Abstract
Seafood processing waste is a potentially rich source of several useful products including chitin (Meanwell and Shama 2008), and has long been generated in large tonnages worldwide (Chang et al. 2007). Chitin is economical and is the second most abundant bio-waste material after cellulose (Shahidi et al. 1999). Annual worldwide chitin production from arthropods (e.g., crustaceans and insects), molluscs (e.g., squid and cuttlefish) and fungi is estimated at about 100 × 109 t (Tharanathan and Kittur 2003). A steady supply of chitinous waste materials from the seafood processing industry has been the major source of commercial products such as chitin and chitosan (Hayes 2012). The increasing consumption of krill oil and mushrooms has also been an additional source for commercial chitin (Nicol and Hosie 1993; Vetter 2007).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Seafood processing waste is a potentially rich source of several useful products including chitin (Meanwell and Shama 2008), and has long been generated in large tonnages worldwide (Chang et al. 2007). Chitin is economical and is the second most abundant bio-waste material after cellulose (Shahidi et al. 1999). Annual worldwide chitin production from arthropods (e.g., crustaceans and insects), molluscs (e.g., squid and cuttlefish) and fungi is estimated at about 100 × 109 t (Tharanathan and Kittur 2003). A steady supply of chitinous waste materials from the seafood processing industry has been the major source of commercial products such as chitin and chitosan (Hayes 2012). The increasing consumption of krill oil and mushrooms has also been an additional source for commercial chitin (Nicol and Hosie 1993; Vetter 2007).
Chitin is a polysaccharide compound dominated by hydroxyl (–OH) and amide (R–CO–NH2) groups. Chitosan is a by-product of the alkaline deacetylation of chitin, wherein the amide group in chitin is hydrolyzed to a primary amine group (R–NH2) to produce chitosan. Unlike chitin, chitosan is soluble in acidic solution, but precipitates into solids at a higher pH. Treating chitosan solution at higher pH has been used to transform it into various physical forms (i.e., membranes, nanoparticles, nanofibres, etc.). Even though the high –NH2 content of chitosan gives the molecule an antimicrobial property (Rabea et al. 2003), it is non-toxic to plants, animals or humans (Baldrick 2010; Kean and Thanou 2010). This lack of toxicity, coupled with its rapid degradability, makes chitosan suitable for several environmental and agricultural uses (Uthairatanakij et al. 2007). Some applications of chitosan include drug delivery in the human gastrointestinal tract (Cook et al. 2013), a role in food processing (Dutta et al. 2009; Romanazzi et al. 2012), biomedical use (Dash et al. 2011; Jayakumar et al. 2010; Khor and Lim 2003; Kim et al. 2007; Muzzarelli 2009), an ingredient in cosmetics (Desbrieres et al. 2010), enzyme immobilization (Krajewska 2004), serving as a heterogeneous catalyst (Guibal 2005), a sorbent for organic and inorganic contaminants (Guibal et al. 2006; Wan Ngah et al. 2011; Wu et al. 2010; Yong et al. 2012), a component of antimicrobial products (Kong et al. 2010), and as an agent to help recover uranium (Muzzarelli 2011).
Soil and water pollution by organic and inorganic contaminants, including metal(loid)s, is of growing concern, because of their potential detrimental effects on human health and the environment (Adriano 2001). With the exception of As, Cr, Hg and Se, most metal(loid)s do not undergo biological or chemical transformation and therefore persist in soils for long periods (Aucott et al. 2010; Bolan et al. 2014). Several methods have been employed to remediate wastewater that is contaminated with toxic metal(loid)s, including chemical precipitation, electrodeposition, ion exchange, membrane separation, and sorption (Geremias et al. 2003). However, these methods are limited by high operational costs (Demirbas 2008) and/or inefficiency for remediation of some toxic metals that exist at trace levels (Juang and Shao 2002).
Renewable chitinous waste materials derived from plants or animals may be a cost-effective approach to remediate wastewater (Niu and Volesky 2007). Crab shells have effectively been used to decontaminate metals (e.g., Pb, Cd, Cu, and Cr) in wastewater (An et al. 2001; Kim 2003). Fungus mycelium biomass has recently emerged as a promising new source of chitinous material to potentially remediate contaminated wastewater (Kamari et al. 2011a, b; Tay et al. 2011a, b, 2012). The commercial value of employing chitosan for environmental applications may be enhanced considerably by modifying its structure and chemical functionalization. For example, by applying a crosslinking process on chitosan both its resistance to acidic solubilization (Hsien and Rorrer 1995) and to microbial degradation are enhanced (Yamamoto and Amaike 1997).
Pillai et al. (2009) and Roberts (2008) have previously described the general applications to which chitin and chitosan have been put. However, to the best of our knowledge, the environmental applications of chitosan or its derivatives have not previously been reviewed in detail. Therefore, it is our aim in this review to close this gap by addressing the latest developments made with chitosan for environmental applications. In particular, we emphasize chitosan’s use for mitigating heavy metals and organic contaminants in soil and wastewater. In addition, we cover chitosan’s general properties, its limitations and prospects for chemically modifying it in ways that enhances its performance and utility for environmental applications.
2 Production and Properties of Chitosan
2.1 Production of Chitosan
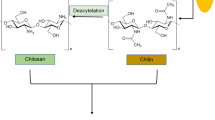
Chitosan is derived from chitin through a deacetylation process, whereby, the acetamide group is hydrolyzed to produce acetate ions and an –NH2 group (Fig. 1). Production of chitosan from raw shells involves four major steps, viz., deproteination, demineralization, bleaching and deacetylation. Chitin is produced by removing protein and calcium carbonate from the raw shells and cuticles via deproteination and demineralization processes, respectively. The amount of chemicals and reaction time needed to produce chitosan depends on the origin of the raw chitinous materials. Harsher reaction conditions are required to demineralize crab and lobster shells (due to their higher calcium carbonate content) than are needed for prawn shells or squid pen.
Crab shells are deproteinized by incubation in a solution of 1 M sodium hydroxide (NaOH) at 90 °C for 24 h, followed by demineralization in 1 M hydrochloric acid (HCl) for 24 h (Peniche et al. 2008). Usually, a complete demineralization of shrimp shells at room temperature is achieved within 15 min by using 0.25 M HCl at a liquor ratio of 40:1. The amount of HCl required for demineralization must be accurately calculated (i.e., from the calcium carbonate content of prawn shells) to minimize the hydrolysis of chitin molecules by residual HCl. The subsequent deproteination stage is relatively mild, and involves incubation in 1 M NaOH at 70 °C for 24 h. A lower NaOH concentration (0.3 M) has also been used to deproteinize various marine chitinous materials at 80–85 °C; however, using NaOH during deproteination may cause browning of chitinous materials (Rhazi et al. 2000). Carotenoids in prawn or shrimp shell may also impart an unwanted reddish color to chitin. Hence, an optional bleaching process may be added prior to deacetylation to decolorize chitin. A simple, but less effective decoloration process is to irradiate chitin with sunlight (Youn et al. 2007). Traditionally, organic solvents (e.g., acetone, ethanol, chloroform and ethyl acetate) and oxidizing agents (e.g., sodium hypochlorite and hydrogen peroxide) have been used to decolorize chitin. Although oxidizing agents are more effective than organic solvents for bleaching chitin, they may decrease the molecular weight of chitin via oxidative depolymerization.
The final step for chitosan production is deacetylation. To date, most chitin deacetylation processing has been conducted through a thermo-chemical reaction, using a 40% (wt/wt) NaOH or KOH solution at 100 °C. The degree of deacetylation of chitosan increases with increasing NaOH concentration, temperature and heating time during the deacetylation process (Methacanon et al. 2003; Santhosh et al. 2010). However, excessive use of NaOH and energy in the thermo-chemical deacetylation process makes it unsustainable due to high cost and its potential adverse impact on the environment. Sodium ions in wastewater from the thermo-chemical deacetylation process may adversely impact soil quality. In addition, the harsh conditions in the thermo-chemical process may degrade chitosan and thereby decrease its molecular weight. To reduce reaction time, temperature and consumption of NaOH, a thermo-chemical deacetylation process has been utilized that employs either microwave (Goycoolea et al. 1997), gamma ray (Tahtat et al. 2007) or ultrasound irradiation treatments (Delezuk et al. 2011). However, such methods still consume a significant amount of energy and NaOH, and may not be economically viable, given that additional energy and wastewater treatment further increase chitosan production costs.
Recently, enzymatic and microbiological fermentation processes have emerged as a promising alternative pathway to produce chitosan economically. In these, the thermo-chemical process is replaced with either enzymatic reactions or fermentation with enzyme-producing microbes (Wang et al. 2011). The latter process utilizes acidic by-products of cheese whey fermentation (i.e., lactic acid and acetic acid) as substitutes for HCl in the demineralization process (Mahmoud et al. 2007). Lactic acid bacteria have also been used to demineralize crab shells (Jung et al. 2005) and prawn shells (Rao and Stevens 2005). A protease enzyme, which is co-produced by lactic-acid-producing bacteria during fermentation, may further deproteinize chitin. Fungi (i.e., Aspergillus niger) have also been used to deproteinize chitin (Mahmoud et al. 2007). The advantages of using enzymatic and microbiological fermentation processes over the conventional thermo-chemical deacetylation process include lower energy consumption, reduced production of wastewater and reduced hydrolysis of the chitosan structure. The milder reaction conditions of the enzymatic and microbiological processes allow preservation of protein by-product, which can then be processed as a value-added food product for human (Coral-Hinostroza and Bjerkeng 2002) or animal consumption (Hirano et al. 1990).
Another small-scale chitosan production method has been employed by utilizing fungal proteolytic enzymes to demineralize and deproteinize prawn shell powder (Teng et al. 2001). Compared to the conventional chitosan production methods, this approach offers two advantages: (a) it provides an additional source of chitin from the mycelium biomass of the fungi, and (b) it utilizes protein in prawn shell waste as a source of nitrogen for fungal growth.
The enzymatic deacetylation process of chitin utilizes chitin deacetylase, which is produced by fungi (e.g., Mucor rouxii, Saccharomyces cerevisiae, etc.), marine bacteria (i.e., Vibrionaceae cholerae) and some insects. A powdered sample of chitin is treated with chitin deacetylase or is fermented with chitin-deacetylase-secreting-microbes. The enzymatic process yields chitosan that possesses a low degree of deacetylation and depolymerization (Tsigos et al. 2000). Araki and Ito (1975) reported that enzymatic deacetylation alone of glycol chitin released about 30% of acetyl groups. However, compared to the thermo-chemical process, the enzymatic deacetylation efficiency of the crystalline chitin substrate to produce partially-deacetylated-chitin is still low. Hence, further treatment of partially-deacetylated-chitin is necessary to obtain chitosan that has a high degree of deacetylation (≥60%). Moreover, the enzymatic process produces a mixture of partially-deacetylated-chitin, calcium salts and hydrolyzed protein, which needs further isolation. In reality, enzymatic deacetylation may be more practical when applied to the more water soluble amorphous chitin substrate (i.e., partially deacetylated chitin, chitin oligomers or glycol chitin). Currently, it is still not economically feasible to deacetylate chitin by using enzymatic processes (Zhao et al. 2010).
2.2 Properties of Chitosan
In general, chitin and chitosan are polysaccharides that have multiple hydroxyl (–OH) and amide (R–CO–NH2) or amine (R–NH2) functional groups. Chitin and chitosan are both co-polymers of glucosamine and N-acetylglucosamine units with varying molecular weights, depending on the extent of degradation of chitin and chitosan polymer. Chitin primarily consists of N-acetylglucosamine units with >40% amide groups, whereas chitosan is mostly glucosamine with ≥60% amine groups (Roberts 1992). The structures of chitin and chitosan are presented in Fig. 2. The physical and chemical properties of chitosan depend on its molecular weight and degree of deacetylation (Wang et al. 2006). The extensive number of –NH2 and –OH groups causes intermolecular interactions, such as hydrogen bonds and other non-specific interactions between chitosan molecules. Compared to chitin, chitosan is more soluble in acidic solution. Bulk chitosan molecules dissolve in monoprotic acids such as formic, acetic, hydrochloric and nitric acids. In acidic solution, chitosan becomes positively charged, when an –NH2 group accepts a proton to form a protonated amine group (–NH3 +). Consequently, the positively charged chitosan particles are separated through repulsive force and become suspended in aqueous acidic solution. The solubility of chitosan increases with the increase in –NH2 groups (Sannan et al. 1976). An acidic chitosan solution, with a concentration of up to 15 g/L has been successfully prepared using 0.2 M acetic acid (Barreiro-Iglesias et al. 2005).
The acidic chitosan solution can be precipitated by a phase inversion process, wherein the solution pH is increased to >6.5 by adding alkali solution (e.g., NaOH, KOH or NH4OH). The hydroxide ions deprotonate the –NH3 + group of the dissolved chitosan to form a chitosan precipitate. This technique has been applied to produce porous hydrogel (Dang et al. 2011), beads (Rorrer et al. 1993), fibers (Suzuki 1993) (1992, patent appl date), membranes (Uragami et al. 1983), and sponges (Denkbaş and Odabaşi 2000). The acidic chitosan solution can be converted to semi-solid phase through a gelation process. Chitosan ionic gel has been produced by mixing chitosan with polyprotic acids such as sulfuric and phosphoric acids. Larger polycarboxylates (e.g., oxalate, malonate, succinate, citrate, and alginate) are also used to engineer chitosan ionic gels (Hamdine et al. 2005). The ionic gelation mechanism relies on forming a strong intermolecular interaction (i.e., ionic bonds) between –NH3 + in chitosan and the anions of polyprotic acids.
