Abstract
Genetic polymorphisms of alcohol dehydrogenase-1B (ADH1B) and aldehyde dehydrogenase-2 (ALDH2) modulate exposure levels to ethanol/acetaldehyde. Endoscopic screening of 6,014 Japanese alcoholics yielded high detection rates of esophageal squamous cell carcinoma (SCC; 4.1 %) and head and neck SCC (1.0 %). The risks of upper aerodigestive tract SCC/dysplasia, especially of multiple SCC/dysplasia, were increased in a multiplicative fashion by the presence of a combination of slow-metabolizing ADH1B*1/*1 and inactive heterozygous ALDH2*1/*2 because of prolonged exposure to higher concentrations of ethanol/acetaldehyde. A questionnaire asking about current and past facial flushing after drinking a glass (≈ 180 mL) of beer is a reliable tool for detecting the presence of inactive ALDH2. We invented a health-risk appraisal (HRA) model including the flushing questionnaire and drinking, smoking, and dietary habits. Esophageal SCC was detected at a high rate by endoscopic mass-screening in high HRA score persons. A total of 5.0 % of 4,879 alcoholics had a history of (4.0 %) or newly diagnosed (1.0 %) gastric cancer. Their high frequency of a history of gastric cancer is partly explained by gastrectomy being a risk factor for alcoholism because of altered ethanol metabolism, e.g., by blood ethanol level overshooting. The combination of H. pylori-associated atrophic gastritis and ALDH2*1/*2 showed the greatest risk of gastric cancer in alcoholics. High detection rates of advanced colorectal adenoma/carcinoma were found in alcoholics, 15.7 % of 744 immunochemical fecal occult blood test (IFOBT)-negative alcoholics and 31.5 % of the 393 IFOBT-positive alcoholics. Macrocytosis with an MCV ≥ 106 fl increased the risk of neoplasia in the entire aerodigestive tract of alcoholics, suggesting that poor nutrition as well as ethanol/acetaldehyde exposure plays an important role in neoplasia.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Acetaldehyde
- Alcohol dehydrogenase
- Alcoholic
- Aldehyde dehydrogenase
- Colorectal neoplasia
- Esophageal cancer
- Head and neck cancer
- Mean corpuscular volume
- Stomach cancer
15.1 Endoscopy and Esophageal Iodine Staining of Screening Japanese Alcoholic Men for Upper Aerodigestive Tract Neoplasia
Alcohol consumption, tobacco smoking, inadequate intake of fruits and vegetables, and low body mass index (BMI) are risk factors for squamous cell carcinoma (SCC) of the upper aerodigestive tract (UADT, i.e., the oral cavity, pharynx, larynx, and esophagus), and many alcoholic patients have all of these risk factors. We introduced an endoscopic screening program at Kurihama Medical and Addiction Center in 1993 [1], and by 2010 initial screening of 6,014 Japanese alcoholic men by endoscopy combined with head and neck inspection and esophageal iodine staining had detected esophageal SCC in 243 (4.0 %) of them and head and neck SCC in 65 (1.1 %) of them. Barrett adenocarcinoma was detected in only two patients. Technical improvements in endoscopes and a growing understanding of the endoscopic findings of early SCC in the UADT [2, 3] have enabled very early detection of SCC in the UADT. Treatment of early SCC in UADT by endoscopic or endoscope-guided mucosectomy has become a widespread practice in Japan and succeeded in improving the outcome of this high-mortality cancer [3, 4].
15.2 ALDH2 Genotype and Alcohol Metabolism
Genetic polymorphism of aldehyde dehydrogenase-2 (ALDH2, rs671) modulates exposure levels to acetaldehyde after drinking (Fig. 15.1). A mutant ALDH2*2 allele encoding an inactive subunit of ALDH2 was carried by Han Chinese as they spread throughout East Asia [5]. About 40 % of Japanese, 7 % being homozygotes and 35 % heterozygotes [6], have the inactive ALDH2*2 allele which acts in a dominant manner. After drinking a small amount of alcohol, people with inactive ALDH2 tend to experience a flushing response that includes facial flushing, palpitations, nausea, and drowsiness [7]. People with inactive ALDH2 [8] or with inactive-ALDH2-associated facial flushing [9] tend to experience a hangover in the morning after drinking a smaller amount of alcohol than people without either of them. Thus presence of inactive ALDH2 in some East Asians tends to prevent them from drinking heavily [6]. Because of their very intense flushing responses ALDH2*2/*2 homozygotes are usually nondrinkers or occasional drinkers. However, the inhibitory effect of being heterozygous for inactive ALDH2 on heavy drinking is influenced by sociocultural factors. The proportion of Japanese alcoholics with the ALDH2*1/*2 genotype increased dramatically from 2.5 % in 1979, to 8.0 % in 1986, and to 13.0 % in 1992 [10], and the proportion continued to increase from 13.0 % in 1996–2000, to 14.0 % in 2001–2005, and to 15.4 % in 2006–2010 [11].
