Abstract
Pain usually occurs as a result of tissue damage and has a role in healing and protection. However, in certain conditions it has no functional purpose and can become chronic and debilitating. A demand for more effective treatments to deal with this highly prevalent problem requires a better understanding of the underlying mechanisms. TRP channels are associated with numerous sensory functions across a wide range of species. Investigation into the expression patterns, electrophysiological properties and the effects of channel deletion in transgenic animal models have produced a great deal of evidence linking these channels to transduction of noxious stimuli as well as signalling within the pain system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Pain and Nociception
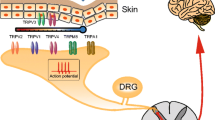
Pain is a complex phenomenon which has been described by the International Association for the Study of Pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (http://www.iasp-pain.org). The perception of pain is usually the result of tissue damage caused by a noxious stimulus and as such has a protective function as well as being important for allowing healing of damaged tissue. Specialised damage-sensing neurons that innervate the skin, muscle and viscera are activated by noxious stimuli, and this initial transduction of pain‐producing stimuli is known as nociception. Noxious stimuli can be mechanical, thermal or chemical, and they activate nociceptors, a type of sensory afferent neuron whose cell bodies lie in the dorsal and trigeminal root ganglia. The two main categories of nociceptor are Aδ and C fibres which are myelinated and unmyelinated, respectively. As a result of their myelination state and their larger diameter, Aδ fibres have a greater conduction velocity than C fibres and are responsible for the so-called first pain, a pinprick sensation which precedes the burning sensation, and the ‘second pain’ mediated by small-diameter unmyelinated C fibres. C fibres can be subdivided into peptidergic and non-peptidergic sets according to their expression profiles; peptidergic C fibres express the TrkA receptor and respond to nerve growth factor (NGF), while non‐peptidergic nociceptors bind the lectin IB4 and are sensitive to glial-derived neurotrophic factor (GDNF) acting through the c‐Ret receptor. Each subtype of sensory neurons expresses a different subset of transient receptor potential (TRP) channels which are linked to a variety of functions (Table 1).
Although pain usually arises from a stimulus, it can occur without noxious input or outlast the initial insult and become chronic. A large-scale survey estimated that approximately 19 % of people in Europe suffer from chronic pain (http://www.britishpainsociety.org/Pain%20in%20Europ%20survey%20report.pdf, British Pain Society 2003). The comorbidities associated with chronic pain are numerous and debilitating, including depression, anxiety and insomnia. It was also reported that 1 in 5 sufferers in Europe have lost, or had to leave, their job as a result of their pain. Chronic pain can occur as a result of either inflammatory or neuropathic processes.
Inflammation associated with sensitisation (reduced pain thresholds) is characterised by redness, swelling and tenderness. Following tissue damage peripheral leucocytes, Schwann cells and endothelia release proinflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and interleukin-1 (IL‐1), which leads to an increase in NGF production by macrophages. In turn, NGF forms part of a positive feedback loop whereby it binds to receptors such as TrkA on the peripheral terminals of nociceptors. As a consequence, tyrosine kinases phosphorylate TRPV1 which then is trafficked into the plasma membrane (Zhang et al. 2005a, b). NGF also activates mast cells which release mediators such as prostaglandins, bradykinin, ATP and serotonin (5‐HT), adding to the inflammatory milieu. This cycle of events via its effect on TRPV1 function leads to nociceptor sensitisation as well as increased excitability at central terminals resulting in symptoms such as thermal and mechanical hyperalgesia. When these proinflammatory effects persist, chronic pain can occur and is seen, for example, in conditions such as osteoarthritis where ongoing pain and increased sensitivity in the affected joints are highly detrimental to normal life.
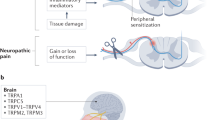
Neuropathic pain is the result of damage, disease or dysfunction within the nervous system. When damage occurs, several key events follow which can lead to spontaneous pain, hyperalgesia (enhanced sensitivity to a noxious stimulus) and allodynia (pain from a normally non‐noxious stimulus). For example, there is a loss of trophic support; usually, NGF and other growth factors such as GDNF are taken up by peripheral neurons and retrogradely transported to the cell somata. When peripheral neurons are damaged, the portion of the cell distal to the injury begins to degenerate, and consequently, the peripheral receptors can no longer respond appropriately to growth factor inputs. Further, the contents of the cell, including growth factors and neurotransmitters, are released into the surrounding area. As a result, not only is there aberrant activity and ectopic transduction in the damaged neuron itself, but there is also an increase in excitability of surrounding neurons. Peripheral nociceptors themselves also undergo an increase in excitability and response to a wider range of stimulus intensities. The barrage of peripheral activity which occurs following damage to the nervous system also leads to sensitisation centrally. It is important to note, however, that while animal models of neuropathic pain (Fig. 1) have elucidated many details of underlying mechanisms of pain consequent to nerve damage, they have also highlighted that each model can undergo different molecular and cellular changes, albeit with an apparently similar nociceptive phenotype (Koltzenburg and Scadding 2001).
Models of neuropathic pain. (1) SNL spinal nerve ligation; tight ligation of L5 and L6 spinal nerves. (2) PSNL partial sciatic nerve ligation; tight ligation of between one third and one half of the sciatic nerve. (3) SNT sciatic nerve transection; complete transection of sciatic nerve. (4) CCI chronic constriction injury; 3–4 loose ligatures around nerve. (5) SNI spared nerve injury; tibial and common peroneal branches of the sciatic nerve are transected distal to its trifurcation
Existing analgesics, centred around opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), are limited in their efficacy and are frequently associated with undesirable side effects and can induce dependence. The development of new, more effective analgesics therefore requires new potential targets and a better understanding of the underlying mechanisms of pain.
2 Heat
A number of TRP channels have been linked to noxious heat and cold transduction. When TRPV1 was first identified, it was named vanilloid receptor subtype 1 (VR1) because of its response to capsaicin, a component of capsicum peppers which is the active ingredient in spicy foods (Caterina et al. 1997). TRPV1 has repeatedly been shown to be activated by noxious thermal stimuli (temperatures >43 °C) and low pH (Caterina et al. 1997, 2000). Interestingly, however, while mice lacking the TRPV1 receptor lose sensitivity to capsaicin as well as low pH, they exhibit almost no loss of sensitivity to acute noxious heat (Davis et al. 2000; Caterina et al. 2000; Woodbury et al. 2004). In contrast, in models of inflammatory pain induced by carrageenan or complete Freund’s adjuvant (CFA), thermal hyperalgesia is lost (Caterina et al. 2000; Davis et al. 2000). Consistent with a deficit in inflammation‐induced thermal hyperalgesia in TRPV1 null mice, tissue damage leads to elevated TRPV1 expression in sensory neurons of the dorsal root ganglia (DRG) and in lamina I and II of the spinal cord (Amaya et al. 2003; Luo et al. 2004). Following these findings, Mishra et al. (2011) developed a conditional mouse model and found that selective ablation by diphtheria toxin of TRPV1‐expressing sensory neurons results in loss of both sensitivity to acute noxious heat and thermal hyperalgesia. Thus the population of TRPV1 expressing neurons is involved in heat sensing, and TRPV1 is principally involved in inflammatory pain.
As TRPV1 has no role in sensing acute noxious heat (Davis et al. 2000), other members of the TRPV family were considered potential candidates for this function. TRPV2, though insensitive to capsaicin, confers sensitivity to high temperatures >52 °C in Xenopus oocytes (Caterina et al. 1999), although the relevance of this finding to mammalian cells has not been demonstrated. TRPV3 and TRPV4 are activated at 33–39 °C and 25–34 °C, respectively, and are expressed in sensory neurons (TRPV3 is co‐expressed with TRPV1) and keratinocytes (Liedtke et al. 2000; Smith et al. 2002; Xu et al. 2002; Peier et al. 2002b; Chung et al. 2004). TRPV4 is sensitive to capsaicin, but TRPV3 is not (Smith et al. 2002), and instead it responds the phenylpropanoid eugenol (which is also detected by TRPV1 and TRPV3).