Chitosan is an excellent sorbent for trace metals due to the excess of –OH and –NH2 groups that it possesses, and the high flexibility of its structure (Miretzky and Cirelli 2009). Sorption of trace metals by chitosan is selective and independent of the size and hardness of metal ions, or the physical form of chitosan (e.g., film, powder and solution) (Rhazi et al. 2002). The metal affinity sequence of the chitosan film was in the order: Cu(II) ≥ Hg(II) > Zn(II) > Cd(II) > Ni(II) > Co(II) ~ Ca (II) for divalent cations; and Eu (III) ≥ Nd (III) > Pr(III) for trivalent cations (Muzzarelli 1973). Kim (2004) reported a similar order of metal affinity sequences for chitosan: Cu(II) > Fe(II) > Zn(II) > Cd(II). Vold et al. (2003) used the selectivity coefficient, k B A (Eq) to compare binding of two different metal ions (represented by A and B) to chitosan:
where X A and X B are the mole fractions of A and B, respectively (X A + X B = 1); and C A and C B are the molar concentrations for A and B at equilibrium. Chitosan showed greater selectivity for Cu(II) with k B A values ranging from 100 to 1,000, when compared to other metals such as Zn(II), Cd(II) and Ni(II) (Vold et al. 2003). Initially, it was proposed that the interaction between metal ions and chitosan was due to ion exchange, sorption and chelation (Muzzarelli et al. 1986). However, Vold et al. (2003) suggested that the metal sorption selectivity of chitosan did not depend upon the ionic strength and pH of the solution. Sorption of uranyl ions by chitosan is independent of pH (Piron and Domard 1998), which indicates that the sorption mechanism of metal ions with chitosan is inclined to complexation, rather than electrostatic attraction. Metal ions are coordinated to the –NH2 groups of chitosan to form a pendant-like metal-chitosan complex (Lü et al. 2008). The stability of metal-chitosan complexes is further enhanced by chelation, whereby the metal ion is coordinated to two –OH groups and two –NH2 groups to form a bridge-like metal-chitosan complex (Klepka et al. 2008).
Solubilization of chitosan using strong and concentrated acid at high temperature may cause glycosidic hydrolysis (Sklyar et al. 1981), producing smaller chitosan fragments having low molecular weight (i.e., chito-oligosaccharide and glucosamine). Thermo-chemical deacetylation also decreases the average molecular weight of chitosan from 1.03 × 106–2.5 × 106 g/mol to 1 × 105–5 × 105 g/mol (Austin et al. 1981). Chito-oligosaccharide and glucosamine have low viscosity and are more soluble than chitosan at relatively neutral pH conditions. Both chito-oligosaccharide and glucosamine are bioactive (e.g., anti-microbial, anti-inflammatory, anti-oxidant, etc.) (Xia et al. 2011), and have applications in medicine (Hirano 2000), textiles (Lim and Hudson 2003) and food preservation (Dutta et al. 2009). The –NH3 + group of dissolved chitosan particles bind with the negatively charged cell walls of microbes, eventually causing leakage of their intracellular constituents (Rabea et al. 2003). However, such leakage is not of concern because chitosan is not persistent in the biosphere and is biodegraded to chito-oligosaccharide and finally to carbon dioxide (Ratajska and Boryniec 1998). In another study, an increase in the degree of deacetylation of chitosan reduced the in vivo (i.e., rats) and in vitro (i.e., egg white lysozyme) biodegradation rates of chitosan film (Tomihata and Ikada 1997).
Chitosan is non-toxic to higher organisms such as animals and humans. The median lethal dose (LD50) of orally administered chitosan for rats and mice is >1,500 mg/kg and 16,000 mg/kg, respectively. The LD50 of orally administered chito-oligosaccharide is >10,000 mg/kg for mice (Baldrick 2010). However, intravenous administration of a high-dose chitosan (50 mg/kg) may be lethal due to blood cell aggregation (Kean and Thanou 2010). Dogs injected with 200 mg/kg of chitosan suffered severe haemorrhagic pneumonia and died after 8 days (Minami et al. 1996). The oral LD50 of chitosan for humans is estimated to be 1,330 mg/kg (Prajapati 2009). Even with the administration of high oral doses (6.75 g daily per human subject for 84 days), no clinically significant adverse symptoms were observed other than mild to transitory nausea and constipation (Baldrick 2010). However, chitosan may possibly be allergenic to humans. Marine chitosan (sourced from crabs and prawns) is likely to cause allergic reactions from the presence of antigens that trigger release of immunoglobin E antibodies (IgE). However, Muzzarelli (2010) suggested that neither chitin nor chitosan is allergenic to humans. To date, there has been no direct evidence that marine chitosan products cause allergic reactions in humans. Results from two pilot studies on shrimp-derived glucosamine supplements (Gray et al. 2004) and a chitosan bandage (Waibel et al. 2011) produced no evidence of allergic reaction in human subjects. Rather, chitosan has been a popular weight management supplement for animals and humans (Walsh et al. 2013), which further suggests that chitosan is safe for environmental applications.
2.3 Chitosan Modification
Attempts have been made to chemically modify chitosan to overcome the issue of its acid instability (due to solubility). Such modification would not change the fundamental skeleton of chitosan, but rather would bring new or improved properties. Guibal et al. (1998) found that enhancing the solubility of chitosan in acidic wastewater (e.g., due to acidic mine drainage) results in low porosity, low hydraulic conductivity and low permeability that can clog pore structures in a wastewater treatment. Acidic wastewater may also cause dissolution of chitosan, resulting in a loss of mass that could lead to structural failure of the chitosan sorbent (Oshita et al. 2002). Hence, the structure and functional groups of chitosan must be optimized to enhance its stability and metal sorption capacity.
Chitosan’s instability under acidic conditions has adversely affected its practical use, particularly when using an acidic eluent to regenerate spent sorbent. Introducing crosslinks to the chitosan structure has successfully enhanced its durability in acidic environments. Crosslinking of chitosan was achieved by using various crosslinking agents, such as glutaraldehyde (GA) (Guibal et al. 1998), epichlorohydrin (ECH) (Baba et al. 1994), ethylene glycol diglycidyl ether (EGDE) (Kamari et al. 2009), and hexamethylene diisocyanate (HMDIC) (Choudhari et al. 2007) (Fig. 3; see Table 1 for a list of abbreviations and acronyms used in this paper). The enhanced durability from crosslinked chitosan was attributed to its enhanced resistance to structural changes under hydrated or acidic conditions (Berger et al. 2004). Hsien and Rorrer (1995) reported that the surface area of chitosan beads increased after crosslinking, although, crosslinking may block sorption sites (i.e., –NH2 groups) and cause a significant decrease of metal sorption capacity (Milot et al. 1998). For example, Cu (II) sorption capacity of GA-crosslinked chitosan was lower than that of non-crosslinked chitosan (Osifo et al. 2008). A crosslinking process that prevents loss of –NH2 groups in chitosan is crucial to maintain high sorption capacity for metal ions. Shin et al. (2011) used benzaldehyde to protect –NH2 groups in chitosan, prior to crosslinking with ECH. The resulting ECH crosslinks were formed at the C6 –OH group in chitosan. Subsequent treatment of the crosslinked chitosan with ethanol hydrochloride decomposed the Schiff base derivative to –NH2 groups, thereby, maintaining the –NH2 group content for metal-ion binding (Sahin et al. 2011).
The stability of complexed metals in chitosan’s structure also affects metal sorption capacity. The Hard Soft Acid Base (HSAB) theory (Pearson 1963) predicts that the –NH2 and –OH groups in chitosan are borderline and hard Lewis bases, respectively. However, any sorbed soft Lewis acids (e.g., Cd(II), Pd(II), Pt(II), etc.) may produce unstable metal-chitosan complexes that decrease the sorption capacity for these metals. To reverse this instability, chitosan has been chemically modified to introduce soft Lewis bases (e.g., sulfide, dithiocarbamate, thiourea, etc.). For example, grafting of chitosan with thiourea derivatives increased sorption capacities for Pt(II), Pd(II), Au(III) (Bratskaya et al. 2011), and Cd(II) (Yong et al. 2013). Similarly, grafting increased the number of negative charged functional groups for metal complexation. Chloroacetic acid has been used to introduce carboxymethyl groups (–CH2COOH) to –NH2 or –OH groups to increase the number of chitosan metal-binding sites (Muzzarelli and Tanfani 1982). Polyprotic acid (i.e., citric acid) has been grafted to create more negatively charged binding sites for metals to improve Pb(II) sorption capacity (Li et al. 2010).
Chitosan has been modified to enhance sorption selectivity for specific metal ions, whereby the intended metal ion is used as a template to create specific cavities in the chitosan structure. The metal templates are removed from the chitosan structure by rinsing with diluted acid solution, leaving specific cavities that are only accessible to the intended metal ions. For example, Ga(III) (Inoue et al. 1996), Cu(II) (Cao et al. 2004) and Pb(II) (Sun et al. 2006; Wang et al. 2010a) ions have been used as templates to form metal-specific chitosan sorbents for wastewater remediation. Cao et al. (2001) further introduced GA crosslinks to the structure of Cu(II)-templated chitosan resin, producing stable chitosan resins in acidic solution that also possess high sorption capacity for Cu(II), Ni(II) and Co(II) ions.
Chitosan has been blended with other materials to form composites that enhance sorption properties (Wan Ngah et al. 2011). Materials with negative surface charge are generally used in chitosan composites, not only to increase binding sites for sorption of metal ions, but also to form a stable ionic composite with the –NH3 + group of chitosan. Alginic acid was blended with chitosan and then crosslinked with GA to produce sorbent beads (Gotoh et al. 2004). Chitosan was also used to coat a montmorillonite clay sorbent for remediation of tungsten (W) from drinking water (Gecol et al. 2006).
3 Chitosan Applications
Chitosan is generally regarded as a non-toxic and biocompatible material for humans and animals, and has been widely used in the fields of agriculture, pharmaceuticals, and industrial food processing. Chitosan has also been used for several environmental applications, including remediation of both organic (e.g., colored dyes, residual oil/solid from palm oil mill effluent) and inorganic contaminants (e.g., toxic trace metal(loid)s) from wastewater and soil. However, there are several challenges when using chitosan for environmental applications, particularly in terms of maintaining its stability and efficacy for remediating contaminated wastewater and soil.
3.1 Remediation of Inorganic Contaminants in Aqueous Systems
Chitosan has been evaluated for remediating heavy metals, such as Cu(II) (Ng et al. 2002), Pb(II) (Ng et al. 2003), Hg(II) (Shafaei et al. 2007), Cd(II) (Rorrer et al. 1993) and Zn(II) (Karthikeyan et al. 2004). It is regarded as one of the best natural chelators for trace metals (Chui et al. 1996). The chitosan molecule is sufficiently flexible to form a helical structure around metal ions (Ogawa et al. 1993), which forms multiple coordination bonds with each ion (Wu et al. 2010). Metal sorption can be augmented by physically modifying the chitosan sorbent. For example, a phase inversion process can be used to produce porous chitosan beads, which may lead to increased metal sorption capacity, possibly from increased surface area (Rorrer et al. 1993).
Sorption of metal ions by chitosan is usually optimum at pH values higher than chitosan’s pKa value (>6.5) (Guzman et al. 2002; Navarro et al. 2003). A high sorption pH value (<6.5) induces deprotonation of –NH3 + to –NH2 groups, thus enabling coordination of metal cations and increasing sorption capacity (Jeon and Höll 2003). Physical optimization of chitosan has also been conducted by mixing it with negatively charged substances. Qi and Xu (2004) precipitated chitosan-tripolyphosphate composite nanoparticles (CS-TPP) (mean sizes = 40–100 nm) from chitosan hydroacetate solution using aqueous sodium tripolyphosphate solution. The CS-TPP was found to have high Pb(II) sorption capacity (398 mg/g), owing to the low crystalinity of CS-TPP and the presence of the TPP ion (Qi and Xu 2004).
Chitosan has been used to economically remediate wastewater resulting from electroplating activities (Coughlin et al. 2006), and to recover precious metals from mining wastewater (Benavente et al. 2011). The abundance of –NH2 groups in chitosan allows it to successfully remediate toxic metal oxyanions (e.g., chromate and arsenate) from wastewater. Metal oxyanions are adsorbed to –NH3 + ions on chitosan through an ion exchange mechanism (Navarro et al. 2003), although the arsenate ion sorption from aqueous systems using chitosan beads was low (<12 mg/g) (Chassary et al. 2004). Mixing chitosan with montmorillonite (Assaad et al. 2007) and alginate (Wan Ngah and Fatinathan 2010) enhanced sorption of metal ions from wastewater. The sorption mechanism involved metal chelation by –NH2 groups, as well as coordination/coagulation of metal ions by polar groups of montmorillonite (i.e., silanol and aluminol) and alginate (i.e., carboxylate) (Assaad et al. 2007; Qi and Xu 2004; Wan Ngah and Fatinathan 2010).
Modifying chitosan with molybdate ions enhanced the sorption capacity of arsenate ions from complexation of arsenate ions to the modified chitosan (Dambies et al. 2002). Similarly, composites of Fe(III) and chitosan were synthesized to enhance sorption of arsenate ions (Gupta et al. 2009; Gupta and Sankararamakrishnan 2010). Pulverized Fe(III)-chitosan nanocomposite was chemically reduced to produce zero-valent iron, which further enhanced sorption of arsenate ions. Arsenate ions were reduced by zero-valent iron to arsenite ions, and were subsequently complexed by the oxidized iron (Fe(III)) (Gupta et al. 2012). Similarly, remediation of chromate-contaminated wastewater may also involve a reduction mechanism. At low pH (4.5), sorbed chromate ions may be partially reduced by chitosan (Dambies et al. 2001). The reduction of chromate ions was possible because of their high reduction potential (E o = (−1.33 V) under acidic conditions.
These observations have inspired numerous chitosan modifications, specifically designed to reduce chromate ions in wastewater, such as introduction of xanthate to chitosan (Sankararamakrishnan et al. 2006), synthesis of chitosan nanoparticles with zero-valent iron (Geng et al. 2009a, b) and entrapment of zero-valent iron nanoparticles in chitosan beads (Liu et al. 2010). GA-crosslinked chitosan also enhanced reduction of chromate ions, possibly from the presence of free aldehyde groups of GA (Vieira et al. 2011). The reduction process helps alleviate the pollution impact by converting toxic Cr(VI) ions to relatively less toxic Cr(III) ions.
Chitosan has also been used to remediate water resources contaminated with non-metal anions (i.e., nitrates, nitrites, phosphates and fluoride). Similar to the mechanism by which nitrate and orthophosphate are sorbed, the –NH3 + ions in chitosan also sorb fluoride ions. Such sorption would be enhanced at low pH, where the amount of –NH3 + groups is high (Chatterjee and Woo 2009). Even though acidic conditions increase the amount of –NH3 + groups, fluoride ions may form hydrofluoride vapor at pH levels <5, which would decrease fluoride ion sorption from wastewater (Miretzky and Cirelli 2011). However, as chitosan is physically unstable at low pH values, sorbent made from chitosan would normally be stabilized by treatment with a crosslinking agent (e.g., GA (Jaafari et al. 2004) or ECH (Chatterjee et al. 2009)). Huang et al. (2012) demonstrated that the protonated form of the GA-crosslinked chitosan particle (CCP) enhanced fluoride ion sorption capacity and stability of chitosan as compared to the deprotonated form of GA-crosslinked chitosan. The enhanced stability of CCP in acid enables regeneration using a 0.1 M HCl solution (Huang et al. 2012). Lanthanum ion (La(III)) has also been chelated to the –NH2 groups of chitosan flakes (Rayalu et al. 2007) and chitosan beads (Bansiwal et al. 2009) that successfully enhanced fluoride sorption.