15.3 ALDH2 Genotype and Squamous Cell Neoplasia in the UADT
In 1996, we first reported that being heterozygous for inactive ALDH2 is a strong risk factor for esophageal cancer in daily drinkers and alcoholics [12]. Since then, epidemiological studies have almost consistently demonstrated a strong association between the ALDH2*1/*2 genotype and the risk of UADT cancer in East-Asian drinkers [13]. A meta-analysis of Asian case–control studies of esophageal cancer showed that the ALDH2*1/*2-associated odds ratio (95 % confidence interval) [OR (95 % CI)] for esophageal cancer was 3.12 (1.95–5.02) in moderate drinkers, 5.64 (1.57–20.25) in ex-drinkers, and 7.12 (4.67–10.86) in heavy drinkers [14]. The ALDH2*1/*2 genotype has been found to be a very strong risk factor for esophageal cancer among Taiwan Chinese and Japanese drinkers (OR = 4.74–6.21 in moderate drinkers and 9.21–9.75 in heavy drinkers), but the OR associated with the ALDH2*1/*2 genotype in the high incidence regions of esophageal cancer of Mainland China was not so high (OR = 1.98 in moderate drinkers and 1.31 in heavy drinkers). The meta-analysis confirmed that being homozygous for the inactive allele, i.e., having the ALDH2*2/*2 genotype greatly increased the risk of esophageal cancer in drinkers, and that the heterozygous genotype, i.e., ALDH2*1/*2 increased the risk of esophageal cancer in a similar manner in both men and women. A meta-analysis of 6 Japanese case–control studies showed that the ALDH2*1/*2-associated risk for head and neck cancer was also greater in heavy drinkers [OR (95 % CI) = 3.57 (1.21–2.77)] than in moderate drinkers [OR (95 % CI) = 1.68 (1.22–2.27)] [15].
The United States National Cancer Institute’s SEER Program reported synchronous multiple cancers and metachronous multiple cancers in only 2 % and 3 %, respectively, of esophageal cancer patients during the 1973–2003 period [16]. The prevalence of multiple organ cancers among esophageal cancer patients treated at the National Cancer Center of Japan increased at an alarming rate between 1969 and 1996 from 6.3 % in 1969–1980, to 22.2 % in 1981–1991, and to 39.0 % in 1992–1996 [17], and the most frequent sites of the other cancers were the head and neck and stomach. The increased proportion of multiple cancers in Japanese esophageal cancer patients may partly explained by a dramatic increase in the proportion of ALDH2*1/*2 heterozygotes among Japanese heavy drinkers during the same period [10]. The ALDH2*1/*2 genotype has consistently been demonstrated to be a strong determinant of the risk of synchronous and metachronous SCC of the UADT in Japanese drinkers [13].
The ORs (95 % CI) associated with the ALDH2*1/*2 genotype in Japanese alcoholics has been found to increase for the very early stages of the esophageal neoplasia, from 2.88 (1.81–4.57) for low-grade intraepithelial neoplasia, to 5.14 (2.87–9.19) for high-grade intraepithelial neoplasia, and to 4.07 (1.97–8.40) for invasive SCC [18]. The presence of multiple esophageal iodine-unstained lesions or a large esophageal dysplasia has been found to be a strong predictor of the development of multiple cancers in the UADT in Japanese [13], and to be associated with the ALDH2*1/*2 genotype [18], p53 alteration [19], and telomere shortening in the esophagus [20] in Japanese alcoholics. Thus, acetaldehyde, which is an established human carcinogen, plays a critical role in the multicentric development of neoplasia throughout the UADT.
15.4 The Simple Flushing Questionnaire
The discovery of ALDH2-associated cancer susceptibility emphasizes the importance of developing screening tests for inactive ALDH2 based on alcohol flushing. We devised a flushing questionnaire to identify person with inactive ALDH2 (Fig. 15.2) [21, 22]. The simple flushing questionnaire consists of two questions: (A) Do you have a tendency to flush in the face immediately after drinking a glass (≈180 mL) of beer? (B) Did you have a tendency to flush in the face immediately after drinking a glass of beer during the first to second year after you started drinking? The results are used to classify the subjects as a current flusher, former flusher, or never flusher. When current flushers or former flushers were assumed to have inactive ALDH2, the simple flushing questionnaire had 90 % sensitivity and 88 % specificity when evaluated in 610 Japanese men 40 years of age or older [22] and 88 % sensitivity and 92 % specificity when evaluated in 381 Japanese women 40 years of age or older [23]. The individuals’ risks of UADT cancer estimated on the basis of the replies to the flushing questionnaire were slightly lower than but essentially comparable to their risks estimated on the basis of ALDH2 genotyping [21–23].
