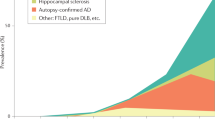
Abstract
Neuropathological hallmarks of Alzheimer’s disease (AD) include tangles (NFT) and beta amyloid (Aβ) plaques. Despite numerous neuropathological studies that assessed the relationship of cognitive decline with neuropathologic lesions, their correlation still remains unclear. NFTs and Aβ plaques have been widely implicated and described in normal aging. The number of NFTs in the CA1 and the entorhinal cortex seems to be more closely related to cognitive status, compared to the amyloid load whose role still remains controversial in the AD. In this review, we refer to our main studies performed in Geneva during the past two decades attempting to assess the correlation of pathology with clinical expression. The theory of cognitive reserve has been proposed for further understanding of interindividual differences in terms of compensation despite the presence of pathological lesions. The increasing prevalence of the AD, the limitations of actual treatments, as well as the high public cost reflect the imperative need for better therapeutic and early diagnosis strategies in the future.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Alzheimer’s disease
- Neurofibrillary tangles
- Aβ plaques
- Paired helical filaments
- Substantia nigra pars compacta
- Mini Mental State Score
- β-Amyloid precursor protein
6.1 Introduction
The population is growing older every year creating a big challenge for public health to cope with the amelioration of life quality with increasing age in the future. Prevention and early detection of heavy pathological entities in terms of public health cost and prognosis, such as the Alzheimer’s disease, are necessary. Therefore, a lot of funding has been invested in the comprehension of pathophysiological mechanisms and early disease detection biomarkers during the past few years.
Cerebral aging is a very complex process involving molecular mechanisms’ alterations, such as reduced mitochondrial function, increased oxidative stress, autophagy, the accumulation of ubiquitylated protein aggregates, as well as impaired signalling of numerous neurotransmitter and neurotrophic factor pathways [1]. The main neuropathological findings in the Alzheimer’s disease have often been described in old individuals without cognitive impairment. Recent research has turned its interest on the trajectory of normal aging in the human brain, suggesting that there is a clinicopathological continuum between healthy aging and dementia. The hypothesis behind this new line of research is that the quantity and localization but not the nature of the lesions characterize cognitive deterioration and progression from intact cognition to mild cognitive impairment and to dementia [2]. We performed a number of clinicopathological studies in order to address the issue of the lesions’ continuum in a very old population. When studying neuropathological correlates of centenarians we found that they were resistant to neurodegenerative process and had mild loss of neurons in the hippocampus, restricted to layer II of the entorhinal cortex [3–5].
Neurofibrillary tangles and amyloid plaques are the principal pathological hallmarks described in both normal aging and neurodegenerative diseases. Distinguishing demented and non-demented participants based on pathology has been revealed to be extremely difficult. We will further overview the basic pathological findings in the aging brain, their overlap with the ones in the Alzheimer’s disease, as well as their clinical correlates.
6.2 Neurofibrillary Tangles: From Healthy Aging to the AD
Paired helical filaments (PHF) are one of the principal constituents in AD pathology. They are found in three distinct intraneuronal regions, forming the major part of the neurofibrillary tangle (NFT) [6]. Two different groups identified the core protein component of the PHFs, the tubule-associated hyperphosphorylated tau protein in 1986 [7–9]. The physiological role of the protein tau involves the microtubules’ stabilization and their interaction with cytoskeletal filaments [10].
Braak and Braak described in 1991 a staging scheme concerning NFT lesions in dementia to differentiate different stages of disease [11]. They suggested that Braak I and II stages include the transentorhinal cortex with mild implication of the hippocampus and no implication of the neocortex, corresponding to clinically silent AD. Stages III and IV include more NFTs in the entorhinal and transentorhinal cortex, in the hippocampus and mild neocortical pathology corresponding to early AD. Stages V and VI involve high density of NFTs in the hippocampus, as well as the neocortex, and are linked to fully developed AD.