Chitosan has been used to immobilize symbiotic microorganisms for bioremediation of orthophosphate and nitrate ions. For example, chitosan was utilized to form aggregates with microalgae Phormidium (Chitosan: Phormidium = 1:2, dry wt basis) to remove 98% of nitrogenous compounds and 88% of phosphate compounds from a secondary effluent during a 24 h period (De La Noue and Proulx 1988). The reduction of orthophosphate and nitrate ion levels in effluent resulted from direct assimilation of orthophosphate and inorganic nitrogen ions by Phormidium, while the presence of chitosan protected the algae from abrasion.
3.2 Remediation of Metal-Contaminated Soils and Sediments
Remediation methods for metal-contaminated soils can be roughly classified into three types: physical, chemical and biological (including phytoremediation) (Zhou and Song 2004). Metal-contaminated soil can be remediated with chitosan either by (1) active removal of metal ions from the contaminated soil through extraction of metal complexes, or (2) immobilization of metal ions (Peng et al. 2009). Generally, treatment of contaminated soil with organic matter (i.e., chitin and chitosan) helps immobilize metals by precipitation, sorption or complexation (Mench et al. 1994; Park et al. 2011). Addition of acid-dissolved, positively charged chitosan changes the physicochemical properties of soil, eventually immobilizing heavy metals therein. Chitosan-coated sand, which had been pre-treated with NaOH, improved the sorption capacity of Cu(II) and reduced its leachability (Wan et al. 2004). This shows that the metal-retention properties of chitosan is only significant when the –NH2 group is not protonated. Conversely, in another study, a sand-packed column had low Cu(II) sorption capacity and high leachable Cu(II) content with the addition of an acidic chitosan solution (Etemadi et al. 2003). Bulk density, cationic exchange capacity (CEC) and pH decreased when an acidic chitosan solution was added to soil, whereas the electrical conductivity (EC) increased (Hu et al. 2006). This appears to be a more realistic scenario, given that Cu and other metal cations tend to sorb onto negative charged surfaces (Srivastava et al. 2005).
Chitosan has been used to treat metal-containing leachate after active remediation of contaminated soil. Bassi et al. (2000) used a citric acid solution as a complexing agent to leach metal ions from contaminated soil. The leachate was then treated with chitosan flakes to remove metal ions. Other complexing agents such as ethylenediamine tetra acetic acid (EDTA), diethylene triamine pentaacetic acid (DTPA) and nitrilotriacetic acid (NTA) were shown to effectively mobilize metal ions from contaminated soil (Barona et al. 1999; Barona and Romero 1996; Elliott and Brown 1989; Saifullah et al. 2010). However, NTA has been reported as a potential carcinogen to humans (Pohanish 2008). Although EDTA and DTPA are themselves relatively non-toxic, their use may cause poisoning by making toxic metals more bioavailable (Barona et al. 2001). As previously mentioned, chitosan is non-toxic to plants, animals and humans (Baldrick 2010; Kean and Thanou 2010), and is therefore a safe complexing agent from the health and safety perspective. Low molecular weight chitosans (i.e., chito-oligosaccharide and glucosamine) produced by acidic hydrolysis or oxidation of chitosan are safe complexing agents for active remediation of contaminated soil. Although chito-oligosaccharide and glucosamine hydrochloride were inferior to EDTA and l-asparaginic-N, N-diacetic acid (ASDA), in terms of their ability to leach Cu(II) ions from vermiculite (Guo and Inoue 2003), they are non-toxic and do not adversely impact the environment. Chitosan has also been used to extract Cd(II) (Li et al. 2008) and Cr(VI) ions (Li et al. 2009) from contaminated soils. Guo et al. (2009) observed enhanced extraction of Cu(II) and Cd(II) ions with chitosan hydrochloride solution in subsurface soil (14–16 and 24–26 cm) after a 7–14 days incubation period. Cd(II) was better extracted than Cu(II), confirming the high affinity of chitosan for Cu(II) ions (Guo et al. 2009).
Chitosan was used in a permeable reactive wall (Liao et al. 2010) (2009, patent appl date) to remediate metal ions in soil. Chitosan lixiviant liquid was pumped into the soil to mobilize toxic metal ions from the contaminated soil, which were then removed by the permeable reactive wall. Table 2 shows examples of chitosan and its derivatives being used as lixiviants for metal mobilization, thereby demonstrating that they augment metal sorption by plants during phytoremediation of contaminated soil. Monomeric chitosan (glucosamine) forms metal complexes that increased the availability of metal ions for plant absorption. Addition of chitosan to soil at pH 2 increased desorption of Pb(II) (Liu et al. 2006a), and led to enhanced uptake of Pb(II) by corn (Zea mays L.) from contaminated soil (Liu et al. 2006b). With the addition of chitosan, the Pb(II) concentration in plant roots was threefold higher than in the control (without chitosan) (Liu et al. 2006b). Similarly, adding chitosan and ethylene diamine disuccinate (EDDS) helped Elsholtzia splendens accumulate Cu(II) and Cd(II) (Weng et al. 2005). Adding chitosan to microbial inocula (Arbuscular mycorrhizal fungi and Penicillium sp.) was found to synergistically increase Zn(II), Pb(II) and Cd(II) accumulation in the shoots of E. splendens. Weng et al. (2005) attributed this synergistic effect to the mobilization of metal ions by complexation with chitosan. Water-soluble metal-chitosan complexes enabled greater plant absorption, possibly from increased partitioning of metal ions to the shoot tissues of the E. splendens plants (Wang et al. 2007).
Modified chitosan has been shown to improve extraction and phytoremediation of metal ions from contaminated soil (Yang et al. 2006). A thiol group was introduced to the –NH2 groups in chitosan to produce thiol-modified chitosan (SCTA-I), which enhanced Pb(II) uptake in corn roots and shoots from a Pb-contaminated soil. The enhanced absorption of Pb(II) possibly resulted from the greater ability of SCTA to extract Pb(II) from soil, compared to citric acid and chitosan (Yang et al. 2006).
Chitosan and its crosslinked derivatives have been used as amendments for immobilizing metals in contaminated soil. The solid form of chitosan can be applied to adsorb metal ions from moist soil, thereby reducing the availability of toxic metal ions to living organisms. Nonetheless, using chitosan alone may not be fully effective for remediating contaminated soil, because of its low affinity for metal ions (i.e., Cd(II), Zn(II), etc.). This issue may be overcome by blending chitosan with materials having different functional groups to form a composite that augments its activity. For example, the chitosan-apatite composite more strongly retains a wide range of metal ions (Knox et al. 2008).
As described previously, stability is a potential issue, when using chitosan to remediate metal ions. Natural degradation may break chitosan down to chito-oligosaccharides (Kwon et al. 2010), and possibly release immobilized metal ions into the environment. Hence, chitosan that employs a crosslinking process may be essential for modulating natural degradation processes that involve chitosan. Kamari et al. (2011a) reported the remediation of metal contaminated soil by using both chitosan and crosslinked chitosan as soil amendments. In these studies, ECH, GA and EDGE were used for crosslinking, and each of these treatments increased the surface area of chitosan. Leaching of the adsorbed metal ions was lower with crosslinked chitosan than for pristine chitosan (Kamari et al. 2011b). Kamari et al. (2012) further reported that the low application rate of chitosan (at 1% wt/wt) enhanced metal sorption by plants, possibly by forming water-soluble metal-chitosan complexes. However, a high application rate of chitosan (at 10% wt/wt) decreased metal sorption by perennial ryegrass (Lolium perenne). The metal sorbed by rapeseed (Brassica napus) was also decreased from the chitosan amendment, regardless of application rate. High application rates of chitosan or crosslinked chitosan amendments may inhibit formation of the water-soluble metal-chitosan complex, thereby reducing metal sorption by plants.
Microbes have been used with chitosan to improve removal of metal ions from contaminated soil. Cuero (1996) enhanced the removal of Zn(II) and Cu(II) ions from contaminated soil by using a mixture of bacteria (Bacillus subtilis) and chitosan in solution. Ideally, microbes immobilized with chitosan show greater stability, remediation efficiency, and regenerability (de-Bashan LE and Bashan 2010). A composite that consists of chitosan, alginate and polyvinyl alcohol was crosslinked with ECH to immobilize inactive Saccharomycetes pombe 806 yeast biomass for sorbing Cd(II) from soil leachate (Lin and Lin 2005). The concentration of Cd(II) in soil leachate decreased from 3.91 to 0.269 mg/L, when using 0.3 g of the yeast-immobilized-chitosan beads.
3.3 Remediation of Organic Contaminants
Soil and wastewater that are contaminated with organic contaminants can be remediated with chitosan, although the success is somewhat dependent on the characteristics of the contaminants. In Table 3, we summarize studies in which chitosan and its derivatives have been applied as a sorbent to remove organic contaminants from wastewater. Chitosan is known to have a high capacity for sorbing oily contaminants (Yong and Wong 2013). Because chitosan is positively-charged at low pH values, it may be an effective coagulant for anionic organic contaminants such as dyes, tannins and humic acids. Recently, toxic organic chemicals (e.g., phenol and bisphenol A (BPA)) have been remediated by using chitosan incorporated with enzymes (Suzuki et al. 2010), clay (Fan et al. 2007) and Cu(II) hydroxide nanoparticles (Jaiswal et al. 2012).
Oily wastewater (e.g., palm oil mill effluent (POME) and vegetable oil mill effluent (VOME)) causes serious environmental problems. POME is a thick brownish colloidal suspension that contains 95–96% water, 0.6–0.7% oil and grease, 4–5% total solids and pH ranging from 4.0 to 5.0 (Rupani et al. 2010). Chitosan may act as a non-toxic coagulant (Ahmad et al. 2006) and sorbent (Ahmad et al. 2005) of residual oil and suspended solids from POME. Ahmad et al. (2006) found that chitosan performed more efficiently and economically than did other coagulants (e.g., aluminum sulfate (alum) and polyaluminum chloride (PAC)) and sorbents (e.g., bentonite and activated carbon).
Toxic p-benzoquinone in treated drinking water is a potential threat to human health (Richardson and Postigo 2012). It is produced from chlorination of acetaminophen in wastewater (Bedner and MacCrehan 2005). Chitosan has been used to detoxify p-benzoquinone in aqueous systems (Park et al. 2000). The non-enzymatic reaction between p-benzoquinone and d-glucosamine (a monomer of chitosan) occurred at an optimum pH (>7.4) and temperature (35 °C), in which free –NH2 groups in chitosan formed covalent bonds with the p-benzoquinone.
Chitosan has been evaluated for remediating anionic organic compounds such as humic substances (Bratskaya et al. 2004) and humate-metal complexes from wastewater (Yan and Bai 2005). Humic substances are widespread natural polymers that (a) adversely affect the aesthetics of waterways; (b) enhance the bioavailability of metal ions by forming metal-humate complexes; and (c) produce carcinogenic by-products with chlorine (e.g., trihalomethanes). Compared to alum, chitosan is more effective and eco-friendly in reducing the chemical oxygen demand (COD) of wastewater that is generated from the food processing industry (Muzzarelli and Muzzarelli 2003). At low pH, the sorption capacity of chitosan for humate decreases by about 25%. The –NH3 + group in chitin and chitosan is responsible for forming ionic complexes with the negatively charged humate ions (Wan Ngah and Musa 1998). In addition to ionic forces, van der Waals forces contribute to chitosan sorption of tannin and humate ions.
Chitosan composites have been synthesized, with the aim of enhancing its sorption capacity for organic contaminants. Blending chitosan (240 g/kg at pH 4) with montmorillonite increased its CEC and capacity to sorb tannic acid (An and Dultz 2007). Recently, a chitosan-montmorillonite nanocomposite was produced for sorbing anionic herbicide clopyralid from wastewater and soil-water suspension (Celis et al. 2012). High sorption capacity for clopyralid occurred at low pH, because of its abundant –NH3 + ions in the chitosan-montmorillonite nanocomposite. Desorption of clopyralid increased with higher salt concentrations, indicating a cation exchange sorption mechanism (Celis et al. 2012). Apart from clay, Cu(II) hydroxide has been blended with chitosan to produce CuCH nanocomposites, for remediation of organophosphorous insecticides (i.e., parathion and methyl parathion) in agricultural runoff (Jaiswal et al. 2012). Unlike the chitosan-montmorillonite nanocomposite, sorption of malathion onto a CuCH nanocomposite occurred through complexation. Chitosan-alginate micro-shells have been synthesized by assembling layers of alginate and chitosan, for decontaminating 2,4-dichlorophenol (DCP) and salicylic acid (SA) in wastewater. The chitosan-alginate micro-shells have enhanced sorption capacity, and have a shorter sorption time for DCP and SA, than do poly-styrenesulfonic acid (PSS) and poly(allylamine hydrochloride) shells (Ding et al. 2009).
BPA is used to harden polycarbonate plastics and epoxy resins. Its presence in waterways may impact normal functions of endocrine hormone in fishes and humans. Chitosan and its derivatives have been used as a sorbent for BPA and its enzymatic oxidation by-products (i.e., quinones). Chitosan is more effective in the bead form than in solution or in powder form, when used to sorb quinones from treated wastewater (Kimura et al. 2012; Suzuki et al. 2010). Sorption of BPA and trichlorophenol (TCP) from wastewater has been investigated using magnetic chitosan-fly ash composite (Pan et al. 2011). Spent sorbent could be recovered by applying magnetic field to the suspension of treated wastewater. In addition, α-ketoglutaric acid has been blended with chitosan to produce composite resins that are useful for sorbing BPA from wastewater (Gong et al. 2010).