15.5 Mass-Screening for SCC of the UADT by Means of Health-Risk Appraisal Models
Based on the results of a case–control study [24], we devised health-risk appraisal (HRA) models for esophageal cancer that include ALDH2 genotype or alcohol flushing [25]. The total risk score is calculated by adding the scores A–E (Fig. 15.3). If a person’s risk score is 11 or more according to the HRA-Flushing model, that person’s risk of esophageal cancer is in the top 10 % of the study population. A cross-validation study predicted that approximately 60 % of the esophageal and hypopharyngeal SCCs in the entire population could be detected by examining only people whose risk scores were in the top 10 % in the HRA models. Follow-up endoscopy with esophageal iodine staining (median follow-up period: 5.0 years) was performed on 404 cancer-free controls and resulted in the diagnosis of six esophageal SCCs and two pharyngeal SCCs [26]. The risk scores of six of these eight cancer patients at baseline were in the top 10 % according to the HRA-Flushing model. The cancer detection rate per 100 person–years in the top 10 % risk group of the cancer-free controls was 2.3 for esophageal cancer and 3.5 for esophageal or pharyngeal cancer. We applied this HRA-Flushing questionnaire to endoscopic mass-screening programs of 2,221 Japanese men during 2008 and 2009 at five cancer screening facilities, and esophageal cancer was diagnosed in 19 persons as a result [27]. The HRA-Flushing score of 5 % of the examinees was 11 or greater, and esophageal cancer was detected in 4.3 % of them, as opposed to in 0.7 % of the other examinees. A receiver operating characteristic curve analysis showed that when we used an cutoff point of an HRA score of ≥9 in the 50–69 age group and of ≥8 in the 70–89 age group to select individuals with a high risk for esophageal cancer, the sensitivity and false-positive rate was 52.6 % and 15.2 %, respectively, and cancer was detected in 2.91 % of the examinees in the high-risk group, as opposed to 0.48 % in the other group. Although the cutoff values for high-risk groups should be changed to achieve better performance of the HRA model according to the population targeted, these figures encouraged using our questionnaire to screen larger populations of Japanese men.
Health-risk appraisal model for esophageal cancer combined with the simple flushing questionnaire. The questionnaire enables makes it possible for people to easily determine their risk of esophageal cancer, and public awareness campaigns that use the questionnaire will help persuade high-risk persons to undergo endoscopic screening or enable them to change their lifestyle to prevent esophageal cancer
Our simple questionnaire makes it possible for many people to identify their risk of UADT cancer very easily, and use of the questionnaire in public awareness campaigns will help persuade high-risk persons to undergo endoscopic screening or enable them to change their lifestyle to prevent UADT cancer. Our HRA model that includes ALDH2 genotype yielded a slightly better positive predictive value [25] and a slightly higher cancer detection rate [26] than the HRA-Flushing model. Genotyping ALDH2 would entail an initial cost, but genotyping needs to be performed only once in a lifetime, the data are always available, and the unit cost would be greatly discounted if a huge number of samples are analyzed.
15.6 ADH1B Genotype, Alcohol Metabolism, and SCC of the UADT
Alcohol dehydrogenase-1B (ADH1B, previously called ADH2) also has a functional genetic polymorphism (rs1229984; Fig. 15.1). The ADH1B*1/*1 genotype, which results in expression of slow-metabolizing ADH1B, is prevalent in Caucasians (present in approximately 90 %), but present in only a small fraction of East Asians (e.g., 7 % of Japanese) [6]. The presence of slow-metabolizing ADH1B is a stronger risk factor for alcoholism and SCC of the UADT in East Asians than the presence of the fast-metabolizing ADH1B because of having the ADH1B*2 allele. Approximately 30 % of Japanese alcoholics have the slow-metabolizing ADH1B, and the slow-metabolizing ADH1B has been found to be more frequent in the younger generations of Japanese alcoholics, probably because the ADH1B*1/*1 genotype accelerates the progression of alcohol dependence [11].
Alcohol challenge tests have failed to demonstrate any associations between ADH1B genotype and the blood ethanol or acetaldehyde concentrations after ingestion of small to moderate doses of ethanol [13]. However, the slow-metabolizing ADH1B has an approximately 40 times lower Vmax in vitro than the fast-metabolizing ADH1B [28], and an experiment in which a clamping technique and intravenous alcohol infusion were used showed a modestly but significantly lower ethanol elimination rate (11–18 %) among Jews with the slow-metabolizing ADH1B than with the fast-metabolizing ADH1B [29]. At the time of their first visit to our Addiction Center, we evaluated associations between ADH1B and ALDH2 genotypes and the blood ethanol levels of 805 Japanese alcoholic men in the morning after drinking within the previous 34 h [30]. The results showed no significant differences in age-adjusted usual alcohol consumption according to ADH1B or ALDH2 genotypes. Higher blood ethanol levels persisted for longer periods in the group with the slow-metabolizing ADH1B who were ADH1B*1/*1 carriers (n = 246) than in the group with the fast-metabolizing ADH1B who were ADH1B*2 carriers (n = 559), and blood ethanol levels ≥0.3 mg/mL (criterion for drunk driving according to Japanese law) after a 12.1–18-h interval since the last drink were observed in a significantly higher proportion of the ADH1B*1/*1 carriers than of the ADH1B*2 carriers (40 % vs. 14–17 %, p < 0.0001). Multivariate analyses showed that the ethanol levels were 0.500 mg/mL higher in the group with the ADH1B*1*1 genotype, and the OR (95 % CI) for an ethanol level ≥0.3 mg/mL in the presence of the ADH1B*1/*1 genotype was 3.44 (2.34–5.04). There were no significant differences in blood ethanol levels according to ALDH2 genotype.