Several clinicopathological studies have examined the correlation of cognitive status and pathological findings [12–14]. Arriagada et al. were one of the first teams to describe the positive correlation between the number of neocortical NFTs and the severity of dementia [15]. Over the past few years the Geneva group has performed a series of clinicopathological analyses with demented and non-demented subjects [16–22]. NFT number was proved to be strongly related to cognitive impairment, especially massive NFT formation in the CA1 field, the entorhinal cortex, and the area 9 of the brain [23]. In line with our studies, other groups with large autopsy series have concluded that the density of NFTs in selected cerebral fields is strongly related to the cognitive status as tested by different neuropsychological tests [24–26].
Tomlinson et al. were the first to describe the presence of NFTs in cognitively intact elderly people [27]. Since then several other studies have indicated the presence of NFTs in normal brain aging [28–31]. Our group has performed a series of autopsies in demented and non-demented population during the past two decades. NFTs were found to exist in areas such as the entorhinal cortex, the CA1 field, as well as the temporal cortex [16]. The localization and number of NFTs correlate with cognitive decline. NFT numbers in the entorhinal cortex or area 9 could predict almost more than 87 % of the MMSE scores’ variability [17].
We studied a particular subset brain in a subgroup of AD patients that develop parkinsonism [20]. They represent a percentage of approximately 30 % and present no Lewy body pathology at autopsy. Our sample included 22 patients with AD, 11 with parkinsonism and 11 without. All cases were carefully selected from a brain bank of 5,278 autopsies performed in the Geneva University Hospitals after neuropathological AD confirmation and parkinsonism. Post-encephalitic, idiopathic PD, potentially drug-induced parkinsonism, cases were excluded. The results showed that parkinsonism in AD is related to an important neuronal loss in the substantia nigra pars compacta (SN) and in the putamen. Tau deposition was a less important factor, with densities of NFTs in the SN correlating with parkinsonism but not in the putamen. These data suggest that a subgroup of patients with AD develop more subcortical extension with a probable worse prognosis in comparison with cortical only AD cases.
6.3 Amyloid Oligomers and Senile Plaques: From Healthy Aging to the AD
Amyloid deposits in the brain are the second pathologic hallmark of the AD. Amyloid β peptide is a 39–43 amino-acid peptide generated by enzymatic cleavages of the β-amyloid precursor protein (β APP) that is very sensitive to the proteolysis by a set of proteases called secretases. Secretases are responsible for the production of Aβ(1–40) peptide or Aβ(1–42/43) with a higher tendency to self-aggregate. Senile plaques consist of amyloid fibrils composed by aggregated Aβ peptides. Before developing fibrils, they aggregate into oligomers. Soluble non-fibrillar oligomeric assemblies are suggested toxic at the synaptic level [32]. To further elucidate the molecular entity underlying cognitive impairment and amyloid deposition we performed a neuropathological study to explore the relationship of mid-molecular weight Aβ oligomers with their regional distribution and their relationship with lesion development in controls and mild-to-severe AD patients. We found that mid-molecular weight Aβ oligomers had the same regional distribution with fibrillar amyloid pathology both in controls and AD. This suggests that the formation of oligomers is perhaps necessary for the formation of stable amyloid plaques (Mid range molecular weight Aβ oligomers in normal aging and AD: regional and clinicopathological correlations. Bouras, personal communication).