Remediating dyes in textile wastewater is vital because of their adverse effects on human and aquatic life (Chequer et al. 2013). The presence of –NH3 + groups in chitosan makes it suitable for remediating dye-containing wastewater. The auxochrome groups that consist of acidic (–COOH, –OH and –SO3H) or basic functional groups (–NH–, >N–, –NH2) augment the color intensity of a dye molecule (Nidheesh et al. 2013). The presence of acidic auxochromes increases the negative charge of the dye molecules, enabling ionic interactions with the –NH3 + groups in chitosan. Chitosan or its derivatives have mainly been applied to remediate acidic or acid-azoic dyes that contain sulfonate groups (–SO3 −) (Table 4). Chitosan has also been used for sorbing congo red (Chatterjee et al. 2007), reactive black 5 (Guibal et al. 2005), reactive red 222 (Wu et al. 2000), direct scarlet B (Annadurai 2000), acid blue 161 (Aksu et al. 2008), acid black 1 and acid violet 5 (Szyguła et al. 2008), acid orange 10, acid red 18, acid red 73 and acid green 25 (Cheung et al. 2007) dyes. In addition, chitosan has been used to immobilize TiO2 photocatalyst for photo oxidation of dye molecules in wastewater (Zainal et al. 2009).
High molecular weight chitosan may be used as a coagulant to complement the action of conventional aluminum and iron coagulants (Guibal et al. 2006). Dye remediation by chitosan relies on surface charge neutralization of dye molecules. The –SO3 − groups in the acidic dye are attracted to –NH3 + groups in chitosan molecule through an electrostatic interaction (Chiou and Li 2003). Moreover, negatively-charged-acid-dyes may be attracted to several –NH3 + groups in chitosan molecule. The so-called bridging mechanism causes coagulation from the increased molecular weight of dye molecules (Guibal and Roussy 2007).
Attempts have been made to sorb basic dye (methylene blue) by using chitosan (Annadurai 2002). Although increasing sorption temperature and pH augmented the capacity of chitosan to sorb methylene blue (Annadurai 2002), the interaction between the basic dye and chitosan might not be sustained because of their similar functional groups. Chitosan and basic dye have primary (–NH2) and tertiary amine groups (>N–), respectively. To enhance the sorption of basic dyes, negatively charged functional groups (i.e., –COOH) have been grafted onto –NH2 and/or –OH groups in chitosan molecules. Grafting of the chitosan molecule with chloroacetic acids has produced monocarboxymethylated chitosan (MCM-chitosan) (Uzun and Güzel 2004), and N, O-carboxymethyl-chitosan (N, O-CMC) (Wang and Wang 2008). Uzun and Güzel (2004) reported an increase in sorption percentage of MCM-chitosan for acid orange 7 (99%) compared to that of chitosan (12%). Wang and Wang (2008) reported higher sorption capacity of N, O-CMC for congo red dye (331 mg/g) than that of chitosan (79 mg/g). The augmented sorption capacity for congo red was attributed to the complexation of dye molecule by –COO−, –NH2 and –OH groups in N, O-CMC (Wang and Wang 2008).
As previously mentioned, chitosan is structurally unstable under acidic conditions and hence its application as a sorbent is problematic, when remediating dye-containing acidic wastewaters. A crosslinking process is required to prevent the solubilization of chitosan sorbent in acidic dye wastewater (see Chitosan Modification). Permanent crosslinks have been introduced to chitosan using EDGE (Kamari et al. 2009) and ECH (Chiou and Li 2003). Non-permanent, ionic crosslinks may also enhance the stability of chitosan in acid, which is formed when chitosan reacts with divalent anions (sulfate) (Xu et al. 2008) or polyvalent anions (citrate) (Trung et al. 2003). Addition of ionic crosslinks also increased the amorphicity of chitosan, thereby contributing to higher sorption capacity for anionic dyes.
Spent chitosan sorbent is regenerated by treatment with alkaline solutions. A decrease in the sorption capacity of chitosan for dye occurs as pH increases. About 20% of dye molecules were desorbed from the chitosan beads when the solution pH was increased to 12 (Chatterjee et al. 2007). Chitosan crosslinked with ECH had greater sorption capacity than GA- and EDGE-crosslinks (Chiou et al. 2003; Chiou and Li 2003). Crosslinked chitosans have been studied for sorption of metanil yellow, reactive blue 15 (Chiou and Chuang 2006); reactive red 2, acid red 14, reactive yellow 2, reactive yellow 86, reactive blue 2, direct red 81, acid orange 7, acid orange 12 (Chiou et al. 2004); reactive black 5 (Guibal et al. 2006); acid blue 25, acid red 37 (Kamari et al. 2009); acid red 18, acid red 73 (Cheung et al. 2007) and acid red 87 (Chatterjee et al. 2005; Du et al. 2008). Crosslinked chitosan may be used in the permeable reactive barriers (PRBs) for remediating dyes in soil (Lazaridis and Keenan 2010). Chitosan beads crosslinked with GA and inoculated with TPP have a greater affinity and retardation factor for reactive black 5 as compared to soil.
Although crosslinking is likely to decrease the content of –NH3 + groups in chitosan’s structure, its sorption capacity for dye may not be decreased, because crosslinked chitosan has an augmented dye sorption capacity, as compared to non-crosslinked chitosan (Chiou et al. 2004). Parameters such as low pH may enhance sorption capacity for dye molecules. Certain interactions between chitosan and dye molecules may also affect dye sorption capacity of chitosan. For instance, dyes interact with high molecular weight chitosan via coagulation mechanisms involving charge neutralization and bridging effects (Szyguła et al. 2008). Remediation of large, planar direct dye molecules may involve van der Waals forces (Mazengarb and Roberts 2009). Sorption of reactive dyes may occur with the C6 –OH group in chitosan (Shukla and Pai 2005). The stability of hydrogen bonds between the dye and the chitosan molecule also depends on the polarity of –NH2 groups, which may be influenced by its spatial arrangement (e.g., –NH2, >N–, –N=) (Ikeda et al. 2008). Sorption degree of dyes is affected by surface and pore diffusion processes in the porous chitosan (Cheung et al. 2007). Dye molecules may form aggregates, even at low dye concentrations, thereby, decreasing sorption capacity of porous chitosan (Walker and Weatherley 2001).
Chitosan and its derivatives have an indirect role in bioremediation of organic contaminants in soil and aqueous systems. Chitosan may be used as (a) an immobilizing material for microbes or enzymes, or (b) a solubilizing agent of organic contaminants. Crosslinked chitosan can also be used as a sole immobilizing material. Other materials have been blended with chitosan to form composites for immobilizing reactive microbes or enzymes (Table 5). For example, fungal tyrosinase enzyme (TYR) has been immobilized using chitosan (Yamada et al. 2005) and chitosan-SiO2 gel (Shao et al. 2007) to remediate phenol compounds in wastewater. TYR catalyzes the oxidation of phenol to o-quinones, which are then adsorbed by chitosan (Ikehata and Nicell 2008). The sorption of o-quinones by chitosan is important if inactivation of TYR is to be avoided. When avoided, TYR can be reused for further remediation of wastewater. Other polymeric materials such as polyvinyl alcohol have also been used together with chitosan in the immobilization of the laccase enzyme to remediate 2,4-dichlorophenol (DCP) (Xu et al. 2013).
Chitosan has been used to immobilize reactive microbes, such as Sphingobium sp. P2 (Khondee et al. 2012) and Rhodococcus corynebacterioides (Gentili et al. 2006), for degrading lubricants and crude oils in water, respectively. Another bacteria, Mycobacterium frederiksbergense was immobilized in chitosan, and was coated onto alginate-polyvinyl alcohol beads for degrading pyrene in silicone oil (Sarma et al. 2011). Furthermore, chitosan may be blended with negatively charged biopolymers, such as carrageenan for immobilizing Pseudomonas putida (NICM 2174) (Jianlong and Yi 1999), alginate for immobilizing a variety of microbes (i.e., Pseudomonas, Bacillus, Zoogloea, Micrococcus, etc.) (Si et al. 2010) (2009, patent appl date), and carboxymethylated biopolymers (i.e., carboxymethyl cellulose and carboxymethyl chitosan) for immobilizing phosphate-solubilizing bacteria (i.e., Bacillus, Pseudomonas, Rhizobium or Agrobacterium), Azospirillum brasilense and Alcaligenes faecalis (Zhang et al. 2011) (2009, patent appl date).
Chitosan is also useful for remediating oil-contaminated soils or seawater by facilitating microbial degradation of oil contaminants. Oily, non-water-soluble contaminants are sorbed and concentrated in chitosan, improving interaction with microbes for effective degradation. Addition of chitin and chitosan significantly reduced degradation time for heavy oils in seawater by Pseudomonas sp. (Setti et al. 1999). The efficiency of chitin and chitosan in the remediation of oil-contaminated beach sediment was compared with a slow-releasing fertilizer (Osmocote). Even though chitosan alone has higher oil sorption capacity (2.2 g/g) than chitin (0.24 g/g), it requires a nutrient supply to induce biodegradation by microbes. Addition of chitosan has increased biodegradation of polyaromatic hydrocarbon (PAH) compounds, possibly from higher availability of PAH to the microbial biomass (Xu et al. 2005).
3.4 Chitosan-Based Sensors
A sensor is a device that provides analytical information about an analyte (e.g., identifying or characterizing the analyte and its concentration) by detecting signals generated from a chemical and/or a biological reaction (Hulanicki et al. 1991). These signals are usually measured by using optical (e.g., absorbance, reflectance or fluorescence spectrophotometry) (Fan et al. 2008), or electrochemical detectors (amperometry, voltametry, conductimetry and potentiometry) (Hanrahan et al. 2004). An amperometric sensor is capable of rapid, real-time detection of contaminants, as compared to colorimetric and spectrophotometric-based sensors (Brett 2001). Incorporating sensing elements in the amperometric sensor enhances detection sensitivity and selectivity for analytes, with greater reproducibility of results (Du et al. 2007c).
Chitosan and its derivatives are mostly biocompatible, making them suitable for immobilizing sensing elements, such as enzymes (e.g., TYR, acetylcholinesterase (AChE), horseradish peroxidise (HRP), organophosphorus hydrolase (OPH), and alkaline phosphatase (ALP)), and nanoparticles (e.g., gold and Fe3O4). Immobization of sensing elements may include a crosslinking process to (a) prevent the loss of incorporated sensing elements, and (b) stabilize metallic/magnetic materials in the sensors (Radhakumary and Sreenivasan 2012; Sugunan et al. 2005) (Table 6). Although GA has been used in crosslinking processes, it is toxic to all living organisms and may decrease the sensitivity of sensors by denaturing the immobilized enzyme (Leung 2001). Non-toxic chemicals have been used to replace GA in the crosslinking process. These chemicals include organic polyprotic anions (e.g., alginate and carageenan) or clays (e.g., layered double hydroxide and laponite) (Fan et al. 2007). Co-immobilization of chitosan and polyprotic anion improved the ionic interactions of –NH3 + groups and negative surfaces of polyanions, thereby, improving the mechanical strength of the host matrix for enzymes (Han et al. 2007). Chitosan is also used as a ‘capping’ agent to stabilize and reduce gold nanoparticles in chemosensors (Radhakumary and Sreenivasan 2012; Sugunan et al. 2005).
Chitosan has been used as a sensing element in chemosensors for detecting metals (Table 6). The –NH2 and –OH groups in the chitosan molecule form complexes with metal-based analytes (Wu et al. 2010). The affinities of metal ions to chitosan are different, depending on the presence of soft, borderline, or hard Lewis bases of pristine or modified chitosan. Thin- film chitosan composites have been modified with polyallylamine, increasing the –NH2 group content in thin film for qualitative detection of metal ions in wastewater (Schauer et al. 2004). Cathell et al. (2008) produced a colorimetric sensor containing thiol groups to chitosan film to enhance the selectivity for Hg(II) ions. Complexation of Hg(II) ions by thiolated groups changes the thickness, refractive index and color of the chitosan film. These optical changes were measured to identify and quantify Hg(II) ions in wastewater (Cathell et al. 2008). The thiolated sensor film may detect other metal ions (in the d-block of the periodic table) that form color complexes with the thiol groups.
Surface plasmon resonance (SPR) is a method commonly applied in colorimetric sensors to measure the monolayer thicknesses of adsorbed contaminant molecules on a conducting metal surface (typically gold or silver) (Eustis and El-Sayed 2006). The principle of SPR is based on the oscillation of charge density arising from the interaction of incidental light with the metal surface. Chitosan-based SPR sensor consists of a thin layer of chitosan, coated onto the adjacent metal surface. Sorption of metal ions by chitosan changes the refractive index of the coated metal surface, and is measured to determine the concentration of metal ions (Yu et al. 2004). Chitosan has been used in the SPR sensor to enhance sensitivity for Hg(II) and Pb(II) (Abdi et al. 2011); Fe(III) (McIlwee et al. 2008); Cu(II), Zn(II) and Mn(II) (Fen et al. 2011); and Cu(II) ions (Lin et al. 2012). However, chitosan is not suitable for detecting other metal(loid) complexes (e.g., Al(III), As(III) and As(VI), etc.) due to the colorless nature of most of the metal(loid)-amine complexes. To overcome this problem, a fluorescent dye is grafted onto the sensor surface to augment signals of the colorimetric detectors. Rhodamine (Meng et al. 2012) and dansyl chloride (Wang et al. 2012) were grafted onto chitosan films to enhance detection of Hg(II) ions. Alternatively, introduction of a thiol group could increase chemisorption of Hg(II) ions to a chitosan sensor, where signal was measured using anodic stripping voltametry (Deng et al. 2010).
A chitosan film has been studied for detecting low level catechol in wastewater (1 mM); the film has a detection limit of about 0.2 mM for a 10 min reaction time (Dykstra et al. 2009). Although chitosan has not yet been applied successfully in a commercial setting for organic contaminant sensing, it has great potential as a molecular-imprinted sensor for detecting specific contaminants. This initial success may pave the way for developing a cheap, enzyme-free sensor that is commercially cost effective.
4 Conclusions and Future Research
We have demonstrated in this review that chitosan and its derivatives show promise for various environmental applications. Such applications range from remediation of organic and inorganic contaminants to development of sensors for soil and water contaminates. The prospects for commercial use of chitosan and its derivatives have grown significantly as their versatility and biodegradability have been enhanced. However, chitosan’s cost effectiveness in comparison to other biomaterials is questionable because processing it for use requires substantial amounts of high-grade alkali. Unless economical and reliable processing and production methods are developed, use of chitosan for environmental applications will remain commercially unviable. Enzymatic or microbial methods for chitosan processing appear to be economic; however, further work is required to demonstrate their commercial economic viability.