We evaluated associations between ADH1B and ALDH2 genotypes and the body weight and BMI of 1,301 Japanese alcoholic men on the day of their first visit [31]. There were no significant differences in usual caloric intake in the form of alcoholic beverages according to ADH1B genotype in any of the age brackets, but the presence of the slow-metabolizing ADH1B was more strongly associated with weight gain in all age brackets. This result links the slower ethanol elimination by the ADH1B*1*/*1 alcoholics with their more efficient utilization of ethanol as an energy source. No effects of ALDH2 genotype on body weight or BMI were observed.
In a study in which we simultaneously measured the blood and salivary ethanol and acetaldehyde levels of Japanese alcoholics in the morning on the day of their first visit [32, 33], we found that ethanol and acetaldehyde remained in the blood and saliva for much longer periods and at much higher levels in the group with the slow-metabolizing ADH1B than in the group with the fast-metabolizing ADH1B, even after adjusting for age, body weight, the amount of alcohol consumed, and interval since the previous drink. Chronic heavy drinking by alcoholics may amplify the modest effect of ADH1B*1/*1 on ethanol metabolism and lead to clear prolongation of the presence of ethanol in the body, including in the UADT. The blood and salivary ethanol levels of the subjects were similar, but the acetaldehyde levels in their saliva were much higher than in their blood because of acetaldehyde production by oral microorganisms. [32, 33]
ADH1B genotype markedly affects alcohol flushing [22, 34]. Alcohol flushing in fast-metabolizing ADH1B carriers is triggered by a rapid initial rise in the blood acetaldehyde concentration, whereas the slow initial rise in the blood acetaldehyde in slow-metabolizing ADH1B carriers may weaken the alcohol flushing [22, 34]. The results of our flushing questionnaire showed that despite the presence of inactive ALDH2 in heterozygotes, 25 % of the slow-metabolizing ADH1B carriers were never flushers and 38 % were former flushers, and thus there was a clear association between alcohol consumption by inactive ALDH2 heterozygotes and their facial flushing categories [22].
The above findings provide clues as to why slow-metabolizing ADH1B increases the risk of both alcoholism and UADT cancer. First, slow-metabolizing ADH1B diminishes the intensity of facial flushing, thereby accounting for the greater susceptibility to heavy drinking. Second, chronic heavy drinking amplifies the modest effect of slow-metabolizing ADH1B on ethanol elimination, which leads to much longer exposure to ethanol and, in turn, results in increasing the risk of developing alcoholism. When ethanol lingers in the body, the UADT is exposed to high levels of acetaldehyde as a result of acetaldehyde production in saliva, and that creates a condition that increases the risk of UADT cancer.
15.7 The ADH1B*1/*1 and ALDH2*1/*2 Genotype Combination and SCC in the UADT
The risk of SCC of the head and neck and esophagus of Japanese and Taiwanese drinkers has been found to be extremely increased in a multiplicative fashion by the combination of slow-metabolizing ADH1B in the ADH1B*1/*1 genotype and inactive ALDH2 in the ALDH2*1/*2 genotype (OR = 22–122 for esophageal SCC) [13, 18, 24, 35–37]. In Japanese alcoholics, the OR (95 % CI) with the ADH1B*1/*1 and ALDH2*1/*2 genotype combination has been found to increase for the very early stages of the esophageal neoplasia, from 4.53 (2.17–9.47) for low-grade intraepithelial neoplasia, to 10.4 (4.34–24.7) for high-grade intraepithelial neoplasia, and 21.7 (7.96–59.3) for invasive SCC [18]. When the two genotypes were combined with other risk factors, based on the multivariate OR for each risk factor the ORs (95 % CI) of Japanese for esophageal SCC increased enormously, by 248 times when combined with consumption of 198–395 g ethanol/week and by 414 times when combined with consumption of ≥396 g ethanol/week [24], in Taiwanese by 382 (47–3,085) times when combined with consumption of >30 g ethanol/day [35], and in Japanese by 189 (95–377) times when combined with both consumption of >96.5 g ethanol/week and smoking [36] and by 357 (105–1,210) times when combined with both drinking and smoking [37].