TRPV2 has been studied less intensively than TRPV1, and TRPV2 knockout mice have been available only since 2011 (Park et al. 2011). In these animals (which have reduced viability and survival into adulthood) no hyperalgesia develops after inflammation or spinal nerve ligation. Further evidence against an acute heat-sensing role for TRPV2 is provided by the fact that 82 % of heat-sensitive neurons in TRPV1 null mice do not express TRPV2 (Woodbury et al. 2004). Moreover, in TRPV1 neuron-depleted mice, TRPV2 expression was still found in spite of the complete loss of heat sensitivity (Mishra et al. 2011).
TRPV3 shows the unusual property of hysteresis. Current is progressively activated at temperatures above 28 °C, but if the preparation is cooled even slightly during the experiment, the current deactivates sharply. Moreover, the channel itself undergoes sensitisation after a train of stimuli, whereby the current is evoked more reliably after the temperature is stepped repeatedly from 21 °C to 45 °C (Liu et al. 2011; Peier et al. 2002b). TRPV3 also supports a secondary current, which develops after channel sensitisation, with increased amplitude, loss of rectification and altered permeability to cations (Chung et al. 2005). These observations plausibly account for some features of peripheral sensitisation; however since the expression of TRPV3 is higher in keratinocytes than in nervous tissue, some of the behavioural effects might be only indirectly neuronal. TRPV3 null mice show deficits in detection of noxious heat but not a complete loss of sensitivity, and mice develop thermal hyperalgesia induced by bradykinin and CFA in the same way as wild‐type controls (Moqrich et al. 2005).
TRPV4 is the mammalian homologue of the C. elegans osmosensor osm‐9. Osmolar and pH changes occur as a result of inflammation, motivating a study (Alessandri‐Haber et al. 2003) on single fibres of rat sensory nerve in hypotonic solution. This treatment activated a proportion of the fibres, an effect that was enhanced by application of the inflammatory mediator prostaglandin but was reduced by antisense to TRPV4. Knockout mice showed a reduced sensitivity to protons and altered thermal preference, whereas response to noxious heat was unimpaired, and thresholds of mechanical sensation were increased (Liedtke and Friedman 2003). The channel is sensitised by an agonist of protease-activated receptor 2/PAR2, which elicits mechanical hyperalgesia in mice but not in TRPV4 knockout animals. In rat spinal cord, PAR2 agonist stimulated release from the dorsal horn of the nociceptive neuropeptide, substance P, an effect presumed to depend on the expression of TRPV4, though this was not shown directly (Grant et al. 2007). In expression systems, TRPV4 is activated by heat, and the extent of activation is increased in hypotonic conditions (Güler et al. 2002) of the kind that might prevail during inflammation.
Since TRPV3 and TRPV4 have overlapping temperature response profiles, Huang et al. (2011) developed TRPV3/TRPV4 double knockout mice. They found no significant differences in the acute thermal responses or in various thermal preference paradigms between wild-type (WT) and TRPV3/4 null mice. Similarly, minimal differences were seen in null mice treated with a TRPV1 antagonist in both acute and inflammatory models of thermal pain suggesting this channel does not mask a role of TRPV3 and TRPV4 (Huang et al. 2011).
Inflammatory mediators (including bradykinin, ATP, cytokines) are released from endothelium and cells of the immune system in the neighbourhood of the nociceptor, and via receptors on the neuron surface, these factors can engage second messengers that act on TRP channels (Amadesi et al. 2006). Since TRPV1 null mice have deficits in thermal hyperalgesia, the role of TRPV1 in inflammatory pain states has been widely investigated. Pro‐inflammatory mediators sensitise TRPV1, leading to induction and maintenance of thermal hyperalgesia, via protein kinase A (PKA) and PKA-mediated phosphorylation of the C‐terminal. PKA accomplishes this in a process mediated by PKA-anchoring proteins (AKAP) leading to enhanced gating and increased TRPV1 translocation to the membrane; for example, inhibition of the AKAP79/150 protein prevents sensitisation of TRPV1 by bradykinin and PGE2 (Zhang et al. 2008). Protein kinase C (PKC) is able to reduce the activation threshold via phosphorylation of TRPV1 leading to enhanced gating and potentiation of channel activity (Premkumar and Ahern 2000; Vellani et al. 2001) and is upregulated in DRG during inflammation (Zhou et al. 2003).
PGE2 and PGI2, released following tissue damage, sensitise TRPV1 via PKC- and PKA-dependent mechanisms and are able to reduce the thermal threshold of TRPV1 to ~35 °C (Moriyama et al. 2005). By a similar mechanism of sensitisation, injection of the chemokine CCL3 in mice-induced thermal hyperalgesia (Zhang et al. 2005a, b), while treating cultured trigeminal ganglia (TG) neurons with 5‐HT, also potentiates TRPV1 activity (Loyd et al. 2011). Bradykinin-mediated thermal hyperalgesia can be inhibited by administration of the TRPV1 antagonist, capsazepine, as well as by blocking activity of phospholipase C (PLC), PKC and PKA (Ferreira et al. 2004). Interestingly, TRPA1 null mice, and neurons in culture, also show attenuated sensitivity to bradykinin in a similar way to TRPV1 null TG cultures and animal models (Bautista et al. 2006). TRPV1 and TRPV4 are both sensitised by ATP, a known mediator of inflammation. ATP reduces the sensitivity of TRPV3 whereas it has no effects on TRPV2 (Phelps et al. 2010). TRPV3 activation in keratinocytes, by agonists or heat, leads to release of PGE2 and appears to cause increased sensitivity to acute noxious heat and thermal hyperalgesia in a TRPV1‐independent manner (Huang et al. 2008). Recruitment of ‘silent’ afferents which previously did not respond is a feature of sensitisation evoked by inflammation; there appears to be a single report on the role of TRP channels in this process, which concluded, on the basis of work in null mutant mice, that TRPV1 is not involved (Koerber et al. 2010). NGF can increase TRPV1 expression by upregulating its transcription and also via PKC-mediated phosphorylation of the channel (Ji et al. 2002); PI3K can also potentiate its activity and induce thermal hyperalgesia (Zhuang et al. 2004), and this requires tyrosine phosphorylation by Src family kinases (Zhang et al. 2005a, b). When retrogradely transported to the DRG, NGF activates the MAP kinase p38 which increases translation and transport of TRPV1 to the periphery where it contributes to maintenance of thermal hyperalgesia in inflammatory and neuropathic pain states (Ji et al. 2002).
TRPV1 expression is downregulated in damaged neurons following partial or total spinal nerve ligation (SNL) and in sciatic nerve transection (SNT), models of neuropathic pain (Hudson et al. 2001; Fukuoka et al. 2002). It is also upregulated in surrounding, undamaged neurons, for example, in L4 neurons following SNL of L5 (Fukuoka et al. 2002). This upregulation correlates with the thermal hypersensitivity profile observed after SNL (Fukuoka et al. 2002). Thermal nocifensive behaviour is reduced by block of NGF following chronic constriction injury (CCI) (Wilson‐Gerwing et al. 2005), an effect which might depend on reduced levels of TRPV1. TRPV1 expression is also altered in human painful neuropathies; a loss of TRPV1-positive neurons in peripheral and sural nerves and in the skin was observed in diabetic and motor neuropathies (Lauria et al. 2006; Facer et al. 2007). An increase in TRPV1 and TRPV3 expression is seen in intact nerves after injury while TRPV4 appears to be unaltered (Facer et al. 2007).