Alternative raw materials such as fungi and insects have been used for chitosan production to overcome seasonal availability of the major source (i.e., seafood-derived chitins). Fungal mycelium is a significant chitin source due to the growing consumption of mushrooms worldwide. The mycelium-containing spent mushroom waste may be enzymatically processed to produce more affordable, low-grade chitosan for large-scale environment applications (Tsigos et al. 2000). However, more research is needed to determine the feasibility of using low-grade fungi-derived chitosan for environmental applications.
Application of chitosan in acidic environments has been challenged by its high solubility, and low structural stability. This limitation can be overcome by modifying chitosan’s structure via crosslinking. Such modifications not only improve the stability of chitosan, but also enhances its physical characteristics, such as surface area, porosity, hydraulic conductivity and permeability, etc.. Because chitosan predominantly contains positively charged groups at low pH, certain crosslinks also introduce negative charges that enhance chitosan’s sorption capacity for metal cations. However, the sorption capacity of modified chitosan varies with the type of crosslink and methodologies used for crosslinking. Further research is required to ascertain what types of crosslinking agents best enhance sorption capacities for various cations.
Chitosan has been used to remediate a vast array of contaminants. Heavy metals, for example, have been remediated by using pure chitosan and modified chitosans, and by combining chitosan with microorganisms. Low molecular weight chitosan derivatives have been effective in enhancing phytoremediation of certain heavy metals. However, the mechanism by which plant uptake of metals is enhanced in the presence chitosan is not clear, and must be clarified by performing additional research. Similarly, chitosan and its derivatives have been effective for remediating anionic organic contaminants such as dyes, tannins and humic acids, due to the presence of having positive surface charges at low pH. Other organic contaminants, such as phenol, bisphenol-A, PCBs, pharmaceuticals, p-benzoquinone, oil-based wastewater, and herbicides have been remediated by using chitosan. Chitosan has the potential to remediate many other organic contaminants, and discovering such contaminants will be an interesting topic for further research.
Organo-clays have been effective in remediating both organic and inorganic contaminants. Chitosan has been blended with some clay minerals such as montmorillonite to enhance the surface area for sorbing both organic and inorganic contaminants. However, limited research has been conducted in this area, and a great potential exists for synthesis of sorbents made up of different organo-clays and chitosan.
Recently, incorporation of chitosan in nano zero valent iron (nZVI) particles has been attempted to form chitosan nano-composites, which have subsequently been used to oxidize certain organic contaminants. However, the production cost of such nano-composites is high. Hence, these composits must be reusable, which has been successfully achieved by incorporating magnetic nanoparticles. As nZVI is increasingly being used in contaminant remediation activities, more research is required to study the suitability of chitosan for minimizing aggregation and oxidation of these nanoparticles. Moreover, little is known about the ecological and human health effects of these new nanoparticles, so the need to acquire safety data must also be met.
Chitosan has been suitable for use in developing sensors for many metals (e.g., Cu, Fe, Hg, Mn, Pb and Zn), organic compounds (e.g., enzymes) and nanoparticles (e.g., Au and Fe) by using immobilization and crosslinking processes. Chitosan could not be successfully applied as a sensor to detect metalloids such as Al(III), As(III) and As(VI), etc. because the metalloid-amine complex is colorless. Certain fluorescent dyes can be added to overcome this issue. For example, detection of Hg was achieved by using rhodamine and dansyl chloride. However, more research is required to investigate the potential of chitosan in sensing these and other metal(loid)s. Although chitosan has not yet been applied successfully for organic contaminant sensing, it is known to have the potential as a molecular-imprinted sensor for detecting specific contaminants.
5 Summary
Chitosan originates from the seafood processing industry and is one of the most abundant of bio-waste materials. Chitosan is a by-product of the alkaline deacetylation process of chitin. Chemically, chitosan is a polysaccharide that is soluble in acidic solution and precipitates at higher pHs. It has great potential for certain environmental applications, such as remediation of organic and inorganic contaminants, including toxic metals and dyes in soil, sediment and water, and development of contaminant sensors.
Traditionally, seafood waste has been the primary source of chitin. More recently, alternative sources have emerged such as fungal mycelium, mushroom and krill wastes, and these new sources of chitin and chitosan may overcome seasonal supply limitations that have existed. The production of chitosan from the above-mentioned waste streams not only reduces waste volume, but alleviates pressure on landfills to which the waste would otherwise go.
Chitosan production involves four major steps, viz., deproteination, demineralization, bleaching and deacetylation. These four processes require excessive usage of strong alkali at different stages, and drives chitosan’s production cost up, potentially making the application of high-grade chitosan for commercial remediation untenable. Alternate chitosan processing techniques, such as microbial or enzymatic processes, may become more cost-effective due to lower energy consumption and waste generation.
Chitosan has proved to be versatile for so many environmental applications, because it possesses certain key functional groups, including –OH and –NH2. However, the efficacy of chitosan is diminished at low pH because of its increased solubility and instability. These deficiencies can be overcome by modifying chitosan’s structure via crosslinking. Such modification not only enhances the structural stability of chitosan under low pH conditions, but also improves its physicochemical characteristics, such as porosity, hydraulic conductivity, permeability, surface area and sorption capacity.
Crosslinked chitosan is an excellent sorbent for trace metals especially because of the high flexibility of its structural stability. Sorption of trace metals by chitosan is selective and independent of the size and hardness of metal ions, or the physical form of chitosan (e.g., film, powder and solution). Both –OH and –NH2 groups in chitosan provide vital binding sites for complexing metal cations. At low pH, –NH3 + groups attract and coagulate negatively charged contaminants such as metal oxyanions, humic acids and dye molecules.
Grafting certain functional molecules into the chitin structure improves sorption capacity and selectivity for remediating specific metal ions. For example, introducing sulfur and nitrogen donor ligands to chitosan alters the sorption preference for metals.
Low molecular weight chitosan derivatives have been used to remediate metal contaminated soil and sediments. They have also been applied in permeable reactive barriers to remediate metals in soil and groundwater. Both chitosan and modified chitosan have been used to phytoremediate metals; however, the mechanisms by which they assist in mobilizing metals are not yet well understood. In addition, microbes have been used in combination with chitosan to remediate metals (e.g., Cu and Zn) in contaminated soils.
Chitosan has also been used to remediate organic contaminants, such as oil-based wastewater, dyes, tannins, humic acids, phenols, bisphenol-A, p-benzoquinone, organo-phosphorus insecticides, among others.
Chitosan has also been utilized to develop optical and electrochemical sensors for in-situ detection of trace contaminants. In sensor technology, naturally-derived chitosan is used primarily as an immobilizing agent that results from its enzyme compatibility, and stabilizing effect on nanoparticles. Contaminant-sensing agents, such as enzymes, microbes and nanoparticles, have been homogeneously immobilized in chitosan gels by using coagulating (e.g., alginate, phosphate) or crosslinking agents (e.g., GA, ECH). Such immobilization maintains the stability of sensing elements in the chitosan gel phase, and prevents inactivation and loss of the sensing agent.
In this review, we have shown that chitosan, an efficient by-product of a waste biomaterial, has great potential for many environmental applications. With certain limitations, chitosan and its derivatives can be used for remediating contaminated soil and wastewater. Notwithstanding, further research is needed to enhance the physicochemical properties of chitosan and mitigate its deficiencies.
References
Abdi MM, Abdullah LC, Sadrolhosseini AR, Yunus WMM, Moksin MM, Tahir PM (2011) Surface plasmon resonance sensing detection of mercury and lead ions based on conducting polymer composite. PLoS One 6:e24578
Abdullah J, Ahmad M, Karuppiah N, Heng LY, Sidek H (2006) Immobilization of tyrosinase in chitosan film for an optical detection of phenol. Sens Actuators B 114:604–609
Adriano DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risk of metals, 2nd edn. Springer, New York
Ahmad A, Sumathi S, Hameed B (2005) Residual oil and suspended solid removal using natural adsorbents chitosan, bentonite and activated carbon: a comparative study. Chem Eng J 108:179–185
Ahmad A, Sumathi S, Hameed B (2006) Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chem Eng J 118:99–105
Aksu Z, Tatlı Aİ, Tunç Ö (2008) A comparative adsorption/biosorption study of Acid Blue 161: effect of temperature on equilibrium and kinetic parameters. Chem Eng J 142:23–39
An J-H, Dultz S (2007) Adsorption of tannic acid on chitosan-montmorillonite as a function of pH and surface charge properties. Appl Clay Sci 36:256–264
An HK, Park BY, Kim DS (2001) Crab shell for the removal of heavy metals from aqueous solution. Water Res 35:3551–3556
Annadurai G (2000) Design of optimum response surface experiments for adsorption of direct dye on chitosan. Bioproc Eng 23:451–455
Annadurai G (2002) Adsorption of basic dye on strongly chelating polymer: batch kinetics studies. Iran Polym J 11:237–244
Araki Y, Ito E (1975) A pathway of chitosan formation in Mucor rouxii. Eur J Biochem 55:71–78
Assaad E, Azzouz A, Nistor D, Ursu A, Sajin T, Miron D, Monette F, Niquette P, Hausler R (2007) Metal removal through synergic coagulation–flocculation using an optimized chitosan–montmorillonite system. Appl Clay Sci 37:258–274
Aucott M, Namboodiripad A, Caldarelli A, Frank K, Gross H (2010) Estimated quantities and trends of cadmium, lead, and mercury in US municipal solid waste based on analysis of incinerator ash. Water Air Soil Pollut 206:349–355
Austin PR, Brine CJ, Castle JE, Zikakis JP (1981) Chitin: new facets of research. Science (NY) 212:749–753
Baba Y, Hirakawa H, Kawano Y (1994) Selective adsorption of precious metals on sulfur-containing chitosan derivatives. Chem Lett 23:117–120
Baldrick P (2010) The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol 56:290–299
Bansiwal A, Thakre D, Labhshetwar N, Meshram S, Rayalu S (2009) Fluoride removal using lanthanum incorporated chitosan beads. Colloids Surf B Biointerfaces 74:216–224
Barona A, Romero F (1996) Fractionation of lead in soils and its influence on the extractive cleaning with EDTA. Environ Technol 17:63–70
Barona A, Aranguiz I, Elias A (1999) Zinc and copper distribution in soils and their removal by chelating extraction. J Chem Technol Biotechnol 74:700–708
Barona A, Aranguiz I, Elıas A (2001) Metal associations in soils before and after EDTA extractive decontamination: implications for the effectiveness of further clean-up procedures. Environ Pollut 113:79–85
Barreiro-Iglesias R, Coronilla R, Concheiro A, Alvarez-Lorenzo C (2005) Preparation of chitosan beads by simultaneous cross-linking/insolubilisation in basic pH: rheological optimisation and drug loading/release behaviour. Eur J Pharm Sci 24:77–84
Bassi R, Prasher SO, Simpson BK (2000) Removal of selected metal ions from aqueous solutions using chitosan flakes. Separ Sci Technol 35:547–560
Bedner M, MacCrehan WA (2005) Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-acetyl-p-benzoquinone imine. Environ Sci Technol 40:516–522
Benavente M, Moreno L, Martinez J (2011) Sorption of heavy metals from gold mining wastewater using chitosan. J Taiwan Inst Chem Eng 42:976–988
Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R (2004) Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm 57:19–34
Bolan NS, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park JE, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize? J Hazard Mater 266:141–166
Bratskaya S, Schwarz S, Chervonetsky D (2004) Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate. Water Res 38:2955–2961
Bratskaya SY, Ustinov AY, Azarova YA, Pestov AV (2011) Thiocarbamoyl chitosan: synthesis, characterization and sorption of Au(III), Pt(IV), and Pd(II). Carbohydr Polym 85:854–861
Brett CMA (2001) Electrochemical sensors for environmental monitoring. Strategy and examples. Pure Appl Chem 73:1969–1977
Cao Z, Ge H, Lai S (2001) Studies on synthesis and adsorption properties of chitosan cross-linked by glutaraldehyde and Cu (II) as template under microwave irradiation. Eur Polym J 37:2141–2143