15.8 ADH1B and ALDH2 Genotype and Liver Disease in Japanese Alcoholic Men
Contrary to results of investigations of the relationships between the ADH1B and ALDH2 genotypes and susceptibility to UADT cancer, a cross-sectional survey of 1,902 Japanese alcoholic men showed that liver cirrhosis was associated with the presence of the ADH1B*2 allele and ALDH2*1/*1 genotype [38]. When non-cirrhotic patients with no or only mild liver fibrosis as controls, the results showed that the OR (95 % CI) of the ADH1B*2 allele increased according to the severity of their liver disease, from 1.67 (1.32–2.11) in the non-cirrhotic group with liver fibrosis, to 1.81 (1.24–2.63) in the Child-Pugh class A cirrhosis group, 2.97 (1.79–4.93) in the Child-Pugh class B cirrhosis group, and 4.32 (1.48–12.6) in the Child-Pugh class C cirrhosis group. Since age-adjusted daily alcohol consumption did not differ according to ADH1B/ALDH2 genotypes in the alcoholics, their ADH1B/ALDH2-associated increase in risk of liver disease cannot be explained by the levels of ethanol and acetaldehyde exposure.
15.9 Gastric Cancer in Japanese Alcoholic Men
The lifetime drinking profiles of Japanese alcoholic men have shown that gastrectomy increases susceptibility to alcoholism [39]. Gastrectomy results in swift passage of ethanol from the small intestine into the systemic circulation. Because of this dynamic change in ethanol delivery, overshoot of blood ethanol levels and subsequent high ethanol exposure have been observed after gastrectomy [40], and they lead to rapid development of alcohol dependence. A large survey of 4,879 Japanese alcoholic men demonstrated that a high proportion of them had a history of gastrectomy, although the proportion decreased from 13.3 to 7.8 % during the 1996–2010 period [11]. Many alcoholic men with a history of gastrectomy had changed their drinking pattern after the gastrectomy and had became alcoholics after a shorter period of heavy drinking and after a lower cumulative alcohol intake than alcoholics with no history of gastrectomy [39]. There were more frequent blackouts in the gastrectomy group, and that may have reflected the sharper rise in their blood ethanol level. Since a history of gastrectomy increases the risk of alcohol dependence, this acquired risk factor increases susceptibility to alcohol dependence in the absence of the alcoholism-susceptibility genotype ADH1B*1/*1, and that explains the lower frequency of ADH1B*1/*1 that was found in a gastrectomy group of Japanese alcoholic men than in a non-gastrectomy group [11].
The prevalence of gastric cancer is extremely high among Japanese alcoholic men. A study of 4,879 Japanese male alcoholic patients revealed that 187 had a history of gastrectomy for gastric cancer and ten had a history of mucosectomy for gastric cancer, and 47 were diagnosed with gastric cancer during the initial endoscopic screening [11]. A total of 244 (5.0 %) of the patients had a history of gastric cancer or were newly diagnosed with gastric cancer.
Inactive ALDH2, macrocytosis, and simultaneous presence of UADT cancer as well as H. pylori-associated atrophic gastritis have been found to be associated with the risk of gastric cancer detected by endoscopic screening of Japanese alcoholic men [41]. This finding partly explains why gastric, esophageal, and head and neck cancers are often concurrent in Japanese alcoholic men. However, the frequency of the ALDH2*1/*2 genotype in alcoholics with a history of gastrectomy for gastric cancer was found to be as low as in alcoholics without a history of gastrectomy [11]. These findings suggest different causal associations between alcoholism and each group of gastric cancer.
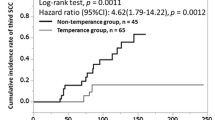
The risk of metachronous gastric cancer is high in Japanese with esophageal SCC, especially among alcoholic men, suggesting a common cause of both cancers. Endoscopic follow-up (median, 47 months) after the initial diagnosis of esophageal SCC was performed in 99 Japanese alcoholic men [42]. A serum pepsinogen test showed a higher seroprevalence of severe chronic atrophic gastritis among the esophageal SCC cases than among age-matched alcoholic controls, whereas their H. pylori status was similar. The accelerated progression of severe chronic atrophic gastritis observed in Japanese alcoholic men with esophageal SCC suggests the existence of a common mechanism by which both esophageal SCC and H. pylori-related severe chronic atrophic gastritis develop in the alcoholics. Metachronous gastric adenocarcinoma was diagnosed in 11 of the 99 gastric cancer-free patients in the same study, and the cumulative rate of metachronous gastric cancer within 5 years was estimated to be 15 %. The hazard ratio [HR (95 % CI)] of metachronous gastric cancer was 7.87 (1.43–43.46) in the group with severe chronic atrophic gastritis in comparison with the group without chronic atrophic gastritis. Inactive heterozygous ALDH2 was not associated with an increased risk of metachronous gastric cancer. Accelerated development of severe chronic atrophic gastritis at least partially explained the very high frequency of development of metachronous gastric cancer in this population of Japanese men with an initial diagnosis of SCC of the esophagus.
