVC that is widely used because of the relative ease of insertion; it is usually placed in the upper arm veins (cephalic or basilic veins) and has a lower rate of mechanical complications. However, the rate of thrombosis may increase with the increasing number of lumens.
-
Implanted CVCs: This type of CVC provides long-term, semi-permanent central venous access. The removal of implanted CVCs is not recommended until there is no more need for the CVC or until complications occur. Two types of implanted CVCs are available, tunneled catheters and completely implantable venous access devices:
-
Tunneled CVCs: contain a subcutaneous tunnel that crosses the catheterized vein and the skin exit site. By this mean the rate of biofilm formation and the infection are lower than the non-tunneled catheters (Dryden et al. 1991). The CVC may be round or flat. Sizes range from 2.7 to 12.5 F (e.g., Hickman, Broviac).
-
Subcutaneous port CVCs: This type of CVC is a completely implanted device that provides long-term venous access through a CVC that is passed from the cannulated vein under the skin and connected to a subcutaneous infusion port or reservoir that is placed in a subcutaneous pocket. The port is accessed through the skin via a needle puncture of the port’s septum. Subcutaneous ports are widely used to administer chemotherapy agents because of their low rates of extravasation and infection (Pegues et al. 1992); on the other hand, it is cosmetically more preferable as it is hidden beneath the skin. The only limiting infusion rate factor of this CVC is that the bore of the access needle is nearly always smaller than the internal diameter of the CVC attached to the port.
10.2 Epidemiology
Despite the utility of catheters, biofilm formation and bloodstream infections are major risks associated with catheter placement. More than five million catheters are inserted each year in the United States (Ruesch et al. 2002), with 250,000–400,000 catheter-related bacteremia and fungaemias cases annually (if entire hospitals are assessed rather than ICUs only) (Maki et al. 2006), The National Healthcare Safety Network reports a rate of 1.5 central line-associated bloodstream infections (CLABSIs) per 1,000 CVC days in the United States (Edwards et al. 2009), with a mortality rate of 12–25 % (Centers for Disease and Prevention 2011; Pronovost et al. 2006). The attributable cost is estimated to be around $34,508–56,000 per infection (Rello et al. 2000; Soufir et al. 1999); the cumulative annual cost of caring for patients with CLABSIs ranges from $296 million to $2.3 billion (Mermel 2000; O’Grady et al. 2002).
CVCs are the source of 87 % of bloodstream infections that occur in intensive care units (Richards et al. 1999), with approximately 80,000 CLABSIs resulting in increased hospitalization stays of approximately 7–12 days (Pittet et al. 1994).
10.3 Adherence and Biofilm Formation
Biofilm is a microbially derived sessile community that is characterized by cells that are irreversibly attached to a substratum, interface, or to one another; they are embedded in a matrix of extracellular polymeric substances (EPS) that they have produced and exhibit an altered phenotype with respect to growth rate and gene expression (Donlan and Costerton 2002). As many as 60 % of the microbial infections treated in developed nations are related to biofilm formation (Costerton et al. 1999); according to the NIH, biofilms are clinically important, accounting for over 80 % of microbial infections in the body (Lewis 2001).
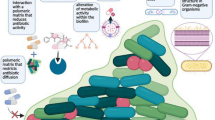
Biofilm formation is a complex developmental process that involves distinct stages, including primary adherence and immobilization of planktonic microbes on a surface, cell-to-cell interaction, microcolony formation, the development of a matured three-dimensional biofilm structure, and detachment of cells from the matured biofilm to colonize new niches under desirable conditions (Fig. 10.1) (O’Toole et al. 2000). Microbes that constitute biofilm communities have extremely complex and heterogeneous physiological characteristics and are different from planktonic microbes.
The initial adhesion of microbes to biomaterial surfaces is influenced by physiochemical and electrostatic interactions between the microbial cell wall and the substrate; this is often conditioned by the fluids to which it is exposed (Dunne 2002), leading to reversible attachment that is strongly influenced by environmental factors, such as nutrient availability, pH, ionic strength, hydrodynamics, and temperature (Danese et al. 2000a). In addition, surface roughness, surface configuration, surface charge, and the hydrophobicity of material surfaces are key factors that influence microbial adherence to biomaterial surfaces (Donlan 2002; Katsikogianni and Missirlis 2004). The ability to perform this initial adherence is also linked to genetic factors, including genes encoding motility, quorum sensing, adhesins, and various metabolic activities (Costerton 1995; O’Toole et al. 2000). Host-specific adhesins that are located on the microbial cell surface or cellular components, such as pili and fimbriae, reinforce the reversible attachment between microbial cells and surfaces, resulting in the irreversible attachment of microbial cells to surfaces (Fig. 10.1).
Biofilm formation is initiated by the attachment of microbial cells to the surfaces of indwelling medical implants or devices such as catheters or infusion parts. The irreversible attachment phase of biofilm formation on a surface is likely to be involved with cell wall-associated proteins, such as microbial surface components that recognize adhesive matrix molecules (Patti et al. 1994). S. aureus produces multiple adhesive factors that can facilitate binding to host factors (Gotz 2002) and eventually mediate microbial attachment to implant surfaces covered by host plasma and other extracellular matrix components. Staphylococci produce several proteins that specifically bind to proteins of the extracellular matrix, including fibrinogen, fibronectin, vitronectin, and collagen (McDevitt et al. 1997; Menzies 2003; Ni Eidhin et al. 1998; Switalski et al. 1993). These host components, including thrombin, platelets, and laminin, may deposit on catheters and foreign body material surfaces and provide specific ligands for microbial adhesins (Shenkman et al. 2002). Fibronectin and fibrinogen are known to influence the adherence of bacteria, especially staphylococci, to biomaterials (Dickinson et al. 1997; Herrmann et al. 1988). Thrombus proteins increase bacterial adherence on catheters and have been associated with catheter-related bloodstream infection; the formation of a fibrin sheath around the catheter greatly increases catheter colonization (Mehall et al. 2002). Furthermore, platelets have been shown to increase the adherence of Staphylococcus aureus in combination with thrombin. Activated platelets also bind to several soluble plasma proteins, including von Willebrand factor, fibronectin, fibrinogen, and thrombospondin, and consequently promoting microbial adhesion (Baumgartner and Cooper 1998). The implants are in direct contact with the bloodstream; thus, the surface becomes coated with blood components that act as a conditioning film to which microbes can attach by expressing specific adhesins.
Irreversible adherence is also performed using attachment organelles to the abiotic surface. Adherence is performed by the type I pili in Escherichia coli (Beloin et al. 2008; Thumbikat et al. 2009; Wellens et al. 2008). Curli, and antigen 43 have been reported to mediate bacterial interactions on biomaterial surfaces, in addition to primary attachment (Cegelski et al. 2009; Ulett et al. 2007). Curli also enables binding to extracellular matrix proteins, such as fibronectin and plasminogen (Cookson et al. 2002; Uhlich et al. 2006). Type IV pili-driven twitching motility has been reported in Pseudomonas aeruginosa that contacts the surface and maintains adherence (Klausen et al. 2003; O’Toole and Kolter 1998).
A plethora of primary attachment adhesins has been reported in gram-positive bacteria as well. A variety of Enterococcal adhesins such as SagA, Ace, Esp, and enterococcal biofilm pili (Ebp) contribute to biofilm formation, endocarditis, and urinary tract infections (Kemp et al. 2007; Kline et al. 2010; Mohamed and Huang 2007; Mohamed et al. 2004, 2006; Nallapareddy et al. 2006). Staphylococcal proteins such as Bap, SasG, Aap, EmbP, FnbpA, and FnbpB are reported to be involved in bacterial adhesion and colonization on biomaterial surfaces (Geoghegan et al. 2010; O’Neill et al. 2008; Potter et al. 2009; Rohde et al. 2007).
Pathogenic microbes require various ion acquisition mechanisms to obtain ions from host tissues and establish infection in the form of biofilm in humans. Metallic cations such as iron, calcium (Ca2+), and magnesium (Mg2+) play roles in microbial adherence and biofilm formation. Numerous studies have shown that intracellular iron concentration plays a crucial role in biofilm formation and development. Iron regulates biofilm formation in many bacterial species, including P. aeruginosa and E. coli (Banin et al. 2005; Wu and Outten 2009). Lactoferrin, the iron chelator in human blood, restricted the maturation of P. aeruginosa biofilm (Singh et al. 2002). Apo-transferrin inhibits the adhesion of S. aureus and Staphylococcus epidermidis to polystyrene, polyurethane, and silicone surfaces (Ardehali et al. 2002), whereas catecholamine inotropes, which extract iron from plasma iron-binding proteins, stimulate biofilm formation by allowing S. epidermidis to adhere to solid surfaces (Lyte et al. 2003). Ca2+ has been reported as a key initiator that binds to extracellular DNA (eDNA) on bacterial surfaces and mediates bacterial aggregation and biofilm formation in both gram-positive and gram-negative bacteria (Das et al. 2014). Mg2+ is also known to be essential for biofilm formation. Mg2+ influenced attachment and subsequent biofilm formation and structure in Pseudomonas species (Song and Leff 2006). The increasing levels of Mg2+ enhanced biofilm production by S. epidermidis (Dunne 2002). Further, the increasing concentrations of potassium, the major intracellular cation, promoted biofilm formation in S. aureus (Beckingsale et al. 2011). Subsequently, flow conditions are considered a leading factor that strongly influences the number of adhered bacteria (Dickinson et al. 1995; Isberg and Barnes 2002) and the biofilm structure and performance (Klapper et al. 2002; Stoodley et al. 1999).