Numerous studies have focused on its role in the normal aging brain and the neurodegenerative disease. NFTs and amyloid depositions are very common in elderly people without any cognitive impairment, particularly in the oldest old [18], reference NFT. While the correlation of NFT number with the severity of cognitive decline has been confirmed by several researchers, a direct link for amyloid deposits still remains unclear. The use of amyloid ligand uptake in PET as one of the most important biomarkers for the early diagnosis of the AD still remains controversial. In fact, in the early stages of AD there is no or scarce deposition of amyloid in the frontal cortex. On the other hand, the soluble oligomers that are detected by PET should be present everywhere in the brain. In our recent study [20] we compared amyloid deposition in 1,599 autopsied cases aged 65 and more (mean age approximately 82.8 years) between 1972 and 2006 in Geneva to detect cohort effects on age- and AD-related neuropathologic changes. Amyloid-to-NFT stage ratios were used to account for possible changes in AD prevalence to severity over time. We found that the brains of older individuals in 2006 were 10 years younger in terms of amyloid deposition compared to people that died earlier. The amyloid/NFT ratio was decreased in both demented and non-demented cases confirming the decreased amyloid in more recent autopsied subjects despite the severity of presence NFT densities.
6.4 Vascular Disease
Vascular disease is the second most common dementia disease after AD [33]. We performed a series of studies to assess the repercussions of vascular burden in brain aging. A high percentage of AD cases (approximately 2/3) were found to present a mixed neuropathological profile with the coexistence of vascular pathology confined to microvascular lesions [34] or lacunes [35]. Cortical microinfarct scores statistically correlated with the clinical variability assessed by the CDR scale. Subcortical lacunes in the thalamus and basal ganglia predicted cognitive decline in the elderly in our second series of autopsy including 72 patients. Periventricular and deep white matter lesions seem to play a very modest role in cognitive decline. A lot of research has been done in the field to assess the criteria for mixed dementia, as well as the potential synergistic role of vascular and neurodegenerative disease when the amount of lesions is not sufficient to provoke disease [36, 37].
6.5 Discussion
Neuropathological lesions of AD have been widely described in cognitive intact elderly people by several authors. Which is the turnover point over which lesions become clinically evident? Why elderly people with extended neuropathological lesions manage to compensate them and remain cognitively intact? Through our experience in the field and the existing literature we notice that the appearance of NFTs and amyloid plaques after the age of 50 is inevitable. Despite this common feature only 10 % of the elderly population (after 65 years) manifests the symptomatology of AD. Each case though presents an individual profile in terms of lesions and clinical symptomatology with cognitive decline depending on the number of healthy remaining neurons. Epigenetics have been proposed as a potential molecular mechanism participating in the AD pathophysiology to explain interindividual differences [38, 39]. APP DNA methylation has been proved to be normally methylated and hypomethylated with age, resulting in increased Aβ production (reference Tohgi et al. reduction with age in methycytosine. Brain Res Mol Brain Res 70:288–292). Histone modifications have also been described in AD hippocampal neurons, as well as other brain regions implicated in the disease [40]. The theory of cognitive reserve [41] has been proposed during the past years as the most plausible hypothesis to explain interindividual variability in pathology and clinical expression of AD. Low educational level and occupational status have been described as risk factors for the AD [42]. Cognitive reserve is thought to be the moderator between pathological brain changes and clinical expression [41, 43]. Its pathophysiological basis still remains unclear. Perhaps the anatomical variability mainly in the entorhinal cortex may play an important role for the preservation of the cognitive functions. FMRI studies in elderly healthy controls could help us to further elucidate the underlying neural mechanisms. The need for more efficient therapeutic strategies is crucial given the actual poor results from clinical trials, as well as the increasing prevalence of the disease.