15.10 Colonoscopic Screening of Japanese Alcoholic Men for Colorectal Neoplasia
The results of colonoscopic screening of Japanese alcoholic men for colorectal neoplasia yielded an extremely high rate of advanced colorectal neoplasia: 15.7 % in the group of 744 subjects with a negative immunochemical fecal occult blood test (IFOBT) and 31.6 % in the group of 393 subjects with a positive IFOBT [43]. Advanced colorectal neoplasia has been reported to have been detected in 2.6 % of an IFOBT-negative group and 16.0 % of an IFOBT-positive group in the Japanese general population [44]. Advanced colorectal neoplasia includes adenomas ≥10 mm, villous and tubulovillous adenomas, high-grade dysplasia, carcinoma-in-situ, and invasive cancers. Thus, screening alcoholic men by the IFOBT alone is inadequate, and colonoscopy should be recommended to the patients. There were no significant associations between ALDH2 genotypes and the risk of advanced colorectal neoplasia [43].
15.11 Association Between a High Mean Corpuscular Volume and Increased Risk of Aerodigestive Tract Neoplasia in Japanese Alcoholic Men
Epidemiological evidence indicates that the red cell mean corpuscular volume (MCV) of inactive ALDH2 carriers is increased by exposure to acetaldehyde [45–51]. Alcoholism, severe acetaldehyde exposure because of the presence of inactive ALDH2, smoking, low BMI, and folate deficiency are associated with both increased MCV and increased risk of aerodigestive tract cancer. The simultaneous presence of a high MCV of 106 or more, ALDH2*1/*2 genotype, and ADH1B*1/*1 genotype in Japanese alcoholic men synergistically increase their risk of esophageal SCC [OR (95 % CI) = 320 (27–>1,000)] [47]. An endoscopic follow-up study of cancer-free Japanese alcoholics revealed that cancer of the UADT developed much more frequently among alcoholics with a high MCV of 106 or more [HR (95 % CI) = 2.52 (1.22–5.22)] [51]. An MCV of 106 or more in Japanese alcoholic men was found to increase their risks of head and neck SCC [OR (95 % CI) = 2.71 (1.42–5.16)] [52], esophageal SCC [OR (95 % CI) = 3.68 (1.96–6.93)] [48], and gastric cancer [OR (95 % CI) = 2.5 (1.2–5.2)] [41], and to increase their risk of advanced colorectal neoplasia in an IFOBT-negative group [ORs (95 % CI) = 1.65 (1.02–2.64)] and an IFOBT-positive group [2.83 (1.15–6.93)] [43] (Table 15.1).
15.12 Conclusions
-
The ADH1B*1/*1 genotype and ALDH2*1/*2 genotype are associated with an increased risk of UADT neoplasia in East-Asian drinkers.
-
Prolonged exposure to high ethanol and acetaldehyde concentrations and inefficient degradation of acetaldehyde in the UADT explains the high risk of UADT neoplasia in people with these genotypes.
-
Health-risk appraisal models that include alcohol flushing or ALDH2 genotype are useful tools for mass-screening for UADT neoplasia.
-
The ADH1B genotype of Japanese alcoholic men affects their body weight and susceptibility to liver disease.
-
Gastric cancer and advanced colorectal neoplasia are more common in Japanese alcoholic men than in nonalcoholic Japanese men.
-
A high MCV in Japanese alcoholic men increases their risk of aerodigestive tract neoplasia.