After the irreversible attachment, the multiplication of microbes on the surface lead cell-to-cell aggregations to form discrete EPS matrix-encased cell communities called microcolonies (Fig. 10.1); these microcolonies hold the cells together in a mass and firmly attach the microbial mass to the underlying surface. The continued growth of microbial cells on a surface leads to the development of mature biofilm that contains millions of tightly packed cells. These cells are assembled into a complex pillar- and mushroom-shaped structure, with towers interspersed with fluid-filled channels that facilitate nutrient supply (Hall-Stoodley et al. 2004). Thus, mature biofilm are complex, highly differentiated three-dimensional structures. Colonization and biofilm formation may occur within 3 days of catheterization (Fig. 10.2) (Anaissie et al. 1995). Raad et al. showed that catheters inserted for less than 10 days tend to have more extensive biofilm on the exterior surface of the catheter; in long-term catheters, biofilms were more extensive on the internal lumen (Fig. 10.3) (Raad 1998; Raad et al. 1993). At the final stage of biofilm development, microbes from the biofilm colony detach and disperse into new environmental sites to initiate another biofilm (Fig. 10.1). Dispersal of biofilms has been reported to be regulated by various environmental signals, signal transduction pathways, and effector molecules (Karatan and Watnick 2009). Such mechanisms could be linked to the cause of bacteremia and infections in new sites from implanted medical devices.
10.4 Extracellular Polymeric Substances
Depending on the species involved in the formation of the microcolonies (single or multiple species), biofilm is usually composed of 10–25 % cells and 75–90 % extracellular polymeric substances (EPS) (Costerton et al. 1987). EPS generally contain polysaccharides, proteins, phospholipids, teichoic acids, eDNA, and other polymeric substances, hydrated to 85–95 % water (Costerton et al. 1981; Sutherland 1983). The biofilm matrix, in the form of EPS, contributes to the overall architecture, maintenance, and resistance phenotypes of biofilms (Branda et al. 2005; Sutherland 2001). Polysaccharides are a major component of the EPS matrix (Flemming and Wingender 2010). Exopolysaccharides are mostly heteropolysaccharides that consist of neutral and charged sugar residues. Many known exopolysaccharides, including alginate, xanthan, and colanic acid, are polyanionic in nature. In addition, polycationic exopolysaccharides, such as polymer N-acetyl glucosamine (PNAG) (which is isolated from S. aureus and S. epidermidis), are reported to be responsible for colonizing medical implants, leading to biofilm-related infections (Gotz 2002).
EPS have been extensively studied in both gram-negative bacteria, gram-positive bacteria, and fungi. Polyglucosamine and colanic acid are the main components that contribute to the architecture of the biofilm in E. coli (Agladze et al. 2005; Danese et al. 2000b; Kostakioti et al. 2013; Wang et al. 2005). Three major exopolysaccharide components, Pel, Psl, and alginate were found to have increased attachment to mucin, airway epithelial cells and stabilizing the biofilm structure in P. aeruginosa. Interestingly, it was recently found that mucoid P. aeruginosa strains also depend on Psl to form biofilms (Ma et al. 2012; Yang et al. 2012). Another EPS component, alginate, is the exopolysaccharide that is expressed by P. aeruginosa clinical isolates from the lungs of CF patients (Govan and Deretic 1996); it plays an important role in structural stability and is associated with superior resistance to antibiotic treatment and host immune defenses during chronic infections (Hentzer et al. 2001; Leid et al. 2005). Furthermore, eDNA plays a critical role in cell-to-cell interactions and stabilization of Pseudomonas biofilm (Whitchurch et al. 2002; Yang et al. 2007). Extracellular proteins and several proteinaceous components are also considered to be matrix components, including type IV pili, flagella, and fimbriae. These components were found to mainly have secondary functions as adhesion factors and structural support in the biofilm formation of P. aeruginosa (Mann and Wozniak 2012).
The EPS of gram-positive bacteria such as staphylococci consist of different secreted polymers, exopolysaccharides, teichoic acids, and specific proteins, as well as eDNA. Majority of the strains of S. aureus utilize PNAG also referred as polysaccharide intercellular adhesin (PIA), to form biofilm (O’Gara 2007). The expression of icaADBC operon, which encodes enzymes that are required for the production of PIA on the surface of S. aureus, is critical to cell-to-cell adhesion and biofilm formation. The same study found that the ica locus is present both in S. epidermidis, S. aureus, and several other streptococcal species (Cramton et al. 1999). PIA plays a critical role in the adherence of S. epidermidis to biomaterials by providing favorable acid-base interactions with the surface (Olson et al. 2006).
EPS also harbor an adhesive protein, Bap, that is required for biofilm formation in S. aureus (Lasa and Penades 2006). Several surface proteins, including Aap and SasG, have also found to promote biofilm formation in S. epidermidis and S. aureus (Geoghegan et al. 2010; Rohde et al. 2007). Methicillin-resistant S. aureus (MRSA) biofilm is promoted by the fibronectin-binding proteins FnBpA, FnBpB, and Embp, as a component of a proteinaceous biofilm (Christner et al. 2010; O’Neill et al. 2008). Teichoic acids are another cell wall component that has been reported to take part in the structure of staphylococcal biofilms (Sadovskaya et al. 2004).
Besides PIA and proteins in the biofilm matrix, eDNA is a major component of biofilm that stimulates S. epidermidis biofilm formation (Qin et al. 2007). eDNA has also been shown to be indispensable for biofilm formation of Streptococcus mutans, Streptococcus intermedius, and Enterococcus faecalis (Thomas et al. 2008; Whitchurch et al. 2002).
Similarly, in bacterial biofilms, the extracellular matrix helps preserve the architectural integrity of fungal biofilms and contributes to antifungal tolerance (Flemming and Wingender 2010; Hawser and Douglas 1995). Andes’s team identified soluble β-1,3-glucans as an important component of the biofilm matrix of Candida albicans in vivo and in vitro (Nett et al. 2007a, b). In addition, EPS of C. albicans biofilm contain chitins and eDNA (Al-Fattani and Douglas 2006; Ramage et al. 2009).
Polysaccharide molecules can interact with themselves or with heterologous molecules to yield gels, often with multivalent cations playing a substantial role in the process (Sutherland 2001). Among several ions, Ca2+ and Mg2+ bind to the majority of the most EPS components in a biofilm matrix (Decho 2010). Ca2+ may play a role in biofilm formation as an ionic cross-bridging matrix molecule in P. aeruginosa (Sarkisova et al. 2005). The presence of Ca2+ affects the mechanical properties of biofilms and serves as ionic cross-bridging of the polysaccharide in the biofilm matrix (Kierek and Watnick 2003). As numerous matrix polymers are anionic in nature, they may also bind to cations and provide essential nutrients. The matrix itself can also act as a carbon and energy reserve (Sutherland 2001).
10.5 Quorum Sensing and Biofilm
Many microbial pathogens communicate through the production of and sensing of auto-induced signaling molecules known as auto-inducing peptides (AIPs) to control the expression of specific genes in response to population density; this is known as quorum sensing (Waters and Bassler 2005). In staphylococci, the quorum-sensing system is encoded by the agr (accessary gene regulator) locus, which consists of agrA, agrC, agrD, and agrB genes that are co-transcribed. Once AIP reach a threshold level, the bacteria respond by activating the expression of sequences of specific cell density-dependent gene. Most staphylococcal products are under the control of agr, which is activated during the transition from the exponential to the stationary growth phase. The E. faecalis fsr quorum-sensing system controls biofilm development (Hancock and Perego 2004). Like gram-positive bacteria, many gram-negative bacteria also use AIP, called N-acetyl homoserine lactone (AHL). P. aeruginosa has AHL-dependent QS systems (LasR/LasI, RhlR/RhlI, and PQS) that mediate biofilm architecture and the production of extracellular polymeric slime (Shih and Huang 2002; Yoon et al. 2002). The quorum-sensing molecules farnesol and tyrosol have also been identified in C. albicans (Chen et al. 2004; Ramage et al. 2009).
10.6 Biofilm Dispersal
Biofilm dispersal is the final stage of biofilm development, in which microbial cells from the matured biofilm detach and disperse into the milieu. This is an essential phase of the biofilm that contributes to biological dispersal, bacterial survival, and disease transmission (Kaplan 2010). Biofilm dispersal can be a complex and dynamic process, including multiple genetic determinants, environmental signals, signal transduction pathways, and effector molecules. Until recently, the mechanisms by which actual microbial dispersal from biofilms occur remained almost completely unexplored, and little was known about the functions or regulatory pathways involved in the release of microbes from biofilms. A better understanding of the mechanism of dispersion reveals the signals, which regulate the dispersal processes that leads to the development of clinically useful agents that inhibit biofilm formation or promote biofilm detachment on medical implants.
Biofilm dispersal or detachment can be split into different phases, such as detachment of microbes from the biofilm colony, translocation of the cells to the new site, and attachment of the cells to a substrate in the new site (Kaplan 2010). Both active and passive types of dispersal processes play a role in the initiation of biofilm detachment, such as nutrient availability (Gjermansen et al. 2005; Hunt et al. 2004; Sauer et al. 2004), enzyme-mediated breakdown of the biofilm matrix (O’Neill et al. 2007; Rohde et al. 2007; Whitchurch et al. 2002), free radical production (Barraud et al. 2006; Webb et al. 2003), surfactant production (Davey et al. 2003; Otto 2014; Periasamy et al. 2012; Wang et al. 2011), the control of quorum-sensing systems (Boles and Horswill 2008; Periasamy et al. 2012; Rice et al. 2005; Wang et al. 2011), and signaling molecules (Morgan et al. 2006).