3 Cold
Recently cell ablation studies have highlighted TRPM8-positive neurons as the key cell types involved in noxious cold perception in mice (Pogorzala et al. 2013). The threshold for noxious cold, as distinct from innocuous cool, is considered to occur at temperatures <15 °C. TRPM8 is sensitive to menthol (a cooling compound) and cold, with a threshold of ~25 °C, and is activated by temperatures encompassing the innocuous cool and noxious cold range (McKemy et al. 2002; Peier et al. 2002a). TRPM8 is expressed in a subset of capsaicin-insensitive, small-diameter neurons in both dorsal root and trigeminal ganglia, though at higher levels in the latter (7.4 % vs. 14.8 %), consistent with greater cold sensitivity in structures of the face and head (McKemy et al. 2002). Since TRPV1 expression is often considered a marker for nociceptors, this would suggest that TRPM8 is not involved in detection of noxious stimuli. Indeed, the use of TRPM8 null mice has firmly established a role for the channel in detection of innocuous cold though its role in detection of noxious stimuli is less conclusive. Knowlton et al. (2013) suggested that the cold-plate technique of measuring cold hypersensitivity, used in many of these investigations, was highly variable across earlier studies. Two groups found there was no difference in the nocifensive responses of TRPM8 null mice when compared to WT in a noxious cold-plate test (Bautista et al. 2007; Dhaka et al. 2007). However these same two groups showed a significant decrease in the response of these mice to application of the noxious cooling chemical acetone on the hind paw. Colburn et al. (2007) found a deficit in cold sensitivity in both of these assays, but this deficit was not reversed upon administration of TRPM8 antisense oligonucleotides.
It has emerged more recently that TRPM8 is upregulated in experimental bowel inflammation and that the TRPM8 agonist icilin, which attenuates chemically induced colitis in normal mice, presumably because the channel mediates a local cooling, brings no relief in the knockout animals (Ramachandran et al. 2013). In other circumstances, however, TRPM8 agonists lead to increases of core temperature, an effect that suggests coupling of central channels to the homeostatic mechanisms, whereas a TRPM8 blocker induces hypothermia (Ma et al. 2012; Knowlton et al. 2011). Once again, this example illustrates the apparently paradoxical effect of channels on somatosensation, when they are activated in different tissues by different experimental paradigms. Because TRPV1 is expressed in TRPM8-positive neurons during development but subsequently downregulated, conditional knockout of TRPV1 in sensory neurons also caused ablation of TRPM8. These animals showed significant deficits in responses to acute noxious cold (Mishra et al. 2011).
TRPA1 was the first candidate cold-sensing TRP channel. This channel is expressed in a subset of capsaicin-sensitive, calcitonin gene-related peptide (CGRP) positive neurons and has an activation threshold of 17 °C (Story et al. 2003) which is close to the cold temperature considered to be painful. Interestingly these neurons do not appear to co‐express TRPM8 (Story et al. 2003).
TRPA1 is also activated by chemicals such as mustard oil (allyl isothiocyanate) which, when applied to the skin, elicits a burning or pricking sensation and causes aversive behaviour which is lost in TRPA1 null mice (Jordt et al. 2004; Bandell et al. 2004; Bautista et al. 2006; Kwan et al. 2006). Jordt et al. (2004) found that the majority (96 %) of cultured trigeminal neurons from rat which were sensitive to the mustard oil component allyl isothiocyanate (AITC) were insensitive to cold and that the remaining 4 % were sensitive to menthol, suggesting a role for TRPM8 in their cold sensitivity (Jordt et al. 2004). Also, when human embryonic kidney (HEK293) cells were transfected with TRPA1, although a response to mustard oil (AITC) was clearly observed, no responses to cold temperature of 5 °C were seen. Furthermore, it was shown that 5 % of the cold-sensitive neurons in culture were insensitive to both menthol and AITC, something corroborated by Babes et al. (2004), indicating another mechanism for noxious cold sensitivity, independent of TRPA1 and TRPM8.
Kwan et al. (2006) reported that mice lacking the TRPA1 channel showed decreased responsiveness to noxious cold temperatures on a cold plate and reduced sensitivity to acetone application when compared with WT mice. Using the same behavioural assays, Bautista et al. (2006) observed no such difference. Furthermore, Bautista et al. (2006) cultured WT and TRPA1 null trigeminal neurons and found there was no difference in the magnitude of current in response to application of a noxious cold stimulus. It has been proposed that this discrepancy may be the result of different techniques and experimental setup (Kwan et al. found a greater deficit in the response of female mice while Bautista et al. only used males). Also, when considering the conflicting evidence provided by these models, the design of the knockout model must be considered. For example, some knockout constructs involve deletion of an entire gene while others may merely result in insertion of a cassette or premature stop codon. The latter may lead to generation of a truncated form of the protein. As a result, two knockout models of the same gene may vary substantially in their expression of that gene which may potentially impact upon the phenotype exhibited by each model. Indeed, TRP channels are known to heteromultimerise, and since the TRPA1 ‘null’ mice used by both aforementioned groups are believed to express a truncated form of the channel, it has been suggested that this truncated form may exert an effect on other implicated channels, thus affecting the phenotype of the null mice (Foulkes and Wood 2007).
The difficulty in clarifying the role of TRPA1 in noxious cold detection led to investigations of its underlying properties. The release of Ca2+ from intracellular stores activates TRPA1 by a PLC-dependent mechanism, while the presence of extracellular Ca2+ is able to augment the response of TRPA1 to agonists such as AITC (Jordt et al. 2004). Zurborg et al. (2007) investigated this further and found that an EF‐hand domain within the channel subunit is required for intracellular Ca2+-mediated activation of TRPA1. They demonstrated that cells expressing TRPA1 showed responses to cold; however, in EF‐hand domain mutants, responses to cold were not abolished but rather were reduced to levels of control cells which did not express TRPA1. They reasoned, therefore, that TRPA1 is activated by an increase in intracellular Ca2+ seen upon cooling rather than being directly activated by cold thus providing a potential explanation for the inconsistencies seen in vitro and in vivo. Subsequently, however, Karashima et al. (2009) used heterologous expression studies to argue that TRPA1 could be activated by cold in the absence of both intra‐ and extracellular Ca2+.
Cold hyperalgesia is a symptom of diseases such as rheumatoid arthritis (Jahanshaki et al. 1989), and cold allodynia is a common feature of many neuropathic pain states including those caused by traumatic nerve injury and post-herpetic neuralgia (Jørum et al. 2003). Since TRPM8 and TRPA1 have both been implicated in sensitivity to innocuous and noxious cold, their roles in these phenomena have been investigated. Caspani et al. (2009) reported nociceptive behaviour in response to menthol, a TRPM8 agonist, in mice following CCI surgery, suggesting a normally cool stimulus mediating a noxious effect via TRPM8 in a neuropathic pain model. Similarly, Colburn et al. (2007) found that acetone application resulted in a reduced response in TRPM8 null mice after CCI surgery and CFA injection when compared to WT; an even greater allodynic phenotype was seen after CCI in mice with ablation of TRPM8 expressing sensory neurons (Knowlton et al. 2013). In spite of this, reports of changes in TRPM8 expression after CCI surgery remain contentious (Proudfoot et al. 2006; Caspani et al. 2009), and no changes in expression following CFA-induced inflammation were seen in one report (Obata et al. 2005). No upregulation of TRPM8 mRNA or protein was seen following SNL, and administration of antisense oligonucleotides to TRPM8 had no effect on SNL-induced cold hyperalgesia, although antisense oligonucleotide block of TRPA1 was able to attenuate this behaviour (Obata et al. 2005; Katsura et al. 2006; Stucky et al. 2009). Indeed, though TRPA1 expression is decreased in rats and mice after CCI (Caspani et al. 2009) and in injured L5 nerves in SNL, it is upregulated in intact L4 nerves and DRG in this model (Obata et al. 2005; Katsura et al. 2006). Cold allodynia resulting from spared nerve injury (SNI) surgery and CFA injection in rats was diminished by administration of TRPA1 antagonists (Stucky et al. 2009; del Camino et al. 2010). TRPA1 null mice exhibited reduced nocifensive responses to formalin, a chemical inducer of inflammation (Macpherson et al. 2007; McNamara et al. 2007; Stucky et al. 2009) and TG cultures from null mice show attenuated responses to bradykinin (Bautista et al. 2006).