References
Yokoyama A, Ohmori T, Makuuchi H et al (1995) Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer 76:928–934
Muto M, Nakane M, Katada C et al (2004) Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer 101:1375–1381
Makuuchi H (2001) Endoscopic mucosal resection for mucosal cancer in the esophagus. Gastrointest Endosc Clin N Am 11:445–458
Sato Y, Omori T, Yokoyama A et al (2006) Treatment of superficial carcinoma in the pharynx and the larynx. Shokaki Naishikyo 18:1407–1416 (in Japanese with English abstract)
Li H, Borinskaya S, Yoshimura K et al (2009) Refined geographic distribution of the Oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet 73:335–345
Higuchi S, Matsushita S, Murayama M et al (1995) Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry 152:1219–1221
Harada S, Agarwal DP, Goedde HW (1981) Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet 2(8253):982
Yokoyama M, Yokoyama A, Yokoyama T et al (2005) Hangover susceptibility in relation to aldehyde dehydrogenase-2 genotype, alcohol flushing, and mean corpuscular volume in Japanese workers. Alcohol Clin Exp Res 29:1165–1171
Yokoyama M, Suzuki N, Yokoyama T et al (2012) Interactions between migraine and tension-type headache and alcohol drinking, alcohol flushing, and hangover in Japanese. J Headache Pain 13:137–145
Higuchi S, Matsushita S, Imazeki H et al (1994) Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet 343:741–742
Yokoyama A, Yokoyama T, Matsui T et al (2013) Trends in gastrectomy and ADH1B and ALDH2 genotypes in Japanese alcoholic men and their gene-gastrectomy, gene-gene and gene-age interactions for risk of alcoholism. Alcohol Alcohol 48:146–152
Yokoyama A, Muramatsu T, Ohmori T et al (1996) Esophageal cancer and aldehyde dehydrogenase-2 genotypes in Japanese males. Cancer Epidemiol Biomarkers Prev 5:99–102
Yokoyama A, Omori T, Yokoyama T (2010) Alcohol and aldehyde dehydrogenase polymorphisms and a new strategy for prevention and screening for cancer in the upper aerodigestive tract in East Asians. Keio J Med 59:115–130
Yang SJ, Yokoyama A, Yokoyama T et al (2010) Relationship between genetic polymorphisms of ALDH2 and ADH1B and esophageal cancer risk: a meta-analysis. World J Gastroenterol 16:4210–4220
Boccia S, Hashibe M, Galli P et al (2009) Aldehyde dehydrogenase 2 and head and neck cancer: a meta-analysis implementing a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev 18:248–254
Hayat MJ, Howlader N, Reichman ME et al (2007) Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 12:20–37
Watanabe H (1998) Present status and management of multiple primary esophageal cancer associated with head and neck cancer. J Jpn Bronchoesophagol Soc 49:151–155 (in Japanese)
Yokoyama A, Hirota T, Omori T et al (2012) Development of squamous neoplasia in esophageal iodine-unstained lesions and the alcohol and aldehyde dehydrogenase genotypes of Japanese alcoholic men. Int J Cancer 130:2949–2960
Yokoyama A, Tanaka Y, Yokoyama T et al (2011) p53 protein accumulation, iodine-unstained lesions, and alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 genotypes in Japanese alcoholic men with esophageal dysplasia. Cancer Lett 308:112–117
Aida J, Yokoyama A, Shimomura N et al (2013) Telomere shortening in the esophagus of Japanese alcoholics: relationships with chromoendoscopic findings, ALDH2 and ADH1B genotypes and smoking history. PLoS One 8:e63860
Brooks PJ, Enoch MA, Doldman D et al (2009) The alcohol flushing response: an unrecognized risk factor of esophageal cancer from alcohol consumption. PLoS Med 6:e50
Yokoyama T, Yokoyama A, Kato H et al (2003) Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev 12:1227–1233
Yokoyama A, Kato H, Yokoyama T et al (2006) Esophageal squamous cell carcinoma and aldehyde dehydrogenase-2 genotypes in Japanese females. Alcohol Clin Exp Res 30:491–500
Yokoyama A, Kato H, Yokoyama T et al (2002) Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferees M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis 23:1851–1859
Yokoyama T, Yokoyama A, Kumagai Y et al (2008) Health Risk Appraisal Models for Mass Screening of Esophageal Cancer in Japanese Men. Cancer Epidemiol Biomarkers Prev 17:2846–2854
Yokoyama A, Kumagai Y, Yokoyama T, Omori T, Kato H, Igaki H, Tsujinaka T, Muto M, Yokoyama M, Watanabe H (2009) Health risk appraisal models for mass screening for esophageal and pharyngeal cancer: an endoscopic follow-up study of cancer-free Japanese men. Cancer Epidemiol Biomarkers Prev 18:651–655
Yokoyama A, Oda J, Iriguchi Y et al (2013) A health-risk appraisal model and endoscopic mass screening for esophageal cancer in Japanese men. Dis Esophagus 26:148–153
Yin SJ, Bosron WF, Magnes LJ et al (1984) Human liver alcohol dehydrogenase: Purification and kinetic characterization of the β2β2, β2β1, αβ2 and β2γ1 ‘Oriental’ isozymes. Biochemistry 23:5847–5853
Neumark YD, Friedlander Y, Durst R et al (2004) Alcohol dehydrogenases polymorphisms influence alcohol-elimination rates in a male Jewish population. Alcohol Clin Exp Res 28:10–14