Sauer and co-workers observed that spent medium from P. aeruginosa cultures induced dispersal of biofilm (Sauer et al. 2004). The RNA-binding protein CsrA (carbon storage regulator A) acts as an activator of biofilm dispersal in E. coli (Jackson et al. 2002). Depletion of oxygen was found to stimulate the production of a specific exopolysaccharide lyase, which degraded the matrix of Pseudomonas fluorescens biofilm and dispersed the bacteria (Allison et al. 1998). Increased production of alginate lyase digests alginate in the biofilm matrix, promoting the detachment of P. aeruginosa biofilms (Boyd and Chakrabarty 1994); a separate study showed that digestion of alginate and eDNA with their respective enzymes enhanced the efficacy of tobramycin, amikacin, and gentamicin against P. aeruginosa biofilm (Alipour et al. 2009). Nitric oxide was reported to induce biofilm dispersal in P. aeruginosa biofilm (Barraud et al. 2006). P. aeruginosa produces extracellular surfactant rhamnolipids that can mediate biofilm dispersal (Boles and Horswill 2008). Cis-2 decanoic acid, an unsaturated fatty acid, is produced by P. aeruginosa, is capable of inducing the dispersion of established biofilm. The exogenous addition of this messenger molecule was also shown to induce the dispersion of biofilms formed by other pathogens, E. coli, Klebsiella pneumonia, S. aureus, and C. albicans (Davies and Marques 2009). Increased levels of cyclic-dimeric GMP, an intracellular signal, resulted in enhanced production of exopolysaccharides, while decreased levels of this molecule induced biofilm dispersal in different bacteria (Kaplan 2010).
It has been documented that activation of the agr quorum-sensing system leads to the up-regulation of extracellular proteases (Aur and Spl) that contribute to S. aureus biofilm detachment (Boles and Horswill 2008). Another study showed that PNAG-degrading enzyme dispersin B was able to release preformed biofilm produced by S. epidermidis (Izano et al. 2008). Deoxyribonuclease has also been implicated in bacterial detachment in S. aureus biofilm (Mann et al. 2009).
In addition to proteases, other agr-regulated factors contribute to biofilm detachment. Surfactant-like molecules such as δ-toxin may exert dispersal effects on the biofilm of S. aureus (Kong et al. 2006). Otto’s group recently identified a family of short staphylococcal peptides, the phenol-soluble modulins (PSMs), that are under the control of agr. The specific secreted surfactant PSMs promote biofilm structuring and detachment in S. aureus and S. epidermidis (Periasamy et al. 2012; Wang et al. 2011).
Fungi have been reported to be involved in biofilm dispersal as well. Yeast wall protein 1 was involved in the dispersal of C. albicans biofilm (Granger et al. 2005). NRG1 is a negative regulator that has been shown to be involved in biofilm detachment in C. albicans biofilm (Uppuluri et al. 2010). Very recently, it has been was reported that the histone deacetylase complex facilitates biofilm dispersal in C. albicans (Nobile et al. 2014).
10.7 Dissemination of Biofilm and Bacteremia
Microbial biofilm formation is the primary mode of growth in most natural and clinical settings. Dispersal plays a vital part in spreading pathogenic microbes from environmental reservoirs to human hosts and spreading infections within a host. Many biofilm-associated infections occur in the nosocomial setting as a result of the contamination of indwelling medical devices from the skin flora of patients or health care workers. The rate of biofilm dispersion is enhanced with increased biofilm thickness and external shear forces of the surrounding medium, such as urine, blood, saliva, and other body fluids. Bacterial biofilm on medical implants can cause bloodstream infections and systemic inflammation, in which biofilm bacteria detach and disperse during biofilm development (Otto 2014; Raad et al. 2008a); in these cases, the infected medical devices should be removed (Raad et al. 2008a).
PSMs contribute to the dissemination from a preformed biofilm to secondary infection sites. In a mouse model of biofilm-associated infections, PSMs of staphylococcal origin facilitated the dissemination of biofilm on CVCs to secondary infection sites (Periasamy et al. 2012; Wang et al. 2011). These studies provide evidence of the significance of such molecules in the dissemination of biofilm-associated infections, motivating us to identify potential therapeutic targets and thus prevent complications and the spread of infections. To achieve this goal as a primary step, Wang et al. generated antibodies against PSMβ1 of S. epidermidis peptides, which inhibit bacterial dissemination from the CVC (Wang et al. 2011).
Dissemination in patients with chronic infections is also mediated by biofilm dispersal, as it allows biofilm bacteria to spread throughout the infected organ or colonize other parts of the body. For instance, transient bacteremias have been diagnosed after dental procedures (Kinane et al. 2005). Nosocomial pneumonia was found to be caused by bacteria released from biofilms in a patient with an endotracheal tube (Adair et al. 1999); a kidney infection was caused by bacteria that detached from a biofilm in a patient’s bladder (Mathoera et al. 2000); and suffering from other biofilm infections, such as endocarditis, (Parsek and Singh 2003). Remarkably, in cystic fibrosis (CF) patients, only Burkholderia species are able to cause systemic infections (Isles et al. 1984). Recently, biofilm dispersal of Streptococcus pneumoniae was documented to result in a significantly increased spread and cause infections to other sites such as the middle ear, lungs, and bloodstream in a mouse model (Marks et al. 2013).
10.8 Multi-species Biofilm and Its Clinical Relevance
Multi-species or mixed species of biofilm are certainly the dominant form in nature and are prominent in human host tissues and medical biomaterials, such as the oral cavity, the lungs of the CF patients, chronic wounds, and catheters. The extensive interaction between different species of microorganisms determines the structural and functional dynamics of multi-species biofilms. Coaggregation interactions are believed to contribute to multi-species biofilm formation in different environments (Rickard et al. 2003).
Several bacterial cell surface protein adhesins play important roles in coaggregation during multi-species biofilm formation. Five specific adhesins are expressed by Streptococcus oralis and aggregate with other species of oral bacteria in dental plaque (Yang et al. 2011). Since protein adhesins are commonly distributed among bacteria, adhesin-mediated coaggregation may be a major strategy for multi-species biofilm development. As protein adhesins are also found in fungi, these adhesins can mediate fungi-bacteria interactions (Li and Palecek 2008; Silverman et al. 2010). Bacterial pili, flagella, and their motilities are also essential for multi-species biofilm formation. P. aeruginosa type IV pili facilitate multi-species microcolony formation with S. aureus in multi-species biofilms (Yang et al. 2011). Further, pili promote multi-species biofilm of E. coli and Citrobacter freundi (Pereira et al. 2010). eDNA also widely exists among multi-species biofilms (Steinberger and Holden 2005). Recently, it was reported to enhance the mixed-species biofilm of S. epidermidis and C. albicans both in vitro and in vivo (Pammi et al. 2013). It has been documented that C. albicans interacts with 12 other species of Candida and bacteria, such as P. aeruginosa and S. epidermidis, in the form of multi-species biofilm in a polystyrene tube model (El-Azizi et al. 2004).
Some quorum-sensing molecules support interspecies communication in multi-species biofilm, enabling the microbes to sense the presence of other species (Waters and Bassler 2005). AHL autoinducers are the most common signaling molecules in bacteria and can influence a broad range of cross-species and cross-genus communications (Federle and Bassler 2003). P. aeruginosa and Burkholderia cepacia, which are sometimes found in the lungs of CF patients, can form mixed biofilms. B. cepacia is capable of perceiving the AHL signals produced by the CF pathogen P. aeruginosa (Riedel et al. 2001). Besides the involvement of signaling molecules in multi-species biofilm, the dispersion mechanism through such signaling molecules within multi-species biofilm can cause polymicrobial bloodstream infections and spread to other parts of the body.
One of the most alarming consequences of multi-species biofilm is that it is more resistant to antimicrobial agents and host immune responses than is mono-species biofilm. Many studies have compared the antibiotic resistance of multi-species and mono-species biofilms; in most cases, mixed-species biofilms were significantly more resistant to antimicrobial treatment. The CF pathogen Stenotrophonomonas maltophilia AHL signaling molecule affects biofilm and polymyxin tolerance in P. aeruginosa (Ryan et al. 2008). The slime produced by S. epidermidis can inhibit the penetration of fluconazole, while C. albicans can protect slime-negative S. epidermidis against vancomycin in the mixed biofilms of C. albicans and S. epidermidis (Adam et al. 2002). In another study, C. albicans induced S. aureus vancomycin resistance in multi-species biofilm (Harriott and Noverr 2010).
Multi-species biofilms have been clinically linked to polymicrobial infections, which themselves are associated with significantly poorer clinical outcomes than are single microbial infections (Brogden et al. 2005; Sutter et al. 2008); they also account for roughly 15 % of infections in immunocompromised cancer patients. CRBSIs have also been reported in young and adults patients (Cairo et al. 2011; Downes et al. 2008). One of the significant risk factors for polymicrobial infections is the presence of indwelling vascular catheters that act as sites for mixed-species biofilm formation (Cairo et al. 2011; Downes et al. 2008). Many types of chronic infection are caused by biofilm-associated microbes; these are hard to eradicate because of the protective biofilm matrix, which may be further enforced if multiple species are present. Recently, it was demonstrated that in vivo wound healing was delayed when patients were coinfected with both S. epidermidis and P. aeruginosa (Pastar et al. 2013). Mixed-species S. epidermidis and C. albicans biofilms facilitate S. epidermidis infection and blood dissemination in the mouse subcutaneous CVC biofilm model; this study may explain the increased clinical mortality and morbidity in the polymicrobial environment.
Researchers have begun study multi-species biofilms to unravel the complexity of inter-species and inter-genus communications and their effect in clinical and environmental settings. The prevalence of mixed-species biofilms and their involvement in various infections highlights the need for a better understanding of the interactions and dynamics within mixed biofilm communities, which is necessary to successfully prevent or treat polymicrobial infections.