Cao ZY, Wei QF, Zhang QX (2004) Template synthesis and adsorption properties of chitosan salicylal Schiff bases. J Central South Univ Technol 11:169–172
Cathell MD, Szewczyk JC, Bui FA, Weber CA, Wolever JD, Kang J, Schauer CL (2008) Structurally coloured thiol chitosan thin films as a platform for aqueous heavy metal ion detection. Biomacromolecules 9:289–295
Celis R, Adelino MA, Hermosín MC, Cornejo J (2012) Montmorillonite–chitosan bionanocomposites as adsorbents of the herbicide clopyralid in aqueous solution and soil/water suspensions. J Hazard Mater 209–210:67–76
Chang W-T, Chen Y-C, Jao C-L (2007) Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Biores Technol 98:1224–1230
Chassary P, Vincent T, Guibal E (2004) Metal anion sorption on chitosan and derivative materials: a strategy for polymer modification and optimum use. React Funct Polym 60:137–149
Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164:1012–1018
Chatterjee S, Chatterjee S, Chatterjee BP, Das AR, Guha AK (2005) Adsorption of a model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. J Colloid Interface Sci 288:30–35
Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK (2007) Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids Surf A Physicochem Eng Asp 299:146–152
Chatterjee S, Lee DS, Lee MW, Woo SH (2009) Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate. J Hazard Mater 166:508–513
Chequer FMD, de Oliveira GAR, Ferraz ERA, Cardoso JC, Zanoni MVB, de Oliveira DP (2013) Textile dyes: dyeing process and environmental impact. In: Günay M (ed) Eco-FRIENDLY TEXTILE DYEING AND FINISHING. InTech, Rijeka, pp 151–176
Cheung WH, Szeto YS, McKay G (2007) Intraparticle diffusion processes during acid dye adsorption onto chitosan. Biores Technol 98:2897–2904
Chiou M-S, Chuang G-S (2006) Competitive adsorption of dye metanil yellow and RB15 in acid solutions on chemically cross-linked chitosan beads. Chemosphere 62:731–740
Chiou M-S, Li H-Y (2002) Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J Hazard Mater 93:233–248
Chiou MS, Li HY (2003) Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 50:1095–1105
Chiou M-S, Kuo W-S, Li H-Y (2003) Removal of reactive dye from wastewater by adsorption using ECH cross-linked chitosan beads as medium. J Environ Sci Health A Toxicol Hazard Subst Environ Eng 38:2621–2631
Chiou MS, Ho PY, Li HY (2004) Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigments 60:69–84
Choudhari SK, Kittur AA, Kulkarni SS, Kariduraganavar MY (2007) Development of novel blocked diisocyanate crosslinked chitosan membranes for pervaporation separation of water–isopropanol mixtures. J Membr Sci 302:197–206
Chui VWD, Mok KW, Ng CY, Luong BP, Ma KK (1996) Removal and recovery of copper(II), chromium(III), and nickel(II) from solutions using crude shrimp chitin packed in small columns. Environ Int 22:463–468
Constantine CA, Mello SV, Dupont A, Cao X, Santos D, Oliveira ON, Strixino FT, Pereira EC, Cheng T-C, Defrank JJ, Leblanc RM (2003) Layer-by-layer self-assembled chitosan/poly(thiophene-3-acetic acid) and organophosphorus hydrolase multilayers. J Am Chem Soc 125:1805–1809
Cook MT, Tzortzis G, Khutoryanskiy VV, Charalampopoulos D (2013) Layer-by-layer coating of alginate matrices with chitosan-alginate for the improved survival and targeted delivery of probiotic bacteria after oral administration. J Mater Chem B 1:52–60
Coral-Hinostroza GN, Bjerkeng B (2002) Astaxanthin from the red crab langostilla (Pleuroncodes planipes): optical R/S isomers and fatty acid moieties of astaxanthin esters. Comp Biochem Physiol B 133:437–444
Coughlin RW, Deshaies MR, Davis EM (2006) Chitosan in crab shell wastes purifies electroplating wastewater. Environ Progr 9:35–39
Cuero RG (1996) Enhanced heavy metal immobilization by a bacterial-chitosan complex in soil. Biotechnol Lett 18:511–514
Dambies L, Guimon C, Yiacoumi S, Guibal E (2001) Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Colloids Surf A Physicochem Eng Asp 177:203–214
Dambies L, Vincent T, Guibal E (2002) Treatment of arsenic-containing solutions using chitosan derivatives: uptake mechanism and sorption performances. Water Res 36:3699–3710
Dang QF, Yan JQ, Li JJ, Cheng XJ, Liu CS, Chen XG (2011) Controlled gelation temperature, pore diameter and degradation of a highly porous chitosan-based hydrogel. Carbohydr Polym 83:171–178
Dash M, Chiellini F, Ottenbrite R, Chiellini E (2011) Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci 36:981–1014
De La Noue J, Proulx D (1988) Biological tertiary treatment of urban wastewaters with chitosan-immunobilized Phormidium. Appl Microbiol Biotechnol 29:292–297
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresource Technol 101:1611–1627
Delezuk JAM, Cardoso MB, Domard A, Campana-Filho SP (2011) Ultrasound-assisted deacetylation of beta-chitin: influence of processing parameters. Polym Int 60:903–909
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229
Deng W, Tan Y, Li Y, Wen Y, Su Z, Huang Z, Huang S, Meng Y, Xie Q, Luo Y, Yao S (2010) Square wave voltammetric determination of Hg(II) using thiol functionalized chitosan-multiwalled carbon nanotubes nanocomposite film electrode. Microchimica Acta 169:367–373
Denkbaş EB, Odabaşi M (2000) Chitosan microspheres and sponges: preparation and characterization. J Appl Polym Sci 76:1637–1643
Desbrieres J, Bousquet C, Babak V (2010) Surfactant-chitosan interactions and application to emulsion stabilization. Cellulose Chem Technol 44:395
Ding Y, Zhao Y, Tao X, Zheng Y-Z, Chen J-F (2009) Assembled alginate/chitosan micro-shells for removal of organic pollutants. Polymer 50:2841–2846
Du D, Ding J, Cai J, Zhang A (2007a) Determination of carbaryl pesticide using amperometric acetylcholinesterase sensor formed by electrochemically deposited chitosan. Colloids Surf B Biointerfaces 58:145–150
Du D, Huang X, Cai J, Zhang A (2007b) Amperometric detection of triazophos pesticide using acetylcholinesterase biosensor based on multiwall carbon nanotube-chitosan matrix. Sensors Actuators B B127:531–535
Du D, Huang X, Cai J, Zhang A, Ding J, Chen S (2007c) An amperometric acetylthiocholine sensor based on immobilization of acetylcholinesterase on a multiwall carbon nanotube-cross-linked chitosan composite. Anal Bioanal Chem 387:1059–1065
Du WL, Xu ZR, Han XY, Xu YL, Miao ZG (2008) Preparation, characterization and adsorption properties of chitosan nanoparticles for eosin Y as a model anionic dye. J Hazard Mater 153:152–156
Dutta PK, Tripathi S, Mehrotra GK, Dutta J (2009) Perspectives for chitosan based antimicrobial films in food applications. Food Chem 114:1173–1182
Dykstra P, Hao J, Koev ST, Payne GF, Yu L, Ghodssi R (2009) An optical MEMS sensor utilizing a chitosan film for catechol detection. Sensors Actuators B 138:64–70
Elliott HA, Brown GA (1989) Comparative evaluation of NTA and EDTA for extractive decontamination of Pb-polluted soils. Water Air Soil Pollut 45:361–369
Etemadi O, Petrisor IG, Kim D, Wan M-W, Yen TF (2003) Stabilization of metals in subsurface by biopolymers: laboratory drainage flow studies. Soil Sediment Contam 12:647–661
Eustis S, El-Sayed MA (2006) Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev 35:209–217
Fahnestock KJ, Manesse M, McIlwee HA, Schauer CL, Boukherroub R, Szunerits S (2009) Selective detection of hexachromium ions by localized surface plasmon resonance measurements using gold nanoparticles/chitosan composite interfaces. Analyst 134:881–886
Fan Q, Shan D, Xue H, He Y, Cosnier S (2007) Amperometric phenol biosensor based on laponite clay–chitosan nanocomposite matrix. Biosens Bioelectron 22:816–821
Fan X, White IM, Shopova SI, Zhu H, Suter JD, Sun Y (2008) Sensitive optical biosensors for unlabeled targets: a review. Anal Chim Acta 620:8–26
Fen YW, Yunus WMM, Yusof NA (2011) Surface plasmon resonance optical sensor for detection of essential heavy metal ions with potential for toxicity: copper, zinc and manganese ions. Sensor Lett 9:1704–1711
Gecol H, Miakatsindila P, Ergican E, Hiibel SR (2006) Biopolymer coated clay particles for the adsorption of tungsten from water. Desalination 197:165–178
Geetha Devi M, Shinoon Al-Hashmi ZS, Chandra Sekhar G (2012) Treatment of vegetable oil mill effluent using crab shell chitosan as adsorbent. Int J Environ Sci Technol 9:713–718
Geng B, Jin Z, Li T, Qi X (2009a) Preparation of chitosan-stabilized Fe0 nanoparticles for removal of hexavalent chromium in water. Sci Tot Environ 407:4994–5000
Geng B, Jin Z, Li T, Qi X (2009b) Kinetics of hexavalent chromium removal from water by chitosan-Fe0 nanoparticles. Chemosphere 75:825–830
Gentili AR, Cubitto MA, Ferrero M, Rodriguéz MS (2006) Bioremediation of crude oil polluted seawater by a hydrocarbon-degrading bacterial strain immobilized on chitin and chitosan flakes. Int Biodeter Biodegrad 57:222–228
Geremias R, Pedrosa R, Benassi J, Favere V, Stolberg J, Menezes C, Laranjeira M (2003) Remediation of coal mining wastewaters using chitosan microspheres. Environ Technol 24:1509–1515
Gong R, Jiang Y, Cai W, Zhang K, Yuan B, Jiang J (2010) Enhanced sorption of bisphenol A on α-ketoglutaric acid-modified chitosan resins by hydrophobic sorption of hemimicelles. Desalination 258:54–58
Gotoh T, Matsushima K, Kikuchi K-I (2004) Preparation of alginate–chitosan hybrid gel beads and adsorption of divalent metal ions. Chemosphere 55:135–140
Goycoolea F-M, Higuera-Ciapara I, Hernandez G, Lizardi J, Garcia K-D (1997) Preparation of chitosan from squid (Loligo spp.) pen by a microwave-accelerated thermochemical process. Adv Chitin Sci 2:78–83
Gray HC, Hutcheson PS, Slavin RG (2004) Is glucosamine safe in patients with seafood allergy? J Allergy CliImmunol 114:459–460
Guibal E (2005) Heterogeneous catalysis on chitosan-based materials: a review. Prog Polym Sci 30:71–109
Guibal E, Roussy J (2007) Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan). React Funct Polym 67:33–42
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Indust Eng Chem Res 37:1454–1463
Guibal E, Touraud E, Roussy J (2005) Chitosan interactions with metal ions and dyes: dissolved-state vs.solid-state application. World J Microbiol Biotechnol 21:913–920
Guibal E, Van VM, Dempsey BA, Roussy J (2006) A review of the use of chitosan for the removal of particulate and dissolved contaminants. Sep Sci Technol 41:2487–2514
Guo XY, Inoue K (2003) Elution of copper from vermiculite with environmentally benign reagents. Hydrometallurgy 70:9–21
Guo Z, Hu X, Ao Y (2009) Effect of chitosan on the available contents and vertical distribution of Cu2+ and Cd2+ in different textural soils. J Hazard Mater 167:1148–1151
Gupta A, Sankararamakrishnan N (2010) Column studies on the evaluation of novel spacer granules for the removal of arsenite and arsenate from contaminated water. Biores Technol 101:2173–2179
Gupta A, Chauhan VS, Sankararamakrishnan N (2009) Preparation and evaluation of iron–chitosan composites for removal of As (III) and As (V) from arsenic contaminated real life groundwater. Water Res 43:3862–3870
Gupta A, Yunus M, Sankararamakrishnan N (2012) Zerovalent iron encapsulated chitosan nanospheres – A novel adsorbent for the removal of total inorganic Arsenic from aqueous systems. Chemosphere 86:150–155
Guzman J, Saucedo I, Navarro R, Revilla J, Guibal E (2002) Vanadium interactions with chitosan: influence of polymer protonation and metal speciation. Langmuir 18:1567–1573
Hamdine M, Heuzey M-C, Bégin A (2005) Effect of organic and inorganic acids on concentrated chitosan solutions and gels. Int J Biol Macromol 37:134–142
Han E, Shan D, Xue H, Cosnier S (2007) Hybrid material based on chitosan and layered double hydroxides: characterization and application to the design of amperometric phenol biosensor. Biomacromolecules 8:971–975
Hanrahan G, Patil DG, Wang J (2004) Electrochemical sensors for environmental monitoring: design, development and applications. J Environ Monitor 6:657–664
Hayes M (2012) Chitin, chitosan and their derivatives from marine rest raw materials: potential food and pharmaceutical applications. In: Hayes M (ed) Marine bioactive compounds: sources, characterization and applications. Springer, Berlin, pp 115–128
Hirano S (2000) Chitin and Chitosan. In: Elvers B, Arpe H-J (eds) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim, pp 471–481
Hirano S, Itakura C, Seino H, Akiyama Y, Nonaka I, Kanbara N, Kawakami T (1990) Chitosan as an ingredient for domestic animal feeds. J Ag Food Chem 38:1214–1217
Hsien T-Y, Rorrer GL (1995) Effects of acylation and crosslinking on the material properties and cadmium ion adsorption capacity of porous chitosan beads. Sep Sci Technol 30:2455–2475
Hu X, Wang R, Ao Y (2006) Effects of chitosan on soil physical and chemical properties. Turang Tongbao 37:68–72
Huang R, Yang B, Liu Q, Ding K (2012) Removal of fluoride ions from aqueous solutions using protonated cross-linked chitosan particles. J Fluorine Chem 141:29–34
Hulanicki A, Głab S, Ingman F (1991) Chemical sensors definitions and classification. Pure Appl Chem 63:1247–1250
Ikeda Y, Poompradub S, Morita Y, Kohjiya S (2008) Preparation of high performance nanocomposite elastomer: effect of reaction conditions on in situ silica generation of high content in natural rubber. J Sol-Gel Sci Technol 45:299–306
Ikehata K, Nicell JA (2008) Color and toxicity removal following tyrosinase-catalyzed oxidation of phenols. Biotechnol Prog 16:533–540
Inoue K, Hirakawa H, Ishikawa Y, Yamaguchi T, Nagata J, Ohto K, Yoshizuka K (1996) Adsorption of metal ions on gallium(III)-templated oxine type of chemically modified chitosan. Sep Sci Technol 31:2273–2285