Yokoyama A, Yokoyama T, Mizukami T et al (2014) Blood ethanol levels of nonabstinent Japanese alcoholic men in the morning after drinking and their ADH1B and ALDH2 genotypes. Alcohol Alcohol 49(1):31–37
Yokoyama A, Yokoyama T, Matsui T et al (2013) Alcohol dehydrogenase-1B genotype (rs1229984) is a strong determinant of the relationship between body weight and alcohol intake in Japanese alcoholic men. Alcohol Clin Exp Res 37:1123–1132
Yokoyama A, Tsutsumi E, Imazeki H et al (2007) Contribution of the alcohol dehydrogenase-1B genotype and oral microorganisms to high salivary acetaldehyde concentrations in Japanese alcoholic men. Int J Cancer 121:1047–1054
Yokoyama A, Tsutsumi E, Imazeki H et al (2010) Polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and the blood and salivary ethanol and acetaldehyde concentrations of Japanese alcoholic men. Alcohol Clin Exp Res 34:1246–1256
Yokoyama A, Yokoyama T, Omori T (2010) Past and current tendency for facial flushing after a small dose of alcohol is a marker for increased risk of upper aerodigestive tract cancer in Japanese drinkers. Cancer Sci 101:2497–2498
Lee CH, Lee JM, Wu DC et al (2008) Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer 122:1347–1356
Cui R, Kamatani Y, Takahashi A et al (2009) Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology 137:1768–1775
Tanaka F, Yamamoto K, Suzuki S et al (2010) Strong interaction between the effects of alcohol consumption and smoking on oesophageal squamous cell carcinoma among individuals with ADH1B and/or ALDH2 risk alleles. Gut 59:1457–1464
Yokoyama A, Mizukami T, Matsui T et al (2013) Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol Clin Exp Res 37:1391–1401
Yokoyama A, Takagi T, Ishii H et al (1995) Gastrectomy enhances vulnerability to the development of alcoholism. Alcohol 12:213–216
Caballeria J, Frezza M, Hernández-Muñoz R et al (1989) Gastric origin of the first-pass metabolism of ethanol in humans: effect of gastrectomy. Gastroenterology 97:1205–1209
Yokoyama A, Yokoyama T, Omori T et al (2007) Helicobacter pylori, chronic atrophic gastritis, inactive aldehyde dehydrogenase-2, macrocytosis and multiple upper aerodigestive tract cancers and the risk for gastric cancer in alcoholic Japanese men. J Gastroenterol Hepatol 22:210–217
Yokoyama A, Omori T, Yokoyama T et al (2009) Chronic atrophic gastritis and metachronous gastric cancer in Japanese alcoholic men with esophageal squamous cell carcinoma. Alcohol Clin Exp Res 33:898–905
Mizukami T, Yokoyama A, Yokoyama T et al (2012) Immunochemical fecal occult blood test and colonoscopic screening in Japanese alcoholic men. Alcohol Clin Exp Res 36(Suppl):94A (Abstract No. P009
Morikawa T, Kato J, Yamaji Y et al (2005) A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 129:422–428
Nomura F, Itoga S, Tamura M et al (2000) Biological markers of alcoholism with respect to genotypes of low-Km aldehyde dehydrogenase (ALDH2) in Japanese subjects. Alcohol Clin Exp Res 24(4 Suppl):30S–33S
Hashimoto Y, Nakayama T, Futamura A et al (2002) Erythrocyte mean cell volume and genetic polymorphism of aldehyde dehydrogenase 2 in alcohol drinkers. Blood 99:3487–3488
Yokoyama M, Yokoyama A, Yokoyama T et al (2003) Mean corpuscular volume and the aldehyde dehydrogenase-2 genotype in male Japanese workers. Alcohol Clin Exp Res 27:1395–1401
Yokoyama A, Yokoyama T, Muramatsu T et al (2003) Macrocytosis, a new predictor for esophageal squamous cell carcinoma in Japanese men. Carcinogenesis 24:1773–1778
Yokoyama A, Yokoyama T, Kumagai Y et al (2005) Mean corpuscular volume, alcohol flushing and the predicted risk of squamous cell carcinoma of the esophagus in cancer-free Japanese men. Alcohol Clin Exp Res 29:1877–1883
Yokoyama T, Saito K, Lwin H et al (2005) Epidemiological evidence that acetaldehyde plays a significant role in the development of decreased serum folate concentration and elevated mean corpuscular volume in alcohol drinkers. Alcohol Clin Exp Res 29:622–630
Yokoyama A, Omori T, Yokoyama T et al (2006) Risk of squamous cell carcinoma of the upper aerodigestive tract in cancer-free alcoholic Japanese men: an endoscopic follow-up study. Cancer Epidemiol Biomarkers Prev 15:2209–2215
Yokoyama A, Omori T, Yokoyama T et al (2010) Risk factors of squamous cell carcinoma in the oropharynx, hypopharynx, and epilarynx of Japanese alcoholic men: A case-control study based on an endoscopic screening program. Stomach Intestine (Tokyo) 45:180–189 (In Japanese with English abstract, tables, and figures)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Yokoyama, A., Mizukami, T., Yokoyama, T. (2015). Genetic Polymorphisms of Alcohol Dehydrogense-1B and Aldehyde Dehydrogenase-2, Alcohol Flushing, Mean Corpuscular Volume, and Aerodigestive Tract Neoplasia in Japanese Drinkers. In: Vasiliou, V., Zakhari, S., Seitz, H., Hoek, J. (eds) Biological Basis of Alcohol-Induced Cancer. Advances in Experimental Medicine and Biology, vol 815. Springer, Cham. https://doi.org/10.1007/978-3-319-09614-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-09614-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09613-1
Online ISBN: 978-3-319-09614-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)