10.9 Diagnosis
Studies have shown that the formation of biofilm in venous lines is universal; quantitative electron microscopy has demonstrated that biofilm formation starts after insertion of the catheter, even with the absence of clinical manifestations of CLABSI (Raad et al. 1993). However, by exceeding certain threshold number of colony-forming units (CFUs) with dissemination, the clinical manifestation of bloodstream infections will be more apparent (Cleri et al. 1980; Sherertz et al. 1990).
The location of the biofilm depends on the type of the catheter (the duration of catheterization); for short-term catheters (<10 days), the external surface is the main site of biofilm formation, whereas for long-term catheters (>30 days), the internal surface of the lumen is the main site (Raad et al. 1993).
The roll-plate, semi-quantitative culture method of Maki et al. allows one to culture the external surface of the catheter (Cicalini et al. 2002). The catheter is rolled back and forth on a Columbia agar plate supplemented with 5 % sheep blood; the plate is then incubated for 3 days (72 h) at temperature of 35 °C with 5 % CO2, and the CFUs of the microorganism are quantitated (Slobbe et al. 2009). This is a convenient method for culturing microorganisms in short-term catheter. However, the roll-plate technique can give high false-negative results in long-term catheters and fails to release biofilm-embedded organisms from the CVC surface (Sherertz et al. 1990). Other limitation of this method is that some catheters come out in an irregular shape, which makes it hard for the laboratory personnel to perform the procedure. This may also lead to false-negative results.
New quantitative methods have been developed to culture the external and internal surface of catheters. These include sonication and vortexing (Bjornson et al. 1982). The method includes placing the catheter in 5 ml of 0.9 % NaCl, sonicating it for 1 min, and vortexing the sonication fluid for 15 s. Subsequently, 50 μl of the sonication fluid is cultured on agar plates, allowing for a detection limit of ≥100 CFU/catheter tip (Slobbe et al. 2009). Other quantitative diagnostic procedures include flushing the catheter with 2 ml of brain-heart infusion broth. After the fluid has been diluted to tenfold, 0.1 ml is streaked onto a blood agar; ≥103 CFUs is considered significant colonization in the catheter lumen (Linares et al. 1985; Cleri et al. 1980).
Quantitative methods are considered superior to semi-quantitative methods, with the highest sensitivity (80–100 %) and specificity (more than 90 %) (Siegman-Igra et al. 1997). The most sensitive individual culture method is sonication of the tip or subcutaneous catheter segments. However, culturing both can increase the test sensitivity by 20 % (Sherertz et al. 1990; Kristinsson et al. 1989; Bjornson et al. 1982).
Studies have shown that using semi-quantitative methods prior to sonication of the catheter will decrease the sensitivity of the sonication; thus, it is not recommended to combine these two methods.
For all the quantitative and semi-quantitative methods described above, removal of the CVC is essential; unfortunately, the unnecessary removal of CVCs will make these methods clinically not useful.
For that reason, new approaches are designed to establish the diagnosis without removing the catheter, depending on the results of the clinical evaluation and confirmatory positive blood or catheter tip cultures.
The presence of positive blood cultures with no other apparent source of infection should raise suspicion for CLABSI, in addition to the clinical manifestations of systemic infection; although fever is the most sensitive indicator of a bloodstream infection, it is not specific. Other signs are chills, hypotension, and inflammation of the catheter insertion site, which is the most specific sign but has low sensitivity (Safdar and Maki 2002).
The Centers for Disease Control (CDC) has introduced a new term: laboratory-confirmed bloodstream infections; (LCBI) these must meet one of the following criteria (Horan et al. 2008):
-
1.
One or more positive blood cultures with no apparent source of infection.
-
2.
Clinical presentation of infection (fever, chills, and hypotension), two or more positive blood cultures collected on two different occasions, and no apparent source of infection or skin contaminant.
-
3.
Patients <1 year of age must have at least one of the following signs or symptoms: fever, hypothermia, apnea, or bradycardia and a positive blood culture with no source of infection or skin contaminant.
A new device called a smart CVC (SCVC) which formed to detect biofilm formation (in vitro) through a biosensor. Information is sent through an antenna to an external base station to detect biofilm formation early; this provides more knowledge about colonization formation and leads to proper treatment. This device is still being researched, but is promising for enhancing the quality of patient care (Paredes et al. 2014).
10.10 Prevention of CLABSIs
CVC infection risk increases with the duration of use, yet routine changing of CVCs, unlike peripherally inserted catheters, is not recommended. Removing the CVC and inserting another in a different site carries high mechanical risks, and exchanging the CVC with a guidewire may increase the risk of bloodstream infection (Cobb et al. 1992). In addition, changing a CVC is not an easy procedure, as it is with a peripheral CVC. For that reason, different kinds of measurements are used to minimize and prevent the incidence of biofilm formation and bloodstream infections. The CDC recommends the following guidelines, which are considered as bundle of septic techniques to follow during CVC insertion (Casanova Vivas 2014):
-
1.
Use gloves after washing hands with antiseptic-containing soap, alcohol gel, or foam.
-
2.
Full sterile barrier precautions must be used during insertion of the catheter, including sterile gloves, a surgical gown, and a mask, with a large sterile sheet drape.
-
3.
Apply skin disinfectant (2 % chlorhexidine) at the CVC insertion site and wait for it to fully air dry before proceeding with the insertion.
-
4.
Remove the CVC once it is no longer needed.
-
5.
Avoid sites with a high incidence of infection (e.g., femoral) (Goetz et al. 1998).
Described below are the most important and widely used modalities for preventing biofilm colonization. CVCs impregnated with antibacterial or antiseptic have been shown, in a huge number of in vitro, animal, and clinical studies, to have substantial efficacy at preventing biofilm colonization and eventually bloodstream infection (Raad and Hanna 2002; Darouiche et al. 1999; Hanna et al. 2004). Two types of impregnated CVCs are used widely in the United States: minocycline-rifampin (M/R) and chlorhexidine-silver sulfadiazine (CHX/SS).
CHX/SS-impregnated catheters, which are now considered the first-generation CVC, showed a twofold decrease in colonization and nearly fivefold decrease in the rate of bloodstream infection (Maki et al. 1997). A meta-analysis of 12 studies (Veenstra et al. 1999) showed that catheters impregnated with CHX/SS were effective at preventing colonization and bloodstream infections. However, in first-generation CHX/SS catheters, only the external surface of the catheter is coated, which compromises its efficacy over the long term (>3 weeks); in long-term catheters, the internal surface is the main source of biofilm formation (Mermel 2000; Raad et al. 1996; Bach et al. 1996). In addition, the antimicrobial durability of all CHX/SS CVCs (first and second generation) in plasma is limited to 1 week (Raad et al. 2012; Darouiche et al. 1999). On the other hand, reports have raised concerns about anaphylaxis shock associated with chlorhexidine. The risk of this complication is low but significant, and it could be genetically related as it was reported only in Japan (where it is prohibited for that cause) (Oda et al. 1997; Fujita et al. 1997).
Second-generation CHX/SS catheters have been developed that have threefold increases in the chlorhexidine concentration, in addition to coating the internal lumen, the hub, and extension lines. A study of 780 patients in intensive care units showed a significant decrease in colonization compared to non-coated catheters; however, there was a non-significant reduction in the rate of CRBSIs (Rupp et al. 2005).
Antibiotic-impregnated CVCs M/R resulted in significantly superior outcomes to those of CHX/SS catheters. In a large randomized, prospective, multi-center clinical trial (Darouiche et al. 1999), these CVCs led to a 12-fold decrease in the rate of bloodstream infection compared with first-generation CHX/SS catheters. The prolonged antimicrobial activity (around 50 days) (Darouiche et al. 2005) and established activity against multi-drug resistant (MDR) VRSA and gram-negative bacteria that are associated with CRBSIs (Raad et al. 2008b) were the reasons for its superior clinical efficacy.
Concerns have been raised regarding the emergence of resistant bacterial strains with the use of antibiotic-coated catheters; however, large clinical studies have failed to demonstrate resistance after prolonged use (up to 7 years) and more than 500,000 CVC days (Raad et al. 1997; Darouiche et al. 1999; Raad and Hanna 2002). In addition, these CVCs led to a decrease in the rate of nosocomial vancomycin-resistant enterococci (VRE)-related bacteremia in critically ill patients (Hanna et al. 2003).
The overuse of antiseptic skin preparations and sterile barrier precautions changed the epidemiological map of the pathogens that cause CLABSIs, shifting them toward resistant gram-negative bacteria, in which both CHX/SS and M/R CVCs have limited activity. For that reason, our team at The University of Texas MD Anderson Cancer Center has developed a new broad-spectrum catheter by adding chlorhexidine to M/R (CHX-M/R). In vitro results showed that it has excellent activity against multidrug-resistant, gram-negative Acinetobacter baumannii, Enterobacter cloacae, E. coli, K. pneumoniae, P. aeruginosa, and S. maltophilia, and is superior to M/R and CHX/SS catheters.
A new in vitro cross-contamination model of exchange study showed that exchanging uncoated CVCs over a guidewire in the presence of bacteremia using CHX-M/R catheters completely prevented cross-contamination by MRSA, P. aeruginosa, and C. albicans biofilm; exchanging them for CHX/SS CVCs reduced but did not prevent cross-contamination by MRSA. Furthermore, CHX-M/R CVCs showed superior activity against P. aeruginosa and C. albicans to M/R catheters and were superior to CHX/SS CVCs against MRSA and P. aeruginosa (Jamal et al. 2014).