References
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535
Serrano-Pozo A, Qian J, Monsell SE, Frosch MP, Betensky RA, Hyman BT (2013) Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J Neuropathol Exp Neurol 72:1182–1192
Giannakopoulos P, Hof PR, Surini M, Michel JP, Bouras C (1993) Quantitative immunohistochemical analysis of the distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of nonagenarians and centenarians. Acta Neuropathol 85:602–610
Giannakopoulos P, Hof PR, Kovari E, Vallet PG, Herrmann FR, Bouras C (1996) Distinct patterns of neuronal loss and Alzheimer's disease lesion distribution in elderly individuals older than 90 years. J Neuropathol Exp Neurol 55:1210–1220
Imhof A, Kovari E, von Gunten A et al (2007) Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci 257:72–79
Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer's disease. Nature 197:192–193
Brion JP, Flament-Durand J, Dustin P (1986) Alzheimer's disease and tau proteins (letter). Lancet 2(8515):1098
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Grundke-Iqbal I, Vorbrodt AW, Iqbal K, Tung YC, Wang GP, Wisniewski HM (1988) Microtubule-associated polypeptides tau are altered in Alzheimer paired helical filaments. Brain Res 464:43–52
Maccioni RB, Cambiazo V (1995) Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev 75:835–864
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Bierer LM, Hof PR, Purohit DP et al (1995) Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol 52:81–88
Crystal H, Dickson D, Fuld P et al (1988) Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology 38:1682–1687
Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P (1995) Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging 16:285–298, discussion 298–304
Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 42:631–639
Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 153:131–135
Giannakopoulos P, Herrmann FR, Bussiere T et al (2003) Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60:1495–1500
Gold G, Bouras C, Kovari E et al (2000) Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol 99:579–582, discussion 583–584
Horvath J, Burkhard PR, Herrmann FR, Bouras C, Kovari E (2014) Neuropathology of parkinsonism in patients with pure Alzheimer's disease. J Alzheimer's Dis 39:115–120
Kovari E, Herrmann FR, Bouras C, Gold G (2014) Amyloid deposition is decreasing in aging brains: an autopsy study of 1,599 older people. Neurology 82:326–331
von Gunten A, Kovari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P (2005) Stereologic analysis of hippocampal Alzheimer's disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol 193:198–206
von Gunten A, Kovari E, Bussiere T et al (2006) Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer's disease. Neurobiol Aging 27:270–277
Giannakopoulos P, Gold G, Kovari E et al (2007) Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol 113:1–12
Duyckaerts C, Bennecib M, Grignon Y et al (1997) Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging 18:267–273
Nelson PT, Jicha GA, Schmitt FA et al (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66:1136–1146
Sabbagh MN, Cooper K, DeLange J et al (2010) Functional, global and cognitive decline correlates to accumulation of Alzheimer's pathology in MCI and AD. Curr Alzheimer Res 7:280–286
Tomlinson BE, Blessed G, Roth M (1968) Observations on the brains of non-demented old people. J Neurol Sci 7:331–356
Ball MJ (1977) Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol 37:111–118
Bennett DA, Schneider JA, Arvanitakis Z et al (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66:1837–1844
Davis DG, Schmitt FA, Wekstein DR, Markesbery WR (1999) Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol 58:376–388
Knopman DS, Parisi JE, Salviati A et al (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med 2:a006338
Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P (2009) Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med 6:e1000180
Kovari E, Gold G, Herrmann FR et al (2004) Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke 35:410–414
Gold G, Kovari E, Herrmann FR et al (2005) Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke 36:1184–1188
Esiri MM, Wilcock GK, Morris JH (1997) Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 63:749–753
Nagy Z, Esiri MM, Jobst KA et al (1997) The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56:165–170
Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD (2009) Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS One 4:e6617
Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J (2011) Epigenetic mechanisms in Alzheimer's disease. Neurobiol Aging 32:1161–1180
Ogawa O, Zhu X, Lee HG et al (2003) Ectopic localization of phosphorylated histone H3 in Alzheimer's disease: a mitotic catastrophe? Acta Neuropathol 105:524–528
Stern Y (2012) Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 11:1006–1012
Meng X, D'Arcy C (2012) Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 7:e38268
Whalley LJ, Deary IJ, Appleton CL, Starr JM (2004) Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev 3:369–382
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Xekardaki, A. et al. (2015). Neuropathological Changes in Aging Brain. In: Vlamos, P., Alexiou, A. (eds) GeNeDis 2014. Advances in Experimental Medicine and Biology, vol 821. Springer, Cham. https://doi.org/10.1007/978-3-319-08939-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-08939-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-08938-6
Online ISBN: 978-3-319-08939-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)