Jaafari K, Elmaleh S, Coma J, Benkhouja K (2004) Equilibrium and kinetics of nitrate removal by protonated cross-linked chitosan. Water SA 27:9–14
Jaiswal M, Chauhan D, Sankararamakrishnan N (2012) Copper chitosan nanocomposite: synthesis, characterization, and application in removal of organophosphorous pesticide from agricultural runoff. Environ Sci Pollut Res 19:2055–2062
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150
Jeon C, Höll WH (2003) Chemical modification of chitosan and equilibrium study for mercury ion removal. Water Res 37:4770–4780
Jianlong W, Yi Q (1999) Microbial degradation of 4-chlorophenol by microorganisms entrapped in carrageenan-chitosan gels. Chemosphere 38:3109–3117
Juang RS, Shao HJ (2002) A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res 36:2999–3008
Jung WJ, Kuk JH, Kim KY, Park RD (2005) Demineralization of red crab shell waste by lactic acid fermentation. Appl Microbiol Biotechnol 67:851–854
Kamari A, Wan Ngah WS, Ken LL (2009) Chitosan and chemically modified chitosan beads for acid dyes sorption. J Environ Sci 21:296–302
Kamari A, Pulford ID, Hargreaves JSJ (2011a) Chitosan as a potential amendment to remediate metal contaminated soil—a characterisation study. Colloids Surf B Biointerfaces 82:71–80
Kamari A, Pulford ID, Hargreaves JSJ (2011b) Binding of heavy metal contaminants onto chitosans—an evaluation for remediation of metal contaminated soil and water. J Environ Manage 92:2675–2682
Kamari A, Pulford I, Hargreaves J (2012) Metal accumulation in Lolium perenne and Brassica napus as affected by application of chitosans. Int J Phytoremed 14:894–907
Karthikeyan G, Anbalagan K, Andal NM (2004) Adsorption dynamics and equilibrium studies of Zn (II) onto chitosan. J Chem Sci 116:119–127
Kean T, Thanou M (2010) Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev 62:3–11
Khondee N, Tathong S, Pinyakong O, Powtongsook S, Chatchupong T, Ruangchainikom C, Luepromchai E (2012) Airlift bioreactor containing chitosan-immobilized Sphingobium sp. P2 for treatment of lubricants in wastewater. J Hazard Mater 213–214:466–473
Khor E, Lim LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24:2339–2349
Kim DS (2003) The removal by crab shell of mixed heavy metal ions in aqueous solution. Biores Technol 87:355–357
Kim HS (2004) Thermodynamic studies of interaction between chitosan and metal ions by isothermal titration calorimetry (I). J Indust Eng Chem 10:273–277
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS (2007) Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv 26:1–21
Kimura Y, Yamamoto M, Shimazaki R, Kashiwada A, Matsuda K, Yamada K (2012) Use of chitosan for removal of bisphenol a from aqueous solutions through quinone oxidation by polyphenol oxidase. J Appl Polymer Sci 124:796–804
Klepka MT, Nedelko N, Greneche J-M, Lawniczak-Jablonska K, Demchenko IN, Slawska-Waniewska A, Rodrigues CA, Debrassi A, Bordini C (2008) Local atomic structure and magnetic ordering of iron in Fe−chitosan complexes. Biomacromolecules 9:1586–1594
Knox AS, Paller MH, Reible DD, Xingmao M, Petrisor IG (2008) Sequestering agents for active caps—Remediation of metals and organics. Soil Sediment Contamination 17:516–532
Konaganti VK, Kota R, Patil S, Madras G (2010) Adsorption of anionic dyes on chitosan grafted poly(alkyl methacrylate)s. Chem Eng J 158:393–401
Kong M, Chen XG, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63
Krajewska B (2004) Application of chitin-and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139
Kwon S, Thomas J, Reed BE, Levine L, Magar VS, Farrar D, Bridges TS, Ghosh U (2010) Evaluation of sorbent amendments for in situ remediation of metal-contaminated sediments. Environ Toxicol Chem 29:1883–1892
Lazaridis NK, Keenan H (2010) Chitosan beads as barriers to the transport of azo dye in soil column. J Hazard Mater 173:144–150
Leung H-W (2001) Ecotoxicology of glutaraldehyde: review of environmental fate and effects studies. Ecotoxicol Environ Saf 49:26–39
Li Y-F, Liu Z-M, Liu Y-L, Yang Y-H, Shen G-L, Yu R-Q (2006) A mediator-free phenol biosensor based on immobilizing tyrosinase to ZnO nanoparticles. Anal Biochem 349:33–40
Li Z, Wang G, Meng Y, Zhang D (2008) Research on the enhanced effect of chitosan on extraction of cadmium from contaminated soil. Anquan Yu Huanjing Xuebao 8:51–54
Li Z, Meng Y, Wang T, Zhang D (2009) Enhanced extraction of chitosan for chromium (VI) from contaminated soil. Turang Tongbao 40:660–663
Li Q, Chai L, Wang Q, Yang Z, Yan H, Wang Y (2010) Fast esterification of spent grain for enhanced heavy metal ions adsorption. Biores Technol 101:3796–3799
Liao Z, Tao T, Guo J, He Q, Zou L, Jiang W, Shen P, Wang P, Yang X, Long B, Zhou J, Yang S (2010) Soil remediation with chitosan and permeable reacting barrier. Chinese patent No. 101829673. Jiangxi JDL Environmental Protection Research Ltd, Nanchang
Lim SH, Hudson SM (2003) Review of chitosan and its derivatives as antimicrobial agents and their uses as textile chemicals. J Macromol Sci Polym Rev 43:223–269
Lin CC, Lin HL (2005) Remediation of soil contaminated with the heavy metal (Cd2+). J Hazard Mater 122:7–15
Lin S, Chang C-C, Lin C-W, Leu T-H, Li J-D, Hong S-W, Wang T-M, Huang S-C, Lu T-W, Ghoshuni M (2012) A reversible optical sensor based on chitosan film for the selective detection of copper ions. Biomed Eng Appl Basis Commun 24:295–305
Liu ZM, Wang H, Yang Y, Yang HF, Hu SQ, Shen GL, Yu RQ (2004) Amperometric tyrosinase biosensor using enzyme-labeled Au colloids immobilized on cystamine/chitosan modified gold surface. Analyt Lett 37:1079–1091
Liu L, Shu J, Yang Z (2006a) Effects of chitosan and EDTA on Lead desorption in Pb contaminated soil. Nongye Huanjing Kexue Xuebao 25:345–348
Liu LD, Zhang WA, Shu JL, Yang ZK, Wang YT (2006b) The potential of corn (Zea mays L.) for phytoremediation of Pb-contaminated soils with the aid of chitosan addition. Shengtai Duli Xuebao 1:271–277
Liu T, Zhao L, Sun D, Tan X (2010) Entrapment of nanoscale zero-valent iron in chitosan beads for hexavalent chromium removal from wastewater. J Hazard Mater 184:724–730
Loh KS, Lee YH, Musa A, Salmah AA, Zamri I (2008a) Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2,4-dichlorophenoxyacetic acid. Sensors 8:5775–5791
Loh SK, Lee HY, Musa A, Salmah AA, Zamri I (2008b) Evaluation of pesticide and heavy metal toxicity using immobilized enzyme alkaline phosphatase with an electrochemical biosensor. Asian J Biochem 3:359–365
Lü R, Cao Z, Shen G (2008) Comparative study on interaction between copper (II) and chitin/chitosan by density functional calculation. J Mol Struct (THEOCHEM) 860:80–85
Mahmoud NS, Ghaly AE, Arab F (2007) Unconventional approach for demineralization of deproteinized crustacean shells for chitin production. Am J Biochem Biotechnol 3:1–9
Mazengarb S, Roberts GA (2009) Studies on the diffusion of direct dyes in chitosan film. In: Jaworska MM (ed) Progress on Chemistry and Application of Chitin and Its derivatives. Media, Lodz, pp 25–32
McIlwee HA, Schauer CL, Praig VG, Boukherroub R, Szunerits S (2008) Thin chitosan films as a platform for SPR sensing of ferric ions. Analyst 133:673–677
Meanwell RJL, Shama G (2008) Production of streptomycin from chitin using Streptomyces griseus in bioreactors of different configuration. Biores Technol 99:5634–5639
Mench MJ, Didier VL, Löffler M, Gomez A, Masson P (1994) A mimicked in-situ remediation study of metal-contaminated soils with emphasis on cadmium and lead. J Environ Qual 23:58–63
Meng Q, He C, Su W, Zhang X, Duan C (2012) A new rhodamine–chitosan fluorescent material for the selective detection of Hg2+ in living cells and efficient adsorption of Hg2+ in natural water. Sens Actuators B 174:312–317
Methacanon P, Prasitsilp M, Pothsree T, Pattaraarchachai J (2003) Heterogeneous N-deacetylation of squid chitin in alkaline solution. Carbohydr Polym 52:119–123
Milot C, McBrien J, Allen S, Guibal E (1998) Influence of physicochemical and structural characteristics of chitosan flakes on molybdate sorption. J Appl Polym Sci 68:571–580
Minami S, Oh-oka M, Okamoto Y, Miyatake K, Matsuhashi A, Shigemasa Y, Fukumoto Y (1996) Chitosan-inducing hemorrhagic pneumonia in dogs. Carbohydr Polym 29:241–246
Miretzky P, Cirelli AF (2009) Hg(II) removal from water by chitosan and chitosan derivatives: a review. J Hazard Mater 167:10–23
Miretzky P, Cirelli AF (2011) Fluoride removal from water by chitosan derivatives and composites: a review. J Fluorine Chem 132:231–240
Muzzarelli RAA (1973) Natural chelating polymers: alginic acid, chitin, and chitosan, 1st edn. Pergamon, Oxford
Muzzarelli R (2009) Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr Polym 77:1–9
Muzzarelli R (2010) Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar Drugs 8:292–312
Muzzarelli R (2011) Potential of chitin/chitosan-bearing materials for uranium recovery: an interdisciplinary review. Carbohydr Polym 84:54–63
Muzzarelli C, Muzzarelli RAA (2003) Chitin related food science today (and two centuries ago). Agro Food Indust Hi Tech 1:39–42
Muzzarelli R, Tanfani F (1982) N-(O-Carboxybenzyl) chitosan, N-carboxymethyl chitosan, and chitosan dithiocarbamate: new chelating derivatives of chitosan. Pure Appl Chem 54:2141–2150
Muzzarelli RAA, Jenieux R, Gooday GW (1986) Chitin in nature and technology. Plenum, New York
Navarro R, Guzmán J, Saucedo I, Revilla J, Guibal E (2003) Recovery of metal ions by chitosan: sorption mechanisms and influence of metal speciation. Macromol Biosci 3:552–561
Ng J, Cheung W, McKay G (2002) Equilibrium studies of the sorption of Cu (II) ions onto chitosan. J Colloid Interface Sci 255:64–74
Ng J, Cheung W, McKay G (2003) Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 52:1021–1030
Nicol S, Hosie GW (1993) Chitin production by krill. Biochem Syst Ecol 21:181–184
Nidheesh P, Gandhimathi R, Ramesh S (2013) Degradation of dyes from aqueous solution by Fenton processes: a review. Environ Sci Pollut Res 20(4):2099–132
Niu CH, Volesky B (2007) Modeling chromium (VI) biosorption by acid washed crab shells. AIChE J 53:1056–1059
Ogawa K, Oka K, Yui T (1993) X-ray study of chitosan-transition metal complexes. Chem Mater 5:726–728
Oshita K, Oshima M, Gao YH, Lee KH, Motomizu S (2002) Adsorption behavior of mercury and precious metals on cross-linked chitosan and the removal of ultratrace amounts of mercury in concentrated hydrochloric acid by a column treatment with cross-linked chitosan. Anal Sci 18:1121–1125
Osifo PO, Webster A, van der Merwe H, Neomagus HWJP, van der Gun MA, Grant DM (2008) The influence of the degree of cross-linking on the adsorption properties of chitosan beads. Biores Technol 99:7377–7382
Pan J, Yao H, Li X, Wang B, Huo P, Xu W, Ou H, Yan Y (2011) Synthesis of chitosan/γ-Fe2O3/fly-ash-cenospheres composites for the fast removal of bisphenol A and 2,4,6-trichlorophenol from aqueous solutions. J Hazard Mater 190:276–284
Park S, Kwon O, Choi C, Kim C-J (2000) Glucosamine-mediated detoxification of p-benzoquinone and its removal by chitosan. Biotechnol Lett 22:21–24
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung J-W (2011) Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J Hazard Mater 185:549–574
Pearson RG (1963) Hard and soft acids and bases. J Am Chem Soc 85:3533–3539
Peng JF, Song YH, Yuan P, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640
Peniche C, Argüelles-Monal W, Goycoolea FM (2008) Chitin and chitosan: major sources, properties and applications. In: Belgacem MN, Gandini A (eds) Monomers. Polymers and Composites from Renewable Resources, Elsevier, Amsterdam, pp 517–542
Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Progr in Polym Sci 34:641–678
Piron E, Domard A (1998) Interaction between chitosan and uranyl ions. Part 2. Mechanism of interaction. Int J Biol Macromol 22:33–40
Pohanish RP (2008) Sittig’s handbook of toxic and hazardous chemicals and carcinogens, 5th edn. William Andrew Inc, New York
Praig VG, McIlwee H, Schauer CL, Boukherroub R, Szunerits A (2009) Localized surface plasmon resonance of gold nanoparticle-modified chitosan films for heavy-metal ions sensing. J Nanosci Nanotechnol 9:350–357
Prajapati BG (2009) Chitosan a marine medical polymer and its lipid lowering capacity. Internet J Health 9:1–7
Qi L, Xu Z (2004) Lead sorption from aqueous solutions on chitosan nanoparticles. Colloids Surf A Physicochem Eng Asp 251:183–190
Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W (2003) Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457–1465
Radhakumary C, Sreenivasan K (2012) Rapid and highly selective dipchecking for cyanide ions in aqueous media. Analyst 137:5387–5391
Rao MS, Stevens WF (2005) Chitin production by Lactobacillus fermentation of shrimp biowaste in a drum reactor and its chemical conversion to chitosan. J Chem Technol Biotechnol 80:1080–1087
Ratajska M, Boryniec S (1998) Physical and chemical aspects of biodegradation of natural polymers. React Funct Polym 38:35–49
Rayalu SS, Kamble SP, Jagtap S, Labhsetwar NK, Thakare D, Godfrey S, Devotta S (2007) Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem Eng J 129:173–180
Rhazi M, Desbrières J, Tolaimate A, Alagui A, Vottero P (2000) Investigation of different natural sources of chitin: influence of the source and deacetylation process on the physicochemical characteristics of chitosan. Polym Int 49:337–344
Rhazi M, Desbrières J, Tolaimate A, Rinaudo M, Vottero P, Alagui A, El Meray M (2002) Influence of the nature of the metal ions on the complexation with chitosan: application to the treatment of liquid waste. Eur Polym J 38:1523–1530
Richardson SD, Postigo C (2012) Drinking water disinfection by-products. Springer, Emerging Organic Contaminants and Human Health, pp 93–137
Roberts GAF (1992) Chitin chemistry. Macmillan, London