Using HEK293 and cultured neurons as well as behavioural models, del Camino et al. (2010) found that even noxious cold temperatures were, alone, unable to evoke significant channel activity but that in the presence of an agonist, such as AITC, even low levels of innocuous cool evoked large currents and nocifensive responses. Moreover, the responses to noxious cold plate were comparable in WT and TRPA1 null mice (del Camino et al. 2010) suggesting that TRPA1 may play a role in mediating cold sensitivity only in pathological conditions, such as those mimicked in animal models of inflammatory and neuropathic pain.
4 Mechanical
The role of TRP channels in mechanosensation has been summarised in a recent review (Eijkelkamp et al. 2013). Very interestingly, TRPV1 global and conditional knockout mice show normal responses to mechanical stimulation even in models of inflammatory pain. A CCI model of neuropathic pain in rats, however, showed ipsilateral upregulation of TRPV1 protein in lamina I and II of the spinal cord and mechanical allodynia behaviour which was attenuated by a specific TRPV1 antagonist, BCTC (Kanai et al. 2005). TRPV3 null mice show no deficits in mechanical responses nor any differences in mechanical hyperalgesia induced by CFA or bradykinin compared to WTs (Moqrich et al. 2005). Though it does not respond to stretching of the membrane in vitro, and thus may not be gated by mechanical stimulation (Strotmann et al. 2000), TRPV4 does appear to play a role in mechanical hyperalgesia in inflammatory and neuropathic pain. In a variety of rat models of neuropathic pain, including diabetic and peripheral neuropathies, the reduction in mechanical threshold was reversed by intrathecal administration of TRPV4 antisense oligonucleotides while TRPV4 null mice did not exhibit mechanical hypersensitivity to the same extent as WTs (Alessandri‐Haber et al. 2008). Interestingly, however, the expression of TRPV4 does not appear to be upregulated in these rat models of neuropathic pain. TRPV4 is frequently co-expressed with TRPC1 and TRPC6 in DRG, and it has been proposed that the channels may act in concert to mediate mechanical hyperalgesia in sensitised nociceptive neurons. Induction of mechanical hyperalgesia in inflammatory and neuropathic pain models was reversed by administration of antisense oligonucleotides to TRPC6 and, in certain models, TRPC1 (Alessandri‐Haber et al. 2009). In contrast with this it has recently been shown that TRPC3 and TRPC6 double knockout mice do not have deficits in sensitivity to noxious mechanical pressure (Quick et al. 2012).
Drosophila larvae lacking expression of the TRPA1 homologue, painless, are insensitive to noxious mechanical stimuli (Tracey et al. 2003); similar to its role in noxious cold, however, the role of TRPA1 in transduction of noxious mechanical stimuli is in debate. Kwan et al. (2006) found a deficit in response to repeated application of ‘high force’ innocuous and noxious mechanical stimulation in TRPA1 null mice though Bautista et al. (2006) focus on their finding that there is no variation in the mechanical thresholds of these mice. A loss of slowly and intermediate adapting currents in small-diameter, non-peptidergic fibres was seen in DRG neurons taken from TRPA1 null mice (Vilceanu and Stucky 2010; Brierley et al. 2011); it had previously been suggested that slowly adapting currents from such neurons are associated with noxious mechanosensation (Drew et al. 2007). Mustard oil-induced mechanical hyperalgesia was inhibited by TRPA1 antisense oligonucleotides and TRPA1 antagonists (Perin‐Martins et al. 2013), while CFA- and SNL-induced mechanical hyperalgesia is attenuated by TRPA1 antagonists (Petrus et al. 2007; Eid et al. 2008) but not by antisense oligonucleotides (Obata et al. 2005). On the basis of transfection of HEK293 cells with TRPA1, however, it seemed that the channel alone is not sufficient to confer mechanical sensitivity (Vilceanu and Stucky 2010); hence, it is possible that TRPA1 does not contribute to acute noxious mechanosensitivity but rather to the maintenance of mechanical hyperalgesia (Petrus et al. 2007). Alternatively, Brierley et al. (2011) suggest that TRPA1 confers mechanical sensitivity to a specific set of small-diameter fibres and that there are other DRG neurons which do not require the channel to confer mechanosensitivity.
A point mutation (N855S) in the S4 transmembrane segment of TRPA1 causes an autosomal dominant heritable pain condition known as familial episodic pain syndrome (FEPS) (Kremeyer et al. 2010). This mutation leads to greater inward currents following channel activation at resting neuronal membrane potentials and manifests as crippling upper body pain which begins in infancy and consists of attacks with a duration of ~1.5 h. Interestingly, the attacks are described as involving a sensation of mechanical pain which is initiated by a number of factors including cold.
5 Central Pain Pathways
The architecture of the central pain-processing pathway is generally agreed (Ossipov et al. 2010; Perl 2011), although refinements of the wiring have been proposed to account for the range of pain sensations evoked by different stimuli (Craig 2003). The primary afferent nociceptor terminates centrally on relay neurons and interneurons of the dorsal horn in the spinal cord, mostly in laminae I and II. Most of these terminals are glutamatergic, but some are peptidergic. Relay neurons within the cord project to spinothalamic neurons, which course through the brainstem and midbrain, and synapse onto different nuclei of the thalamus (Fig. 2). Lateral branches of the ascending pathway also terminate within brainstem structures, the periaqueductal grey (PAG) and the rostroventral medulla (RVM). Thalamic neurons are wired to cortical regions mediating sensory and emotive aspects of pain, respectively, the somatosensory cortex and anterior cingulate cortex. A spinoparabrachial pathway travels from the spinal cord to limbic structures. Descending pathways (Fig. 2) originating in the cortex and amygdala, and modulated by outputs of the PAG, medial RVM and locus ceruleus, affect the activity of the dorsal horn neurons via neurotransmitters including GABA and serotonin, which can antagonise or facilitate the sensation of pain, in some cases leading to the generation of efferent signals along the nociceptor into the periphery.
TRP channels of the A, C, V and M subfamilies are detectable in the spinal cord and brain. Topical and systemic application of capsaicin dominates the study of central TRP channels. TRPV1 may occur postsynaptically in lamina II of the dorsal horn, especially in lumbar segments, in glia as well as in neurons, and co‐localises with substance P (Spicarová and Palecek 2008). TRPV1 activity in the cord causes release of substance P, ultimately antagonising peripheral inputs by activating interneurons (Ferrini et al. 2007). The inflammatory mediator bradykinin is released from endothelia and glia following injury (Hausmann 2003) and mediates pain via sensitisation of TRPA1 co-localised with B2R receptors on DRG neurons (Wang et al. 2008), but there is no report of a similar co‐expression in central neurons.