Recently, several studies have been conducted using locking solutions instead of the usual heparin lock. These solutions are mostly a combination of an antimicrobial and an anticoagulant (Campos et al. 2011).
Minocycline combined with EDTA (M-EDTA) showed high efficacy in preventing bloodstream infection in chronic hemodialysis patients (Campos et al. 2011; Raad et al. 2008a). This combination was highly active against S. epidermidis, S. aureus, and C. albicans that were embedded in biofilm (Raad et al. 2003). Randomized clinical trials show at least a threefold reduction in the occurrence of bacteremia (McIntyre et al. 2004; Dogra et al. 2002; Betjes and van Agteren 2004; Bleyer et al. 2005). On the other hand, a study by Bleyer et al. showed that minocycline/EDTA lock solution was significantly effective at preventing CLABSIs in patients with a history of recurrent bacteremia and a high risk of infection (Feely et al. 2007). Furthermore, this solution was used to prevent infection in an implantable port in children with cancer (Chatzinikolaou et al. 2003).
A new antimicrobial lock solution that was developed recently by Raad et al. contains 7 % citrate; this has been approved as an anticoagulant heparin-free catheter lock, 20 % ethanol; for its antimicrobial activity, plus 0.01 glyceryl trinitrate (GTN); which is well known for its intravenous use in treating hypertension. In vitro results show that these components rapidly and fully eradicate biofilms in all organisms tested (MRSA, methicillin-resistant S. epidermidis, P. aeruginosa, and C. albicans), in synergistic manner (Rosenblatt et al. 2013).
10.11 Management of Catheter Related Biofilm
The conventional treatment for all foreign body-associated biofilm infections is to remove the foreign body. However, removing the CVC and reinserting another in a different vascular access site carries a risk of iatrogenic mechanical complications; it is also time consuming and relatively expensive (Dimick et al. 2001). On the other hand, systemic antibiotics alone are not sufficient for treating biofilm bacteria because the extracellular materials in the biofilm, with their high concentrations of metal ions and low pH, cause metabolic inactivation of the antibiotics (Hoiby et al. 2010). These factors can contribute to the biofilm bacteria becoming 1,000 times more resistant than planktonic cells (Fig. 10.2) (Hoiby et al. 2010; Kostakioti et al. 2013). For that reason, many strategies have been designed to treat biofilm formation, either by killing the bacteria or dissolving the biofilm by targeting different developmental stages of biofilm formation.
Some biofilm disruption and treatment strategies are described below:
-
Chelating Agents: Metal cations, such as calcium, iron, and magnesium, play an essential role in maintaining biofilm matrix integrity and inhibiting bacterial growth by affecting bacterial membrane stability (Patrauchan et al. 2005; Sarkisova et al. 2005; Raad et al. 2008a). Chelating agents can destabilize the biofilm matrix architecture, thus helping to dissolve it. Sodium citrate is one of the chelators that has an inhibitory effect on Staphylococcus species biofilm (Shanks et al. 2006). On the other hand, in vitro studies show high efficacy of tetra sodium-EDTA in eradicating biofilm (Kite et al. 2004; Percival et al. 2005). The combination of disodium-EDTA and antimicrobial agents such as tigecycline or gentamicin is effective at reducing biofilm formation in both staphylococcus species and P. aeruginosa (Bookstaver et al. 2009). Raad et al. showed a synergetic effect of Minocycline-EDTA (M-EDTA) in preventing colonization and biofilm formation (Raad et al. 2007). This combination was effective in organisms embedded in both fresh biofilm (in vitro) and mature biofilm (ex vivo) (Raad et al. 2003). To our knowledge, this is the only biofilm-disrupting and -dissolving treatment and technology with a large number of successful clinical studies (Raad and Bodey 2011; Raad et al. 2002, 2007; Chatzinikolaou et al. 2003).
-
Phage Therapy: An abundant, easily isolated, self-replicating, organism with a high mutation rate easily adapts to any given environment. One promising alternative to antibiotics is encoding phages with EPS-degrading enzymes (Hughes et al. 1998; Sutherland et al. 2004; Sillankorva et al. 2004), resulting in fast destruction of the bacterial cell wall. No clinical studies of this approach are available to confirm its efficacy and safety in humans.
-
Antimicrobial Peptides: Cathelcidins are one of the most important antimicrobial peptide classes; as they show activity in activating the innate immune system response, they can be considered a possible strategy for treating biofilm formation (Pompilio et al. 2011). Pompilio et al. compared the activity of tobramycin, which is considered a first-choice treatment for P. aeruginosa in CF patients, with that of cathelcidins. The results show that cathelcidins peptides have faster kinetics and rapid bactericidal activity, while the overall extent of bacterial killing is greater with tobramycin. More precise and advanced studies are needed of the mechanisms involved before cathelcidins can be considered a treatment strategy (Pompilio et al. 2011; Kostakioti et al. 2013).
-
Polysaccharides: Cell-to-surface and cell-to-cell interactions are mediated by exopolysaccharides, which is an essential step in biofilm formation. Mutations in polysaccharide synthesis cause instability in the biofilm structure, which makes it highly susceptible to antibiotics and immune defense (Rendueles et al. 2013). On the other hand, the results of new studies show that bacterial exopolysaccharides can interfere with the biofilm formation of other bacterial species, For example, P. aeruginosa exopolysaccharides can inhibit the biofilm formation of Staphylococcus species (Qin et al. 2009; Rendueles et al. 2013).
-
Signal Transduction Interference: A promising new strategy involves targeting the bacterial signaling cascades. By inhibiting these signals, we can deprogram optimal gene expression without killing the bacteria or increasing the risk of bacterial resistance (Cegelski et al. 2008). An example of this model of treatment is targeting the QseBC two-component system that is common in biofilm-forming, gram-negative pathogens (Huang et al. 2006; Kostakioti et al. 2009; Khajanchi et al. 2012). Clinical data are required to demonstrate the clinical efficacy and safety of this approach.
References
Adair CG, Gorman SP, Feron BM, Byers LM, Jones DS, Goldsmith CE, Moore JE, Kerr JR, Curran MD, Hogg G, Webb CH, Mccarthy GJ, Milligan KR (1999) Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med 25:1072–1076
Adam B, Baillie GS, Douglas LJ (2002) Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol 51:344–349
Agladze K, Wang X, Romeo T (2005) Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J Bacteriol 187:8237–8246
Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008
Alipour M, Suntres ZE, Omri A (2009) Importance of DNase and alginate lyase for enhancing free and liposome encapsulated aminoglycoside activity against Pseudomonas aeruginosa. J Antimicrob Chemother 64:317–325
Allison DG, Ruiz B, SanJose C, Jaspe A, Gilbert P (1998) Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol Lett 167:179–184
Anaissie E, Samonis G, Kontoyiannis D, Costerton J, Sabharwal U, Bodey G, Raad I (1995) Role of catheter colonization and infrequent hematogenous seeding in catheter-related infections. Eur J Clin Microbiol Infect Dis 14:134–137
Ardehali R, Shi L, Janatova J, Mohammad SF, Burns GL (2002) The effect of apo-transferrin on bacterial adhesion to biomaterials. Artif Organs 26:512–520
Bach A, Schmidt H, Bottiger B, Schreiber B, Bohrer H, Motsch J, Martin E, Sonntag HG (1996) Retention of antibacterial activity and bacterial colonization of antiseptic-bonded central venous catheters. J Antimicrob Chemother 37:315–322
Banin E, Vasil ML, Greenberg EP (2005) Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci U S A 102:11076–11081
Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS (2006) Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol 188:7344–7353
Baumgartner JN, Cooper SL (1998) Influence of thrombus components in mediating Staphylococcus aureus adhesion to polyurethane surfaces. J Biomed Mater Res 40:660–670
Beckingsale TB, Page JE, Jennings A, Fawcett T (2011) Increased sodium and potassium concentrations lead to increased penicillin resistance and increased biofilm formation in Stapylococcus aureus. J Bone Joint Surg Br 93-B:319
Beloin C, Roux A, Ghigo JM (2008) Escherichia coli biofilms. Curr Top Microbiol Immunol 322:249–289
Betjes MG, Van Agteren M (2004) Prevention of dialysis catheter-related sepsis with a citrate-taurolidine-containing lock solution. Nephrol Dial Transplant 19:1546–1551
Bjornson HS, Colley R, Bower RH, Duty VP, Schwartz-Fulton JT, Fischer JE (1982) Association between microorganism growth at the catheter insertion site and colonization of the catheter in patients receiving total parenteral nutrition. Surgery 92:720–727
Bleyer AJ, Mason L, Russell G, Raad II, Sherertz RJ (2005) A randomized, controlled trial of a new vascular catheter flush solution (minocycline-EDTA) in temporary hemodialysis access. Infect Control Hosp Epidemiol 26:520–524
Boles BR, Horswill AR (2008) Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052
Bookstaver PB, Williamson JC, Tucker BK, Raad II, Sherertz RJ (2009) Activity of novel antibiotic lock solutions in a model against isolates of catheter-related bloodstream infections. Ann Pharmacother 43:210–219
Boyd A, Chakrabarty AM (1994) Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol 60:2355–2359
Branda SS, Vik S, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13:20–26
Brogden KA, Guthmiller JM, Taylor CE (2005) Human polymicrobial infections. Lancet 365:253–255
Cairo J, Hachem R, Rangaraj G, Granwehr B, Raad I (2011) Predictors of catheter-related gram-negative bacilli bacteraemia among cancer patients. Clin Microbiol Infect 17:1711–1716