Roberts GAF (2008) Thirty years of progress in chitin and chitosan. In: Jaworska MM (ed), Progress on chemistry and application of chitin and its derivatives, pp 7-15
Romanazzi G, Lichter A, Gabler FM, Smilanick JL (2012) Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol Technol 63:141–147
Rorrer GL, Hsien TY, Way JD (1993) Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from wastewater. Indust Eng Chem Res 32:2170–2178
Rupani PF, Singh RP, Ibrahim MH, Esa N (2010) Review of current palm oil mill effluent (POME) treatment methods: vermicomposting as a sustainable practice. World Appl Sci J 11:70–81
Sahin M, Kocak N, Arslan G, Ucan H (2011) Synthesis of crosslinked chitosan with epichlorohydrin possessing two novel polymeric ligands and Its use in metal removal. J Inorg Organometallic Polym Mater 21:69–80
Saifullah, Ghafoor A, Zia MH, Murtaza G, Waraich EA, Bibi S, Srivastava P (2010) Comparison of organic and inorganic amendments for enhancing soil lead phytoextraction by wheat (Triticum aestivum L.). Int J Phytoremediation 12:633–649
Sankararamakrishnan N, Dixit A, Iyengar L, Sanghi R (2006) Removal of hexavalent chromium using a novel cross linked xanthated chitosan. Biores Technol 97:2377–2382
Sannan T, Kurita K, Iwakura Y (1976) Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromolekulare Chemie 177:3589–3600
Santhosh S, Sini TK, Mathew PT (2010) Variation in properties of chitosan prepared at different alkali concentrations from squid pen and shrimp shell. Int J Polym Mater 59:286–291
Sarma SJ, Pakshirajan K, Mahanty B (2011) Chitosan-coated alginate–polyvinyl alcohol beads for encapsulation of silicone oil containing pyrene: a novel method for biodegradation of polycyclic aromatic hydrocarbons. J Chem Technol Biotechnol 86:266–272
Schauer CL, Chen M-S, Price RR, Schoen PE, Ligler FS (2004) Colored thin films for specific metal ion detection. Environ Sci Technol 38:4409–4413
Setti L, Mazzieri S, Pifferi PG (1999) Enhanced degradation of heavy oil in an aqueous system by a Pseudomonas sp. in the presence of natural and synthetic sorbents. Biores Technol 67:191–199
Shafaei A, Ashtiani FZ, Kaghazchi T (2007) Equilibrium studies of the sorption of Hg (II) ions onto chitosan. Chem Eng J 133:311–316
Shahidi F, Arachchi JKV, Jeon Y-J (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10:37–51
Shao J, Ge H, Yang Y (2007) Immobilization of polyphenol oxidase on chitosan–SiO2 gel for removal of aqueous phenol. Biotechnol Lett 29:901–905
Shukla SR, Pai RS (2005) Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Separ Purif Technol 43:1–8
Si Y, Zhang J, Si X, Peng J (2010) Microbe-immobilized granules for remediation of pesticide-contaminated soil. Chinese patent No. 101701216. Anhui Agricultural University
Sklyar AM, Gamzazade AI, Rogovina LZ, Titkova LV, Pavlova SSA, Rogozhin SV, Slonimskii GL (1981) Study of rheological properties of dilute and moderately concentrated solutions of chitosan. Polymer Sci (USSR) 23:1546–1554
Srivastava P, Singh B, Angove M (2005) Competitive adsorption behavior of heavy metals on kaolinite. J Colloids Interface Sci 290:28–38
Sugunan A, Thanachayanont C, Dutta J, Hilborn JG (2005) Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci Technol Adv Mater 6:335–340
Sun S, Wang L, Wang A (2006) Adsorption properties of crosslinked carboxymethyl-chitosan resin with Pb(II) as template ions. J Hazard Mater 136:930–937
Suzuki M (1993) Complex of chitosan and cellulose fibers. Japanese patent No. 05228176. Nippon Kyushutai Gijutsu Kenky
Suzuki M, Sugiyama T, Musashi E, Kobiyama Y, Kashiwada A, Matsuda K, Yamada K (2010) Use of chitosan for removal of bisphenol A and bisphenol derivatives through tyrosinase-catalyzed quinone oxidation. J Appl Polym Sci 118:721–732
Szyguła A, Guibal E, Ruiz M, Sastre AM (2008) The removal of sulphonated azo-dyes by coagulation with chitosan. Colloids Surf A Physicochem Eng Asp 330:219–226
Tahtat D, Uzun C, Mahlous M, Güven O (2007) Beneficial effect of gamma irradiation on the N-deacetylation of chitin to form chitosan. Nucl Instrum Methods Phys Res B 265:425–428
Tang L, Zeng G-M, Shen G-L, Li Y-P, Zhang Y, Huang D-L (2008) Rapid detection of Picloram in agricultural field samples using a disposable immunomembrane-based electrochemical sensor. Environ Sci Technol 42:1207–1212
Tay CC, Liew HH, Redzwan G, Yong SK, Surif S, Abdul-Talib S (2011a) Pleurotus ostreatus spent mushroom compost as green biosorbent for nickel (II) biosorption. Water Sci Technol 64:2425–2432
Tay CC, Liew HH, Yin C-Y, Abdul-Talib S, Surif S, Suhaimi A, Yong SK (2011b) Biosorption of cadmium ions using & Pleurotus ostreatus: growth kinetics, isotherm study and biosorption mechanism. Kor J Chem Eng 28:825–830
Tay CC, Liew HH, Yong SK, Surif S, Redzwan G, Abdul-Talib S (2012) Cu(II) removal onto fungal derived biosorbents: biosorption performance and the half saturation constant concentration approach. Int J Res Chem Environ 2:138–143
Tee Y-H, Bhattacharyya D (2008) Chitosan membranes with nanoparticles for remediation of chlorinated organics. In: Li NN, Fane AG, Ho WSW, Matsuura T (eds) Advanced membrane technology and applications. Wiley, Hoboken, pp 189–216
Teng WL, Khor E, Tan TK, Lim LY, Tan SC (2001) Concurrent production of chitin from shrimp shells and fungi. Carbohydr Res 332:305–316
Tharanathan RN, Kittur FS (2003) Chitin—the undisputed biomolecule of great potential. Crit Rev Food Sci Nutr 43:61–87
Tomihata K, Ikada Y (1997) In vitro and in vivo degradation of films of chitin and its deacetylated derivatives. Biomaterials 18:567–575
Trung T, Ng C-H, Stevens W (2003) Characterization of decrystallized chitosan and its application in biosorption of textile dyes. Biotechnol Lett 25:1185–1190
Tsigos I, Martinou A, Kafetzopoulos D, Bouriotis V (2000) Chitin deacetylases: new, versatile tools in biotechnology. Trends Biotechnol 18:305–312
Ummadisingu A, Gupta S (2012) Characteristics and kinetic study of chitosan prepared from seafood industry waste for oil spills cleanup. Desalination Water Treatment 44:44–51
Uragami T, Yoshida F, Sugihara M (1983) Studies of synthesis and permeabilities of special polymer membranes. LI. Active transport of halogen ions through chitosan membranes. J Appl Polym Sci 28:1361–1370
Uthairatanakij A, da Silva JAT, Obsuwan K (2007) Chitosan for improving orchid production and quality. Orchid Sci Biotechnol 1:1–5
Uzun İ, Güzel F (2004) Kinetics and thermodynamics of the adsorption of some dyestuffs and p-nitrophenol by chitosan and MCM-chitosan from aqueous solution. J Colloid Interface Sci 274:398–412
Vetter J (2007) Chitin content of cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem 102:6–9
Vieira RS, Oliveira MLM, Guibal E, Rodríguez-Castellón E, Beppu MM (2011) Copper, mercury and chromium adsorption on natural and crosslinked chitosan films: an XPS investigation of mechanism. Colloids Surf A Physicochem Eng Asp 374:108–114
Vold IMN, Vårum KM, Guibal E, Smidsrød O (2003) Binding of ions to chitosan–selectivity studies. Carbohydr Polym 54:471–477
Waibel KH, Haney B, Moore M, Whisman B, Gomez R (2011) Safety of chitosan bandages in shellfish allergic patients. Mil Med 176:1153–1156
Walker GM, Weatherley LR (2001) Adsorption of dyes from aqueous solution — the effect of adsorbent pore size distribution and dye aggregation. Chem Eng J83:201–206
Walsh AM, Sweeney T, Bahar B, O’Doherty JV (2013) Multi-functional roles of chitosan as a potential protective agent against obesity. PLoS One 8:e53828
Wan Ngah WS, Fatinathan S (2010) Pb(II) biosorption using chitosan and chitosan derivatives beads: equilibrium, ion exchange and mechanism studies. J Environ Sci 22:338–346
Wan Ngah WS, Musa A (1998) Adsorption of humic acid onto chitin and chitosan. J Appl Polym Sci 69:2305–2310
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Wan MW, Petrisor IG, Lai HT, Kim D, Yen TF (2004) Copper adsorption through chitosan immobilized on sand to demonstrate the feasibility for in situ soil decontamination. Carbohydr Polym 55:249–254
Wang L, Wang A (2008) Adsorption properties of congo red from aqueous solution onto N, O-carboxymethyl-chitosan. Biores Technol 99:1403–1408
Wang QZ, Chen XG, Liu N, Wang SX, Liu CS, Meng XH, Liu CG (2006) Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr Polym 65:194–201
Wang FY, Lin XG, Yin R (2007) Role of microbial inoculation and chitosan in phytoextraction of Cu, Zn, Pb and Cd by Elsholtzia splendens—a field case. Environ Pollut 147:248–255
Wang S, Tan Y, Zhao D, Liu G (2008) Amperometric tyrosinase biosensor based on Fe3O4 nanoparticles–chitosan nanocomposite. Biosens Bioelectron 23:1781–1787
Wang L, Xing R, Liu S, Qin Y, Li K, Yu H, Li R, Li P (2010a) Studies on adsorption behavior of Pb(II) onto a thiourea-modified chitosan resin with Pb(II) as template. Carbohydr Polym 81:305–310
Wang S, Lei Y, Zhang Y, Tang J, Shen G, Yu R (2010b) Hydroxyapatite nanoarray-based cyanide biosensor. Anal Biochem 398:191–197
Wang SL, Liang TW, Yen YH (2011) Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials. Carbohydr Polym 84:732–742
Wang X, Su Y, Yang H, Dong Z, Ma J (2012) Highly sensitive fluorescence probe based on chitosan nanoparticle for selective detection of Hg2+ in water. Colloids Surf A Physicochem Eng Asp 11:88–93
Weng G, Wang Z, Wu L, Luo Y, Song J, Qian W, Lin Q, Wang F, Jiang Y, Dai X, Qiu X (2005) Effect of degradable chelate and microbial preparation on the phytoremediation of contaminated soil by Elsholtzia splendens. Turang 37:152–157
Wu FC, Tseng RL, Juang RS (2000) Comparative adsorption of metal and dye on flake- and bead-types of chitosans prepared from fishery wastes. J Hazard Mater 73:63–75
Wu FC, Tseng RL, Juang RS (2010) A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J Environ Manage 91:798–806
Xia W, Liu P, Zhang J, Chen J (2011) Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids 25:170–179
Xu R, Yong LC, Lim YG, Obbard JP (2005) Use of slow-release fertilizer and biopolymers for stimulating hydrocarbon biodegradation in oil-contaminated beach sediments. Mar Pollut Bull 51:1101–1110
Xu D, Hein S, Loo SL, Wang K (2008) The fixed-bed study of dye removal on chitosan beads at high pH. Indust Eng Chem Res 47:8796–8800
Xu R, Zhou Q, Li F, Zhang B (2013) Laccase immobilization on chitosan/poly(vinyl alcohol) composite nanofibrous membranes for 2,4-dichlorophenol removal. Chem Eng J 222:321–329
Yamada K, Akiba Y, Shibuya T, Kashiwada A, Matsuda K, Hirata M (2005) Water purification through bioconversion of phenol compounds by tyrosinase and chemical adsorption by chitosan beads. Biotechnol Prog 21:823–829
Yamamoto H, Amaike M (1997) Biodegradation of cross-linked chitosan gels by a microorganism. Macromolecules 30:3936–3937
Yan WL, Bai R (2005) Adsorption of lead and humic acid on chitosan hydrogel beads. Water Res 39:688–698
Yang ZK, Shu JL, Liu LD (2006) Enhanced phytoremediation of lead-contaminated soils by chitosan chelating agent. Nongye Huanjing Kexue Xuebao 25:86–89
Yong SK, Wong TW (2013) Chitosan for body weight management: current issues and future directions. In: Kim S-K (ed) Marine nutraceuticals: prospects and perspect. CRC, Boca Raton, pp 151–168
Yong SK, Bolan NS, Lombi E, Skinner W, Guibal E (2012) Sulfur-containing chitin and chitosan derivatives as trace metal adsorbents: a review. Crit Rev Environ Sci Technol 43:1741–1794
Yong SK, Bolan NS, Lombi E, Skinner W (2013) Synthesis and characterization of thiolated chitosan beads for removal of Cu(II) and Cd(II) from wastewater. Water Air Soil Pollut 224:1–12
Youn DK, No HK, Prinyawiwatkul W (2007) Physical characteristics of decolorized chitosan as affected by sun drying during chitosan preparation. Carbohydr Polym 69:707–712
Yu JCC, Lai EPC, Sadeghi S (2004) Surface plasmon resonance sensor for Hg(II) detection by binding interactions with polypyrrole and 2-mercaptobenzothiazole. Sens Actuators B 101:236–241
Zainal Z, Hui LK, Hussein MZ, Abdullah AH, Hamadneh IR (2009) Characterization of TiO2—Chitosan/Glass photocatalyst for the removal of a monoazo dye via photodegradation—adsorption process. J Hazard Mater 164:138–145
Zhang Y, Ji C (2010) Electro-induced covalent cross-linking of chitosan and formation of chitosan hydrogel films: its application as an enzyme immobilization matrix for use in a phenol sensor. Anal Chem 82:5275–5281
Zhang L, Li Q, Huang D, Li Y, Hou X, Deng X (2011) Soil remediation using chitosan composite material. Chinese patent No. 101705099. Environment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences; Sichuan University
Zhao Y, Park RD, Muzzarelli R (2010) Chitin deacetylases: properties and applications. Mar Drugs 8:24–46
Zhou QX, Song YF (2004) Remediation of contaminated soils: principles and methods. Science, Beijing, pp 1–489
Acknowledgements
Soon Kong Yong would like to thank the University of South Australia for the UniSA President Scholarship award and Universiti Teknologi MARA for the UiTM Staff Scholarship award. Manoj Shrivastava acknowledges the Australian Government’s Endeavour Research Fellowship award for conducting this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yong, S.K., Shrivastava, M., Srivastava, P., Kunhikrishnan, A., Bolan, N. (2015). Environmental Applications of Chitosan and Its Derivatives. In: Whitacre, D. (eds) Reviews of Environmental Contamination and Toxicology Volume 233. Reviews of Environmental Contamination and Toxicology, vol 233. Springer, Cham. https://doi.org/10.1007/978-3-319-10479-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-10479-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10478-2
Online ISBN: 978-3-319-10479-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)