TRPV1 shows robust in vitro response to inflammatory mediators, but the special characteristics of the central nervous system (CNS) immune response—generally weaker, involving a different repertoire of cell types and excluding many serum proteins by reason of the blood–spinal cord and blood–brain barriers—imply that the signalling pathways will differ from those in the periphery (Hausmann 2003). Nonetheless, microglial activation has been correlated with peripheral pain states including those caused by peripheral nerve trauma and bone cancer pain (Watkins et al. 2001a, b; Xu et al. 2006), and TRPV1 activity leads to microglial cell death (Kim et al. 2006), suggesting a coupling of central pain and inflammatory pathways. In the same way, spinal cord trauma leads to release of factors, some of which are not found after peripheral nerve damage, to mediate oxidative stress, inflammatory, energetic, apoptotic and lipid metabolic change (Kuner 2010; Yip and Malaspina 2012). It is not clear which if any of these factors subserve nociceptive functions by acting on TRP channels or in which direction the effect occurs. TRPV1 may stand upstream of some inflammatory responses, as suggested by the finding that systemic capsaicin upregulated B1R expression on spinal cord microglia (Talbot et al. 2012). The secondary injury to the blood capillaries that follows spinal cord trauma is preceded by upregulation of the TRPM4 channel on the vessels (Gerzanich et al. 2009). Capillary fragmentation does not necessarily contribute to pain sensation, though it impairs neurological function. This channel overexpressed in HEK293 cells shows a graduated response to temperatures between 15 °C and 35 °C (Talavera et al. 2005) but must respond to other, unidentified outputs from injury in vivo to mediate the reported effects on haemorrhage. TRPV1 is implicated in the central response to a plantar injection of Freund’s adjuvant; within substantia gelatinosa of spinal cord isolated after CFA treatment, synaptic transmission is inhibited by a TRPV1-selective antagonist, as though the channel had become activated by the inflammatory stimulus (Lappin et al. 2006). Intrathecal application of TRV1 antagonists alleviates the response to paw injection of formalin (Kanai et al. 2006), supporting the same conclusion. Oral administration of TRPV1 antagonists with different degrees of CNS permeance shows that central TRP channels contribute to pain responses (Cui et al. 2006). Intrathecal application of the TRPV1 agonist RTX produces analgesia, attributable to selective ablation of TRPV1‐expressing central nerve terminals (Jeffry et al. 2009).
TRPV1 channels at the central terminals of nociceptors are exposed to CNS modulators of activity. The endogenous ligands of TRPV1 include 12‐HPETE (an arachidonic acid derivative), AEA (anandamide) and NADA (N‐arachidonoyl dopamine) (O’Neill et al. 2012). The endocannabinoid anandamide promotes calcium entry through TRPV1, via a mechanism that differs from that of capsaicin (Fischbach et al. 2007). The affinity of anandamide for the CB1 receptor is higher, and it is unclear what sequence of events would lead to effects on TRPV1 function in situ.
Neurotransmitters and peptides (e.g. substance P) with modulatory effect on the circuitry of relay neurons and interneurons are released by the central terminals of nociceptors. A family of lipid mediators, the resolvins, lowers transmission probability across spinal synapses via effects on TRPV1 and TRPA1 (Park et al. 2011). Activation of central TRPV1 channels by capsaicin is correlated with altered spinal plasticity as well as hyperalgesic behaviour. This altered plasticity is a likely component of central sensitisation, whereby innocuous inputs are interpreted as noxious (Willis 2009). Sensitisation of spinal—and perhaps supraspinal—responses is proposed to account for a sensation of pain that persists beyond the initial stimulus (Park et al. 1995: Sang et al. 1996). This pain might be ectopic (secondary hyperalgesia) or evoked in response to innocuous stimuli (allodynia). The injured afferent may additionally re‐route, projecting to a synapse on a different lumbar segment, to facilitate input from undamaged afferents (Campbell and Meyer 2006). The relevant biological mechanism leading to facilitation is likely to be nociceptor burst activity, repeatedly stimulating the central synapse, following peripheral injury (Campbell and Meyer 2006); this proposed peripheral origin does not exclude the action of mediators within the cord sensitising TRP channels. Following synaptic plasticity, upregulation of cyclooxygenase (COX)‐2 and other neuronal signalling pathways leads to ongoing pain beyond the initial stimulus (Rivat et al. 2010). Distinct features of central sensitisation include secondary hyperalgesia and mechanical allodynia, whereby the abnormally enhanced response occurs in undamaged tissue. The TRPV1 agonist gingerol can relieve secondary hyperalgesia after central application in rat model of spinal nerve injury (Gauthier et al. 2012).
The reactive metabolite methylglyoxal (MG) occurs in all cell types as a by-product of glycolysis and lipid peroxidation and might be an endogenous ligand of the TRPA1 channel. MG is normally detoxified by a glyoxalase pathway, but deficiency of this route is suggested by the high MG plasma levels in diabetes mellitus patients. The effect of MG on depressing the compound action potential of the sciatic nerve preparation is abolished in TRPA1 null mice (Eberhardt et al. 2012). While diabetes patients show reduced conduction velocity in the peroneal nerve (Hyllienmark et al. 2013), and patients with diabetic neuropathy can experience an increase in sensitivity to mechanical and thermal stimuli, the relevance of the MG observations to pathology is currently unclear.
Pain‐associated plastic changes are best documented in spinothalamic tract neurons of the spinal cord (Willis 2002), but thalamic and cortical plasticity also is believed to contribute to sensitisation (Fu et al. 2008). Although potentiation of spinal transmission can be demonstrated, this is not conclusively a cellular analogue of pain sensation. Nonetheless, the data of Hjornevik et al. (2010) suggest that high-frequency stimulation of the spine produces both LTP and alterations in opioid receptor activity in higher brain areas.
Supraspinal involvement of TRP channels in pain are harder to demonstrate. Nonetheless, a coherent body of work with channel agonists and antagonists, applied centrally or peripherally, suggests a role for TRPV1 in descending modulation. TRPV1 mRNA occurs on cell bodies and synapses in brainstem structures involved in pain, namely the PAG and the RVM. Binding sites for the TRPV1 radioligand [3H]RTX occur in these locations as well as in the cortex, thalamus, hypothalamus, cerebellar cortex, locus ceruleus and spinal cord (Roberts et al. 2004) and the somatosensory cortex, anterior cingulate and amygdala (Steenland et al. 2006). Radioligand affinity for its target varies between structures (Szabo et al. 2002), which implies that accessory factors may be required for receptor availability. LTP in the amygdala is reported to be TRPV1 dependent (Zschenderlein et al. 2011). On the other hand, a recent publication on a TRPV1 reporter mouse claims expression restricted to the hippocampus, PAG, hypothalamus and midbrain only (Cavanaugh et al. 2011).
Accumulating data also indicate a role for TRPC channels in synaptic plasticity. LTP elicited at hippocampal interneuron synapses can be blocked by a nonspecific TRP channel inhibitor, and accompanying evidence on the calcium dependence of these channels implicates the TRPC family (Topolnik et al. 2006). RNAi experiments in hippocampal slice culture have shown that TRPC3 is required for dendritic spine formation in the presence of brain-derived neurotrophic factor (BDNF) (Amaral and Pozzo-Miller 2007). The influx of sodium and calcium ions through TRPC3 is proposed to stand upstream of NMDA receptor activation, leading to enhanced synaptic transmission in the form of LTP (Minichiello 2009). In hippocampal neuron culture, TRPC5 and TRPC6 activity mediates the phosphorylation of Akt by BDNF; this may have consequences for synaptic plasticity, since both Akt phosphorylation and LTP induction protocols can lead to the transient insertion of calcium‐permeable AMPA receptors in the neuronal membrane (Fortin et al. 2012). Finally, the TRPC5 knockout mouse, which resists pilocarpine induction of seizures, is defective in LTP induction (Phelan et al. 2013), suggesting that plasticity and regulated excitability might be coupled outputs of TRP‐mediated calcium homeostasis.