Campos RP, Do Nascimento MM, Chula DC, Riella MC (2011) Minocycline-EDTA lock solution prevents catheter-related bacteremia in hemodialysis. J Am Soc Nephrol 22:1939–1945
Casanova Vivas S (2014) Recommendations from CDC for the prevention of catheter-related infections (2013 update). Rev Enferm 37:28–33
Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ (2008) The biology and future prospects of antivirulence therapies. Nat Rev Microbiol 6:17–27
Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Aberg V, Walker JN, Seed PC, Almqvist F, Chapman MR, Hultgren SJ (2009) Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol 5:913–919
Centers for Disease Control and Prevention (2011) Vital signs: central line-associated blood stream infections – United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 60:243–248
Chatzinikolaou I, Zipf TF, Hanna H, Umphrey J, Roberts WM, Sherertz R, Hachem R, Raad I (2003) Minocycline-ethylenediaminetetraacetate lock solution for the prevention of implantable port infections in children with cancer. Clin Infect Dis 36:116–119
Chen H, Fujita M, Feng Q, Clardy J, Fink GR (2004) Tyrosol is a quorum-sensing molecule in Candida albicans. Proc Natl Acad Sci U S A 101:5048–5052
Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P, Kroll G, Schulze C, Buck F, Mack D, Aepfelbacher M, Rohde H (2010) The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol Microbiol 75:187–207
Cicalini S, Palmieri F, Noto P, Boumis E, Petrosillo N (2002) Diagnosis of intra vascular catheter-related infection. J Vasc Access 3:114–119
Cleri DJ, Corrado ML, Seligman SJ (1980) Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis 141:781–786
Cobb DK, High KP, Sawyer RG, Sable CA, Adams RB, Lindley DA, Pruett TL, Schwenzer KJ, Farr BM (1992) A controlled trial of scheduled replacement of central venous and pulmonary-artery catheters. N Engl J Med 327:1062–1068
Cookson AL, Cooley WA, Woodward MJ (2002) The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int J Med Microbiol 292:195–205
Costerton JW (1995) Overview of microbial biofilms. J Ind Microbiol 15:137–140
Costerton JW, Irvin RT, Cheng KJ (1981) The bacterial glycocalyx in nature and disease. Annu Rev Microbiol 35:299–324
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F (1999) The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433
Danese PN, Pratt LA, Dove SL, Kolter R (2000a) The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol 37:424–432
Danese PN, Pratt LA, Kolter R (2000b) Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol 182:3593–3596
Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris RL, Mayhall G (1999) A comparison of two antimicrobial-impregnated central venous catheters. Catheter Study Group. N Engl J Med 340:1–8
Darouiche RO, Berger DH, Khardori N, Robertson CS, Wall MJ Jr, Metzler MH, Shah S, Mansouri MD, Cerra-Stewart C, Versalovic J, Reardon MJ, Raad II (2005) Comparison of antimicrobial impregnation with tunneling of long-term central venous catheters: a randomized controlled trial. Ann Surg 242:193–200
Das T, Sehar S, Koop L, Wong YK, Ahmed S, Siddiqui KS, Manefield M (2014) Influence of calcium in extracellular DNA mediated bacterial aggregation and biofilm formation. PLoS One 9:e91935
Davey ME, Caiazza NC, O’Toole GA (2003) Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol 185:1027–1036
Davies DG, Marques CN (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403
Decho AW (2010) Overview of biopolymer-induced mineralization: what goes on in biofilms? Ecol Eng 36:137–144
Dickinson RB, Nagel JA, Mcdevitt D, Foster TJ, Proctor RA, Cooper SL (1995) Quantitative comparison of clumping factor- and coagulase-mediated Staphylococcus aureus adhesion to surface-bound fibrinogen under flow. Infect Immun 63:3143–3150
Dickinson RB, Nagel JA, Proctor RA, Cooper SL (1997) Quantitative comparison of shear-dependent Staphylococcus aureus adhesion to three polyurethane ionomer analogs with distinct surface properties. J Biomed Mater Res 36:152–162
Dimick JB, Pelz RK, Consunji R, Swoboda SM, Hendrix CW, Lipsett PA (2001) Increased resource use associated with catheter-related bloodstream infection in the surgical intensive care unit. Arch Surg 136:229–234
Dogra GK, Herson H, Hutchison B, Irish AB, Heath CH, Golledge C, Luxton G, Moody H (2002) Prevention of tunneled hemodialysis catheter-related infections using catheter-restricted filling with gentamicin and citrate: a randomized controlled study. J Am Soc Nephrol 13:2133–2139
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Downes KJ, Metlay JP, Bell LM, Mcgowan KL, Elliott MR, Shah SS (2008) Polymicrobial bloodstream infections among children and adolescents with central venous catheters evaluated in ambulatory care. Clin Infect Dis 46:387–394
Dryden MS, Samson A, Ludlam HA, Wing AJ, Phillips I (1991) Infective complications associated with the use of the Quinton ‘Permcath’ for long-term central vascular access in haemodialysis. J Hosp Infect 19:257–262
Dunne WM Jr (2002) Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 15:155–166
Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC (2009) National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control 37:783–805
El-Azizi MA, Starks SE, Khardori N (2004) Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Appl Microbiol 96:1067–1073
Federle MJ, Bassler BL (2003) Interspecies communication in bacteria. J Clin Invest 112:1291–1299
Feely T, Copley A, Bleyer AJ (2007) Catheter lock solutions to prevent bloodstream infections in high-risk hemodialysis patients. Am J Nephrol 27:24–29
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Fujita S, Sumita S, Kawana S, Iwasaki H, Namiki A (1997) Two cases of anaphylactic shock induced by chlorhexidine. Masui 46:1118–1121
Geoghegan JA, Corrigan RM, Gruszka DT, Speziale P, O’Gara JP, Potts JR, Foster TJ (2010) Role of surface protein SasG in biofilm formation by Staphylococcus aureus. J Bacteriol 192:5663–5673
Gjermansen M, Ragas P, Sternberg C, Molin S, Tolker-Nielsen T (2005) Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environ Microbiol 7:894–906
Goetz AM, Wagener MM, Miller JM, Muder RR (1998) Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol 19:842–845
Gotz F (2002) Staphylococcus and biofilms. Mol Microbiol 43:1367–1378
Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574
Granger BL, Flenniken ML, Davis DA, Mitchell AP, Cutler JE (2005) Yeast wall protein 1 of Candida albicans. Microbiology 151:1631–1644
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108
Hancock LE, Perego M (2004) The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J Bacteriol 186:5629–5639
Hanna HA, Raad II, Hackett B, Wallace SK, Price KJ, Coyle DE, Parmley CL, MD Anderson Catheter Study Group (2003) Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest 124:1030–1038
Hanna H, Benjamin R, Chatzinikolaou I, Alakech B, Richardson D, Mansfield P, Dvorak T, Munsell MF, Darouiche R, Kantarjian H, Raad I (2004) Long-term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter-related bloodstream infection in cancer patients: a prospective randomized clinical trial. J Clin Oncol 22:3163–3171
Harriott MM, Noverr MC (2010) Ability of Candida albicans mutants to induce Staphylococcus aureus vancomycin resistance during polymicrobial biofilm formation. Antimicrob Agents Chemother 54:3746–3755
Hawser SP, Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39:2128–2131
Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR (2001) Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183:5395–5401
Herrmann M, Vaudaux PE, Pittet D, Auckenthaler R, Lew PD, Schumacher-Perdreau F, Peters G, Waldvogel FA (1988) Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis 158:693–701
Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332
Huang YH, Ferrieres L, Clarke DJ (2006) The role of the Rcs phosphorelay in Enterobacteriaceae. Res Microbiol 157:206–212
Hughes KA, Sutherland IW, Jones MV (1998) Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144(Pt 11):3039–3047
Hunt SM, Werner EM, Huang B, Hamilton MA, Stewart PS (2004) Hypothesis for the role of nutrient starvation in biofilm detachment. Appl Environ Microbiol 70:7418–7425
Isberg RR, Barnes P (2002) Dancing with the host; flow-dependent bacterial adhesion. Cell 110:1–4
Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H (1984) Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr 104:206–210
Izano EA, Amarante MA, Kher WB, Kaplan JB (2008) Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol 74:470–476
Jackson DW, Suzuki K, Oakford L, Simecka JW, Hart ME, Romeo T (2002) Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J Bacteriol 184:290–301
Jamal MA Jr, Jiang Y, Hachem R, Chaftari A-M, Raad II (2014) Prevention of transmission of multidrug-resistant organisms during catheter exchange using antimicrobial catheters. Antimicrob Agents Chemother 58:5291–5296
Kaplan JB (2010) Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res 89:205–218
Karatan E, Watnick P (2009) Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev 73:310–347
Katsikogianni M, Missirlis YF (2004) Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cell Mater 8:37–57
Kemp KD, Singh KV, Nallapareddy SR, Murray BE (2007) Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect Immun 75:5399–5404
Khajanchi BK, Kozlova EV, Sha J, Popov VL, Chopra AK (2012) The two-component QseBC signalling system regulates in vitro and in vivo virulence of Aeromonas hydrophila. Microbiology 158:259–271
Kierek K, Watnick PI (2003) The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2 +-dependent biofilm development in sea water. Proc Natl Acad Sci U S A 100:14357–14362
Kinane DF, Riggio MP, Walker KF, Mackenzie D, Shearer B (2005) Bacteraemia following periodontal procedures. J Clin Periodontol 32:708–713
Kite P, Eastwood K, Sugden S, Percival SL (2004) Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J Clin Microbiol 42:3073–3076
Klapper I, Rupp CJ, Cargo R, Purvedorj B, Stoodley P (2002) Viscoelastic fluid description of bacterial biofilm material properties. Biotechnol Bioeng 80:289–296
Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T (2003) Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol 48:1511–1524
Kline KA, Dodson KW, Caparon MG, Hultgren SJ (2010) A tale of two pili: assembly and function of pili in bacteria. Trends Microbiol 18:224–232
Kong KF, Vuong C, Otto M (2006) Staphylococcus quorum sensing in biofilm formation and infection. Int J Med Microbiol 296:133–139
Kostakioti M, Hadjifrangiskou M, Pinkner JS, Hultgren SJ (2009) QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol Microbiol 73:1020–1031
Kostakioti M, Hadjifrangiskou M, Hultgren SJ (2013) Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306
Kristinsson KG, Burnett IA, Spencer RC (1989) Evaluation of three methods for culturing long intravascular catheters. J Hosp Infect 14:183–191
LASA I, PENADES JR (2006) Bap: a family of surface proteins involved in biofilm formation. Res Microbiol 157:99–107
Leid JG, Willson CJ, Shirtliff ME, Hassett DJ, Parsek MR, Jeffers AK (2005) The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J Immunol 175:7512–7518
Lewis K (2001) Riddle of biofilm resistance. Antimicrob Agents Chemother 45:999–1007
Li F, Palecek SP (2008) Distinct domains of the Candida albicans adhesin Eap1p mediate cell-cell and cell-substrate interactions. Microbiology 154:1193–1203
Linares J, Sitges-Serra A, Garau J, Perez JL, Martin R (1985) Pathogenesis of catheter sepsis: a prospective study with quantitative and semiquantitative cultures of catheter hub and segments. J Clin Microbiol 21:357–360
Lyte M, Freestone PP, Neal CP, Olson BA, Haigh RD, Bayston R, Williams PH (2003) Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet 361:130–135
Maki DG, Stolz SM, Wheeler S, Mermel LA (1997) Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med 127:257–266
Maki DG, Kluger DM, Crnich CJ (2006) The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171
Ma L, Wang S, Wang D, Parsek MR, Wozniak DJ (2012) The roles of biofilm matrix polysaccharide Psl in mucoid Pseudomonas aeruginosa biofilms. FEMS Immunol Med Microbiol 65:377–380
Mann EE, Wozniak DJ (2012) Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol Rev 36:893–916
Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, Tsang LH, Smeltzer MS, Horswill AR, Bayles KW (2009) Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One 4:e5822
Marks LR, Davidson BA, Knight PR, Hakansson AP (2013) Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio 4:e00438–13
Mathoera RB, Kok DJ, Nijman RJ (2000) Bladder calculi in augmentation cystoplasty in children. Urology 56:482–487
Mcdevitt D, Nanavaty T, House-Pompeo K, Bell E, Turner N, Mcintire L, Foster T, Hook M (1997) Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem 247:416–424
Mcgee DC, Gould MK (2003) Preventing complications of central venous catheterization. N Engl J Med 348:1123–1133
Mcintyre CW, Hulme LJ, Taal M, Fluck RJ (2004) Locking of tunneled hemodialysis catheters with gentamicin and heparin. Kidney Int 66:801–805
Mehall JR, Saltzman DA, Jackson RJ, Smith SD (2002) Fibrin sheath enhances central venous catheter infection. Crit Care Med 30:908–912
Menzies BE (2003) The role of fibronectin binding proteins in the pathogenesis of Staphylococcus aureus infections. Curr Opin Infect Dis 16:225–229
Mermel LA (2000) Prevention of intravascular catheter-related infections. Ann Intern Med 132:391–402
Mohamed JA, Huang DB (2007) Biofilm formation by enterococci. J Med Microbiol 56:1581–1588
Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE (2004) Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect Immun 72:3658–3663
Mohamed JA, Teng F, Nallapareddy SR, Murray BE (2006) Pleiotrophic effects of 2 Enterococcus faecalis sagA-like genes, salA and salB, which encode proteins that are antigenic during human infection, on biofilm formation and binding to collagen type i and fibronectin. J Infect Dis 193:231–240
Morgan R, Kohn S, Hwang SH, Hassett DJ, Sauer K (2006) BdlA, a chemotaxis regulator essential for biofilm dispersion in Pseudomonas aeruginosa. J Bacteriol 188:7335–7343
Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE (2006) Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 116:2799–2807
Nett J, Lincoln L, Marchillo K, Andes D (2007a) Beta -1,3 glucan as a test for central venous catheter biofilm infection. J Infect Dis 195:1705–1712
Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, Vanhandel M, Andes D (2007b) Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother 51:510–520
Ni Eidhin D, Perkins S, Francois P, Vaudaux P, Hook M, Foster TJ (1998) Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol Microbiol 30:245–257
Nobile CJ, Fox EP, Hartooni N, Mitchell KF, Hnisz D, Andes DR, Kuchler K, Johnson AD (2014) A histone deacetylase complex mediates biofilm dispersal and drug resistance in Candida albicans. MBio 5:e01201–e01214
O’Gara JP (2007) ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett 270:179–188
O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, Mccormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA (2002) Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S. Pediatrics 110:e51
O’Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O’Gara JP (2007) Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 45:1379–1388
O’Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O’Gara JP (2008) A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 190:3835–3850
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304
O’Toole G, Kaplan HB, Kolter R (2000) Biofilm formation as microbial development. Annu Rev Microbiol 54:49–79
Oda T, Hamasaki J, Kanda N, Mikami K (1997) Anaphylactic shock induced by an antiseptic-coated central venous [correction of nervous] catheter. Anesthesiology 87:1242–1244
Olson ME, Garvin KL, Fey PD, Rupp ME (2006) Adherence of Staphylococcus epidermidis to biomaterials is augmented by PIA. Clin Orthop Relat Res 451:21–24
Otto M (2014) Phenol-soluble modulins. Int J Med Microbiol 304:164–169
Pammi M, Liang R, Hicks J, Mistretta TA, Versalovic J (2013) Biofilm extracellular DNA enhances mixed species biofilms of Staphylococcus epidermidis and Candida albicans. BMC Microbiol 13:257
Paredes J, Alonso-Arce M, Schmidt C, Valderas D, Sedano B, Legarda J, Arizti F, Gomez E, Aguinaga A, Del Pozo JL, Arana S (2014) Smart central venous port for early detection of bacterial biofilm related infections. Biomed Microdevices 16:365–374
Parsek MR, Singh PK (2003) Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 57:677–701
Pastar I, Nusbaum AG, Gil J, Patel SB, Chen J, Valdes J, Stojadinovic O, Plano LR, Tomic-Canic M, Davis SC (2013) Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 8:e56846
Patrauchan MA, Sarkisova S, Sauer K, Franklin MJ (2005) Calcium influences cellular and extracellular product formation during biofilm-associated growth of a marine Pseudoalteromonas sp. Microbiology 151:2885–2897
Patti JM, Allen BL, Mcgavin MJ, Hook M (1994) MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol 48:585–617
Pegues D, Axelrod P, Mcclarren C, Eisenberg BL, Hoffman JP, Ottery FD, Keidan RD, Boraas M, Weese J (1992) Comparison of infections in Hickman and implanted port catheters in adult solid tumor patients. J Surg Oncol 49:156–162
Percival SL, Kite P, Eastwood K, Murga R, Carr J, Arduino MJ, Donlan RM (2005) Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect Control Hosp Epidemiol 26:515–519
Pereira AL, Silva TN, Gomes AC, Araujo AC, Giugliano LG (2010) Diarrhea-associated biofilm formed by enteroaggregative Escherichia coli and aggregative Citrobacter freundii: a consortium mediated by putative F pili. BMC Microbiol 10:57
Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M (2012) How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A 109:1281–1286
Pittet D, Tarara D, Wenzel RP (1994) Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 271:1598–1601
Pompilio A, Scocchi M, Pomponio S, Guida F, Di Primio A, Fiscarelli E, Gennaro R, Di Bonaventura G (2011) Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 32:1807–1814
Potter A, Ceotto H, Giambiagi-Demarval M, Dos Santos KR, Nes IF, Bastos Mdo C (2009) The gene bap, involved in biofilm production, is present in Staphylococcus spp. strains from nosocomial infections. J Microbiol 47:319–326
Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355:2725–2732
Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D (2007) Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153:2083–2092
Qin Z, Yang L, Qu D, Molin S, Tolker-Nielsen T (2009) Pseudomonas aeruginosa extracellular products inhibit staphylococcal growth, and disrupt established biofilms produced by Staphylococcus epidermidis. Microbiology 155:2148–2156
Raad I (1998) Intravascular-catheter-related infections. Lancet 351:893–898
Raad I, Bodey GP Sr (2011) Novel antimicrobial catheter lock solution: a new direction in which chelators replace heparin. Crit Care Med 39:875–876
Raad II, Hanna HA (2002) Intravascular catheter-related infections: new horizons and recent advances. Arch Intern Med 162:871–878
Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP (1993) Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis 168:400–407
Raad I, Darouiche R, Hachem R, Mansouri M, Bodey GP (1996) The broad-spectrum activity and efficacy of catheters coated with minocycline and rifampin. J Infect Dis 173:418–424
Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, Robertson C, Shenaq S, Curling P, Burke T, Ericsson C (1997) Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med 127:267–274
Raad I, Hachem R, Tcholakian RK, Sherertz R (2002) Efficacy of minocycline and EDTA lock solution in preventing catheter-related bacteremia, septic phlebitis, and endocarditis in rabbits. Antimicrob Agents Chemother 46:327–332
Raad I, Chatzinikolaou I, Chaiban G, Hanna H, Hachem R, Dvorak T, Cook G, Costerton W (2003) In vitro and ex vivo activities of minocycline and EDTA against microorganisms embedded in biofilm on catheter surfaces. Antimicrob Agents Chemother 47:3580–3585
Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R (2007) Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob Agents Chemother 51:78–83
Raad II, Fang X, Keutgen XM, Jiang Y, Sherertz R, Hachem R (2008a) The role of chelators in preventing biofilm formation and catheter-related bloodstream infections. Curr Opin Infect Dis 21:385–392
Raad I, Reitzel R, Jiang Y, Chemaly RF, Dvorak T, Hachem R (2008b) Anti-adherence activity and antimicrobial durability of anti-infective-coated catheters against multidrug-resistant bacteria. J Antimicrob Chemother 62:746–750
Raad I, Mohamed JA, Reitzel RA, Jiang Y, Raad S, Al Shuaibi M, Chaftari AM, Hachem RY (2012) Improved antibiotic-impregnated catheters with extended-spectrum activity against resistant bacteria and fungi. Antimicrob Agents Chemother 56:935–941
Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J (2009) Our current understanding of fungal biofilms. Crit Rev Microbiol 35:340–355
Rello J, Ochagavia A, Sabanes E, Roque M, Mariscal D, Reynaga E, Valles J (2000) Evaluation of outcome of intravenous catheter-related infections in critically ill patients. Am J Respir Crit Care Med 162:1027–1030
Rendueles O, Kaplan JB, Ghigo JM (2013) Antibiofilm polysaccharides. Environ Microbiol 15:334–346
Rice SA, Mcdougald D, Kumar N, Kjelleberg S (2005) The use of quorum-sensing blockers as therapeutic agents for the control of biofilm-associated infections. Curr Opin Investig Drugs 6:178–184
Richards MJ, Edwards JR, Culver DH, Gaynes RP (1999) Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 27:887–892
Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS (2003) Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol 11:94–100
Riedel K, Hentzer M, Geisenberger O, Huber B, Steidle A, Wu H, Hoiby N, Givskov M, Molin S, Eberl L (2001) N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147:3249–3262
Rohde H, Burandt EC, Siemssen N, Frommelt L, Burdelski C, Wurster S, Scherpe S, Davies AP, Harris LG, Horstkotte MA, Knobloch JK, Ragunath C, Kaplan JB, Mack D (2007) Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 28:1711–1720
Rosenblatt J, Reitzel R, Dvorak T, Jiang Y, Hachem RY, Raad II (2013) Glyceryl trinitrate complements citrate and ethanol in a novel antimicrobial catheter lock solution to eradicate biofilm organisms. Antimicrob Agents Chemother 57:3555–3560
Ruesch S, Walder B, Tramer MR (2002) Complications of central venous catheters: internal jugular versus subclavian access – a systematic review. Crit Care Med 30:454–460
Rupp ME, Lisco SJ, Lipsett PA, Perl TM, Keating K, Civetta JM, Mermel LA, Lee D, Dellinger EP, Donahoe M, Giles D, Pfaller MA, Maki DG, Sherertz R (2005) Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med 143:570–580
Ryan RP, Fouhy Y, Garcia BF, Watt SA, Niehaus K, Yang L, Tolker-Nielsen T, Dow JM (2008) Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol Microbiol 68:75–86
Sadovskaya I, Vinogradov E, Li J, Jabbouri S (2004) Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr Res 339:1467–1473
Safdar N, Maki DG (2002) Inflammation at the insertion site is not predictive of catheter-related bloodstream infection with short-term, noncuffed central venous catheters. Crit Care Med 30:2632–2635
Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ (2005) Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J Bacteriol 187:4327–4337
Sauer K, Cullen MC, Rickard AH, Zeef LA, Davies DG, Gilbert P (2004) Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J Bacteriol 186:7312–7326
Shanks RM, Sargent JL, Martinez RM, Graber ML, O’Toole GA (2006) Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant 21:2247–2255
Shenkman B, Varon D, Tamarin I, Dardik R, Peisachov M, Savion N, Rubinstein E (2002) Role of agr (RNAIII) in Staphylococcus aureus adherence to fibrinogen, fibronectin, platelets and endothelial cells under static and flow conditions. J Med Microbiol 51:747–754
Sherertz RJ, Raad II, Belani A, Koo LC, Rand KH, Pickett DL, Straub SA, Fauerbach LL (1990) Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol 28:76–82
Shih PC, Huang CT (2002) Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J Antimicrob Chemother 49:309–314
Siegman-Igra Y, Anglim AM, Shapiro DE, Adal KA, Strain BA, Farr BM (1997) Diagnosis of vascular catheter-related bloodstream infection: a meta-analysis. J Clin Microbiol 35:928–936
Sillankorva S, Oliveira R, Vieira MJ, Sutherland IW, Azeredo J (2004) Bacteriophage Phi S1 infection of Pseudomonas fluorescens planktonic cells versus biofilms. Biofouling 20:133–138
Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF (2010) Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect Immun 78:4644–4652
Singh PK, Parsek MR, Greenberg EP, Welsh MJ (2002) A component of innate immunity prevents bacterial biofilm development. Nature 417:552–555
Slobbe L, El Barzouhi A, Boersma E, Rijnders BJ (2009) Comparison of the roll plate method to the sonication method to diagnose catheter colonization and bacteremia in patients with long-term tunnelled catheters: a randomized prospective study. J Clin Microbiol 47:885–888
Song B, Leff LG (2006) Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol Res 161:355–361
Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S (1999) Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol 20:396–401
Steinberger RE, Holden PA (2005) Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl Environ Microbiol 71:5404–5410
Stoodley P, Lewandowski Z, Boyle JD, Lappin-Scott HM (1999) Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: an in situ investigation of biofilm rheology. Biotechnol Bioeng 65:83–92
Sutherland IW (1983) Microbial exopolysaccharides – their role in microbial adhesion in aqueous systems. Crit Rev Microbiol 10:173–201
Sutherland IW (2001) The biofilm matrix–an immobilized but dynamic microbial environment. Trends Microbiol 9:222–227
Sutherland IW, Hughes KA, Skillman LC, Tait K (2004) The interaction of phage and biofilms. FEMS Microbiol Lett 232:1–6
Sutter D, Stagliano D, Braun L, Williams F, Arnold J, Ottolini M, Epstein J (2008) Polymicrobial bloodstream infection in pediatric patients: risk factors, microbiology, and antimicrobial management. Pediatr Infect Dis J 27:400–405
Switalski LM, Patti JM, Butcher W, Gristina AG, Speziale P, Hook M (1993) A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol 7:99–107
Thomas VC, Thurlow LR, Boyle D, Hancock LE (2008) Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol 190:5690–5698
Thumbikat P, Berry RE, Zhou G, Billips BK, Yaggie RE, Zaichuk T, Sun TT, Schaeffer AJ, Klumpp DJ (2009) Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog 5:e1000415
Uhlich GA, Cooke PH, Solomon EB (2006) Analyses of the red-dry-rough phenotype of an Escherichia coli O157: H7 strain and its role in biofilm formation and resistance to antibacterial agents. Appl Environ Microbiol 72:2564–2572
Ulett GC, Valle J, Beloin C, Sherlock O, Ghigo JM, Schembri MA (2007) Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract. Infect Immun 75:3233–3244
Uppuluri P, Pierce CG, Thomas DP, Bubeck SS, Saville SP, Lopez-Ribot JL (2010) The transcriptional regulator Nrg1p controls Candida albicans biofilm formation and dispersion. Eukaryot Cell 9:1531–1537
Veenstra DL, Saint S, Saha S, Lumley T, Sullivan SD (1999) Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA 281:261–267
Wang X, Dubey AK, Suzuki K, Baker CS, Babitzke P, Romeo T (2005) CsrA post-transcriptionally represses pgaABCD, responsible for synthesis of a biofilm polysaccharide adhesin of Escherichia coli. Mol Microbiol 56:1648–1663
Wang R, Khan BA, Cheung GY, Bach TH, Jameson-Lee M, Kong KF, Queck SY, Otto M (2011) Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J Clin Invest 121:238–248
Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346
Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, Givskov M, Kjelleberg S (2003) Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol 185:4585–4592
Wellens A, Garofalo C, Nguyen H, Van Gerven N, Slattegard R, Hernalsteens JP, Wyns L, Oscarson S, De Greve H, Hultgren S, Bouckaert J (2008) Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One 3:e2040
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS (2002) Extracellular DNA required for bacterial biofilm formation. Science 295:1487
Wu Y, Outten FW (2009) IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J Bacteriol 191:1248–1257
Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen T (2007) Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153:1318–1328
Yang L, Liu Y, Wu H, Hoiby N, Molin S, Song ZJ (2011) Current understanding of multi-species biofilms. Int J Oral Sci 3:74–81
Yang L, Hengzhuang W, Wu H, Damkiaer S, Jochumsen N, Song Z, Givskov M, Hoiby N, Molin S (2012) Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol Med Microbiol 65:366–376
Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, Dekievit TR, Gardner PR, Schwab U, Rowe JJ, Iglewski BH, Mcdermott TR, Mason RP, Wozniak DJ, Hancock RE, Parsek MR, Noah TL, Boucher RC, Hassett DJ (2002) Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell 3:593–603
Acknowledgements
Dr. Raad is co-inventor of technology related to minocycline and rifampin-coated catheters. This technology is the property of The University of Texas MD Anderson Cancer Center and the Baylor College of Medicine and is licensed to Cook, Inc. Dr. Raad is also a co-inventor of technology related to minocycline-EDTA Lock. This technology is licensed to Novel Anti-infective Technology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yousif, A., Jamal, M.A., Raad, I. (2015). Biofilm-Based Central Line-Associated Bloodstream Infections. In: Donelli, G. (eds) Biofilm-based Healthcare-associated Infections. Advances in Experimental Medicine and Biology, vol 830. Springer, Cham. https://doi.org/10.1007/978-3-319-11038-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-11038-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-11037-0
Online ISBN: 978-3-319-11038-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)