TRPM, the melastatin family of TRP channels, currently includes eight members. TRPM3 is highly expressed in the kidney, brain, spinal cord and testis and in some experimental preparations works as an osmosensor (Grimm et al. 2003). This function would be consistent with a role in kidney physiology. TPRM3 in beta cells is moreover activated by pregnenolone sulphate (Wagner et al. 2008), a cholesterol derivative synthesised in the adrenal and the brain, and standing upstream of the glucocorticoids, mineralocorticoids and sex steroids. Pregnenolone sulphate (PS) was proposed as a pain mediator in the same year that TRPM3 was cloned, on quite independent grounds related to expression of enzymes in the spinal cord (Patte-Mensah et al. 2003). The association of the channel and the steroid motivated a study that showed heat activation of TRPM3 expressed in HEK cells and the potentiation of this effect by low doses of PS. In the tail immersion test, TRPM3 -/- mice tolerated temperatures up to 57 °C to a greater extent than wild-type mice. A different behavioural response to PS was not reported by this study (Vriens et al. 2011), and so the relevance of the steroid as an endogenous ligand for TRPM3-mediated nociception remains to be demonstrated.
The PAG is connected monosynaptically to the RVM, in which the activity of a population of pain modulatory OFF cells is correlated with analgesia. Injection of TRPV1 agonists into the PAG enhances the excitability of the OFF cells and suppresses thermoception in the plantar test (Maione et al. 2006). This is discrepant with the effects of capsaicin in the periphery. Consistent with this report, infusions of a TRPV1 antagonist into the PAG enhance the response of rats to thermal stimulation of the paw (Starowicz et al. 2007). The activation of TRP channels in the PAG plausibly modulates pain responses, since RVM neurons project to the dorsal horn, synapsing with the primary nociceptive afferents as well as with the relay and interneurons. The antinociceptive projections are GABAergic and glycinergic, but it is unclear that they originate from the OFF cells (Ossipov et al. 2010). The effect of capsaicin on excitation in the ventrolateral PAG depends on glutamate receptors (Palazzo et al. 2002). McGaraughty et al. (2003) recorded neuronal responses to infused capsaicin simultaneously with tail‐flick latencies at 52 °C. They reported that the OFF neurons in dorsal PAG are active during analgesia only after some two hours of ON neuronal activity and pain‐avoiding behaviour and suggested that pain reduction occurred only after desensitisation of TRPV1 by capsaicin.
Capsaicin microinjected into the ACC increases the activity of selected neurons, while repressing others (Steenland et al. 2006). The correlation of this TRPV1‐mediated neuronal activity with behaviourally relevant stimuli is unknown, but since ACC is connected to the PAG, a descending effect on nociception is presumed.
Systemic injection of capsaicin increases excitability within the locus ceruleus, a structure that responds to noxious stimuli (Hajós et al. 1987), is reciprocally connected with the RVM and sends a noradrenergic descending projection to the dorsal horn (Ossipov et al. 2010). The noradrenergic output can be suspended after peripheral nerve injury (Rahman et al. 2008), and this presumably facilitates pain sensation, but the involvement of TRP channels in this process is not known.
6 Analgesics
The roles of TRP channels in mediating sensitivity to noxious stimuli make them attractive targets for analgesics. Most drug development studies have focussed on TRPV1 and TRPA1, which are clearly linked to aspects of peripheral nociceptor function. For example, Honore et al. (2000) found repeated dosing of two distinct TRPV1 antagonists was able to abolish spontaneous pain and thermal and mechanical behaviour responses in animal models of inflammatory and neuropathic pain. Capsaicin causes sensitisation of TRPV1; however, application of high-dose patches of ~8 % to the skin can be used to cause desensitisation and is used as a treatment for patients suffering with neuropathic pain. A review of six studies involving 2,073 participants with post-herpetic neuralgia or HIV neuropathy found these high-concentration capsaicin patches were effective at inducing pain relief (Derry et al. 2013). Pain ratings of sufferers of chronic lower back pain, a common and notoriously difficult to treat condition, are reduced by application of a capsicum plaster (Frerick et al. 2003). The minimal side effects resulting from this topically applied TRPV1-mediated analgesic provide further support for the potential role of TRP channels in the development of improved analgesics. Low doses of icilin and menthol, both TRPM8 agonists, are able to reduce mechanical and thermal hypersensitivity caused by peripheral nerve injury (Proudfoot et al. 2006). Antagonism of TRPV1 and TRPA1 during the acute phase of pancreatitis reduced pain behaviour and inflammation as well as preventing progression of the pathological changes seen in chronic pancreatitis though these treatments were unable to reverse pain behaviours in models of established chronic pancreatitis (Schwartz et al. 2013). TRPA1 has been shown to play a role in hyperalgesia, and the antagonist HC‐030031 attenuates inflammatory‐ and neuropathy‐induced mechanical hypersensitivity (Eid et al. 2008).
In spite of the evidence that TRP channels are involved in sensing noxious stimuli, their potential as analgesic drug targets has not been fulfilled in a substantial way. The widespread expression of TRP channels, together with their ability to heteromultimerise increases the likelihood that such drugs may also induce a number of off‐target effects.
References
Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD (2003) Hypotonicity induces TRPV4‐mediated nociception in rat. Neuron 39:497–511
Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD (2008) Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 28:1046–1057
Alessandri-Haber N, Dina OA, Chen X, Levine JD (2009) TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci 29:6217–6228
Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas‐Lopez C, Vanner S, Vergnolle N, Bunnett NW (2006) Protease‐activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilonand A‐dependent mechanisms in rats and mice. J Physiol 575:555–571
Amaral MD, Pozzo-Miller L (2007) TRPC3 channels are necessary for brain‐derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J Neurosci 27:5179–5189
Amaya F, Oh‐hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M (2003) Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res 963:190–196
Babes A, Zorzon D, Reid G (2004) Two populations of cold‐sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci 20:2276–2282
Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41:849–857
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124:1269–1282
Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–208
Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA (2011) TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol 589:3575–3593
British Pain Society (2003) Pain in Europe Survey, Janet Fricker for Mundipharma International Ltd. http://www.britishpainsociety.org/Pain%20in%20Europ%20survey%20report.pdf
Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52:77–92
Caspani O, Zurborg S, Labuz D, Heppenstall PA (2009) The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One 4:e7383
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat‐activated ion channel in the pain pathway. Nature 389:816–824
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999) A capsaicin‐receptor homologue with a high threshold for noxious heat. Nature 398:436–441
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–13
Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI (2011) Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31:5067–5077
Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ (2004) TRPV3 and TRPV4 mediate warmth‐evoked currents in primary mouse keratinocytes. J Biol Chem 279:21569–21575
Chung MK, Güler AD, Caterina MJ (2005) Biphasic currents evoked by chemical or thermal activation of the heat‐gated ion channel, TRPV3. J Biol Chem 280:15928–15941
Colburn RW, Lubin ML, Stone DJ, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54:379–386
Craig AD (2003) Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26:1–30
Cui M, Honore P, Zhong C, Gauvin D, Mikusa J, Hernandez G, Chandran P, Gomtsyan A, Brown B, Bayburt EK, Marsh K, Bianchi B, McDonald H, Niforatos W, Neelands TR, Moreland RB, Decker MW, Lee CH, Sullivan JP, Faltynek CR (2006) TRPV1 receptors in the CNS play a key role in broad‐spectrum analgesia of TRPV1 antagonists. J Neurosci 26:9385–9393
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA (2000) Vanilloid receptor‐1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–187
del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM (2010) TRPA1 contributes to cold hypersensitivity. J Neurosci 30:15165–15174
Derry S, Sven-Rice A, Cole P, Tan T, Moore RA (2013) Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2, CD007393
Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A (2007) TRPM8 is required for cold sensation in mice. Neuron 54:371–378
Drew LJ, Rugiero F, Cesare P, Gale JE, Abrahamsen B, Bowden S, Heinzmann S, Robinson M, Brust A, Colless B, Lewis RJ, Wood JN (2007) High‐threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure‐evoked pain. PLoS One 2:e515
Eberhardt MJ, Filipovic MR, Leffler A, de la Roche J, Kistner K, Fischer MJ, Fleming T, Zimmermann K, Ivanovic-Burmazovic I, Nawroth PP, Bierhaus A, Reeh PW, Sauer SK (2012) Methylglyoxal activates nociceptors through transient receptor potential channel A1 (TRPA1): a possible mechanism of metabolic neuropathies. J Biol Chem 287:28291–28306
Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO (2008) HC‐030031, a TRPA1 selective antagonist, attenuates inflammatory‐ and neuropathy‐induced mechanical hypersensitivity. Mol Pain 4:48
Eijkelkamp N, Quick K, Wood JN (2013) Transient receptor potential channels and mechanosensation. AnnuRev Neurosci 36:519–546
Elg S, Marmigere F, Mattsson JP, Ernfors P (2007) Cellular subtype distribution and developmental regulation of TRPC channel members in mouse dorsal root ganglion. J Comp Neurol 503(1):35–46
Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, Sinisi M, Birch R, Anand P (2007) Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol 7:11
Ferreira J, da Silva GL, Calixto JB (2004) Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol 141:787–794
Ferrini F, Salio C, Vergnano AM, Merighi A (2007) Vanilloid receptor‐1 (TRPV1)‐dependent activation of inhibitory neurotransmission in spinal substantia gelatinosa neurons of mouse. Pain 129:195–209
Fischbach T, Greffrath W, Nawrath H, Treede RD (2007) Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive drg neurons of rats. J Neurophysiol 98:929–938
Fortin DA, Srivastava T, Dwarakanath D, Pierre P, Nygaard S, Derkach VA, Soderling TR (2012) Brain‐derived neurotrophic factor activation of CaM‐kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1‐containing calcium‐permeable AMPA receptors. J Neurosci 32:8127–8137
Foulkes T, Wood JN (2007) Mechanisms of cold pain. Channels (Austin) 1:154–160
Frerick H, Keitel W, Kuhn U, Schmidt S, Bredehorst A, Kuhlmann M (2003) Topical treatment of chronic low back pain with a capsicum plaster. Pain 106:59–64
Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V (2008) PKA and ERK, but not PKC, in the amygdala contribute to pain‐related synaptic plasticity and behavior. Mol Pain 4:26
Fukuoka T, Tokunaga A, Tachibana T, Dai Y, Yamanaka H, Noguchi K (2002) VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain 99:111–120
Gauthier ML, Beaudry F, Vachon P (2013) Intrathecal [6]-Gingerol administration alleviates peripherally induced neuropathic pain in male Sprague-Dawley Rats. Phytother Res 27(8):1251–1254
Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, Freichel M, Simard JM (2009) De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med 15:185–191
Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, Geppetti P, Bunnett NW (2007) Protease‐activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 578:715–733
Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278(24):21493–21501
Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M (2002) Heat-evoked activation of the ion channel, TRPV4. J Neurosci 22:6408–6414
Hajós M, Jancsó G, Engberg G (1987) Capsaicin‐induced excitation of locus coeruleus neurons. Acta Physiol Scand 129:415–420
Hausmann ON (2003) Post‐traumatic inflammation following spinal cord injury. Spinal Cord 41:369–378
Hjornevik T, Schoultz BW, Marton J, Gjerstad J, Drzezga A, Henriksen G, Willoch F (2010) Spinal long‐term potentiation is associated with reduced opioid neurotransmission in the rat brain. Clin Physiol Funct Imaging 30:285–293
Honore P, Chandran P, Hernandez G, Gauvin DM, Mikusa JP, Zhong C, Joshi SK, Ghilardi JR, Sevcik MA, Fryer RM, Segreti JA, Gomtsyan A, Lee C-H, Kort ME, Reilly RM, Surowy CS, Kym PR, Mantyh PW, Sullivan JP, Jarvis MF, Faltynek CR (2000) Repeated dosing of ABT-102, a potent and selective TRPV1 antagonist, enhances TRPV1-mediated analgesic activity in rodents, but attenuates antagonist‐induced hyperthermia. Pain 99:111–120
Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, Coulombe PA, Walker JM, Caterina MJ (2008) Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci 28:13727–13737
Huang SM, Li X, Yu Y, Wang J, Caterina MJ (2011) TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 7:37
Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J (2001) VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci 13:2105–2114
Hyllienmark L, Alstrand N, Jonsson B, Ludvigsson J, Cooray G, Wahlberg-Topp J (2013) Early electrophysiological abnormalities and clinical neuropathy: a prospective study in patients with type 1 diabetes. Diabetes Care doi:10.2337/dc12-2226
Jahanshaki M, Pitt P, Williams I (1989) Pain avoidance in rheumatoid arthritis. J Psychosomat Res 33:579–589
Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar LS (2009) Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One 4:e7021
Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ (2002) p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36:57–68
Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Jørum E, Warncke T, Stubhaug A (2003) Cold allodynia and hyperalgesia in neuropathic pain: the effect of N methyl-D-aspartate (NMDA) receptor antagonist ketamine-a double-blind, cross-over comparison with alfentanil and placebo. Pain 101:229–235
Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A (2005) Involvement of an increased spinal TRPV1 sensitization through its up‐regulation in mechanical allodynia of CCI rats. Neuropharmacology 49:977–984
Kanai Y, Hara T, Imai A (2006) Participation of the spinal TRPV1 receptors in formalin-evoked pain transduction: a study using a selective TRPV1 antagonist, iodo-resiniferatoxin. J Pharm Pharmacol 58:489–493
Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T (2009) TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A 106:1273–1278
Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K (2006) Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol 200:112–123
Kim SR, Kim SU, Oh U, Jin BK (2006) Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+‐mediated mitochondrial damage and cytochrome c release. J Immunol 177:4322–4329
Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD (2011) Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 6:e25894
Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD (2013) A sensory-labeled line for cold: TRPM8‐expressing sensory neurons define the cellular basis for cold, cold pain, and cooling mediated analgesia. J Neurosci 33:2837–2848
Koerber HR, McIlwrath SL, Lawson JJ, Malin SA, Anderson CE, Jankowski MP, Davis BM (2010) Cutaneous C-polymodal fibers lacking TRPV1 are sensitized to heat following inflammation, but fail to drive heat hyperalgesia in the absence of TPV1 containing C‐heat fibers. Mol Pain 6:58
Koltzenburg M, Scadding J (2001) Neuropathic pain. Curr Opin Neurol 14:641–647
Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, Villegas A, Acosta N, Pineda-Trujillo NG, Ramírez JD, Zea J, Burley MW, Bedoya G, Bennett DL, Wood JN, Ruiz-Linares A (2010) A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66:671–680
Kuner R (2010) Central mechanisms of pathological pain. Nat Med 16:1258–1266
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP (2006) TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 50:277–289
Lappin SC, Randall AD, Gunthorpe MJ, Morisset V (2006) TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur J Pharmacol 540:73–81
Lauria G, Morbin M, Lombardi R, Capobianco R, Camozzi F, Pareyson D, Manconi M, Geppetti P (2006) Expression of capsaicin receptor immunoreactivity in human peripheral nervous system and in painful neuropathies. J Peripher Nerv Syst 11:262–271
Liedtke W, Friedman JM (2003) Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A 100:13698–13703
Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Liu B, Yao J, Zhu MX, Qin F (2011) Hysteresis of gating underlines sensitization of TRPV3 channels. J Gen Physiol 138:509–520
Loyd DR, Weiss G, Henry MA, Hargreaves KM (2011) Serotonin increases the functional activity of capsaicinsensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain 152:2267–2276
Luo H, Cheng J, Han JS, Wan Y (2004) Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund's adjuvant in rats. Neuroreport 15:655–658
Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, Jin R, Ma L, Wang P, Zhu Z, Li L, Zhong J, Liu D, Nilius B (2012) Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol 4:88–96
Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A (2007) An ion channel essential for sensing chemical damage. J Neurosci 27:11412–11415
Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, Petrosino S, Guglielmotti V, Rossi F, Di Marzo V (2006) Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther 316:969–982
McGaraughty S, Chu KL, Bitner RS, Martino B, El Kouhen R, Han P, Nikkel AL, Burgard EC, Faltynek CR, Jarvis MF (2003) Capsaicin infused into the PAG affects rat tail flick responses to noxious heat and alters neuronal firing in the RVM. J Neurophysiol 90:2702–2710
McKemy DD, Neuhausser WM, Julius D (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416:52–58
McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (2007) TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A 104:13525–30
Minichiello L (2009) TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10:850–860
Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA (2011) TRPV1‐lineage neurons are required for thermal sensation. EMBO J 30:582–593
Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A (2005) Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307:1468–1472
Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M (2005) Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 1:3
O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH (2012) Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev 64:939–971
Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 115:2393–2401
Ossipov MH, Dussor GO, Porreca F (2010) Central modulation of pain. J Clin Invest 120:3779–3787
Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, Maione S (2002) Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol 439:69–75
Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ (1995) Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain 63:163–172
Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR (2011) Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci 31:18433–18438
Patte-Mensah C, Kappes V, Freund-Mercier MJ, Tsutsui K, Mensah-Nyagan AG (2003) Cellular distribution and bioactivity of the key steroidogenic enzyme, cytochrome P450side chain cleavage, in sensory neural pathways. J Neurochem 86:1233–1246
Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A (2002a) A TRP channel that senses cold stimuli and menthol. Cell 108:705–715
Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A (2002b) A heat‐sensitive TRP channel expressed in keratinocytes. Science 296:2046–2049
Perin-Martins A, Teixeira JM, Tambeli CH, Parada CA, Fischer L (2013) Mechanisms underlying transient receptor potential ankyrin 1 (TRPA1)-mediated hyperalgesia and edema. J Peripher Nerv Syst 18:62–74
Perl ER (2011) Pain mechanisms: a commentary on concepts and issues. Prog Neurobiol 94:20–38
Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A (2007) A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain 3:40
Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F (2013) Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol 83:429–438
Phelps CB, Wang RR, Choo SS, Gaudet R (2010) Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem 285:731–740
Pogorzala LA, Mishra SK, Hoon MA (2013) The cellular code for mammalian thermosensation. J Neurosci 33(13):5533–5541
Premkumar LS, Ahern GP (2000) Induction of vanilloid receptor channel activity by protein kinase C. Nature 408:985–990
Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R (2006) Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol 16:1591–1605
Quick K, Zhao J, Eijkelkamp N, Linley JE, Rugiero F, Cox JJ, Raouf R, Gringhuis M, Sexton JE, Abramowitz J, Taylor R, Forge A, Ashmore J, Kirkwood N, Kros CJ, Richardson GP, Freichel M, Flockerzi V, Birnbaumer L, Wood JN (2012) TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol 2:120068
Rahman W, D’Mello R, Dickenson AH (2008) Peripheral nerve injury-induced changes in spinal alpha(2)-adrenoceptor-mediated modulation of mechanically evoked dorsal horn neuronal responses. J Pain 9:350–359
Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, Ghosh S, McKemy DD, Vergnolle N, Beck PL, Altier C, Hollenberg MD (2013) TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A 110:7476–7481
Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ (2010) Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long‐lasting anxiety‐induced hyperalgesia. Pain 150:358–368
Roberts JC, Davis JB, Benham CD (2004) [3H]Resiniferatoxin autoradiography in the CNS of wild‐type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res 995:176–183
Sang CN, Gracely RH, Max MB, Bennett GJ (1996) Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories. Evidence for a central mechanism. Anesthesiology 85:491–496
Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF (2013) TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci 33:5603–5611
Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB (2002) TRPV3 is a temperature‐sensitive vanilloid receptor‐like protein. Nature 418:186–190
Spicarová D, Palecek J (2008) The role of spinal cord vanilloid (TRPV1) receptors in pain modulation. Physiol Res 57(Suppl 3):S69–S77
Starowicz K, Maione S, Cristino L, Palazzo E, Marabese I, Rossi F, de Novellis V, Di Marzo V (2007) Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J Neurosci 27:13739–13749
Steenland HW, Ko SW, Wu LJ, Zhuo M (2006) Hot receptors in the brain. Mol Pain 2:34
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP‐like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702
Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM (2009) Roles of transient receptor potential channels in pain. Brain Res Rev 60:2–23
Szabo T, Biro T, Gonzalez AF, Palkovits M, Blumberg PM (2002) Pharmacological characterization of vanilloid receptor located in the brain. Brain Res Mol Brain Res 98:51–57
Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y, Margolskee RF, Nilius B (2005) Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438:1022–1025
Talbot S, Dias JP, Lahjouji K, Bogo MR, Campos MM, Gaudreau P, Couture R (2012) Activation of TRPV1 by capsaicin induces functional kinin B(1) receptor in rat spinal cord microglia. J Neuroinflammation 9:16
Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC (2006) mGluR1/5 subtype‐specific calcium signalling and induction of long‐term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol 575(Pt 1):115–131
Tracey WD, Wilson RI, Laurent G, Benzer S (2003) painless, a Drosophila gene essential for nociception. Cell 113:261–273
Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA (2001) Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534:813–825
Vilceanu D, Stucky CL (2010) TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS One 5:e12177
Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, Benoit M, Xue F, Janssens A, Kerselaers S, Oberwinkler J, Vennekens R, Gudermann T, Nilius B, Voets T (2011) TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 70:482–494
Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, Düfer M, Lis A, Flockerzi V, Philipp SE, Oberwinkler J (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol Dec 10(12):1421–1430
Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K (2008) Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 131:1241–1251
Watkins LR, Milligan ED, Maier SF (2001a) Glial activation: a driving force for pathological pain. Trends Neurosci 24:450–455
Watkins LR, Milligan ED, Maier SF (2001b) Spinal cord glia: new players in pain. Pain 93:201–205
Willis WD (2002) Long‐term potentiation in spinothalamic neurons. Brain Res Brain Res Rev 40:202–214
Willis WD (2009) The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp Brain Res 196:5–11
Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VM (2005) Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci 25:758–67
Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM (2004) Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 24:6410–6415
Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE (2002) TRPV3 is a calcium‐permeable temperature‐sensitive cation channel. Nature 418:181–186
Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG (2006) The role of tumor necrosis factor‐alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain 123:306–321
Yip PK, Malaspina A (2012) Spinal cord trauma and the molecular point of no return. Mol Neurodegener 7:6
Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ (2005a) A proinflammatory chemokine, CCL3, sensitizes the heat‐ and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci U S A 102:4536–4541
Zhang X, Huang J, McNaughton PA (2005b) NGF rapidly increases membrane expression of. TRPV1 heat‐gated ion channels EMBO J 24(24):4211–4223
Zhang X, Li L, McNaughton PA (2008) Proinflammatory mediators modulate the heat‐activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59:450–461
Zhou Y, Li GD, Zhao ZQ (2003) State‐dependent phosphorylation of epsilon‐isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J Neurochem 85:571–580
Zhuang ZY, Xu H, Clapham DE, Ji RR (2004) Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 24:8300–8309
Zschenderlein C, Gebhardt C, von Bohlen Und Halbach O, Kulisch C, Albrecht D (2011) Capsaicin‐induced changes in LTP in the lateral amygdala are mediated by TRPV1. PLoS One 6:e16116
Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA (2007) Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10:277–279
Acknowledgments
We thank the Wellcome Trust and the Medical Research Council for generous support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sexton, J.E., Vernon, J., Wood, J.N. (2014). TRPs and Pain. In: Nilius, B., Flockerzi, V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, vol 223. Springer, Cham. https://doi.org/10.1007/978-3-319-05161-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-05161-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05160-4
Online ISBN: 978-3-319-05161-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)